Abstract
With increasingly explored ideologies and technologies for potential applications of artificial intelligence (AI) in oncology, we here describe a holistic and structured concept termed intelligent oncology. Intelligent oncology is defined as a cross-disciplinary specialty which integrates oncology, radiology, pathology, molecular biology, multi-omics and computer sciences, aiming to promote cancer prevention, screening, early diagnosis and precision treatment. The development of intelligent oncology has been facilitated by fast AI technology development such as natural language processing, machine/deep learning, computer vision, and robotic process automation. While the concept and applications of intelligent oncology is still in its infancy, and there are still many hurdles and challenges, we are optimistic that it will play a pivotal role for the future of basic, translational and clinical oncology.
Keywords: Artificial intelligence, Oncology, Cancer prevention, Cancer screening, Deep learning, Machine learning
1. The emerging concept of intelligent oncology
Artificial intelligence (AI) refers to the use of a computer, robot or other machine to conduct human-like intelligent behavior.1 In recent years, increasingly advanced AI technologies have shown great applications in medical practices, and AI has quickly become an integral part of healthcare.2 There are no doubts that these medical applications will have major impacts on the future healthcare.
As critical scientific issues constantly being addressed and the use of AI technologies in the context of clinical oncology is increasing, the concept of intelligent oncology has emerged. We define it as an inter-disciplinary integration of clinical oncology, radiology, pathology, molecular biology, multi-omics with AI. Broadly speaking, intelligent oncology entails subjects of preclinical and clinical medicine, public health, and computer sciences. Specifically, intelligent oncology is intended to employ core AI techniques such as nature language processing, machine/deep learning, computer vision, biometric identification, and robotic process automation, to establish the intelligently ecological chain in the process of cancer care. Ultimately, intelligent oncology will improve the accuracy and efficiency of cancer management in prevention and screening, early diagnosis and treatment, prognosis and risk stratification.
Compared with precision medicine and personalized medicine in oncology, the core connotation of intelligent oncology focuses on the development, improvement and application of AI technologies in tumor screening, diagnosis, treatment and prognosis prediction, which could assist clinicians to meet clinical needs. Statistical analysis, omics analysis and clinical trials with more medical attributes in precision medicine and personalized medicine are not included in the discipline of intelligent oncology.
2. The content of intelligent oncology
2.1. Core technologies of Al in intelligent oncology
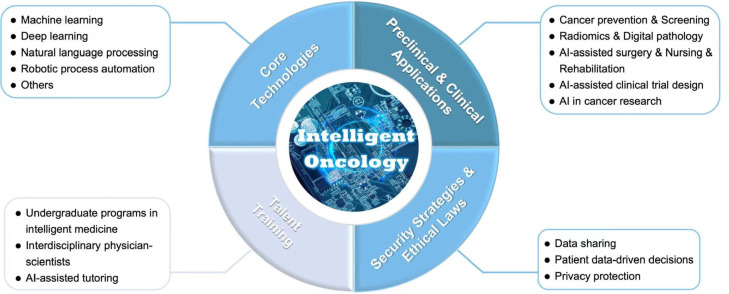
Intelligent oncology employs some of the core AI techniques to establish an intelligently ecological chain for the whole process of cancer care (Fig. 1). These technologies include:
Fig. 1.
The content of intelligent oncology. AI, artificial intelligence.
2.1.1. Machine learning (ML)
ML is a type of algorithms that enable machines have learning, predicting and cognizing ability through a priori knowledge and data mining.2 According to different construction methods, ML can be divided into supervised learning and unsupervised learning.3 The main difference between them is whether the training dataset contains the target label or not. Supervised learning trains an optimal model by data-label pairs, then uses the model to predict possible labels or values for unknown data. The typical task, suitable for supervised ML algorithms, is classification and regression. There are many supervised learning tools for intelligent oncology, such as Naive Bayes, logistic regression, k-nearest neighbors (KNN), decision tree (DT), support vector machines (SVM), and random forest (RF).4, 5, 6 In unsupervised learning algorithms, the input data have no corresponding labels, and an optimization model needs to be computed based on the similarity between training samples. Unsupervised learning is essentially a statistical tool, which is a learning method for clustering unlabeled original data by calculating the potential structures or features. A lot of unsupervised learning methods are used for cancer screening, prognosis analyzing, key-marker extracting, feature dimension reduction and gene representation clustering in oncology, such as principal component analysis (PCA), singular value decomposition (SVD), k-means, mean-shift, hierarchical clustering, DBSCAN, optics clustering et al.2,6,7
2.1.2. Deep learning (DL)
DL is a branch of ML based on the muti-layer neural network structure.1,8 Compared to conventional ML models, DL algorithms can handle huge datasets and extract potential advanced semantic features through multilayer nonlinear transformations.9,10 Although these semantic features are hard to understand and interpret, they enable very accurate performance of the target task, making machine intelligent. DL is an effective tool for precisely resolving tumor-related tasks, it makes the research of intelligent oncology to be widely applicable in the clinical setting. The convolutional neural networks (CNN), recurrent neural network (RNN), long short-term memory (LSTM), fully convolutional networks (FCN), generative adversarial network (GAN) et al.11, 12, 13, 14, 15 become the backbone modes in DL techniques which guarantee the basic performance of applications. Recently, some innovative network structures have been proposed and used for intelligent oncology research, such as graph convolutional networks (GCN), attention, muti-head attention, transformer, vsion-transformer (ViT), auto-encoder (AE), variational auto-encoder (VAE), deepclustering et al. In addition, to solve the problem of high cost of annotation in medical data and to make the most of every raw data, the contrastive learning methods have been also widely used in intelligent oncology task. Especially in the hard to annotation research area, the contrastive learning algorithm is an effective tool for learning latent information automatically from none-annotation data by self-supervised method, such as digital pathology, single cell omics, spatial transcriptomics et al.
2.1.3. Natural language processing (NLP)
NLP is one of the key branches in AI, as it makes computers to have the ability to understand human language from text, audio and video.16 It is useful for oncology research and clinical application. There are three main steps in NLP algorithms, receiving, transforming and generating. Conventionally, NLP applications usually perform tasks using two families of approaches: symbolic and statistical. Symbolic approaches consist of a set of rules, which are designed hand-crafted or learned automatically, by modeling different linguistic phenomena. Statistical approaches typically use ML algorithms to learn language phenomena. With the wide application of DL, NLP algorithms based on multi-layer networks have become mainstream models. There are many successful models for NLP,17, 18, 19 such as ULMFiT, bidirectional encoder representations from transformer (BERT), Transformer-XL, Google PaLM, GPT-3 et al. In the clinical treatment, NLP can improve the efficiency of doctors through generating standard medical records automatically. In cancer research, NLP tools can automatically extract key information from non-structured text data. It can process free text documentation, including reports in pathology and radiology reports, and oncological clinical notes.20,21
2.1.4. Robotic process automation (RPA)
RPA are integrated intelligence combining various technologies such as sensors, automation and AI,22 which are widely used in research and clinical treatment of intelligent oncology, such as surgery, radiation oncology, oncology nursing and rehabilitation.23,24
2.1.5. Telemedicine and cloud computing
Telemedicine seeks to improve a patient's health by permitting two-way, real-time interactive communications between the patient, and the physician or practitioner at a distant site. Based on the 5th generation (5G) technology, large-scale medical images such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET-CT), endoscopy, and pathological whole slide images can be transmitted in real time, which is the basis of realizing remote diagnosis, remote surgery and remote teaching. Combined with AI, telemedicine can build virtual reality (VR) scenes and blend them into real scenes to establish augmented reality (AR) scenes for remote surgery. Cloud computing is the on-demand availability of computer system resources, especially data storage (cloud storage) and computing power. AI requires significant amounts of computing resources, and cloud computing is a suitable solution. Through the sharing of cloud computing resources, it provides core foundation for the robustness and versatility of intelligent oncology applications.
