Highlights
-
•
Occult breast cancer is rare, and its best treatment is unknown.
-
•
Comparison of ALND + radiotherapy and mastectomy + ALND in OBC.
-
•
ALND + radiotherapy improved the OS of pN3 OBC and those receiving chemotherapy.
-
•
It is the optimal treatment for patients with imaging-negative OBC.
Keywords: Occult breast cancer; Axillary lymph node dissection; Radiotherapy; Mastectomy; Surveillance, Epidemiology, and End Results
Abstract
Background
Because of the rarity of occult breast cancer (OBC) and limited experience in OBC treatment, the optimal treatment strategy is unknown. This study aimed to compare the efficacy of axillary lymph node dissection (ALND) plus radiotherapy with that of mastectomy plus ALND in patients with OBC.
Methods
Relevant clinical data between January 2004 and December 2015 were retrospectively collected from the Surveillance, Epidemiology, and End Results database. The clinical characteristics and prognoses of patients who underwent ALND plus radiotherapy or mastectomy plus ALND were compared before and after propensity score matching.
Results
Overall, 569 eligible patients with OBC were included in this study. Of these, 247 patients underwent ALND plus radiotherapy and 322 underwent mastectomy plus ALND. The 5-year overall survival (OS) rates in the ALND plus radiotherapy group and the mastectomy plus ALND group were 89.2% and 80.6%, respectively; and the corresponding 5-year breast cancer-specific survival (BCSS) rates were 95.2% and 93.0%, respectively. After propensity score matching, the OS in the ALND plus radiotherapy group was significantly better than that in the mastectomy plus ALND group. In addition, further subgroup analyses revealed that ALND plus radiotherapy prolonged OS in the pN3 subgroup. Among patients receiving adjuvant chemotherapy, those who underwent ALND plus radiotherapy had better BCSS and OS than those who underwent mastectomy plus ALND.
Conclusions
ALND plus radiotherapy could improve the OS of patients with OBC, especially those with pN3 disease and those receiving chemotherapy. ALND combined with radiotherapy is the optimal treatment strategy for patients with imaging-negative OBC.
1. Introduction
Occult breast cancer (OBC) is a rare pathological subtype of breast cancer that accounts for less than 1% of all breast tumors.1 OBC presents as axillary lymph node metastasis without an identifiable primary site in physical examination, imaging, or postoperative pathological evaluation.2 Therefore, patients with OBC usually undergo axillary lymph node dissection (ALND) and mastectomy or whole-breast radiotherapy (WBRT) as local treatment. However, owing to the rarity of the disease and limited experience in OBC treatment, the optimal treatment modality is unclear.
The current National Comprehensive Cancer Network (NCCN) guidelines recommend mastectomy plus ALND or ALND plus WBRT with or without lymph node irradiation for patients with OBC. It has been demonstrated that patients who undergo mastectomy with ALND or radiotherapy (RT) with ALND have significantly better local control than patients who undergo ALND alone.3 However, few studies have compared mastectomy plus ALND and ALND plus RT for OBC, and there is controversy over which treatment option provides more survival benefit.4, 5, 6 Traditionally, mastectomy has been considered to have a higher frequency of detection of the primary breast tumor, and the addition of mastectomy to ALND may provide more effective local control. Nevertheless, there is no significant difference in survival between patients who undergo mastectomy plus ALND and those who receive ALND plus RT.3 In addition, advances in magnetic resonance imaging (MRI) and positron emission tomography (PET)-computed tomography have improved the sensitivity of breast tumor detection.7 For cases with no MRI findings, the probability of OBC detection in specimens obtained during mastectomy is low,8 and OBC may arise from ectopic breast tissue in the axillary lymph nodes.9 Therefore, more robust evidence is needed to determine the optimal treatment option.
Therefore, we conducted a large population-based study using data from the Surveillance, Epidemiology, and End Results (SEER) database. The purpose of this study was to compare the efficacy of ALND plus RT and mastectomy plus ALND in patients with OBC. Our results will help clarify the clinical value of these treatment regimens and provide a theoretical basis for the standardized treatment of OBC.
2. Materials and methods
2.1. Study population
The data of patients with OBC who underwent ALND between January 2004 and December 2015 were retrospectively retrieved from the SEER database.
The inclusion criteria were as follows: 1) female patients; 2) age ≥18 years; 3) breast-adjusted American Joint Committee on Cancer sixth edition TNM stage of pT0N13M0; 4) at least one positive regional node; and 5) underwent mastectomy or RT. The exclusion criteria were as follows: 1) repeated medical records; 2) aspiration or core biopsy of regional nodes but not removal of regional nodes; 3) unavailable information on RT or surgery of the primary site; 4) distant metastasis; and 5) underwent breast-conserving surgery. The flow diagram of the patient selection process is shown in Supplementary Fig. 1.
