Abstract
Background
Colonoscopy is a widely used diagnostic and therapeutic modality. A large proportion of the population is likely to undergo colonoscopy for diagnosis and treatment of colorectal diseases, or when participating in colorectal cancer screening programs. To reduce pain, water infusion instead of traditional air insufflation during the insertion phase of the colonoscopy has been proposed, thereby improving patients’ acceptance of the procedure. Moreover, the water infusion method may improve early detection of precancerous neoplasms.
Objectives
To compare water infusion techniques with standard air insufflation, specifically evaluating technical quality and screening efficacy, as well as patients’ acceptance of the water infusion procedure.
Search methods
We searched the Cochrane Colorectal Cancer Group Specialized Register (February 2014), the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 1), Ovid MEDLINE (1950 to February 2014), Ovid EMBASE (1974 to February 2014), and ClinicalTrials.gov (1999 to February 2014) for eligible randomised controlled trials.
Selection criteria
We included randomised controlled trials comparing water infusion (water exchange or water immersion methods) against standard air insufflation during the insertion phase of the colonoscopy.
Data collection and analysis
Two review authors independently assessed the studies for inclusion and extracted data from eligible studies. We performed analysis using Review Manager software (RevMan 5).
Main results
We included 16 randomised controlled trials consisting of 2933 colonoscopies. Primary outcome measures were cecal intubation rate and adenoma detection; secondary outcomes were time needed to reach the cecum, pain experienced by participants during the procedure, completion of cecal intubation without sedation/analgesia, and adverse events. Completeness of colonoscopy, that is cecal intubation rate, was similar between water infusion and standard air insufflation (risk ratio 1.00, 95% confidence interval (CI) 0.97 to 1.03, P = 0.93). Adenoma detection rate, that is number of participants with at least one detected adenoma, was slightly improved with water infusion (risk ratio 1.16, 95% CI 1.04 to 1.30, P = 0.007). Assuming the fraction of patients undergoing screening colonoscopy who had one or more adenomas detected was 20 per 100 with standard colonoscopy, the use of water colonoscopy may increase the fraction to 23 per 100 individuals. From our findings, it is possible that up to 68,000 more of the 1.7 million outpatient screening colonoscopies performed annually in the United States, could detect adenomas if water infusion colonoscopy was used. In addition, with water infusion participants experienced significantly less pain (mean difference in pain score on a 0 to 10 scale: ‐1.57, 95% CI ‐2.00 to ‐1.14, P < 0.00001) and a significantly lower proportion of participants requested on‐demand sedation or analgesia, or both (risk ratio 1.20, 95% CI 1.14 to 1.27, P < 0.00001). Qualitative analysis suggests that water infusion colonoscopy was not associated with a markedly increased rate of adverse events compared with the standard procedure.
Authors' conclusions
Completeness of colonoscopy, that is cecal intubation rate, was not improved by water infusion compared with standard air insufflation colonoscopy. However, adenoma detection, assessed with two different measures (that is adenoma detection rate and number of detected adenomas per procedure), was slightly augmented by the water infusion colonoscopy. Improved adenoma detection might be due to the cleansing effects of water infusions on the mucosa. Detection of premalignant lesions during standard colonoscopy is suboptimal, and so improvements in adenoma detection by water infusion colonoscopy, although small, may help to reduce the risk of interval colorectal carcinoma. The most obvious benefit of water infusion colonoscopy was reduction of procedure‐related abdominal pain, which may enhance the acceptance of screening/surveillance colonoscopy.
Keywords: Humans, Water, Water/administration & dosage, Abdominal Pain, Abdominal Pain/etiology, Abdominal Pain/prevention & control, Adenoma, Adenoma/diagnosis, Colonoscopy, Colonoscopy/adverse effects, Colonoscopy/methods, Colorectal Neoplasms, Colorectal Neoplasms/diagnosis, Insufflation, Insufflation/methods, Randomized Controlled Trials as Topic
Plain language summary
Water infusion versus air insufflation for colonoscopy
Colonoscopy is a visual examination of the inner lining of the large intestine with a camera on a flexible tube passed through the anus. Colonoscopy can provide a visual diagnosis of colorectal diseases. In particular, colonoscopies are an important tool in screening for colorectal cancer, and are the gold standard for the early detection of tumours and polyps located in the intestinal wall. Polyps may develop into colorectal cancer and a standard procedure is to remove these if identified, Colonoscopy allows the removal of polyps, sparing the need for open surgery.
The degree of colorectal cancer protection offered by colonoscopy depends on the technical performance. For example, it is important that colonoscopy is performed through the whole length of the colon, allowing for examination of all the colonic segments. Another measure of the quality of a colonoscopy is whether it can detect a lesion in the colon, such as adenomas.
Standard colonoscopy uses air insufflations to expand the lumen for better visualisation and facilitating advancement of the colonoscope through the large intestine. Recent clinical trials have proposed that using water instead of air insufflation may minimise patient discomfort and facilitate passage of the colonoscope through difficult segments of the large intestine. Moreover, water colonoscopy may remove residual faeces, thereby improving the view and the detection of adenomas.
The purpose of this review was to compare the effectiveness of the novel water infusion colonoscopy with the standard air colonoscopy.
We included 16 trials encompassing 2933 colonoscopies in this review. The review showed that completeness of colonoscopy was similar between water infusion and standard air insufflation, and that adenoma detection (participants with at least one adenoma detected) was improved with water colonoscopy (36% versus 31% in the air group).
In addition, participants experienced significantly less pain with water colonoscopy compared with the standard procedure.
Detection of cancer and precancerous lesions during standard colonoscopy is far from perfect. Improvements in adenoma detection by water infusion colonoscopy, although small, may help to increase the rate of adenoma detection. This may reduce the risk of colorectal cancer development after a colonoscopy without abnormal findings.
Summary of findings
Summary of findings for the main comparison. Primary outcomes.
| Primary outcomes | ||||||
|
Patient or population: Participants with colonoscopy Settings: Participants undergoing diagnostic, screening, or surveillance colonoscopy Intervention: Water infusion colonoscopy (water immersion or water exchange) Comparison: Standard colonoscopy with air insufflation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Primary outcomes | |||||
| Cecal intubation rate (intention‐to‐treat analysis) | Study population | RR 1 (0.97 to 1.03) | 2933 (16 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 936 per 1000 | 936 per 1000 (907 to 964) | |||||
| Moderate | ||||||
| 964 per 1000 | 964 per 1000 (935 to 993) | |||||
| Adenoma detection rate (endoscopists with all levels of experience) | Study population | RR 1.16 (1.04 to 1.30) | 2457 (12 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 309 per 1000 | 359 per 1000 (322 to 399) | |||||
| Moderate | ||||||
| 309 per 1000 | 358 per 1000 (321 to 399) | |||||
| Adenoma detection rate (experienced endoscopists only) | Study population | RR 1.17 (1.05 to 1.32) | 2200 (12 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 311 per 1000 | 364 per 1000 (326 to 410) | |||||
| Moderate | ||||||
| 314 per 1000 | 367 per 1000 (330 to 414) | |||||
| Number of adenomas per participant | The mean number of adenomas per participant in the intervention groups was 0.13 higher (0.01 to 0.25 higher) | 902 (2 studies) | ⊕⊕⊕⊝ moderate1 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 because a high risk of bias for 1 or more of the included studies was found for this outcome. 2 Downgraded by 1 because heterogeneity existed between trials, which may be explained by different experience levels of endoscopists, premedication, indications for colonoscopy, or age and sex of participants, although other explanations cannot be ruled out.
Background
Description of the condition
Colonoscopy is considered the gold standard for the diagnosis of colonic diseases and as an important therapeutic modality (Häfner 2007; Rex 2015). For example, in the case of inflammatory bowel disease, colonoscopy is useful in making an initial diagnosis, distinguishing ulcerative colitis from Crohn’s disease that affects the large bowel, assessing disease extent and activity, and providing endoscopic treatment, such as stricture dilation (Annese 2013). Colonoscopy is also a preferred modality for colorectal cancer prevention (U.S. Preventive Services Task Force 2008; Smith 2015). Colonoscopy can detect both cancer and premalignant neoplasms, offering the advantages of complete visualisation of the entire colon, detection and removal of polyps, and diagnostic sampling of cancers (Rex 2015; Lieberman 2012; Smith 2015).
The degree of colorectal cancer protection offered by colonoscopy depends strictly on the identification of these precancerous lesions. However, colonoscopy is far from perfect and does not completely prevent mortality from colorectal cancer. Several studies have suggested that colonoscopy reduces colorectal cancer‐related mortality by 60% to 70%, and that the benefit for distal colorectal cancer is much larger than for proximal colorectal cancer (Baxter 2009; Singh 2010; Winawer 1993). One likely reason why colonoscopy cannot prevent all colorectal cancers is that it can miss significant lesions. It has been estimated that colonoscopy misses up to 22% of adenomas, irrespective of size, and up to 2% to 3% of adenomas equal to or greater than 10 mm (van Rijn 2006). It has been estimated that 86% of all postcolonoscopy colorectal cancers could be explained by procedural factors, especially missed lesions and thus are preventable (le Clercq 2014).
Since the adenoma detection rate has been shown to be inversely correlated with the risk of interval colorectal cancer (that is the diagnosis of colorectal cancer after screening colonoscopy and before the scheduled time of surveillance colonoscopy), there have been significant efforts to improve the detection of adenomas and, therefore, to maximize the protective effect of colonoscopy. The effectiveness of colonoscopy in reducing colon cancer incidence depends on adequate visualisation of the entire colon, diligence in examining the mucosa, and patient acceptance of the procedure.
Colonoscopy is invasive, with 3 to 5 serious adverse events per 1000 examinations (e.g. intestinal bleeding or perforation and cardiorespiratory problems due to analgesics and sedatives) (Harris 2007; Whitlock 2008), and is usually perceived as a painful procedure. Fear of pain during the examination seems to be an important barrier that limits people's willingness to undergo screening (Seip 2010). Sedatives or analgesics, or both are commonly given to achieve a variable degree of conscious sedation to improve patient satisfaction with the procedure (Froehlich 2006). While sedation use decreases anxiety, minimizes discomfort, and improves the tolerance and acceptance of the examination, it is responsible for up to 50% of endoscopy‐related complications due to haemodynamic and ventilatory side effects (Ladas 2010). Sedation is also associated with increased cost due to the lengthy induction and recovery periods (Froehlich 2006). National and cultural differences, as well as patient wishes and the endoscopist’s attitude about the examination, may influence the decision to use sedatives/analgesics (Ladas 2010).
To improve the quality and effectiveness of colonoscopy, great attention has been focused on bowel preparation, training of endoscopists, technological developments, and procedural issues (for example withdrawal time and technique, position changes, or administration of antispasmodic drugs) in the last few years (Rondonotti 2014). Recently a specific procedural modification was put forward, namely the replacement of air insufflation with warm water infusion to distend the colon and reduce colonic spasms during the insertion phase (Leung FW 2008a; Leung FW 2008b; Leung FW 2011). It has been postulated that the water infusion method minimises patient discomfort and facilitates passage of the colonoscope through difficult segments of the colon, thereby enhancing cecal intubation, particularly in potentially difficult colonoscopy (for example in patients with prior abdominal or pelvic surgery).
