Abstract
Background
Association between cannabis use and metabolic syndrome (MetS) has been documented; yet variation by race/ethnicity is understudied. We examined cannabis use and MetS by race/ethnicity among emerging adults (18-25 years old), the age group with the highest prevalence of cannabis use.
Methods
Data from 18- to 25-year-olds who completed the National Health and Nutrition Examination Survey (2009-2018) were analyzed. Current cannabis use was defined as ≥1 day of use in the last 30 days. MetS was defined using standardized guidelines as ≥3 of the following: elevated fasting glucose, triglycerides, systolic (SBP) and/or diastolic blood pressure (DPB), waist circumference, and/or low high-density lipoprotein (HDL) cholesterol. Logistic regression was used to examine the association between current cannabis use (CCU) and MetS, adjusting for covariates.
Results
Of 3974 respondents, 48.8% were female, mean age 21.1 years (SD = 2.4), 56.7% non-Hispanic white, 20.4% Hispanic, and 14.0% non-Hispanic black (NHB). Hispanics had the highest MetS prevalence (7.9%) and lowest CCU prevalence (23.5%). NHB had highest CCU prevalence (33.4%, P < .0001) and lowest MetS prevalence (4.8%, P = .2543). CCUs had a higher mean SBP (P = .020) and Hispanics (P = .002) than never users. Conversely, NHB CCUs exhibited lower mean SBP than NHB never users (P = .008). CCUs had 42% reduced odds of MetS than never users (AOR: 0.58, 95% CI: 0.35-0.95). Among NHB, CCUs had 78% lower likelihood of having MetS than never users (AOR: 0.22, 95% CI: 0.06-0.81).
Conclusions
Cannabis use impacts MetS and blood pressure differently by race/ethnicity. Current cannabis use was associated with lower odds of MetS overall and among NHB. Further research is warranted to investigate how administration routes, dosages, and usage duration affect MetS.
Keywords: Blood pressure, BMI, Cannabis, Marijuana, Metabolic syndrome, NHANES, Race/ethnicity
Introduction
Metabolic syndrome (MetS) is a cluster of risk factors for cardiometabolic disease that includes hypertension, central obesity, insulin resistance, and atherogenic dyslipidemia.1 MetS is associated with a 2-fold higher risk of cardiovascular disease2 and 5-fold greater risk of type 2 diabetes.3 In the United States, population-based estimates suggest that the prevalence of MetS among adults ≥18 years of age has increased by 35% between 1988 and 2012, increasing from 25.3% to 34.2%.4 A pooled analysis of 34 studies from 17 countries reported prevalence of MetS in young adults ranging from 4.8% to 7.0%.5 Emerging adulthood (18-25 years) is crucial for weight control, with the highest rate of weight gain during this period and the prevalence of overweight and obesity contributing to MetS, which is estimated to be 40% among emerging adults.6 Population-based studies in the United States have documented differences in the prevalence of MetS by race/ethnicity and gender in the general population, with highest prevalence among Hispanic (35.4%), followed by non-Hispanic whites (33.4%) and blacks (32.7%). Likewise, there is a significantly higher prevalence in women compared to men (35.6% vs 30.3%).7, 8, 9, 10, 11 However, lifestyle behaviors, such as cannabis use, have not been examined thoroughly within this context, especially considering the anticipated increase in the prevalence of obesity and diabetes among emerging adults.12
Emerging adults6 have the highest prevalence of current cannabis use (22.1%) compared to adults over the age of 26 years (8.6%) in the United States.13 The prevalence of cannabis use has increased over the past decade and is not likely to decrease due to the rise in accessibility and legalization.14 Prior studies have found an association between current cannabis use and lower prevalence of MetS,15 lower glucose and insulin levels,16 and abnormal blood pressure17; however, none have examined variations in these associations by race/ethnicity among emerging adults. Cannabis contains various chemical compounds known as cannabinoids, each contributing to its diverse effects. Two main types of cannabis plants are Cannabis sativa and Cannabis indica, and their hybrids are also used. These plants produce different combinations and concentrations of cannabinoids, primarily tetrahydrocannabinol (THC) and cannabidiol (CBD).18 Additionally, synthetic cannabinoids are artificially created compounds designed to mimic the effects of natural cannabinoids, especially THC found in the cannabis plant.19,20 Prior evidence suggests that THC may influence appetite and metabolism, potentially leading to increased food intake, weight gain, and changes in insulin sensitivity.21 However, the evidence is not entirely consistent, and more research is needed to fully understand these effects. Additionally, individual responses to cannabinoids can vary due to the endocannabinoid system (ECS), and the overall impact of cannabis on metabolic health may be influenced by factors such as dosage, frequency of use, and individual characteristics.18 Therefore, stratification by race/ethnicity is essential to understand potential disparities, as MetS and cannabis use prevalence estimates both vary among these groups.
While reviews have identified the need to examine the impact of cannabis use on cardiometabolic disease risk,22,23 current estimates do not focus on emerging adults overall and stratification by race/ethnicity. Previous studies show that emerging adults have the highest prevalence of cannabis use13 and lower prevalence of MetS.15 Understanding the association between current cannabis use and other potential risk factors including cigarette smoking for MetS is critical to identify underlying mechanisms, which may be related to the energy and metabolic homeostasis–related ECS.24, 25, 26, 27 Cigarette smoking promotes the development and progression of MetS by increasing insulin resistance through elevated levels of insulin-antagonistic hormones, including cortisol, catecholamines, and growth hormones.28 This study aims to examine the association between current cannabis use and MetS in a nationally representative US sample of emerging adults by race/ethnicity, considering potential confounders such as cigarette smoking.