2.2. Applications of Al technologies in cancer
A key component of intelligent oncology consists of combination of AI with oncology for clinical applications (Fig. 1). Although many of the studies are still in method development and generalization stages, AI technologies have multiple application scenarios in clinical oncology, including cancer prevention, screening, early diagnosis, prognosis prediction, drug discovery and development, clinical trials, protein structure prediction, surgery, radiotherapy, nursing and rehabilitation. We will provide examples and new development of these applications in Section 3.
2.3. Security strategies, ethical laws for Al technologies
Intelligent oncology contains a large quantity of big data and predictive analytics in medical decision-making processes, therefore the data security strategies and ethics regulation of AI technologies in the oncology setting, specifically whether data-driven decisions might risk dehumanizing patients are of great concerns,25 despite that these issues are not specific to oncology. Due to large amounts of personal data contained, the risk of privacy violation is also high. Protecting patient data is a core responsibility of healthcare providers, therefore the content of intelligent oncology also includes data security strategies and ethical laws for relevant Al technologies (Fig. 1).
2.4. Talent training
The development of intelligent oncology also relies on cultivating talents specialized in oncology together with computer sciences, and on promoting interdisciplinary integration and innovation in training of the next generation of physician-scientists (Fig. 1). Higher education institutes worldwide have established undergraduate programs such as intelligent medicine. In addition, AI technologies have been used in tutoring in medical training in various scenarios. For example, randomized clinical trials have shown superior performance outcome and skill transfer for an AI based surgical simulation training.26, 27, 28 Meanwhile, interdisciplinary collaborations focusing on intelligent oncology have been a mainstream direction in oncology-specialized institutions.29 In addition, the talent training includes continued medical education, e-learning, self-learning, mentorship programs and more.
3. Key applications of intelligent oncology
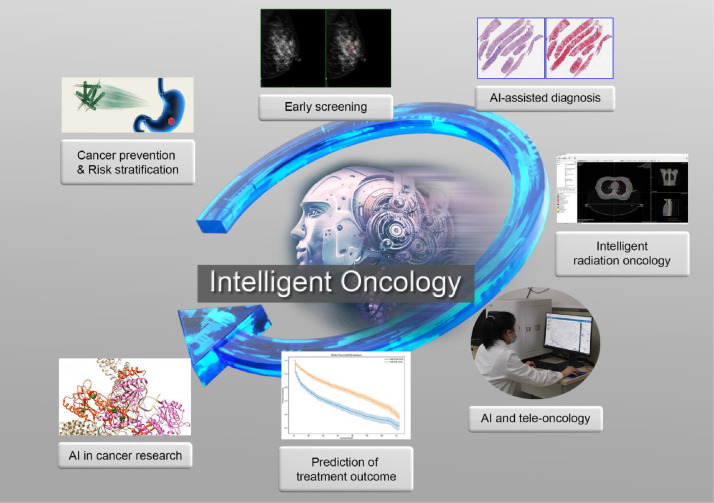
For intelligent oncology, AI techniques have been explored almost in the whole process of cancer care and preclinical research (Fig. 2). In PubMed, there are more than 25,000 publications since 2002, and the number has been exponentially growing in recent years. In the following sessions, we summarize some of the major directions of these applications.
Fig. 2.
An intelligently ecological chain in the process of cancer care. AI, artificial intelligence.
3.1. Cancer prevention and screening
Data generated from epidemiological studies, combining multi-omics and molecular cancer biology can be extracted by ML to build up models for primary prevention of many cancers. Incorporation of relevant cancer control programs would allow to establish precision prevention platforms. Such AI platforms include some of the primary prevention practices, such as monitoring healthy lifestyle choices and physical activities.
AI technologies are also playing increasingly important roles in cancer screening, in which high-risk populations are analyzed by radiological or pathological methods in order to catch signs of cancer early. There are at least four cancer types showing clear benefits for screening. These cancers include breast, lung, colorectal and cervical cancers. AI-assisted methods have been used for breast cancer (ultrasound30,31 and X-ray mammogram32), lung cancer (low-dose CT33, 34, 35, 36, 37), colorectal cancer (colonoscopy38, 39, 40, 41, 42 and MRI43), and cervical cytology.44 In other cancers, retrospective studies have explored using DL based algorithms for their values in screening.45 Future optimization of the methods might improve the screening efficiency and eventually benefit these high-risk populations.
3.2. AI-assisted diagnosis
Intelligent oncology has some best examples showing successful applications of ML/DL-based algorithms for fast and accurate diagnosis of cancers using radiological and pathological images, owing to the superior power of ML/DL in performing image-recognition tasks.46,47 In radiology, AI-based technologies have been integrated into daily practices in recent years, and methods range from convolutional neural networks to VAE.48,49 VAE is a powerful generative model, which has been improved from AE. Like AE, it also contains an encoder and a decoder network. The encoder network translates the original high-dimension input into the latent low-dimensional code. The decoder network recovers the data from the code, likely with larger and larger output layers. Compared to AE, VAE maps the input data into a distribution, assumed as multi-Gaussian distribution, and the recover vectors are sampled from the distribution. For applications with VAE, they not only can extract the discrimination representation, but also can generate diverse samples. Industries have been approved for licensees for relevant software. For example, there are at least 11 lung nodule assistant diagnosis software approved by National Medical Products Administration (NMPA) in China. These products can automatically identify lung nodules and categorizing them as benign or malignant. In addition, multi-task algorithms have been developed in lung cancer for automatically fast-tracking tumor lesions and classifying corresponding histological subtypes.50 Meanwhile, digital pathology has accelerated the advancement of clinical pathology, leading to more efficient, economic and accurate diagnosis. Compared to traditional pathology, digital pathology stores images that can be viewed with a computer monitor or mobile device. Several cancer types have been benefited by these technologies. For instance, newly developed AI algorithms can automatically diagnose a large amount of specimens for neoplastic and non-neoplastic cervical cytological slides.44 A research team from Harvard University built an algorithm based on a DL method named tumor origin assessment via deep learning (TOAD) which can be used to differentiate the origin of the primary tumor using conventionally obtained histological sections. Besides, TOAD can also be used as an adjunctive tool to differentiate between complex metastatic tumors and cancer of unknown primary (CUP) cases and can be used in combination with or in place of adjunctive or extensive diagnostic tests to reduce the occurrence of CUP.51
3.3. Prediction of treatment outcome
