Abstract
Background
Natural compounds can positively impact health, and various studies suggest that they regulate glucose‒lipid metabolism by influencing short-chain fatty acids (SCFAs). This metabolism is key to maintaining energy balance and normal physiological functions in the body. This review explores how SCFAs regulate glucose and lipid metabolism and the natural compounds that can modulate these processes through SCFAs. This provides a healthier approach to treating glucose and lipid metabolism disorders in the future.
Methods
This article reviews relevant literature on SCFAs and glycolipid metabolism from PubMed and the Web of Science Core Collection (WoSCC). It also highlights a range of natural compounds, including polysaccharides, anthocyanins, quercetins, resveratrols, carotenoids, and betaines, that can regulate glycolipid metabolism through modulation of the SCFA pathway.
Results
Natural compounds enrich SCFA-producing bacteria, inhibit harmful bacteria, and regulate operational taxonomic unit (OTU) abundance and the intestinal transport rate in the gut microbiota to affect SCFA content in the intestine. However, most studies have been conducted in animals, lack clinical trials, and involve fewer natural compounds that target SCFAs. More research is needed to support the conclusions and to develop healthier interventions.
Conclusions
SCFAs are crucial for human health and are produced mainly by the gut microbiota via dietary fiber fermentation. Eating foods rich in natural compounds, including fruits, vegetables, tea, and coarse fiber foods, can hinder harmful intestinal bacterial growth and promote beneficial bacterial proliferation, thus increasing SCFA levels and regulating glucose and lipid metabolism. By investigating how these compounds impact glycolipid metabolism via the SCFA pathway, novel insights and directions for treating glucolipid metabolism disorders can be provided.
Keywords: Natural compounds, Short-chain fatty acids, Glucose metabolism, Lipid metabolism
Introduction
Glucose metabolism and lipid metabolism are important processes in the maintenance of energy homeostasis and normal physiological functions and play crucial roles in maintaining intracellular homeostasis and energy balance [1]. The molecular mechanism of glucose metabolism involves multiple steps such as glucose uptake, glycogen synthesis, glycogenolysis, glycolysis, and the tricarboxylic acid cycle [2]. During fasting, glycogenolysis is the main source of glucose released into the bloodstream [3]. Disorders of glucose metabolism are characterized mainly by hyperglycemia, dyslipidemia, fatty liver, and atherosclerosis [4]. Lipids, including triglycerides, phospholipids and steroids, are important components of the body [5]. The regulation of lipid metabolism, such as lipid uptake, synthesis and hydrolysis, is essential for the maintenance of cellular homeostasis [6]. Dysregulation of lipid metabolism in the human body can cause a variety of diseases, such as hyperlipidemia [7], osteoporosis [8], atherosclerosis [8], obesity and diabetes [9].
Natural compounds are widely distributed in plants and their derived materials [10]. Natural compounds are secondary metabolites of plants [11], and they include nutrients essential for health, such as proteins, carbohydrates, vitamins, minerals and other chemicals, such as phenolic acids, flavonoids and other phenolic substances [12]. The ability of natural compounds to prevent chronic diseases by preventing oxidative stress and inflammation, inducing autophagy, and interacting with the gut microbiota, among other signaling pathways, and their nutritional effects have been widely studied [13]. Natural compounds have been shown to regulate the body’s metabolism and reduce the risk of chronic diseases such as type 2 diabetes, atherosclerosis and cancer [14]. Recent studies have indicated that many natural compounds affect the production of short-chain fatty acids (SCFAs), thereby ameliorating disorders of glucolipid metabolism [15, 16].
SCFAs are produced by anaerobic fermentation of dietary fiber and resistant starch by the microbiota in the gut [17]. SCFAs are a type of saturated fatty acid, among which acetic, propionic and butyric acids are the most abundant SCFAs in the human body and can maintain the integrity of the intestinal barrier and influence the production of gastrointestinal mucus [18]. Studies in recent years have shown that SCFAs play important roles in human energy metabolism and energy supply [19]. As a microbial metabolite, they can maintain host health through mechanisms related to the regulation of gut barrier function, microbial activity, and glucose homeostasis [20]. Short-chain fatty acids can prevent and manage diabetes by increasing insulin sensitivity, improving glucose homeostasis and inhibiting hepatic gluconeogenesis [21]. SCFAs also inhibit the production of fat [22]. Acetate reduces lipid accumulation, inhibits the lipolysis of white adipose tissue and induces browning of white adipose tissue, which reduces body fat by increasing thermogenesis [23]. Increasing SCFA levels may be an effective therapeutic modality against dysglycolipidemia [24]. SCFAs may regulate glucose homeostasis by decreasing glucose production [25], increasing glucose uptake and glycogen synthesis in the liver [26], increasing pancreatic β-cell mass and regulating insulin secretion [27]. In regulating lipid metabolism, short-chain fatty acids may improve lipid metabolism by decreasing inflammation in adipose tissue [28], increasing the lipid buffering capacity of adipose tissue, and enhancing fatty acid oxidation and mitochondrial function in the liver and skeletal muscle [24].
In this paper, we comprehensively review the relevant roles of SCFAs in glycolipid metabolism; introduce potential natural compounds that may ameliorate the dysregulation of glycolipid metabolism by modulating the SCFA pathway, such as polysaccharides, anthocyanins, quercetin, resveratrol, carotenoids, and betaines; and briefly discuss the ways in which each of these compounds influences glycolipid metabolism through the SCFA pathway, with the goal of providing new ideas for the treatment of glycolipid metabolism dysregulation. We briefly discuss how each compound affects glycolipid metabolism through SCFAs to provide new ideas for the treatment of dysglycolipidemia.
Generation of SCFAs
SCFAs are produced by the fermentation of indigestible dietary components, including complex carbohydrates [29] and dietary fiber, by the gut microbiota. The colon is the primary site of SCFA production within the human body, as it harbors the highest density of the gut microbiota [30]. While SCFAs are derived primarily from the fermentation of microbially accessible carbohydrates (MACs), they can also be byproducts of bacterial amino acid metabolism. The relative contribution of amino acid metabolism to overall SCFA production is not well understood, but the total intake of protein and fiber are considered influential factors [31]. Owing to the physiological pH range of the colonic lumen, which typically falls between 5.6 and 6.6, the majority of SCFAs exist in their anionic form, rendering simple diffusion challenging [32]. After production, SCFAs are primarily transported from the colonic lumen into the colonic cells through passive diffusion and/or carrier-mediated transport [33]. SCFA concentrations vary along the length of the colon, with the highest levels observed in the cecum and proximal colon and a decline in concentration toward the distal colon. This gradient is likely due to the increased absorption of SCFAs mediated by the sodium-coupled monocarboxylate transporter SLC5A8 and the low-affinity H+-coupled monocarboxylate transporter SLC16A1 [34]. It may also be due to the greater availability of carbohydrates and water in the proximal portion of the colon than in the distal portion. The total amount of SCFAs in the proximal colon is estimated to be 70–140 mM, whereas the total amount of SCFAs in the distal colon decreases to 20–70 mM [35, 36].
SCFAs are carboxylic acids containing 1–6 carbon atoms, including acetic, propionic, butyric, valeric, and caproic acids, of which acetic (C2), propionic (C3), and butyric (C4) acids are the most abundant and are produced by anaerobic fermentation of dietary fiber (DF) in the intestines [35, 37]. The molar ratio of C2, C3 and C4 is approximately 60:20:20 [38]. Acetate is the major anion in human intestinal contents, accounting for more than 50-60% of short-chain fatty acids, followed by propionate and butyrate in roughly equal amounts, with small amounts of the branched-chain fatty acids isobutyric and isovaleric acids, as well as small amounts of lactic acid and succinic acid [35, 39].
The acetic acid production pathway is widely distributed in the bacterial community, whereas the propionic, butyric, and lactic acid production pathways are more conserved and substrate specific [40]. The main dietary sources of acetate are acetate-containing foods such as pickles, cheese and other dairy products; processed meats; bread; wine; beer; and bacterial breakdown of dietary fibers such as resistant starch, indigestible oligosaccharides and other plant polysaccharides. Although fructose is mainly absorbed from the small intestine, unabsorbed fructose reaches the colon, where it can be converted by the microbiota to acetic acid. Bacteria such as A. muciniphila and Bacteroides spp. produce acetic acid by digesting food fibers in the colon. The gut bacteria that produce acetic acid also include Bifidobacterium spp., Akkermansia muciniphila, Pravotella spp. and Ruminococcus spp. After dietary fiber reaches the intestinal tract, the intestinal microbiota breaks down the dietary fiber through two metabolic pathways, glycolytic fermentation or acetogenesis, to produce acetate. Most of these species of gut bacteria produce acetic acid by fermenting pyruvic acid through acetyl-CoA. In addition, acetic acid-producing bacteria can also produce acetic acid from CO2 and H2 via the Wood–Ljungdahl pathway. Acetate can also be derived from the metabolism of the oxidative breakdown of alcohol in the liver. Acetic acid may also be produced from microbial fermentation of residual peptides and fats [35, 41–45].
Propionic acid is produced mainly by Bifidobacterium spp. and mucin-degrading bacteria such as Akkermansia muciniphila [37]. There are three pathways for propionate formation in human gut bacteria: the succinate pathway, the acrylate pathway, and the propylene glycol pathway [46]. Among them, the phylum Bacteroidetes utilizes the succinate pathway to produce propionic acid via methyl propionyl coenzyme A. Negative bacteria in the Firmicutes phylum also utilize the succinate pathway to produce propionic acid. In addition, propionic acid can be formed from organic acids, such as the succinic acid in organic acids. In addition, Megasphaera elsdenii produces butyric acid when it is grown on glucose but produces propionic acid when it is grown on lactate. Some bacteria can also produce 1,2-propanediol from oligosaccharides, dihydroalcoholic phosphate or lactic acid, which is then further metabolized to propionic acid. Propionate is generated from fucose via propylene glycol in Roseburia inulinivorans from the human gut [47].
