Abstract
Objective
To investigate the optimal management of patients with epidermal growth factor receptor gene (EGFR) mutant locally advanced non-small cell lung cancer (LA-NSCLC).
Methods
Patients with unresectable stage III lung adenocarcinoma (LAC) harboring EGFR mutations from 2012 to 2018 were analyzed retrospectively, and were categorized into three groups according to the primary treatment: chemoradiotherpy (CRT) (group 1), combined radiation therapy (RT) and EGFR-tyrosine kinase inhibitors (TKI) with/without chemotherapy (group 2), and EGFR-TKI alone until tumor progression (group 3). Inverse probability of multiple treatment weighting (IPTW) of propensity score was used to compare overall survival (OS) and progression free survival (PFS) between treatments and account for confounding.
Results
A total of 104, 105, and 231 patients were categorized into groups 1, 2, and 3, respectively. After IPTW adjustment, the median PFS for each group was 12.4, 26.2, and 16.2 months (log-rank P < 0.001), and the median OS was 51.0, 67.4 and 49.3 months (log-rank P = 0.084), respectively. Compared with those in group 1, patients in group 2 had significantly improved PFS [adjusted hazard ratio HR (aHR), 0.40; 95% confidence interval (CI): 0.29, 0.54; P < 0.001] and OS (aHR, 0.61; 95% CI: 0.38, 0.98; P = 0.039). Patients in group 3 had prolonged PFS (aHR, 0.66; 95% CI: 0.50, 0.87; P = 0.003), but not OS (aHR, 0.90; 95% CI: 0.62, 1.32; P = 0.595). Doubly robust IPTW analysis and multivariable Cox regression analysis yielded similar findings.
Conclusions
EGFR-TKIs after chemoradiation or combined with radiation alone correlated with the longest PFS and OS (versus CRT or TKIs alone) in patients with EGFR-mutant unresectable LA-NSCLC. Well-designed prospective trials were warranted.
Keywords: Non-small cell lung cancer, Radiotherapy, Epidermal growth factor receptor kinase, Protein kinase inhibitor
1. Introduction
Non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) activating mutations forms a distinct subgroup, accounting for 10% of the Caucasian patients with stage-IV lung adenocarcinoma (LAC) and up to 50% of Asian patients.1 It has a unique biology with a high response rate (∼70%) to targeted therapy with EGFR-tyrosine kinase inhibitors (TKIs), more sensitive to radiation therapy, and a poor response to immunotherapy.2, 3, 4 Therefore, genotype-directed therapy has been established in routine clinical practice for patients with stage-IV NSCLC.
Approximately 30% of patients with NSCLC have locally advanced disease (LA-NSCLC).5 In contrast to stage-IV NSCLC, ∼15–20% of patients with LA-NSCLC treated with chemoradiotherapy (CRT) are curable. Concurrent CRT is currently the standard care, and consolidation immunotherapy is recommended for those without progression after concurrent CRT.6 No evidence has supported genotype-driven treatment in an LA-NSCLC cohort because most of the patients enrolled in randomized phase-3 trials were current or former smokers, who rarely have EGFR mutations. For example, only 6.0% of the patients had EGFR mutations in the PACIFIC study, and no significant benefit with durvalumab was observed in this prespecified subgroup. Therefore, whether overall survival (OS) benefit favored durvalumab in EGFR-mutant LA-NSCLC remained inconclusive. Several retrospective small studies (n < 40) indicate that these patients might have distinct clinical features, including a shorter progression-free survival (PFS) after CRT and a higher likelihood of distant metastases,7, 8, 9, 10, 11, 12, 13, 14 which highlight the potential benefit of early use of EGFR-TKI targeted therapy.
In China, CRT is used to treat unresectable stage-III EGFR-mutant LAC according to the current National Comprehensive Cancer Network (NCCN) guidelines.15 However, considering the lower toxicity profile and better health-related quality of life of stage-IV patients receiving EGFR-TKI, some Chinese patients with EGFR-mutant LA-NSCLC choose target therapy alone and refuse CRT as their primary treatment. Currently, there is no evidence at all to either support or discourage EGFR-TKI monotherapy for patients with LA-NSCLC suitable for thoracic RT. Recognizing the paucity of data for patients with unresectable stage-III NSCLC harboring EGFR mutations, we conducted a retrospective analysis (REFRACT program: radiotherapy in EGFR-mutant locally-advanced lung cancer study) using individual patient data from 12 Chinese academic cancer institutions to address this evidence gap.
2. Materials and methods
2.1. Study design and participants
We retrospectively collected data from 12 Chinese academic cancer institutions between January 2012 and December 2018 for patients with LA-NSCLC harboring EGFR mutations (Supplementary Table 1). Patients with anaplastic lymphoma kinase (ALK) gene rearrangements were excluded. Available baseline data and clinical and imaging follow-up data (for > 6 months) were required to assess the outcomes.
