Abstract
Tomato spotted wilt virus (TSWV) was shown to use alfalfa mosaic virus (AMV) RNAs as cap donors in vivo during a mixed infection in Nicotiana benthamiana. By use of nested reverse transcription-PCR, TSWV N and NSs mRNAs provided with capped leader sequences derived from all four AMV RNAs could be cloned and sequenced. The sequence specificity of the putative TSWV endonuclease involved is discussed.
Like all negative-strand RNA viruses with segmented genomes, bunyaviruses use the mechanism of “cap snatching” to initiate transcription of their mRNAs (1–3, 8, 12, 15). During this process, cap structures are cleaved from host mRNAs by a virus-encoded endonuclease and subsequently used to prime transcription; this process was first described for influenza A virus (11, 13). For tomato spotted wilt virus (TSWV), it has been shown that the viral mRNAs produced during plant infection contain capped leader sequences of nonviral origin and of 12 to 21 nucleotides (nt) (9, 16). In this study, we have investigated whether TSWV would use one or more of the alfalfa mosaic virus (AMV) RNAs as cap donors during a mixed infection. Such a study would provide further insight into the cap-snatching process of TSWV, and the demonstration of the use of AMV leaders during the natural TSWV infection process would open the possibility of studying in vivo the sequence specificity of the endonuclease involved in mixed infections with specific mutants of AMV.
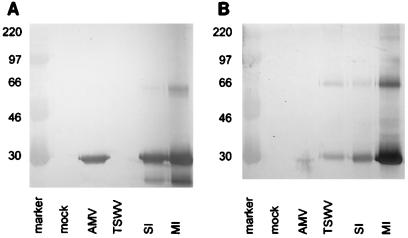
For this purpose, 5-week-old Nicotiana benthamiana plants were mechanically inoculated with both TSWV (Brazilian strain BR-01) and AMV (Leiden strain 425) in two ways, and subsequently it was determined which leaf material became successfully coinfected with both viruses. Plants were inoculated either with TSWV and AMV on separate leaves of the same plant (separate infection [SI]) or with a mixed inoculum on the same leaf (mixed infection [MI]). At 15 days (Fig. 1) postinoculation (p.i.), samples of systemically infected top leaves were analyzed by Western immunoblot analyses with antisera directed against the TSWV nucleoprotein and the AMV capsid protein as described previously (10). Both viruses clearly coreplicated in both SI and MI plants (Fig. 1, lanes SI and MI), and reverse transcription (RT)-PCR analyses were performed with RNA from leaves of SI plants 15 days p.i.
FIG. 1.
Western immunodetection of AMV and TSWV in single and mixed infections of N. benthamiana 15 days p.i. (A) Detection with anti-AMV antiserum. (B) Detection with anti-TSWV antiserum. Mock, mock-infected plants; AMV, AMV-infected plants; TSWV, TSWV-infected plants. Numbers at left are molecular size markers (in kilodaltons).
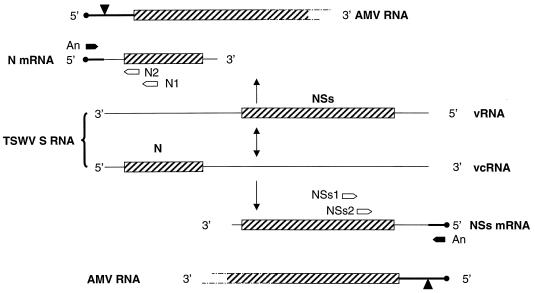
To test whether TSWV would use AMV RNAs as cap donors during a mixed infection, RT-PCR amplification was performed with total RNA extracts made from systemically infected leaf material as described by Gurr and McPherson (7). Primers were chosen to amplify the leader sequences of the TSWV N and NSs mRNAs, both derived from the ambisense S RNA segment (Fig. 2) (4). First-strand cDNA was synthesized from 10 μg of total RNA extract with primers N1 (nt positions 2595 to 2609: 5′-GGAATGTCAGACATG-3′) and NSs1 (nt positions 455 to 439: 5′-GGGCAGGAGACAAAACC-3′) and Superscript reverse transcriptase (GIBCO BRL) according to the manufacturer’s procedures. Subsequently, a nested PCR was performed with primers N2 (nt positions 2634 to 2651; 5′-CCCGGATCCGTTCGATGTTTTCCAGAC-3′) and NSs2 (nt positions 384 to 367; 5′-CCCGGATCCGATAGTGCCAGAACAGAG) in combination with primers matching the 5′ termini of AMV RNAs (A12, matching both AMV RNA 1 and AMV RNA 2: 5′-CCCGGATCCGTTTTTATCTT-3′; A3: 5′-CCCGGATCCGTATTAATACC-3′; A4: 5′-CCCGGATCCGTTTTTATTTT-3′). The latter primers were designed to match the first 11 nt of the AMV sequences (Fig. 2), as the sizes of the snatched sequences found varied between 12 and 21 nt and added sequences shorter than 12 nt thus far have not been detected at the 5′ ends of TSWV mRNAs (9, 16).