One of the major research directions in intelligent oncology is to utilize ML/DL-based algorithms to predict the cancer therapeutic response. Compared to traditional Cox-based prediction methods, AI algorithms based on DL are more self-adaptive and suitable for non-linear representation, which has a more precise predict performance and applicable in cancer prognosis prediction. Shown in Table 1 are some major studies related to cancer prognostic prediction based on AI approaches in top medical journals from 2020 to 2022. These studies cover a wide range of cancer types, including cancer from lung, breast, colorectal, liver, prostate, et al., and also include major cancer therapies such as neo-adjuvant therapy, radiation therapy, targeted therapy, and immunotherapy. To address the issue of treatment evaluation in colorectal cancer, four papers in Lancet have explored outcome prediction based on deep network models for neo-adjuvant chemo-radiotherapy, adjuvant therapy, immunotherapy and conventional treatment. Yamashita R, et al.52 used whole slide image of hematoxylin-eosin (H&E)-stained to construct a MSINet model based on CNN and residual networks (ResNet), which can predict the microsatellite stability state of colorectal cancer and thus the outcome of immunotherapy. It achieved an area under the curve (AUC) of 0.931 for microsatellite instability (MSI) prediction and AUC of 0.865 for treatment prediction. Skrede OJ, et al.53 and Bilal M, et al.54 created deep neural network models based on the whole slide image of H&E-stained to predict adjuvant therapy and conventional therapy effectiveness respectively. On the basis of it, Feng L, et al.55 proposed a muti-module method for neo-adjuvant chemo-radiotherapy outcome prediction of rectal cancer. They built a radio-pathomics integrated prediction system (RAPIDS) through the integration of radiomic and pathomic signatures from the MRI (T2-weighted imaging, contrast-enhanced T1-weighted imaging, and diffusion-weighted imaging) image and H&E-stained whole-slide images. There are three main algorithms in RAPIDS, visual geometry group19 (VGG19), elastic network, and SVM. VGG19 is used to extract features from the whole slide images (WSIs) and elastic network are used to evaluate the features extracted by radiomics. All the two types of omics feature would combine in the SVM, which was trained for predicting pathological complete response (pCR) of neo-adjuvant chemo-radiotherapy in locally advanced rectal cancer. In the retrospective study, RAPIDS received a prediction result with AUC 0.872 in the validation setting. Further, in a prospective validation study, it achieved AUC 0.812. Although image data are the most common data for cancer treatment outcome prediction, the clinical text and other types of data also can add values. Arbour KC, et al.56 constructed a DL model trained by the data of radiology text reports. They used the model to estimate response evaluation criteria in solid tumors (RECIST)-defined outcomes in non-small cell lung cancer. The test result reveals the non-small cell lung cancer (NSCLC) outcome prediction by the AI method is effectiveness, the best performance is AUC 0.9.
Table 1.
Recent literature on prediction of cancer treatment outcome using ML/DL.
| Author | Cancer type | Therapy method | Application task | AI method | Data type | Journal |
|---|---|---|---|---|---|---|
| Arbour K C56 | Non-small cell lung cancer |
Immunotherapy | Estimate immunotherapy response | Deep learning model | Radiology text reports | Cancer Discov |
| McIntosh C98 | Prostate cancer | Radiation therapy | Radiation therapy treatment plan assessment | Random forest | Radiation therapy treatment plans | Nature Med |
| Feng L55 | Locally advanced rectal cancer | Neoadjuvant chemoradiotherapy | Predict pathological complete response | Radiomics+VGG19+SVM | Radiation medical image + pathology whole slide image | Lancet Digital Health |
| Bilal M54 | Colorectal cancer | Conventional treatment | Optimal therapeutic decision | ResNet18+adapted ResNet34 | Pathology whole slide image | Lancet Digital Health |
| Skrede O J99 | Colorectal cancer | Adjuvant therapy | Improved markers of adjuvant therapy |
DoMore-v1-CRC | Pathology whole slide image | Lancet |
| Yamashita R52 | Colorectal cancer | Immunotherapy | Predict outcome of immunotherapy | MSINet | Pathology whole slide image | Lancet Oncol |
| Saillard C100 | Liver cancer | Conventional treatment | Improve therapeutic strategies | ResNet+attention | Pathology whole slide image | Hepatology |
| Lin Lu101 | Metastatic colorectal cancer | Anti-vascular endothelial growth factor therapies | Characterize tumor morphological change for response assessment | Deep learning network | VELOUR trial | Nat Commun |
| Wei Mu102 | Non-small cell lung cancer | Immunotherapy response |
Predict the PD-L1 expression and the immunotherapy response |
small residual-convolutional-network |
PET/CT images | J Immunother Cancer |
| Cheng Jin103 | Rectal cancer | Neoadjuvant chemoradiotherapy | Predict pathologic complete response |
Multi-task deep learning | MRI and blood-based tumor markers | Nat Commun |
| Jie-Yi Shi104 | Hepatocellular carcinoma | Conventional therapy | Discover the prognostic indicators | Deep learning method | WSI, multi-omics data | Gut |
| Sammut S J105 | Breast cancer | Immunotherapy | Build response predictors of treatment |
LR+ENR+SVM | Muti-omics data | Nature |
Abbreviations: CT, computed tomography; CRC, colorectal cancer; DL, deep learning; ENR, ElasticNet regression; ML, machine learning; LR, logistic regression; MRI, magnetic resonance imaging; MSINet, deep learning model for the prediction of microsatellite instability in colorectal cancer; PET, positron emission tomography; ResNet, residual networks; SVM, support vector machines; VGG19, visual geometry group19; WSI, whole slide image.
3.4. Intelligent radiation oncology
Intelligent oncology has penetrated into each step of radiotherapeutic practices and is expected to significantly enhance the efficiency, accuracy and quality of radiation therapy. In the initial step of radiotherapy planning workflow, AI has been applied to image acquisition, processing. For instance, AI can be adopted to generate synthetic CT images from MRI images. The differences of dose calculation using synthetic CT and true CT is only 0.5%,57 which could meet the criterion of a clinical use.58 Image registration is another preparation step for radiotherapy planning. Utilizing registration algorithms has achieved superior accuracy and robustness than other advanced registration methods59 and could mitigate image and motion artefacts.60 Moreover, automatic segmentation and contouring tumor volume and organs at risk could reduce workload and boost efficiency.61
AI has been integrated into the step of planning design in two main aspects: one is setting up planning parameters, and the other predicting the optimal dose distribution. For the first aspect, AI is adopted to learn the relationship between image features and the patient's anatomical shape to infer the dose distribution in tumor volume and organs at risks, then to generate clinically applicable treatment plans without manual intervention. For the other aspect, predicting the optimal dose distribution could reduce repeated optimization for planning, improve the communication between physicians and physicists, and facilitate clinical decision-making. In addition, AI can be used to help generate biological target volume-based planning. For instance, lung ventilation metrics is generated by AI using CT images.62 Incorporating lung function into treatment planning to decrease dose to highly functioning areas of lung could potentially reduce the incidence of radiation pneumonitis. Moreover, AI is applied to motion management. Park et al.63 reported that intra- and inter-fraction fuzzy DL might achieve real-time estimation and tracking for respiratory associated tumor movements. In addition, AI has also been used in the quality control for radiotherapy equipment. Together, AI has the potential to substantially improve the entire process of radiotherapy, paving a path to intelligent radiation oncology.
