Abstract
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has led to a diverse pattern of myocardial injuries, including myocarditis, which is linked to adverse outcomes in patients. Research indicates that myocardial injury is associated with higher mortality in hospitalized severe COVID-19 patients (75.8% vs 9.7%). Cardiovascular Magnetic Resonance (CMR) has emerged as a crucial tool in diagnosing both ischaemic and non-ischaemic myocardial injuries, providing detailed insights into the impact of COVID-19 on myocardial tissue and function. This review synthesizes existing studies on the histopathological findings and CMR imaging patterns of myocardial injuries in COVID-19 patients. CMR imaging has revealed a complex pattern of cardiac damage in these patients, including myocardial inflammation, oedema, fibrosis, and ischaemic injury, due to coronary microthrombi. This review also highlights the role of LLC criteria in diagnosis of COVID-related myocarditis and the importance of CMR in detecting cardiac complications of COVID-19 in specific groups, such as children, manifesting multisystem inflammatory syndrome in children (MIS-C) and athletes, as well as myocardial injuries post-COVID-19 infection or following COVID-19 vaccination. By summarizing existing studies on CMR in COVID-19 patients and highlighting ongoing research, this review contributes to a deeper understanding of the cardiac impacts of COVID-19. It emphasizes the effectiveness of CMR in assessing a broad spectrum of myocardial injuries, thereby enhancing the management and prognosis of patients with COVID-19 related cardiac complications.
Keywords: CMR, COVID-19, myocarditis, SARS-CoV-2
Background
Since the onset of the COVID-19 pandemic in December 2019 in Wuhan, China, up to October 2023, there have been approximately 771 million confirmed cases and nearly 7 million associated deaths.1 COVID-19 is caused by the SARS-CoV-2 virus, which belongs to the Coronaviridae family.2 Notably, MERS-CoV, SARS-CoV, and SARS-CoV-2, members of this family, are probably zoonotic viruses responsible for severe respiratory illnesses.3 COVID-19 is a multisystemic disease that predominantly affects the respiratory and cardiovascular systems. This is attributed to the fact that angiotensin-converting enzyme 2 (ACE2) receptors are abundant in these systems and serve as the entry receptors for SARS-CoV-2.4,5 Studies have identified that the primary risk factors for mortality in COVID-19 patients include advanced age (over 60 years), male gender, and the presence of comorbidities such as hypertension, obesity, and myocardial injury.6,7 Shaobo et al have revealed that 15.8% of all admitted patients have had myocardial injury based on high blood levels of troponin I (cTnI). Of note, patients who died had suffered more often from myocardial injury during hospitalization compared with survivors (75.8% vs 9.7%).8 Cardiovascular magnetic resonance has emerged as a reference imaging tool for assessing myocardial function and tissue characterization. The American College of Cardiology, the European Society of Cardiology, and the Society for CMR all emphasize that CMR is a useful diagnostic tool for patients with COVID-19 who show evidence of myocardial injury and cardiac dysfunction.9–12
Myocardial injury in COVID-19
Myocardial injury can be broadly categorized into two types: ischaemic and non-ischaemic.
Ischaemic myocardial injury primarily results from acute plaque rupture or erosion,13,14 but cases of myocardial infarction without obstructive coronary arteries (MINOCA) have been reported in the context of acute COVID-19 due to triggers like direct viral endothelial cell infection (endothelitis)14,15 and pro-thrombotic effects from the massive cytokine release associated with COVID-19.16
Conversely, non-ischaemic myocardial injury may occasionally arise from direct viral infection of cardiomyocytes17 or, indirectly, via immune responses and systemic hyperinflammation, as reported in multisystem inflammatory syndrome (MIS-C), particularly in children.18–20 Infrequently, COVID-19 can also lead to stress-induced cardiomyopathy (Takotsubo) with reversible myocardial injury,21,22 also potentially leading to acute heart failure.23
Histopathological findings of COVID-19-related myocardial injury
The mechanisms underlying myocardial injury in patients with COVID-19 are varied and not yet fully understood.
Most of our understanding of the histopathologic manifestations of COVID-related myocardial injury stems from post-mortem examination of deceased hospitalized patients. There is growing evidence highlighting that vascular leakage and tissue oedema are principal contributors to myocardial injury in severe COVID-19 cases.24 Several mechanisms explain the development of myocardial oedema: First, direct invasion of endothelial cells by SARS-CoV-2, causing endothelitis characterized by endothelial dysfunction and subsequent cellular necrosis.24,25 Second, the downregulation of the ACE2 receptor may lead to increased angiotensin 2 and activation of the kallikrein-bradykinin pathway, resulting in increased vascular permeability4; and third, a surge in inflammatory cytokines and vasoactive molecules, disrupting inter-endothelial junctions.26
Sang et al27 reported having frequently observed myocardial fibrosis (80%), hypertrophy (72.0%), and coronary microthrombi (66.0%) in deceased COVID-19 patients post-mortem.
Halushka et al reviewed findings from 277 cardiac autopsies, revealing that non-myocarditis inflammatory infiltrate and single cell ischemia were the predominant cardiac findings, present in 12.6% and 13.7% of cases, respectively, while classical myocarditis based on Dallas criteria was identified in only 1.4% of the cases.28
A review analysing immunohistochemical data from 209 cardiac autopsies of severe SARS-CoV-2 patients revealed myocardial infiltration of CD3+, CD8+ cytotoxic lymphocytes, and CD68+ macrophages. The presence of CD3+ lymphocytes emphasizes that cellular immunity is an essential part of the host response during COVID-19 infection.29 Another proposed mechanism underlying myocardial damage is ischaemic injury.30 Upon examination of 40 hearts, Pellegrini et al identified microthrombus formation predominantly in small vessels as the main pathological cause of myocyte necrosis, accounting for 78.6% of cases.31
Imaging assessment of COVID-19 induced cardiac injury
Although endomyocardial biopsy is by many considered the gold standard for diagnosing acute or chronic inflammatory cardiac disorders, it is recommended only in patients with complicated presentations or significant clinical deterioration despite proper treatment, including high-grade heart block or ventricular arrhythmia, once obstructive coronary artery disease is excluded.32,33 According to the ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults, transthoracic echocardiography (TTE) is the cardiac imaging modality of choice in patients presenting with cardiac symptoms (chest pain, dyspnoea, palpitations, syncope); other markers are elevated cardiac troponin (cTn); and abnormal electrocardiographic (diffuse T-wave inversion, ST-segment elevation without reciprocal ST-segment depression, prolongation of the QRS complex duration).32
Dweck et al analysed data from an international survey on the clinical use of TTE in 1216 patients hospitalized with COVID-19. Their findings revealed that LV abnormalities were observed in approximately half of the patients, and imaging findings led to change in management in one-third of cases (33%).34 While TTE is a clinically extremely useful non-invasive tool with excellent accessibility and bedside applicability, especially for assessing ventricular function, it is not the optimal choice for ruling out myocardial injury in this group of patients. This limitation can be related to echocardiography’s inability to provide significant information on the myocardial tissue characteristics associated with COVID-19.
