Abstract
Management of immune thrombocytopenia (ITP) in dogs and cats is evolving, but there are no evidence‐based guidelines to assist clinicians with treatment decisions. Likewise, the overall goals for treatment of ITP have not been established. Immunosuppressive doses of glucocorticoids are the first line treatment, but optimal treatment regimens beyond glucocorticoids remain uncertain. Additional options include secondary immunosuppressive drugs such as azathioprine, modified cyclosporine, and mycophenolate mofetil, usually selected based on clinician preference. Vincristine, human IV immunoglobulin (hIVIg), and transfusion of platelet or red blood cell–containing products are often used in more severe cases. Splenectomy and thrombopoietin receptor agonists are usually reserved for refractory cases, but when and in which patient these modalities should be employed is under debate. To develop evidence‐based guidelines for individualized treatment of ITP patients, we asked 20 Population Intervention Comparison Outcome (PICO) format questions. These were addressed by 17 evidence evaluators using a literature pool of 288 articles identified by a structured search strategy. Evidence evaluators, using panel‐designed templates and data extraction tools, summarized evidence and created guideline recommendations. These were integrated by treatment domain chairs and then refined by iterative Delphi survey review to reach consensus on the final guidelines. In addition, 19 non‐PICO questions covering scenarios in which evidence was lacking or of low quality were answered by expert opinion using iterative Delphi surveys with panelist integration and refinement. Commentary was solicited from multiple relevant professional organizations before finalizing the consensus. The rigorous consensus process identified few comparative treatment studies, highlighting many areas of ITP treatment requiring additional studies. This statement is a companion manuscript to the ACVIM Consensus Statement on the Diagnosis of Immune Thrombocytopenia in Dogs and Cats.
Keywords: glucocorticoids, immunoglobulin, immunosuppressive, platelet, transfusion, vincristine
Abbreviations
- ABCB1
ATP binding cassette subfamily B member 1
- CBC
complete blood cell count
- CR
complete response
- DOGiBAT
daily canine bleeding assessment tool
- hIVIg
human IV immunoglobulin
- IMHA
immune‐mediated hemolytic anemia
- ITP
immune thrombocytopenia
- MDR1
multidrug resistance 1
- MMF
mycophenolate mofetil
- NR
no response
- NSAID
nonsteroidal anti‐inflammatory drug
- PICO
Population Intervention Comparison Outcome
- pITP
primary immune thrombocytopenia
- PR
partial response
- sITP
secondary immune thrombocytopenia
- TEG
thromboelastography
- TPE
therapeutic plasma exchange
- TPO
thrombopoietin
1. INTRODUCTION
Immune thrombocytopenia (ITP) is an acquired immune‐mediated disorder that can result in hemorrhage because of a failure of primary hemostasis. The disorder is common in dogs, rare in cats, and is associated with substantial morbidity, and mortality rates of up to 27% in dogs. 1
The pathogenesis of ITP involves antiplatelet autoantibody formation that can result in platelet clearance 2 , 3 and complement‐mediated platelet destruction. 4 Platelet destruction by cytotoxic T‐cells contributes to thrombocytopenia development 5 , 6 , 7 and can occur in the absence of detectable platelet surface–associated immunoglobulins. 8 Platelet production may be inhibited by antibodies and T‐cells that target megakaryocytes 3 and through inappropriately low thrombopoietin (TPO) concentrations. 9 , 10 Clinically, ITP is a heterogeneous disease with limited association between thrombocytopenia severity and bleeding signs. Some animals have subclinical disease despite severe thrombocytopenia, whereas others with similar platelet counts experience life‐threatening hemorrhage. 11
Treatment for primary (spontaneous, nonassociative) ITP (pITP) typically involves nonspecific immune suppression using glucocorticoids and other immunosuppressive drugs, such as azathioprine, modified cyclosporine, or mycophenolate mofetil (MMF), adjunctive treatment with vincristine and human IV immunoglobulin (hIVIg), and transfusion with blood products as needed. Treatment of secondary (associative) ITP (sITP) aims to eliminate disease triggers and, in some cases, provide treatment for the associated immune‐mediated disorder. The diagnosis of ITP, including investigation for potential triggers of the disease, is systematically reviewed in the American College of Veterinary Internal Medicine (ACVIM) Consensus Statement on the Diagnosis of Immune Thrombocytopenia in Dogs and Cats. 12
Large randomized clinical trials evaluating therapeutic options for ITP are lacking, resulting in variation in clinical practice and ongoing debate regarding optimal treatment. Here, we summarize the available evidence, formulate management recommendations for ITP in dogs and cats, and highlight knowledge gaps to inform future study design. This consensus statement not only focuses on immunosuppressive treatment and transfusion but also considers supportive and emerging treatment modalities, during both the initial stabilization of patients and their long‐term management. We have designed 2 algorithms for the initial and long‐term management of dogs and cats with ITP incorporating the recommendations laid out in the text. The objective of this consensus statement is to present recommendations for the treatment of ITP in dogs and cats resulting from a systematic review of the available veterinary evidence while recognizing that only expert opinion can be provided in the absence of evidence. Wherever possible, guidelines were developed using standardized Population Intervention Comparison Outcome (PICO) questions. We also provide recommendations for assessing response to ITP treatment and ITP treatment goals as informed by guidelines used in human medicine 13 and author expertise. Although not evidence‐based, these definitions of response are included to aid standardization for future studies and provide clinicians with guidance on reasonable treatment goals.
2. MATERIALS AND METHODS
A schematic overview of the consensus statement process is shown in Figure 1. A comprehensive literature search strategy (Supporting Information 1) was developed to identify all articles relevant to the treatment of ITP in dogs and cats. Retrieved article records were imported into an online systematic review software (Covidence, Melbourne, Australia) for deduplication and initial screening of title and abstract for relevance. Studies were considered relevant if they described dogs or cats or both, asked and answered a question relevant to the treatment of ITP, and provided primary empirical evidence in any language. Patents, proceedings, reviews, abstracts, dissertations, theses, and letters to the editor were excluded. Full texts of potentially relevant articles were screened by 2 panelists, with a 3rd panelist serving as a tie breaker; after this screening, 273 complete articles were included (see Supporting Information 2 for a bibliography of the included articles).
FIGURE 1.
Overview of the methodology of the treatment domain. EE, evidence evaluator; PICO Population Intervention Comparison Outcome.
Panelists identified and recruited suitably qualified specialists, based on their publications relevant to ITP in dogs and cats, to serve as evidence evaluators. Willing participants were assigned to medical treatment or transfusion domains. The medical treatment domain was co‐chaired by B. Kohn and A. J. Mackin and included 15 evidence evaluators (E.H. Appleman, T.M. Archer, D. Bianco, S.L. Blois, B.M. Brainard, M.B. Callan, C.L. Fellman, A.S. Hale, A.A. Huang, J.M. Lucy, S.K. O'Marra, E.A. Rozanski, J.M. Thomason, J.E. Walton, and H.E. Wilson). The treatment‐transfusion domain was chaired by R. Goggs and included 4 evidence evaluators (A. Abrams‐Ogg, J.M. Haines, A.S. Hale, and J.E. Walton).
Domain chairs developed 20 clinical questions using a PICO format to investigate whether, in dogs and cats with primary ITP (P), treatment with a specific intervention (I), compared to a stated alternative intervention (C), improved the patient‐centered outcomes survival to discharge, duration of hospitalization, blood product usage, time to platelet recovery, response to first line treatment, and relapse (O). Nineteen additional questions were formulated that could not readily use the PICO format. Termed non‐PICO questions, these additional queries were generated to minimize gaps in the guidelines that were ultimately intended to inform clinical practice.
At least 2 evidence evaluators were assigned to each PICO. Evaluators were provided with detailed instructions on how to approach PICOs systematically (Supporting Information 3). To maximize completeness of the systematic review, a seminal paper from those prescreened was nominated by each evidence evaluator for every assigned PICO question and forward citation searches of these articles were performed using Scopus to uncover previously unidentified references (n = 11); 4 additional references were identified by evidence evaluator knowledge. A total of 288 references informed the final treatment guidelines. Evidence evaluators answered each PICO question by (i) selecting and refining a list of relevant articles from within the prescreened references through discussion with domain chairs; (ii) extracting data in a standardized manner using an electronic spreadsheet (Supporting Information 4 and 5); and (iii) scoring and summarizing evidence to generate draft summary statements utilizing structured evidence summary templates (Supporting Information 6). Additional targeted searches were used to screen for potentially relevant literature where few or no studies were initially identified that addressed the PICO question. Clinical diagnostic algorithms developed for ITP in dogs and cats as described in the accompanying diagnosis manuscript 12 were used by evidence evaluators to assess diagnostic certainty and adjudicate applicability of evidence to treatment guidance. Domain chairs reviewed PICO responses and integrated them into a single consensus response to each PICO question.
The PICO responses were subjected to 2 to 3 iterative rounds of Delphi surveys with post‐survey review, discussion, and revision until consensus, or near complete consensus, was reached. Unresolved differences of opinion are indicated in the text of the consensus statement. Responses to non‐PICO questions and suggestions for treatment response guidelines were obtained from panelists and evidence evaluators using anonymous surveys, compiled by panelists, and then revised among panelists with reference to any relevant literature until consensus was reached. One non‐PICO question (#10) was assessed by the comorbidity group and subsequently relocated here for consistency. The consensus statement was drafted and edited by B. Kohn, D.N. LeVine, R. Goggs, and A.J. Mackin. The draft of the consensus statement was then reviewed by all the panelists before submission to the American College of Veterinary Internal Medicine for review by all members and to other affiliate colleges and organizations as detailed in Supporting Information 1. Consistent feedback from colleagues was integrated and then utilized by the panelists to produce the final consensus statement.
3. RESULTS
3.1. Defining treatment response
Definitions of response to ITP treatment and the recommended treatment goal were established based on surveys of evidence evaluators and panelists, followed by refinement by panelists until consensus was reached. Definitions were guided by American Society of Hematology ITP guidelines. 13
No response (NR) was defined as a platelet count <30 000/μL or ongoing bleeding at least 2 weeks after initiating treatment.
Partial response (PR) was defined as a platelet count ≥30 000/μL and < 100 000/μL, combined with a >2‐fold increase in platelet count from diagnosis, and the absence of bleeding.
Complete response (CR) was defined as a platelet count ≥100 000/μL, without bleeding.
Full remission was defined as platelet count ≥100 000/μL, without bleeding in the absence of ongoing treatment.
The recommended treatment goal, either on treatment or once treatment was withdrawn, was a platelet count ≥100 000/μL with no evidence of bleeding.
Figures 2 and 3 show the overall treatment algorithms integrating the results of all PICO and non‐PICO questions.
FIGURE 2.
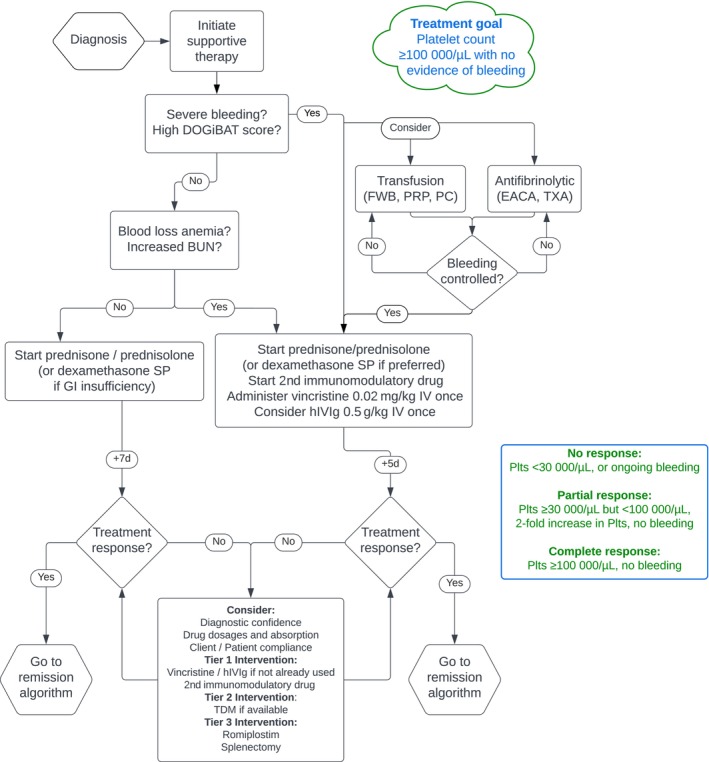
Treatment goals and an algorithm for the initial management of dogs and cats with immune thrombocytopenia. BUN, blood urea nitrogen; DOGiBAT, daily canine bleeding assessment tool; EACA, epsilon aminocaproic acid; FWB, fresh whole blood; GI, gastrointestinal; hIVIg, human IV immunoglobulin; PC, platelet concentrate; Plts, platelet count; PRP, platelet‐rich plasma; TDM, therapeutic drug monitoring; TXA, tranexamic acid.
FIGURE 3.
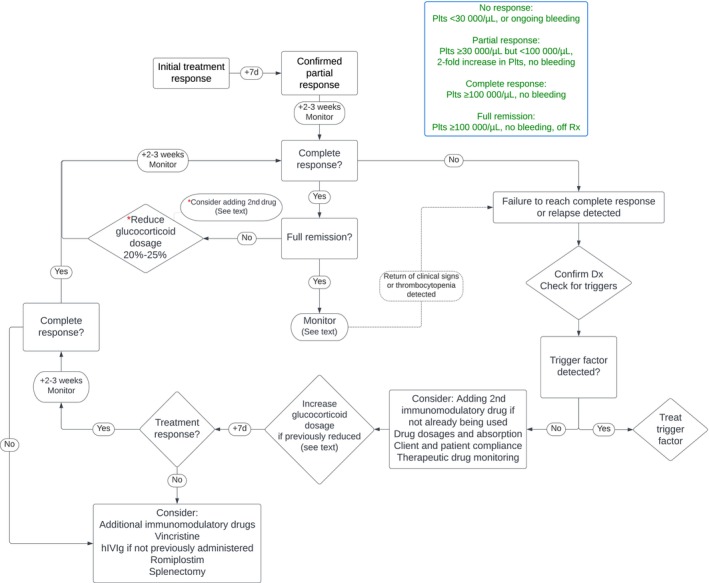
Treatment goals and an algorithm for the management of drug withdrawal, remission and relapse in dogs and cats with immune thrombocytopenia. Dx, diagnosis; hIVIg, human IV immunoglobulin; Plts, platelet count; Rx, treatment; TDM, therapeutic drug monitoring.
3.2. Guidelines for ITP treatment and evidence summaries
1. In dogs and cats with pITP (P), is combined treatment with glucocorticoids and vincristine (I) compared with use of glucocorticoids alone (C) associated with different primary or secondary outcomes (O)?
Dogs:
There is moderately strong evidence that a single IV administration of 0.02 mg/kg vincristine to dogs with pITP and clinically relevant bleeding, in conjunction with immunosuppressive dosages of glucocorticoids, accelerates initial platelet count recovery and shortens hospitalization time.
- We recommend vincristine as a first line emergency adjunctive treatment for dogs with ITP and clinically relevant bleeding.
- We suggest that vincristine should, however, be used with caution, if at all, in dog breeds with a high incidence of the ABCB1 (MDR1) gene mutation, including smooth/rough collies, Shetland sheepdogs, Australian shepherds and long‐haired whippets.
- We suggest that, if modified cyclosporine is considered as a potential concurrent immunosuppressive drug, commencement of cyclosporine should be delayed for several days after administration of vincristine to minimize the risk of drug‐induced neutropenia.
- We recommend that neutrophil counts be monitored in dogs receiving vincristine at recommended dosages, particularly if administered in conjunction with cyclosporine.
Level of evidence: Moderate. Strength of recommendation: Moderate. Degree of consensus: 39/40 Delphi Round 2. One evidence evaluator suggested that caution must be taken to prevent extravasation.
Evidence summary:
Vincristine is inexpensive, readily available, and easy to use. 14 , 15 In a prospective study, 14 the time to reach ≥40 000 platelets/μL was 4.9 ± 1.1 days in the vincristine/prednisone group compared with 6.8 ± 4.5 days in dogs that received prednisone alone. Duration of hospitalization decreased in the vincristine/prednisone group (5.4 ± 0.3 days), compared with the prednisone‐only group (7.3 ± 0.5 days). Limitations included use of surrogate endpoints, small sample size, lack of randomization, nonblinded design and lack of placebo controls. The time to reach ≥40 000 platelets/μL in the vincristine/prednisone group was slightly shorter (2.5; 1‐4 days) in a subsequent randomized clinical trial. 15 Neither study observed immediate or delayed adverse reactions.