3.5. AI and tele-oncology
Telemedicine technologies have been advanced rapidly due to the fast development of the 5G technology. The most noticeable has been internet hospitals as well as the emergence and widely applications of wearable health devices to monitor vital signs. Telemedicine can provide equitable distribution of advanced healthcare resources, reducing healthcare disparities. The COVID-19 pandemic has accelerated the implementation of telemedicine, which has helped reduce unnecessary visits and infection risks.64 For cancer survivors, telemedicine interventions can significantly improve the life quality.65,66
Advances in wireless technology, smartphones have help develop novel approaches of collecting patient-generated health data. AI helps create data-driven approaches to identifying and intervening in early-on treatment toxicity and cancer progression.67 Meanwhile, AI with advanced telecommunications can facilitate healthy lifestyle behaviors, symptom management, and medication adherence by close contact with patients outside of clinic.67
Moreover, VR technologies are capable to support cancer care, as we are seeing mega-verse entering healthcare. VR and AR in complex surgery such as oncologic liver surgery and cranial tumor surgery are evolving technologies that can improve pre-operative planning and intraoperative navigation.68,69 Furthermore, a meta-analysis has reported that VR-based interventions can reduce symptoms of anxiety, depression, pain, and cognitive function for cancer patients.70 VR games with high fidelity components can relieve pain for pediatric cancer patients.71
The 5G-based telecommunications enable better quality and reliable video-consultations and real-time examinations. Using advanced AI computational and mathematical approaches to processing massive data generated from the internet of things could promote self-management and clinical decisions.72
3.6. AI in cancer research
A critical component of intelligent oncology consists of a wide range of research directions from basic to translational and clinical research.
3.6.1. Prediction of gene mutations
AI has made new breakthroughs in mutation prediction using ML algorithms. For example, a radiomics signature can be used to discriminate EGFR positive and EGFR negative images for lung cancer.73 Further, studies have found six of ten genes that are most commonly mutated in lung adenocarcinoma (LUAD): STK11, EGFR, FAT1, SETBP1, KRAS and TP53-can be well predicted from pathology images.74 It has been reported that a weakly supervised learning technique to train a deep neural networks (DNN) to predict BRAF mutational status, in H&E-stained images of thyroid cancer tissue without regional annotations.75
3.6.2. Multi-omics studies
ML and DL algorithms have made possible to globally profile genes (genomics and epigenomics), RNAs (RNA transcriptomics), proteins (proteomics and phospho-proteomics), and metabolites (metabolomics). ML-based technologies are useful to integrate these multi-omics data and identify critical components for tumor initiation and progression, and their mechanisms associated with heterogeneous therapeutic responses.
3.6.3. Protein structure prediction
In 2020, the AlphaFold Protein Structure Database was launched, highlighting AlphaFold's application to the entire human proteome. This neural network architecture yields the 3D structure of proteins from any given amino acid sequence.76
3.6.4. Protein-protein interactions
A human interactome map of human binary protein interactions, also called ‘HuRI’ was recently launched.77 With approximately 53,000 protein–protein interactions, HuRI has approximately four times as many interactions as there are high-quality curated interactions from small-scale studies. The integration of HuRI with genome, transcriptome and proteome data enables a cellular function to be studied within most physiological or pathological cellular contexts. The utility of HuRI can identify specific subcellular roles of protein–protein interactions.77
3.6.5. Drug discovery and development
Drug discovery and development requires lengthy processes to identify therapeutically targetable elements of a disease, and they depend on extensively high-quality datasets to produce a safe and efficacious drug. Owing to the AI-based fast understanding of target structures and the availability of supercomputer powers, cancer drug discovery has been accelerated exponentially. ML not only provides a high-throughput approach to data analysis and storage, it also increases the likelihood of developing a successful product. In addition, researchers have developed AI-based clinical decision support systems to identify eligible candidates for clinical trials. Compared to manual screening for clinical trials, these systems offer the potential to increase screening efficiency and accuracy by excluding ineligible patients for clinical trials.78,79 An increasing number of studies have also adopted AI to predict drug responses.80
4. The impact of intelligent oncology
With the development of intelligent oncology, AI will be integrated into the clinical work of tumor diagnosis and treatment. For screening and diagnostic purposes, AI technologies can be implemented into medical diagnostic devices to improve the accuracy and efficiency. For therapeutic purposes, AI technologies have been explored in clinical decision making, surgery and radiotherapy, and it can assist predicting the therapeutic response and patient outcome. As we continuously invest significant efforts to explored AI ideologies and technologies for potential applications in oncology, the impact on intelligent oncology can be felt at three levels: physician, patient and hospital.
4.1. Physician level
The major impact at the healthcare provider level is that, applications of AI technologies can reduce repetitive work and human errors. More importantly, it can significantly improve the efficiency, accuracy, consistency and accessibility of oncologists' diagnosis and treatment of cancers.81, 82, 83, 84, 85 With advancement of technology and the development of medical equipment, the medical data during the diagnosis and treatment of cancer patients have been rapidly improved in multiple dimensions. Exponentially increasing data can come from any part of the clinical cancer process and applicable to diverse AI methods and models. AI can provide more accurate and sufficient medical evidence through this process, thereby helping clinicians build a comprehensive, personalized view of each patient and assist them in making judgments and decisions.
4.2. Patient level
In intelligent oncology, AI can learn patterns for precise patient groups and guide diagnosis and treatment.86 For instance, AI has been applied to extract and analyze unstructured text that prostate cancer patients published on an online health support platform.87 Patient behaviors, demographics, clinical factors, emotions are analyzed by AI. Results demonstrated that the platform empowered patients in terms of individualized decision-making, clinical and emotional needs, which is facilitative of proposing a healthcare measure of extending and applying online health support platforms to patients.87 Abidi proposed an information technology (IT) info-structure that could automatically transform healthcare data and generate knowledge-driven decision-support services.88 In addition, Hung et al. developed a health index using DL on a large-scale and longitudinal population based electronic health record.89 Moreover, while cancer survivors often experience depression, AI can be used to identify cues of depression in language, speech, and facial expression domains, which could facilitate the early detection of depression and potentially provide better support to cancer survivors.90 At the patient level, intelligent wearable medical devices are capable to collect, analyze and display the physiological data of human through sensors, allowing users to monitor and respond to changes of their physical signs in real time.
4.3. Hospital level
There has been a trend to build smart hospitals worldwide. The boost of AI technologies and data production materials will digitize the development of oncology-specialized hospitals, accelerate hospital's constructions of digital medical care, centralized storage of patient data and data cooperation between hospitals will make hospital a huge data processing center. New AI systems can integrate hospital operations, doctors and patients, and scientific research. In this regard, intelligent oncology aims at building smart oncology clinics through upgrading systems, such as electronic medical record systems and supply chains. For example, the NLP approach can be developed to synthesize structured electronic medical records and unstructured clinical notes. In addition, with the use of telemedicine, intelligent oncology will play an important role in reducing health disparities. Meantime, cloud-based AI may assist the hierarchical diagnosis and treatment in oncology.
