Abstract
Immune thrombocytopenia (ITP) is the most common acquired primary hemostatic disorder in dogs. Immune thrombocytopenia less commonly affects cats but is an important cause of mortality and treatment‐associated morbidity in both species. Immune thrombocytopenia remains a diagnosis of exclusion for which diagnostic guidelines are lacking. Primary, or non‐associative, ITP refers to autoimmune platelet destruction. Secondary, or associative, ITP arises in response to an underlying disease trigger. However, evidence for which comorbidities serve as ITP triggers has not been systematically evaluated. To identify key diagnostic steps for ITP and important comorbidities associated with secondary ITP, we developed 12 Population Evaluation/Exposure Comparison Outcome (PECO) format questions. These questions were addressed by evidence evaluators utilizing a literature pool of 287 articles identified by the panelists using a structured search strategy. Evidence evaluators, using panel‐designed templates and data extraction tools, summarized evidence and created guideline recommendations that then were integrated by diagnosis and comorbidity domain chairs. The revised PECO responses underwent a Delphi survey process to reach consensus on final guidelines. A combination of panel expertise and PECO responses were employed to develop algorithms for diagnosis of ITP in dogs and cats, which also underwent 4 iterations of Delphi review. Comorbidity evidence evaluators employed an integrated measure of evidence (IME) tool to determine evidence quality for each comorbidity; IME values combined with evidence summaries for each comorbidity were integrated to develop ITP screening recommendations, which also were subjected to Delphi review. Commentary was solicited from multiple relevant professional organizations before finalizing the consensus. The final consensus statement provides clinical guidelines for the diagnosis of, and underlying disease screening for, ITP in dogs and cats. The systematic consensus process identified numerous knowledge gaps that should guide future studies. This statement is a companion manuscript to the ACVIM Consensus Statement on the Treatment of Immune Thrombocytopenia.
Keywords: autoimmune, hemostasis, immune‐mediated, platelet, thrombopoietin
Abbreviations
- AAFP
American Association of Feline Practitioners
- EE
evidence evaluator
- FeLV
feline leukemia virus
- FIV
feline immunodeficiency virus
- GPs
glycoproteins
- IME
integrated measure of evidence
- IMHA
immune‐mediated hemolytic anemia
- IPF
immature platelet fraction
- ITP
immune thrombocytopenia
- MPV
mean platelet volume
- PECO
population evaluation/exposure comparison outcome
- PSAIG
platelet surface‐associated immunoglobulin
- TPO
thrombopoietin
1. INTRODUCTION
Immune thrombocytopenia (ITP) is the most common acquired primary hemostatic disorder in dogs, 1 and although less common, ITP does occur in cats. The mortality rate of ITP in dogs and cats ranges from 10% to 30% and substantial immunosuppressant treatment‐related morbidity occurs. 2 , 3 , 4 , 5 In severe ITP in humans, mortality results equally from secondary infections associated with immunosuppression and from refractory hemorrhage. 6 Because ITP is potentially fatal and managed with potent immunosuppressants, rapid and accurate disease diagnosis is critical. Presently, ITP in dogs and cats is a diagnosis of exclusion that lacks definitive diagnostic criteria. A similar diagnostic ambiguity exists for ITP in humans, precluding adoption of well‐defined human ITP guidelines.
The lack of a diagnostic test for ITP can be attributed to ITP's heterogenous nature, with interpatient variability in pathogenesis, disease course, and response to treatment. In people, the pathogenesis of ITP involves autoantibodies targeting platelet surface glycoproteins (GPs) resulting in Fc gamma receptor (FcγR)‐mediated platelet clearance by the mononuclear phagocytic system. In addition, antibody‐triggered platelet desialylation may result in Ashwell‐Morell receptor‐mediated removal by hepatocytes or clearance by hepatic Kupffer cells. 7 , 8 , 9 , 10 Complement‐mediated platelet destruction also may contribute in some cases. 11 Cytotoxic T cell‐mediated platelet destruction may occur in the absence of detectable platelet autoantibodies. 7 , 12 , 13 , 14 , 15 Antibodies and T cells also can target megakaryocytes, inhibiting platelet production. 7 Thrombopoietin (TPO) is the major regulator of platelet production and is necessary for survival, proliferation, and differentiation of megakaryocytes to platelets. 16 , 17 , 18 Inappropriately low TPO concentrations are common in human ITP patients, contributing to decreased platelet production. 16 , 17 , 18 Platelet‐ and megakaryocyte‐associated antibodies have been documented in dogs with ITP using assays that have variable sensitivity and specificity (see Section 4.1.7 below). 19 , 20 , 21 It is likely that antibody‐independent mechanisms of immune‐mediated destruction occur, as described in humans. 15 Variable mechanisms of platelet destruction contribute to ITP's diagnostic complexity.
Immune thrombocytopenia can be spontaneous (primary, or non‐associative) or induced by a putative trigger (secondary, or associative). We chose to use “primary” and “secondary” in keeping with the standard nomenclature in human medicine. Secondary ITP pathogenesis and presentation varies with the underlying cause, resulting in disease heterogeneity. Treatment of secondary ITP aims to eliminate disease triggers, but inciting causes of ITP in dogs and cats have not been systematically reviewed. Guidelines for investigation of potential ITP triggers are needed to improve case management.
The clinical presentation of primary ITP is also variable, with limited association between thrombocytopenia severity and clinical signs of bleeding. Many dogs and cats remain subclinical despite severe thrombocytopenia, whereas others with similar platelet counts experience life‐threatening hemorrhage. 22 Biomarkers of disease severity are needed to guide individualized treatment.
We designed a diagnostic algorithm for systematic exclusion of other causes of thrombocytopenia as an essential aid in ITP diagnosis. Informed by systematic review of the available veterinary evidence, we also aimed to develop guidelines on (a) the diagnostic approach to ITP in dogs and cats; (b) comorbidity screening in dogs and cats with ITP; and (c) diagnostic testing to inform prognosis and guide intensity of treatment. Guidelines were developed using a standardized Population Exposure/Evaluation Comparison Outcome (PECO) question format. Knowledge gaps in the available evidence were identified to inform future study design.
2. MATERIALS AND METHODS
An overview of the consensus statement process is shown schematically in Figure 1 and described below. A comprehensive literature search strategy was developed to identify all manuscripts relevant to ITP in dogs and cats (detailed in Supporting Information 1). Clinical diagnostic algorithms for ITP in dogs and cats were developed and used by evidence evaluators in their literature reviews. Draft algorithms were developed (OAG, LK) based on the collective expertise of the consensus panel members and refined by panel members using a Delphi review process. The diagnostic algorithms were reviewed a final time after completion of the PECO question process.
FIGURE 1.
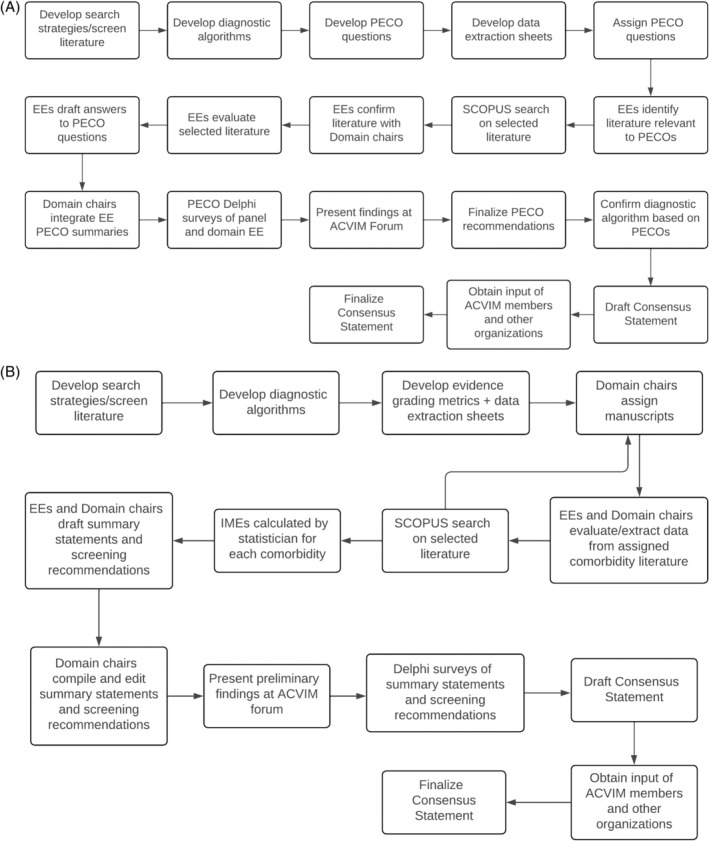
(A) Overview of the methodology of the Diagnosis Domain. (B) Overview of the methodology of the Comorbidity Domain. EE, evidence evaluator; IME, integrated metric of evidence; PECO, Population, Exposure/Evaluation, Comparison, Outcome question.
The literature review was divided into 2 domains: Diagnosis (co‐chairs MBB and DNL; evidence evaluators JA, PWC, BG, HM, MCN, LNN, SS, AKV) and Comorbidity (co‐chairs LK and OAG; evidence evaluators AJB, MAF, ASH, KFL, EL, KFL, KMM, XR, ES). Supporting Information 1 includes extended details of the methods, whereas Supporting Information 2 and 3 includes bibliographies of the manuscripts reviewed by the Diagnosis and Comorbidity domains, respectively. Supporting Information 4 includes the instruction guide sent to all Diagnosis evidence evaluators. Supporting Information 5 includes the instruction guide sent to all Comorbidity evidence evaluators.
The Diagnosis domain chairs generated clinical questions using a PECO format to investigate whether in dogs and cats with thrombocytopenia (P), evaluation by a diagnostic test (E) compared with platelet count alone (C) improved differentiation of ITP from non‐immune thrombocytopenia (O). To investigate disease severity, population referred to dogs and cats diagnosed with primary ITP and outcome referred to disease severity encompassing bleeding risk, blood product usage, duration of hospitalization, time to platelet recovery, response to first‐line treatment, or relapse. Finalized PECO questions were answered by the evidence evaluators using the identified references and structured data extraction and summary templates (Supporting Information 6 and 7). Evaluators' PECO responses (n = 2/PECO) were reviewed by domain chairs and synthesized into a single consensus response to each PECO that subsequently underwent Delphi review. Consensus, or near complete consensus, was reached after 2 Delphi rounds. The remaining disagreements are indicated below.
The comorbidity PECO, “In a population of dogs or cats (P), what is the effect of exposure (E) to comorbidity X compared with a lack of exposure (C) on the development of ITP (O)?” was answered by the evidence evaluators in the Comorbidity domain using a quality assessment tool (Integrated Metric of Evidence; IME) and data extraction tool we adapted from the IMHA diagnosis consensus statement (Supporting Information 8 and 9). 23 Threshold IME values were computed to allow comorbidities to be designated as negligible, low, intermediate, or high evidence for a causal relationship with ITP. For the Comorbidity domain, manuscripts, not PECOs, were assigned to 2 or 3 evidence evaluators for data extraction, evidence evaluation, and quality assessment. Evidence summaries and screening recommendations were drafted by evidence evaluators for each comorbidity in each manuscript; these summaries and recommendations then were consolidated by LK and subjected to 3 to 4 rounds of Delphi review until 100% consensus was reached.
3. ALGORITHM FOR THE DIAGNOSIS OF ITP
Consensus on final versions of the algorithms (Figures 2 and 3) was reached after 4 rounds of revision and review. The first step of the diagnostic algorithms, “Platelet count <100 000/μL confirmed by blood smear examination,” warrants further emphasis. The diagnosis of ITP requires determination of platelet count. Although beyond the scope of this discussion, clinicians must consider the biologic, pre‐analytic, and analytic variables that influence platelet count. 24 , 25 , 26 Importantly, platelet activation during blood collection or processing can result in spurious thrombocytopenia. 27 , 28 Pseudothrombocytopenia is especially common in cats because of variable platelet size and relative hyper‐reactivity of feline platelets compared with those of other species. 29 Automated hematology analyzers employ different test principles, and all are subject to inherent imprecision. 30 Regardless of instrumentation, low platelet count should be interpreted critically in the context of allowable errors of up to 25%. 26 Confirmation of thrombocytopenia must include slide examination, and replicate platelet count determinations often are warranted. An appropriate diagnosis of ITP is based on persistent thrombocytopenia, and platelet count monitoring over time is best performed using consistent methodology. 30
FIGURE 2.
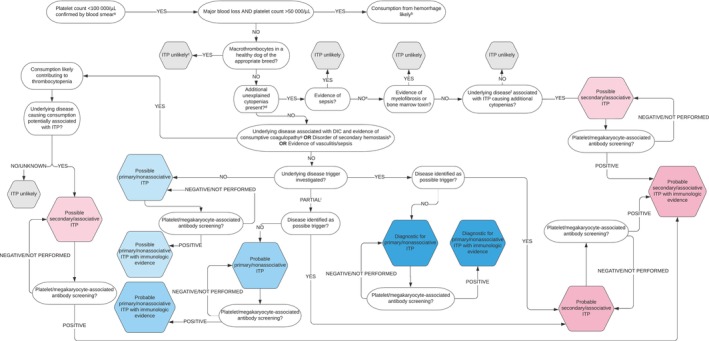
An algorithm for the diagnosis of ITP in dogs. aThrombocytopenia should be confirmed with a slide assessment. In brief, to perform a platelet estimate, first assess the slide body and feathered edge under low magnification for clumps, the presence of which suggests that the platelet count is falsely low. Resampling is then warranted, if possible. If there are no clumps, a platelet count is estimated by calculating the mean number of platelets per 10 oil immersion fields (×100) in the body of the smear where erythrocytes are spread in monolayer and multiplying this number by 15 000 to 20 000 to obtain the number of platelets per microliter; bthe magnitude of thrombocytopenia is consistent with consumption from major hemorrhage. However, it is possible that DIC, vasculitis, sequestration, or ITP may be contributing; cconsider genetic testing; dexcludes additional cytopenias with plausible explanations for example, pre‐regenerative anemia in the face of acute hemorrhage or hemolysis or lymphopenia in an ill/stressed patient; esampling of bone marrow by aspiration, core biopsy, or both, is undertaken; ffor example, lymphoreticular neoplasia or ehrlichiosis; gat least 2 of 5 parameters abnormal in addition to thrombocytopenia: prothrombin time (PT), activated partial thromboplastin time (aPTT), D‐dimers > reference interval (RI); antithrombin, fibrinogen < RI; hPT or aPTT >25% control value or upper bound of RI; ipartial screening (ie, not exhaustive) for potential trigger factors undertaken and negative.
FIGURE 3.
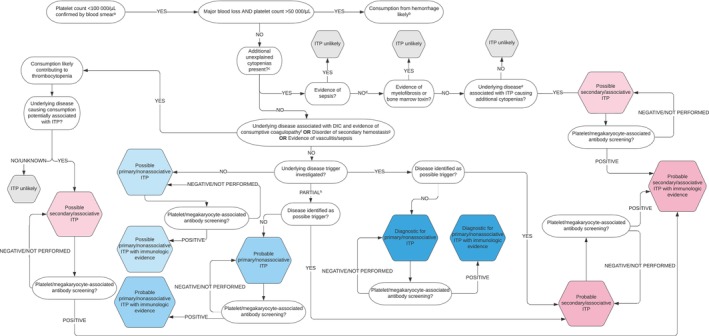
An algorithm for the diagnosis of ITP in cats. aThrombocytopenia should be confirmed with a slide assessment. In brief, to perform a manual count first assess the slide's feathered edge under low magnification for clumps, the presence of which suggests that the platelet count is falsely low. Resampling is then warranted, if possible. If there are no clumps, a platelet count is estimated by calculating the mean number of platelets per 10 oil immersion fields (×100) and multiplying this number by 15 000 to 20 000 to obtain the number of platelets per microliter; bthe magnitude of thrombocytopenia is consistent with consumption from major hemorrhage; this is a rare association in cats. However, it is possible that DIC, vasculitis, sequestration, or ITP may be contributing; cexcludes additional cytopenias with plausible explanations for example, pre‐regenerative anemia in the face of acute hemorrhage or hemolysis or lymphopenia in an ill/stressed patient; dsampling of bone marrow by aspiration, core biopsy, or both, is undertaken; efor example, lymphoreticular neoplasia or feline immunodeficiency virus infection; fat least 2 of 4 parameters abnormal in addition to thrombocytopenia: prothrombin time (PT), activated partial thromboplastin time (aPTT), D‐dimers > reference interval (RI); fibrinogen < RI; gPT or aPTT >25% control value; hpartial screening (ie, not exhaustive) for potential trigger factors undertaken and negative.
The final versions of the algorithm delineate 6 levels of diagnostic certainty for primary ITP: Possible, Possible with immunologic evidence, Probable, Probable with immunologic evidence, Diagnostic, and Diagnostic with immunologic evidence. Although we considered that immunologic evidence such as identification of platelet surface‐associated immunoglobulin (PSAIG) or megakaryocyte‐associated immunoglobulin strengthened the diagnosis, a confirmatory diagnosis of ITP could be made without such testing. For secondary ITP, diagnostic certainty levels include Possible, Probable, or Probable with immunologic evidence. Coexistence of more than 1 mechanism of thrombocytopenia in secondary ITP precludes definitive diagnosis of ITP in this context.