Butyric acid is produced by intestinal bacteria of the genera Faecalibacterium prausnitzii, Eubacterium rectale and Roseburia spp. [48] and by Ruminococcaceae, Lachnospiraceae, Anaerobutyricum hallii and Anaerostipes spp. [49]. Butyric acid is the preferred energy source for colonic epithelial cells and plays an important role in their metabolism and normal development [50]. Butyric acid is derived from carbohydrates through glycolysis and produced from the combination of two molecules of acetyl coenzyme A to form acetoacetyl coenzyme A, which is then progressively reduced to butyryl coenzyme A. There are two different pathways for the formation of butyric acid from butyryl coenzyme A, either by butyryl coenzyme A, acetate coenzyme transferase or phosphotransbutyrylase and butyric acid kinase [51]. Resistant starch (RS) is the main source of butyrate [52]. A study by Venkataraman et al. [53]. reported that dietary supplementation with resistant starch increases fecal butyrate concentrations. A study by Louis et al. [54]. revealed that although Faecalibacterium prausnitzii accounts for approximately 10% of human fecal bacteria, it accounted for only 4% of the butyl coenzyme A transferase sequences identified in their study, suggesting that only a few strains of Fraecalibacterium prausnitzii have the butyryl coenzyme A and acetate coenzyme transferase genes and that the majority of strains are not butyrate producers.
SCFAs and human glucose and lipid metabolism
Short-chain fatty acids constitute approximately 10% of human caloric needs [35] and play important roles in the regulation of glucose metabolism and lipid metabolism [19]. Glycometabolism refers mainly to the way blood glucose is metabolized, which is derived from intestinal absorption, hepatic glycogenolysis, and gluconeogenesis [55]. Under fed conditions, carbohydrates in food are digested and processed by various glucosidases in the digestive tract, and the resulting monosaccharides are transported to various tissues as the main fuel for ATP production [56]. The liver plays a major role in controlling glucose homeostasis by controlling various pathways of glucose metabolism, such as gluconeogenesis, glycogenolysis, glycolysis, and gluconeogenesis, with glycolysis being essential for most of the cells where glucose catabolism for energy production is crucial [57]. Glucose is phosphorylated by hexose kinase to form glucose 6-phosphate, which enters the glycolytic pathway and undergoes a series of enzyme-catalyzed reactions to produce pyruvate, along with ATP for energy or glycogen storage [19]. Whereas lipid metabolism refers mainly to the way in which lipids are metabolized, lipids are stored mainly in adipose and other tissues or in other nonadipose tissues; excessive accumulation of triacylglycerol (TAG) and cholesteryl esters (CE) leads to abnormalities in lipid metabolism [55], and inactivation of the synthesis of TAG, a major energy substrate stored in adipose tissues, leads to temporal and spatial variations in fat absorption and a reduction in postprandial triglyceridemia, postprandial changes in gut hormone levels, and resistance to diet-induced obesity in rodents [58]. In contrast, elevated levels of cholesterol in the circulation are among the major risk factors for atherosclerosis [59].
SCFAs are able to regulate glucose and lipid metabolism by acting on the G protein-coupled receptors GPR43 and GPR41 in the terminal ileum and colon [60], with propionate being the most potent agonist of GPR41 and GPR43. Acetic acid is more selective for GPR43, whereas butyric and isobutyric acids are more active for GPR41 [61]. Andrew J. Brown et al. [62]. reported that valerate (C5) activated GPR41 more effectively than did acetic acid, but acetic acid activated GPR43 more effectively than did valeric acid. GPR43 is also known as free fatty acid receptor 2 (FFAR2). Ikuo Kimura et al. [63]. reported that short-chain fatty acid-mediated activation of GPR43 could inhibit lipid accumulation and ameliorate obesity. GPR41 is also known as free fatty acid receptor 3 (FFAR3). Ikuo by Kimura et al. [64] reported that propionate was able to maintain metabolic homeostasis and body energy expenditure by directly modulating sympathetic nervous system (SNS) activity through GPR41 at the sympathetic ganglion level.
Glucoregulatory functions of SCFAs in different metabolic tissues
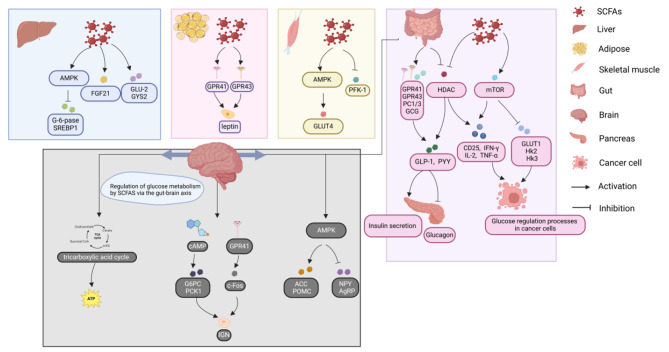
In the liver, Shoji Sakakibara et al. [65]. reported that sodium acetate directly activates AMPK by neutralizing acetic acid (AcOH), which in turn reduces the expression of genes such as glucose-6-phosphatase (G-6-pase) and sterol regulatory element-binding protein-1 (SREBP1) in rat liver cells. Research by Huating Li et al. [66] indicated that butyrate can increase fibroblast growth factor 21 (FGF21) levels in the liver, stimulating the oxidation of long-chain fatty acids and decreasing glucose levels. Short-chain fatty acids (SCFAs) are capable of increasing the mRNA expression of glucose transporter 2 (GLUT-2) and glycogen synthase 2 (GYS2) in the liver, thereby reducing hepatic glycolysis and gluconeogenesis while increasing glycogen synthesis [17, 67].
In adipose tissue, SCFAs can promote the release of adiponectin by increasing the expression of GPR41 and GPR43 [68, 69], and adiponectin can increase glucose metabolism during glycogen breakdown in the liver, skeletal muscle, and brown adipose tissue (BAT) [70]. A study by Ikuo Kimura et al. [71] revealed that GPR43 activation mediated by short-chain fatty acids could inhibit insulin signaling in adipocytes, whereas acetate could suppress glucose uptake in adipocytes and promote glucose metabolism in other tissues.
Skeletal muscle is considered the largest organ in the body and is responsible for approximately 80% of insulin-stimulated glucose uptake [72]. An increase in SCFAs can activate AMP-activated protein kinase (AMPK), leading to improved insulin sensitivity and increased glucose metabolism in skeletal muscle [73]. This phenomenon is likely attributed to the ability of AMPK to regulate the expression of GLUT4 [74], which is the predominant glucose transporter in skeletal muscle cells [19]. Glucose is transported into muscle cells through the GLUT4 transporter, thereby influencing glucose uptake [74]. Furthermore, a study by T Fushimi et al. [75] indicated that in skeletal muscle, acetate may inhibit glycolysis by suppressing the activity of phosphofructokinase-1 (PFK-1).
In the gut, SCFAs act as promoters for two crucial gut hormones, glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) [17, 76]. SCFAs can increase the secretion of plasma GLP-1 by upregulating the expression of GPR41, GPR43, PC1/3, and GCG. Subsequently, GLP-1 induces insulin secretion and inhibits glucagon secretion, effectively regulating and controlling blood glucose metabolism [77, 78]. SCFAs increase the expression of PYY in enteroendocrine cells within the gut through two distinct pathways. Acetate, propionate, and butyrate stimulate GPR43, resulting in a slight increase in PYY mRNA levels. Additionally, propionate and butyrate induce a significant increase in PYY mRNA levels by inhibiting histone deacetylases (HDACs). PYY plays a critical role in regulating food intake and insulin secretion [79].
SCFAs can also increase the sense of satiety through the gut–brain axis. Acetate can inactivate AMP-activated protein kinase (AMPK) in the hypothalamus, leading to increased activity of acetyl-CoA carboxylase (ACC). This process, in turn, stimulates the expression of proopiomelanocortin (POMC) and predominantly GABAergic neurotransmission in the hypothalamus while reducing the expression of neuropeptide Y (NPY) and agouti-related peptide (AgRP). This cascade ultimately decreases appetite and food intake, preventing weight gain and reducing the risk of type 2 diabetes mellitus (T2DM) [17, 80]. SCFAs can also control glucose and energy homeostasis through the gut–brain neural axis. Propionate activates the fatty acid receptor FFAR3 (GPR41), leading to increased c-Fos expression (a well-recognized marker of neuronal activation) and the activation of intestinal gluconeogenesis (IGN) gene expression through neural pathways, such as the dorsal vagal complex (DVC), the C1 segment of the spinal cord, the parabrachial nucleus (PBN), and the hypothalamus. Butyrate, conversely, increases the expression of cyclic adenosine monophosphate (cAMP), which induces upregulation of the glucose-6-phosphatase catalytic subunit (G6PC) and phosphoenolpyruvate carboxykinase 1 (PCK1) genes, directly activating IGN gene expression in intestinal cells [81]. Acetate can also provide fuel for the tricarboxylic acid (TCA) cycle in central nervous system (CNS) microglia. Studies have shown that germ-free (GF) mice have significantly lower acetate levels, possibly because microglia utilize acetate to produce oxaloacetic acid (OAA), activating ATP-citrate lyase (ACL)-mediated conversion of citrate to acetyl-coenzyme A (Ac-CoA) as a carbon donor in the TCA cycle, the production of citrate and the generation of ATP [82].
Glycolysis is a primary means by which normal cells obtain energy under aerobic conditions. In the presence of oxygen, normal cells generate energy through mitochondrial oxidative phosphorylation (OXPHOS). However, when oxygen is limited, cells rely on glycolysis to produce ATP. In contrast, cancer cells undergo metabolic reprogramming to prioritize glycolysis for energy production, even in the presence of sufficient oxygen [83, 84]. Histone deacetylases (HDACs) are evolutionarily conserved enzymes that remove acetyl modifications from histones, playing crucial roles in epigenetic gene silencing [85]. HDAC inhibitors can suppress c-Myc protein levels and increase the expression of proliferator-activated receptor γ coactivator 1α (PGC1α) and peroxisome proliferator-activated receptor δ (PPARδ), driving oxidative energy metabolism. This process leads to an increase in fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS) while weakening glycolysis and reducing ATP levels [86]. As HDAC enzyme inhibitors, short-chain fatty acids (SCFAs) can epigenetically regulate cellular metabolism [34]. Valerate and butyrate can increase the activity of mTOR (mechanistic target of rapamycin) and inhibit class I HDAC enzymes, leading to increased expression of CD25, interferon gamma (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-α) in cytotoxic T lymphocytes (CTLs) through the glycolytic pathway, thereby enhancing cellular antitumor immunity [87]. Additionally, mTORC1-driven glutamine uptake can suppress the expression of glycolytic genes, such as Slc2a1 (glucose transporter 1), the hexokinase isoforms Hk2 and Hk3, and glucose metabolism in cancer cells [88].
