Abstract
Background
This study aimed to provide a detailed analysis of the temporal trends of cancer incidence rates for individuals aged 0–19 years in selected regions globally from 1978 to 2012.
Methods
Data were obtained from Volumes V-XI of Cancer Incidence in Five Continents (CI5), published by the International Agency for Research on Cancer. A total of 53 registries in 23 regions from the Americas, Asia, Europe, and Oceania that contained information on cancer incidence throughout 1978-2012 (35 years) were included in this study. Joinpoint regression was used for the analysis of trends.
Results
Most regions showed increasing trends in overall childhood cancer among children (aged 0-14 years) and adolescents (aged 15-19 years). Nearly all regions showed rising trends in childhood and adolescent leukemia incidence rates, whereas the incidence of lymphoma among children generally decreased. Only France, Australia, and New Zealand showed decreasing trends for malignant central nervous system (CNS) tumors among adolescents. Kidney cancer and bone cancer incidence rates remained stable for most regions. The incidence of thyroid cancer among adolescents increased in most regions and that of testicular cancer decreased in approximately one-half of the regions studied.
Conclusion
The international temporal trends of cancer incidents among children and adolescents are varied by region, cancer type, age group, and gender, and have changed over time.
Keywords: Incidence, Trend, Childhood, Adolescent, Cancer
1. Introduction
Cancer continues to be one of the leading causes of death among children (0-14 years) and adolescents (15-19 years).1,2 Recent evidence suggests that changes in incidence rates over time might vary significantly between regions and populations.3 For instance, while some studies have shown stabilizing of childhood and/or adolescent cancer incidence rates in Japan and in some parts of Europe,4,5 other studies have found an increase in the United States, Australia, India, and some Nordic countries.6, 7, 8, 9 In addition, different magnitudes of change or even their direction have been observed for some individual cancer subtypes.3
The majority of early studies on childhood and/or adolescent cancer have focused on trends in cancer incidence within a single country or geographic location, such as China, India, and Europe.10, 11, 12 Only a few previous studies, attempting to capture geographic variations in cancer incidence, have reported global cancer incidence for children and/or adolescents.1,13, 14, 15, 16 In Europe, trends for overall cancer incidence are similar in four regions (East, North, South, and West); 3 however, cancer-type-specific trends were different between the four regions: decreasing incidence of childhood lymphomas in eastern Europe, increasing incidence of childhood central nervous system (CNS) tumors in western Europe, and increasing leukemia incidence in the west, the east, and the south, compared with the stable trends in other regions. 3 As the limited number of studies failed to provide a solid summary of global childhood cancer temporal trends, it is important to investigate whether temporal trends of specific types of cancer vary across regions globally. Furthermore, the temporal trends might not be linear, though previous studies usually make this assumption in estimating long-term trends. In addition, different methodologies in previous studies (e.g., data sources, models, time frames) might hamper a direct comparison of temporal trends in cancer incidence across countries, based on reports from individual studies. It also remains unclear whether geographic variations in temporal trends exist for adolescent cancer, and whether such variations exist for different types of cancers globally.
To address these challenges, we examined childhood and adolescent cancer incidence data over the 35-year period from 1978 to 2012 for 23 selected regions in the Americas, Asia, Europe, and Oceania, to understand how childhood and adolescent cancer incidence (overall and type-specific) has changed over time (allowing non-linear trends) across different populations globally.
2. Materials and methods
2.1. Data sources
Population-based cancer incidence data were obtained from Volumes V-XI of Cancer Incidence in Five Continents (CI5). The CI5 volumes comprised cancer data on 5-year incidence reported by selected population-based cancer registries covering regions within five continents (Volume V: 1978-1982, 36 regions; Volume VI: 1983-1987, 52 regions; Volume VII: 1988-1992, 53 regions; Volume VIII: 1993-1997, 60 regions; Volume IX: 1998-2002, 63 regions; Volume X: 2003-2007, 74 regions; Volume XI: 2008-2012, 71 regions).17 Comparability, completeness, and validity of submitted datasets in all registries in the CI5 have previously been ensured by the International Agency for Research on Cancer (IARC).18
Registries were included for the analysis if they reported data for the full period of 1978 to 2012, except for the registries from Kuwait, where the data were unavailable for the year 1978. Altogether, 23 regions from America, Asia, Europe, and Oceania with 53 cancer registries containing data for children (aged 0 to 14 years) and adolescents (aged 15 to 19 years) met the eligibility criteria and were included in the present study (Supplementary Table 1). No registry from the continent of Africa, however, met the inclusion criteria. Although the “CI5 plus” dataset contained annual incidence rates for 124 selected populations from 108 cancer registries published in CI5, only 33 registries in 15 regions contained annual incidence rates for at least 35 years. To cover more regions in our study, we chose CI5 Volumes V-XI instead of the “CI5 plus” dataset. Not all regions in the CI5 dataset had a national coverage by cancer registries for the full period of 1978-2012. The current analysis for each region included only registries that reported data for the full period.
Each individual registry contained information on related characteristics, including sex, age at diagnosis (shown as five-year age group, such as 4, and 5–9), number of cases, person-years at risk, as well as cancer site. The anatomic cancer site was based on the International Classification of Diseases, ninth revision (ICD-9 for volumes 5-7) and tenth revision (ICD-10 for volumes 8-11).
2.2. Statistical analysis
Various numbers of registries were included in different regions. To obtain cancer incidence rates for each region, we first calculated age-standardized incidence rates for children (0-14 years) at registry level using direct standardization to the World Health Organization's (WHO) New World (WHO 2000-2025) Standard Population.19 Because the adolescent group contained only one age group (15-19 years), incidence rates for adolescents were calculated as the quotient of the number of cases and the number of person-years in the 15-19 year age group at each registry for a specified time period without age standardization. The region-specific rates were estimated as a weighted average based on the population size (children or adolescents) at each registry. All incidence rates were expressed per million person-years. We further assessed changes over time using the Joinpoint Regression Program (version 4.7.0) applied for log-transformed rates in the analyses for both the overall and type-specific cancer incidence rates in children and adolescents, respectively. We set a maximum of three joinpoints to test whether an apparent change in trend was statistically significant, and then calculated the period percentage change (PPC) and corresponding 95% confidence interval (CI) for each joinpoint segment. Average period percent change (APPC), a weighted average of PPCs, was used to describe changes in cancer incidence rates over the 35-year study period. We ranked incidence rates during the most recent time period of all types of cancer and analyzed the top five cancers for children and adolescents, respectively. The top five childhood cancers included leukemia, CNS tumors, lymphoma, kidney cancer, and bone cancer, while the top five adolescent cancers included lymphoma, leukemia, thyroid cancer, CNS tumors, and testicular cancer. We also stratified all analyses by sex. An alpha level of 0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and Joinpoint Regression Program (SEER, version 4.7.0).
3. Results
3.1. Childhood cancers
3.1.1. Overall cancers
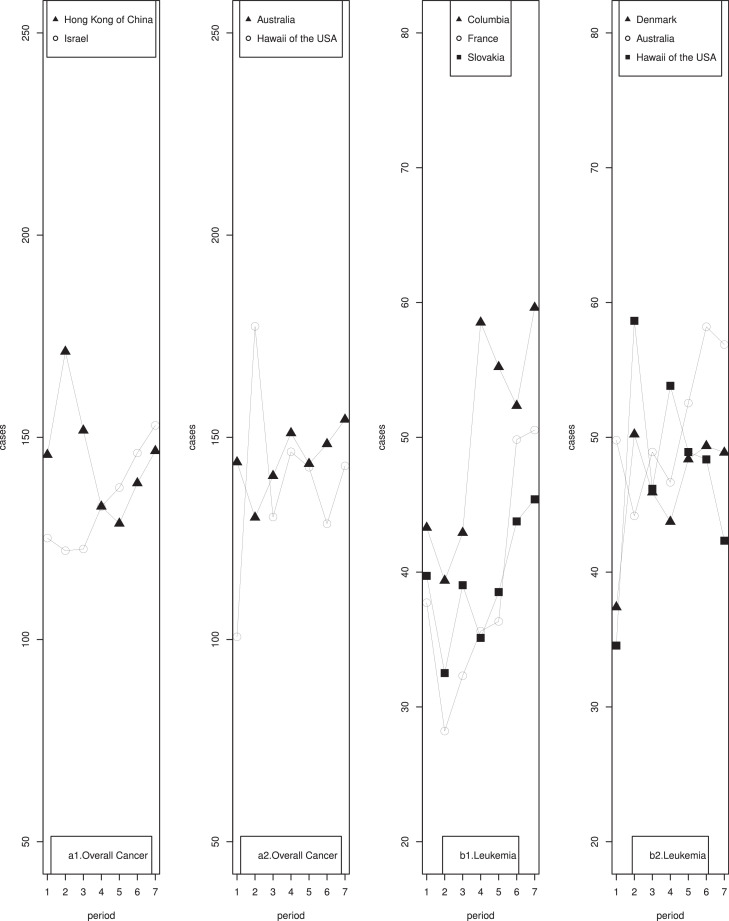
The most recent (i.e., 2008-2012) age-standardized incidence rates of overall childhood cancer showed considerable variation by region, ranging from 172.47 in Slovakia to 88.88 per million person-years in India (Table 1). Age-standardized incidence rates significantly increased from 1978 to 2012 in 11 regions, namely the USA (excluding Hawaii), Colombia, Chinese mainland, India, Israel, France, Italy, Slovakia, Spain, the United Kingdom (UK), and Australia (Table 1). The largest statistically significant APPC was observed in Italy followed by India. Considerable decreases in age-standardized incidence rates were observed in the Hong Kong region of China. Notably, while the trends showed monotonic increases or decreases for the majority of these regions, cancer incidence rates in Hawaii of the USA, Hong Kong of China, Israel, and Australia showed statistically significant directional changes over time (Fig. 1, a1, a2).
