Abstract
Tat activation-response region (TAR) decoys have been developed for use in gene therapy for people infected with human immunodeficiency virus type 1 (HIV-1). When a TAR RNA decoy is overexpressed, it will bind Tat, thus leaving less of this crucial protein to bind to and activate the natural transcriptional promoter of HIV-1. Previous TAR decoy constructs have used HIV-1 TAR. However, recent epidemiological and biological data began to suggest that the TAR region from the human immunodeficiency virus type 2 (HIV-2) may suppress HIV-1 transcription and hence replication. We created a vector which overexpresses TAR-2 under the control of the human U6 small nuclear RNA gene promoter and here show that the U6–TAR-2 decoy construct potently inhibits both HIV-2 and HIV-1 gene expression. Further, this decoy construct is able to markedly suppress HIV-1 replication. Thus, we have directly proven that TAR-2 can suppress HIV-1 replication and suggest that the HIV-2 TAR decoy may prove useful for combating HIV-1 infection.
AIDS is caused by infection with either human immunodeficiency virus type 1 (HIV-1) or HIV-2. The two types of HIV are related in origin but differ in rates of transmission and disease progression (reviewed in reference 31). Despite the most aggressive pharmaceutical treatment of HIV-infected patients, HIV remains in the immune system, and long-term therapy is necessary (18, 42). Thus, the use of gene therapy to combat HIV pathogenesis is an attractive concept. For this reason, much work has been dedicated to finding genetic constructs that would render the cells of the immune system resistant to HIV replication (reviewed in references 22 and 27).
Gene therapy approaches to treating HIV-1 infection have focused on inhibiting the regulation of viral gene expression by the viral proteins Rev and Tat. Rev and Tat are key regulators of HIV gene expression and are required for effective replication (6, 14–16, 19, 29). Both Tat and Rev proteins mediate their regulatory effects through RNA targets on the HIV transcripts: the Tat activation-response region (TAR) and the Rev-response element. The function of these two key viral regulatory proteins has been blocked by the expression of dominant negative mutant proteins (9, 41). However, though these dominant negative mutant proteins demonstrate promise for suppressing Tat and Rev function in vivo, expression of foreign proteins or peptides in host cells has several drawbacks from a gene therapy perspective (discussed in reference 27). The Tat and Rev proteins can also be functionally inactivated by sequestering them from the HIV RNA through the overexpression of the respective RNA-response elements in the cell, to act as molecular decoys (reviewed in reference 32). Tat-mediated transactivation of HIV-1 gene expression has been targeted by using expression constructs that constitutively express the TAR region of HIV-1 (TAR-1) to function as a molecular decoy. Early decoy experiments with constructs expressing TAR-1 (26, 28, 30) or circular TAR-1 RNA decoys (7) were reasonably effective against HIV-1. The mechanism of action of such TAR-1 decoys was found to be consistent with sequestration of Tat (8).
The current TAR RNA decoy approaches to decrease Tat activity have all focused on TAR-1, which is structurally divergent from the TAR region of HIV-2 (TAR-2) (35). Likewise, the HIV-1 and HIV-2 Tat transactivating proteins (Tat-1 and Tat-2, respectively) exhibit functional differences (reviewed in references 10 and 21). Although Tat-1 and Tat-2 show considerable similarity, and Tat-1 can transactivate HIV-2 gene expression through the TAR-2 element, Tat-2 is unable to effectively interact with TAR-1 to transactivate the HIV-1 promoter (reviewed in reference 10). This nonreciprocal activity of the Tat-TAR interactions, while perhaps due to a more stringent RNA site requirement for the Tat-2 protein than for the Tat-1 protein (12), may also suggest that TAR-2 is a more versatile structure for functional interactions with Tat proteins than is TAR-1. Further, there is in vitro binding (20, 35) and in vivo functional (17) data to support interaction between at least two of the hairpin structures of TAR-2 with Tat, suggesting an avidity of interaction that would increase the effectiveness of a TAR-2 decoy relative to that of the current TAR-1-based systems.
