Abstract
Purpose
This study aimed to examine the effect of radiation esophagitis (RE) and the dynamics of RE on subsequent survival in non-small cell lung cancer (NSCLC) patients who underwent radiotherapy.
Experimental Design
Patients with NSCLC treated with fractionated thoracic radiotherapy enrolled in prospective trials were eligible. RE was graded prospectively according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0 per protocol requirement weekly during-RT and 1 month after RT. This study applied conditional survival assessment which has advantage over traditional survival analysis as it assesses the survival from the event instead of from the baseline. P-value less than 0.05 was considered to be significant. The primary endpoint is overall survival.
Results
A total of 177 patients were eligible, with a median follow-up of 5 years. The presence of RE, the maximum RE grade, the evolution of RE and the onset timing of RE events were all correlated with subsequent survival. At all conditional time points, patients first presented with RE grade1 (initial RE1) had significant inferior subsequent survival (multivariable HRs median: 1.63, all P-values<0.05); meanwhile those with RE progressed had significant inferior subsequent survival than those never develop RE (multivariable HRs median: 2.08, all P-values<0.05). Multivariable Cox proportional-hazards analysis showed significantly higher C-indexes for models with inclusion of RE events than those without (all P-values<0.05).
Conclusion
This study comprehensively evaluated the impact of RE with conditional survival assessment and demonstrated that RE is associated with inferior survival in NSCLC patients treated with RT.
Keywords: Radiotherapy, Radiation esophagitis, Conditional survival, Non-small cell lung cancer
1. Introduction
Radiation esophagitis (RE) is a common acute toxicity which occurs in non-small cell lung cancer (NSCLC) patients treated with radiation therapy (RT). Classically, RE starts 2 to 4 weeks after RT initiation and persists for several weeks after the completion of RT1. In patients treated with concurrent chemoradiation, approximately 50% of patients develop grade 2 or higher RE2 and 25% grade 3 or higher RE3. Symptoms of acute RE include odynophagia, dysphagia, and retrosternal pain, or some combination of these3,4. Endoscopic evaluation often reveals erythema and ulceration which result from various inflammatory changes and radiation-related atrophy of endothelial and stromal cells5. Severe RE can impair nutrition and necessitate a treatment break, which is associated with inferior oncologic outcomes6,7.
Although there are a number of studies on predictive factors for RE1, 2, 3,8, 9, 10, yet few studies have examined the effect on long-term survival. Randomized Phase III Trial RTOG 9410 revealed that with a median follow-up time of 11 years, the rates of acute grade 3–5 non-hematologic toxicities were higher with concurrent than sequential therapy, but late toxicities were similar11. A retrospective by Holgersson et al.12 reported that RE can be a prognostic factor in patients with lung cancer. It is unclear whether the RE has an effect on long-term outcome of these patients and there are interactions between RE and subsequent survival. In addition, tumor stage and other clinical information at baseline can only provide limited prognostic information, treatment side effects like the RE events after treatment initiation may help improve prognostication.
Common survival analysis may not be suitable in such study because the RE event is a changing variable after RT initiation. Conditional survival, defined as the probability of surviving further t years, given a patient has already survived s years after treatment initiation, is a preferable method. Conditional survival provides dynamic assessment at various time point after treatment initiation and has a potential to provide more accurate prediction on prognosis at later times13,14. Using conditional survival method, this study aimed to study the effects of RE events and dynamics on patients’ subsequent survival in patients with locally advanced NSCLC treated with fractionated radiotherapy.
2. Materials and methods
2.1. Study design and inclusion criteria
This was a secondary analysis of four prospective trials (UMCC 2003.073, UMCC 2003.076, NCT00603057, NCT01190527) in patients with NSCLC as shown in Supplemental Table 1. Patients who were eligible for these studies included those with fluorodeoxyglucose (FDG)-avid tumors (maximum standard uptake value (SUV)≥4.0, from positron emission tomography (PET) scan of any date, any scanner) and histologically or cytologically proven NSCLC. There were no clinical exclusion criteria for these clinical studies. Patients were treated with between March 11, 2004 and March 21, 2013 and were followed until Sep 12, 2016. Baseline clinicopathologic variables were collected, and serial evaluations of treatment response and toxicity were performed during and after therapy. University of Michigan ethics committee approved the research. All participants gave written informed cosent to participate in the study.
2.2. Radiation treatment
All patients were treated with fractionated conformal RT of thorax with or without concurrent or sequential chemotherapy (Supplemental Table 1). The gross tumor volume (GTV) included the primary tumor and any involved hilar or mediastinal lymph nodes, as determined by tissue diagnosis and/or PET-CT. Due to the dose/fraction variation among patients in the four prospective trials, biologically effective dose (BED) was computed to normalize doses by using the linear-quadratic model with alpha/beta ratio of 10 Gy.
