Abstract
Background
The prognosis of individual dogs with meningoencephalomyelitis of unknown etiology (MUE) remains difficult to predict. MUE cases with no lesions detected by magnetic resonance imaging (MRI) occur, but it is unknown whether this finding is associated with prognosis.
Hypothesis
MUE cases without detectable lesions on MRI have a better outcome than cases with detectable lesions.
Animals
Study included 73 client‐owned dogs with MUE presenting to Purdue University Veterinary Hospital from 2010 to 2020.
Methods
Retrospective study. Dogs with a clinical diagnosis of MUE were identified by medical record search. MRI reports were reviewed for presence or absence of lesions consistent with MUE. Clinical findings at presentation, treatment, disease‐specific survival, and outcomes including rates of remission and relapse were compared between cases with normal MRI or abnormal MRI.
Results
Overall, 54 dogs (74%) were classified as abnormal MRI, and 19 dogs (26%) were classified as normal MRI cases. Death caused by MUE occurred in 1/19 (5%) normal MRI dogs and 18/54 (33%) abnormal MRI dogs (P = .016). Median survival was >107 months in both groups, but survival was significantly longer in the normal MRI group (P = .019). On multivariate analysis, abnormal MRI was significantly related to death (hazard ratio, 7.71; 95% confidence interval 1.03‐58.00, P = .0470), whereas significant relationships with death were not identified for either the use of secondary immunosuppressive medications or cerebrospinal fluid nucleated cell count.
Conclusions
MUE dogs with no detectable lesions on MRI have reduced disease‐related death compared with dogs with abnormal MRI. The presence or absence of MRI lesions in MUE dogs is prognostically relevant.
Keywords: brain, canine, encephalitis, GME, meningitis, necrotizing encephalitis
Abbreviations
- CSF
cerebrospinal fluid
- GME
granulomatous meningoencephalitis
- MRI
magnetic resonance imaging
- MUE
meningoencephalomyelitis of unknown etiology
- NME
necrotizing meningoencephalitis
- TNCC
total nucleated cell count
- T2W
T2‐weighted
- T1W
T1‐weighted
1. INTRODUCTION
Meningoencephalomyelitis of unknown etiology (MUE) is a common disease in dogs. The prognosis for dogs with MUE is highly variable, with life‐threatening neurologic deterioration in some but prolonged remission in others. 1 , 2 Few prognostic indicators exist to predict outcome. The presence of mass effect on magnetic resonance imaging (MRI), is associated with early (<3 month) death, suggesting a possible relationship between MRI findings and outcome. 3
Normal MRI is observed routinely in human patients with noninfectious encephalitis. 4 , 5 , 6 , 7 , 8 Abnormal MRI is not required to diagnose 1 of the group of diseases referred to as autoimmune encephalitis. 4 This is particularly the case for leucine‐rich glioma‐inactivated 1 (LGI1) antibody‐associated encephalitis and anti‐N‐methyl‐D‐aspartate receptor encephalitis, for which only about half of patients have abnormal MRI. 6 , 7 , 8 Similarly, normal MRI is repeatedly reported in dogs with MUE, 1 , 2 , 9 , 10 , 11 , 12 , 13 but the outcome and survival of this subset of dogs is not reported. Other reported prognostic factors for MUE include cerebrospinal fluid (CSF) total nucleated cell count (TNCC), mentation change, body weight, and seizures. 11 , 14 , 15 , 16
The primary goal of the current study was to determine whether any difference in survival exists between MUE dogs with and without MRI lesions. The secondary goal of our study was to investigate various other outcome measures, in addition to survival. We hypothesized that in dogs diagnosed with MUE, normal MRI cases would have improved survival and other outcome measures compared with abnormal MRI cases.
2. METHODS
2.1. Case selection
Medical records from Purdue University Veterinary Hospital's database from 2010 to 2020 were searched for client‐owned dogs. To be included in the study, dogs had to have a clinical diagnosis of MUE, and meet all of the following criteria 9 :
Age between 6 months and 10 years at diagnosis.
Brain or spine MRI performed.
Cerebrospinal fluid TNCC >5 cells/μL.
Infectious diseases tests performed.
Minimum follow‐up (until the date of death or euthanasia, or for at least 3 months past the date of MUE diagnosis).
We additionally included dogs with MRI and a histologic diagnosis of granulomatous meningoencephalitis (GME), necrotizing meningoencephalitis (NME), or necrotizing leukoencephalitis, regardless of whether they met all the criteria above. We then excluded dogs diagnosed with any of the following:
Infectious meningoencephalitis/meningomyelitis.
Eosinophilic meningoencephalitis, corticosteroid responsive tremor syndrome or steroid‐responsive meningitis arteritis.
2.2. Clinical data collection
Clinical information obtained from the medical records of each dog included: signalment, presence/absence of seizures (at presentation for MRI), neurolocalization, spinal cord‐only neurolocalization (myelitis‐only), MRI findings, CSF TNCC, histopathologic findings (when available), corticosteroid dose in the 1st month of treatment, adjunctive treatment (used at any time during treatment), whether remission was achieved (neurologic status remaining improved or normal after discontinuing corticosteroids; some cases were still receiving adjunctive medications on the date corticosteroids were discontinued), whether relapse occurred (recurrence of neurologic clinical signs, further characterized as occurring before or after discontinuing corticosteroids), date of last contact, date of death if applicable, and whether death occurred because of MUE (including death caused by MUE treatment complications). For 39 dogs, the date and cause of death were retrieved from the Purdue medical record. For 34 cases, follow‐up information was obtained via phone call to the primary veterinarian's office and reviewing their medical records. Details recorded included the date of the last appointment, if the dog was dead, the date of death, the cause of death or euthanasia, and details of all medications (current or at the time of death). Additional questions were asked as necessary, to ascertain whether the cause of death was caused by MUE (including MUE treatment complications), or unrelated to MUE. When necessary, the owner was then contacted to confirm whether a dog was still alive, and if not, their date and cause of death.
