Abstract
Mouse strains with null mutations in the gamma interferon gene (Ifng) or the gamma interferon receptor gene (Ifngr) have been engineered. The use of these strains as animal models of viral and bacterial infections has enhanced our understanding of the role of gamma interferon (IFN-γ) in the host immune response. However, direct comparisons between Ifng−/− (GKO) and Ifngr−/− (RGKO) mice have been problematic because previously available strains of these mice have had different genetic backgrounds (i.e., C57BL/6 and BALB/c for GKO mice and 129/Sv//Ev for RGKO mice). To enable direct comparison of herpes simplex virus type 1 (HSV-1) infections in GKO and RGKO mice, we introduced the IFN-γ null mutation into the 129/Sv//Ev background. We report that, after HSV-1 inoculation, mortality was significantly greater in RGKO mice than in GKO mice (38 versus 23%, P = 0.0001). Similarly, the mortality from vaccinia virus challenge was significantly greater in RGKO mice than in GKO mice. With differences in genetic background excluded as a confounding issue, these results are consistent with the existence of an alternative ligand(s) for the IFN-γ receptor that is also capable of mediating protection against viral challenge.
Gamma interferon (IFN-γ) was originally referred to as immune interferon in recognition of its antiviral activity. However, IFN-γ is currently better known for the remarkable spectrum of pleiotropic effects it elicits in diverse tissues and particularly for its unique immunoregulatory properties which distinguish it from IFN-α/β, other antiviral proteins in the interferon family. The antiviral effects of IFN-γ are not as well understood, but they appear to be especially important for long-term control of viral infections (4, 38). IFN-γ also mediates protection against intracellular microbes, including certain viruses, by activating macrophages and other mononuclear phagocytes to produce nitrous oxide (2, 4). IFN-γ and IFN-α/β bind to distinct receptors, and no cross-reactivity has been observed. IFN-γ binds to the α chain of the high-affinity IFN-γ receptor that is ubiquitously expressed on different cell types, and it is the only known ligand for this receptor. A low-affinity IFN-γ receptor has been identified on human macrophages, but its physiological significance is unknown (1, 13).
Recent studies of null-mutant mice have confirmed the importance of IFN-γ in the host immune responses to several bacterial and viral pathogens, including herpes simplex virus type 1 (HSV-1), vaccinia virus, measles virus, and Theiler’s virus (5, 9, 16, 17, 24). Studies with these IFN-γ mutant strains have also provided evidence, albeit controversial at times, supporting and refuting the involvement of IFN-γ in autoimmune diseases such as experimental allergic encephalomyelitis, experimental autoimmune myasthenia gravis, and insulin-dependent diabetes mellitus (2, 15, 25, 40). Controversy has arisen from the fact that different results have been obtained from various studies depending on whether the null mutation is in the IFN-γ gene (Ifng) (GKO mice) (10) or in the IFN-γ receptor gene (Ifngr) (RGKO mice) (24). Previously, GKO and RGKO mice have been available only in different genetic backgrounds (i.e., C57BL/6 and BALB/c for GKO and 129/Sv//Ev for RGKO).
Previously we reported a prolonged inflammatory response in the trigeminal ganglion after ocular inoculation with HSV-1 (7). We showed the CD4+ and CD8+ T cells associated with the large amounts of IFN-γ which were tightly surrounding neurons (some of which were latently infected with HSV-1 as shown by in situ hybridization). These observations led to speculation that IFN-γ might play a role during HSV-1 infection, and we proposed the use of GKO and RGKO strains to directly test this hypothesis. However, the strain restrictions of these mice were problematic, since it is known that different mouse strains vary in susceptibility to HSV-1, with the C57BL/6 strain being relatively resistant to the virus (26). To circumvent the influence on experimental outcome of the different genetic backgrounds of the mutant mice, the IFN-γ null mutation was introduced into the 129/Sv//Ev background. This enabled the direct comparison of the outcomes of HSV-1 infections of GKO and RGKO mice, which differed genetically only at the mutant locus.
Isolation of the IFN-γ null mutation in the 129/Sv//Ev background.