There is limited data on the utility of molecular imaging for diagnosing acute myocardial injury related to COVID-19. However, a recent study by Hanneman et al showed evidence of myocardial inflammation on fluorodeoxyglucose—positron emission tomography (FDG-PET) in 17% of 47 patients recently recovered from COVID-19.35
Assessment of myocardial injury using CMR
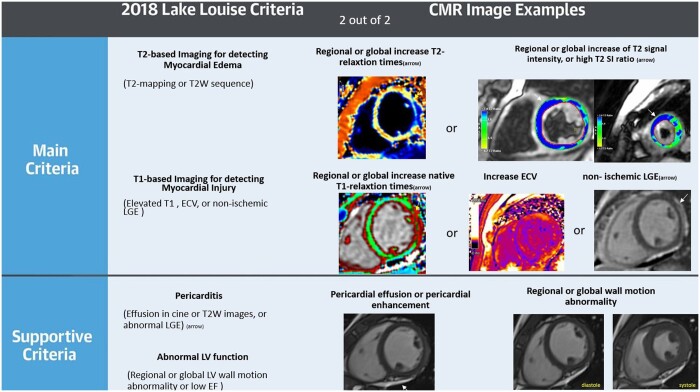
Cardiovascular magnetic resonance has been established as the non-invasive gold standard tool for the in vivo diagnosis of ischaemic and non-ischaemic myocardial injury. Cine CMR imaging offers a high spatial and temporal resolution for an accurate analysis of both left and right ventricular regional wall motion and global function. Additionally, strain imaging can detect subtle and potentially subclinical myocardial functional abnormalities.36 Furthermore, CMR has the unique capability to verify or rule out myocardial oedema by T2-weighted imaging or T2 mapping; it also provides markers from native and post contrast T1 maps for diffuse interstitial fibrosis via estimation of extracellular volume (ECV).12,37 Late gadolinium enhancement (LGE) imaging is used for detecting of replacement fibrosis in the myocardium. The patterns of LGE help to differentiate between ischaemic and non-ischaemic causes of myocardial fibrosis.38 Generally, ischaemic myocardial injury often leads to LGE in a subendocardial or transmural pattern in coronary arteries distribution, while non-ischaemic injury affects subepicardial or mid-myocardial layers.39,40 The 2018 consensus criteria for CMR in myocardial inflammation, known as the Lake Louise Criteria (LLC), recommend utilizing at least one T2-based criterion for myocardial oedema (either a global or regional elevation in myocardial T2 relaxation time or heightened signal intensity on T2-weighted CMR images) in conjunction with at least one T1-based criterion for myocardial injury (elevated myocardial T1, high ECV fraction, or LGE41Figure 1). Furthermore, CMR can also be used to assess myocardial perfusion using stress perfusion imaging42,43 or to study coronary vascular function by tracking changes of myocardial oxygenation during vasoactive interventions using Oxygenation-Sensitive CMR.44
Figure 1.
The LLC. The 2018 LLC diagnostic consensus guideline includes two primary criteria: T1- and T2-based. A positive T1-based criterion is indicated by prolonged native T1 relaxation times, elevated ECV, or non-ischaemic LGE marked by a white arrowhead. The T2-based criterion is considered positive with elevated T2 relaxation times, localized areas of high signal intensity on T2-weighted images identified by white arrows, or an increased overall T2 signal intensity. Of note, in case with very suggestive clinical presentation, even one criterion would support the diagnosis of myocarditis. Abbreviations: SI = signal intensity, T2-W = T2 weighted, LGE = late gadolinium enhancement, ECV = extracellular volume. Ferreira VM, et al. Journal of the American College of Cardiology. 2018 Dec 18;72(24):3158–76. with permission (41).