4. GUIDELINES FOR DIAGNOSIS OF ITP
The diagnostic guidelines below are presented under their respective PECO questions with their evidence summaries. Guidelines for dogs and cats are presented separately. Guidelines for PECO questions for which minimal or no evidence was identified (common for cats) are included in Supporting Information 11.
4.1.
4.1.1. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone (C) do platelet indices (eg, mean platelet volume [MPV], immature platelet fraction [IPF], reticulated platelets, plateletcrit) (E) improve differentiation of ITP from non‐immune thrombocytopenia (O)?
Guidelines (dogs)
There is conflicting evidence regarding changes in MPV in dogs with ITP compared with other causes of thrombocytopenia or healthy dogs.
In dogs with thrombocytopenia, increased reticulated platelets may help differentiate ITP from non‐immune thrombocytopenia, but not between dogs with primary and secondary ITP.
There is insufficient evidence to make recommendations regarding use of plateletcrit and IPF in the diagnosis of ITP in dogs.
In thrombocytopenic dogs, use of plateletcrit, IPF, and MPV as routine diagnostic tests for primary ITP are not recommended.
In thrombocytopenic dogs, we suggest that increased reticulated platelets (when measurement is available) can be considered to support a diagnosis of primary or secondary ITP.
Level of evidence (dogs): Low. Strength of recommendation: Weak.
Evidence summary (dogs)
Of the 21 observational studies in dogs reviewed for this PECO question, 14 studies with some relevance were identified, and none directly addressed the question. 19 , 21 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42
Reticulated platelets
Of the available platelet indices, most evidence exists for use of reticulated platelets to distinguish primary ITP from non‐immune thrombocytopenia, but overall, the evidence is weak. In a prospective cross‐sectional study including 17 dogs with probable primary ITP, increased reticulated platelet percentage helped differentiate primary ITP from non‐immune thrombocytopenia, whereas platelet count did not. 19 In this study, a reticulated platelet threshold of 8% combined with PSAIG was sensitive (94.1%) but not specific (27.6%) for discriminating primary ITP from other causes of thrombocytopenia. 19 However, neither platelet count nor percentage of reticulated platelets differentiated primary from secondary ITP. 19 In another prospective cross‐sectional study 36/45 (80%) thrombocytopenic dogs had increased reticulated platelets. All 6 dogs with ITP had increased reticulated platelets, but so did many other dogs with non‐immune thrombocytopenia, and sub‐groups were not directly compared. 31 Smaller studies have observed increased reticulated platelets in dogs with ITP 41 and thrombocytopenia in general. 32 Reticulated platelets decrease when platelet counts increase, 33 , 34 but do not correlate with bone marrow megakaryocyte numbers. 35
MPV
Seven studies evaluating MPV in ITP were reviewed with conflicting results. 21 , 36 , 37 , 38 , 39 , 40 , 42 In dogs with ITP, both decreased to normal MPV 21 , 42 and increased MPV have been observed. 37 , 38 In a retrospective case series of 83 thrombocytopenic dogs, those with ITP (primary and secondary combined, n = 37) and with primary ITP (n = 17) had lower MPV than dogs with non‐immune thrombocytopenia. 21 However, the diagnostic algorithm could not be completely applied to this study, decreasing diagnostic certainty. 21 Most studies compared dogs with ITP to healthy controls rather than to dogs with non‐immune thrombocytopenia. 38 , 39 For example, a retrospective case‐control study determined that dogs diagnosed with primary ITP (n = 49) have increased MPV compared with healthy control dogs (n = 46), 38 a finding corroborated by a retrospective cross‐sectional study. 39 A retrospective case series of dogs with PSAIG showed that MPV was lower in dogs with primary ITP (n = 21) than in dogs with secondary ITP (n = 24). 36 In contrast, another case series described increased MPV in 4/5 dogs with primary ITP and low to normal MPV in dogs with secondary ITP. 37 Finally, a retrospective case series of 60 thrombocytopenic dogs observed that MPV was significantly associated with a bone marrow response, but did not assess the utility of MPV for differentiating the cause of thrombocytopenia. 40
Plateletcrit
No studies evaluating the use of plateletcrit or IPF for differentiating the cause of thrombocytopenia in dogs were identified. In a retrospective case‐control study, plateletcrit was decreased in dogs with primary ITP compared with healthy control dogs, but no dogs with thrombocytopenia of other causes were assessed. 38
Link to diagnostic algorithm (Figure 2): Although not included in our systematic review, we recommend that identification of macrothrombocytes, particularly in healthy dogs lacking signs of a primary hemostatic defect and breeds, or mixed breeds, with hereditary macrothrombocytopenia, should prompt genetic testing early in the diagnostic evaluation. 43 , 44
Guidelines and evidence summary (cats)
See Supporting Information 11.
4.1.2. In dogs or cats with primary ITP (P), do platelet indices (E) compared with platelet count alone (C) impact predictions of disease severity, including bleeding risk, blood product requirement, hospitalization duration, time to platelet recovery, response to first‐line treatment, survival, or relapse (O)?
Guidelines (dogs)
In dogs with primary ITP, there is weak evidence to suggest that plateletcrit as opposed to platelet count is more sensitive to platelet recovery.
In dogs with primary ITP, there is insufficient evidence to assess the utility of MPV, reticulated platelets, or IPF for prediction of bleeding severity, duration of hospitalization, blood product requirement, or platelet count recovery.
In dogs with primary ITP, use of MPV, reticulated platelets, and IPF as routine prognostic tools is not recommended.
We suggest that serial plateletcrits be considered as adjunctive parameters to platelet counts in assessing response to treatment in dogs with ITP.
Level of evidence (dogs): Low. Strength of recommendation: Weak.
Evidence summary (dogs)
No studies were identified that directly assessed platelet indices as severity metrics, but 3 studies provided indirect information. 34 , 38 , 45 One retrospective case‐control study observed that in dogs with primary ITP (n = 49) plateletcrit increased before platelet count, 38 but did not compare the prognostic value of platelet count and plateletcrit values. Two single case reports suggested that increased reticulated platelet percentage at the time of diagnosis might be associated with treatment response, but confirmatory evidence is lacking. 34 , 45
Guidelines and evidence summary (cats)
See Supporting Information 11.
4.2.
4.2.1. In dogs/cats with confirmed thrombocytopenia (P), does severe thrombocytopenia (E) compared with mild to moderate thrombocytopenia (C), improve differentiation of ITP from non‐immune thrombocytopenia (O)?
Guidelines (dogs)
Most evidence suggests that dogs with ITP have more severe thrombocytopenia compared with non‐immune causes of thrombocytopenia, but there is overlap between diagnostic groups.
In dogs with thrombocytopenia, evaluation of the severity of thrombocytopenia may help differentiate ITP from non‐immune thrombocytopenia.
We suggest that a platelet count <20 000/μL supports a diagnosis of ITP in dogs but is insufficient to independently make such a diagnosis.
Level of evidence (dogs): Moderate. Strength of recommendation: Moderate.
Evidence summary (dogs)
Five studies were identified that suggest thrombocytopenia severity has diagnostic relevance. 21 , 22 , 36 , 46 , 47 Two were prospective, 22 , 46 but did not specifically address the PECO question. In 4 studies, dogs with primary ITP had more severe thrombocytopenia than dogs with non‐immune thrombocytopenia. 21 , 22 , 46 , 47 In 3 studies, dogs with primary ITP had more severe thrombocytopenia than dogs with secondary ITP. 21 , 22 , 36 In all 5 studies, platelet counts overlapped between groups, precluding use of platelet count alone for primary ITP diagnosis, but suggesting platelet count may aid diagnosis when combined with other data. One retrospective study reported that thrombocytopenia <20 000/μL was consistent with ITP, particularly when accompanied by decreased MPV. However, a cutoff of 40 000 platelets/μL was not discriminating for ITP. 42
Two opposing studies were identified. In 1 retrospective study, dogs with ITP and disseminated intravascular coagulation (DIC) had lower platelet counts than dogs with thrombocytopenia from other causes, but thrombocytopenia severity was not discriminating. 48 Another retrospective study of dogs with thrombocytopenia observed only mild thrombocytopenia (78 000‐125 000/μL) in dogs with ITP, 49 whereas dogs with DIC and other non‐immune causes of thrombocytopenia had lower platelet counts. Statistical comparisons were not performed.
Most studies evaluated were retrospective, 21 , 36 , 42 , 47 , 48 , 49 many lacked long‐term follow‐up, and some were hampered by case selection bias precluding strong recommendations. Overall, the available evidence suggests that severe thrombocytopenia compared with mild or moderate thrombocytopenia may help differentiate ITP from other causes of thrombocytopenia, but platelet count cannot be used as the sole criterion.
Guidelines (cats)
There is weak evidence suggesting that cats with ITP have more severe thrombocytopenia compared with those with non‐immune causes of thrombocytopenia.
In cats with thrombocytopenia, evaluation of the severity of thrombocytopenia may help differentiate ITP from non‐immune thrombocytopenia.
We suggest that a platelet count <20 000/μL supports a diagnosis of ITP in cats but is insufficient to independently make such a diagnosis.
Level of evidence (cats): Low. Strength of recommendation: Weak.
Evidence summary (cats)
No studies were identified that directly addressed the PECO question, but 4 studies provided indirect and weakly supportive evidence. 4 , 5 , 50 , 51 A retrospective study of 194 cats with thrombocytopenia observed that cats with probable primary ITP had the most severe thrombocytopenia, but platelet counts <50 000/μL also were seen in cats with neoplasia, infection, bone marrow disease, or traumatic hemorrhage. 50 Bone marrow disease was the most common cause of platelet counts <50 000/μL. 50 A retrospective study of thrombocytopenia in 41 cats identified 1 case of primary ITP, but insufficient information was provided to apply the diagnostic algorithm. This cat had 1 of the lowest platelet counts in the study, but the result was similar to those found in infectious causes of thrombocytopenia. 51 In a case series of 4 cats with confirmed primary ITP, all had platelet counts <20 000/μL at diagnosis. 5 Similarly, in 5 cats with confirmed primary ITP, observed platelet counts ranged from 4 to 46 000/μL. 4
Link to diagnostic algorithms (Figures 2 and 3): Our diagnostic algorithm begins with a confirmed platelet count of <100 000/μL, consistent with ITP definitions in human medicine, and a relevant threshold for likely ITP diagnoses in animals. 52 Some animals with ITP may have higher platelet counts at diagnosis, but this threshold increases the likelihood of an ITP diagnosis. Humans with mild thrombocytopenia (100 000‐150 000/μL) have only a 6.9% 10‐year probability of developing more severe thrombocytopenia. 53
4.2.2. In dogs/cats with primary ITP (P), does severe thrombocytopenia (E) compared with mild to moderate thrombocytopenia (C) impact prediction of bleeding severity, response to first line treatment, relapse, survival, hospitalization duration, blood product requirement, or time to platelet count recovery (O)?
Guidelines (dogs)
In dogs with primary ITP with moderate to severe thrombocytopenia (platelet count <50 000/μL), there is moderate evidence to suggest that admission platelet count does not impact disease outcome or response to treatment but may be related to signs of hemorrhage at presentation.
In dogs with primary ITP with moderate to severe thrombocytopenia (platelet count <50 000/μL), we suggest that admission platelet count alone should not be employed to predict disease outcome or guide treatment.
No studies were identified that compared outcomes in dogs with primary ITP with mild (platelet count >75 000/μL) versus moderate to severe thrombocytopenia (platelet count <50 000/μL).
Level of evidence (dogs): Moderate. Strength of recommendation: Moderate.
Evidence summary (dogs)
One prospective observational study was identified that addressed the PECO question, but was considered neutral because all dogs diagnosed with primary ITP had platelet counts <50 000/μL. 22 Platelet count and bleeding severity score (DOGiBAT bleeding assessment tool) were negatively correlated in dogs with primary ITP, suggesting a relationship between these variables, but platelet count was not associated with blood product administration, duration of hospitalization, or survival to discharge.
Five studies indirectly addressed the PECO question and were considered opposed to it. 46 , 54 , 55 , 56 , 57 In a prospective cross‐sectional study of 28 dogs with immunologically confirmed ITP and thrombocytopenia ≤60 000/μL, admission platelet count did not predict treatment response. 46 A retrospective cohort study of 73 dogs with probable primary ITP and thrombocytopenia <50 000/μL observed that dogs with melena had decreased survival and higher transfusion requirements than those without melena. 54 Platelet counts were similar between dogs with and without melena, suggesting that platelet count did not impact bleeding severity. Furthermore, the initial platelet count and lowest recorded platelet count were not associated with survival to discharge. 54 A prospective study evaluating the use of vincristine in 24 dogs with probable primary ITP and thrombocytopenia <15 000/μL did not observe a relationship between initial platelet count and time for platelet count recovery. 55 A retrospective case series of 65 dogs with probable primary ITP and thrombocytopenia <40 000/μL determined that platelet count at admission did not differ between dogs that relapsed and those that did not. 56 Likewise, a retrospective case series of 15 dogs with ITP and thrombocytopenia <50 000/μL also found no relationship between initial platelet count and relapse. 57
Two studies were reviewed that described more bleeding in dogs with severe thrombocytopenia but did not rigorously address patient outcomes related to platelet count. In 1 retrospective case series of 30 dogs with probable primary ITP with immunologic confirmation, thrombocytopenia <30 000/μL was positively correlated with spontaneous bleeding. 3 Most dogs in the study had thrombocytopenia <30 000/μL, but some had platelet counts up to 110 000/μL. All dogs that received transfusions had thrombocytopenia ≤20 000/μL. 3 In another retrospective case series of 54 dogs with possible primary ITP, all dogs with bleeding had platelet counts ≤30 000/μL and 80% of bleeding dogs had platelet counts ≤10 000/μL, 58 but the range of platelet counts was not reported. 58
Prospective studies with standardized treatment that assess the association between thrombocytopenia severity and outcome are lacking. It is possible that dogs with severe thrombocytopenia were treated more aggressively in the reviewed studies, masking an impact of platelet count on disease outcome.
Guidelines and evidence summary (cats)
See Supporting Information 11.
4.3.
4.3.1. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone (C) does the addition of bone marrow examination (E) help differentiate ITP from non‐immune thrombocytopenia (O)?
Guidelines (dogs)
There is insufficient evidence to determine whether bone marrow examination improves the diagnosis of primary ITP in dogs.
Bone marrow examination is not recommended as a routine diagnostic test for primary ITP in dogs.
We suggest that bone marrow examination be considered to characterize ill‐defined cytopenias, recognizing that there is no bone marrow abnormality diagnostic for primary ITP.
Level of evidence (dogs): Low. Strength of recommendation: Weak.
Evidence summary (dogs)
No studies were identified that directly addressed the PECO question. Several case reports and case series described bone marrow examination in dogs with thrombocytopenia. In a study of 55 dogs with thrombocytopenia that underwent bone marrow examinations, diagnostically relevant cytologic abnormalities were less common in dogs with platelet counts <20 000/μL compared with platelet counts between 20 000 and 200 000/μL. 59 However, causes of thrombocytopenia were not differentiated. Megakaryocytic hyperplasia, hypoplasia, and normal megakaryocyte numbers all are described in dogs with ITP, 21 , 54 , 58 , 60 , 61 but these studies did not analyze or compare bone marrow findings between dogs with ITP and those with non‐immune causes of thrombocytopenia.
Guidelines and evidence summary (cats)
See Supporting Information 11.
Link to diagnostic algorithms (Figures 2 and 3): Bone marrow evaluation is only recommended if otherwise unexplained additional cytopenias are present, consistent with current American Society of Hematology guidelines. 62
4.3.2. In dogs/cats with primary ITP (P) does bone marrow evaluation (E) compared with platelet count alone (C) improve prediction of bleeding severity, response to first‐line therapy, survival, blood product requirement, duration of hospitalization, days to platelet recovery, or ITP relapse (O)?
Guidelines (dogs)
Available evidence is contradictory regarding the utility of megakaryocyte hypoplasia for prediction of disease severity in dogs with primary ITP.
In dogs with primary ITP, bone marrow examination is not recommended as a routine prognostic tool.
Level of evidence (dogs): Low. Strength of recommendation: Weak.
Evidence summary (dogs)
No studies were identified that directly addressed the PECO question. In a retrospective case series of 34 dogs with primary ITP, anemia, clinical bleeding, transfusion, and poor survival were associated with megakaryocytic hypoplasia or dysplasia (no statistical analysis performed). 63 In 2 case reports, dogs with primary ITP and megakaryocytic hypoplasia had clinical bleeding and delayed responses to treatment. 64 , 65 In contrast, a retrospective cohort study of 73 dogs with probable primary ITP that included 11 dogs with bone marrow examination found no relationship between megakaryocyte hypoplasia and days to platelet recovery, or survival to discharge. 54 A case series of dogs with thrombocytopenia, including 13 dogs with primary ITP, found that 10 had clinical signs of bleeding, and all 9 dogs with bone marrow evaluation had megakaryocytic hyperplasia but how many of those dogs experienced bleeding was not stated. 21 Finally, all 7 dogs with megakaryocyte hypoplasia survived to discharge in a case series of dogs with thrombocytopenia that included primary ITP cases. 59 Some evidence suggests that megakaryocyte hypoplasia may be related to more severe disease, but contradictory evidence on megakaryocyte response also exists; hence, the prognostic value of bone marrow examination could not be determined.