Consistent with the above observations, preliminary findings suggest that TAR-2 may be capable of inhibiting Tat-1 in vivo. For example, results of epidemiological studies in Senegal suggested that infection with HIV-2 provides protection against subsequent infection with HIV-1 (though findings from Gambian cohort studies have recently disputed this effect [4, 38, 39]). The trans inhibition of HIV-1 by HIV-2 was also investigated in vitro, and it was suggested, although not directly demonstrated, that HIV-2 suppresses the activity of the HIV-1 long terminal repeat due to differences in the TAR RNAs of HIV-1 and HIV-2 (34). This trans inhibition was also examined by another group, and the results suggested that inhibition may occur by competition between the viral RNAs for Tat and cellular factors (5). Further, HIV-2 infection has been shown to decrease HIV-1 replication in primary human monocytes (3), further suggesting that trans inhibition does indeed occur in vivo.
The above findings led us to believe that a decoy construct expressing TAR-2 could be a potent inhibitor of HIV-1 replication. To test this, we cloned the coding sequence for the entire TAR-2 superstructure into an expression vector demonstrated to express high levels of structured RNA molecules, synthesized by RNA polymerase III from the human U6 small nuclear RNA promoter, and to target their delivery to the cell nucleus (23). The targeting signals serve to concentrate the transcripts in the nucleus and thus decrease the chance of any toxic effects of the RNA on cytoplasmic functions such as translation. Since the transcripts are not translated, the construct generates no potentially immunogenic proteins and should therefore not elicit an adverse immune response. Here we show that the resulting TAR-2 decoy construct was highly effective in decreasing HIV gene expression and suppressing HIV-1 replication. This inhibition was of greater potency than that of other constructs that we tested, thus directly demonstrating the ability of TAR-2 to interfere with HIV-1 replication and suggesting that TAR-2 is a highly effective decoy molecule.
TAR-2 decoy potently inhibits HIV gene expression.
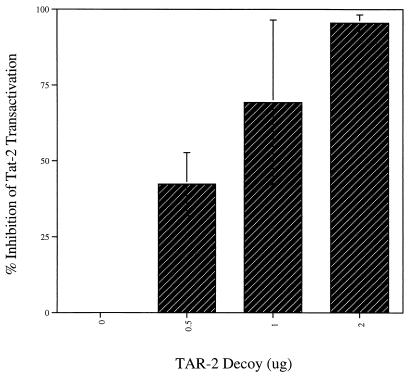
To test the ability of the TAR-2 decoy to inhibit transactivation of the HIV-2 promoter by Tat-2, we generated plasmid pTZU6+19 TAR-2. The DNA coding sequence for the entire TAR-2 region (from +1 to +160) was amplified by PCR from the HIV-2 CAT (chloramphenicol acetyltransferase) plasmid (15), using primers which introduced restriction sites for SalI and XbaI on the 5′ and 3′ ends, respectively. The TAR-2 coding region was then cloned into the pTZU6+19 RNA polymerase III expression vector to generate pTZU6+19 TAR-2 (Fig. 1; reference 23). This construct was then tested for its effectiveness in inhibiting transactivation by Tat in cotransfection experiments using an HIV-2 promoter-driven reporter plasmid (15). The Tat-2 expression construct (37) was used at the concentration which gave effective transactivation in our previous studies (10). The TAR-2 decoy plasmids strongly inhibited Tat-2 transactivation of the parental HIV-2 promoter in a dose-dependent manner (Fig. 2). Promoter activity in the presence of Tat-2 was reduced to basal levels in the presence of 2 μg of the decoy vector.
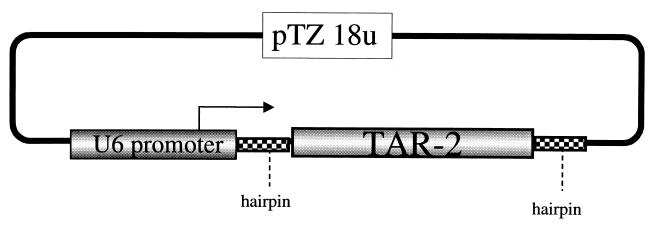
FIG. 1.