Methods
Data Source
This study included data from 5 continuous 2-year cycles (2009-2018) of the NHANES (National Health and Nutrition Examination Survey), a US population–based health and nutrition status assessment program29 managed by the Centers for Disease Control and Prevention. NHANES uses stratified and multilevel probability cluster sampling30 to provide a series of information on demographics, health-related behaviors, and dietary and biomedical indicators. Specific populations, such as pregnant women, low-income groups, Mexican Americans, and non-Hispanic blacks (NHB), were oversampled to ensure the statistical reliability of survey data.29 We extracted deidentified data of participants aged 18-25 years who completed the drug questionnaire and participated in the physical and medical examinations. Overall, 3974 emerging adults were included in the analyses. The survey protocols were approved by the National Center for Health Statistics (NCHS) Ethics Review Board and informed consent was obtained from participants.
Cannabis Use
Cannabis use was ascertained via self-report to the following: “Have you ever, even once, used cannabis or hashish?” (Yes/No) and “During the past 30 days, on how many days did you use cannabis or hashish?” (0-31 days). Categories were: never users (no lifetime cannabis use), current users (lifetime use and ≥1 day in the past 30 days), and past users (lifetime use but not in the past 30 days).15,31,32 Computer-assisted self interview software was used to administer the drug use questionnaire.33
Metabolic Syndrome and Individual Risk Factors
Anthropometry and phlebotomy were conducted at Mobile Examination Center (MEC).34 The presence of MetS was affirmed according to the ATP III definition,35 ie, a cluster of ≥3 of following: waist circumference exceeding 102 cm (men) or 88 cm (women), blood pressure above130/85 mmHg, fasting triglycerides (TG) level above 150 mg/dL, fasting high-density lipoprotein (HDL) cholesterol below 40 mg/dL (men) or 50 mg/dL (women), and fasting blood sugar above 100 mg/dL.
Covariates
Age and binary gender were self-reported. Cigarette smoking status was determined by response to: “Have you smoked at least 100 cigarettes in your/his/her entire life?” and “Do you now smoke cigarettes every day/some days/not at all?”. Smoking categories were: current smoker (at least 100 cigarettes in lifetime and consuming every day/some days), past smoker (at least 100 cigarettes in lifetime and not currently consuming every day), and nonsmokers (have not consumed 100 cigarettes in lifetime). The family income-to-poverty ratio was a proxy indicating socioeconomic status.36 A new race/ethnicity variable was created from NHANES's original 5-level race/ethnicity variable combining Mexican Americans and other Hispanics into a single category, resulting in four categories: non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic, and others.
Statistical Analysis
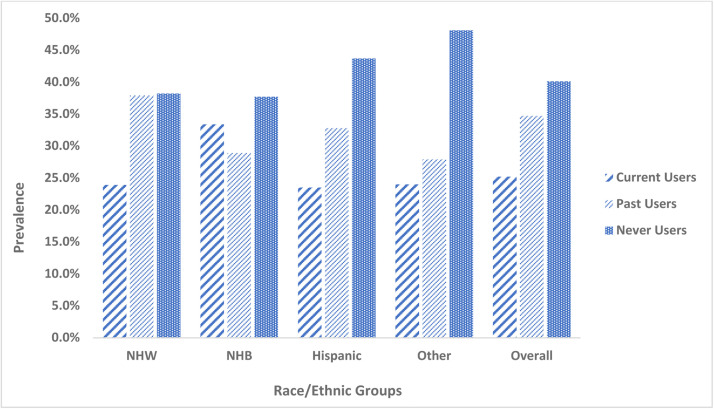
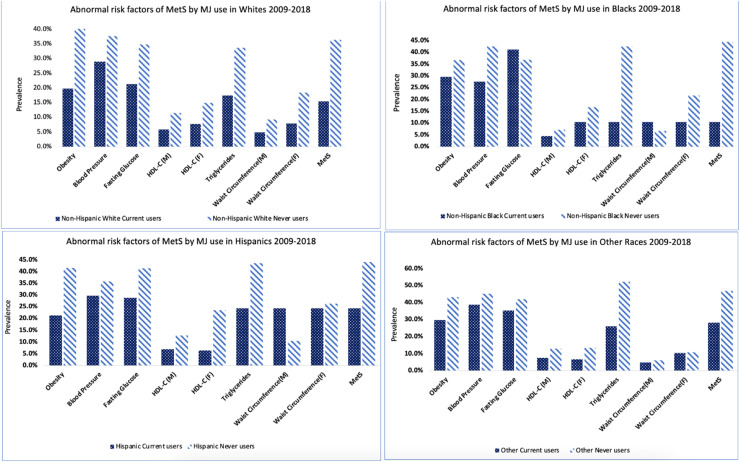
NHANES provided sampling weights for survey cycles to account for disproportionate population selection and nonresponses. Weights served as a poststratification adjustment to the US population.30 Analytic weights were constructed for combined survey cycles (2009-2018) as per NHANES instructions.37 All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). Weighted descriptive statistics was employed by cannabis use status for the overall sample and within race/ethnicity (Table 1). Prevalence of cannabis use by race/ethnicity (Figure 1) as well as presence of MetS and components/risk factors of MetS were examined by combination of race/ethnicity and cannabis use status, as depicted (Figure 2 and Table 2). ANOVA tests were conducted to compare means with never users as the reference category, followed by posthoc testing to examine significant differences. Pearson and Rao-Scott Chi-square tests were used to test the significance of proportions.
Table 1.