2.3. Radiation esophagitis
RE, defined as either dysphagia or odynophagia, was assessed and graded prospectively by the treating physicians according to a predefined grade system of odynophagia or dysphagia, according to the Common Terminology Criteria for Adverse Events (CTCAE) v3.015. Patients were evaluated weekly during RT, with follow-up evaluations at 1,3, 6, 9, 12, 18, and 24 months after completion of RT. Detailed history and physical examination as well as a chest computed tomography (CT) scan were performed at each visit except for the 1-month follow-up visit.
2.4. Statistical analysis
Conditional overall survival estimates were computed for every additional time survived after the initiation of RT, conditional on being alive at the beginning of that subsequent time (subsequent survival)13,14. Total 7 conditional time points were selected (1-week, 0.5-month, 1-month, 1.5-month, 2-month, 2.5-month, 3-month following the initiation of RT), correspondingly, 7 subgroups’ conditional overall survivals were estimated.
Under conditional overall survival analysis, the univariate and multivariable Cox proportional-hazards models including dynamic RE grade or RE onset timing were performed. Significant clinical variables (at the 0.05 p level) were also included in multivariable models. Model discrimination was evaluated by the concordance index (C-index)16. The paired t-test was applied to compare the C-index to understand whether RE event was an independent prognostic factor of subsequent survival. P-value <0.05 was considered statistically significant. All statistical analyses were conducted with R, version 3.6.117.
The effects of RE were studied under five different scenarios: 1) Initial RE grade, classified into no RE (RE0, and these patients never got RE during the whole course of their diseases), initially experienced RE grade=1 (initial RE1) or initially experienced RE grade≥2 (initial RE2); 2) maximum RE grade, also with classifications of no RE (RE0), experienced maximum grade=1 RE (maximum RE1) or grade≥2 (maximum RE2) before the conditional time point; 3) RE evolution, classified into the following scenarios: presented with any grade of RE that either progressed to higher grade RE or not resolved till 3 months (RE progressed); presented with any grade of RE, and RE finally resolved (RE resolved); using RE0 as the reference; 4) The onset timing of the first RE (initial RE-timing, 0 for RE0); and 5) The timing of the maximum grade of RE presented before the conditional time point (maximum RE-timing).
3. Results
3.1. Baseline clinical characteristics
A total of 177 patients from four prospective trials were retrospectively analyzed. After a median follow-up time of 5.0 years (range, 2 days to 11.2 years), 134 (75.7%) patients died and 43 (24.3%) patients were censored. Table 1 presents the baseline clinical characteristics of the entire cohort. The median age was 65.9 years, 75.7% were male, and 66.1% patients received both RT and chemotherapy (all except one received concurrent therapy). Karnofsky Performance Status (KPS), Clinical American Joint Committee on Cancer (AJCC) Stage, Stereotactic Body Radiation Therapy (SBRT) and Biologically Effective Dose (BED) were significant for overall survival as shown in Table 1.
Table 1.
Baseline clinical characteristics of all patients and their univariate correlations with overall survival.
| Clinical characteristics | No. (%) | HR | 95%CI | P-value |
|---|---|---|---|---|
| Gender | ||||
| Female Male |
43 (24.3) 134 (75.7) |
Ref. 1.46 |
0.96–2.24 |
0.07 |
| Age | ||||
| Median (1st – 3rd Qu) | 65.9 (60.1–73.5) | 1.01 | 0.99–1.03 | 0.16 |
| KPS | ||||
| <80 ≥80 |
36 (20.3) 141 (79.7) |
Ref. 0.55 |
0.37–0.83 |
0.004 • |
| Smoking status | ||||
| No Yes |
6 (3.3) 171 (96.7) |
Ref. 3.99 |
0.96–16.19 |
0.06 |
| Clinical AJCC Stage | ||||
| I II III |
35 (19.8) 23 (13.0) 119 (67.2) |
Ref. 2.25 1.58 |
1.21–4.14 0.99–2.52 |
0.01 • 0.05 |
| T stage | ||||
| 1 2 3 4 |
41 (23.2) 43 (24.3) 49 (27.7) 44 (24.8) |
Ref. 1.33 1.47 1.35 |
0.79–2.21 0.9–2.41 0.88–2.37 |
0.27 0.12 0.14 |
| N stage | ||||
| 0 1 2 3 |
59 (33.3) 20 (11.3) 61 (34.5) 27 (20.9) |
Ref. 1.52 1.47 1.58 |
0.83–2.76 0.97–2.23 0.98–2.357 |
0.17 0.07 0.06 |
| Chemotherapy | ||||
| No Yes |
60 (33.9) 117 (66.1) |
Ref. 1.21 |
0.84–1.74 |
0.3 |
| SBRT | ||||
| No | 152 (85.9) | Ref. | ||
| Yes | 25 (24.1) | 0.49 | 0.26–0.91 | 0.02 • |
| BED (Gy) | ||||
| Low Median (1st – 3rd Qu) High Median (1st – 3rd Qu) |
78.0 (72.0–79.7) 105.0 (92.3–109.9) |
Ref. 0.56 |
0.4–0.8 |
0.001 • |
Abbreviation: KPS, Karnofsky Performance Status; SBRT, Stereotactic Body Radiation Therapy; BED, Biologically Effective Dose; Qu, quartile; HR, hazard ratio; CI, confident interval.