Several non-invasive imaging modalities are available to assess microvascular dysfunction by measuring myocardial blood flow (MBF) and myocardial perfusion reserve (MPR), characterized by the ratio of maximum hyperaemic response to resting coronary blood flow.45 While cardiac PET is acknowledged as the standard method for MBF quantification,46 CMR has emerged as a reliable alternative. CMR employs stress-induced first-pass perfusion following the administration of a vasodilator agent such as adenosine or regadenoson to measure peak MBF, followed by an assessment at rest.45,47 Various CMR techniques have been applied to myocardial perfusion measurement, including semi-quantitative and fully quantitative methods. Semiquantitative methods can be used to analyse variations in myocardial signal intensity during the passage of contrast media, whereas fully quantitative techniques require a measurable relationship between myocardial signal intensity variation and underlying coronary blood flow, offering the advantage of calculating a broader range of perfusion indexes.45,48 The accuracy of the semiquantitative methods can be influenced by the pharmacodynamic and pharmacokinetic properties of the contrast agents used. Most quantitative analyses necessitate the mathematical deconvolution of measured blood (arterial input function) and tissue (tissue function) enhancement data, from which myocardial perfusion is computed.45,46,49 Recent advancements have validated the accuracy of automated quantitative CMR MBF maps.50
Although the efficacy of CMR using the LLC for a non-invasive diagnosis of acute myocarditis is well-established, CMR remains underutilized in COVID-19. This underuse can be attributed in part to the limited accessibility of cardiac MRI in routine clinical settings,51 the difficulty of patients with lung involvement to hold their breath, and concerns about procedure-related infections.9
Pattern of cardiac injury in CMR images
There are various patterns of cardiac injury secondary to SARS-CoV-2 infection, including myocarditis (Figure 2), pericarditis, myopericarditis, inducible ischaemia, infarction, and less commonly, stress-induced cardiomyopathy (Figure 3). The LLC are frequently used to diagnose COVID-19-related myocarditis32,37 (Figure 2). Several studies found a more global myocardial injury as evident by an elevated myocardial T1, consistent with diffuse myocardial fibrosis (or oedema), and high T2 values, indicating myocardial oedema.52–54 However, other studies have demonstrated more focal findings in a non-ischaemic pattern of myocardial injury, such as subepicardial, mid-wall, or patchy fibrosis, as evidenced in LGE images.12,55,56 A controversial study reported myocardial LGE in 30% and pericardial enhancement in 22% of 100 patients who had recently recovered from COVID-19,57 while in a large international multicentric study involving 56 963 hospitalized COVID-19 patients, Ammirati et al found a much lower prevalence of acute myocarditis, ie, between 2.4 and 4.1 per 1000 patients.58
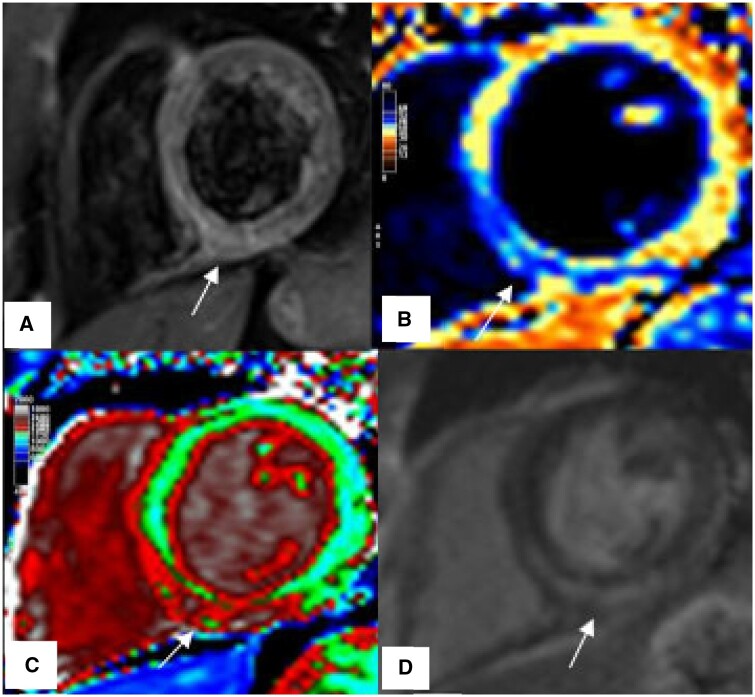
Figure 2.
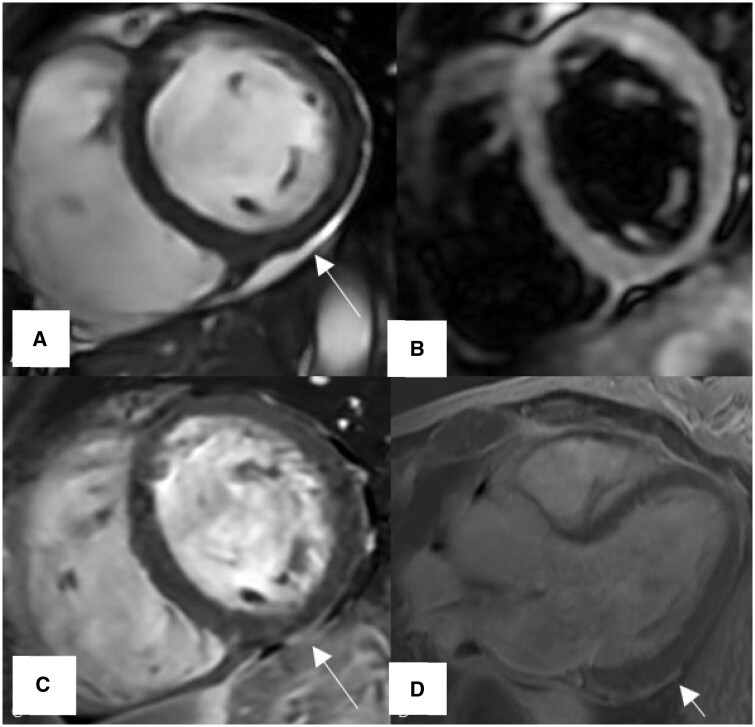
Example of classic myocarditis according to LLC during acute COVID-19, patient presented with chest pain and elevated troponin levels. (A) T2-W, Regional increase myocardial signal intensity in the mid inferoseptal, inferior wall consistent with myocardial edema (arrow), (B) T2 map, Regional high T2-times in the inferoseptal segment (blue), (C) T1 map, Regional increase native T1-times in the inferoseptal and inferior segments (red), (D) LGE, Linear subepicardial enhancement in the mid inferoseptal, inferior and inferolateral segments as well as mid-myocardial enhancement involving the septum (arrow).
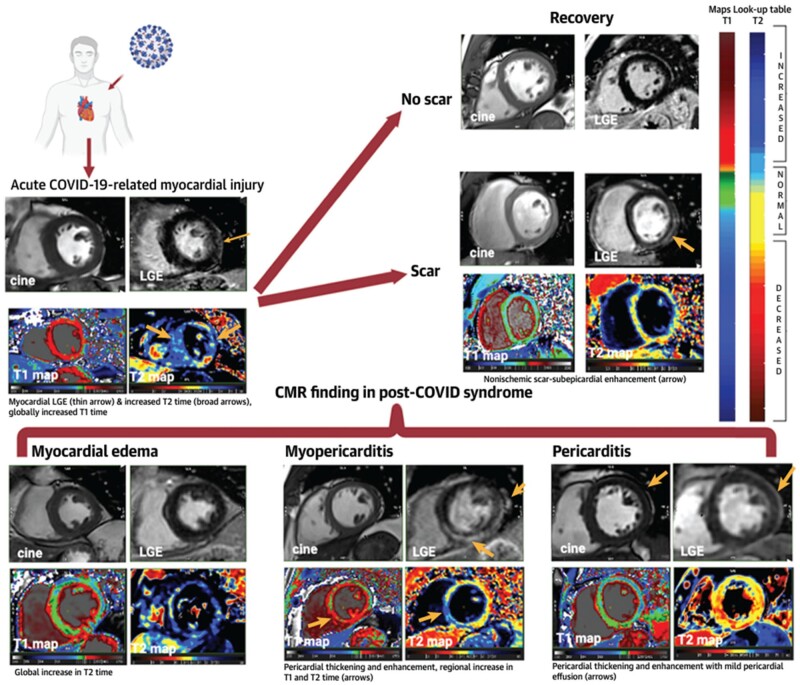
Figure 3.