Characteristics of Study Sample by Race/Ethnicity and Cannabis Use, National Health and Nutrition Examination Surveys, 2009-2018 (N = 3974).
| Overall (N = 3974) |
Non-Hispanic white (N = 1241) |
Non-Hispanic black (N = 910) |
Hispanic (N = 1081) |
Other (N = 562) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never user | Current user | Past user | Never user | Current user | Past user | Never user | Current user | Past user | Never user | Current user | Past user | Never user | Current user | Past user | |
| Age, mean (SD) | |||||||||||||||
| 21.4 (0.07) | 21.5 (0.10) | 21.8 (0.01) | 21.5 (0.11) | 21.5 (0.15) | 22.0 (0.12) | 21.4 (0.16) | 21.4 (0.15) | 21.5 (0.14) | 21.3 (0.13) | 21.2 (0.17) | 21.5 (0.14) | 21.4 (0.16) | 21.7 (0.19) | 21.7 (0.24) | |
| Gender, n (%) | |||||||||||||||
| Male | 747 (36.5) | 571 (29.1) | 576 (34.4) | 229 (35.6) | 182 (27.3) | 207 (36.1) | 146 (31.4) | 171 (38.2) | 130 (30.4) | 214 (38.3) | 137 (27.4) | 170 (34.3) | 158 (46.1) | 81 (30.1) | 69 (23.8) |
| Female | 907 (43.9) | 394 (21.1) | 599 (34.9) | 229 (41.0) | 125 (20.3) | 239 (38.7) | 208 (43.8) | 128 (28.8) | 127 (27.4) | 296 (49.5) | 94 (19.3) | 170 (31.2) | 144 (50.0) | 47 (18.0) | 63 (32.0) |
| Cigarette use, n (%) | |||||||||||||||
| Never | 1265 (50.0) | 444 (17.3) | 731 (32.7) | 369 (50.3) | 105 (14.6) | 240 (35.1) | 260 (43.4) | 150 (25.5) | 176 (31.1) | 385 (50.3) | 122 (18.3) | 228 (31.4) | 251 (58.0) | 67 (16.7) | 87 (225.3) |
| Past | 33 (11.7) | 76 (32.8) | 104 (55.5) | 13 (8.8) | 57 (30.8) | 103 (60.4) | 2 (8.1) | 18 (59.1) | 10 (32.8) | 13 (19.0) | 16 (26.8) | 32 (54.2) | 5 (31.1) | 9 (49.0) | 5 (19.9) |
| Current | 81 (13.4) | 301 (48.5) | 219 (38.1) | 38 (13.9) | 125 (44.5) | 117 (41.6) | 12 (9.8) | 89 (66.6) | 34 (23.6) | 19 (15.9) | 50 (50.2) | 38 (33.9) | 12 (11.3) | 37 (48.5) | 30 (40.2) |
| Income, n (%) | |||||||||||||||
| 0-9999 | 156 (43.1) | 91 (27.6) | 97 (29.3) | 36 (41.1) | 23 (27.4) | 28 (31.5) | 47 (33.6) | 48 (39.6) | 34 (26.8) | 44 (57.7) | 10 (15.2) | 22 (27.1) | 29 (47.6) | 10 (19.6) | 13 (32.8) |
| 10,000-19,999 | 184 (35.5) | 146 (29.1) | 154 (35.4) | 58 (34.4) | 53 (28.7) | 56 (36.9) | 52 (39.3) | 46 (34.8) | 35 (25.9) | 53 (31.8) | 37 (26.5) | 57 (41.7) | 21 (48.2) | 10 (26.8) | 9 (25.0) |
| 20,000-34,999 | 333 (37.6) | 199 (27.5) | 248 (34.9) | 82 (32.6) | 67 (28.5) | 93 (38.9) | 67 (32.3) | 61 (34.2) | 59 (33.5) | 141 (49.0) | 47 (21.4) | 74 (29.6) | 43 (42.3) | 24 (26.8) | 22 (30.9) |
| 35,000-54,999 | 262 (38.8) | 174 (26.7) | 198 (34.5) | 72 (36.1) | 48 (25.1) | 77 (38.8) | 45 (35.9) | 55 (39.1) | 34 (24.8) | 94 (40.3) | 50 (23.6) | 73 (36.1) | 51 (57.2) | 21 (28.3) | 14 (14.5) |
| 55,000-74,999 | 130 (41.2) | 71 (22.1) | 96 (36.7) | 43 (41.7) | 19 (19.7) | 37 (38.6) | 31 (40.5) | 20 (29.1) | 21 (30.3) | 35 (43.5) | 20 (22.6) | 20 (33.9) | 21 (35.6) | 12 (26.9) | 18 (37.5) |
| Education, n (%) | |||||||||||||||
| 6-8 grade | 12 (46.9) | 4 (36.4) | 3 (16.6) | 0 | 2 (71.1) | 2 (28.9) | 1 (100) | 0 | 0 | 9 (70.2) | 2 (18.7) | 1 (11.1) | 2 (100) | 0 | 0 |
| 9-12 grade | 225 (51.8) | 104 (23.9) | 108 (24.3) | 57 (52.5) | 23 (25.1) | 27 (22.4) | 50 (45.3) | 37 (29.9) | 32 (24.8) | 88 (52.3) | 33 (20.4) | 42 (27.3) | 30 (59.8) | 11 (16.0) | 7 (24.2) |
| >High school | 431 (46.6) | 216 (26.0) | 231 (27.4) | 114 (46.9) | 64 (27.5) | 67 (25.6) | 101 (46.7) | 63 (30.1) | 50 (23.2) | 124 (44.7) | 56 (20.5) | 84 (34.8) | 92 (48.1) | 33 (23.8) | 30 (28.1) |
Figure 1.
Prevalence of cannabis use in the overall sample and by race/ethnicity, National Health and Nutrition Examination Surveys, 2009-2018 (N = 3974). Abbreviations: NHW (Non-Hispanic White); NHB (Non-Hispanic Black). ⁎⁎Never users-(participants with no report of lifetime cannabis use, even once); Current users (participants who used cannabis at least once in the last 30 days); Past users (participants who used cannabis before in lifetime but not in the last 30 days).