All MRI studies were performed using a 1.5 Tesla magnet (GE Signa LXI, GE Medical Systems, Milwaukee, Wisconsin) and images were retrieved using an image analysis workstation. Images were contemporaneously reviewed by a board‐certified neurologist and a board‐certified radiologist who were aware of the dog's clinical status.
Abnormal MRI was defined as T2‐hyperintense or contrast‐enhancing lesions or both, in the brain, spinal cord, and/or meninges, consistent with noninfectious inflammatory disease. Normal MRI was defined as the absence of such lesions. For the purposes of our study, concurrent lesions unrelated to MUE (e.g., caudal occipital malformation syndrome) were not considered when classifying MRI status.
A neurology resident reviewed the MRI report of each dog to obtain the following MRI data: site imaged (brain, spine, or both); sequences performed; abnormal or normal MRI; and the presence of the following signs of mass effect: midline shift, loss of sulci, or transforaminal herniation. 3 , 11 The number of these features of mass effect present (0, 1, 2, or 3) was then tabulated for each MRI.
2.3. Outcome measures
All dogs were allocated to the normal MRI group or the abnormal MRI group. The primary outcome measure was disease‐specific survival, measured from the date of the 1st MRI until date of death caused by MUE.
The secondary outcome measures were death rate because of MUE (percentage of each group dying because of MUE), remission rate, disease‐free interval (months), relapse while taking corticosteroids (early relapse), relapse after discontinuation of corticosteroids (late relapse; including dogs who relapsed after discontinuing corticosteroids but before discontinuing secondary medications), death in hospital, and death within the first 3 months.
2.4. Data analysis and statistical methods
Disease‐specific survival time was defined as the total time from the date of MRI until the time of death specifically caused by MUE. Dogs were censored on the date they died from non‐MUE causes, and on the date of last follow‐up for dogs who were still alive. Disease‐specific survival was compared between groups (normal MRI and abnormal MRI) using a log rank test and displayed using Kaplan‐Meier curves.
To determine the effects of discrepant therapeutic protocols on outcomes, the highest prednisone dose received within the 1st month of diagnosis was recorded for all dogs as either lower dosage (<1.5 mg/kg per day) or higher dosage (≥1.5 mg/kg per day). Dogs receiving dexamethasone had the dose converted to prednisone‐equivalent (dose divided by 7). Other variables were also compared with investigate factors between the normal MRI and abnormal MRI groups. Numerical variables of median follow‐up time, CSF TNCC, body weight, and age were compared between the 2 groups using Wilcoxon rank sum tests. Proportional data of mass effect on MRI, death rate caused by MUE, death in hospital, and death in first 3 months were compared using Fisher's exact tests because of low expected frequencies. The percentage of dogs with mentation change, myelitis‐only (spinal cord‐only neurolocalization), seizures at presentation, and use of secondary immunosuppressive medications were compared using Pearson chi‐squared tests.
Variables significantly associated with outcomes after univariable analyses were placed into multivariable Cox proportional hazard regression analyses with Breslow method for ties. A P value ≤.05 was considered statistically significant for all analyses.
3. RESULTS
3.1. Study sample
A total of 314 dogs with meningoencephalomyelitis were identified within the study period (Figure 1). After excluding dogs who did not meet all inclusion and exclusion criteria, a total of 73 dogs with MUE remained. This included 19 normal MRI dogs and 54 abnormal MRI dogs. Table 1 compares clinical characteristics of the normal and abnormal MRI groups and Table 2 compares secondary outcome measures between the 2 groups.
FIGURE 1.
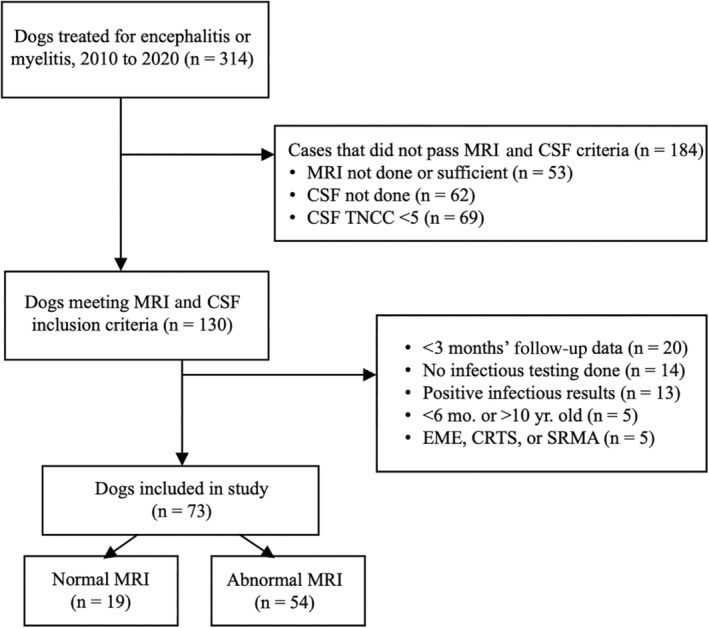
Case selection for inclusion in the current study. Flow chart demonstrating criteria for inclusion of 73 dogs in the study. CRTS, corticosteroid‐responsive tremor syndrome; CSF, cerebrospinal fluid; EME, eosinophilic meningoencephalitis; MRI, magnetic resonance imaging; SRMA, steroid responsive meningitis‐arteritis; TNCC, total nucleated cell count.
TABLE 1.
