Abstract
Introduction
Cirrhosis-associated immune dysfunction (CAID) is a chronic vasodilatory state with hyperdynamic circulation and alterations in thermoregulation that may make patients more susceptible to and mask underlying infection. This study aims to determine whether SIRS criteria are an accurate tool for predicting bloodstream infection (BSI) in cirrhosis.
Methods
In our retrospective chart review, study population included patients with cirrhosis that were 18 years or older. For all study patients, model for end-stage liver disease (MELD) scores and values for each SIRS variable at the time of admission and blood culture data were recorded. Univariable and multivariable logistic regression analysis was performed to identify any associations between dichotomized SIRS variables that fulfill SIRS positivity and BSI.
Results
Significantly more patients without BSI met positivity criteria for WBC counts (30% vs 13% p < .001). In the analysis of the SIRS variables as continuous variables in prediction of BSI, the AUC curves generated were all unsatisfactory with the temperature (36-38°C) and WBC count (4 × 103 to 12 × 103 mcL) at the time of admission having the highest areas under the ROC curve (0.52 and 0.55, respectively). Looking at the SIRS variables dichotomized (according to whether fulfilling SIRS criteria or not) in univariable logistic regression, only WBC counts meeting SIRS criteria were significantly associated with BSI OR 0.37 (0.18-0.77); p = .008, but this was an inverse association. This association was true even in the multivariable model OR 0.38 (0.18-0.80); p = .01.
Conclusion
Our study shows that SIRS criteria are a poor predictor of BSI among patients with cirrhosis.
Keywords: Bloodstream infection, Cirrhosis, Cirrhosis-associated immune dysfunction, MELD, SIRS
Introduction
Cirrhosis is the final pathological outcome of chronic liver disease of various etiologies in a process that represents the crescendo of cytokine production and hepatic stellate cell activation, repeat cycles of apoptosis and regeneration of hepatocytes, and defenestration and capillarization of sinusoidal endothelial cells.1 A 2015 epidemiological study placed the prevalence of cirrhosis at 0.27% in the United States, corresponding to approximately 634,000 Americans.2 As the compensatory mechanisms of the liver fail, it is well known that uncontrolled portal hypertension results in ascites and variceal hemorrhage, diminished synthetic function results in coagulopathy, and metabolic derangements result in jaundice, encephalopathy, hepatorenal, and hepatopulmonary syndromes. A far more indolent but equally perilous mediator of morbidity in cirrhosis is cirrhosis-associated immune dysfunction (CAID).
CAID refers to the state of simultaneous systemic inflammation and immunodeficiency seen in cirrhotic patients. Immunodeficiency results both from decreased capacity for immune surveillance secondary to reticuloendothelial system damage and from reduced innate immunity due to decreased hepatic synthesis of immunoglobulins.3 In fact, it is estimated that bacterial infection is present on admission or develops during hospitalization in 30% of patients with cirrhosis, carrying a mortality rate of 38%. In patients with cirrhosis who develop septic shock, the mortality is estimated to be >70%.4 Being able to accurately recognize infectious complications in patients with cirrhosis can result in significant reduction in mortality.
The most widespread method for early detection of systemic infection, including bacteremia, identifies features of the systemic inflammatory response syndrome (SIRS), which is defined as ≥2 of the following: temperature >38°C or <36°C, heart rate >90 bpm, respiratory rate >20/min, and white blood cell (WBC) count >12 × 103 or <4 × 103/mcL. However, it appears that many of these physiological parameters are inherently affected in patients with cirrhosis, due to either the underlying pathophysiology or the disease-modifying therapies utilized in this population. For example, thermoregulation may be impaired in the setting of CAID, bradycardia may result from beta-blockers used in the primary prevention of variceal bleeding, tachypnea may result from encephalopathy, and a spectrum of WBC counts may be observed in CAID, producing a phenotype that mimics SIRS. As such, we hypothesized that SIRS criteria are a poor predictor of bloodstream infection (BSI) in patients with cirrhosis and are likely to produce higher rates of false alarms in EMRs equipped with sepsis alerts triggered by SIRS criteria. The aim of this study was to evaluate the utility of SIRS criteria in detecting BSI in cirrhotic patients.