• P-value < 0.05.
3.2. RE characteristics
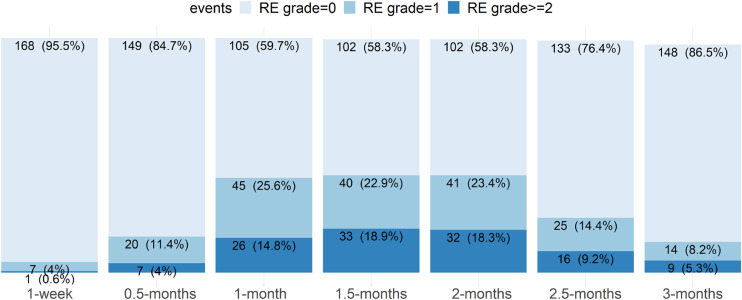
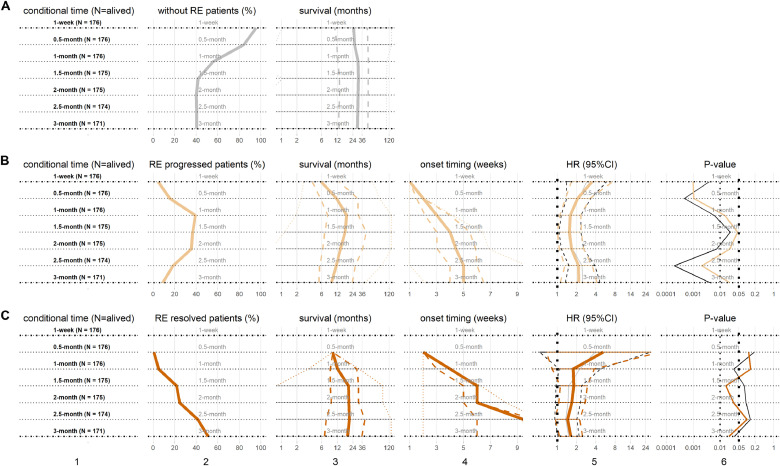
In this study, RE events referred to any RE, i.e. grade=1 or ≥2 which included grade 2 and 3, as there was no grade 4 or 5 in this study. As shown in Fig. 1, total 176 patients were alive at 1 month following the initiation of RT and 171 remained alive after 3 months. A total of 105 patients presented with events of RE grade=1 or RE grade≥2; RE in 24 patients worsened and RE in 87 patients resolved from RT initiation to the end of 3-month. However, the total number of RE events were much more than the numbers shown in Fig. 1, because resolved RE events were not counted in at later conditional time points. The exact numbers or percentages of each kind of RE events at each time point were listed in Section 3.3 to 3.6 and shown in Fig. 2-5 (subplots of patients’ percentages).
Fig. 1.
The distribution of radiation esophagitis (RE). x-axis shows the conditional time points from RT commencement, y-axis represents the patient numbers (percentages) with different RE grades.
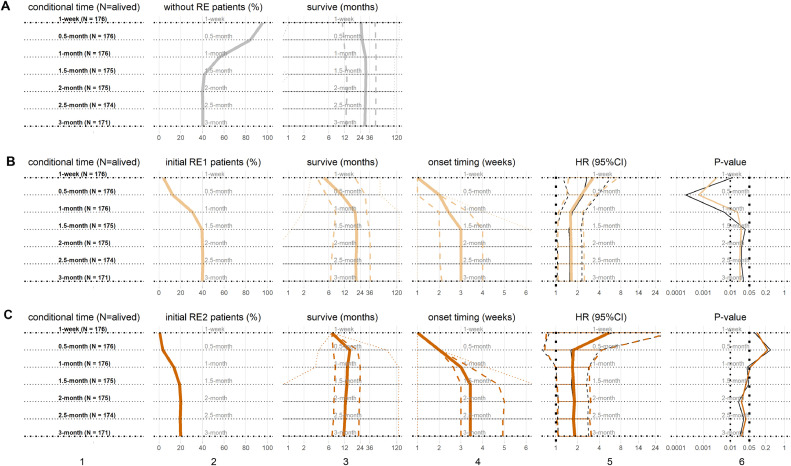
Fig. 2.