Most dogs with ITP attain a platelet count between 50 and 100 000/μL within 7 days of commencing immunosuppressive glucocorticoid treatment, 16 , 17 potentially suggesting a modest effect size for vincristine. Median times to platelet count recovery in dogs treated with glucocorticoids alone in other studies are 5 days (2‐14, n = 27), 18 4 days (2‐9, n = 7), 19 and 5 and 7 days for 2 dogs. 20 In a retrospective study of 30 dogs, the median time to reach a platelet count ≥50 000/μL in 6 dogs treated with prednisolone/vincristine was 4 days (2‐7), whereas the median was 5 days (4‐11) in 14 dogs treated with prednisolone alone. 21 In another retrospective study of dogs with pITP, the median time to achieve a platelet count >40 000/μL in dogs that received vincristine in addition to glucocorticoids was 4 days (2‐10, n = 8), and the median time for normalization of platelet count was 10 days (3‐42). 22
Vincristine also may accelerate platelet recovery time in dogs unresponsive to glucocorticoids or in those with severe disease requiring blood transfusions. 23 , 24 , 25 , 26 , 27 A case report 28 and a case series 29 both suggest potential beneficial effects of vincristine‐loaded platelets for treatment of refractory and severe presumed pITP. The study designs of these reports limit their utility, but they also support a temporal association between vincristine or vincristine‐loaded platelet administration and platelet count recovery.
Transient neutropenia was reported in 19/127 dogs with presumed pITP treated with vincristine (0.02 mg/kg IV) within multidrug protocols. Administration of cyclosporine, but not other immunomodulatory drugs or hIVIg, was associated with the development of neutropenia. This effect was hypothesized to be caused by effects of cyclosporine on vincristine's metabolism or excretion. 30 It might be prudent to delay initiating cyclosporine by several days after vincristine administration. For dogs receiving vincristine, the median time until platelet count reached ≥40 000/μL was 4 days (1‐14) postvincristine for the dogs that became neutropenic and 3 days (0‐48) for those that did not. Four neutropenic dogs and 7 non‐neutropenic dogs had no recorded platelet count ≥40 000/μL. Survival to discharge was 95% in both groups, but duration of hospitalization was longer for dogs that became neutropenic, with a median of 6 days (3‐22) versus 4 days (2‐15).
Most studies suggest that platelet function after vincristine administration is not impaired in healthy dogs, but 1 study found decreased platelet aggregation after vincristine in dogs with lymphoma. 31 , 32 , 33 , 34
Cats:
There is insufficient evidence to determine with certainty if vincristine affects patient‐centered outcomes in cats with pITP, but the limited evidence that is available suggests that vincristine may not be effective.
In cats with pITP, vincristine is not recommended until more evidence becomes available.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 39/40 Delphi Round 2. Some evidence evaluators cautioned against overinterpreting the evidence because 2/3 cats that received vincristine had refractory disease and received vincristine late in the course of treatment; future study is needed.
Evidence summary:
Weak evidence from 2 case reports and 1 case series with 4 cats suggests that vincristine at 0.4 mg/m2, 0.025 mg/kg, and 0.02 mg/kg is ineffective or has minimal effect on platelet counts in cats with presumed ITP. 35 , 36 , 37 In 1 cat with ITP treated with multiple immunosuppressive drug protocols over time, a single IV injection of vincristine (0.025 mg/kg) on day 121 was followed by a small, temporary increase in platelet count on day 129. 35 In a case report of a cat with presumed ITP treated with multiple immunosuppressive drugs, vincristine (0.4 mg/m2) was given on day 41 and platelet count increased to 20 000/μL 6 days later. 36 In the case series, 1 cat was given dexamethasone (0.3 mg/kg IV q12h) and 1 dose of vincristine (0.02 mg/kg IV) but failed to respond and was euthanized. 37
Non‐PICO 1: What dosage of vincristine should be used?
We suggest a vincristine dosage of 0.02 mg/kg IV given once, with a maximum dosage of 0.5 mg/m2 for dogs >25 kg, although a recent study did not find evidence of an association between neutropenia and increased vincristine dosages. 30 We suggest that vincristine should, however, be used with caution, if at all, in dog breeds with a high incidence of the ABCB1 (MDR1) gene mutation.
Non‐PICO 2: Is there rationale to use vincristine alone versus in combination with glucocorticoids?
No studies suggest that vincristine alone, without glucocorticoids, is effective and safe for pITP. Vincristine alone might provide a transient increase in circulating platelets, but sustained immunosuppression provided by glucocorticoids is required for long‐term management. Use of vincristine alone might be considered in actively bleeding patients if glucocorticoid administration must be delayed (eg, recent nonsteroidal anti‐inflammatory drug [NSAID] treatment). Human IVIg or romiplostim have more sustained effects and might be preferred to vincristine if immunosuppression is contraindicated.
2. In dogs and cats with P, is treatment with combined glucocorticoids and hIVIg (I) compared with use of glucocorticoids alone (C) associated with different primary or secondary outcomes (O)?
Dogs:
There is strong evidence that a single IV hIVIg infusion (0.5 g/kg) over 6‐12 hours in dogs with presumed pITP, in conjunction with immunosuppressive dosages of glucocorticoids, accelerates initial platelet count recovery and shortens hospitalization time, compared with glucocorticoids alone.
- Intravenous hIVIg can be considered, in combination with glucocorticoids, as a first line emergency adjunctive treatment in dogs with pITP and clinically relevant bleeding, but vincristine typically is preferred in such circumstances (PICO 3).
- We recommend the use of IV hIVIg as an emergency adjunctive treatment in dogs with presumed ITP and clinically relevant bleeding where vincristine has greater potential for adverse effects, such as dog breeds with a high incidence of the ABCB1 (MDR1) gene mutation, including smooth/rough collies, Australian shepherds, Shetland sheepdogs and long‐haired whippets, or where vincristine has been ineffective.
- Consideration of the use of hIVIg may be influenced by product cost and availability.
No studies were identified that evaluated the combined use of vincristine and hIVIg in dogs with ITP, but this approach can be considered in animals with life‐threatening bleeding.
Level of evidence: High. Strength of recommendation: Strong. Degree of consensus: 39/40 Delphi Round 2.
Evidence summary:
Two randomized controlled trials (RCTs) support the use of hIVIg in dogs with pITP. 15 , 38 At a minimum dosage of 0.5 g/kg, hIVIg in addition to glucocorticoids accelerated platelet count recovery and shortened the hospitalization time compared with glucocorticoids alone. 38 Median platelet recovery time was 3.5 days (2‐7), and median hospitalization time was 4 days (2‐8) compared with 7.5 days (3‐12) and 8 days (4‐12), respectively, in the corticosteroid‐only group. The outcomes for the hIVIg treatment group in a subsequent clinical trial 15 were very similar: median platelet recovery time was 2.5 days, and median hospitalization time was 5 days. Compared with glucocorticoids alone, administration of hIVIg to dogs with ITP did not significantly affect survival to discharge, transfusion requirements, cost of hospitalization, or long‐term survival. 38 This may be because of the postulated short‐term effects of hIVIg in dogs. 39 , 40 Several case reports and case series support the use of hIVIg to accelerate platelet recovery in dogs with refractory pITP and those with clinically relevant bleeding. 39 , 41 , 42 , 43 Dogs that respond to hIVIg infusion can experience platelet count recovery during the hIVIg infusion itself or ≤72 hours after administration. Nonresponders typically experience platelet count recovery >72 hours after hIVIg infusion, which may result from the use of additional immunosuppressive or immunomodulatory drugs. As for vincristine, the effect size of hIVIg administration may be modest (see PICO 1 above). Single hIVIg infusions appear relatively safe, because few adverse reactions have been reported. 15 , 38 , 39 , 41 , 42 , 43 One case report described acute hemolytic anemia that was suspected to be secondary to hIVIg administration. 44 Studies in healthy dogs observed a hypercoagulable state in association with hIVIg infusion, 45 and hypercoagulability has been observed in dogs treated for ITP. 46 As such, dogs receiving hIVIg for ITP should be closely monitored for thrombotic complications as the platelet count rebounds. Infusion of hIVIg especially should be considered as an adjunctive treatment for severely affected, hospitalized animals because the product has limited availability, high cost, and there are potential ethical concerns surrounding human product use. Moreover, hemodynamically stable pITP dogs without anemia or active bleeding have a good prognosis. 21 , 47
Cats:
Based on the descriptions of several cats with presumed pITP that may have responded to hIVIg after failure to respond to glucocorticoids alone, there is weak evidence that hIVIg in combination with glucocorticoids may induce recovery of platelet counts in individual cats compared with the use of glucocorticoids alone.
We suggest that hIVIg in conjunction with glucocorticoids be considered as an adjunctive emergency treatment option in cats with clinically relevant bleeding or refractory pITP.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 39/40 Delphi Round 2.
Evidence summary:
Use of hIVIg in cats with ITP has been described in 2 case reports. 48 , 49 In 1 cat with precursor immune‐mediated anemia and thrombocytopenia refractory to glucocorticoids, a single IV infusion of hIVIg (1.21 g/kg) was associated with reticulocytosis and platelet count recovery within 72 hours. 48 In another report of a cat with presumed pITP unresponsive to prednisolone and cyclosporine, a single hIVIg infusion was associated with a temporary increase in platelet count. 49
Non‐PICO 3: What dosage of IVIg should be used?
We suggest a hIVIg dosage of 0.5‐1.0 g/kg IV given once, with dosage adjustments based on vial and patient size as needed. Repeated administration of IVIg is not recommended because the efficacy and safety of repeated treatment beyond 3 days has not been assessed in dogs or cats.
3. In dogs and cats with pITP (P), is treatment with combined glucocorticoids and IVIg (I) compared with use of glucocorticoids and vincristine (C) associated with different primary or secondary outcomes (O)?
Dogs:
There is moderately strong evidence that the use of a single IV administration of vincristine at the appropriate dosage of 0.02 mg/kg or a single hIVIg infusion at a minimum dosage of 0.5 g/kg IV over 6‐12 hours in dogs with presumed pITP, in conjunction with immunosuppressive dosages of glucocorticoids, accelerates initial platelet count recovery and shortens hospitalization time.
- Owing to substantially lower cost, ready availability, and ease of administration, vincristine should be used as a first line emergency adjunctive treatment in preference to hIVIg for dogs with presumed ITP and clinically relevant bleeding.
- We suggest that vincristine should, however, be used with caution, if at all, in dog breeds with a high incidence of the ABCB1 (MDR1) gene mutation, including smooth/rough collies, Shetland sheepdogs and long‐haired whippets.
- We also suggest that, if modified cyclosporine is considered as a potential concurrent immunosuppressive drug, the commencement of cyclosporine be delayed for several days after administration of vincristine to minimize the risk of drug‐induced neutropenia.
Level of evidence: Moderate. Strength of recommendation: Moderate. Degree of consensus: 35/39 Delphi Round 2. Some evaluators felt that because there was clinical equivalence, cost should not factor into the recommendation.
Evidence summary:
One RCT directly addressed the PICO question, 15 2 other prospective studies partially addressed it, 14 , 38 and several other reports provided additional relevant information. 23 , 24 , 25 , 26 , 27 , 47 , 50 , 51 The RCT supports use of either vincristine or hIVIg in addition to glucocorticoids in dogs with pITP and clinically relevant bleeding to accelerate platelet count recovery and shorten hospitalization time. 15 In that study, the median time to reach ≥40 000 platelets/μL was 2.5 days (1‐4) in the vincristine/prednisone group, and 2.5 days (0‐10) in the hIVIg/prednisone group. Results from both intervention groups in the RCT were very similar to comparable groups in previous reports. 14 , 38 In the RCT, no differences in transfusion requirements or survival to discharge, or survival to 6 or 12 months after study entry, were observed between interventions, but the trial was underpowered to detect differences in these outcomes. In the RCT, however, cost of treatment was significantly higher in the hIVIg/prednisone compared with the vincristine/prednisone group.
Cats:
There is insufficient evidence to make a strong recommendation for use of vincristine or hIVIg in cats with pITP, but limited available data from case reports and series favor hIVIg over vincristine as an adjunctive emergency treatment option in conjunction with immunosuppressive dosages of glucocorticoids in cats with ITP and clinically relevant bleeding.
We suggest that, in addition to glucocorticoids, hIVIg should be considered in preference to vincristine as an adjunctive emergency treatment option in cats with pITP.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 38/39 Delphi Round 2. Some evidence evaluators cautioned against overinterpreting the evidence against vincristine because 2/3 cats that received vincristine had refractory disease and received vincristine late in the course of treatment; future study is needed.
Evidence summary:
See PICO 1 and 2 above.
4. In dogs and cats with pITP (P), is treatment with glucocorticoids combined with a second immunosuppressive drug (I) compared with use of glucocorticoids alone (C) associated with different primary or secondary outcomes (O)?
Dogs:
There is insufficient evidence to determine if combining glucocorticoids with a 2nd immunosuppressive drug is associated with different outcomes versus use of glucocorticoids alone.
- We suggest a 2nd immunosuppressive drug be used in combination with glucocorticoids in the following situations:
- No response within 5‐7 days of starting glucocorticoids.
- Development or expected development of severe adverse effects to glucocorticoids.
- Relapse during tapering of glucocorticoid dosage after a CR.
- Early use of a 2nd immunosuppressive drug may be considered in
- Dogs >25 kg to allow more rapid tapering of glucocorticoid dosages.
- Dogs with severe bleeding because of anticipated delayed onset of action of secondary drugs.
If a 2nd immunosuppressive drug is to be used, reasonable options include (alphabetically): azathioprine, cyclosporine (modified), leflunomide, and MMF.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 34/39 Delphi Round 2.
Evidence summary:
No prospective controlled studies addressing the PICO question were identified. Seven retrospective studies included dogs treated with glucocorticoids alone or treated with glucocorticoids and at least 1 second immunosuppressive drug, with 2nd drugs often evaluated as a group rather than individual medications. No study supported or refuted the benefit of a 2nd immunosuppressive drug for initial response to treatment 52 or rate of relapse, 53 but all were likely underpowered. 1 , 16 , 20 , 21 , 47 , 52 , 53 Some studies did not directly compare treatment groups and outcomes, 16 , 20 or dogs were treated with >1 second drug, precluding any conclusions regarding efficacy. 1 , 21 , 47 , 53 Proprietary or generic microemulsified “modified” cyclosporine is the recommended formulation of the drug, because nonmodified formulations may have suboptimal bioavailability. 54 , 55
All animals in a case series of 5 dogs with ITP treated with MMF as a sole immunosuppressive drug achieved complete remission. 56 This suggests that MMF might be an effective, single‐drug treatment for ITP in dogs, although spontaneous remission cannot be ruled out.
Progressively higher frequency of glucocorticoid adverse effects with increasing body weight has been described in dogs. 57 This outcome might warrant more rapid glucocorticoid dosage tapering, through dosage reduction or dosing interval extension, in larger dogs, which may be facilitated by early institution of a 2nd immunosuppressive drug. See non‐PICO 14 for more discussion of drug tapering.
There is no evidence that adding a 3rd immunosuppressive drug improves outcomes whereas further immunosuppression can result in adverse effects. 47 Administration of more than 2 immunosuppressive drugs is not recommended, but changing the 2nd immunosuppressive drug can be considered.
Cats:
There is insufficient evidence to determine if combining glucocorticoids with a 2nd immunosuppressive drug is associated with different outcomes versus use of glucocorticoids alone.
- We suggest a 2nd immunosuppressive drug be used in combination with glucocorticoids in the following situations:
- No response within 5‐7 days of starting glucocorticoids.
- Development or expected development of severe adverse effects to glucocorticoids.
- Relapse during tapering of glucocorticoid dosage after a CR.
Early use of a 2nd immunosuppressive drug may be considered in cats with severe bleeding because of anticipated delayed onset of action of secondary drugs.
- If a 2nd immunosuppressive drug is to be used, modified cyclosporine and chlorambucil can be considered.
- Azathioprine should not be used in cats because of their inability to effectively metabolize it and risk of hematological toxicity.
- Chlorambucil can be considered but has been associated with thrombocytopenia when used chronically.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 37/39 Delphi Round 2.
Evidence Summary:
No prospective controlled studies addressing the PICO question were identified. Among 11 cats with ITP reported in the literature across 2 case series and 2 case reports, 35 , 36 , 37 , 58 there are no consistent findings regarding efficacy of specific 2nd immunosuppressive drugs or of specific glucocorticoids. 36 , 37 , 58 In individual cats that responded to treatment, both chlorambucil 58 and cyclosporine have been used. 35 , 37 Chlorambucil has been associated with myelosuppression and with thrombocytopenia when given chronically for management of gastrointestinal (GI) lymphoma in cats. 59 , 60 Azathioprine is poorly metabolized by cats 61 and can cause fatal myelosuppression. 62 The use of MMF as an adjunctive immunosuppressive drug has been reported in 2 cats with primary immune‐mediated hemolytic anemia, 63 but its use in ITP has not been described to date. In cats, MMF has variable pharmacokinetics, 64 and data on efficacy and safety are limited at present. 65 A single case report of a cat with pITP that underwent splenectomy for treatment‐refractory ITP suggests that cyclophosphamide may have some efficacy for treatment of ITP in cats. 49
Non‐PICO 4: In dogs and cats with pITP, should we individualize treatment using bleeding scoring systems such as daily canine bleeding assessment tool (DOGiBAT)?