In summary, SCFAs in different metabolic tissues are able to regulate glucose metabolism by decreasing glycolysis and gluconeogenesis, increasing insulin secretion, and increasing glycogen synthesis (Fig. 1).
Fig. 1.
Glucose metabolism is affected by SCFAs
SCFAs have systemic effects on glucose metabolism to varying degrees in different tissues. The major metabolic pathways include the liver, adipocytes, skeletal muscle, intestine, pancreas and gut-brain axis, impacting the human liver through glucose transport, FGF21 and AMPK and adipocytes through GPR41 and GPR43; skeletal muscle through AMPK and PFK-1; and the human intestine via GPR41, GPR43, PC1/3 and GCG to subsequently affect the brain via the brain‒gut axis.
Lipid regulatory functions of SCFAs in different metabolic tissues
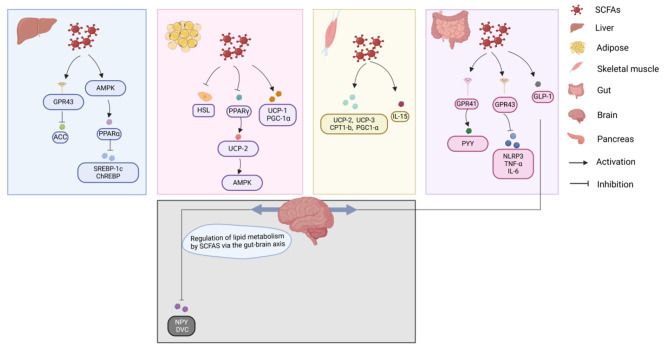
In the context of the liver, a study by Hua Zhou et al. [67] demonstrated that the exogenous provision of short-chain fatty acids (SCFAs) can increase the protein levels of GPR43 and reduce the protein levels of ACC within hepatic tissue. Concurrently, SCFA supplementation was observed to upregulate the expression of AMPK, collectively contributing to the improvement of lipid metabolism. Furthermore, there is evidence suggesting that acetate can induce the phosphorylation of AMPKα, which in turn leads to the induction of PPARα expression in hepatocytes. This cascade of events ultimately suppresses the expression of SREBP-1c and ChREBP, thereby inhibiting the mRNA expression of lipogenic genes and reducing fatty acid synthesis in the liver [89, 90].
In the context of adipose tissue, a study by Johan W E Jocken et al. [91] revealed that acetate could attenuate the phosphorylation of hormone-sensitive triglyceride lipase (HSL) in adipocytes, thereby modulating lipid metabolism in human adipose tissue. Furthermore, research conducted by Zhanguo Gao et al. [92] revealed that butyrate could increase the expression of two thermogenesis-related genes, PGC-1α and UCP-1, in brown adipose tissue (BAT). Additionally, a study by Gijs den Besten et al. [93] demonstrated that supplementation with short-chain fatty acids (SCFAs) could reduce the expression of peroxisome proliferator-activated receptor γ (PPARγ) and increase the expression of UCP-2 in adipocytes, ultimately stimulating the oxidative metabolism of adipose tissue through activation of AMPK. Importantly, existing evidence [94] suggests that mitochondrial uncoupling in adipocytes plays a crucial role in the regulation of lipid metabolism and obesity, with mitochondrial uncoupling protein 1 (UCP-1) being the most significant marker of BAT. Upregulation of UCP-1 expression can promote lipid consumption and heat generation [95]. Furthermore, mitochondrial uncoupling protein 2 (UCP-2) is expressed in various tissues, including the spleen, kidneys, immune system, pancreas, and central nervous system, and it can also contribute to the promotion of lipid metabolism [96].
UCP3 is the predominant UCP isoform in skeletal muscle [97]. Mitochondrial dysfunction in skeletal muscle may be a contributing factor to impaired lipid oxidation, which is associated with decreased expression of the UCP3 protein in muscle [98]. A study by Jian Hong et al. [99] revealed that butyrate could increase the content of muscle by increasing the expression of UCP-2, UCP-3, and fatty acid oxidation enzymes such as recombinant carnitine palmitoyltransferase 1b (CPT1-b) and peroxisome proliferator-activated receptor gamma coactivator-1α (PGC1-α) in skeletal muscle. Furthermore, SCFAs can increase the production of muscle in children by increasing the expression of interleukin-15 (IL-15), a myokine associated with muscle growth [100].
In the intestinal tract, SCFAs act on the GPR41 receptor to increase the release of the satiety hormone PYY in the colon, which has been shown to increase fasting lipid oxidation [101, 102]. Interestingly, a study by Zhuang Li et al. [103]] demonstrated that the ingestion of butyrate can stimulate the secretion of GLP-1 in the intestine, thereby activating GLP-1 receptor signaling in the vagus nerve. This process, in turn, reduces the activity of the orexigenic neuropeptide Y (NPY) in the hypothalamus and the neurons in the nucleus of the solitary tract (NTS) and the dorsal vagal complex (DVC) through the gut–brain axis, leading to decreased food intake and increased satiety, ultimately preventing obesity and fat accumulation. Additionally, Yibing Zhou et al. [104] reported that valerate can increase the concentration of GPR43 in the colon, which in turn reduces the expression of the NLRP3 inflammasome, TNF-α, and IL-6, thereby decreasing lipid deposition.
In conclusion, SCFAs in different metabolic tissues are able to regulate lipid metabolism by promoting lipid oxidation, reducing lipid synthesis, decreasing lipid deposition and increasing thermogenesis (Fig. 2).
Fig. 2.
Lipid metabolism is influenced by SCFAs
SCFAs have different systemic effects on lipid metabolism at different levels. The main metabolic pathways include the liver, adipocytes, skeletal muscle, intestine, pancreas and gut-brain axis, impacting the human liver via GPR43 and AMPK. Additionally, adipocytes are affected via HSL, PPARγ, UCP-1, UCP-2, PGC-1ɑ and AMPK; skeletal muscle is affected via UCP-2, UCP-3, CPT1-b, PGC1-α and IL-15; and the gut is affected via GPR41, GPR43, and GLP-1 to subsequently impact the brain via the brain‒gut axis.
Natural compounds that regulate glucolipid metabolism
Compounds from various plants are widely used as regulators of human glucose and lipid metabolism and play important roles in the treatment of diabetes, hyperglycemia, obesity, and other diseases [105]. Moreover, natural compounds are widely used in the development of new drugs and play key therapeutic roles in the fields of cancer, infectious diseases, cardiovascular diseases, and multiple sclerosis [106]. In this section, we assess the role of several phytochemicals in regulating human glycolipid metabolism through SCFAs. These phytochemicals include polysaccharides, anthocyanins, quercetin, resveratrol, carotenoids, and betaine. Their basic sources, roles in different metabolic tissues and effects on SCFAs are shown in Table 1.
Table 1.