2.2. Study endpoints
In this study, the primary endpoints were overall survival (OS) and breast cancer-specific survival (BCSS). Among them, OS was defined as the time from pathological diagnosis to death or the final follow-up. BCSS was defined as the time from pathological diagnosis to breast cancer-related death or the last follow-up.
2.3. Statistical analyses
All analyses were performed using SPSS (version 23.0; Chicago, IL, USA) and R Studio (version 23.0). Figures were created using GraphPad Prism (version 9.0; San Diego, CA, USA). Patients were divided into two cohorts according to treatment modality: ALND + RT and mastectomy + ALND ± RT. Propensity score matching (PSM) (1:1) was used to eliminate uneven distributions of baseline characteristics and interference of confounders. The general characteristics of the patients in both cohorts were comprehensively assessed using the χ2 test before and after PSM. Survival analysis was performed using the Kaplan-Meier method, and differences in survival were assessed using the log-rank test. Univariate and multivariate Cox proportional hazard analyses were used to identify factors affecting OS and BCSS. Furthermore, subgroup analyses were performed to explore the beneficiary populations in both cohorts. Hazard ratios (HRs) and 95% confidence intervals (CIs) for each subgroup are displayed in forest plots. A two-tailed P < 0.05 was considered statistically significant in all analyses.
3. Results
3.1. Clinicopathological characteristics
A total of 569 patients with OBC were included in this study. In the entire cohort, the median age at diagnosis was 58 years old. Regarding N stage, 58.5%, 21.3%, and 20.2% of the patients were diagnosed with N1, N2, and N3 disease, respectively. Patients with estrogen receptor (ER)-positive tumors accounted for 55.9% of the overall study population, and 49% had progesterone receptor (PR)-positive tumors. Of the 243 patients with available human epidermal growth factor receptor 2 (HER2) data, 73.7% had HER2-negative tumors. A total of 32 (5.6%) patients had well-differentiated or moderately differentiated tumors (grades I-II), whereas 113 (19.9%) patients had poorly differentiated or undifferentiated tumors (grades III-IV). Approximately 70% of the patients had more than 10 examined regional lymph nodes. Regarding therapeutic options, 247 patients underwent ALND plus RT and 322 patients underwent mastectomy plus ALND with or without RT. 46% (148/322) of patients who underwent mastectomy plus ALND received postoperative RT. Furthermore, 451 of 569 patients received postoperative adjuvant chemotherapy. The demographic and clinicopathological characteristics of all patients are shown in Table 1; they were not balanced between the ALND plus RT and mastectomy plus ALND groups. Patients younger than 60 years at diagnosis were more likely to undergo mastectomy plus ALND (χ2 = 4.382, P = 0.036); there were no significant differences in any other baseline features between the two groups (all P > 0.05).
Table 1.
Characteristics of patients with OBC before and after PSM.
| Characteristics | Before PSM |
After PSM |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 569) | ALND +radiotherapy (n = 247) | Mastectomy + ALND (n = 322) | P | Total (n = 494) | ALND +radiotherapy (n = 247) | Mastectomy + ALND (n = 247) | P | |||