Fig. 1.
Changing patterns of cancer incidence rates among children aged 0-14 years during the period of 1978-2012 (continued).
Table 1.
Age-standardized incidence rates (per 1,000,000 person-years) of overall cancer among children aged 0-14 years during the period of 1978-2012 by country or region.
| 1978-1982 | 1983-1987 | 1988-1992 | 1993-1997 | 1998-2002 | 2003-2007 | 2008-2012 | APPC | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Americas | ||||||||||
| USA (excluding Hawaii) | 129.29 | 137.13 | 142.79 | 135.52 | 154.03 | 149.31 | 151.20 | 2.7 | 0.5-6.0 | |
| Hawaii, USA | 100.66 | 177.44 | 130.29 | 146.41 | 142.61 | 128.62 | 142.93 | 1.2 | -2.4-4.9 | |
| Canada | 137.24 | 152.71 | 139.84 | 143.52 | 142.26 | 149.61 | 167.52 | 1.6 | -0.9-4.1 | |
| Colombia | 117.24 | 122.46 | 118.77 | 134.90 | 133.98 | 145.39 | 148.56 | 4.2 | 3.6-4.9 | |
| Asia | ||||||||||
| Chinese mainland | 106.53 | 110.46 | 106.42 | 114.91 | 100.02 | 103.68 | 106.92 | 3.2 | 1.5-4.9 | |
| Hong Kong, China | 145.69 | 171.20 | 151.69 | 132.89 | 128.68 | 138.67 | 146.63 | -3.1 | -5.2–1 | |
| India | 70.33 | 78.76 | 79.87 | 90.32 | 96.97 | 88.36 | 88.88 | 4.5 | 1.5-7.6 | |
| Israel | 125.09 | 121.96 | 122.39 | 132.87 | 137.56 | 146.12 | 152.95 | 3.7 | 3.2-4.1 | |
| Japan | 110.05 | 124.55 | 128.54 | 127.96 | 111.05 | 118.18 | 123.89 | 0.6 | -3.4-4.9 | |
| Kuwait | 88.28 | 108.59 | 111.02 | 101.79 | 205.28 | 127.00 | 110.00 | 4.7 | -9.1-20.5 | |
| Europe, others | ||||||||||
| France | 123.04 | 117.83 | 134.18 | 134.46 | 136.83 | 135.76 | 158.42 | 4.2 | 0.8-7.6 | |
| Germany | 122.86 | 146.54 | 118.27 | 166.21 | 122.50 | 138.75 | 138.22 | 0.5 | -7.3-8.9 | |
| Italy | 138.70 | 147.64 | 137.98 | 166.91 | 200.50 | 178.04 | 171.37 | 6.1 | 4.6-7.6 | |
| Slovakia | 123.20 | 128.19 | 130.12 | 126.25 | 128.94 | 136.84 | 172.47 | 3.9 | 2.9-4.9 | |
| Slovenia | 110.16 | 109.11 | 113.17 | 139.40 | 142.79 | 138.17 | 135.47 | 0.0 | -2.8-2.9 | |
| Spain | 140.42 | 129.29 | 134.41 | 159.48 | 173.23 | 154.82 | 159.53 | 3.8 | 0.5-7.2 | |
| Switzerland | 108.92 | 151.60 | 127.47 | 168.90 | 169.07 | 129.10 | 138.44 | 2.7 | -3.1-8.9 | |
| UK | 124.61 | 123.41 | 133.09 | 131.07 | 130.17 | 134.00 | 130.75 | 0.9 | 0.2-1.5 | |
| Europe, Scandinavian Countries | ||||||||||
| Denmark | 122.04 | 150.98 | 138.23 | 141.26 | 131.01 | 133.85 | 146.86 | 0.7 | -2.7-4.2 | |
| Iceland | 115.67 | 126.73 | 110.09 | 140.55 | 110.96 | 109.45 | 128.82 | 0.0 | -7.6-8.3 | |
| Norway | 131.05 | 150.47 | 129.06 | 149.14 | 127.90 | 144.30 | 146.43 | 0.4 | -4.2-5.3 | |
| Oceania | ||||||||||
| Australia | 143.85 | 130.17 | 140.47 | 151.05 | 143.45 | 148.35 | 154.40 | 2.0 | 0-4.1 | |
| New Zealand | 140.32 | 161.97 | 161.86 | 154.37 | 145.24 | 139.03 | 148.50 | -0.7 | -3.4-2 | |
Aberration: APPC, average period percentage change.
Fig. 1.
Changing patterns of cancer incidence rates among children aged 0-14 years during the period of 1978-2012.
Boys generally experienced higher incidence rates than girls across all study periods and all regions except Switzerland, where girls experienced higher incidence rates than boys during the two time periods: 1978-1982 and 2008-2012 (Table 2). Significant increasing trends were observed among both boys and girls in the USA (excluding Hawaii), Colombia, India, Israel, and France; among boys only in Hong Kong of China, Italy, and the UK; among girls only in Germany, Slovakia, and Switzerland.
Table 2.
Age-standardized incidence rates (per 1,000,000 person-years) of overall cancer among children aged 0-14 years during the period of 1978-2012 by gender.
| 1978-1982 | 1983-1987 | 1988-1992 | 1993-1997 | 1998-2002 | 2003-2007 | 2008-2012 | APPC | 95% CI | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |||||||
| Americas | ||||||||||||||||||||||||
| USA (excluding Hawaii) | 133.40 | 125.01 | 145.13 | 128.81 | 152.24 | 132.9 | 146.16 | 124.37 | 160.14 | 147.61 | 158.88 | 139.31 | 158.74 | 143.31 | 2.8 | 2.9 | 0.7-5.1 | 2.1-3.7 | ||||||
| Hawaii, USA | 113.14 | 87.45 | 183.56 | 178.87 | 136.72 | 123.41 | 159.71 | 132.29 | 157.51 | 126.86 | 132.65 | 124.17 | 162.05 | 122.80 | 1.7 | 0.5 | -4.4-8.1 | -0.2-1.1 | ||||||
| Canada | 147.44 | 126.52 | 167.38 | 137.29 | 150.78 | 128.35 | 149.97 | 136.73 | 152.83 | 131.17 | 161.08 | 137.51 | 170.71 | 164.14 | 0.80 | 2.5 | 0-1.7 | -1.9-7.2 | ||||||
| Colombia | 127.77 | 106.81 | 135.16 | 110.10 | 125.84 | 111.40 | 135.85 | 133.87 | 140.93 | 126.78 | 150.76 | 139.92 | 150.52 | 146.51 | 2.50 | 5.6 | 1.2-3.8 | 2.4-8.9 | ||||||
| Asia | ||||||||||||||||||||||||
| Chinese mainland | 112.91 | 99.76 | 124.66 | 95.41 | 117.44 | 95.28 | 128.03 | 101.14 | 106.85 | 92.91 | 106.57 | 100.67 | 117.24 | 95.86 | -1.20 | 0.1 | -6.6-4.4 | -2.7-2.9 | ||||||
| Hong Kong, China | 177.74 | 111.34 | 185.43 | 155.80 | 168.51 | 133.52 | 138.35 | 127.00 | 145.54 | 110.54 | 163.63 | 111.96 | 152.86 | 139.97 | 2.40 | -3.9 | 0-6.8 | -8.4-5.6 | ||||||
| India | 86.61 | 53.16 | 93.44 | 63.41 | 94.94 | 64.48 | 106.41 | 73.19 | 111.96 | 80.78 | 104.49 | 70.75 | 106.14 | 69.90 | 3.40 | 5.2 | 0.1-6.9 | 0.8-9.8 | ||||||
| Israel | 139.29 | 110.12 | 132.76 | 110.54 | 133.59 | 110.59 | 149.83 | 115.01 | 153.20 | 121.09 | 155.86 | 135.85 | 168.07 | 137.06 | 3.50 | 3.9 | 2.2-4.8 | 2-5.9 | ||||||
| Japan | 122.05 | 97.40 | 133.19 | 115.43 | 138.65 | 117.92 | 138.54 | 116.86 | 124.14 | 97.31 | 124.63 | 111.40 | 125.86 | 121.82 | -0.20 | 1.7 | -2.7-2.4 | -4.3-8 | ||||||
| Kuwait | 102.03 | 74.08 | 122.58 | 93.66 | 122.37 | 99.60 | 117.31 | 85.70 | 214.18 | 195.65 | 146.99 | 106.06 | 125.57 | 93.62 | 4.60 | 4.7 | -7.9-18.9 | -10.8-22.9 | ||||||
