Abstract
B cell malignancies pose challenges due to therapeutic resistance and repeated relapse. Advances in adoptive cellular therapies including chimeric antigen receptor (CAR)-T cells have the potential to transform the treatment landscape in hematological and solid tumor cancers. Improvements in constructs of CAR-T have improved specificity in targeting malignant cells. Multiple clinical trials have demonstrated the efficacy of CAR-T and other cellular treatments. In spite of advances in cellular therapies, hurdles in managing toxicities and lingering resistance remain. This review aims to summarize current innovations in adoptive cellular therapies and introduces future paths of discovery that will enhance these therapies in the era of precision oncology.
Keywords: Chimeric antigen receptor, Adoptive cell therapy, B cell malignancies
1. Introduction
The natural course of B cell malignancies is a repetitive process of remission and relapse. During this course of therapeutic pressure, mutations continue to accumulate causing progressive increases in drug resistance which leads to rapidly deteriorating efficacy until t we lose the patient to B cell malignancies. In certain B cell malignancies such as large cell lymphoma, 60% of patients achieve long term remission, but the other 40% of large cell lymphomas behave like the majority of B cell malignancy with the debilitating natural course described above. Therapies for B cell malignancies have evolved from chemotherapy to chemo-free targeted therapies, to immunotherapies and now to genetic cellular therapies. We have now learned that chemotherapies, targeted therapies and immunotherapies can only cure a small fraction of B cell malignancies. When B cell malignancies become resistant to multiple therapies, the risk of mortality is very high and the patient's survival is usually less than a year1, 2, 3. The “magic” of chimeric antigen receptor (CAR)-T cell therapy is that it can rescue patients from high risk terminal B cell malignancies, rendering a portion of them into long term survivors with prolonged remissions. More than ever, this has excited scientists, clinicians and translational researchers. Although these therapies induce long term remissions in some patients, many patients relapse after CAR-T cell therapy4 or suffer severe toxicities and adverse events5, suggesting that there is still space for innovation.
This review will focus on cellular therapies in B cell malignancies, where most advances in cellular therapy have occurred. We must know and we should understand while we advance in cellular therapies, we continue to grow other therapy types including less toxic more efficient chemotherapies, targeted therapies and immunotherapies. We'll also continue to explore the best ways to integrate all these therapeutic modalities for the best clinical outcome for our patients with B cell malignancies. Since many excellent reviews on cellular therapies exist, we shall focus on critical points and new ideas.
2. CAR-T design and strategies for utilization
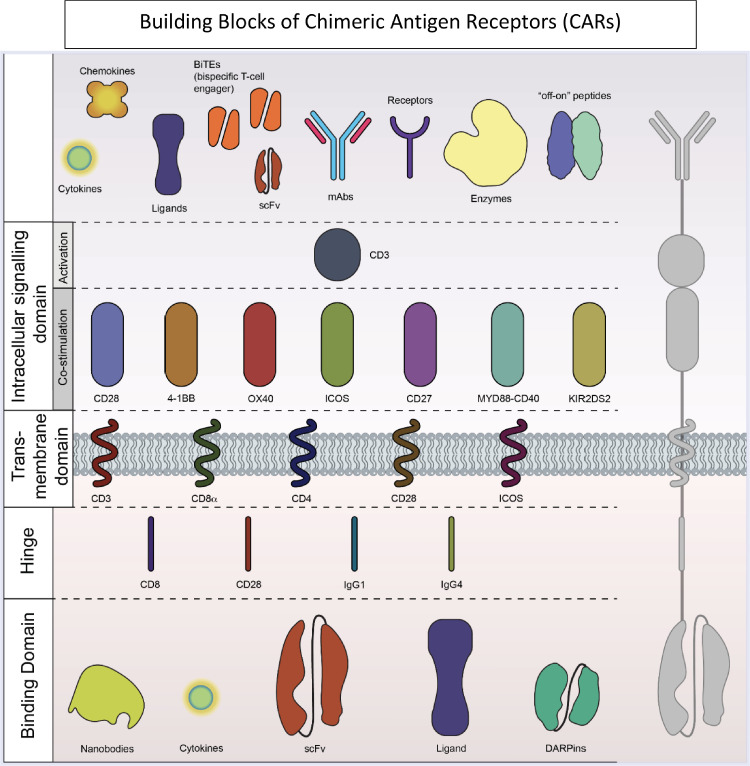
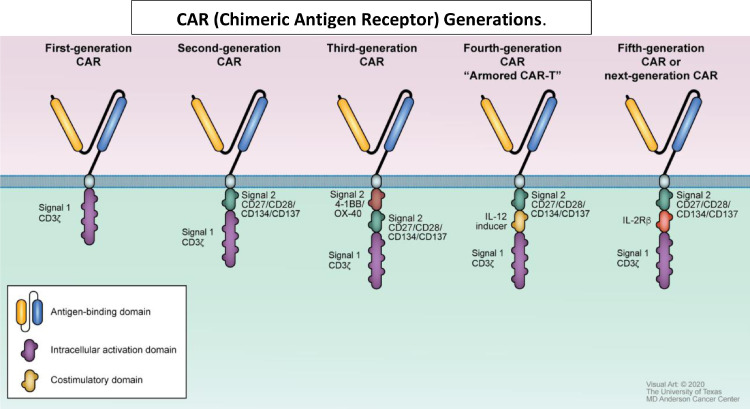
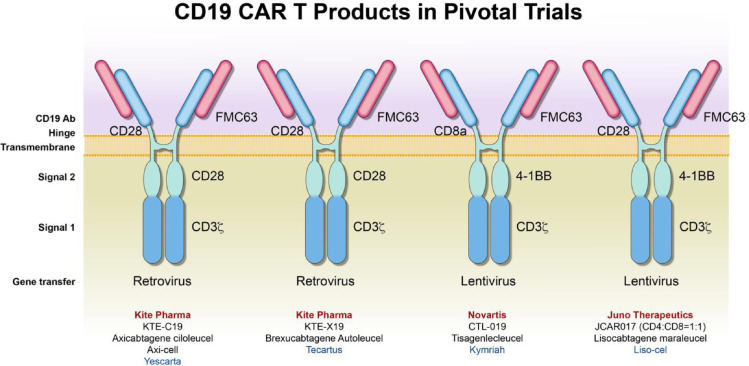
Many building blocks are used to compose the CAR-T construct and include signaling domains and antigen recognition domains (Fig. 1). To date, five generations of CAR-T designs have been reported (Fig. 2). The first generation of CAR-T cells was a simple design with an extracellular antigen recognition unit and one intracellular CD3 delta functional unit. Although some patients achieved remissions in clinical trials with the first-generation CAR-T cells, a substantial percentage of patients had severe toxicities correlated with serum inflammatory cytokine levels.6 Second generation CAR-T cells have improved upon the original construct. This generation included costimulatory molecules, either CD28 or 4-1BB. All the clinically-approved CAR-T therapies belong to the second generation of construct designs 7, 8, 9, 10, 11, 12, 13, 14. CAR-T products used in clinical trials leading to these approved therapies are represented in Fig. 3. Clinical trials are ongoing with third generation CAR-T products that have 2 costimulatory molecules, and may have both CD28 and 4-1BB. The third generation has not improved efficacy and toxicity thus far. 12,13 Fourth and fifth generation CARs attempt to overcome barriers related to the tumor microenvironment15 and reduce off target immune responses which increases the risk of toxicities16. These advances may allow CAR-T therapy to be used to target solid tumors and may also improve treatment of hematological malignancies. Constructs improving on third generation CARs are still in preclinical stages of validation.