In animals with pITP, we suggest that treatment be individualized based on disease severity (Figure 2). Animals with suspected pulmonary, central nervous system, or overt GI bleeding should receive vincristine or hIVIg, and co‐administration of a 2nd immunosuppressive drug in addition to glucocorticoids should be considered. In contrast, we suggest that patients with minimal or no bleeding be managed with glucocorticoids alone unless they are predicted to be glucocorticoid intolerant. Bleeding severity scores (eg, DOGiBAT) might help individualize treatment, but further validation is required before such systems are used to guide treatment.
Non‐PICO 5: In dogs and cats with pITP, what are the best predictors of disease severity?
In animals with pITP, the clinical signs of bleeding and anatomic site of bleeding are markers of disease severity. The presence of GI bleeding, specifically melena, and central nervous system or pulmonary hemorrhage are the most common indicators of severe ITP. 11 High blood urea nitrogen concentration and anemia necessitating transfusion are also indicators of severe ITP. 47
Non‐PICO 6: In dogs and cats with pITP, when should a 2nd immunosuppressant drug beyond glucocorticoids be added?
We suggest 2nd immunosuppressive drugs be considered in the following scenarios:
Severe hemorrhage requiring multiple transfusions, with the 2nd drug given either immediately or within 1‐3 days of diagnosis. It may be prudent to delay initiating cyclosporine by several days after vincristine administration to minimize the risk of vincristine‐related neutropenia.
Active, refractory hemorrhage, including GI bleeding, if initial rapid control measures besides glucocorticoids (hIVIg, vincristine) are unsuccessful within 1‐3 days of diagnosis.
Inadequate response to glucocorticoids (no substantial increase in platelet count), typically within 5 days of commencing initial treatment.
When adverse effects associated with glucocorticoids are a concern (eg, large dogs), are unacceptable for the owner, or concurrent disease is present that would necessitate rapid glucocorticoid dosage reduction (eg, diabetes mellitus, hyperadrenocorticism, urinary incontinence, severe cardiac disease, prior thromboembolic events).
When ITP relapse has occurred while receiving glucocorticoids.
5. In dogs and cats with P, is treatment with dexamethasone (I) compared with use of prednisone or prednisolone (C) associated with different primary or secondary outcomes (O)?
See Supporting Information 7.
6. In dogs and cats with P, is treatment with hIVIg alone (I) compared with use of prednisone or prednisolone (C) associated with different primary or secondary outcomes (O)?
There is insufficient evidence to make treatment recommendations regarding use of hIVIg alone in dogs and cats with pITP compared with the use of glucocorticoids alone.
- Use of hIVIg as the sole treatment in dogs and cats with pITP not receiving glucocorticoids or any other immunosuppressive drug is not recommended.
- Use of hIVIg can be considered in patients in which glucocorticoids or other immunosuppressants are contraindicated.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 38/38 Delphi Round 2.
Evidence summary (Dogs):
No studies were identified describing the use of hIVIg alone for treatment of pITP in dogs. In contrast, there is evidence supporting use of glucocorticoids as sole drugs. 1 , 14 , 16 , 19 , 20 , 21 , 38 , 47 One case report described successful treatment of immune‐mediated hemolytic anemia (IMHA) and presumed ITP in a dog with diabetes mellitus using a single hIVIg infusion (1.3 g/kg IV over 8 hours) combined with leflunomide (2 mg/kg PO q12h). 66 The platelet count was 2000/μL at presentation, 51 000/μL immediately after the hIVIg infusion, and 116 000/μL 24 hours later. Current American Society of Hematology guidelines suggest glucocorticoids in preference to IVIg in children with ITP and non–life‐threatening mucosal bleeding. 67
Evidence summary (Cats):
Use of hIVIg in cats with ITP has been described in 2 case reports. 48 , 49 Both cats received concurrent glucocorticoids, and 1 cat also received cyclosporine and was splenectomized. As such, the utility of hIVIg as a sole treatment is unknown. The total number of cats with pITP described is low, 35 , 36 , 37 , 58 , 68 , 69 , 70 but glucocorticoids alone, or in combination with other immunosuppressive drugs, may be effective for the treatment of cats with pITP. 35 , 36 , 37 , 58 , 69 In a case series of 4 cats with presumed pITP, 3 cats developed adverse effects associated with chronic glucocorticoid administration including diabetes mellitus and recurrent bacterial urinary tract infections. 37 Adverse effects should be considered when planning chronic treatment of cats with presumed pITP.
7. In dogs and cats with pITP (P), is initial treatment with high dosages of prednisolone or prednisone (>2 mg/kg/day; I) compared with more conservative dosages (2 mg/kg or dosing based on m 2 as in IMHA; C) associated with different primary or secondary outcomes (O)?
See Supporting Information 7.
8. In dogs and cats with pITP (P), is a maintenance treatment with glucocorticoids and a 2nd immunosuppressive drug (I) superior to glucocorticoids alone (C) to prevent relapse (O)?
Dogs:
There is insufficient evidence to determine if maintenance treatment with glucocorticoids and a 2nd immunosuppressive drug is superior to glucocorticoids alone to prevent relapse.
- For maintenance treatment of pITP in dogs, use of a 2nd immunosuppressive drug in combination with glucocorticoids can be considered in the following situations:
- Relapse after a CR when the glucocorticoid dosage is tapered.
- Development or expectation of severe adverse effects related to the use of glucocorticoids.
- Dogs weighing >25 kg to enable more rapid tapering of glucocorticoid dosages.
Reasonable 2nd immunosuppressive drug options include (alphabetically) azathioprine, cyclosporine (modified), leflunomide, or MMF.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 38/40 Delphi Round 2.
Evidence summary:
No prospective studies addressed the PICO question. Three retrospective studies that compared rate of relapse among dogs treated with glucocorticoids alone with those receiving combined glucocorticoids and additional immunosuppressive drugs neither support nor refute the benefit of additional immunosuppressive drugs in preventing relapse. 1 , 21 , 53 In a study observing dogs with pITP for ≥1 year, the relapse rate was 31% (14 of 45 dogs), but no difference in relapse rate among dogs treated with prednisone alone (n = 8) compared with regimens combining prednisone with other immunosuppressive drugs (n = 37) was observed. 53 Overall relapse rates in 2 other studies were 26% 21 and 39%, 1 but neither study observed any difference in relapse rates among groups of dogs managed with different immunosuppressive drug regimens.
Increasing body weight is associated with progressively higher incidence of glucocorticoid adverse effects in dogs. 57 Addition of a 2nd immunosuppressive drug could allow more rapid glucocorticoid tapering.
Cats:
There is no evidence to determine if maintenance treatment with glucocorticoids and a 2nd immunosuppressive drug is superior to glucocorticoids alone to prevent relapse.
- For maintenance treatment of pITP in cats, use of a 2nd immunosuppressive drug in combination with glucocorticoids can be considered in the following situations:
- Relapse after a CR when the glucocorticoid dosage is tapered.
- Development or expectation of severe adverse effects related to the use of glucocorticoids.
- Reasonable 2nd immunosuppressive drug options include (alphabetically) chlorambucil and modified cyclosporine.
- Chlorambucil can be considered but has been associated with thrombocytopenia when used chronically.
Level of evidence: None. Strength of recommendation: Weak. Degree of consensus: 36/39 Delphi Round 2.
Evidence summary:
No prospective studies addressed the PICO question. No publication on cats with ITP compared glucocorticoids alone with multidrug regimens. 35 , 36 , 37 , 58 In 1 case series, the 3 cats that survived to discharge were managed with glucocorticoids alone and relapsed several times during the follow‐up period; 1 cat received a glucocorticoid and cyclosporine to maintain remission. 37 Similarly, another case report described a cat with probable ITP that relapsed during prednisolone tapering, appeared to respond to the addition of cyclosporine and subsequently had changes in platelet count that corresponded with changes in immunosuppressive drug dosages. 35 In another case series, 3 of the 4 cats that survived to discharge were treated with glucocorticoids alone and did not relapse within 12 to 120 days. 58 One cat treated with prednisolone and chlorambucil relapsed on day 36 after both drugs had been tapered and responded to increased dosages. 58 For further discussion of additional immunosuppressive drugs in cats, please see PICO 4 above.
Non‐PICO 7: In dogs and cats with pITP, how often and why do relapses occur?
Reported relapse rates range from 9% to 47%, with most occurring earlier in the disease (median, 79 days in 1 study). 1 , 16 , 20 , 21 , 47 , 53 , 71 Potential causes include: rapid tapering or cessation of immunosuppression, 72 occurrence of a new comorbidity triggering an autoimmune response, or the persistent effect of an occult comorbidity that escaped detection during the initial diagnostic evaluation. It is uncertain if time to recovery of platelet count is associated with relapse. 20 , 53 Similarly, treatment duration (finite vs indefinite) and treatment regimen have not been consistently identified to impact relapse, 1 , 53 although rapidity of prednisone tapering may be associated with relapse. 72
Non‐PICO 8: In dogs and cats with pITP experiencing a relapse, what tests or diagnostic evaluation should be performed?
We suggest the following be considered: history including drug and travel exposures, CBC with blood smear examination, serum biochemical profile, and urinalysis. Imaging studies, cytologic examination, and infectious or neoplastic disease screening may be indicated based on geographic location and infectious disease risk. 12 Therapeutic drug monitoring might be useful. The extent of investigation should be adjusted based on the interval from initial diagnosis and any recent changes in drug treatment (ie, less investigation if relapse occurs during immunosuppressive drug tapering).
Non‐PICO 9: How should dogs and cats with pITP that experience relapse be managed?
If relapse is suspected, we recommend that the diagnosis be reconfirmed using the diagnostic criteria and approach described in the Consensus Statement on Diagnosis of ITP, 12 recognizing that some test results may be affected by recent or current immunosuppressive drug administration. Assessments should be made for potential trigger factors with particular attention paid to infectious triggers if the relapse occurred during immunosuppressive treatment. Identification of a trigger factor warrants initiation of disease‐specific treatment with or without concurrent immunosuppression (see non‐PICO 10). As shown in Figure 3, if no trigger factor is identified, and the relapse occurred while tapering immunosuppressive drugs, we suggest that the dosage of immunosuppressive drugs be increased. A relapse of mild disease can be managed by returning the drug dosages to their most recent previous dosages. In contrast, a relapse of severe disease should be managed by restarting the induction protocol. Depending on severity of relapse, vincristine and hIVIg could be administered, with hIVIg only being given if it was not previously used. After re‐establishing a treatment response, all future immunosuppressive drug tapering should be undertaken more gradually (eg, doubling time intervals or decreasing the decrements in drug dosages). Lifelong immunosuppressive treatment, at the lowest achievable dosage, may be necessary if recurrent relapses occur despite careful, gradual tapering. Continuous immunosuppressive treatment or repeated relapse might prompt consideration for use of romiplostim or splenectomy provided potential infectious disease triggers have been excluded.
9. In dogs and cats with pITP (P), is treatment with glucocorticoids and any 2nd drug (I) compared with treatment with glucocorticoids and any other 2nd drug (C) associated with different primary or secondary outcomes (O)?
Dogs:
There are insufficient data to allow comparison of efficacy of any 2nd immunosuppressive drugs in improving treatment outcomes in dogs with ITP.
- If use of a 2nd immunosuppressive drug in combination with glucocorticoids is deemed to be indicated, any of the options listed under PICO 4 (azathioprine, modified cyclosporine, leflunomide, or MMF) can be considered, because there are no data documenting improved efficacy of a specific 2nd drug compared to another.
- The preferred drug might be determined by patient size, because modified cyclosporine, as a drug approved for veterinary use, is available in formulations suitable for dosing smaller dogs without the need for compounding.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 39/40 Delphi Round 2.
Evidence summary:
One retrospective study of 37 dogs with pITP compared prednisone and cyclosporine (n = 17) with prednisone and MMF (n = 20). 73 Baseline patient characteristics were not statistically compared, but larger dogs appeared to more frequently receive MMF. No outcome differences were apparent including duration of hospitalization (both median, 3 days), 30‐day survival (17/20 dogs for MMF vs 15/17 for cyclosporine), and 60‐day survival (16/20 dogs for MMF vs 14/17 for cyclosporine). The authors observed that fewer adverse events occurred in the MMF group (9/20 for MMF vs 11/17 for cyclosporine), but statistical comparisons were not made. No conclusions regarding the comparative efficacy of MMF versus cyclosporine for treatment of presumed pITP could be drawn. Four other retrospective studies included dogs that received a glucocorticoid and a 2nd immunosuppressive drug, but they were not designed to determine the effect of 1 second drug compared to another in dogs receiving glucocorticoids. 1 , 21 , 47 , 53 Use of cyclosporine has been associated with increased risk of bacterial infection 74 and with opportunistic, potentially life‐threatening, fungal infections. 75
Cats:
No data allow comparison of efficacy of any 2nd immunosuppressive drugs in improving treatment outcomes in cats with ITP.
- If use of a 2nd immunosuppressive drug in combination with glucocorticoids is deemed to be indicated in cats, either chlorambucil or modified cyclosporine can be considered.
- Azathioprine should not be used in cats because of their inability to effectively metabolize it and the risk of hematological toxicity.
- Chlorambucil can be considered but has been associated with thrombocytopenia when used chronically.
Level of evidence: None. Strength of recommendation: Weak. Degree of consensus: 38/40 Delphi Round 2.
Evidence summary:
No studies directly addressing the PICO question were identified. Various 2nd immunosuppressive drugs including cyclosporine (n = 2), chlorambucil (n = 1), and cyclophosphamide (n = 1) have been administered to cats with ITP, but no conclusions can be drawn from these case reports regarding drug efficacy. 35 , 36 , 37 , 49 , 58
Non‐PICO 10: What is the role of immunosuppression, if any, in the treatment of ITP associated with a reversible comorbidity such as an infection, and does it vary with the comorbidity or individual animal?
In patients with probable sITP and severe, life‐threatening thrombocytopenia, short‐term immunosuppression may be considered in conjunction with the administration of specific treatment while the results of diagnostic tests are pending, when treating the underlying comorbidity is not expected to result in rapid resolution of thrombocytopenia, or when the perceived risk of life‐threatening hemorrhage exceeds the risks of immunosuppression. In patients with probable sITP and moderate thrombocytopenia, definitive treatment for the comorbidity and monitoring platelet count is recommended. Where surgery is required to eliminate the comorbidity, potential consequences of immunosuppression on healing should be considered. Platelet products should be provided for hemostasis according to the transfusion guidelines.
Whether immunosuppression is needed to resolve sITP is likely to vary with the comorbidity and the individual patient. Both comorbidity and patient factors are likely to determine the mechanism of immune‐mediated platelet destruction, which in turn might influence the need for immunosuppression. Some comorbidities may induce a true autoimmune response where antibodies specifically target platelet antigens, resulting in Fc or complement‐mediated phagocytosis and destruction. 76 , 77 , 78 , 79 Antibodies targeting comorbidity antigens or hapten (rather than self‐antigen) may also cause immune‐mediated platelet destruction when antigen, haptens, or circulating immune complexes bind platelet membranes. 22 , 76 , 77 , 80 , 81 , 82 The formation of platelet‐leukocyte aggregates also contributes to thrombocytopenia during some infections, suggesting that innate immunity is involved. 83 The individual patient immune milieu when the comorbidity develops may impact whether or not persistent immune dysregulation that requires immunosuppression occurs. 78
Few studies have investigated the mechanisms of immune‐mediated platelet destruction or evaluated the impact of immunosuppression on outcomes in dogs and cats with sITP. The consequences of immunosuppression are most important to consider with an infectious trigger because it may worsen infection or enable organism persistence. In many patients, eliminating the comorbidity without immunosuppression resolves ITP. 84 , 85 , 86 , 87 , 88 , 89 , 90 By eliminating the comorbidity, platelet autoantibody production may decrease even when platelets, rather than comorbidity antigen, are targeted. 79 For others, immunosuppression may be necessary, but controlled studies are lacking. 84 , 88 , 89 , 90 Importantly, thrombocytopenia associated with vector‐borne disease agents usually rapidly responds to appropriate antimicrobial treatment alone. Pending results of diagnostic testing, empirical treatment with doxycycline is warranted in any patient (without contraindications) where doxycycline responsive vector‐borne disease agents are potential triggers. Definitively ruling out infection can be challenging, and testing guidelines should be followed to ensure infection is not overlooked. 12 Further research investigating whether, and in what circumstances, immunosuppression is required to resolve sITP is necessary.
10. In dogs and cats with pITP (P), is treatment with melatonin (I) compared with no melatonin treatment (C) associated with different primary or secondary outcomes (O)?
See Supporting Information 7.
