Abstract
Objective
Mathematical modeling and simulation is a useful research method to inform decision-making. This article aims to describe the National Cancer Center (NCC) modeling framework and how well it reproduces observed empirical data for six major cancers.
Methods
We developed the NCC modeling framework for six major cancers in China (lung, liver, stomach, colorectal, esophageal, and breast), which simulates the life-histories represented by states among normal, precancerous lesion, stage-specific invasive cancer, and death for six cancers separately. Each NCC simulation model could be illustrated as an integrated framework of 3 modules: a demography module, natural history module, and screening module. Combined with costs and health utilities data, the models could have many detailed outputs for informing decisions, including the harm of screening (e.g., false positives, complications, and overdiagnosis), healthcare costs, and benefits (quality-adjusted life years gained, cancer incidence and mortality, and investment returns). We calibrated the models to Chinese population-based observations on cancer incidence, mortality, and stage distribution. All models are validated by comparing model simulated results to data observed from nationwide cancer registration and a large prospective cohort study.
Results
The simulated results from the calibrated models consistently match the epidemiological patterns in six major cancer incidence, mortality, and stage distributions in China. Model projected age-specific cancer incidence and mortality were close to the observed data in the national cancer registration. The NCC modeling framework reproduced the cumulative cancer cases and deaths observed in the prospective cohort study at 7.0 and 10.8 years of follow-up. Model estimated net survival rates also consistent with population-based statistics.
Conclusion
The NCC modeling framework's ability to reproduce the observed population-level cancer statistics and the cancer cases in a prospective cohort study suggests its results are reliable to inform decision-making related to six major cancers in China.
Keywords: Cancer simulation, Modeling study, Natural history, Screening, China
1. Introduction
Per the data from the National Cancer Center (NCC), there were 4.06 million new cancer cases and 2.41 million cancer deaths in China in 2016, and these numbers were about 130,000 and 60,000 more than that of the previous year, respectively.1,2 The top five causes of cancer deaths in China were lung cancer, liver cancer, stomach cancer, colorectal cancer, and esophageal cancer, accounting for 69.3% of all cancer deaths.1 Breast cancer ranks sixth in the cancer deaths, accounting for approximately 3.0% of all cancer deaths.1 While among females, breast cancer was the most common cancer which account for 16.7% of all female cancer incident cases.1 Activities for prevention and treatment of the six major cancers is the key to reducing the burden of cancer in China. Except cervical cancer, the current nationwide cancer screening program in China is targeted to the six major cancer types that are the leading causes of cancer deaths.3 Therefore, extensive evidences are needed to inform decision-making on six major cancers, especially those comparative studies that integrate multiple sources of evidences.
Cancer related decisions ideally should base on empirical evidence; clinical trials remain the standard of evidence to evaluate the efficacy of screening technologies and cancer treatments. Cancer registration and several large cohorts could provide primary data for decision-making.4,5 Nevertheless, trade-off between benefits and harms requires more comprehensive evidences from health technology assessment (HTA) and high-quality data about multiple interventions.6 Unfortunately, it is generally not feasible within a single randomized clinical trial to evaluate efficacy for multiple inventions or technologies.7 Health policy and healthcare decision-making typically need to consider the long-term or lifetime consequences of health interventions while real-world study can generally only provide data about short-term outcomes of interventions.8 As a consequence, there is always limited information on long-term outcomes or direct comparisons among relevant alternative strategies. In this situation, simulation modeling can be a useful research method to synthesize existing data and compare a broader range of alternatives.9 A good illustration of cancer modeling is the Cancer Intervention and Surveillance Modeling Network (CISNET), which is a consortium established by the National Cancer Institute.10 The CISNET includes comparative modeling approach to improve understanding of the impact of interventions on cancer incidence and mortality.10,11 The CISNET models can be used to project future trends, aid in the development of optimal cancer control strategies, and guide public health research and priorities.12, 13, 14
In China, rapidly emerging evidence and real-world data has put pressure on the health system for the adoption of new technologies and policies. Single study only provides results that apply to enrolled participants and may not translate to impact on population-level health benefits. As such, it is urgent to develop a reliable cancer modeling framework to aid six major cancers decision-making. However, excepting few newly constructed models based on regional data,15, 16, 17, 18 China has not yet established an extensively validated cancer modeling framework for six major cancers. We previously developed a hybrid model for cervical cancer and successfully applied it the cervical cancer elimination policy marking in China.19, 20, 21 Based on the modeling experience of cervical cancer, we developed a Chinese adapted modeling framework for six major cancers, which could be used to simulate the lifetime consequences of multiple interventions (e.g., prevention, screening, and treatment) and facilitate HTAs for emerging technology targeted to six major cancers. In this paper, we describe the structure, assumptions, parameters, and calibration and verification results of NCC modeling framework, providing methodological details for future decision-making using these models.
2. Methods
2.1. Model overview
The NCC mathematical modeling framework is a collection of simulation models for six major cancers (breast, colorectal, esophageal, gastric, liver, and lung) decision-making in China. Each cancer was modelled separately to simulate the life-histories represented by states among normal, precancerous lesion, stage-specific invasive cancer, and death (Fig. 1). The models simulate males (except for breast cancer) and females per single year age cohort, from birth or the age at the beginning of simulation and continues on an annual time-step until death or 85 years of age, whichever occurs first. Neonates were assumed to be in the normal state at birth, and competing non-cancer mortality (i.e., risk of death from causes other than the specific cancer) was present in all states. As the simulated population ages, precancerous lesions may arise, and some can subsequently progress to preclinical cancer. Symptoms may be present at any time during the development of the disease, and preclinical cancers may be diagnosed.
Fig. 1.
Model structure. (A) NCC-Breast; (B) NCC-Colorectal; (C) NCC-Esophageal; (D) NCC-Gastric; (E) NCC-Liver; (F) NCC-Lung. NCC, National Cancer Center.
Each NCC simulation model could be illustrated as an integrated framework of 3 modules separately (not physically separated): a demography module, natural history module, and screening module (Table 1). The demography module generates people at the initial age at the beginning of simulation, and a date of competing death without the specific cancer for each individual (in microsimulation model) or population cohort (in cohort model) simulated, creating a life-history without the simulated cancer. The natural history module generates a precancerous lesion where cancer initiation present, and progresses into preclinical cancer as cancer development. Cancer may be diagnosed because of symptoms at any stage of TNM stages I to IV and results in cancer death before competing death (without the specific cancer) would have occurred. In the screening module a screening examination is simulated. During this examination the precancerous lesion and preclinical cancer may be detected, and as a result cancer cases and cancer death may prevent.
Table 1.