Description of the intervention
One of the first reports of a water‐related method for aiding colonoscope insertion was published by Falchuk and Griffin (Falchuk 1984). The authors found that water instillation into the sigmoid colon facilitated colonoscope insertion through difficult segments of severe diverticulosis (Falchuk 1984). Fifteen years later, Baumann conducted a randomised controlled trial (RCT) comparing the traditional technique with water injection into the distal sigmoid colon (Baumann 1999). The main result was that the use of water significantly reduced the cecal intubation time (Baumann 1999). Of note, these initial trials used water at room temperature. Instillation of water warmed to body temperature into the colon by means of the accessory channel of the colonoscope was first investigated by Church as a method of overcoming colonic spasms during colonoscopy (Church 2002). Study participants experienced significantly less discomfort compared with control participants (Church 2002). In several subsequent studies, warm water at body temperature was instilled through the accessory channel into difficult colonic segments and was combined with air insufflation to distend the lumen for visualisation and advancement of the colonoscope (water loading method).
These initial studies were performed as hybrid procedures, with the water infusion being combined with air insufflation, while more recent studies applied the water‐only technique (Leung FW 2008b). The latter method largely eliminates the use of air insufflation during insertion of the colonoscope, that is water is infused intermittently in lieu of air to distend the colon and identify the lumen (Leung FW 2011). There are two ways to apply the water‐only technique, which are distinguished by the timing of removal of infused water: predominantly during withdrawal (water immersion) or during insertion (water exchange) (Leung FW 2012). Both water immersion and water exchange methods used water warmed to room temperature (approximately 24°C) or body temperature (approximately 37°C). In cases of suboptimal bowel preparation, the dirty water is suctioned and followed by clean‐water infusion to improve the view and facilitate the advancement of the colonoscope. Irrespective of the specific insertion technique, the withdrawal phase is always done with air insufflation similar to the traditional air method.
How the intervention might work
Several mechanisms by which water infusion may facilitate insertion of the colonoscope have been proposed:
Water infusion produces local distension to facilitate passage of the colonoscope. With the patient in the left lateral position, the water infused into the sigmoid colon flows into the lower‐lying descending colon, opening a passage through the loops and bends. The weight of the water in the left colon also straightens the sigmoid colon (Baumann 1999).
When warm water is used, the warmth of the water may minimise spasm (Church 2002).
Water instillation does not elongate the colon as much as air insufflation (Leung FW 2011).
The water infusion may also improve the quality of colonoscopy in terms of adenoma detection. Inadequate bowel preparation can result in cancer or adenoma hidden from view by faeces. With simultaneous infusion of water and suction of residual faeces, the turbulence in the vicinity of the tip of the endoscope dislodges faeces from the adjacent mucosa (Leung FW 2011). The water infusion technique may salvage a suboptimal bowel preparation (Leung FW 2011). Water colonoscopy may have a cleansing effect, thereby improving the view during withdrawal and increasing the detection rate of adenoma.
Why it is important to do this review
Colonoscopy is a widely used diagnostic and therapeutic modality. A large proportion of the population is likely to undergo colonoscopy for diagnosis and treatment of colorectal diseases, or when participating in colorectal cancer screening programs. The warm water method has been claimed to:
improve the quality with respect to the cecal intubation rate and adenoma/polyp detection rates;
alleviate the pain of a colonoscopy, thereby improving the acceptance of the procedure, particularly for repeat colonoscopies;
enable colonoscopy in unsedated or minimally sedated patients, which can lead to shorter recovery times with less risk of side effects; and
reduce the costs of the procedure.
There is growing interest in the water technique, as revealed, for example, by the growing number of RCTs investigating the method registered at ClinicalTrials.gov. A thorough review of the literature and a meta‐analysis of the data are required to determine whether the promises of the water infusion technique have been realised.
Objectives
To compare water infusion techniques with standard air insufflation, specifically evaluating technical quality and screening efficacy, as well as patients’ acceptance of the water infusion procedure.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) comparing water infusion (water exchange or water immersion) against standard air insufflation during the insertion phase of colonoscopy. We included RCTs irrespective of language and publication status.
Types of participants
We included adult male and female participants undergoing colonoscopy, regardless of the indication (screening, surveillance, symptoms).
Types of interventions
We included studies that compared colonoscopy with water infusion in lieu of air (water exchange or water immersion) during insertion to standard colonoscopy with air insufflation. We excluded studies with water‐related methods as adjuncts to usual air insufflation. For example, we excluded studies where water was loaded into the sigmoid colon or was instilled into difficult colonic segments to facilitate advancement of the colonoscope through this segment, but air insufflation was used to distend the lumen for visualisation and advancement of the colonoscope during insertion (water loading methods).
We did not discriminate between water immersion and water exchange methods, mainly because the critical distinctions between the two methods were only made recently. Most study reports do not clearly state whether the water immersion or the water exchange method (or a combination of the two) was applied. To facilitate interpretation of our results, we tried to define for each study which of the water infusion methods was primarily used (based on the descriptions provided in the study reports). However, our classification may not be accurate in all cases, particularly if the endoscopist did not clearly discriminate between the two methods.
Types of outcome measures
Colonoscopy can reduce the incidence of and mortality from colorectal cancer by detecting and removing precancerous lesions (that is adenomas). As the degree of colorectal cancer protection depends strictly on completeness of colonoscopy and the identification of these precancerous lesions, we evaluated the completeness of colonoscopy, as evidenced by the cecal intubation rate and adenoma detection as primary outcomes. With respect to adenoma detection, we considered the proportion of participants with at least one detected adenoma a primary outcome variable because it is a widely used indicator of colonoscopy technical quality and screening efficacy. We also investigated the mean number of detected adenomas per procedure, which may be a more appropriate primary‐outcome measure to evaluate whether water infusion facilitates the inspection of colonic mucosa, although this outcome is reported less frequently.
Primary outcomes
Cecal intubation rate
-
Adenoma detection
Number of participants with at least one adenoma detected (adenoma detection rate)
Number of adenomas detected per participant
Secondary outcomes
The time needed to reach the cecum
Maximum pain score reported by the participants
Completing cecal intubation without sedation/analgesia
Adverse events (side effects from sedatives/analgesics used or procedure‐related complications)
Search methods for identification of studies
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished randomised controlled trials. We used no language restriction. We searched the following electronic databases to identify potential studies:
Cochrane Colorectal Cancer Group Specialized Register (searched February 2014)
Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 1) (Appendix 1);
Ovid MEDLINE (1950 to February 2014) (Appendix 2);
Ovid EMBASE (1974 to February 2014) (Appendix 3);
ClinicalTrials.gov (1999 to February 2014) (Appendix 4).
Searching other resources
We inspected the references from all identified studies as well as review articles on this topic for more eligible trials. We screened published meeting abstracts of international scientific conferences such as the Digestive Disease Week, the United European Gastroenterology Week, the European Crohn’s and Colitis Organisation meeting, and the annual meeting of the American College of Gastroenterology to identify studies published in abstract form only.
Data collection and analysis
Selection of studies
Two review authors independently inspected each identified reference and applied the inclusion criteria. We further assessed full papers of the selected references for inclusion or exclusion. We resolved disagreements by discussion or by review of a third review author if necessary. We documented justification for study exclusion.
Data extraction and management
Two review authors (SH and OZ) independently extracted data from the included studies using a data extraction form. In case of disagreement between the two review authors, a third review author was consulted (KZ). We discussed the data extraction, documented decisions, and, when necessary, contacted the authors of the original studies for clarification of data or additional information, or both. We extracted, verified, and recorded the following data:
Characteristics and methods of trials
Setting of trial
Blinding of participants
Blinding of outcome assessors
Intervention model
A priori calculation of sample size
Characteristics of participants
Recruitment period
Number of participants enrolled (total, per study arm)
Dropouts/withdrawals
Inclusion criteria
Exclusion criteria
Nationality
Age
Sex ratio (females:males)
Comparability of groups at baseline
Characteristics of interventions
Type of water infusion technique
Use of sedatives/analgesics
Experience of endoscopists
Characteristics of outcome measures
Procedure‐ and participant‐related outcomes studied
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Random sequence generation
'Low risk' if the investigators described a random component in the sequence generation process.
'High risk' if the investigators described a non‐random component in the sequence generation process.
'Unclear risk' if insufficient information about the sequence generation process was provided.
Allocation sequence concealment
'Low risk' if participants and investigators enrolling participants could not foresee assignment.
'High risk' if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias.
'Unclear risk' if the method of concealment was not described or not described in sufficient detail to allow a definite judgement.
Blinding of participants and personnel
'Low risk' if blinding of participants and personnel ensured, and it was unlikely that the blinding could have been broken.
'High risk' if (a) no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding or (b) blinding of participants attempted, but it was likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
'Unclear risk' if insufficient information was provided to allow a definite judgement.
Due to the nature of the intervention, blinding of the endoscopist and the assistance personnel was not possible and thus the risk of bias must be classified 'high risk'.
Blinding of outcome assessment
'Low risk' if (a) blinding of outcome assessors ensured, and it was unlikely that the blinding could have been broken.
'High risk' if (a) no blinding of outcome assessors, and the outcome was likely to be influenced by lack of blinding or (b) blinding of interviewers attempted, but it was likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
'Unclear risk' if insufficient information was provided to allow a definite judgement.
Due to the nature of the intervention, for most outcome items blinding of outcome assessors was not possible and thus the risk of bias for these outcomes must be classified 'high risk'.
Incomplete outcome data
'Low risk' if no outcome data were missing, particularly if there were no post‐randomisation dropouts or withdrawals.
'High risk' if reason for missing outcome data was likely related to true outcome.
'Unclear risk' if insufficient reporting of attrition/exclusions to permit judgement.
Selective reporting
'Low risk' particularly if (a) the study protocol was available, and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way or (b) the study protocol was not available, but it was clear that the published reports included all expected outcomes, including those that were prespecified.
'High risk' particularly if (a) not all of the study’s prespecified primary outcomes have been reported, (b) one or more outcomes of interest in the review were reported incompletely so that they cannot be entered in a meta‐analysis, or (c) the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
'Unclear risk' if insufficient information was provided to allow a definite judgement.
Other bias
'Low risk' if the study appeared to be free of other sources of bias (e.g. the conduct of the study is affected by interim results, design‐specific risk of bias that does not fit into the categories above, fraud).
'High risk' if there was at least one other important risk of bias.
'Unclear risk' if there may be a risk of bias, but there was either insufficient information to assess whether an important risk of bias existed or insufficient rationale or evidence that an identified problem would introduce bias.
Measures of treatment effect
We performed analysis using Review Manager software (RevMan 5). We used risk ratio and its respective 95% confidence interval as summary measure of association for trials with dichotomous primary outcomes. We analysed continuous data by calculating mean differences and 95% confidence intervals.
Unit of analysis issues
All RCTs eligible for our meta‐analysis were carried out with parallel group design. The number of observations in the analyses matched the number of 'units' (participants) that were randomised. We collected and analysed a single measurement for each outcome from each participant.
Dealing with missing data
If data were missing or incomplete, we contacted the study authors for help finding relevant information for this review. Two papers reported only median values or mean values without measures of dispersion of relevant outcome variables and we asked the authors to provide the respective mean ± SD values. The authors of Falt 2012 and Radaelli 2010 provided the data requested.
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test (we regarded a P value of less than 0.10 as statistically significant). We used the l2 statistic to estimate the degree of heterogeneity. This measure describes the percentage of total variation across studies that results from heterogeneity rather than chance. We considered a value of 25% as indicating low heterogeneity, 50% moderate heterogeneity, and 75% high heterogeneity. We also used graphical displays, namely Galbraith plots, to examine the heterogeneity and to detect outliers. The Galbraith plot is a scatter plot of standardised intervention estimates (intervention effect divided by its standard error) against the reciprocal of the standard errors. Each study is represented by a single dot, and a regression line running centrally through the plot is calculated. Parallel to the regression line, at a 2‐standard‐deviation distance, two lines create an interval in which we would expect most dots to fall if the studies were estimating a single, fixed parameter.
Assessment of reporting biases
We investigated potential publication bias using the funnel plot. As inspection of funnel plots did not reveal signs of asymmetry, we did not perform additional tests such as Egger's linear regression test (Egger 1997).