The initial grade of radiation esophagitis (RE) and subsequent survival. From top row to bottom row, subplots are 7 time points of patients with (A) RE0 as the reference, (B) initial RE1, and (C) initial RE2. From left column to right column, subplots are (1) subgroups at each conditional time point with the number of the still alive patients, (2) patient percentages (%) with RE events, (3) subsequent survivals (months) in (median: solid, inter-quartile range (IQR): dash, range: dot), (4) onset timing of initial RE1 and initial RE2 (weeks) in (median: solid, IQR: dash, range: dot), (5) the hazard ratios (HRs) of initial RE1 and initial RE2 in (median: solid, 95%confidence interval (CI): dash), including univariate HRs (black) and multivariable HRs (color), and (6) P-values of HRs, including univariate P-values (black) and multivariable P-values (color). The same horizon refers to the results at the same conditional time points.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
The onset timing of radiation esophagitis (RE) and subsequent survival. This figure shows the results of Cox regression models with (A) the onset timing of first RE event and (B) the timings of the maximum RE grades. Similarly in (A) and (B), from left column to right column, subplots are (1) subgroups at each conditional time point with patient number, (2) patient percentages, (3) subsequent survivals in (median: solid, IQR: dash, range: dot), (4) the onset timing of RE events in (median: solid, IQR: dash, range: dot), (5) the HRs of initial RE-timing/maximum RE-timing, in (median: solid, 95%CI: dash), including univariate HRs (black) and multivariable HRs (color), (6) P-values of HRs, including univariate P-values (black) and multivariable P-values (color). The same horizon refers to the results at same conditional time points.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
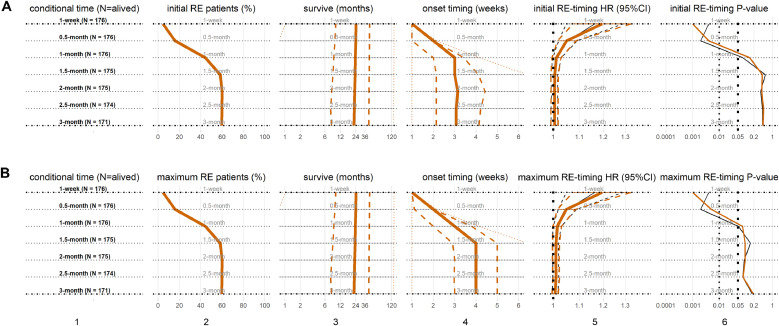
3.3. Initial RE grade and survival
As shown in Fig. 1, at 1-week after RT treatment initiation, 7 (4.0%) and 1 (0.6%) patients had initial RE1 and initial RE2, respectively. At the end of 3 months, total 68 (39.8%) and 34 (19.9%) developed RE1 and RE2, respectively. Counting up to the end of 3-month, the median onset of initial RE1 was 3 weeks, while the initial RE2 was 3.4 weeks. The median subsequent survival of patients were 19.9 months and 11.5 months for initial RE1 and RE2, respectively, comparing to the median subsequent survival of 29.1 months for patients without RE (RE0).
Conditional overall survivals of the initial RE grades are shown in Fig. 2. In each conditional time points, univariate Cox proportional-hazards model was established with initial RE; multivariable model was established with initial RE and other significant clinical factors (KPS, Stage, SBRT and BED), which was similar in other scenarios. Interestingly, at all conditional time points from 1-week to 3-month following the initiation of RT, the patients with initial RE1 had significant inferior subsequent survival (univariate HRs median: 1.56; multivariable HRs median: 1.63; all P-values < 0.05). Patients with initial RE2 also had inferior subsequent survival (univariate HRs median: 1.7, P-values median: 0.04; multivariable HRs median: 1.75, P-values median: 0.04) than RE0. Initial RE2 in 1-month were not significant for inferior subsequent survival, likely due to the fact that less than 10% of patients had initial RE2 in 1-month.