Figure 2.
Prevalence of abnormal components of metabolic syndrome by cannabis use within each race/ethnicity, National Health and Nutrition Examination Surveys, 2009-2018 (N = 3974). Abbreviations:HDL (High Density Lipoprotein). ⁎⁎Never users-(participants with no report of lifetime cannabis use, even once); Current users (participants who used cannabis at least once in the last 30 days); past users (participants who used cannabis before in lifetime but not in the last 30 days).
Table 2.
Mean Estimates of Metabolic Syndrome Risk Factors by Cannabis Use Status in the Overall Sample and Within Race/Ethnicity, National Health and Nutrition Examination Surveys, 2009-2018 (N = 3974).
| Overall (N = 3974) |
Non-Hispanic white (N = 1241) |
Non-Hispanic black (N = 910) |
Hispanic (N = 1081) |
Other (N = 562) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Never user | Current user | Past user | Never user | Current user | Past user | Never user | Current user | Past user | Never user | Current user | Past user | Never user | Current user | Past user | |
| Waist circumference (cm)* | |||||||||||||||
| Male | 91.9 (1.0) | 89.0† (1.0) | 92.3 (1.0) | 93.2 (1.4) | 89.8 (1.7) | 91.1 (1.3) | 87.8 (1.7) | 84.8 (1.2) | 91.1 (2.0) | 93.6 (1.6) | 91.0 (1.6) | 97.4 (1.4) | 86.5 (1.4) | 88.1 (1.9) | 88.9 (2.4) |
| Female | 89.5 (0.9) | 89.9 (1.1) | 88.2 (1.1) | 89.7 (1.3) | 88.6 (1.7) | 88.3 (1.6) | 91.8 (1.7) | 91.0 (2.1) | 93.0 (1.8) | 91.5 (1.2) | 91.3 (2.8) | 91.5 (1.7) | 81.2 (1.6) | 92.7† (4.0) | 85.9 (3.3) |
| HDL cholesterol (mg/dL)* | |||||||||||||||
| Male | 46.9 (0.7) | 50.2† (0.7) | 48.6 (0.8) | 46.6 (0.9) | 49.9† (0.9) | 48.9 (1.1) | 50.1 (1.3) | 53.9† (1.2) | 53.0 (1.8) | 45.7 (1.0) | 48.6† (1.3) | 45.2 (0.9) | 47.6 (1.4) | 49.0 (1.5) | 49.2 (1.6) |
| Female | 55.2 (0.5) | 55.6 (0.7) | 56.4 (0.8) | 55.0 (0.8) | 55.6 (1.4) | 56.8 (1.2) | 55.5 (1.3) | 56.4 (1.3) | 56.9 (1.5) | 53.1 (0.9) | 54.8 (1.1) | 54.6 (0.9) | 60.0 (1.8) | 54.9† (2.2) | 56.5 (2.0) |
| Body mass index (kg/m2) | |||||||||||||||
| 27.2 (0.2) | 26.4† (0.3) | 27.1 (0.3) | 27.1 (0.4) | 25.9† (0.4) | 26.4 (0.5) | 28.1 (0.4) | 26.9† (0.4) | 28.8 (0.6) | 27.8 (0.4) | 27.2 (0.6) | 28.5 (0.5) | 25.1 (0.7) | 26.7 (0.6) | 25.8 (0.9) | |
| Systolic blood pressure (mmHg) | |||||||||||||||
| 112.8 (0.3) | 114.2† (0.5) | 112.9 (0.4) | 113.0 (0.5) | 114.2 (0.7) | 113.1 (0.6) | 115.5 (0.6) | 113.4† (0.7) | 115.4 (0.8) | 111.2 (0.6) | 114.1† (0.8) | 112.0 (0.7) | 111.7 (0.7) | 115.3 (1.5) | 110.3 (1.2) | |
| Diastolic blood pressure (mmHg) | |||||||||||||||
| 65.0 (0.5) | 64.1 (0.6) | 65.3 (0.5) | 65.7 (0.6) | 64.7 (0.9) | 66.1 (0.7) | 65.7 (0.9) | 63.0† (0.9) | 63.7 (1.0) | 62.3 (0.6) | 63.5 (1.0) | 63.7 (0.8) | 66.2 (0.6) | 64.4 (1.1) | 66.0 (1.1) | |
| Fasting glucose (mg/dL) | |||||||||||||||
| 94.2 (0.6) | 95.9 (0.8) | 95.7 (0.7) | 93.5 (0.9) | 96.1 (1.3) | 96.0† (0.9) | 94.0 (1.6) | 93.2 (0.8) | 92.0 (0.8) | 95.0 (0.6) | 98.0 (1.6) | 96.3 (0.9) | 96.0 (2.2) | 96.8 (1.1) | 96.5 (2.1) | |
| Triglycerides (mg/dL) | |||||||||||||||
| 90.6 (2.4) | 91.9 (3.9) | 89.7(3.5) | 88.0 (3.0) | 89.9 (4.8) | 90.4(5.2) | 75.2(4.4) | 67.0 (3.1) | 76.8 (5.8) | 104.3 (5.3) | 115.2 (11.4) | 99.1 (4.6) | 92.4 (11.6) | 107.6 (11.3) | 72.9 (5.3) | |
HDL = high-density lipoprotein.
*Have sex-/gender-specific cut-offs.
†Statistically significant (P ≤ .05 level).
Note:Never users (participants with no report of lifetime cannabis use, even once); current users (participants who used cannabis at least once in the last 30 days); past users (participants who used cannabis before in lifetime but not in the last 30 days).