Fig. 1.
Building Blocks of Chimeric Antigen Receptors. CARs contain a binding domain and an intracellular signaling domain, and can be modified to target specific antigens using co-stimulatory domains. Additional components may include “off-on” switches, additional receptors, mAbs and BiTEs.
Fig. 2.
CAR (Chimeric Antigen Receptor) Generations. First generation CARs. consist of an antigen-binding domain and an intracellular activation domain.6 The design of second-generation CARs improved by adding a costimulatory domain that can be specific for CD 27, 28, 134 or 137.14,33 Third-generation CARs added a 2nd costimulatory domain increasing targeting capability.12,13,18 “Armored” or fourth-generation CARs, sometimes referred to as TRUCKs include an IL-12 inducer that allows autocrine stimulation and enhances cytotoxic effector functions of the T-cells; these allow the CAR-T cells to overcome a hostile tumor microenvironment.15,82 Fifth-generation CARs or “next-generation” CARs may contain costimulatory molecules that may produce various specific cytokines and localize treatment to a tumor; IL-2R is shown in this example, but other inducers may be used (scFv to block PD-1 for example); this approach is thought to improve the toxicity profile of CAR-T therapy.16
Fig. 3.
CAR-T Products in Pivotal Trials Leading to Clinical Use Approval. Varied CAR constructs were used in clinical trials for hematological malignancies. These products have been approved for clinical application. Although axicabtagene clioleucel (KTE-C19) and brexucabatgene autoleucel (KTE-X19) are similar constructs with CD28 costimulatory signals, their manufacturing procedure differs: KTE C19 utilizes T-cell selection and lymphocyte enrichment. Both KTE-C19 and KTE-X19 use retroviral gene transfer. Tisagenlecleucel (CTL-019) and lisocabtagene maraleucel (JCAR017) utilize 4-1BB costimulatory signals and a lentiviral gene transfer. These two products differ by hinges: CD8a and CD28 respectively.
Simultaneous infusion of a second-generation anti-CD19 CAR-T cells (containing the CD28 domain) and third-generation CAR-T cells (containing both the CD28 and 4-1BB domains) in a phase I trial of 16 non-Hodgkin lymphoma (NHL) patients revealed that the third-generation designs had superior expansion and persistence 17. This was confirmed in previous in vitro and in vivo studies by the Ramos group, demonstrating the benefit of adding the 4-1BB kinetic to CD28 containing CD19-targeted T cells. The same study, reported the inclusion of 4-1BB in addition to CD28 is associated with superior expansion and persistence which can lead to significant clinical responses, including sustained complete responses (CRs), in patients with relapsed or resistant NHL. 17 Other reports, however were less optimistic when measuring the robustness and efficacy of these constructs; however, they reported excellent toxicity profiles 13,18.
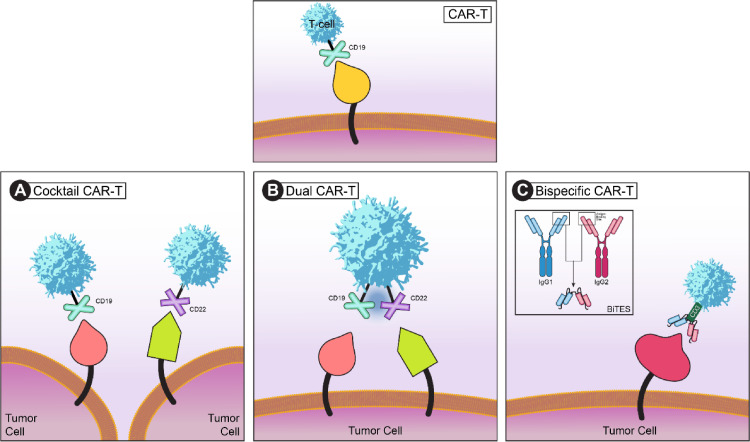
Different modalities of incorporating CAR-T therapies have been proposed (Fig. 4). Cocktail CAR-T employs a combination of CAR-T cells that have two or more different targets (i.e. CD19 and CD22 in acute lymphoblastic leukemia)19. Bispecific CARs are engineered to prevent tumor resistance due to a loss in targeted antigens, such as CD19, expressed on the surface of malignant B cells. CARs expressing single-chain variable fragments (scFvs) that are specific for two different antigens on a single-chain are defined as bispecific CARs, also known as “tandem CARs.” Like the single-chain bispecific “OR-gate” CAR, dual-CARs and CAR-pool are alternative strategies to fulfill bispecific targeting. Dual CARs co-express two full-length receptors specific for cognate antigens with two individual signals on the same single cells 20. For example, two CARs can target CD19 and CD22 respectively.21 Cocktail-CARs or CAR-pools simply combine two or more single-inputs of CAR-T cell products in the “cocktail”. Cocktail-CARs involve the sequential administration of two different antigen-targeting CAR-T cell populations and has been used in clinical trials 22. In a recent study, CD19 CAR-Tcells were administered in pediatric ALL patients followed by an infusion of CD22 CAR-T. All 20 study participants achieved remission defined by negative minimal residual disease (MRD).23
Fig. 4.
Cocktail, dual, and bispecific CAR-T cell structures. A normal CAR-T cell structure is depicted in the top panel, with CD19 as the target. (A) Cocktail CAR-T cell is used when a combination of CAR-T cells with two or more different targets are needed for therapy (i.e. targeting both CD19 and CD22 simultaneously in acute lymphoblastic leukemia). (B) When CAR-T cells are made to express two tumor-associated antigen receptors simultaneously, a dual CAR-T cell therapy is used. This reduces the possibility of the T cells attacking non-tumor cells. (C) In bispecific CAR-T cells (BiTES), bispecific molecules adapted from two antibodies targets both an antigen and CD3 on the surface of the T cells. By doing so ensures that the T cells cannot be activated unless they are in close proximity to the tumor cell.
Bispecific CARs or CAR pools are engineered to prevent antigen escape and improve the efficacy of CAR-T therapy. With expert design and optimization, these bispecific receptors can serve as an “OR-gate” CARs that enable T cells to effectively target tumor cells that express either antigen A or antigen B, thus tumor cells can only escape T cell detection when both antigens are lost.24 Interestingly, among the bispecific approaches, the OR-gate CAR approach appears to be the most effective. To overcome the limitation of CD19 CAR-T therapy in CD-19 negative relapse, an investigation led by the Dai group demonstrated that bispecific CD19/CD22 CAR-T cells can trigger robust cytolytic activity against target cells. Complete remission with no residual disease was reported in all 6 patients25.