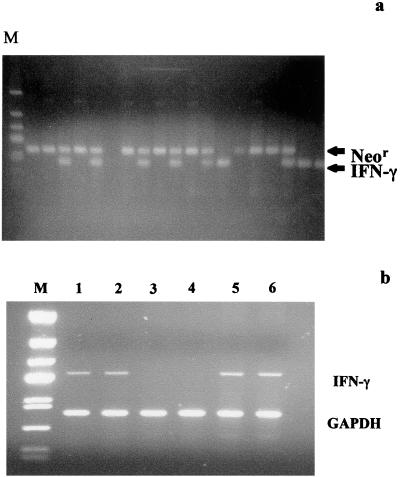
The AB-1 embryonic stem (ES) cell clone 97E of the 129/Sv//Ev strain, heterozygous for a mutation in Ifng (9), was obtained from Tim Stewart (Genentech), expanded, and injected into blastocysts of the C57BL/6 strain. Chimeric founder males were mated to 129/Sv//EvTac female mice (Taconic Farms, Inc., Germantown, N.Y.) to generate 129/Sv//Ev mice heterozygous for the IFN-γ null mutation. Offspring resulting from germ line transmission of the ES cell genome were identified by glucose phosphate isomerase-1 (Gpi-1s) isozyme assay, type A (distinct from type AB) mice (22) were selected, and of these selected mice, those carrying the mutation were identified by PCR. The IFN-γ mutation was thereby retained in the 129/Sv//Ev genetic background. Ifng+/− heterozygous mice were intercrossed to produce Ifng−/− homozygous, GKO mutant mice which were identified by PCR analysis of DNA extracted from their tails for IFN-γ and Neor gene sequences. In Fig. 1a, PCR genotyping results for several offspring from matings of heterozygous Ifng+/− mice are presented. GKO mice homozygous for the IFN-γ null mutation (Ifng−/−) are identified by the exclusive presence of the 375-bp Neor band and the absence of the 220-bp IFN-γ band (Fig. 1a). The PCR genotyping results were confirmed by probing Southern blots of BamHI-digested tail DNA from the same mice with an Ifng cDNA probe (not shown) (9), and from these latter mice, a breeding colony was derived and maintained. Reverse transcriptase (RT)-PCR analysis of GKO spleen cells stimulated in vitro with concanavalin A (ConA) failed to detect IFN-γ mRNAs, whereas these transcripts were readily detected in ConA-stimulated spleen cells from RGKO and control 129/Sv//Ev mice, confirming the GKO null-mutant phenotype (Fig. 1b).
FIG. 1.
(a) PCR genotyping of GKO mice. Tail DNA from offspring of matings of heterozygous 129/Sv//Ev mice were analyzed by PCR for IFN-γ and Neor gene sequences to identify mice homozygous for the IFN-γ null mutation. The upper band is the Neor gene amplification product, and the lower band is the IFN-γ gene amplification product. PCR primers for IFN-γ gene are as follows: forward, 5′-AGAAGTAAGTGGAAGGGCCCAGAAG, and reverse, 5′-AGGGAAACTGGGAGAGGAGAAATAT, giving a 220-bp product. Primers for the Neor gene are as follows: forward, 5′-TCAGCGCAGGGGCGCCCGGTTCTTT, and reverse, 5′-ATCGACAAGACCGGCTTCCATCCGA, giving a 375-bp product. (b) RT-PCR for Ifng mRNA. cDNA preparations from ConA-stimulated spleen cells isolated from 129/Sv//Ev mice (lanes 1 and 2), GKO mice (lanes 3 and 4), and RGKO mice (lanes 5 and 6) were amplified with Ifng-specific primers. M, markers; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Breeding pairs of mice homozygous for the null mutation in Ifngr (129/Sv//Ev, RGKO) were obtained from Michel Aguet (Institute of Molecular Biology, University of Zurich) (23). RGKO and GKO mice were bred under specific-pathogen-free conditions in a vivarium at the City of Hope National Medical Center. 129/Sv//EvTac control mice (referred to herein as 129/Sv//Ev mice) were purchased from Taconic Farms, Inc. Experiments were conducted in strict compliance with the Guidelines on the Care and Use of Laboratory Animals in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. All experiments with mice were approved by the Institutional Research and Animal Care Committee, and ocular experimental procedures were conducted according to the Association for Research in Vision and Ophthalmology resolution on the use and care of laboratory animals.