In addition, intelligent oncology is committed to improving medical data sharing and ensuring data security with the assistance of AI. AI systems are employed in the process to guarantee data quality, ensure images loading easily, and reduce the specialists’ workload.91 Furthermore, ML can be used to integrate large-scale patient data from multiple hospitals, enhancing the size of database and generalization power. A recent study has incorporated data of colorectal cancer patients from different hospitals while overcoming feature misalignment and distribution divergence by employing hybrid semi-supervised transfer learning models from multi-centers, in combination with the DNN and neural decision forest model. They demonstrate that the approach has superior generalizability regardless of the data heterogeneity.92
5. Obstacles and challenges of intelligent oncology
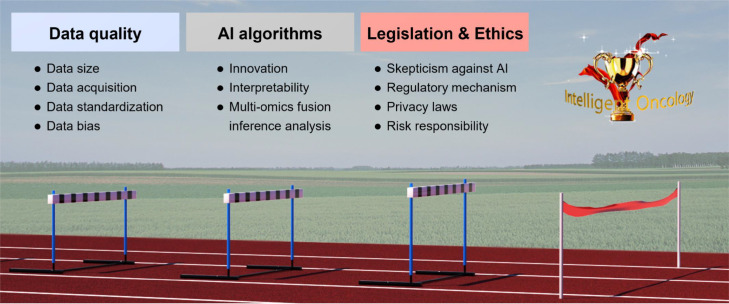
Through concerted efforts in above-mentioned aspects, intelligent oncology is expected to contribute significantly to the future success of basic, translational and clinical oncology. While we are optimistic on the future intelligent oncology, there are a range of obstacles and challenges to advancing the new specialty (Fig. 3).
Fig. 3.
The challenges and hurdles in intelligent oncology. AI, artificial intelligence.
5.1. The quality of clinical data for algorithm training and adaptation
The data quality is considered one major barrier to the smooth development of AI applications in intelligent oncology.86 The issues include data aggregation, bias, curation, reliability, and transparency.93 Intelligent oncology requires a large volume of high-quality data to train and to evaluate a model. However, the adequate high-quality data acquisition in medicine is more difficult than in other industries. Firstly, the size of data source is a key issue. Limited by any single institution, the number of original data and the sampling range are not sufficient to cover the distribution of a patient population, resulting in potential bias in the data used for training and modeling. Secondly, the quality of data is low in many institutions, due to the complexity of the diseases, treatment options, standardizations and follow-ups. A common phenomenon is that there exists a significant disparity between training data and validation data or real-world data.94 In addition, due to sensitivity of medical data and limited sharing mechanisms, there are difficulties in data acquisition. Thirdly, healthcare data are often recorded and stored in highly heterogeneous and unstructured ways.93 Data standardization is a solution for data interpretation, but it should be designed before data collection. In addition, due to dynamic changes in source data, AI algorithms need to have automatic changes to update results after a certain period.95 Therefore, how to reduce data bias to ensure that an AI-generated model becomes reliable and can be used in real world remains to be a challenging topic in the future.
5.2. AI algorithms
For Intelligent oncology, innovative AI algorithms should be developed and optimized to facilitate multi-model data stream input, output, as well as architectural and parameter design. One major challenge is the interpretability of explainable AI (XAI) approaches. To gain the trust of doctors, regulators, and patients, any medical care system must be understandable and explainable.96 Ideally, it should be able to explain to all parties involved the complete logic of making a decision. Due to the inherent nature of medical data, building interpretable DL models for analysis is different from other applications. The diversity of tumors and the complexity of human biology make AI algorithm designs more difficult. Although many of medical practices are initially based on empirical evidence, for new technologies as such AI-assisted medical devices, the mandatory nature of XAI should be maintained within reasonable limits to maximize both the benefits to the patient and the healthy development of XAI itself. In the future of intelligent oncology, multi-omics fusion inference analytical techniques are expected to play a key role. This poses higher requirement for AI algorithms and greater demand for integrated medical and AI talents.
5.3. Legislation and ethics
As the traditional diagnosis and treatment models have been deeply rooted in the public, patients and doctors instinctively have a skeptical attitude towards the addition of AI. In addition to technology, the smooth development of intelligent oncology also involves ethical, philosophical, moral and economic issues. At present, many countries have introduced relevant laws and policies to promote the reasonable applications of AI in healthcare systems. However, for intelligent oncology, it still has a long way ahead to overcome the traditional concept based on humanistic ethics and to optimize the supervision mechanism. The intervention of AI has brought some changes to the traditional doctor-patient relationship, and the legal relationship will increase one party in the subject aspect, as well as a variety of uncontrollable problems and unknown risks.97 Data protection and privacy such as consent, data anonymization, and de-identification also draw wide-spread public attentions. 2021, the US Food and Drug Administration (FDA) has identified a workflow for AI and ML-based software as medical device (SaMD).102 There are 5 actions: (1) tailored regulatory framework for AI/ML-based SaMD; (2) good machine learning practice (GMLP); (3) patient-centered approach incorporating transparency to users; (4) regulatory science methods related to algorithm bias & robustness; (5) real-world performance (RWP). The AI/ML-based SaMD action plan is intended as a multi-pronged approach to further advance the agency's oversight of these technologies. It should be noted that there are significant differences among government regulation worldwide. In addition, existing regulations/laws still have loopholes in the protection of AI in information security, and the risk responsibility system in the process of research and development is not mature enough.
6. Conclusions
Currently, the concept and applications of intelligent oncology is still in its infancy stage. Most research has focused on proof-of-principal and method development, rather than on implementing the methods in clinical practice. As indicated, AI technologies such as ML and DL have their own limitations, and aggressive clinical trials should be used to verify any AI-driven results and implement the generalization of algorithms. In this regard, there is an urgent need to have more scientists/engineers and clinicians to recognize intelligent oncology as a new specialty and educate them about the strengths and weaknesses of AI technologies. Furthermore, we need to rigorously assess the benefits regarding clinical outcomes, patient experience, and costs. As we look forward to the future of medicine, we believe that intelligent oncology will play a pivotal role for oncology care. With implementation of intelligent oncology, more accurate, efficient, and economical cancer care are expected, from which all parties involved will benefit.
Declaration of competing interest
The authors declare that they have no conflict of interests..
Acknowledgments
Acknowledgments
We thank all members of the Chinese Society of Artificial Intelligence, China Anti-Cancer Association for helpful discussions. This work was supported by the National Natural Science Foundation of China (grant numbers: 81974464, 61906022), Chongqing Natural Science Foundation (grant number: cstc2020jcyj-msxmX0482) and Chongqing University Research Fund (grant number: 2021CDJXKJC004).
Author contributions
Conceptualization: BX; Data curation and formal analysis: BL,ZT, YM and XY; Funding acquisition: BX, YL and BL; Supervision: BX and YL, Writing - original draft:BX, BL, ZT, YM, XY, and YL; Writing - review & editing: BX, YL and BL.
Contributor Information
Yajie Liu, Email: Anthea1966@163.com.
Bo Xu, Email: xubo731@cqu.edu.cn.