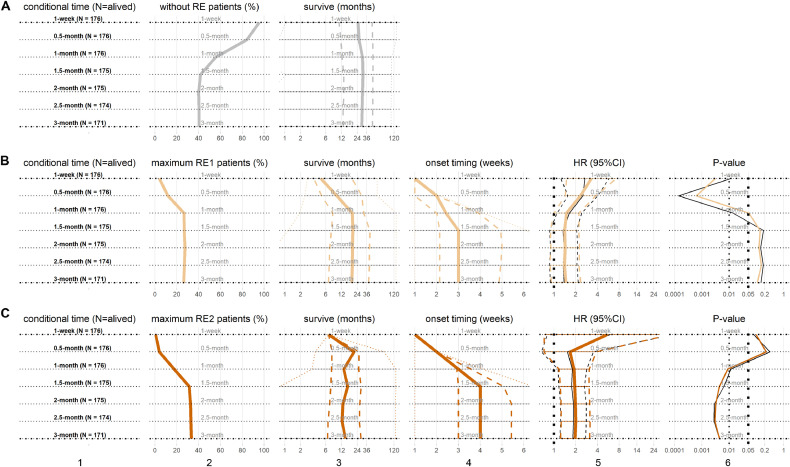
3.4. Maximum RE grade and conditional survival
As shown in Fig. 3, the profile of maximum RE at 1-week is similar to that of above initial RE, while some patients with RE grade 1 evolved to RE grade 2 later. Before the end of 3-month following RT initiation, total 45 (26.3%) and 57 (33.4%) patients had maximum RE1 and maximum RE2, respectively. The median onset timing of maximum RE1 or maximum RE2 were 3 weeks or 4 weeks, respectively; the corresponding median subsequent survivals were 18.7 months or 14.1 months respectively; meanwhile the median subsequent survival of patients with RE0 were 29.1 months.
Fig. 3.
The maximum grades of radiation esophagitis (RE) and conditional survival. From top row to bottom row, subplots are for patients at 7 time points (A) the RE0 as reference, (B) maximum RE1, and (C) maximum RE2. From left column to right column, subplots are (1) subgroups at each conditional time point with patient number, (2) patient percentages, (3) subsequent survivals in (median: solid, IQR: dash, range: dot), (4) onset timing of maximum RE1 and maximum RE2 in (median: solid, IQR: dash, range: dot), (5) the HRs of maximum RE1 and maximum RE2 in (median: solid, 95%CI: dash), including univariate HRs (black) and multivariable HRs (color), and (6) P-values of HRs, including univariate P-values (black) and multivariable P-values (color). The same horizon refers to the results at same conditional time points.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Conditional overall survival analyses incorporating the maximum grades of RE experienced were also performed as shown in Fig. 3. Comparing to RE0 patients, the patients with maximum RE1 had inferior subsequent survival (univariate HRs median: 1.39, P-values median: 0.14; multivariable HRs median: 1.47, P-values median: 0.1); similarly, the patients with maximum RE2 also did worse (univariate HRs median: 1.82, P-values median: 0.006; multivariable HRs median: 1.96, P-values median: 0.004). The maximum RE1 presented earlier than 1-month (early onset) were significant for subsequent survival comparing to all other patients; meanwhile after 1-month, the maximum RE2 were significant with subsequent survival comparing to RE0 patients.
3.5. RE evolution and conditional survival
In addition, analysis considering the effects of the evolution RE on subsequent survival were also performed by conditional survival method. As shown in Fig. 4, at 1-week after RT treatment initiation, total 8 (4.6%) patients had already experienced RE (RE progressed). At 0.5-month, 27 patients (15.3%) had developed RE or RE progressed to higher grades (RE progressed); and 1 patient (0.6%) had initial RE resolved. Because of dynamic RE and death, at the end of 3-month, there were 15 (8.8%) and 87 (50.9%) patients with RE progressed and RE resolved respectively, while the remaining 69 patients (40.3%) never experienced any RE.
Fig. 4.
The evolution of radiation esophagitis (RE) and subsequent survival. Cox regression models were used to estimate the hazard ratios (HRs). From top row to bottom row, subplots are for patients at 7 time points for (A) the RE0 as reference, (B) RE progressed, (C) RE resolved. From left column to right column, subplots are (1) subgroups at each conditional time point with patient number, (2) patient percentages, (3) subsequent survivals in (median: solid, IQR: dash, range: dot), (4) the newest onset timing of RE progressed and RE resolved in (median: solid, IQR: dash, range: dot), (5) the HRs of RE progressed and RE resolved in (median: solid, 95%CI: dash), including univariate HRs (black) and multivariable HRs (color), and (6) P-values of HRs, including univariate P-values (black) and multivariable P-values (color). The same horizon refers to the results at same conditional time points.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The median onset timing of RE progressed and RE resolved were 1 and 2.5 months, respectively; and the median subsequent survival were 9.2 months and 18.9 months, respectively. Meanwhile the median subsequent survival with RE0 as reference were longer as 29.1 months.
The conditional overall survival assessment examining the RE evolution was shown in Fig. 4. At all conditional time points, the patients with RE progressed had significant inferior subsequent survival (univariate HRs median: 2.16; multivariable HRs median: 2.08; all P-values < 0.05). The patients with RE resolved had borderline significant inferior subsequent survival (univariate HRs median: 1.51, P-values median: 0.08; multivariable HRs median: 1.73, P-values median: 0.06) than those with RE0.
