Abstract
The phytochemical profile of essential oils is influenced by genetic and paragenetic factors. In this research, we studied the essential oils of Lavandula angustifolia and Lavandula x intermedia cultivated in Lebanon. The latter is a cross hybrid between Lavandula angustifolia and Lavandula latifolia and is also known as lavandin and Lavandula hybrida. Specifically, the chemical composition and biological activities (antibacterial, antioxidant, anticancer, and hemolytic) of the essential oils were assessed. GC-MS results showed marked differences in the chemical compositions of the oils. For example, linalool was more abundant in L. x intermedia (44.15%) than in L. angustifolia (32%), while an opposite trend was observed for the percentages of 1,8-cineole (8.6% in L. angustifolia and 4.0% in L. x intermedia). FTIR analysis confirmed the richness of both oils in monoterpenes and sesquiterpenes. In terms of antioxidant activity, L. angustifolia essential oil demonstrated significantly better activity (IC50= 5.24 ± 1.20 mg/mL) compared to L. x intermedia oil in the DPPH radical scavenging assay. MTT cell viability assays revealed that L. angustifolia essential oil was a slightly more potent antiproliferative agent than L. x intermedia oil on human colorectal (HCT-116) and human breast (MCF-7) cancer cells. The antibacterial activity of the essential oils was tested against Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, and Serratia marcescens. Both oils showed good antibacterial activities with MIC values of 0.174 and 0.169 mg/mL for L. angustifolia and L. x intermedia oils, respectively. MBC determinations revealed that the antibacterial activity was bactericidal against all bacteria, except Staphylococcus aureus. Furthermore, both essential oils did not exhibit notable hemolytic activity on red blood cells. Overall, Lebanese L. angustifolia and L. x intermedia essential oils have promising industrial and medicinal values.
1. Introduction
Worldwide essential oil production has experienced an exponential increase over the last two decades. In fact, the global essential oils market was valued at USD 11.2 billion in 2022 and is expected to reach a value of USD 18.8 billion by 2028, growing at a compound annual growth rate of 8.7%.1 The global increase in demand for essential oils has led to a subsequent increase in the price, which has caused producers to cultivate essential oil-producing plants and engineer hybrids with distinct characteristics and notes. Increased awareness toward preventive healthcare, improved living standards, and rising demand for aromatherapy contributed to the growth of the essential oils market. Unfortunately, the essential oil industry faces major challenges related to the widespread adulteration in essential oils by synthetic adulterants that can be hazardous and cause permanent damage to humans.2 Therefore, routine chemical analysis of essential oils becomes a necessity to meet the standards set by international governing bodies.3,4
The unprecedented popularity of essential oils in the food and beverage industry, cosmetics, personal care products, aromatherapy, and pharmaceuticals, to name a few, can be attributed to their safety profile where they are classified as Generally Recognized as Safe (GRAS) by the US Food and Drug Administration (FDA).5 Essential oils are used alone or as additives in formulations. The therapeutic scheme of essential oils is attributed to its richness in hundreds of bioactive phytochemicals with a broad spectrum of biological and pharmacological activities,6 the most important of which are terpenoids (monoterpenoids and sesquiterpenoids) and phenylpropanoids.7 Aromatic components are generally obtained from the leaves, stems, flowers, bark, roots, and other parts of a plant through various methods of extraction. Essential oils have demonstrated strong antioxidant, antimicrobial, anti-inflammatory,8 antiallergic,9 anticonvulsant,10 antidepressant,11 contraceptive,12 antimutagenic,13 analgesic,14 antitumor,15 and antidiabetic properties,16 as well as a role in preventing the development of cardiovascular17 and degenerative diseases.18
Lavandula, of the Lamiaceae family, is divided into 39 species according to the shape of the leaves, corolla morphology, flower staples, and bract in the plant.19 Although lavender is native to the Mediterranean, where it is cultivated in France, Italy, Spain, and several Middle Eastern countries, it is currently grown worldwide, particularly in countries that have a climate similar to the Mediterranean region.20 In fact, the world’s leading producer of lavender oil is Bulgaria, followed by France and China. Lavender essential oil is mainly produced by glands present on the surface of the aromatic flowers and the leaves of the plant.21 The quality of the essential oil is influenced by the nature and percentage of its chemical constituents, which in turn is influenced by several factors such as the chosen species, environmental factors, cultivation and harvesting practices, and extraction methods.22,23 An oil of industrial value should have high levels of linalool and linalyl acetate (>20%) and low levels of camphor (<5%) if it is destined for perfumery and cosmetic applications. Among the essential oils of plants of the Lavandula genus, lavender oils are particularly renowned for their use in the food and beverage industry, fragrance sector, and personal care products as well as in the pharmaceutical industry. Lavender essential oils are traditionally believed to have sedative, carminative, antimicrobial, anticancer, antidepressive, and anti-inflammatory properties. In addition, it has recognized qualities in the treatment of anxiety, allergies, insomnia, hypertension, eczema, nausea, and menstrual cramps. It is also used to treat several skin conditions, such as acne, wrinkles, and psoriasis.24−29
Situated on the Eastern shore of the Mediterranean at the meeting point between the Mediterranean Basin and Fertile Crescent region, Lebanon covers a total space of 10 452 km2, most of which is mountainous, and benefits from a very rich biodiversity. Much of Lebanon’s natural wealth is made up of medicinal and aromatic plants (MAPs) with more than 300 species, a high number of which is endemic and threatened species.30,31
The important aromatic components of lavender essential oil are secondary metabolites, whose concentrations can be influenced by several genetic and paragenetic factors, environmental conditions, soil, and harvest times, thus affecting the quality of the oil and its notes. Therefore, in the present study, we analyzed the essential oils of L. angustifolia and L. x intermedia cultivated in Lebanon. The chemical composition of the oils was determined by GC-MS and fingerprinted by FTIR. The in vitro evaluation of the biological properties of both oils was addressed in terms of antibacterial, antioxidant, anticancer, and hemolytic activities. To the best of the authors’ knowledge, this is the first comparative report assessing the chemical composition and biological characteristics of Lebanese L. angustifolia and L. x intermedia.
2. Materials and Methods
2.1. Materials
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Acros Organics, and rutin was obtained from Merck. Sodium carbonate and sodium bicarbonate were purchased from Allia. Quercetin was obtained from Fluka. Gallic acid, butanol, Folin-Ciocalteu, Dulbecco′s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Sigma-Aldrich. Triton X-100 was purchased from Himedia. All chemicals were used as received from the supplier without further purification. Essential oils were obtained from a local producer who extracted the oils by hydro-distillation of lavender plants harvested during the last week of July 2021. Deionized water was used in all experiments.
2.2. Chemical Composition Analysis
2.2.1. GC-MS Analysis
The essential oils were refrigerated at 4 °C and maintained away from light sources until analysis. The GC/EI-MS analyses were performed with an Agilent 7890B gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an Agilent HP-5MS capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and Agilent 5977B single quadruple mass detector. The essential oils were diluted to 5% in HPLC-grade n-hexane before the injection. The analytical conditions were set as follows: oven temperature ramp from 60 to 240 °C at 3 °C/min, injector temperature was 220 × C, transfer line temperature was 240 × C, and the carrier gas used was helium with a flow rate of 1 mL/min. The injection volume was 1 μL, with a split ratio of 1:25. The acquisition parameters were full scan, with a scan range of 30–300 m/z, and the scan time was 1.0 s. The identification of the constituents was based on a comparison of the retention times with those of the authentic samples, comparing their linear retention indices relative to those of the series of n-hydrocarbons. Computer matching was also used against commercial (NIST 14 and ADAMS 2007) and laboratory-developed mass spectra libraries built up from pure substances and components of commercial essential oils of known composition and MS literature data.
2.2.2. FTIR Analysis
FTIR spectra were obtained on a FTIR NICOLET iS5 (Thermo Fisher Scientific) in the range 400–4000 cm–1. Samples were prepared by mixing ∼1 mg of material and 100 mg of ground anhydrous KBr, followed by pressing to form a pellet.32
2.3. Assessing the Antioxidant Activity
The antioxidant activity of the essential oils was measured using the DPPH radical scavenging assay according to the method described by Manzocco and coworkers.33 Serial dilutions of the oils were prepared from stock solutions. Two mL of oil solution was mixed with 2 mL of 60 mM DPPH methanolic solution in sealed test tubes wrapped with aluminum foil. The mixture was shaken and incubated at room temperature for 30 min. The absorbance was then measured at 517 nm on a JASCO V-670 spectrophotometer. A 1:1 mixture of methanol:water was used as a blank. The negative control was prepared by mixing 2 mL of methanol with 2 mL of DPPH. Quercetin, a known natural antioxidant, was used as a positive control. The percentage of DPPH scavenging activity, expressed as the percentage of inhibition of DPPH reduction, was calculated according to the following equation:
where Ac is the absorbance of the control, and As is the absorbance of the sample.
The percentage of inhibition was plotted against sample concentration, and the dose–response curve was used to determine the IC50 value, defined as the concentration of the oil that resulted in 50% of DPPH scavenging.
2.4. Assessing the Antibacterial Activity
The antibacterial activity of the lavender essential oils was tested against five clinically significant bacterial strains: three Gram positive bacteria (Staphylococcus aureus, Enterococcus faecalis, and Staphylococcus epidermidis), and two Gram negative bacteria (Serratia marcescens, and Escherichia coli). All of the isolates were kindly provided by the microbiology research laboratory at the Faculty of Science at Beirut Arab University. The isolates were acquired from a local hospital (Mount Lebanon hospital) and phenotypically identified as described by the Bergey’s manual of determinative bacteriology.34 No analytical profile index (API) was performed for further classification of the microorganisms.