| Year of diagnosis | 1.172 | 0.279 | 0.835 | 0.361 | ||||||
| 2004 - 2010 | 331 (58.2) | 150 (60.7) | 181 (56.2) | 290 (58.7) | 150 (60.7) | 140 (56.7) | ||||
| 2011 - 2015 | 238 (41.8) | 97 (39.3) | 141 (43.8) | 204 (41.3) | 97 (39.3) | 107 (43.3) | ||||
| Age at diagnosis | 4.382 | 0.036 | 0.008 | 0.928 | ||||||
| < 60 | 298 (52.4) | 117 (47.4) | 181 (56.2) | 233 (47.2) | 117 (47.4) | 116 (47) | ||||
| ≥ 60 | 271 (47.6) | 130 (52.6) | 141 (43.8) | 261 (52.8) | 130 (52.6) | 131 (53) | ||||
| ER | 0.506 | 0.776 | 0.528 | 0.768 | ||||||
| Negative | 197 (34.6) | 87 (35.2) | 110 (34.2) | 180 (36.4) | 87 (35.2) | 93 (37.7) | ||||
| Positive | 318 (55.9) | 139 (56.3) | 179 (55.6) | 270 (54.7) | 139 (56.3) | 131 (53) | ||||
| Unknown | 54 (9.5) | 21 (8.5) | 33 (10.2) | 44 (8.9) | 21 (8.5) | 23 (9.3) | ||||
| PR | 2.301 | 0.316 | 1.212 | 0.546 | ||||||
| Negative | 279 (49) | 114 (46.2) | 165 (51.2) | 238 (48.2) | 114 (46.2) | 124 (50.2) | ||||
| Positive | 224 (39.4) | 106 (42.9) | 118 (36.6) | 200 (40.5) | 106 (42.9) | 94 (38.1) | ||||
| Unknown | 66 (11.6) | 27 (10.9) | 39 (12.1) | 56 (11.3) | 27 (10.9) | 29 (11.7) | ||||
| HER2 | 2.767 | 0.251 | 3.836 | 0.147 | ||||||
| Negative | 179 (31.5) | 83 (33.6) | 96 (29.8) | 160 (32.4) | 83 (33.6) | 77 (31.2) | ||||
| Positive | 64 (11.2) | 22 (8.9) | 42 (13.1) | 58 (11.7) | 22 (8.9) | 36 (14.6) | ||||
| Unknown | 326 (57.3) | 142 (57.5) | 184 (57.1) | 276 (55.9) | 142 (57.5) | 134 (54.3) | ||||
| Grade | 2.417 | 0.299 | 1.922 | 0.383 | ||||||
| I-II | 32 (5.6) | 10 (4.1) | 22 (6.8) | 27 (5.5) | 10 (4) | 17 (6.9) | ||||
| III-IV | 113 (19.9) | 47 (19.0) | 66 (20.5) | 93 (18.8) | 47 (19) | 46 (18.6) | ||||
| Unknown | 424 (74.5) | 190 (76.9) | 234 (72.7) | 374 (75.7) | 190 (76.9) | 184 (74.5) | ||||
| Lymph node | 0.597 | 0.742 | 0.113 | 0.945 | ||||||
| N1 | 333 (58.5) | 146 (59.1) | 187 (58.1) | 290 (58.7) | 146 (59.1) | 144 (58.3) | ||||
| N2 | 121 (21.3) | 49 (19.8) | 72 (22.4) | 101 (20.4) | 49 (19.8) | 52 (21.1) | ||||
| N3 | 115 (20.2) | 52 (21.1) | 63 (19.6) | 103 (20.9) | 52 (21.1) | 51 (20.6) | ||||
| RNE | 0.215 | 0.643 | 0.144 | 0.704 | ||||||
| < 10 | 183 (32.2) | 82 (33.2) | 101 (31.4) | 168 (34.0) | 82 (33.2) | 86 (34.8) | ||||
| ≥ 10 | 386 (67.8) | 165 (66.8) | 221 (68.6) | 326 (66.0) | 165 (66.8) | 161 (65.2) | ||||
| Chemotherapy | 0.065 | 0.799 | 0 | 1 | ||||||
| Yes | 451 (79.3) | 197 (79.8) | 254 (78.9) | 394 (79.8) | 197 (79.8) | 197 (79.8) | ||||
| No | 118 (20.7) | 50 (20.2) | 68 (21.1) | 100 (20.2) | 50 (20.2) | 50 (20.2) | ||||
Abbreviations: ALND, axillary lymph node dissection; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; OBC, occult breast cancer; PR, progesterone receptors; PSM, propensity score matching; RNE, regional lymph node examined.
3.2. Survival outcomes and prognostic factors
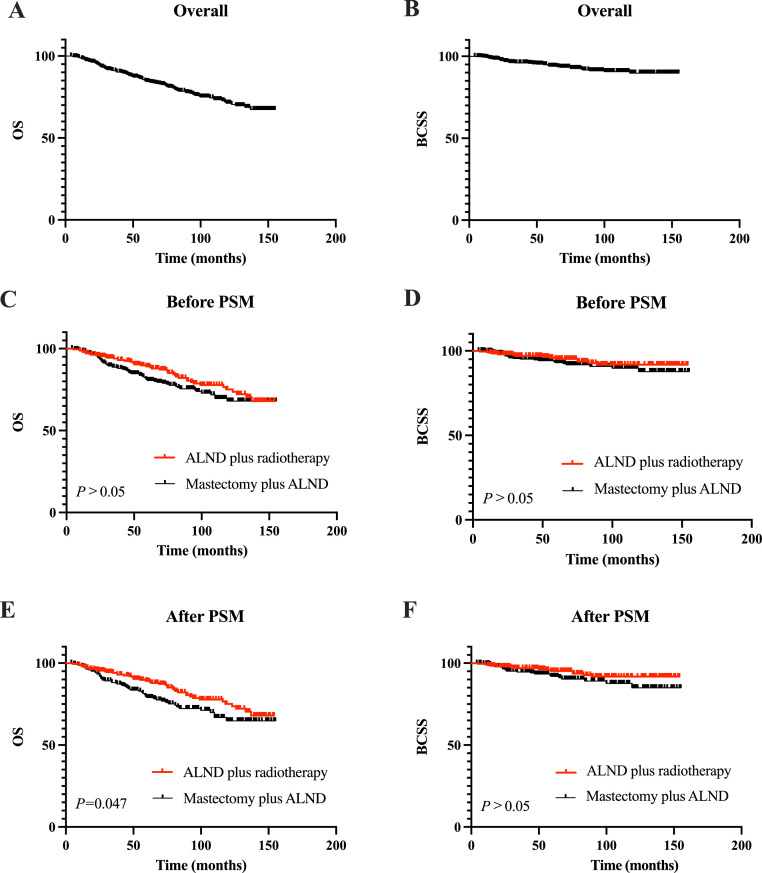
The median follow-up time was 79 months (range: 4–155 months). The survival curves of the overall population are presented in Fig. 1A and B. The 5-year OS rates of the patients who underwent ALND plus RT and mastectomy plus ALND were 89.2% and 80.6%, respectively. The corresponding 5-year BCSS rates were 95.2% and 93.0%, respectively (Fig. 1C and D).