Guidelines and evidence summary (cats)
See Supporting Information 11.
4.4.
4.4.1. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone (C) do platelet/megakaryocyte‐associated antibody assays (E) help differentiate ITP from non‐immune thrombocytopenia (O)?
Guidelines (dogs)
We suggest that in thrombocytopenic dogs, positive platelet/megakaryocyte‐associate antibody tests indicate that an immune component is contributing to thrombocytopenia but are not diagnostic for ITP.
In dogs with thrombocytopenia, routine measurement of platelet/megakaryocyte‐associated antibodies is not recommended.
Level of evidence (dogs): Low. Strength of recommendation: Weak.
Evidence summary (dogs)
Eighteen studies were reviewed that employed platelet or megakaryocyte antibody testing, all neutral to the PECO question. 19 , 20 , 21 , 32 , 36 , 41 , 46 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 In total, the evidence suggests that the presence of PSAIG indicates an immune component to the thrombocytopenia, but is not diagnostic for ITP.
One prospective cross‐sectional study describing a direct radioimmunoassay presented sufficient data to allow calculation of assay diagnostic metrics. 20 The PSAIG radioimmunoassay and platelet count <40 000/μL performed similarly for primary ITP diagnosis (Table 1). The PSAIG assay did not reliably differentiate ITP from non‐immune causes of thrombocytopenia because 9/17 dogs with non‐immune thrombocytopenia had PSAIG concentrations above the cut‐off. 20 It was also possible to calculate the positive predictive value (PPV) of PSAIG and platelet count from a retrospective case series, 21 with both PSAIG and platelet count <20 000/μL performing similarly (Table 1). Although 12/13 dogs with ITP were PSAIG‐positive, dogs with non‐immune causes similarly were positive. 21 Diagnostic data provided were inconsistent, precluding application of the diagnostic algorithm. These studies provide weak evidence suggesting that PSAIG and platelet count perform similarly for differentiating ITP from non‐immune thrombocytopenia.
TABLE 1.
Diagnostic performance of various assays for platelet surface‐associated immunoglobulins (PSAIG).
| Study | PSAIG assay | Metric | Primary ITP vs other causes of thrombocytopenia | ITP (primary and secondary) vs non‐immune thrombocytopenia | ||||
|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | Sens | Spec | |||
| Bachman et al 19 | Flow cytometry | Direct IgG | 29% | 76% | 28% | 79% | ||
| Direct/indirect IgM or IgG | 77% | 66% | ||||||
| Direct/indirect IgG | 46% | 71% | ||||||
| Scott et al 20 | Radioimmunoassay | PSAIG | 100% | 47% | 72% | 100% | ||
| PLT <40 K/μL | 96% | 76% | 85% | 93% | ||||
| Dircks et al 21 | Flow cytometry | PSAIG | 32% | |||||
| PLT <20 K/μL | 34% | |||||||
Abbreviations: ITP, immune thrombocytopenia; NPV, negative predictive value; PLT, platelet count; PPV, positive predictive value; PSAIG, platelet surface‐associated immunoglobulin; Sens, sensitivity; Spec, specificity.
A prospective cross‐sectional study provided information regarding the diagnostic utility of a flow cytometric PSAIG assay, but did not compare assay utility with platelet count. 19 The study of 115 dogs, 17 of which had probable primary ITP, reported moderate sensitivities and specificities (Table 1). 19 When dogs with ITP (primary and secondary) were compared with those with non‐immune causes, there was no difference in percentage of direct or indirect IgG, direct or indirect IgM, and direct and indirect IgG/IgM combined. 19 However, direct and indirect IgG combined were higher in dogs with ITP compared with dogs with non‐immune thrombocytopenia. 19 Another prospective cross‐sectional study similarly found no difference in PSAIG between dogs with primary ITP and those with non‐immune thrombocytopenia. 71 Several other studies also suggest that PSAIG do not reliably differentiate primary from secondary ITP. 41 , 46 , 68 , 71
Fourteen additional studies were assessed, including 11 observational, cross‐sectional studies; 2 retrospective case series; and 1 experimental study (total n = 709 dogs; 275 ITP dogs; 208 primary ITP dogs). 32 , 41 , 46 , 66 , 67 , 68 , 69 , 70 , 72 , 73 , 74 , 75 , 76 None of these studies directly compared PSAIG assays with platelet count for differentiating ITP from non‐immune thrombocytopenia. Additionally, in many of these studies, the presence of PSAIG was a criterion for a diagnosis of ITP, limiting assessments of assay diagnostic performance. Regardless, studies that report diagnostic performance data consistently suggest that direct PSAIG assays are sensitive (median, 94%; range, 29.4%‐100%), but less specific (median, 75.9%; range, 47%‐100%) for differentiating ITP from non‐immune thrombocytopenia, with low to moderate PPV (median, 48%; range, 28%‐72%) and moderate to very good negative predictive value (NPV; median, 100%; range, 79%‐100%) for ITP.
Only 2 studies provided data on megakaryocyte‐associated antibody assays for ITP diagnosis, but included no concurrent platelet count data. 67 , 72 Consequently, there is insufficient information to assess the utility of megakaryocyte‐associated antibody assays for ITP diagnosis.
Guidelines and evidence summary (cats)
See Supporting Information 11.
Link to diagnostic algorithms (Figures 2 and 3): The panel considered immunologic testing as supportive evidence for an immune component to platelet destruction but platelet/megakaryocyte antibody testing is not required for a diagnosis of ITP, consistent with current American Society of Hematology guidelines. 62
Lack of consensus: The 13/14 evidence evaluators agreed with guideline 4.4.1, whereas 1/14 stated that PSAIG assays should be performed if available.
4.4.2. In dogs/cats with primary ITP (P), do platelet or megakaryocyte‐associated antibody determinations (E) compared with platelet count alone (C) impact prediction of bleeding severity, response to first‐line treatment, relapse, survival, hospitalization duration, blood product requirement, or time to platelet count recovery (O)?
Guidelines (dogs)
In dogs with primary ITP, evaluation of platelet/megakaryocyte‐associated antibodies for outcome prediction is not recommended.
In dogs with primary ITP, we suggest that serial monitoring of platelet/megakaryocyte‐associated antibodies might help identify disease relapse.
Level of evidence (dogs):Low. Strength of recommendation: Weak.
Evidence summary (dogs)
One prospective cross‐sectional study was identified that directly assessed the PECO question. The study evaluated whether the magnitude of PSAIG measured using a flow cytometric assay influenced the following disease outcomes: (a) response to initial treatment, (b) survival to hospital discharge, and (c) relapse. 46 The study described 28 dogs with primary ITP and reported that neither the initial percentage of PSAIG‐positive platelets nor initial platelet count discriminated for response to initial treatment or survival to hospital discharge. 46 Changes in PSAIG positivity and platelet count for the first 4 weeks of treatment did not predict response to treatment at 6 weeks. 46 These comparisons provide evidence that neither initial magnitude of PSAIG positivity nor initial platelet count predict response to initial treatment or survival to discharge. However, for dogs with primary ITP that entered remission, recurrence of PSAIG positivity was associated with relapse in 2 dogs. 46
Two studies indirectly assessed bleeding and presence of PSAIG, but not all dogs had primary ITP. 21 , 72 One retrospective case series found more bleeding in dogs with PSAIG, but statistical analyses were only performed for all causes of thrombocytopenia. 21 An additional 4 studies were reviewed: 2 observational, cross‐sectional studies and 2 retrospective case series (total n = 252 dogs; 203 ITP dogs; 91 primary ITP dogs). None presented data allowing comparison of autoantibody detection with platelet count for any PECO severity metric. 3 , 20 , 36 , 77
Overall, weak evidence suggests that platelet/megakaryocyte‐associated antibody assays are equally ineffective as initial platelet count for prediction of response to initial treatment and survival to hospital discharge. There is weak evidence that recurrence of PSAIG positivity is associated with ITP relapse. There is insufficient information in the reviewed literature to assess the potential of platelet/megakaryocyte‐associated antibody to predict bleeding severity, duration of hospitalization, blood product requirement, and platelet count recovery in dogs with primary ITP.
Guidelines and evidence summary (cats)
See Supporting Information 11.
4.5.
4.5.1. In dogs/cats with confirmed thrombocytopenia (P), compared with platelet count alone (C) does the addition of hemostasis testing (eg, coagulation testing, platelet function testing, viscoelastic testing, fibrinolysis testing, D‐dimer concentration) (E) help differentiate ITP from non‐immune thrombocytopenia (O)?
Guidelines (dogs)
In dogs with thrombocytopenia, we recommend performing coagulation testing (activated partial thromboplastin time and prothrombin time) during the routine diagnostic evaluation.
In dogs with thrombocytopenia, measurement of fibrinolysis markers (fibrin degradation products [FDP], D‐dimer) should be considered.
Level of evidence (dogs): Moderate. Strength of recommendation: Strong.
Evidence summary (dogs)
Five observational studies or retrospective cases series reported coagulation testing in dogs with thrombocytopenia. 49 , 76 , 78 , 79 , 80 Overall, these studies support the use of coagulation testing to differentiate patients with consumptive and toxic coagulopathies from patients with primary ITP. Coagulation testing aided identification of dogs with thrombocytopenia associated with disseminated intravascular coagulation (DIC) and anticoagulant rodenticide ingestion. 49 , 76 , 78 , 80 Limited data support the use of fibrinolysis markers (eg, D‐dimer) because these markers were inconsistently measured. One study described decreased platelet aggregation in dogs with ITP, but no comparisons were made with non‐immune thrombocytopenia, precluding any determination of diagnostic utility. 79
Guidelines (cats)
In thrombocytopenic cats, we recommend performing coagulation testing (activated partial thromboplastin time [aPTT] and prothrombin time [PT]) during the routine diagnostic evaluation.
In thrombocytopenic cats, measurement of fibrinolysis markers (eg, FDP, D‐dimer) should be considered.
Level of evidence (cats): Moderate. Strength of recommendation: Strong.
Evidence summary (cats)
Five retrospective cases series or case reports describing cats with thrombocytopenia were reviewed. 51 , 81 , 82 , 83 , 84 These reports support the use of coagulation testing to differentiate cats with primary ITP from other systemic disorders associated with thrombocytopenia. In a case series of 85 ill cats, thrombocytopenia was present in 34%, and in 58% of cats with coagulation abnormalities, 84 whereas a second case series of cats with thrombocytopenia identified only 1 cat with primary ITP. 51 Thrombocytopenia was present in 50% of cats with DIC in 1 case series. Prolongation of the aPTT was present in all DIC cats, whereas additional tests (eg, FDP, antithrombin) were useful to further characterize DIC. 81 A case series of 69 cats with at least 1 abnormal hemostasis test result indicated that thrombocytopenia was present in 57% of cats with DIC, with neoplasia, liver failure, and feline infectious peritonitis being the most common inciting diseases. 83 One case report described the use of thrombelastography to characterize a bleeding diathesis in a cat with thrombocytopenia, but the cause was not determined. 82
Link to diagnostic algorithms (Figures 2 and 3): The panel integrated coagulation testing early in the diagnostic algorithm to rule out consumptive causes of thrombocytopenia before undertaking additional investigation.
Lack of consensus: While 13/14 evidence evaluators agreed with guideline 4.5.1 in dogs, 1/14 suggested that ITP dogs with severe melena can have mildly increased aPTT, D‐dimers and FDP. Although 13/14 evidence evaluators agreed with guideline 4.5.1 in cats, 1/14 felt the literature insufficiently supported the recommendation.
4.5.2. In dogs/cats with primary ITP (P), compared with determination of platelet count alone (C) does determination of bleeding severity score (E) improve prediction of bleeding severity, response to first‐line treatment, survival, blood product requirement, duration of hospitalization, days to platelet recovery, or ITP relapse (O)?
Guidelines (dogs)
In dogs with primary ITP, we recommend severity scoring be performed to aid assessment of disease severity.
Level of evidence (dogs): Moderate. Strength of recommendation: Moderate.
Evidence summary (dogs)
Five studies relevant to the PECO question were reviewed. 22 , 46 , 54 , 63 , 85 Evidence from cohort studies indicates an association between severity score or anatomic site of bleeding with transfusion requirement, duration of hospitalization, or survival. In a cohort study of 34 dogs with primary ITP, a bleeding severity score (DOGiBAT bleeding assessment tool) directly correlated with transfusion requirement and duration of hospitalization, whereas platelet count was not associated with these metrics. 22 In a retrospective study including 73 dogs with probable primary ITP, 54 and a prospective study with 28 primary ITP cases, 46 the presence of melena was associated with poor survival. Gastrointestinal bleeding is a component of the DOGiBAT bleeding assessment tool score. 22
Guidelines and evidence summary (cats)
See Supporting Information 11.
4.6.
4.6.1. In dogs/cats with primary ITP(P), compared with platelet count alone (C) do CBC or biochemistry panel abnormalities (E) improve prediction of bleeding severity, response to first‐line treatment, survival, blood product requirement, duration of hospitalization, days to platelet recovery, or ITP relapse (O)?
Guidelines (dogs)
In dogs with primary ITP, we recommend that CBC and biochemistry panels be performed to aid assessment of disease severity.
Level of evidence (dogs): Moderate. Strength of recommendation: Moderate.
Evidence summary (dogs)
Nine studies were reviewed, but none directly addressed the PECO question. 3 , 21 , 37 , 54 , 56 , 58 , 63 , 86 , 87 Evidence from case reports and case series indicates that biochemistry panel abnormalities (high blood urea nitrogen concentration) and CBC abnormalities (low hematocrit) may be associated with increased disease severity in dogs with primary ITP. A retrospective study of 55 dogs with primary ITP found an association between high blood urea nitrogen concentration and poor survival. 54 An association between low hematocrit and transfusion was reported in 2 case series of dogs with primary ITP, but in 1 of these studies the most anemic dogs had amegakaryocytic thrombocytopenia. 37 , 63 Other case series identified a variable association or did not evaluate the relationship between these variables and severity metrics. 3 , 21 , 86 , 87 Thrombocytopenia severity was related to transfusion requirements or bleeding in 2 retrospective case series, 3 , 58 but no assessments of other hematologic variables were described.
Guidelines and evidence summary (cats)
See Supporting Information 11.
5. GUIDELINES FOR COMORBIDITY SCREENING IN ITP
The first part of this section summarizes the evidence for a causal role of various comorbidities in ITP; the second part presents guidelines for comorbidity screening based on the evidence accrued. Evidence summaries and guideline recommendations are presented for comorbidities represented by ≥1 study with a high level of evidence. The remainder, including all studies in cats, are included in Supporting Information 12.
Overall, 165 manuscripts met inclusion criteria for review (Supporting Information 1 and 3). Integrated metric of evidence values could be calculated for 59 comorbidities from 48 manuscripts (Figure 4, Supporting Information 10). 41 , 61 , 70 , 73 , 76 , 78 , 85 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 For the remainder, IME values could not be calculated because the number of animals with ITP or the comorbidity could not be discerned, >1 comorbidity was present, ITP was ultimately deemed “unlikely” or “primary” based on the diagnostic algorithm, the diagnostic algorithm could not be applied, or patient data were already captured in another study.
FIGURE 4.
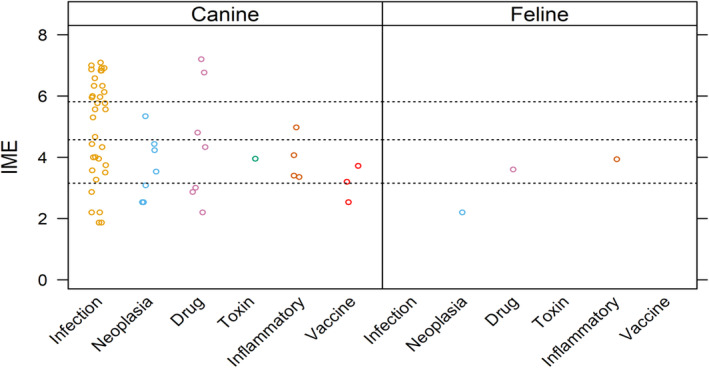
Summary of evidence for the causal role of comorbidities as a trigger for ITP in dogs and cats. Level of evidence for a specific comorbidity as a cause of ITP was assessed by means of a published Integrated Metric of Evidence (IME) value, which captures information on study design, quality of reporting, confidence of comorbidity diagnosis, likelihood of a causal link between the comorbidity and ITP, confidence of the ITP diagnosis, and the number of patients with a given comorbidity (excluding those patients with more than 1 comorbidity). Horizontal dotted lines indicate threshold IME values between negligible and low (3.15), low and intermediate (4.57), and intermediate and high (5.81) levels of evidence based on predetermined hypothetical thresholds (Supporting Information 8). An IME value of 0 indicates that the study directly demonstrated a lack of evidence that a comorbidity caused ITP.