2.4.1. Bacterial Susceptibility Test
The susceptibility of the bacterial strains to the lavender essential oils was first assessed by the plate-well diffusion assay as described by Kudi and coworkers.35 A sterile cork-borer (8 mm diameter) was used to make the wells in the agar plates. 1.5 × 108 CFU/mL inoculum, equivalent to 0.5 McFarland was prepared, and 25 μL was swabbed over the surface of Müller-Hinton agar plate. 26.2 μg of the essential oil solutions was added to each well, and the plates were incubated for 24 h at 37 °C. The diameters of the growth inhibition zones were then measured in millimeters.
2.4.2. Determination of MIC and MBC
The antibacterial efficacy of the essential oils was evaluated using the broth microdilution assay as recommended by the Clinical and Laboratory Standards Institute36 and described in previous work.37 In brief, serial dilutions of the essential oils (100 μL) were loaded into the wells of a 96 well-plate containing growth medium (95 μL) and a bacterial suspension (5 μL of 5 × 105 CFU/mL). Positive control wells (no test compound added, resulting in maximal bacterial growth) were prepared by mixing growth medium (195 μL) and a bacterial suspension (5 μL). The commercial antibiotic, levofloxacin, was used as a negative control (minimal bacterial growth) where 100 μL of serially diluted solutions of levofloxacin was added instead of the essential oil. The plates were incubated for 18 h at 37 °C. The minimum inhibition concentration (MIC), defined as the minimum concentration of the oil to visually inhibit the growth of the microorganisms, was determined. The minimum bactericidal concentration (MBC) for the essential oils was determined by subculturing the contents (25 μL) of the wells that showed no bacterial growth in the MIC assay onto the surface of new agar plates, which were then incubated at 37 °C for 24 h. The minimum concentration of the test substance (essential oil or levofloxacin) that killed all bacteria, as inferred from the absence of bacterial growth on the agar, represented the MBC. Tests were performed in triplicate, and mean values were presented. The MIC and MBC were determined for all strains.
2.5. Assessing the Antiproliferative Activity
2.5.1. Cell Culture
HCT-116 human colorectal cancer cells (ATCC, CCL-247) and MCF-7 human breast cancer cell lines (ATCC, HTB-22) were cultured in DMEM medium (Sigma) supplemented with 10% FBS and 100U of penicillin/streptomycin at 37 °C and 5% CO2 in a humidified chamber.
2.5.2. MTT Cell Viability Assay
Cells were seeded into 96-well plates at a concentration of 1 × 104 cells/well. Cells were treated with serial dilutions of the essential oils or cisplatin, which were used a positive control. Wells used as negative controls received growth media only. The plates were incubated for 48 h at 37 °C and 5% CO2 in a humidified chamber. All wells then received 10 μL of MTT reagent (Sigma), and the plates were incubated for an additional 2 h. Quantification was performed colorimetrically at 570 nm using an ELISA microplate reader (BioTek, EL800). The absorbance of the blank wells was subtracted from that of the corresponding sample well. The percentage of cell viability was determined according to the following equation, and results were normalized to the corresponding controls.38
where Ac is the absorbance of the control wells (no test substance added), As is the absorbance of the wells that received test sample, and Ab is the absorbance of the background wells (media only).
2.6. Hemolytic Activity of the Essential Oils
The hemolysis assay was performed according to the method of Henkelman et al. with some modifications.39 Blood, obtained from a healthy volunteer, was centrifuged at 2500 rpm for 5 min, and the supernatant (plasma) was removed. The erythrocytes were successively washed with 1X phosphate-buffered saline (PBS, pH 7.4) until the supernatant became clear. A 2% erythrocyte suspension in PBS was prepared and used for further analysis. Stock solutions of the essential oils were prepared in DMSO, and serial dilutions were prepared in PBS by successive dilutions of the stock solution.
The hemolytic activity was evaluated by mixing 100 μL of the 2% erythrocyte suspension with 150 μL of oil solutions. 2% Triton X-100 in PBS was used as a positive control. The tubes were gently shaken, and the reaction mixture was incubated at 37 °C for 1 h. The tubes were then centrifuged at 2500 rpm for 3 min. The absorbance of the supernatant was measured using a 96-well plate reader at 450 nm. Experiments were performed in triplicates, and the average hemolytic activity was calculated according to the following equation:
where H represents hemolytic activity, As is the absorbance of the sample, and Ac is the absorbance of the positive control.
3. Results and Discussion
3.1. Chemical Composition
3.1.1. GC-MS Analysis
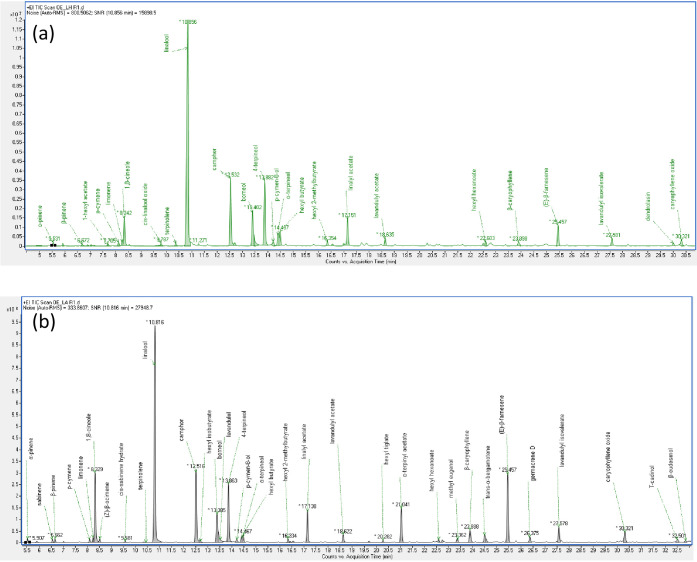
Phytochemical analysis of the essential oils of L. angustifolia and L. x intermedia grown in Lebanon by GC-MS identified 48 components, as summarized in Table 1. The corresponding total ion chromatograms (TICs) are depicted in Figure 1, showing the high resolution of the peaks and therefore the suitability of the method employed in the analysis. GC-MS results showed that the main constituents of the two essential oils under study are linalool (32.5% for L. angustifolia and 43.0% for lavandin), linalyl acetate (4.2% for L. angustifolia and 4.0% for lavandin), 1,8-cineole also known as eucalyptol (8.8% for L. angustifolia and 4.0% for lavandin), borneol (4.2% for L. angustifolia and 6.1% for lavandin), 4-terpineol (7.6% for L. angustifolia and 9.5% for lavandin), (E)-β-farnesene (8.7% for L. angustifolia and 3.6% for lavandin), and camphor (9.9% for L. angustifolia and 10.8% for lavandin). L. angustifolia contained α-terpinyl acetate with a remarkable amount of 4.4%; however, L. x intermedia oil lacked this oxygenated monoterpene. Furthermore, L. x intermedia essential oil is richer in oxygenated monoterpenes compared to L. angustifolia essential oil. Such monoterpenes include camphor and linalool. On the other hand, L. angustifolia essential oil is significantly richer in sesquiterpene hydrocarbons such as trans-α-bergamotene, (E)-β-farnesene, and germacrene D and contains slightly higher amounts of monoterpene hydrocarbons, oxygenated sesquiterpenes (e.g., caryophyllene oxide and T-cadinol), and phenylpropanoids (e.g., methyl eugenol).
Table 1. Results of the GC-MS Analysis of Lebanese L. x intermedia and L. angustifoliaa.