Data synthesis
We analysed data using Review Manager 5. We combined data from individual trials for meta‐analysis if the interventions, participant groups, and outcomes were sufficiently similar (as determined by consensus). We calculated the pooled risk ratio and 95% confidence interval for dichotomous outcomes. For continuous outcomes, we calculated the pooled mean difference and 95% confidence interval.
Subgroup analysis and investigation of heterogeneity
In cases where we detected significant heterogeneity, we attempted to identify the source of the heterogeneity. We generally used the fixed‐effect model. We have chosen random‐effect models if we anticipated variation among our studies for a specific outcome variable. Variation among the studies may be due for example to differences in the experience level of the endoscopist or sedation practice. The anticipated variation among our studies was confirmed by results of the I2 test for heterogeneity. Random‐effect models are not suitable with sparse datasets, such as the analysis of ‘the number of adenomas per patient’, where a fixed‐effect model was used. In cases of heterogeneity, and if we identified a sufficient number of randomised trials, we planned to perform subgroup analyses. We conducted the following subgroup analyses:
The outcome 'maximum pain score' was stratified according to the use of sedation and analgesia.
The outcomes 'time to cecum' and 'adenoma detection rate' were re‐analysed including only those colonoscopies that were performed by experienced endoscopists.
Sensitivity analysis
We performed a sensitivity analysis to check the robustness of results when omitting studies with high risk of bias or to investigate whether the meta‐analysis result was heavily determined by outlier studies. The Galbraith plot was used to detect potential outliers.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Overall, we identified 236 potentially matching records through database searching until 17 February 2014. Figure 1 displays the article selection process. Through screening of the titles and abstracts, we found 214 articles that did not meet the minimum inclusion criteria, which we therefore excluded. We reviewed full manuscripts of 22 articles, 6 of which we excluded (see Characteristics of excluded studies) and 16 of which we included (see Characteristics of included studies and Table 2). We also screened published meta‐analyses for more RCTs eligible for inclusion in our review. Through this search we did not identify additional articles.
1.
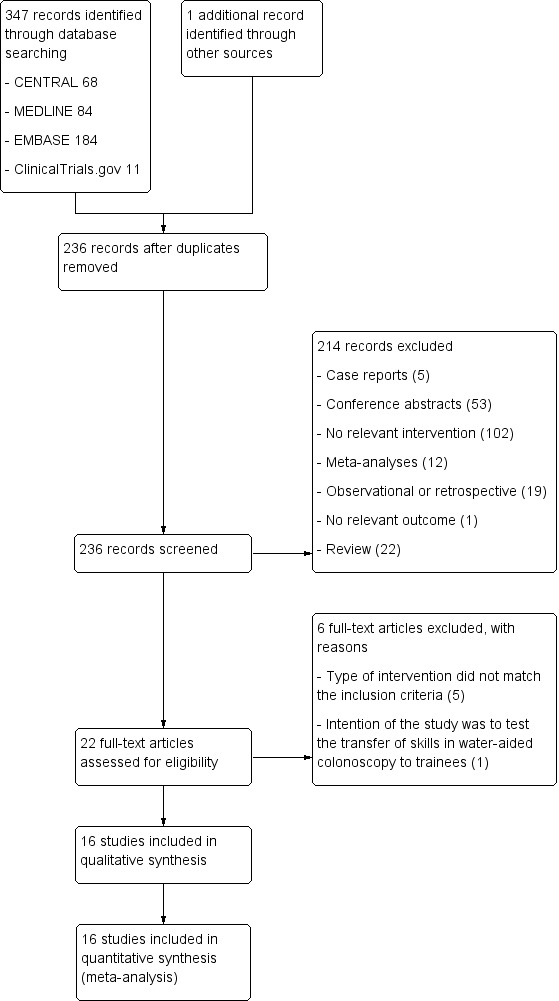
Results of searching for studies for inclusion in the review.
1. Summary of the characteristics of included studies.
| Study | Participants | Mean age (yrs) | Male participants (%) | Intervention | Initial sedation | On‐demand sedation | Endoscopists (level of experience) |
| Amato A, 2013 | Outpatients with routine indications for colonoscopy | 60.5 | 64 | Water immersion | No | Yes | 4 (experienced) |
| Bayupurnama P, 2013 | Symptomatic patients with indications for diagnostic colonoscopy, no screening cases | 50.7 | 65 | Water immersion | No | No | ? (experienced) |
| Cadoni S, 2014 | Consecutive patients aged 18 to 85 years presenting for open‐access colonoscopy | 59.0 | 60 | Water immersion & water exchange | No | Yes | 5 (experienced) |
| Falt P, 2012 | Diagnostic outpatient colonoscopy | 59.1 | 52 | Water immersion | Yes | Yes | 4 (experienced) |
| Hsieh YH, 2011a | Patients with indications for diagnostic and surveillance colonoscopy | 55.3 | 57 | Water immersion | Yes | No | 2 (experienced) |
| Hsieh YH, 2013 | Patients with indications for screening, surveillance, or diagnostic colonoscopy | 55.7 | 62 | Water immersion & water exchange | Yes | Yes | 1 (experienced) |
| Leung CW, 2010 | Elective outpatient colonoscopy for screening or surveillance | 62.5 | 100 | Water immersion | Yes | Yes | ? (trainees and experienced) |
| Leung FW, 2010 | Patients with indications for screening, surveillance, or diagnostic colonoscopy | 66.4 | 100 | Water immersion | No | No | 1 (experienced) |
| Leung J, 2011 | Patients with indications for screening or surveillance colonoscopy | 59.5 | 99 | Water immersion | No | Yes | 2 (experienced) |
| Leung JW, 2009 | Patients with indications for screening or surveillance colonoscopy | 59.5 | 91 | Water immersion | Yes | Yes | 2 (experienced) |
| Leung JW, 2013 | Patients with indications for (scheduled) screening or surveillance colonoscopy | 60.5 | 97 | Water exchange | No | No | 2 (experienced) |
| Luo H, 2013 | Outpatients with prior abdominal or pelvic surgery undergoing unsedated diagnostic, screening, or surveillance colonoscopy | 56.2 | 31 | Water exchange | No | No | 2 (experienced) |
| Pohl J, 2011 | Outpatients presenting for screening, surveillance, or diagnostic colonoscopy | 62.2 | 73 | Water immersion | No | Yes | 2 (experienced) |
| Portocarrero DJ, 2012 | Patients undergoing elective outpatient screening colonoscopy | 68.0 | 30 | Water immersion | Yes | Yes | 1 (experienced) |
| Radaelli F, 2010 | Outpatients presenting for screening, surveillance, or diagnostic endoscopy | 58.6 | 58 | Water immersion | No | Yes | 2 (experienced) 1 (trainee) |
| Ramirez FC, 2011 | Patients undergoing screening colonoscopy | 59.6 | 96 | Water exchange | Yes | Yes | 1 (experienced) |
Included studies
Sixteen RCTs met the inclusion criteria. Description of the intervention and outcomes were found for each of the included studies. All studies except three (Hsieh 2011a, Portocarrero 2012, Ramirez 2011) were registered at clinicaltrials.gov or the German Clinical Trials Register (DRKS), where a summary of the study protocol was provided. The number of participants in each trial ranged from 22 to 818, with 2718 participants included in our analysis. Two studies enrolled only men (Leung CW 2010; Leung FW 2010), and four other studies included almost exclusively male participants (Leung JW 2009; Leung J 2011; Leung JW 2013; Ramirez 2011). In two studies, female participants were overrepresented (Luo 2013; Portocarrero 2012). Male and female participants were more evenly distributed in the other eight studies. The mean age of the study participants ranged from 51 to 66 years. Screening and surveillance were the most frequent indications for colonoscopy. Only five clearly stated which of the two major water‐aided methods, water immersion or water exchange, was used (Cadoni 2014; Hsieh 2013; Leung CW 2010; Leung JW 2013; Luo 2013). For the remaining studies, we classified the interventions as water immersion or water exchange based on the description of the procedure in the articles (see Characteristics of included studies).
Excluded studies
We excluded five trials because the type of intervention did not match the inclusion criteria (Brocchi 2008; Hamamoto 2002; Hsieh 2011b; Ryu 2012; Wang 2014). We excluded one study because the primary intention was to test the transfer of skills used in water‐aided colonoscopy to trainees (Ransibrahmanakul 2010).
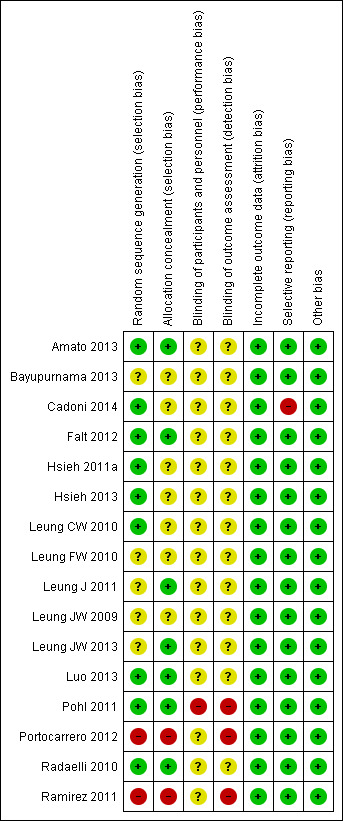
Risk of bias in included studies
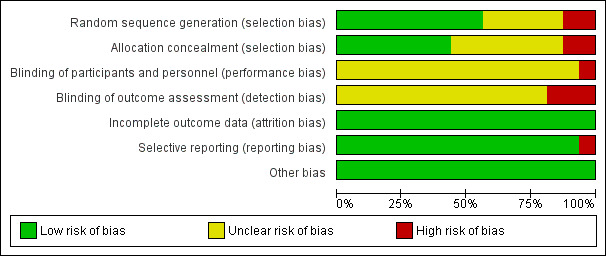
We analysed and reported risk of bias for each study in the 'Risk of bias' tables (see Characteristics of included studies). Figure 2 and Figure 3 provide a graphical overview.
2.
3.
Allocation
Investigators of two studies described a non‐random component in the sequence generation process, namely sequence generated by the participant’s Social Security number (Ramirez 2011) and by the odd or even day (Portocarrero 2012). Information about random sequence generation was missing in five studies (Bayupurnama 2013; Leung FW 2010; Leung J 2011; Leung JW 2009; Leung JW 2013).
Blinding
Due to the nature of the intervention, it was not possible to blind the endoscopists and assisting nurses. In most studies (Cadoni 2014; Falt 2012; Leung CW 2010; Leung FW 2010; Leung J 2011; Leung JW 2009; Leung JW 2013; Luo 2013; Portocarrero 2012; Radaelli 2010) attempts were made to blind the participants. There was risk of detection bias due to knowledge of the allocated intervention by outcome assessors because, due to the nature of the intervention, the endoscopist and assistants were not blinded. However, nine studies (Amato 2013; Bayupurnama 2013; Cadoni 2014; Hsieh 2011a; Hsieh 2013; Leung FW 2010; Leung J 2011; Leung JW 2013; Radaelli 2010) reported blinding of interviewers assessing, for instance, participants' pain and tolerance scores.
Incomplete outcome data
Follow‐up time was short and therefore losses to follow‐up did not occur in any study. If participants were excluded from the analysis, the number of exclusions and the reasons for exclusions where reported.
Selective reporting
During their study, Cadoni et al. switched from the water immersion to the water exchange technique. In their paper, however, they only reported results obtained from comparing the water exchange method with standard air insufflation during insertion of the colonoscope (Cadoni 2014). For some outcome measures, Falt et al. and Radaelli et al. reported only median values or mean values without measures of dispersion (for example 95% confidence interval (CI), standard deviation, or standard error of the mean) (Falt 2012; Radaelli 2010). We contacted the authors, and they provided the requested data. We did not note any discrepancy between pre‐specified outcomes and reported outcomes.