Although CD19 CAR-T cell therapy is clinically effective against relapsed/refractory (R/R) B cell lymphomas, this efficacy may be improved by combining the CD19 construct with additional immunotherapies such as programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) inhibitors.26 CARs may also be engineered to include a PD-1 receptor to target diverse immunosuppressive pathways. Liu et al. generated a novel anti-CD19 CAR expressing PD-1/CD28 chimeric switch-receptor (CD19-PD-1/CD28-CAR). Following a phase 1b study evaluating the safety and efficacy of CD19-PD-1/CD28-CAR-T cells in the treatment of PD-L1+ B cell lymphoma, safety and efficacy was demonstrated in early trial analyses providing proof of principle for this unique therapy.27
A study conducted by Shah et al., addressed CD19 downregulation mediated relapse through a first-in-human trial that used bispecific, non-cryopreserved anti-CD20, anti-CD19 (LV20.19) manufactured using the CliniMACS Prodigy device at a dose of 2.5 × 106 cells per kg for R/R B cell NHL and chronic lymphocytic leukemia (CLL) 28. Lower rates of toxicity were reported using this manufacturing process. Grade 3–4 cytokine release syndrome (CRS) occurred in one (5%) patient, and grade 3–4 neurotoxicity (NT) occurred in three (14%) patients. Eighteen (82%) patients achieved an overall response at day 28, while 14 (64%) had a complete response (CR), and 4 (18%) had a partial response (PR). The overall response rate to the dose of 2.5 × 106 cells per kg with non-cryopreserved infusion (n = 12) was 100% (CR, 92%; PR, 8%). Given the low toxicity and high efficacy of the bispecific CAR described in this study, this modality could become a potential method of improving clinical response by mitigating target antigen downregulation as a mechanism of relapse (NCT03019055).
Lymphodepleting therapy could enhance CAR-T cell responses by eradication of T regulatory cells, elimination of other immune cells that consume homeostatic cytokines.29,30 It is very important to note the role of conditioning therapies in the majority of CAR-T cell trials, which is essential to the efficacy of any CAR construct.31 This was evidenced in the recent ZUMA 2 and 3 trials which employed conditioning chemotherapy.10,32
3. Clinical studies of CAR-T therapies in hematological malignancies
Several CAR-T products have been studied and approved for clinical use. These studies and approvals are summarized in Table 1.
Table 1.
FDA approved CAR-T Cell therapies
| CAR Product | Lead Investigator(s) | Trial | Year | Major Findings | |
|---|---|---|---|---|---|
| ALL | |||||
| Tisagenlecleucel (tisa-cell) | Maude and Grupp | ELIANA33 | 2017 | ORR 81%, EFS 73%, OS 90% | |
| DLBCL | |||||
| Axicabtagene Clioleucel (axicell) | Locke and Neelapu | ZUMA 183 | 2017 | ORR 83%, CR 58% | |
| Tisagenlecleucel (tisa-cell) | Schuster | JULIET8 | 2019 | ORR 52%, CR 40% | |
| Lisocabtagene Maraleucel (liso-cel) | Abramson | TRANSCEND-NHL11 | 2021 | ORR 73% CR 53% | |
| FL | |||||
| Axicabtagene Clioleucel (axicell) | Jacobson and Salles | ZUMA-538 | 2021 | ORR 91%, CR 74% | |
| MCL | |||||
| Brexcabtagene Autoleucel | Wang | ZUMA 210 | 2000 | ORR 93%, CR 67% |
Abbreviation: ALL, acute lymphoblastic leukemia; CR, complete response; DLBCL, diffuse large B cell lymphoma; EFS, event-free survival; FL, follicular lymphoma; MCL, mantle cell lymphoma; ORR, overall response rate; OS, overall survival.
3.1. Acute lymphoblastic leukemia (ALL)
CAR-T products are studied and used extensively in acute lymphoblastic leukemia (ALL). The first Food and Drug Administration (FDA) approval for CAR-T therapy was tisagenlecleulcel (Kymriah) for pediatric (up to 25 years) relapsed or refractory ALL in 2017. Both the safety and efficacy of this therapy were demonstrated in a multicenter clinical trial that consisted of 75 pediatric and young adult ALL patients. 81% achieved remission within 3 months of the treatment and were also MRD negative when tested via flow cytometry. However, the side effects of CAR-T can be extreme, which include CRS, neurological effects, acute kidney injury, and hypoxia. A single infusion of tisagenlecleucel gave durable remission and persisted in a long-term manner among ALL patients 33.
A hurdle in wide utilization of CAR-T in ALL is the long manufacturing time, which may range from 2 to 3 weeks. In a recent clinical trial, a “FasT CAR” requires only one day to transduce T cells. In FasT CAR trials the response rate in ALL is high with 20/20 responding to therapy and an objective response rate (ORR) of 100%.34 However, without consolidation chemotherapy or transplantation with only CAR-T cells, median overall survival (OS) in ALL is only about 13 months 35. More strategies and studies are needed to further improve the outcome. Currently, consolidative stem cell transplantation, either auto or allo, should be used to consolidate the CAR-T cell response for long-term remissions. 33
3.2. Diffuse large B cell lymphoma (DLBCL)
DLBCL has been the most studied cancer type in CAR-T cell therapy. Three CD-19 CAR products are approved for clinical use in DLBCL: axicabtagene clioleucel (axicell, Yescarta)9, tisagenlecleucel (tisa-cell, Kymirah)8 and lisocabtagene maraleucel (liso-cel, Breyanzi). 36
The TRANSCEND trial includes 268 patients with various types of R/R large B cell lymphoma. These include patients with DLBCL, primary mediastinal B cell lymphoma, and follicular lymphomas. The TRANSCEND trial is an open label, multicenter, phase I study 36. Results published in September 2020 were promising. An objective response was achieved by 186 (73%, 95% CI: 66.8-78.0) patients and a CR was seen in 136 patients (53%, 95% CI: 46.8-59.4). Adverse events of grade 3 or higher were neutropenia (60%), anemia (37%), thrombocytopenia (27%), whereas CRS occurred in 113 patients.
3.3. Mantle cell lymphoma (MCL)
On July 24, 2020, during the COVID-19 pandemic, the FDA approved Brexucabatagene Autoleucel for MCL. The enrollment for ZUMA-1, Axi-cel and the ZUMA-2 trials, both started in 2015. However, with MCL being a rare disease and far fewer cases, an FDA approval of Brexucabatagene Autoleucel fell behind the FDA approval of Axi-cel by 3 years. During these 3 years, while many large cell lymphoma patients enjoyed the Axi-cel and Liso-cel therapeutic benefits, many MCL patients continued to die. Juno CAR-T cell has also developed a cohort for MCL FDA approval. The initial result was reported at American Society of Clinical Oncology (ASCO) in 2019. The follow-up results presented at ASCO in 2020 showed promising results with a tolerable toxicity profile. ORR was 78%, whereas 44% had serious treatment-emergent adverse events (TEAEs). 56% had grade 3/4 TEAEs, primarily anemia, neutropenia, and hypophosphatemia. 33% had CRS, all of which were grade 1 37, this will be most likely the second product approved for MCL. Please note that the ZUMA-2 used CD28 while Juno used 41BB as co-stimulatory molecule in the construct design.