Methods
Study Design, Participants, and Data Collection
This study was a single-center retrospective analysis looking at patients with cirrhosis over 18 years of age admitted to Einstein Medical Center Philadelphia, an academic urban tertiary care hospital, between January 1, 2017, and December 31, 2018. Patients were identified from a Department of Hepatology database of patients admitted to or consulted on by the inpatient hepatology service. Patients were included if they were >18 years of age with cirrhosis admitted during the study period. Patients were excluded if they had undergone liver transplantation, were pregnant, were immunocompromised, had malignancy, or had HIV infection with CD4 count < 200.
The electronic medical record (EMR) of each subject was reviewed to determine if the patient had BSI during the admission and was assessed for the presence of SIRS criteria at the time infection was suspected. The following data were collected for each patient at the time of admission and at the time blood cultures were drawn: SIRS criteria (temperature, heart rate, respiratory rate, WBC count) and MELD-Na Score components (sodium, INR, total bilirubin, creatinine). BSI was defined as bacterial growth in blood cultures of microbiologic species not suggestive of contamination as determined by an infectious disease consultant during the admission. Other key data points that were collected were specific types of pathogens that were identified.
Statistical Analysis
Demographic and clinical data were presented using descriptive statistics, frequencies, and percentages. For continuous variables that were not normally distributed, median (IQR) was used and Wilcoxon signed ranked tests were used, while chi-square tests were used for categorical data in comparison to demographic data. SIRS variables were analyzed as continuous and dichotomous variables. SIRS variables as continuous variables were analyzed with receiver operating characteristic (ROC) curves, and area under the curve (AUC) was used to analyze these variables as predictors of BSI. In this study, the AUC values were defined as follows: 0.9-1 = excellent accuracy; 0.8-0.9 = good accuracy; 0.7-0.8 = fair accuracy; 0.6-0.7 = poor accuracy; 0.5-0.6 = fail.5 If the AUC is >0.7, Youden's index will be calculated to identify the cutoff value that maximizes the sensitivity and specificity of each predictor, and sensitivities and specificities will be computed. Dichotomized SIRS variables (whether fulfilling positive SIRS criteria or not) individually and in different combinations of temperature, heart rate, respiratory rate, and WBC counts and association with BSI were explored using univariable and multivariable logistic regression; 95% confidence intervals were presented when appropriate. A P-value of <.05 was considered statistically significant. Analysis was done using Stata (Version 17; StataCorp, College Station, TX).
Results
After EMR review of 572 inpatient charts identified by query of the Department of Hepatology database and exclusion criteria were exercised, a total sample size of 420 patients was included in our final analysis (Figure 1). Please refer to Table 1 for a demographic breakdown of the 420 patients included in the final study sample.
Figure 1.
The total number of patients that were admitted during the study period and how many were then actually included in the study population once applying exclusion criteria. n = number of patients within said category.
Table 1.
Demographics of Patient Population.
| Non-BSI (n = 353) | BSI (n = 67) | P-value | |
|---|---|---|---|
| Gender—no. (%) | |||
| Male | 205 (58) | 31 (46) | .074 |
| Female | 148 (42) | 36 (54) | |
| Median age—years (IQR) | 60 (52-65) | 61 (55-69) | .09 |
| Median BMI—kg/m2 (IQR) | 28 (25-33) | 29 (24-36) | .42 |
| Ethnicity—no. (%) | .06 | ||
| Caucasian | 182 (52) | 44 (66) | |
| African American | 81 (23) | 15 (22) | |
| Hispanic | 32 (9) | 6 (9) | |
| Other/unknown* | 58 (16) | 2 (3) | |
| SIRS criteria at time of presentation—mean ± SD | |||
| Temperature (°C) | 36.7 ± 0.05 | 36.9 ± 0.07 | .18 |
| Heart rate (bpm) | 84.0 ± 0.93 | 80.3 ± 2.13 | .11 |
| Respiratory Rate (brpm) | 19.0 ± 0.22 | 17.5 ± 0.28 | .003 |
| White blood cell count (cells/L) | 7.78 ± 0.28 × 109 | 8.05 ± 0.60 × 109 | .70 |
| SIRS criteria positive (%) | 61 (17) | 6 (9) | .09 |
| (+) SIRS for temperature | 12(3) | 3(4) | .66 |
| (+) SIRS for heart rate | 116(33) | 16(24) | .15 |
| (+) SIRS for RR | 45(13) | 3(4) | .05 |
| (+) SIRS for WBC count | 105(30) | 9(13) | .006 |
| SIRS criteria at time of positive blood culture collection—mean | |||
| Temperature (°C) | n/a | 37.2 | |
| Heart rate (bpm) | n/a | 83 | |
| Respiratory rate (brpm) | n/a | 19 | |
| White blood cell count (cells/L) | n/a | 8.72 × 109 | |
| MELD score | |||
| At time of presentation | 21.1 ± 0.40 | 17.2 ± 0.90 | <.001 |
| At time of positive blood culture collection | n/a | 17.5 |
bpm = beats/minute; brpm = breaths/minute; °C = degrees Celsius
Other: Asian, declined to identify.