| Europe, others | ||||||||||||||||||||||||
| France | 132.25 | 113.33 | 138.59 | 95.99 | 139.29 | 128.83 | 159.49 | 108.23 | 148.55 | 124.41 | 130.40 | 141.42 | 167.26 | 149.18 | 4.40 | 6.1 | 2.8-6 | 4.1-8.2 | ||||||
| Germany | 137.89 | 107.29 | 171.63 | 120.45 | 127.72 | 108.33 | 178.62 | 153.11 | 164.77 | 78.15 | 164.66 | 111.59 | 147.09 | 128.99 | 0.50 | 10.5 | -1.3-2.4 | 4.9-16.3 | ||||||
| Italy | 143.50 | 133.53 | 170.43 | 123.59 | 160.98 | 113.80 | 171.55 | 161.88 | 184.54 | 217.40 | 177.13 | 179.05 | 190.37 | 151.19 | 3.80 | 6.3 | 1.8-5.9 | -4.1-18 | ||||||
| Slovakia | 135.73 | 110.13 | 136.63 | 119.41 | 142.44 | 117.27 | 139.86 | 112.00 | 135.32 | 122.22 | 144.18 | 129.11 | 195.07 | 148.69 | 0.90 | 7.7 | -0.4-2.3 | 5.4-10.1 | ||||||
| Slovenia | 127.71 | 91.43 | 126.34 | 90.78 | 122.32 | 103.50 | 138.21 | 140.64 | 156.95 | 127.74 | 143.13 | 132.93 | 144.70 | 125.69 | 3.20 | 0.9 | -0.8-7.3 | -3.5-5.4 | ||||||
| Spain | 172.95 | 105.84 | 138.75 | 119.22 | 144.54 | 123.58 | 186.30 | 130.87 | 164.44 | 182.66 | 181.06 | 127.06 | 172.77 | 145.50 | 2.60 | 4.0 | -0.9-6.1 | -5.9-15.1 | ||||||
| Switzerland | 95.93 | 122.48 | 168.88 | 133.64 | 141.98 | 112.28 | 194.43 | 141.97 | 180.86 | 156.73 | 132.58 | 125.46 | 122.95 | 154.74 | 2.40 | 7.7 | -2.2-7.2 | 4.3-11.2 | ||||||
| UK | 130.84 | 118.05 | 131.65 | 114.75 | 144.02 | 121.59 | 146.07 | 115.37 | 143.45 | 116.21 | 143.28 | 124.26 | 140.50 | 120.52 | 1.00 | 0.4 | 0.4-1.7 | -0.8-1.6 | ||||||
| Europe, Scandinavian Countries | ||||||||||||||||||||||||
| Denmark | 134.37 | 109.12 | 157.39 | 144.28 | 149.06 | 126.89 | 157.94 | 123.75 | 139.31 | 122.28 | 138.61 | 128.86 | 154.48 | 138.84 | 0.30 | 1.2 | -4.4-5.2 | -0.6-3 | ||||||
| Iceland | 131.00 | 99.61 | 130.26 | 123.14 | 141.65 | 77.21 | 142.72 | 138.15 | 114.33 | 107.57 | 149.05 | 68.26 | 146.32 | 110.37 | -0.20 | -1.8 | -1.6-1.2 | -15.7-14.4 | ||||||
| Norway | 147.25 | 114.01 | 157.65 | 142.91 | 138.06 | 119.60 | 157.93 | 139.88 | 138.03 | 117.24 | 146.71 | 141.77 | 153.24 | 139.25 | -0.50 | 1.5 | -4.4-3.6 | -4.3-7.7 | ||||||
| Oceania | ||||||||||||||||||||||||
| Australia | 163.90 | 122.87 | 141.38 | 118.53 | 150.25 | 130.18 | 163.73 | 137.71 | 154.23 | 132.12 | 157.92 | 138.27 | 169.26 | 138.74 | 1.40 | 2.8 | -1.2-4.1 | 1.3-4.4 | ||||||
| New Zealand | 152.89 | 127.20 | 180.24 | 142.77 | 171.28 | 152.00 | 161.14 | 147.22 | 165.65 | 123.68 | 133.96 | 144.33 | 165.02 | 131.14 | -1.30 | 0.1 | -7.7-5.6 | -5.2-5.7 | ||||||
Abbreviation: AAPC, average period percentage change.
3.1.2. Leukemia
Colombia had the highest incidence rate during the most recent time period, while India had the lowest rate (Supplementary Table 2.1). Most regions showed significantly increasing trends in leukemia over time. Kuwait, followed by Hong Kong of China, Israel, and Colombia showed the highest increase in leukemia incidence rates. Significant directional changes in incidence rates were observed in Hawaii of the USA, Columbia, France, Slovakia, Denmark, and Australia (Fig. 1, b1, b2).
Girls had lower incidence rates than boys with the exception of those from Switzerland (Supplementary Table 3.1). Increasing trends were observed among both girls and boys in Canada, India, and Australia; among boys only in Hawaii of the USA, Chinese mainland, Israel, Kuwait, France, Italy, Slovakia, Switzerland, and Denmark; and among girls only in Colombia, Germany, and the UK.
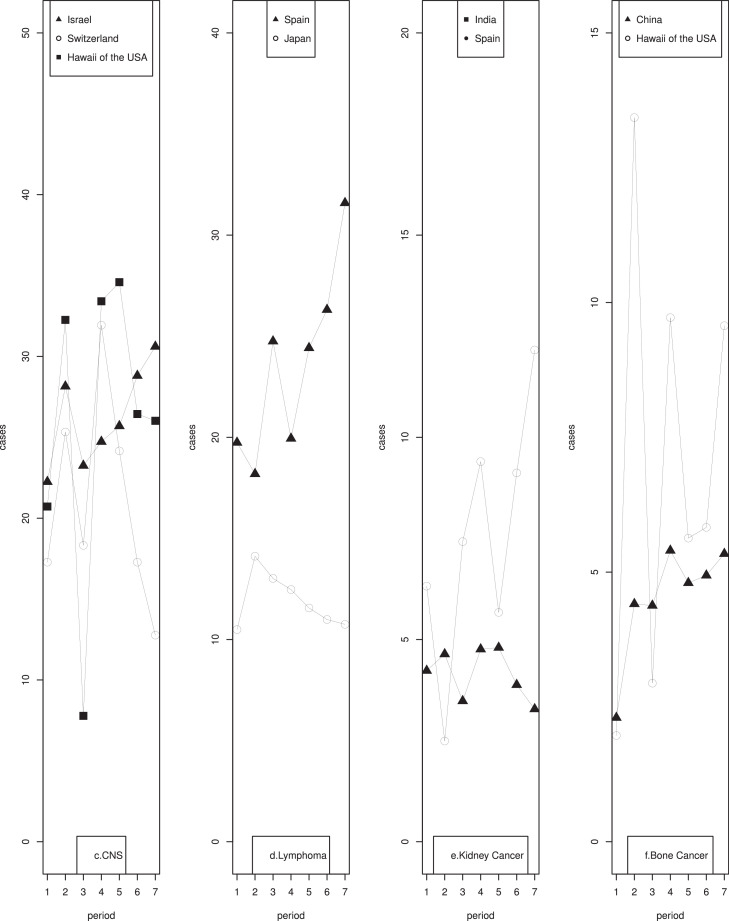
3.1.3. CNS tumors
The highest age-standardized incidence rate of CNS tumors based on the most recent 5 years of CI5 data was observed in Slovakia (36.41 per million person-years) and the lowest in Kuwait (10.91 per million person-years) (Supplementary Table 2.2). Significantly rising trends were seen in Israel, the USA (excluding Hawaii), Kuwait, and India. A significant decreasing trend occurred in five regions, namely Switzerland, Australia, Spain, France, and New Zealand. Fluctuating patterns of incidence rates were observed in Hawaii of the USA, Israel, and Switzerland (Fig. 1, c). Significantly increasing trends in India and significantly decreasing trends in Spain and New Zealand were observed in both girls and boys (Supplementary Table 3.2). Additionally, significantly increasing trends were observed among boys only in the USA (excluding Hawaii), Israel, and Slovakia, and among girls only in Hawaii of the USA, Colombia, and France; while decreasing trends among boys only in Chinese mainland and Denmark.
3.1.4. Lymphoma
The highest age-standardized incidence rate of childhood lymphoma among the most recent time period was 31.59 per million person-years in Spain, while the lowest rate of 9.64 per million person-years was observed in Chinese mainland (Supplementary Table 2.3). Relatively stable trends were observed in the majority of the regions, while an increasing trend was noted for France, Spain, and Norway, and a decreasing trend was observed for Hong Kong of China, Germany, and Japan. Significant directional changes were observed for Japan and Spain (Fig. 1, d).
After stratification by gender, significant decreasing trends were observed among boys in Hong Kong of China and Slovakia, and girls in Japan and New Zealand; while significant increasing trends were found among girls in the USA (excluding Hawaii), Hawaii of the USA, Israel, Italy, Norway, and Australia; and among boys in Spain, Switzerland, and Norway (Supplementary Table 3.3). Lymphoma incidence rates were much higher among boys than girls across all regions and time periods.