11. In dogs and cats with pITP (P), is treatment with TPO receptor agonists (I) compared with no TPO receptor agonist treatment (C) associated with different primary or secondary outcomes (O)?
Dogs:
Limited evidence, based on a small number of cases, suggests that the TPO receptor agonist romiplostim may be associated with improved platelet counts in dogs with treatment‐refractory ITP.
- In dogs with refractory ITP with substantial risk for clinical bleeding, romiplostim may be considered as a potentially safe and effective treatment option.
- The current very high cost of romiplostim may limit routine usage.
- Use of romiplostim could be considered in patients where glucocorticoids or other immunosuppressants are contraindicated.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 38/40 Delphi Round 2.
Evidence summary:
The use of romiplostim, a TPO receptor agonist, has been described in 5 dogs with ITP (3 pITP and 2 sITP) 91 and in a case report of a dog with refractory amegakaryocytic ITP. 92 Five dogs had improved outcomes after initiation of romiplostim, without adverse effects. One dog with ITP secondary to chronic ehrlichiosis had an increased platelet count only after repeated romiplostim administration in conjunction with glucocorticoids. Study limitations include low case numbers, no external controls, and concurrent immunosuppressive drug administration. Although expensive, romiplostim may carry lower risk than treatments such as splenectomy or therapeutic plasma exchange (TPE). Eltrombopag, another TPO receptor agonist used to treat ITP in humans is, based on experimental studies utilizing canine platelets, unlikely to be effective in dogs. 93
Cats:
There are no reports of TPO receptor agonist use in cats with pITP.
In cats with pITP, TPO receptor agonists are not recommended until evidence is available.
Level of evidence: None. Strength of recommendation: Weak. Degree of consensus: 39/40 Delphi Round 2.
Evidence summary:
There are no reports of TPO receptor agonist use in cats with pITP. Predicted amino acid sequence homology between feline and human TPO suggests that romiplostim may bind feline TPO receptors and have functional activity. 94 Eltrombopag is unlikely to be effective in cats. 93
12. In dogs and cats with pITP (P), is treatment with splenectomy (I) compared with no splenectomy (C) associated with different primary or secondary outcomes (O)?
Dogs:
- In dogs with ITP, splenectomy may lead to increased platelet counts in some animals refractory to standard medical treatment and lead to sustained remission without medical treatment in some individuals.
- Future relapse of thrombocytopenia after splenectomy is common.
- Splenectomy is well‐tolerated in most dogs, provided vector‐borne disease is ruled out before surgery.
- In dogs, for management of pITP, routine use of splenectomy can be neither recommended nor not recommended.
- Splenectomy may be considered in dogs with ITP refractory to medical treatment alone or when adverse drug effects necessitate discontinuation of immunosuppressive treatment.
- Patients should be carefully screened for locally relevant infectious disease before splenectomy.
- Owners should be counseled that long‐term response rates to splenectomy are variable.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 38/40 Delphi Round 2.
Evidence summary:
Three retrospective studies addressed the PICO question and are equivocal regarding the efficacy of splenectomy for ITP in dogs. 16 , 20 , 95 A retrospective study of 15 dogs with ITP suggested a decreased relapse rate with splenectomy compared with medical treatment alone. Five dogs with recurrent ITP underwent splenectomy, of which 4 experienced complete remission without ongoing medical treatment. 20 A case series of 9 dogs that underwent splenectomy for treatment‐refractory immune‐mediated hematologic disease included 3 dogs with ITP, of which 1 achieved complete remission. 95 An older retrospective case‐control study of 54 dogs with ITP suggested that splenectomy did not decrease relapse frequency versus medical treatment alone. Eight dogs underwent splenectomy to prevent recurrence; 1 died perioperatively, 1 achieved complete remission, and 6 experienced relapse. 16 One retrospective study supported the PICO, wherein 7 dogs with probable ITP underwent splenectomy, 3 of which achieved complete remission and 3 achieved PRs. One dog initially responded but later relapsed. 96 One dog with probable pITP, considered refractory to multiple immunosuppressive drugs and hIVIg, achieved lasting remission (>2 years) after splenectomy. 97 Various case reports and case series include thrombocytopenic dogs that underwent splenectomy but none specifically referenced dogs with ITP or provided sufficient data to evaluate efficacy. 1 , 96 , 98 , 99 , 100 Combining data from the available studies with clear outcome information, 23/24 dogs with ITP that underwent splenectomy survived to discharge, of which 10 experienced complete remission, 4 experienced PR, 7 experienced relapse, and 2 failed to improve.
Cats:
There is insufficient evidence to determine if splenectomy affects patient‐centered outcomes in cats with pITP.
In cats with pITP, routine splenectomy is not recommended.
Splenectomy may be considered in cats with ITP that remain refractory to standard medical treatment, but owners should be counseled that the likelihood of success is unknown, and ongoing medical treatment may still be needed.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 40/40 Delphi Round 2.
Evidence summary:
One case report of a cat with pITP that underwent splenectomy for treatment‐refractory ITP was identified. 49 The cat failed to respond to treatment with prednisolone, cyclosporine, and hIVIg. After splenectomy, cyclophosphamide was added to the treatment regimen. The cat was still alive after 6 months, but experienced relapse when cyclophosphamide was discontinued, calling into question the contribution of splenectomy to disease remission.
13. In dogs and cats with pITP (P), is treatment with TPE (I) compared with no TPE (C) associated with different primary or secondary outcomes (O)?
Dogs:
There is insufficient evidence to determine if TPE affects patient‐centered outcomes in dogs with pITP.
Routine TPE is not recommended in dogs with pITP.
We suggest that in dogs with pITP refractory to standard medical treatment, TPE can be considered for severely affected dogs.
Cost and risks associated with TPE must be weighed against the cost and risks of standard medical treatments to determine whether TPE should be used routinely in the management of ITP.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 40/40 Delphi Round 2.
Evidence summary:
Two retrospective studies describe the use of TPE in dogs with immune‐mediated hematological disease but neither suggests a definitive therapeutic benefit. The technique was generally considered safe with complications including filter clotting, hypersensitivity reactions, hypovolemia, and hypocalcemia reported in up to 35% of the treatments. 101 , 102 One study, which also included dogs with IMHA, compared dogs with ITP treated with membrane‐based TPE (n = 10) to conventionally treated controls (n = 66). Descriptions of ITP diagnosis and management were limited. No difference in survival to discharge between treatment groups was observed, although dogs undergoing TPE were more severely affected than controls. 101 Dogs in the TPE group were hospitalized longer and incurred higher costs. In a case series, 4 dogs with pITP that had been transfused and had NR to treatment for >4 days, underwent 3 sequential centrifugal TPE sessions. 102 Three dogs survived to discharge; time to platelet count ≥40 000 μL was 1 day, 5 days, and 6 days. One dog was euthanized because of persistent thrombocytopenia and transfusion dependence.
Cats:
There is insufficient evidence to determine if TPE affects patient‐centered outcomes in cats with pITP.
Therapeutic plasma exchange is not recommended in cats with pITP.
Level of evidence: None. Strength of recommendation: Weak. Degree of consensus: 38/40 Delphi Round 2.
Evidence summary:
No studies evaluating TPE in cats with immune‐mediated hematologic disorders were identified.
14. In dogs and cats with ITP undergoing treatment (P), does administration of an antithrombotic (I) as opposed to no antithrombotic treatment (C) improve any outcomes (O)?
Available data are contradictory regarding the risk of thrombosis in dogs undergoing treatment for ITP; no relevant studies in cats were identified.
In dogs or cats with ITP, the use of antithrombotic drugs is generally not indicated but the use of antithrombotics may be considered in specific circumstances where the patient is at low risk for hemorrhage and preexisting comorbidities predispose to thrombosis.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 17/17 Delphi Round 3.
Evidence summary:
No studies were identified that addressed the PICO question. Two studies identified thrombosis in dogs with ITP receiving immunosuppressive prednisone dosages, 47 , 103 whereas 3 did not. 16 , 21 , 53 In 1 study, venous thrombosis was documented in 2/73 dogs with ITP. 47 Both events occurred during acute treatment within 2 weeks of hospital discharge. Four other dogs in the study died because of acute respiratory distress; differential diagnoses included pulmonary hemorrhage, pulmonary thromboembolism, or acute lung injury. An additional 5 dogs developed neurologic dysfunction that could have resulted from intracranial bleeding or thrombosis. A follow‐up study using thromboelastography (TEG) to assess dogs with ITP suggested a transient hypercoagulable state as platelet counts normalized, but clinical thrombosis was not identified. 46 Potential mechanisms of a prothrombotic state in these patients include release of reactive immature (reticulated) platelets and inflammation associated with hospitalization and transfusion. Concomitant glucocorticoid administration might also lead to a hypercoagulable state. 104 , 105 , 106 The clinical relevance or corresponding thrombotic risk of hypercoagulable TEG tracings is unknown. One cat in a case series of 5 cats with pITP was euthanized during hospitalization with clinical signs of acute respiratory distress. 58 Pulmonary thromboembolism was suspected but not confirmed. There are no data upon which to base recommendations for the use of antithrombotics in the setting of ITP. If comorbidities that are recognized risk factors for thrombosis are present, 107 , 108 thromboprophylaxis can be considered in dogs with ITP as the platelet count begins to rebound and the patient is no longer at risk of spontaneous bleeding. 109
15. In dogs and cats with ITP undergoing treatment (P), does development of coagulation test abnormalities (not just thrombocytopenia; I) versus normal coagulation test results (C) worsen any outcomes (O)?
In dogs and cats with ITP undergoing treatment, there are no studies assessing the effect of development of coagulation test abnormalities in addition to thrombocytopenia on patient outcomes.
- Routine measurement of clotting tests (such as prothrombin time and activated partial thromboplastin time) is not indicated in dogs and cats with ITP undergoing treatment.
- Clotting times could be measured in dogs and cats that have bleeding into body cavities (not typical of ITP) or that develop bleeding after improvement in platelet numbers.
- Clotting times should be measured as part of the diagnosis of ITP to rule out other causes of thrombocytopenia (see Consensus Statement for Diagnosis of ITP in Dogs and Cats). 12
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 17/17 Delphi Round 3.
Evidence summary:
No studies were identified with follow‐up monitoring of coagulation tests after initial diagnosis. In a small prospective observational study, hypercoagulability was identified using TEG, 46 but thrombosis was not identified in any dog. As such, the available evidence suggests that coagulation abnormalities are uncommon in patients diagnosed with ITP and undergoing treatment. In general, development of a coagulopathy would be expected to worsen outcome, but the specific risks for dogs or cats with ITP remain unknown.
16. In dogs and cats with pITP (P), is the use of proton‐pump inhibitors, sucralfate or other gastroprotectants (I) compared with no gastroprotectant treatment (C) associated with different primary or secondary outcomes or less evidence of gastric erosion or ulceration or GI bleeding (O)?
Dogs:
There is insufficient evidence to determine if the use of proton‐pump inhibitors or sucralfate or other gastroprotectants affects patient‐centered outcomes in dogs with pITP.
- We suggest that in dogs with pITP, the use of gastroprotectants be considered in the presence of observed or suspected melena.
- This suggestion is based on the premise that, although GI bleeding can occur without loss of mucosal integrity in dogs with thrombocytopenia, it is impossible to exclude with certainty the possibility of GI ulceration, especially in the face of glucocorticoid treatment.
- Specific gastroprotectant drug and dosage recommendations are provided by the 2018 ACVIM Consensus Statement Support for Rational Administration of GI Protectants to Dogs and Cats. 110
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 36/40 Delphi Round 2. A few evidence evaluators disagreed, stating there was insufficient evidence for the efficacy of gastroprotectants in pITP patients to support their use, and that sucralfate, if given concurrently with other drugs, could inhibit absorption of essential ITP medications. In humans, proton‐pump inhibitors have been associated with decreased GI absorption of mycophenolate. 111 It is unknown if this effect can also occur in dogs and cats.
Evidence summary:
Melena is common dogs with ITP, 15 , 38 , 47 increases the need for blood transfusions, 112 and is associated with nonsurvival. 47 Melena does not necessarily indicate compromised GI mucosal integrity in animals with ITP, because GI bleeding can occur despite an intact mucosa in animals with thrombocytopenia. Gastroprotectants are frequently administered to dogs with ITP, 1 , 21 , 113 , 114 but evidence of efficacy is limited. One retrospective cohort study that addressed the PICO question was identified. 47 Of 73 dogs included, 62 (85%) received a gastroprotectant, but no association with survival to discharge was observed. However, treatment was not standardized, and the study was not designed to answer the PICO question. Another retrospective study of dogs with combined IMHA and thrombocytopenia also found no association of gastroprotectant use with outcome, but again the study design was limiting. 115 No studies were identified that evaluated the effect of gastroprotectants on GI bleeding, erosion, or ulceration in dogs with ITP. One study, available only in abstract form, did not identify a benefit of gastroprotectants in dogs treated with immunosuppressive dosages of glucocorticoids. 116 In addition, even twice‐daily IV proton‐pump inhibitor treatment is unlikely to achieve a prolonged intragastric pH >6 that would allow for optimal platelet aggregration. 117 , 118 There is conflicting evidence regarding the risk of GI ulceration in dogs receiving glucocorticoids. 119 , 120 , 121 , 122 Meta‐analyses in human medicine generally agree that the risk of GI bleeding associated with glucocorticoid administration is low in outpatients not concurrently receiving NSAIDs, but glucocorticoids may increase GI bleeding risk in hospitalized patients. 123
Cats:
There are currently no studies available to support or refute the claim that the use of gastroprotectants improves outcome in cats with pITP.
We suggest that in cats with pITP, the use of gastroprotectants be considered in the presence of observed or suspected melena.
Level of evidence: None. Strength of recommendation: Weak. Degree of consensus: 37/40 Delphi Round 2.
Evidence summary:
Evidence regarding use of gastroprotectants in cats with ITP is limited to a single case series, precluding assessments of efficacy. 58 Although glucocorticoid treatment might be a risk factor for GI perforation in cats with concurrent diseases, it is unknown how this risk translates to cats with ITP. 124 Proton‐pump inhibitors are unlikely to increase intragastric pH to a level at which platelet aggregation can readily occur. 125
Non‐PICO 11: Is there rationale to employ probiotics in treatment of dogs and cats with pITP?
Routine administration of probiotics to animals with ITP is not currently recommended because our understanding of the role of the microbiome in disease pathogenesis is nascent. Evidence from humans suggests a role for dysbiosis in the development of ITP, 126 , 127 with 1 study identifying a link between alterations in the microbiome and treatment response. 128 Drugs such as vincristine have been reported to cause dysbiosis. 129 One case of fecal microbiota transplantation for management of ITP in a human has been reported, and a clinical trial evaluating the efficacy of probiotics in human ITP patients is ongoing. 130 One study observed that dogs with ITP have alterations in the GI microbiome including enrichment of potential pathogens (Clostridium septicum and Escherichia coli). 131 Probiotics could be considered in animals with GI signs, especially if these are a sequelae of the treatment.
17. In dogs and cats with pITP (P), are vaccinations after ITP diagnosis (I) compared with no vaccinations (C) associated with a higher rate of ITP relapse (O)?
There are very limited data in dogs and no data in cats regarding the association between vaccination and ITP disease relapse.
For dogs and cats that have recovered from an immune‐mediated disease associated with vaccination, we recommend that public health considerations and the risk of disease exposure be weighed against the unknown and presumed very small chance of relapse of immune‐mediated disease, or revaccination may be avoided by monitoring titers. Alternatively, cats could be housed indoors in an environment where vaccination may not be required.
It is unclear if the same degree of caution is needed in dogs or cats with ITP that was not originally associated with vaccination.
Recommendations should be individualized for dogs and cats considering vaccination history, lifestyle factors, treatment (eg, immunosuppressive drugs), and the potential consequences of failing to vaccinate.
Level of evidence: None. Strength of recommendation: Weak. Degree of consensus: 38/40 Delphi Round 2.
Evidence summary:
Vaccine‐associated ITP is rare in dogs, but can occur and evidence that vaccination is associated with ITP in dogs is limited, with a single small study demonstrating a lack of association. 12 , 22 There are very limited data regarding the association of vaccination with pITP relapse in dogs or cats. One study (available only in abstract form) evaluated the association between vaccination and relapse risk in dogs with pITP. 132 The study included 21 dogs with pITP that were successfully tapered off all immunosuppressive drugs, 12 of which received at least 1 vaccination ≥1 month after immunosuppressive drug discontinuation; none experienced relapse of their ITP.
Guidelines from the World Small Animal Veterinary Association 133 suggest that core revaccination might be avoided in dogs that have recovered from an immune‐mediated disease by monitoring titers. Noncore vaccines should be selected after weighing the risk of disease exposure against the risk of immune‐mediated disease relapse. 133 For animals in remission, it may be reasonable to vaccinate only for life‐threatening conditions (eg, leptospirosis) and for diseases where there is a legal requirement (eg, rabies). The American Society of Hematology 2019 guidelines recommend evaluating vaccine titers in children with suspected vaccine‐related ITP to aid decision‐making regarding repeat vaccination. Where protective titers are present, repeat vaccination is not necessary, whereas in children with inadequate titers, reimmunization is recommended. 67
Non‐PICO 12: In dogs and cats with pITP, what recommendations should be made about vaccinations in animals after treatment?