FIG. 2.
Locations of primers for RT-PCR on TSWV mRNAs containing 5′-terminal AMV sequences. Both the viral (v) and the viral complementary (vc) strands of the ambisense TSWV S RNA (4) are shown; open reading frames are indicated as hatched bars, and caps on AMV RNAs are indicated as black circles. An, primers for AMV RNAs 1 and 2 (A12), AMV RNA 3 (A3), or AMV RNA 4 (A4); N1, primer for RT reaction on TSWV N mRNA; N2, nested primer for PCR on TSWV N mRNA; NSs1, primer for RT reaction on TSWV NSs mRNA; NSs2, nested primer for PCR on TSWV NSs mRNA.
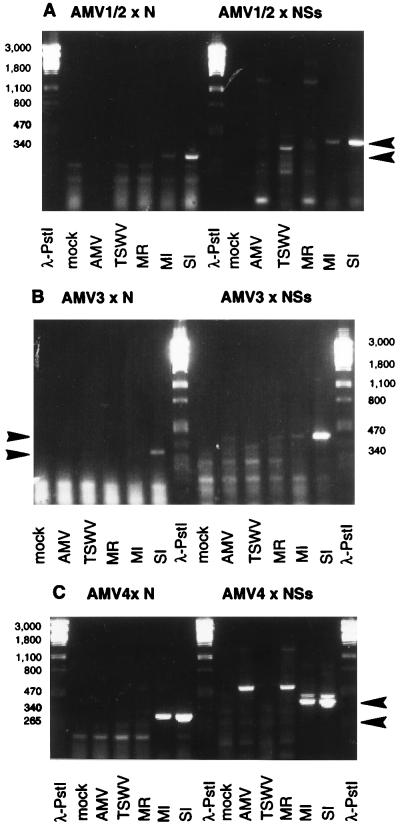
PCR amplification was performed with the synthesized first-strand cDNA (5 min at 94°C; 5 cycles of 30 s at 94°C, 30 s at 25°C, and 30 s at 72°C; 35 cycles of 30 s at 94°C, 30 s at 55°C, and 30 s at 72°C; and 7 min at 72°C), and products of the predicted sizes were observed for N mRNA (∼315 bp) and NSs mRNA (∼420 bp) in MI plants and especially in SI plants (Fig. 3).
FIG. 3.
RT-PCR products from N and NSs mRNAs provided with leader sequences derived from AMV RNA 1 and RNA 2 (A), RNA 3 (B), and RNA 4 (C). Total RNA was isolated from infected N. benthamiana at 15 days p.i. The RT primers used were N1 and NSs1, and the PCR primers used were A12 (A), A3 (B), and A4 (C) in combination with N2 and NSs2 (Fig. 2). PCR products of ∼315 bp represent specific TSWV N mRNAs, and PCR products of ∼420 bp represent TSWV NSs mRNAs. Products of primers A12 and N2 or NSs2 contain both AMV RNA 1- and RNA 2-derived leaders because the 5′-terminal 11-nt sequences of these AMV RNAs are identical and therefore are both recognized by primer A12. λ-PstI, molecular size (numbers at left, in base pairs) markers; mock, mock-infected plants; AMV, AMV-infected plants; TSWV, TSWV-infected plants; MR, in vitro mixed RNA of singly (AMV or TSWV) infected plants. Arrowheads indicate specific PCR products from N mRNAs (∼315 bp) and NSs mRNAs (∼420 bp).
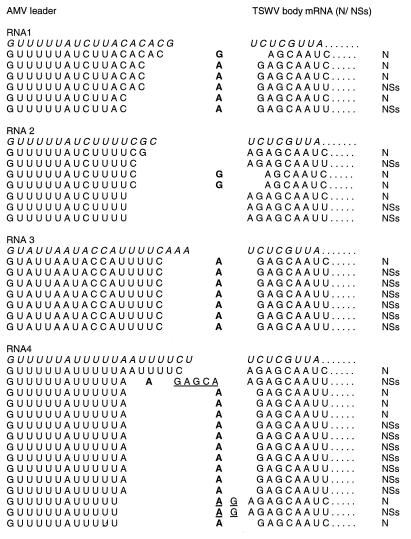
To determine the fusion sites of AMV sequences to TSWV mRNAs, fragments obtained from different RT-PCRs were cloned and analyzed by sequence analysis. This analysis showed that all clones contained 5′ sequences of AMV origin fused to TSWV N or NSs mRNAs and that apparently all four AMV RNAs were used as cap donors. Moreover, these findings imply that the transcription of TSWV, a negative-strand RNA virus, takes place in the same subcellular compartment as that in which the RNAs of AMV, a positive-strand RNA virus, are found. Whereas the AMV leader sequences varied in length from 12 to 19 nt (Fig. 4), the majority of the clones analyzed contained leader sequences of 13 to 15 nt. However, this result was not independent of the cap donor RNA that was used, as RNA 3 was exclusively cleaved after C17. These findings suggest that the length of the leader sequence may be an important factor but is not the only contributor to the cleavage specificity of the putative viral endonuclease.