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
See: Table 1
Eleven studies used a water‐based intubation technique that corresponded to the water immersion method (Amato 2013; Bayupurnama 2013; Falt 2012; Hsieh 2011a; Leung JW 2009; Leung CW 2010; Leung FW 2010; Leung J 2011; Pohl 2011; Portocarrero 2012; Radaelli 2010); three studies used the water exchange method (Leung JW 2013; Luo 2013; Ramirez 2011); and two studies used both techniques (Cadoni 2014; Hsieh 2013). The protocols of most studies were strictly designed and did not allow any air insufflation during insertion in the water group. Three studies, however, allowed the use of air if the colonic lumen could not be opened or visualisation of the colonic lumen could not be achieved with water infusion only (Amato 2013; Hsieh 2013; Radaelli 2010). The use of sedation/analgesia differed substantially across the studies. In seven studies premedication was routinely applied (Falt 2012; Hsieh 2011a; Hsieh 2013; Leung JW 2009; Leung CW 2010; Portocarrero 2012; Ramirez 2011), and in five of these studies participants were offered additional sedation/analgesia, as needed (Falt 2012; Leung JW 2009; Leung CW 2010; Portocarrero 2012; Ramirez 2011). Five studies were carried out in unsedated participants, who had the option of on‐demand sedation/analgesia during the procedure (Amato 2013; Cadoni 2014; Leung J 2011; Pohl 2011; Radaelli 2010), while four studies were performed without any sedation/analgesia (Bayupurnama 2013; Leung FW 2010; Leung JW 2013; Luo 2013). The protocol of the latter studies deviated from standard practice adopted in most Western countries, where sedation/analgesia is usually provided to achieve cecal intubation. In two studies, the anticholinergic drug hyoscine‐N‐butylbromide was administered to the participants as a premedication to reduce gastrointestinal spasm during the endoscopic procedure (Bayupurnama 2013; Hsieh 2011a). Colonoscopy itself is operator‐dependent. Fourteen studies involved only experienced endoscopists (Amato 2013; Bayupurnama 2013; Cadoni 2014; Falt 2012; Hsieh 2011a; Hsieh 2013; Leung JW 2009; Leung FW 2010; Leung J 2011; Leung JW 2013; Luo 2013; Pohl 2011; Portocarrero 2012; Ramirez 2011). In two studies, both experienced endoscopists and endoscopists‐in‐training performed colonoscopies (Leung CW 2010; Radaelli 2010).
Primary outcomes
Rate of successful cecal intubation
Sixteen studies (water infusion: 1490 participants, air insufflation: 1443 participants) were eligible for evaluation of the rate of successful cecal intubation (Analysis 1.1). In four studies with rather small sample size, no events of intubation failure were observed either in the water intervention group or in the air control group (Leung JW 2009; Leung CW 2010; Leung J 2011; Portocarrero 2012). Results of the remaining studies were highly heterogenous (heterogeneity: Chi² = 53.35, df = 15 (P < 0.00001); I² = 72%). Heterogeneity might be due to between‐study differences in (i) study populations (age, sex), (ii) indications for colonoscopy (screening, diagnostic, surveillance), (iii) premedication (initial or on‐demand analgesia/sedation, smooth muscle relaxation), (iv) technique of water‐based cecal intubation (water immersion versus water exchange, water at room temperature versus water at 37°C), or (v) the level of experience of the endoscopist. Subgroup analyses (as specified in the Methods section) did not reveal sedation/analgesia (whether or not participants were offered sedation/analgesia) or the experience of the endoscopist (qualified attending staff versus trainees) as the specific source of heterogeneity.. In particular, the water infusion technique (water exchange versus water immersion), sedation/analgesia (whether or not participants were offered sedation/analgesia), or the experience of the endoscopist (qualified attending staff versus trainees), evaluated separately, could not explain the heterogeneity. Overall, we did not observe a significant difference in the rate of cecal intubation between water infusion and air insufflation (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.97 to 1.03).
1.1. Analysis.
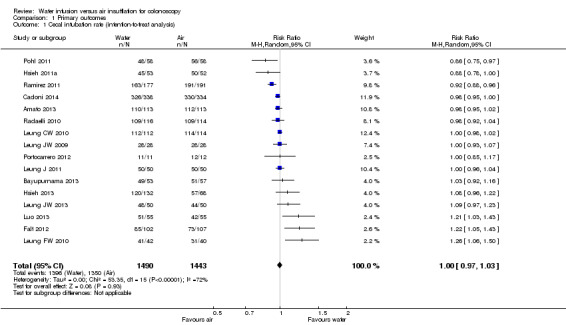
Comparison 1 Primary outcomes, Outcome 1 Cecal intubation rate (intention‐to‐treat analysis).
Adenoma detection
To assess whether the water method was superior to air insufflation regarding adenoma detection, we analysed the number of participants having at least one adenoma detected during colonoscopy (adenoma detection rate) (Analysis 1.2). Twelve studies were eligible for analysis (Amato 2013; Cadoni 2014; Falt 2012; Hsieh 2011a; Leung CW 2010; Leung FW 2010; Leung J 2011; Leung JW 2013; Pohl 2011; Portocarrero 2012; Radaelli 2010; Ramirez 2011). All but one study (Radaelli 2010) found increased adenoma detection rates in the water infusion group compared to the air control group. The meta‐analysis involved 2457 colonoscopies; at least one adenoma was detected in 831 colonoscopies. Overall, the adenoma detection rate was 16% higher in the water infusion group compared to the air insufflation group (36.3% versus 31.4%, respectively; RR 1.16, 95% CI 1.04 to 1.30, P = 0.007). However, as adenoma detection may depend on the experience of the endoscopist, we re‐analysed the data by including only procedures performed by experienced, staff endoscopists and excluding procedures performed by junior‐level gastroenterology trainees (Analysis 1.3). While heterogeneity was reduced (I² = 35% versus I² = 21%), the overall effect remained similar (RR 1.17, 95% CI 1.05 to 1.32, P = 0.006).
1.2. Analysis.
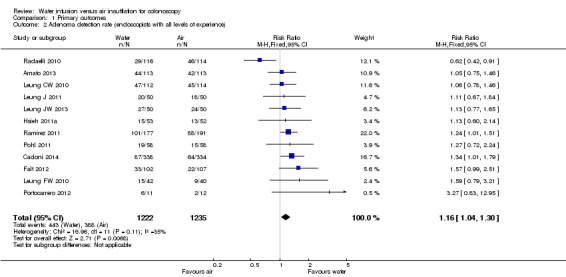
Comparison 1 Primary outcomes, Outcome 2 Adenoma detection rate (endoscopists with all levels of experience).
1.3. Analysis.
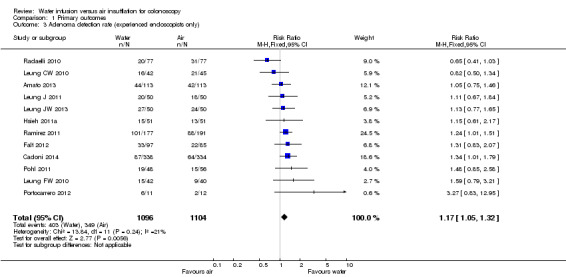
Comparison 1 Primary outcomes, Outcome 3 Adenoma detection rate (experienced endoscopists only).
Three studies reported the mean number of adenomas per participant (Cadoni 2014; Leung FW 2010; Radaelli 2010). Radaelli et al. found no significant difference between water infusion and air insufflation colonoscopies (1.70 versus 1.74, P = 0.46), whereas Cadoni et al. detected more adenomas per participant in the water group (0.40 versus 0.26, P < 0.0005). Leung et al. did not report standard deviation values or perform a statistical analysis. Combined quantitative analysis of the former two studies suggests that water infusion may slightly increase the number of adenomas detected per participant (mean difference (MD) 0.13, 95% CI 0.01 to 0.25, P = 0.03) (Analysis 1.4).
1.4. Analysis.
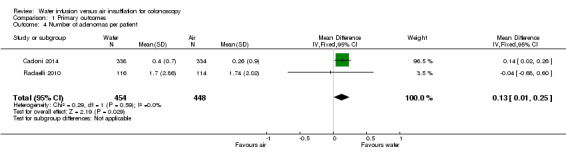
Comparison 1 Primary outcomes, Outcome 4 Number of adenomas per patient.
With respect to the primary outcome adenoma detection rate, the Galbraith plots for investigating heterogeneity revealed that the study by Radaelli et al. (Radaelli 2010) might be an outlier (Figure 4). Moreover, we identified studies with a rather high risk of bias due to 'quasi‐randomisation' (Portocarrero 2012; Ramirez 2011) and blinding issues (Pohl 2011; Portocarrero 2012; Ramirez 2011) (Figure 2). Exclusion of any single one of these problematic studies did not qualitatively change the results of the primary outcomes (cecal intubation rate: RR between 1.00 (95% CI 0.98 to 1.03) and 1.01 (95% CI 0.98 to 1.04), all not significant; adenoma detection rate: RR between 1.14 (96% CI 1.00 to 1.30) and 1.24 (95% CI 1.10 to 1.38), all significant). Also exclusion of all four of these studies did not change the overall results (cecal intubation rate: RR 1.02 (95% CI 0.99 to 1.06), P = 0.22; adenoma detection rate: RR 1.22 (95% CI 1.06 to 1.40), P = 0.006).
4.
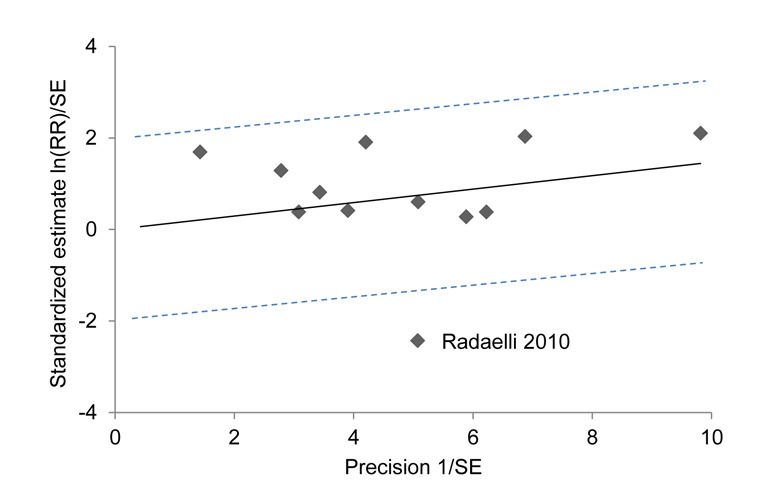
Galbraith plot analysis for the outcome adenoma detection rate (endoscopists with all level of experience). For each study, ratio of the ln(relative risk) to its standard error (y‐axis) is plotted against reciprocal of the standard error (x‐axis). The 95% confidence interval is between the two outer parallel dotted lines at two units above and below the regression line. Eleven studies were inside the 95% bounds, while one study was the outlier.
Secondary outcomes
Time needed to reach the cecum
All studies reported the cecal intubation time (Analysis 2.1). Mean cecal intubation times varied between 5.6 and 34 min in participants with water‐aided colonoscopy and between 4.6 and 37 min in participants with standard air colonoscopy (MD 0.61 min, 95% CI ‐0.34 to 1.56, P = 0.21). This huge between‐study variability translates into high heterogeneity of the MD values (heterogeneity: Chi² = 73.85, df = 15 (P < 0.00001); I² = 80%). Sensitivity analysis revealed that the result was heavily determined by one trial that had a particularly long procedure time (at least three times longer than the other trials) (Leung FW 2010). Exclusion of this study reduced heterogeneity markedly (Chi² = 19.13, df = 14 (P = 0.16); I² = 27%) and changed analysis result suggesting that cecal intubation was prolonged with water infusion compared to air insufflation (MD 1.33 min, 95% CI 0.83 to 1.84, P < 0.00001).