3.1.5. Kidney cancers
The highest and the lowest incidence rates of childhood kidney cancer were 12.16 per million person-years (Spain) and 1.53 per million person-years (Hawaii), respectively (Supplementary Table 2.4). Considerable increases in childhood kidney cancer occurred in registries from India, Kuwait, Italy, Spain, and Australia. Decreasing trends were observed in Japan and France. Fluctuating trends were observed in India and Spain (Fig. 1, e). Significantly increasing trends were observed among boys in Kuwait, Germany, Switzerland, Iceland, and Australia, and girls in Kuwait, Slovenia, Spain, Denmark, and Australia (Supplementary Table 3.4). While significantly decreasing trends were found among both boys and girls in Hawaii of the USA, Hong Kong of China, and Japan; among boys only in Norway, and New Zealand; and among girls only in Iceland.
3.1.6. Bone cancers
The highest age-standardized incidence rate of bone cancer based on the most recent 5 years of CI5 data was observed in Germany (11.06 per million person-years) and the lowest in Japan (4.32 per million person-years) (Supplementary Table 2.5). Significantly rising trends were seen in Canada, Chinese mainland, India, Slovakia, Denmark, Norway, Australia, and New Zealand, and the trends of other countries remained stable. A fluctuating trend was observed in Hawaii of the USA and Chinese mainland over time (Fig. 1, f). Stratified by sex, significant increasing trends were observed among boys in Hong Kong of China, India, Israel, Germany, Italy, Slovakia, UK, Denmark, and New Zealand; and among girls in Colombia, Chinese mainland, Slovakia, Iceland, Australia, and New Zealand (Supplementary Table 3.5). Decreasing trends occurred among boys in Canada and Iceland and among girls in Spain.
3.2. Adolescent cancers
3.2.1. Overall cancers
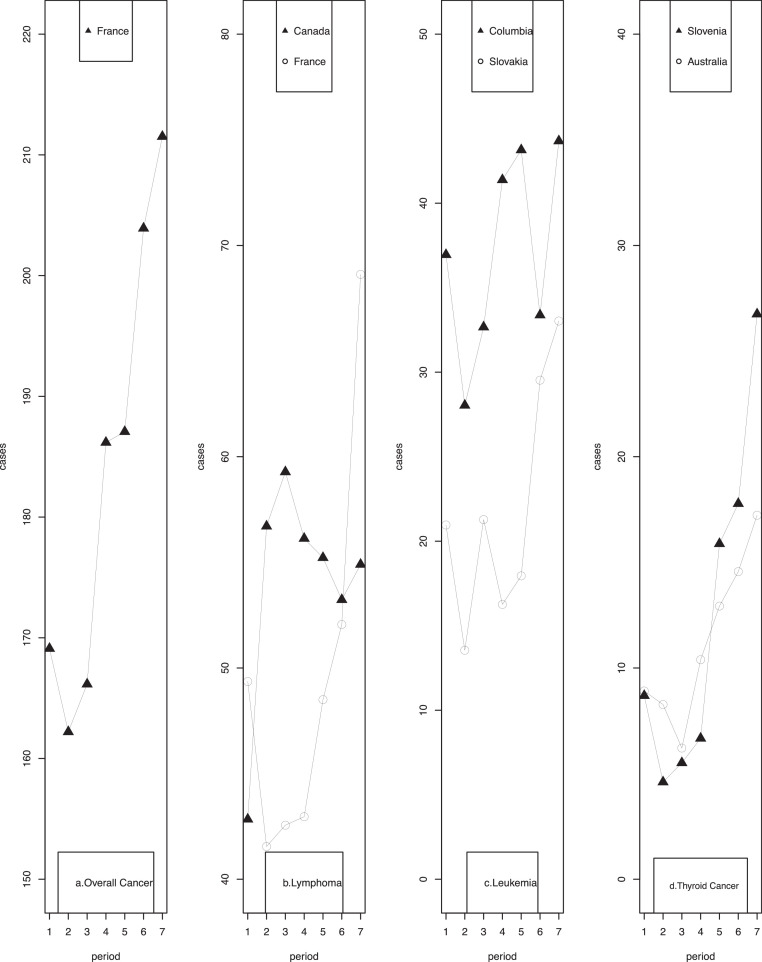
Incidence rates for all cancers in adolescents (15-19 years) had been on increasing trends for most regions, with the largest increase taking place in Spain, followed by Slovenia and Ireland (Table 3). Stable trends were shown in Colombia, Chinese mainland, Hong Kong of China, India, Israel, Kuwait, Germany, the UK, and New Zealand. A significantly directional change in incidence rates was observed in France (Fig. 2, a). Focusing on the most recent time period, Italy had the highest adolescent cancer incidence rate (263.06 per million person-years), whereas India had the lowest (94.54 per million person-years).
Fig. 2.
Changing patterns of cancer incidence rates among adolescents during the period of 1978-2012 (continued).
Table 3.
Incidence rates (per 1,000,000 person-years) of overall cancer among adolescents aged 15-19 years during the period of 1978-2012.
| 1978-1982 | 1983-1987 | 1988-1992 | 1993-1997 | 1998-2002 | 2003-2007 | 2008-2012 | APPC | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Americas | ||||||||||
| USA (excluding Hawaii) | 183.63 | 201.04 | 204.31 | 187.82 | 206.64 | 217.01 | 227.94 | 4.4 | 1-7.9 | |
| Hawaii, USA | 144.90 | 164.45 | 183.55 | 203.69 | 164.10 | 218.03 | 207.35 | 7.5 | 3.9-11.2 | |
| Canada | 177.77 | 207.07 | 193.87 | 194.44 | 199.52 | 205.09 | 228.42 | 2.6 | 0.6-4.6 | |
| Colombia | 153.50 | 144.21 | 115.01 | 145.46 | 184.17 | 164.74 | 174.72 | 1.6 | -3.9-7.5 | |
| Asia | ||||||||||
| Chinese mainland | 125.72 | 116.77 | 140.50 | 112.23 | 134.46 | 182.76 | 142.87 | 5.6 | -6.9-19.8 | |
| Hong Kong, China | 162.17 | 196.62 | 196.86 | 169.99 | 143.98 | 144.67 | 158.66 | -3.4 | -8.4-2 | |
| India | 85.37 | 95.88 | 95.71 | 97.47 | 102.03 | 87.29 | 94.54 | 0.9 | -3.3-5.3 | |
| Israel | 176.95 | 168.56 | 184.41 | 233.17 | 225.12 | 221.99 | 208.67 | 3.7 | -0.7-8.2 | |
| Japan | 84.94 | 94.36 | 96.62 | 97.63 | 92.17 | 99.89 | 118.07 | 5.9 | 1.6-10.4 | |
| Kuwait | 172.30 | 96.12 | 121.47 | 165.61 | 133.69 | 131.91 | 150.31 | -10.0 | -30.3-16.3 | |
| Europe, others | ||||||||||
| France | 169.11 | 162.21 | 166.16 | 186.18 | 187.07 | 203.92 | 211.52 | 4.4 | 3-5.9 | |
| Germany | 131.16 | 171.79 | 203.27 | 241.66 | 217.71 | 173.81 | 200.37 | 6.6 | -0.1-13.8 | |
| Italy | 183.54 | 173.97 | 202.60 | 234.50 | 193.92 | 252.55 | 263.06 | 7.0 | 0.4-14.1 | |
| Slovakia | 139.93 | 139.62 | 163.77 | 166.03 | 176.78 | 191.73 | 223.20 | 7.9 | 5.8-10.1 | |
| Slovenia | 116.44 | 131.13 | 139.05 | 157.47 | 203.53 | 198.85 | 227.35 | 12.2 | 10.4-14 | |
| Spain | 133.06 | 157.23 | 177.44 | 253.48 | 198.11 | 235.43 | 249.86 | 14.0 | 8.6-19.7 | |
| Switzerland | 163.87 | 182.80 | 205.26 | 221.98 | 209.04 | 216.80 | 232.21 | 5.7 | 2.9-8.5 | |
| UK | 141.95 | 159.42 | 177.31 | 198.77 | 176.64 | 207.02 | 183.58 | 4.4 | -0.2-9.3 | |
| Europe, Scandinavian Countries | ||||||||||
| Denmark | 154.28 | 178.09 | 178.42 | 206.46 | 184.14 | 207.07 | 252.31 | 9.3 | 4.4-14.5 | |
| Iceland | 156.21 | 170.14 | 160.38 | 313.61 | 262.59 | 356.09 | 178.97 | 11.8 | 3.1-21.2 | |
| Norway | 179.68 | 186.42 | 181.62 | 216.57 | 208.70 | 224.47 | 209.23 | 3.6 | 2.2-5.1 | |
| Oceania | ||||||||||
| Australia | 186.22 | 193.91 | 205.08 | 230.31 | 224.87 | 229.60 | 233.45 | 4.4 | 3.2-5.6 | |
| New Zealand | 217.14 | 200.07 | 191.86 | 199.67 | 201.43 | 228.59 | 218.96 | 0.8 | -2.3-3.9 | |
Abbreviation: AAPC, average period percentage change.
Fig. 2.
Changing patterns of cancer incidence rates among adolescents during the period of 1978-2012.