Of the 420 patients with cirrhosis, mean age was 59.1 ± 10.9. Totally, 44% were female, 54% were Caucasian, 23% were African American, and 9% were Hispanic; the remainder of the demographic and clinical parameters stratified by presence of BSI are in Table 1. BSI occurred in 67 patients (16%). Escherichia coli was the most common pathogen isolated (16%), followed by methicillin-resistant Staphylococcus aureus (MRSA) (14%) and coagulase-negative staphylococci (13%) (Table 2). Most cases were thought to be due to gut translocation (please refer to Table 3 for other sources of BSI). Only 6 of the 67 patients (9%) with BSI were SIRS positive; therefore, 61 of the 67 patients with BSI were SIRS negative (Table 1). On the other hand, 61 of the 353 patients without BSI (17%) were SIRS positive (Table 1).
Table 2.
Isolated Organisms Identified as BSI.
| Organism | Number of Patients (n) | Percentage (%) |
|---|---|---|
| Escherichia coli | 12 | 18 |
| Methicillin-resistant Staphylococcus aureus | 10 | 15 |
| Coagulase-negative Staphylococcus spp. | 10 | 15 |
| Methicillin-sensitive Staphylococcus aureus | 7 | 10 |
| Klebsiella pneumoniae | 4 | 6 |
| Pseudomonas aeruginosa | 4 | 6 |
| Enterococcus faecium | 4 | 6 |
| Other bacteria* | 16 | 24 |
Other bacteria: Aerococcus spp., Viridans Streptococcus spp., Enterobacter aerogenes, Group B streptococcus, Enterobacter cloacae, Serratia marcescens, vancomycin-resistant Enterococcus, Morganella morganii, Klebsiella oxytoca, Candida glabrata, Acinetobacter baumannii, Actinomyces spp., Proteus spp., Enterococcus faecalis.
Table 3.
Identified Sources of Culprit Organisms.
| Source of bacteremia | Number of patients (n) | Percentage (%) |
|---|---|---|
| Gut translocation | 36 | 54 |
| Prosthetic orthopedic device | 17 | 25 |
| Urinary tract | 6 | 9 |
| Infective endocarditis | 2 | 3 |
| Undetermined | 6 | 9 |
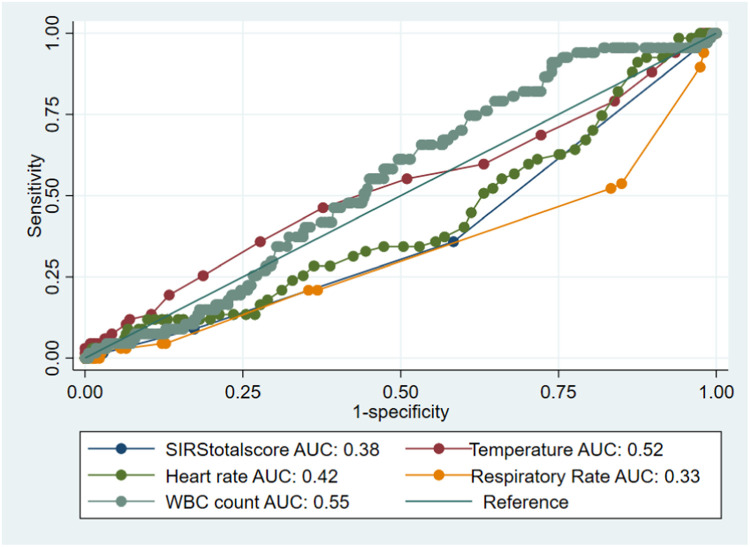
Interestingly, significantly more patients without BSI met positivity criteria for WBC counts (30% vs 13% P < .001) (Table 1). In the analysis of the SIRS criteria as continuous variables in the prediction of BSI, only respiratory rate was significantly inversely associated with BSI, even on the multivariable model (OR 0.76 [0.66-0.88]; p < .001). However, the AUC curves generated were all unsatisfactory, with the temperature (36-38°C) and WBC count (4 × 103 to 12 × 103/mcL) at the time of admission having the highest areas under the ROC curve (0.52 and 0.55, respectively) (Figure 2). Looking at the SIRS variables dichotomized according to fulfilling SIRS criteria vs those not, in univariable logistic regression only WBC count meeting the SIRS criteria was significantly associated with BSI (OR 0.37 [0.18-0.77]; P = .008), but this was also an inverse association. This association persisted even in the multivariable model (OR 0.38 [0.18-0.80]; P = .01) (Table 4). In that accord, no specific pair of positive SIRS variables were significantly associated with BSI (Table 5).