5.1. Infections
In humans, several infectious agents are associated with ITP, including Helicobacter pylori, Plasmodium spp., severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), Hepatitis C Virus, and Human Immunodeficiency Virus. 129 , 130 , 131 , 132 , 133 , 134 , 135 Evidence suggests that mechanisms of immune‐mediated platelet destruction include molecular mimicry, immune‐targeting of bound or expressed platelet antigens, and immune‐complex binding of platelet FcγRII receptors. 129 , 132 , 133 , 136 , 137 Genetic and environmental factors that affect the host immune milieu, and genetic and phenotypic differences among strains of microorganisms, also influence whether ITP develops or not. 129 , 132 , 136 , 137 , 138 In this study, IME values were calculated for 34 infections as potential causes of ITP in dogs and cats (Figure 5 and Supporting Information 10).
FIGURE 5.
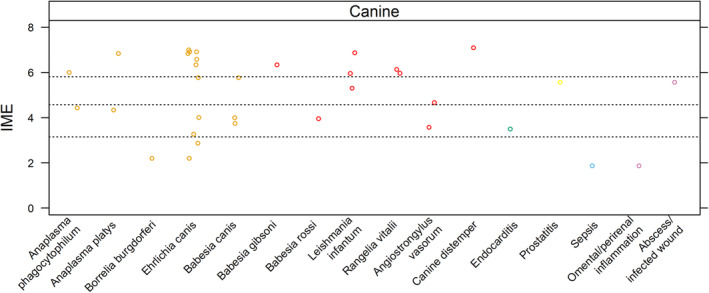
Summary of evidence for the causal role of infection as a trigger for ITP in dogs. Level of evidence for infection with a specific pathogen (or location/type of infection) as a cause of ITP was assessed by means of a published Integrated Metric of Evidence (IME) value. Horizontal dotted lines indicate threshold IME values between negligible and low (3.15), low and intermediate (4.57), and intermediate and high (5.81) levels of evidence based on predetermined thresholds (Supporting Information 8). An IME value of 0 indicates that the study directly demonstrated a lack of eidence that a comorbidity caused ITP.
5.1.1. Canine vector‐borne infections
Ehrlichia spp.
Consensus summary statement
Overall, there is a high level of evidence that infection with E. canis causes ITP in dogs. Mechanisms in addition to immune‐mediated destruction contribute to thrombocytopenia. Further study is needed to determine whether species or strain differences, as well as host or environmental factors, contribute to the development of ITP.
Evidence summary
Twenty‐seven studies were evaluated. 3 , 21 , 61 , 70 , 74 , 85 , 93 , 95 , 97 , 100 , 103 , 104 , 107 , 114 , 116 , 117 , 123 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 Integrated metric of evidence values (median, 6.33; range, 2.2‐7.0) were calculated for 11 studies. 70 , 85 , 93 , 100 , 103 , 104 , 107 , 114 , 116 , 117 , 123 Of these 11 studies, evidence was negligible in 18% (2/11), 85 , 93 low in 18% (2/11), 114 , 116 intermediate in 9% (1/11), 107 and high in 50% (6/11) 70 , 100 , 103 , 104 , 117 , 123 that infection with E. canis causes ITP in dogs. Integrated metric of evidence values could not be calculated for the remaining 16 studies. 3 , 21 , 61 , 74 , 95 , 97 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148
Of the 11 studies with an IME value, 10 specifically studied E. canis. 70 , 85 , 93 , 100 , 103 , 104 , 114 , 116 , 117 , 123 One study did not indicate the Ehrlichia species involved, 107 but E. canis was presumed based on geographic locale. 149 Overall, high level evidence suggests immune‐mediated platelet destruction contributes to thrombocytopenia in dogs with ehrlichiosis. In 7 studies (median IME, 6.45; range, 3.27‐7.00; n = 50) dogs were experimentally infected. 70 , 100 , 103 , 104 , 114 , 117 , 123 PSAIG was demonstrated using flow cytometry in 4 of these studies. 70 , 103 , 117 , 123 Because PSAIG was demonstrated in some dogs as early as day 7, it has been hypothesized they might represent naturally occurring autoantibodies. 103 , 140 Of note, experimental infections involved infusing infected canine blood, which presumably contained platelets, into naïve recipients.
Levels of evidence varied for 4 additional studies that involved dogs naturally infected with E. canis (median IME, 3.22; range, 2.20‐5.77; n = 8), 85 , 93 , 107 , 116 including 2 case reports, 93 , 116 1 study comparing platelet transfusion products in which 1 dog was E. canis‐seroreactive, 85 and 1 study documenting 5 dogs with severe thrombocytopenia and PSAIG. 107 An IME value could not be calculated for 1 additional study that included 7 E. canis‐seroreactive dogs with thrombocytopenia and PSAIG. 3
In addition to PSAIG, splenic sequestration and removal of platelets, 104 vasculitis, hypercoagulability, and myelosuppression all may contribute to thrombocytopenia in ehrlichiosis. 70 , 150 , 151 , 152
There were no studies of other species of Ehrlichia for which IME values could be calculated. In 1 study, no significant difference was found in mean platelet count for 3 dogs infected with E. chaffeensis compared with uninfected controls. 144 This finding contrasted with E. canis‐infected dogs, in which platelet counts significantly decreased during the study. In a retrospective study of 41 dogs PCR‐positive for E. ewingii, 16 were thrombocytopenic (range, 5‐189 000 platelets/μL) and 2 were reported to have ITP, although the ITP diagnostic criteria were not described. 147
Leishmania spp.
Consensus summary statement
Overall, high‐level evidence suggests that Leishmania infantum infection causes ITP in dogs. Mechanisms other than immune‐mediated destruction also contribute to thrombocytopenia in dogs with leishmaniosis.
Evidence summary
Seven studies were evaluated. 3 , 21 , 96 , 97 , 107 , 121 , 153 Integrated metric of evidence values of 5.30 to 6.87 (median 5.9) were calculated for 3 studies. 96 , 97 , 121 Thirty‐three percent (1/3) 97 of the studies demonstrated an intermediate level of evidence and 67% (2/3) demonstrated a high level of evidence that Leishmania causes ITP. 96 , 121 In a study of 33 dogs naturally infected with L. infantum (IME, 5.95), platelet surface‐associated IgM and IgG were detected by flow cytometry in 64% (21) of dogs. 121 All 21 dogs had platelet surface‐associated IgM, and 9 had both IgM and IgG. Platelet surface‐associated immunoglobulin was demonstrated in 8 of 9 thrombocytopenic dogs and antibody presence was significantly associated with severity of illness. Three of the 9 thrombocytopenic dogs had platelet counts <100 000/μL. In a prospective case‐control study (IME, 6.87) of naturally infected dogs, 96 PSAIG was documented by flow cytometry in 19/20 Leishmania‐infected dogs with thrombocytopenia, 13/24 Leishmania‐infected dogs with normal platelet counts, and 0/10 healthy controls. In Leishmania‐infected dogs, the presence of PSAIG was significantly associated with thrombocytopenia, suggesting that immune‐mediated platelet destruction contributes to thrombocytopenia. A third study (IME, 5.30) also suggested that leishmaniosis causes ITP. 97 This study investigated the prevalence of PSAIG in 10 dogs with leishmaniosis, 10 dogs with ehrlichiosis, 10 dogs coinfected with Ehrlichia and Leishmania, and 10 control dogs. 97 Platelet surface‐associated immunoglobulin was documented in 5/10 dogs with leishmaniosis, 6/10 dogs with ehrlichiosis, and 7/10 coinfected dogs. Only 1 Leishmania‐infected dog had a platelet count <100 000/μL; PSAIG was not detected in this dog (the “n” used in the IME calculation was based on this dog). For 4 additional studies, IME values could not be calculated because of coinfection, inadequate data, reported platelet counts >100 000/μL, and presence of pancytopenia. 3 , 21 , 107 , 153 In addition to immune‐mediated destruction, leishmaniosis in dogs may cause thrombocytopenia as a consequence of vasculitis, bleeding diatheses, and bone marrow infection. 154 In a mouse model of visceral leishmaniosis, decreased thrombopoietin, decreased bone marrow megakaryocytic maturation, and increased splenic and hepatic removal of opsonized and desialylated platelets all contributed to thrombocytopenia. 155
Anaplasma spp.
Consensus summary statement
Overall, there is an intermediate level of evidence that Anaplasma spp. cause ITP in dogs. Immunologic mechanisms of platelet destruction were clearly documented only for A. phagocytophilum. Whether antibody‐mediated platelet destruction occurs during A. platys infection is not known. Other mechanisms may contribute to the development of thrombocytopenia in dogs with anaplasmosis. Further study is needed to determine if species and strain differences, or host or environmental factors, affect whether ITP develops during infection.
Evidence summary
Eleven studies were evaluated for evidence that Anaplasma spp. cause ITP in dogs. 21 , 46 , 88 , 90 , 94 , 100 , 117 , 144 , 148 , 156 , 157 Integrated metric of evidence values were calculated for 4 studies (median, 5.2; range, 4.34‐6.83; n = 32). 88 , 90 , 94 , 100 Fifty percent (2/4) demonstrated low‐level evidence, 88 , 90 and 50% (2/4) demonstrated high‐level evidence 94 , 100 that Anaplasma spp. cause ITP in dogs.
Two studies documented thrombocytopenia associated with Anaplasma platys infection. 88 , 100 In dogs simultaneously or sequentially infected with Ehrlichia canis and A. platys, or either organism alone, (n = 6 per group), A. platys infection resulted in thrombocytopenia, particularly when combined with E. canis. 100 The IME value for non‐coinfected dogs was 6.83. 100 In a separate study, 3 dogs with thrombocytopenia were documented to be infected with A. platys using cytology and PCR (IME, 4.34). 88
Two additional studies documented thrombocytopenia in dogs infected with A. phagocytophilum. 90 , 94 In a study of 63 dogs (IME, 6.0) PCR‐positive for A. phagocytophilum, 54 (86%) were thrombocytopenic, including 21 classified as severe (<30 000/μL) and 21 classified as moderate (30 000‐100 000/μL). 94 Thirty‐six dogs were tested for PSAIG, of which 16 (44%) were positive. The platelet counts in these dogs ranged from 0 to 95 800/μL (median, 16 700/μL). A case report (IME, 4.43) described a dog actively infected with A. phagocytophilum with severe thrombocytopenia, PSAIG, IMHA, and polyarthritis. 90
Seven additional studies for which IME values could not be calculated documented thrombocytopenia in dogs with anaplasmosis. 21 , 46 , 117 , 144 , 148 , 156 , 157 In a study of 18 dogs PCR‐positive for A. phagocytophilum, 16 were thrombocytopenic. 156 Six of the 9 dogs tested were positive for PSAIG, suggesting an immune‐mediated pathogenesis. An IME value could not be calculated for this study because the number of dogs with platelet counts <100 000/μL could not be determined. Another study documented thrombocytopenia after confirmed experimental infection of A. platys in 4 dogs and A. phagocytophilum in 3 dogs, but an IME value could not be calculated because the number of dogs that became thrombocytopenic after infection was not reported. 144
Piroplasms
Babesia spp.
Consensus summary statement
Overall, the evidence that Babesia spp. cause ITP is low, although indirect study design affected some IME values. Two studies showed intermediate and high levels of evidence, respectively, that Babesia spp. may cause ITP. Further study is required to determine whether there are species differences in the ability of Babesia spp. to induce immune‐mediated platelet destruction. Mechanisms other than immune‐mediated destruction also may contribute to thrombocytopenia in dogs with babesiosis.
Evidence summary
Fifteen studies were evaluated for evidence that Babesia spp. cause ITP in dogs. 3 , 21 , 41 , 101 , 107 , 109 , 111 , 117 , 145 , 158 , 159 , 160 , 161 , 162 , 163 Integrated metric of evidence values could be calculated for 5 studies (median, 4.00; range, 3.74‐6.33; n = 56). 41 , 101 , 107 , 109 , 111 Sixty percent (3/5) provided low‐level evidence, 101 , 109 , 111 20% (1/5) provided intermediate, 107 and 20% (1/5) provided high‐level evidence. 41 For B. canis, 3 studies had IME values of 3.74, 111 4.00, 109 and 5.77 107 (for 1 of these studies, species was presumed based on geographic locale). 107 , 149 A high level of evidence was provided by a study in which 4 dogs experimentally infected with B. gibsoni developed severe thrombocytopenia and PSAIG (IME, 6.33). 41 One study of 24 dogs naturally infected with B. rossi provided low‐level evidence that B. rossi causes ITP (IME, 3.95). 101 This study did not assess whether platelet‐ or megakaryocyte‐associated antibodies were present, but did find that infected dogs had significantly higher levels of platelet‐leukocyte aggregates than uninfected dogs, suggesting that innate immunity mediates consumption of platelets during infection. Notably, B. rossi can cause DIC, 101 suggesting that multiple mechanisms may contribute to thrombocytopenia in dogs with babesiosis. The 10 studies for which an IME value could not be calculated, and several other studies not captured by the search strategy, documented that thrombocytopenia is common in dogs infected with, or exposed to, many Babesia species. 21 , 41 , 117 , 145 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168
Rangelia spp.
Consensus summary statement
Two available studies provide high‐level evidence that Rangelia spp. cause ITP in dogs.
Evidence summary
Two studies were evaluated, 99 , 112 and provided high‐level evidence (IME, 5.97 and 6.13) that Rangelia causes ITP in dogs. In a study of dogs with naturally occurring R. vitalli infections diagnosed using light microscopy, infected dogs had lower platelet counts than uninfected controls (34 100 ± 27 918/μL versus 259 900 ± 61 050/μL) and more platelets with surface‐associated IgM. 99 Another study demonstrated severe thrombocytopenia 10 and 20 days post‐experimental infection, but did not test for PSAIG. 112 Mechanisms other than immune‐mediated destruction also likely contribute to thrombocytopenia in rangeliosis. 112
Borrelia burgdorferi
See Supporting Information 12.
Rickettsia rickettsii
See Supporting Information 12.
Angiostrongylus vasorum
See Supporting Information 12.
5.1.2. Canine viral infections
Canine distemper virus
Consensus summary statement
One study provided high‐level evidence that canine distemper virus strain R252 causes ITP, potentially through phagocytosis of platelets with surface‐bound virus‐antibody immune complexes and decreased platelet production because of megakaryocyte infection with distemper virus. Further study is required to determine the occurrence and clinicopathological course of ITP after natural infections.
Evidence summary
A single study (IME, 7.09) documented ITP in 2 gnotobiotic dogs experimentally infected with canine distemper virus (strain R252). 126 Thrombocytopenia developed on day 1 post‐infection and peaked on day 10. Platelet count recovery was observed without immunosuppressive or other treatment until the end of the study on day 15 post‐infection. In the same study, 17 additional dogs were inoculated with distemper virus and similarly developed transient thrombocytopenia, albeit with platelet counts >100 000/μL. These dogs were not included in the IME calculation. The study provided mechanistic insights and identified viral antigen and anti‐virus IgG on platelet surfaces of infected dogs from day 7 post‐infection using immunocytochemistry. Serum anti‐virus IgM was detected by ELISA on days 8 and 9. Immune complex‐mediated platelet phagocytosis by hepatic reticuloendothelial cells was demonstrated by electron microscopy from day 5. Immunocytochemistry identified viral megakaryocyte infection starting on day 8 post‐infection. A second study of 987 dogs with thrombocytopenia described the finding of canine distemper inclusion bodies. 47 The study design did not allow evaluation of the magnitude of platelet counts or the distribution of underlying diseases and an IME value therefore was not calculated.
5.1.3. Other infections in dogs
See Supporting Information 12
5.1.4. Vector‐borne infections in cats
See Supporting Information 12
5.1.5. Other infections in cats
See Supporting Information 12
5.2. Drugs
Mechanisms of drug‐induced ITP have been investigated extensively in human medicine. 169 , 170 , 171 Drug‐induced immune dysregulation may result in both cellular and humoral immune targeting of platelets. 171 Immune‐mediated platelet destruction occurs when antibodies that target drug bound to platelet membranes or drug complexed with surface glycoproteins cause Fc‐mediated phagocytosis or complement activation and platelet destruction. Destruction may also occur when drugs bind and cause a conformational change in the complementarity‐determining region of specific antibodies allowing the antibody to target platelet glycoproteins. 169 , 170 , 172 Drugs also can induce autoantibodies targeting platelets or megakaryocytes directly. 169 , 172
5.2.1. Dogs
Overall, the evidence that drugs cause ITP in dogs is low, but high‐level evidence suggests that cefazedone and gold salts, and intermediate‐level evidence suggests that sulfonamide drugs, ITP. A lack of evidence for other drugs does not preclude the possibility of a drug‐triggering ITP. Drug‐induced ITP in dogs may be underreported.