Based on the primary treatment pattern, patients were categorized as follows: group 1: concurrent or sequential CRT; group 2: EGFR-TKIs after CRT or combined with RT alone; group 3: EGFR-TKIs alone until tumor progression. Generally, patients were followed up every 3 months for the first year, then every 3 to 6 months thereafter. Intensity modulated radiation therapy (IMRT) was used preferred. The gross tumor volume (GTV) included the primary tumor as well any involved regional lymph node (GTV-nd). Clinical target volume (CTV) included GTV plus a 6–8 mm margin, ipsilateral hilum and involved lymph node regions, the planning target volume (PTV) included the CTV plus 5 mm.
Molecular pathology associated with EGFR mutations was evaluated using either polymerase chain reaction (PCR) amplification or next generation sequencing. Cell-free DNA testing was performed in specific circumstances if insufficient tissue was available for molecular analysis.
2.2. Outcomes
Both PFS and OS were calculated from the date of diagnosis. The efficacy of salvage targeted therapy was assessed by determining the PFS2 as the interval between the first progression and the second progression, death, or last follow-up.
2.3. Statistical analysis
Patient and tumor characteristics were compared using the χ2 test or Fisher's exact test. OS, PFS, and PFS2 were estimated using the Kaplan-Meier method. The roles of different treatment regimens and the baseline characteristics in OS and PFS were explored using log-rank tests and the Cox proportional hazard regression model. Formal test16 and Scaled Schoenfeld residuals17 were used to assess the proportional hazards assumptions when necessary. To properly evaluate the failure patterns, the first site of recurrence (local regional or distant) was analyzed by considering death as a competing risk, respectively.18 Cumulative incidence curves and Fine-Gray regression models for subdistribution hazard were then used to summarize and evaluate the first recurrences in different treatment groups.
To reduce the effects of potential confounding factors in the comparison of different clinical outcomes among the three treatment groups while maximizing the effective sample sizes, inverse-probability of treatment weighting (IPTW) based on a multinomial propensity score model was used. The multinomial propensity score was estimated using generalized boosted regression model19 included the following potential confounders: age, sex, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, American Joint Committee on Cancer (AJCC) stages, positron emission tomography-computed tomography (PET-CT) staging, and mutation type. Standardized mean differences in covariate values were used to assess the covariate balance in the IPTW sample. Both unadjusted and IPTW-adjusted Kaplan-Meier estimates of OS and PFS rates, as well as cumulative incidences of failure patterns were reported. For sensitivity analyses, conventional multivariable Cox regression analyses, as well as doubly-robust IPTW analysis using Cox model,20 were both performed to adjust potential residual confounding. A two-sided P < 0.05 was considered statistically significant, except for OS per the study design. All analyses were performed using STATA software, version 16.0 (StataCorp, College Station, TX, USA) and R software (version 3.4.2).
3. Results
3.1. Patient characteristics
440 patients were included to assess the outcomes, accounting for 24.1% of all the patients with unresectable stage III lung adenocarcinoma screened. 104 patients (23.6%) were assigned to group 1, 105 (23.9%) were assigned to group 2, and 231 (52.5%) were assigned to group 3. As shown in Table 1, the baseline characteristics were well balanced except that patients receiving EGFR-TKIs alone were older (P = 0.001). 57.0% patients used PCR and 30.7% was next generation sequencing. In the induction phase, platinum-based doublet agents regimens recommended by NCCN guideline of NSCLC were used (including pemetrexed, paclitaxel, or gemcitabine and platinum). In the concurrent chemotherapy, pemetrexed and cisplatin, or etoposide and cisplatin were used. 98.2% of the patients first-line use of TKIs (group 2 and 3) received gefitinib, erlotinib, or Icotinib. 82.4% of the patients progressed in group 1 received salvage EGFR-TKIs, among whom 90.7% were treated with gefitinib, erlotinib, or Icotinib.