Fig. 1.
Survival analyses of the overall population and by treatment group. (A) OS of overall population. (B) BCSS of overall population. (C) Comparison of OS between the two treatment groups before PSM. (D) Comparison of BCSS between the two treatment groups before PSM. (E) Comparison of OS between the two treatment groups after PSM. (F) Comparison of BCSS between the two treatment groups after PSM. ALND, axillary lymph node dissection; BCSS, breast cancer-specific survival; OS, overall survival; PSM, propensity score matching.
Univariate analyses showed that pN stage was associated with both OS and BCSS (all P<0.05). In addition, age and chemotherapy were closely related to OS, and ER status were closely related to BCSS (all P<0.05). In multivariate analysis, pN stage, chemotherapy, local treatment modality were independent prognostic factors affecting OS (all P<0.05, Supplementary Table 1). The OS was significantly longer in patients in the ALND plus RT group compared with those in the mastectomy plus ALND group (P = 0.043, HR: 0.667, 95% CI: 0.451-–0.987). In addition, we found that pN stage was also an independent prognostic factor for BCSS (all P<0.05). However, there were no statistically significant differences in BCSS between the ALND plus RT group and the mastectomy plus ALND group (Supplementary Table 1).
3.3. Propensity score matching analysis
After the PSM analysis, patients were divided into two groups according to the therapies they received, namely, ALND plus RT (n = 247) and mastectomy plus ALND (n = 247). As shown in Table 2, there were no significant differences between the two groups in the year of diagnosis, age at diagnosis, pN stage, number of examined regional lymph nodes, ER status, PR status, HER2 status, histological grade, or administration of chemotherapy.
Table 2.
Univariate and multivariate analyses for BCSS and OS after PSM.
| Characteristic | BCSS |
OS |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Year of diagnosis | ||||||||
| 2004–2010 | 1 | 1 | 1 | 1 | ||||
| 2011–2015 | 0.913 (0.391–2.133) | 0.833 | 1.085 (0.292–4.036) | 0.903 | 0.624 (0.348–1.119) | 0.113 | 0.658 (0.284–1.523) | 0.328 |
| Age at diagnosis (year) | ||||||||
| < 60 | 1 | 1 | 1 | 1 | ||||
| ≥ 60 | 1.215 (0.622–2.375) | 0.568 | 1.229 (0.609–2.479) | 0.564 | 1.497 (0.999–2.243) | 0.050 | 1.301 (0.853–1.983) | 0.222 |
| ER | ||||||||
| Negative | 1 | 1 | 1 | 1 | ||||
| Positive | 0.431 (0.211–0.883) | 0.021 | 0.467 (0.177–1.236) | 0.125 | 0.813 (0.539–1.225) | 0.322 | 0.604 (0.336–1.086) | 0.092 |
| Unknown | 0.613 (0.182–2.063) | 0.429 | 2.454 (0.072–83.3) | 0.618 | 0.749 (0.353–1.592) | 0.453 | 0.29 (0.077–1.088) | 0.067 |
| PR | ||||||||
| Negative | 1 | 1 | 1 | 1 | ||||
| Positive | 0.48 (0.222–1.038) | 0.062 | 0.793 (0.285–2.209) | 0.658 | 0.909 (0.595–1.389) | 0.660 | 1.28 (0.705–2.326) | 0.418 |
| Unknown | 0.544 (0.163–1.813) | 0.321 | 0.309 (0.009–10.316) | 0.511 | 0.97 (0.517–1.819) | 0.924 | 1.967 (0.665–5.813) | 0.221 |
| HER2 | ||||||||
| Negative | 1 | 1 | 1 | 1 | ||||
