Abstract
The sequenced gammaherpesviruses each contain a single viral bcl-2 homolog (v-bcl-2) which may encode a protein that functions in preventing the apoptotic death of virus-infected cells. Epstein-Barr virus (EBV), a gammaherpesvirus associated with several lymphoid and epithelial malignancies, encodes the v-Bcl-2 homolog BHRF1. In this report the previously uncharacterized BALF1 open reading frame in EBV is identified as having significant sequence similarity to other v-bcl-2 homologs and cellular bcl-2. Transfection of cells with a BALF1 cDNA conferred apoptosis resistance. Furthermore, a recombinant green fluorescent protein-BALF1 fusion protein suppressed apoptosis and associated with Bax and Bak. These results indicate that EBV encodes a second functional v-bcl-2.
Apoptosis is important in the elimination of malignant or virally infected cells through a genetic program of characteristic enzymatic and morphologic events (10, 12, 23, 40). Bcl-2 family members control tissue homeostasis and development via the programmed cell death process that is also known as apoptosis (reviewed in reference 40). The proto-oncogene bcl-2, which prevents apoptosis, was first identified by virtue of its overexpression in follicular lymphoma (41). bcl-2 knockout mice are lymphopenic, and their lymphoid cells undergo apoptosis at a much higher rate than in normal mice (18, 28, 42). Bax is a proapoptotic Bcl-2 family member that heterodimerizes with Bcl-2. Lymphoid hyperproliferation occurs in bax knockout mice (19). Thus, Bcl-2 family members are central to lymphoid homeostasis.
Gammaherpesviruses produce Bcl-2 protein homologs (v-Bcl-2s) (9, 13, 29, 33) that are hypothesized to contribute to immune evasion and to promote tumorigenesis by preventing apoptosis in response to either virus infection or cytotoxic immune effectors (reviewed by reference 40). Epstein-Barr virus (EBV) possesses a homolog of the Bcl-2 family, BHRF1, which suppresses apoptosis (13). BHRF1 is expressed primarily during lytic infection (11), is dispensable for lymphocyte transformation (24, 26), and is not expressed in posttransplantation lymphomas (27). These findings suggest that BHRF1 functions primarily to increase the life span of cells undergoing viral replication. Although it is not expressed in all EBV-associated malignancies (6, 27, 35), latent membrane protein 1 (LMP-1) of EBV has been shown to be required for EBV-induced lymphocyte transformation (4), and LMP-1 induces cellular Bcl-2 (14). In this report, we characterize a novel antiapoptotic v-Bcl-2 encoded by the EBV genome.
The BALF1 ORF predicts a novel v-Bcl-2.
A search for viral Bcl-2 homologs was conducted in sequenced viruses. Using FASTA (32) and the amino acid sequence of Bcl-xl as a search sequence, we determined that the E4 open reading frame (ORF) in equine herpesvirus 2 (38) encodes a protein with 20% identity to Bcl-xl and contains two Bcl-2 homology (BH) domains, BH1 and BH2, believed to be essential for the dimerization, heterodimerization, and function of the antiapoptotic protein Bcl-2 (43). A BLAST search to identify additional structural homologs (1) revealed a similar ORF in EBV. This reading frame, the BALF1 ORF, is 0.7 kb in size and predicts a 220-amino-acid protein in a region of early EBV transcripts (2).
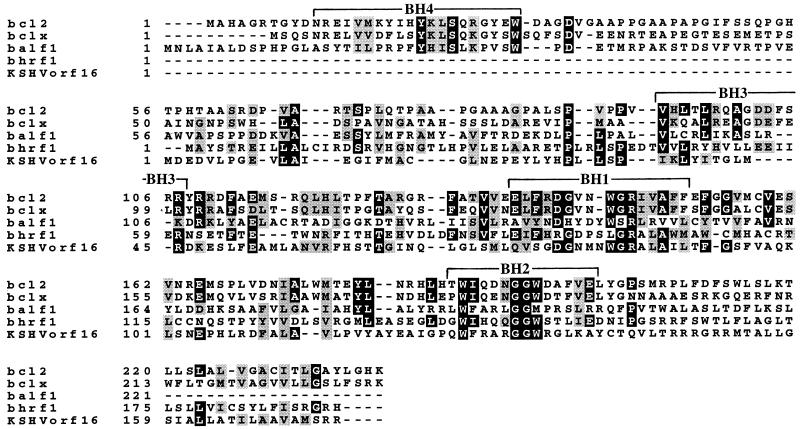
Analysis of BALF1 reveals several structural features that define it as a Bcl-2 family member. These features include sequence homology in the functionally important BH domains BH1 to BH4 (reviewed in reference 21). Interestingly, there is closer similarity between BALF1, Bcl-xl, and Bcl-2 than between the known EBV-encoded Bcl-2 homolog BHRF1 (13), Bcl-xl, and Bcl-2 (Fig. 1). A MAST (3) analysis based on motifs generated by both the Bcl-2 family members shown and the Caenorhabditis elegans Bcl-2 homolog, CED-9, demonstrated a 1-in-10 billion probability that the similarity between BALF1 and other Bcl-2s is due to chance.