FIG. 4.
5′-Terminal sequences of individual TSWV N and NSs mRNAs containing AMV cap structures. Nontemplate nucleotides which were inserted between the AMV and TSWV sequences are underlined. Nucleotides which could be derived from either AMV or TSWV RNA are in bold type. Original AMV (5′ → 3′) leader sequences and TSWV (3′ → 5′) terminal template sequences are in italic type.
A total of 35 clones were analyzed; 3 clones lacked the first nucleotide (A) of the TSWV genomic sequence, 2 clones contained an additional AG, and 1 clone even contained a repeat of the first 6 nt of the TSWV genomic sequence. These insertions may have arisen from polymerase slippage on the template viral RNA, as has been suggested previously for snowshoe hare bunyavirus (1). Alternatively, these insertions may have arisen by means of resnatching, in which an already AMV-capped TSWV mRNA is reused as a cap-donor but is cleaved further downstream from the original fusion site.
Because, in this study, the leader sequences used could be identified as being derived from each of the individual AMV RNAs, the sequence data could provide information on preferential cleavage sites in terms of sequence specificity and distance to the cap structure. There are, however, two possible interpretations of the results, as in 29 of the 35 sequences, the residue at the fusion site could equally well represent the first residue of the TSWV body mRNA or the last residue of the AMV leader (Fig. 4, residues indicated in bold). In the former interpretation, endonucleolytic cleavage would occur after a C residue in 49% (17 of 35) of the studied sequences, after an A residue in 31% (11 of 35), after a U residue in 17% (6 of 35), and after a G residue in the remaining single sequence. In the latter interpretation, however, which would allow for base pairing of the 3′ ultimate residue of the AMV-derived primer sequence to the 3′ ultimate or penultimate residue of the TSWV template, as was found for influenza A virus (13), the cleavage preference would be different: 74% (26 of 35) after an A residue, 11% (4 of 35) after a G residue, 9% (3 of 35) after a U residue, and 6% (2 of 35) after a C residue. Base pairing between cap donor RNA and TSWV template RNA would explain not only the apparent preference for endonucleolytic cleavage after an A residue but also the loss of the first nucleotide of the TSWV sequence in three of the clones analyzed; in those clones, it is likely that the G residue preceding the cleavage site base paired with the TSWV template RNA at the penultimate residue, resulting in a loss of the 3′ ultimate residue. Furthermore, the base-pairing requirement also could explain the difference in lengths observed between the snatched leader sequences derived from AMV RNA 3 (18 nt) and leader sequences derived from AMV RNAs 1, 2, and 4 (13 to 15 nt): AMV RNA 3 is not cleaved to generate leader sequences of the preferential length of 13 to 15 nt because the nucleotide sequence at these positions does not allow base pairing, resulting in a scan for a cleavage site further downstream (position 18 nt) that does allow base pairing.
For several members of the family Bunyaviridae and the genus Tenuivirus, a “prime-and-realign” mechanism has been proposed for the initiation of transcription (5, 6, 8). Such a mechanism, if involved during the initiation of transcription for TSWV as well, could account for the presence of additional nucleotides (AG or AGAGCA) between the AMV and TSWV sequences in three of the clones analyzed. To decide between these alternative possibilities, further studies with mutagenized AMV donor RNAs are required.
The data presented in this paper show the feasibility of exploiting the in vivo coinfection approach, with the advantage that the endonuclease involved can be tested directly in vivo. The value of this in vivo approach and its broader applicability for other viruses that use cap snatching during the initiation of transcription are supported by similar results obtained for the tenuivirus maize stripe virus in a coinfection with barley stripe mosaic virus and published during the course of our investigations (5). For AMV RNAs 2, 3, and 4, cleavage by the cap-snatching machinery of TSWV appears to take place preferentially after a UUUUC/A(A) sequence motif (the residue in parentheses depending on which of the two priming mechanisms is used; see above). However, this location may be only accidental, as AMV RNA 1 does not contain such a motif in the region in which endonucleolytic cleavage takes place and yet can be used as a cap donor, and among the host mRNA-derived leaders previously sequenced (16), this motif is absent. Of the four AMV RNAs cannibalized by TSWV, only RNA 3 is cleaved at a single position (Fig. 4). Therefore, this RNA is possibly the best starting point at which to explore the sequence requirements of the TSWV endonuclease more precisely by mutational analysis. Such an approach will also shed light on whether the priming reaction does or does not require base pairing of the 3′-terminal leader residue to the TSWV template RNA.