| Positive | 0.299 (0.038–2.361) | 0.252 | 0.245 (0.03–1.998) | 0.189 | 0.278 (0.065–1.194) | 0.085 | 0.28 (0.065–1.218) | 0.090 |
| Unknown | 0.849 (0.379–1.902) | 0.691 | 0.798 (0.229–2.789) | 0.724 | 1.1 (0.648–1.869) | 0.723 | 0.805 (0.379–1.713) | 0.574 |
| Grade | ||||||||
| I-II | 1 | 1 | 1 | 1 | ||||
| III-IV | 1.289 (0.282–5.895) | 0.744 | 0.784 (0.154–4.005) | 0.770 | 0.84 (0.338–2.085) | 0.707 | 0.884 (0.337–2.324) | 0.803 |
| Unknown | 0.779 (0.183–3.308) | 0.735 | 0.558 (0.12–2.598) | 0.457 | 0.791 (0.344–1.819) | 0.581 | 0.891 (0.371–2.139) | 0.796 |
| Lymph node | ||||||||
| N1 | 1 | 1 | 1 | 1 | ||||
| N2 | 2.994 (1.246–7.195) | 0.014 | 3.192 (1.263–8.063) | 0.014 | 2.12 (1.327–3.388) | 0.002 | 2.204 (1.352–3.591) | 0.002 |
| N3 | 4.321 (1.941–9.62) | 0.000 | 6.249 (2.56–15.254) | <0.001 | 1.912 (1.185–3.084) | 0.008 | 2.125 (1.261–3.579) | 0.005 |
| RNE | ||||||||
| < 10 | 1 | 1 | 1 | 1 | ||||
| ≥ 10 | 1.044 (0.519–2.098) | 0.904 | 0.557 (0.255–1.216) | 0.142 | 1.158 (0.755–1.776) | 0.503 | 0.995 (0.627–1.579) | 0.984 |
| Chemotherapy | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.901 (0.393–2.065) | 0.806 | 0.739 (0.294–1.856) | 0.520 | 0.45 (0.297–0.684) | <0.001 | 0.379 (0.237–0.604) | <0.001 |
| Local treatment | ||||||||
| Mastectomy + ALND | 1 | 1 | 1 | 1 | ||||
| ALND +radiotherapy | 0.588 (0.299–1.158) | 0.125 | 0.591 (0.297–1.174) | 0.133 | 0.669 (0.45–0.994) | 0.047 | 0.622 (0.414–0.932) | 0.021 |
Abbreviations: ALND, axillary lymph node dissection; CI, confidence interval; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; OS, overall survival; PR, progesterone receptors; PSM, propensity score matching; RNE, regional lymph node examined.
In the univariate analyses after PSM correction, ALND plus RT was associated with better OS than mastectomy plus ALND (P = 0.047, HR: 0.669, 95% CI: 0.450–0.994). However, no significant difference in BCSS was observed between ALND plus RT group and mastectomy plus ALND group (P = 0.125, HR: 0.588, 95% CI: 0.299–1.158) (Fig. 1E and F). Subsequently, in the Cox multivariate analyses, there was no statistically significant difference in BCSS between the ALND plus RT and mastectomy plus ALND groups (P = 0.133, HR: 0.591, 95% CI: 0.297–1.174). Notably, the OS in the ALND plus RT group was significantly better than that in the mastectomy plus ALND group (P = 0.021, HR: 0.622; 95% CI: 0.414–0.932). Detailed results of univariate and multivariate analyses of BCSS and OS are presented in Table 2.
3.4. Subgroup analysis
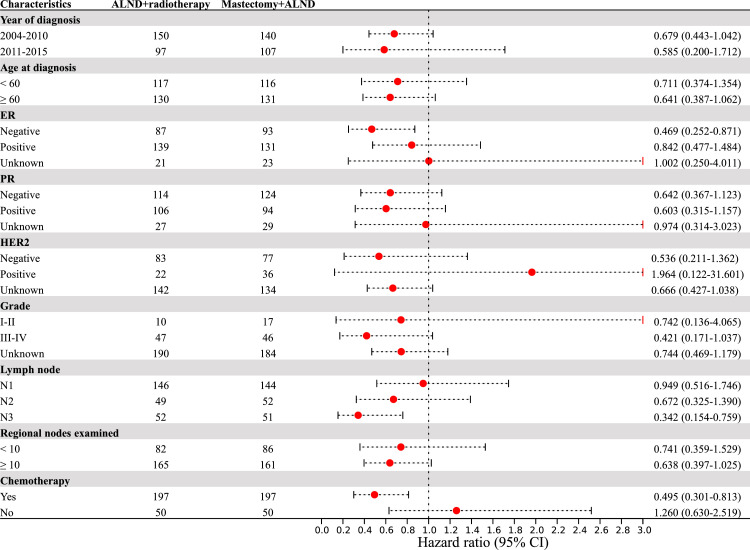
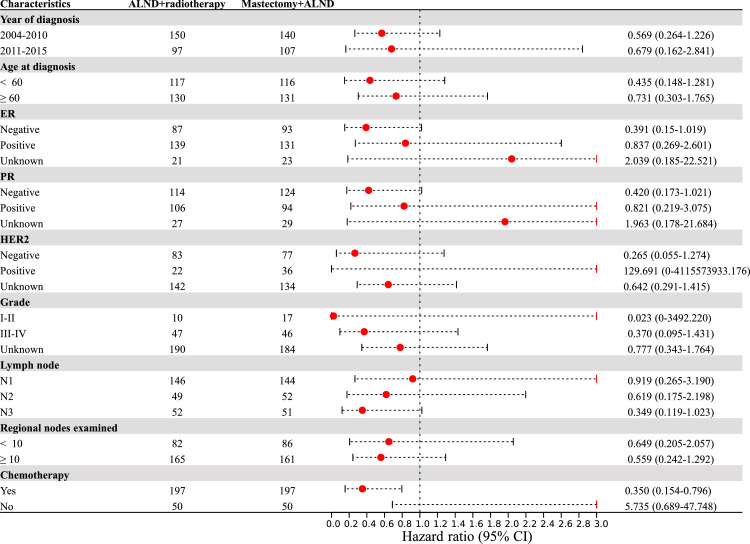
In order to identify whether specific subgroups would benefit from ALND plus RT, subgroup analyses were further conducted on the PSM cohort. Interestingly, we found that in the pN3 subgroup, ALND plus RT significantly prolonged OS compared to mastectomy plus ALND (P = 0.008; HR: 0.342; 95% CI: 0.154–0.759). Among patients who received adjuvant chemotherapy, those who received ALND plus RT had better BCSS and OS than those who underwent mastectomy plus ALND (all P < 0.05) (Fig. 2-3).