Evidence summary
Twenty‐four studies were evaluated. 3 , 21 , 38 , 46 , 74 , 91 , 98 , 115 , 120 , 122 , 124 , 128 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 Integrated metric of evidence values were calculated for 7 drugs in 7 studies (median, 4.33; range, 2.20‐7.20; Figure 6). 91 , 98 , 115 , 120 , 122 , 124 , 128 Forty‐three percent (3/7) demonstrated negligible, 98 , 115 , 124 14% (1/7) demonstrated low, 122 14% (1/7) demonstrated intermediate, 120 and 28% (2/7) demonstrated high 91 , 128 levels of evidence that a drug caused ITP in dogs.
FIGURE 6.
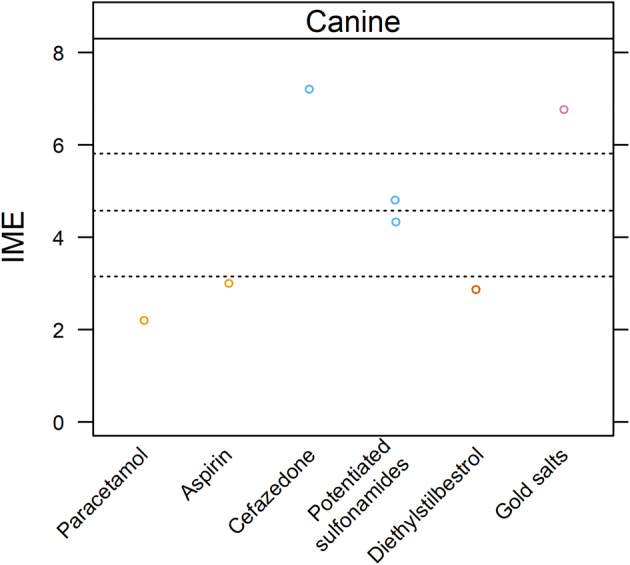
Summary of evidence for the causal role of drugs as a trigger for ITP in dogs. Level of evidence for drugs as a cause of ITP was assessed by means of a published Integrated Metric of Evidence (IME) value. Horizontal dotted lines indicate threshold IME values between negligible and low (3.15), low and intermediate (4.57), and intermediate and high (5.81) levels of evidence based on predetermined thresholds (Supporting Information 8). An IME value of 0 indicates that a study directly demonstrted a lack of evidence that a comorbidity caused ITP.
A high level of evidence for drug‐induced ITP was found in 2 studies. 91 , 128 In an experimental study (IME, 7.20) of high‐dose (≥540 mg/kg/day) IV cefazedone given over 6 to 17 weeks, thrombocytopenia developed in 11/14 dogs. 128 Six of the 11 thrombocytopenic dogs had platelet counts <100 000/μL, all of which had PSAIG. Thrombocytopenia resolved after cessation of cefazedone in 1 dog and spontaneously resolved despite continued treatment in 2 dogs. In another experimental study (IME, 6.77) gold salt administration induced thrombocytopenia in 5/28 dogs after administration of PO auranofin or IM gold sodium thiomalate for 45 to 72 months. 91 PSAIG was documented in 4 thrombocytopenic dogs, and platelet counts were < 100 000/μL in 4 dogs, 3 of which had PSAIG. Thrombocytopenia resolved in 3 dogs after withdrawal of gold salts and in 2 dogs in response to prednisolone. Platelet surface‐associated immunoglobulin and platelet count were inversely correlated in 1 dog with relapsing ITP.
One case report yielded an intermediate IME value of 4.80. 120 Thrombocytopenia was documented in a dog treated for 7 days with trimethoprim/sulfadiazine, and thrombocytopenia resolved after the drug was discontinued. An immune‐mediated mechanism was proposed since plasma from the treated dog caused fragmentation of healthy canine platelets. 120
The remaining reported cases were associated with low or negligible evidence supporting drug‐induced ITP in dogs. 98 , 115 , 122 , 124 In one case report (IME 4.33), thrombocytopenia, protein‐losing nephropathy, lymphadenopathy, and polyarthritis were documented after administration of sulfadimethoxine/ormetoprim that resolved rapidly upon drug discontinuation. 122 In another case report (IME, 3.00), a dog was treated with aspirin at 10 mg/kg PO q8h for 3 weeks, then at 10 mg/kg PO q24h, before developing IMHA with thrombocytopenia. 124 The dog improved with immunosuppression but subsequently was euthanized because of dyspnea. Another case report (IME, 2.87) described a dog that received diethylstilbestrol (2.5 mg/kg weekly) for 3 to 4 years that developed severe thrombocytopenia. 98 In a case report of a dog with IMHA and severe thrombocytopenia after acetaminophen administration (IME, 2.20), the thrombocytopenia resolved rapidly after acetaminophen discontinuation and an unspecified dose of prednisolone. 115
For 17 studies, IME values could not be calculated because of the administration of multiple drugs, presence of multiple comorbidities, incomplete data reporting, or an inability to apply ITP diagnostic criteria. 3 , 21 , 38 , 46 , 74 , 120 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 One study explored an immune‐mediated mechanism for sulfonamide‐induced ITP and documented PSAIG in 19/21 dogs. 179 All 11 thrombocytopenic dogs with clinical bleeding had documented PSAIG. However, thrombocytopenia was defined as <175 000 platelets/μL, and individual values were not reported, precluding calculation of an IME value.
5.2.2. Cats
See Supporting Information 12
5.3. Cancer
See Supporting Information 12
5.4. Vaccination
See Supporting Information 12
5.5. Toxins
See Supporting Information 12
5.6. Inflammatory DIsease
See Supporting Information 12
6. CONSENSUS SCREENING RECOMMENDATIONS
6.1. Global screening recommendations for dogs and cats
Testing should be performed according to the diagnostic algorithm (Figures 2 and 3) and the written recommendations presented in the preceding sections to confirm that consumption, sequestration, or bone marrow disease (other than that affecting megakaryocytes because of ITP) are not the sole cause of thrombocytopenia.
To differentiate primary from secondary ITP, a thorough history documenting vaccinations, drugs, toxins, travel, exposure to fleas, ticks and other vectors, flea and tick prevention, and heartworm testing and prevention is recommended. A thorough physical examination including retinal examination and examination of the skin, lymph nodes, joints, bones, digital examination of the rectum, and in male dogs, the prostate gland, should be performed. Laboratory screening should include a CBC with blood film examination by a board‐certified clinical pathologist (or equivalently trained hematologist), serum biochemical panel, and routine urinalysis. Urine culture and fecal flotation with centrifugation also should be considered. Imaging and other diagnostic tests to screen for cancer and potential foci of inflammation or infection should be performed at the discretion of the attending clinician based on their likelihood in the individual animal. In intact male dogs, prostatic ultrasound examination should be considered.
6.2. Specific screening recommendations for dogs
Dogs with suspected ITP should be specifically screened for infection with Ehrlichia spp., Babesia spp., and Anaplasma spp. using combined testing with serology and PCR. Repeat testing by means of PCR or convalescent serologic testing or both should be performed in all dogs originally testing negative but with a high risk of infection based on breed or exposure risk. 144 , 185 , 186 , 187 , 188 , 189
Dogs with suspected ITP and other signs of leishmaniosis living in or with a history of travel to enzootic areas, and at‐risk breeds in non‐enzootic areas, should be screened for leishmaniosis using microscopy, serology, PCR or some combination of these tests. Dogs with suspected ITP living in or traveling to enzootic areas should be screened for infection with Rangelia spp. using combined testing with microscopy and PCR. Repeat testing by means of PCR should be performed in all dogs originally testing negative but with a high risk of infection. In dogs with ITP living in or traveling to enzootic areas, and especially those with concurrent cardiopulmonary disease, screening for Angiostrongylus vasorum using fecal sedimentation, antigen assay, or additional testing such as tracheal lavage fluid cytologic examination and PCR can be considered.
Further study to determine how and if other vector‐borne disease agents cause ITP is required before definitive screening recommendations can be made for additional organisms. However, screening for additional vector‐borne pathogens should be considered in dogs with exposure risk and clinical abnormalities consistent with infection. Screening for canine distemper virus should be considered if dogs are non‐ or under‐vaccinated, living in enzootic areas, and have clinical findings consistent with infection. Evidence of systemic (eg, endocarditis, leptospirosis) or focal infections identified during initial screening should be further investigated, and changes in platelet count in response to treatment monitored.
6.3. Specific screening recommendations for cats
All sick cats should be tested for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) infection, according to American Association of Feline Practitioners (AAFP) retrovirus management guidelines. Although the role of infections in the development of ITP is unclear, identifying infections with a minimum database of CBC, serum biochemical panel, urinalysis, imaging, and specific testing as appropriate for a given case context, is warranted in thrombocytopenic cats with suspected ITP given the implications of immunosuppression and the possible role of infection as a trigger for ITP in an individual cat. In cats with suspected ITP living in enzootic areas or with exposure risk, screening for A. phagocytophilum, Ehrlichia spp., and B. felis should be considered.
7. LIMITATIONS
A limitation of the literature evaluated for both diagnostic and comorbidity components of this consensus statement is that platelet counts were not universally or uniformly verified in the evaluated studies, such that the prevalence and magnitude of thrombocytopenia might have been overestimated. In addition, there was inherent subjectivity and therefore variability in how the diagnostic algorithm was applied by evidence evaluators and, for comorbidities, in evaluating study indirectness by evidence evaluators. For several PECO questions, the level of evidence for the use of a diagnostic test was “low” and the strength of recommendation was “weak.” Similarly, evidence that a comorbidity caused ITP was often “negligible” or “low.”
For some comorbidities, it is possible that mechanisms other than immune‐mediated platelet destruction contributed to the magnitude of thrombocytopenia. For example, infections can cause decreased platelet production by targeting bone marrow or decreasing hepatic TPO production, platelet sequestration from vasculitis or splenomegaly, platelet consumption from DIC, or by causing platelet desialylation and removal. 137 , 155 , 190 Applying the algorithm helped to determine whether these processes contributed to the thrombocytopenia, but the extent of their effects could not always be determined. This approach could have affected causation scores and overall IME values.
Many comorbidities were assigned low or negligible IME values because studies did not specifically address whether a comorbidity causes ITP (indirectness). These studies were designated as “Descriptive Association” for the study design (Supporting Information 9). Therefore, low or negligible IME values should not be interpreted as a lack of causation.
Finally, although some studies were designed to determine if a comorbidity caused ITP, IME values could not be calculated because of the way platelet counts were reported or inability to apply the diagnostic algorithm. When identified, these articles were captured in the evidence summaries and are reflected in the evidence summary statements and screening recommendations.
8. FUTURE DIRECTIONS
Given the lack of available literature to answer many of the PECO questions, appropriately designed outcomes‐based studies that directly ask and answer these questions are needed to make more robust guidelines for the diagnosis of ITP in dogs and cats. Additional platelet indices such as immature platelet fraction recently have been introduced to veterinary medicine and may have diagnostic and prognostic utility. 191 Diagnostic guidelines regarding the utility of certain tests may be revised in the future based on the results of studies designed to answer the PECO questions.
Mechanistic studies investigating whether PSAIG causes FcγR dependent or independent clearance, the role of antibody‐independent immune‐mediated platelet destruction, and whether and how comorbidity or self‐antigen is targeted by the immune system may help in the development of tests that definitively diagnose ITP and individualize treatment strategies. Similarly, future research may demonstrate, as it has in murine models and human patients, that the mechanism of platelet clearance varies with the platelet antigen being targeted, which may in turn impact the ideal treatment modality. 192
AUTHOR CONTRIBUTIONS
Dana N. LeVine, Linda Kidd and Oliver A. Garden are co‐chairs on the consensus statement and share corresponding author status. Dana N. LeVine, Linda Kidd, Oliver A. Garden, Marjory B. Brooks, and Robert Goggs share first authorship. Marjory B. Brooks, Robert Goggs, Barbara Kohn, and Andrew J. Mackin are panel members on the consensus statement. Erin Eldermire, Yu‐Mei Chang, Julie Allen, Peter W. Christopherson, Barbara Glanemann, Haruhiko Maruyama, Maria C. Naskou, Lise N. Nielsen, Sarah Shropshire, Austin K. Viall, Adam J. Birkenheuer, Marnin A. Forman, Andrew S. Hanzlicek, Kathrin F. Langner, Erin Lashnits, Katharine F. Lunn, Kelly M. Makielski, Xavier Roura, and Eva Spada are advisory task force members on the consensus statement.
CONFLICT OF INTEREST DECLARATION
Dana LeVine has received relevant research funding from the American Kennel Club Canine Health Fund, BodeVet, Morris Animal Foundation, and Veterinary and Comparative Clinical Immunology Society (VCCIS) and honoraria for speaking engagements from the ACVIM Forum, ECVIM‐CA Congress, ACVIM Advanced Continuing Education, CVMA, and ACVP/ACSVP, and CanWest, and is also a consultant for Cellphire Therapeutics; Linda Kidd was a Veterinary Field Specialist for Zoetis Diagnostics August 2022‐July 2023, is an Associate Editor for the Journal of Veterinary Internal Medicine and has received speaking honoraria from the ACVIM and IDEXX; OIiver Garden has received relevant research funding from the Kennel Club (UK) and Okava Pharmaceuticals, and speaking honoraria from the ACVIM Forum, ECVIM‐CA Congress, and ACVP/ACSVP Annual Meeting. He has served as a consultant for Okava Pharmaceuticals and is the President of the Veterinary & Comparative Clinical Immunology Society; Marjory Brooks has received relevant research funding from the American Kennel Club and honoraria for speaking engagements from ACVIM Advanced Continuing Education, University of North Carolina Chapel Hill Symposium of Hemostasis, and the Society of Toxicologic Pathology's Symposium; Robert Goggs has received relevant research funding from the American Kennel Club Canine Health Fund, Aurin Biotech, BodeVet, Morris Animal Foundation, and Okava Pharmaceuticals and honoraria for speaking engagements from ACVIM Forum, EVECC Congress, IVECCS, PacVet, UnisVet and VCCIS; Julie Allen has served as a member of the Zoetis IMAGYST advisory board since 2022; Austin Viall has received relevant research funding from Morris Animal Foundation and VCCIS and has received honoraria for speaking engagements at ECVIM‐CA Congress, CanWest, CVMA, IVMA, and SDVMA; Erin Lashnits is a key opinion leader and research collaborator with IDEXX and Elanco Animal Health and research collaborator with Galaxy Diagnostics; Andrew Hanzlicek is an employee of MiraVista Diagnostics; Xavier Roura consults with Elanco, Idexx, Hill's, Purina, Affinity, Zoetis, Royal Canin, Merck Sharp and Dohme, Leti, Dechra, Bioiberica, Pharmacross, Nano1Health, Vetgenomics, Ecuphar, SantéVet, Fatro, Virbac, and Boehringer Ingelheim; Barbara Kohn has held lectures, had research collaborations and acted as a consultant for veterinary pharmaceutical and diagnostic companies. No other authors declare a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1. Summary of evidence for the causal role of neoplasia as a trigger for ITP in dogs. Level of evidence for neoplasia as a cause of ITP was assessed by means of a published Integrated Metric of Evidence (IME) value. Horizontal dotted lines indicate threshold IME values between negligible and low (3.15), low and intermediate (4.57), and intermediate and high (5.81) levels of evidence based on predetermined thresholds (Supporting Information 8). An IME value of 0 indicates that the study directly demonstrated a lack of evidence that a comorbidity caused ITP.
Figure S2. Summary of evidence for the causal role of inflammatory disease as a trigger for ITP in dogs. Level of evidence for inflammatory disease as a cause of ITP was assessed by means of a published Integrated Metric of Evidence (IME) value. Horizontal dotted lines indicate threshold IME values between negligible and low (3.15), low and intermediate (4.57), and intermediate and high (5.81) levels of evidence based on predetermined thresholds (Supporting Information 8). An IME value of 0 indicates that the study directly demonstrated a lack of evidence that a comorbidity caused ITP. SLE, systemic lupus erythematosus.
Figure S3. An algorithm for the diagnosis of ITP in dogs. aThrombocytopenia should be confirmed with a slide assessment. In brief, to perform a platelet estimate, first assess the slide body and feathered edge under low magnification for clumps, the presence of which suggests that the platelet count is falsely low. Resampling is then warranted, if possible. If there are no clumps, a platelet count is estimated by calculating the mean number of platelets per 10 oil immersion fields (×100) in the body of the smear where erythrocytes are spread in monolayer and multiplying this number by 15 000 to 20 000 to obtain the number of platelets per microliter; bthe magnitude of thrombocytopenia is consistent with consumption from major hemorrhage. However, it is possible that DIC, vasculitis, sequestration, or ITP may be contributing; cconsider genetic testing; dexcludes additional cytopenias with plausible explanations for example, pre‐regenerative anemia in the face of acute hemorrhage or hemolysis or lymphopenia in an ill/stressed patient; esampling of bone marrow by aspiration, core biopsy, or both, is undertaken; ffor example, lymphoreticular neoplasia or ehrlichiosis; gat least 2 of 5 parameters abnormal in addition to thrombocytopenia: prothrombin time (PT), activated partial thromboplastin time (aPTT), D‐dimers > reference interval (RI); antithrombin, fibrinogen < RI; hPT or aPTT >25% control value or upper bound of RI; ipartial screening (ie, not exhaustive) for potential trigger factors undertaken and negative.