Mortality of vaccinia virus-infected GKO, RGKO, and control mice.
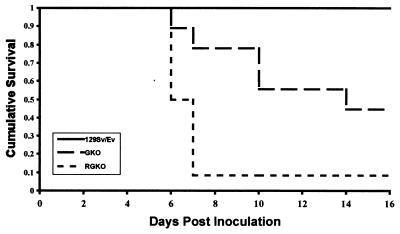
Prior studies with normal mice and mice unresponsive to IFN-γ due to a null mutation in Ifng or Ifngr have established that interferon is crucial for survival after inoculation with poxviruses such as vaccinia virus (31). To confirm the susceptibility of GKO mice with the 129/Sv//Ev background to vaccinia virus and to directly compare the response of the GKO and RGKO strains, mutant and control male mice were challenged with vaccinia virus by tail vein inoculation. The results presented in Fig. 2 confirm that the control 129/Sv//Ev mice were completely resistant to vaccinia virus infection as expected (9, 31, 39). Compared to the mortality rates of control mice, the mortality rates in vaccinia virus-infected GKO mice were high (56%) (P = 0.002) and even higher (92%) in RGKO mice (P = 0.0001). The difference in mortality rates between RGKO and GKO mice was significant (P = 0.03) and corresponded to a 3.3-fold increase in the relative risk of death for RGKO mice inoculated with HSV-1 compared to similarly inoculated GKO mice (95% confidence limit).
FIG. 2.
Survival of vaccinia virus-infected 129/Sv//Ev, GKO, and RGKO mice. Deeply anesthetized, 6- to 8-week-old male mice were inoculated intravenously with 107 PFU of vaccinia virus. There were 12 mice in each group, except that the GKO group had 9 mice. Mice were monitored daily over a 20-day period, and mice with symptoms of life-threatening disease were euthanatized.
Mortality of HSV-1 Infected GKO, RGKO, and control mice.
To determine the role of IFN-γ during the acute stage of HSV-1 infection, deeply anesthetized, 6- to 8-week-old male GKO, RGKO, and 129/Sv//Ev mice were inoculated with HSV-1 F strain (American Type Culture Collection, Rockville, Md.) by gently massaging a 4-μl drop containing 105 to 107 PFU of virus into the scarified cornea of the right eye (7). Mice were monitored twice daily for signs of morbidity and development of encephalitis, and mice with symptoms indicative of imminent death due to encephalitis were euthanatized. Clinical signs of HSV-1 infection, including corneal clouding (an early sign of herpes stromal keratitis), eyelid disease, ruffled coat, and signs of encephalitis, such as circling behavior and impaired coordination and movement, were first seen in the IFN-γ mutant mice and were generally more severe and prolonged in the IFN-γ mutant mice than in the 129/Sv//Ev mice. The most noticeable sign of HSV-1 infection in these mice (and particularly in the RGKO strain) was periocular skin disease characterized, in its most severe stage, by the complete loss of facial hair around the inoculated eye and extending down the snout and up to, and including, the forehead and often accompanied by bleeding and skin lacerations. Whereas most of the GKO and control mice recovered, most of the RGKO mice showed progressive deterioration.
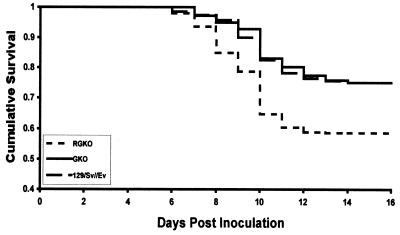
The survival data for GKO, RGKO, and 129/Sv//Ev mice inoculated with HSV-1 are presented in Fig. 3. Mortality rates were calculated based on the total number of deaths occurring after inoculation with HSV-1. Figure 3 summarizes mortality data accrued from multiple experiments involving HSV-1 infection of large numbers of mice (150 129/Sv//Ev, 244 GKO, and 380 RGKO) used in the course of studying the role of IFN-γ during acute and latent infection, and therefore, the statistical significance of the data is very high. Univariate Cox regression (8) was performed to identify the risk factors that increased rates of mouse mortality. Possible risk factors evaluated included gender, mouse strain, and inoculum dose. All significance testing was performed at the 0.05 level (two sided).