Fig. 2.
The forest plot of overall survival. ALND, axillary lymph node dissection; CI, confidential interval; ER, estrogen receptor; HER, human epidermal growth factor receptor; HR, hazard ratio; PR, progesterone receptors.
Fig. 3.
The forest plot of breast cancer-specific survival. ALND, axillary lymph node dissection; CI, confidential interval; ER, estrogen receptor; HER, human epidermal growth factor receptor; HR, hazard ratio; PR, progesterone receptors.
4. Discussion
Knowledge of OBC is scarce given its low morbidity rate. In this study, we performed a large-scale population analysis to comprehensively describe the characteristics of patients with OBC and further assess more appropriate therapeutic strategies for this population. Our results found that patients in the ALND plus RT group had longer OS compared with the mastectomy plus ALND group, both before and after PSM. More importantly, subgroups that could benefit from ALND plus RT were identified. For OBC patients with pN3 disease, ALND plus RT was superior to mastectomy plus ALND, which has important guiding significance for the clinical practice.
Once histopathology and immunohistochemistry of a biopsied axillary lymph node confirm metastasis of adenocarcinoma from the breast, physical examination, breast ultrasonography, and mammography can be used to identify the primary tumor site. When traditional examination methods cannot identify the primary breast lesion, contrast-enhanced MRI of the breast can further improve the detection rate.10, 11, 12, 13 The American College of Radiology also recommends the use of MRI for the diagnosis of OBC in cases in which mammography and ultrasonography cannot detect the primary breast tumor. Comprehensive imaging evaluation, including MRI, is required for the diagnosis of radiological OBC.2 However, no standardized or unified definition of OBC was used in previous studies. Many previous studies used negative breast ultrasonography and/or mammography results for the diagnosis of radiological OBC; however, with older imaging technologies, the detection sensitivity of primary breast lesions was low. Therefore, there might be many false-negative results in the preoperative assessment.
The current NCCN and European Society for Medical Oncology guidelines suggest that OBC, a subtype of carcinoma of unknown primary site with a favorable prognosis, can be treated similarly to stage II–III breast cancer. ALND is the standard treatment for patients with OBC, and adjuvant chemotherapy, endocrine therapy, and targeted therapy can also be selected based on the tumor stage, immunohistochemical results, and other risk factors. However, the optimal management of the ipsilateral breast in OBC is controversial and varies between studies.
Previous reports have shown that mastectomy can reduce local recurrence and improve patient survival compared with no local treatment.11, 14, 15 The advantage of mastectomy is that the primary breast lesion can be further identified by a detailed pathological examination. Therefore, mastectomy may be an appropriate option for OBC with a non-detectable underlying primary lesion. However, as we noted earlier, almost all studies on OBC treatment are retrospective studies with small sample sizes that do not meet the diagnostic criteria for radiological OBC.14, 16 With the development of imaging technologies, breast MRI has become the most sensitive imaging method for cancer detection, with a specificity of up to 88%.17 PET can further improve preoperative disease staging and is an effective tool for OBC diagnosis, with a sensitivity and specificity of 95% and 65%, respectively.18, 19 The declining trend in OBC incidence also indicates that imaging improves the detection rate of primary breast cancer. Further, although many patients undergo mastectomy, no primary breast cancer can be detected on pathological examination.3 OBC may originate from ectopic breast tissue in the axillary lymph nodes, in which case mastectomy may not be necessary.9 Therefore, further studies are needed to identify the origin of OBC and reformulate the treatment strategy based on more precise diagnosis.