Figure S4. An algorithm for the diagnosis of ITP in cats. aThrombocytopenia should be confirmed with a slide assessment. In brief, to perform a manual count first assess the slide's feathered edge under low magnification for clumps, the presence of which suggests that the platelet count is falsely low. Resampling is then warranted, if possible. If there are no clumps, a platelet count is estimated by calculating the mean number of platelets per 10 oil immersion fields (×100) and multiplying this number by 15 000 to 20 000 to obtain the number of platelets per microliter; bthe magnitude of thrombocytopenia is consistent with consumption from major hemorrhage; this is a rare association in cats. However, it is possible that DIC, vasculitis, sequestration, or ITP may be contributing; cexcludes additional cytopenias with plausible explanations for example, pre‐regenerative anemia in the face of acute hemorrhage or hemolysis or lymphopenia in an ill/stressed patient; dsampling of bone marrow by aspiration, core biopsy, or both, is undertaken; efor example, lymphoreticular neoplasia or feline immunodeficiency virus infection; fat least 2 of 4 parameters abnormal in addition to thrombocytopenia: prothrombin time (PT), activated partial thromboplastin time (aPTT), D‐dimers > reference interval (RI); fibrinogen < RI; gPT or aPTT >25% control value; hpartial screening (ie, not exhaustive) for potential trigger factors undertaken and negative.
Supporting Information 1. Extended materials and methods.
Supporting Information 2. Bibliography of studies for diagnosis.
Supporting Information 3. Bibliography of studies for comorbidities.
Supporting Information 4. Evidence evaluator instructions for Diagnosis PECO questions.
Supporting Information 5. Evidence evaluator instructions for Comorbidity PECO questions.
Supporting Information 6. Data extraction template for Diagnosis PECO questions
Supporting Information 7. Diagnosis PECO evidence summary template.
Supporting Information 8. Derivation of Integrated Metric of Evidence (IME) scores (D, Q, C, L, I, N).
Supporting Information 9. Data extraction template for Comorbidity PECO questions.
Supporting Information 10. Integrated Metric of Evidence (IME) calculations for comorbidity manuscripts.
Supporting Information 11. Evidence summaries and consensus recommendations for feline PECOs with minimal evidence.
Supporting Information 12. Consensus recommendations and evidence summaries for additional comorbidities.
ACKNOWLEDGMENT
No funding was received for this study.
LeVine DN, Kidd L, Garden OA, et al. ACVIM consensus statement on the diagnosis of immune thrombocytopenia in dogs and cats. J Vet Intern Med. 2024;38(4):1958‐1981. doi: 10.1111/jvim.16996
Consensus Statements of the American College of Veterinary Internal Medicine (ACVIM) provide the veterinary community with up‐to‐date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ACVIM Board of Regents oversees selection of relevant topics, identification of panel members with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence‐based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ACVIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited before publication. The authors are solely responsible for the content of the statements.
REFERENCES
- 1. LeVine DN, Brooks MB. Immune thrombocytopenia (ITP): pathophysiology update and diagnostic dilemmas. Vet Clin Pathol. 2019;48(Suppl 1):17‐28. [DOI] [PubMed] [Google Scholar]
- 2. Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. J Vet Intern Med. 1996;10:207‐218. [DOI] [PubMed] [Google Scholar]
- 3. Putsche JC, Kohn B. Primary immune‐mediated thrombocytopenia in 30 dogs (1997‐2003). J Am Anim Hosp Assoc. 2008;44:250‐257. [DOI] [PubMed] [Google Scholar]
- 4. Wondratschek C, Weingart C, Kohn B. Primary immune‐mediated thrombocytopenia in cats. J Am Anim Hosp Assoc. 2010;46:12‐19. [DOI] [PubMed] [Google Scholar]
- 5. Bianco D, Armstrong PJ, Washabau RJ. Presumed primary immune‐mediated thrombocytopenia in four cats. J Feline Med Surg. 2008;10:495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Portielje JE, Westendorp RG, Kluin‐Nelemans HC, et al. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97:2549‐2554. [DOI] [PubMed] [Google Scholar]
- 7. Consolini R, Legitimo A, Caparello MC. The centenary of immune thrombocytopenia – part 1: revising nomenclature and pathogenesis. Front Pediatr. 2016;4:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrington WJ, Minnich V, Hollingsworth JW, et al. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med. 1951;38:1‐10. [PubMed] [Google Scholar]
- 9. Tiemeyer KH, Kuter DJ, Cairo CW, Hollenhorst MA. New insights into the glycobiology of immune thrombocytopenia. Curr Opin Hematol. 2023;30:210‐218. [DOI] [PubMed] [Google Scholar]
- 10. Audia S, Mahevas M, Nivet M, et al. Immune thrombocytopenia: recent advances in pathogenesis and treatments. Hemasphere. 2021;5:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peerschke EI, Andemariam B, Yin W, et al. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148:638‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang F, Chu X, Wang L, et al. Cell‐mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2006;76:427‐431. [DOI] [PubMed] [Google Scholar]
- 13. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6:16.28208757 [Google Scholar]
- 14. Olsson B, Andersson PO, Jernas M, et al. T‐cell‐mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123‐1124. [DOI] [PubMed] [Google Scholar]
- 15. Zhao C, Li X, Zhang F, Wang L, Peng J, Hou M. Increased cytotoxic T‐lymphocyte‐mediated cytotoxicity predominant in patients with idiopathic thrombocytopenic purpura without platelet autoantibodies. Haematologica. 2008;93:1428‐1430. [DOI] [PubMed] [Google Scholar]
- 16. Aledort LM, Hayward CP, Chen MG, et al. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol. 2004;76:205‐213. [DOI] [PubMed] [Google Scholar]
- 17. Chang M, Qian JX, Lee SM, et al. Tissue uptake of circulating thrombopoietin is increased in immune‐mediated compared with irradiated thrombocytopenic mice. Blood. 1999;93:2515‐2524. [PubMed] [Google Scholar]
- 18. Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell‐Morell receptor regulates hepatic thrombopoietin production via JAK2‐STAT3 signaling. Nat Med. 2015;21:47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bachman DE, Forman MA, Hostutler RA, Corn S, Lin JM, Kociba GJ. Prospective diagnostic accuracy evaluation and clinical utilization of a modified assay for platelet‐associated immunoglobulin in thrombocytopenic and nonthrombocytopenic dogs. Vet Clin Pathol. 2015;44:355‐368. [DOI] [PubMed] [Google Scholar]
- 20. Scott MA, Kaiser L, Davis JM, Schwartz KA. Development of a sensitive immunoradiometric assay for detection of platelet surface‐associated immunoglobulins in thrombocytopenic dogs. Am J Vet Res. 2002;63:124‐129. [DOI] [PubMed] [Google Scholar]
- 21. Dircks BH, Schuberth HJ, Mischke R. Underlying diseases and clinicopathologic variables of thrombocytopenic dogs with and without platelet‐bound antibodies detected by use of a flow cytometric assay: 83 cases (2004‐2006). J Am Vet Med Assoc. 2009;235:960‐966. [DOI] [PubMed] [Google Scholar]
- 22. Makielski KM, Brooks MB, Wang C, Cullen JN, O'Connor AM, LeVine DN. Development and implementation of a novel immune thrombocytopenia bleeding score for dogs. J Vet Intern Med. 2018;32:1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garden OA, Kidd L, Mexas AM, et al. ACVIM consensus statement on the diagnosis of immune‐mediated hemolytic anemia in dogs and cats. J Vet Intern Med. 2019;33:313‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wasserkrug‐Naor A. Platelet kinetics and laboratory evaluation of thrombocytopenia. In: Brooks MB, Harr KE, Seelig DM, Wardrop KJ, Weiss DJ, eds. Schalm's Veterinary Hematology. 7th ed. Hoboken NJ, Wiley‐Blackwell; 2022:675‐685. [Google Scholar]
- 25. Flatland B, Baral RM, Freeman KP. Current and emerging concepts in biological and analytical variation applied in clinical practice. J Vet Intern Med. 2020;34:2691‐2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nabity MB, Harr KE, Camus MS, Flatland B, Vap LM. ASVCP guidelines: allowable total error hematology. Vet Clin Pathol. 2018;47:9‐21. [DOI] [PubMed] [Google Scholar]
- 27. Stokol T, Erb HN. A comparison of platelet parameters in EDTA‐ and citrate‐anticoagulated blood in dogs. Vet Clin Pathol. 2007;36:148‐154. [DOI] [PubMed] [Google Scholar]
- 28. Mylonakis ME, Leontides L, Farmaki R, Kostoulas P, Koutinas AF, Christopher M. Effect of anticoagulant and storage conditions on platelet size and clumping in healthy dogs. J Vet Diagn Invest. 2008;20:774‐779. [DOI] [PubMed] [Google Scholar]
- 29. Norman EJ, Barron RC, Nash AS, et al. Prevalence of low automated platelet counts in cats: comparison with prevalence of thrombocytopenia based on blood smear estimation. Vet Clin Pathol. 2001;30:137‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baccini V, Genevieve F, Jacqmin H, et al. Platelet counting: ugly traps and good advice. Proposals from the French‐Speaking Cellular Hematology Group (GFHC). J Clin Med. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiss DJ, Townsend E. Evaluation of reticulated platelets in dogs. Comp Haematol Int. 1998;88:166‐170. [Google Scholar]
- 32. Zmigrodzka M, Guzera M, Winnicka A. Evaluation of reticulated platelets in dogs with breed‐related thrombocytopenia. Pol J Vet Sci. 2014;17:137‐142. [DOI] [PubMed] [Google Scholar]
- 33. Pankraz A, Bauer N, Moritz A. Comparison of flow cytometry with the Sysmex XT2000iV automated analyzer for the detection of reticulated platelets in dogs. Vet Clin Pathol. 2009;38:30‐38. [DOI] [PubMed] [Google Scholar]
- 34. Hanahachi A, Kano R, Watari T, Hasegawa A. Thiazole orange‐positive platelets in a dog with idiopathic thrombocytopenic purpura. Vet Rec. 2002;150:48‐49. [DOI] [PubMed] [Google Scholar]
- 35. Silva LF, Golim MA, Takahira RK. Measurement of thrombopoietic activity through the quantification of megakaryocytes in bone marrow cytology and reticulated platelets. Res Vet Sci. 2012;93:313‐317. [DOI] [PubMed] [Google Scholar]
- 36. Dircks B, Schuberth HJ, Mischke R. Clinical and laboratory‐diagnosed parameters in 21 dogs with primary immune‐mediated thrombocytopenia. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2011;39:17‐24. [PubMed] [Google Scholar]
- 37. Kohn B, Engelbrecht R, Leibold W, et al. Clinical findings, diagnostics and treatment results in primary and secondary immune‐mediated thrombocytopenia in the dog. Kleintierpraxis. 2000;45:893‐907. [Google Scholar]
- 38. Schwartz D, Sharkey L, Armstrong PJ, Knudson C, Kelley J. Platelet volume and plateletcrit in dogs with presumed primary immune‐mediated thrombocytopenia. J Vet Intern Med. 2014;28:1575‐1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith JR, Smith KF, Brainard BM. Platelet parameters from an automated hematology analyzer in dogs with inflammatory clinical diseases. Vet J. 2014;201:406‐411. [DOI] [PubMed] [Google Scholar]
- 40. Sullivan PS, Manning KL, McDonald TP. Association of mean platelet volume and bone marrow megakaryocytopoiesis in thrombocytopenic dogs: 60 cases (1984‐1993). J Am Vet Med Assoc. 1995;206:332‐334. [PubMed] [Google Scholar]
- 41. Wilkerson MJ, Shuman W, Swist S, Harkin K, Meinkoth J, Kocan AA. Platelet size, platelet surface‐associated IgG, and reticulated platelets in dogs with immune‐mediated thrombocytopenia. Vet Clin Pathol. 2001;30:141‐149. [DOI] [PubMed] [Google Scholar]
- 42. Northern J, Tvedten HW. Diagnosis of microthrombocytosis and immune‐mediated thrombocytopenia in dogs with thrombocytopenia: 68 cases (1987‐1989). J Am Vet Med Assoc. 1992;200:368‐372. [PubMed] [Google Scholar]
- 43. Gelain ME, Bertazzolo W, Tutino G, Pogliani E, Cian F, Boudreaux MK. A novel point mutation in the beta1‐tubulin gene in asymptomatic macrothrombocytopenic Norfolk and Cairn Terriers. Vet Clin Pathol. 2014;43:317‐321. [DOI] [PubMed] [Google Scholar]
- 44. Davis B, Toivio‐Kinnucan M, Schuller S, Boudreaux MK. Mutation in beta1‐tubulin correlates with macrothrombocytopenia in Cavalier King Charles Spaniels. J Vet Intern Med. 2008;22:540‐545. [DOI] [PubMed] [Google Scholar]
- 45. Michimoto T, Okamura T, Suzuki K, et al. Thiazole orange positive platelets in a dog with Evans' syndrome. J Vet Med Sci. 2004;66:1305‐1306. [DOI] [PubMed] [Google Scholar]
- 46. Shropshire S, Dow S, Lappin M. Detection and dynamics of anti‐platelet antibodies in thrombocytopenic dogs with and without idiopathic immune thrombocytopenia. J Vet Intern Med. 2020;34:700‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grindem CB, Breitschwerdt EB, Corbett WT, Jans HE. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases. Vet Clin Pathol. 1991;20:38‐43. [DOI] [PubMed] [Google Scholar]
- 48. Botsch V, Kuchenhoff H, Hartmann K, et al. Retrospective study of 871 dogs with thrombocytopenia. Vet Rec. 2009;164:647‐651. [DOI] [PubMed] [Google Scholar]
- 49. Cockburn C, Troy GC. A retrospective study of sixty‐two cases of thrombocytopenia in the dog. Southwest Vet. 1986;37:133‐141. [Google Scholar]
- 50. Ellis J, Bell R, Barnes DC, Miller R. Prevalence and disease associations in feline thrombocytopenia: a retrospective study of 194 cases. J Small Anim Pract. 2018;59:531‐538. [DOI] [PubMed] [Google Scholar]
- 51. Jordan HL, Grindem CB, Breitschwerdt EB. Thrombocytopenia in cats: a retrospective study of 41 cases. J Vet Intern Med. 1993;7:261‐265. [DOI] [PubMed] [Google Scholar]
- 52. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386‐2393. [DOI] [PubMed] [Google Scholar]
- 53. Stasi R, Amadori S, Osborn J, Newland AC, Provan D. Long‐term outcome of otherwise healthy individuals with incidentally discovered borderline thrombocytopenia. PLoS Med. 2006;3:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346‐352. [DOI] [PubMed] [Google Scholar]
- 55. Rozanski EA, Callan MB, Hughes D, Sanders N, Giger U. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune‐mediated thrombocytopenia in dogs. J Am Vet Med Assoc. 2002;220:477‐481. [DOI] [PubMed] [Google Scholar]
- 56. Simpson K, Chapman P, Klag A. Long‐term outcome of primary immune‐mediated thrombocytopenia in dogs. J Small Anim Pract. 2018;59:674‐680. [DOI] [PubMed] [Google Scholar]
- 57. Jans HE, Armstrong PJ, Price GS. Therapy of immune mediated thrombocytopenia. A retrospective study of 15 dogs. J Vet Intern Med. 1990;4:4‐7. [DOI] [PubMed] [Google Scholar]
- 58. Williams DA, Maggio‐Price L. Canine idiopathic thrombocytopenia: clinical observations and long‐term follow‐up in 54 cases. J Am Vet Med Assoc. 1984;185:660‐663. [PubMed] [Google Scholar]
- 59. Miller MD, Lunn KF. Diagnostic use of cytologic examination of bone marrow from dogs with thrombocytopenia: 58 cases (1994‐2004). J Am Vet Med Assoc. 2007;231:1540‐1544. [DOI] [PubMed] [Google Scholar]
- 60. Busse L, Mischke R. Megakaryopoiesis in bone marrow of dogs with autoimmune‐induced thrombocytopenia and Ehrlichia canis infection. Tierarztl Umsch. 1998;53:703‐708. [Google Scholar]
- 61. Lachowicz JL, Post GS, Moroff SD, Mooney SC. Acquired amegakaryocytic thrombocytopenia—four cases and a literature review. J Small Anim Pract. 2004;45:507‐514. [DOI] [PubMed] [Google Scholar]
- 62. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190‐4207. [DOI] [PubMed] [Google Scholar]
- 63. Cooper SA, Huang AA, Raskin RE, Weng HY, Scott‐Moncrieff JC. Clinical data, clinicopathologic findings and outcome in dogs with amegakaryocytic thrombocytopenia and primary immune‐mediated thrombocytopenia. J Small Anim Pract. 2016;57:142‐147. [DOI] [PubMed] [Google Scholar]