2.1. Analysis.
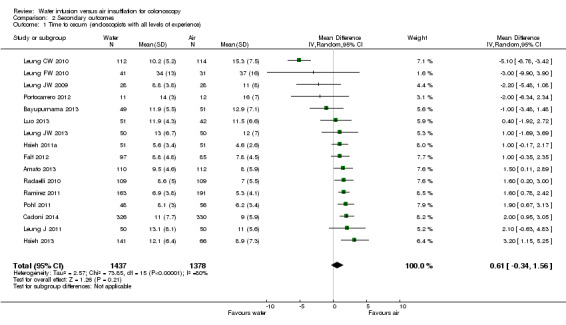
Comparison 2 Secondary outcomes, Outcome 1 Time to cecum (endoscopists with all levels of experience).
As the cecal intubation time may depend on the experience level of the endoscopist, we re‐analysed the data by including only those participants investigated by experienced, staff endoscopists and excluding participants investigated by junior‐level gastroenterology trainees (Analysis 2.2). Heterogeneity was markedly reduced, although still present (heterogeneity: Chi² = 25.62, df = 15 (P = 0.04); I² = 41%). Overall, cecal intubation was prolonged with water infusion compared to air insufflation with a MD of 1.09 min (95% CI 0.51 to 1.68, P = 0.0002).
2.2. Analysis.
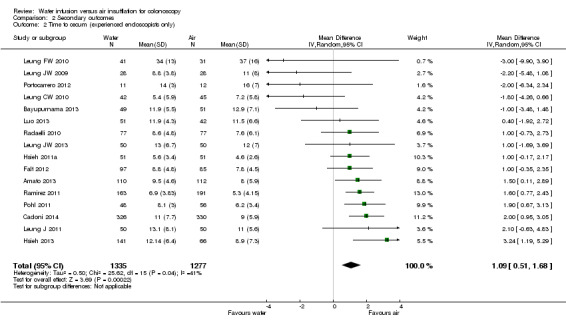
Comparison 2 Secondary outcomes, Outcome 2 Time to cecum (experienced endoscopists only).
Maximum pain score
Eleven studies reported maximum pain scores measured using the 0–10 visual analogue or numeric rating scales (0 no pain, 10 most severe or worst pain) (Cadoni 2014; Hsieh 2011a; Hsieh 2013; Leung JW 2009; Leung J 2011; Leung JW 2013; Luo 2013; Pohl 2011), 100 mm visual analogue scale (0 no pain, 100 worst pain) (Amato 2013; Radaelli 2010), or 7‐point Likert scale (0 no pain, 6 intolerable pain) (Falt 2012). We included data in the quantitative meta‐analysis after transformation to a uniform 0 to 10 scale. Because pain scores most likely differ between participants with and without sedation/analgesia, we performed a subgroup analysis stratified according to premedication: procedures with initial analgesia/sedation with or without on‐demand analgesia/sedation, procedures with on‐demand analgesia/sedation only, and procedures without any analgesia/sedation (Analysis 2.3). Of note, drugs and their doses differed between studies, which among other factors may contribute to the observed heterogeneity (heterogeneity: Chi² = 31.48, df = 10 (P = 0.0005); I² = 68%). Moreover, in some studies participants received the spasmolytic drug hyoscine‐N‐butylbromide (Bayupurnama 2013; Hsieh 2011a). In all included studies, pain scores were lower with water infusion compared with air insufflation. The MD in maximum pain scores ranged from ‐0.50 (95% CI ‐1.48 to 0.48) to ‐2.80 (95% CI ‐4.22 to ‐1.38). The MD in maximum pain scores was smallest in participants with initial analgesia/sedation (‐1.37, 95% CI ‐2.15 to ‐0.59, P = 0.0006) and greatest in participants without any analgesia/sedation (‐2.38, 95% CI ‐2.94 to ‐1.81, P < 0.00001). There were significant subgroup differences (Chi² = 6.65, df = 2, P = 0.04). The meta‐analysis of all 1922 participants revealed a reduction in maximum pain score by ‐1.57 (95% CI ‐2.00 to ‐1.14, P < 0.00001) in the water infusion group compared with the air insufflation group.
2.3. Analysis.
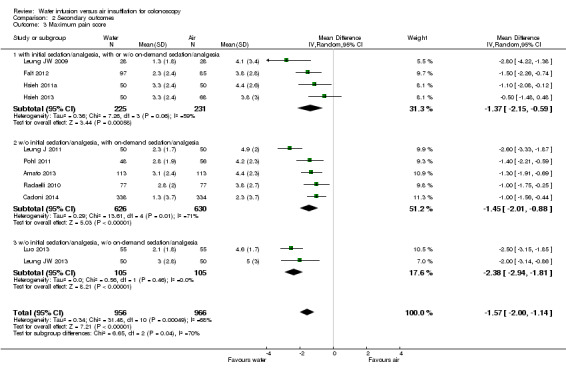
Comparison 2 Secondary outcomes, Outcome 3 Maximum pain score.
Completing cecal intubation without sedation/analgesia
The observation of a lower degree of pain during colonoscopy using the water infusion methods raises the question of whether fewer patients with water infusion require sedation/analgesia compared with those having air insufflation. Five studies in participants accepting on‐demand sedation/analgesia were eligible to address this question (Amato 2013; Cadoni 2014; Leung J 2011; Pohl 2011; Radaelli 2010). In these studies, the number of participants requiring on‐demand sedation/analgesia was consistently lower in the water infusion group than in the air insufflation group, although in one study the difference just failed to reach statistical significance (Radaelli 2010). In the combined analysis, including 665 and 667 participants in the water and the air groups, respectively, the risk ratio for requiring sedation/analgesia during colonoscopy was 1.20 (95% CI 1.14 to 1.25, P < 0.00001) in favour of water infusion (Analysis 2.4).
2.4. Analysis.
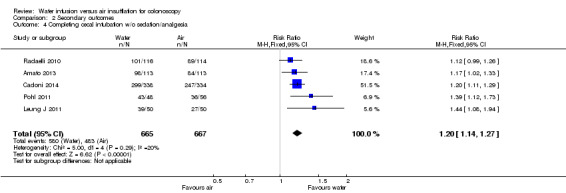
Comparison 2 Secondary outcomes, Outcome 4 Completing cecal intubation w/o sedation/analgesia.
Adverse effects
Eight studies reported data on adverse events (Table 3). The total rate of adverse events was 10 per 914 for water infusion and 22 per 915 for air insufflation. In detail, oxygen desaturation, which was related to the use of sedatives and/or analgesics, was more frequent in the air insufflation group (13 with air insufflation versus 6 with water infusion). Other reported adverse events included vagal reactions (one with water infusion versus four with air insufflation), unspecified cardiopulmonary events (zero with water infusion versus two with air insufflation), postpolypectomy bleeding (three with water infusion versus two with air insufflation), and one bowel perforation, which occurred in a participant allocated to the air group.
2. Adverse events.
| Water | Air | |||
| Study | Events | Total | Events | Total |
| Amato A, 2013 | 2 (2: oxygen desaturation) | 113 | 5 (4: oxygen desaturation, 1: bowel perforation) | 113 |
| Cadoni S, 2013 | 3 (1: vagal reactions, 2: postpolypectomy bleeding) | 338 | 3 (2: vagal reactions, 1: postpolypectomy bleeding) | 334 |
| Falt P, 2012 | 0 | 102 | 0 | 107 |
| Leung CW, 2010 | 0 | 112 | 0 | 114 |
| Leung J, 2011 | 0 | 50 | 1 (1: cardiopulmonary event) | 50 |
| Leung JW, 2009 | 0 | 28 | 1 (1: cardiopulmonary event) | 28 |
| Luo H, 2013 | 0 | 55 | 0 | 55 |
| Radaelli F, 2010 | 5 (4: oxygen desaturation, 1: postpolypectomy bleeding) | 116 | 12 (9: oxygen desaturation, 2: vagal reactions, 1: postpolypectomy bleeding) | 114 |
| TOTAL | 10 | 914 | 22 | 915 |
Discussion
The quality of colonoscopy is mainly defined by the success of the procedure (that is insertion of the endoscope to the cecum) and by its capacity to detect (and then remove) potentially harmful bowel tumours or precancerous lesions. Therefore 'cecal intubation failure' and 'adenoma detection rate' are important endpoints of our analysis and must be considered as leading parameters when comparing different methods of colonoscopy.
Summary of main results
Primary outcomes
Rate of successful cecal intubation
Compared with the standard air insufflation technique, water infusion did not significantly change the rate of successful cecal intubations. The total number of successful cecal intubations (intention‐to‐treat analysis) was 93.7% for water infusion and 93.6% for air insufflation.
Adenoma detection
Colonoscopy can reduce the incidence of and mortality from colorectal cancer by detecting and removing precancerous lesions (that is adenomas). Therefore, the degree of colorectal cancer protection offered by colonoscopy depends strictly on the identification of these precancerous lesions.
The adenoma detection rate was significantly higher with water infusion than with air insufflation: in 443 of 1222 examinations (36%), at least one adenoma was found in the water group versus 388 of 1235 examinations (31%) in the air group. All studies included in this meta‐analysis except one reported the advantage of water infusion over air insufflation. The heterogeneity of the trials, although low (I² = 35%), might be due to the varying levels of experience of the endoscopists. In fact, heterogeneity was reduced (I² = 21%) when we included only colonoscopies performed by experienced endoscopists in the quantitative analysis and excluded procedures performed by trainees. Both analyses consistently suggested a significantly increased adenoma detection rate by more than 15% with water infusion versus air insufflation. The result can easily be explained by the observation that intermittent water infusions help wash off remaining faeces covering the colonic mucosa, thereby improving detection of possible lesions.
Secondary outcomes
Time needed to reach the cecum
The time needed to reach the cecum is likely an important colonoscopy performance variable when the endoscopist is considering whether to accept or reject a new technique. In large trials involving experienced endoscopists, the mean time for insertion of the colonoscope into the cecum typically ranges from 4 to 10 minutes (Kim 2000). However, one study far exceeded this time range, with mean insertion times between 34 and 37 minutes in the water infusion group and the air insufflation group, respectively (Leung FW 2010). In this study, experienced endoscopists performed colonoscopies in unsedated participants. Several factors may lead to difficulties advancing the colonoscope, thus delaying cecal intubation. These could include participant characteristics (for example prior surgery, female sex, low or extremely high body mass index, abdominal pain or history of irritable bowel syndrome, the use of sedatives or analgesics, and degree of colon cleanliness) and technical aspects (for example the experience and skill of the endoscopist and technicians; and type, length, diameter, and stiffness of the colonoscope) (Kim 2000). The level of experience of the endoscopist was identified as a factor that largely explains the interstudy heterogeneity of the results. Considering only colonoscopies performed by experienced, staff endoscopists and excluding procedures performed by junior‐level gastroenterology trainees, the meta‐analysis suggested that time‐to‐cecum is marginally prolonged by about 1 min in the water group compared to the air insufflation group (P = 0.0002). Prolonged cecal intubation time may reflect poor bowel preparation or limited experience of the endoscopist and thus might be used an indirect quality indicator for colonoscopy. The one minute difference however, although being statistically significant, is not expected to be of clinical significance.