FIG. 1.
Similarity of the EBV transcripts BALF1 and BHRF1 to Kaposi’s sarcoma-associated herpesvirus ORF16 (KSHVorf16) and the cellular genes bcl-x and bcl-2. Sequences were aligned via the PIMA multiple sequence alignment program, using the sequential branching clustering method (36). Areas of identity are shown in black; conserved regions are shown in gray (BOXSHADE, Isrec, Switzerland).
The predicted amino acid sequence of BALF1 reveals three unique features for a v-Bcl-2.
First, in BALF1 the BH1 domain glycine (position 149 in the alignment in Fig. 1) is replaced by serine, whereas in virtually all Bcl-2 family members there is a critical BH1 domain glycine (43). Mutation of the BH1 domain glycine in Bcl-2 or CED-9 to alanine abolishes antiapoptotic function (43). Second, whereas all other characterized gammaherpesvirus v-Bcl-2s possess hydrophobic C termini capable of insertion in organellar membranes, BALF1 lacks a hydrophobic C terminus, as do E1B and A179L, (5, 9, 13, 21, 22, 26, 29, 33). Finally, in contrast to the divergence in the BH4 domains of other v-Bcl-2s compared with human Bcl-2 (9), BALF1’s similarity to human Bcl-2 and Bcl-xl is conserved (Fig. 1). Thus, there are several structural features unique to BALF1, which is itself distinct among v-Bcl-2s (5, 9, 13, 21, 22, 26, 29, 33) in that BALF1 occurs in a virus that contains another Bcl-2 homolog.
BALF1 suppresses apoptosis.
To analyze whether BALF1 was a functional v-Bcl-2, vectors encoding green fluorescent protein (GFP) fusion proteins were transfected into HeLa cells. Briefly, pEGFP-BALF1 was constructed by PCR amplification of BALF1 DNA (from the BamHI A fragment of the B95-8 EBV genome) with the 5′-HindIII- and 3′-SacII-encoding primers 5′-CCCAAGCTTGGGATGAACCTGGCCATTGCT-3′ and 5′-TCCTCCCCGCGGCAAAGATTTCAG-3′, containing sequences from the 5′ and 3′ portions, respectively, of the BALF1 gene. pEGFP-bcl-xl was constructed by PCR amplification of pSFFV-bcl-xl DNA (4) with primers containing a 5′-SacI site (5′-GCAGCAGCAGAGCTCATGTCTCAGAGCAACCGG-3′) or a 3′-PstI site (5′-TGCTGCTGCCTGCAGTTTCCGACTGAAGAGTGA-3′) which also complemented the 5′ or 3′ bcl-xl cDNA of pSFFVbcl-xl. We ligated the digested PCR products into the appropriate sites in the pEGFP-N1 vectors, generating pEGFP-BALF1 and pEGFP-bcl-xl. This was followed by confirmatory sequencing of the two new plasmid inserts.
The resultant vectors pEGFP-N1, pEGFP-BALF1, and pEGFP-bcl-xl, encoding GFP, GFP-BALF1, and GFP–Bcl-xl, respectively, were transfected into HeLa cells by using a Bio-Rad GenePulser, set at 960 μF and 0.34 kV. HeLa transfectants were single-cell cloned in standard media (25), and clones possessing green fluorescence were expanded in G418 (400 μg/ml). Flow cytometric analysis was performed to indicate that the subclones had equivalent levels of fluorescence.