In our study, a population-based analysis was conducted to explore the clinical value of ALND plus RT and mastectomy plus ALND. The initial results of our study suggested that mastectomy plus ALND did not provide more benefit than ALND plus RT. It is worth noting that RT is indicated for patients with OBC with a primary lesion that is not detected on imaging, whereas for patients who undergo mastectomy, OBC should be pathologically confirmed. Theoretically, mastectomy involves more thorough tumor removal and usually a better prognosis, although this is not always true in practice. Previous reports from the SEER database also suggested this. In the study of Walker GV, et al.,14 the locoregional treatment type was not significantly associated with better clinical outcomes in patients with OBC. Johnson H, et al.4 also found that the type of local therapy was not related to BCSS for those with OBC. In addition, Wu et al.20 studied the value of additional local treatment strategies (combined RT, combined surgery, and combined RT plus surgery) for OBC after ALND and found no significant difference in BCSS and OS among the groups. A meta-analysis showed a similar conclusion, indicating no significant differences in the local recurrence rate, distant metastasis rate, or mortality between the mastectomy plus ALND and ALND plus RT groups.21 Furthermore, after matching the baseline characteristics of the two groups in our study, we found that patients in the ALND plus RT group had longer OS than those in the mastectomy plus ALND group. However, there was no significant difference in BCSS between the two groups. Similarly, in the study by Hessler et al.,6 patients who received ALND plus radiotherapy had improved OS compared with modified radical mastectomy. These results suggest that ALND plus RT may be the optimal local treatment for OBC owing to its OS benefit and better cosmetic effect.
To date, there have been no clinical trials examining the optimal dose of RT for patients with OBC, and the selection of the irradiation field and specific dose still needs to be explored prospectively. In clinical practice, prophylactic RT is often used for OBC, which helps eliminate potential microscopic lesions.6, 22 A prophylactic dose is sufficient for MRI-negative cases.22 In the subgroup analysis, ALND plus RT resulted in longer survival than mastectomy plus ALND in patients with pN3 disease. This may be related to the fact that these patients received RT to the axillary lymph nodes and breasts. Notably, we did not have information regarding the sites and doses of RT, which is a study limitation. However, our findings support the fact that ALND plus RT is not inferior to mastectomy plus ALND in cases without radiological detection of primary breast lesions. In addition, ALND plus RT can improve the OS of patients with pN3 OBC and those who received chemotherapy. It is important to emphasize that ALND plus RT can preserve the breasts, which is beneficial for the patient's appearance and mental health.
This study has several limitations, including its retrospective nature. First, there was no detailed treatment information on the radiation site, radiation dose, or fractionation in the SEER database, which imposes some limitations on the detailed exploration of the effect of ALND plus RT in the treatment of OBC, and more data are needed to further validate our results. In addition, some information not recorded in this public database, such as performance status, comorbidities, neurological invasion and endovascular invasion, may also have some influence on treatment decisions. In the future, prospective clinical trials are necessary to explore the optimal treatment strategies for patients with OBC.
5. Conclusion
Our results suggest that ALND plus RT may be a favorable management strategy for patients with OBC. ALND plus RT could improve the OS of patients with OBC, particularly those with pN3 disease and those who underwent chemotherapy. Our study clarified the clinical value of ALND plus RT in OBC patients and the patients who will derive the greatest benefit, which is important for individualized treatment.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Ethics statement
Because all data could be obtained from public databases with patient anonymity, institutional ethics committee approval and written consent were not required.
Acknowledgments
The authors acknowledge the efforts of the SEER program tumor registries in the creation of the SEER database and thank the patients analyzed in this study.
Author contributions
L.L., D.Z. and F.M. designed this study and provided critical revisions. L.L. and D.Z. extracted the data and analyzed the results. L.L., D.Z., T.W., Y.W., D.L., J.Z. were involved in writing this manuscript. F.M. supervised the whole process. L.L., D.Z. and F.M. revised the manuscript. All authors read and agreed to the published version of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jncc.2022.09.001.