Table 1.
Demographic and baseline clinical characteristics of patients.
| Unweighted, number (%) |
Weighed, mean (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Overall (n = 440) |
CRT (n = 104) |
EGFR-TKI+RT (n = 105) |
EGFR-TKI (n = 231) |
P | EGFR-TKI+RT vs. CRT | EGFR-TKI vs. CRT | EGFR-TKI+RT vs. EGFR-TKI |
| Age | ||||||||
| Median (range) | 61 (30–89) | 58 (30–82) | 56 (37–80) | 63 (30–89) | 0.001 | |||
| ≥ 60 | 225 (51.1%) | 46 (44.2%) | 42 (40.0%) | 137 (59.3%) | 49.3 | 48.3 | 49.3 | |
| < 60 | 215 (48.9%) | 58 (55.8%) | 63 (60.0%) | 94 (40.7%) | 50.7 | 51.7 | 50.7 | |
| Sex | ||||||||
| Female | 265 (60.2%) | 54 (51.9%) | 62 (59.0%) | 149 (64.5%) | 0.090 | 60.8 | 60.4 | 60.8 |
| Male | 175 (39.8%) | 50 (48.1%) | 43 (41.0%) | 82 (35.5%) | 39.2 | 39.6 | 39.2 | |
| Smoking | ||||||||
| Yes | 130 (29.5%) | 37 (35.6%) | 25 (23.8%) | 68 (29.4%) | 0.176 | 26.3 | 29.4 | 26.3 |
| No | 310 (70.5%) | 67 (64.4%) | 80 (76.2%) | 163 (70.6%) | 73.7 | 70.6 | 73.7 | |
| ECOG PS | ||||||||
| < 2 | 417 (94.8%) | 99 (95.2%) | 103 (98.1%) | 215 (93.1%) | 0.155 | 96.9 | 95.0 | 96.9 |
| ≥ 2 | 23 (2.8%) | 5 (4.8%) | 2 (1.9%) | 16 (6.9%) | 3.1 | 5.0 | 3.1 | |
| Stage | ||||||||
| III A | 155 (35.2%) | 30 (28.8%) | 44 (41.9%) | 81 (35.1%) | 0.142 | 33.6 | 34.3 | 33.6 |
| III B | 285 (64.8%) | 74 (71.2%) | 61 (58.1%) | 150 (64.8%) | 66.4 | 66.7 | 66.4 | |
| PET-CT | ||||||||
| Yes | 126 (28.6%) | 38 (36.5%) | 38 (36.2%) | 50 (21.6%) | 0.003 | 70.6 | 71.3 | 70.6 |
| No | 314 (71.4%) | 66 (63.5%) | 67 (63.8%) | 181 (78.4%) | 29.4 | 28.7 | 29.4 | |
| EGFR mutation | ||||||||
| 19DEL | 217 (49.3%) | 53 (51.0%) | 53 (50.5%) | 111 (48.1%) | 0.559 | 49.1 | 49.8 | 49.1 |
| L858R | 186 (42.3%) | 39 (37.5%) | 43 (41.0%) | 104 (45.0%) | 43.9 | 43.3 | 43.9 | |
| Uncommon | 37 (8.4%) | 12 (11.5%) | 9 (8.3%) | 16 (6.9%) | 6.9 | 6.9 | 6.9 | |
Abbreviations: CRT, chemoradiation therapy; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; PET-CT, positron emission tomography-computed tomography; RT, radiotherapy; TKI, tyrosine kinase inhibitor.
3.2. Univariate analysis of survival outcomes
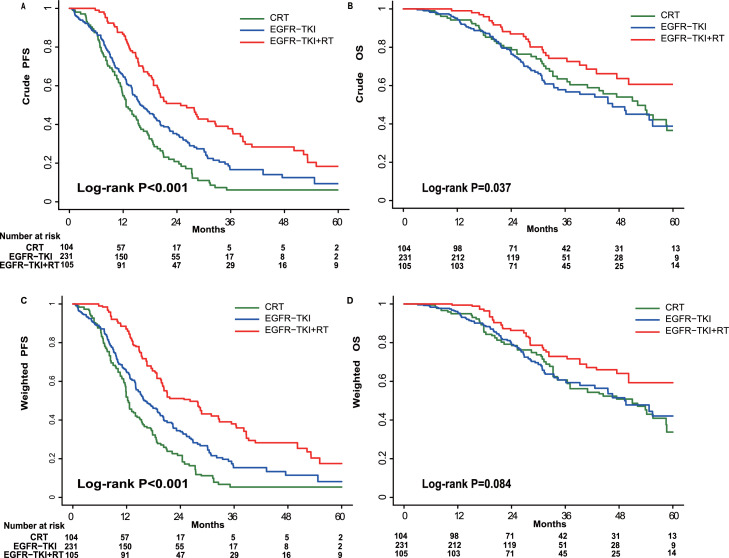
For the entire cohort with a median follow-up of 35.9 months [interquartile range (IQR): 23.8–53.6], the crude median PFS and OS were 16.9 months [95% confidence interval (CI): 15.3, 18.5] and 53.9 months (95% CI: 46.6, 61.1), respectively.
The median PFSs for groups 1–3 were 12.6 months (95% CI: 10.5, 14.7), 24.8 months (95% CI: 17.6, 32.1), and 15.9 months (95% CI: 13.1, 18.6), respectively (log-rank P < 0.001; Fig. 1A). The median OS for groups 1–3 were 52.1 months (95% CI: 41.7, 57.5), 67.4 months (95% CI: 56.8, 78.0), and 46.5 months (95% CI: 35.5, 57.6), respectively (log-rank P = 0.037; Fig. 1B). The 3 and 5-year OS rates were 63.5% (95% CI: 52.4%, 72.7%) and 33.8% (95% CI: 21.1%, 47.0%) for group 1, 74.2% (95% CI: 63.0%, 82.5%) and 60.6% (95% CI: 46.6%, 72.1%) for group 2, and 57.8% (95% CI: 49.3%, 65.5%) and 38.8% (95% CI: 27.0%, 50.5%) for group 3, respectively. Notably, the OS was similar in group 2 with or without chemotherapy (CT) [hazard ratio (HR), 0.70; 95% CI: 0.32,1.53; P = 0.367]. In group 1, the PFS and OS was similar for the concurrent versus sequential irradiation and chemotherapy (P = 0.904; P = 0.896) respectively (Supplementary Fig. 1).The PFS2 of salvage EGFR-TKI therapy for patients receiving CRT was 10.2 months (95% CI: 7.6, 12.8).
Fig. 1.
Progression-free survival (PFS) and overall survival (OS) evaluated using the Kaplan–Meier method before (A, B) and after (C, D) inverse-probability of treatment weighting analysis for overall corhorts. CRT, chemoradiation therapy; EGFR, epidermal growth factor receptor; RT, radiation therapy; TKI, tyrosine kinase inhibitor.