Weighted logistic regression was performed to examine the association between cannabis use and MetS. A stepwise procedure was used to control for confounders and covariates (age, gender, poverty-to-income ratio, cigarette smoking, and survey cycle). Score and Wald tests were used to test the null hypothesis. Fisher's scoring was applied as the optimization technique, and Taylor series linearization was used to estimate the variance to account for differential weighting and within-cluster correlation.30 Collinearity diagnostics was utilized to detect multicollinearity between variables. Adjusted odds ratio (AOR) and 95% confidence interval (CI) were reported based on the Clopper-Pearson CI.38 All analyses considered a P-value of < .05 statistically significant.
Results
Sample Characteristics
Of the overall sample (N = 3794; mean age 21.1 years [SD = 2.4]), 48.8% were female, 20.4% Hispanic, 14.0% NHB, 56.7% NHW, and 8.9% other. Prevalence of cannabis use stratified by race/ethnicity is illustrated in Figure 1. Overall, 25.2% were current cannabis users, 34.7% past users, and 40.1% never users. NHB had the highest prevalence of current cannabis use (33.4%) compared to ∼24% of NHW, Hispanics, and others (P < .0001).
Participant demographics are displayed by race/ethnicity and cannabis use (Table 1). Overall, 58.9% of cannabis users were male, 50.4% smoked cigarettes in addition to cannabis, and 70.0% had high school diploma or higher. Only 8.2% of never users overall were past cigarette smokers. The prevalence of cigarette smoking among cannabis users was significantly different by race/ethnicity. NHW cannabis users had the highest prevalence of cigarette use (58.4%) compared to NHB (42.4%), Hispanics (37.8%), and others (47.0%) (P < .0001).
Mean Estimates of Individual Components of Metabolic Syndrome
Means for each component of MetS and their standard errors were estimated overall and by race/ethnicity (Table 2).
Blood pressure. Overall, SBP levels were higher among current cannabis users than never users (114.2 mmHg vs 112.8 mmHg, P = .02) as well as among Hispanics (114.1 mmHg vs 111.2 mmHg, P = .002). Conversely, among NHB lower SBP was observed in current cannabis users than never users (113.4 mmHg vs 115.5 mmHg, P = .001). For DBP, a significant difference was only observed among NHB, where current cannabis users had a lower DBP than never users (63.0 mmHg vs 65.7 mmHg, P = .013).
Fasting glucose. Among NHW, there was a significant difference in fasting glucose levels between never users and past users (93.5 mg/dL vs 96.0 mg/dL, P = .0395).
HDL cholesterol. Among males, HDL levels were significantly higher in current cannabis users than never users (50.2 mg/dL vs 46.9 mg/dL, P = .0013). Similar significant differences were found between male current cannabis users and never users among NHW (49.9 mg/dL vs 46.6 mg/dL, P = .022) and NHB (53.9 mg/dL vs 50.1 mg/dL, P = .043). Likewise, male Hispanic current cannabis users also had higher levels of HDL compared to nonusers, though not statistically significant (48.6 mg/dL vs 45.7 mg/dL, P = .054).
Triglycerides. There was no statistical difference in triglyceride levels among cannabis users compared to never users in any racial/ethnic group.
Waist circumference. Among males overall, current users had significantly lower waist circumference than never users (89.0 cm vs 91.9 cm, P = .022). No statistically significant difference was found with cannabis use status within male NHW, NHB, and Hispanic populations. Conversely, in the other race/ethnicity category, female current cannabis users had greater waist circumference (92.7 cm vs 81.2 cm, P = .020) than never users.
Body mass index (BMI) (kg/m2). Overall, current cannabis users exhibited statistically significant lower BMI than never users (26.4 kg/m2 vs 27.2 kg/m2, P = .0316). Likewise, lower levels of BMI were observed within NHW (25.9 kg/m2 vs 27.1 kg/m2, P = .0270) and NHB (26.9 kg/m2 vs 28.1 kg/m2, P = .047), comparing current cannabis users to never users in respective race/ethnic categories.
Prevalence of Abnormal Metabolic Syndrome Risk Factors
Figure 2 illustrates the prevalence of MetS and its individual components in each race/ethnicity by cannabis use status. Overall, the prevalence of MetS was more than twice among never users than current users (40.1% vs 18.3%; P = .05). No statistically significant difference was found when examined across race/ethnicities. However, 15.4% of NHW current users presented with MetS compared to 36.4% of NHW never users (P = .071); 20.6% of NHB current users compared to 44.4% of NHB never users (P = .207); and 20.4% of Hispanic current users compared to 44.1% Hispanic never users (P = .804).
Odds of Metabolic Syndrome by Race/Ethnicity and Cannabis Use
In the overall sample, current use of cannabis was significantly associated with lower odds of MetS (AOR = 0.58, 95% CI: 0.35-0.95) after adjusting for confounders. Variables were tested for multicollinearity and education had a collinearity with a set of variables and a high collinearity with income (Condition Index = 155>30); therefore, education level was excluded from the model estimation and income was adjusted instead to account for participants’ socioeconomic status. Prior studies have also indicated that both income and education reflect a measure of socioeconomic status and income inequality as an important variable influencing cannabis use.39,40 After controlling for age, sex, income, smoking status, and survey cycle year, the observed association was also statistically significant within NHB (AOR = 0.22; 95% CI: 0.06-0.82). There were no significant differences in the other race/ethnic groups, nor among past cannabis users.