Boys generally experienced higher incidence rates than girls (Table 4). Significantly increasing trends were observed among both boys and girls in Colombia, India, Israel, and France; among boys only in the USA (excluding Hawaii), Hong Kong of China, Italy, and the UK; and among girls only in Germany, Slovakia, and Switzerland.
Table 4.
Incidence rates (per 1,000,000 person-years) of overall cancer among adolescents aged 15-19 years during the period of 1978-2012 by gender.
| 1978-1982 | 1983-1987 | 1988-1992 | 1993-1997 | 1998-2002 | 2003-2007 | 2008-2012 | APPC | 95% CI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | ||
| Americans | |||||||||||||||||||
| USA (excluding Hawaii) | 192.10 | 174.95 | 211.36 | 190.41 | 213.07 | 195.20 | 191.00 | 184.48 | 214.11 | 198.74 | 225.10 | 208.51 | 229.25 | 226.56 | 3.8 | 4.7 | -0.4-8.3 | 1.9-7.6 | |
| Hawaii, USA | 141.16 | 149.14 | 132.30 | 199.56 | 164.41 | 204.38 | 228.84 | 176.82 | 184.59 | 141.64 | 257.85 | 173.40 | 190.95 | 224.99 | 8.5 | 7.0 | 1.6-15.9 | -2.9-17.8 | |
| Canada | 194.74 | 160.06 | 210.4 | 203.58 | 196.88 | 190.71 | 203.1 | 185.28 | 204.52 | 194.24 | 207.71 | 202.31 | 241.4 | 214.73 | 3.8 | 3.0 | 0.2-7.4 | 2.7-3.4 | |
| Colombia | 178.41 | 134.7 | 159.28 | 132.16 | 137.04 | 97.12 | 169.61 | 125.62 | 198.13 | 170.25 | 194.69 | 136.35 | 179.73 | 169.54 | 2.5 | 2.0 | -2.9-8.2 | -2.9-7.2 | |
| Asia | |||||||||||||||||||
| Chinese mainland | 132.38 | 118.92 | 121.89 | 111.2 | 151.06 | 128.79 | 124.83 | 101.05 | 140.06 | 128.78 | 174.58 | 191.18 | 124.7 | 161.3 | -0.8 | 9.4 | -7.6-6.6 | -6.7-28.2 | |
| Hong Kong, China | 184.53 | 138.18 | 201.44 | 191.42 | 197.77 | 195.87 | 184.13 | 154.83 | 146.81 | 141.01 | 145.08 | 144.25 | 163.46 | 153.58 | -4.5 | -1.9 | -11-2.5 | -5.1-1.4 | |
| India | 96.62 | 71.76 | 104.2 | 86.17 | 106.82 | 83 | 107.65 | 85.55 | 110.88 | 90.92 | 95.11 | 77.13 | 98.09 | 89.99 | 0.1 | 2.0 | -3.4-3.7 | -3.4-7.8 | |
| Israel | 192.23 | 160.82 | 185.55 | 150.46 | 184.43 | 184.4 | 232.34 | 234.05 | 223.88 | 226.43 | 230.88 | 212.67 | 213.53 | 203.56 | 2.4 | 5.2 | -0.8-5.7 | -0.8-11.6 | |
| Japan | 97.36 | 72.01 | 107.64 | 80.34 | 102.19 | 90.74 | 107.51 | 87.29 | 103.98 | 79.88 | 99.38 | 100.43 | 125.8 | 109.98 | 4.6 | 7.7 | -0.5-9.9 | 3.9-11.5 | |
| Kuwait | 178.65 | 164.9 | 111.34 | 79.61 | 134.21 | 108.74 | 162.45 | 168.99 | 123.59 | 144.63 | 125.55 | 138.83 | 171.93 | 126.46 | -0.2 | -11 | -11.4-12.3 | -34.8-21.4 | |
| Europe, others | |||||||||||||||||||
| France | 185.17 | 152.82 | 188.91 | 134.69 | 179.1 | 152.83 | 195.42 | 176.62 | 198.26 | 175.57 | 207.64 | 200.12 | 187.56 | 236.08 | 1.4 | 8.2 | 0.2-2.7 | 4.3-12.2 | |
| Germany | 155.62 | 105.33 | 196.89 | 145.55 | 195.01 | 211.93 | 266.31 | 215.95 | 222.01 | 213.16 | 179.12 | 168.21 | 255.85 | 142.02 | 6.2 | 7.6 | -5.6-19.4 | 2.7-12.8 | |
| Italy | 205.69 | 160.49 | 211.78 | 134.41 | 225.43 | 178.62 | 262.92 | 204.66 | 199.25 | 188.31 | 276.95 | 226.59 | 257.35 | 269.14 | 4.1 | 10.3 | 1.4-6.9 | 4.8-16.1 | |
| Slovakia | 153.48 | 125.74 | 160.09 | 118.25 | 177.95 | 149.04 | 182.07 | 149.34 | 198.55 | 154.05 | 222.04 | 160.15 | 270.68 | 173.53 | 9.3 | 2.7 | 5.3-13.4 | -4.1-10.1 | |
| Slovenia | 106.34 | 126.82 | 151.81 | 109.8 | 172.55 | 104.1 | 207.71 | 104.34 | 220.08 | 186.2 | 211.03 | 186 | 222.84 | 232.12 | 12.6 | 11.6 | 10.5-14.8 | -0.2-24.8 | |
| Spain | 135.59 | 130.42 | 177.82 | 135.78 | 182.52 | 172.11 | 246.77 | 260.58 | 185.89 | 211.13 | 255.26 | 214.25 | 239.12 | 261.27 | 9.8 | 12.1 | -0.3-20.9 | 4.3-20.5 | |
| Switzerland | 212.41 | 118.38 | 188.42 | 177.34 | 255.92 | 153.75 | 241.06 | 202.52 | 233.59 | 183.74 | 191.31 | 243.17 | 247.03 | 216.79 | -1.3 | 10.0 | -9.7-7.8 | 2-18.6 | |
| UK | 149.48 | 134.12 | 178.13 | 139.86 | 187.46 | 166.73 | 192.89 | 204.94 | 179.76 | 173.43 | 189.27 | 225.71 | 203.43 | 162.87 | 5.0 | 4.1 | 2.7-7.5 | 1-7.4 | |
| Europe, Scandinavian Countries | - | - | |||||||||||||||||
| Denmark | 187.79 | 119.2 | 208.84 | 145.68 | 212.65 | 142.63 | 254.71 | 156.01 | 218.44 | 148.4 | 238.22 | 174.2 | 255.98 | 248.45 | 4.8 | 12.9 | -0.2-10 | 5.6-20.7 | |
| Iceland | 165.48 | 146.47 | 240.95 | 96.45 | 240.3 | 77.07 | 298.45 | 329.35 | 349.59 | 172.15 | 302.62 | 412.39 | 249.58 | 104.83 | 8.7 | 3.2 | 0.3-17.9 | -24.6-41.3 | |
| Norway | 194.95 | 163.58 | 203.39 | 168.55 | 203.24 | 159.02 | 230.69 | 201.79 | 230.51 | 185.75 | 240.04 | 208.08 | 230.19 | 186.96 | 3.8 | 3.4 | 2.7-5 | 1.4-5.5 | |
| Oceania | - | - | |||||||||||||||||
| Australia | 198.84 | 173.08 | 210.62 | 176.36 | 226.04 | 183.17 | 244.65 | 215.22 | 244.05 | 204.77 | 251.89 | 206.2 | 262.18 | 203.08 | 4.7 | 3.3 | 4-5.3 | 1.4-5.2 | |
| New Zealand | 214.98 | 219.4 | 201.7 | 198.36 | 207.8 | 175.44 | 219.35 | 179.39 | 199.1 | 203.89 | 232.1 | 224.92 | 229.29 | 208.08 | 1.5 | 0.1 | -2.6-5.7 | -4.6-5 | |
Abbreviation: AAPC, average period percentage change.
3.2.2. Lymphoma
The highest rate of adolescent lymphoma was observed in Italy (100.01 per million person-years), while the lowest rate was found in India (14.63 per million person-years). Significant rising trends were identified in Hawaii of the USA, Canada, Chinese mainland, Japan, Australia, New Zealand, and most European countries (Supplementary Table 4.1). Substantial fluctuations in incidence rates were observed in Canada and France (Fig. 2, b). After stratification by gender (Supplementary Table 5.1), significantly increasing trends were evident for girls in most regions. In contrast, few regions including Japan, Spain, Iceland, Australia, and New Zealand showed significantly increasing trends in boys.
3.2.3. Leukemia
The USA (excluding Hawaii), Canada, France, Slovakia, Slovenia, Norway, Australia, and New Zealand showed significantly increasing trends in leukemia incidence rates (Supplementary Table 4.2). In contrast, Hong Kong of China, Italy, and Spain had significantly decreasing trends in adolescent leukemia incidence. Fluctuations in incidence rates were also observed in Colombia and Slovakia (Fig. 2, c). Germany had the highest incidence rate during the most recent time period (54.65 per million person-years), while Switzerland had the lowest rate (7.42 per million person-years). The observed significant trends remained the same among both boys and girls in Canada, Slovenia, and Australia (Supplementary Table 5.2). Significantly increasing trends among boys were seen in Hawaii of the USA, France, Germany, Slovakia, Norway, and New Zealand, and among girls in the USA (excluding Hawaii), Hawaii of the USA, and India. A significantly decreasing trend was observed among boys in Switzerland. Incidence rates were higher in boys than in girls in most regions and during most of the time periods.