3.3. IPTW analysis of survival outcomes
The propensity score model consisted of age, sex, ECOG performance status, smoking status, disease stage, PET-CT staging, and the EGFR mutation type. As shown in Table 1, IPTW adjustment resulted in an excellent balance of baseline characteristics among treatment groups (Supplementary Table 2 and 3).
The IPTW-adjusted median PFS were 12.4 months (95% CI: 11.4, 15.5), 26.2 months (95% CI: 19.8, 36.4), and 16.2 months (95% CI:14.1, 19.5) (log-rank P < 0.001), and median OS were 51.0 months (95% CI: 36.4, 60.7), 67.4 months [95% CI: 50.1, not reached (NR)], and 49.3 months (95% CI: 39.3, NR) (log-rank P = 0.084) in groups 1, 2, and 3, respectively (Fig. 1C and D). As summarized in Table 2, consistent with the results for the overall study population, compared to patients who received CRT, patients receiving TKIs after CRT or combined with RT showed significantly improved PFS [adjusted HR (aHR), 0.40; 95% CI: 0.29, 0.54; P < 0.001) and OS (aHR, 0.61; 95% CI: 0.38, 0.98; P = 0.039). TKI treatment alone prolonged the PFS (aHR, 0.66; 95%CI: 0.50, 0.87; P = 0.003) but not OS (aHR, 0.90; 95% CI: 0.62, 1.32; P = 0.595).
Table 2.
Summary of unadjusted and propensity score-weighted analysis results.
| Treatment | Hazard ratio (95% CI) | P |
|---|---|---|
| PFS | ||
| Unadjusted | ||
| EGFR-TKI+RT vs CRT | 0.43 (0.32–0.59) | < 0.001 |
| EGFR-TKI vs CRT | 0.69 (0.54–0.89) | 0.005 |
| EGFR-TKI+RT vs EGFR-TKI | 0.63 (0.48–0.82) | 0.001 |
| Propensity score adjusted | ||
| EGFR-TKI+RT vs CRT | 0.40 (0.29–0.54) | < 0.001 |
| EGFR-TKI vs CRT | 0.66 (0.50–0.87) | 0.003 |
| EGFR-TKI+RT vs EGFR-TKI | 0.60 (0.46–0.79) | < 0.001 |
| OS | ||
| Unadjusted | ||
| EGFR-TKI+RT vs CRT | 0.62 (0.40–0.96) | 0.035 |
| EGFR-TKI vs CRT | 1.04 (0.73–1.48) | 0.875 |
| EGFR-TKI+RT vs EGFR-TKI | 0.59 (0.39–0.90) | 0.017 |
| Propensity score adjusted | ||
| EGFR-TKI+RT vs CRT | 0.61 (0.38–0.98) | 0.039 |
| EGFR-TKI vs CRT | 0.90 (0.62–1.32) | 0.595 |
| EGFR-TKI+RT vs EGFR-TKI | 0.68 (0.43–1.06) | 0.089 |
Abbreviations: CI, confidence interval; CRT, chemoradiation therapy; EGFR, epidermal growth factor receptor; OS, overall survival; PFS, progression-free survival; Propensity score adjusted, score adjusted based on the inverse probability of multiple treatment weighting method; RT, radiotherapy; TKI, tyrosine kinase inhibitor.
Similar results were found by doubly robust IPTW analysis and multivariable Cox regression analysis (Supplementary Table 4 and 5). TKIs after CRT or combined with RT significantly improved both the PFS (aHR, 0.39; 95% CI: 0.29, 0.54; P < 0.001) and OS (aHR, 0.60; 95% CI: 0.37, 0.97; P = 0.036) of patients. TKI treatment alone prolonged the PFS (aHR, 0.69; 95%CI: 0.52, 0.90; P = 0.007) but not the OS (aHR, 0.91; 95% CI: 0.63,1.32; P = 0.630).
Because uncommon EGFR mutations (such as exon 20 insertion) were with lower likelihood of response to EGFR-TKI, a sensitivity analysis was conducted by excluding patients with uncommon EGFR mutations (Supplementary Fig. 2). Among those who had activating EGFR mutations [exon 19 deletion (19DEL) or L858R], the effect sizes of receiving RT + TKI with/without CT vs CRT alone are similar to those in full cohort, e.g., PFS aHR was 0.37 (95% CI: 0.26, 0.52; P < 0.001), OS aHR was 0.61 (95% CI: 0.45, 0.82; P = 0.082), with expected wider CIs due to reduced number of PFS and OS events.