Acknowledgments
We thank John Bol, Leiden University, for providing AMV strain 425 Leiden.
This research was supported by The Netherlands Foundation for Chemical Sciences (C.W.) with financial aid from The Netherlands Organization for Scientific Research (N.W.O.).
REFERENCES
- 1.Bishop D H L, Gay M E, Matsuoko Y. Nonviral heterogeneous sequences are present at the 5′ ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983;11:6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouloy M, Pardigon N, Vialat P, Gerbaud S, Girard M. Characterization of the 5′ and 3′ ends of viral messenger RNAs isolated from BHK21 cells infected with Germiston virus (bunyavirus) Virology. 1990;175:50–58. doi: 10.1016/0042-6822(90)90185-t. [DOI] [PubMed] [Google Scholar]
- 3.Collett M S. Messenger RNA of the M segment RNA of Rift Valley fever virus. Virology. 1986;151:151–156. doi: 10.1016/0042-6822(86)90114-5. [DOI] [PubMed] [Google Scholar]
- 4.De Haan P, Wagemakers L, Peters D, Goldbach R. The S RNA segment of tomato spotted wilt virus has an ambisense character. J Gen Virol. 1990;71:1001–1007. doi: 10.1099/0022-1317-71-5-1001. [DOI] [PubMed] [Google Scholar]
- 5.Estabrook E M, Tsai J, Falk B W. In vivo transfer of barley stripe mosaic hordeivirus ribonucleotides to the 5′ terminus of maize stripe tenuivirus RNAs. Proc Natl Acad Sci USA. 1998;95:8304–8309. doi: 10.1073/pnas.95.14.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcin D, Lezzi M, Dobbs M, Elliott R M, Schmaljohn C, Kang C Y, Kolakofsky D. The 5′ ends of Hantaan virus (Bunyaviridae) RNAs suggest a prime-and-realign mechanism for the initiation of RNA synthesis. J Virol. 1995;69:5754–5762. doi: 10.1128/jvi.69.9.5754-5762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurr S J, McPherson M J. Nucleic acids techniques. In: Gurr S J, McPherson M J, Bowles D J, editors. Molecular plant pathology: a practical approach. I. Oxford, United Kingdom: IRL Press; 1992. pp. 112–113. [Google Scholar]
- 8.Jin H, Elliott R M. Characterization of bunyamwera virus S RNA that is transcribed and replicated by the L protein expressed from recombinant vaccinia virus. J Virol. 1993;67:1396–1404. doi: 10.1128/jvi.67.3.1396-1404.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kormelink R, van Poelwijk F, Peters D, Goldbach R. Non-viral heterogeneous sequences at the 5′ ends of tomato spotted wilt virus mRNAs. J Gen Virol. 1992;73:2795–2804. doi: 10.1099/0022-1317-73-8-2125. [DOI] [PubMed] [Google Scholar]
- 10.Kormelink R, Kitajima E W, de Haan P, Zuidema D, Peters D, Goldbach R. The nonstructural protein (NSs) encoded by the ambisense S RNA segment of tomato spotted wilt virus is associated with fibrous structures in infected plant cells. Virology. 1994;181:459–468. doi: 10.1016/0042-6822(91)90878-f. [DOI] [PubMed] [Google Scholar]
- 11.Krug R M, Alonso-Caplen F V, Julkunen I, Katze M G. Expression and replication of the influenza virus genome. In: Krug R M, editor. The influenza viruses. New York, N.Y: Plenum Press; 1989. pp. 89–152. [Google Scholar]
- 12.Patterson J L, Kolakofsky D. Characterization of La Crosse virus small-genome transcripts. J Virol. 1984;49:680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotch S J, Bouloy M, Ulmanen I, Krug R M. A unique cap (m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23:847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- 14.Shih S-R, Krug R M. Surprising function of the three influenza viral polymerase proteins: selective protection of viral mRNAs against the cap-snatching reaction catalyzed by the same polymerase proteins. Virology. 1996;226:430–435. doi: 10.1006/viro.1996.0673. [DOI] [PubMed] [Google Scholar]
- 15.Simons J F, Pettersson R F. Host-derived 5′ ends and overlapping complementary 3′ ends of the two mRNAs transcribed from the ambisense S segment of Uukuniemi virus. J Virol. 1991;65:4741–4748. doi: 10.1128/jvi.65.9.4741-4748.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Poelwijk F, Kolkman J, Goldbach R. Sequence analysis of the 5′ ends of tomato spotted wilt virus N mRNAs. Arch Virol. 1996;141:177–184. doi: 10.1007/BF01718599. [DOI] [PubMed] [Google Scholar]