Figure 2.
The ROC curve for all SIRS criteria variables (binary classifiers) in order to plot their true and false positive rates to help detect BSI based on the set threshold.
Table 4.
Analysis of SIRS Variables Both as Continuous and Dichotomous (Fulfilling SIRS Criteria) Using Univariable and Multivariable Logistic Regression in the Prediction of BSI.
| SIRS variables as continuous | Univariable: odds ratio (95% CI); P-value | Multivariable model: odds ratio (95% CI); P-value |
|---|---|---|
| Temperature | OR 1.45 (0.88-2.39); P = .14 | OR 1.56 (0.92-2.67); P = .10 |
| Heart rate | OR 0.99 (0.97-1.00); P = .12 | OR 0.99 (0.98-1.01); P = .43 |
| Respiratory rate | OR 0.75 (0.65-0.87); P < .001 | OR 0.76 (0.66-0.88); P < .001 |
| WBC count | OR 1.01 (0.96-1.06); P = .70 | OR 1.02 (0.97-1.07); P = .41 |
| SIRS variables as dichotomous (SIRS+) | ||
| Temperature meeting SIRS criteria | OR 1.33 (0.37-4.85); P = .66 | OR 1.28 (0.34-4.79); P = .71 |
| Heart rate meeting SIRS criteria | OR 0.64 (0.35-1.17); P = .15 | OR 0.68 (0.37-1.27); P = .23 |
| Respiratory rate meeting SIRS criteria | OR 0.32 (0.10-1.06); P = .06 | OR 0.40 (0.12-1.35); P = .14 |
| WBC count meeting SIRS criteria | OR 0.37 (0.18-0.77); P = .008 | OR 0.38 (0.18-0.80); P = .01 |
Table 5.
Analysis of Paired Positive SIRS Criteria with P-Value of <.05 Being Statistically Significant in Prediction of BSI in Patient with Cirrhosis.
| Paired SIRS variables | Odds ratio and confidence interval |
|---|---|
| Positive for Both Fever + Heart Rate | OR 1.05 (95% CI 0.12-9.17; P = .96) |
| Positive Fever + Respiratory Rate | OR 0 (P = .999) |
| Positive Fever + White Blood Cell Count | OR 2.66 (95% CI 0.24-29.75; P = .43) |
| Positive Respiratory Rate + Heart Rate | OR 0.62 (95% CI 0.18-2.10; P = .44) |
| Positive Respiratory Rate + White Blood Cell Count | OR 0.28 (95% CI 0.04-2.15; P = .22) |
| Positive Heart Rate + White Blood Cell Count | OR 0.31 (95% CI 0.07-1.32; P = .11) |
No single or combination of SIRS variables was significantly associated with prediction of BSI (see Tables 4 and 5).
Discussion
Cirrhosis is characterized by a pathological milieu of CAID encompassing systemic inflammation, immunodeficiency, and a hyperdynamic hemodynamic state that often mimics signs of infection. To date, the SIRS criteria have been regarded as the seminal criteria in detecting early signs of systemic infection.
In this study, we demonstrate that SIRS criteria variables—independent or paired—were not associated with BSI in cirrhotic patients. On univariate analysis performed on pairs of SIRS parameters, none achieved significance in predicting BSI. The SIRS parameters that demonstrated the strongest independent predictive values for infection were WBC count and temperature at time of admission; while these parameters did not achieve significance in the study, it is possible that they were closest in predicting infection because they were least effected by the “hyperdynamic circulatory syndrome” phenotype that affects the other parameters defining SIRS. It is important to note that the average MELD-Na score of our study population was 17, indicating advanced disease and an immunophenotype that likely reflects a trend toward decompensation with a hyperdynamic circulatory syndrome.