3.4. Initial failure patterns and the efficacy of salvage EGFR-TKI after CRT
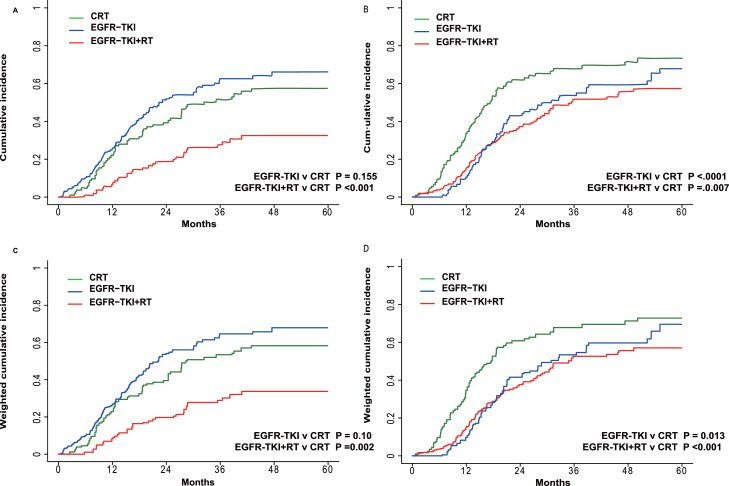
Using an IPTW-adjusted Fine-Gray regression model for competing risks, RT + TKIs with/without CT was associated with a lower probability of local regional recurrence, with an aHR of 0.48 (95% CI: 0.31, 0.77; P = 0.002; Fig. 2A and C; Supplementary Table 6). Both RT + TKIs with/without CT and TKIs alone correlated with a lower probability of initial distant metastasis with aHRs of 0.62 (95% CI: 0.42, 0.90; P = 0.013) and 0.56 (95% CI: 0.39, 0.79; P < 0.001), respectively (Fig. 2B and D; Supplementary Table 7). Similar findings were found in multivariable competing risk analyses.
Fig. 2.
The incidence of locoregional failure and distant progression determined by competing risk analysis before (A, B) and after (C, D) inverse-probability of treatment weighting analysis. CRT, chemoradiation therapy; EGFR, epidermal growth factor receptor; RT, radiation therapy; TKI, tyrosine kinase inhibitor.
4. Discussion
We detected genomic EGFR alterations in approximately 24% of the tested Chinese patients with unresectable stage III LAC, which was significantly lower than that reported for stage IV Asian patients.21 Similar to finds with advanced patients,22 EGFR mutations were more prevalent in females and non-smokers in a locally advanced setting. More T1-T2 lesions were present in these patients than patients with in wild-type EGFR. These findings are important for understanding the biological features of EGFR-mutant NSCLC.
The efficacy of consolidation durvalumab after CRT remained unsatisfactory in this patient population. the distant relapse occurred more frequently (especially brain metastases), suggesting that an effective systemic consolidation regimen should be developed for this population. Moreover, in PACIFIC, the median PFS of 10.3 months among EGFR-mutant patients was significant shorter as compared with the overall cohort (17.2 months). Similarly, in a recent retrospective, multicenter institution series, patients with EGFR-mutant LA-NSCLC had a median PFS of 10.3 months and might experience a high frequency of immune-related adverse events (irAEs).23 For stage III NSCLC, longer PFS or disease-free survival (DFS) was demonstrated in response to the combination of EGFR-TKI and local therapy (surgery or radiotherapy), versus combined local therapy and chemotherapy, in subgroup analyses of two phase III studies.24,25 Similary to our findings, Aredo JV, et al. recently reported that CRT and EGFR-TKI consolidation was associated with a significantly longer median PFS (26.1 months) compared to durvalumab following CRT or CRT alone (log-rank P = 0.023).23 However, it remains unknown whether the combination of genotype-driven EGFR-TKI and local therapy can improve OS. In our series, a median OS of 67.4 months in the RT + TKI group was observed, which was longer compared to the CRT group (51.0 months) and the TKI-alone group (49.3 months). The median OS of the CRT group in our study was in line with similar historical data (median OS, 34.6–51.1 months; Table 3). Using doubly robust propensity score IPTW analysis and multivariable Cox-regression analysis, the survival differences were still significant among all three treatment groups, suggesting that the improved OS seen in the TKIs after CRT or combined with RT group was likely due to effective treatment, after accounting for potential selection bias or confounding factors. Similarly, a population-based retrospective study also revealed that thoracic RT after EGFR-TKI treatment was an independent prognostic factor for OS in patients with stage IIIB EGFR-mutant NSCLC.26
Table 3.
Comparison with different treatments studies for EGFR mutant LA-NSCLC.