FIG. 3.
Survival of HSV-1-infected 129/Sv//Ev, GKO, and RGKO mice. Male, 6- to 8-week-old mice were inoculated in the right eye by ocular scarification with doses of HSV-1 ranging from 105 to 107 PFU. Mice were monitored daily over a 20-day period for signs of encephalitis, and mice with pronounced symptoms indicative of imminent death were euthanatized. As no statistically significant difference was found between groups given inocula of 105, 106, or 107 PFU, the data from these three groups were pooled and analyzed together.
Two significant findings emerged from the studies that compared HSV-1 infection in GKO and RGKO mice to infection in 129/Sv//Ev mice. The first is that disruption of Ifng did not affect rates of mortality in HSV-1-infected mice compared to the mortality rates in control 129/Sv//Ev mice (Fig. 3). The mortality rate was 23% in both groups. The second, and most interesting, finding in our study was that mice with a disrupted Ifngr gene had a twofold increase in risk of death (at the 95% confidence limit) after HSV-1 inoculation compared to GKO and 129/Sv//Ev mice (Fig. 3). The overall mortality rate was 38% in RGKO mice, compared to 23% in GKO mice (P = 0.0001) and 23% in the 129/Sv//Ev mice (P = 0.0003). The difference in susceptibility of RGKO and GKO mice to HSV-1 essentially confirms the results obtained after vaccinia virus challenge (Fig. 2) and suggests that the difference in viral susceptibility of RGKO and GKO mice might be more general. These results show that IFN-γ fails to confer protection against mortality induced by HSV-1 infection, while, in contrast, the presence of the IFN-γ receptor (as in GKO mice) was associated with a significant protective effect.
Time course of HSV-1 infection in RGKO and GKO mice.
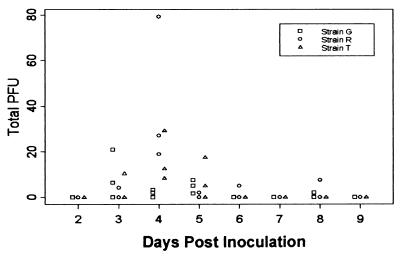
The increased mortality of RGKO compared to GKO and 129/Sv//Ev mice suggested the possibility of enhanced replication and/or persistence of HSV-1 in the RGKO mice. We have previously reported a statistically significant higher mean titer in the trigeminal ganglia and brain stem cells of RGKO than in 129/Sv//Ev mice 3 to 4 days after HSV-1 inoculation; however, the difference in titer was slight (<0.5 log) (7). More recently, we determined the daily viral titers in trigeminal ganglia and brain stem cells from days 2 to 7 after HSV-1 inoculation. The results showed that, contrary to expectation, there was no significant difference in either the kinetics of HSV-1 replication or clearance in the trigeminal ganglia of RGKO, GKO, and 129/Sv//Ev mice (6). More relevant to the mortality results presented here, Fig. 4 shows a time course of daily brain stem HSV-1 titers in the RGKO, GKO, and 129/Sv//Ev mice. For statistical comparison of HSV-1 titers, the total plaque counts for the three mouse groups were fitted to an overdispersed Poisson model. Statistical analysis of HSV-1 titers in the eye indicated a significantly greater likelihood of HSV-1 persistence in the eyes of GKO and RGKO mice than in 129/Sv//Ev mice (not shown), similar to the study of Bouley et al., which showed persistence of HSV-1 in the tear film of GKO, but not control, mice (5). Persistence of HSV-1 in the eyes of IFN-γ mutant mice shows that IFN-γ-dependent mechanisms are crucial for clearance of HSV-1 from the eye, as was previously shown for skin infections (21, 35). However, we found no significant difference in brain stem viral titers over the course of the infection (Fig. 4). All the mice surviving inoculation with HSV-1 were confirmed as being latently infected.
FIG. 4.