This study adds to a growing body of evidence demonstrating the decreased utility of the SIRS criteria in detecting infection and defining BSI. A prior large-scale retrospective cohort study investigating the SIRS criteria in defining severe sepsis in the general adult population demonstrated that 12.1% of patients were SIRS negative despite having documented infection complicated by organ failure.6 By comparison, 91% of the bacteremic patients in our study had SIRS-negative BSI, highlighting the inefficacy of the SIRS criteria to detect true infection in the cirrhotic population. Our study adds to a prior study by Borgonovo et al. which assessed the utility of SIRS in predicting infection in patients hospitalized for acute decompensated cirrhosis, finding that it was of no prognostic value.7 Other work assessing predictive models of sepsis (Sepsis-3 criteria, quick-SOFA score, and National Early Warning Score [NEWS]) in patients with cirrhosis have focused on an endpoint of mortality rather than prediction of true infection at the time SIRS parameters are met and have failed to correlate SIRS with worse survival on multivariate Cox regression analyses.7, 8, 9, 10 To the best of our knowledge, this study is the first to assess the utility of the SIRS criteria in predicting bacteremia in a cirrhotic population.
As such, our study has important implications for both the prompt identification of bacteremia and the downstream effects of a potentially unnecessary infectious workup. Because many EMR systems have automated alert systems to notify users when patients meet SIRS criteria, it prompts to encourage the workup and empiric treatment for BSI that may be inappropriate for the patient with advanced cirrhosis. Following such direction can be expected to produce increased length of hospital stay, increased antibiotic exposure (which in turn increases the risk for Clostridium difficile infection), and increased healthcare-associated costs. For cirrhotic patients awaiting liver transplantation, meeting SIRS criteria can also inactivate them from the transplant list pending results of the workup.11 And lastly, for those who are, in fact, septic with bacteremia, our study identified a clear deficit in the diagnostic accuracy of SIRS criteria.
In summary, cirrhosis represents a distinct physiologic state where infection cannot reliably be detected with existing SIRS parameters. While our study is a single-center retrospective analysis limited by a moderate sample size, inability to time SIRS parameters to the exact time blood cultures were ordered, and a population with advanced liver disease, it adds to converging evidence that identifying SIRS is of limited utility in patients with cirrhosis. Future work is needed to create predictive models that are sensitive enough to detect infection in the setting of the immunodeficient state of cirrhosis, specific enough to differentiate infection from the systemic inflammation that is chronically present, and versatile enough to adapt to the evolving immunophenotype of progressive hepatic dysfunction.
Author Contributions
Nandakumar Mohan: Corresponding and primary author, principal investigator, data collection and analysis.
Samir Shah, Atif Nehvi, Edward Bley: Secondary authors, data collection and analysis.
Kevin Bryan Lo: Secondary attending physician, primary statistician for data analysis.
Sarah Perloff: Primary attending physician and project supervisor.
Declaration of Competing Interest
We have no conflicts of interest to disclose with this original piece of research.
References
- 1.Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20(23):7312–7324. doi: 10.3748/wjg.v20.i23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scaglione S, Kliethermes S, Cao G, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49(8):690–696. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 3.Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56(Suppl 1):S1–S12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 5.Safari S, Baratloo A, Elfil M, Negida A. Evidence based emergency medicine; part 5 receiver operating curve and area under the curve. Emerg Tehran Iran. 2016;4(2):111–113. [PMC free article] [PubMed] [Google Scholar]
- 6.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 7.Borgonovo A, Baldin C, Maggi DC, et al. Systemic inflammatory response syndrome in patients hospitalized for acute decompensation of cirrhosis. Can J Gastroenterol Hepatol. 2021;2021 doi: 10.1155/2021/5581587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brink A, Alsma J, Verdonschot RJCG, et al. Predicting mortality in patients with suspected sepsis at the emergency department; A retrospective cohort study comparing qSOFA, SIRS and National Early Warning Score. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piano S, Bartoletti M, Tonon M, et al. Assessment of Sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut. 2018;67(10):1892–1899. doi: 10.1136/gutjnl-2017-314324. [DOI] [PubMed] [Google Scholar]
- 10.Müller M, Schefold JC, Leichtle AB, et al. qSOFA score not predictive of in-hospital mortality in emergency patients with decompensated liver cirrhosis. Med Klin Intensivmed Notfallmed. 2019;114(8):724–732. doi: 10.1007/s00063-018-0477-z. [DOI] [PubMed] [Google Scholar]
- 11.Cárdenas A, Gustot T. Delisting of liver transplant candidates because of bacterial sepsis. Liver Transpl. 2015;21(7):866–867. doi: 10.1002/lt.24174. [DOI] [PubMed] [Google Scholar]