Summarized illustration of CMR findings of cardiac involvement in COVID-19.37
The LLC have been validated with classic histopathologic evidence of myocarditis per the Dallas criteria.37,41 Their efficacy in diagnosing myocardial injury after SARS-CoV-2 infection, mainly assessed in patients recovered from an acute episode59 and those with long COVID-19 with persistent cardiac symptoms, however, is less well established.12,60 Considering the moderate sensitivity of CMR, even one T1 or T2-based criterion may still support the diagnosis in cases with a very suggestive clinical presentation, especially when associated with supportive criteria like pericarditis (enhanced pericardium and/or evidence of pericardial effusion) (Figure 4) or left ventricular dysfunction.12,37
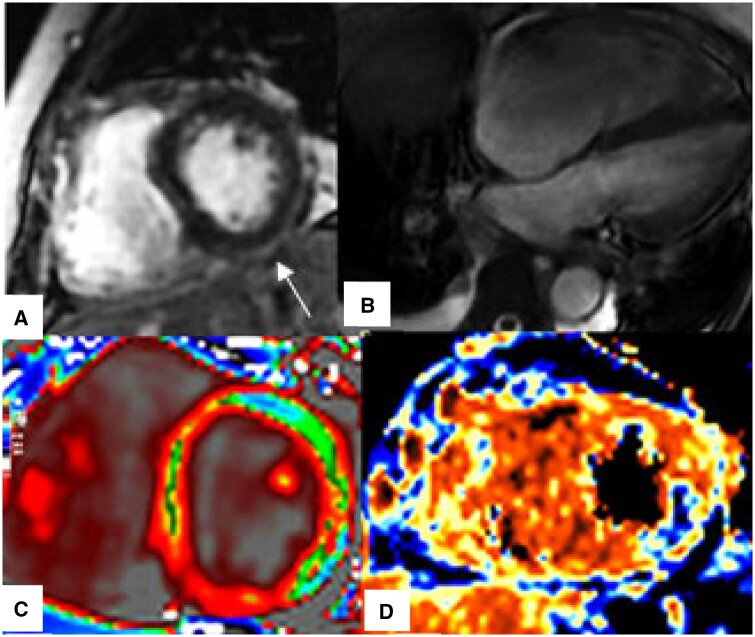
Figure 4.
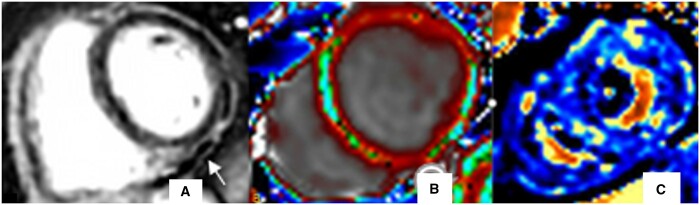
CMR findings of myocardial injury in patients with acute severe COVID-19. (A) LGE, Non-ischaemic subepicardial enhancement involving inferior and inferolateral segments (arrow), (B) Four-chamber SSFP, Dilated right ventricle, (C) T1 map, Diffusely increased T1-relaxation times, (D) T2 map, Focal high T2-values in the mid inferolateral wall (blue colour).
Reports on the clinical utility of CMR to evaluate myocardial injury in the early, hyperacute phase of severe COVID-19 are scarce, with initial findings derived from small cohorts or single-centre studies only. This limitation is largely due to the infrequent use of CMR in patients experiencing hemodynamic instability or requiring mechanical ventilation.
There are case reports that have highlighted abnormal myocardial T1 and T2-relaxation times, as well as non-ischaemic myocardial injury on LGE images54,61 (Figure 2). Galea et al, in a small cohort of 27 patients with active COVID-19 and suspected cardiac involvement, found that prolonged T2-relaxation was the most prevalent abnormality correlated with hs-cTn values.62 In a recent comprehensive, multicentric prospective study (COVID-HEART study), 519 hospitalized COVID-19 patients with elevated troponin levels underwent CMR to assess acute myocardial injury. A significant proportion of 42% of patients demonstrated myocardial scarring on LGE images, six times more than COVID-19 patients with normal troponin levels. The LGE patterns were diverse, encompassing ischaemic, non-ischaemic, mixed, and nonspecific presentations. Moreover, the extent of the scarring was linked to adverse cardiac remodelling.63 Interestingly, global myocardial T2 values were not elevated in these patients. However, this can be explained by the fact that CMR scans were performed on average 30 days after admission, when acute inflammation with associated oedema typically would have receded.
A study involving 148 patients hospitalized with severe acute respiratory syndrome from COVID-19 and elevated troponin levels revealed that about one-third showed a myocarditis-like pattern on LGE images. In addition, 22% showed evidence of infarction and/or ischaemia, and 6% had evidence of mixed ischaemic and non-ischaemic injury during the early post-infection recovery period.64 Finally, in patients with a high pre-test probability for acute myocardial injury, CMR may improve diagnostic specificity, guide management decisions, and affect the prognosis12,37 (Figure 5).
Figure 5.
41-year-old woman with COVID infection presented with dyspnoea, palpitation and fatigue and mild perimyocardial involvement. (A) short axis SSFP, small pocket of pericardial fluid as high signal intensity located adjacent to the basal inferior wall of the LV (arrow). (B) T2-weighted, Regional signal increase in the mid anterior, anterolateral segments to suggest myocardial oedema. (C, D) LGE images, Regional subepicardial and adjacent pericardial enhancement of the basal inferior, inferolateral segments, adjacent to a pocket of effusion (white arrows).