Basic sources of six phytochemicals, their roles in different metabolic tissues and their effects on SCFAs
| Compound Name | Dietary Source | Role in different metabolic organizations | Effects on SCFAs |
|---|---|---|---|
| Polysaccharides | Seeds |
Gut: Altering the concentration of the gut microbiota [110–117]. Downregulation of the expression of TNF-α, and upregulation of the expression of MUC-2 and GPRs [112]. Upregulation of the expression of GLP-1 and PYY [116]. Downregulation of the expression of IL-1β, IL-6, and TNF-α [117]. |
Upregulation of the concentration of D. vulgaris, llobaculum, Lactococcus, Muribaculaceae_unclassified, Bifidobacterium, Alistipes, Rikenellaceae_RC9_gut_group, Bacteroides Ruminococcus_bromii, Anaerotruncus_colihominis, Clostridium_methylpentosum, Alistipes and Odoribacter. Downregulation of the concentration of Proteobacteria, Lactobacillus, Firmicutes_unclassified, Dubosiella Bilophila, and Streptococcus. |
| Stem and leaf tissues | |||
| Herbal plants | |||
| Animal body fluids | |||
| Cell walls | |||
| Anthocyanins | Vegetative vesicle |
Gut: Altering the concentration of the gut microbiota [123–126, 128]. Upregulation of the expression of FFAR2, FFAR3, and TJ [123]. Upregulation of the expression of TJs and NF-κB pathway protein [127]. |
Upregulation of the concentration of Ruminococcaceae, Akkermansia, Bacteroide, Odoribacter, Roseburia, Faecalibaculum, Parabacteroides, Ruminococcus, Intestinimonas, and Clostridium_XVIII. Reduction of the Firmicutes/Bacteroide ratio. |
| Quercetin | Tea, buckwheat |
Gut: Altering the concentration of the gut microbiota [133, 134]. Downregulation of the expression of TRPV1, AQP3, and iNOS. Upregulation of the expression of GDNF and c-Kit [133]. |
Upregulation of the concentration of Coprococcus, Ruminiclostridium and Roseburia. Downregulation of the concentration of Enterococcus and Enterobacter. |
| Cranberries, apples | |||
| Lettuce, radish leaves, cilantro and onions | |||
| Resveratrol | Grapes and wine |
Gut: Altering the concentration of the gut microbiota [142–145]. Downregulation of the expression of IL-6 and IL-1β [143]. Liver: Upregulation of the expression of Nrf2, SOD, and CAT [142]. |
Upregulation of the concentration of S24-7, Adlercreutzia, Faecalibaculum, and Lactobacillus. Downregulation of the concentration of Allobaculum, Blautia, Lactobacillaceae, and Prevotella. |
| Peanuts, soybeans and berries | |||
| Carotenoids | Vegetables and fruits |
Gut: Altering the concentration of the gut microbiota [154]. Liver: Downregulation of the expression of NLRP3, Pro-Caspase-1, Caspase-1, and NF-κB [154]. |
Upregulation of the concentration of Allobaculum. Downregulation of the concentration of Lachnospiraceae_NK4A136_group, Desulfovibrio, and Alistipes. |
| Betaine | Beta vulgaris |
Gut: Altering the concentration of the gut microbiota [159, 160]. Liver: Upregulation of the hepatic lipid oxidation genes PPARα and CPT1α and the hepatic lipid transporter gene FATP2 expression [160]. Adipose: Downregulation of the expression of the adipogenic genes Fas and ACC [160]. |
Upregulation of the concentration of A. muciniphila, Ruminococcus, Oscillospira, Lactobacillus, and Lactobacillus paracasei. Downregulation of the concentration of Aspergillus, Desulfovibrio, and Ruminococcus. |
| Bran, Wheat Germ, Spinach |
Polysaccharides
Polysaccharides are naturally occurring macromolecular polymers that usually consist of more than 10 monosaccharides through linear glycosidic or branched chains [107]. Polysaccharides are found in almost all living things in nature, including seeds, stem and leaf tissues, herbal plants, animal body fluids and cell walls [108]. Polysaccharides can lower blood glucose and lipids by repairing pancreatic islet cells, improving insulin resistance, regulating the intestinal flora, enhancing antioxidant capacity, and regulating the activity of key enzymes in glucose and lipid metabolism [109]. A study by Ying Hong et al. [110]. revealed that astragalus polysaccharide was able to increase the content of D. vulgaris strains in the midgut of mice fed a high-fat diet, whereas D. vulgaris strains were able to significantly increase the content of acetic acid, regulate hepatic lipid metabolism, and effectively attenuate hepatic steatosis in mice. A study by Doudou Li et al. [111]. revealed that acetic acid levels were elevated in high-fat chow-fed mice after 12 weeks of LBP supplementation, and the administration of moderate and high doses of LBP significantly lowered blood glucose and increased fasting serum insulin levels. These findings suggested that acetic acid may bind to receptors on intestinal neurons and regulate duodenal hypercontractility, thereby improving glucose homeostasis. A study by Xinyi Tian et al. [112]. revealed that polysaccharides increase the relative abundance of Allobaculum and Lactococcus, decrease the relative abundance of Proteobacteria, and increase the level of SCFAs and the expression of related G-protein-coupled receptors in the intestinal bacterial community of mice fed a high-sugar and high-fat diet. A study by Jinli Xie et al. [113]. revealed that Ganoderma lucidum polysaccharides significantly increased the levels of propionic acid and butyric acid in the small intestine and cecum of rats, thereby enhancing intestinal immunity and reducing inflammatory reactions. A study by Ying Lan et al. [114]. revealed that Seabuckthorn polysaccharides increase the proportions of Muribaculaceae_unclassified, Bifidobacterium, Alistipes, and Bacteroides in the intestines of high-fat diet-induced obese mice; and decrease the proportions of intestinal Lactobacillus, Firmicutes_unclassified, Dubosiella Bilophila, and Streptococcus, which are also able to increase the content of SCFAs in feces and regulate hepatic lipid metabolism by modulating changes in the gut microbiome and the SCFA content. A study by Liman Luo et al. [115]. revealed that inulin-type fructans could increase fecal and serum acetate concentrations, reducing mitochondrial dysfunction and toxic glucose metabolite levels. A study by Ye Yao et al. [116]. revealed that srychnine polysaccharides were able to increase the production of SCFAs by SCFA-producing bacteria, such as Ruminococcus bromii, Anaerotruncus_colihominis, and Clostridium_methylpentosum, and upregulate GLP-1 and PYY to improve glucose metabolism in rats. A study by Ciliang Guo et al. [117]. revealed that hawthorn polysaccharides increased the proportions of Alistipes and Odoribacter in the intestinal tract microbiota, increased the contents of acetic acid and propionic acid, and inhibited the expression of inflammatory cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α).
Polyphenols
Polyphenols are among the most abundant and widely distributed natural products in the plant kingdom [118]. They are phytochemicals that are synthesized from plants with one aromatic ring and one hydroxyl group and are found in large quantities in various natural plants, including fruits and vegetables. Polyphenols promote health and prevent various types of chronic diseases. They have the ability to regulate signaling pathways and exert antioxidant activities, which can regulate processes such as oxidative stress, inflammation and apoptosis [119]. According to their different chemical structures, polyphenols can be categorized into phenolic acids, flavonoids, and polyphenol amides, among which anthocyanins and quercetin are flavonoids, and resveratrol is a nonflavonoid polyphenol found in grapes and red wine [120].
Anthocyanins
Anthocyanins are glycosylanthocyanidins that are widely distributed in plant vesicles, and their color depends on the pH of the environment [121]. A study reported that [122] anthocyanins can improve obesity, control diabetes, and prevent cardiovascular disease and cancer. Baoming Tian et al. [123]. reported that anthocyanins can increase the production of SCFAs by SCFA-producing bacteria, such as Ruminococcaceae, Akkermansia, Bacteroides and Odoribacter, and attenuate HFD-induced intestinal barrier damage by activating SCFA receptors, such as FFAR2 and FFAR3, and by upregulating TJ proteins. A study by Xu Si al [124]. revealed that blueberry anthocyanins attenuated the high-fat diet-induced oxidative stress state in mice. The levels of SCFAs in the intestine are promoted by increasing the SCFA-producing bacteria Roseburia, Faecalibaculum, and Parabacteroides, and increases in of SCFAs were shown to reduce hepatic steatosis and to improve the status of hippocampal neurons in mice. A study by Jiebiao Chen et al. [125]. showed that anthocyanins from common berries such as blackberries, black goji berries, strawberries, mulberries, prunes, raspberries, and red goji berries were able to increase the level of SCFAs in the intestine, improve the internal antioxidant status of mice, alleviate body weight gain and inhibit food intake by enriching the SCFA-producing bacteria Ruminococcus, Intestinimonas, and Clostridium_XVIII, among others. A study by Telma Angelina Faraldo Corrêa et al. [126]. showed that the intake of blood orange juice anthocyanins affected the abundance of operational taxonomic units (OTUs) in the intestinal flora, significantly increasing the levels of propionic acid and isobutyric acid in the intestinal tract of overweight females, reducing fasting blood glucose and insulin levels, and improving insulin resistance. A study by Ting Chen et al. [127]. found that purple red rice bran anthocyanins could promote the production of SCFAs in the intestinal tract and upregulate the expression of tight junction proteins (TJs) and nuclear factor kappa B (NF-κB) pathway proteins to improve the intestinal barrier function and dysbiosis of the intestinal flora in mice. A study by Yun Zhang et al. [128]. revealed that cactus anthocyanins significantly increased the content of SCFAs in the cecum of mice by altering the microbial diversity and flora composition of the intestinal tract, with the greatest increases observed in the contents of acetic acid, propionic acid and butyric acid.
Quercetin
Quercetin is a plant pigment that is widely found in tea, lettuce, radish leaves, cranberries, apples, buckwheat, cucumber, and onion and exists in plants as quercetin-3-O-glucoside, which provides color to a wide variety of vegetables and fruits [129, 130]. It has antiallergic, anti-inflammatory, cardiovascular protective, antitumor, antiviral, antidiabetic, immunomodulatory and antihypertensive effects [131]. Quercetin is also able to modulate metabolic disorders through different mechanisms, such as by increasing lipocalin, decreasing leptin, decreasing insulin resistance, increasing insulin levels, and blocking calcium channels [132]. A study by Wenhui Liu et al. [133]. reported that quercetin reduced the expression of transient receptor potential vanilloid 1 (TRPV1), aquaporin 3 (AQP3), and inducible nitric oxide synthase (iNOS) in the intestine and increased the expression of glial cell line-derived neurotrophic factor (GDNF), c-Kit, and stem cell factor (SCF). These changes inhibited the growth and reproduction of Enterococcus and Enterobacter in the intestine, increased the intestinal acetic acid, propionic acid and butyric acid contents in the intestine, improved gastrointestinal peristalsis, and increased the intestinal transit rate. Quercetin increases the abundance of Coprococcus spp. Ruminiclostridium spp. and Roseburia spp. in the mouse intestine [134] and the amount of these gut microbes that can produce SCFAs [135], which improves glucose and lipid metabolism in the body.
Resveratrol
Resveratrol was first isolated by Takaoka in 1939 from the flower Quercus serrata [136]. This phenolic substance is found not only in grapes and wine but also in peanuts, soybeans and berries [137]. It has powerful regenerative, antioxidant, protein-regulating and anticancer properties [138]. Early studies have shown that resveratrol inhibits the oxidation of low-density lipoprotein (LDL) in humans [139], reduces insulin resistance in animal models [140], and reduces adipocyte size and the inflammatory response in adipose tissue [141]. A study by Jin-Xian Liao et al. [142]. showed that resveratrol increased the abundance of S24-7 and Adlercreutzia; decreased the abundance of Allobaculum, Blautia, Lactobacillaceae, and Prevotella; increased the concentration of acetic and propionic acids in the intestine; and regulated hepatic antioxidant capacity by increasing the expression of NF-E2-related factor 2 (Nrf2) in the liver to promote HO-1 transcription as well as the expression of superoxide dismutase (SOD) and Catalase (CAT). A study by Yu Zhuang et al. [143]. reported that resveratrol supplementation decreased expression of the inflammatory cytokines IL-6 and IL-1β; increased the expression of propionic acid, isobutyric acid, butyric acid and isovaleric acid in the intestinal tract; and affected the metabolism of the intestinal flora by regulating amino acid metabolism and lipid metabolism to improve intestinal health in mice. A study by Hongjia Yan et al. [144]. revealed that resveratrol ameliorated the progression of diabetic kidney disease by increasing the abundance of Faecalibaculum and Lactobacillus bacteria in the intestinal tract, increasing the concentration of acetic acid in the feces, and modulating the gut microbiota-SCFA axis. A study by Le-Feng Wanget al [145]. reported that resveratrol lowered the intestinal pH, promoted the growth and proliferation of probiotics in the intestinal tract, and significantly increased the content of isobutyric acid in the colons of aging mice.