Model description.
| NCC-Lung | NCC-Colorectal | NCC-Breast | NCC-Esophageal | NCC-Gastric | NCC-Liver | |
|---|---|---|---|---|---|---|
| Population | Male and female separately | Male and female separately | Female only | Male and female separately | Male and female separately | Male and female cirrhosis patients, separately |
| Natural history | ||||||
| Cancer initiation | Two-stage clonal expansion model, influenced by smoking | Small adenoma, percentage of progressive adenomas influenced by age of initiation | DCIS or pre-clinical undetectable lesions | Mild dysplasia | Low-grade intraepithelial neoplasia | Induced by viral or non-viral hepatitis |
| Cancer development | From malignant nodules, after a lag time | Form progressive adenoma ≥ 6mm | From screen-detectable DCIS or progressive lesions | From severe dysplasia/carcinoma in situ | From high-grade intraepithelial neoplasia/carcinoma in situ | From decompensated or compensated cirrhosis |
| Stage progression model | Markov state-transition by histology | Markov state-transition by progressive characteristic | Markov state-transition | Markov state-transition | Markov state-transition | Markov state-transition by treatment status |
| Modeling approach and rational | Microsimulation; individualized smoking history and histology | Microsimulation; individualized progressive characteristic and number of adenomas | Cohort; transition determined by disease status | Cohort; transition determined by disease status | Cohort; transition determined by disease status | Cohort; transition determined by disease status |
| Cancer Epidemiology | ||||||
| Secular cancer incidence trends | AAPC model 2012–2016; −0.1% for male and 4.6% for female | AAPC model 2012–2016; 1.3% for male and 0.0% for female | AAPC model 2012–2016; 1.4% for female | AAPC model 2012–2016; −4.5% for male and −7.5% for female | AAPC model 2012–2016; −3.4% for male and −2.9% for female | AAPC model 2012–2016; −3.0% for male and −3.7% for female |
| Stage at clinical diagnosis without screening | Stage I to IV: 11.6%, 15.7%, 27.9%, 44.8% for male and 28.0%, 14.3%, 16.2%, 41.5% for female | Stage I to IV: 14.9%, 33.3%, 33.2%, 18.6% for male and 15.7%, 32.4%, 34.1%, 17.9% for female | Stage I to IV: 27.6%, 50.7%, 16.9%, 4.7% for female | Stage I to IV: 16.9%, 27.7%, 44.0%, 11.4% for male and 24.3%, 35.9%, 32.3%, 7.5% for female | Stage I to IV: 20.5%, 14.2%, 44.4%, 20.9% for male and 22.9%, 14.2%, 40.3%, 22.6% for female | Stage I to IV: 18.2%, 26.9%, 29.5%, 25.4% for male and 14.6%, 20.3%, 28.7%, 36.4% for female |
| Cancer survival | Cure and cancer specific death probability by age and stage | Cure and cancer specific death probability by age and stage | Cure and cancer specific death probability by age and stage | Cure and cancer specific death probability by age and stage | Cure and cancer specific death probability by age and stage | Cure and cancer specific death probability by age and stage |
| Competing non-cancer mortality | Sex, age, and cohort-specific through 2020, modifications for lung cancer mortality | Sex, age, and cohort-specific through 2020, modifications for colorectal cancer mortality | Age, and cohort-specific through 2020, modifications for female breast cancer mortality | Sex, age, and cohort-specific through 2020, modifications for esophageal cancer mortality | Sex, age, and cohort-specific through 2020, modifications for gastric cancer mortality | Sex, age, and cohort-specific through 2020, modifications for liver cancer mortality |
| Calibration | ||||||
| Calibration parameters | Stage-specific probability of symptoms, age-specific cure fraction, dose-response relative risk of smoking | Stage-specific probability of symptoms, age-specific cure fraction, age-specific adenoma incidence | Stage-specific probability of symptoms, age-specific cure fraction, age-specific probability of invasive cancer from normal tissue | Stage-specific probability of symptoms, age-specific cure fraction, age-specific mild dysplasia incidence | Stage-specific probability of symptoms, age-specific cure fraction, age-specific low-grade intraepithelial neoplasia incidence | Stage-specific probability of symptoms, age-specific cure fraction, age-specific cirrhosis prevalence |
| Data sources used for calibration | Age-specific incidence and mortality from NCC population-based cancer registration (2013–2017); stage and histology distribution from NCC hospital-based cancer registration (2016–2017) | Age-specific incidence and mortality from NCC population-based cancer registration (2013–2017); stage distribution from NCC hospital-based cancer registration (2016–2017) | Same as NCC-Colorectal | Same as NCC-Colorectal | Same as NCC-Colorectal | Same as NCC-Colorectal |
| Model verification | Nationwide age-specific cancer incidence and mortality; cumulative cancer cases and deaths from CKB cohort study; 5-year net survival rate | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung |
| Screening | ||||||
| Screening effectiveness mechanism | Combination cure model and stage-shift model | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung |
| Lead-time bias adjustment | Stage-shift model | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung |
| Length bias adjustment | Cure model; screen screen-detected cancer has higher cure fraction and lower cancer specific death probability within a stage | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung |
| Positive screening follow-up algorithm | Implicit based on follow-up rates | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung |
| Output | ||||||
| General outputs | Counts of risk assessments, screenings, false positive results, screen-detected and nonscreen-detected incident cases, prevalent cases and cancer deaths by stages, non-cancer deaths, and surviving population for each age and calendar year combination | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung | Same as NCC-Lung |
| Special outputs | Counts of initiated cells, malignant nodules and cancer cases by histology | Counts of progressive and non-progressive adenomas | Counts of DCISs | Counts of mild dysplasia, moderate dysplasia, and severe dysplasia | Counts of low-grade intraepithelial neoplasia and high-grade intraepithelial neoplasia | Counts of compensated and decompensated cirrhosis (with or without treatment) |
Abbreviations: AAPC, average annual percent change; CKB, China Kadoorie Biobank; DCIS, ductal carcinoma in situ; NCC, National Cancer Center.
All models are constructed using the R software version 4.1.3. The computationally intensive simulations in microsimulation model are compiled by C++ using “Rcpp” package. Multiple cohort simulation and Monte Carlo sampling are speeded up by parallel computing approach using “doSNOW” package.
2.2. Model inputs
Model parameters and inputs were synthesized from various sources (Table 2). Natural history parameters were obtained from several recent large-scale pooled analyses or high-quality meta-analyses of studies in China. When unavailable, we adapted parameters from well-established models included in the CISNET. We used sex- and age-specific values for parameters may sensitive to sex and age, such as transition probabilities for esophageal and gastric cancers, smoking related parameters and cancer survival estimates (Table 1 and 2). Male and female populations per single age were extracted from the 2020 China Population Census. The number of neonates from 2021 onwards was based on the fertility rate for women aged 15 to 49, which were extracted from the United Nations' World Population Prospects 2022. All-cause mortality rates were extracted from the National Mortality Surveillance Report. Cancer statistic data were collected by the NCC nationwide population-based cancer registration and hospital-based cancer registration.1,4,22,23
Table 2.