Univariate analyses for potential confounding variables between normal MRI and abnormal MRI groups, in dogs diagnosed with meningoencephalomyelitis of unknown etiology.
| Variable | Normal MRI (n = 19) | Abnormal MRI (n = 54) | P value |
|---|---|---|---|
| Demographic characteristics | |||
| Sex | .12 | ||
| Male neutered (intact) | 4 (6) | 15 (5) | |
| Female neutered (intact) | 8 (1) | 30 (4) | |
| Age (years) | 4.2 (0.5‐10.6) | 4.2 (1.0‐10.0) | .70 |
| Median (range) | |||
| Body weight (kg) | 5.6 (1.2‐47.6) | 7.0 (1.4‐33.4) | .35 |
| Median (range) | |||
| Clinical presentation | |||
| Mentation change | 4 (21%) | 16 (30%) | .47 |
| Seizures at presentation | 5 (26%) | 13 (24%) | .84 |
| Myelitis‐only | 5 (26%) | 13 (24%) | .84 |
| Diagnostics | |||
|
CSF TNCC (cells/μL) median (range) |
24 (6‐1405) | 98 (4‐7650) | .04 |
| Mass effect on MRI | .16 | ||
| Present | 0 (0%) | 11 (20%) | |
| 1 feature | 0 (0%) | 9 (17%) | |
| 2 features | 0 (0%) | 2 (4%) | |
| 3 features | 0 (0%) | 0 (0%) | |
Note: Data are presented as count (percentage) of cases unless otherwise specified. Cases were included if TNCC >5, or if necropsy confirmed granulomatous meningoencephalitis or necrotizing encephalitis. P values ≤ 0.05 were considered significant.
Abbreviations: CSF TNCC, cerebrospinal fluid total nucleated cell count; MRI, magnetic resonance imaging.
TABLE 2.
Secondary outcome measures in dogs with normal and abnormal MRI.
| Outcome measure | Normal MRI | Abnormal MRI | P value |
|---|---|---|---|
| (n = 19) | (n = 54) | ||
| Remission | |||
| Remission achieved | 13 (68%) | 29 (53%) | .26 |
| Subsequent disease‐free interval (months) | 25 | 19 | .38 |
| Relapse | |||
| Early relapse | 5 (26%) | 13 (24%) | .84 |
| Late relapse | 2 (11%) | 11 (20%) | .49 |
| Death | |||
| Death in hospital | 0 (0%) | 6 (11%) | .33 |
| Death in first 3 months | 1 (5%) | 7 (13%) | .67 |
| Death caused by disease | 1 (5%) | 18 (33%) | .02 |
Note: The outcome of dogs with meningoencephalitis of unknown etiology with normal and abnormal magnetic resonance imaging (MRI) is presented as count (percentage) of cases. Early relapse, relapse while still receiving prednisone. Late relapse, relapse after discontinuing prednisone. Remission was achieved if a dog discontinued prednisone and remained neurologically improved to normal. P values ≤ 0.05 were considered significant.
There were 30 male (19 neutered) and 43 female (38 neutered) dogs. This included 12 mixed breeds, 10 Maltese, 7 Chihuahuas, 6 shih tzus, 4 Yorkshire terriers, 2 each of pugs, French bulldogs, Havanese, miniature schnauzers, Pomeranians, Boston terriers, and Airedale terriers, and 1 each of 20 other breeds.
3.2. Clinical data
A definitive diagnosis based on histopathology was obtained in 7 dogs (6 GME and 1 NME). All 7 had abnormal MRI. For 1 of these dogs, CSF analysis was not available.
MRI studies performed on the brain only (43 cases), spine only (12 cases), or both (18 cases) were available for review. For brain MRI, multiplanar T2‐weighted (T2W), T1‐weighted (T1W), and postcontrast T1W images of the entire brain were obtained in all cases. Transverse T2W fluid attenuation inversion recovery, T2*‐weighted gradient echo, and diffusion weighted images were variably performed. All spine studies included multiplanar T2W, T1W, and postcontrast T1W images, except for 2 cases that lacked postcontrast images. T2W short tau inversion recovery and half Fourier acquisition single‐shot turbo spin‐echo images were variably performed.
The duration of follow‐up was ≤3 months for 14/73 (19%) dogs, 4 to 12 months for 12/73 (16%) dogs and >12 months for 47/73 (64%) dogs. The median follow‐up was 9 months (range, 1‐105) in normal MRI cases and 12 months (range, 0‐65) in abnormal MRI cases (P = .64).
3.3. Primary outcome measure: survival
Disease‐specific survival time significantly differed between the normal MRI group and the abnormal MRI group (P = .02). Median survival was not reached in either group in the Kaplan‐Meier survival analysis (Figure 2) and was >107 months in both groups.
FIGURE 2.
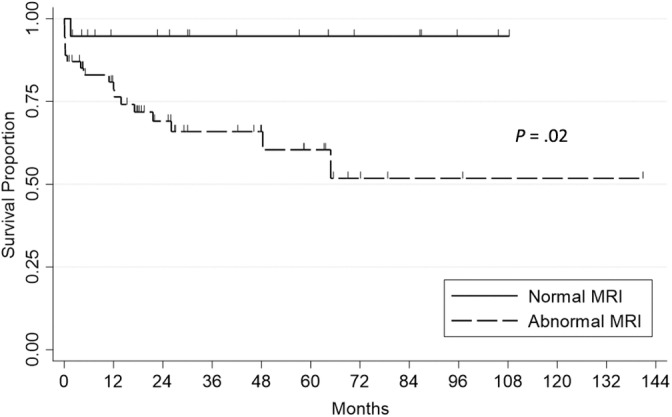
Disease‐specific survival. Kaplan‐Meier analysis of 73 dogs with meningoencephalitis of unknown etiology, comparing cases with normal magnetic resonance imaging (MRI) (n = 19) and abnormal MRI (n = 54). Each vertical tick mark represents a censored dog that was either lost to follow‐up or deceased because of causes unrelated to meningoencephalomyelitis of unknown etiology. Median survival because of disease was not reached in either group. Survival time because of disease differed by group (P = .02, log rank test).
3.4. Secondary outcome measures
Death caused by MUE occurred in 1/19 (5%) dogs with normal MRI and 18/54 (33%) dogs with abnormal MRI, which was significantly different (P = .02; Table 2).
There were no significant differences between the normal MRI and abnormal MRI groups in the percentage of dogs experiencing remission, disease‐free interval, early relapse (while still receiving prednisone), or late relapse (after discontinuing prednisone). Remission (remaining neurologically improved to normal despite discontinuing steroids) was achieved in 68% (13/19) of the normal MRI group after a median of 25 months and in 53% (29/54) of the abnormal MRI group after a median of 19 months. No dogs with normal MRI died in hospital, whereas 11% of dogs with abnormal MRI died during hospitalization (1 within 24 hours and another 5 before discharge). This difference was also not significant (P = .33). The total number of dogs dying within 3 months of diagnosis was 1 dog (5%) in the normal MRI group and 7 dogs (13%) in the abnormal MRI group (P = .67). Of these 8 deaths, 6 dogs died, and 2 were euthanized because of failure to respond to treatment.