Carotenoids
Carotenoids are a general term for yellow, red and orange pigments containing long-chain hydrocarbons with conjugated double bonds. More than 1,100 carotenoids exist in nature, and humans can obtain them from vegetables and fruits [146]. Carotenoids can reduce free radical damage to cells [147] and are an important natural antioxidant. One molecule of beta-carotene can inhibit the activity of 1000 oxygen molecules [148]. Additionally, carotenoids can also be metabolized in the intestine and other tissues into vitamin A-like bioactive compounds, which provide immunomodulation and enhance the immune response [149]. Carotenoid supplementation activates the AMPK signaling pathway, which in turn activates upstream kinases, upregulates transcription factors, induces the discoloration of white adipose tissue, and blocks adipogenesis [150, 151]. Lycopene is a carotenoid [152] with antioxidant, anti-inflammatory, apoptotic and cellular communication-modulating effects [153]. A study by Xiang Gao et al. [154]. revealed that lycopene can decrease the expression of proteins such as NLRP3, Pro-Caspase-1, Caspase-1, and NF-κB in the liver; decrease the abundance of Lachnospiraceae_NK4A136_group, Desulfovibrio, and Alistipes in the gut microbiota; and increase the abundance of Allobaculum microbiota, thereby increasing the content of SCFAs, inhibiting the NF-κB/NLRP3 inflammatory pathway, and ameliorating nonalcoholic fatty liver disease (NAFLD). However, intake of beta carotene has been a question worth exploring, with studies concluding that 20 mg/day or more beta carotene is contraindicated for heavy smokers; the Panel on Nutrition, Dietetic Products, Novel Food and Allergy of the Norwegian Scientific Committee for Food Safety has set a tentative upper limit (TUL) of 4 mg/day for beta carotene supplementation; and the Panel on Vitamins and Minerals has set a safe upper limit of 7 mg/day for lifetime beta carotene supplementation for the general population (excluding smokers) and has discouraged concomitant use of beta carotene supplements by smokers [155].
Betaine
Betaine is a stable and nontoxic natural substance that was first discovered in the plant Beta vulgaris and subsequently found in relatively high concentrations in several other organisms, such as wheat bran, wheat germ, spinach, and sugar beet [156]. Betaine [157] is a trimethyl derivative of the amino acid glycine that promotes glucose uptake through GLUT-4 expression, directly increases ATP production while helping to stimulate glucose utilization in myocytes, and enhances energy production by increasing mitochondrial biogenesis. Betaine can also play an important role as an antioxidant and protect the liver from oxidative stress [158]. A study by Jingjing Du et al. [159] revealed that betaine supplementation increases acetate- and butyrate-producing intestinal flora such as A. muciniphila, Ruminococcus, Oscillospira, and Lactobacillus in the gut, which in turn increases acetate and butyrate levels and improves the prevention of obesity and obesity-related metabolic comorbidities. A study by Liuqiao Sun et al. [160] revealed that betaine supplementation upregulated expression of hepatic lipid oxidation genes, such as PPARα and CPT1α, and the hepatic lipid transporter gene FATP2; downregulated expression of the adipogenic genes fatty acid synthase (Fas) and ACC in adipose tissue; decreased the relative abundance of Proteus mirabilis, Vibrio desulfuricans, and Ruminococcus ruminanti in the intestine; and increased the relative abundance of Lactobacillus and Lactobacillus paracasei in the intestine. Increasing the concentration of SCFAs in the feces can modulate the hepatic triglyceride content and improve NAFLD.
Conclusions
Glucose and fatty acids are the main sources of energy in the human body. Under normal metabolic conditions, glucose metabolism and lipid metabolism pathways can meet the body’s normal activity needs, and the two metabolic pathways can affect each other; for example, glucose can be converted to fatty acids and cholesterol through the lipid biosynthesis pathway in glucose and lipid metabolism disorders, cardiovascular disease, diabetes, fatty liver and other serious diseases [161]. SCFAs modulate the structure of the gut microbiota [162], enhance the intestinal epithelial barrier [163], and can slow the onset and progression of disease [164]. By regulating the levels of SCFAs in the gut, it is able to exert beneficial effects on human glucose and lipid metabolism. Natural compounds in plants continue to be a hot topic in current research because of their safe composition, wide range of sources, and ability to treat a variety of diseases, such as cancer, diabetes, heart disease and Alzheimer’s disease [165]. Here, we review the role of SCFAs in glucose and lipid metabolism and describe the mechanism by which natural compounds in plants, such as polysaccharides, anthocyanins, quercetin, resveratrol, carotenoids, and betaines, modulate human glucose and lipid metabolism by increasing the content of SCFAs. Research has shown that these natural compounds can increase the number of beneficial bacteria, such as Alistipes and Odoribacter, thereby helping to maintain intestinal health. Moreover, they can also reduce potentially harmful bacteria, such as Lactobacillus, and lower their content in the intestine. Natural compounds can also regulate the content of short-chain fatty acids by affecting the abundance of OTUs in the gut microbiota and the intestinal transport rate. This regulatory effect helps to maintain the stability of the gut microbiota and prevent excessive proliferation of harmful bacteria. By using natural compounds, we can adjust the structure of the gut microbiota, maintain the stability of the intestinal environment, increase the content of SCFAs in the intestine, and promote human health. Further research on the effects of natural compounds on human SCFAs can help develop more beneficial products and methods for improving human health. Although the mechanisms by which natural compounds in plants regulate human glucose and lipid metabolism have been widely studied, the shortcomings of the results reported to date are that most were conducted in animal, with very few clinical trials. Moreover, the variety of natural compounds with SCFAs as unique targets of action is still limited. As a next step, preclinical and clinical studies are needed to understand and identify natural compounds that can play a role in regulating human glucose and lipid metabolism by modulating SCFAs. Additionally, targeted studies are needed to develop natural compounds in plants that provide new therapeutic options for the treatment of glucose and lipid metabolism disorders.
Acknowledgements
We would also like to thank the author of this article for contributing to the article.
Abbreviations
- SCFAs
Short-chain fatty acid
- SLC5A8
Anti-SLC5A8 rabbit polyclonal antibody
- SLC16A1
Human solute carrier family 16, member 1
- ATP
Adenosine triphosphate
- GPR43
G protein coupled receptor 43
- GPR41
G protein coupled receptor 41
- G-6-pase
Glucose-6-phosphatase
- SREBP1
Sterol regulatory element-binding protein 1
- FGF21
Fibroblast growth factor 21
- GLU-2
Glucose transporter 2
- GYS2
Glycogen synthase 2
- BAT
Brown adipose tissue
- AMPK
Adenosine 5‘-monophosphate (AMP)-activated protein kinase
- GLUT4
Glucose transporter 4
- PFK-1
phosphofructokinase-1
- GLP-1
Glucagon-like peptide-1
- PYY
Peptide YY
- PC1/3
Prohormone convertase 1/3
- GCG
Recombinant Glucagon
- HDAC
Histone deacetylase
- ACC
Acetyl coenzyme A carboxylase
- POMC
Proopiomelanocortin
- NPY
Neuropeptide Y
- AgRP
Agouti-related peptide
- T2DM
Type 2 diabetes mellitus
- IGN
Intestinal gluconeogenesis
- cAMP
Cyclic Adenosine Monophosphate
- G6PC
Glucose-6-phosphatase catalytic subunit
- PCK1
Phosphoenolpyruvate carboxykinase 1
- PGC1α
Proliferator–activated Receptor γ Coactivator 1α
- PPARδ
Peroxisome Proliferator–activated Receptorδ
- FAO
Fatty Acid 0xidation
- mTOR
Mechanistic Target of Rapamycin
- CTLs
Cytotoxic T lymphocytes
- IFN-γ
Interferon Gamma
- IL-2
Interleukin-2
- TNF-α
Tumor Necrosis Factor Alpha
- Slc2a1
Glucose Transporter 1
- PPARα
Peroxisome proliferators-activated receptors Alpha
- ChREBP
Carbohydrate response element binding protein
- HSL
Hormone-sensitive triglyceride lipase
- UCP-1
Uncoupling protein 1
- PPARγ
Peroxisome Proliferator-activated Receptorγ
- UCP-2
Uncoupling protein 2
- UCP-3
Uncoupling protein 3
- CPT1-b
Carnitine Palmitoyltransferase 1b
- IL-15
Interleukin-15
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- TNF-α
Tumor necrosis factor alpha
- OTU
Operational Taxonomic Unit
- HFD
High-fat diet
- TJs
Tight junction proteins
- NF-κB
Nuclear factor kappa B
- TRPV1
Transient Receptor Potential Vanilloid 1
- AQP3
Aquaporin 3
- iNos
inducible Nitric Oxide Synthase
- GDNF
Glial Cell Line-derived Neurotrophic Factor
- SCF
Stem Cell Factor
- Nrf2
NF-E2-related Factor 2
- SOD
Superoxide Dismutase
- CAT
Catalase
- CPT1ɑ
Carnitine lipoyltransferase Iα
- FATP2
Fatty acid transport protein 2
Author contributions
LJR, ZJY contributed to the conception and design of the study. TCX organized the database. DLS, KZZ, WJS, ZS embellished the article images. LM, TXL refined the design ideas for the article. LJR, ZJY, TCX wrote the first draft of the manuscript. All authors were involved in revising the manuscript, reading and approving the submitted version.
Funding
This work was supported by the Clinical Research Center Construction Project of Guang’anmen Hospital under Grant number 2022LYJSZX01 ~ 2022LYJSZX29 and the [High Level Chinese Medical Hospital Promotion Project] under Grant number HLCMHPP2023084.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study does not require ethical approval. Because this article does not involve research on animals or humans.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiarui Li, Jinyue Zhao and Chuanxi Tian contributed equally to this work.
Contributor Information
Min Li, Email: limin-72114@163.com.
Xiaolin Tong, Email: tongxiaolin@vip.163.com.