Brexucabtagene autoleucel (Tecartus) is the first CAR-T cell therapy approved for patients with R/R MCL. The FDA granted this approval in 2020 following the results from the clinical trial, Zuma 2. Zuma 2 assessed brexucabtagene autoleucel in 60 patients who had received up to 5 prior MCL therapies. 93% responded following a single infusion, with 67% showing CR status. This trial showed CAR-T cell therapy's ability of overcoming prior treatment resistant MCL.10
3.4. Follicular lymphoma (FL)
CAR-T therapy was approved in FL in 2021 after the successful ZUMA-5 trials. Investigators reported 91% ORR and 74% CR. 38
3.5. Hodgkin's lymphoma (HL)
Two studies have examined the efficacy of CAR-T therapy in HL. These studies utilized a CD30 CAR-T product (ATLCAR). The first enrolled 18 patients; at 6 months 7 patients achieved PR, and progression free survival (PFS) was also 6 months.39 The second study enrolled 41 patients and reported an ORR of 62%, CR of 51%, PFS of 36% and a 1-year OS of 94%.40 More studies are needed to determine if CAR-T is an effective treatment in HL.
4. CAR-T cells and combination therapy in B cell malignancies
Lenalidomide, acalabrutinib, ibrutinib and checkpoint inhibitor have all been reported to enhance CAR-T cell function and expansion. A caveat is that while the enhanced CAR-T cell expansion may increase function, it can also increase toxicity. To increase CAR-T treatment efficacy in multiple myeloma in vivo, CS1 specific CAR-T cells were transduced and expanded in the presence of lenalidomide. When compared to mice that received CAR-T cell treatment without lenalidomide, mice that received the combination were found to have enhanced immune functions of the CAR-T cells, which include cytotoxicity, Th1 cytokine production, and immune synapse formation. 41 In CLL, a defect in the formation of an immunological formation between T and CLL cells is present. Lenalidomide has been shown to reverse this defect in vitro when administered as a single agent, which is the underlying reasoning behind the aim of combining it in a low-dose fashion with CAR-T cell therapy. Once both treatments were injected into a patient derived xenograft (PDX) murine model, it resulted in an improved survival when compared to mice that were injected with lenalidomide as a single agent (P<0.03). Thus, adding low doses of lenalidomide alongside CAR-T cell therapy in CLL was shown to give positive and improved outcomes 42. Studies in both cell lines and PDX models also occurred in MCL, where ibrutinib was used with anti-CD19 CAR-T cell therapy. Targeting and killing of MCL cells were enhanced when ibrutinib was added to CAR-T cells and this was further supported in the PDX models as mice only receiving CAR-T cell as a monotherapy eventually relapsed. Overall, 80-100% mice injected with both treatments remained in long-term remission compared to 0-20% of mice with CAR-T cell only (P<0.05). In addition to lenalidomide, ibrutinib was shown to be an effective adjuvant therapy option as well 43 In a more recent study, the functionality of the anti-CD19 CAR of lisocabtagene maraleucel (liso-cel) in combination with ibrutinib or acalabrutinib was assessed both in vitro and in vivo. RNA-seq showed that only ibrutinib resulted in CAR-T cells gene expression change that was consistent with a memory-like, Th1, and Bruton's Tyrosine Kinase (BTK) phenotype. However, both BTK inhibitors improved tumor clearance and increased survival when combined with CAR-T cells 44.
Recent clinical data shows improved efficacy when combining CAR-T and ibrutinib in CLL. In a pilot study of 19 R/R patients, ORR was 83% and 61% of patients achieved negative MRD status.45
4.1. Toxicity management
Toxicities associated with CAR-T therapies can be apparent in multiple organ systems, but typically include CRS, NT and B cell aplasia. Currently, we rarely lose a patient to CRS or NT in experienced major academic centers thanks to insights learned from previous CAR-T studies 46. With more cases treated and longer follow up, we have gradually discovered that B cell aplasia related immunocompromised host status including leukopenia and hypogammaglobinemia could lead to fatal infections and sometimes could kill patients in more numbers than CRS and NT 46. Cardiovascular complications could also hinder therapeutic outcome by resulting in unacceptable morbidity and mortality. In a retrospective analysis of 145 adult patients undergoing treatment with CAR-T cell therapy, 31 patients reported major adverse cardiovascular events. These included 22 heart failure events in 21 patients (15%), 12 episodes of atrial fibrillation in 11 patients (7.5%), 2 events of other arrhythmias (supraventricular tachycardia, non-sustained ventricular tachycardia), 2 episodes of acute coronary syndrome, and 2 cardiac deaths.47
CRS is typically treated with Tocilizumab which is a humanized monoclonal antibody against the IL-6 receptor that has been FDA approved and has been very effective11. Siltuximab (CNTO 328) is an IL-6 antagonist, a chimeric monoclonal antibody against the IL-6 protein molecule which binds directly to the cytokine and could also diminish CRS symptoms.48 The IL-1 antibody, Anakinra which is a drug used to treat rheumatoid arthritis and is a modified version of IL-1 has also been effective to help control CRS49,50.
Steroids are a common treatment modality in the management of toxicities and adverse events associated with CAR-T cell therapy although their usage remains controversial and must be weighed for each patient individually 51. A recent study examined the impact of steroid usage on clinical outcomes in patients with R/R large B cell lymphoma who were being treated with commercial anti-CD 19 CAR-T cell therapy. 100 patients were studied, 60 of whom received steroids for various CAR-T cell therapy adverse events. The median dose given was 186 mg (8-1803 mg) with a median duration of 9 days (1-30 days). Steroids were started between day 0 and day 7 in the majority of patients (75%). After a median follow-up of 10 months (95% CI: 8-12 months), use of higher cumulative dose of corticosteroids was associated with significantly shorter PFS. The results from this study suggest that steroid use in CAR-T cell therapy patients should be administered at the lowest dose for short duration. Additionally, the use of steroids should be delayed whilst managing CAR-T cell associated toxicities. 52 There is one report recently, showing that early use of high-dose steroids could hinder the function of CAR-T cells compromising efficacy 53,54.
At the American Society of Hematology (ASH) in 2019, we reported one case where a patient with R/R MCL undergoing treatment with anti-CD19 CAR-T cell therapy, developed CAR-T cell therapy-associated grade 4 cerebral edema with concomitant CRS which resolved following a multimodality clinical intervention including anti-thymocyte globulin (ATG) administration. Ventriculostomy was initiated rapidly with dramatic successful recovery. Although difficult to conclude whether ATG administration was solely responsible for the amelioration of symptoms, from the timeline of events it suggests that ATG contributed to the patient's improvement in neurological symptoms while also dampening CAR-T levels in the blood. Pharmacokinetic and pharmacodynamic results indicated that ATG might have contributed to the resolution of cerebral edema, along with other clinical interventions, including corticosteroids, IL-6 or IL-6 receptor blockade with siltuximab or tocilizumab, and a ventriculostomy.55
Subgroups of patients treated with CD19 CAR-T cells display neurological events following infusion which includes fatal cerebral edema. Using single cell RNA sequencing, Parker et al., found that brain mural cells express CD19, which is critical for the blood brain barrier integrity 56. They suggested a possible on-target mechanism for CD19 CAR-T cell-mediated NT, which may be caused by the previously unrecognized expression of CD19 in mural cells in the human brain. Such studies depict the importance of developing a comprehensive human single-cell atlas. Certain cell types might be missed in measurements of bulk tissue due to their low frequency, but which may be critically important and have clinical repercussions for targeted therapy. This will become even more crucial with future generations of CAR-T cells which may distinguish between combination of target antigens in order to improve specificity.