3.2.4. Thyroid cancer
In general, there were significant trends towards an increase in adolescent thyroid cancer incidence rates in half of the regions with, the largest annual increases occurring in France, followed by Slovakia, and Chinese mainland (Supplementary Table 4.3). Significant directional changes were found in Slovenia and Australia (Fig. 2, d). Incidence rates during the most recent time period ranged from 0 to 36.96 per million person-years, with the highest rate in Italy, followed by Chinese mainland, and the USA (excluding Hawaii). Girls experienced much higher incidence rates than boys across almost all registries and all time periods (Supplementary Table 5.3).
3.2.5. CNS tumors
Incidence rates of malignant CNS tumors in adolescents remained stable in the vast majority of regions except for Chinese mainland, India, France, Australia, and New Zealand (Supplementary Table 4.4). Registries from Chinese mainland and India reported increasing incidence rates over time, while registries from France, Australia, and New Zealand showed downward trends. No significant directional changes in overall incidence rates were observed. However, trends for decreasing incidence rates were observed among both girls and boys in Australia, but only among boys in France and New Zealand (Supplementary Table 5.4). Significantly increasing trends were observed among girls in India, Germany, and Italy, and among boys in Hong Kong of China, Italy, and Slovakia.
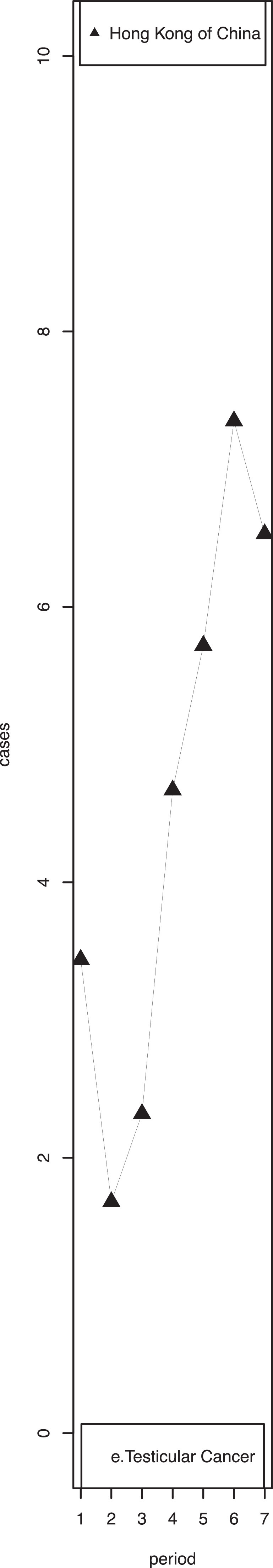
3.2.6. Testicular cancer
Eleven regions [the USA (excluding Hawaii), Hawaii of the USA, Canada, Colombia, Chinese mainland, Hong Kong of China, Israel, Kuwait, Italy, Slovenia, and Australia] showed significantly increasing trends in testicular cancer incidence rates (Supplementary Table 4.5). The highest increase in testicular cancer incidence was found among adolescents in Hong Kong of China. Fluctuations in incidence rates were also observed in Hong Kong of China (Fig. 2, e). Slovakia had the highest incidence rate during the most recent time period (64.61 per million person-years), while India had the lowest rate (2.96 per million person-years).
4. Discussion
The cross-region trends in childhood and adolescent cancer incidence rates during the period of 1978-2012, estimated using high-quality population-based registry data obtained from the CI5 covering 23 regions around the world, indicated that the international temporal trends of cancer incidence rates varied by region, cancer type, age group, and gender, and had changed over time.
The observed increasing trends of overall cancer incidence among children and adolescents in most regions in our study were consistent with many early studies, including those using data from the Automated Childhood Cancer Information System (ACCIS) 10,20, 21, 22 and CI5 plus database.23 A French study reported a stable trend of childhood overall cancer incidence during 2000-2014,24 while our study observed an increasing trend. The Swiss Childhood Cancer Registry study suggested an increase in the overall childhood cancer incidence rate from 1985 to 2014,25 while the present study showed a stable trend. Differences in covering registries between our study and the French and Swiss studies might be a reason for the inconsistent results.
A 28-year-long study reported no significant increase in childhood leukemia incidence in Germany,26 which was consistent with our findings. The observed increasing trends of leukemia in children and adolescents in many regions in our study were found consistent with another report.27 The findings of lymphoma in our study were similar to a study that using CI5 data reported a significantly increasing incidence of lymphoma in Northern and Southern European and Oceania countries among children aged 10-19 years during 1988-2012.28 No significant changes were seen in incidence rates of childhood and adolescent CNS tumors in most regions in our study, which was similar to a previous study that analyzed CI5 data among populations aged 0-24 years.29 A recent study analyzed individual CNS tumors using CI5 data and reported a decreasing trend of astrocytic tumors and an increasing trend of other CNS tumors from 1988 to 2012.30 The observed increasing trends of kidney cancer in India, Kuwait, Italy, Spain, and Australia and stable trends in more than half of the studied regions in the current study were consistent with other reports.10,21,31,32 In a study conducted from Germany a slightly increasing trend was reported for childhood bone cancer.33 Our study showed a nonsignificant increasing trend of childhood bone cancer. The observed trends of thyroid cancer in our study were similar to those in a study that used the International Incidence of Childhood Cancer Volume 3 (ICCC3) database and reported rapid increases in thyroid cancer incidence in almost all countries among adolescents, particularly in girls.34 Since most testicular cancers are germ cell tumors, we compared our testicular cancer results to a study of ovarian germ cell cancer and consistently reported increasing trends in Eastern Asia, Western Europe, Southern Europe, North America, and Oceania.35
Reasons for the apparent rises in these incidence rates are poorly understood. The increasing trends for cancer incidence rates among children and adolescents may reflect improved population-level data collection.36 Differences in criteria and clinical practices related to the detection of childhood and adolescent cancers serve as one plausible explanation for the observed trends in some regions.37 A report from the USA observed increased incidence rates of radiographically-confirmed diagnoses cancers such as malignant glioma and pilocytic astrocytoma.38 Overdiagnosis of thyroid cancer might have also contributed to some of the observed increasing trends of thyroid cancer among children and adolescents as adult thyroid cancers.34,39 However, as screening is generally not practiced among children and adolescents, the dramatic increase in incidence rates in childhood and adolescent cancer may not be accounted to large-scale population screening that leads to increased detection. The intercountry consistency regarding incidence trends specifically in childhood and adolescent thyroid cancer and leukemia might be the result of adverse impacts of harmful environmental agents that exist worldwide and modifiable individual risk factors. For instance, frequent exposure to ionizing radiation, which has been linked to the development of childhood and adolescent leukemia, thyroid cancer, and bone cancer40, 41, 42, might contribute to the rising trends in cancer to some extent. It has been a long time since the latest atomic-bomb explosion and nuclear fallout, but medical radiation might pose new threats to global health. Diagnostic and/or therapeutic radiation can increase the risk of acute leukemia, thyroid cancer, and bone cancer.43, 44, 45 Other increasingly prevalent population risk factors, such as childhood obesity have become a global epidemic and have been linked to the pathogenesis of many types of cancers, including thyroid cancer.46 Additionally, common use of the cesarean section, observed in many parts of the world,47 might help explain some of the rising rates in childhood leukemia, as delivery by cesarean section has been suggested to be associated with a significantly elevated risk of childhood leukemia.48,49 Children with high birth weight had an increased risk of leukemia,50 CNS tumors,51,52 kidney cancer51, and osteosarcoma.53 Increased height, which was found to be associated with osteosarcoma and testicular cancer, 53,54 might be due to high-calorie intake during childhood that promotes the development of intrauterine testicular germ cell tumor precursors.54 Girls with high fetal growth had an increased risk of Wilms tumor, especially before the age of 5 years.55 Furthermore, early age at puberty has also been reported to be correlated with an increased incidence of testicular cancer.54 Recent studies have also reported associations between persistent organic pollutants and increased risks for childhood cancers. For example, exposures to air toxics (or pollutants) and pesticides and benzene have been linked to leukemia.56, 57, 58, 59, 60 Bisphenol A (BPA), polychlorinated biphenyls (PCB), and polybrominated diphenyl ethers (PBDEs) have been implicated in the etiology of leukemia, thyroid cancer, and testicular cancer. 61, 62, 63, 64, 65 An increased exposure to these environmental chemicals might be responsible for at least part of the morbidity burden that we observed for childhood and adolescent leukemia, thyroid cancer, and testicular cancer.
In addition, scientific advances related to the prevention of certain types of cancer are likely to contribute to the geographical and temporal variations in cancer incidence rates. Declines in cancer incidence rates in certain regions are likely attributable to improved cancer prevention efforts. For example, the significant drop in childhood lymphoma incidence in Japan, especially since the year 1998, is likely due to better control of the transmission of Epstein-Barr virus (EBV),66 which is an established risk factor for non-Hodgkin Lymphoma in children.67 In the USA, improved maternal folic acid intake during pregnancy might have contributed to the observed decrease in CNS tumor incidence through nationwide recommendations for the consumption of folic acid supplements and fortified foods.68 Nevertheless, the stabilization or even decline in cancer incidence in high-resource populations could also be due to an incidence “ceiling”, 69 which represents a mature phase in the epidemic. Relatively low-resource populations are still in the early phase, with increases anticipated in the future.