3.6. RE onset timings are prognostics
The onset timings of RE events, including any RE and maximum RE as defined in Section 2.4, and their subsequent survivals are shown in Fig. 5(A) and 5(B), respectively. At 1-month following the initiation of RT, both initial RE-timing and maximum RE-timing were significant associated with subsequent survival under either univariate or multivariable analysis (all P-values: <0.05). The initial RE-timing had significant inferior subsequent survival (univariate HR: 1.16, P-value: 0.004; multivariable HR: 1.2, P-value: 0.001) at 1-week; (univariate HR: 1.05, P-value: 0.002; multivariable HR: 1.05, P-value: 0.004) at 0.5-month. The maximum RE-timing had the same results as initial RE-timing.
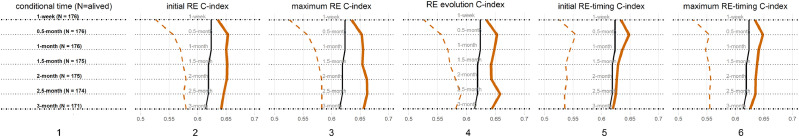
3.7. C-index
To understand if RE is an independent biomarker of survival, the comparison of C-index was applied. At all conditional time points, the multivariable Cox proportional-hazards models incorporating significant clinical variables with inclusion of initial RE grade had the discrimination power (C-indexes median: 0.65); maximum RE grade (C-indexes median: 0.64), RE evolution (C-indexes median: 0.64); initial RE-timing (C-indexes median: 0.63); or maximum RE-timing (C-indexes median: 0.64). These models improved C-indexes from the multivariable models with only significant clinical variables (C-indexes median: 0.62, all comparing P-values: <0.05) and greater than the univariate Cox regressions with only RE events (all comparing P-values: <0.01) (Fig. 6).
Fig. 6.
C-indexes of Radiation Esophagitis (RE) events. This figure shows the C-indexes of Cox regression models with (1) patients numbers at each of the 7 time points, (2) initial RE grade, (3) maximum RE grade, (4) RE evolution, (5) the onset timing of first RE event and (6) the timings of the maximum RE grades. From (2) to (6), C-indexes of multivariable of RE events incorporating with significant clinical variables (color), C-indexes of multivariable only with significant clinical variables (black), C-indexes of univariate models (dashed color). The same horizon refers to the results at same conditional time points.(For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This study, representing a comprehensive conditional survival assessment, demonstrated at first time that the presence of RE events, their dynamic (progression or resolution within 3 months) and onset timing were significantly associated with subsequent survival in the NSCLC patients treated with thoracic radiotherapy.
The method of conditional survival analysis used in this study is a dynamic study of subsequent survival. Conditional survival analysis has been implemented for several cancer sites including gastrointestinal, prostate, retroperitoneal sarcoma, kidney and the ovaries 18, 19, 20, 21, 22, 23, 24. The main clinical utility of conditional survival analysis is in counselling patients who have already survived a certain period of time after their treatment. In this study, we analyzed the RE toxicities which started and evolved after the initiation of RT and even continued after the end of RT by conditional survival assessments. The descriptive data on survival outcome are informative to the practice physicians for better patient counselling.
To the best of our knowledge, this is the first comprehensive study to systematically investigate the effect of RE on survival. Interestingly, this study demonstrated that (1) the presence of any RE grade was significant for inferior subsequent survival. (2) The survival impact of maximum RE grade was at least, if not more, important than initial RE grade as previously reported 25. (3) the patients with RE progressed had significant inferior subsequent survival than those who did not progress, while the RE resolved events were also significant for inferior survival than those never had RE. (4) The onset timing of acute RE in 1-month was significantly associated with inferior subsequent survival. One hypothesis is that early RE may present in those patients with higher intrinsic normal tissue radiosensitivity. Another hypothesis is that although the RE symptoms resolved from medical management, some of the normal tissue damage already induced may not be repaired completely, thus leading to worse additional survival.
In addition, all kinds of RE features were associated with overall survival. The predictive C-indexes of the multivariable Cox regression including initial RE grade and significant clinical variables were 0.65 (IRQ: 0.64 to 0.65), comparable to other models reported in the literatures. For example, Aerts et al.26 found that the four prognostic radiomic signatures computed from baseline CT scans of the primary tumor had a good performance of predicting OS in an independent validation cohort of stage I-III NSCLC patients (C-index, 0.65); while de Jong et al.27 using same model showed suboptimal C-index (C-index, 0.58) in their cohort.