| Study | Type | Center | No. of patients |
Treatment | mFU (months) | ORR | mPFS (months) | MST (months) |
Pneumonitis incidence |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G2 | G3-G4 | G5 | |||||||||
| CRT | |||||||||||
| Kosuke Tanaka, et al. (2015)5 | R | S | 29 | CCRT | 35 | 74% | 9.8 | 51.1 | NA | NA | NA |
| Yu Jin Lim, et al. (2017)6 | R | S | 26 | CCRT | 22.4 | 89% | NA | 3 years: 68% | NA | NA | NA |
| Song Ee Park, et al. (2019)7 | R | S | 36 | CCRT | 66.5 | 72.2% | 8.9 | 34.6 | NA | NA | NA |
| Masaki Nakamura, et al. (2019)8 | R | S | 34 | CCRT/SCRT | 36.0 | NA | 3 years: 15% | 3 years: 46% | NA | NA | NA |
| Shigehiro Yagishita, et al. (2014)9 | R | S | 34 | CCRT/SCRT | 29.0 | 79% | NA | 46.9 | NA | NA | NA |
| Hiroaki Akamatsu, et al. (2014)10 | R | S | 13 | CCRT | NA | 76.9% | 9.6 | 57.9 | NA | NA | NA |
| HIDETOSHI HAYASHI, et al. (2012)11 | R | S | 11 | CCRT/SCRT | 20.7 | 90.9% | 13.1 | 67.5 | NA | NA | NA |
| Hidehito Horinouchi, et al. (2020)12 | R | M | 29 | CCRT/SCRT | 31.6 | NA | 16.9 | NA | NA | NA | NA |
| Jacqueline V. Aredo, et al. (2021)20 | R | M | 16 | CCRT | 14.5 | NA | 6.9 | NA | 8.3% | 16.7% | 0 |
| Our Study | R | M | 104 | CCRT/SCRT | 49.3 | NA | 12.4 | 51.0 | NA | NA | 0 |
| CRT/RT+TKI | |||||||||||
| LOGIK0902/OLCSG0905 (2021)32 | P | M | 20 | TKI +CCRT | 47.5 | 75% | 2 years: 37% | 2 years: 90% | NA | 0 | 0 |
| RECEL (2021)26 | P | M | 20 | RT+TKI | 21.3 | 70% | 24.5 | NA | NA | 16.7% | 0 |
| WJOG6911L (2021)27 | P | M | 28 | RT+TKI | 51.8 | 81.5% | 18.6 | 61.1 | 30% | 4% | 0 |
| Jacqueline V Aredo, et al. (2021)20 | R | M | 8 | CRT+TKI | 14.5 | NA | 26.1 | NR | NA | NA | NA |
| Our Study | R | M | 105 | CRT/RT+TKI | 38.1 | NA | 26.2 | 67.4 | NA | NA | 0 |
| TKI alone | |||||||||||
| Ranpu Wu, et al. (2021)33 | R | S | 63 | TKI | NA | NA | 13.87 | 41.47 | NA | NA | NA |
| Our Study | R | M | 231 | TKI | 30.7 | NA | 16.2 | 49.3 | NA | NA | 0 |
Abbreviations: CCRT, concurrent chemoradiotherapy; CRT, chemoradiation therapy; EGFR, epidermal growth factor receptor; LA-NSCLC, locally advanced non-small cell lung cancer; M, multi-center; mFU, median follow-up time; mPFS, median progression-free survival; MST, median overall survival time; NA, not available; ORR, objective response rate; P, perspective; R, retrospective; RT, radiation therapy; S, single center; SCRT, sequential chemoradiotherapy; TKI, Tyrosine kinase inhibitor.
Improved OS in patients who received RT + TKI might be explained by the effective control of both local regional and distant diseases. Definitive RT may control intrathoracic tumors, whereas EGFR-TKI simultaneously control potentially micrometastatic disease, resulting in prolonged survival. Using a competing-risks regression model, RT + TKI was associated with the lowest probabilities of both local regional progression (aHR, 0.48; 95% CI: 0.31, 0.77; P = 0.002) and distant metastasis progression (aHR, 0.62; 95% CI: 0.42, 0.90; P < 0.001) in our study. Multiple findings have shown that EGFR-mutant NSCLC is highly radiosensitive in both preclinical and clinical settings.27,28 However, locoregional failure became the most dominant failure pattern in the TKI alone group, resulting in shorter survival, demonstrating that initial definitive thoracic RT is indispensable for LA-NSCLC.
It remains unknown how to optimally administer EGFR-TKI with RT for improved efficacy and minimized toxicity in unresectable stage III EGFR-mutant NSCLC. A multicenter, randomized phase 2 trial (RECEL) comparing erlotinib (up to 2 years) with concurrent RT (E+RT) versus concurrent chemoradiation (NCT01714908) found that a significantly longer PFS (median: 24.5 vs 9.0 months; HR, 0.104; 95% CI: 0.028, 0.389; P < 0.001) for E+RT versus concurrent CRT.29 The results of a single arm phase-II study (West Japan Oncology Group 6911L) demonstrated that gefitinib treatment (up to 2 years) with concurrent RT (total of 64 Gy) resulted in an encouraging median PFS of 18.6 months (95%CI: 12.0, 24.5) and a median OS of 61.1 months (95%CI: 38.1, NR), respectively. Only 2 patients (7.4%) failed within radiation field. The most common site of initial recurrence was the brain, which provides support for investigation of third-generation EGFR-TKI in this patients population.30
The lung toxicity of the concurrent use of RT and EGFR‐TKI is a particular concern. In the recent RECEL study, 16.7% of patients developed grades 3 to 4 radiation pneumonitis (RP) in the concurrent RT and erlotinib group, and no grade 5 RP was observed.29 In the WJOG6911L study, all events of pneumonitis were mild (grade 2: 30.0%; no grade 3–5) when patients were treated with concurrent gefitinib and RT.30 Xu KP, et al. retrospectively analyzed 45 patients treated with concurrent RT and EGFR-TKI during 2012 to 2017. 37.7% of these patients developed symptomatic RP (grades 2+) and 6.7% had severe (grade 3) RP. However, no grade 4 to 5 adverse events and limited late lung toxicity were observed.31 These results demonstrated the feasibility of thoracic RT plus concurrent EGFR-TKI with the improvements in the RT techniques. However, pulmonary toxicity should be carefully monitored.