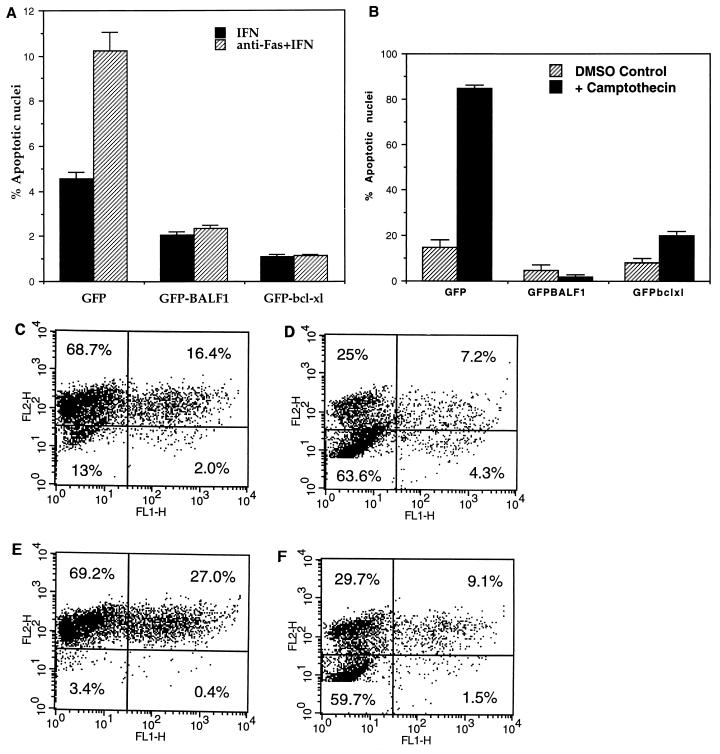
Apoptosis was induced in susceptible clones with the sensitizing agent gamma interferon (IFN-γ) and anti-Fas antibody, using modifications of published methods (25). Briefly, 100,000 cells/ml were incubated in triplicate overnight at 37°C in medium containing either IFN-γ (Endogen, Woburn, Mass.) at 20 ng/ml or IFN-γ plus anti-Fas antibody CH11 (400 ng/ml; MBL International, Nagoya, Japan). Control cells were treated with IFN-γ alone. Transfectants were evaluated for apoptosis by flow cytometric analysis of apoptotic subdiploid nuclei (30). After treatment with IFN-γ plus anti-Fas, the survival of Bcl-xl and BALF1 transfectants was increased (Fig. 2A) compared with that of GFP transfectants. This experiment demonstrates that BALF1 suppresses apoptosis when stably expressed in anti-Fas-treated HeLa cells. Similar results were obtained with IFN-γ plus anti-Fas when CMXRosamine staining (7) was used to visualize apoptosis (Fig. 2C shows an example of this method) and when tumor necrosis factor alpha plus cycloheximide was used to induce apoptosis (data not shown).
FIG. 2.
Inhibition of apoptosis by Bcl-xl and BALF1 in stable transfectants. (A) HeLa cells were transfected with vectors encoding GFP, GFP-BALF1 and GFP–Bcl-xl. GFP-positive transfectants were selected and then treated with IFN-γ and anti-Fas antibody or with control IFN-γ alone, using modifications of published methods (25). Transfectants were evaluated for apoptosis by flow cytometric analysis of apoptotic subdiploid nuclei (30). (B) GFP, GFP–Bcl-xl, and GFP-BALF1 clones were treated with 2.5 μM camptothecin in 2.5% DMSO. Controls were treated with 2.5% DMSO alone. The day after each series of treatments, cells were evaluated for apoptosis by flow cytometric analysis as described above. (C to F) HeLa cells were transiently cotransfected with pEGFPN1 (C and D) or with a plasmid encoding BALF1 cDNA, pcDNA3.1BALF1, and pEGFPN1 (at a pEGFPN1:BALF1 ratio of 1:2) (E and F). Following treatment with camptothecin (D and F) or DMSO (C and E) at the concentration used for panel B, the effect of BALF1 on apoptosis (E and F) was assessed by loss of fluorescence of CMXRosamine (FL2) in the GFP-cotransfected cells (FL1). Percentages indicate the percentage of cells gated in each quadrant.