| no. | constituents | LRI | L. angustifolia | L.x intermedia |
|---|---|---|---|---|
| 1 | α-pinene | 941 | 0.5 | 0.4 |
| 2 | camphene | 955 | 0.1 | 0.3 |
| 3 | sabinene | 977 | 0.4 | - |
| 4 | β-pinene | 982 | 0.6 | 0.3 |
| 5 | 3-octanone | 987 | 0.2 | |
| 6 | myrcene | 992 | 0.2 | 0.1 |
| 7 | butyl butyrate | 995 | - | 0.1 |
| 8 | δ-3-carene | 1010 | - | 0.1 |
| 9 | 1-hexyl acetate | 1011 | - | 0.2 |
| 10 | p-cymene | 1028 | 0.4 | 0.5 |
| 11 | limonene | 1032 | 0.6 | 0.7 |
| 12 | 1,8-cineole | 1034 | 8.8 | 4.0 |
| 13 | (Z)-β-ocimene | 1052 | 0.7 | 0.3 |
| 14 | cis-sabinene hydrate | 1071 | 0.2 | 0.1 |
| 15 | 1-octanol | 1072 | - | 0.2 |
| 16 | cis-linalool oxide (furanoid) | 1075 | - | 0.6 |
| 17 | terpinolene | 1090 | 0.1 | - |
| 18 | trans-linalool oxide (furanoid) | 1091 | - | 0.3 |
| 19 | linalool | 1101 | 32.5 | 43.0 |
| 20 | cis-p-menth-2-en-1-ol | 1123 | - | 0.2 |
| 21 | camphor | 1146 | 9.9 | 10.8 |
| 22 | hexyl isobutyrate | 1148 | 0.6 | 0.7 |
| 23 | borneol | 1168 | 4.2 | 6.1 |
| 24 | lavandulol | 1169 | 1.6 | 1.1 |
| 25 | 4-terpineol | 1179 | 7.6 | 9.5 |
| 26 | p-cymen-8-ol | 1185 | 0.5 | 0.9 |
| 27 | (E)-2.6-dimethyl-3,7-diene2,6-diol | 1190 | - | 2.3 |
| 28 | α-terpineol | 1191 | 0.7 | - |
| 29 | hexyl butyrate | 1192 | 0.9 | 2.2 |
| 30 | hexyl 2-methylbutyrate | 1236 | 0.5 | 0.5 |
| 31 | cumin aldehyde | 1241 | 0.2 | |
| 32 | hexyl isovalerate | 1242 | 0.2 | 0.1 |
| 33 | linalyl acetate | 1259 | 4.2 | 4.0 |
| 34 | lavandulyl acetate | 1291 | 0.9 | 1.0 |
| 35 | hexyl tiglate | 1332 | 0.3 | - |
| 36 | α-terpinyl acetate | 1353 | 4.4 | - |
| 37 | geranyl acetate | 1385 | - | 0.4 |
| 38 | hexyl hexanoate | 1386 | 0.4 | 0.8 |
| 39 | methyl eugenol | 1403 | 0.6 | - |
| 40 | β-caryophyllene | 1419 | 2.0 | 0.8 |
| 41 | trans-α-bergamotene | 1437 | 0.5 | - |
| 42 | (E)-β-farnesene | 1459 | 8.7 | 3.6 |
| 43 | germacrene D | 1482 | 0.8 | - |
| 44 | lavandulyl isovalerate | 1509 | 1.9 | 1.3 |
| 45 | dendrolasin | 1575 | 0.5 | |
| 46 | caryophyllene oxide | 1582 | 1.7 | 1.4 |
| 47 | T-cadinol | 1641 | 0.5 | - |
| 48 | β-eudesmol | 1650 | 0.3 | - |
| monoterpene hydrocarbons | 3.6 | 2.7 | ||
| oxygenated monoterpenes | 77.6 | 85.6 | ||
| sesquiterpene hydrocarbons | 12.0 | 4.4 | ||
| oxygenated sesquiterpenes | 2.5 | 1.9 | ||
| phenylpropanoids | 0.6 | 0.0 | ||
| nonterpene derivatives | 2.9 | 5.0 | ||
| Total identified | 99.2 | 99.6 |
Two repetitions were performed for oil. Important phytochemicals essential for oil quality assessment are highlighted in bold black.
Figure 1.
Total ion chromatogram (TIC) of L. change x intermedia (a) and L. angustifolia(b) essential oils.
It has been reported in numerous studies that lavender oil contains more than 100 compounds, the most important of which are linalyl acetate, linalool, terpinen-4-ol, lavandulol, lavandulyl acetate, 1,8-cineole, limonene, and cis- and trans-β-ocimene.20,40 The studied lavender essential oil is characterized by a high content of linalool and linalyl acetate, a moderate amount of terpinene-4-ol, lavandulyl acetate, and lavandulol, and variable levels of 1,8-cineole and camphor. Several factors can contribute to the variations in the chemical composition of lavender oil, including plant genotype, environmental factors, cultivation practices, the part of the plant extracted, postharvest processing procedures, and extraction techniques.41−43
Therefore, it is crucial to continuously monitor the essential oil quality, in terms of the identity of phytochemicals and their percentages in the oil. The chemical composition of L. angustifolia essential oil is regulated by the European Pharmacopeia4 and other international standardization bodies, such as the International Standards Organization (ISO).3
In fact, essential oil producers do not have a clear set of criteria for assessing the quality of the oil. Although lavender oil composition is regulated by many international standards, such as those mentioned above, these international bodies rely on different acceptable limits for some components, as summarized in Table 2. For example, Ph. Eur. sets minimum limits for 3-octanone, terpinene-4-ol, lavandulyl acetate, and lavandulol as characteristic components. According to ISO 3515:2002 (other origin), no lower limits are required for these compounds.3 In addition, the ISO has a unique set of criteria for oils of different origins. While it is necessary for some components to be present in high levels in oils of a specific origin, it might be a requisite that these same constituents exist in minimal levels in oils of different origin. However, this is not an issue with the Ph. Eur. that has only one set of specifications regardless of the origin of the oil.
Table 2. Comparative requirements of Ph. Eur. (10th Edition), ISO 3515:2002 for the essential oil of L. angustifolia, and ISO 8902:2009 for L. x intermedia Grosso (French type) essential oil.28.
| requirements |
this
study |
||||
|---|---|---|---|---|---|
| component | Ph. Eur. | ISO3515:2002 (other origin)a | ISO8902:2009(L. x intermediaGrosso)a | L. angustifolia | L. x intermedia |
| limonene | ≤1% | ≤1% | 0.5–1.5% | 0.6% | 0.6% |
| 1,8-cineoleb | ≤2.5% | ≤3% | 4.0–8.0% | 8.6% | 4.0% |
| β-phellandreneb | - | ≤1% | - | - | - |
| cis-β-ocimene | - | 1–10% | 0.5–1.5% | 0.6% | 0.3% |
| trans-β-ocimene | - | 0.5–6% | Not detectable-1.0% | - | - |
| 3-octanone | 0.1–5% | ≤3% | - | - | 0.2% |
| camphor | ≤1.2% | ≤1.5% | 6–8% | 9.8% | 10.6% |
| linalool | 20–45% | 20–43% | 24–35% | 32% | 44% |
| linalyl acetate | 25–47% | 25–47% | 28–38% | 4.2% | 3.9% |
| terpinene-4-ol | 0.1–8% | ≤8% | 1.5–5.0% | 7.7% | 9.7% |
| lavandulyl acetate | min. 0.2% | ≤8% | 1.5–3.5% | 1% | 1% |
| lavandulol | min. 0.1% | ≤3% | 0.2–1.0% | 1.7% | 1.4% |
| α-terpineol | ≤2% | ≤2% | 0.3–1.3% | 0.7% | - |
ISO provides different specifications depending on origin.
1,8-cineole and β-phellandrene can be coeluted.
Many of the phytochemicals identified in the essential oils of Lebanese L. x intermedia and L. angustifolia meet the ISO 3515:2002 (for L. angustifolia),3 ISO 8902:2009 (for L. x intermedia Grosso, French type),44 and Ph. Eur.4 standards, as summarized in Table 2. For example, linalool, limonene, cis-β-ocimene, 3-octanone, terpinene-4-ol, α-terpineol, lavandulyl acetate, and lavandulol are all within the limits set by the above bodies, thus highlighting the good commercial value of Lebanese lavender oils. On the other hand, other components such as linalyl acetate, 1,8-cineole, and camphor did not satisfy the benchmark set by the standardization bodies. A key aspect of the oils evaluated in this study is the low linalyl acetate content (∼4%), which is well below the range set by ISO 3515:2002, ISO8902:2009, and Ph. Eur. This is probably a characteristic feature of these cultivars. Lavender essential oils with a low linalyl acetate content have been previously reported in the literature. For example, Lane and coworkers investigated the chemical composition of tenessential oil samples of L. angustifolia grown in British Columbia, Canada and reported that linalyl acetate levels ranged from 0 to 14%.45 Afifia and colleagues studied the chemical composition of essential oils of L. angustifolia Mill. grown in Jordan, and linalyl acetate levels were between 2.61 and 8.73%.46
A study conducted by Pljevljakušić & Drinić investigated the quality of L. x intermedia essential oil by GC-MS, and results showed that the oil contained 23.07% linalool, 14.55% 1,8-cineole, 16.29% camphor, and 5.23% borneol.47 Another study showed that the essential oil had 11.4% to 46.7% linalool and 7.4% to 44.2% linalyl acetate as principal components.28 However, due to the high content of α-terpineol (0.5–4.5%) and/or terpinene-4-ol (1.2–18.7%), the samples did not meet the Ph. Eur. and ISO 3515:2002 requirements. The oil analyzed by the authors was of lower quality than those we studied in terms of levels of key chemical components that affect the quality of lavender oils. For example, the essential oils from Lebanon contained lower amounts of camphor, which increases their qualities and medical significance since high amounts of camphor may cause skin irritation and seizures. In addition, a characteristic feature of Lebanese L. angustifolia essential oil is its richness in 1,8-cineole, a component with many biological benefits.48
3.1.2. FTIR Analysis of the Essential Oils
Classical FTIR or attenuated total reflectance (ATR)-FTIR spectroscopy are routinely used to chemically fingerprint essential oils, including lavender oils.32,49 Therefore, the characteristic functional groups of the chemical constituents that make up the Lebanese L. angustifolia and L. x intermedia essential oils were characterized by FTIR spectroscopy, and the corresponding spectra are depicted in Figure 2. The collected FTIR spectra for the oils under study were very similar. The characteristic band at 3600–3200 cm–1 was assigned to the O–H stretching, while the bands between 3080 and 3020 cm–1 and 2990–2850 cm–1 corresponded to the alkene HC= and alkane C–H stretching bands, respectively. The strong intensity of the band between 2990 and 2850 cm–1 can be attributed to the richness of both essential oils in sesquiterpenes. The distinctive small band at 3085 cm–1 is characteristic of the C–H stretching of vinyl groups (CH2=CH−) commonly encountered in monoterpenes cis-ocimene, linalool, and linalyl acetate. Additional bands that further reinforced the vinyl group are the in plane C–H bending band at 1413 cm–1 and the out-of-plane C–H bending bands at 995 and 919 cm–1. The unique band at 2730 cm–1 was attributed to the C–H stretching of an aldehyde functional group (CHO), thus strongly suggesting the presence of aldehyd-based molecules in the essential oils. The strong and sharp band at 1737 cm–1 signaled the presence of carbonyl groups (C=O) possibly of aldehydes and esters. The existence of the ester functionality is further supported by the C–O stretching band at 1167 cm–1. The presence of C=C in the essential oils was supported by the appearance of the band at 1640 cm–1 that corresponds to C=C stretching bands. In addition, the collection of bands between 1000 and 1300 cm–1 indicated the presence of primary (1052 cm–1), secondary (1111 cm–1), and tertiary (1213 cm–1) alcohols, as well as phenolic hydroxyl groups (1250 cm–1). These vibrations are consistent with the presence of linalool (tertiary alcohol), geraniol, and citronellol (primary alcohols). The bands between 700 and 1000 cm–1 resulted from the out-of-plane bending vibrations of alkenyl =C—H functionality. The presence of a long-chain linear aliphatic structure is sustained by the appearance of several low-frequency absorption bands at 1450 cm–1 (CH2 bending), 1375 cm–1 (CH3 bending), 1167 cm–1 (skeletal C–C vibrations), and 751 cm–1 (CH2 rocking). In conclusion, the collective analysis of the FTIR spectra highlighted that the essential oils under investigation are dominated by monoterpenes and sesquiterpenes. The characteristic bands observed in the FTIR spectra are consistent with the GC-MS results, thus providing further confirmation of the various molecules identified.