To our knowledge, the present study is the first to assess and compare temporal trends in both childhood and adolescent cancers across 23 regions by sex over an extended period of time, allowing for a comprehensive evaluation of changes in overall and type-specific cancer incidence rates over 35 years. This study also provides the first analysis on the time trend of thyroid cancer globally, the fastest increasing cancer among adolescents but less studied compared to other types of cancers that are commonly seen in children and adolescents. The present study is additionally strengthened by the incorporation of population-level high-quality CI5 data.
However, historical data on cancer incidence among children and adolescents are rarely available for low-resource areas such as Africa, thereby limiting our ability to investigate changes occurring in cancer incidence rates in these parts of the world. The estimated trends regarding overall cancer incidence rates in some countries might also be undermined when local registries are treated as proxies of nationwide cancer profiles due to limited data availability. The disease patterns in the areas covered were not necessarily typical of those across the whole country. As the representativeness of these local registries has not been tested, our results should be interpreted with caution. A potential bias might be produced by selective diagnosis and treatment of cancers in male children in contrast to female children. A further limitation of the study is that in CI5 cancers were classified according to ICD rather than International Classification of Diseases for Oncology (ICD-O). This makes it impossible to perform analyses for neuroblastoma and soft-tissue sarcomas, two of the most frequent cancer types in children. Finally, it is difficult to gauge the extent to which the changes in cancer incidence over time truly reflect changes in cancer risk or other sociodemographic, clinical, or environmental factors.
5. Conclusion
We observed a substantial increase in the incidence of most types of childhood and adolescent cancers from 1978 through 2012 in majority regions examined in this study, except for childhood lymphoma, childhood leukemia, and adolescent malignant CNS tumors. Future etiological and epidemiological research should focus on both genetic and environmental factors that might help explain diverse global patterns and trends in cancer risk. Comprehensive preventative strategies should also be developed and implemented to reduce the morbidity and mortality burden posed by cancer in young populations.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Ethics approval and consent to participate
The study was exempt from institutional review board review by the Yale Human Research Protection Program and Beijing Children's Hospital.
Acknowledgments
Acknowledgments
The authors wish to thank the Cancer Incidence in Five Continents for providing the dataset. The study was sponsored by the National Major Science and Technology Projects of China (2018ZX09721003).
Authors' contributions
Y.Z. and X.N. designed this study and provided critical revisions. Y.Z. and J.X. were involved in analyzing and writing this manuscript. P.S. analyzed the dataset. P.S. and L.J. performed the image analysis. N.M., Z.L., G.F., N.D., and X.M. helped in reviewing the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jncc.2022.02.001.
Contributor Information
Xin Ni, Email: nixin@bch.com.cn.
Yawei Zhang, Email: zhangya69@foxmail.com.
Appendix. Supplementary materials
References
- 1.Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001–10: A population-based registry study. Lancet Oncol. 2017;18(6):719–731. doi: 10.1016/s1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the United States. N Engl J Med. 2018;379(25):2468–2475. doi: 10.1056/NEJMsr1804754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steliarova-Foucher E, Fidler MM, Colombet M, et al. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991–2010 (Automated Childhood Cancer Information System): A population-based study. Lancet Oncol. 2018;19(9):1159–1169. doi: 10.1016/s1470-2045(18)30423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishihara H, Ohno Y, Fujii M, et al. Epidemiological analysis of childhood cancer in Japan based on population-based cancer registries, 1993-2009. Jpn J Clin Oncol. 2017;47(7):660–663. doi: 10.1093/jjco/hyx041. [DOI] [PubMed] [Google Scholar]
- 5.AIRTUM Working Group; CCM; AIEOP Working Group Italian cancer figures, report 2012: Cancer in children and adolescents. Epidemiol Prev. 2013;37(1 Suppl 1):1–225. [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 7.Karim-Kos HE, Hackl M, Mann G, et al. Trends in incidence, survival and mortality of childhood and adolescent cancer in Austria, 1994-2011. Cancer Epidemiol. 2016;42:72–81. doi: 10.1016/j.canep.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Satyanarayana L, Asthana S, Labani SP. Childhood cancer incidence in India: A review of population-based cancer registries. Indian Pediatr. 2014;51(3):218–220. doi: 10.1007/s13312-014-0377-0. [DOI] [PubMed] [Google Scholar]
- 9.Grabas MR, Kjaer SK, Frederiksen MH, et al. Incidence and time trends of childhood cancer in Denmark, 1943-2014. Acta Oncol. 2020;59(5):588–595. doi: 10.1080/0284186X.2020.1725239. [DOI] [PubMed] [Google Scholar]
- 10.Kaatsch P, Steliarova-Foucher E, Crocetti E, et al. Time trends of cancer incidence in European children (1978-1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):1961–1971. doi: 10.1016/j.ejca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Zheng R, Peng X, Zeng H, et al. Incidence, mortality and survival of childhood cancer in China during 2000-2010 period: A population-based study. Cancer Lett. 2015;363(2):176–180. doi: 10.1016/j.canlet.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Arora RS, Eden T, Kapoor G. Epidemiology of childhood cancer in India. Indian J Cancer. 2009;46(4):264–273. doi: 10.4103/0019-509X.55546. [DOI] [PubMed] [Google Scholar]
- 13.Breslow NE, Langholz B. Childhood cancer incidence: Geographical and temporal variations. Int J Cancer. 1983;32(6):703–716. doi: 10.1002/ijc.2910320609. [DOI] [PubMed] [Google Scholar]
- 14.Parkin DM, Stiller CA, Draper GJ, et al. The international incidence of childhood cancer. Int J Cancer. 1988;42(4):511–520. doi: 10.1002/ijc.2910420408. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard AK, Spector LG, Fortuna G, et al. Trends in international incidence of pediatric cancers in children under 5 years of age: 1988-2012. JNCI Cancer Spectr. 2019;3(1):pkz007. doi: 10.1093/jncics/pkz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiller CA, Nectoux J. International incidence of childhood brain and spinal tumours. Int J Epidemiol. 1994;23(3):458–464. doi: 10.1093/ije/23.3.458. [DOI] [PubMed] [Google Scholar]
- 17.Bray F, Ferlay J, Laversanne M, et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–2071. doi: 10.1002/ijc.29670. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127(12):2918–2927. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad OB, Boschi Pinto C, Lopez AD. Age standardization of rates: A new WHO standard. GPE Discussion Paper Series: No 31. 2001:10–12. [Google Scholar]
- 20.Stiller CA, Desandes E, Danon SE, et al. Cancer incidence and survival in European adolescents (1978-1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2006–2018. doi: 10.1016/j.ejca.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): An epidemiological study. Lancet. 2004;364(9451):2097–2105. doi: 10.1016/s0140-6736(04)17550-8. [DOI] [PubMed] [Google Scholar]
- 22.Steliarova-Foucher E, Fidler MM, Colombet M, et al. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991-2010 (Automated Childhood Cancer Information System): A population-based study. Lancet Oncol. 2018;19(9):1159–1169. doi: 10.1016/S1470-2045(18)30423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Harper A, Ruan Y, et al. International trends in the incidence of cancer among adolescents and young adults. J Natl Cancer Inst. 2020;112(11):1105–1117. doi: 10.1093/jnci/djaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goujon S, Kyrimi E, Faure L, et al. Spatial and temporal variations of childhood cancers: Literature review and contribution of the French national registry. Cancer Med. 2018;7(10):5299–5314. doi: 10.1002/cam4.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommer G, Schindler M, Redmond S, et al. Temporal trends in incidence of childhood cancer in Switzerland, 1985-2014. Cancer Epidemiol. 2019;61:157–164. doi: 10.1016/j.canep.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Wellbrock M, Spix C, Grabow D, et al. 28-year incidence and time trends of childhood leukaemia in former East Germany compared to West Germany after German reunification: A study from the German Childhood Cancer Registry. Cancer Epidemiol. 2021;73 doi: 10.1016/j.canep.2021.101968. [DOI] [PubMed] [Google Scholar]
- 27.Linet MS, Brown LM, Mbulaiteye SM, et al. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0-19 years. Int J Cancer. 2016;138(8):1862–1874. doi: 10.1002/ijc.29924. [DOI] [PubMed] [Google Scholar]
- 28.Chun GYC, Sample J, Hubbard AK, et al. Trends in pediatric lymphoma incidence by global region, age and sex from 1988-2012. Cancer Epidemiol. 2021;73 doi: 10.1016/j.canep.2021.101965. [DOI] [PubMed] [Google Scholar]
- 29.Raja N, Hayes L, Basta N, et al. International trends in the incidence of brain tumours in children and young-adults and their association with indicators of economic development. Cancer Epidemiol. 2021;74 doi: 10.1016/j.canep.2021.102006. [DOI] [PubMed] [Google Scholar]