3.5. Confounding factors between normal MRI and abnormal MRI groups
Between the normal MRI and abnormal MRI groups of dogs, there was no significant difference in sex distribution, age, body weight, or the percentage of dogs with each of mentation change, seizures at presentation, or myelitis‐only (Table 1). There was also no significant difference in the percentage of dogs receiving ≥1.5 mg/kg/day of prednisone (Table 3).
TABLE 3.
Use of immunosuppressive medications in dogs with normal and abnormal MRI.
| Treatment | Normal MRI | Abnormal MRI | P value |
|---|---|---|---|
| (n = 19) | (n = 54) | ||
| Prednisone ≥1.5 mg/kg/day | 12/19 (63%) | 33/54 (61%) | .88 |
| Secondary immunosuppressive (n = 31) | 3/19 (16%) | 28/54 (52%) | .01 |
| Number of dogs receiving: | |||
| Cytosine arabinoside | 1 | 14 | |
| Mycophenolate | 1 | 11 | |
| Cyclosporine | 1 | 9 | |
| Procarbazine | 3 | ||
| CCNU | 2 | ||
| Leflunomide | 1 | ||
| Chlorambucil | 1 | ||
Note: Treatment of dogs with meningoencephalomyelitis of unknown etiology is presented as the count (percentage) of cases. Some dogs in the abnormal magnetic resonance imaging (MRI) group received more than 1 secondary medication. P values ≤ 0.05 were considered significant.
There was a significant difference in CSF TNCC. Abnormal MRI dogs had a higher median TNCC (98 cells/μL, range, 4‐7650) than normal MRI dogs (24 cells/μL, range, 6‐1405; P = .04).
Mass effect was present in 11 dogs in the abnormal MRI group, comprised of 9 dogs with 1 feature of mass effect, 2 dogs with 2 features of mass effect, and no dogs with all 3 features of mass effect (Table 1). In line with the study design, there were no cases with mass effect in the normal MRI group. This difference in mass effect between the 2 groups was not significant (P = .16). Survival of all dogs grouped by mass effect was not significantly different (Figure 3).
FIGURE 3.
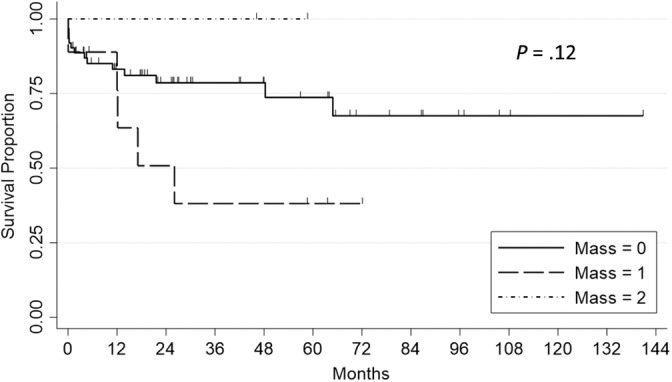
Survival in dogs with and without magnetic resonance imaging (MRI) features of mass effect. Survival in 73 dogs with meningoencephalitis of unknown etiology, with 0, 1, or 2 features of mass effect on MRI. Features of mass effect evaluated were midline shift, loss of sulci, and transforaminal herniation. There were only 2 dogs with 2 features of mass effect. Survival was not significantly different between groups (P = .12, log rank test). Mass = 2, 2 features of mass effect present. Mass = 1, 1 features of mass effect present. Mass = 0, no features of mass effect present.
3.6. Influence of immunosuppressive treatment
All dogs received corticosteroids, at a dosage of <1.5 mg/kg/day of prednisone (n = 28) or ≥1.5 mg/kg/day (n = 45) in the 1st month of treatment. Some dogs received additional immunosuppressive medications (Table 3). Compared with lower dose prednisone, higher dose prednisone was not statistically significantly related to any outcome measure, including death caused by MUE, achievement of disease remission, occurrence of relapse before or after discontinuing prednisone, or disease‐free interval (P > .35, for each analysis).
Dogs with an abnormal MRI were significantly more likely to receive 1 or more secondary immunosuppressive drugs (P = .01).
3.7. Multivariate analyses
In univariate analyses (above), there were significant differences between the normal MRI and abnormal MRI groups in the percentage of dogs dying because of MUE, the median CSF TNCC, and the use of secondary immunosuppressive medications.
In Cox proportional hazard models, the abnormal MRI group was significantly related to death caused by disease (hazard ratio, 7.71; 95% confidence interval, 1.03‐58.00, P = 0.047), whereas the use of secondary immunosuppressive medications (P = .60) and CSF TNCC (P = .67) were not significant.
4. DISCUSSION
The most important finding of our study was that dogs in the abnormal MRI group had significantly more deaths compared with the normal MRI group, in univariate and multivariate analyses. In univariate analyses, both the CSF TNCC and the percentage of dogs receiving secondary immunosuppressive medications were significantly higher in the abnormal MRI group. Both of these factors were assessed because they are prognostic indicators in some studies. 9 , 11 , 14 However, in our sample, multivariate analyses confirmed the significant association between abnormal MRI and death, discounting a significant relationship between both CSF TNCC and secondary medications with death.
4.1. Survival data
There were significant differences in survival between normal MRI and abnormal MRI dogs with MUE. Both the primary outcome measure (survival time) and 1 of the secondary outcome measures (percentage of cases dying) were significantly worse in the abnormal MRI group. Multivariate analyses confirmed the significance of abnormal MRI, discounting both CSF TNCC and the use of secondary immunosuppressive medications. Taken together, these relationships suggest abnormal MRI truly carries a worse prognosis. The large hazard ratio of abnormal MRI for death (7.71; 95% confidence interval, 1.03‐58.00) suggested that the relationship is clinically important as well as statistically significant. The prognosis of dogs with MUE having normal MRI findings has not yet been well described, and our findings suggest that these dogs have better survival.