Model parameters and inputs.
| Input parameter | Value | Source |
|---|---|---|
| Breast cancer | ||
| Ratio of DCIS incidence to invasive breast cancer incidence | 0.12 | 28 |
| Rate ratio of invasive cancer from DCIS | 2.02 | 28 |
| Stage I to II | 0.06 | 28 |
| Stage II to III | 0.11 | 28 |
| Stage III to IV | 0.15 | 28 |
| Colorectal cancer | ||
| Normal to small (≤ 5 mm) adenomas | 0.013 | 53 |
| Small to medium (6–9 mm) adenomas | 0.061 | 53 |
| Medium to large (≥ 10 mm) adenomas | 0.051 | 53 |
| Medium to stage I | 0.003 | 53 |
| Large to stage I | 0.015 | 53 |
| Stage I to II | Median dwell 2.5 years | 18 |
| Stage II to III | Median dwell 2.5 years | 18 |
| Stage III to IV | Median dwell 3.7 years | 18 |
| Percentage of non-progressive adenomas | 86% before age 65 years, decreases gradually to 63% at age 75 years and to 4% at age 100 years | 54,55 |
| Esophageal cancer | ||
| Normal to mD | 0.012 | 31,32 |
| mD to normal | 0.05 | 31,32 |
| mD to MD | 0.05 | 31,32 |
| MD to mD | 0.08 | 31,32 |
| MD to SD/CIS | 0.12 | 31,32 |
| SD/CIS to MD | ||
| Before age 45 | 0.17 | 31,32 |
| Age 45–49 | 0.15 | 31,32 |
| Age 50–54 | 0.14 | 31,32 |
| Age 55–59 | 0.12 | 31,32 |
| Age 60–64 | 0.11 | 31,32 |
| Age 65 and older | 0.09 | 31,32 |
| SD/CIS to stage I | ||
| Before age 45 | 0.08 | 31,32 |
| Age 45–49 | 0.10 | 31,32 |
| Age 50–54 | 0.12 | 31,32 |
| Age 55–59 | 0.14 | 31,32 |
| Age 60–64 | 0.16 | 31,32 |
| Age 65 and older | 0.18 | 31,32 |
| Stage I to II | Median dwell 2 years | 56,57 |
| Stage II to III | Median dwell 1 years | 56,57 |
| Stage III to IV | Median dwell 1 years | 56,57 |
| Gastric cancer | ||
| Normal to LGIN | 0.007 | 32 |
| LGIN to normal | 0.04 | 32 |
| LGIN to HGIN/CIS | 0.03 | 32 |
| HGIN/CIS to LGIN | ||
| Before age 45 | 0.17 | 32 |
| Age 45–49 | 0.15 | 32 |
| Age 50–54 | 0.14 | 32 |
| Age 55–59 | 0.12 | 32 |
| Age 60–64 | 0.11 | 32 |
| Age 65 and older | 0.09 | 32 |
| HGIN/CIS to Stage I | ||
| Before age 45 | 0.08 | 32 |
| Age 45–49 | 0.10 | 32 |
| Age 50–54 | 0.12 | 32 |
| Age 55–59 | 0.14 | 32 |
| Age 60–64 | 0.16 | 32 |
| Age 65 and older | 0.18 | 32 |
| Stage I to II | Median dwell 3 years | 56,58 |
| Stage II to III | Median dwell 2 years | 56,58 |
| Stage III to IV | Median dwell 1 years | 56,58 |
| Liver cancer | ||
| Without treatment | ||
| Compensated to decompensated cirrhosis | 0.058 | 33 |
| Compensated cirrhosis to HCC | 0.0316 | 59 |
| Decompensated cirrhosis to HCC | 0.034 | 59 |
| Compensated cirrhosis death | 0.031 | 34 |
| Decompensated cirrhosis death | 0.170 | 34 |
| With treatment | ||
| Compensated to decompensated cirrhosis | 0.019 | 34 |
| Compensated cirrhosis to HCC | 0.020 | 34 |
| Compensated cirrhosis death | 0.017 | 34 |
| Decompensated cirrhosis to HCC | 0.024 | 34 |
| Decompensated cirrhosis death | 0.095 | 34 |
| Stage I to II | Median dwell 2 years | 56,60 |
| Stage II to III | Median dwell 1 years | 56,60 |
| Stage III to IV | Median dwell 1 years | 56,60 |
| Lung cancer | ||
| No smoking | ||
| Normal stem cells to initiated cells - male | 0.024 | 24 |
| Normal stem cells to initiated cells - female | 0.036 | 24 |
| Net initiated cells proliferation rate | 0.0973 | 24 |
| Initiated cells to malignant cell | 0.0000000758 (i.e., 7.58e-8) | 24 |
| Regular smoking | ||
| RR for incident lung cancer - male | 2.51 | 50 |
| RR of < 15 cigarettes per day for incident lung cancer - male | 1.90 | 50 |
| RR of 15–24 cigarettes per day for incident lung cancer - male | 2.68 | 50 |
| RR of > 25 cigarettes per day for incident lung cancer - male | 3.59 | 50 |
| RR for incident lung cancer - female | 2.28 | 50 |
| Median lag time from first malignant cell to lung cancer detection | 3 years | 25 |
| Stage I to II | Median dwell 2 years | 56,61 |
| Stage II to III | Median dwell 1 years | 56,61 |
| Stage III to IV | Median dwell 1 years | 56,61 |
| Adeno or large cell proportion - male | 0.464 | 62 |
| Squamous cell proportion - male | 0.355 | 62 |
| Small cell carcinoma proportion - male | 0.181 | 62 |
| Adeno or large cell proportion - female | 0.813 | 62 |
| Squamous cell proportion - female | 0.079 | 62 |
| Small cell carcinoma proportion - female | 0.108 | 62 |
Abbreviations: CIS, carcinoma in situ; DCIS, ductal carcinoma in situ; HCC, hepatocellular carcinoma; HGIN, high-grade intraepithelial neoplasia; LGIN, low-grade intraepithelial neoplasia; MD, moderate dysplasia; mD, mild dysplasia; RR, risk ratio; SD, severe dysplasia.
2.3. Demography module
The demography module simulates individual (in microsimulation model) or population cohort (in cohort model) life histories without the evaluated cancer for all mainland Chinese population (expect breast cancer that only consider females). Using cross-sectional demographic statistics, fertility and mortality rate tables representative of the population in mainland China, the model assigns competing death without the specific cancer for each simulated individual or cohort. Our model framework used 2020 as the beginning of simulation, therefore, the model simulates multiple birth cohorts born from 1935 (i.e., the people aged 85 years at 2020) to the final year of simulation, to reflect annual 86 population age groups (people aged 0, 1, 2, …, 84, and 85 years and older) from 2020 onwards.