References
- 1.Tian H, Zhao X, Zhang Y, Xia Z. Abnormalities of glucose and lipid metabolism in myocardial ischemia-reperfusion injury. Volume 163. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2023. p. 114827. [DOI] [PubMed]
- 2.Mulukutla BC, Yongky A, Le T, Mashek DG, Hu WS. Regulation of glucose metabolism - A perspective from cell bioprocessing. Trends Biotechnol. 2016;34(8):638–51. doi: 10.1016/j.tibtech.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Adeva-Andany MM, Pérez-Felpete N, Fernández-Fernández C, Donapetry-García C. Pazos-García C. Liver glucose metabolism in humans. Biosci Rep. 2016;36(6). [DOI] [PMC free article] [PubMed]
- 4.Xiong P, Zhang F, Liu F, Zhao J, Huang X, Luo D, et al. Metaflammation in glucolipid metabolic disorders: Pathogenesis and treatment. Volume 161. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2023. p. 114545. [DOI] [PubMed]
- 5.Natesan V, Kim SJ. Lipid metabolism, disorders and therapeutic drugs - review. Biomolecules Ther. 2021;29(6):596–604. doi: 10.4062/biomolther.2021.122. [DOI] [Google Scholar]
- 6.Röhrig F, Schulze A. The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer. 2016;16(11):732–49. doi: 10.1038/nrc.2016.89. [DOI] [PubMed] [Google Scholar]
- 7.Natesan V, Kim SJ. Diabetic Nephropathy - a review of risk factors, progression, mechanism, and Dietary Management. Biomolecules Ther. 2021;29(4):365–72. doi: 10.4062/biomolther.2020.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoon H, Shaw JL, Haigis MC, Greka A. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol Cell. 2021;81(18):3708–30. doi: 10.1016/j.molcel.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuo SC, Nasir HM, Mohd-Setapar SH, Mohamed SF, Ahmad A, Wani WA, et al. A glimpse into the extraction methods of active compounds from plants. Crit Rev Anal Chem. 2022;52(4):667–96. doi: 10.1080/10408347.2020.1820851. [DOI] [PubMed] [Google Scholar]
- 11.Tandoro Y, Chen BK, Ali A, Wang CK. Review of Phytochemical Potency as a natural Anti-helicobacter Pylori and Neuroprotective Agent. Molecules. 2023;28(20). [DOI] [PMC free article] [PubMed]
- 12.Liu RH. Dietary bioactive compounds and their health implications. J Food Sci. 2013;78(Suppl 1):A18–25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Li H, Zhang B, Deng Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit Rev Food Sci Nutr. 2022;62(20):5658–77. doi: 10.1080/10408398.2021.1888693. [DOI] [PubMed] [Google Scholar]
- 14.Kaulmann A, Bohn T. Carotenoids, inflammation, and oxidative stress–implications of cellular signaling pathways and relation to chronic disease prevention. Nutr Res (New York NY) 2014;34(11):907–29. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Yao Y, Liu Y, Xu Q, Mao L. Short chain fatty acids: essential weapons of Traditional Medicine in treating inflammatory bowel disease. Molecules. 2024;29(2). [DOI] [PMC free article] [PubMed]
- 16.Fan Xie WY, Mingxia Xing H, Zhang. Lianzhong Ai Natural polyphenols-gut microbiota interactions and effects on glycolipid metabolism via polyphenols-gut-brain axis: a state-of-the-art review. Trends in Food Science & Technology; 2023.
- 17.Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23(3). [DOI] [PMC free article] [PubMed]
- 18.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Reviews Gastroenterol Hepatol. 2019;16(8):461–78. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 19.He J, Zhang P, Shen L, Niu L, Tan Y, Chen L et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17). [DOI] [PMC free article] [PubMed]
- 20.Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and Cardiovascular Health. Curr Nutr Rep. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong RG, Zhou DD, Wu SX, Huang SY, Saimaiti A, Yang ZJ, et al. Health benefits and Side effects of short-chain fatty acids. Foods (Basel Switzerland) 2022;11:18. doi: 10.3390/foods11182863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah S, Fillier T, Pham TH, Thomas R, Cheema SK. Intraperitoneal Administration of Short-Chain fatty acids improves lipid metabolism of Long-Evans rats in a sex-specific manner. Nutrients. 2021;13(3). [DOI] [PMC free article] [PubMed]
- 23.Sahuri-Arisoylu M, Brody LP, Parkinson JR, Parkes H, Navaratnam N, Miller AD et al. Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. International journal of obesity (2005). 2016;40(6):955 – 63. [DOI] [PubMed]
- 24.Tang R, Li L. Modulation of Short-Chain Fatty Acids as Potential Therapy Method for Type 2 Diabetes Mellitus. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale. 2021;2021:6632266. [DOI] [PMC free article] [PubMed]
- 25.Yoshida H, Ishii M, Akagawa M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch Biochem Biophys. 2019;672:108057. doi: 10.1016/j.abb.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Zhang WQ, Zhao TT, Gui DK, Gao CL, Gu JL, Gan WJ, et al. Sodium Butyrate improves liver glycogen metabolism in type 2 diabetes Mellitus. J Agric Food Chem. 2019;67(27):7694–705. doi: 10.1021/acs.jafc.9b02083. [DOI] [PubMed] [Google Scholar]
- 27.Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab. 2017;19(2):257–65. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- 28.Al-Lahham S, Rezaee F. Propionic acid counteracts the inflammation of human subcutaneous adipose tissue: a new avenue for drug development. Daru: J Fac Pharm Tehran Univ Med Sci. 2019;27(2):645–52. doi: 10.1007/s40199-019-00294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Reviews Endocrinol. 2015;11(10):577–91. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 30.Tan JK, Macia L, Mackay CR. Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol. 2023;151(2):361–70. doi: 10.1016/j.jaci.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Sci (New York NY) 2018;362(6416):776–80. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 32.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16(4):684–95. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- 33.Fredericks E, Theunissen R, Roux S. Short chain fatty acids and monocarboxylate transporters in irritable bowel syndrome. Turkish J Gastroenterology: Official J Turkish Soc Gastroenterol. 2020;31(12):840–7. doi: 10.5152/tjg.2020.19856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–45. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 35.Ziętek M, Celewicz Z, Szczuko M. Short-chain fatty acids, maternal microbiota and metabolism in pregnancy. Nutrients. 2021;13(4). [DOI] [PMC free article] [PubMed]
- 36.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-Mediated gut epithelial and Immune Regulation and its relevance for inflammatory Bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7(1):17–44. doi: 10.3390/nu7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–7. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernández MAG, Canfora EE, Jocken JWE, Blaak EE. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11:8. doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schug ZT, Vande Voorde J, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;16(11):708–17. doi: 10.1038/nrc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuchmann K, Müller V. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria. Nat Rev Microbiol. 2014;12(12):809–21. doi: 10.1038/nrmicro3365. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Jian YP, Zhang YN, Li Y, Gu LT, Sun HH, et al. Short-chain fatty acids in diseases. Cell Communication Signaling: CCS. 2023;21(1):212. doi: 10.1186/s12964-023-01219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579(7800):586–91. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8(6):1323–35. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott KP, Martin JC, Campbell G, Mayer CD, Flint HJ. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium Roseburia inulinivorans. J Bacteriol. 2006;188(12):4340–9. doi: 10.1128/JB.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: a double-edged Sword for Health? Advances in nutrition (Bethesda. Md) 2018;9(1):21–9. doi: 10.1093/advances/nmx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12(10). [DOI] [PMC free article] [PubMed]
- 50.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66(4):1654–61. doi: 10.1128/AEM.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- 52.Champ MM. Physiological aspects of resistant starch and in vivo measurements. J AOAC Int. 2004;87(3):749–55. doi: 10.1093/jaoac/87.3.749. [DOI] [PubMed] [Google Scholar]
- 53.Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome. 2016;4(1):33. doi: 10.1186/s40168-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12(2):304–14. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 55.Gao S, Feng Q. The Beneficial effects of Geniposide on glucose and lipid metabolism: a review. Drug Des Devel Ther. 2022;16:3365–83. doi: 10.2147/DDDT.S378976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 57.Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48(3):e218. doi: 10.1038/emm.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yen CE, Nelson DW, Yen MI. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. J Lipid Res. 2015;56(3):489–501. doi: 10.1194/jlr.R052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo S, Lu J, Zhuo Y, Xiao M, Xue X, Zhong S, et al. Endogenous cholesterol ester hydroperoxides modulate cholesterol levels and inhibit cholesterol uptake in hepatocytes and macrophages. Redox Biol. 2019;21:101069. doi: 10.1016/j.redox.2018.101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–32. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 62.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 63.Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in Health and Disease. Physiol Rev. 2020;100(1):171–210. doi: 10.1152/physrev.00041.2018. [DOI] [PubMed] [Google Scholar]
- 64.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci USA. 2011;108(19):8030–5. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem Biophys Res Commun. 2006;344(2):597–604. doi: 10.1016/j.bbrc.2006.03.176. [DOI] [PubMed] [Google Scholar]