The pTZU6 TAR-2 decoy expression plasmid. The expression cassette that uses the RNA polymerase III promoter for U6 RNA has been described elsewhere (23). The DNA coding sequence for the entire TAR-2 element was cloned downstream of the +19 site of the expression plasmid. The integrity of the insert was confirmed by DNA sequencing.
FIG. 2.
The TAR-2 decoy inhibits the response of the HIV-2 promoter to Tat-2. Jurkat T cells were transfected with 5 μg of HIV-2 CAT and 0.5 μg of the Tat-2 expression vector, plus 0, 0.5, 1, or 2 μg of the TAR-2 decoy construct pTZU6+19 TAR-2 and 5, 4.5, 4, or 3 μg of the empty vector, respectively. Percent inhibition was calculated in comparison to 5 μg of HIV-2 CAT cotransfected with 0.5 μg of Tat-2 expression vector, no decoy vector, and 5 μg of the empty pTZU6+19 vector. CAT assays were normalized for protein concentration and performed as described elsewhere (10). The data presented are compiled from three separate experiments.
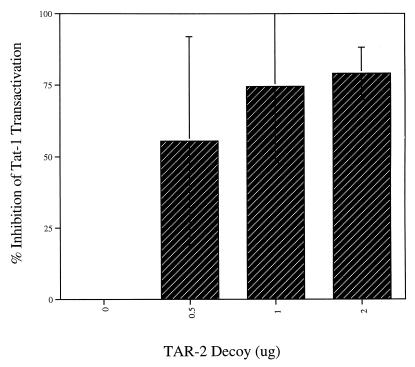
The TAR-2 decoy was also able to potently inhibit transactivation of the HIV-1 promoter by Tat-1 (Fig. 3). Inhibition to near basal levels again occurred in the presence of 2 μg of the pTZU6+19 TAR-2 vector (Fig. 3). Such potent inhibition of Tat transactivation suggested that the pTZU6+19 TAR-2 decoy would be effective at lowering virus production during infection; therefore, it was then tested in cotransfection experiments with HIV-1 infectious clones.
FIG. 3.
The TAR-2 decoy construct inhibits HIV-1 promoter activity in Jurkat T cells. Transfections were performed as for Fig. 2 except that HIV-1 CAT was substituted for HIV-2 CAT and an expression vector for Tat-1 (10) was used. The data presented are compiled from three separate experiments.
The TAR-2 decoy construct is a potent inhibitor of HIV-1 replication.
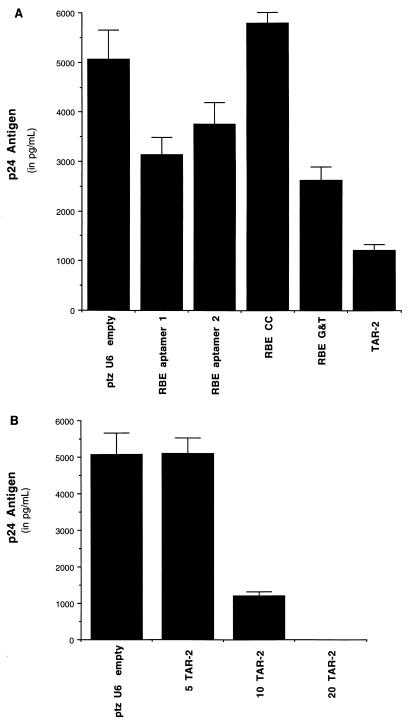
The TAR-2 decoy construct was tested for efficacy in inhibiting HIV-1 replication in the 293 human kidney cell line, which is a readily transfectable and highly productive host for HIV-1. The 293 cells were transfected by using the Lipofectamine reagent (GibcoBRL), according to the manufacturer’s protocol, with 2 μg of decoy or carrier plasmid DNA, 200 ng of the HIV-1 infectious clone pNL4-3 (1) (acquired from the AIDS Repository), and 6 μl of Lipofectamine reagent per well. The transfections were performed in triplicate, and clarified supernatants were harvested 40 h later. The TAR-2 decoy showed potent inhibition of HIV-1 replication, as indicated by decreased p24 antigen production (kit from SAIC Frederick) (Fig. 4A). Compared to several other recently developed decoy RNA expression constructs which effectively sequester the HIV-1 Rev protein (23), the TAR-2 decoy showed superior inhibition of HIV-1 replication (Fig. 4A). Further, the TAR-2 decoy inhibited HIV-1 in a dose-dependent manner, with HIV-1 production falling below detectable levels when 20 μg of the TAR-2 decoy was used (Fig. 4B). Such inhibition is particularly impressive, as the replication of HIV-1 in 293 cells is generally more refractory to inhibitors than is replication in T cells and monocytic cells (36).