References
- 1.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 2.Van Calster B, Wynants L. Machine learning in medicine. N Engl J Med. 2019;380(26):2588. doi: 10.1056/NEJMc1906060. -2588. [DOI] [PubMed] [Google Scholar]
- 3.Obermeyer Z, Emanuel EJ. Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med. 2016;375(13):1216–1219. doi: 10.1056/NEJMp1606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng WY, Yang THO, Anastassiou D. Development of a prognostic model for breast cancer survival in an open challenge environment. Sci Transl Med. 2013;5(181):181ra50. doi: 10.1126/scitranslmed.3005974. [DOI] [PubMed] [Google Scholar]
- 5.Jordan MI, Mitchell TM. Machine learning: Trends, perspectives, and prospects. Science. 2015;349(6245):255–260. doi: 10.1126/science.aaa8415. [DOI] [PubMed] [Google Scholar]
- 6.Wong D, Yip S. Machine learning classifies cancer. Nature. 2018;555(7697):446–447. doi: 10.1038/d41586-018-02881-7. [DOI] [PubMed] [Google Scholar]
- 7.Kumar RD, Swamidass SJ, Bose R. Unsupervised detection of cancer driver mutations with parsimony-guided learning. Nat Genet. 2016;48(10):1288–1295. doi: 10.1038/ng.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouyanfar S, Sadiq S, Yan YL, et al. A survey on deep learning: algorithms, techniques, and applications. Acm Computing Surveys. 2019;51(5):1–36. doi: 10.1145/3234150. [DOI] [Google Scholar]
- 9.Chiu YC, Zheng SY, Wang LJ, et al. Predicting and characterizing a cancer dependency map of tumors with deep learning. Sci Adv. 2021;7(34):eabh1275. doi: 10.1126/sciadv.abh1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coudray N, Tsirigos A. Deep learning links histology, molecular signatures and prognosis in cancer. Nat Cancer. 2020;1(8):755–757. doi: 10.1038/s43018-020-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Si X, Hu C, et al. A review of recurrent neural networks: LSTM cells and network architectures. Neural Comput. 2019;31(7):1235–1270. doi: 10.1162/neco_a_01199. [DOI] [PubMed] [Google Scholar]
- 12.Shin H-C, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285–1298. doi: 10.1109/TMI.2016.2528162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong W, Dong ZY, Jia Y, et al. Short-term residential load forecasting based on LSTM recurrent neural network. Kong, Weicong et al. IEEE Transactions on Smart Grid. 2019;10(1):841–851. doi: 10.1109/TSG.2017.2753802. [DOI] [Google Scholar]
- 14.Jain A, Zamir AR, Savarese S, et al. Structural-RNN: deep learning on spatio-temporal graphs. 2016:5308–5317. doi: 10.1109/CVPR.2016.573. [DOI]
- 15.Cho K, Van Merriënboer B, Gulcehre C, et al. Learning phrase representations using RNN encoder-decoder for statistical machine translation. 2014; arXiv:1406.1078.
- 16.Li Z, Zhang Z, Zhao H, et al. Text compression-aided transformer encoding. IEEE Trans Pattern Anal Mach Intell. 2021;44(7):3840–3857. doi: 10.1109/tpami.2021.3058341. [DOI] [PubMed] [Google Scholar]
- 17.Han K, Wang Y, Chen H, et al. A survey on vision transformer. IEEE Trans Pattern Anal Mach Intell. 2022 doi: 10.1109/tpami.2022.3152247. [DOI] [PubMed] [Google Scholar]
- 18.Lin C-H, Lucey S. Inverse compositional spatial transformer networks. 2017; doi: 10.1109/CVPR.2017.242. [DOI]
- 19.Devlin J, Chang M-W, Lee K, et al. Bert: Pre-training of deep bidirectional transformers for language understanding. 2018; arXiv:1810.04805.
- 20.Zeng JM, Gensheimer MF, Rubin DL, et al. Uncovering interpretable potential confounders in electronic medical records. Nat Commun. 2022;13(1):1014. doi: 10.1038/s41467-022-28546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajpurkar P, Chen E, Banerjee O, Topol EJ. AI in health and medicine. Nat Med. 2022;28(1):31–38. doi: 10.1038/s41591-021-01614-0. [DOI] [PubMed] [Google Scholar]
- 22.Harari YN. Reboot for the AI revolution. Nature. 2017;550(7676):324–327. doi: 10.1038/550324a. [DOI] [PubMed] [Google Scholar]
- 23.Zemmar A, Lozano AM, Nelson BJ. The rise of robots in surgical environments during COVID-19. Nat Mach Intell. 2020;2(10):566–572. doi: 10.1038/s42256-020-00238-2. [DOI] [Google Scholar]
- 24.Kinross JM, Mason SE, Mylonas G, et al. Next-generation robotics in gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2020;17(7):430–440. doi: 10.1038/s41575-020-0290-z. [DOI] [PubMed] [Google Scholar]
- 25.Jobin A, Ienca M, Vayena E. The global landscape of AI ethics guidelines. Nat Mach Intell. 2019;1(9):389–399. doi: 10.1038/s42256-019-0088-2. [DOI] [Google Scholar]
- 26.Yao X, Rushlow DR, Inselman JW, et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med. 2021;27(5):815–819. doi: 10.1038/s41591-021-01335-4. [DOI] [PubMed] [Google Scholar]
- 27.Fazlollahi AM, Bakhaidar M, Alsayegh A, et al. Effect of artificial intelligence tutoring vs expert instruction on learning simulated surgical skills among medical students: a randomized clinical trial. JAMA Netw Open. 2022;5(2) doi: 10.1001/jamanetworkopen.2021.49008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Cruz Rivera S, Moher D, et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364–1374. doi: 10.1038/s41591-020-1034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpless NE, Kerlavage AR. The potential of AI in cancer care and research. Biochim Biophys Acta Rev Cancer. 2021;1876(1) doi: 10.1016/j.bbcan.2021.188573. [DOI] [PubMed] [Google Scholar]
- 30.Wu GG, Zhou LQ, Xu JW, et al. Artificial intelligence in breast ultrasound. World J Radiol. 2019;11(2):19–26. doi: 10.4329/wjr.v11.i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Choi Y, Kim E, et al. Deep learning-based computer-aided diagnosis in screening breast ultrasound to reduce false-positive diagnoses. Sci Rep. 2021;11(1):395. doi: 10.1038/s41598-020-79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacilè S, Lopez J, Chone P, et al. Improving breast cancer detection accuracy of mammography with the concurrent use of an artificial intelligence tool. Radiol Artif Intell. 2020;2(6) doi: 10.1148/ryai.2020190208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu YC, Tsai YH, Weng HH, et al. Artificial neural networks improve LDCT lung cancer screening: a comparative validation study. BMC Cancer. 2020;20(1):1023. doi: 10.1186/s12885-020-07465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamberlin J, Kocher MR, Waltz J, et al. Automated detection of lung nodules and coronary artery calcium using artificial intelligence on low-dose CT scans for lung cancer screening: accuracy and prognostic value. BMC Med. 2021;19(1):55. doi: 10.1186/s12916-021-01928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui S, Ming S, Lin Y, et al. Development and clinical application of deep learning model for lung nodules screening on CT images. Sci Rep. 2020;10(1):13657. doi: 10.1038/s41598-020-70629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le NQK, Kha QH, Nguyen VH, et al. Machine learning-based radiomics signatures for EGFR and KRAS mutations prediction in non-small-cell lung cancer. Int J Mol Sci. 2021;22(17):9254. doi: 10.3390/ijms22179254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lancaster HL, Zheng S, Aleshina OO, et al. Outstanding negative prediction performance of solid pulmonary nodule volume AI for ultra-LDCT baseline lung cancer screening risk stratification. Lung Cancer. 2022;165:133–140. doi: 10.1016/j.lungcan.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad OF, Soares AS, Mazomenos E, et al. Artificial intelligence and computer-aided diagnosis in colonoscopy: current evidence and future directions. Lancet Gastroenterol Hepatol. 2019;4(1):71–80. doi: 10.1016/s2468-1253(18)30282-6. [DOI] [PubMed] [Google Scholar]