Maximum pain score
Colon cancer screening by colonoscopy lags behind other forms of cancer screening for participation rates. Concerns that relate specifically to colonoscopy and influence acceptability of the procedure include anticipated pain (Condon 2008; Ussui 2013). One reason for the emerging interest in water‐based colonoscopy is that this technique is thought to reduce pain. Consequently, most trials focused primarily on whether water infusion colonoscopy might be less painful than the standard air insufflation technique. The studies consistently demonstrated a significant reduction in maximum pain during colonoscopy when using water infusion compared with air insufflation. The meta‐analysis confirmed a significant reduction in the maximum pain score by about 1.5 units on a scale from 0 to 10. The MD in the maximum pain score between water infusion and air insufflation was even greater, by about 2.4 units, when colonoscopy was performed in unsedated patients. The minimum clinically significant change in the pain score has been estimated as 0.9‐1.1 units on a 0 to 10‐point scale, irrespective of gender, age, and the cause of pain (Wolfe 2007; Kelly 1998). Thus, the changes in pain score observed in the meta‐analysis are expected to be clinically significant.
Completing cecal intubation without sedation/analgesia
Consistent with the finding of pain reduction, the proportion of examinations completed without use of sedatives or analgesics was significantly higher in the water group (87.2%) than in the air group (72.4%). The probability to complete cecal intubation without sedation/analgesia was about 20% higher with water infusion compared to air insufflation. We derived these data from only five studies because most of the protocols routinely provided initial sedation/analgesia to the participants.
Adverse events
The exploration of complications was not part of most study protocols; only eight of the 16 RCTs reported adverse events as endpoints. We therefore cannot definitively conclude that in the other eight studies complications did not occur. A total of 32 adverse events were reported (Table 3).
In general, cardiovascular and pulmonary complications are the most frequent adverse events during colonoscopy, occurring approximately 19 times per 1000 colonoscopies (Day 2011). In our meta‐analysis, the mean incidence of cardiovascular and pulmonary events per 1000 colonoscopies was 7.6 and 20.8, in the water infusion and air insufflation groups, respectively. The difference in the number of cardiopulmonary events between these groups has been attributed to the less frequent on‐demand use of analgesics/sedatives in the water infusion group (Radaelli 2010). Although the incidence of cardiopulmonary events in the water infusion group was only half that of the air insufflation group, the statistical power of our analysis was not sufficient to prove that water infusion is safer than air insufflation. Our meta‐analysis also did not have enough power to compare the frequencies of rare but severe complications, such as bleeding and perforation.
Overall completeness and applicability of evidence
We included all RCTs satisfying the inclusion criteria in the analysis, so that as of the date of publication, this review reflects the current state of knowledge of this topic. As the water infusion method is a topic of high interest, further studies will be published in the near future that will build upon this analysis.
Quality of the evidence
The quality of evidence found in the trials included in this meta‐analysis appear to be moderate or even low because of potential bias in some studies (Figure 3; Table 1).
Potential biases in the review process
We conducted comprehensive searches of journal and conference databases to ensure that we identified all published and unpublished trials. We did not limit the searches to a particular language. One study written in Chinese was translated. Where necessary, we contacted authors to ask for additional data. Two review authors independently extracted data from the included studies.
Potential bias may result from factors outside the control of the review authors. With colonoscopy, there is no way to conduct double‐blind studies because the endoscopist must operate using the screen and can see which method is applied. As the endoscopist also usually assesses the examination outcome, the risk of bias is high.
Some studies included both male and female participants, whereas other studies included only male participants. In our meta‐analysis we included all studies irrespective of differences in the sex ratio. Subgroup analyses for sex differences may provide insight into the basis for individual outcome differences and may provide future directions for research. However, we were unable to perform such subgroup analyses, because none of the studies including both male and female participants reported the outcomes by sex.
Agreements and disagreements with other studies or reviews
In 2012, a meta‐analysis of nine RCTs with 1283 participants comparing warm‐water infusion and traditional air insufflation found a 4‐fold higher risk of cecal intubation failure with the 'water only' technique, but no significant differences when a brief use of air was allowed with the water infusion method (Rabenstein 2012). Cecal intubation times and adenoma detection rates were similar, but the maximum pain scores and need of sedation/analgesia were significantly lower with warm water than with air.
A subsequent meta‐analysis performed by Jun et al. included seven studies involving 872 participants to compare water infusion with air insufflation (Jun 2013). The combined analysis revealed that participants in the water group required less abdominal compression or position change, had lower mean and maximum pain scores, and needed less on‐demand sedation compared with the air insufflation group. Cecal intubation rate, cecal intubation time, total procedure time, and adenoma detection rate did not differ between protocols.
Lin et al. pooled the results from nine RCTs (1414 participants) comparing standard air insufflation colonoscopy with water‐aided insertion methods. The authors found no differences in cecal intubation rate and time and adenoma detection rate, but observed lower pain scores and less on‐demand sedation or analgesia in the water group (Lin 2013).
In a meta‐analysis of 18 RCTs with 2797 participants, water‐aided colonoscopy was associated with a significantly higher cecal intubation rate than standard air colonoscopy, a significantly lower visual analogue scale score for abdominal pain, and greater willingness of the participants to repeat colonoscopy (Hu 2013). The intubation time was similar between both groups.
The principal result of the meta‐analysis performed by Leung et al., which pooled results from 11 RCTs (1573 participants), was that water immersion and water exchange methods significantly reduced insertion pain (Leung FW 2013). No significant difference in overall adenoma detection rate was observed between water‐aided methods and air insufflation.
A major limitation of many of these meta‐analyses (for example Hu 2013; Leung FW 2013; Lin 2013) is that they pooled highly heterogenous types of interventions. In particular, studies with water‐related methods as adjuncts to traditional air insufflation were combined with water‐only methods. In our meta‐analysis, we focused on the water‐only methods of luminal distension during insertion. Several new trials comparing colonoscopy with water infusion in lieu of air (water exchange or water immersion) versus standard air insufflation colonoscopy were recently published and included in our analysis. The number of included studies and participants (16 studies, 2718 participants) in our review was therefore quite high.
Another limitation of some previous meta‐analyses was that they did not apply strictly to the intention‐to‐treat principle, for example the analysis by Hu et al. (Hu 2013).
Overall, previous meta‐analyses were inconsistent with respect to cecal intubation and adenoma detection rates, reporting no differences or benefits with water‐aided insertion methods versus air insufflation. Differences in the statistical power (that is the number of included studies) and in the definition of the intervention of interest, and compliance/non‐compliance with the intention‐to‐treat principle most likely contribute to the disparate findings. There is, however, general consensus that water infusion colonoscopy significantly reduces insertion pain when compared with air insufflation colonoscopy.
Authors' conclusions
Implications for practice.
Water infusion did not improve completeness of colonoscopy, that is cecal intubation rate, compared with standard air insufflation colonoscopy.
Water infusion colonoscopy, however, improved adenoma detection by 16%. This is expected to be clinically relevant, because the adenoma detection rate is an important quality indicator with the strongest association to post‐colonoscopy colorectal cancer or “missed” colorectal cancer. The current recommendation of the American Society for Gastrointestinal Endoscopy / American College of Gastroenterology Task Force on Quality in Endoscopy is that individual Colonoscopists should identify one or more adenomas in at least 25% of a male/female population (at least 30% for men and 20% for women) ≥50 years undergoing screening colonoscopy (Rex 2015). Provided that the 1.7 million outpatient screening colonoscopies performed annually in the United States (Rex 2015) meet the current quality standards, it can be assumed that about 425.000 people have adenomas detected during conventional colonoscopy annually. Our findings suggest that there would be 68,000 extra cases detected per year in the United States if water infusion colonoscopy was used. Based on data from more than 300,000 colonoscopies performed by 136 gastroenterologists in the United States, Corley et al. found that there was a 3% reduction in the incidence of colorectal cancer and a 5% reduction in cancer mortality for each 1% increase in the adenoma detection rate (Corley 2014). Combining these previous data with our findings, screening colonoscopy using water infusion compared with standard technique may save a few thousand extra lives per year in the United States, where colorectal cancer is expected to cause about 50,000 deaths every year.
Improved adenoma detection might be due to the cleansing effects of water infusions on the mucosa. Overall, by applying some of the most important indicators of colonoscopy, namely technical quality and screening efficacy, our meta‐analysis suggested that water infusion colonoscopy may provide some small benefits. The most obvious benefit is the reduction of procedure‐related abdominal pain. This benefit alone may enhance the acceptance of screening/surveillance colonoscopy and may help to reduce the needed dose of analgesics/sedatives.
It is reasonable to apply the water infusion method in practice because it is superior to air insufflation in terms of pain reduction and acceptance by patients. Detection of neoplasia during standard colonoscopy is suboptimal; improvements in adenoma detection by water infusion colonoscopy, although small, may nevertheless reduce the risk of interval colorectal carcinoma. With respect to procedural outcomes, the water infusion method seems to be largely equivalent to standard air insufflation colonoscopy.
Implications for research.
In the past few years the water infusion technique has been refined. To date, two different water infusion techniques have been described: the original water immersion and the more recent water exchange method (Leung FW 2012). These are distinguished by the timing of removal of infused water: predominantly during withdrawal (water immersion) or during insertion (water exchange) (Leung FW 2012). Whether the timing of removal of infused water has a major impact on endoscopic performance and quality indicators, for example cecal intubation and adenoma detection rates, is currently unclear. Only one comparative study (water exchange versus water immersion) has been performed so far (Hsieh 2013).
To further improve upon the quality of the evidence presented, future studies should adhere to specific aspects of the colonoscopy protocol in order to render more comparable results between studies, especially the time points of pain score assessment (during the procedure, immediately afterwards, or 24 hours later, etc.) and the definition and reporting of adverse events. Mechanisms of pain reduction by water versus air are not yet fully understood and must be investigated further in order to optimise the colonoscopy procedure. Cluster randomisation in future studies may reduce the risk of bias due to the lack of blinding of the investigator/outcome assessor. Cluster‐randomization is most effective with large numbers of clusters to balance potentially confounding characteristics in the treatment and control arms of the study. However, regulatory challenges and financial constraints may limit large‐scale, multisite studies.