Another, albeit less frequent imaging manifestation in patients with acute COVID-19 can be focal or global inducible perfusion deficits during stress CMR.43,65 This may be related to microvascular dysfunction, endothelial inflammation, or micro- and macrovascular fibrin thrombosis, observed in moderate to severe acute COVID-19.65–68 Another suggested mechanism for myocardial ischaemia may be impaired flow-mediated epicardial coronary dilatation.69
Estimating the prevalence of myocardial infarction during acute COVID-19 is challenging. However, several studies have indicated an increased risk of myocardial infarction during the acute phase of COVID-19.70,71 Saad et al demonstrated that myocardial infarctions associated with COVID-19 have higher mortality rates compared to cases without COVID-19 evidence.72 In a retrospective multicentre study in 1047 patients with PCR-confirmed COVID-19 infection, Vidula et al demonstrated that 6.7% of participants had ischaemic myocardial injury, with 1.9% showing an acute ischaemic pattern on CMR.73 CMR evidence of COVID-related ischaemic myocardial injury with characteristic subendocardial or transmural LGE was similar to other causes.41,56,73
Biventricular thrombus formation, as a rare cardiovascular complication of acute COVID-19, has been described after myocardial infarction or a prothrombotic state.74,75 While cardiac thrombi are typically detected in echocardiography images, contrast-enhanced CMR has the highest sensitivity and specificity for LV thrombus detection, surpassing TTE, and transoesophageal echocardiography.12,76
Right ventricular dysfunction with cavity dilation, impaired RV strain, or depressed systolic function can be identified using CMR or echocardiography in about 40% of patients with COVID-19.77 RV impairment is correlated with an increased incidence of myocardial damage in COVID-19 and has been recognized as a predictor for adverse outcomes.78–80 Although CMR is the gold standard method for assessing RV size and function (Figure 4), multiparametric CMR may be useful for identifying RV myocardial inflammation during acute myocarditis.81 RV dysfunction has been shown to indicate an impaired prognosis in myocarditis.82
Several studies have indicated a significant increase in the prevalence of stress-induced cardiomyopathy or Takotsubo syndrome (TTS) during the COVID-19 pandemic.83 Researchers found that during the pandemic, 7.75% of patients with acute coronary syndrome were diagnosed with TTS, in contrast with only 1.5%-1.8% before the pandemic.84 TTS is characterized by a characteristic pattern of acute, transient regional left ventricular systolic dysfunction. The aetiology of TTS has been attributed to psychological or physical stress leading to a surge of catecholamines, causing an acute inflammatory response in tissues with a dense presence of adrenergic receptors, with myocardial oedema being a hallmark feature of Takotsubo cardiomyopathy.21,85 Multiparametric CMR, including cine images, LGE, as well as T1- and T2-mapping, is more accurate than echocardiography (or other imaging modalities), in effectively distinguishing TTS from conditions like acute myocardial infarction and myocarditis.86
CMR in athletes after recovery from COVID-19
Given that acute myocarditis accounts for approximately 10% of sudden cardiac deaths in young and active adults (aged < 35 years),87 there is a significant concern regarding myocardial injury and subsequent risk of adverse cardiovascular events in competitive athletes following COVID-19 infection. Available data shows lower rates of CMR findings of cardiac injury in the athletes.37 In a study of 145 student-athletes with mild to moderate symptoms during acute infection, only two patients (1.4%) had CMR findings consistent with myocarditis according to the updated LLC55 (Figure 6). A comprehensive multicentric study by Daniels et al,88 encompassing 13 universities, involved 2461 athletes, with 1597 undergoing CMR. Clinically, myocarditis was diagnosed in 37 out of the 1597 athletes (2.3%). Of these 37, 31 exhibited CMR findings consistent with the LLC. Interestingly, the rate of abnormal CMR results ranged from 0% to 7.6% across the participating institutions.88 These results suggest that, while the prevalence of myocarditis-like manifestations on CMR in competitive athletes following COVID-19 is relatively low, there is uncertainty about the actual numbers, given the significant variation in reported rates across different studies, ranging from 0% to 15%.37,89 A contributing factor to false-positive CMR findings in endurance athletes may be the interpretation of LGE at the RV insertion, a non-specific finding with a prevalence ranging from 0% to 26%,37,90 as post-inflammatory injury.
Figure 6.
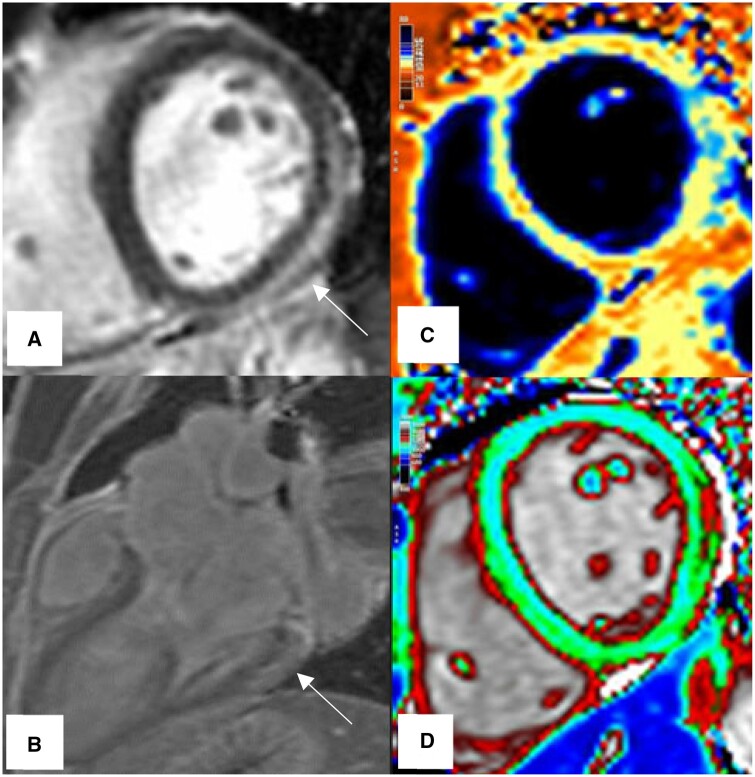
CMR findings in a young male athlete after recovery from COVID-19, clinically presented with persistent fatigue, malaise and shortness of breath. The CMR shows signs of persisting inflammation. (A) LGE-PSIR, Small pockets of pericardial effusion as low signal intensity region (arrow) along the mid-lateral and inferior walls associated with enhancement of the adjacent pericardium, mild subepicardial enhancement of the mid-inferior and inferolateral segments is visible. (B) T1 map, Increase in native T1-times in the anterior, anterolateral, and inferoseptal segments (red colour). (C) T2 map, Globally prolonged T2-relaxtaion times (blue colour). Abbreviation: PSIR = Phase Sensitive Inversion Recovery.