This study had a few limitations. Although we attempt to balance most baseline characteristics using IPTW method and achieved well balance, the selection bias could not be excluded owing to our retrospective design and our results needed to be discussed critically. First, although the OS of the CRT group was in line with historical data with higher PET/CT staging utility, only approximately 30% patients had a baseline PET scan staging. Second, the details of toxicities could not be determined except for no treatment-related deaths (G5 toxicities). Third, there were no control on the assessment schedules, while the magnitude of PFS benefits is large enough (more than 2 scheduled visits) to conclude the PFS benefits should be robust. Moreover, recent studies suggested that co-occurring TP53 mutations might result in poor response to EGFR-TKI therapy. Although we excluded patients with concurrent ALK rearrangement, information regarding co-curring mutations of other important genes, such as TP53 or KRAS, were not available in our database. Finally, because the ethnic susceptibility of patients with EGFR mutations is unclear, our results should be validated with different ethnic populations.
5. Conclusions
To the best of our knowledge, this study is the largest multi-center cohort analysis of patients with EGFR-mutant LA-NSCLC, and found that the use of CRT followed by EGFR-TKIs or EGFR-TKIs combined with RT correlated with the longest PFS and OS in both doubly robust propensity score IPTW analysis and multivariable Cox-regression analysis, suggesting that the improved outcomes might be likely due to effective treatment. Well designed prospective trials are warranted. The randomized phase III LAURA trial (NCT03521154) is underway to assess the efficacy of osimertinib consolidation after concurrent CRT. The other randomized, multicenter phase III trial (ChiCTR2000040590) evaluating almonertinib (HS-10296, a novel third-generation EGFR-TKI) with RT versus concurrent CRT is ongoing in China. The findings of these phase III studies will shed more light on the standard care for patients with EGFR-mutant unresectable LA-NSCLC.
Declaration of competing interest
The authors declare that they have no conflict of interests. Part of this study was oral presented at 2020 World Conference on Lung Cancer (WCLC), Worldwide Virtual Event, Jan 28–31, 2021.
Acknowledgments
Ethics statement
It was conducted in compliance with the principles of the Declaration of Helsinki and approved by the ethics review boards and the study was registered with Clinical Trials.gov. (Trial number: NCT04304638). Written informed consent was waived since this is a retrospective analysis.
Acknowledgments
This trial was founded by the National Natural Sciences Foundation Key Program (grant number: 81572971); CAMS Innovation Fund for Medical Sciences (grant number: 2017-I2M-1-005); Sanming Project of Medicine in Shenzhen (grant number: SZSM201612063); National Natural Sciences Foundation Key Program of China (grant number: 81572971) and National Key R&D Program of China (grant number: 2018YFC1312104).
Data sharing
After deidentification, individual participant data will be made available to investigators who provide a methodologically sound proposal for individual patient based meta-analyses. Proposals should be directed to Luhua Wang (wlhwq@yahoo.com). Data requestors should sign a data access agreement.
Author contributions
N.B., K.X., C.H. and L.W. designed the study, analyzed the data, and wrote the manuscript. N.B., K.X., H.G., M.C., M.E., L.H., J.C., X.H., X.D., B.X., L.Z., L.H., J.L. contributed to data collection and enrolled patients. N.B., K.X., and C.H. did the statistical analysis. All authors approved the final report to submit for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jncc.2022.11.003.
Contributor Information
Chen Hu, Email: chu22@jhmi.edu.
Luhua Wang, Email: wlhwq@yahoo.com.