In a second series of experiments (Fig. 2B), GFP, GFP–Bcl-xl, and GFP-BALF1 clones were treated with the topoisomerase inhibitor camptothecin at a final concentration of 2.5 μM in 2.5% dimethyl sulfoxide (DMSO). Controls were treated with 2.5% DMSO alone. As previously demonstrated, camptothecin induced significant levels of apoptotic cell death (16), as evidenced by quantitation of subdiploid nuclei (30) in the GFP clones but not in the GFP–Bcl-xl and GFP-BALF1 clones (Fig. 2B). These data (Fig. 2A and B) demonstrate that BALF1 is an antiapoptotic protein, using two independent methods of inducing apoptotic cell death.
To test BALF1’s function independently of the fusion protein moiety, HeLa cells were transiently cotransfected both with a plasmid encoding BALF1 cDNA, pcDNA3.1BALF1, as well as with a separate plasmid encoding GFP, pEGFN1 (at a pEGFN1:BALF1 ratio of 1:2, to be certain that only cells transfected with BALF1 were analyzed). Forty-eight hours later, transfectants were treated with camptothecin to induce apoptosis as described above. The transfectants were analyzed by staining with CMXRosamine to measure loss of mitochondrial integrity (7). Loss of CMXRosamine staining (FL2 in Fig. 2E and F) indicates a loss of mitochondrial integrity that is an early apoptotic event (7). Following treatment with camptothecin or DMSO as described in the legend to Fig. 2, the effect of BALF1 cotransfection on apoptosis was assessed by fluorescence of CMXRosamine in the GFP-cotransfected cells. Note that more than 50% of cells are in the lower left quadrant of each graph in Fig. 2D and F. These untransfected (GFP-negative) groups demonstrate a significant loss of mitochondrial integrity (a predecessor of apoptosis) following camptothecin treatment. A smaller percentage of EGFPN1:BALF1 cotransfectants (1.5%) than of the EGFPN1 control transfectants (4.3%) is apoptotic (Fig. 2F versus 2D). These results demonstrate that BALF1 functions independently of the GFP fusion moiety to prevent apoptosis and that BALF1 is an antiapoptotic protein, using two independent methods of detecting apoptotic cell death.
BALF1 and Bcl-xl coimmunoprecipitate with Bak and Bax.
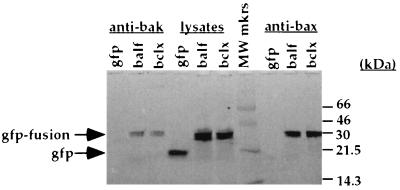
In most cases the antiapoptotic function of Bcl-2 family members is achieved through their heterodimerization with a proapoptotic Bcl-2 homolog such as Bak, Bax, Bik, or Bad (reviewed in reference 21). To determine whether BALF1 associates with Bak and Bax in HeLa cells, we performed coimmunoprecipitation with anti-Bax and anti-Bak antibodies (Fig. 3). Briefly, 107 cells per sample were washed in phosphate-buffered saline and lysed on ice for 1 h in 100 μl of a lysis buffer composed of 0.5% Triton X-100, 142 mM NaCl, 10 mM HEPES (pH 7.6), 5 mM MgCl2, and 1× Complete protease inhibitor cocktail (Boehringer Mannheim). Following centrifugation for 20 min at 14,000 rpm in an Eppendorf 5415C centrifuge at 4°C, lysates were precleared with 100 μl of 10% (wt/vol) protein A-Sepharose beads (Pharmacia) for 30 min at 4°C. Lysates were normalized for green fluorescence on a STORM FluorImager (Molecular Dynamics). Immunoprecipitation of GFP fusion proteins complexed to proapoptotic proteins was performed with anti-Bax (Santa Cruz Biotechnology) and anti-Bak (Pharmingen) polyclonal antibodies immobilized on protein A-Sepharose beads for 2 h at 4°C. Samples were washed three times and eluted from beads by incubation in 1% sodium dodecyl sulfate (SDS) 0.1 M Tris (pH 10.5) for 15 min at 37°C. Then the entire eluate was run on a 10% non-GFP-denaturing SDS-polyacrylamide gel, using a modification of previously described techniques (31). The gel was directly visualized (Fig. 3) on a STORM FluorImager with the photomultiplier tube set at 700 V in the blue fluorescence mode to determine if GFP was present.