Figure 2.
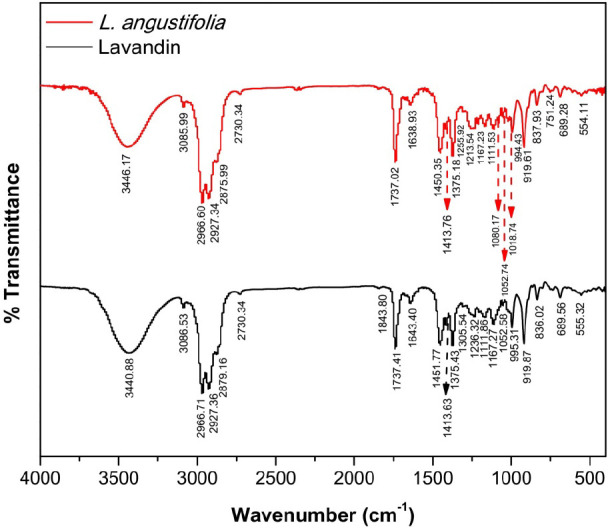
FTIR spectra of L. angustifolia and L. x intermedia (Lavandin) essential oils.
3.2. Antioxidant Activity of Lavender Essential Oils
The DPPH radical scavenging assay was used to evaluate the antioxidant potential. The dose–response curves are presented in Figure 3. L. angustifolia essential oil showed stronger antioxidant activity than L. x intermedia, with good IC50 value of 5.24 ± 1.20 mg/mL. On the other hand, the essential oil of L. x intermedia showed a weak DPPH radical scavenging activity with only 26% inhibition achieved at the highest concentration tested (20 mg/mL). At the same concentration, L. angustifolia essential oil achieved a maximum of 60% DPPH scavenging. Quercetin, a known natural antioxidant used as a positive control in this assay, showed an IC50 value of 1.60 ± 0.07 μg/mL. The notable difference in the antioxidant activity of the two oils can be attributed, at least in part, to the presence of minor constituents with hydrogen donating tendencies in L. angustifolia essential oil, such as phenylpropanoids and α-terpineol that are not present in L. x intermedia oil. These phytochemicals may contribute to the observed overall antioxidant activity by acting alone50,51 or synergistically to enhance the activity of the major chemical constituents in the complex oil.52,53
Figure 3.
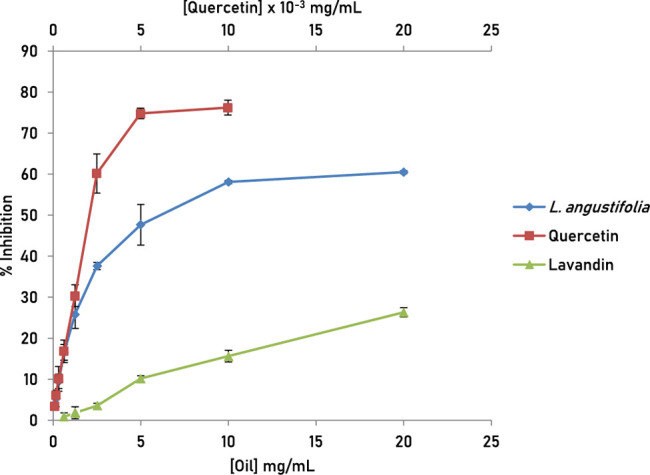
DPPH radical scavenging activity of quercetin, L. angustifolia, and L. x intermedia essential oils. Experiments were performed in triplicates and results are reported as mean ± standard deviation.
The antioxidant activity of essential oils of different lavender species has been reported in the literature by different groups. For example, Kıvrak evaluated the essential oil compositions and antioxidant activities of six L. angustifolia cultivars and two L. x intermedia in Turkey.54 Interestingly, the antioxidant activity varied depending on the different species and the type of assay conducted. In the DPPH assay, L. x intermedia Super A cultivars showed the highest inhibition activity (IC50 = 89.81 ± 0.17 μg/mL). In the ABTS.+ assay, L. angustifolia Sevtopolis cultivar displayed the highest radical scavenging activity, with inhibition value of 61.23 ± 0.11 μg/mL. Another study by Gharib and coworkers examined the antioxidant activity of L. angustifolia grown in Egypt and reported an IC50 value of 60.53 ± 0.21 μg/mL in the DPPH assay.55 Slimani and colleagues investigated the antioxidant activity of the essential oil of Moroccan L. angustifolia using a DPPH radical scavenging assay. The authors showed that the essential oil (IC50 = 1.6 ± 0.34 mg/mL) exhibited an antioxidant activity comparable to that of the natural antioxidant ascorbic acid (IC50 = 1.35 ± 0.02 mg/mL), and slightly higher than that of the aqueous extract of L. angustifolia (IC50 = 1.86 ± 0.23 mg/mL).56 Different factors affect the outcome of DPPH assay such as chemical composition of the oils, pH, solvent used, extraction method, dilution factor, duration of the assay, and activity calculation method, rendering the direct comparison between results from different studies improper.57,58 Nevertheless, the lavender essential oils investigated in this report were less potent antioxidants compared to the activities reported for essential oils from other origins.
3.3. Antibacterial Activity
The antibacterial activity of L. angustifolia and L. x intermedia essential oils was tested on five different bacteria, namely S. aureus (Gram positive), S. epidermidis(Gram positive), E. faecalis (Gram positive), S. marcescens (Gram negative) and E. coli (Gram negative). First, a preliminary study was conducted using the well diffusion assay to assess the susceptibility of the bacteria under investigation to the oils. Results were compared with those of the commercial antibiotic levofloxacin used as a negative control in the experiments. Zones of growth inhibition were measured, and the results are shown in Figure 4 and Table 3. Interestingly, all tested bacteria were susceptible to lavender essential oils as indicated by the visual zones of growth inhibition, thus highlighting the good antibacterial potential of the essential oils used against Gram positive and Gram negative bacteria.
Figure 4.
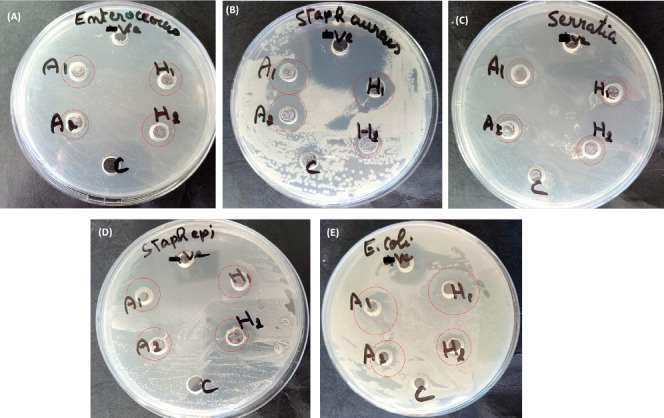
Agar well diffusion results confirming the susceptibility of the tested bacteria to L. angustifolia and L. x intermedia essential oils. (a) E. faecalis; (b) S. aureus; (c) S. marcescens; (d) S. epidermidis; (e) E. coli. C = Positive control (no test compound added); -ve = Negative control (Levofloxacin); A = L. angustifolia essential oil; H = L. x intermedia essential oil.
Table 3. Zones of Growth Inhibition, MIC, and MBC for Essential Oils and Levofloxacin against the Organisms under Testa.
| diameter
of inhibition zone (mm) |
MIC(mg/mL) |
MBC(mg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| microorganism | L. angustifolia | L.x intermedia | levofloxacin | L. angustifolia | L. x intermedia | levofloxacin | L. angustifolia | L. x intermedia | levofloxacin |
| S. aureus | 17.7 ± 0.5 | 16.1 ± 0.6 | 45.0 ± 0.2 | 0.174 | 0.169 | 0.25 | bND | bND | bND |
| S. epidermidis | 14.5 ± 0.3 | 14.5 ± 0.5 | 51.4 ± 0.4 | 0.174 | 0.169 | 0.25 | 0.174 | 0.169 | 15.62 |
| E. faecalis | 14.5 ± 0.2 | 14.0 ± 0.6 | 48.9 ± 0.8 | 0.174 | 0.169 | 0.25 | 0.174 | 0.169 | 3.19 |
| S. marcescens | 12.9 ± 0.5 | 12.9 ± 0.4 | 38.6 ± 0.6 | 0.174 | 0.169 | 0.25 | 0.174 | 0.169 | 3.19 |
| E. coli | 13.9 ± 0.4 | 15.2 ± 0.3 | 31.8 ± 0.7 | 0.174 | 0.169 | 0.25 | 0.174 | 0.169 | 15.62 |
All experiments were performed in triplicates.