FIG. 4.
(A) The TAR-2 decoy construct is a potent inhibitor of HIV-1 replication in 293 cells compared to other U6-based anti-HIV constructs. The RBE constructs express Rev-binding decoy RNAs. The human embryonal kidney cell line 293 was transfected with 200 ng of the pNL4-3 HIV-1 infectious clone and 10 μg of the plasmids indicated. The cell supernatants were harvested 40 h posttransfection and assayed for p24 antigen. The data are from triplicate samples, and the standard deviations were used to determine the error bars. (B) The TAR-2 decoy inhibits HIV-1 replication in a dose-dependent manner. Transfections were performed as for panel A except that 5, 10, and 20 μg of the TAR-2 decoy construct were used where indicated.
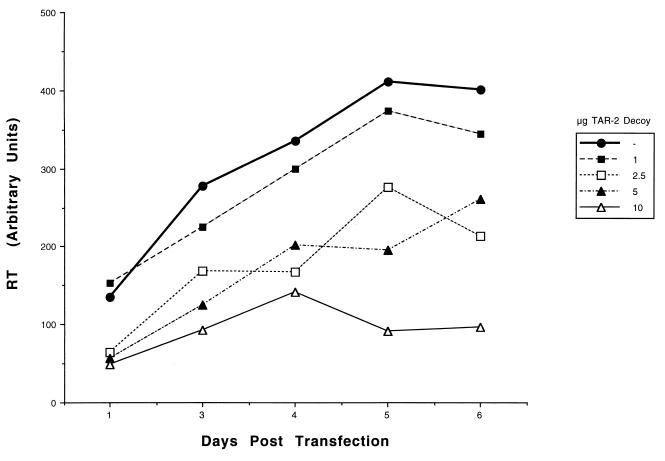
The TAR-2 decoy was next assessed in cotransfection experiments using the more physiologically relevant U937 monocytic cell line with a different HIV-1 infectious clone, pHXB3 (24). The inhibition of HIV-1 production, as measured by reverse transcriptase (RT) activity (2), was dose dependent and occurred during the entire time course of the infection (Fig. 5). Ten micrograms of the decoy construct reduced the RT activity to almost baseline levels throughout the course of the experiment. Thus, the TAR-2 decoy is able to potently inhibit the replication of at least two different clones of HIV-1. This effect is seen in the 293 cell line, which has been shown to be an extremely high producer of HIV-1 in previous experiments (11), and in the more physiologically relevant monocytic cells.
FIG. 5.
The TAR-2 decoy construct is a potent inhibitor of HIV-1 replication in U937 monocytic cells. Monocytic cells were cotransfected with 1 μg of the pHXB3 HIV-1 infectious clone DNA and 0, 1, 2.5, 5, or 10 μg of the TAR-2 decoy vector and 10, 9, 7.5, 5, or 0 μg of the empty pTZU6+19 vector, respectively, to normalize for DNA content. The cells were plated out in duplicate wells, and cellular supernatants were harvested every 2 days for assessment of RT activity. Peak RT activity is displayed in arbitrary units.
Discussion.
The vector for expressing the decoy presented in these studies has several benefits over other current anti-HIV gene therapy constructs. The RNA polymerase III promoter for U6 RNA is a highly productive promoter capable of generating complex RNA structures with high integrity, as the natural role of RNA polymerase III in cells is to transcribe tRNA and other highly structured RNA molecules. Further, the presence of U6 upstream flanking sequences allows for the TAR-2 decoy molecules to be targeted to the cell nucleus, where they can more effectively compete for nuclear transcription factors and the viral transactivator protein Tat. In addition, the terminal hairpin serves to protect the TAR RNA from degradation by cellular RNases (23).