2.4. Lung cancer natural history module
The NCC-Lung model is a Chinese adapted microsimulation model similar to MISCAN-Lung (or model E) within the CISNET Lung Working Group (Fig. 1).11,24 NCC-Lung model is based on a two-stage clonal expansion (TSCE) carcinogenesis process influenced by the smoking component. The TSCE process assumes carcinogenic process consists of three phases: initiation, promotion, and malignant conversion.11,25 Normal stem cells may mutate to create initiated cells. Initiated cells divide, die or differentiate stochastically, and a very small number of them may mutate to create a malignant cell. After a lag time distribution, malignant nodule or clinically diagnosable lung cancer may develop from the malignant cell. One person may form more than one malignant nodule during a life history. Base rates of initiation, promotion, malignant transformation in TSCE process could be modified when an individual expose to smoking. A smoke generator is employed to generate sex-specific smoking history (i.e., starting age, stopping age, and smoking intensity), and an adjustment factor (i.e., the risk ratio) is specified for each level of smoking intensity to account for the risk of smoking. Malignant nodule and lung cancer progression is described with transition probabilities in a Markov model representing consecutive preclinical and clinical disease states. Three lung cancer histology types are distinguished: squamous cell carcinoma, adenocarcinoma and large cell carcinoma combined, and small cell carcinoma.11,24
2.5. Colorectal cancer natural history module
The NCC-Colorectal model is a Chinese adapted microsimulation model similar to MISCAN-Colon within the CISNET Colorectal Working Group (Fig. 1).18,26,27 NCC-Colorectal model simulates the development of colorectal cancer through the adenoma carcinoma sequence. As an individual ages, one or more small (≤ 5 mm) adenomas may arise and some can progress to medium (6–9 mm) and large (≥10 mm) adenomas. These adenomas can be either progressive or non-progressive, only progressive adenomas can develop into preclinical cancer. Some medium and large progressive adenomas develop into preclinical cancer, and may be diagnosed because of symptoms. An individual's risk of developing adenomas depends on the individual's age and a personal risk index. As such, most individuals develop no adenomas while some develop many. The median dwell time between the adenoma and preclinical cancer stages were drawn from exponential distributions.26 Durations within the adenoma phase and within the preclinical cancer phase were assumed to be 100% associated with each other.26
2.6. Breast cancer natural history module
The NCC-Breast model is a state-transition Markov model previously used in Chinese population (Fig. 1).28,29 NCC-Breast considers that women with ductal carcinoma in situ (DCIS) are at a higher risk of developing invasive breast cancer than healthy women. Healthy women can transition to DCIS or stage I cancer, or remain free of cancer. In following yearly cycles, women with DCIS can remain in that state, develop clinically detectable invasive breast cancer at stage I, or die of competing causes other than breast cancer. Undetected breast cancer at stage I can progress to stage II, stage III and stage IV in turn. Detected breast cancer may remain in that state with disease, move to clinical or statistical cured state after effective treatment,30 or die of breast cancer or other causes.
2.7. Esophageal cancer natural history module
The NCC-Esophageal model is a state-transition Markov model we previously constructed to simulate esophageal cancer natural history in Chinese population (Fig. 1).31,32 The precancerous conditions in the NCC-Esophageal model are grouped into mild, moderate, and severe dysplasia. Healthy people may develop mild dysplasia, and may progress through moderate dysplasia, severe dysplasia, and preclinical cancer stages I to IV. Before preclinical cancer develops, people with precancerous lesions are allowed to regress to less-advanced precancerous conditions. Severe dysplasia and each stage of cancer may be diagnosed because of symptoms. People diagnosed with severe dysplasia may be cured by receiving treatment or progress to preclinical cancer when absence of effective treatment. Detected esophageal cancer may remain in that state with disease, move to clinical or statistical cured state after effective treatment,30 or die of esophageal cancer or other causes.
2.8. Stomach cancer natural history module
Similar to the structure of NCC-Esophageal model, the NCC-Gastric model is a state-transition Markov model we previously constructed to simulate stomach cancer natural history in Chinese population (Fig. 1). Unlike esophageal cancer, the precancerous conditions in the NCC-Gastric model are grouped into low-grade and high-grade intraepithelial neoplasia. Healthy people may develop stomach cancer through low-grade and high-grade intraepithelial neoplasia in turn.
2.9. Liver cancer natural history module
The NCC-Liver model is a Chinese adapted state-transition Markov model, which was constructed to simulate liver cancer that develops from decompensated or compensated cirrhosis (Fig. 1).33,34 Healthy people may develop chronic hepatitis (implicitly simulated) in the presence of risk factors (such as hepatitis virus infection, alcohol abuse or non-alcoholic steatohepatitis), and subsequently some may progress to cirrhosis and hepatocellular carcinoma.35,36 Patients with compensated and decompensated cirrhosis may clinically diagnosed after developing clinical symptoms and some may receive treatment. Both compensated and decompensated cirrhosis have the probability of malignant conversion and then liver cancer may arise, regardless of the detect and treatment status. Cirrhosis patients receiving treatment have lower rates of malignant conversion and cirrhosis-related deaths than those who remained untreated or undetected. Successful treatment for cirrhosis and hepatocellular carcinoma, such as resection and liver transplantation, may drive the patients to a state of clinical or statistical cured.30
2.10. Screening module
The screening module will alter some of the simulated life histories. With screening, cancer may either be prevented at the precancerous lesion phase or detected at an earlier stage with a more favorable prognosis. Thus, the incidence and/or mortality rate of cancer may be reduced. More favorable outcomes on screen-detected cancer could be attributed to several factors, including lead-time (stage shift) and length bias (less-aggressive tumors), which can cause a spurious improvement of survival in the screened population.37 Application of stage-specific survival to screen-detected cancer reduces the magnitude of lead-time bias that is caused by stage shift. However, the stage-specific survival of screen-detected cancer was substantially more favorable than that of clinically detected cancer, even after correcting for lead-time bias and length bias.26,37,38 Therefore, we assumed that within-stage survival is slightly better for screen-detected cancers versus symptomatically detected cancers.39, 40, 41, 42, 43, 44 Besides modeling positive health effects of screening, the models also consider false-positive results, screen-related complications, over-diagnosis and over-treatment of cancer (i.e., the detection and treatment of cancers that would not have been diagnosed without screening).
Characteristics of organized screening programs, such as screening ages, intervals, screening modality, attendance by first and subsequent screens, and sensitivity and specificity of screening can be incorporated into the model directly. The screening dissemination that reflects the historic opportunistic screening patterns observed in the special cancer can also be simulated. Timing of screenings can follow an invitational schedule or an opportunistic pattern. Follow-up algorithm for positive screening results is implicitly modelled according to the predefined follow-up rates. Screening sensitivity depends on which screening technology is in use and the exact stage of precancerous lesion and cancers. The number of false-positive screens is calculated based on the total number of screenings performed in the people without any cancer-related conditions and the screening specificity. We simulate the life histories of same population twice, under the scenarios with or without screening, to quantify overdiagnosis due to screening.