The finding of improved survival in normal MRI dogs could be used early in the disease course to predict the prognosis of individual dogs. Negative prognostic factors for MUE include mentation change, seizures at presentation, and body weight, 15 , 16 as well as MRI features of mass effect. 3 We accounted for each of these confounding factors, finding no significance difference in their prevalence between the normal and abnormal MRI groups. We also accounted for another negative prognostic indicator, increased TNCC 11 , 14 (see below).
The overall outcome in our sample (normal and abnormal MRI dogs) was similar to findings in other samples for outcome measures such as relapse rate. 14 , 17 , 18 , 19 However, the rate of death in the first 3 months, remission rate, median survival time, 5‐year survival rate, and the rate of death caused by MUE appeared improved compared with previous reports (Table 4). Given that the rates of MUE‐related death in our study were significantly lower in the normal MRI dogs compared with abnormal MRI dogs, it is plausible that the inclusion of normal MRI dogs is an important reason for the improved outcome compared with previous studies. In fact, the presence of focal or multifocal MRI lesions has been a criterion for inclusion in most previous studies of MUE. When conducting future MUE studies that include normal MRI dogs, it will therefore be important to account for the potential for normal MRI to exert an independent effect on the outcome of the study sample.
TABLE 4.
Outcome comparison between current and previous studies.
| Outcome measure | Current study | Previous findings | References |
|---|---|---|---|
| MST (days) | >3240 | >1095 | Stee et al, 2020 20 |
| >1‐year survival rate | 48/73 (66%) | Between 57% and 74% | Pausova et al, 2021, 21 Kaczmarska et al, 2020, 22 Goncalves et al, 2022, 23 , a Lowrie et al, 2016 24 |
| >5‐year survival rate | 16/73 (22%) | 19/182 (10%) | Pausova et al, 2021 21 |
| Remission achieved | 42/73 (58%) | 9/45 (20%) | Portero et al, 2019 12 , b |
| Relapse rate | 31/73 (42%) | Between 23% and 51% | Brady et al, 18 Wong et al, 19 Kaczmarska et al, 2020, 22 Pausova et al, 2021, 21 Song et al, 14 Goncalves et al, 2023, 25 Stee et al, 2020 20 |
| Death in first 3 months | 8/73 (11%) | Between 22% and 56% | Stee et al, 2020, 20 Lowrie et al, 2016, 24 Lowrie et al, 2013 3 |
| Total deaths caused by MUE | 19/73 (26%) | Between 40% and 55% | Brady et al, 2020, 18 Kaczmarska et al, 2020, 22 Portero et al, 2019 12 |
Note: Comparison of outcomes in dogs with meningoencephalitis of unknown etiology. All of these studies had abnormal MRI findings as an inclusion criterion, except Portero et al. 11 Remission achieved, neurologic status remaining improved or normal after discontinuing corticosteroids.
Abbreviations: MRI, magnetic resonance imaging; MST, median survival time; MUE, meningoencephalitis of unknown etiology.
65.3% of all dogs with inflammatory CNS disease alive at 1 year; no difference in short or long‐term survival between infectious and noninfectious causes.
9/45 dogs had a normal neurologic examination while remaining treatment‐free for 1 year or longer.
Even when considering only the abnormal MRI group in our current study, the proportions of deaths in the first 3 months and total proportion of deaths caused by MUE (13% and 33%, respectively) were somewhat lower than that of previous studies (22%‐56% and 40%‐55%; Tables 2 and 4). Possible explanations for this finding might include the relatively longer follow‐up time provided, differences in disease severity or patient samples between institutions, differences in treatment protocols, and differences in decisions made by pet owners. In addition, the number of dogs in both the current study and previous studies are fewer than ideal, which can be a reason for apparent differences in outcome.
Our primary and secondary outcomes of dogs with MUE should be assessed carefully for several reasons. First, survival data can be prone to average values that are poorly representative of the population as a whole, particularly when sample sizes are small or the number of outliers is high. Second, discrepancies in study period duration make it difficult to compare results between studies, and follow‐up that is prematurely ended could result in underestimation of survival times. Lastly, the requirement of CSF values for the clinical diagnosis of MUE risks excluding the most severely affected dogs from the dataset. This is the case in our study as well as most other studies of MUE, because dogs with signs of increased intracranial pressure on MRI have a high risk of brain herniation and death during CSF collection. 15 , 16 , 17
4.2. Inclusion of normal MRI cases in studies of dogs with MUE
There have been multiple MUE case series that included some dogs with normal MRI. 1 , 9 , 10 , 11 , 12 , 13 Abnormal CSF analysis or lesions on T2W images or both have been used as inclusion criteria, rather than requiring abnormal MRI for every case. 12 Up to 7% of MUE dogs show no T2W abnormalities, with 31% showing no abnormal contrast‐enhancement. 1 , 9 Review articles state that it can be acceptable to include normal MRI cases in MUE studies. 1 It is largely accepted that some MUE dogs will have a normal MRI; it is rare for any diagnostic test to be 100% sensitive, and this certainly applies to MRI of MUE. 9 In human medicine, it is well‐accepted that MRI can be normal in the group of diseases referred to as autoimmune encephalitis. 4 The diagnostic criteria for the autoimmune encephalitis require only 1 of the following: new focal central nervous system (CNS) findings, new‐onset seizures, CSF pleocytosis, and MRI suggestive of encephalitis. 4 Many such diseases are confirmed by autoantibody confirmation. In LGI1 antibody‐associated encephalitis, MRI is usually normal. 5 Pretreatment MRI was abnormal in only 43% of cases. 6 18F‐FDG positron emission tomography‐computed tomography, which appears to be a more sensitive form of imaging, was abnormal in every case. Similarly, for anti‐N‐methyl‐D‐aspartate receptor encephalitis, MRI was abnormal in only 56% or 57% of patients. 7 , 8
4.3. Confounding factors between normal MRI and abnormal MRI groups
Other than MRI findings, the only significant difference between the normal MRI and abnormal MRI groups at the time of presentation was a higher median CSF TNCC in the latter. We considered it important to compare TNCC between the 2 groups because previous studies on the relationship between CSF TNCC and survival yielded conflicting results. 11 , 14 , 15 , 16 , 17 Although a higher CSF TNCC was correlated to shorter survival in 2 studies, the strength of the influence was weak. 11 , 14 In 1 of these, the relative hazard ratio of TNCC in predicting mortality was only 1.004. 14 Furthermore, 3 other studies failed to demonstrate an association between CSF TNCC and survival time. 15 , 16 , 17 Ours is the 2nd study to find a higher TNCC in the subgroup with more pronounced MRI findings. 11 It is possible that both TNCC and MRI abnormalities increase in severity as the disease progresses, but putting together all the studies to date, MRI lesions appear to be more useful in predicting prognosis.