Our experiments show that the use of TAR-2, rather than TAR-1, as the RNA decoy for Tat and cellular cofactors may prove a significant advance in the design of gene therapy constructs for HIV. This finding is compatible with very recent epidemiological data (38, 39), molecular experiments (5, 34), and cellular assays (3) which had begun to suggest that TAR-2 may be an effective decoy construct. Further, we have shown that Tat-1 is able to interact functionally with TAR-2 even when the RNA contains substantial mutations in primary sequence and structure (10), suggesting that the interaction between the Tat-1 protein and TAR-2 RNA is very robust. The studies presented here demonstrate that the TAR-2 decoy is, indeed, a highly potent inhibitor of HIV-1 gene expression and replication. In direct comparisons, it appears to be at least as effective as other vectors used to inhibit HIV-1 replication and may prove superior (Fig. 4A and reference 36).
The potency of TAR-2 RNA as a molecular decoy for inhibition of HIV replication could stem from its multiple sites for interaction with Tat and/or its ability to sequester cellular factors. The importance of cellular factors for the transactivation of HIV by Tat, particularly cyclin-dependent kinases and their associated cyclins (13, 25, 33, 40, 43, 44), has been clearly demonstrated (reviewed in references 10 and 21). Multiple binding sites for human cellular proteins have been identified on the TAR RNA structure (discussed in reference 10). The TAR-2 decoy could potentially function by competing with the viral nascent transcript for association with any of these cellular factors in addition to the viral protein Tat. The TAR-2 superstructure possesses three separate loop regions and may therefore more effectively compete with the single stem-loop structured TAR-1 for loop-binding cellular factors. Lack of interaction with cellular cofactors for Tat might also explain why a minimal TAR-1 decoy, expressed from the same polymerase III promoter construct as our TAR-2 decoy, did not inhibit HIV-1 replication (23).
In this study, we have shown that expression of TAR-2 RNA in trans can inhibit HIV-1 replication. The pTZU6 TAR-2 decoy expression construct generated in this study is a very potent inhibitor of Tat activation of both the HIV-1 and HIV-2 promoters in both monocytic and T-cell lines, which represent the key cellular hosts during the course of HIV-1 infection. Further, we have shown that the TAR-2 decoy is highly effective at blocking HIV-1 replication in at least two human cell lines. These data demonstrate that use of the TAR-2 decoy is a promising new stratagem in the effort to control HIV pathogenesis by gene therapy approaches. Further studies are under way to determine the scope and duration of HIV inhibition by this construct and assess its performance in combination with other gene therapy modalities.
Acknowledgments
We thank Michael Imperiale, David Friedman, Victor DiRita, and Clive Woffendin for helpful discussions and training.
This work was supported by grants AI-36685 and HL-57885 to D.M.M. from the National Institutes of Health. C.M.B. was supported by fellowships from the Cellular Biotechnology Training Program (5 T32 GM08353) and the Cancer Biology Training Program (T32 CA09676) of the University of Michigan. L.C. and J.R. were supported by grant NIH AI38592 from the National Institutes of Health.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldovini A, Walker B D. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. [Google Scholar]
- 3.Al-Harthi L, Owais M, Arya S K. Molecular inhibition of HIV type 1 by HIV type 2: effectiveness in peripheral blood mononuclear cells. AIDS Res Human Retroviruses. 1998;14:59–64. doi: 10.1089/aid.1998.14.59. [DOI] [PubMed] [Google Scholar]
- 4.Ariyoshi K, Schim van der Loeff M, Sabally S, Cham F, Corrah T, Whittle H. Does HIV-2 infection provide cross-protection against HIV-1 infection? AIDS. 1997;11:1053–1053. [PubMed] [Google Scholar]