In dogs and cats with pITP, the risk of vaccination should be individually assessed, considering the risk of infectious disease exposure, local laws, and, for rabies, consequences of non‐adherence to local laws in the event of exposure. Measuring titers for canine distemper virus, canine adenovirus, canine parvovirus, and feline panleukopenia virus as surrogate markers for protection can be considered, in accordance with American Animal Hospital Association vaccination guidelines. 134 Administering only 1 vaccine per visit is recommended, and only after immunosuppressive drugs are discontinued or limited to anti‐inflammatory dosages of glucocorticoids (eg, 0.5 mg/kg prednisone q24h) if glucocorticoid discontinuation is not anticipated. Measuring platelet count 2 and 5 weeks postvaccination should be considered.
18. In dogs and cats with pITP (P), does treatment with any platelet‐containing transfusion product (I), compared with no platelet‐containing products (C), improve any outcomes (O)?
Dogs:
There is insufficient evidence to determine if transfusion of platelet‐containing products affects patient‐centered outcomes in dogs with pITP.
In dogs with pITP, routine transfusion of platelet‐containing products is not recommended.
We suggest that in dogs with pITP, transfusion of platelet‐containing products be considered if there is evidence of severe or life‐threatening bleeding.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 13/13 Delphi Round 1.
Evidence summary:
One retrospective case‐control study was identified that directly addressed the PICO question. 135 This study described 43 dogs that received a cryopreserved platelet product for thrombocytopenic bleeding and 43 control dogs that did not receive a platelet‐containing transfusion product. The study included dogs with probable pITP and demonstrated that cryopreserved platelet transfusions can increase recipient platelet count. There was no effect on clinical bleeding or survival. Notably, transfused dogs had more severe disease, confounding assessment of association between the intervention and patient‐centered outcomes. There is no indication from this study, or from the literature, that routine platelet transfusion is essential for management of ITP. Producing platelet transfusion products is challenging and costly, 136 , 137 , 138 and without clear evidence of efficacy and the potential for allogenic transfusion reactions, 136 , 139 routine platelet transfusion in dogs with ITP is not recommended.
Six additional prospective studies were reviewed describing the use of platelet transfusion products in dogs, but none described dogs with ITP. 136 , 140 , 141 , 142 , 143 , 144 These studies reported transfusion of fresh platelet concentrates, 136 , 140 , 141 , 142 cryopreserved platelet products, 143 or lyophilized platelet products 144 in nonimmune disease conditions including radiation‐induced pancytopenia, 140 , 141 , 142 , 143 coronary artery bypass grafting models, 144 and bone marrow transplantation. 136 These 6 studies suggest that transfusion of platelet products is safe, increases platelet count, and decreases clinical bleeding in dogs with non‐ITP. Although these studies are not of ITP, they suggest that platelet transfusion in dogs with clinical bleeding secondary to ITP is a reasonable treatment option. One retrospective study of 149 dogs, including 39 (26%) with probable pITP, that received fresh platelet concentrates was reviewed. 145 The study design precluded an assessment of the impact of platelet transfusion on outcome. This study compared pretransfusion and posttransfusion platelet counts and observed a significant increase in platelet count after transfusion in dogs with pITP. The median change in platelet count was 2000 platelets/μL (range, −5000 to +75 000). This finding suggests that platelet concentrates, if accessible, are a reasonable choice for treatment of dogs with pITP and will in some cases effect a meaningful increase in platelet count that could be lifesaving. However, on average, the increment in platelet count after transfusion is minimal. These data reinforce the perspective that transfusion treatment in dogs with pITP is an adjunctive therapeutic strategy only.
Cats:
There is insufficient evidence to determine if transfusion of platelet‐containing products affects patient‐centered outcomes in cats with pITP.
In cats with pITP, routine transfusion of platelet‐containing products is not recommended.
We suggest that in cats with pITP, transfusion of platelet‐containing product only be considered if there is evidence of severe or life‐threatening bleeding.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 13/13 Delphi Round 1.
Evidence summary:
No studies were identified that directly addressed the PICO question. One study described administration of fresh feline platelet concentrates for correction of prolonged oral mucosal bleeding times in cats with Chediak‐Higashi syndrome, 146 a storage pool disorder that abrogates platelet adenosine diphosphate and serotonin release. 147 , 148 , 149 Transfusion to a platelet count of 40‐60 000/μL normalized oral mucosal bleeding times, which became prolonged again as transfused platelets were eliminated, indicating that the transfused feline platelets were hemostatic. It cannot be inferred from this study that transfusion of feline platelets to cats with ITP would control bleeding or improve patient outcomes, but it does suggest that transfusion of feline platelets can be considered in cats with ITP that are experiencing clinical bleeding. Various case series and case reports describe feline whole blood transfusion to cats with ITP, typically for management of anemia potentially in combination with thrombocytopenia and clinical bleeding. 35 , 48 , 58 , 150 , 151 , 152 Transfused cats may have received viable, hemostatic platelets within these whole blood transfusions, but the impact of this treatment on outcome cannot be determined. It is assumed from studies in other species that fresh whole blood contains functional platelets, but this has not been reported in cats.
19. In dogs and cats with pITP (P), does treatment with 1 platelet‐containing product (I), compared with any other platelet‐containing products (C), improve any outcomes (O)?
Dogs:
There is insufficient evidence to determine if any platelet‐containing product is superior to another for treatment of dogs with pITP.
We suggest that when determining which platelet‐containing product to use in dogs with pITP, factors including availability, safety, product volume, and platelet particle number be considered.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 13/13 Delphi Round 1.
Evidence summary:
Two multicenter clinical trials were identified that directly addressed the PICO question. 114 , 153 Most, but not all, dogs in these studies had probable pITP but rigorous diagnostic evaluations were not performed and outcomes in dogs with pITP were not specifically compared. Both studies compared patient‐centered outcomes between interventions, but both were underpowered to detect survival differences. In the 1st study, dogs with thrombocytopenic bleeding were randomized to receive either fresh canine platelet concentrates or formaldehyde‐stabilized lyophilized canine platelets. 153 Outcome measures included bleeding scores, transfusion requirements, duration of hospitalization, survival to discharge, and survival to 28 days after study entry. This study confirmed the feasibility and safety of transfusion of both products in clinical patients, but no differences in any outcome measure between groups were identified. The 2nd study was a noninferiority study comparing dimethyl sulfoxide (DMSO)‐stabilized cryopreserved canine platelets with trehalose‐stabilized lyophilized canine platelets for management of bleeding in dogs with thrombocytopenia. 114 More stringent study entry criteria might have increased specificity for ITP, but extensive diagnostic investigations were not universally performed. Outcome measures included pretransfusion to posttransfusion change in bleeding score, platelet count and hematocrit, the need for additional red blood cell (RBC) transfusion, and all‐cause mortality. Overall, the lyophilized platelets were noninferior to the DMSO‐stabilized cryopreserved platelet control product for all of the primary outcome measures. The lyophilized platelets were superior for change in hematocrit at 1‐hour posttransfusion, but this effect disappeared by 24 hours, and its clinical relevance is questionable. Taken together, these trials suggest that none of the 4 canine platelet products tested are superior and any could therefore reasonably be used to manage thrombocytopenic bleeding in dogs with ITP. The choice of platelet product might be informed by availability, safety, product volume, and platelet particle number.
Cats:
No evidence was available to determine if any platelet‐containing product is superior to another for treatment of cats with pITP.
We suggest that when determining which platelet‐containing product to use in cats with pITP factors including availability, safety, product volume, and platelet particle number be considered.
Level of evidence: None. Strength of recommendation: Weak. Degree of consensus: 13/13 Delphi Round 1.
Evidence summary:
No studies were identified that addressed the PICO question. The study in cats with Chediak‐Higashi syndrome (PICO 18), 146 suggested that platelet concentrates are hemostatically active, but availability of feline platelet concentrates is limited. Fresh whole blood may be the only widely available platelet‐containing product for cats, but efficacy is unknown.
20. In dogs and cats with pITP (P), does treatment with any RBC‐containing transfusion product (I), compared with no RBC‐containing products (C), improve any outcomes (O)?
Dogs:
There is insufficient evidence to determine if transfusion of any RBC‐containing product affects patient‐centered outcomes in dogs with pITP.
We suggest that in dogs with pITP, use of RBC‐containing transfusion products for management of clinically relevant anemia or hemorrhagic hypovolemia should be considered.
We suggest that when determining which RBC‐containing product to use in dogs with pITP, factors including availability, safety, product volume, and presence of platelets be considered.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 13/13 Delphi Round 1.
Evidence summary:
No studies that directly addressed the PICO question were identified, potentially because of widespread availability of blood products for dogs and ethical concerns about managing dogs with symptomatic anemia or hemorrhagic hypovolemia without transfusion. Studies describing hemoglobin‐based oxygen carrying solution administration were excluded. Six studies were reviewed: 5 were retrospective case series, 1 , 47 , 112 , 115 , 154 and 1 was a prospective longitudinal cohort study. 155 None of these studies compared whole blood with packed RBCs, and individual product usage appeared to reflect availability and clinician preference. In the 2 studies focused on probable ITP cases, 1 , 47 RBC transfusions were administered in 17% (10/58) of cases 1 and 36% (26/73) of cases. 47 The impact of transfusion on outcome was not assessed in either study. In 1 study, 6/10 dogs that received RBC transfusions did not survive to discharge, 1 whereas the overall survival rate in the other study was 84% (61/73). Although the influence of transfusion on survival was not assessed, 26/73 dogs received transfusions, 14 of which received multiple transfusions. 47
In a study of 86 dogs with Babesia rossi infection and probable sITP, 32 dogs were transfused and 54 were not. No difference in outcome was identified between these 2 groups, although only 4/86 patients died. 155 In a case series of 12 dogs with IMHA and severe thrombocytopenia (<15 000/μL), RBC transfusion was performed in 8/12 cases. 115 No difference in survival frequency was identified between the dogs transfused and those not transfused, but the utility of this analysis is questionable because overall survival to discharge was 75% (9/12), and only 4 cases did not receive RBCs. Of note, in a study of dogs with GI bleeding, 16/55 dogs had probable ITP, 112 and these dogs required significantly more RBC transfusions than those with GI bleeding secondary to other causes.
Transfusion of RBC‐containing products is common in dogs with ITP and is likely performed for management of anemia, hypovolemia, and in some cases, concurrent platelet administration. There may also be some additional hemostatic benefit associated with RBC administration in anemic patients, 156 but this possibility is not an indication for RBC transfusion. It is generally recognized that RBC transfusion is necessary to manage symptomatic anemia and clinically relevant bleeding in dogs with ITP. 157 , 158 When RBC transfusion is being considered, factors such as product availability and age, 159 recipient blood type, 160 , 161 prior transfusion history, 162 , 163 , 164 potential for transfusion‐related adverse effects, 165 , 166 , 167 and presence of platelets in the product 168 , 169 should be considered.
Cats:
There is insufficient evidence to determine if transfusion of any RBC‐containing product affects patient‐centered outcomes in cats with pITP.
We suggest that in cats with pITP, use of RBC‐containing transfusion products for management of clinically relevant anemia or hemorrhagic hypovolemia be considered.
We suggest that when determining which RBC‐containing product to use in cats with pITP factors, including availability, safety, product volume, and presence of platelets be considered.
Level of evidence: Low. Strength of recommendation: Weak. Degree of consensus: 13/13 Delphi Round 1.
Evidence summary:
No studies were identified that directly addressed the PICO question. Two retrospective case series were identified that reported probable pITP cases (total n = 9) and described transfusion of RBC‐containing products in a subset of cases (total n = 5), 37 , 58 but the effect of a RBC‐containing transfusion on patient‐centered outcomes was not directly evaluated, and no comparisons were made between individuals or groups of individual animals. The overall survival rates in these studies were 3/4 37 and 4/5. 58 In 1 study, an increase in pretransfusion to posttransfusion hematocrit was noted in 1 cat. 37 In addition to the small numbers of animals included in these reports, the need for a RBC transfusion in the setting of ITP might indicate greater illness severity or duration, confounding assessments of association between transfusion and outcome. As in dogs, RBC‐containing transfusions are indicated for the management of clinically relevant anemia or hemorrhagic hypovolemia in cats. 170 , 171 When transfusion of RBC‐containing products is being considered, factors such as product availability and age, 172 recipient blood type, 173 , 174 , 175 prior transfusion history, 163 potential for transfusion‐related adverse effects, 165 , 166 , 167 and presence of platelets in the product 176 should be considered.
Non‐PICO 13: In dogs and cats with pITP, what supportive treatments should be provided beyond transfusion?
In patients with pITP, separate from specific treatments, we suggest that all possible steps be taken to minimize iatrogenic harm and promote recovery. Patients with ITP should be strictly rested and handled to the minimum extent necessary to provide care. Anxiolytics may be required to facilitate strict rest in a busy clinical environment. Exercise should be restricted, and food should be soft (eg, canned or soaked, with no hard chew toys available to minimize gingival self‐trauma). Unnecessary procedures should be avoided, and all procedures should be performed by experienced personnel using gentle restraint (eg, use of harnesses). All blood samples should be collected by experienced, skilled operators, avoiding large blood vessels and those in non‐compressible locations. Medications should not be administered by SC or IM injection. Sampling of friable, vascular organs (eg, liver, spleen, bladder cystocentesis) should only be performed if essential and consideration given to prophylactic platelet transfusion if such procedures are required. Nasal cannulae and nasoesophageal or nasogastric tubes should be avoided unless essential. Antiplatelet drugs and anticoagulants should be avoided while patients are severely thrombocytopenic. If disseminated intravascular coagulation is ruled out, then antifibrinolytic drugs (aminocaproic acid or tranexamic acid) can be considered for life‐threatening bleeding. Available evidence on the use of aminocaproic acid in dogs with ITP suggests antifibrinolytics may increase clot strength, but associations with transfusion requirements, duration of hospitalization, or survival have not been demonstrated. 177 Similarly, use of tranexamic acid did not alter outcomes in a small cohort study but was associated with an increased incidence of vomiting. 178 When determining the duration of hospitalization, clinicians should consider the nature, location and extent of bleeding, the stability of the patient's hematocrit and need for RBC transfusion, and the trend of the patient's platelet count. It is reasonable to consider managing thrombocytopenic patients with stable hematocrits as outpatients.
Non‐PICO 14: In dogs and cats with pITP, how should prednisolone or prednisone be tapered if animals are in remission?
We suggest decreasing the glucocorticoid dosage by approximately 25% every 2‐4 weeks provided platelet count is stable and confirmed immediately before each dosage reduction. More rapid reductions can be performed initially if the initial dosage is high. Dosage reduction also may involve decreasing the frequency of dosing. Glucocorticoid treatment is generally continued for several months, but tapering may be accelerated in individual patients based on adverse glucocorticoid effects, concurrent administration of other immunosuppressive drugs, and the presence of concurrent diseases that cause glucocorticoid intolerance. Glucocorticoid treatment duration may be extended in patients with a history of relapse.
Non‐PICO 15: In dogs and cats with pITP, how should 2nd immunosuppressive drugs be tapered if animals are in remission?
Evidence for the best approach is lacking, and consensus was not reached. Some co‐authors decrease the dosage or discontinue glucocorticoids before tapering the 2nd immunosuppressive drug, whereas others alternate dosage reductions among immunosuppressive drugs. There was also no consensus regarding tapering versus abrupt discontinuation of the 2nd immunosuppressive drug, but most panelists (5/7) taper the 2nd immunosuppressive drug. Any gradual, measured, and deliberate dosage reduction and drug discontinuation protocol is considered reasonable. The tapering schedule may change in individual patients based on the drugs administered, adverse effects, concurrent medications, presence of concurrent diseases, and response to treatment, but several months generally will be required to taper both the glucocorticoid and 2nd immunosuppressive drug to the point of discontinuation of both.
Non‐PICO 16: Are outcomes different for dogs and cats with pITP compared with megakaryocytic hypoplasia or aplasia?
No consensus was obtained, but respondents generally considered that delayed responses to treatment were more common in patients with megakaryocytic hypoplasia than pITP. Some respondents suggested that megakaryocytic hypoplasia was associated with poor outcome. The existing literature is conflicting and further research is needed. 18 , 47
Non‐PICO 17: In dogs and cats with pITP, how should we monitor treatment, including for adverse drug effects, and during inpatient, outpatient, and remission phases?