2.11. Model calibration
Some parameters, such as the probability of symptoms, cure fraction, precancerous condition incidence rate (Table 1), are obtained by optimizing the goodness of fit between simulated data and observed data (i.e., the model calibration). All models were calibrated to the target data provided by the National Cancer Registry, including sex- and age-specific cancer incidence and mortality from population-based cancer registration, and stage and histology distribution from NCC hospital-based cancer registration.1,22,23 We used the simulated annealing algorithm to search for an optimum parameter solution, because it performs quite well in speeding up the computations.45 Weighted sum of squared differences between observed and simulated data are obtained by repeated evaluation of different sets of parameter values. In the calibration process, each step in the optimization algorithms is based on output from previous simulation runs, to find the maximum likelihood estimates allowing the simulated results to be consistent with the target observations. Recalibration will be undertaken for the parameters of the model component that is being changed. For instance, some emerging screening technologies may alter the stage-specific probability of symptoms, and some may require changes in the model structure to explicitly simulate the triage or follow-up process.
The baseline prevalence rates of each natural history state in 2020 were informed by calibrated outputs, because all birth cohorts except those at births are assigned to a specific Markov state at the beginning of simulation. During the model calibration, the period trends of cancer incidence were not considered, as the models are calibrated in a cross-sectional population where the temporal risk of cancer is remains unchanged. However, period effects driven by the identified or unidentified cancer risk factors are not negligible when simulate cancers with substantive average annual percent change (AAPC) of incidence rates.1 Therefore, with consideration of the secular trends in incidence and mortality for each cancer type, sex-specific AAPC in cancer incidence were incorporated into the calibrated models by adjusting the probability of precancerous conditions incidence rates (i.e., implicitly simulate the effects of risk factors) and the baseline prevalence rates of each natural history state.
2.12. Model validation
We used several approaches to validate the models collected in the NCC modeling framework. First, we compared model projections of incidence, mortality, and stage distribution to those reported by the NCC nationwide cancer registration. We also external validated the models against the cancer incidence and mortality observed from the China Kadoorie Biobank (CKB) prospective cohort study.5 Cumulative cancer cases and deaths of each cancer site measured at 7.0 years and 10.8 years of follow-up were used.46, 47, 48 We compared the cohort observed cumulative cases and deaths to that predicted from model simulation, using the matched age distortion at baseline and the person-years of follow-up. As the age-specific cancer incidence was much higher in populations included in the CKB cohort than that from national cancer registration, we adjust age-specific cancer incident cases predicted from the NCC model framework, using the sex-, site-, and age-specific incidence ratio between the CKB cohort and national cancer registration.46,47 Finally, we compared the survival rates after diagnosis to the observed net survival rates from 17 population-based cancer registration.22
3. Results
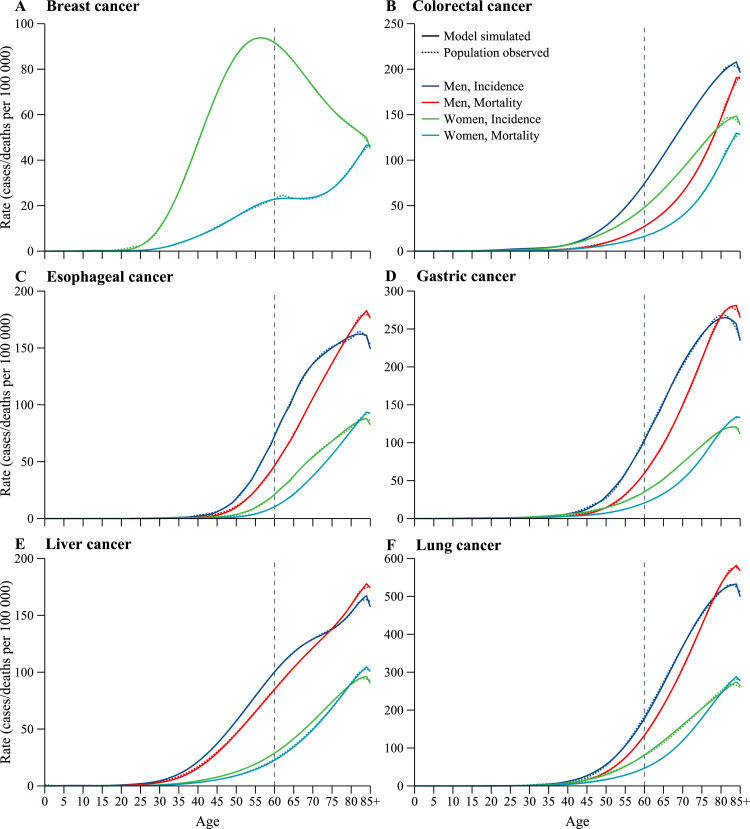
The age-specific cancer incidence and mortality estimated from the calibrated NCC mathematical modeling framework were close to the observed data from nationwide cancer registration, both for male and female (Fig. 2). NCC-Breast replicates the observed breast cancer incidence rates and trend over the age, which is increase with age and then decrease gradually, with the peaked incidence rate estimated at age of 50–59 years. For breast cancer mortality, NCC-Breast simulated rates are slightly lower than that reported from cancer registration in women aged 60–64 years, because an approximately bimodal age distribution for breast cancer mortality was presented, with the first smaller peaked rates observed at age of 60–64 years. Age-specific cancer incidence and mortality for other five cancers are gradually increase before 85 years old, and the model simulated rates are generally matched with the increasing trends. However, some subtle differences still identifiable in some age ranges, such as liver cancer mortality in men aged 30–45 years and lung cancer incidence in men aged 40–50 years, because our calibration process was designed not to over fit the model. Model simulated cancer incidence and mortality for people aged 85 years and older are apparently lower than that for people aged 84 years, because the competing deaths without specific cancer are increased dramatically from age 84 to age 85. For some cancers with high case-fatality rate (e.g., esophageal, gastric, liver and lung cancer), large numbers of patients dying in older age groups than they were in the time at diagnosis. And as a consequence, the mortality rate in older age group may exceed the incidence rate, with the mortality-to-incidence ratio exceed 1.0. Additionally, sex disparities in stage at diagnosis reflect the differences in awareness and interpretation of cancer symptoms, as males and females are simulated separately in all NCC modeling framework to allow for inconsistent probabilities of symptoms. NCC modeling framework generates similar outputs for cancer stage distribution across each of six major cancers, thus, the calibrated stage distribution is not shown.
Fig. 2.
Model calibration. (A) Breast cancer. (B) Colorectal cancer. (C) Esophageal cancer. (D) Gastric cancer. (E) Liver cancer. (F) Lung cancer. Shown are age-specific cancer incidence and mortality drawn from model simulation and cancer registration.