CMR findings in children with multisystem inflammatory syndrome after COVID-19
Multisystem inflammatory syndrome in children has been recognized as a severe complication in a small proportion of children a few weeks after SARS-CoV-2 infection.20,91 Similar to Kawasaki’s Disease, it is characterized by a severe clinical presentation, often with circulatory shock, myocardial depression, and coronary involvement.92 Several studies have shown that CMR may be an important diagnostic tool to identify a subset of patients at risk for cardiac sequelae and more prone to myocardial damage.18,20,92 A large multicentre study found that out of 111 patients who met the WHO criteria for MIS-C and showed clinical signs of cardiac involvement, 20 (18%) fulfilled the LLC, which was associated with a worse prognosis.18 Another important cardiac complication related to MIS-C is coronary artery dilatation or aneurysm, which has been reported in 6%-24% of cases.20,93,94 CMR has been established to detect the presence of coronary artery aneurysms, wall motion abnormalities, reversible ischaemia, and myocardial infarction without the use of radiation or invasive procedures.95
COVID-19-vaccine-related cardiac injury
Following the initial case series of myocarditis in young men in Israel found after Pfizer-BioNTech mRNA SARS-CoV-2 vaccination, numerous international studies have shown an increased rate of myopericarditis following mRNA-based COVID-19 vaccination.96–98 A systematic review reported an overall incidence of myopericarditis post mRNA COVID-19 vaccination of 18 cases per million doses, with significant variations depending on age, sex, and vaccine type and dose.99 The highest risk of cardiac injury is observed among male adolescents and adults aged 18-25 years, specifically after their second COVID-19 mRNA vaccine dose within 1-7 days of vaccination.100,101 The pathophysiology of vaccine-induced myopericarditis remains unclear. It has been suggested that vaccine-induced cardiac injury might be due to a hypersensitivity reaction, given its typical development post-second vaccine dose, or molecular mimicry between the SARS-CoV-2 spike protein and cardiac self-antigens.102 CMR has a key role in the diagnosis of vaccination-related cardiac injury, and a systemic review showed that more than two-thirds of patients with clinically suspected post-COVID-19-vaccination myocarditis meet the LLC, consistent with acute myocardial inflammation.103 Common CMR characteristics observed in patients with myocarditis post-COVID-19 vaccination were similar to those seen in myocarditis from other aetiologies. This includes subepicardial LGE, predominantly involving the basal inferolateral wall and associated myocardial oedema (Figure 7). In addition, small pericardial effusion may also be present, sometimes accompanied by enhancement of the adjacent pericardium.56,104,105 While most patients with myocarditis following COVID-19 vaccination demonstrate favourable short to mid-term outcomes, data on long-term follow-up are scarce. A small case series by Patel et al indicates that acute myocarditis following mRNA-based COVID-19 vaccination showed CMR-based evidence of myocardial recovery within 3-6 months, while some mild abnormalities may persist.106
Figure 7.
CMR imaging findings in a 27-year-old man with myocarditis after a second COVID-19 mRNA vaccine dose. (A, B) LGE images, Subepicardial enhancement involving basal to mid inferior, infero and anterolateral segments (white arrow). (C) T2 map, with focal myocardial inflammation manifesting as high T2-values in the mid-lateral wall (blue colour). (D) T1 map, with focal high T1-value in the subepicardial part of the mid lateral wall (red colour).
Contributor Information
Moezedin Javad Rafiee, Department of Medicine, McGill University Health Centre, Montreal, Quebec H4A3J1, Canada; Department of Diagnostic Radiology, McGill University Health Centre, Montreal, Quebec H4A3J1, Canada.
Matthias G Friedrich, Department of Medicine, McGill University Health Centre, Montreal, Quebec H4A3J1, Canada; Department of Diagnostic Radiology, McGill University Health Centre, Montreal, Quebec H4A3J1, Canada.
Funding
None declared.
Conflicts of interest
None declared.
References
- 1. WHO Coronavirus (COVID-19) Dashboard [Internet]. Accessed October 15, 2023. https://covid19.who.int
- 2. Zhang S-F, Tuo J-L, Huang X-B, et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010-2015 in Guangzhou. PLoS One. 2018;13(1):e0191789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D.. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281-292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han Y, Chen T, Bryant J, et al. Society for cardiovascular magnetic resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn Reson. 2020;22(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rudski L, Januzzi JL, Rigolin VH, et al. Multimodality imaging in evaluation of cardiovascular complications in patients with COVID-19: JACC scientific expert panel. J Am Coll Cardiol. 2020;76(11):1345-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Task Force for the management of COVID-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: part 2-care pathways, treatment, and follow-up. Eur Heart J. 2022;43(11):1059-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferreira VM, Plein S, Wong TC, et al. Cardiovascular magnetic resonance for evaluation of cardiac involvement in COVID-19: recommendations by the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Magn Reson. 2023;25(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with covid-19 - a case series. N Engl J Med. 2020;382(25):2478-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheth AR, Grewal US, Patel HP, et al. Possible mechanisms responsible for acute coronary events in COVID-19. Med Hypotheses. 2020;143:110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burkert FR, Niederreiter L, Dichtl W, et al. Case report of a COVID-19-associated myocardial infarction with no obstructive coronary arteries: the mystery of the phantom embolus or local endothelitis. Eur Heart J Case Rep. 2021;5(2):ytaa521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cytokine Release Syndrome in COVID-19: mechanisms and Management | Frontiers Research Topic [Internet]. Accessed September 22, 2023. https://www.frontiersin.org/research-topics/24736/cytokine-release-syndrome-in-covid-19-mechanisms-and-management
- 17. Doyen D, Moceri P, Ducreux D, Dellamonica J.. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet (London, England). 2020;395(10235):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aeschlimann FA, Misra N, Hussein T, et al. Myocardial involvement in children with post-COVID multisystem inflammatory syndrome: a cardiovascular magnetic resonance based multicenter international study-the CARDOVID registry. J Cardiovasc Magn Reson. 2021;23(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chakraborty A, Philip R, Santoso M, Naik R, Merlocco A, Johnson JN.. Cardiovascular magnetic resonance in children with multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19: institutional protocol-based medium-term follow-up study. Pediatr Cardiol. 2022;43(8):1879-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A.. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Osch D, Asselbergs FW, Teske AJ.. Takotsubo cardiomyopathy in COVID-19: a case report. Haemodynamic and therapeutic considerations. Eur Heart J Case Rep. 2020;4(FI1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moady G, Atar S.. Stress-induced cardiomyopathy—considerations for diagnosis and management during the COVID-19 pandemic. Medicina. 