We suggest that animals be monitored using physical examination and CBC at a frequency based on patient characteristics and client wishes. During physical examination, close attention should be paid to abnormalities of primary hemostasis, (eg, mucocutaneous petechiae, ecchymoses, retinal hemorrhage, melena), signs of secondary infection (eg, urinary signs, skin changes), and drug‐related adverse effects (eg, GI signs, hepatotoxicity, bone marrow suppression, calcinosis cutis, diabetes mellitus). Thorough histories should be taken during follow‐up visits to identify drug‐related adverse effects or changes in clinical status.
Inpatient monitoring should include daily physical examination and CBC at least every 2‐3 days until bleeding ceases, and the platelet count exceeds 40‐50 000/μL. More intensive and frequent monitoring is warranted for all patients with active bleeding, anemia, or in the event of clinical deterioration.
On an outpatient basis, with platelet counts >40‐50 000/μL and no signs of bleeding, monitoring frequency may be decreased. A suggested schedule is once every 1‐2 weeks for a month, then every 2‐4 weeks until durable remission is achieved. During treatment tapering, a physical examination, CBC, and blood smear immediately should precede any dosage reduction and should be followed by a planned reevaluation, typically within 1‐3 weeks. Contingent on the immunosuppressive drugs used, serum biochemical profiles should be considered at least monthly throughout treatment to monitor for potential adverse drug effects, such as effects on liver or kidney function. Routine urinalysis should be performed where concerns exist relating to kidney function. Urine bacterial culture and susceptibility testing should be performed as necessary according to previously published guidelines. 179
Non‐PICO 18: In dogs and cats with pITP, what monitoring is recommended for animals in remission and not receiving drug treatment?
We suggest patients in remission be monitored monthly through history, physical examination, and CBC for 3 months, with subsequent gradual extensions in the assessment interval. Platelet counts should be measured before any procedure or vaccination. Clients should monitor their animals closely for signs of relapse.
Non‐PICO 19: In dogs and cats with pITP, what is the role of measuring platelet or megakaryocyte surface associated immunoglobulins in monitoring treatment?
We do not currently recommend monitoring response to treatment based on platelet or megakaryocyte surface associated immunoglobulin assays. One study reported an association between ITP relapse and antibody recurrence, 71 but limited assay availability, lag time for results, and lack of assay standardization preclude routine use of these assays for patient monitoring.
4. FUTURE DIRECTIONS
The lack of available literature to answer many of the PICO questions highlights the need for appropriately designed studies that directly answer these questions. Of particular importance, adequately powered prospective RCTs are needed to determine the utility of 2nd immunosuppressive drugs in the management of ITP and whether any 2nd immunosuppressive drug is superior to any other. The use of TPO receptor agonists has revolutionized ITP management in people, obviating the need for 2nd immunosuppressive drugs in most human ITP patients. Randomized controlled trials evaluating the efficacy of romiplostim in dogs and cats should be prioritized. Optimizing use of interventions like TPE and splenectomy in veterinary ITP patients also will require prospective, RCTs. Ideally, the results of future ITP studies will be readily comparable by standardized reporting of consistent diagnostic criteria and outcome measures. To that end, we have developed suggested reporting guidelines for future studies investigating ITP in dogs and cats (Supporting Information 8).
Additional current and emerging treatments in human ITP patients may have future roles in the treatment of dogs and cats with ITP. Rituximab, a chimeric anti‐CD20 monoclonal antibody, is a standard 2nd‐line treatment for humans with ITP. 67 Unfortunately, no anti‐CD20 antibodies are commercially available for dogs or cats, precluding this treatment approach in veterinary patients. Development of canine and feline anti‐CD20 immunotherapies and their trial in dogs and cats with ITP should be considered. Spleen tyrosine kinase inhibitors, designed to inhibit macrophage phagocytosis, are promising novel therapeutics for human ITP patients that may have potential utility in veterinary medicine. 180 These inhibitors, through inhibition of specific platelet activation pathways necessary for thrombosis but not hemostasis, might have antithrombotic benefits in the recovery phase of ITP. 180
Further research into the diverse pathogenesis of ITP in individual patients could enable targeted ITP treatments. Recently, it has been demonstrated in murine models and human patients that antibodies targeting the glycoprotein Ibα complex sometimes activate platelet neuraminidase. Neuraminidase desialylates platelets, leading to their premature clearance by the hepatic Ashwell‐Morell receptor. 181 In these patients, hIVIg is ineffective because platelet clearance is independent of macrophages, but patients can respond to treatment with neuraminidase inhibitors such as oseltamivir. Future research is needed to determine whether platelet clearance mechanisms vary with platelet antigen target in dogs and cats with ITP. Ideally, future ITP treatments will be individualized using our understanding of platelet clearance mechanisms and predictors of disease severity that remain to be discovered.
CONFLICT OF INTEREST DECLARATION
Dana LeVine has received relevant research funding from American Kennel Club Canine Health Fund, BodeVet, Morris Animal Foundation, and the Veterinary & Comparative Clinical Immunology Society (VCCIS) and honoraria for speaking engagements from the ACVIM Forum, ECVIM‐CA Congress, ACVIM ACE, CVMA, and CanWest and is also a consultant for Cellphire Therapeutics. Robert Goggs has received relevant research funding from the American Kennel Club Canine Health Fund, Aurin Biotech, BodeVet, Morris Animal Foundation, and Okava Pharmaceuticals and honoraria for speaking engagements from ACVIM Forum, EVECC Congress, IVECCS, PacVet, UnisVet, and VCCIS. Barbara Kohn has held lectures, had research collaborations, and acted as a consultant for veterinary pharmaceutical and diagnostic companies. Linda Kidd was a Veterinary Field Specialist for Zoetis Diagnostics August 2022 to July 2023 and is an Associate Editor of the Journal of Veterinary Internal Medicine. OIiver A. Garden has received relevant research funding from the Kennel Club (UK) and Okava Pharmaceuticals and speaking honoraria from the ACVIM Forum, ECVIM‐CA Congress, and ACVP/ACSVP Annual Meeting. He has served as a consultant for Okava Pharmaceuticals and is the President of VCCIS. Marjory B. Brooks has received relevant research funding from the American Kennel Club and honoraria for speaking engagements from ACVIM Advanced Continuing Education, University of North Carolina Chapel Hill Symposium of Hemostasis, and the Society of Toxicologic Pathology's Symposium. Benjamin M. Brainard has received sponsorship from BodeVet Inc. for studies analyzing the properties of the lyophilized StablePlate RX or related products and has consulted for Cellphire, Inc. Anne S. Hale has equity in VetStem and was previously employed as the Chief Development Officer.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC, or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1. Extended materials and methods.
Data S2. Bibliography of studies for treatment.
Data S3. Evidence evaluator instructions for medical and transfusion management of ITP PICOs.
Data S4. Data extraction—medical.
Data S5. Data extraction—transfusion.
Data S6. PICO evidence summary template for the ACVIM Consensus Statement on the Treatment of Immune Thrombocytopenia in Dogs and Cats.
Data S7. Evidence summaries and consensus recommendations PICOs with minimal evidence.
Data S8. Immune thrombocytopenia diagnosis, treatment, and outcome reporting guidelines.
ACKNOWLEDGMENT
We acknowledge the work of the comorbidity task force and their contributions to the non‐PICO question regarding treatment of ITP patients with comorbidities: Adam J. Birkenheuer, Marnin A. Forman, Andrew S. Hanzlicek, Kathrin F. Langner, Erin Lashnits, Katharine F. Lunn, Kelly M. Makielski, Xavier Roura, and Eva Spada. We also gratefully acknowledge Dan Fletcher and the RECOVER Co‐Chairs for their input and assistance with data gathering and assessment resources.
LeVine DN, Goggs R, Kohn B, et al. ACVIM consensus statement on the treatment of immune thrombocytopenia in dogs and cats. J Vet Intern Med. 2024;38(4):1982‐2007. doi: 10.1111/jvim.17079
Consensus Statements of the American College of Veterinary Internal Medicine (ACVIM) provide the veterinary community with up‐to‐date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ACVIM Board of Regents oversees selection of relevant topics, identification of panel members with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence‐based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ACVIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine, where it is edited prior to publication. The authors are solely responsible for the content of the statements.
Dana N. LeVine, Linda Kidd, and Oliver A. Garden are co‐chairs on the Consensus Statement.
Dana N. LeVine, Robert Goggs, Barbara Kohn, and Andrew J. Mackin share first authorship.
Marjory B. Brooks, Robert Goggs, Barbara Kohn, and Andrew J. Mackin are panel members on the Consensus Statement.
Erin R. B. Eldermire, Anthony Abrams‐Ogg, Elizabeth H. Appleman, Todd M. Archer, Domenico Bianco, Shauna L. Blois, Benjamin M. Brainard, Mary Beth Callan, Claire L. Fellman, Jillian M. Haines, Anne S. Hale, Alice A. Huang, John M. Lucy, Shana K. O'Marra, Elizabeth A. Rozanski, John M. Thomason, Jenny E. Walton, and Helen E. Wilson are advisory task force members on the Consensus Statement.
REFERENCES
- 1. Scuderi MA, Snead E, Mehain S, Waldner C, Epp T. Outcome based on treatment protocol in patients with primary canine immune‐mediated thrombocytopenia: 46 cases (2000‐2013). Can Vet J. 2016;57:514‐518. [PMC free article] [PubMed] [Google Scholar]
- 2. Harrington W, Minnich V, Hollingsworth J, Moore C. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med. 1951;38:1‐10. [PubMed] [Google Scholar]
- 3. Consolini R, Legitimo A, Caparello MC. The centenary of immune thrombocytopenia—part 1: revising nomenclature and pathogenesis. Front Pediatr. 2016;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peerschke EI, Andemariam B, Yin W, Bussel JB. Complement activation on platelets correlates with a decrease in circulating immature platelets in patients with immune thrombocytopenic purpura. Br J Haematol. 2010;148:638‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olsson B, Andersson PO, Jernås M, et al. T‐cell‐mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat Med. 2003;9:1123‐1124. [DOI] [PubMed] [Google Scholar]
- 6. Zhang F, Chu X, Wang L, et al. Cell‐mediated lysis of autologous platelets in chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2006;76:427‐431. [DOI] [PubMed] [Google Scholar]
- 7. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6:6.28067796 [Google Scholar]
- 8. Zhao C, Li X, Zhang F, Wang L, Peng J, Hou M. Increased cytotoxic T‐lymphocyte‐mediated cytotoxicity predominant in patients with idiopathic thrombocytopenic purpura without platelet autoantibodies. Haematologica. 2008;93:1428‐1430. [DOI] [PubMed] [Google Scholar]
- 9. Aledort LM, Hayward CPM, Chen MG, Nichol JL, Bussel J, ITP Study Group . Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol. 2004;76:205‐213. [DOI] [PubMed] [Google Scholar]
- 10. Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell‐Morell receptor regulates hepatic thrombopoietin production via JAK2‐STAT3 signaling. Nat Med. 2015;21:47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makielski KM, Brooks MB, Wang C, Cullen JN, O'Connor AM, LeVine DN. Development and implementation of a novel immune thrombocytopenia bleeding score for dogs. J Vet Intern Med. 2018;32:1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LeVine DN, Kidd L, Garden OA, et al. ACVIM consensus statement on the diagnosis of immune thrombocytopenia in dogs and cats. J Vet Intern Med. 2024. doi: 10.1111/(ISSN)1939-1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190‐4207. [DOI] [PubMed] [Google Scholar]
- 14. Rozanski EA, Callan MB, Hughes D, Sanders N, Giger U. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune‐mediated thrombocytopenia in dogs. J Am Vet Med Assoc. 2002;220:477‐481. [DOI] [PubMed] [Google Scholar]
- 15. Balog K, Huang AA, Sum SO, Moore GE, Thompson C, Scott‐Moncrieff JC. A prospective randomized clinical trial of vincristine versus human intravenous immunoglobulin for acute adjunctive management of presumptive primary immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 2013;27:536‐541. [DOI] [PubMed] [Google Scholar]
- 16. Williams DA, Maggio‐Price L. Canine idiopathic thrombocytopenia: clinical observations and long‐term follow‐up in 54 cases. J Am Vet Med Assoc. 1984;185:660‐663. [PubMed] [Google Scholar]
- 17. Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. J Vet Intern Med. 1996;10:207‐218. [DOI] [PubMed] [Google Scholar]
- 18. Cooper SA, Huang AA, Raskin RE, Weng HY, Scott‐Moncrieff JC. Clinical data, clinicopathologic findings and outcome in dogs with amegakaryocytic thrombocytopenia and primary immune‐mediated thrombocytopenia. J Small Anim Pract. 2016;57:142‐147. [DOI] [PubMed] [Google Scholar]
- 19. Kohn B, Engelbrecht R, Leibold W, Giger U. Clinical findings, diagnostics and treatment results in primary and secondary immune‐mediated thrombocytopenia in the dog. Kleintierpraxis. 2000;45:893‐907. [Google Scholar]
- 20. Jans HE, Armstrong PJ, Price GS. Therapy of immune mediated thrombocytopenia. A retrospective study of 15 dogs. J Vet Intern Med. 1990;4:4‐7. [DOI] [PubMed] [Google Scholar]
- 21. Putsche JC, Kohn B. Primary immune‐mediated thrombocytopenia in 30 dogs (1997‐2003). J Am Anim Hosp Assoc. 2008;44:250‐257. [DOI] [PubMed] [Google Scholar]
- 22. Huang AA, Moore GE, Scott‐Moncrieff JC. Idiopathic immune‐mediated thrombocytopenia and recent vaccination in dogs. J Vet Intern Med. 2012;26:142‐148. [DOI] [PubMed] [Google Scholar]
- 23. Greene CE, Scoggin J, Thomas JE, Barsanti JA. Vincristine in the treatment of thrombocytopenia in five dogs. J Am Vet Med Assoc. 1982;180:140‐143. [PubMed] [Google Scholar]
- 24. Wolf AM. Vincristine therapy of chronic thrombocytopenia in the dog. Southwest Vet. 1983;35:209‐215. [Google Scholar]
- 25. Niebauer WG. A case of autoimmune thrombocytopenia in the dog and its treatment with vincristine. Wien Tierarztl Monatsschr. 1983;70:170‐172. [Google Scholar]
- 26. Davis WM. Hapten‐induced, immune‐mediated thrombocytopenia in a dog. J Am Vet Med Assoc. 1984;184:976‐977. [PubMed] [Google Scholar]
- 27. Murtaugh RJ, Jacobs RM. Suspected immune‐mediated megakaryocytic hypoplasia or aplasia in a dog. J Am Vet Med Assoc. 1985;186:1313‐1315. [PubMed] [Google Scholar]
- 28. Helfand SC, Jain NC, Paul M. Vincristine‐loaded platelet therapy for idiopathic thrombocytopenia in a dog. J Am Vet Med Assoc. 1984;185:224‐226. [PubMed] [Google Scholar]
- 29. Park HJ, Kim JW, Song KH, Seo KW. Application of vincristine‐loaded platelet therapy in three dogs with refractory immune‐mediated thrombocytopenia. J Vet Sci. 2015;16:127‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LaQuaglia KA, Robertson JB, Lunn KF. Neutropenia in dogs receiving vincristine for treatment of presumptive immune‐mediated thrombocytopenia. J Vet Intern Med. 2021;35:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mackin AJ, Allen DG, Johnston IB. Effects of vincristine and prednisone on platelet numbers and function in clinically normal dogs. Am J Vet Res. 1995;56:100‐108. [PubMed] [Google Scholar]
- 32. Grau‐Bassas ER, Kociba GJ, Couto CG. Vincristine impairs platelet aggregation in dogs with lymphoma. J Vet Intern Med. 2000;14:81‐85. [DOI] [PubMed] [Google Scholar]