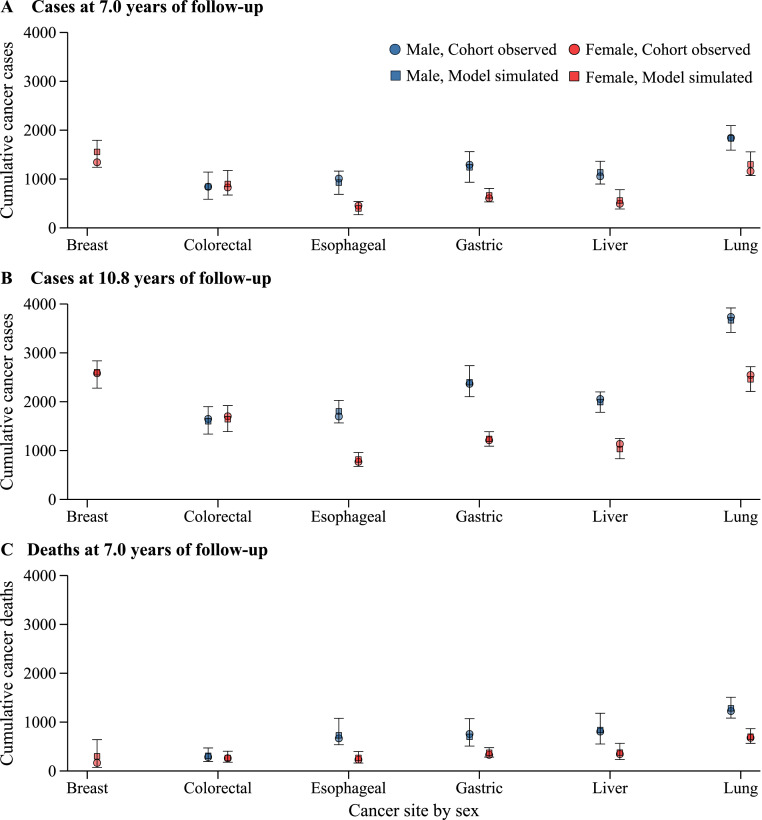
In our external validation exercise, we estimated the cumulative cancer cases and deaths for participants included in the CKB prospective cohort study. The CKB observed cumulative cancer cases and deaths were within the uncertainty intervals of NCC modeling framework projections (Fig. 3). Although the uncertainty interval of the simulation results is wide, the point estimates are close to the observations from CKB, indicating that our models have acceptable external generalizability. When estimating the cumulative cancer cases at 7.0 years of follow-up, NCC-Breast and NCC-Lung estimated more cases than the CKB cohort observations for breast cancer (1557 vs. 1343) and female lung cancer (1299 vs. 1159), but the estimates were more similar at the 10.8 years of follow-up. Model projected cancer deaths were almost identical to the CKB cohort observations at 7.0 years of follow-up, expect for female breast cancer (model simulated 298 deaths vs. cohort observed 165 deaths). Model estimated 5-year net survival rates are consistent to that observed from population-based cancer registration. For example, the NCC-Lung estimated an 17.4% and 27.0% of net survival rate for male and female lung cancer, respectively.
Fig. 3.
Model validation. (A) Breast cancer. (B) Colorectal cancer. (C) Esophageal cancer. (D) Gastric cancer. (E) Liver cancer. (F) Lung cancer. Shown are cohort observed and model simulated cumulative cancer cases and deaths for the China Kadoorie Biobank study.
4. Discussion
This paper provides an overview of the NCC modeling framework inputs, assumptions, structures, and model calibration and validation results. When simulating incidence, mortality, and stage at diagnosis of six major cancers, model estimates were close to the observations reported in the national cancer registration. The model's ability to reproduce the observed cumulative cancer cases and deaths from a large perspective cohort study increases the confidence of using the model results to inform decision-making.
The calibrated and validated NCC modeling framework would be a useful toolkit to inform decision-making in major six cancers in China. Our model can be used to investigate questions related to major cancer screening policies, such as cost-effectiveness analysis and targeting screening ages. Combined with the costs and health utilities data, the models have many detailed outputs for informing policy decisions, including the harm of screening (e.g., false positives, complications, and overdiagnosis), healthcare costs, and benefits (quality-adjusted life years gained, cancer incidence and mortality, and investment returns). The models can also be used to guide primary prevention and clinical treatment for six major cancers, such as the long-term effects for controlling cancer risk factors based on a cohort study and health economic evaluation for new cancer treatment regimens based on a randomized clinical trial.
Model calibration and validation results suggest that the NCC modeling framework is reliable to simulate cancer epidemiology in the Chinese population. Model simulated outputs are consistent with that observed from national cancer registration and a large perspective cohort. Modeling results from NCC-Breast were higher that from CKB cohort at 7.0 years follow-up, mainly driven by the different pattern in breast cancer age distribution.46,49 The NCC modeling framework and nationwide cancer registration data only identified a unimodal distribution, with peaked incidence rate observed at women aged 50–59.49 However, the CKB cohort study found a typical bimodal distribution for breast cancer age-specific incidence, with peaked incidence rates observed in the premenopausal and postmenopausal women.46,47 As the mean age of women included in the CKB cohort study was 51.5 years old,5 NCC-Breast model predicted more cumulative breast cancer cases and deaths than the observations from CKB cohort.
The NCC modeling framework has several limitations. First, NCC-Lung and NCC-Colorectal are simulation models built using the adapted structures from the CISNET and parameters from worldwide; some natural history parameters of other countries may not fully represent the Chinese people. As the cigarette epidemic are more advanced in western countries, we used smoking dose-responses risk ratios from China rather than that from western countries.50,51 Second, the current NCC modeling framework is a base version that need continuously update and expansions, to enable the model to answer more specific questions related to cancer screening and control. Third, our model validation exercise did not reflect the prevalence of precancerous lesions. The NCC modeling framework is a multipurpose cancer simulation toolkit and could be used in many scenarios, therefore, it would not be feasible to validate all its possible simulations against observed data, especially when the verified observation value is difficult to be measured accurately. Finally, the NCC cancer modeling framework focuses on six major cancers in China only, therefore, our modeling results may not directly comparable to that estimated from models in the CISNET. We did not include our previously built cervical cancer model into the NCC modeling framework, because cervical cancer model uses a different model structure (i.e., a two-stage hybrid model).19,20
The NCC modeling framework does not need to be updated annually or every time it is used to address emerging issues. However, a cancer specific model will be updated when the parameters of the model component that is being changed, to ensure the relevance for supporting decision-marking. The updated model will be recalibrated and validated with emerging real-world data and refines the model based on new evidence for natural history. Further enhancements will be included in future updated version of the NCC models. First, explicitly incorporate other risk factors except smoking in lung cancer in current version, such as body mass index and polygenic risk scores. Second, separate the population by urban-rural region and high-risk or non-high-risk areas. Similar to our cervical cancer model,19 the hierarchical models might be worthwhile for cancers with substantial geographical disparities on incidence rates. Third, consider the impact of comorbidity on cancer-specific survival. Finally, contribute comparative modeling approach explores differences between models in a systematic way and improves model transparency.10,52 When consensus across all models can be reached, comparative modeling approach greatly enhances the credibility of modeling results by highlighting their reproducibility.11, 12, 13, 14
5. Conclusions
The NCC modeling framework is a collection of simulation models for six major cancers of lung, colorectal, breast, esophageal, gastric, and liver. All models are developed using the extensively validated structures and calibrated to Chinese nationwide cancer epidemiological data. Each NCC model reproduces cancer statistics in the cancer registration and cancer cases observed in a large prospective cohort in China. As such, the NCC cancer modeling framework would be a reliable toolkit to inform decision-making related to six major cancers in China.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Acknowledgments
Acknowledgments
This study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number: 2021-I2M-1-033), the Jing-jin-ji Special Project for Basic Research Cooperation (grant number: J200017) and the National Natural Science Foundation of China (grant number: 82273721).