Although it was less studied on the significant correlation between RE and survival, it was well accepted that RE was a side effector accompanied with radiation therapy. Some reasons behind the occurrence of RE have been studied. For example, Harder EM, et al.28 found that BED was a strong predictor of RE grade≥2 (P<0.05); Xu T, et al.29 found that high serum miR-155 and miR-221 during the first 2 weeks of treatment were associated with RE (miR-155: OR=1.53, 95% CI: 1.04–2.25, P = 0.03; miR-221: OR=2.07, 95% CI: 1.17–3.64, P = 0.012); Our group30 combined baseline level of IL-8 in plasma and esophagus generalized equivalent uniform dose to predict the RE grade≥2 (AUC=0.78). In this study, we also found KPS, cancer stage, N stage, with chemotherapy and whether SBRT are significant clinical characteristics with the development of RE while BED is not significant. It still need many studies to do to clarify the cause of RE and the correlations behind RE and survival in the future.
There are some limitations in this study. First, RE toxicities are symptoms of radiation damage to the esophagus which could have some correlation with damage of other adjacent organs that deserves further study. Secondly, because only a limited number of patients developed grade 3 or higher toxicities, these patients could not be analyzed separately. Thirdly, we did not consider the clinical characteristics like the position of tumor, histology and weight loss in this study because of their lots of missing data when the collection started more than ten years ago. Fourth, patients in this analysis were from four prospective trials with different radiation treatment strategies including patients treated conventional or dose escalated RT, and this study simplified the RT dose variable as BED in the multivariable Cox proportional hazards models. Lastly, this study emphasized the effect of RE on overall survival, did not intend to include all clinical significant factors like comorbidity to develop a comprehensive model for overall survival, which is a topic of our future study with large number of patients.
Overall, this study systematically quantified the effect size of RE on subsequence survival by estimating any RE, RE onset-timing and RE dynamics, and comparing their C-indexes performances by conditional survival assessment. Critical detailed information with regard to RE may be helpful for guiding subsequent patient care. While findings of this study warrant independent validation, these findings call for a more stratified dose constraint to limit toxicity in normal organs to avoid undue toxicity in radiotherapy for lung cancer. Investigations in a larger number of patients are urgently needed for independent validation and to evaluate the effects of RE events among survivors of lung cancer.
Declaration of Competing Interest
The authors declare that they have no conflict of interests.
Acknowledgement
This work was financially supported by Shenzhen Fundamental Research Program (JCYJ2020109150427184), Shenzhen Science and Technology Program (KQTD20180411185028798) and Shenzhen Fundamental Research Program (JCYJ20180508153249223). The funders had no role in the initiation or design of the study, collection of samples, analysis, interpretation of data, writing of the paper, or the submission for publication. The study and researchers are independent of the funders.
Footnotes
Given her role as Associate Editor, Feng-Ming (Spring) Kong had no involvement in the peer-review of this article and had no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Huan He.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jncc.2021.02.003.
Contributor Information
Hao Yu, Email: yuhao_zju@163.com.
Feng-Ming (Spring) Kong, Email: kong0001@hku.hk, fxk132@case.edu.
Appendix. Supplementary materials
References
- 1.Mehmood Q., Sun A., Becker N., et al. Predicting radiation esophagitis using 18F-FDG PET during chemoradiotherapy for locally advanced non-small cell lung cancer. J Thorac Oncol. 2016;11:213–221. doi: 10.1016/j.jtho.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Palma D.A., Senan S., Oberije C., et al. Predicting esophagitis after chemoradiation therapy for non-small cell lung cancer: an individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;87:690–696. doi: 10.1016/j.ijrobp.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Rose J., Rodrigues G., Yaremko B., et al. Systematic review of dose-volume parameters in the prediction of esophagitis in thoracic radiotherapy. Radiother Oncol. 2009;91:282–287. doi: 10.1016/j.radonc.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Werner-Wasik M., Yorke E., Deasy J., et al. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76:S86–S93. doi: 10.1016/j.ijrobp.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins P.G., Boonstra P.S., Hobson S.T., et al. Prediction of radiation esophagitis in non-small cell lung cancer using clinical factors, dosimetric parameters, and pretreatment cytokine levels. Transl Oncol. 2018;11:102–108. doi: 10.1016/j.tranon.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murro D., Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827–830. doi: 10.5858/arpa.2014-0111-RS. [DOI] [PubMed] [Google Scholar]