Time course of HSV-1 infection in 129/Sv//Ev, GKO, and RGKO mice. The right eyes of 6- to 8-week-old male mice were inoculated with 106 PFU of HSV-1 by ocular scarification. Three mice from each group were sacrificed daily from day 2 through day 7, and HSV-1 titers in brain stem cells were determined. The whole-tissue HSV-1 titers were plotted against day postinoculation for 129/Sv//Ev (strain T), GKO (strain G), and RGKO (strain R) mice. There was no statistically significant difference in HSV-1 titers in the brain stem cells for the three groups of mice.
At face value, the equivalent titers are surprising, since cell culture studies have demonstrated inhibition of HSV-1 by IFN-γ (11, 14). However, the results from our time course study are in agreement with an earlier study of GKO mice from another group which showed no difference in mean HSV-1 titers in nervous system tissue at set times after HSV-1 inoculation (19). Indeed, other studies using an IFN-γ-transgenic model showed that IFN-γ protected against HSV-1-induced mortality without significantly affecting HSV-1 titers in the nervous system of transgenic, compared to nontransgenic, mice (18). Similarly, in studies with murine gamma herpesvirus 68, there was no difference in viral load or rate of virus clearance in the lungs of infected RGKO mice compared to 129/Sv//Ev mice (12). In contrast to the apparent dispensability of IFN-γ in the control of murine gamma herpesvirus 68 in the lung, severe structural damage and elevated latency loads were seen in the spleens of RGKO, but not 129/Sv//Ev, mice.
Contrasting its role in acute infection, we have recently demonstrated a role for IFN-γ in the control of reactivated HSV-1 whereby it contributes to the maintenance of biological latency (i.e., the absence of infectious HSV-1 in ganglionic homogenates or HSV-1 antigens in ganglionic sections) (6). Because infectious HSV-1 has never been found in mice not subjected to hyperthermia to induce reactivation, we suggested that IFN-γ does not maintain molecular latency (i.e., it does not block the reactivation process itself). Considered together, the results of our studies assessing the role of IFN-γ during acute and latent infection are consistent with the postulated role of IFN-γ in the long-term control of viral infections (38).
Possible explanations for the phenotypic difference between RGKO and GKO mice.
We do not think that minor differences in genetic background can explain the higher mortality rates of RGKO compared to GKO mice (3, 20). This is because the GKO strain used in this study was derived from the same 129/Sv//Ev background as was the RGKO strain. Although the 129/Sv//Ev strain used to derive the GKO strain was Gpi1sc/Gpi1sc, we selected Gpi1sa/Gpi1sa animals for propagation because RGKO mice, also derived with AB-1 ES cells, are Gpi1sa/Gpi1sa (unpublished observation). For the GKO strain, the Gpi1sa allele originated from the AB-1 ES cell clone that carried the targeted mutation of Ifng (24). We typed two independent AB-1 ES clones, including the clone used here, and found that they were both Gpi1sa/Gpi1sa and not Gpi1sc/Gpi1sc, as previously reported for AB-1 ES cells (34). Based on this analysis, we believe the GKO and RGKO strains to be isogenic except for the mutant locus and to differ from the control 129/Sv//Ev strain at Gpi1s. Such minor differences in histocompatibility loci between the IFN-γ knockout strains (GKO and RGKO mice) and 129/Sv//Ev would not be expected to influence the results reported here. However, prior studies of HSV-1 infection in GKO mice of different genetic backgrounds have produced dramatically different results. Thus, Geiger et al. (19) reported no mortality in C57BL/6 control or C57BL/6 GKO mice inoculated with HSV-1 strain F, while, in contrast, Bouley et al. (5) reported that BALB/c GKO mice died after inoculation with a dose of HSV-1 which was sublethal when routinely used to induce herpes stromal keratitis in control BALB/c mice. Aside from differences in the genetic background of GKO strain studied, differences in HSV-1 strain, route of inoculation, age, and gender of the mice used could all contribute to the discrepant mortality rates of the different GKO strains (3, 34). For example, we found that gender strongly influenced the outcome of HSV-1 infection in both IFN-γ mutant and control mice, with males being more susceptible than females (20a).