Safety mechanisms to halt toxicities such as “suicide or safety switches” have been proposed. The incorporation of safety-switches with genes such as caspase-9 and truncated epidermal growth factor are under investigation for the improvement of patient safety and the management of life-threatening toxicities. 57 In a phase 1 and 2 trial of CAR-natural killer (NK) cells, a caspase-9 safety switch was included in the construct design. However, given a lack of serious CRS, NT (NT) or hemophagocytic lymphocytosis the caspase-9 safety switch was not activated 58.
4.2. Predictive biomarkers and clinical factors of efficacy and toxicity
Vitale and Strati report baseline high tumor burden is indicated by either an elevated lactate dehydrogenase (LDH) level or high total metabolic tumor volume measured by position emission tomography (PET) scans.53 This has been shown to be associated with a lower ORR in a correlative analysis study in tisagenleclecucel-treated R/R DLBCL patients in the Juliet trial 59. In ZUMA-1, outcomes were also associated with the baseline high tumor burden. 60
Early steroids may also play a role in toxicity and response. ZUMA-1 found that early steroid use for patients who had grade 1 NT and CRS had a lower incidence of toxicity at the median follow-up; however, liberal use of steroids is not encouraged as it has a potential negative impact on CAR-T cells efficacy 54.
Cytokines may be predictive of toxicity and efficacy. The progression of CRS can be generalized into 4 stages: 1) CAR-T cell local expansion stage, 2) CAR-T cell overflow and inflammatory cytokine surge stage, 3) CAR-T cell redistribution and organ damage stage and 4) Recovery stage (immune reconstruction). The first stage occurs between days 0-5 post CAR-T infusion, where sustained intra-tumoral expansion CAR-T cells are retained within tumor mass while a few other cells recirculate in the peripheral blood (PB). Activated CAR-T cells release cytokines in a large surge, followed by a local inflammatory response. In a range of days 3-12, the second stage occurs where both CAR-T cells and cytokines enter the circulatory system. There's a rapid increase in CAR-T cells and IL-6 in the PB. 61
When evaluating relapse following treatment with CD19, it is important to evaluate tumor relapse due to mutation in CD19 which can reduce the efficacy of CAR-T cell therapy. Zhang et al., analyzed CAR-T and CD19+ B cells from peripheral blood or bone marrow using flow cytometry 62. Genomic sequencing was conducted to identify the molecular characteristics of CAR-T and CD19+ B cells from both pre-relapse and post relapse samples. CD19 CARs comprising scFv from antibody clones (e.g. FMC63 or 21D4) were constructed. Their analysis showed that FMC63 CAR-T cells exhibited limited cytotoxic efficacy in patients with CD19 mutations, leading to relapse after the CAR-T cell therapy. Conversely, 21D4 CAR-T cells could exert cytotoxic effect on B cells with CD19 mutations both in vitro and in vivo, which could be a potential alternative strategy for treatment for lymphoma patients with CD19 mutations. This paper is a clear example of how point mutation can both facilitate CAR-T relapse and also effectively eradicate mutated B cells allowing for an individualized treatment approach for patients with relapsed lymphoma.
Correlative analyses on CAR-T cell therapy and responses are limited and have mainly focused on toxicity and immune programs associated with CAR-T cell therapy. There is a lack of data on mechanisms of treatment resistance, including target antigen loss seen in a subset of responding patients. A recent study was initiated to analyze biomarker data from ZUMA-1 in a statistical analysis plan for correlations of durable response and parameters differentially associated with efficacy and toxicities. Through univariable and multivariable analyses, it was found that rapid CAR-T cell expansion is correlated with pretreatment tumor burden, the number of CD8 and CCR7 CD45RA T cells infused, and host systemic inflammation63.
Another correlative study in a modified intent to treat (mITT) population from ZUMA-1 yielded similar rates of overall response (OR) and toxicities from the trial in 122 patients regardless of eligibility for the study. CR and duration of response (DOR) were higher in those enrolled in ZUMA-1 64. This also revealed that bridging therapy (rendering some ineligible for the trial) may be a useful strategy in CAR-T since OR and toxicities are relatively the same. Several markers were also collected to assess response and T cell expansion.
Cellular characteristics of the CAR-T product themselves may be implicated in variable efficacy and toxicity. Green et al., conducted a study to determine why responses to CAR-T cell therapy are so varied and diverse65. They performed single-cell RNA sequencing of axicabtagene clioleucel products from 24 patients with large B cell lymphoma. The results from sequencing were correlated with clinical responses at 3 months. 50% progressive disease (PD), 38 % CR and one patient died before the 3-month evaluation
From their analysis of the cell types in the infusion they found central memory CD8+ T cells to be more abundant in patients who achieved complete remission status. Conversely, patients whose infusion products had a large quantity of exhausted CD8+ and CD4+ T cells were more likely to experience PD or achieve only partial remission status.
CAR-T cells’ impact on side effects were also examined. They evaluated the cell characteristics of patients who developed grade 3 or 4 immune effector cell-associated neurotoxicity syndrome (ICANS). They found their infusions to contain numerous cells with gene expression pattern characteristic of monocytes which produced cytokines such as IL-6 promoting ICANS.
Future research will be conducted to identify the nature of the cells in the CAR-T cell preparations. Directions as such have the potential for making CAR-T cells more effective. Implementing ctDNA may prove beneficial for choosing personalized CAR-T treatments for patients.
5. Novel cellular therapies
5.1. CAR-NK cells
NK cells have recently been modified to express an anti-CD19 CAR which have the potential to overcome the toxicity and adverse events associated with CAR-T cell therapy. Ongoing CAR NK trials are summarized in Table 2. Phase 1 and 2 trials were conducted to evaluate the use of anti-CD19 CAR NK cells in patients with R/R NHL or CLL.66 NK cells were transduced with a retroviral vector expressing genes that encode anti-CD19 CAR, interleukin-15, and inducible caspase 9 as a safety switch. The cells were expanded ex vivo and administered in a single infusion at one of three doses (1 × 105, 1 × 106, or 1 × 107 CAR-NK cells per kilogram of body weight) after lymphodepleting chemotherapy.
Table 2.