- 7.Kong F.M., Hayman J.A., Griffith K.A., et al. Final toxicity results of a radiation-dose escalation study in patients with non-small cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 8.Werner-Wasik M., Paulus R., Curran W.J.Jr, et al. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non–small-cell lung cancer: analysis of the Radiation Therapy Oncology Group (RTOG) database. Clin Lung Cancer. 2011;12:245–251. doi: 10.1016/j.cllc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Wang S., Campbell J., Stenmark M.H., et al. A model combining age, equivalent uniform dose and IL-8 may predict radiation esophagitis in patients with non-small cell lung cancer. Radiother Oncol. 2018;126:506–510. doi: 10.1016/j.radonc.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan S.T., Ellingrod V.L., Schipper M., et al. Genetic variations in TGFβ1, tPA and ACE and radiation induced thoracic toxicities in patients with non-small cell lung cancer. J Thorac Oncol. 2013;8:208–213. doi: 10.1097/JTO.0b013e318274592e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran W.J.Jr, Paulus R., Langer C.J., et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holgersson G., Bergström S., Liv P., et al. Effect of increased radiotoxicity on survival of patients with non-small cell lung cancer treated with curatively intended radiotherapy. Anticancer Res. 2015;35:5491–5497. [PubMed] [Google Scholar]
- 13.Haydu L.E., Scolyer R.A., Lo S., et al. Conditional survival: an assessment of the prognosis of patients at time points after initial diagnosis and treatment of locoregional melanoma metastasis. J Clin Oncol. 2017;35:1721–1729. doi: 10.1200/jco.2016.71.9393. [DOI] [PubMed] [Google Scholar]
- 14.Hieke S., Kelber M., Konig C., et al. Conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res. 2015;21:1530–1536. doi: 10.1158/1078-0432.Ccr-14-2154. [DOI] [PubMed] [Google Scholar]
- 15.Trotti A., Colevas A.D., Setser A., et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/s1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 16.F. Harrell. Regression Modeling Strategies: with Applications to Linear Models Logistic Regression, and Survival Analysis 1-571, 2001 New York, NY Springer Crossref, Google Scholar.
- 17.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and Environment for Statistical Computing.https://www.R-project.org/ URL. [Google Scholar]
- 18.Rowell N.P., O’Rourke N.P., et al. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2004;4:CD002140. doi: 10.1002/14651858.CD002140.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Choi M., Fuller C.D., Thomas C.R.Jr., et al. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109:203–209. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Zamboni B.A., Yothers G., Choi M., et al. Conditional survival and the choice of conditioning set for patients with colon cancer: an analysis of NSABP trials C-03 through C-07. J Clin Oncol. 2010;28:2544–2548. doi: 10.1200/jco.2009.23.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott A.M., Habermann E.B., Parsons H.M., et al. Prognosis for primary retroperitoneal sarcoma survivors: a conditional survival analysis. Cancer. 2012;118:3321–3329. doi: 10.1002/cncr.26665. [DOI] [PubMed] [Google Scholar]
- 22.Harshman L.C., Xie W., Bjarnason G.A., et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol. 2012;13:927–935. doi: 10.1016/s1470-2045(12)70285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karakiewicz P.I., Suardi N., Capitanio U., et al. Conditional survival predictions after nephrectomy for renal cell carcinoma. J Urol. 2009;182:2607–2612. doi: 10.1016/j.juro.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 24.Tan M.C., Butte J.M., Gonen M., et al. Prognostic significance of early recurrence: a conditional survival analysis in patients with resected colorectal liver metastasis. HPB (Oxford) 2013;15:803–813. doi: 10.1111/hpb.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Multidisciplinary Larynx Cancer Working Group Conditional survival analysis of patients with locally advanced laryngeal cancer: construction of a dynamic risk model and clinical nomogram. Sci Rep. 2017;7:43928. doi: 10.1038/srep43928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aerts H.J., Velazquez E.R., Leijenaar R.T., et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jong E.E.C., van Elmpt W., Rizzo S., et al. Applicability of a prognostic CT-based radiomic signature model trained on stage I-III non-small cell lung cancer in stage IV non-small cell lung cancer. Lung Cancer. 2018;124:6–11. doi: 10.1016/j.lungcan.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Harder E.M., Chen Z.J., Park H.S., et al. Dose-volume predictors of esophagitis after thoracic stereotactic body radiation therapy. Am J Clin Oncol. 2017;40:477–482. doi: 10.1097/coc.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 29.Xu T., Liao Z., O’Reilly M.S., et al. Serum inflammatory miRNAs predict radiation esophagitis in patients receiving definitive radiochemotherapy for non-small cell lung cancer. Radiother Oncol. 2014;113:379–384. doi: 10.1016/j.radonc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Wang S., Campbell J., Stenmark M.H., et al. A model combining age, equivalent uniform dose and IL-8 may predict radiation esophagitis in patients with non-small cell lung cancer. Radiother Oncol. 2018;126:506–510. doi: 10.1016/j.radonc.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.