- 66.Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J, et al. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes. 2012;61(4):797–806. doi: 10.2337/db11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou H, Yu B, Sun J, Liu Z, Chen H, Ge L, et al. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J Anim Sci Biotechnol. 2021;12(1):61. doi: 10.1186/s40104-021-00581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazhar M, Zhu Y, Qin L. The interplay of Dietary fibers and intestinal microbiota affects type 2 diabetes by Generating short-chain fatty acids. Foods (Basel, Switzerland). 2023;12(5). [DOI] [PMC free article] [PubMed]
- 69.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101(4):1045–50. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pereira S, Cline DL, Glavas MM, Covey SD, Kieffer TJ. Tissue-specific effects of Leptin on glucose and lipid metabolism. Endocr Rev. 2021;42(1):1–28. doi: 10.1210/endrev/bnaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frampton J, Murphy KG, Frost G, Chambers ES. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metabolism. 2020;2(9):840–8. doi: 10.1038/s42255-020-0188-7. [DOI] [PubMed] [Google Scholar]
- 73.Toejing P, Khat-Udomkiri N, Intakhad J, Sirilun S, Chaiyasut C, Lailerd N. Putative mechanisms responsible for the antihyperglycemic action of Lactobacillus paracasei HII01 in experimental type 2 Diabetic rats. Nutrients. 2020;12(10). [DOI] [PMC free article] [PubMed]
- 74.Richter EA, Hargreaves M, Exercise GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93(3):993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 75.Fushimi T, Tayama K, Fukaya M, Kitakoshi K, Nakai N, Tsukamoto Y, et al. Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. J Nutr. 2001;131(7):1973–7. doi: 10.1093/jn/131.7.1973. [DOI] [PubMed] [Google Scholar]
- 76.Zhang C, Fang B, Zhang N, Zhang Q, Niu T, Zhao L et al. The Effect of Bifidobacterium animalis subsp. lactis MN-Gup on glucose metabolism, gut microbiota, and their metabolites in type 2 Diabetic mice. Nutrients. 2024;16(11). [DOI] [PMC free article] [PubMed]
- 77.Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi XH. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Volume 125. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2020. p. 109914. [DOI] [PubMed]
- 78.Wang J, An G, Peng X, Zhong F, Zhao K, Qi L, et al. Effects of three Huanglian-derived polysaccharides on the gut microbiome and fecal metabolome of high-fat diet/streptozocin-induced type 2 diabetes mice. Int J Biol Macromol. 2024;273(Pt 1):133060. doi: 10.1016/j.ijbiomac.2024.133060. [DOI] [PubMed] [Google Scholar]
- 79.Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. 2018;8(1):74. doi: 10.1038/s41598-017-18259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 82.Erny D, Dokalis N, Mezö C, Castoldi A, Mossad O, Staszewski O, et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metabol. 2021;33(11):2260–e767. doi: 10.1016/j.cmet.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 83.Callao V, Montoya E. Toxohormone-like factor from microorganisms with impaired respiration. Sci (New York NY) 1961;134(3495):2041–2. doi: 10.1126/science.134.3495.2041. [DOI] [PubMed] [Google Scholar]
- 84.Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20(1):28. doi: 10.1186/s12943-021-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–8. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen TTT, Zhang Y, Shang E, Shu C, Torrini C, Zhao J, et al. HDAC inhibitors elicit metabolic reprogramming by targeting super-enhancers in glioblastoma models. J Clin Investig. 2020;130(7):3699–716. doi: 10.1172/JCI129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12(1):4077. doi: 10.1038/s41467-021-24331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593(7858):282–8. doi: 10.1038/s41586-021-03442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, Chen H, Guan Y, Li X, Lei L, Liu J, et al. Acetic acid activates the AMP-activated protein kinase signaling pathway to regulate lipid metabolism in bovine hepatocytes. PLoS ONE. 2013;8(7):e67880. doi: 10.1371/journal.pone.0067880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yap F, Craddock L, Yang J. Mechanism of AMPK suppression of LXR-dependent Srebp-1c transcription. Int J Biol Sci. 2011;7(5):645–50. doi: 10.7150/ijbs.7.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jocken JWE, González Hernández MA, Hoebers NTH, van der Beek CM, Essers YPG, Blaak EE, et al. Short-chain fatty acids differentially affect intracellular lipolysis in a human White Adipocyte Model. Front Endocrinol. 2017;8:372. doi: 10.3389/fendo.2017.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–17. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-chain fatty acids protect Against High-Fat Diet-Induced obesity via a PPARγ-Dependent switch from Lipogenesis to Fat Oxidation. Diabetes. 2015;64(7):2398–408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 94.Kopecký J, Rossmeisl M, Flachs P, Bardová K, Brauner P. Mitochondrial uncoupling and lipid metabolism in adipocytes. Biochem Soc Trans. 2001;29(Pt 6):791–7. doi: 10.1042/bst0290791. [DOI] [PubMed] [Google Scholar]
- 95.Xiong W, Xiong Z, Song A, Lei C, Ye C, Zhang C. Relieving lipid accumulation through UCP1 suppresses the progression of acute kidney injury by promoting the AMPK/ULK1/autophagy pathway. Theranostics. 2021;11(10):4637–54. doi: 10.7150/thno.56082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18(1):52–8. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Della Guardia L, Luzi L, Codella R. Muscle-UCP3 in the regulation of energy metabolism. Mitochondrion. 2024;76:101872. doi: 10.1016/j.mito.2024.101872. [DOI] [PubMed] [Google Scholar]
- 98.Garvey WT. Uncoupling protein 3 and human metabolism. J Clin Endocrinol Metab. 2006;91(4):1226–8. doi: 10.1210/jc.2006-0133. [DOI] [PubMed] [Google Scholar]
- 99.Hong J, Jia Y, Pan S, Jia L, Li H, Han Z, et al. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget. 2016;7(35):56071–82. doi: 10.18632/oncotarget.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Visuthranukul C, Leelahavanichkul A, Tepaamorndech S, Chamni S, Mekangkul E, Chomtho S. Inulin supplementation exhibits increased muscle mass via gut-muscle axis in children with obesity: double evidence from clinical and in vitro studies. Sci Rep. 2024;14(1):11181. doi: 10.1038/s41598-024-61781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 102.Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7(1):2360. doi: 10.1038/s41598-017-02546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67(7):1269–79. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Y, Chen Z, Zhou H, Niu B, Liu J, Li Y, et al. ACT001 alleviates chronic kidney injury induced by a high-fat diet in mice through the GPR43/AMPK pathway. Lipids Health Dis. 2023;22(1):198. doi: 10.1186/s12944-023-01949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Borah AK, Sharma P, Singh A, Kalita KJ, Saha S, Chandra Borah J. Adipose and non-adipose perspectives of plant derived natural compounds for mitigation of obesity. J Ethnopharmacol. 2021;280:114410. doi: 10.1016/j.jep.2021.114410. [DOI] [PubMed] [Google Scholar]
- 106.Atanasov AG, Zotchev SB, Dirsch VM, Orhan IE, Banach M, Rollinger JM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discovery. 2021;20(3):200–16. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie JH, Jin ML, Morris GA, Zha XQ, Chen HQ, Yi Y, et al. Advances on Bioactive polysaccharides from Medicinal plants. Crit Rev Food Sci Nutr. 2016;56(Suppl 1):S60–84. doi: 10.1080/10408398.2015.1069255. [DOI] [PubMed] [Google Scholar]
- 108.Basavarajappa GM, Priyanka KM, Goudanavar P, Narasimha LG, Naveen NR, Gowthami B, et al. A spotlight on application of microwave-assisted modifications of plant derived polymers in designing novel drug delivery systems. Des Monomers Polym. 2023;26(1):106–16. doi: 10.1080/15685551.2023.2194176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding M, Wang G, Yuan P, He S, Shao T, Liu C, et al. [Research progress in the role and mechanism of polysaccharides in regulating glucose and lipid metabolism] Nan Fang Yi Ke da xue xue bao = J South Med Univ. 2021;41(3):471–5. doi: 10.12122/j.issn.1673-4254.2021.03.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hong Y, Sheng L, Zhong J, Tao X, Zhu W, Ma J, et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes. 2021;13(1):1–20. doi: 10.1080/19490976.2021.1930874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li D, Zhang X, Fan Y, Zhang Y, Tao X, Yang J. Lycium barbarum Polysaccharides Improved glucose metabolism in prediabetic mice by regulating duodenal contraction. Nutrients. 2023;15(20). [DOI] [PMC free article] [PubMed]
- 112.Tian X, Dong W, Zhou W, Yan Y, Lu L, Mi J, et al. The polysaccharides from the fruits of Lycium barbarum ameliorate high-fat and high-fructose diet-induced cognitive impairment via regulating blood glucose and mediating gut microbiota. Int J Biol Macromol. 2023;258(Pt 2):129036. doi: 10.1016/j.ijbiomac.2023.129036. [DOI] [PubMed] [Google Scholar]
- 113.Xie J, Liu Y, Chen B, Zhang G, Ou S, Luo J et al. Ganoderma Lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr Res. 2019;63. [DOI] [PMC free article] [PubMed]
- 114.Lan Y, Sun Q, Ma Z, Peng J, Zhang M, Wang C, et al. Seabuckthorn polysaccharide ameliorates high-fat diet-induced obesity by gut microbiota-SCFAs-liver axis. Food Funct. 2022;13(5):2925–37. doi: 10.1039/D1FO03147C. [DOI] [PubMed] [Google Scholar]
- 115.Luo L, Luo J, Cai Y, Fu M, Li W, Shi L, et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacol Res. 2022;183:106367. doi: 10.1016/j.phrs.2022.106367. [DOI] [PubMed] [Google Scholar]
- 116.Yao Y, Yan L, Chen H, Wu N, Wang W, Wang D. Cyclocarya paliurus polysaccharides alleviate type 2 diabetic symptoms by modulating gut microbiota and short-chain fatty acids. Phytomedicine: Int J Phytotherapy Phytopharmacology. 2020;77:153268. doi: 10.1016/j.phymed.2020.153268. [DOI] [PubMed] [Google Scholar]
- 117.Guo C, Wang Y, Zhang S, Zhang X, Du Z, Li M, et al. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int J Biol Macromol. 2021;181:357–68. doi: 10.1016/j.ijbiomac.2021.03.137. [DOI] [PubMed] [Google Scholar]