- 5.Arya S K, Gallo R C. Human immunodeficiency virus (HIV) type 2-mediated inhibition of HIV type 1: a new approach to gene therapy of HIV-infection. Proc Natl Acad Sci USA. 1996;93:4486–4491. doi: 10.1073/pnas.93.9.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arya S K, Guo C, Josephs S F, Wong-Staal F. Transactivator gene of human T-lymphotropic virus type III (HTLV-III) Science. 1985;229:69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- 7.Bohjanen P R, Colvin R A, Puttaraju M, Been M D, Garcia-Blanco M A. A small circular TAR RNA decoy specifically inhibits Tat-activated HIV-1 transcription. Nucleic Acids Res. 1996;24:3733–3738. doi: 10.1093/nar/24.19.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohjanen P R, Liu Y, Garcia-Blanco M A. TAR RNA decoys inhibit tat-activated HIV-1 transcription after preinitiation complex formation. Nucleic Acids Res. 1997;25:4481–4481. doi: 10.1093/nar/25.22.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonyhadi M L, Moss K, Voytovich A, Auten J, Kalfoglou C, Plavec I, Forestell S, Su L, Bohnlein E, Kaneshima H. RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem-progenitor cells inhibit human immunodeficiency virus replication. J Virol. 1997;71:4707–4716. doi: 10.1128/jvi.71.6.4707-4716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browning C, Hilfinger J M, Rainier S, Lin V, Hedderwick S, Smith M, Markovitz D M. The sequence and structure of the 3′ arm of the first stem-loop of the human immunodeficiency virus type 2 trans-activation responsive region mediate Tat-2 transactivation. J Virol. 1997;71:8048–8055. doi: 10.1128/jvi.71.10.8048-8055.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cagnon L, Cucchiarini M, Lefebvre J C, Doglio A. Protection of a T-cell line from human immunodeficiency virus replication by the stable expression of a short antisense RNA sequence carried by a shuttle RNA molecule. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;9:349–358. [PubMed] [Google Scholar]
- 12.Chang Y N, Jeang K T. The basic RNA-binding domain of HIV-2 Tat contributes to preferential trans-activation of a TAR2-containing LTR. Nucleic Acids Res. 1992;20:5465–5472. doi: 10.1093/nar/20.20.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cujec T P, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan D O, Peterlin B M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11(20):2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayton A I, Sodroski J G, Rosen C A, Goh W C, Haseltine W A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 15.Emerman M, Guyader M, Montagnier L, Baltimore D, Muesing M A. The specificity of the human immunodeficiency virus type 2 transactivator is different from that of human immunodeficiency virus type 1. EMBO J. 1987;6:3755–3760. doi: 10.1002/j.1460-2075.1987.tb02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 17.Fenrick R, Malim M H, Hauber J, Le S Y, Maizel J, Cullen B R. Functional analysis of the Tat trans activator of human immunodeficiency virus type 2. J Virol. 1989;63:5006–5012. doi: 10.1128/jvi.63.12.5006-5012.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 19.Fisher A G, Feinberg M B, Josephs S F, Harper M E, Marselle L M, Reyes G, Gonda M A, Aldovini A, Debouk C, Gallo R C. The trans-activator gene of HTLV-III is essential for virus replication. Nature. 1986;320:367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Martinez L F, Mavankal G, Peters P, Wu-Baer F, Gaynor R B. Tat functions to stimulate the elongation properties of transcription complexes paused by the duplicated TAR RNA element of human immunodeficiency virus 2. J Mol Biol. 1995;254:350–363. doi: 10.1006/jmbi.1995.0622. [DOI] [PubMed] [Google Scholar]
- 21.Gaynor R B. Regulation of HIV-1 gene expression by the transactivator protein Tat. Curr Top Microbiol Immunol. 1995;193:51–77. doi: 10.1007/978-3-642-78929-8_3. [DOI] [PubMed] [Google Scholar]