- 33. Campbell O, MacDonald VS, Dickinson RM, Gagnon J. Evaluation of the effect of vincristine on platelet count in dogs with lymphoma. J Small Anim Pract. 2019;60:734‐738. [DOI] [PubMed] [Google Scholar]
- 34. Allen EC, Tarigo JL, LeVine DN, Barber JP, Brainard BM. Platelet number and function in response to a single intravenous dose of vincristine. J Vet Intern Med. 2021;35:1754‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garon CL, Scott MA, Selting KA, Cohn LA. Idiopathic thrombocytopenic purpura in a cat. J Am Anim Hosp Assoc. 1999;35:464‐470. [DOI] [PubMed] [Google Scholar]
- 36. Tasker S, Mackin AJ, Day MJ. Primary immune‐mediated thrombocytopenia in a cat. J Small Anim Pract. 1999;40:127‐131. [DOI] [PubMed] [Google Scholar]
- 37. Bianco D, Armstrong PJ, Washabau RJ. Presumed primary immune‐mediated thrombocytopenia in four cats. J Feline Med Surg. 2008;10:495‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bianco D, Armstrong PJ, Washabau RJ. A prospective, randomized, double‐blinded, placebo‐controlled study of human intravenous immunoglobulin for the acute management of presumptive primary immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 2009;23:1071‐1078. [DOI] [PubMed] [Google Scholar]
- 39. Scott‐Moncrieff JC, Reagan WJ. Human intravenous immunoglobulin therapy. Semin Vet Med Surg Small Anim. 1997;12:178‐185. [DOI] [PubMed] [Google Scholar]
- 40. Reagan WJ, Scott‐Moncrieff C, Christian J, Snyder P, Kelly K, Glickman L. Effects of human intravenous immunoglobulin on canine monocytes and lymphocytes. Am J Vet Res. 1998;59:1568‐1574. [PubMed] [Google Scholar]
- 41. Bianco D, Armstrong PJ, Washabau RJ. Treatment of severe immune‐mediated thrombocytopenia with human IV immunoglobulin in 5 dogs. J Vet Intern Med. 2007;21:694‐699. [DOI] [PubMed] [Google Scholar]
- 42. Back FP, Lacerda LA, Ventura FVC. Human intravenous immunoglobulin for the treatment of immune‐mediated thrombocytopenia in dog—case report. Acta Vet Bras. 2013;7:535‐536. [Google Scholar]
- 43. Fathi E, Jamshidi S. Case report: follow‐up, diagnosis, clinical evidence, laboratory evaluation, and treatment of idiopathic thrombocytopenia using human intravenous Immunglobulin in a terrier dog. Iran J Vet Sci Technol. 2014;6:64‐70. [Google Scholar]
- 44. Koo Y, Yun T, Chae Y, et al. Suspected human intravenous immunoglobulin‐induced acute haemolytic anaemia in a dog. J Small Anim Pract. 2022;63:482‐485. [DOI] [PubMed] [Google Scholar]
- 45. Tsuchiya R, Akutsu Y, Ikegami A, et al. Prothrombotic and inflammatory effects of intravenous administration of human immunoglobulin G in dogs. J Vet Intern Med. 2009;23:1164‐1169. [DOI] [PubMed] [Google Scholar]
- 46. O'Marra SK, Shaw SP, deLaforcade AM. Investigating hypercoagulability during treatment for immune‐mediated thrombocytopenia: a pilot study. J Vet Emerg Crit Care. 2012;22:126‐130. [DOI] [PubMed] [Google Scholar]
- 47. O'Marra SK, deLaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346‐352. [DOI] [PubMed] [Google Scholar]
- 48. Zini E, Hauser B, Meli ML, Glaus TM. Immune‐mediated erythroid and megakaryocytic aplasia in a cat. J Am Vet Med Assoc. 2007;230:1024‐1027. [DOI] [PubMed] [Google Scholar]
- 49. Ano H, Fujino M, Katamoto H. Case of feline idiopathic immune‐mediated thrombocytopenia effectively treated with cyclophosphamide after splenectomy. J Japan Vet Med Assoc. 2014;67:269‐273. [Google Scholar]
- 50. Jain NC, Switzer JW. Autoimmune thrombocytopenia in dogs and cats. Vet Clin North Am Small Anim Pract. 1981;11:421‐434. [DOI] [PubMed] [Google Scholar]
- 51. Atkinson M. IMT in spaniel after vaccination. Vet Times. 2011;41:10‐11. [Google Scholar]
- 52. Dircks B, Schuberth HJ, Mischke R. Clinical and laboratory‐diagnosed parameters in 21 dogs with primary immune‐mediated thrombocytopenia. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2011;39:17‐24. [PubMed] [Google Scholar]
- 53. Simpson K, Chapman P, Klag A. Long‐term outcome of primary immune‐mediated thrombocytopenia in dogs. J Small Anim Pract. 2018;59:674‐680. [DOI] [PubMed] [Google Scholar]
- 54. Kovalik M, Taszkun I, Pomorski Z, et al. Evaluation of a human generic formulation of ciclosporin in the treatment of canine atopic dermatitis with in vitro assessment of the functional capacity of phagocytic cells. Vet Rec. 2011;168:537. [DOI] [PubMed] [Google Scholar]
- 55. Vargo C, Austel M, Banovic F. Comparison of whole blood concentrations of oral human generic modified ciclosporin capsules with microemulsified ciclosporin capsules approved for canine atopic dermatitis following a single oral administration to healthy dogs. Vet Dermatol. 2023;34:156‐160. [DOI] [PubMed] [Google Scholar]
- 56. Yau VK, Bianco D. Treatment of five haemodynamically stable dogs with immune‐mediated thrombocytopenia using mycophenolate mofetil as single agent. J Small Anim Pract. 2014;55:330‐333. [DOI] [PubMed] [Google Scholar]
- 57. Sri‐Jayantha LS, Doornink MT, Urie BK. Increased risk of select glucocorticoid adverse events in dogs of higher body weight. Can Vet J. 2022;63:32‐38. [PMC free article] [PubMed] [Google Scholar]
- 58. Wondratschek C, Weingart C, Kohn B. Primary immune‐mediated thrombocytopenia in cats. J Am Anim Hosp Assoc. 2010;46:12‐19. [DOI] [PubMed] [Google Scholar]
- 59. Stein TJ, Pellin M, Steinberg H, Chun R. Treatment of feline gastrointestinal small‐cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc. 2010;46:413‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lingard AE, Briscoe K, Beatty JA, et al. Low‐grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg. 2009;11:692‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Salavaggione OE, Yang C, Kidd LB, et al. Cat red blood cell thiopurine S‐methyltransferase: companion animal pharmacogenetics. J Pharmacol Exp Ther. 2004;308:617‐626. [DOI] [PubMed] [Google Scholar]
- 62. Beale KM, Altman D, Clemmons RR, Bolon B. Systemic toxicosis associated with azathioprine administration in domestic cats. Am J Vet Res. 1992;53:1236‐1240. [PubMed] [Google Scholar]
- 63. Bacek LM, Macintire DK. Treatment of primary immune‐mediated hemolytic anemia with mycophenolate mofetil in two cats. J Vet Emerg Crit Care. 2011;21:45‐49. [DOI] [PubMed] [Google Scholar]
- 64. Slovak JE, Rivera SM, Hwang JK, Court MH, Villarino NF. Pharmacokinetics of mycophenolic acid after intravenous administration of mycophenolate mofetil to healthy cats. J Vet Intern Med. 2017;31:1827‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Slovak JE, Hwang JK, Rivera SM, Villarino NF. Pharmacokinetics of mycophenolic acid and its effect on CD4(+) and CD8(+) T cells after oral administration of mycophenolate mofetil to healthy cats. J Vet Intern Med. 2019;33:2020‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bianco D, Hardy RM. Treatment of Evans' syndrome with human intravenous immunoglobulin and leflunomide in a diabetic dog. J Am Anim Hosp Assoc. 2009;45:147‐150. [DOI] [PubMed] [Google Scholar]
- 67. Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829‐3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Joshi BC, Raplee RG, Powell AL, Hancock F. Autoimmune thrombocytopenia in a cat. J Am Anim Hosp Assoc. 1979;15:585‐588. [Google Scholar]
- 69. Jordan HL, Grindem CB, Breitschwerdt EB. Thrombocytopenia in cats: a retrospective study of 41 cases. J Vet Intern Med. 1993;7:261‐265. [DOI] [PubMed] [Google Scholar]
- 70. Ellis J, Bell R, Barnes DC, Miller R. Prevalence and disease associations in feline thrombocytopenia: a retrospective study of 194 cases. J Small Anim Pract. 2018;59:531‐538. [DOI] [PubMed] [Google Scholar]
- 71. Shropshire S, Dow S, Lappin M. Detection and dynamics of anti‐platelet antibodies in thrombocytopenic dogs with and without idiopathic immune thrombocytopenia. J Vet Intern Med. 2020;34:700‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morishita K, Takamura D, Osuga T, Sasaki N, Ohta H, Takiguchi M. Rapid decrease in prednisolone dosage can cause early recurrence of immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 2018;32:2254. [Google Scholar]
- 73. Cummings FO, Rizzo SA. Treatment of presumptive primary immune‐mediated thrombocytopenia with mycophenolate mofetil versus cyclosporine in dogs. J Small Anim Pract. 2017;58:96‐102. [DOI] [PubMed] [Google Scholar]
- 74. High EJ, Olivry T. The prevalence of bacterial infections during cyclosporine therapy in dogs: a critically appraised topic. Can Vet J. 2020;61:1283‐1289. [PMC free article] [PubMed] [Google Scholar]
- 75. McAtee BB, Cummings KJ, Cook AK, Lidbury JA, Heseltine JC, Willard MD. Opportunistic invasive cutaneous fungal infections associated with sdministration of cyclosporine to dogs with immune‐mediated disease. J Vet Intern Med. 2017;31:1724‐1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takeuchi H, Okamoto A. Helicobacter pylori infection and chronic immune thrombocytopenia. J Clin Med. 2022;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abadi U, Yarchovsky‐Dolberg O, Ellis MH. Immune thrombocytopenia: recent progress in pathophysiology and treatment. Clin Appl Thromb Hemost. 2015;21:397‐404. [DOI] [PubMed] [Google Scholar]
- 78. Kuwana M. Helicobacter pylori‐associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20:714‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Satoh T, Uojima H, Wada N, et al. Introduction of direct‐acting antiviral agents alters frequencies of anti‐GPIIb/IIIa antibody‐producing B cells in chronic hepatitis C patients with thrombocytopenia. Platelets. 2023;34:2161498. [DOI] [PubMed] [Google Scholar]
- 80. Kelton JG, Keystone J, Moore J, et al. Immune‐mediated thrombocytopenia of malaria. J Clin Invest. 1983;71:832‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Franchini M, Veneri D, Lippi G. Thrombocytopenia and infections. Expert Rev Hematol. 2017;10:99‐106. [DOI] [PubMed] [Google Scholar]
- 82. Lerner W, Caruso R, Faig D, Karpatkin S. Drug‐dependent and non‐drug‐dependent antiplatelet antibody in drug‐induced immunologic thrombocytopenic purpura. Blood. 1985;66:306‐311. [PubMed] [Google Scholar]
- 83. Goddard A, Leisewitz AL, Kristensen AT, Schoeman JP. Platelet activation and platelet‐leukocyte interaction in dogs naturally infected with Babesia rossi . Vet J. 2015;205:387‐392. [DOI] [PubMed] [Google Scholar]
- 84. Bloom JC, Blackmer SA, Bugelski PJ, Sowinski JM, Saunders LZ. Gold‐induced immune thrombocytopenia in the dog. Vet Pathol. 1985;22:492‐499. [DOI] [PubMed] [Google Scholar]
- 85. Bloom JC, Thiem PA, Sellers TS, Lewis HB, Deldar A. Cephalosporin‐induced immune cytopenia in the dog: demonstration of erythrocyte‐, neutrophil‐, and platelet‐associated IgG following treatment with cefazedone. Am J Hematol. 1988;28:71‐78. [DOI] [PubMed] [Google Scholar]
- 86. Sullivan PS, Arrington K, West R, McDonald T. Thrombocytopenia associated with administration of trimethoprim/sulfadiazine in a dog. J Am Vet Med Assoc. 1992;201:1741‐1744. [PubMed] [Google Scholar]
- 87. Vasilopulos RJ, Mackin A, Lavergne SN, Trepanier LA. Nephrotic syndrome associated with administration of sulfadimethoxine/ormetoprim in a dobermann. J Small Anim Pract. 2005;46:232‐236. [DOI] [PubMed] [Google Scholar]
- 88. Helfand SC, Couto CG, Madewell BR. Immune‐mediated thrombocytopenia associated with solid tumors in dogs. J Am Anim Hosp Assoc. 1985;21:787‐794. [Google Scholar]
- 89. Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. 2018;59:112‐120. [DOI] [PubMed] [Google Scholar]
- 90. Kohn B, Galke D, Beelitz P, Pfister K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J Vet Intern Med. 2008;22:1289‐1295. [DOI] [PubMed] [Google Scholar]
- 91. Kohn B, Bal G, Chirek A, Rehbein S, Salama A. Treatment of 5 dogs with immune‐mediated thrombocytopenia using romiplostim. BMC Vet Res. 2016;12:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Polydoros T, Ioannidi OM, Korsavvidis I, Stefanidis S, Antoniadis T, Mylonakis ME. Romiplostim as adjunctive treatment of refractory amegakaryocytic immune thrombocytopenia in a dog. Top Companion Anim Med. 2021;42:100488. [DOI] [PubMed] [Google Scholar]
- 93. Erickson‐Miller CL, DeLorme E, Tian SS, et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Exp Hematol. 2005;33:85‐93. [DOI] [PubMed] [Google Scholar]
- 94. Matsushiro H, Kato H, Tahara T, et al. Molecular cloning and functional expression of feline thrombopoietin. Vet Immunol Immunopathol. 1998;66:225‐236. [DOI] [PubMed] [Google Scholar]
- 95. Feldman BF, Handagama P, Lubberink AA. Splenectomy as adjunctive therapy for immune‐mediated thrombocytopenia and hemolytic anemia in the dog. J Am Vet Med Assoc. 1985;187:617‐619. [PubMed] [Google Scholar]
- 96. Bestwick JP, Skelly BJ, Swann JW, et al. Splenectomy in the management of primary immune‐mediated hemolytic anemia and primary immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 2022;36:1267‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hisasue M, Hukunaga D, Akaike K, et al. A canine case of idiopathic thrombocytopenic purpura successfully treated by intravenous immunoglobulin therapy and splenectomy. J Japan Vet Med Assoc. 2008;61:223‐226. [Google Scholar]
- 98. Kim SM, Kim GN, Jeong SW, Kim JH. Multiple splenic infarctions in a dog with immune‐mediated hemolytic anemia: therapeutic implications. Iran J Vet Res. 2020;21:65‐69. [PMC free article] [PubMed] [Google Scholar]
- 99. Vargo CL, Taylor SM, Haines DM. Immune mediated neutropenia and thrombocytopenia in 3 giant schnauzers. Can Vet J. 2007;48:1159‐1163. [PMC free article] [PubMed] [Google Scholar]
- 100. Akiyoshi M, Hisasue M, Neo S, Akiyoshi M. Presumptive hemophagocytic syndrome associated with immune‐mediated anemia in two miniature dachshunds. J Vet Med Sci. 2021;83:689‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Francey T, Etter M, Schweighauser A. Evaluation of membrane‐based therapeutic plasma exchange as adjunctive treatment for immune‐mediated hematologic disorders in dogs. J Vet Intern Med. 2021;35:925‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kopecny L, Palm CA, Naylor S, Kirby J, Cowgill LD. Application of therapeutic plasma exchange in dogs with immune‐mediated thrombocytopenia. J Vet Intern Med. 2020;34:1576‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dorrestein E, Peda A, Thrall MA, Illanes O. Fatal pulmonary thromboembolism in a two‐year‐old dog receiving long‐term corticosteroid therapy. Vet Rec Case Rep. 2019;7:e000701. [Google Scholar]
- 104. Flint SK, Abrams‐Ogg AC, Kruth SA, Bersenas AM, Wood RD. Independent and combined effects of prednisone and acetylsalicylic acid on thromboelastography variables in healthy dogs. Am J Vet Res. 2011;72:1325‐1332. [DOI] [PubMed] [Google Scholar]
- 105. O'Kell AL, Grant DC, Panciera DL, Troy GC, Weinstein NM. Effects of oral prednisone administration with or without ultralow‐dose acetylsalicylic acid on coagulation parameters in healthy dogs. Am J Vet Res. 2012;73:1569‐1576. [DOI] [PubMed] [Google Scholar]
- 106. Romão F, Campos EF, Mattoso CR, Takahira R. Hemostatic profile and thromboembolic risk in healthy dogs treated with prednisone: a randomized controlled trial. BMC Vet Res. 2013;9:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. deLaforcade A, Bacek L, Blais MC, Goggs R, Lynch A, Rozanski E. Consensus on the rational use of antithrombotics in veterinary critical care (CURATIVE): Domain 1‐defining populations at risk. J Vet Emerg Crit Care. 2019;29:37‐48. [DOI] [PubMed] [Google Scholar]
- 108. deLaforcade A, Bacek L, Blais MC, et al. 2022 update of the Consensus on the Rational Use of Antithrombotics and Thrombolytics in Veterinary Critical Care (CURATIVE) Domain 1‐ defining populations at risk. J Vet Emerg Crit Care. 2022;32:289‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Goggs R, Blais MC, Brainard BM, et al. American College of Veterinary Emergency and Critical Care (ACVECC) Consensus on the Rational Use of Antithrombotics in Veterinary Critical Care (CURATIVE) guidelines: Small animal. J Vet Emerg Crit Care. 2019;29:12‐36. [DOI] [PubMed] [Google Scholar]