ND: Not determined. The test compound did not exhibit a bactericidal effect on the microorganism.
Next, the minimum bactericidal concentration (MIC) was determined for the essential oils after overnight incubation with the bacteria, and the results are presented in Table 3. L. angustifolia and L. x intermedia essential oils exhibited comparable MIC values of 0.174 and 0.169 mg/mL, respectively, against all tested microorganisms. The lavender oils were more potent than the commercial antibiotic levofloxacin that had a MIC value of 0.25 mg/mL against all bacteria. It is important to note that the bacteria showing the largest inhibition zones by diffusion method are not always those exhibiting the lowest MIC and MBC values, due to the fact that oil solubility and volatility affect the diameter of the growth inhibition zone.59
The minimum inhibition concentration (MBC) was subsequently determined, and the results are summarized in Table 3. MBC is defined as the lowest concentration of an antibacterial agent required to kill a bacterium over a fixed, somewhat extended period, such as 18 or 24 h, under a specific set of conditions. It can be determined by broth dilution of MIC tests by subculturing on agar plates that do not contain the test agent. Valuable information on the bactericidal or bacteriostatic effect of the antibacterial agent can be obtained from the MBC values. The MBC values of the essential oils and levofloxacin against S. aureus were not determined, indicating that these substances exhibited bacteriostatic rather than bactericidal effects on the microorganism. On the other hand, bactericidal effects were observed for the test compounds against other microorganisms. The essential oils exerted bactericidal effects at lower MBC values than that of the commercial antibiotic levofloxacin. L. angustifolia and L. x intermedia essential oils had MBC values of 0.174 and 0.169 mg/mL against the microorganisms under study, respectively. Levofloxacin, on the other hand, had higher MBC values of 3.91 mg/mL against S. marcescens and E. faecalis, and 15.62 mg/mL against S. epidermidis and E. coli.
The antimicrobial activity of lavender essential oils has been well documented in the literature. This property has been attributed to the presence of several chemical constituents with proven antimicrobial activity, such as eucalyptol, linalool, terpinen-4-ol, borneol, α-pinene, β-pinene, 1,8-cineole, lavandulol, lavandulyl acetate, and α-terpineol. In particular, linalool, present in high percentages in the essential oils evaluated in this study, is the most potent antimicrobial constituent in lavender essential oils against a wide range of microorganisms. Borneol was shown to exhibit strong antiparasitic activity, while linalool, α-pinene, β-pinene, 1,8-cineol, and α-terpineol had strong antifungal activity. All of these phytochemicals are present in the Lebanese L. angustifolia and L. x intermedia essential oils investigated in this study. The levels of these chemicals, and consequently the antimicrobial potential of the oil sample, are influenced by many factors including the geographic origin of the oil.60,61 An important indicator of good antimicrobial characteristics of lavender oils is the ratio of linalool and linalyl acetate: it is desirable to be higher than 1, accompanied by high levels of these constituents. In addition, it has been reported that a good antimicrobial activity requires that the ratio of the content of the sum of linalyl acetate with linalool to the content of terpinen-4-ol in lavender essential oil is higher than 13.60 However, this ratio is close to 5 in our study. Due to their lipophilic nature, essential oils, including lavender ones, exert their antimicrobial action by binding and disrupting the cell membrane of the microorganisms, thus increasing their permeability and inducing the leakage of vital cellular components, resulting in cell death.62 Furthermore, it has been reported that lavender essential oils can inhibit the synthesis of essential biomacromolecules in the cell, such as RNA, DNA, proteins, and polysaccharides. In fungi, lavender oils have been found to work by inhibiting the production of certain enzymes.62,63
Numerous studies reported the antibacterial activity of the essential oils extracted from different lavender species. For example, Ciocarlan and coworkers evaluated the antimicrobial activity of lavender essential oils obtained from seven producers from different regions of the Republic of Moldova.64 The authors indicated that the tested essential oils showed good antibacterial and antifungal activity against Bacillus subtilis, Pseudomonas fluorescens, Xanthomonas campestris, Erwinia carotovora with an MFC/MBC value of 300 μg/mL, and Erwinia amylovora, Candida utiliz (MFC/MBC of 150 μg/mL). A study by Bajalan and coworkers investigated the chemical composition and antibacterial activity of Iranian L. x intermedia essential oil obtained by hydro-distillation.65 The in vitro antibacterial activity was evaluated by disc diffusion method against S. agalactiae, S. aureus, E. coli, and Klebsiella pneumoniae. The inhibition zones ranged between 9.36 mm for S. aureus to 23.30 mm for E. coli. Different oils from plants obtained from different collection sites display altered activity against the microorganism under study. Knowing that the obtained essential oils contained no or little linalool or linalyl acetate, the authors attributed the observed activity mainly to the presence of high levels of 1,8-cineole, (31.64 to 47.94%), borneol (17.11 to 26.14%), and camphor (8.41 to 12.68%). Gharib et al. evaluated the antibacterial activity of an Egyptian L. angustifolia essential oil by determining its MIC.55 The oil was tested on two Gram negative bacteria (Escherichia coli and Salmonella), three Gram positive bacteria (Bacillus cereus, Bacillus subtilis, and Staphylococcus aureus), and one yeast (Saccharomyces cerevisiae). The essential oil showed activity against all bacterial strains, with MIC values ranging between 10 μL/mL (Bacillus cereus) and 50 μL/mL for Salmonella. The observed activity was significantly correlated to the high concentration of linalool in the oil.
It is worth mentioning that the antibacterial activity of essential oils is not solely attributed to the phytochemicals present at high concentrations in the sample such as linalool and linalyl acetate. In fact, even chemical constituents present at low levels can contribute significantly to the observed activity, either by increasing the effect through synergy66 or by abolishing it through antagonism.67 Therefore, the antibacterial activity as well as other biological properties of essential oils should be addressed and analyzed as one entity of phytochemicals rather than as individual constituents.
3.4. Antiproliferative Activity
The antiproliferative activity of Lebanese L. angustifolia and L. x intermedia essential oils was evaluated by MTT cell viability assay against two cancer cell lines, namely, MCF-7 human breast cancer cells and HCT-116 human colorectal cancer cells. The dose–response curves are depicted in Figures 5, and IC50 values, defined as the concentration of the oil that inhibited the proliferation of 50% of cells, were extracted from the curves of Figure 5 and summarized in Table 4. While both oils displayed good antiproliferative activity, L. angustifolia essential oil was slightly more active, with IC50 values of 1.12 and 1.73 μg/mL against HCT-116 and MCF-7 cell lines, respectively. On the other hand, the essential oil of L. x intermedia exhibited IC50 values of 1.38 and 2.25 μg/mL against HCT-116 and MCF-7 cell lines, respectively. Interestingly, the tested essential oils demonstrated comparable antiproliferative activities to the commercial clinical anticancer drug cisplatin that exhibited IC50 values of 1.79 ± 0.093 and 0.90 ± 0.051 μg/mL against HCT-116 and MCF-7 cell lines, respectively.
Figure 5.
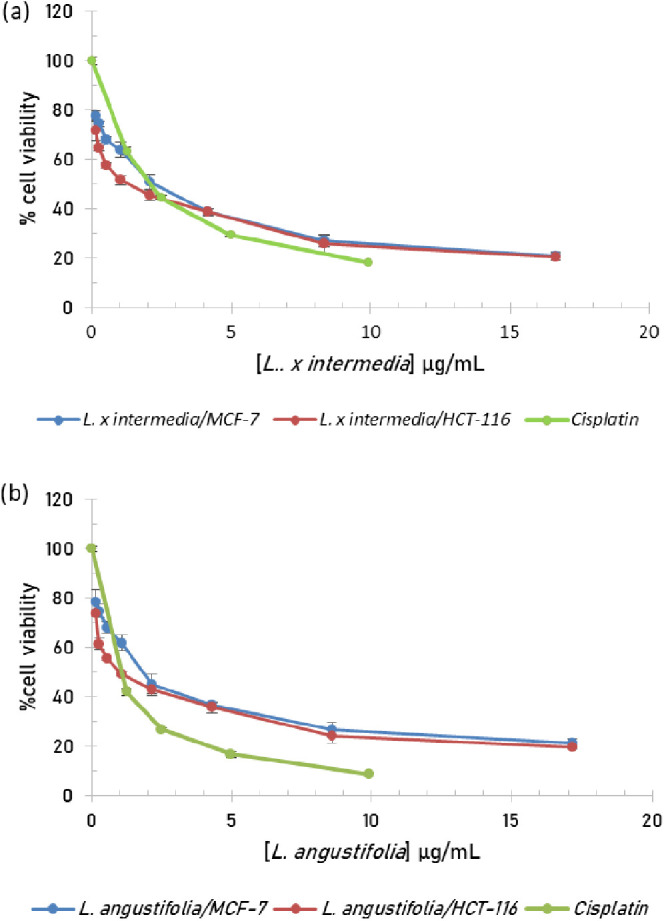
Cell viability assay of (a) L. x intermedia and (b) L. angustifolia essential oils. Experiments were performed in triplicates, and results are expressed as mean ± standard deviation.