- 39.Barua I, Vinsard DG, Jodal HC, et al. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021;53(3):277–284. doi: 10.1055/a-1201-7165. [DOI] [PubMed] [Google Scholar]
- 40.Murakami D, Yamato M, Arai M, et al. Artificial intelligence in colonoscopy. Lancet Gastroenterol Hepatol. 2021;6(12):984–985. doi: 10.1016/s2468-1253(21)00379-4. [DOI] [PubMed] [Google Scholar]
- 41.Misawa M, Kudo SE, Mori Y, et al. Artificial intelligence-assisted polyp detection for colonoscopy: initial experience. Gastroenterology. 2018;154(8):2027–2029. doi: 10.1053/j.gastro.2018.04.003. e3. [DOI] [PubMed] [Google Scholar]
- 42.Hassan C, Spadaccini M, Iannone A, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93(1):77–85. doi: 10.1016/j.gie.2020.06.059. e6. [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Yu Q, Gao Y, et al. Identification of metastatic lymph nodes in MR imaging with faster region-based convolutional neural networks. Cancer Res. 2018;78(17):5135–5143. doi: 10.1158/0008-5472.Can-18-0494. [DOI] [PubMed] [Google Scholar]
- 44.Kanavati F, Hirose N, Ishii T, et al. A deep learning model for cervical cancer screening on liquid-based cytology specimens in whole slide images. Cancers (Basel). 2022;14(5):1159. doi:10.3390/cancers14051159 [DOI] [PMC free article] [PubMed]
- 45.Bulten W, Pinckaers H, van Boven H, et al. Automated deep-learning system for Gleason grading of prostate cancer using biopsies: a diagnostic study. Lancet Oncol. 2020;21(2):233–241. doi: 10.1016/s1470-2045(19)30739-9. [DOI] [PubMed] [Google Scholar]
- 46.Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18(8):500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bera K, Schalper KA, Rimm DL, et al. Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019;16(11):703–715. doi: 10.1038/s41571-019-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han K, Wen H, Shi J, et al. Variational autoencoder: An unsupervised model for encoding and decoding fMRI activity in visual cortex. NeuroImage. 2019;198:125–136. doi: 10.1016/j.neuroimage.2019.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sandfort V, Yan K, Graffy PM, et al. Use of variational autoencoders with unsupervised learning to detect incorrect organ segmentations at CT. Radiol Artif Intell. 2021;3(4) doi: 10.1148/ryai.2021200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi J, Deng Z, Sun G, et al. One-step algorithm for fast-track localization and multi-category classification of histological subtypes in lung cancer. Eur J Radiol. 2022;154 doi: 10.1016/j.ejrad.2022.110443. [DOI] [PubMed] [Google Scholar]
- 51.Lu MY, Chen TY, Williamson DFK, et al. AI-based pathology predicts origins for cancers of unknown primary. Nature. 2021;594(7861):106–110. doi: 10.1038/s41586-021-03512-4. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita R, Long J, Longacre T, et al. Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. Lancet Oncol. 2021;22(1):132–141. doi: 10.1016/S1470-2045(20)30535-0. [DOI] [PubMed] [Google Scholar]
- 53.Skrede OJ, De Raedt S, Kleppe A, et al. Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet. 2020;395(10221):350–360. doi: 10.1016/s0140-6736(19)32998-8. [DOI] [PubMed] [Google Scholar]
- 54.Bilal M, Raza SEA, Azam A, et al. Development and validation of a weakly supervised deep learning framework to predict the status of molecular pathways and key mutations in colorectal cancer from routine histology images: a retrospective study. Lancet Digit Health. 2021;3(12):e763–e772. doi: 10.1016/s2589-7500(21)00180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng L, Liu Z, Li C, et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. Lancet Digit Health. 2022;4(1):e8–e17. doi: 10.1016/s2589-7500(21)00215-6. [DOI] [PubMed] [Google Scholar]
- 56.Arbour KC, Luu AT, Luo J, et al. Deep learning to estimate RECIST in patients with NSCLC treated with PD-1 blockade. Cancer Discov. 2021;11(1):59–67. doi: 10.1158/2159-8290.CD-20-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cusumano D, Lenkowicz J, Votta C, et al. A deep learning approach to generate synthetic CT in low field MR-guided adaptive radiotherapy for abdominal and pelvic cases. Radiother Oncol. 2020;153:205–212. doi: 10.1016/j.radonc.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 58.Huynh E, Hosny A, Guthier C, et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol. 2020;17(12):771–781. doi: 10.1038/s41571-020-0417-8. [DOI] [PubMed] [Google Scholar]
- 59.Liao R, Miao S, Tournemire PD, et al. An artificial agent for robust image registration. 2016; arXiv:1611.10336.
- 60.Hou B, Alansary A, Mcdonagh S, et al. Predicting slice-to-volume transformation inPresence of arbitrary subject motion. 2017; arXiv:1702.08891.
- 61.Liu X, Li KW, Yang R, et al. Review of deep learning based automatic segmentation for lung cancer radiotherapy. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.717039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cazoulat G, Balter JM, Matuszak MM, et al. Mapping lung ventilation through stress maps derived from biomechanical models of the lung. Med Phys. 2021;48(2):715–723. doi: 10.1002/mp.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park S, Lee SJ, Weiss E, et al. Intra- and inter-fractional variation prediction of lung tumors using fuzzy deep learning. IEEE J Transl Eng Health Med. 2016;4 doi: 10.1109/JTEHM.2016.2516005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q, Zhang L, Yin R, et al. Effectiveness of telephone-based interventions on health-related quality of life and prognostic outcomes in breast cancer patients and survivors-A meta-analysis. Eur J Cancer Care (Engl) 2018;27(1) doi: 10.1111/ecc.12632. [DOI] [PubMed] [Google Scholar]
- 66.Cox A, Lucas G, Marcu A, et al. Cancer survivors' experience with telehealth: a systematic review and thematic synthesis. J Med Internet Res. 2017;19(1):e11. doi: 10.2196/jmir.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jim HSL, Hoogland AI, Brownstein NC, et al. Innovations in research and clinical care using patient-generated health data. CA Cancer J Clin. 2020;70(3):182–199. doi: 10.3322/caac.21608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quero G, Lapergola A, Soler L, et al. Virtual and augmented reality in oncologic liver surgery. Surg Oncol Clin N Am. 2019;28(1):31–44. doi: 10.1016/j.soc.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Mazur T, Mansour TR, Mugge L, et al. Virtual reality-based simulators for cranial tumor surgery: a systematic review. World Neurosurg. 2018;110:414–422. doi: 10.1016/j.wneu.2017.11.132. [DOI] [PubMed] [Google Scholar]
- 70.Zeng Y, Zhang JE, Cheng ASK, et al. Meta-analysis of the efficacy of virtual reality-based interventions in cancer-related symptom management. Integr Cancer Ther. 2019;18 doi: 10.1177/1534735419871108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yap KY, Koh DWH, Lee VSJ, et al. Use of virtual reality in the supportive care management of paediatric patients with cancer. Lancet Child Adolesc Health. 2020;4(12):899–908. doi: 10.1016/s2352-4642(20)30240-6. [DOI] [PubMed] [Google Scholar]