- 118.Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005;81(1):S223–9. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- 119.Mohd Nor NA, Budin SB, Zainalabidin S, Jalil J, Sapian S, Jubaidi FF et al. The role of Polyphenol in modulating Associated genes in Diabetes-Induced Vascular disorders. Int J Mol Sci. 2022;23(12). [DOI] [PMC free article] [PubMed]
- 120.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–46. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9(5):416–31. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res. 2012;56(1):159–70. doi: 10.1002/mnfr.201100526. [DOI] [PubMed] [Google Scholar]
- 123.Tian B, Zhao J, Zhang M, Chen Z, Ma Q, Liu H, et al. Lycium Ruthenicum anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol Nutr Food Res. 2021;65(8):e2000745. doi: 10.1002/mnfr.202000745. [DOI] [PubMed] [Google Scholar]
- 124.Si X, Bi J, Chen Q, Cui H, Bao Y, Tian J, et al. Effect of Blueberry Anthocyanin-Rich extracts on peripheral and hippocampal antioxidant defensiveness: the analysis of the serum fatty acid species and Gut Microbiota Profile. J Agric Food Chem. 2021;69(12):3658–66. doi: 10.1021/acs.jafc.0c07637. [DOI] [PubMed] [Google Scholar]
- 125.Chen J, Shu Y, Chen Y, Ge Z, Zhang C, Cao J et al. Evaluation of antioxidant capacity and gut microbiota Modulatory effects of different kinds of berries. Antioxid (Basel Switzerland). 2022;11(5). [DOI] [PMC free article] [PubMed]
- 126.Corrêa TAF, Tobaruela EC, Capetini VC, Quintanilha BJ, Cortez RV, Taddei CR, et al. Blood orange juice intake changes specific bacteria of gut microbiota associated with cardiometabolic biomarkers. Front Microbiol. 2023;14:1199383. doi: 10.3389/fmicb.2023.1199383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen T, Shen M, Yu Q, Chen Y, Wen H, Lu H, et al. Purple red rice anthocyanins alleviate intestinal damage in cyclophosphamide-induced mice associated with modulation of intestinal barrier function and gut microbiota. Food Chem. 2022;397:133768. doi: 10.1016/j.foodchem.2022.133768. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y, Chang H, Shao S, Zhao L, Zhang R, Zhang S. Anthocyanins from Opuntia ficus-indica modulate gut microbiota composition and improve short-chain fatty acid production. Biology. 2022;11(10). [DOI] [PMC free article] [PubMed]
- 129.Singh P, Arif Y, Bajguz A, Hayat S. The role of quercetin in plants. Plant Physiol Biochemistry: PPB. 2021;166:10–9. doi: 10.1016/j.plaphy.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 130.Shabbir U, Rubab M, Daliri EB, Chelliah R, Javed A, Oh DH. Curcumin, Quercetin, catechins and metabolic diseases: the role of gut microbiota. Nutrients. 2021;13(1). [DOI] [PMC free article] [PubMed]
- 131.Lakhanpal P, Rai DK. Quercetin: a versatile flavonoid. Internet J Med Update. 2007;2(2):22–37. [Google Scholar]
- 132.Hosseini A, Razavi BM, Banach M, Hosseinzadeh H. Quercetin and metabolic syndrome: a review. Phytother Res. 2021;35(10):5352–64. doi: 10.1002/ptr.7144. [DOI] [PubMed] [Google Scholar]
- 133.Liu W, Zhi A. The potential of Quercetin to protect against loperamide-induced constipation in rats. Food Sci Nutr. 2021;9(6):3297–307. doi: 10.1002/fsn3.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ju S, Ge Y, Li P, Tian X, Wang H, Zheng X, et al. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle (Georgetown Tex) 2018;17(1):53–63. doi: 10.1080/15384101.2017.1387701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Z, Liang H, Hu Y, Lu L, Zheng C, Fan Y, et al. Gut bacterial profiles in Parkinson’s disease: a systematic review. CNS Neurosci Ther. 2023;29(1):140–57. doi: 10.1111/cns.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takaoka M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. Nippon Kagaku Kaishi. 1939;60(11):1090–100. doi: 10.1246/nikkashi1921.60.1090. [DOI] [Google Scholar]
- 137.Breuss JM, Atanasov AG, Uhrin P. Resveratrol and its effects on the Vascular System. Int J Mol Sci. 2019;20(7). [DOI] [PMC free article] [PubMed]
- 138.Stokes Iii J, Vinayak S, Williams J, Malik S, Singh R, Manne U, et al. Optimum health and inhibition of cancer progression by microbiome and resveratrol. Front Bioscience (Landmark Edition) 2021;26(3):496–517. doi: 10.2741/4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Frankel E, Waterhouse A, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341(8852):1103–4. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 140.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metabol. 2013;18(4):533–45. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liao JX, Chen YW, Shih MK, Tain YL, Yeh YT, Chiu MH et al. Resveratrol Butyrate Esters Inhibit BPA-Induced Liver damage in male offspring rats by modulating antioxidant capacity and gut microbiota. Int J Mol Sci. 2021;22(10). [DOI] [PMC free article] [PubMed]
- 143.Zhuang Y, Huang H, Liu S, Liu F, Tu Q, Yin Y, et al. Resveratrol improves growth performance, intestinal morphology, and Microbiota Composition and Metabolism in mice. Front Microbiol. 2021;12:726878. doi: 10.3389/fmicb.2021.726878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yan H, Zhang Y, Lin X, Huang J, Zhang F, Chen C et al. Resveratrol improves diabetic kidney disease by modulating the gut microbiota-short chain fatty acids axis in db/db mice. Int J Food Sci Nutr. 2024:1–13. [DOI] [PubMed]
- 145.Wang LF, Li WJ, Zhang XY, Zhang YC, Chen GF, Zhou XY, et al. Resveratrol prevents age-related heart impairment through inhibiting the Notch/NF-κB pathway. Food Sci Nutr. 2024;12(2):1035–45. doi: 10.1002/fsn3.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Terao J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. 2023;14(17):7799–824. doi: 10.1039/D3FO02330C. [DOI] [PubMed] [Google Scholar]
- 147.Biard C, Hardy C, Motreuil S, Moreau J. Dynamics of PHA-induced immune response and plasma carotenoids in birds: should we have a closer look? J Exp Biol. 2009;212(Pt 9):1336–43. doi: 10.1242/jeb.028449. [DOI] [PubMed] [Google Scholar]
- 148.Olson JA. Molecular actions of carotenoids. Ann N Y Acad Sci. 1993;691:156–66. doi: 10.1111/j.1749-6632.1993.tb26167.x. [DOI] [PubMed] [Google Scholar]
- 149.Krinsky NI. Carotenoids as chemopreventive agents. Prev Med. 1989;18(5):592–602. doi: 10.1016/0091-7435(89)90032-7. [DOI] [PubMed] [Google Scholar]
- 150.Ferreira YAM, Jamar G, Estadella D, Pisani LP. Role of carotenoids in adipose tissue through the AMPK-mediated pathway. Food Funct. 2023;14(8):3454–62. doi: 10.1039/D2FO03781E. [DOI] [PubMed] [Google Scholar]
- 151.Gu M, Luo L, Fang K. Crocin inhibits obesity via AMPK-dependent inhibition of adipocyte differentiation and promotion of lipolysis. Biosci Trends. 2018;12(6):587–94. doi: 10.5582/bst.2018.01240. [DOI] [PubMed] [Google Scholar]
- 152.Przybylska S, Tokarczyk G. Lycopene in the Prevention of Cardiovascular diseases. Int J Mol Sci. 2022;23(4). [DOI] [PMC free article] [PubMed]
- 153.Thies F, Mills LM, Moir S, Masson LF. Cardiovascular benefits of lycopene: fantasy or reality? The Proceedings of the Nutrition Society. 2017;76(2):122-9. [DOI] [PubMed]
- 154.Gao X, Zhao X, Liu M, Zhao H, Sun Y. Lycopene prevents non-alcoholic fatty liver disease through regulating hepatic NF-κB/NLRP3 inflammasome pathway and intestinal microbiota in mice fed with high-fat and high-fructose diet. Front Nutr. 2023;10:1120254. doi: 10.3389/fnut.2023.1120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Turck D, Bohn T, Castenmiller J, de Henauw S, Hirsch-Ernst KI, Knutsen HK, et al. Scientific opinion on the tolerable upper intake level for preformed vitamin A and β-carotene. EFSA J Eur Food Saf Auth. 2024;22(6):e8814. doi: 10.2903/j.efsa.2024.8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao G, He F, Wu C, Li P, Li N, Deng J, et al. Betaine in inflammation: mechanistic aspects and applications. Front Immunol. 2018;9:1070. doi: 10.3389/fimmu.2018.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma J, Meng X, Kang SY, Zhang J, Jung HW, Park YK. Regulatory effects of the fruit extract of Lycium chinense and its active compound, betaine, on muscle differentiation and mitochondrial biogenesis in C2C12 cells. Volume 118. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie; 2019. p. 109297. [DOI] [PubMed]
- 158.Meng X, Peng L, Xu J, Guo D, Cao W, Xu Y, et al. Betaine attenuate chronic restraint stress-induced changes in testicular damage and oxidative stress in male mice. Reproductive biology and endocrinology: RB&E; 2022. p. 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Du J, Zhang P, Luo J, Shen L, Zhang S, Gu H, et al. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes. 2021;13(1):1–19. doi: 10.1080/19490976.2020.1862612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun L, Tan X, Liang X, Chen H, Ou Q, Wu Q et al. Maternal betaine supplementation mitigates maternal high Fat Diet-Induced NAFLD in offspring mice through gut microbiota. Nutrients. 2023;15(2). [DOI] [PMC free article] [PubMed]
- 161.Chen L, Chen XW, Huang X, Song BL, Wang Y, Wang Y. Regulation of glucose and lipid metabolism in health and disease. Sci China Life Sci. 2019;62(11):1420–58. doi: 10.1007/s11427-019-1563-3. [DOI] [PubMed] [Google Scholar]
- 162.Matsushita M, Fujita K, Hayashi T, Kayama H, Motooka D, Hase H, et al. Gut microbiota-derived short-chain fatty acids promote prostate Cancer Growth via IGF1 Signaling. Cancer Res. 2021;81(15):4014–26. doi: 10.1158/0008-5472.CAN-20-4090. [DOI] [PubMed] [Google Scholar]
- 163.Guo Y, Yu Y, Li H, Ding X, Li X, Jing X, et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur J Nutr. 2021;60(4):2217–30. doi: 10.1007/s00394-020-02414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. New York, NY: Science; 2018. pp. 1151–6. [DOI] [PubMed] [Google Scholar]
- 165.Nasim N, Sandeep IS, Mohanty S. Plant-derived natural products for drug discovery: current approaches and prospects. Nucleus: Int J Cytol Allied Top. 2022;65(3):399–411. doi: 10.1007/s13237-022-00405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.