CAR NK cell therapies for hematological malignancies under investigation
| Disease | Target | Source | Principal Investigator/location | Phase | Clinical Trial Identifier |
|---|---|---|---|---|---|
| MM | BCMA | NK-92 | Department of Hematology, Wuxi People's Hospital, Nanjing Medical University Wuxi, Jiangsu, China, 214000 | Phase I/II | NCT03940833 |
| ALL, CLL, NHL | CD19 | CD NK cells | University of Texas MD Anderson Cancer Center, Houston, Texas, United States | Phase I/II | NCT03056339 |
| Refractory B Cell Lymphoma | CD22 | Unknown | Allife Medical Science and Technology, Beijing, China | Early Phase I | NCT03692767 |
| Refractory B Cell Lymphoma | CD19 | Unknown | Allife Medical Science and Technology, Beijing, China | Early Phase I | NCT03690310 |
| Refractory B Cell Lymphoma | CD19/CD22 | Unknown | Allife Medical Science and Technology, Beijing, China | Early Phase I | NCT03824964 |
| B Cell Lymphoma, CLL | CD19 | iPSC | Wayne Chu, MD Fate Therapeutics San Diego Ca | Phase I | NCT04245722 |
Abbreviation: ALL, acute lymphocytic leukemia; CD, cord derived; CLL, chronic lymphocytic leukemia; iPSC,induced pluripotent stem cell; MM, multiple myeloma; NHL, non-Hodgkin lymphoma.
Promising results were found with the administration of CAR-NK cells. There was no association with the development of CRS, NT or graft versus host disease (GVHD). Additionally, there were no increases in the levels of inflammatory cytokines when compared to baseline levels. 73% had a response; of which 7 (4 with lymphoma and 3 with CLL) had a complete remission.
5.2. Cancer specific T cell receptor (TCR)
Adoptive transfer of T cells genetically modified to express a cancer-specific TCR has shown remarkable therapeutic potential for both hematological and solid tumors. While CAR-based adoptive cell therapies are already showing great promise, a key constraint of most CARs is that conventional CAR-T cells recognize antigens expressed on the cell surface, limiting potential for using any intracellular tumor proteins as targets 67 . TCRs have the advantage of targeting any peptide including those derived from intracellular proteins processed through proteasome degradation 68. Unlike CAR-T cells that recognize proteins expressed on the surface, TCRs can recognize all types of tumor-specific proteins processed into peptides and presented on major histocompatibility complex MHC molecules, including intracellular proteins that remarkably increases the number of potential peptide targets. A unique TCR expressed on each T cell enables the cell to scan for antigens presented on MHC molecules on the tumor cell surface. These tumor antigens can be divided into two categories: non-mutated common antigens, including tissue-specific or cancer-testis (CT) antigens that are aberrantly expressed in cancerous cells and patient unique neoantigens resulting from non-synonymous somatic mutations within the cancerous cells.69 TCRs are engineered to recognize a tumor-specific peptide/MHC combination,70 leading to TCR-T therapy which has the advantage to recognize most of tumor antigens as they are able to recognize both extracellular and intracellular antigens.71 To generate TCR-engineered T cells, one or more tumor antigens are to be first identified as therapeutic targets. Genes encoding TCRs can be isolated from high avidity T cells that recognize cancer antigens, and then introduced into patient or donor-derived T cells, using genetic engineering techniques. Alternatively, TCRs from these patient's tumor infiltrating cell (TIL) could be transferred into autologous peripheral blood T cells with a younger phenotype and administered as treatment. TCR-T cells that specifically recognize tumor antigens are expanded in vitro and then reinfused to patients to kill tumor cells.72
A recent report on TCR T clinical trials showed that affinity improved TCR T delivered 80% clinical responses in patients with advanced myeloma 73,74. The remarkable capability of TCR-T has also been illustrated by the complete cure of a metastatic melanoma patient with NY-ESO-1 specific CD4+ T cells isolated directly from the same patient 75. Numerous trials have shown objective clinical responses, targeting the melanocyte differentiation antigen, MART-1/Melan-A.76 Clinical trials using TCRs for adoptive T cell therapy have had some successes in eradicating both solid and hematological tumors, which target AHNAK and ERBB2 in melanoma (via the Sleeping Beauty transposon system)77, p53 in various cancers 78, MDM2 in CLL. 79
TCR-T therapy is now being developed at an accelerating speed, led by NCI, Medigene, Zeopharm Oncology (Sleeping Beauty TCR-T Cell Therapy), PACT Pharm, Juno Therapeutics, and Genentech, among many others. Future innovations in genomics, genetic engineering, and cell manufacturing will make individualized TCR‐based therapies targeting multiple private neoantigens and dramatically enhance clinical efficacy of adoptively transferred T cells expressing TCRs.
5.3. FT596
In 2019, a clinical-stage biopharmaceutical company dedicated to the development of programmed cellular immunotherapies for cancer and immune disorders, announced new in vivo preclinical data for FT596, its off-the-shelf, multi-antigen targeting NK cell product candidate derived from a clonal master engineered induced pluripotent stem cell (iPSC) line. Data for the FT596 were presented at 61st ASH meeting and exposition. The data highlighted the potential of next generation cancer immunotherapies to overcome the current limitations of patient specific CAR-T cell therapy. Currently CAR-T cell therapies are programmed to recognize only one antigen and patients often succumb to relapse due to antigen escape mechanisms.80 To date, FT596 is the only cellular immunotherapy engineered with three active anti-tumor components under clinical investigation by the FDA.
5.4. Dominant negative CAR-T cell construct
Many groups have reported upregulation of PD-L1 and cytotoxic T-lymphocyte-associated antigen (CTLA) thereby inhibiting CAR-T cell function by accelerating the exhaustion of the CAR-T cells. This is especially relevant in targeting solid tumors. Dominant negative TGF- receptors in new constructs could enhance CAR-T proliferation, increase cytokine activity and induce solid tumor eradication.81 This approach could also prevent exhaustion of transduced CAR-T cells and reduce induced regulatory T cell (Treg). This phenomenon has been observed in patient derived xenografts (PDX) and cell-line derived xenografts (CDX)82. While this technique is priming CAR-T products for solid tumor targets, better understanding the reaction of the tumor microenvironment, especially the activation of immune and stromal cells, may improve CAR-T in B cell malignancies.
5.5. The future of genetic cellular products
In our current cellular therapy era, numerous sophisticated constructs have been generated to increase efficacy, decrease toxicity, and prolong the duration of efficacy. Three major categories of constructs are being made and emerging in publications. Category 1 is to modify extracellular CAR-T protein product. Category 2 is to modify the intracellular portion. Category 3 is to improve the microenvironment for the CAR-T cells. All categories of construct innovations could enhance and improve current available adoptive cellular products for B cell malignancies.
Additionally, the molecular precision medicine era is dawning when multi-omics and biological mechanistic studies may reveal drivers leading to drug resistance in tumors and individual patients. We could then attack this “Achilles' heel” of tumor resistance with personalized molecular precision therapies knocking out the driver drug resistance mechanism. Precision approaches combined with novel cellular therapies have enormous potential in curing the now incurable.
Cellular therapy has rapidly accelerated the curative fraction of patients of B cell malignancies. Our generation enjoys the best and most advanced science technology in the history of mankind. Therefore, it is both our fortune and responsibility to take advantage of available scientific breakthroughs and translate them into clinical outcomes that benefit patients.