The interpretation that we favor for these results is that some novel ligand induced by viral infection elicits a protective response against HSV-1 and vaccinia virus infections in GKO mice by virtue of its interaction with the IFN-γ receptor which is functional in GKO, but not in RGKO, mice (9, 24). Although not formally excluded, the notion that the mere presence of the receptor, independent of ligand binding, can somehow protect against viral challenge is much less attractive, because it is difficult to envisage how such a mechanism could affect virus infection. We considered potential developmental effects of the Ifng−/− and Ifngr−/− mutations but could not conceive an explanation that could account for our results without the presence of an alternative ligand for the receptor. As an explanation for our results, we also considered the possibility that IFN-γ signaling through the low-affinity IFN-γ receptor (1, 13) somehow enhances susceptibility to viral infections in RGKO mice. But, even if this mechanism exists, it would not explain the relative resistance to viral infection that we observed in GKO mice. We even considered the possibility that a prior, inapparent, bacterial or viral infection in our IFN-γ mutant colonies could, through effects on the host immune response, have altered the outcome of a subsequent virus infection, as was recently shown for selected virus pairs (33). Since both colonies of mice were housed together, it is likely that both would have been infected with the unknown agent (if such an infection had occurred), and hence, it is difficult to explain the relative resistance of GKO mice compared to RGKO mice on this basis. Finally, our hypothesis is consistent with the conclusions drawn in other systems where similar phenotypic discrepancies between ligand and receptor null mutants have been found. Invariably, the proposed, and in some instances verified, explanation for the discrepancy has been that the receptor was indeed being utilized by another, closely related but previously unrecognized, ligand(s) (10, 28, 29).
A candidate for the novel ligand in the GKO mice is the neuronal IFN-γ immunoreactive molecule (N-IFN-γ), which has recently been purified from rat trigeminal ganglia by using two monoclonal antibodies (DB1 and DB16) that recognize distinct epitopes in rat IFN-γ (30). Although distinct from authentic IFN-γ, N-IFN-γ shares many of its biological properties, including induction of major histocompatibility complex class I and II antigens on macrophages and cultured skeletal muscle cells, direct antiviral activity, and growth-stimulatory activity for Trypanosoma brucei. The proposed existence of N-IFN-γ is controversial, though, because no other reports confirming the isolation of this protein have been made. However, a recent independent study, combining whole-cell patch-clamp electrophysiology with single-cell RT-PCR and confocal laser immunocytochemistry, has demonstrated the expression of IFN-γ in cultured, fetal-rat, dorsal root ganglion neurons (27). Because the same DB1 antibody was used for immunostaining, this neuron-expressed IFN-γ may be identical to N-IFN-γ (30). The putative N-IFN-γ is an attractive candidate as the novel ligand for the IFN-γ receptor, since it is thought to be expressed in sensory ganglionic neurons, the primary site of HSV-1 replication and latency (32, 36). After ocular inoculation, HSV-1 spreads by retrograde axonal transport to sensory ganglionic and central nervous system neurons, where compensatory upregulation of inducible N-IFN-γ expression may occur, conferring resistance to infection to GKO mice. The validity of this hypothesis can be tested once the putative N-IFN-γ gene is isolated, as detailed characterization and elucidation of the pattern of expression of the protein will then be possible. Derivation of the IFN-γ null mutation in the same 129/Sv//Ev background as the IFN-γ receptor null mutation should prove useful for examining the diverse biological roles of IFN-γ. In particular, these strains can be used to determine the mechanisms underlying the differences in susceptibilities to autoimmune diseases and infections previously reported for IFN-γ null mutant mice (15, 25, 37, 41).
Acknowledgments
We thank Michel Aguet for supplying the RGKO mouse strain and Tim Stewart for supplying the ES cell clone carrying the null mutation for Ifng.
This work was supported by Public Health Service grant MH55784 from the National Institute of Mental Health.