Table 4. IC50 Values of L. angustifolia and L. x intermedia Essential Oils against HCT-116 and MCF-7 Cancer Cell Lines.
|
IC50(μg/mL) |
||
|---|---|---|
| test compound | HCT-116 | MCF-7 |
| L. angustifolia | 1.05 ± 0.076 | 1.81 ± 0.26 |
| L.x intermedia | 1.17 ± 0.16 | 2.20 ± 0.25 |
| Cisplatin | 1.79 ± 0.093 | 0.90 ± 0.051 |
The antiproliferative activity of the essential oils of the two lavender species has been described in several reports. An elegant study by Zhao and coworkers investigated the antiproliferation efficacy of L. angustifolia essential oil and its major constituents (linalool and linalyl acetate) on prostate cancer cells both in vitro and in vivo.68 Potent in vitro cytotoxicity was achieved by the essential oils, linalool, and linalyl acetate in the MTT cell viability assay. The IC50 of the essential oil treatment of DU145 and PC-3 prostate cancer cells was 0.199 ± 0.026% and 0.037 ± 0.011% (v/v), respectively. The IC50 of linalool and linalyl acetate were 7.22 ± 0.28 μM and 11.74 ± 0.62 μM, in DU145, and 3.06 ± 0.22 μM and 4.98 ± 0.31 μM, respectively, in PC-3. In addition, all tested compounds significantly inhibited the migration of DU145 and PC-3 cells in the wound healing assay in both dose- and time-dependent manners, thus highlighting the great potential of the L. angustifolia essential oil in preventing the migration, invasion, and metastasis of cancer cells and its possible use for the treatment of advanced metastatic prostate cancer. By examining the mechanism of action of the essential oil, the authors indicated that it arrested cell cycle in the G2/M phase in PC-3 cells and in the S phase in the DU145 cells and suggested different cellular targets for the natural oil in these cell lines. In addition, the essential oil increased the expression levels of two death receptors in DU145 cells, namely DR4 and DR5, which are known to increase TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. In the xenograft tumor model, lavender essential oil and linalool significantly suppressed tumor growth in vivo by inhibiting tumor cell proliferation.
In another study, Tabatabaei and coworkers studied the cytotoxic activity of Lavandula x intermedia against MCF-7 human breast cancer cells.69 The effect was dose dependent as determined by the MTT assay, with an IC50 of 800 μg/mL. Nikšić et al. examined the antiproliferative activity of L. angustifolia essential oil obtained from plants grown in Bosnia and Herzegovina against MCF-7 (breast adenocarcinoma), NCI-H460 (lung carcinoma), and MOLT-4 (acute lymphoblastic leukemia).27 The oil showed significant antiproliferative activity with IC50 values of 17, 94, and 97 μg/mL against MOLT-4, NCI-H460, and MCF-7 cancer cells, respectively. In a parallel study conducted on L. angustifolia essential oil from Turkey, Gezici proved that lavender oil exerted strong anticancer and cytotoxic activity against all treated cancer cells, namely A549 (lung carcinoma), H1299 (nonsmall cell lung cancer), and C6 (glioma) cancer cells, but not against the nontumor HUVEC (human umbilical vein endothelial cells).70 The authors claimed that the observed decrease in cell growth was mediated by the induction of apoptosis and necrosis. Tayarani-Najaran and coworkers conducted a comprehensive study to assess the antiproliferative activity of L. angustifolia essential oil from Iran against HeLa and MCF-7 cell lines.71 The authors reported strong cytotoxic and apoptotic effects on both cancer cell lines with the induction of apoptosis as a plausible mechanism of action.
The antiproliferative activity of essential oils has been attributed to their ability to induce apoptosis by activating proapoptotic mediators, arrest the cell cycle at various phases, and interfere with the normal functioning of vital cellular organelles. This is accompanied by an increase in membrane fluidity of the affected cell, a reduced adenosine triphosphate (ATP) generation, alteration in pH gradient, and a loss of mitochondrial potential, which are the major precursors of cell death.72
3.5. Hemolytic Activity
The hemolytic activity of Lebanese L. angustifolia and L. x intermedia essential oils was evaluated at different concentrations, and the results are reported in Figure 6. Interestingly, the oils did not show significant hemolytic activity. At the highest test concentration (10 mg/mL), L. angustifolia and L. x intermedia induced only 7.93% and 5.19% hemolysis of HRBC, respectively, and thus were considered safe to consume within the studied range (Figure 6).
Figure 6.
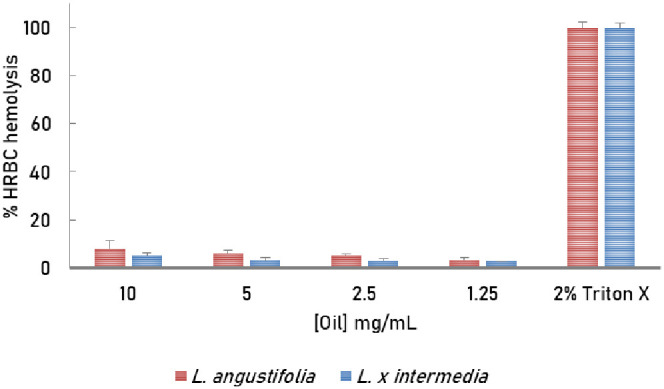
Hemolytic activity of Lebanese L. x intermedia and L. angustifolia essential oils. Experiments were performed in triplicates, and results are reported as mean ± standard deviation.
These findings are in good agreement with literature reports regarding the toxicity of lavender oils. In general, lavender oils are considered toxic if swallowed in large amounts, either accidentally or on purpose. The major toxic substances present in the oils are linalool and linalyl acetate. It has been reported by ECHA that the oral LD50 of the L. angustifolia essential oil is 6.2 ± 0.8 mL/kg bw for male rats and at 5.0 ± 0.9 mL/kg bw for female rats.73 In another study conducted by the same European agency to estimate the acute dermal toxicity of L. angustifolia essential oil, the reported LD50 was >5000 mg/kg bw in a rabbit model, where no deaths or clinical signs of toxicity occurred during the observation period. Dermal reactions noted were slight redness (9/10 rabbits), slight edema (1/10 rabbit), and moderate edema (2/10 rabbits) at the site of application. These data highlight the relatively safe therapeutic profile of lavender essential oils. In a similar recent study by Mekonnen et al. who evaluated the oral toxicity of Ethiopian L. angustifolia essential oil in mice and skin irritation in rabbit,74 the acute oral toxicity test revealed LD50 value higher than 2000 mg/kg. In a subacute toxicity test in which animals received 2000 mg/kg of the oil orally for 21 days, no death was observed in either sex of mice and no statistically significant change in the animals’ body weight, food consumption, and biochemical parameters related to liver and kidney functions. No histopathological changes were observed in the livers and kidneys of the treated and untreated groups. In addition, no toxicity was detected in the acute skin irritation test conducted on rabbits.
Despite the relatively safe toxicity profile of lavender essential oils, it is strongly recommended to analyze each oil sample individually because of its potential toxicity due to the wide variation in the chemical composition of the oils of plants of the same species but different origins. In addition, determining the cytotoxicity of the oil through assays other than the hemolysis is also critical to evaluate any adverse effects that may be accompanied by the use of this plant material.
4. Conclusion
In conclusion, lavender essential oil represents an attractive and viable candidate as a natural alternative medicine and an effective substitute to synthetic drugs, especially after the failure of many synthetic and chemical remedies currently used to challenge modern diseases and viruses, which are in continuous mutation. However, many factors may affect the quality of any essential oil, such as geographic origin, cultivation practices, extraction process, and the species of MAP utilized. Therefore, continued research and future studies regarding the chemical quality and biological efficacy of essential oils are highly recommended. All lavender species are medicinally interesting and could be professionally and abundantly cultivated in Lebanon. In fact, this study provided encouraging scientific evidence in favor of cultivating commercially valuable MAPs, such as lavender, as a support measure to the deteriorating Lebanese economy. The findings of this study will inspire us to further investigate the essential oils of lavenders cultivated in Lebanon. These investigations include, but are not limited to, assessing the anti-inflammatory and anti-Alzheimer properties of the oils, determining its mechanisms of action, and conducting in vivo experiments to validate the in vitro results.
Acknowledgments
The authors would like to thank the microbiology laboratory at Beirut Arab University for assisting in the antimicrobial assays and also the authors would like to thank the Ministry of Higher Education and Scientific Research (Laboratory LR14ES08), Tunisia, for its financial support to this research work.
Author Contributions
M.H.E.-D. contributed to conceptualization; M.B., G.F., and R.I.M. contributed to methodology; M.H.E.-D., M.B., G.F., and R.I.M. contributed to vaildation; R.I.M. contributed to formal analysis; R.I.M., H.A., and A.Z. contributed to investigation; R.I.M. contributed to writing—original draft; M.H.E.-D., M.B., and G.F. contributed to writing—review and editing; M.H.E.-D. contributed to supervision.
The authors did not receive specific funding for this study.
The authors declare no competing financial interest.