4.4. Influence of immunosuppressive treatment
The percentage of dogs in the current study that died because of MUE did not significantly differ between dogs receiving lower dosage (<1.5 mg/kg/day) and immunosuppressive (≥1.5 mg/kg/day) doses of prednisone. Similarly, there was no significant difference in the achievement of disease remission, early relapse (relapse while taking steroids), or late relapse (relapse when not taking steroids).
Treatment of MUE with glucocorticoids at initial doses less than 2 mg/kg/day is reported sparsely, 26 and studies comparing glucocorticoid doses are lacking. Given that prednisone dose in the current study did not significantly impact the primary or secondary outcome measures in dogs with MUE, and considering the adverse effects associated with high doses of glucocorticoids, further studies to evaluate anti‐inflammatory dose glucocorticoids for the treatment of MUE are indicated.
In the present study, abnormal MRI dogs were significantly more likely to receive a secondary immunosuppressive treatment than normal MRI dogs (52% vs 16%, P = 0.047). In retrospective studies, it is possible that more severe cases can receive more aggressive treatment, resulting in a loss of apparent significance of the impact of treatment choices. As such, it is possible that the differences in treatment protocols between groups could be exerting its own effect on outcome. Considering that several studies suggest improved outcomes in dogs treated with polytreatment, 14 , 17 , 18 , 19 , 27 , 28 , 29 available literature does not provide any evidence that dogs receiving glucocorticoid monotreatment have improved survival over polytreatment‐treated dogs. Therefore, in our study it is likely that the normal MRI group truly had a better outcome, and despite the abnormal MRI group receiving putatively more aggressive treatment.
In general, conflicting data exist regarding the efficacy of treatment outcomes with monotreatment versus polytreatment, and median survival varies profoundly between studies. 14 , 17 , 27 , 28 , 29
4.5. Relationship between mass effect and survival
In 1 study, mass effect was associated with 1 specific measure of survival (survival to 3 months). 3 We therefore analyzed whether the prevalence of mass effect was different between the 2 groups (normal MRI and abnormal MRI) to account for any confounding effect, finding no significant difference. There was also no significant relationship between mass effect and survival, but by requiring CSF collection, we might have excluded the worst mass effect cases. One possible interpretation of our findings combined with the previous study 3 is that there is a spectrum of decreasing survival from cases with no MRI lesions, to MRI lesions, to MRI lesions including mass effect.
4.6. Limitations
There are several limitations to our study. Follow‐up data was obtained for some cases by contacting owners, which relies upon owner recall. This is less precise than data obtained from medical records. The retrospective nature and lack of standardized treatment protocol between dogs means that outside variables (e.g., medications used, owner finances) could influence outcomes. Being a retrospective study, we were not able to investigate the mechanisms through which MRI changes might influence survival.
By requiring abnormal CSF results for inclusion, we likely excluded some dogs with clinical or imaging signs of brain herniation where the clinician chose not to attempt CSF collection. Because mass effect reduces 3‐month survival, 3 this might have skewed our study to include less severe cases not fully representative of all MUE cases encountered in practice. However, requiring CSF pleocytosis is typical in MUE studies 3 , 10 , 15 , 16 , 17 , 19 , 27 , 28 , 29 , 30 and is recommended as a routine inclusion criterion. 1 , 2 , 9 , 13 , 26 , 31 In addition, because our study was specifically investigating MUE cases with normal MRI, requiring abnormal CSF was deemed important to heighten the likelihood of a correct diagnosis of MUE.
In the past 2 decades, it has become routine to use antemortem diagnostic tests (MRI, CSF, etc.) as inclusion criteria in MUE studies, and to not require histology. Only one tenth of our cases were histologically confirmed. There has been concern that the survival time of those cases that undergo necropsy vastly underestimates the prognosis of a typical MUE case. MUE studies must be interpreted in light of which dogs are included/excluded, which varies from study to study. As is now routine in studies reliant upon antemortem diagnosis, it is certainly possible that some of our cases were misdiagnosed. There is a known diagnostic challenge, differentiating between MUE and diseases with a worse prognosis (eg, glioma) and diseases with a better prognosis (e.g., cerebrovascular accident). 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 Although we required pleocytosis, TNCC >5 is routinely seen in neoplasia, cerebrovascular accident and other diseases. There is no ideal TNCC value to discriminate between inflammatory and noninflammatory diseases. Regardless of the TNCC cutoff value chosen, there is always some likelihood of inadvertently excluding MUE cases while including noninflammatory cases. Although multiple other studies have included MUE or GME cases with normal MRI, 9 , 10 , 12 including such cases could increase the misdiagnosis rate. Infectious disease testing was not standardized in this retrospective study, and in addition misdiagnosis because of false negative results is possible. The prolonged survival of most cases in the face of immunosuppressive treatment, and the histologic diagnosis of GME or NME in all necropsied dogs, suggests that the inadvertent inclusion of infectious or neoplastic diseases was mitigated. Given the prolonged survival, unintended inclusion of benign diseases such as cerebrovascular accident is conceivable. It is difficult to postulate whether this could have preferentially affected the normal MRI group or the abnormal MRI group.