- 30.Williams LA, Hubbard AK, Scheurer ME, et al. Trends in paediatric central nervous system tumour incidence by global region from 1988 to 2012. Int J Epidemiol. 2021;50(1):116–127. doi: 10.1093/ije/dyaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tulla M, Berthold F, Graf N, et al. Incidence, trends, and survival of children with embryonal tumors. Pediatrics. 2015;136(3):e623–e632. doi: 10.1542/peds.2015-0224. [DOI] [PubMed] [Google Scholar]
- 32.Nakata K, Colombet M, Stiller CA, et al. Incidence of childhood renal tumours: An international population-based study. Int J Cancer. 2020;147(12):3313–3327. doi: 10.1002/ijc.33147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaatsch P, Strothotte J, Becker C, et al. Pediatric bone tumors in Germany from 1987 to 2011: Incidence rates, time trends and survival. Acta Oncol. 2016;55(9-10):1145–1151. doi: 10.1080/0284186X.2016.1195509. [DOI] [PubMed] [Google Scholar]
- 34.Vaccarella S, Lortet-Tieulent J, Colombet M, et al. Global patterns and trends in incidence and mortality of thyroid cancer in children and adolescents: A population-based study. Lancet Diabetes Endocrinol. 2021;9(3):144–152. doi: 10.1016/S2213-8587(20)30401-0. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard AK, Poynter JN. Global incidence comparisons and trends in ovarian germ cell tumors by geographic region in girls, adolescents and young women: 1988-2012. Gynecol Oncol. 2019;154(3):608–615. doi: 10.1016/j.ygyno.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroll ME, Carpenter LM, Murphy MF, et al. Effects of changes in diagnosis and registration on time trends in recorded childhood cancer incidence in Great Britain. Br J Cancer. 2012;107(7):1159–1162. doi: 10.1038/bjc.2012.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honjo S, Doran HE, Stiller CA, et al. Neuroblastoma trends in Osaka, Japan, and Great Britain 1970-1994, in relation to screening. Int J Cancer. 2003;103(4):538–543. doi: 10.1002/ijc.10859. [DOI] [PubMed] [Google Scholar]
- 38.Withrow DR, Berrington ADG, Lam CJK, et al. Trends in pediatric central nervous system tumor incidence in the United States, 1998-2013. Cancer Epidemiol Biomarkers Prev. 2019;28(3):522–530. doi: 10.1158/1055-9965.EPI-18-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Udelsman R, Zhang Y. The epidemic of thyroid cancer in the United States: The role of endocrinologists and ultrasounds. Thyroid. 2014;24(3):472–479. doi: 10.1089/thy.2013.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liubarets TF, Shibata Y, Saenko VA, et al. Childhood leukemia in Ukraine after the Chornobyl accident. Radiat Environ Biophys. 2019;58(4):553–562. doi: 10.1007/s00411-019-00810-4. [DOI] [PubMed] [Google Scholar]
- 41.Zablotska LB, Nadyrov EA, Rozhko AV, et al. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997-2008) of a cohort of children and adolescents from Belarus exposed to radioiodines after the Chernobyl accident. Cancer. 2015;121(3):457–466. doi: 10.1002/cncr.29073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: A report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys. 2012;84(1):224–230. doi: 10.1016/j.ijrobp.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikkila A, Raitanen J, Lohi O, et al. Radiation exposure from computerized tomography and risk of childhood leukemia: Finnish register-based case-control study of childhood leukemia (FRECCLE) Haematologica. 2020;105(3):849–850. doi: 10.3324/haematol.2019.245704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Chen Y, Huang H, et al. Diagnostic radiography exposure increases the risk for thyroid microcarcinoma: A population-based case-control study. Eur J Cancer Prev. 2015;24(5):439–446. doi: 10.1097/CEJ.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: A report from the childhood cancer survivor study. J Natl Cancer Inst. 2007;99(4):300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohira T, Ohtsuru A, Midorikawa S, et al. External radiation dose, obesity, and risk of childhood thyroid cancer after the Fukushima Daiichi nuclear power plant accident: The Fukushima health management survey. Epidemiology. 2019;30(6):853–860. doi: 10.1097/EDE.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 47.Betran AP, Torloni MR, Zhang JJ, et al. WHO statement on caesarean section rates. BJOG. 2016;123(5):667–670. doi: 10.1111/1471-0528.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang R, Wiemels JL, Metayer C, et al. Cesarean section and risk of childhood acute lymphoblastic leukemia in a population-based, record-linkage study in California. Am J Epidemiol. 2017;185(2):96–105. doi: 10.1093/aje/kww153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenbaum S, Sheiner E, Wainstock T, et al. Cesarean delivery and childhood malignancies: A single-center, population-based cohort study. J Pediatr. 2018;197:292–296. doi: 10.1016/j.jpeds.2017.12.049. e3. [DOI] [PubMed] [Google Scholar]
- 50.Paltiel O, Tikellis G, Linet M, et al. Birthweight and childhood cancer: Preliminary findings from the International Childhood Cancer Cohort Consortium (I4C) Paediatr Perinat Epidemiol. 2015;29(4):335–345. doi: 10.1111/ppe.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kate O'Neill, Michael, et al. Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol. 2015;44(1):153–168. doi: 10.1093/ije/dyu265. [DOI] [PubMed] [Google Scholar]
- 52.Georgakis MK, Kalogirou EI, Liaskas A, et al. Anthropometrics at birth and risk of a primary central nervous system tumour: A systematic review and meta-analysis. Eur J Cancer. 2017;75:117–131. doi: 10.1016/j.ejca.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 53.Mirabello L, Pfeiffer R, Murphy G, et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011;22(6):899–908. doi: 10.1007/s10552-011-9763-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forman D, Pike MC, Davey G, et al. Aetiology of testicular cancer: Association with congenital abnormalities, age at puberty, infertility, and exercise. Testicular Cancer Study Group. BMJ. 1994;308(6941):1393-1399. doi: https://doi.org/10.1136/bmj.308.6941.1393. [PMC free article] [PubMed]
- 55.Crump C, Sundquist J, Sieh W, et al. Perinatal risk factors for Wilms tumor in a Swedish national cohort. Eur J Epidemiol. 2014;29(3):191–197. doi: 10.1007/s10654-014-9880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinceti M, Rothman KJ, Crespi CM, et al. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case-control study in an Italian population. Eur J Epidemiol. 2012;27(10):781–790. doi: 10.1007/s10654-012-9727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Symanski E, Tee LPG, Chen TY, et al. Air toxics and early childhood acute lymphocytic leukemia in Texas, a population-based case-control study. Environ Health. 2016;15(1):70. doi: 10.1186/s12940-016-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crosignani P, Tittarelli A, Borgini A, et al. Childhood leukemia and road traffic: A population-based case-control study. Int J Cancer. 2004;108(4):596–599. doi: 10.1002/ijc.11597. [DOI] [PubMed] [Google Scholar]
- 59.Ding G, Shi R, Gao Y, et al. Pyrethroid pesticide exposure and risk of childhood acute lymphocytic leukemia in Shanghai. Environ Sci Technol. 2012;46(24):13480–13487. doi: 10.1021/es303362a. [DOI] [PubMed] [Google Scholar]
- 60.Menegaux F, Baruchel A, Bertrand Y, et al. Household exposure to pesticides and risk of childhood acute leukaemia. Occup Environ Med. 2006;63(2):131–134. doi: 10.1136/oem.2005.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang H, Sjodin A, Chen Y, et al. Polybrominated diphenyl ethers, polybrominated biphenyls, and risk of papillary thyroid cancer: A nested case-control study. Am J Epidemiol. 2019;189(2):120–132. doi: 10.1093/aje/kwz229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Guo GL, Han X, et al. Do polybrominated diphenyl ethers (PBDEs) increase the risk of thyroid cancer? Biosci Hypotheses. 2008;1(4):195–199. doi: 10.1016/j.bihy.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward MH, Colt JS, Deziel NC, et al. Residential levels of polybrominated diphenyl ethers and risk of childhood acute lymphoblastic leukemia in California. Environ Health Perspect. 2014;122(10):1110–1116. doi: 10.1289/ehp.1307602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ward MH, Colt JS, Metayer C, et al. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect. 2009;117(6):1007–1013. doi: 10.1289/ehp.0900583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paoli D, Giannandrea F, Gallo M, et al. Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J Endocrinol Invest. 2015;38(7):745–752. doi: 10.1007/s40618-015-0251-5. [DOI] [PubMed] [Google Scholar]
- 66.Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond B Biol Sci. 2017;372(1732) doi: 10.1098/rstb.2016.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeuchi K, Tanaka-Taya K, Kazuyama Y, et al. Prevalence of Epstein-Barr virus in Japan: Trends and future prediction. Pathol Int. 2006;56(3):112–116. doi: 10.1111/j.1440-1827.2006.01936.x. [DOI] [PubMed] [Google Scholar]
- 68.Linabery AM, Johnson KJ, Ross JA. Childhood cancer incidence trends in association with US folic acid fortification (1986-2008) Pediatrics. 2012;129(6):1125–1133. doi: 10.1542/peds.2011-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurney JK, Florio AA, Znaor A, et al. International trends in the incidence of testicular cancer: Lessons from 35 years and 41 countries. Eur Urol. 2019;76(5):615–623. doi: 10.1016/j.eururo.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.