Appendix. Supplementary materials
References
- 1.Hirsch F.R., Bunn P.A., Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol. 2009;10(5):432–433. doi: 10.1016/s1470-2045(09)70110-x. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R., Carcereny E., Gervais R., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/s1470-2045(11)70393-x. [DOI] [PubMed] [Google Scholar]
- 3.Mok T.S., Wu Y.L., Thongprasert S., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Negrao M.V., Skoulidis F., Montesion M., et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J Immunother Cancer. 2021;9(8) doi: 10.1136/jitc-2021-002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang P., Allen M.S., Aubry M.C., et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128(1):452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 6.Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi: 10.1200/jco.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K., Hida T., Oya Y., et al. EGFR mutation impact on definitive concurrent chemoradiation therapy for inoperable stage III adenocarcinoma. J Thorac Oncol. 2015;10(12):1720–1725. doi: 10.1097/jto.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 8.Lim Y.J., Chang J.H., Kim H.J., et al. Superior treatment response and in-field tumor control in epidermal growth factor receptor-mutant genotype of stage III nonsquamous non-small cell lung cancer undergoing definitive concurrent chemoradiotherapy. Clin Lung Cancer. 2017;18(3):e169–e178. doi: 10.1016/j.cllc.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Park S.E., Noh J.M., Kim Y.J., et al. EGFR mutation is associated with short progression-free survival in patients with stage III non-squamous cell lung cancer treated with concurrent chemoradiotherapy. Cancer Res Treat. 2019;51(2):493–501. doi: 10.4143/crt.2018.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura M., Kageyama S.I., Niho S., et al. Impact of EGFR mutation and ALK translocation on recurrence pattern after definitive chemoradiotherapy for inoperable stage III non-squamous non-small-cell lung cancer. Clin Lung Cancer. 2019;20(3):e256–e264. doi: 10.1016/j.cllc.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Yagishita S., Horinouchi H., Katsui Taniyama T., et al. Epidermal growth factor receptor mutation is associated with longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;91(1):140–148. doi: 10.1016/j.ijrobp.2014.08.344. [DOI] [PubMed] [Google Scholar]
- 12.Akamatsu H., Kaira K., Murakami H., et al. The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who recieved concurrent chemoradiotherapy. Am J Clin Oncol. 2014;37(2):144–147. doi: 10.1097/COC.0b013e31826e04f9. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi H., Okamoto I., Kimura H., et al. Clinical outcomes of thoracic radiotherapy for locally advanced NSCLC with EGFR mutations or EML4-ALK rearrangement. Anticancer Res. 2012;32(10):4533–4537. [PubMed] [Google Scholar]
- 14.Horinouchi H., Atagi S., Oizumi S., et al. Real-world outcomes of chemoradiotherapy for unresectable Stage III non-small cell lung cancer: the SOLUTION study. Cancer Med. 2020;9(18):6597–6608. doi: 10.1002/cam4.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettinger D.S., Wood D.E., Aggarwal C., et al. NCCN guidelines insights: non-small cell lung cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
- 16.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. doi: 10.2307/2337123. [DOI] [Google Scholar]
- 17.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–241. doi: 10.1093/biomet/69.1.239. [DOI] [Google Scholar]
- 18.Kalbfleisch J.D., Prentice R.L. 2nd Edition. Technometrics. John Wiley & Sons Inc; 2002. The Statistical Analysis of Failure Time Data. [Google Scholar]
- 19.Friedman J., Hastie T., Tibshirani R. Additive logistic regression: a statistical view of boosting (with discussion and a rejoinder by the authors) Ann Stat. 2000;28(2):337–407. [Google Scholar]
- 20.Lunceford J.K., Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y., Au J.S., Thongprasert S., et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9(2):154–162. doi: 10.1097/jto.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellanos E., Feld E., Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12(4):612–623. doi: 10.1016/j.jtho.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Aredo J.V., Mambetsariev I., Hellyer J.A., et al. Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 2021;16(6):1030–1041. doi: 10.1016/j.jtho.2021.01.1628. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y.L., Tsuboi M., He J., et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 25.Zhong W.Z., Wang Q., Mao W.M., et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139–148. doi: 10.1016/s1470-2045(17)30729-5. [DOI] [PubMed] [Google Scholar]
- 26.Yen Y.C., Hsu H.L., Chang J.H., et al. Efficacy of thoracic radiotherapy in patients with stage IIIB-IV epidermal growth factor receptor-mutant lung adenocarcinomas who received and responded to tyrosine kinase inhibitor treatment. Radiother Oncol. 2018;129(1):52–60. doi: 10.1016/j.radonc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Huang S.M., Li J., Armstrong E.A., et al. Modulation of radiation response and tumor-induced angiogenesis after epidermal growth factor receptor inhibition by ZD1839 (Iressa) Cancer Res. 2002;62(15):4300–4306. [PubMed] [Google Scholar]
- 28.Bianco C., Tortora G., Bianco R., et al. Enhancement of antitumor activity of ionizing radiation by combined treatment with the selective epidermal growth factor receptor-tyrosine kinase inhibitor ZD1839 (Iressa) Clin Cancer Res. 2002;8(10):3250–3258. [PubMed] [Google Scholar]
- 29.Xing L., Wu G., Wang L., et al. Erlotinib versus etoposide/cisplatin with radiation therapy in unresectable stage III epidermal growth factor receptor mutation-positive non-small cell lung cancer: a multicenter, randomized, open-label, phase 2 trial. Int J Radiat Oncol Biol Phys. 2021;109(5):1349–1358. doi: 10.1016/j.ijrobp.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Akamatsu H., Murakami H., Harada H., et al. Gefitinib with concurrent thoracic radiotherapy in unresectable locally advanced NSCLC with EGFR mutation; West Japan oncology group 6911L. J Thorac Oncol. 2021;16(10):1745–1752. doi: 10.1016/j.jtho.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Xu K., Liang J., Zhang T., et al. Clinical outcomes and radiation pneumonitis after concurrent EGFR-tyrosine kinase inhibitors and radiotherapy for unresectable stage III non-small cell lung cancer. Thorac Cancer. 2021;12(6):814–823. doi: 10.1111/1759-7714.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hotta K., Saeki S., Yamaguchi M., et al. Gefitinib induction followed by chemoradiotherapy in EGFR-mutant, locally advanced non-small-cell lung cancer: LOGIK0902/OLCSG0905 phase II study. ESMO Open. 2021;6(4) doi: 10.1016/j.esmoop.2021.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu R., Yu S., Ye J., et al. A multicenter retrospective study on the prognosis of stage III unresectable mutant non-small cell lung cancer with tyrosine kinase inhibitors therapy. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.692703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.