In addition, it is possible the dogs with clinical signs and MRI abnormalities limited to the spinal cord could have a different disease entity to those with encephalitis. The occurrence of spinal‐only meningoencephalomyelitis of unknown etiology has been previously described and is postulated, but not proven, to overlap with the histopathologic subtypes of meningoencephalitis of unknown etiology. 36 , 37 , 38 , 39 As it remains ambiguous whether immune‐mediated meningoencephalitis, encephalomyelitis, and myelitis should be considered a spectrum of 1 disease process or different clinical entities, the findings of our study should be interpreted considering this caveat.
Finally, there is an inherent dramatic variability in the outcome of MUE cases, and in small studies, one must be cautious ascribing apparent survival differences between groups to factors such as diagnostic findings or treatment, when random variation in the disease itself appears to be a major factor.
5. CONCLUDING COMMENTS
Our results demonstrate that dogs diagnosed with MUE with normal MRI have reduced disease‐related death compared with dogs with abnormal MRI, confirming our hypothesis. There was no significant difference in the other measures of outcome (e.g., death during first 3 months); however, consideration of other outcome measures has yielded useful information in previous studies, and we might have not had enough cases to detect true differences.
Based on our findings, the presence or absence of MRI lesions in MUE dogs is prognostically relevant and suggests a potential role for MRI as a biomarker of disease severity.
CONFLICT OF INTEREST DECLARATION
Georg E. Moore serves as Consulting Editor for Experimental Design and Statistics for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript. No other authors declare a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Ostrager A, Bentley RT, Lewis MJ, Moore GE. Survival in dogs with meningoencephalomyelitis of unknown etiology with and without lesions detected by magnetic resonance imaging. J Vet Intern Med. 2024;38(4):2204‐2213. doi: 10.1111/jvim.17109
REFERENCES
- 1. Cornelis I, Van Ham L, Gielen I, De Decker S, Bhatti SFM. Clinical presentation, diagnostic findings, prognostic factors, treatment and outcome in dogs with meningoencephalomyelitis of unknown origin: a review. Vet J. 2019;244:37‐44. [DOI] [PubMed] [Google Scholar]
- 2. Jeffery N, Granger N. New insights into the treatment of meningoencephalomyelitis of unknown origin since 2009: a review of 671 cases. Front Vet Sci. 2023;10:1114798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lowrie M, Smith PM, Garosi L. Meningoencephalitis of unknown origin: investigation of prognostic factors and outcome using a standard treatment protocol. Vet Rec. 2013;172(20):527. [DOI] [PubMed] [Google Scholar]
- 4. Graus F, Titulaer MJ, Balu R. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jia Y, Wang H, Zhang M. LGI1 antibody‐associated encephalitis without evidence of inflammation in CSF and brain MRI. Acta Neurol Belg. 2023;123(3):849‐856. [DOI] [PubMed] [Google Scholar]
- 6. Sadaghiani MS, Roman S, Diaz‐Arias LA, et al. Comparison of quantitative FDG‐PET and MRI in anti‐LGI1 autoimmune encephalitis. Neuroradiology. 2023;65(8):1225‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang C, Hao Y, Huang G, et al. Hypometabolism of the left middle/medial frontal lobe on FDG‐PET in anti‐NMDA receptor encephalitis: comparison with MRI and EEG findings. CNS Neurosci Ther. 2023;29(6):1624‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Q, Xie Q, Liu L, Meng C, Wang J. Factors influencing prognosis and relapse in patients with anti‐N‐methyl‐D‐aspartate receptor encephalitis. Mult Scler Relat Disord. 2023;74:104697. [DOI] [PubMed] [Google Scholar]
- 9. Granger N, Smith PM, Jeffery ND. Clinical findings and treatment of non‐infectious meningoencephalomyelitis in dogs: a systematic review of 457 published cases from 1962 to 2008. Vet J. 2010;184(3):290‐297. [DOI] [PubMed] [Google Scholar]
- 10. Lamb CR, Croson PJ, Cappello R, Cherubini GB. Magnetic resonance imaging findings in 25 dogs with inflammatory cerebrospinal fluid. Vet Radiol Ultrasound. 2005;46(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 11. Oliphant BJ, Barnes Heller HL, White JM. Retrospective study evaluating associations between midline brain shift on magnetic resonance imaging and survival in dogs diagnosed with meningoencephalitis of unknown etiology. Vet Radiol Ultrasound. 2017;58(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 12. Portero M, Martínez de Merlo E, Pérez C, Benito M, Daza MA, Fragio C. Cerebrospinal fluid and blood lactate concentrations as prognostic biomarkers in dogs with meningoencephalitis of unknown origin. Vet J. 2019;254:105395. [DOI] [PubMed] [Google Scholar]
- 13. Talarico LR, Schatzberg SJ. Idiopathic granulomatous and necrotising inflammatory disorders of the canine central nervous system: a review and future perspectives. J Small Anim Pract. 2010;51(3):138‐149. [DOI] [PubMed] [Google Scholar]
- 14. Song JH, Yu DH, Lee HC, et al. Evaluation of treatment with a combination of mycophenolate mofetil and prednisolone in dogs with meningoencephalomyelitis of unknown etiology: a retrospective study of 86 cases (2009–2017). BMC Vet Res. 2020;16(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cornelis I, Volk HA, Van Ham L, De Decker S. Prognostic factors for 1‐week survival in dogs diagnosed with meningoencephalitis of unknown aetiology. Vet J. 2016;214:91‐95. [DOI] [PubMed] [Google Scholar]
- 16. Coates JR, Barone G, Dewey CW, Vitale CL, Holloway‐Azene NM, Sessions JK. Procarbazine as adjunctive therapy for treatment of dogs with presumptive antemortem diagnosis of granulomatous meningoencephalomyelitis: 21 cases (1998–2004). J Vet Intern Med. 2007;21(1):100‐106. [DOI] [PubMed] [Google Scholar]