REFERENCES
- 1.Bach E A, Aguet M, Schreiber R D. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 2.Balasa B, Deng C, Lee J, Bradley L M, Dalton D K, Christadoss P, Sarvetnick N. Interferon gamma (IFN-gamma) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med. 1997;186:385–391. doi: 10.1084/jem.186.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banbury Conference on Genetic Background in Mice. Viewpoint: mutant mice and neuroscience: recommendations concerning genetic background. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 4.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 5.Bouley D M, Kanangat S, Wire W, Rouse B T. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 6.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantin E M, Hinton D R, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox D R. Regression models and life tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 9.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.DeChiara T M, Vejsada R, Poueymirou W T, Acheson A, Suri C, Conover J C, Friedman B, McClain J, Pan L, Stahl N, et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–322. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 11.De Stasio P R, Taylor M W. Specific effect of interferon on the herpes simplex virus type 1 transactivation event. J Virol. 1990;64:2588–2593. doi: 10.1128/jvi.64.6.2588-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutia B M, Clarke C J, Allen D J, Nash A A. Pathological changes in the spleens of gamma interferon receptor-deficient mice infected with murine gammaherpesvirus: a role for CD8 T cells. J Virol. 1997;71:4278–4283. doi: 10.1128/jvi.71.6.4278-4283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrar M A, Schreiber R D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 14.Feduchi E, Alonso M A, Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989;63:1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferber I A, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman C G. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 16.Fiette L, Aubert C, Muller U, Huang S, Aguet M, Brahic M, Bureau J F. Theiler’s virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finke D, Brinckmann U G, ter Meulen V, Liebert U G. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiger K, Howes E, Gallina M, Huang X J, Travis G H, Sarvetnick N. Transgenic mice expressing IFN-gamma in the retina develop inflammation of the eye and photoreceptor loss. Investig Ophthalmol Vis Sci. 1994;35:2667–2681. [PubMed] [Google Scholar]
- 19.Geiger K D, Gurushanthaiah D, Howes E L, Lewandowski G A, Reed J C, Bloom F E, Sarvetnick N E. Cytokine-mediated survival from lethal herpes simplex virus infection: role of programmed neuronal death. Proc Natl Acad Sci USA. 1995;92:3411–3415. doi: 10.1073/pnas.92.8.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. . (Erratum, 19:271.) [DOI] [PubMed] [Google Scholar]
- 20a.Han, X., A. Ansari, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. Submitted for publication.
- 21.Henderson S, Rowe M, Gregory C, Croom-Carter D, Wang F, Longnecker R, Kieff E, Rickinson A. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 22.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 23.Huang P L, Dawson T M, Bredt D S, Snyder S H, Fishman M C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 25.Krakowski M, Ownes T. Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 26.Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975;258:152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- 27.Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. Interferon gamma gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noguchi M, Yi H, Rosenblatt H M, Filipovitch A H, Adelstein S, Modi W S, McBride O W, Leonard W J. Interleukin-2 receptor γ chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 29.Nowak R. “Bubble boy” paradox resolved. Science. 1993;262:1818. doi: 10.1126/science.8266068. [DOI] [PubMed] [Google Scholar]
- 30.Olsson T, Kelic S, Edlund C, Bakhiet M, Hojeberg B, van der Meide P H, Ljungdahl A, Kristensson K. Neuronal interferon-gamma immunoreactive molecule: bioactivities and purification. Eur J Immunol. 1994;24:308–314. doi: 10.1002/eji.1830240205. [DOI] [PubMed] [Google Scholar]
- 31.Ramshaw I A, Ramsay A J, Karupiah G, Rolph M S, Mahalingam S, Ruby J C. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 32.Roizman B, Sears A E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–577. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- 33.Selin L K, Varga S M, Wong I C, Welsh R M. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 35.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-γ (IFN-γ) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 36.Stevens J G. Human herpesviruses: a consideration of the latent state. Microbiol Rev. 1989;53:318–332. doi: 10.1128/mr.53.3.318-332.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swihart K, Fruth U, Messmer N, Hug K, Behin R, Huang S, Del Giudice G, Aguet M, Louis J A. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med. 1995;181:961–971. doi: 10.1084/jem.181.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone M B. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 39.van den Broek M F, Muller U, Huang S, Zinkernagel R M, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang B, Andre I, Gonzalez A, Katz J D, Aguet M, Benoist C, Mathis D. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z E, Reiner S L, Zheng S, Dalton D K, Locksley R M. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]