- 64. Murtaugh RJ, Jacobs RM. Suspected immune‐mediated megakaryocytic hypoplasia or aplasia in a dog. J Am Vet Med Assoc. 1985;186:1313‐1315. [PubMed] [Google Scholar]
- 65. Polydoros T, Ioannidi OM, Korsavvidis I, Stefanidis S, Antoniadis T, Mylonakis ME. Romiplostim as adjunctive treatment of refractory amegakaryocytic immune thrombocytopenia in a dog. Top Companion Anim Med. 2021;42:100488. [DOI] [PubMed] [Google Scholar]
- 66. Campbell KL, George JW, Greene CE. Application of the enzyme‐linked immunosorbent assay for the detection of platelet antibodies in dogs. Am J Vet Res. 1984;45:2561‐2564. [PubMed] [Google Scholar]
- 67. Kristensen AT, Weiss DJ, Klausner JS, Laber J, Christie DJ. Detection of antiplatelet antibody with a platelet immunofluorescence assay. J Vet Intern Med. 1994;8:36‐39. [DOI] [PubMed] [Google Scholar]
- 68. Lewis DC, McVey DS, Shuman WS, Muller WB. Development and characterization of a flow cytometric assay for detection of platelet‐bound immunoglobulin G in dogs. Am J Vet Res. 1995;56:1555‐1558. [PubMed] [Google Scholar]
- 69. Lewis DC, Meyers KM. Studies of platelet‐bound and serum platelet‐bindable immunoglobulins in dogs with idiopathic thrombocytopenic purpura. Exp Hematol. 1996;24:696‐701. [PubMed] [Google Scholar]
- 70. Shropshire S, Dow S, Lappin M. Validation of a clinically applicable flow cytometric assay for the detection of immunoglobulin associated platelets in dogs. Vet Immunol Immunopathol. 2018;202:109‐114. [DOI] [PubMed] [Google Scholar]
- 71. Brooks MB, Maruyama H, Cremer SE, et al. Preliminary evaluation of a flow cytometric assay with microsphere controls for the detection of platelet‐bound antibodies in canine immune thrombocytopenia. Vet Clin Pathol. 2022;51:330‐338. [DOI] [PubMed] [Google Scholar]
- 72. Joshi BC, Jain NC. Detection of antiplatelet antibody in serum and on megakaryocytes of dogs with autoimmune thrombocytopenia. Am J Vet Res. 1976;37:681‐685. [PubMed] [Google Scholar]
- 73. Kristensen AT, Weiss DJ, Klausner JS, Laber J, Christie DJ. Comparison of microscopic and flow cytometric detection of platelet antibody in dogs suspected of having immune‐mediated thrombocytopenia. Am J Vet Res. 1994;55:1111‐1114. [PubMed] [Google Scholar]
- 74. Lewis DC, Meyers KM, Callan MB, Bücheler J, Giger U. Detection of platelet‐bound and serum platelet‐bindable antibodies for diagnosis of idiopathic thrombocytopenic purpura in dogs. J Am Vet Med Assoc. 1995;206:47‐52. [PubMed] [Google Scholar]
- 75. McVey DS, Shuman WS. Detection of antiplatelet immunoglobulin in thrombocytopenic dogs. Vet Immunol Immunopathol. 1989;22:101‐111. [DOI] [PubMed] [Google Scholar]
- 76. Zenker I, Keller L, Meichner K, Unterer S, Hartmann K. Immune mediated destruction of platelets in dogs with heat stroke: a prospective study. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2009;37:314‐318. [Google Scholar]
- 77. Wilkins RJ, Hurvitz AI, Dodds‐Laffin WJ. Immunologically mediated thrombocytopenia in the dog. J Am Vet Med Assoc. 1973;163:277‐282. [PubMed] [Google Scholar]
- 78. Helfand SC. Neoplasia and immune‐mediated thrombocytopenia. Vet Clin North Am Small Anim Pract. 1988;18:267‐270. [PubMed] [Google Scholar]
- 79. Kristensen AT, Weiss DJ, Klausner JS. Platelet dysfunction associated with immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 1994;8:323‐327. [DOI] [PubMed] [Google Scholar]
- 80. Lewis DC, Bruyette DS, Kellerman DL, Smith SA. Thrombocytopenia in dogs with anticoagulant rodenticide‐induced hemorrhage: eight cases (1990‐1995). J Am Anim Hosp Assoc. 1997;33:417‐422. [DOI] [PubMed] [Google Scholar]
- 81. Estrin MA, Wehausen CE, Jessen CR, et al. Disseminated intravascular coagulation in cats. J Vet Intern Med. 2006;20:1334‐1339. [DOI] [PubMed] [Google Scholar]
- 82. Öberg J, Tvedten H. Thromboelastography in a cat with steroid responsive thrombocytopenia. Comp Clin Pathol. 2010;19:429‐431. [Google Scholar]
- 83. Peterson JL, Couto CG, Wellman ML. Hemostatic disorders in cats: a retrospective study and review of the literature. J Vet Intern Med. 1995;9:298‐303. [DOI] [PubMed] [Google Scholar]
- 84. Thomas JS, Green RA. Clotting times and antithrombin III activity in cats with naturally developing diseases: 85 cases (1984‐1994). J Am Vet Med Assoc. 1998;213:1290‐1295. [PubMed] [Google Scholar]
- 85. Davidow EB, Brainard B, Martin LG, et al. Use of fresh platelet concentrate or lyophilized platelets in thrombocytopenic dogs with clinical signs of hemorrhage: a preliminary trial in 37 dogs. J Vet Emerg Crit Care (San Antonio). 2012;22:116‐125. [DOI] [PubMed] [Google Scholar]
- 86. Martinez C, Mooney CT, Shiel RE, Tang PK, Mooney L, O'Neill EJ. Evaluation of red blood cell distribution width in dogs with various illnesses. Can Vet J. 2019;60:964‐971. [PMC free article] [PubMed] [Google Scholar]
- 87. Orcutt ES, Lee JA, Bianco D. Immune‐mediated hemolytic anemia and severe thrombocytopenia in dogs: 12 cases (2001‐2008). J Vet Emerg Crit Care (San Antonio). 2010;20:338‐345. [DOI] [PubMed] [Google Scholar]
- 88. Antognoni MT, Veronesi F, Morganti G, Mangili V, Fruganti G, Miglio A. Natural infection of Anaplasma platys in dogs from Umbria region (Central Italy). Vet Ital. 2014;50:49‐56. [DOI] [PubMed] [Google Scholar]
- 89. Atkinson M. IMT in spaniel after vaccination. Vet Times. 2011;41:10‐11. [Google Scholar]
- 90. Bexfield NH, Villiers EJ, Herrtage ME. Immune‐mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J Small Anim Pract. 2005;46:543‐548. [DOI] [PubMed] [Google Scholar]
- 91. Bloom JC, Blackmer SA, Bugelski PJ, Sowinski JM, Saunders LZ. Gold‐induced immune thrombocytopenia in the dog. Vet Pathol. 1985;22:492‐499. [DOI] [PubMed] [Google Scholar]
- 92. Cain GR, Cain JL, Turrel JM, Theilen G, Jain NC. Immune‐mediated hemolytic anemia and thrombocytopenia in a cat after bone marrow transplantation. Vet Pathol. 1988;25:161‐162. [DOI] [PubMed] [Google Scholar]
- 93. Chethan GE, Thakur N, Madhesh E, et al. Therapeutic management of Ehrlichia canis induced pancytopaenia and hepatopathy in a dog. J Vet Parasitol. 2016;30:28‐31. [Google Scholar]
- 94. Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. 2018;59:112‐120. [DOI] [PubMed] [Google Scholar]
- 95. Codner EC, Roberts RE, Ainsworth AG. Atypical findings in 16 cases of canine ehrlichiosis. J Am Vet Med Assoc. 1985;186:166‐169. [PubMed] [Google Scholar]
- 96. Cortese L, Sica M, Piantedosi D, et al. Secondary immune‐mediated thrombocytopenia in dogs naturally infected by Leishmania infantum . Vet Rec. 2009;164:778‐782. [DOI] [PubMed] [Google Scholar]
- 97. Cortese L, Terrazzano G, Piantedosi D, et al. Prevalence of anti‐platelet antibodies in dogs naturally co‐infected by Leishmania infantum and Ehrlichia canis . Vet J. 2011;188:118‐121. [DOI] [PubMed] [Google Scholar]
- 98. Davis WM. Hapten‐induced, immune‐mediated thrombocytopenia in a dog. J Am Vet Med Assoc. 1984;184:976‐977. [PubMed] [Google Scholar]
- 99. Franca RT, Pillat MM, da Silva CB, et al. Surface immunoglobulins of erythrocytes and platelets in dogs naturally infected by Rangelia vitalii . Microb Pathog. 2018;121:245‐251. [DOI] [PubMed] [Google Scholar]
- 100. Gaunt S, Beall M, Stillman B, et al. Experimental infection and co‐infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasites Vectors. 2010;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Goddard A, Leisewitz AL, Kristensen AT, Schoeman JP. Platelet activation and platelet‐leukocyte interaction in dogs naturally infected with Babesia rossi . Vet J. 2015;205:387‐392. [DOI] [PubMed] [Google Scholar]
- 102. Gould SM, McInnes EL. Immune‐mediated thrombocytopenia associated with Angiostrongylus vasorum infection in a dog. J Small Anim Pract. 1999;40:227‐232. [DOI] [PubMed] [Google Scholar]
- 103. Harrus S, Waner T, Weiss DJ, Keysary A, Bark H. Kinetics of serum antiplatelet antibodies in experimental acute canine ehrlichiosis. Vet Immunol Immunopathol. 1996;51:13‐20. [DOI] [PubMed] [Google Scholar]
- 104. Harrus S, Waner T, Keysary A, Aroch I, Voet H, Bark H. Investigation of splenic functions in canine monocytic ehrlichiosis. Vet Immunol Immunopathol. 1998;62:15‐27. [DOI] [PubMed] [Google Scholar]
- 105. Helfand SC, Couto CG, Madewell BR. Immune‐mediated thrombocytopenia associated with solid tumors in dogs. J Am Anim Hosp Assoc. 1985;21:787‐794. [Google Scholar]
- 106. Jo'neill E, Acke E, Tobin E, et al. Immune‐mediated thrombocytopenia associated with angiostrongylus vasorum infection in a Jack Russell terrier. Ir Vet J. 2010;63:434‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kohn B, Engelbrecht R, Leibold W, et al. Klinische befunde, diagnostik und behandlungsdrfolge bei der primaren und sekundaren immunbedingten Thrombozytopenie beim Hund. Kleintierpraxis. 2000;45:889‐956. [Google Scholar]
- 108. Kopecny L, Palm CA, Naylor S, Kirby J, Cowgill LD. Application of therapeutic plasma exchange in dogs with immune‐mediated thrombocytopenia. J Vet Intern Med. 2020;34:1576‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mathe A, Voros K, Papp L, et al. Clinical manifestations of canine babesiosis in Hungary (63 cases). Acta Vet Hung. 2006;54:367‐385. [DOI] [PubMed] [Google Scholar]
- 110. Nakamura RK, Fenty RK, Bianco D. Presumptive immune‐mediated thrombocytopenia secondary to massive Africanized bee envenomation in a dog. J Vet Emerg Crit Care (San Antonio). 2013;23:652‐656. [DOI] [PubMed] [Google Scholar]
- 111. Oines O, Storli K, Brun‐Hansen H. First case of babesiosis caused by Babesia canis canis in a dog from Norway. Vet Parasitol. 2010;171:350‐353. [DOI] [PubMed] [Google Scholar]
- 112. Paim CB, Paim FC, da Silva AS, et al. Thrombocytopenia and platelet activity in dogs experimentally infected with Rangelia vitalii . Vet Parasitol. 2012;185:131‐137. [DOI] [PubMed] [Google Scholar]
- 113. Peterson ME, Kintzer PP, Hurvitz AI. Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med. 1988;2:150‐157. [DOI] [PubMed] [Google Scholar]
- 114. Pierce KR, Marrs GE, Hightower D. Acute canine ehrlichiosis: platelet survival and factor 3 assay. Am J Vet Res. 1977;38:1821‐1825. [PubMed] [Google Scholar]
- 115. Raguvaran R, Mondal RSK, Mondal DB. Management of immune mediated hemolytic anemia (IMHA) secondary to paracetamol toxicity in a dog. Intas Polivet. 2019;20:180‐181. [Google Scholar]
- 116. Sangeetha SG, Ajith Y, Dixit SK, et al. PCR based diagnosis and clinical management of ehrlichiosis in a dog. Intas Polivet. 2017;18:187‐191. [Google Scholar]
- 117. Sato M, Veir JK, Shropshire SB, Lappin MR. Ehrlichia canis in dogs experimentally infected, treated, and then immune suppressed during the acute or subclinical phases. J Vet Intern Med. 2020;34:1214‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Senturk S, Temizel M. Treatment of immune‐mediated hemolytic anemia and thrombocytopenia with vincristine in a dog. J Fac Vet Med. 2001;20:85‐91. [Google Scholar]
- 119. Singh PT, Panagawkar GR. Haemostatic disorders in dogs infected with transmissible venereal tumour. Indian Vet J. 1998;75:879‐881. [Google Scholar]
- 120. Sullivan PS, Arrington K, West R, et al. Thrombocytopenia associated with administration of trimethoprim/sulfadiazine in a dog. J Am Vet Med Assoc. 1992;201:1741‐1744. [PubMed] [Google Scholar]
- 121. Terrazzano G, Cortese L, Piantedosi D, et al. Presence of anti‐platelet IgM and IgG antibodies in dogs naturally infected by Leishmania infantum . Vet Immunol Immunopathol. 2006;110:331‐337. [DOI] [PubMed] [Google Scholar]
- 122. Vasilopulos RJ, Mackin A, Lavergne SN, Trepanier LA. Nephrotic syndrome associated with administration of sulfadimethoxine/ormetoprim in a dobermann. J Small Anim Pract. 2005;46:232‐236. [DOI] [PubMed] [Google Scholar]
- 123. Waner T, Leykin I, Shinitsky M, et al. Detection of platelet‐bound antibodies in beagle dogs after artificial infection with Ehrlichia canis. Vet Immunol Immunopathol. 2000;77:145‐150. [DOI] [PubMed] [Google Scholar]
- 124. Waner T, Lurie S. Aspirin implicated immune‐mediated haemolytic anaemia and thrombocytopenia in a dog—a case report. Refuah Vet. 1992;47:112‐115. [Google Scholar]
- 125. Weiss B. Selective dysmegakaryopoiesis in thrombocytopenic dogs (1996‐2002). Comp Clin Path. 2004;13:24‐28. [Google Scholar]
- 126. Axthelm MK, Krakowka S. Canine distemper virus‐induced thrombocytopenia. Am J Vet Res. 1987;48:1269‐1275. [PubMed] [Google Scholar]
- 127. Ridgway J, Jergens AE, Niyo Y. Possible causal association of idiopathic inflammatory bowel disease with thrombocytopenia in the dog. J Am Anim Hosp Assoc. 2001;37:65‐74. [DOI] [PubMed] [Google Scholar]
- 128. Bloom JC, Thiem PA, Sellers TS, Lewis HB, Deldar A. Cephalosporin‐induced immune cytopenia in the dog: demonstration of erythrocyte‐, neutrophil‐, and platelet‐associated IgG following treatment with cefazedone. Am J Hematol. 1988;28:71‐78. [DOI] [PubMed] [Google Scholar]
- 129. Frydman GH, Davis N, Beck PL, Fox JG. Helicobacter pylori eradication in patients with immune thrombocytopenic purpura: a review and the role of biogeography. Helicobacter. 2015;20:239‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Alhomoud M, Alhobayb T, Armitage K. COVID‐19 infection triggering thrombotic thrombocytopenic purpura. IDCases. 2021;26:e01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Santhosh S, Malik B, Kalantary A, Kunadi A. Immune thrombocytopenic purpura (ITP) following natural COVID‐19 infection. Cureus. 2022;14:e26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kuwana M. Helicobacter pylori‐associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20:714‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Takahashi T, Yujiri T, Shinohara K, et al. Molecular mimicry by Helicobacter pylori CagA protein may be involved in the pathogenesis of H. pylori‐associated chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2004;124:91‐96. [DOI] [PubMed] [Google Scholar]
- 134. Satoh T, Uojima H, Wada N, et al. Introduction of direct‐acting antiviral agents alters frequencies of anti‐GPIIb/IIIa antibody‐producing B cells in chronic hepatitis C patients with thrombocytopenia. Platelets. 2023;34:2161498. [DOI] [PubMed] [Google Scholar]
- 135. Kelton JG, Keystone J, Moore J, et al. Immune‐mediated thrombocytopenia of malaria. J Clin Invest. 1983;71:832‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009;46:S2‐S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Franchini M, Veneri D, Lippi G. Thrombocytopenia and infections. Expert Rev Hematol. 2017;10:99‐106. [DOI] [PubMed] [Google Scholar]
- 138. Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG. Emerging concepts in immune thrombocytopenia. Front Immunol. 2018;9:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Collicutt NB, Garner B. Erythrocyte dysplasia in peripheral blood smears from 5 thrombocytopenic dogs treated with vincristine sulfate. Vet Clin Pathol. 2013;42:458‐464. [DOI] [PubMed] [Google Scholar]
- 140. Grindem CB, Breitschwerdt EB, Perkins PC, Cullins LD, Thomas TJ, Hegarty BC. Platelet‐associated immunoglobulin (antiplatelet antibody) in canine Rocky Mountain spotted fever and ehrlichiosis. J Am Anim Hosp Assoc. 1999;35:56‐61. [DOI] [PubMed] [Google Scholar]
- 141. Harrus S, Day MJ, Waner T, Bark H. Presence of immune‐complexes, and absence of antinuclear antibodies, in sera of dogs naturally and experimentally infected with Ehrlichia canis . Vet Microbiol. 2001;83:343‐349. [DOI] [PubMed] [Google Scholar]
- 142. Kohn B, Bal G, Chirek A, Rehbein S, Salama A. Treatment of 5 dogs with immune‐mediated thrombocytopenia using Romiplostim. BMC Vet Res. 2016;12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Moraes LF, Takahira RK, Golim M, et al. Avaliacao das alteracoes hemostaticas e do risco tromboembolico em caes com AHIM. Pesq Vet Bras. 2016;36:405‐411. [Google Scholar]