FIG. 3.
BALF1 associates with Bak and Bax. HeLa cell transfected with GFP, GFP-BALF1, or GFP–Bcl-xl were lysed, immunoprecipitated with anti-Bax and anti-Bak, analyzed on a 4 to 20% non-GFP-denaturing SDS-polyacrylamide gel, and scanned on a FluorImager. A 33-kDa band (indicated by the arrow) in the GFP-BALF1 and GFP–Bcl-xl lanes and a 20-kDa band in the GFP lane were recognized, in accord with other studies (31). GFP-BALF1 or GFP–Bcl-xl was present in the eluate from the immunoprecipitation on an SDS–12% polyacrylamide gel when anti-Bak (lanes 2 and 3) or anti-Bax was used (lanes 9 and 10), while GFP was not (lanes 1 and 8). MW mkrs, molecular weight markers.
GFP migrates at 20 kDa in this non-GFP-denaturing gel, in accord with other studies (31). The predicted molecular size for GFP is 13 kDa higher than the observed molecular size due to the ability of GFP to resist denaturation and the resultant alteration in gel mobility (31). GFP (Fig. 3, lane 4) migrated faster than lysates from GFP-BALF1 or GFP–Bcl-xl (lanes 5 and 6). These data demonstrate that GFP migrates at the appropriate size and is seen in the lysate lane but not in the coimmunoprecipitate lanes. GFP-BALF1 or GFP–Bcl-xl was present in the eluate from the immunoprecipitation on an SDS–12% polyacrylamide gel when anti-Bax or anti-Bak was used (lanes 2, 3, 9, and 10), while GFP was not (lanes 1 and 8). This result indicates that coimmunoprecipitation by anti-Bak and anti-Bax is specific for GFP-BALF1 and GFP–Bcl-xl. Therefore, BALF1 associates with the proapoptotic proteins Bax and Bak, providing a mechanism for the antiapoptotic effect of BALF1.
BALF1’s structure and its location on a viral genome containing another v-Bcl-2 contrasts significantly with other v-Bcl-2s in four ways. First, BALF1 possesses a polymorphism (S149) at a position in its BH1 domain that contains a highly conserved glycine residue in other Bcl-2 family members (43). Mutations of this glycine dramatically alter interactions between Bcl-2 family members and CED-4 (37), which is a homolog of the Apaf-1 human activator of caspases (44). The C. elegans Bcl-2 homolog, CED-9, gains antiapoptotic function, resulting in an accumulation of anterior pharyngeal cells when its BH1 domain glycine is mutated to an aspartate (15). Second, amino acid sequence analysis indicates (Fig. 1) that BALF1, like Bcl-2 and Bcl-xl, may possess a BH4 domain. This domain is hypothesized to be important for the functions of many Bcl-2 family members (34), including part of their antiapoptotic activity. The lack of conservation of the BH4 domain in BHRF1 (and indeed in the v-Bcl-2s of other viruses [9]) suggests that BALF1 may have a function distinct from that of BHRF1. As is true for other v-Bcl-2s, the putative BH4 domain of BALF1 lacks a DXXD motif suggested to be important in the regulation of Bcl-2 (8) by caspases. Third, the sequenced gammaherpesviruses contain a single ORF which encodes Bcl-2 family members. Why is EBV apparently unique in having two bcl-2-homologous ORFs? Since no v-FLICE-inhibitory proteins are found in the EBV genome, perhaps BALF1 compensates for the missing antiapoptotic effect of these proteins, which are expressed in other gammaherpesviruses (39). Finally, BALF1 is present on the EBV genome with another v-bcl-2 gene, BHRF1, which is expressed in cells during lytic infection. On the basis of the known expression of BHRF1, we hypothesize that the v-Bcl-2s BALF1 and BHRF1 may sometimes act at different stages in the viral life cycle.