2022;58(2):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong N, Cai J, Zhou Y, Liu J, Li F.. End-stage heart failure with COVID-19: strong evidence of myocardial injury by 2019-nCoV. JACC Heart Fail. 2020;8(6):515-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rafiee MJ, Babaki Fard F, Friedrich MG.. COVID-19, myocardial edema and dexamethasone. Med Hypotheses. 2020;145:110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teuwen LA, Geldhof V, Pasut A, Carmeliet P.. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sang CJ, Burkett A, Heindl B, et al. Cardiac pathology in COVID-19: a single center autopsy experience. Cardiovasc Pathol. 2021;54:107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halushka MK, , Vander Heide RS.. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maiese A, Frati P, Del Duca F, et al. Myocardial pathology in COVID-19-associated cardiac injury: a systematic review. Diagnostics (Basel). 2021;11(9):1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones EAV. Mechanism of COVID-19-induced cardiac damage from patient, in vitro and animal studies. Curr Heart Fail Rep. 2023;20(5):451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pellegrini D, Kawakami R, Guagliumi G, et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143(10):1031-1042. [DOI] [PubMed] [Google Scholar]
- 32. Gluckman TJ, Bhave NM, Allen LA, et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;79(17):1717-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seferović PM, Tsutsui H, Mcnamara DM, et al. Heart failure association, heart failure society of America, and Japanese heart failure society position statement on endomyocardial biopsy. J Card Fail. 2021;27(7):727-743. [DOI] [PubMed] [Google Scholar]
- 34. Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanneman K, Houbois C, Schoffel A, et al. Combined cardiac fluorodeoxyglucose–positron emission tomography/magnetic resonance imaging assessment of myocardial injury in patients who recently recovered from COVID-19. JAMA Cardiol. 2022;7(3):298-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rajiah PS, Kalisz K, Broncano J, et al. Myocardial strain evaluation with cardiovascular MRI: physics, principles, and clinical applications. Radiographics. 2022;42(4):968-990. [DOI] [PubMed] [Google Scholar]
- 37. Petersen SE, Friedrich MG, Leiner T, et al. Cardiovascular magnetic resonance for patients with COVID-19. JACC Cardiovasc Imaging. 2022;15(4):685-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jenista ER, Wendell DC, Azevedo CF, et al. Revisiting how we perform late gadolinium enhancement CMR: insights gleaned over 25 years of clinical practice. J Cardiovasc Magn Reson. 2023;25(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK.. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29(1):89-103. [DOI] [PubMed] [Google Scholar]
- 40. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ.. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26(15):1461-1474. [DOI] [PubMed] [Google Scholar]
- 41. Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158-3176. [DOI] [PubMed] [Google Scholar]
- 42. Patel AR, Salerno M, Kwong RY, Singh A, Heydari B, Kramer CM.. Stress cardiac magnetic resonance myocardial perfusion imaging: JACC review topic of the week. J Am Coll Cardiol. 2021;78(16):1655-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thornton GD, Shetye A, Knight DS, et al. Myocardial perfusion imaging after severe COVID-19 infection demonstrates regional ischemia rather than global blood flow reduction. Front Cardiovasc Med. 2021;8:764599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hillier E, Covone J, Friedrich MG.. Oxygenation-sensitive cardiac MRI with vasoactive breathing maneuvers for the non-invasive assessment of coronary microvascular dysfunction. J Vis Exp. 2022;(186):e64149. [DOI] [PubMed] [Google Scholar]
- 45. Mathew RC, Bourque JM, Salerno M, Kramer CM.. Cardiovascular imaging techniques to assess microvascular dysfunction. JACC Cardiovasc Imaging. 2020;13(7):1577-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bratis K, Mahmoud I, Chiribiri A, Nagel E.. Quantitative myocardial perfusion imaging by cardiovascular magnetic resonance and positron emission tomography. J Nucl Cardiol. 2013;20(5):860-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharrack N, Biglands JD, Plein S, Broadbent DA.. Chapter 15 - perfusion MRI in the heart: first-pass perfusion. In: Cheng HLM, Strijkers GJ, eds. Quantitative Perfusion MRI. vol.11. Advances in Magnetic Resonance Technology and Applications [Internet]. Academic Press; 2023:367-404. Accessed March 10, 2024. https://www.sciencedirect.com/science/article/pii/B9780323952095000192 [Google Scholar]
- 48. Hsu LY, Rhoads KL, Holly JE, Kellman P, Aletras AH, Arai AE.. Quantitative myocardial perfusion analysis with a dual-bolus contrast-enhanced first-pass MRI technique in humans. J Magn Reson Imaging. 2006;23(3):315-322. [DOI] [PubMed] [Google Scholar]
- 49. Zorach B, Shaw PW, Bourque J, et al. Quantitative cardiovascular magnetic resonance perfusion imaging identifies reduced flow reserve in microvascular coronary artery disease. J Cardiovasc Magn Reson. 2018;20(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hsu L-Y, Jacobs M, Benovoy M, et al. Diagnostic performance of fully automated pixel-wise quantitative myocardial perfusion imaging by cardiovascular magnetic resonance. JACC Cardiovasc Imaging. 2018;11(5):697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patriki D, Gresser E, Manka R, Emmert MY, Lüscher TF, Heidecker B.. Approximation of the incidence of myocarditis by systematic screening with cardiac magnetic resonance imaging. JACC Heart Fail. 2018;6(7):573-579. [DOI] [PubMed] [Google Scholar]
- 52. Adeboye A, Alkhatib D, Butt A, Yedlapati N, Garg N.. A review of the role of imaging modalities in the evaluation of viral myocarditis with a special focus on COVID-19-related myocarditis. Diagnostics (Basel). 2022;12(2):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Panchal A, Kyvernitakis A, Mikolich JR, Biederman RWW.. Contemporary use of cardiac imaging for COVID-19 patients: a three center experience defining a potential role for cardiac MRI. Int J Cardiovasc Imaging. 2021;37(5):1721-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luetkens JA, Isaak A, Öztürk C, et al. Cardiac MRI in suspected acute COVID-19 myocarditis. Radiol Cardiothorac Imaging. 2021;3(2):e200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6(8):945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sánchez Tijmes F, Marschner CA, de Matos JFRG, et al. Imaging acute and chronic cardiac complications of COVID-19 and after COVID-19 vaccination. Radiographics. 2023;43(9):e230044. [DOI] [PubMed] [Google Scholar]