Discussion
To our knowledge this is the first study to examine the relationship between cannabis use with MetS and its individual components among emerging adults overall and by race/ethnicity utilizing a population-based US dataset. Our results suggest that current cannabis users have a lower prevalence of MetS compared to never users. Findings also indicate that Hispanics had the highest prevalence of MetS and the lowest prevalence of current cannabis use. Conversely, NHB had the highest prevalence of current cannabis use and a relatively lower prevalence of MetS. Furthermore, the regression analysis in the overall sample showed that current cannabis users had 42% lower odds of having MetS as compared to never users. Likewise, within NHB, this association was significant, where current cannabis users had 78% lower odds of having MetS than their never user counterparts. These findings hold clinical and public health importance by shedding light on the potential relationship between cannabis use, MetS, and race/ethnicity, thereby guiding targeted interventions and promoting equitable health strategies.
NHB and Hispanic current cannabis users had lower mean SBP as compared to their respective never users. However, only NHB current cannabis users had significantly lower mean DBP compared to NHB never users. These observed differences in BP may be influenced by a complex interplay of genetic variations, physiologic responses to cannabinoids, and lifestyle factors.41,42 Likewise, social determinants, cultural dynamics, and environmental contexts might contribute to the observed disparities by race/ethnicity. Further research is needed to elucidate the nuanced effects of cannabis on cardiovascular health within diverse populations. Specifically, NHBs had the highest prevalence of female current cannabis users than the other races/ethnicities. Male current cannabis users overall, and within NHW, and NHB categories exhibited higher levels of HDL as compared to never users in the respective racial/ethnic groups. A recent cross-sectional study of adults who used cannabis 4-7 days per week suggests that there is a differential relationship with HDL cholesterol by use status.43 Studies summarized in a review have identified synthetic cannabinoids to increase HDL due to the ECS receptor binding.43,44 Findings are mixed and warrant further research.
Among males, current cannabis users had lower waist circumference than never users. Overall, current cannabis users had lower BMI compared to never users. Similarly, within NHW and NHB, current cannabis users showed significantly lower BMI compared to never users. These findings are consistent with other research studies that have reported lower BMI and waist circumference associated with cannabis use.45,46 Furthermore, there was a smaller proportion of NHB who reported the concomitant use of cigarettes compared to NHW, while Hispanics presented with the lowest prevalence of current cigarette use; our findings from regression analysis were adjusted for cigarette use. At this time, there are no other population-based studies by race/ethnicity on MetS published to make direct comparisons to our results. A previous study16 identified a lower prevalence of MetS among current cannabis users than nonusers; however, results were not presented among emerging adults and by race/ethnicity.
Results indicating lower prevalence and association of MetS among current cannabis users may be considered within the context of the ECS, regulating energy and metabolic homeostasis.47 Cannabis has been noted to increase appetite and has been long recognized as a food intake stimulant.47,48 However, some research indicates a significant association between cannabis use and a smaller waist circumference16,49 in both males and females. The biological mechanism of the ECS could potentially explain this paradox based on the effects of THC and CBD18,50 on the ECS via the CB1 and CB2 receptors.50,51 THC and CBD have been noted to have antiinflammatory effects that can improve MetS,51, 52, 53 characterized as an inflammatory disease.54 Future studies should explore the impact of dosage, route, and form of cannabis use on the relationship between cannabis use and MetS.
Strengths and Limitations
The current study has both strengths and limitations. A notable strength is the use of nationally representative datasets. Combining 5 survey cycles augmented sample size and statistical robustness. Our focused approach on emerging adults, the age group with highest cannabis use prevalence, enhances findings relevant to this demographic, distinct from studies spanning all age groups. Next, cigarette smoking was included in the adjusted model a priori, as more than half of current cannabis users concurrently smoked or ever smoked cigarettes, which has been shown to contribute to increased risks of MetS55,56 and individual MetS components.57
Despite the strengths, there are limitations to note. A causal relationship cannot be established due to the cross-sectional NHANES design. Data on cannabis use route, formulations, and duration were lacking. THC and CBD metabolite levels in blood/urine were also unavailable. Furthermore, dietary information and other chronic conditions that may affect the relationship between cannabis use and MetS were not considered. An investigation of the dietary factors among emerging adult current cannabis users by race/ethnicity would be helpful to explore potential explanations of our results. We chose not to exclude pregnant women, despite previous studies suggesting that pregnancy may influence the metabolism of cannabis components and triglycerides. Excluding pregnant women would have limited the scope of our study (due to NHANES pregnancy variable only consisting of 20- to 44-year-olds) focusing on emerging adults (18-25 years). Self-reported cannabis use and operationalization of cannabis use categories, though based on prior research, may introduce nondifferential misclassification bias, potentially biasing results toward the null hypothesis (Table 3).
Table 3.
Crude and Adjusted* Odds Ratios of Metabolic Syndrome by Cannabis Use Category in the Overall Sample Within Race/Ethnicity among Emerging Adults, National Health and Nutrition Examination Surveys, 2009-2018 (N = 3974).
| Metabolic Syndrome Adjusted Odds Ratio (95% CI) |
|||||
|---|---|---|---|---|---|
| Overall | Non-Hispanic white | Non-Hispanic black | Hispanic | Other | |
| Past users† | |||||
| Crude Model | 1.20 (0.83, 1.67) | 1.29 (0.78, 2.15) | 0.93 (0.46, 1.86) | 0.98 (0.56, 1.71) | 1.20 (0.37, 3.90) |
| Adjusted* Model | 1.00 (0.69, 1.50) | 1.23 (0.70, 2.23) | 0.71 (0.28, 1.79) | 0.80 (0.44, 1.44) | 0.84 (0.25, 2.82) |
| Current users** | |||||
| Crude Model | 0.67 (0.43, 1.0) | 0.58 (0.28, 1.23) | 0.42(0.15, 1.12) | 0.83 (0.39, 1.80) | 1.60 (0.55, 4.65) |
| Adjusted* Model | 0.58 (0.35, 0.95) | 0.59 (0.26, 1.32) | 0.22 (0.06, 0.81) | 0.61 (0.27, 1.37) | 0.85 (0.33, 2.15) |
Reference Group = Cannabis Never Users (defined as participants with no report of lifetime cannabis use, even once).