Suppression of apoptosis appears necessary for the completion of several viral life cycles. Deletion of baculovirus p35 (17), and possibly African swine fever virus v-Bcl-2 (5), abrogates viral replication. One hypothesized mechanism to explain this observation is that apoptosis aborts the viral life cycle (17). Antiapoptotic genes from several other viruses are important to viral pathogenesis in vitro (5, 17), and the EBV v-Bcl-2, BHRF1, is hypothesized to be important in facilitating cell survival during lytic replication in vivo (14). Other studies demonstrate that EBV infection, but not BHRF1, LMP-1, or EBNA1, confers a malignant phenotype and apoptosis resistance, suggesting that EBV encodes another antiapoptotic protein (20). Since BALF1 is an antiapoptotic v-Bcl-2, our data suggest that by associating with proapoptotic proteins, BALF1 could interrupt the apoptotic pathway for elimination of EBV-infected cells.
Acknowledgments
W. Marshall and C. Yim contributed equally to this work.
We are grateful to Justine Milligan and Maris Handley for assistance with flow cytometry, Paul Morrison and Christine Bogle for sequencing, Junko Kato for help with the graphics, and Rakesh Datta for helpful comments on procedures and the manuscript and for generously providing reagents such as pSFFVbcl-xl.
This work was supported by grants from the NIH (AI28691-06 and NIH P30 CA 06516-33 to R.W.F.; NIH RO1 DE12186 to J.D.F.; P30-CA06516-33 and AI01078-05 to W.L.M.) and from the Novartis Drug Discovery Program to R.W.F.
REFERENCES
- 1.Altschul S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Baer R, Bankier A, Biggin M, Deininger P, Farrell P, Gibson T, Hatfull G, Hudson G, Satchwell S, Seguin C, Tuffnell P, Barrell B. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 3.Bailey T, Elkan C. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology. Menlo Park, Calif: AAAI Press; 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers; pp. 28–36. [PubMed] [Google Scholar]
- 4.Boise L, Gonzalez-Garcia M, Postema C, Ding L, Lindsten T, Turka L, Mao X, Nunez G, Thompson C. Bcl-x, a bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 5.Brun A, Rivas C, Esteban M, Escribano J, Alonso C. African swine fever virus gene A179L, a viral homologue of bcl-2, protects cells from programmed cell death. Virology. 1996;225:227–230. doi: 10.1006/viro.1996.0592. [DOI] [PubMed] [Google Scholar]
- 6.Carbone A, Tirelli U, Golghini A, Volpe R, Boicchi M. Human immunodeficiency virus-associated systematic lymphomas may be subdivided into two main groups according to Epstein-Barr viral latent gene expression. J Clin Oncol. 1993;11:1674–1681. doi: 10.1200/JCO.1993.11.9.1674. [DOI] [PubMed] [Google Scholar]
- 7.Castedo M, Hirsch T, Susin S A, Zamzami N, Marchetti P, Macho A, Kroemer G. Sequential acquisition of mitochondrial and plasma membrane alterations during early lymphocyte apoptosis. J Immunol. 1996;157:512–521. [PubMed] [Google Scholar]
- 8.Cheng E, Kirsch D, Clem R, Ravi R, Kastan M, Bedi A, Ueno K, Hardwick J. Conversion of bcl-2 to a bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 9.Cheng E, Nicholas J, Bellows D, Hayward G, Guo H, Reitz M, Hardwick J. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen G. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrell P J. Epstein-Barr genome. In: Klein G, editor. Advances in viral oncology; tumorigenic DNA viruses. Vol. 8. New York, N.Y: Raven Press, Ltd.; 1989. pp. 103–132. [Google Scholar]
- 12.Ghayur T, Hugunin M, Talanian R, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharabanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci USA. 1993;90:8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 15.Hengartner M, Horvitz H. Activation of C. elegans cell death protein CED-9 by an amino-acid substitution in a domain conserved in bcl-2. Nature. 1994;369:318–320. doi: 10.1038/369318a0. [DOI] [PubMed] [Google Scholar]
- 16.Hennequin C, Giocanti N, Balosso J, Favaudon V. Interaction of ionizing radiation with the topoisomerase I poison Camptothecin in growing V-79 and HeLa cells. Cancer Res. 1994;54:1720–1728. [PubMed] [Google Scholar]