Acknowledgements
The review authors would like to thank Dr. Hao Zhang, MD, Department of Internal Medicine ‐ Angiology, Westpfalz Hospital, Kusel, Germany, for the translation of the Chinese publication.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 1) #1 MeSH descriptor: [Colonoscopy] explode all trees #2 colonoscop*:ti,ab,kw #3 #1 or #2 #4 MeSH descriptor: [Water] explode all trees #5 "water":ti,ab,kw #6 #4 or #5 #7 MeSH descriptor: [Air] explode all trees #8 air:ti,ab,kw (Word variations have been searched) #9 #7 or #8 #10 #3 and #6 and #9
Appendix 2. MEDLINE search strategy
Ovid MEDLINE [1950 to 17 Feb 2014] 1. exp Colonoscopy/ 2. colonoscop*.mp. 3. 1 or 2 4. exp Water/ 5. water.mp. 6. 4 or 5 7. exp Air/ 8. air.mp. 9. 7 or 8 10. 3 and 6 and 9
Appendix 3. EMBASE search strategy
Ovid EMBASE [1974 to 17 Feb 2014] 1. exp colonoscopy/ 2. colonoscop*.mp. 3. 1 or 2 4. exp water/ 5. water.mp. 6. 4 or 5 7. exp air/ 8. air.mp. 9. 7 or 8 10. 3 and 6 and 9
Appendix 4. ClinicalTrials.gov search strategy
water AND colonoscopy
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cecal intubation rate (intention‐to‐treat analysis) | 16 | 2933 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.97, 1.03] |
| 2 Adenoma detection rate (endoscopists with all levels of experience) | 12 | 2457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.04, 1.30] |
| 3 Adenoma detection rate (experienced endoscopists only) | 12 | 2200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.05, 1.32] |
| 4 Number of adenomas per patient | 2 | 902 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.01, 0.25] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to cecum (endoscopists with all levels of experience) | 16 | 2815 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.34, 1.56] |
| 2 Time to cecum (experienced endoscopists only) | 16 | 2612 | Mean Difference (IV, Random, 95% CI) | 1.09 [0.51, 1.68] |
| 3 Maximum pain score | 11 | 1922 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [0.00, ‐1.14] |
| 3.1 with initial sedation/analgesia, with or w/o on‐demand sedation/analgesia | 4 | 456 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐2.15, ‐0.59] |
| 3.2 w/o initial sedation/analgesia, with on‐demand sedation/analgesia | 5 | 1256 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐2.01, ‐0.88] |
| 3.3 w/o initial sedation/analgesia, w/o on‐demand sedation/analgesia | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐2.38 [‐2.94, ‐1.81] |
| 4 Completing cecal intubation w/o sedation/analgesia | 5 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.14, 1.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amato 2013.
| Methods | Randomised controlled trial | |
| Participants | Outpatients with routine indications for colonoscopy, 113 water immersion, 113 air insufflation, no dropouts, mean age 60.5 years, 64% male participants, country: Italy | |
| Interventions | Water immersion: During colonoscope insertion, water (37°C) was intermittently infused through an auxiliary water channel of the colonoscope to obtain lumen distention. At the discretion of the endoscopist, short air insufflation (≤ 10 s/episode, ≤ 2 episodes/colonoscopy) was allowed. During the withdrawal phase, water and stool residuals were suctioned. No initial sedation/analgesia. On‐demand sedation/analgesia during colonoscopy (2.5 mg midazolam + 50 mg pethidine). Endoscopists: 4 experienced |
|
| Outcomes | Primary: number of participants undergoing complete unsedated colonoscopy Secondary: evaluation of pain and tolerability scores; procedural outcomes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence (mixed block size), taking into account 4 different endoscopists |
| Allocation concealment (selection bias) | Low risk | Participants and investigators could not foresee assignment because sealed envelopes were used to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participants) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of interviewers assessing the participants' pain and tolerance scores; no blinding of endoscopists and nurses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Bayupurnama 2013.
| Methods | Randomised controlled trial | |
| Participants | Symptomatic patients with indications for diagnostic colonoscopy, no screening cases, 53 water immersion, 57 air insufflation, no dropouts, mean age 50.7 years, 65% male participants, country: Indonesia | |
| Interventions | Water immersion: The air pump of the endoscopy machine was turned off before the colonoscope tip was inserted. The colonoscope was advanced by intermittent water (at room temperature) infusion through the biopsy channel of the colonoscope. Suspended residual faeces obscuring the view were suctioned when encountered. Most of the water infused was aspirated predominantly during withdrawal. No initial sedation/analgesia; initial muscle relaxation: hyoscine‐N‐butylbromide. No on‐demand sedation/analgesia. Endoscopists: experienced |
|
| Outcomes | Primary: discomfort during insertion Secondary: cecal intubation, duration of the examination, willingness of the participants to repeat |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After informed consent consecutive patients were randomized", no mention of how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (quote: "participants were not informed about the method at the time of examination") |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of interviewers assessing participants' pain (medical residents), no blinding of endoscopist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Cadoni 2014.
| Methods | Randomised controlled trial | |
| Participants | Consecutive patients presenting for open‐access colonoscopy, ages 18 to 85 years old. 72 water immersion, 338 water exchange, 406 air insufflation, 144 (72 water immersion/72 air insufflation) excluded from analysis, mean age 59.0 years, 60% male participants, country: Italy | |
| Interventions | Water immersion: Infused water (37°C) was used to facilitate insertion. Infusions were performed using flushing pumps. Air was not used during insertion. Water and air were occasionally removed by suction, but the infused water was removed predominantly during withdrawal. Water exchange: After reaching the rectosigmoid junction, the colon was irrigated with water (37°C) to facilitate insertion. Removal of residual colonic air pockets and faeces and suction of infused water occurred predominantly during insertion to minimize distention of the colonic lumen. No initial sedation/analgesia. On‐demand sedation/analgesia during colonoscopy (midazolam). Endoscopists: 5 experienced |
|
| Outcomes | Primary: cecal intubation with pain scores of ≤ 2 and no sedation or ≤ 2 mg midazolam Secondary: pain score at discharge, cecal intubation rate and time, and adenoma detection rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participant) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of interviewers recording overall pain score at discharge, no blinding of endoscopists and assistant nurses during colonoscopy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for withdrawals were described |
| Selective reporting (reporting bias) | High risk | Analysis of the water immersion method was not reported |
| Other bias | Low risk | None |
Falt 2012.
| Methods | Randomised controlled trial | |
| Participants | Diagnostic outpatient colonoscopy, 102 water immersion and air insufflation during withdrawal, 107 air insufflation during insertion and withdrawal, 105 water immersion and CO2 insufflation during withdrawal, 107 CO2 insufflation during insertion and withdrawal, 16 dropouts, mean age 59.1 years, 52% male participants, country: Czech Republic | |
| Interventions | Water immersion: Water (room temperature) was infused throughout the colon to distend the lumen just to facilitate scope insertion. If unavoidable, residual stool was suctioned and replaced by clean water. As soon as the cecum was reached, the air pump was turned on and the colon was fully distended for scope withdrawal and mucosa inspection. Water and residual stool was removed predominantly during the withdrawal. Initial sedation/analgesia (2 mg midazolam IV). On‐demand sedation/analgesia: midazolam Endoscopists: 4 experienced |
|
| Outcomes | Primary: success rate of minimal‐sedation colonoscopy Secondary: procedural measures, participant comfort during the procedure and during first 24 hours after procedure |
|
| Notes | Participants/groups included in our meta‐analysis: 102 water immersion and air insufflation during withdrawal, 107 air insufflation during insertion and withdrawal, 8 dropouts, mean age 59.4 years, 53% male participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, stratified block (sex and age groups) randomisation method |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed and kept in an envelope for each participant and opened before the procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participant) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of endoscopists and nurses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers of and reasons for withdrawals in all intervention and control groups were described |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Hsieh 2011a.
| Methods | Randomised controlled trial | |
| Participants | Patients with indications for diagnostic or surveillance colonoscopy, 53 water immersion (entire colon), 54 water immersion (rectum and sigmoid colon), 52 air insufflation, 6 dropouts, mean age 55.3 years, 57% male participants, country: Taiwan | |
| Interventions | Water immersion: Water infusion was used throughout the entire colon during the insertion phase with the air pump turned off. Tap water at room temperature was infused through the accessory channel of the colonoscope using a foot switch‐controlled water pump. Air insufflation was used when the cecum was reached. Infused water was removed predominantly during withdrawal. Minimal initial sedation; initial muscle relaxation: hyoscine‐N‐butylbromide IV No on‐demand sedation/analgesia. Endoscopists: 2 experienced |
|
| Outcomes | Primary: pain scores during insertion Secondary: procedural measures; overall satisfaction score; polyp, adenoma, and carcinoma detection rates |
|
| Notes | Participants/groups included in our meta‐analysis: 53 water immersion (entire colon), 52 air insufflation, 3 dropouts, mean age 54.4 years, 57% male participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized randomization" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participant) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assistant unaware of the randomisation status administered the questionnaires to the participants; no blinding of endoscopist and nurses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers of and reasons for withdrawals in all intervention and control groups were described |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Hsieh 2013.
| Methods | Randomised controlled trial | |
| Participants | Patients with indications for screening, surveillance, or diagnostic colonoscopy, 64 water immersion, 68 water exchange, 68 air insufflation, mean age 55.7 years, 62% male participants, country: Taiwan | |
| Interventions | Water immersion: Water (room temperature) was infused through the accessory channel of the colonoscope during insertion and removed during withdrawal. The water was used mainly to open the lumen, without attempting to clear the colon contents. Air insufflation was used when the cecum was reached. However, if the lumen could not be opened with water after attempting for 5 minutes in the insertion phase, the air pump was turned on. Water exchange: Water (room temperature) was infused (through the accessory channel of the colonoscope) and removed at the same time throughout the entire colon during the insertion phase with the air pump turned off. If turbid water obscured the view because of suspended residual fecal matter, the dirty water was suctioned, followed by clean water infusion to improve the view to facilitate advancement of the colonoscope. Suctioning of all residual air was performed during insertion to minimize angulations of the flexures to facilitate insertion. Initial sedation/analgesia: 25 mg pethidine IM. On‐demand sedation/analgesia. Endoscopists: 1 experienced |
|
| Outcomes | Primary: proportion of participants without pain during colonoscope insertion Secondary: procedural measures, participant pain score, patient satisfaction score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerized randomization" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding of participants; the endoscopist was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assistant unaware of the randomisation status administered the questionnaires to the subjects |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Leung CW 2010.
| Methods | Randomised controlled trial | |
| Participants | Elective outpatient colonoscopy (screening and surveillance), 114 water immersion, 115 air insufflation, 3 dropouts, mean age 62.5 years, 100% male participants, country: USA | |
| Interventions | Water immersion: 300 mL of warm water was infused into the rectum through the auxiliary water channel of the colonoscope. At the descending colon, additional water was injected as necessary to distend the lumen enough to safely insert the colonoscope. Air was insufflated when the cecum was reached. Infused water was suctioned during withdrawal. Initial sedation/analgesia: 2 mg midazolam IV On‐demand sedation/analgesia: fentanyl/midazolam Endoscopists: trainees and experienced |
|
| Outcomes | Primary: success of minimal‐sedation colonoscopy Secondary: time to cecum, patient satisfaction |
|
| Notes | Participants/groups included in our meta‐analysis: Participants investigated by experienced endoscopists: 42 water immersion, 45 air insufflation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation, stratified for experience levels of the endoscopists, age groups of the participants, and alcohol consumption of the participants |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participant) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of endoscopists and nurses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Leung FW 2010.
| Methods | Randomised controlled trial | |
| Participants | Patients with indications for screening, surveillance, or diagnostic colonoscopy, 42 water immersion, 40 air insufflation, no dropouts, mean age 66.4 years, 100% male participants, country: USA | |
| Interventions | Water immersion: Warm water loading of the sigmoid colon and warm‐water irrigation for dealing with colonic spasms; water infusion in lieu of air insufflation. No initial sedation/analgesia. No on‐demand sedation/analgesia. Endoscopists: 1 experienced |
|
| Outcomes | Pain and discomfort scores, procedure‐related measures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participant) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of interviewers assessing the participants' overall discomfort after colonoscopy; no blinding of endoscopists and assistant during colonoscopy and of interviewers assessing the participants' maximum discomfort during colonoscopy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Leung J 2011.
| Methods | Randomised controlled trial | |
| Participants | Patients with indications for screening or surveillance colonoscopy, 50 water immersion, 50 air insufflation, no dropouts, mean age 59.5 years, 99% male participants, country: USA | |
| Interventions | Water immersion: Air pump was in the off position until the cecum was reached. Warm water was used, and the volume was unrestricted. If turbid water obscured the view because of suspended residual fecal matter, the dirty water was suctioned, followed by clean‐water infusion to improve the view to facilitate advancement of the colonoscope or visualisation of the appendix opening. Suctioning of all residual air was performed during insertion to minimize angulations of the flexures to facilitate insertion. No initial sedation/analgesia. On‐demand sedation/analgesia: fentanyl/midazolam Endoscopists: 2 experienced |
|
| Outcomes | Primary: proportion of participants completing colonoscopy without sedation Secondary: cecal intubation rate, medication requirement, maximum discomfort, procedure‐related and participant‐related outcomes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how the allocation sequence was generated |
| Allocation concealment (selection bias) | Low risk | A sealed envelope opened immediately before colonoscopy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participants) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of endoscopists; blinding of nurse who queried the participants' willingness to repeat the examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Leung JW 2009.