- 72.Chung AE, Jensen RE, Basch EM. Leveraging emerging technologies and the "internet of things" to improve the quality of cancer care. J Oncol Pract. 2016;12(10):863–866. doi: 10.1200/jop.2016.015784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rios Velazquez E, Parmar C, Liu Y, et al. Somatic mutations drive distinct imaging phenotypes in lung cancer. Cancer Res. 2017;77(14):3922–3930. doi: 10.1158/0008-5472.CAN-17-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559–1567. doi: 10.1038/s41591-018-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anand D, Yashashwi K, Kumar N, et al. Weakly supervised learning on unannotated H&E-stained slides predicts BRAF mutation in thyroid cancer with high accuracy. J Pathol. 2021;255(3):232–242. doi: 10.1002/path.5773. [DOI] [PubMed] [Google Scholar]
- 76.Wardah W, Khan MGM, Sharma A, et al. Protein secondary structure prediction using neural networks and deep learning: a review. Comput Biol Chem. 2019;81:1–8. doi: 10.1016/j.compbiolchem.2019.107093. [DOI] [PubMed] [Google Scholar]
- 77.Luck K, Kim DK, Lambourne L, et al. A reference map of the human binary protein interactome. Nature. 2020;580(7803):402–408. doi: 10.1038/s41586-020-2188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Georgiou A, Magrabi F, Hyppönen H, et al. The safe and effective use of shared data underpinned by stakeholder engagement and evaluation practice. Yearb Med Inform. 2018;27(1):25–28. doi: 10.1055/s-0038-1641194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haddad T, Helgeson JM, Pomerleau KE, et al. Accuracy of an artificial intelligence system for cancer clinical trial eligibility screening: retrospective pilot study. JMIR Med Inform. 2021;9(3):e27767. doi: 10.2196/27767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paton C, Kobayashi S. An open science approach to artificial intelligence in healthcare. Yearb Med Inform. 2019;28(1):47–51. doi: 10.1055/s-0039-1677898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nogales A, García-Tejedor Á J, Monge D, et al. A survey of deep learning models in medical therapeutic areas. Artif Intell Med. 2021;112 doi: 10.1016/j.artmed.2021.102020. [DOI] [PubMed] [Google Scholar]
- 82.Spadaccini M, Iannone A, Maselli R, et al. Computer-aided detection versus advanced imaging for detection of colorectal neoplasia: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(10):793–802. doi: 10.1016/s2468-1253(21)00215-6. [DOI] [PubMed] [Google Scholar]
- 83.Storås AM, Strümke I, Riegler MA, et al. Artificial intelligence in dry eye disease. Ocul Surf. 2022;23:74–86. doi: 10.1016/j.jtos.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Xu F, Wan C, Zhao L, et al. Predicting post-therapeutic visual acuity and OCT images in patients with central serous chorioretinopathy by artificial intelligence. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.649221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20(7):938–947. doi: 10.1016/s1470-2045(19)30333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kann BH, Hosny A, Aerts H. Artificial intelligence for clinical oncology. Cancer Cell. 2021;39(7):916–927. doi: 10.1016/j.ccell.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Silva D, Ranasinghe W, Bandaragoda T, et al. Machine learning to support social media empowered patients in cancer care and cancer treatment decisions. PloS One. 2018;13(10) doi: 10.1371/journal.pone.0205855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abidi SS. Knowledge management in healthcare: towards 'knowledge-driven' decision-support services. Int J Med Inform. 2001;63(1-2):5–18. doi: 10.1016/s1386-5056(01)00167-8. [DOI] [PubMed] [Google Scholar]
- 89.Hung CY, Chen HY, Wee LJ, et al. Deriving a novel health index using a large-scale population based electronic health record with deep networks. 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 2020;2020:5872–5875. doi:10.1109/embc44109.2020.9176454 [DOI] [PubMed]
- 90.Smrke U, Mlakar I, Lin S, et al. Language, speech, and facial expression features for artificial intelligence-based detection of cancer survivors' depression: scoping meta-review. JMIR Ment Health. 2021;8(12):e30439. doi: 10.2196/30439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang Y, Kim YJ, Park S, et al. Development and operation of a digital platform for sharing pathology image data. BMC Med Inform Decis Mak. 2021;21(1):114. doi: 10.1186/s12911-021-01466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J, Tian Y, Li R, et al. Improving prediction for medical institution with limited patient data: leveraging hospital-specific data based on multicenter collaborative research network. Artif Intell Med. 2021;113 doi: 10.1016/j.artmed.2021.102024. [DOI] [PubMed] [Google Scholar]
- 93.Thompson RF, Valdes G, Fuller CD, et al. Artificial intelligence in radiation oncology: a specialty-wide disruptive transformation? Radiother Oncol. 2018;129(3):421–426. doi: 10.1016/j.radonc.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreno-Torres JG, Raeder T, Alaiz-Rodríguez R, et al. A unifying view on dataset shift in classification. 2012;45(1):521–530. doi: 10.1016/j.patcog.2011.06.019
- 95.Hwang TJ, Kesselheim AS, Vokinger KN. Lifecycle regulation of artificial intelligence- and machine learning-based software devices in medicine. JAMA. 2019;322(23):2285–2286. doi: 10.1001/jama.2019.16842. [DOI] [PubMed] [Google Scholar]
- 96.Linardatos P, Papastefanopoulos V, Kotsiantis S., Explainable AI. A review of machine learning interpretability methods. Entropy (Basel) 2020;23(1):18. doi: 10.3390/e23010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hagendorff T. The ethics of ai ethics: an evaluation of guidelines. Minds Mach. 2020;30:99–120. doi: 10.1007/s11023-020-09517-8. [DOI] [Google Scholar]
- 98.McIntosh C, Conroy L, Tjong MC, et al. Clinical integration of machine learning for curative-intent radiation treatment of patients with prostate cancer. Nat Med. 2021;27(6):999–1005. doi: 10.1038/s41591-021-01359-w. [DOI] [PubMed] [Google Scholar]
- 99.Skrede OJ, De Raedt S, Kleppe A, et al. Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet. 2020;395(10221):350–360. doi: 10.1016/S0140-6736(19)32998-8. [DOI] [PubMed] [Google Scholar]
- 100.Saillard C, Schmauch B, Laifa O, et al. Predicting survival after hepatocellular carcinoma resection using deep learning on histological slides. Hepatology. 2020;72(6):2000–2013. doi: 10.1002/hep.31207. [DOI] [PubMed] [Google Scholar]
- 101.Lu L, Dercle L, Zhao B, Schwartz LH. Deep learning for the prediction of early on-treatment response in metastatic colorectal cancer from serial medical imaging. Nat Commun. 2021;12(1):6654. doi: 10.1038/s41467-021-26990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mu W, Jiang L, Shi Y, et al. Non-invasive measurement of PD-L1 status and prediction of immunotherapy response using deep learning of PET/CT images. J Immunother Cancer. 2021;9(6) doi: 10.1136/jitc-2020-002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jin C, Yu H, Ke J, et al. Predicting treatment response from longitudinal images using multi-task deep learning. Nat Commun. 2021;12(1):1851. doi: 10.1038/s41467-021-22188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi JY, Wang X, Ding GY, et al. Exploring prognostic indicators in the pathological images of hepatocellular carcinoma based on deep learning. Gut. 2021;70(5):951–961. doi: 10.1136/gutjnl-2020-320930. [DOI] [PubMed] [Google Scholar]
- 105.Sammut SJ, Crispin-Ortuzar M, Chin SF, et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature. 2022;601(7894):623–629. doi: 10.1038/s41586-021-04278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]