- 17.Hershberger P, LaCount D, Friesen P. The apoptotic suppressor p35 is required early during baculovirus replication and is targeted to the cytosol of infected cells. J Virol. 1994;68:3467–3477. doi: 10.1128/jvi.68.6.3467-3477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 19.Knudson C, Tung K, Tourtellotte W, Brown G, Korsmeyer S J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 20.Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt’s lymphoma cell line Akata. J Virol. 1998;72:9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroemer G. The proto-oncogene bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 22.Lakshmi R, Debbas M, Sabbatini, Hockenberry D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Berenstein A. Apoptosis, cancer, and the p53 tumor suppressor gene. Cancer Metastasis Rev. 1995;14:149–161. doi: 10.1007/BF00665797. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Yates J. BHRF1 of Epstein-Barr virus, which is homologous to human proto-oncogene bcl2, is not essential for transformation of B cells or for virus replication in vitro. J Virol. 1992;66:1899–1906. doi: 10.1128/jvi.66.4.1899-1906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandal M, Maggirwar S, Sharma N, Kaufmann S, Sun S, Kumar R. Bcl-2 prevents CD95 (Fas/APO-1)-induced degradation of lamin B and poly(ADP-ribose) polymerase and restores the NF-kappaB signaling pathway. J Biol Chem. 1996;271:30354–30354. doi: 10.1074/jbc.271.48.30354. [DOI] [PubMed] [Google Scholar]
- 26.Marchini A, Tomkinson B, Cohen J, Kieff E. BHRF1, the Epstein-Barr virus gene with homology to bcl-2, is dispensable for B-lymphocyte transformation and virus replication. J Virol. 1991;65:5991–6000. doi: 10.1128/jvi.65.11.5991-6000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray P, Swinnen L, Constandinou C, Pyle J, Carr T, Hardwick J, Ambinder R. Bcl-2 but not its EBV-encoded homologue, BHRF1, is commonly expressed in postransplantation lymphoproliferative disorders. Blood. 1996;87:706–711. [PubMed] [Google Scholar]
- 28.Nakayama K, Negishi I, Kuida K, Sawa H, Loh D. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nava V, Cheng E, Veliuona M, Zou S, Clem R, Mayer M, Hardwick J. Herpesvirus saimiri encodes a functional homolog of the human bcl-2 oncogene. J Virol. 1997;71:4118–4122. doi: 10.1128/jvi.71.5.4118-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicoletti I, Migliorati G, Pagliacc M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 31.Park S-Y, Raines R. Green fluorescent protein as a signal for protein-protein interactions. Protein Sci. 1997;6:2344–2349. doi: 10.1002/pro.5560061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson W, Lipman D. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarid R, Sato T, Bohenzky R, Russo J, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 34.Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 35.Shibata M, Wiess L, Hernandez A, Nathwani B, Bernstein L, Levine A. Epstein-Barr virus-associated non-Hodgkin’s lymphoma in patients with the human immunodeficiency virus. Blood. 1993;81:2102–2109. [PubMed] [Google Scholar]
- 36.Smith R, Smith T F. Pattern-induced multi-sequence alignment algorithm employing secondary structure-dependent gap penalties for use in comparative protein modeling. Protein Eng. 1992;5:35–41. doi: 10.1093/protein/5.1.35. [DOI] [PubMed] [Google Scholar]
- 37.Spector M, Desnoyers S, Hoeppner D, Hengartner M. Interaction between the C. elegans cell-death regulators CED-9 and CED-4. Nature. 1994;385:653–656. doi: 10.1038/385653a0. [DOI] [PubMed] [Google Scholar]
- 38.Telford E, Watson M, Aird H, Perry J, Davison A. The DNA sequence of equine herpesvirus 2. J Mol Biol. 1995;249:520–528. doi: 10.1006/jmbi.1995.0314. [DOI] [PubMed] [Google Scholar]
- 39.Thome M, Schneider P, Hofman K, Fickenscher H, Meini E, Neipel F, Mattman C, Burns K, Bodmer J-L, Schroter M, Scaffidi C, Krammer P, Peter M, Tschopp J. Viral FLICE-inhibitory proteins (FLIPS) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 40.Thompson C. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 41.Tsujimoto Y, Finger L, Yunis J, Nowell P, Croce C. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;266:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 42.Veis D, Sorenson C, Shutter J, Korsmeyer S. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 43.Yin X-M, Oltvai Z, Korsmeyer S. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 44.Zou H, Henzel W, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]