Appendix. Supplementary materials
References
- 1.Baron P.L., Moore M.P., Kinne D.W., et al. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. 1990;125(2):210–214. doi: 10.1001/archsurg.1990.01410140088014. [DOI] [PubMed] [Google Scholar]
- 2.Ofri A., Moore K. Occult breast cancer: where are we at? Breast. 2020;54:211–215. doi: 10.1016/j.breast.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He M., Tang L.C., Yu K.D., et al. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. Eur J Surg Oncol. 2012;38(11):1022–1028. doi: 10.1016/j.ejso.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Johnson H.M., Irish W., Vohra N.A., et al. The effect of local therapy on breast cancer-specific mortality of women with occult breast cancer and advanced nodal disease (N2/N3): a population analysis. Breast Cancer Res Treat. 2019;177(1):155–164. doi: 10.1007/s10549-019-05285-x. [DOI] [PubMed] [Google Scholar]
- 5.Woo S.M., Son B.H., Lee J.W., et al. Survival outcomes of different treatment methods for the ipsilateral breast of occult breast cancer patients with axillary lymph node metastasis: a single center experience. J Breast Cancer. 2013;16(4):410–416. doi: 10.4048/jbc.2013.16.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessler L.K., Molitoris J.K., Rosenblatt P.Y., et al. Factors influencing management and outcome in patients with occult breast cancer with axillary lymph node involvement: analysis of the national cancer database. Ann Surg Oncol. 2017;24(10):2907–2914. doi: 10.1245/s10434-017-5928-x. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan C.L., Morris E.A., Dorn P.L., et al. Utility of breast magnetic resonance imaging in patients with occult primary breast cancer. Ann Surg Oncol. 2005;12(12):1045–1053. doi: 10.1245/ASO.2005.03.520. [DOI] [PubMed] [Google Scholar]
- 8.McCartan D.P., Zabor E.C., Morrow M., et al. Oncologic outcomes after treatment for MRI occult breast cancer (pT0N+) Ann Surg Oncol. 2017;24(11):3141–3147. doi: 10.1245/s10434-017-5965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terada M., Adachi Y., Sawaki M., et al. Occult breast cancer may originate from ectopic breast tissue present in axillary lymph nodes. Breast Cancer Res Treat. 2018;172(1):1–7. doi: 10.1007/s10549-018-4898-4. [DOI] [PubMed] [Google Scholar]
- 10.Obdeijn I.M., Brouwers-Kuyper E.M., Tilanus-Linthorst M.M., et al. MR imaging-guided sonography followed by fine-needle aspiration cytology in occult carcinoma of the breast. AJR Am J Roentgenol. 2000;174(4):1079–1084. doi: 10.2214/ajr.174.4.1741079. [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Zhao Y., Cao X. Clinical benefits of mastectomy on treatment of occult breast carcinoma presenting axillary metastases. Breast J. 2010;16(1):32–37. doi: 10.1111/j.1524-4741.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 12.Sohn G., Son B.H., Lee S.J., et al. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: a nationwide retrospective study. J Surg Oncol. 2014;110(3):270–274. doi: 10.1002/jso.23644. [DOI] [PubMed] [Google Scholar]
- 13.Orel S.G., Weinstein S.P., Schnall M.D., et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999;212(2):543–549. doi: 10.1148/radiology.212.2.r99au40543. [DOI] [PubMed] [Google Scholar]
- 14.Walker G.V., Smith G.L., Perkins G.H., et al. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer. 2010;116(17):4000–4006. doi: 10.1002/cncr.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pentheroudakis G., Lazaridis G., Pavlidis N. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): a systematic review of published evidence. Breast Cancer Res Treat. 2010;119(1):1–11. doi: 10.1007/s10549-009-0554-3. [DOI] [PubMed] [Google Scholar]
- 16.Varadarajan R., Edge S.B., Yu J., et al. Prognosis of occult breast carcinoma presenting as isolated axillary nodal metastasis. Oncology. 2006;71(5–6):456–459. doi: 10.1159/000107111. [DOI] [PubMed] [Google Scholar]
- 17.Schoub P.K. Understanding indications and defining guidelines for breast magnetic resonance imaging. SA J Radiol. 2018;22(2):1353. doi: 10.4102/sajr.v22i2.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avril N., Dose J., Jänicke F., et al. Assessment of axillary lymph node involvement in breast cancer patients with positron emission tomography using radiolabeled 2-(fluorine-18)-fluoro-2-deoxy-D-glucose. J Natl Cancer Inst. 1996;88(17):1204–1209. doi: 10.1093/jnci/88.17.1204. [DOI] [PubMed] [Google Scholar]
- 19.Liu M., Liu B., Song Y., et al. FDG PET/CT reveals the primary tumor in a patient with occult breast carcinoma undetected by other modalities. Clin Nucl Med. 2014;39(8):755–757. doi: 10.1097/RLU.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 20.Wu S.G., Zhang W.W., Sun J.Y., et al. Comparable survival between additional radiotherapy and local surgery in occult breast cancer after axillary lymph node dissection: a population-based analysis. J Cancer. 2017;8(18):3849–3855. doi: 10.7150/jca.21217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macedo F.I., Eid J.J., Flynn J., et al. Optimal surgical management for occult breast carcinoma: a meta-analysis. Ann Surg Oncol. 2016;23(6):1838–1844. doi: 10.1245/s10434-016-5104-8. [DOI] [PubMed] [Google Scholar]
- 22.Barton S.R., Smith I.E., Kirby A.M., et al. The role of ipsilateral breast radiotherapy in management of occult primary breast cancer presenting as axillary lymphadenopathy. Eur J Cancer. 2011;47(14):2099–2106. doi: 10.1016/j.ejca.2011.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.