Author contributions
C.X. did the literature review, acquired the data, and built the models in R software. W.C. verified the data and did the model validation. C.X. drafted and finalized the manuscript. W.C. provided input to the manuscript, and approved the final manuscript for submission.
References
- 1.Zheng R., Zhang S., Zeng H., et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2(1):1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [Google Scholar]
- 2.Chen W., Zheng R., Zhang S., et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. doi: 10.1016/j.canlet.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Cao M., Li H., Sun D., et al. Cancer screening in China: the current status, challenges, and suggestions. Cancer Lett. 2021;506:120–127. doi: 10.1016/j.canlet.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Wei W., Zeng H., Zheng R., et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21(7):e342–e349. doi: 10.1016/s1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z., Chen J., Collins R., et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40(6):1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sculpher M.J., Claxton K., Drummond M., et al. Whither trial-based economic evaluation for health care decision making? Health Econ. 2006;15(7):677–687. doi: 10.1002/hec.1093. [DOI] [PubMed] [Google Scholar]
- 7.Mutubuki E.N., El Alili M., Bosmans J.E., et al. The statistical approach in trial-based economic evaluations matters: get your statistics together! BMC Health Serv Res. 2021;21(1):475. doi: 10.1186/s12913-021-06513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger M.L., Sox H., Willke R.J., et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on real-world evidence in health care decision making. Value Health. 2017;20(8):1003–1008. doi: 10.1016/j.jval.2017.08.3019. [DOI] [PubMed] [Google Scholar]
- 9.Caro J.J., Briggs A.H., Siebert U., et al. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force–1. Value Health. 2012;15(6):796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 10.NCI's Division of Cancer Control and Population Sciences. The Cancer Intervention and Surveillance Modeling Network (CISNET). 2022. Available from: https://cisnet.cancer.gov/.
- 11.Meza R., ten Haaf K., Kong C.Y., et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer. 2014;120(11):1713–1724. doi: 10.1002/cncr.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meza R., Jeon J., Toumazis I., et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US preventive services task force. JAMA. 2021;325(10):988–997. doi: 10.1001/jama.2021.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudsen A.B., Zauber A.G., Rutter C.M., et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US preventive services task force. JAMA. 2016;315(23):2595–2609. doi: 10.1001/jama.2016.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Criss S.D., Cao P., Bastani M., et al. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171(11):796–804. doi: 10.7326/m19-0322. [DOI] [PubMed] [Google Scholar]
- 15.Sun C., Zhang X., Guo S., et al. Determining cost-effectiveness of lung cancer screening in urban Chinese populations using a state-transition Markov model. BMJ Open. 2021;11(7) doi: 10.1136/bmjopen-2020-046742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu B., Wang L., Lu M., et al. Microsimulation Model for Prevention and Intervention of Coloretal Cancer in China (MIMIC-CRC): development, calibration, validation, and application. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.883401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Z., Du L., Li Y., et al. Cost-effectiveness of lung cancer screening using low-dose computed tomography based on start age and interval in China: modeling study. JMIR Public Health Surveill. 2022;8(7):e36425. doi: 10.2196/36425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cenin D., Li P., Wang J., et al. Optimising colorectal cancer screening in Shanghai, China: a modelling study. BMJ Open. 2022;12(5) doi: 10.1136/bmjopen-2020-048156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia C., Hu S., Xu X., et al. Projections up to 2100 and a budget optimisation strategy towards cervical cancer elimination in China: a modelling study. Lancet Public Health. 2019;4(9):462–472. doi: 10.1016/s2468-2667(19)30162-8. [DOI] [PubMed] [Google Scholar]
- 20.Xia C., Xu X., Zhao X., et al. Effectiveness and cost-effectiveness of eliminating cervical cancer through a tailored optimal pathway: a modeling study. BMC Med. 2021;19(1):62–74. doi: 10.1186/s12916-021-01930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Health Commission. Key Works of Healthy China Action in 2022. 2022. Available from: http://www.nhc.gov.cn/guihuaxxs/s7788/202204/67cb879e0afd44ba916912367de56170.shtml.
- 22.Zeng H., Chen W., Zheng R., et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. doi: 10.1016/s2214-109x(18)30127-x. [DOI] [PubMed] [Google Scholar]
- 23.Zeng H., Ran X., An L., et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6(12):e877–e887. doi: 10.1016/s2468-2667(21)00157-2. [DOI] [PubMed] [Google Scholar]
- 24.Schultz F.W., Boer R., de Koning H.J. Chapter 7: description of MISCAN-lung, the Erasmus MC Lung Cancer microsimulation model for evaluating cancer control interventions. Risk Anal. 2012;32(Suppl 1):S85–S98. doi: 10.1111/j.1539-6924.2011.01752.x. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazelton W.D., Jeon J., Meza R., et al. Chapter 8: the FHCRC lung cancer model. Risk Anal. 2012;32(Suppl 1):S99–S116. doi: 10.1111/j.1539-6924.2011.01681.x. (Suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helsingen L.M., Vandvik P.O., Jodal H.C., et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline. BMJ. 2019;367:l5515. doi: 10.1136/bmj.l5515. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen A.B., Rutter C.M., Peterse E.F.P., et al. Colorectal cancer screening: an updated modeling study for the US preventive services task force. JAMA. 2021;325(19):1998–2011. doi: 10.1001/jama.2021.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L., Legood R., Sadique Z., et al. Cost-effectiveness of risk-based breast cancer screening programme, China. Bull World Health Organ. 2018;96(8):568–577. doi: 10.2471/blt.18.207944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong I.O., Kuntz K.M., Cowling B.J., et al. Cost effectiveness of mammography screening for Chinese women. Cancer. 2007;110(4):885–895. doi: 10.1002/cncr.22848. [DOI] [PubMed] [Google Scholar]
- 30.Xia C., Yu X.Q., Chen W. Measuring population-level cure patterns for cancer patients in the United States. Int J Cancer. 2022 doi: 10.1002/ijc.34291. [DOI] [PubMed] [Google Scholar]
- 31.Xia R., Li H., Shi J., et al. Cost-effectiveness of risk-stratified endoscopic screening for esophageal cancer in high-risk areas of China: a modeling study. Gastrointest Endosc. 2022;95(2):225–235. doi: 10.1016/j.gie.2021.08.008. .e20. [DOI] [PubMed] [Google Scholar]