- 22.Gilboa E, Smith C. Gene therapy for infectious diseases: the AIDS model. Trends Genet. 1994;10:139–144. doi: 10.1016/0168-9525(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 23.Good P D, Krikos A J, Li S X, Bertrand E, Lee N S, Giver L, Ellington A, Zaia J A, Rossi J J, Engelke D R. Expression of small, therapeutic RNAs in human cell nuclei. Gene Ther. 1997;4:45–54. doi: 10.1038/sj.gt.3300354. [DOI] [PubMed] [Google Scholar]
- 24.Hahn B H, Shaw G M, Arya S K, Popovic M, Gallo R C, Wong-Staal F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984;312:166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S W, Gallardo H F, Gaspar O, Smith C, Gilboa E. Inhibition of HIV-1 in CEM cells by a potent TAR decoy. Gene Ther. 1995;2:377–384. [PubMed] [Google Scholar]
- 27.Lever A M. Gene therapy for HIV infection. Br Med Bull. 1995;51:149–166. doi: 10.1093/oxfordjournals.bmb.a072944. [DOI] [PubMed] [Google Scholar]
- 28.Lisziewicz J, Sun D, Smythe J, Lusso P, Lori F, Louie A, Markham P, Rossi J, Reitz M, Gallo R C. Inhibition of human immunodeficiency virus type 1 replication by regulated expression of a polymeric Tat activation response RNA decoy as a strategy for gene therapy in AIDS. Proc Natl Acad Sci USA. 1993;90:8000–8004. doi: 10.1073/pnas.90.17.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malim M H, Bohnlein S, Hauber J, Cullen B R. Functional dissection of the HIV-1 Rev trans-activator-derivation of a trans-dominant repressor of Rev function. Cell. 1989;58:205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 30.Marciniak R A, Calnan B J, Frankel A D, Sharp P A. HIV-1 Tat protein trans-activates transcription in vitro. Cell. 1990;63:791–802. doi: 10.1016/0092-8674(90)90145-5. [DOI] [PubMed] [Google Scholar]
- 31.Markovitz D M. Infection with the human immunodeficiency virus type 2. Ann Intern Med. 1993;118:211–218. doi: 10.7326/0003-4819-118-3-199302010-00010. [DOI] [PubMed] [Google Scholar]
- 32.Nakaya T, Iwai S, Fujinaga K, Otsuka E, Ikuta K. Inhibition of HIV-1 replication by targeting the Rev protein. Leukemia. 1997;11(Suppl. 3):134–137. [PubMed] [Google Scholar]
- 33.Parada C A, Roeder R G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 34.Rappaport J, Arya S K, Richardson M W, Baier-Bitterlich G, Klotman P E. Inhibition of HIV-1 expression by HIV-2. J Mol Med. 1995;73:583–589. doi: 10.1007/BF00196351. [DOI] [PubMed] [Google Scholar]
- 35.Rhim H, Rice A P. Functional significance of the dinucleotide bulge in stem-loop1 and stem-loop2 of HIV-2 TAR RNA. Virology. 1994;202:202–211. doi: 10.1006/viro.1994.1336. [DOI] [PubMed] [Google Scholar]
- 36.Rossi, J. Unpublished observations.
- 37.Tong-Starksen S E, Baur A, Lu X B, Peck E, Peterlin B M. Second exon of Tat of HIV-2 is required for optimal trans-activation of HIV-1 and HIV-2 LTRs. Virology. 1993;195:826–830. doi: 10.1006/viro.1993.1438. [DOI] [PubMed] [Google Scholar]
- 38.Travers K U, Eisen G E, Marlink R G, Essex M E, Hsieh C C, Mboup S, Kanki P J. Protection from HIV-1 infection by HIV-2. AIDS. 1998;12:224–225. . (Letter; comment.) [PubMed] [Google Scholar]
- 39.Travers K, Mboup S, Marlink R, Gueye-Ndiaye A, Silby T, Thior I, Traore I, Dieng-Sarr A, Sankale J-L, Mullins C, Ndoye I, Hsieh C-C, Essex M, Kanki P. Natural protection against HIV-1 infection provided by HIV-2. Science. 1995;268:1612–1615. doi: 10.1126/science.7539936. [DOI] [PubMed] [Google Scholar]
- 40.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 41.Woffendin C, Ranga U, Yang Z, Xu L, Nabel G J. Expression of a protective gene-prolongs survival of T cells in human immunodeficiency virus-infected patients. Proc Natl Acad Sci USA. 1996;93:2889–2894. doi: 10.1073/pnas.93.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Gold M O, Tang D N, Lewis D E, Aguilar-Cordova E, Rice A P, Herrmann C H. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA. 1997;94:12331–12336. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Y, Pe-ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]