Declaration of conflict interest
The authors declare that they have no conflict of interests.
Acknowledgements
Illustrated figures were provided by David Aten from The University of Texas MD Anderson Cancer Center - Medical Illustration
References
- 1.Jain P, Kanagal-Shamanna R, Zhang S, et al. Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol. 2018;183(4):578–587. doi: 10.1111/bjh.15567. [DOI] [PubMed] [Google Scholar]
- 2.Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–1563. doi: 10.1182/blood-2015-10-673145. [DOI] [PubMed] [Google Scholar]
- 3.Epperla N, Hamadani M, Cashen AF, et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma—a “real world” study. Hematol Oncol. 2017;35(4):528–535. doi: 10.1002/hon.2380. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Sun Q, Liang X, et al. Mechanisms of Relapse After CD19 CAR T-Cell Therapy for Acute Lymphoblastic Leukemia and Its Prevention and Treatment Strategies. Front Immunol. 2019;10:2664. doi: 10.3389/fimmu.2019.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain P, Zhang S, Kanagal-Shamanna R, et al. Genomic profiles and clinical outcomes of de novo blastoid/pleomorphic MCL are distinct from those of transformed MCL. Blood Adv. 2020;4(6):1038–1050. doi: 10.1182/bloodadvances.2019001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10(5):267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL, Pulsipher MA, Boyer MW, et al. Efficacy and Safety of CTL019 in the First US Phase II Multicenter Trial in Pediatric Relapsed/Refractory Acute Lymphoblastic Leukemia: Results of an Interim Analysis. Blood. 2016;128(22) 2801-2801. [Google Scholar]
- 8.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 9.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramson JS, Palomba ML, Gordon LI, et al. Pivotal Safety and Efficacy Results from Transcend NHL 001, a Multicenter Phase 1 Study of Lisocabtagene Maraleucel (liso-cel) in Relapsed/Refractory (R/R) Large B Cell Lymphomas. Blood. 2019;134(Supplement_1):241. [Google Scholar]
- 12.George P, Dasyam N, Giunti G, et al. Third-generation anti-CD19 chimeric antigen receptor T-cells incorporating a TLR2 domain for relapsed or refractory B-cell lymphoma: a phase I clinical trial protocol (ENABLE) BMJ Open. 2020;10(2):e034629. doi: 10.1136/bmjopen-2019-034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enblad G, Karlsson H, Gammelgård G, et al. A Phase I/IIa Trial Using CD19-Targeted Third-Generation CAR T Cells for Lymphoma and Leukemia. Clin Cancer Res. 2018;24(24):6185–6194. doi: 10.1158/1078-0432.CCR-18-0426. [DOI] [PubMed] [Google Scholar]
- 14.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 2021;20(7):531–550. doi: 10.1038/s41573-021-00189-2. [DOI] [PubMed] [Google Scholar]
- 16.Yu S, Yi M, Qin S, et al. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer. 2019;18(1):125. doi: 10.1186/s12943-019-1057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos CA, Rouce R, Robertson CS, et al. In Vivo Fate and Activity of Second- versus Third-Generation CD19-Specific CAR-T Cells in B Cell Non-Hodgkin's Lymphomas. Mol Ther. 2018;26(12):2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert M-L, Schmitt A, Neuber B, et al. Third-Generation CAR T Cells Targeting CD19 Are Associated with an Excellent Safety Profile and Might Improve Persistence of CAR T Cells in Treated Patients. Blood. 2019;134(Supplement_1):51. [Google Scholar]
- 19.Wang N, Hu X, Cao W, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood. 2020;135(1):17–27. doi: 10.1182/blood.2019000017. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Jiang P, Zhang X, et al. Anti-CD19/CD22 Dual CAR-T Therapy for Refractory and Relapsed B-Cell Acute Lymphoblastic Leukemia. Blood. 2019;134(Supplement_1) 284-284. [Google Scholar]
- 21.Zhang E, Yang P, Gu J, et al. Recombination of a dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy. J Hematol Oncol. 2018;11(1) doi: 10.1186/s13045-018-0646-9. 102-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han X, Wang Y, Wei J, et al. Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy. J Hematol Oncol. 2019;12(1):128. doi: 10.1186/s13045-019-0813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan J, Zuo S, Deng B, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. 2020;135(5):387–391. doi: 10.1182/blood.2019003293. [DOI] [PubMed] [Google Scholar]
- 24.Zah E, Lin MY, Silva-Benedict A, et al. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res. 2016;4(6):498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai H, Wu Z, Jia H, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):30. doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao Y, Lu W, Sun R, et al. Anti-CD19 Chimeric Antigen Receptor T Cells in Combination With Nivolumab Are Safe and Effective Against Relapsed/Refractory B-Cell Non-hodgkin Lymphoma. Front Oncol. 2019;9:767. doi: 10.3389/fonc.2019.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Lei W, Zhang C, et al. A phase I trial using CD19 CAR-T expressing PD-1/CD28 chimeric switch-receptor for refractory or relapsed B-cell lymphoma. J Clin Oncol. 2019;37(15_suppl):7557. [Google Scholar]
- 28.Shah NN, Johnson BD, Schneider D, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–1575. doi: 10.1038/s41591-020-1081-3. [DOI] [PubMed] [Google Scholar]
- 29.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corrigan-Curay J, Kiem HP, Baltimore D, et al. T-cell immunotherapy: looking forward. Mol Ther. 2014;22(9):1564–1574. doi: 10.1038/mt.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neelapu SS. CAR-T efficacy: is conditioning the key? Blood. 2019;133(17):1799–1800. doi: 10.1182/blood-2019-03-900928. [DOI] [PubMed] [Google Scholar]
- 32.Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491–502. doi: 10.1016/S0140-6736(21)01222-8. [DOI] [PubMed] [Google Scholar]
- 33.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, He J, Zhang X, et al. A Feasibility and Safety Study of a New CD19-Directed Fast CAR-T Therapy for Refractory and Relapsed B Cell Acute Lymphoblastic Leukemia. Blood. 2019;134(Supplement_1) 825-825. [Google Scholar]
- 35.Park JH, Rivière I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Gordon LI, Palomba ML, et al. Safety and preliminary efficacy in patients (pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL) receiving lisocabtagene maraleucel (Liso-cel) in TRANSCEND NHL 001. J Clin Oncol. 2019;37(15_suppl):7516. [Google Scholar]
- 38.Jacobson CA, Chavez JC, Sehgal AR, et al. Interim analysis of ZUMA-5: A phase II study of axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed/refractory indolent non-Hodgkin lymphoma (R/R iNHL) J Clin Oncol. 2020;38(15_suppl):8008. [Google Scholar]
- 39.Wang CM, Wu ZQ, Wang Y, et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin Cancer Res. 2017;23(5):1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 40.Ramos CA, Grover NS, Beaven AW, et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J Clin Oncol. 2020;38(32):3794–3804. doi: 10.1200/JCO.20.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Walter M, Urak R, et al. Lenalidomide Enhances the Function of CS1 Chimeric Antigen Receptor–Redirected T Cells Against Multiple Myeloma. Clin Cancer Res. 2018;24(1):106–119. doi: 10.1158/1078-0432.CCR-17-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertilaccio M, Tettamanti S, Attianese GG, et al. Combining CD23 chimeric antigen receptor immunotherapy and lenalidomide as a novel therapeutic strategy for chronic lymphocytic leukemia. Cytotherapy. 2014;16(4):S35. [Google Scholar]