- 110. Marks SL, Kook PH, Papich MG, Tolbert MK, Willard MD. ACVIM Consensus Statement: Support for rational administration of gastrointestinal protectants to dogs and cats. J Vet Intern Med. 2018;32:1823‐1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gabardi S, Olyaei A. Evaluation of potential interactions between mycophenolic acid derivatives and proton pump inhibitors. Ann Pharmacother. 2012;46:1054‐1064. [DOI] [PubMed] [Google Scholar]
- 112. Waldrop JE, Rozanski EA, Freeman LM, Rush JE. Packed red blood cell transfusions in dogs with gastrointestinal hemorrhage: 55 cases (1999‐2001). J Am Anim Hosp Assoc. 2003;39:523‐527. [DOI] [PubMed] [Google Scholar]
- 113. Goggs R, Boag AK, Chan DL. Concurrent immune‐mediated haemolytic anaemia and severe thrombocytopenia in 21 dogs. Vet Rec. 2008;163:323‐327. [DOI] [PubMed] [Google Scholar]
- 114. Goggs R, Brainard BM, LeVine DN, et al. Lyophilized platelets versus cryopreserved platelets for management of bleeding in thrombocytopenic dogs: A multicenter randomized clinical trial. J Vet Intern Med. 2020;34:2384‐2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Orcutt ES, Lee JA, Bianco D. Immune‐mediated hemolytic anemia and severe thrombocytopenia in dogs: 12 cases (2001‐2008). J Vet Emerg Crit Care. 2010;20:338‐345. [DOI] [PubMed] [Google Scholar]
- 116. Chong SK, Adamantos S. Effect of co‐administration of gastroprotectants in reducing the risk of gastrointestinal side effects in dogs treated with immunosuppressive doses of corticosteroids. BSAVA Congress Proceedings; 2015:458. [Google Scholar]
- 117. Kuhl A, Odunayo A, Price J, et al. Comparative analysis of the effect of IV administered acid suppressants on gastric pH in dogs. J Vet Intern Med. 2020;34:678‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med. 2011;25:47‐54. [DOI] [PubMed] [Google Scholar]
- 119. Culbert LA, Marino DJ, Baule RM, VW3rd K. Complications associated with high‐dose prednisolone sodium succinate therapy in dogs with neurological injury. J Am Anim Hosp Assoc. 1998;34:129‐134. [DOI] [PubMed] [Google Scholar]
- 120. Boag AK, Otto CM, Drobatz KJ. Complications of methylprednisolone sodium succinate therapy in dachshunds with surgically treated intervertebral disc disease. J Vet Emerg Crit Care. 2001;11:105‐110. [Google Scholar]
- 121. Swann JW, Szladovits B, Threlfall AJ, et al. Randomised controlled trial of fractionated and unfractionated prednisolone regimens for dogs with immune‐mediated haemolytic anaemia. Vet Rec. 2019;184:771. [DOI] [PubMed] [Google Scholar]
- 122. Whittemore JC, Mooney AP, Price JM, Thomason J. Clinical, clinicopathologic, and gastrointestinal changes from administration of clopidogrel, prednisone, or combination in healthy dogs: a double‐blind randomized trial. J Vet Intern Med. 2019;33:2618‐2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Narum S, Westergren T, Klemp M. Corticosteroids and risk of gastrointestinal bleeding: a systematic review and meta‐analysis. BMJ Open. 2014;4:e004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bernardin F, Martinez Rivera L, Ragetly G, Gomes E, Hernandez J. Spontaneous gastrointestinal perforation in cats: a retrospective study of 13 cases. J Feline Med Surg. 2015;17:873‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ryan P, Odunayo A, Price J, et al. Comparative analysis of the effect of PO administered acid suppressants on gastric pH in healthy cats. J Vet Intern Med. 2020;34:1879‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhang X, Gu S, You L, et al. Gut microbiome and metabolome were altered and strongly associated with platelet count in adult patients with primary immune thrombocytopenia. Front Microbiol. 2020;11:1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Liu C, Cheng L, Ji L, et al. Intestinal microbiota dysbiosis play a role in pathogenesis of patients with primary immune thrombocytopenia. Thromb Res. 2020;190:11‐19. [DOI] [PubMed] [Google Scholar]
- 128. Wang Y, Liu F, Zhang G, et al. Gut microbiome alterations and its link to corticosteroid resistance in immune thrombocytopenia. Sci China Life Sci. 2021;64:766‐783. [DOI] [PubMed] [Google Scholar]
- 129. Jugan MC, Wouda RM, Higginbotham ML. Preliminary evaluation of probiotic effects on gastrointestinal signs in dogs with multicentric lymphoma undergoing multi‐agent chemotherapy: A randomised, placebo‐controlled study. Vet Rec Open. 2021;8:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Borody T, Campbell J, Torres M, Nowak A, Leis S. Reversal of idiopathic thrombocytopenic purpura [ITP] with fecal microbiota transplantation [FMT]: 941. Am J Gastroenterol. 2011;106:S352. [Google Scholar]
- 131. Liu PY, Xia D, McGonigle K, et al. Immune‐mediated hematological disease in dogs is associated with alterations of the fecal microbiota: a pilot study. Anim Microbiome. 2023;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ellis J, Ward PM, Foale RD. Evaluation of the risk of relapse of canine immune‐mediated thrombocytopenia after routine vaccination. J Vet Intern Med. 2016;30:1467. [Google Scholar]
- 133. Day MJ, Crawford C, Marcondes M, Squires RA. Recommendations on vaccination for Latin American small animal practitioners: a report of the WSAVA vaccination guidelines group. J Small Anim Pract. 2020;61:E1‐e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. AAHA . 2022 AAHA canine vaccination guidelines. American Animal Hospital Association; 2022. [DOI] [PubMed] [Google Scholar]
- 135. Ng ZY, Stokes JE, Alvarez L, Bartges JW. Cryopreserved platelet concentrate transfusions in 43 dogs: a retrospective study (2007‐2013). J Vet Emerg Crit Care. 2016;26:720‐728. [DOI] [PubMed] [Google Scholar]
- 136. Abrams‐Ogg AC, Kruth SA, Carter RF, Valli VE, Kamel‐Reid S, Dubé ID. Preparation and transfusion of canine platelet concentrates. Am J Vet Res. 1993;54:635‐642. [PubMed] [Google Scholar]
- 137. Allyson K, Abrams‐Ogg AC, Johnstone IB. Room temperature storage and cryopreservation of canine platelet concentrates. Am J Vet Res. 1997;58:1338‐1347. [PubMed] [Google Scholar]
- 138. Callan MB, Appleman EH, Sachais BS. Canine platelet transfusions. J Vet Emerg Crit Care. 2009;19:401‐415. [DOI] [PubMed] [Google Scholar]
- 139. Haines JM, Ngwenyama TR, Martin LG, Wardrop KJ. Development and implementation of a hemovigilance program at a university veterinary teaching hospital. J Vet Emerg Crit Care. 2022;32:315‐321. [DOI] [PubMed] [Google Scholar]
- 140. Brecher G, Cronkite EP. The effects of platelet transfusions in dogs made pancytopenic by x‐radiation. N Y State J Med. 1953;53:544‐547. [PubMed] [Google Scholar]
- 141. Cronkite EP, Brecher G. The experimental therapy of the hemorrhagic phase of the radiation syndrome with platelet transfusions. Acta Radiol Suppl. 1954;116:376‐380. [PubMed] [Google Scholar]
- 142. Cronkite EP, Brecher G, Wilbur KM. Development and use of a canine blood donor colony for experimental purposes: I. Leukocyte and platelet transfusions in irradiation aplasia of the dog bone marrow. Mil Surg. 1954;114:359‐365. [PubMed] [Google Scholar]
- 143. Valeri CR, Feingold H, Melaragno AJ, Vecchione JJ. Cryopreservation of dog platelets with dimethyl sulfoxide: therapeutic effectiveness of cryopreserved platelets in the treatment of thrombocytopenic dogs, and the effect of platelet storage at −80 degrees C. Cryobiology. 1986;23:387‐394. [DOI] [PubMed] [Google Scholar]
- 144. Bode AP, Lust RM, Read MS, Fischer TH. Correction of the bleeding time with lyophilized platelet infusions in dogs on cardiopulmonary bypass. Clin Appl Thromb Hemost. 2008;14:38‐54. [DOI] [PubMed] [Google Scholar]
- 145. Saint‐Pierre LM, Farrell KS, Hopper K, Reagan KL. Retrospective evaluation of fresh platelet concentrate administration in dogs: patient characteristics, outcomes, and transfusion practices in 189 transfusion episodes (2008‐2019). J Vet Emerg Crit Care. 2023;33:360‐370. [DOI] [PubMed] [Google Scholar]
- 146. Cowles BE, Meyers KM, Wardrop KJ, Menard M, Sylvester D. Prolonged bleeding time of Chediak‐Higashi cats corrected by platelet transfusion. Thromb Haemost. 1992;67:708‐712. [PubMed] [Google Scholar]
- 147. Meyers KM, Seachord CL, Holmsen H, Prieur DJ. Evaluation of the platelet storage pool deficiency in the feline counterpart of the Chediak‐Higashi syndrome. Am J Hematol. 1981;11:241‐253. [DOI] [PubMed] [Google Scholar]
- 148. Kramer JW, Davis WC, Prieur DJ. The Chediak‐Higashi syndrome of cats. Lab Invest. 1977;36:554‐562. [PubMed] [Google Scholar]
- 149. Prieur DJ, Collier LL. Inheritance of the Chediak‐Higashi syndrome in cats. J Hered. 1981;72:175‐177. [DOI] [PubMed] [Google Scholar]
- 150. Gaschen FP, Smith Meyer B, Harvey JW. Amegakaryocytic thrombocytopenia and immune‐mediated haemolytic anaemia in a cat. Comp Haematol Int. 1992;2:175‐178. [Google Scholar]
- 151. Breitschwerdt EB, Abrams‐Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis‐like infection in cats. J Vet Intern Med. 2002;16:642‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kohn B, Linden T, Leibold W. Platelet‐bound antibodies detected by a flow cytometric assay in cats with thrombocytopenia. J Feline Med Surg. 2006;8:254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Davidow EB, Brainard B, Martin LG, et al. Use of fresh platelet concentrate or lyophilized platelets in thrombocytopenic dogs with clinical signs of hemorrhage: a preliminary trial in 37 dogs. J Vet Emerg Crit Care. 2012;22:116‐125. [DOI] [PubMed] [Google Scholar]
- 154. Langhorn R, Bochsen L, Willesen JL, Sørensen TM, Kristensen AT. Thromboelastography‐guided transfusion in dogs with hypocoagulable disorders: a case series. Acta Vet Scand. 2019;61:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Scheepers E, Leisewitz AL, Thompson PN, Christopher MM. Serial haematology results in transfused and non‐transfused dogs naturally infected with Babesia rossi . J S Afr Vet Assoc. 2011;82:136‐143. [DOI] [PubMed] [Google Scholar]
- 156. Thakar S, Gabarin N, Gupta A, Radford M, Warkentin TE, Arnold DM. Anemia‐induced bleeding in patients with platelet disorders. Transfus Med Rev. 2021;35:22‐28. [DOI] [PubMed] [Google Scholar]
- 157. Tocci LJ. Transfusion medicine in small animal practice. Vet Clin North Am Small Anim Pract. 2010;40:485‐494. [DOI] [PubMed] [Google Scholar]
- 158. Kuo KW, McMichael M. Small animal transfusion medicine. Vet Clin North Am Small Anim Pract. 2020;50:1203‐1214. [DOI] [PubMed] [Google Scholar]
- 159. Maglaras CH, Koenig A, Bedard DL, Brainard BM. Retrospective evaluation of the effect of red blood cell product age on occurrence of acute transfusion‐related complications in dogs: 210 cases (2010‐2012). J Vet Emerg Crit Care. 2017;27:108‐120. [DOI] [PubMed] [Google Scholar]
- 160. Zaremba R, Brooks A, Thomovsky E. Transfusion medicine: an update on antigens, antibodies and serologic testing in dogs and cats. Top Companion Anim Med. 2019;34:36‐46. [DOI] [PubMed] [Google Scholar]
- 161. Mangiaterra S, Rossi G, Antognoni MT, et al. Canine blood group prevalence and geographical distribution around the world: an updated systematic review. Animals (Basel). 2021;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Marshall H, Blois SL, Abrams‐Ogg ACG, Bersenas AM, Ruotsalo K, Monteith G. Accuracy of point‐of‐care crossmatching methods and crossmatch incompatibility in critically ill dogs. J Vet Intern Med. 2021;35:245‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hourani L, Weingart C, Kohn B. Alloimmunisation in transfused patients: serial cross‐matching in a population of hospitalised cats. J Feline Med Surg. 2017;19:1231‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Goulet S, Blais MC. Characterization of anti‐dal alloantibodies following sensitization of two dal‐negative dogs. Vet Pathol. 2018;55:108‐115. [DOI] [PubMed] [Google Scholar]
- 165. Davidow EB, Blois SL, Goy‐Thollot I, et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Transfusion Reaction Small Animal Consensus Statement (TRACS). Part 1: Definitions and clinical signs. J Vet Emerg Crit Care. 2021;31:141‐166. [DOI] [PubMed] [Google Scholar]
- 166. Davidow EB, Blois SL, Goy‐Thollot I, et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Transfusion Reaction Small Animal Consensus Statement (TRACS). Part 2: Prevention and monitoring. J Vet Emerg Crit Care. 2021;31:167‐188. [DOI] [PubMed] [Google Scholar]
- 167. Odunayo A, Nash KJ, Davidow EB, et al. Association of Veterinary Hematology and Transfusion Medicine (AVHTM) Transfusion Reaction Small Animal Consensus Statement (TRACS). Part 3: Diagnosis and treatment. J Vet Emerg Crit Care. 2021;31:189‐203. [DOI] [PubMed] [Google Scholar]
- 168. Abrams‐Ogg AC. Triggers for prophylactic use of platelet transfusions and optimal platelet dosing in thrombocytopenic dogs and cats. Vet Clin North Am Small Anim Pract. 2003;33:1401‐1418. [DOI] [PubMed] [Google Scholar]
- 169. Edwards TH, Darlington DN, Pusateri AE, et al. Hemostatic capacity of canine chilled whole blood over time. J Vet Emerg Crit Care. 2021;31:239‐246. [DOI] [PubMed] [Google Scholar]
- 170. Barfield D, Adamantos S. Feline blood transfusions: a pinker shade of pale. J Feline Med Surg. 2011;13:11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Taylor S, Spada E, Callan MB, et al. 2021 ISFM consensus guidelines on the collection and administration of blood and blood products in cats. J Feline Med Surg. 2021;23:410‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Martinez‐Sogues L, Blois SL, Manzanilla EG, Abrams‐Ogg AO, Cosentino P. Exploration of risk factors for non‐survival and for transfusion‐associated complications in cats receiving red cell transfusions: 450 cases (2009 to 2017). J Small Anim Pract. 2020;61:177‐184. [DOI] [PubMed] [Google Scholar]
- 173. McClosky ME, Cimino Brown D, Weinstein NM, et al. Prevalence of naturally occurring non‐AB blood type incompatibilities in cats and influence of crossmatch on transfusion outcomes. J Vet Intern Med. 2018;32:1934‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Binvel M, Arsenault J, Depré B, Blais MC. Identification of 5 novel feline erythrocyte antigens based on the presence of naturally occurring alloantibodies. J Vet Intern Med. 2021;35:234‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Cannavino A, LeVine D, Blais MC. Characterization of post‐transfusion anti‐FEA 1 alloantibodies in transfusion‐naive FEA 1‐negative cats. J Feline Med Surg. 2022;24:e124‐e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Spada E, Perego R, Baggiani L, Proverbio D. Haematological and morphological evaluation of feline whole blood units collected for transfusion purposes. J Feline Med Surg. 2019;21:732‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wolf J, Ruterbories LK, Handel I, Hansen B. The effect of ε‐aminocaproic acid on blood product requirement, outcome and thromboelastography parameters in severely thrombocytopenic dogs. J Vet Intern Med. 2024;38:1013‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Olivares G, Sharman M, Miller R, Kisielewicz C, Seth M. Use of tranexamic acid in dogs with primary immune thrombocytopenia: a feasibility study. Front Vet Sci. 2023;10:946127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Weese JS, Blondeau J, Boothe D, et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet J. 2019;247:8‐25. [DOI] [PubMed] [Google Scholar]
- 180. Cooper N, Ghanima W, Hill QA, Nicolson PLR, Markovtsov V, Kessler C. Recent advances in understanding spleen tyrosine kinase (SYK) in human biology and disease, with a focus on fostamatinib. Platelets. 2023;34:2131751. [DOI] [PubMed] [Google Scholar]
- 181. Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of fc‐independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6:7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Extended materials and methods.
Data S2. Bibliography of studies for treatment.
Data S3. Evidence evaluator instructions for medical and transfusion management of ITP PICOs.
Data S4. Data extraction—medical.
Data S5. Data extraction—transfusion.
Data S6. PICO evidence summary template for the ACVIM Consensus Statement on the Treatment of Immune Thrombocytopenia in Dogs and Cats.
Data S7. Evidence summaries and consensus recommendations PICOs with minimal evidence.
Data S8. Immune thrombocytopenia diagnosis, treatment, and outcome reporting guidelines.