Adjusted for age, sex/gender, poverty-to-income ratio, cigarette smoking status, and survey cycle year.
Defined as participants who used cannabis before in their lifetime but not in the last 30 days.
Defined as participants who used cannabis at least once in the last 30 days.
Conclusion
There was a differential relationship between current cannabis use and the prevalence of MetS by race/ethnicity among emerging adults. Current cannabis users had a lower prevalence of MetS, predominantly noted among NHB, the group with the highest prevalence of current cannabis use. There were also notable differences in SBP and DBP as well as HDL levels and BMI among current cannabis users in all racial/ethnic groups when compared to never users. Future prospective studies are warranted to examine the role of specific cannabinoids on MetS by race/ethnicity.
CRediT authorship contribution statement
Amrit Baral: Data curation, Formal analysis, Writing – review & editing, Supervision, Project administration, Visualization. Jingxin Liu: Formal analysis, Writing – original draft. Sandra Garcia-Davis: Writing – review & editing. Bria-Necole A. Diggs: Writing – review & editing. Lizelh Ayala: Writing – review & editing. Anurag Aka: Writing – review & editing, Visualization. Yash S. Agrawal: Writing – review & editing. Sarah E. Messiah: Conceptualization, Writing – review & editing. Denise C. Vidot: Conceptualization, Project administration, Supervision, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215–225. doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109(4):551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 3.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin. 2014;43(1):1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Moore JX, Chaudhary N, Akinyemiju T. Peer reviewed: Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, Dalleck LC. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: A pooled analysis. Prev Med Rep. 2017;7:211–215. doi: 10.1016/j.pmedr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanoye A, Brown KL, LaRose JG. The transition into young adulthood: a critical period for weight control. Curr Diab Rep. 2017;17(11):114. doi: 10.1007/s11892-017-0938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 8.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult US population, 1999-2010. J Am Coll Cardiol. 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AM, Gurka MJ, DeBoer MDJP. Trends in metabolic syndrome severity and lifestyle factors among adolescents. Pediatrics. 2016;137(3) doi: 10.1542/peds.2015-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker S, Gurka M, Oliver M, Johns D, DeBoer M. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22(2):141–148. doi: 10.1016/j.numecd.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurka MJ, Ice CL, Sun SS, DeBoer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:1–10. doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lear SA, Gasevic D. Ethnicity and metabolic syndrome: implications for assessment, management and prevention. Nutrients. 2019;12(1):15. doi: 10.3390/nu12010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Results from the 2018 National Survey on Drug Use and Health: Detailed Tables Substance Abuse and Mental Health Services Administration (SAMSHA); 2020. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2018R2/NSDUHDetailedTabsTOC2018.htm#toc.
- 14.Lapham GT, Matson TE, Bobb JF, et al. Prevalence of cannabis use disorder and reasons for use among adults in a US state where recreational cannabis use is legal. JAMA Netw Open. 2023;6(8) doi: 10.1001/jamanetworkopen.2023.28934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vidot DC, Prado G, Hlaing WM, Florez HJ, Arheart KL, SE Messiah. Metabolic syndrome among marijuana users in the United States: an analysis of national health and nutrition examination survey data. Am J Med. 2016;129(2):173–179. doi: 10.1016/j.amjmed.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med. 2013;126(7):583–589. doi: 10.1016/j.amjmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Vidot DC, Powers M, Gonzalez R, et al. Blood pressure and marijuana use: results from a decade of NHANES data. Am J Health Behav. 2019;43(5):887–897. doi: 10.5993/AJHB.43.5.2. [DOI] [PubMed] [Google Scholar]
- 18.Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012;2(6):241–254. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fordjour E, Manful CF, Sey AA, et al. Cannabis: a multifaceted plant with endless potentials. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1200269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May B, Naqi HA, Tipping M, et al. Synthetic cannabinoid receptor agonists detection using fluorescence spectral fingerprinting. Anal Chem. 2019;91(20):12971–12979. doi: 10.1021/acs.analchem.9b03037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farokhnia M, McDiarmid GR, Newmeyer MN, et al. Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: a randomized, double-blind, placebo-controlled, human laboratory study. Transl Psychiatry. 2020;10(1):71. doi: 10.1038/s41398-020-0756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franz CA, Frishman WH. Marijuana use and cardiovascular disease. Cardiol Rev. 2016;24(4):158–162. doi: 10.1097/CRD.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 23.Vidot DC, Prado G, Hlaing WM, Arheart KL, Messiah SE. Emerging issues for our nation's health: the intersection of marijuana use and cardiometabolic disease risk. J Addict Dis. 2014;33(1):1–8. doi: 10.1080/10550887.2014.882718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flores LE, Alzugaray ME, Cubilla MA, et al. Islet cannabinoid receptors: cellular distribution and biological function. Pancreas. 2013;42(7):1085–1092. doi: 10.1097/MPA.0b013e31828fd32d. [DOI] [PubMed] [Google Scholar]
- 25.SCJTAJoM Woods. The endocannabinoid system: mechanisms behind metabolic homeostasis and imbalance. The American Journal of Medicine. 2007;120(2):S9–S17. doi: 10.1016/j.amjmed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Silvestri C, V Di Marzo. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17(4):475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Batkai S. Endocannabinoids and the control of energy homeostasis. J Biol Chem. 2008;283(48):33021–33025. doi: 10.1074/jbc.R800012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balhara YPS. Tobacco and metabolic syndrome. Indian J Endocrinol Metab. 2012;16(1):81–87. doi: 10.4103/2230-8210.91197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NHANES Datasets and Related Documentation. CDC, National Center for Health Statistics 2006. https://wwwn.cdc.gov/nchs/nhanes/.