- 32.Xia R., Zeng H., Liu W., et al. Estimated cost-effectiveness of endoscopic screening for upper gastrointestinal tract cancer in high-risk areas in China. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter H.E., Jeffrey G.P., Ramm G.A., et al. Cost-effectiveness of a serum biomarker test for risk-stratified liver ultrasound screening for hepatocellular carcinoma. Value Health. 2021;24(10):1454–1462. doi: 10.1016/j.jval.2021.04.1286. [DOI] [PubMed] [Google Scholar]
- 34.Su S., Wong W.C., Zou Z., et al. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health. 2022;10(2):e278–e287. doi: 10.1016/s2214-109x(21)00517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujiwara N., Friedman S.L., Goossens N., et al. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68(3):526–549. doi: 10.1016/j.jhep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 37.Mook S., Van 't Veer L.J., Rutgers E.J., et al. Independent prognostic value of screen detection in invasive breast cancer. J Natl Cancer Inst. 2011;103(7):585–597. doi: 10.1093/jnci/djr043. [DOI] [PubMed] [Google Scholar]
- 38.Lew J.B., St John D.J.B., Xu X.M., et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Public Health. 2017;2(7):e331–e340. doi: 10.1016/s2468-2667(17)30105-6. [DOI] [PubMed] [Google Scholar]
- 39.Gierada D.S., Pinsky P.F. Survival following detection of stage I lung cancer by screening in the National Lung Screening Trial. Chest. 2021;159(2):862–869. doi: 10.1016/j.chest.2020.08.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill M.D., Bramble M.G., Hull M.A., et al. Screen-detected colorectal cancers are associated with an improved outcome compared with stage-matched interval cancers. Br J Cancer. 2014;111(11):2076–2081. doi: 10.1038/bjc.2014.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walbaum B., Puschel K., Medina L., et al. Screen-detected breast cancer is associated with better prognosis and survival compared to self-detected/symptomatic cases in a Chilean cohort of female patients. Breast Cancer Res Treat. 2021;189(2):561–569. doi: 10.1007/s10549-021-06317-1. [DOI] [PubMed] [Google Scholar]
- 42.Offman J., Pesola F., Fitzgerald R.C., et al. Impact of Barrett oesophagus diagnoses and endoscopies on oesophageal cancer survival in the UK: a cohort study. Cancer Med. 2022;11(4):1160–1171. doi: 10.1002/cam4.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luu X.Q., Lee K., Jun J.K., et al. Effect of gastric cancer screening on long-term survival of gastric cancer patients: results of Korean national cancer screening program. J Gastroenterol. 2022;57(7):464–475. doi: 10.1007/s00535-022-01878-4. [DOI] [PubMed] [Google Scholar]
- 44.Choi D.T., Kum H.C., Park S., et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976–987. doi: 10.1016/j.cgh.2018.10.031. .e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong C.Y., McMahon P.M., Gazelle G.S. Calibration of disease simulation model using an engineering approach. Value Health. 2009;12(4):521–529. doi: 10.1111/j.1524-4733.2008.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan R., Zhu M., Yu C., et al. Cancer incidence and mortality: a cohort study in China, 2008-2013. Int J Cancer. 2017;141(7):1315–1323. doi: 10.1002/ijc.30825. [DOI] [PubMed] [Google Scholar]
- 47.Pan R. Nanjing Medical University; Nanjing, China: 2017. Analysis of Cancer Incidence and Mortality in the China Kadoorie Biobank Prospective Cohort Study. [Google Scholar]
- 48.Kakkoura M.G., Du H., Guo Y., et al. Dairy consumption and risks of total and site-specific cancers in Chinese adults: an 11-year prospective study of 0.5 million people. BMC Med. 2022;20(1):134. doi: 10.1186/s12916-022-02330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei S., Zheng R., Zhang S., et al. Breast cancer incidence and mortality in women in China: temporal trends and projections to 2030. Cancer Biol Med. 2021;18(3):900–909. doi: 10.20892/j.issn.2095-3941.2020.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z.M., Peto R., Iona A., et al. Emerging tobacco-related cancer risks in China: a nationwide, prospective study of 0.5 million adults. Cancer. 2015;121(Suppl 17):3097–3106. doi: 10.1002/cncr.29560. (Suppl 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thun M., Peto R., Boreham J., et al. Stages of the cigarette epidemic on entering its second century. Tob Control. 2012;21(2):96–101. doi: 10.1136/tobaccocontrol-2011-050294. [DOI] [PubMed] [Google Scholar]
- 52.Eddy D.M., Hollingworth W., Caro J.J., et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–7. Value Health. 2012;15(6):843–850. doi: 10.1016/j.jval.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Rutter C.M., Savarino J.E. An evidence-based microsimulation model for colorectal cancer: validation and application. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1992–2002. doi: 10.1158/1055-9965.Epi-09-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loeve F., Brown M.L., Boer R., et al. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92(7):557–563. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 55.Wieszczy P., Kaminski M.F., Løberg M., et al. Estimation of overdiagnosis in colorectal cancer screening with sigmoidoscopy and faecal occult blood testing: comparison of simulation models. BMJ Open. 2021;11(4) doi: 10.1136/bmjopen-2020-042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broder M.S., Ailawadhi S., Beltran H., et al. Estimates of stage-specific preclinical sojourn time across 21 cancer types. J Clin Oncol. 2021;39(15_suppl) doi: 10.1200/JCO.2021.39.15_suppl.e18584. e18584-e18584. [DOI] [Google Scholar]
- 57.Li H., Zhang S., Zhou J., et al. Endoscopic surveillance for premalignant esophageal lesions: a community-based multicenter, prospective cohort study. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.04.039. S1542-3565(22)00511-0. [DOI] [PubMed] [Google Scholar]
- 58.Choi I.J. Endoscopic gastric cancer screening and surveillance in high-risk groups. Clin Endosc. 2014;47(6):497–503. doi: 10.5946/ce.2014.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Shi J.F., Wang L., et al. Cost-effectiveness analysis of hepatitis B vaccine booster in children born to HBsAg-positive mothers in rural China. Int J Infect Dis. 2019;78:130–139. doi: 10.1016/j.ijid.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 60.Cucchetti A., Trevisani F., Pecorelli A., et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J Hepatol. 2014;61(2):333–341. doi: 10.1016/j.jhep.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 61.Chien C.R., Chen T.H. Mean sojourn time and effectiveness of mortality reduction for lung cancer screening with computed tomography. Int J Cancer. 2008;122(11):2594–2599. doi: 10.1002/ijc.23413. [DOI] [PubMed] [Google Scholar]
- 62.Yang J., Li H., Zheng R.S., et al. Analysis of the clinical characteristics of 8 081 primary lung cancer. Chin J Oncol. 2019;41(6):471–476. doi: 10.3760/cma.j.issn.0253-3766.2019.06.014. [DOI] [PubMed] [Google Scholar]