- 144. Nair AD, Cheng C, Ganta CK, et al. Comparative experimental infection study in dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum . PloS One. 2016;11:e0148239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Neelawala D, Dissanayake DRA, Prasada DVP, Silva ID. Analysis of risk factors associated with recurrence of canine babesiosis caused by Babesia gibsoni . Comp Immunol Microbiol Infect Dis. 2021;74:101572. [DOI] [PubMed] [Google Scholar]
- 146. Ploneczka‐Janeczko K, Rypula K. Application of a flow cytometry in differentiation of antibodies accompanying platelets in Ehrlichia canis and Borrelia burgdorferi infections in dogs. Cent Eur J Immunol. 2011;36:174‐179. [Google Scholar]
- 147. Qurollo BA, Buch J, Chandrashekar R, et al. Clinicopathological findings in 41 dogs (2008‐2018) naturally infected with Ehrlichia ewingii . J Vet Intern Med. 2019;33:618‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Tami J, Martinez I, Tami M, et al. Identification of Ehrlichia species in blood smear. Infect Dis Clin Pract. 1996;5:555‐557. [Google Scholar]
- 149. Birkenheuer AJ, Buch J, Beall MJ, Braff J, Chandrashekar R. Global distribution of canine Babesia species identified by a commercial diagnostic laboratory. Vet Parasitol Reg Stud Rep. 2020;22:100471. [DOI] [PubMed] [Google Scholar]
- 150. Reardon MJ, Pierce KR. Acute experimental canine ehrlichiosis. I. Sequential reaction of the hemic and lymphoreticular systems. Vet Pathol. 1981;18:48‐61. [DOI] [PubMed] [Google Scholar]
- 151. Reardon MJ, Pierce KR. Acute experimental canine ehrlichiosis. II. Sequential reaction of the hemic and lymphoreticular system of selectively immunosuppressed dogs. Vet Pathol. 1981;18:384‐395. [DOI] [PubMed] [Google Scholar]
- 152. Mylonakis ME, Day MJ, Siarkou V, Vernau W, Koutinas AF. Absence of myelofibrosis in dogs with myelosuppression induced by Ehrlichia canis infection. J Comp Pathol. 2010;142:328‐331. [DOI] [PubMed] [Google Scholar]
- 153. Greisen A. Leishmania as a cause of Evans syndrome in a Greek street dog. Dansk Veterinaertidsskrift. 2008;91:8‐11. [Google Scholar]
- 154. Koutinas AF, Koutinas CK. Pathologic mechanisms underlying the clinical findings in canine leishmaniasis due to Leishmania infantum/chagasi . Vet Pathol. 2014;51:527‐538. [DOI] [PubMed] [Google Scholar]
- 155. Rani GF, Preham O, Ashwin H, Brown N, Hitchcock IS, Kaye PM. Dissecting pathways to thrombocytopenia in a mouse model of visceral leishmaniasis. Blood Adv. 2021;5:1627‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kohn B, Galke D, Beelitz P, Pfister K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J Vet Intern Med. 2008;22:1289‐1295. [DOI] [PubMed] [Google Scholar]
- 157. Mazepa AW, Kidd LB, Young KM, Trepanier LA. Clinical presentation of 26 anaplasma phagocytophilum‐seropositive dogs residing in an endemic area. J Am Anim Hosp Assoc. 2010;46:405‐412. [DOI] [PubMed] [Google Scholar]
- 158. Inokuma H, Okuda M, Yoshizaki Y, et al. Clinical observations of Babesia gibsoni infection with low parasitaemia confirmed by PCR in dogs. Vet Rec. 2005;156:116‐118. [DOI] [PubMed] [Google Scholar]
- 159. Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000‐2003). J Am Vet Med Assoc. 2005;227:942‐947. [DOI] [PubMed] [Google Scholar]
- 160. Gonde S, Chhabra S, Lingla LD, et al. Clinico‐haemato‐biochemical changes in naturally occurring canine babesiosis in Punjab, India. Malaysian J Vet Res. 2017;8:37‐44. [Google Scholar]
- 161. Tuttle AD, Birkenheuer AJ, Juopperi T, Levy MG, Breitschwerdt EB. Concurrent bartonellosis and babesiosis in a dog with persistent thrombocytopenia. J Am Vet Med Assoc. 2003;223:1280‐1301. [DOI] [PubMed] [Google Scholar]
- 162. Vijayalakshmi P, Srinivasan SR, Vairamuthu S, et al. Clinico pathological features in dogs associated with Babesiosis . Indian Vet J. 2014;91:21‐24. [Google Scholar]
- 163. Zygner W, Gojska‐Zygner O, Szmidt K. Vincristine and cyclophosphamide in the treatment of persistent anemia and thrombocytopenia related to Babesia canis infection in a dog. Zycie Wet. 2011;86:374‐378. [Google Scholar]
- 164. Schoeman T, Lobetti RG, Jacobson LS, Penzhorn BL. Feline babesiosis: signalment, clinical pathology and concurrent infections. J S Afr Vet Assoc. 2001;72:4‐11. [DOI] [PubMed] [Google Scholar]
- 165. Birkenheuer AJ, Neel J, Ruslander D, Levy MG, Breitschwerdt EB. Detection and molecular characterization of a novel large Babesia species in a dog. Vet Parasitol. 2004;124:151‐160. [DOI] [PubMed] [Google Scholar]
- 166. Sikorski LE, Birkenheuer AJ, Holowaychuk MK, McCleary‐Wheeler AL, Davis JM, Littman MP. Babesiosis caused by a large Babesia species in 7 immunocompromised dogs. J Vet Intern Med. 2010;24:127‐131. [DOI] [PubMed] [Google Scholar]
- 167. Barash NR, Thomas B, Birkenheuer AJ, Breitschwerdt EB, Lemler E, Qurollo BA. Prevalence of Babesia spp. and clinical characteristics of Babesia vulpes infections in north American dogs. J Vet Intern Med. 2019;33:2075‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Birkenheuer AJ, Levy MG, Savary KC, Gager RB, Breitschwerdt EB. Babesia gibsoni infections in dogs from North Carolina. J Am Anim Hosp Assoc. 1999;35:125‐128. [DOI] [PubMed] [Google Scholar]
- 169. Vayne C, Guery EA, Rollin J, et al. Pathophysiology and diagnosis of drug‐induced immune thrombocytopenia. J Clin Med. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bakchoul T, Marini I. Drug‐associated thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2018;2018:576‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Xie W, Hu N, Cao L. Immune thrombocytopenia induced by immune checkpoint inhibitors in lung cancer: case report and literature review. Front Immunol. 2021;12:790051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lerner W, Caruso R, Faig D, Karpatkin S. Drug‐dependent and non‐drug‐dependent antiplatelet antibody in drug‐induced immunologic thrombocytopenic purpura. Blood. 1985;66:306‐311. [PubMed] [Google Scholar]
- 173. Cain GR, Champlin R, Jain N. Immune thrombocytopenia in dogs after fetal liver hematopoietic cell transplantation. Exp Hematol. 1989;17:287‐291. [PubMed] [Google Scholar]
- 174. Fathi E, Jamshidi S. Case report: follow‐up, diagnosis, clinical evidence, laboratory evaluation, and treatment of idiopathic thrombocytopenia using human intravenous immunoglobulin in a terrier dog. Iran J Vet Sci Technol. 2014;6:64‐70. [Google Scholar]
- 175. Feldman BF. Demographics of canine immune‐mediated haemolytic anaemia in the southeastern United States. Comp Haematol Int. 1996;6:42‐45. [Google Scholar]
- 176. Goggs R, Boag AK, Chan DL. Concurrent immune‐mediated haemolytic anaemia and severe thrombocytopenia in 21 dogs. Vet Rec. 2008;163:323‐327. [DOI] [PubMed] [Google Scholar]
- 177. Jackson ML, Kruth SA. Immune‐mediated hemolytic anemia and thrombocytopenia in the dog: a retrospective study of 55 cases diagnosed from 1979 through 1983 at the Western College of Veterinary Medicine. Can Vet J. 1985;26:245‐250. [PMC free article] [PubMed] [Google Scholar]
- 178. Larkin HA, Collins JD, Lambert NH. A fatal case of idiopathic thrombocytopaenic purpura in a dog. Ir Vet J. 1972;26:25‐28. [Google Scholar]
- 179. Lavergne SN, Trepanier LA. Anti‐platelet antibodies in a natural animal model of sulphonamide‐associated thrombocytopaenia. Platelets. 2007;18:595‐604. [DOI] [PubMed] [Google Scholar]
- 180. Mellor PJ, Roulois AJ, Day MJ, et al. Neutrophilic dermatitis and immune‐mediated haematological disorders in a dog: suspected adverse reaction to carprofen. J Small Anim Pract. 2005;46:237‐242. [DOI] [PubMed] [Google Scholar]
- 181. Scott TN, Bailin HG, Jutkowitz LA, Scott MA, Lucidi CA. Bone marrow, blood, and clinical findings in dogs treated with phenobarbital. Vet Clin Pathol. 2021;50:122‐131. [DOI] [PubMed] [Google Scholar]
- 182. Trepanier LA, Danhof R, Toll J, Watrous D. Clinical findings in 40 dogs with hypersensitivity associated with administration of potentiated sulfonamides. J Vet Intern Med. 2003;17:647‐652. [DOI] [PubMed] [Google Scholar]
- 183. Vargo CL, Taylor SM, Haines DM. Immune mediated neutropenia and thrombocytopenia in 3 giant schnauzers. Can Vet J. 2007;48:1159‐1163. [PMC free article] [PubMed] [Google Scholar]
- 184. Woods JP, Johnstone IB, Bienzle D, Balson G, Gartley CJ. Concurrent lymphangioma, immune‐mediated thrombocytopenia, and von Willebrand's disease in a dog. J Am Anim Hosp Assoc. 1995;31:70‐76. [DOI] [PubMed] [Google Scholar]
- 185. Kidd L, Maggi R, Diniz PP, et al. Evaluation of conventional and real‐time PCR assays for detection and differentiation of spotted fever group rickettsia in dog blood. Vet Microbiol. 2008;129:294‐303. [DOI] [PubMed] [Google Scholar]
- 186. Kidd L, Qurollo B, Lappin M, et al. Prevalence of vector‐borne pathogens in Southern California dogs with clinical and laboratory abnormalities consistent with immune‐mediated disease. J Vet Intern Med. 2017;31:1081‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Maggi RG, Birkenheuer AJ, Hegarty BC, Bradley JM, Levy MG, Breitschwerdt EB. Comparison of serological and molecular panels for diagnosis of vector‐borne diseases in dogs. Parasit Vectors. 2014;7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Starkey LA, Barrett AW, Beall MJ, et al. Persistent Ehrlichia ewingii infection in dogs after natural tick infestation. J Vet Intern Med. 2015;29:552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Starkey LA, Barrett AW, Chandrashekar R, et al. Development of antibodies to and PCR detection of Ehrlichia spp. in dogs following natural tick exposure. Vet Microbiol. 2014;173:379‐384. [DOI] [PubMed] [Google Scholar]
- 190. Riswari SF, Tunjungputri RN, Kullaya V, et al. Desialylation of platelets induced by Von Willebrand factor is a novel mechanism of platelet clearance in dengue. PLoS Pathog. 2019;15:e1007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Jornet‐Rius O, Mesalles‐Naranjo M, Pastor J. Performance of the Sysmex XN‐V hematology analyzer in determining the immature platelet fraction in dogs: a preliminary study and reference values. Vet Clin Pathol. 2023;52:433‐442. [DOI] [PubMed] [Google Scholar]
- 192. Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc‐independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Summary of evidence for the causal role of neoplasia as a trigger for ITP in dogs. Level of evidence for neoplasia as a cause of ITP was assessed by means of a published Integrated Metric of Evidence (IME) value. Horizontal dotted lines indicate threshold IME values between negligible and low (3.15), low and intermediate (4.57), and intermediate and high (5.81) levels of evidence based on predetermined thresholds (Supporting Information 8). An IME value of 0 indicates that the study directly demonstrated a lack of evidence that a comorbidity caused ITP.
Figure S2. Summary of evidence for the causal role of inflammatory disease as a trigger for ITP in dogs. Level of evidence for inflammatory disease as a cause of ITP was assessed by means of a published Integrated Metric of Evidence (IME) value. Horizontal dotted lines indicate threshold IME values between negligible and low (3.15), low and intermediate (4.57), and intermediate and high (5.81) levels of evidence based on predetermined thresholds (Supporting Information 8). An IME value of 0 indicates that the study directly demonstrated a lack of evidence that a comorbidity caused ITP. SLE, systemic lupus erythematosus.
Figure S3. An algorithm for the diagnosis of ITP in dogs. aThrombocytopenia should be confirmed with a slide assessment. In brief, to perform a platelet estimate, first assess the slide body and feathered edge under low magnification for clumps, the presence of which suggests that the platelet count is falsely low. Resampling is then warranted, if possible. If there are no clumps, a platelet count is estimated by calculating the mean number of platelets per 10 oil immersion fields (×100) in the body of the smear where erythrocytes are spread in monolayer and multiplying this number by 15 000 to 20 000 to obtain the number of platelets per microliter; bthe magnitude of thrombocytopenia is consistent with consumption from major hemorrhage. However, it is possible that DIC, vasculitis, sequestration, or ITP may be contributing; cconsider genetic testing; dexcludes additional cytopenias with plausible explanations for example, pre‐regenerative anemia in the face of acute hemorrhage or hemolysis or lymphopenia in an ill/stressed patient; esampling of bone marrow by aspiration, core biopsy, or both, is undertaken; ffor example, lymphoreticular neoplasia or ehrlichiosis; gat least 2 of 5 parameters abnormal in addition to thrombocytopenia: prothrombin time (PT), activated partial thromboplastin time (aPTT), D‐dimers > reference interval (RI); antithrombin, fibrinogen < RI; hPT or aPTT >25% control value or upper bound of RI; ipartial screening (ie, not exhaustive) for potential trigger factors undertaken and negative.
Figure S4. An algorithm for the diagnosis of ITP in cats. aThrombocytopenia should be confirmed with a slide assessment. In brief, to perform a manual count first assess the slide's feathered edge under low magnification for clumps, the presence of which suggests that the platelet count is falsely low. Resampling is then warranted, if possible. If there are no clumps, a platelet count is estimated by calculating the mean number of platelets per 10 oil immersion fields (×100) and multiplying this number by 15 000 to 20 000 to obtain the number of platelets per microliter; bthe magnitude of thrombocytopenia is consistent with consumption from major hemorrhage; this is a rare association in cats. However, it is possible that DIC, vasculitis, sequestration, or ITP may be contributing; cexcludes additional cytopenias with plausible explanations for example, pre‐regenerative anemia in the face of acute hemorrhage or hemolysis or lymphopenia in an ill/stressed patient; dsampling of bone marrow by aspiration, core biopsy, or both, is undertaken; efor example, lymphoreticular neoplasia or feline immunodeficiency virus infection; fat least 2 of 4 parameters abnormal in addition to thrombocytopenia: prothrombin time (PT), activated partial thromboplastin time (aPTT), D‐dimers > reference interval (RI); fibrinogen < RI; gPT or aPTT >25% control value; hpartial screening (ie, not exhaustive) for potential trigger factors undertaken and negative.
Supporting Information 1. Extended materials and methods.
Supporting Information 2. Bibliography of studies for diagnosis.
Supporting Information 3. Bibliography of studies for comorbidities.
Supporting Information 4. Evidence evaluator instructions for Diagnosis PECO questions.
Supporting Information 5. Evidence evaluator instructions for Comorbidity PECO questions.
Supporting Information 6. Data extraction template for Diagnosis PECO questions
Supporting Information 7. Diagnosis PECO evidence summary template.
Supporting Information 8. Derivation of Integrated Metric of Evidence (IME) scores (D, Q, C, L, I, N).
Supporting Information 9. Data extraction template for Comorbidity PECO questions.
Supporting Information 10. Integrated Metric of Evidence (IME) calculations for comorbidity manuscripts.
Supporting Information 11. Evidence summaries and consensus recommendations for feline PECOs with minimal evidence.
Supporting Information 12. Consensus recommendations and evidence summaries for additional comorbidities.