- 30.NHANES Analytic Guidelines. CDC, National Center for Health Statistics 2006.https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx.
- 31.Ong LQ, Bellettiere J, Alvarado C, Chavez P, VJHrj Berardi. Cannabis use, sedentary behavior, and physical activity in a nationally representative sample of US adults. Harm Reduct J. 2021;18(1):1–10. doi: 10.1186/s12954-021-00496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okafor CN, Li M, Paltzer JJB. Self-reported cannabis use and biomarkers of inflammation among adults in the United States. Brain Behav Immun Health. 2020;7 doi: 10.1016/j.bbih.2020.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NHANES 2013-2014 Questionnaire Data Overview. Centers for Disease Control and Prevention; 2014. https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/OverviewQuex.aspx?BeginYear=2013.
- 34.National Health and Nutrition Examination Survey . Centers for Disease Control and Prevention (CDC); 2009. Health Tech/Blood Pressure Procedures Manual.http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/BP.pdf [Google Scholar]
- 35.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.How the Census Bureau Measures Poverty. United States Census Bureau; 2018. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html.
- 37.Weighting Module . When and How to Construct Weights When Combining Survey Cycles. Centers for Disease Control and Prevention, National Center for Health Statistics; 2023. https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx [Google Scholar]
- 38.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413. [Google Scholar]
- 39.Benny C, Steele BJ, Patte KA, Leatherdale ST, Pabayo R. Income inequality and daily use of cannabis, cigarettes, and e-cigarettes among Canadian secondary school students: Results from COMPASS 2018-19. Int J Drug Policy. 2023;115 doi: 10.1016/j.drugpo.2023.104014. [DOI] [PubMed] [Google Scholar]
- 40.Jeffers AM, Glantz S, Byers A, Keyhani S. Sociodemographic characteristics associated with and prevalence and frequency of cannabis use among adults in the US. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.36571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abrahamowicz AA, Ebinger J, Whelton SP, Commodore-Mensah Y, Yang E. Racial and ethnic disparities in hypertension: barriers and opportunities to improve blood pressure control. Curr Cardiol Rep. 2023;25(1):17–27. doi: 10.1007/s11886-022-01826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitdumrongthum S, Trachootham D. An individuality of response to cannabinoids: challenges in safety and efficacy of cannabis products. Molecules. 2023;28(6):2791. doi: 10.3390/molecules28062791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cusihuaman S, Moya-Salazar J, Wong-Salgado P, et al. Changes in high-density lipoprotein (HDL), low-density lipoprotein (LDL) and cholesterol concentration in heavy cannabis users: a single-centre study in Cusco, Peru. Processes. 2022;10(8):1597. [Google Scholar]
- 44.Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9(4):413–430. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark TM, Jones JM, Hall AG, Tabner SA, Kmiec RL. Theoretical explanation for reduced body mass index and obesity rates in cannabis users. Cannabis Cannabinoid Res. 2018;3(1):259–271. doi: 10.1089/can.2018.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross JM, Pacheco-Colón I, Hawes SW, Gonzalez RJC, Research C. Bidirectional longitudinal associations between cannabis use and body mass index among adolescents. Cannabis Cannabinoid Res. 2020;5(1):81–88. doi: 10.1089/can.2019.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch S, Tam J. Cannabis: from a plant that modulates feeding behaviors toward developing selective inhibitors of the peripheral endocannabinoid system for the treatment of obesity and metabolic syndrome. Toxins. 2019;11(5):275. doi: 10.3390/toxins11050275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon V, D Cota. Mechanisms in endocrinology: endocannabinoids and metabolism: past, present and future. Toxins. 2017;176(6):R309–R324. doi: 10.1530/EJE-16-1044. [DOI] [PubMed] [Google Scholar]
- 49.Scheffler F, Kilian S, Chiliza B, et al. Effects of cannabis use on body mass, fasting glucose and lipids during the first 12 months of treatment in schizophrenia spectrum disorders. Schizophr Res. 2018;199:90–95. doi: 10.1016/j.schres.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 50.Dibba P, Li AA, Cholankeril G, Ali Khan M, Kim D, Ahmed A. Potential mechanisms influencing the inverse relationship between cannabis and nonalcoholic fatty liver disease: a commentary. Nutr Metab Insights. 2019;12 doi: 10.1177/1178638819847480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleiner D, Ditrói K. The potential use of cannabidiol in the therapy of metabolic syndrome. Orvosi Hetilap. 2012;153(13):499–504. doi: 10.1556/OH.2012.29308. [DOI] [PubMed] [Google Scholar]
- 52.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18(8):727. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mastinu A, Premoli M, Ferrari-Toninelli G, et al. Cannabinoids in health and disease: pharmacological potential in metabolic syndrome and neuroinflammation. Horm Mol Biol Clin Investg. 2018;36(2):20180013. doi: 10.1515/hmbci-2018-0013. [DOI] [PubMed] [Google Scholar]
- 55.Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS One. 2012;7(10):e47791. doi: 10.1371/journal.pone.0047791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carnethon MR, Loria CM, Hill JO, Sidney S, Savage PJ, Liu K. Risk factors for the metabolic syndrome: the Coronary Artery Risk Development in Young Adults (CARDIA) study, 1985-2001. Diabetes Care. 2004;27(11):2707–2715. doi: 10.2337/diacare.27.11.2707. [DOI] [PubMed] [Google Scholar]
- 57.Tresserra-Rimbau A, Medina-Remón A, Lamuela-Raventós RM, et al. Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br J Nutr. 2015;113(S2):S121–S130. doi: 10.1017/S0007114514003262. [DOI] [PubMed] [Google Scholar]