- 57. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ammirati E, Lupi L, Palazzini M, et al. Prevalence, characteristics, and outcomes of COVID-19–associated acute myocarditis. Circulation. 2022;145(15):1123-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2330-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roca-Fernandez A, Wamil M, Telford A, et al. Cardiac abnormalities in long COVID 1-year post-SARS-CoV-2 infection. Open Heart. 2023;10(1):e002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Galea N, Marchitelli L, Pambianchi G, et al. T2-mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID-19: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2021;23(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Artico J, Shiwani H, Moon JC, et al. Myocardial involvement after hospitalization for COVID-19 complicated by troponin elevation: a prospective, multicenter, observational study. Circulation. 2023;147(5):364-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kotecha T, Knight DS, Razvi Y, et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42(19):1866-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Drakos S, Chatzantonis G, Bietenbeck M, et al. A cardiovascular magnetic resonance imaging-based pilot study to assess coronary microvascular disease in COVID-19 patients. Sci Rep. 2021;11(1):15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cenko E, Badimon L, Bugiardini R, et al. Cardiovascular disease and COVID-19: a consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc Res. 2021;117(14):2705-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Evans PC, Rainger GE, Mason JC, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC council of basic cardiovascular science. Cardiovasc Res. 2020;116(14):2177-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bois MC, Boire NA, Layman AJ, et al. COVID-19–associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143(3):230-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verma A, Ramayya T, Upadhyaya A, et al. Post COVID-19 syndrome with impairment of flow-mediated epicardial vasodilation and flow reserve. Eur J Clin Invest. 2022;52(12):e13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Caldeira D, Pinto FJ.. COVID-19 and myocardial infarction. Lancet. 2021;398(10315):1963-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fanaroff AC, Garcia S, Giri J.. Myocardial infarction during the COVID-19 pandemic. JAMA. 2021;326(19):1916-1918. [DOI] [PubMed] [Google Scholar]
- 72. Saad M, Kennedy KF, Imran H, et al. Association between COVID-19 diagnosis and in-hospital mortality in patients hospitalized with ST-segment elevation myocardial infarction. JAMA. 2021;326(19):1940-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vidula MK, Rajewska-Tabor J, Cao JJ, et al. Myocardial injury on CMR in patients with COVID-19 and suspected cardiac involvement. JACC Cardiovasc Imaging. 2023;16(5):609-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Philip AM, George LJ, John KJ, et al. A review of the presentation and outcome of left ventricular thrombus in coronavirus disease 2019 infection. J Clin Transl Res. 2021;7(6):797-808. [PMC free article] [PubMed] [Google Scholar]
- 75. Karikalan S, Sharma M, Chandna M, et al. Intracardiac thrombus in coronavirus disease-2019. Cureus. 2022;14(3):e22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: A comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152(1):75-84. [DOI] [PubMed] [Google Scholar]
- 77. Lan Y, Liu W, Zhou Y.. Right ventricular damage in COVID-19: association between myocardial injury and COVID-19. Front Cardiovasc Med. 2021;8:606318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2287-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McErlane J, McCall P, Willder J, Berry C, Shelley B, COVID-RV Investigators. Right ventricular free wall longitudinal strain is independently associated with mortality in mechanically ventilated patients with COVID-19. Ann Intensive Care. 2022;12(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Willder JM, McCall P, Messow CM, Gillies M, Berry C, Shelley B.. Study protocol for COVID-RV: a multicentre prospective observational cohort study of right ventricular dysfunction in ventilated patients with COVID-19. BMJ Open. 2021;11(1):e042098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Aquaro GD, Negri F, De Luca A, et al. Role of right ventricular involvement in acute myocarditis, assessed by cardiac magnetic resonance. Int J Cardiol. 2018;271:359-365. [DOI] [PubMed] [Google Scholar]
- 82. Diaz-Arocutipa C, Saucedo-Chinchay J, Argulian E.. Association between right ventricular dysfunction and mortality in COVID-19 patients: a systematic review and meta-analysis. Clin Cardiol. 2021;44(10):1360-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shah RM, Shah M, Shah S, Li A, Jauhar S.. Takotsubo syndrome and COVID-19: associations and implications. Curr Probl Cardiol. 2021;46(3):100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jabri A, Kalra A, Kumar A, et al. Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3(7):e2014780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ghadri J-R, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39(22):2047-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Singh T, Khan H, Gamble DT, Scally C, Newby DE, Dawson D.. Takotsubo syndrome: pathophysiology, emerging concepts, and clinical implications. Circulation. 2022;145(13):1002-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Corrado D, Basso C, Thiene G.. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res. 2001;50(2):399-408. [DOI] [PubMed] [Google Scholar]
- 88. Daniels CJ, Rajpal S, Greenshields JT, et al. Big Ten COVID-19 Cardiac Registry Investigators. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the big ten COVID-19 cardiac registry. JAMA Cardiol. 2021;6(9):1078-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Juhász V, Csulak E, Szabó L, et al. Retrospective study of COVID-19 experiences in elite multinational aquatic athletes. Sci Rep. 2023;13(1):13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Domenech-Ximenos B, Sanz-de la Garza M, Prat-González S, et al. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J Cardiovasc Magn Reson. 2020;22(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bartoszek M, Małek ŁA, Barczuk-Falęcka M, Brzewski M.. Cardiac magnetic resonance follow-up of children after pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 with initial cardiac involvement. J Magn Reson Imaging. 2022;55(3):883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Theocharis P, Wong J, Pushparajah K, et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021;22(8):896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P.. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tacke CE, Kuipers IM, Groenink M, Spijkerboer AM, Kuijpers TW.. Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with Kawasaki disease. Circ Cardiovasc Imaging. 2011;4(6):712-720. [DOI] [PubMed] [Google Scholar]
- 96. Husby A, Køber L.. COVID-19 mRNA vaccination and myocarditis or pericarditis. Lancet. 2022;399(10342):2168-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Naveed Z, Li J, Wilton J, et al. Comparative risk of myocarditis/pericarditis following second doses of BNT162b2 and mRNA-1273 coronavirus vaccines. J Am Coll Cardiol. 2022;80:20. Accessed September 26, 2023. https://pubmed.ncbi.nlm.nih.gov/36357091/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wong H-L, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022;399(10342):2191-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ling RR, Ramanathan K, Tan FL, et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022;10(7):679-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Patone M, Mei XW, Handunnetthi L, et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation. 2022;146(10):743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Heymans S, Cooper LT.. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jain SS, Steele JM, Fonseca B, et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148(5):e2021053427. [DOI] [PubMed] [Google Scholar]
- 104. Chelala L, Jeudy J, Hossain R, Rosenthal G, Pietris N, White CS.. Cardiac MRI findings of myocarditis after COVID-19 mRNA vaccination in adolescents. AJR Am J Roentgenol. 2022;218(4):651-657. [DOI] [PubMed] [Google Scholar]
- 105. Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6(12):1446-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Patel YR, Shah NR, Lombardi K, et al. Follow-up cardiovascular magnetic resonance findings in patients with COVID-19 vaccination-associated acute myocarditis. JACC Cardiovasc Imaging. 2022;15(11):2007-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]