References
- Market Research Report, Essential Oils Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023–2028; Imarcgroup, 2023, Report ID: SR112023A3434. (accessed July 15, 2023). [Google Scholar]
- Capetti F.; Marengo A.; Cagliero C.; Liberto E.; Bicchi C.; Rubiolo P.; Sgorbini B. Adulteration of essential oils: A multitask issue for quality control. Three case studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) cheel. Molecules 2021, 26, 5610. 10.3390/molecules26185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 3515:2002. Oil of Lavender (Lavandula angustifolia Mill.).; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- European Pharmacopoeia, 10th Council of Europe; European Pharmacopoeia: Strasbourg, France, 2020. [Google Scholar]
- US Food and Drug Administration, Department of Health and Human Services CFR - Code of Federal Regulations Title 21-Food and drugs. PART 182: substances generally recognized as safe. Section 182.20: essential oils, oleoresins (solvent-free), and natural extractives (including distillates) US Food and Drug Administration; 2023. Vol. 3. [Google Scholar]
- Jini D.Biological Applications of Essential Oil. In Essential Oils, Inamuddin; Wiley, 2023, pp. 361–380. [Google Scholar]
- Zuzarte M.; Salgueiro L.. Essential Oils Chemistry. In. In Bioactive Essential Oils and Cancer, de Sousa D. P. Eds.; Springer International Publishing: Cham, 2015; pp. 19–61.. [Google Scholar]
- Luo W.; Du Z.; Zheng Y.; Liang X.; Huang G.; Zhang Q.; Liu Z.; Zhang K.; Zheng X.; Lin L.; et al. Phytochemical composition and bioactivities of essential oils from six Lamiaceae species. Ind. Crops Prod. 2019, 133, 357–364. 10.1016/j.indcrop.2019.03.025. [DOI] [Google Scholar]
- Mitoshi M.; Kuriyama I.; Nakayama H.; Miyazato H.; Sugimoto K.; Kobayashi Y.; Jippo T.; Kuramochi K.; Yoshida H.; Mizushina Y. Suppression of allergic and inflammatory responses by essential oils derived from herbal plants and citrus fruits. Int. J. Mol. Med. 2014, 33, 1643–1651. 10.3892/ijmm.2014.1720. [DOI] [PubMed] [Google Scholar]
- da Fonsêca D. V.; da Silva Maia Bezerra Filho C.; Lima T. C.; de Almeida R. N.; de Sousa D. P. Anticonvulsant essential oils and their relationship with oxidative stress in epilepsy. Biomolecules 2019, 9 (12), 835. 10.3390/biom9120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca E. C. M.; Ferreira L. R.; Figueiredo P. L. B.; Maia C. D. S. F.; Setzer W. N.; Da Silva J. K. R. Antidepressant Effects of Essential Oils: A Review of the Past Decade (2012–2022) and Molecular Docking Study of Their Major Chemical Components. Int. J. Mol. Sci. 2023, 24 (11), 9244. 10.3390/ijms24119244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S.; Kang S. C. In vitro determination of the contraceptive spermicidal activity of essential oil of Trachyspermum ammi (L.) Sprague ex Turrill fruits. New Biotechnol. 2011, 28, 684–690. 10.1016/j.nbt.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Toscano-Garibay J. D.; Arriaga-Alba M.; Sánchez-Navarrete J.; Mendoza-García M.; Flores-Estrada J. J.; Moreno-Eutimio M. A.; Espinosa-Aguirre J. J.; González-Ávila M.; Ruiz-Pérez N. J. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017, 7 (1), 11479. 10.1038/s41598-017-11818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento-Neto J. F.; Do Nascimento L. G.; Felipe C. F.; De Sousa D. P. Analgesic potential of essential oils. Molecules 2016, 21, 20. 10.3390/molecules21010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M.; Grewal K.; Jandrotia R.; Batish D. R.; Singh H. P.; Kohli R. K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. 10.1016/j.biopha.2021.112514. [DOI] [PubMed] [Google Scholar]
- Tahir H. U.; Sarfraz R. A.; Ashraf A.; Adil S. Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). Int. J. Food Prop. 2016, 19, 2156–2164. 10.1080/10942912.2015.1110166. [DOI] [Google Scholar]
- Saljoughian S.; Roohinejad S.; Bekhit A. E.-D. A.; Greiner R.; Omidizadeh A.; Nikmaram N.; Mousavi Khaneghah A. The effects of food essential oils on cardiovascular diseases: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1688–1705. 10.1080/10408398.2017.1279121. [DOI] [PubMed] [Google Scholar]
- Ayaz M.; Sadiq A.; Junaid M.; Ullah F.; Subhan F.; Ahmed J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. 10.3389/fnagi.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle J.; Lis-Balchin M.. Lavender: history of usage of Lavandula species; CRC Press, 2002; pp. 49–64.. [Google Scholar]
- Giray F. H. An analysis of world lavender oil markets and lessons for Turkey. J. Essent. Oil-Bear. Plants 2018, 21, 1612–1623. 10.1080/0972060X.2019.1574612. [DOI] [Google Scholar]
- Lis-Balchin M.Lavender: the Genus Lavandula (Medicinal and Aromatic Plants - Industrial Profiles), 1st ed. ed.; Taylor & Francis: London, UK, 2002. [Google Scholar]
- Hassiotis C.; Ntana F.; Lazari D.; Poulios S.; Vlachonasios K. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Ind. Crops Prod. 2014, 62, 359–366. 10.1016/j.indcrop.2014.08.048. [DOI] [Google Scholar]
- Babu G. K.; Thakur V.; Singh B. Variability in the composition of Lavandula angustifolia extracts due to extraction methods. J. Herbs Spices Med. Plants 2016, 22, 173–182. 10.1080/10496475.2015.1136979. [DOI] [Google Scholar]
- Cardia G. F. E.; Silva-Comar F. M. D. S.; Rocha E. M. T. D.; Silva-Filho S. E.; Zagotto M.; Uchida N. S.; Amaral V. D.; Bersani-Amado C. A.; Cuman R. K. N. Pharmacological, medicinal and toxicological properties of lavender essential oil: A review. Res. Soc. Dev. 2021, 10, e23310514933 10.33448/rsd-v10i5.14933. [DOI] [Google Scholar]
- Cavanagh H.; Wilkinson J. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- López V.; Nielsen B.; Solas M.; Ramírez M. J.; Jäger A. K. Exploring pharmacological mechanisms of Lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 2017, 8, 280. 10.3389/fphar.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikšić H.; Kovač-Bešović E.; Makarević E.; Durić K.; Kusturica J.; Muratovic S. Antiproliferative, antimicrobial, and antioxidant activity of Lavandula angustifolia Mill. essential oil. J. Health Sci. 2017, 7, 35–43. 10.17532/jhsci.2017.412. [DOI] [Google Scholar]
- Pokajewicz K.; Czarniecka-Wiera M.; Krajewska A.; Maciejczyk E.; Wieczorek P. P. Lavandula × intermedia—A bastard lavender or a plant of many values? Part I. Biology and chemical composition of lavandin. Molecules 2023, a (28), 2943. 10.3390/molecules28072986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokajewicz K.; Czarniecka-Wiera M.; Krajewska A.; Maciejczyk E.; Wieczorek P. P. Lavandula x intermedia—A Bastard Lavender or a Plant of Many Values? Part II. Biological Activities and Applications of Lavandin. Molecules 2023, 28 (7), 2986. 10.3390/molecules28072986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun S.; Chalak L.; Dalleh H.; Arnold N. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J. Ethnopharmacol 2015, 173, 139–156. 10.1016/j.jep.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Deeb T.; Kino K.; Shinwari Z. K.; Kreydiyyeh S.; Baydoun E. Survey of medicinal plants currently used by herbalists in Lebanon. Pak. J. Bot. 2013, 45, 543–555. [Google Scholar]
- Agatonovic-Kustrin S.; Ristivojevic P.; Gegechkori V.; Litvinova T. M.; W. Morton D. Essential oil quality and purity evaluation via FT-IR spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10 (20), 7294. 10.3390/app10207294. [DOI] [Google Scholar]
- Manzocco L.; Anese M.; Nicoli M. C. Antioxidant Properties of Tea Extracts as Affected by Processing. LWT - Food Sci. Technol. 1998, 31, 694–698. 10.1006/fstl.1998.0491. [DOI] [Google Scholar]
- Holt J. G.; Krieg N. R.. Bergey’s Manual of Determinative Bacteriology, 9th ed.; The Williams & Wilkins Co: Baltimore, 1994. [Google Scholar]
- Kudi A. C.; Umoh J. U.; Eduvie L. O.; Gefu J. Screening of some Nigerian medicinal plants for antibacterial activity. J. Ethnopharmacol. 1999, 67, 225–228. 10.1016/S0378-8741(98)00214-1. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data 2ndClinical and Laboratory Standards Institute (CLSI)Wayne, PA, 2006, Approved guideline M39-A2 [Google Scholar]
- Fayoumi L.; Khalil M.; Ghareeb D.; Chokr A.; Bouaziz M.; El-Dakdouki M. H. Phytochemical constituents and therapeutic effects of the essential oil of rose geranium (Pelargonium hybrid) cultivated in Lebanon. S. Afr. J. Bot. 2022, 147, 894–902. 10.1016/j.sajb.2022.03.039. [DOI] [Google Scholar]