- 43.Ruella M, Kenderian SS, Shestova O, et al. The Addition of the BTK Inhibitor Ibrutinib to Anti-CD19 Chimeric Antigen Receptor T Cells (CART19) Improves Responses against Mantle Cell Lymphoma. Clin Cancer Res. 2016;22(11):2684–2696. doi: 10.1158/1078-0432.CCR-15-1527. [DOI] [PubMed] [Google Scholar]
- 44.Qin JS, Johnstone TG, Baturevych A, et al. Antitumor Potency of an Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy, Lisocabtagene Maraleucel in Combination With Ibrutinib or Acalabrutinib. J Immunother. 2020;43(4):107–120. doi: 10.1097/CJI.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gauthier J, Hirayama AV, Purushe J, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650–1660. doi: 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lefebvre B, Kang Y, Frey N, et al. 16207: Cardiovascular Effects of Chimeric Antigen Receptor T-Cell (CART Cell) Therapy in Adults. Circulation. 2019;140(Suppl_1) A16207-A16207. [Google Scholar]
- 48.Chen F, Teachey DT, Pequignot E, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. doi: 10.1016/j.jim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta P, Cron RQ, Hartwell J, et al. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2(6):e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy–associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–3127. doi: 10.1182/bloodadvances.2020002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobson CA. CD19 Chimeric Antigen Receptor Therapy for Refractory Aggressive B-Cell Lymphoma. J Clin Oncol. 2018;37(4):328–335. doi: 10.1200/JCO.18.01457. [DOI] [PubMed] [Google Scholar]
- 52.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitale C, Strati P. CAR T-Cell Therapy for B-Cell non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Clinical Trials and Real-World Experiences. Front Oncol. 2020;10:849. doi: 10.3389/fonc.2020.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Topp M, Van Meerten T, Houot R, et al. Earlier Steroid Use with Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory Large B Cell Lymphoma. Blood. 2019;134(Supplement_1) 243-243. [Google Scholar]
- 55.MLea Wang. Management of a Patient with Mantle Cell Lymphoma who Developed Severe Neurotoxicity After tChimeric Antigen Receptor T-Cell Therapy in ZUMA-2. J Immunother Cancer. 2020;8(2):e001114. doi: 10.1136/jitc-2020-001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker KR, Migliorini D, Perkey E, et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell. 2020;183(1) doi: 10.1016/j.cell.2020.08.022. 126-142.e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duong MT, Collinson-Pautz MR, Morschl E, et al. Two-Dimensional Regulation of CAR-T Cell Therapy with Orthogonal Switches. Mol Ther Oncolytics. 2018;12:124–137. doi: 10.1016/j.omto.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rezvani K, Rouce R, Liu E, et al. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther. 2017;25(8):1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westin JR, Tam CS, Borchmann P, et al. Correlative Analyses of Patient and Clinical Characteristics Associated with Efficacy in Tisagenlecleucel-Treated Relapsed/Refractory Diffuse Large B-Cell Lymphoma Patients in the Juliet Trial. Blood. 2019;134(Supplement_1) 4103-4103. [Google Scholar]
- 60.Dean E, Lu H, Lazaryan A, et al. Association of high baseline metabolic tumor volume with response following axicabtagene ciloleucel in refractory large B-cell lymphoma. J Clin Oncol. 2019;37(15_suppl):7562. [Google Scholar]
- 61.Wei J, Liu Y, Wang C, et al. The model of cytokine release syndrome in CAR T-cell treatment for B-cell non-Hodgkin lymphoma. Signal Transduct Target Ther. 2020;5(1):134. doi: 10.1038/s41392-020-00256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Z, Chen X, Tian Y, et al. Point mutation in CD19 facilitates immune escape of B cell lymphoma from CAR-T cell therapy. J Immunother Cancer. 2020;8(2):e001150. doi: 10.1136/jitc-2020-001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898–4911. doi: 10.1182/bloodadvances.2020002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J Clin Oncol. 2020;38(27):3095–3106. doi: 10.1200/JCO.19.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng Q, Han G, Puebla-Osorio N, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. 2020;26(12):1878–1887. doi: 10.1038/s41591-020-1061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat Med. 2019;25(10):1488–1499. doi: 10.1038/s41591-019-0596-y. [DOI] [PubMed] [Google Scholar]
- 68.Chrusciel E, Urban-Wojciuk Z, Arcimowicz L, et al. Adoptive Cell Therapy-Harnessing Antigen-Specific T Cells to Target Solid Tumours. Cancers (Basel) 2020;12(3) doi: 10.3390/cancers12030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183(3):725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris EC, Stauss HJ. Optimizing T-cell receptor gene therapy for hematologic malignancies. Blood. 2016;127(26):3305–3311. doi: 10.1182/blood-2015-11-629071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garber K. Driving T-cell immunotherapy to solid tumors. Nat Biotechnol. 2018;36(3):215–219. doi: 10.1038/nbt.4090. [DOI] [PubMed] [Google Scholar]
- 72.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16(4):457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.D'Angelo SP, Melchiori L, Merchant MS, et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018;8(8):944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson LA, Heemskerk B, Powell DJ, Jr, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177(9):6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Deniger DC, Pasetto A, Tran E, et al. Stable, Nonviral Expression of Mutated Tumor Neoantigen-specific T-cell Receptors Using the Sleeping Beauty Transposon/Transposase System. Mol Ther. 2016;24(6):1078–1089. doi: 10.1038/mt.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malekzadeh P, Yossef R, Cafri G, et al. Antigen Experienced T Cells from Peripheral Blood Recognize p53 Neoantigens. Clin Cancer Res. 2020;26(6):1267–1276. doi: 10.1158/1078-0432.CCR-19-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mayr C, Bund D, Schlee M, et al. MDM2 is recognized as a tumor-associated antigen in chronic lymphocytic leukemia by CD8+ autologous T lymphocytes. Exp Hematol. 2006;34(1):44–53. doi: 10.1016/j.exphem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 80.Goodridge JP, Mahmood S, Zhu H, et al. FT596: Translation of First-of-Kind Multi-Antigen Targeted Off-the-Shelf CAR-NK Cell with Engineered Persistence for the Treatment of B Cell Malignancies. Blood. 2019;134(Supplement_1):301. [Google Scholar]
- 81.Kloss CC, Lee J, Zhang A, et al. Dominant-Negative TGF-β Receptor Enhances PSMA-Targeted Human CAR T Cell Proliferation And Augments Prostate Cancer Eradication. Mol Ther. 2018;26(7):1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang N, Cheng C, Zhang X, et al. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 2020;5(4):e133977. doi: 10.1172/jci.insight.133977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]