- 17. Paušová TK, Tomek A, Šrenk P, Belašková S. Clinical presentation, diagnostic findings, and long‐term survival time in 182 dogs with meningoencephalitis of unknown origin from central Europe that were administered glucocorticosteroid monotherapy. Top Companion Anim Med. 2021;44:100539. [DOI] [PubMed] [Google Scholar]
- 18. Brady S, Woodward A, le Chevoir M. Survival time and relapse in dogs with meningoencephalomyelitis of unknown origin treated with prednisolone and ciclosporin: a retrospective study. Aust Vet J. 2020;98(10):491‐498. [DOI] [PubMed] [Google Scholar]
- 19. Wong MA, Hopkins AL, Meeks JC, Clarke JD. Evaluation of treatment with a combination of azathioprine and prednisone in dogs with meningoencephalomyelitis of undetermined etiology: 40 cases (2000–2007). J Am Vet Med Assoc. 2010;237(8):929‐935. [DOI] [PubMed] [Google Scholar]
- 20. Stee K, Broeckx BJG, Targett M, Gomes SA, Lowrie M. Cytosine arabinoside constant rate infusion without subsequent subcutaneous injections for the treatment of dogs with meningoencephalomyelitis of unknown origin. Vet Rec. 2020;187(11):e98. [DOI] [PubMed] [Google Scholar]
- 21. Paušová TK, Tomek A, Šrenk P, Belašková S. Clinical presentation, diagnostic findings, and long‐term survival time in 182 dogs with meningoencephalitis of unknown origin from central Europe that were administered glucocorticosteroid monotherapy. Top Companion Anim Med. 2021;44:100539. [DOI] [PubMed] [Google Scholar]
- 22. Kaczmarska A, José‐López R, Czopowicz M, et al. Postencephalitic epilepsy in dogs with meningoencephalitis of unknown origin: clinical features, risk factors, and long‐term outcome. J Vet Intern Med. 2020;34(2):808‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonçalves R, De Decker S, Walmsley G, Butterfield S, Maddox TW. Inflammatory disease affecting the central nervous system in dogs: a retrospective study in England (2010–2019). Front Vet Sci. 2022;8:819945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowrie M, Thomson S, Smith P, Garosi L. Effect of a constant rate infusion of cytosine arabinoside on mortality in dogs with meningoencephalitis of unknown origin. Vet J. 2016;213:1‐5. [DOI] [PubMed] [Google Scholar]
- 25. Gonçalves R, Maddox TW, Phillipps S, et al. Development of a reliable clinical assessment tool for meningoencephalitis in dogs: the neurodisability scale. J Vet Intern Med. 2023;37(3):1111‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beasley MJ, Shores A. Perspectives on pharmacologic strategies in the management of meningoencephalomyelitis of unknown origin in dogs. Front Vet Sci. 2023;10:1167002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnoon I, Shamir MH, Aroch I, et al. Retrospective evaluation of combined mycophenolate mofetil and prednisone treatment for meningoencephalomyelitis of unknown etiology in dogs: 25 cases (2005–2011). J Vet Emerg Crit Care. 2016;26(1):116‐124. [DOI] [PubMed] [Google Scholar]
- 28. Mercier M, Barnes Heller HL. Efficacy of glucocorticoid monotherapy for treatment of canine meningoencephalomyelitis of unknown etiology: a prospective study in 16 dogs. Vet Med Sci. 2015;1(1):16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith PM, Stalin CE, Shaw D, Granger N, Jeffery ND. Comparison of two regimens for the treatment of meningoencephalomyelitis of unknown etiology. J Vet Intern Med. 2009;23(3):520‐526. [DOI] [PubMed] [Google Scholar]
- 30. Cornelis I, Volk HA, De Decker S. Clinical presentation, diagnostic findings and long‐term survival in large breed dogs with meningoencephalitis of unknown aetiology. Vet Rec. 2016;179(6):147. [DOI] [PubMed] [Google Scholar]
- 31. Coates JR, Jeffery ND. Perspectives on meningoencephalomyelitis of unknown origin. Vet Clin North Am Small Anim Pract. 2014;44(6):1157‐1185. [DOI] [PubMed] [Google Scholar]
- 32. Wolff CA, Holmes SP, Young BD, et al. Magnetic resonance imaging for the differentiation of neoplastic, inflammatory, and cerebrovascular brain disease in dogs. J Vet Intern Med. 2012;26(3):589‐597. [DOI] [PubMed] [Google Scholar]
- 33. Young BD, Fosgate GT, Holmes SP, et al. Evaluation of standard magnetic resonance characteristics used to differentiate neoplastic, inflammatory, and vascular brain lesions in dogs. Vet Radiol Ultrasound. 2014;55(4):399‐406. [DOI] [PubMed] [Google Scholar]
- 34. Bentley RT. Magnetic resonance imaging diagnosis of brain tumors in dogs. Vet J. 2015;205(2):204‐216. [DOI] [PubMed] [Google Scholar]
- 35. Cervera V, Mai W, Vite CH, Johnson V, Dayrell‐Hart B, Seiler GS. Comparative magnetic resonance imaging findings between gliomas and presumed cerebrovascular accidents in dogs. Vet Radiol Ultrasound. 2011;52(1):33‐40. [PubMed] [Google Scholar]
- 36. Cornelis I, Volk HA, Van Ham L, et al. Clinical presentation, diagnostic findings and outcome in dogs diagnosed with presumptive spinal‐only meningoencephalomyelitis of unknown origin. J Small Anim Pract. 2017;58:174‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Griffin JF, Levine JM, Levine GJ, et al. Meningomyelitis in dogs: a retrospective review of 28 cases (1999 to 2007). J Small Anim Pract. 2008;49:509‐517. [DOI] [PubMed] [Google Scholar]
- 38. Santifort KM, Garosi L, Weerts EAWS. Case report: Necrotizing leukomyelitis and meningitis in a Pomeranian. Front Vet Sci. 2024;11:1303084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park ES, Uchida K, Nakayama H. Comprehensive immunohistochemical studies on canine necrotizing meningoencephalitis (NME), necrotizing leukoencephalitis (NLE), and granulomatous meningoencephalomyelitis (GME). Vet Path. 2012;49(4):682‐692. [DOI] [PubMed] [Google Scholar]