- Zeiz A.; Kawtharani R.; Elmasri M.; Khawaja G.; Hamade E.; Habib A.; Ayoub A. J.; Abarbri M.; El-Dakdouki M. H. Molecular properties prediction, anticancer and anti-inflammatory activities of some pyrimido[1,2-b]pyridazin-2-one derivatives. Bioimpacts 2024, 14, 27688–27688. 10.34172/bi.2023.27688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkelman S.; Rakhorst G.; Blanton J.; van Oeveren W. Standardization of incubation conditions for hemolysis testing of biomaterials. Mater. Sci. Eng., C 2009, 29, 1650–1654. 10.1016/j.msec.2009.01.002. [DOI] [Google Scholar]
- Nedeltcheva-Antonova D.; Gechovska K.; Bozhanov S.; Antonov L. Exploring the chemical composition of Bulgarian Lavender absolute (Lavandula angustifolia Mill.) by GC/MS and GC-FID. Plants 2022, 11, 3150. 10.3390/plants11223150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Détár E.; Németh É. Z.; Gosztola B.; Demján I.; Pluhár Z. Effects of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula x intermedia Emeric ex Loisel.). Biochem. Syst. Ecol. 2020, 90, 104020. 10.1016/j.bse.2020.104020. [DOI] [Google Scholar]
- Sałata A.; Buczkowska H.; Nurzyńska-Wierdak R. Yield, essential oil content, and quality performance of Lavandula angustifolia leaves, as affected by supplementary irrigation and drying methods. Agriculture 2020, 10, 590. 10.3390/agriculture10120590. [DOI] [Google Scholar]
- Dobros N.; Zawada K.; Paradowska K. Phytochemical profile and antioxidant activity of Lavandula angustifolia and Lavandula x intermedia cultivars extracted with different methods. Antioxidants 2022, 11, 711–726. 10.3390/antiox11040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO. Oil of lavandin Grosso (Lavandula angustifolia Mill. × Lavandula latifolia Medik.), French type; ISO, 2009. ISO 8902:2009 [Google Scholar]
- Lane W. A.; Mahmoud S. S. Composition of essential oil from Lavandula angustifolia and L. Intermedia varieties grown in British Columbia, Canada. Nat. Prod. Commun. 2008, 3, 1361–1366. 10.1177/1934578X0800300822. [DOI] [Google Scholar]
- Afifia F. F. GC-MS composition and antiproliferative activity of Lavandula angustifolia Mill. essential oils determined by hydro-distillation, SFE and SPME. Arab. J. Med. Aromat. Plants 2016, 2, 71–85. 10.48347/IMIST.PRSM/ajmap-v2i2.6691. [DOI] [Google Scholar]
- Pljevljakušić D.; Drinić Z. GC/MS chemical analysis of lavandin (Lavandula x intermedia) hydrolat: successive extraction fractions. Nat. Med. Mater. 2020, 40, 33–39. 10.5937/leksir2040033P. [DOI] [Google Scholar]
- Cai Z.-M.; Peng J.-Q.; Chen Y.; Tao L.; Zhang Y.-Y.; Fu L.-Y.; Long Q.-D.; Shen X.-C. 1,8-Cineole: a review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. 10.1080/10286020.2020.1839432. [DOI] [PubMed] [Google Scholar]
- Truzzi E.; Marchetti L.; Bertelli D.; Benvenuti S. Attenuated total reflectance–Fourier transform infrared (ATR–FTIR) spectroscopy coupled with chemometric analysis for detection and quantification of adulteration in lavender and citronella essential oils. Phytochem. Anal. 2021, 32, 907–920. 10.1002/pca.3034. [DOI] [PubMed] [Google Scholar]
- Khaleel C.; Tabanca N.; Buchbauer G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. 10.1515/chem-2018-0040. [DOI] [Google Scholar]
- Sharma U. K.; Sharma A. K.; Pandey A. K. Medicinal attributes of major phenylpropanoids present in cinnamon. BMC Complement. Altern. Med. 2016, 16 (1), 156. 10.1186/s12906-016-1147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavegowda N.; Baek K.-H. Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications. Biomolecules 2021, 11, 1267. 10.3390/biom11091267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar L. K.; Cech N. B. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. 10.1039/C9NP00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kıvrak Ş. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crops Prod. 2018, 117, 88–96. 10.1016/j.indcrop.2018.02.089. [DOI] [Google Scholar]
- Gharib F. A.; Badr S. E. A.; Al-Ghazali B. A. S.; Zahran M. K. Chemical composition, antioxidant and antibacterial activities of Lavender and Marjoram essential oils. Egypt. J. Chem. 2013, 56, 1–24. [Google Scholar]
- Slimani C.; Sqalli H.; Rais C.; Farah A.; Lazraq A.; Ghadraoui L. E. L.; Belmalha S.; Echchgadda G. Chemical composition and evaluation of biological effects of essential oil and aqueous extract of Lavandula angustifolia L. Not. Sci. Biol. 2022, 14, 11172. 10.15835/nsb14111172. [DOI] [Google Scholar]
- Bolling B. W.; Chen Y.-Y.; Kamil A. G.; Oliver Chen C.-Y. Assay dilution factors confound measures of total antioxidant capacity in polyphenol-rich juices. J. Food Sci. 2012, 77 (2), H69–H75. 10.1111/j.1750-3841.2011.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri M.; Gianotti A.; Tassoni A. Optimisation of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J. Food Compos. Anal. 2013, 30, 94–101. 10.1016/j.jfca.2013.02.004. [DOI] [Google Scholar]
- Bouhdid S.; Skali S.; Idaomar M.; Zhiri A.; Baudoux D.; Amensour M.; Abrini J. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar]
- Białoń M.; Krzyśko-Łupicka T.; Nowakowska-Bogdan E.; Wieczorek P. P. Chemical composition of two different Lavender essential oils and their effect on facial skin microbiota. Molecules 2019, 24, 3270. 10.3390/molecules24183270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danh L. T.; Han L. N.; Triet N. D. A.; Zhao J.; Mammucari R.; Foster N. Comparison of chemical composition, antioxidant and antimicrobial activity of Lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food Bioproc. Technol. 2013, 6, 3481–3489. 10.1007/s11947-012-1026-z. [DOI] [Google Scholar]
- Kalemba D.; Kunicka A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- Trytek M.; Paduch R.; Fiedurek J.; Kandefer-Szerszen M. Monoterpenes–Old compounds, new applications, and biotechnological methods of their production. Biotechnologia 2007, 76, 135–155. [Google Scholar]
- Ciocarlan A.; Lupascu L.; Aricu A.; Dragalin I.; Popescu V.; Geana E.-I.; Ionete R. E.; Vornicu N.; Duliu O. G.; Hristozova G.; et al. Chemical composition and assessment of antimicrobial activity of Lavender essential oil and some by-products. Plants 2021, 10, 1829. 10.3390/plants10091829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajalan I.; Rouzbahani R.; Pirbalouti A. G.; Maggi F. Chemical composition and antibacterial activity of Iranian Lavandula × hybrida. Chem. Biodivers. 2017, 14 (7), e1700064 10.1002/cbdv.201700064. [DOI] [PubMed] [Google Scholar]
- Valero M.; Francés E. Synergistic bactericidal effect of carvacrol, cinnamaldehyde or thymol and refrigeration to inhibit Bacillus cereus in carrot broth. Food Microbiol. 2006, 23, 68–73. 10.1016/j.fm.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Goodner K. L.; Mahattanatawee K.; Plotto A.; Sotomayor J. A.; Jordán M. J. Aromatic profiles of Thymus hyemalis and Spanish T. vulgaris essential oils by GC–MS/GC–O. Ind. Crops Prod. 2006, 24, 264–268. 10.1016/j.indcrop.2006.06.006. [DOI] [Google Scholar]
- Zhao Y.; Chen R.; Wang Y.; Qing C.; Wang W.; Yang Y. In vitro and in vivo efficacy studies of Lavender angustifolia essential oil and its active constituents on the proliferation of human prostate cancer. Integr. Cancer Ther. 2017, 16, 215–226. 10.1177/1534735416645408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabaei S. M.; Kianinodeh F.; Nasiri M.; Tightiz N.; Asadipour M.; Gohari M. In vitro inhibition of MCF-7 human breast cancer Cells by essential oils of Rosmarinus officinalis, Thymus vulgaris L., and Lavender x intermedia. Arch. Breast Cancer 2018, 5, 81–89. 10.19187/abc.20185281-89. [DOI] [Google Scholar]
- Gezici S. Promising anticancer activity of lavender (Lavandula angustifolia Mill.) essential oil through induction of both apoptosis and necrosis. Ann. Phytomed. 2018, 7, 38–45. 10.21276/ap.2018.7.2.5. [DOI] [Google Scholar]
- Tayarani-Najaran Z.; Amiri A.; Karimi G.; Emami S. A.; Asili J.; Mousavi S. H. Comparative studies of cytotoxic and apoptotic properties of different extracts and the essential oil of Lavandula angustifolia on malignant and normal Cells. Nutr. Cancer 2014, 66, 424–434. 10.1080/01635581.2013.878736. [DOI] [PubMed] [Google Scholar]
- Gautam N.; Mantha A. K.; Mittal S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed. Res. Int. 2014, 2014, 154106. 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Chemicals Agency (ECHA) Lavender, Lavandula angustifolia, ext. Endpoint summary. https://echa.europa.eu/registration-dossier/-/registered-dossier/21156/7/3/1, 1989, (accessed 18 July 2023).
- Mekonnen A.; Tesfaye S.; Christos S. G.; Dires K.; Zenebe T.; Zegeye N.; Shiferaw Y.; Lulekal E. Evaluation of skin irritation and acute and subacute oral toxicity of Lavandula angustifolia essential oils in rabbit and mice. J. Toxicol. 2019, 2019, 5979546. 10.1155/2019/5979546. [DOI] [PMC free article] [PubMed] [Google Scholar]