| Methods | Randomised controlled trial | |
| Participants | Patients with indications for screening or surveillance colonoscopy, 28 water immersion, 28 air insufflation, no dropouts, mean age 59.5 years, 91% male participants, country: USA | |
| Interventions | Water immersion: The air pump was turned off before colonoscope insertion. Water (37°C) was infused through the biopsy channel. If turbid water obscured the view, it was suctioned and clean water was infused to improve the endoscopic view and to facilitate colonoscope advancement. After cecal intubation, air was insufflated, and residual colonic content was suctioned on withdrawal. Initial sedation/analgesia: 25 µg fentanyl, 1 mg midazolam, 50 mg diphenhydramine. On‐demand sedation/analgesia: yes Endoscopists: 2 experienced |
|
| Outcomes | Primary: increments of medications used for sedation Secondary: pain scores during colonoscopy, willingness to repeat colonoscopy, procedural outcomes such as cecal intubation rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how the allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | A sealed envelope, opened after premedication and before colonoscopy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participants) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Leung JW 2013.
| Methods | Randomised controlled trial | |
| Participants | Patients with indications for (scheduled) screening or surveillance colonoscopy, 50 water exchange, 50 air insufflation, no dropouts, mean age 60.5 years, 97% male participants, country: USA | |
| Interventions | Water exchange: The air pump was in the off position until the cecum was reached. The volume of water (temperature?) was unrestricted. If turbid water resulting from suspended residual fecal matter obscured the lumen, the dirty water was suctioned followed by clean water infusion to improve the view to facilitate advancement of the colonoscope and visualisation of the appendix opening (cecal intubation). Suctioning of all residual air was performed during insertion to minimize angulations of the flexures to facilitate insertion. No initial sedation/analgesia. No on‐demand sedation/analgesia. Endoscopists: 2 experienced |
|
| Outcomes | Primary: cecal intubation rate Secondary: procedural measures, adenoma detection rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how allocation sequence was generated |
| Allocation concealment (selection bias) | Low risk | A sealed envelope opened immediately before colonoscopy |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participants, towel was placed over participants' eyes) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of endoscopists |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Luo 2013.
| Methods | Randomised controlled trial | |
| Participants | Outpatients with prior abdominal or pelvic surgery undergoing unsedated diagnostic, screening, or surveillance colonoscopy, 55 water exchange, 55 air insufflation, no dropouts, mean age 56.2 years, 31% male participants, country: China | |
| Interventions | Water exchange: The air pump was turned off before colonoscopy. During insertion, residual air in the lumen was suctioned, and water (37°C) was infused through the biopsy channel to obtain lumen visualisation. Turbid luminal water resulting from residual faeces was suctioned and replaced by clean water until the colon lumen was clearly visualised again. Infused water was thus removed predominantly during the insertion phase. The total volume of water was not restricted. During withdrawal in both groups, residual water and faeces were suctioned and air was insufflated sufficiently to facilitate inspection. No initial sedation/analgesia. No on‐demand sedation/analgesia. Endoscopists: 2 experienced |
|
| Outcomes | Primary: cecal intubation rate Secondary: the maximum and mean pain scores during insertion in the right‐side, transverse, and left‐side colon; polyp detection rate; participant willingness to undergo a repeat unsedated colonoscopy; insertion time (from rectum to cecum), withdrawal time (from cecum to rectum, excluding time for biopsy and polypectomy); volume of water infused; number of abdominal compressions, position changes, and stiffness variations used during the insertion phase; and Boston Bowel Preparation Scale scores. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation list was not accessible to the endoscopists or assistants; participants were assigned to groups immediately before examination |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participant) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of endoscopists |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Pohl 2011.
| Methods | Randomised controlled trial | |
| Participants | Outpatients presenting for screening, surveillance, or diagnostic colonoscopy, 58 water immersion, 58 air insufflation, no dropouts, mean age 62.2 years, 73% male participants, country: Germany | |
| Interventions | Water immersion: The air supply was turned off until the cecum was reached, so as to completely omit air insufflation during insertion. Warm water was stored in 1L bottles that were maintained at 37°C using a water bath. A peristaltic flushing pump was used to infuse the warm water intermittently at the endoscopist’s discretion. If sufficient visualisation of the colonic lumen could not be achieved with water infusion within 5 minutes and the endoscopist judged it risky to advance the colonoscope further, the water pump was switched off and the procedure was completed with air. These cases were recorded as failures. No initial sedation/analgesia. On‐demand sedation/analgesia: midazolam/pethidine. Endoscopists: 2 experienced |
|
| Outcomes | Primary: completion of cecal intubation without sedation Secondary: pain scores; willingness to repeat the colonoscopy; procedure times; and adenoma detection rates. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened immediately before colonoscopy; participants and investigators could not foresee assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Portocarrero 2012.
| Methods | Randomised controlled trial | |
| Participants | Patients undergoing elective outpatient screening colonoscopy, 11 water immersion, 12 air insufflation, no dropouts, mean age 68.0 years, 30% male participants, country: USA | |
| Interventions | Water immersion: Upon insertion of the colonoscope into the rectum, all air was suctioned out. A peristaltic pump for infusion of room‐temperature sterile water through the water channel in lieu of air insufflation, combined with suction of residual air to minimize angulations at flexures and water exchange to clear residual faeces for luminal viewing, was used to navigate through the colon. Initial sedation/analgesia: 25 µg fentanyl IV, 1 mg midazolam On‐demand sedation/analgesia: yes Endoscopists: 1 experienced |
|
| Outcomes | Cecal intubation rate; total procedure time; pain scores; willingness to repeat colonoscopy; polyp/adenoma detection rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotation: "quasi‐randomized ‐ odd days (water), even days (air)". Investigators described a non‐random component in the sequence generation process, namely sequence generated by odd or even day |
| Allocation concealment (selection bias) | High risk | Participants or investigators enrolling participants could possibly foresee assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participants) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Radaelli 2010.
| Methods | Randomised controlled trial | |
| Participants | Outpatients presenting for screening, surveillance, or diagnostic endoscopy, 116 water immersion, 114 air insufflation, no dropouts, mean age 58.6 years, 58% male participants, country: Italy | |
| Interventions | Water immersion: Air insufflation was used just at instrument introduction until the rectosigmoid junction was reached, and then it was turned off until the cecum was reached. During colonoscope insertion, water (37°C) was intermittently infused through an auxiliary water channel of the colonoscope. There was no restriction on overall volume of water. If the visualisation of colonic lumen could not be achieved with water infusion and the endoscopist judged it unsafe to advance the colonoscope, air was briefly (lasting no more than 10 seconds) turned on. Any turning on of air was specifically recorded in the participant data sheet. If 3 or more episodes of air were needed, the water pump was turned off and the procedure was completed with air. In this case, the cecal intubation outcome was recorded as a failure. During the withdrawal phase, water and stool residuals were suctioned and air was used to obtain an adequate distention of the colonic lumen. No initial sedation/analgesia. On‐demand sedation/analgesia: 2.5 mg midazolam, 50 mg pethidine Endoscopists: 2 experienced, 1 trainee |
|
| Outcomes | Primary: proportion of participants requiring on‐demand sedation/analgesia Secondary: pain and tolerance scores; willingness to repeat the colonoscopy; procedure times and total hospital stay; cecal intubation rate |
|
| Notes | Participants/groups included in our meta‐analysis: Participants investigated by experienced endoscopists, 77 water immersion, 77 air insufflation, no dropouts, mean age 58.6 years, 58% male participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence (mixed block size) |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes", participants and investigators could not foresee assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participants) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of interviewers assessing the participants' pain and tolerance scores; no blinding of endoscopists and nurses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Ramirez 2011.
| Methods | Randomised controlled trial | |
| Participants | Patients undergoing screening colonoscopy, 177 water exchange, 191 air insufflation, no dropouts, mean age 59.6 years, 96% male participants, country: USA | |
| Interventions | Water exchange: The air pump was turned off and water (room temperature) was infused by a pedal pump connected to the base of the colonoscope. The colonoscope was advanced by intermittent water infusion. Suspended residual faeces obscuring the view had to be suctioned and replaced by clean water. During withdrawal, air insufflation was used to distend the colon for inspection, biopsy, and polypectomy. Initial sedation/analgesia: fentanyl, midazolam On‐demand sedation/analgesia: yes Endoscopists: 1 experienced |
|
| Outcomes | Primary: adenoma detection rate Secondary: success of cecal intubation; intubation and withdrawal time; use of external compression; patient position change; additional sedation medication during colonoscopy; polyp detection rate |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quotation: "Patients were assigned at the time of endoscopy, using quasi‐randomization allocation to treatment based on the last digit of the patient’s social security number ‐ air (odd number) or water (even number)". Investigators described a non‐random component in the sequence generation process, namely sequence generated by the patient’s Social Security number. |
| Allocation concealment (selection bias) | High risk | Participants or investigators enrolling participants could possibly foresee assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded (participant) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brocchi 2008 | Type of intervention did not match the inclusion criteria: Air was used during insertion, although water was instilled into the sigmoid colon at the beginning of the colonoscopy. |
| Hamamoto 2002 | Type of intervention did not match the inclusion criteria: Air was used during insertion, although water was instilled into the colon by enema at the beginning of the colonoscopy. |
| Hsieh 2011b | Type of intervention did not match the inclusion criteria: Air was used during insertion, although water was instilled into the rectum and sigmoid colon at the beginning of the colonoscopy. |
| Ransibrahmanakul 2010 | The intention of the study was to test the transfer of skills in water‐aided colonoscopy to trainees. |
| Ryu 2012 | Type of intervention did not match the inclusion criteria: Air was used during insertion, although water was instilled into the sigmoid colon at the beginning of the colonoscopy. |
| Wang 2014 | Type of intervention did not match the inclusion criteria: Air was used during insertion. Water infusion was used only to facilitate advancement of the colonoscope through the sigmoid colon and left colic flexure. |
Differences between protocol and review
Since publication of the original protocol, the water infusion technique has been refined. To date, two different water infusion techniques have been described: the original water immersion and the more recent water exchange method (Leung FW 2012). These are distinguished by the timing of removal of infused water: predominantly during withdrawal (water immersion) or during insertion (water exchange) (Leung FW 2012). Whether the timing of removal of infused water has a major impact on endoscopic performance and quality indicators, for example cecal intubation and adenoma detection rates, is currently unclear. A comparative study with a small number of participants suggested that the cecal intubation rate was unchanged, but that the cecal intubation was prolonged and pain score was reduced in the water exchange group compared with the water immersion group (Hsieh 2013).
We meant the original protocol to compare only water infusion trials that used warm water. When searching the literature, we noted that some protocols did not state the temperature of the water used, and other studies used water at room temperature. Falt et al. compared warm (37°C) water and water at room temperature in a double‐blind randomised trial (Falt 2013). As the authors did not observe a significant effect of water temperature on cecal intubation time, intubation failure, participant discomfort, and adenoma detection rate, we decided to extend the eligibility criteria by including all studies comparing standard air insufflation with water infusion techniques (water immersion or water exchange), irrespective of the temperature of the infused water.
We changed the primary and secondary outcomes for our analysis and provided a justification for the choice of cecal intubation rate, adenoma detection rate, and adenomas per procedure as primary outcomes. Accordingly, we also changed the title.
Contributions of authors
Drafted the protocol: KZ, OZ, TR Performed the literature search: SH, JO, OZ Extracted data: SH, JO, KZ, OZ Performed statistical analyses: SH, OZ Drafted the review: SH, KZ, TR, FR, OZ
Declarations of interest
The review authors have no conflicts of interest.
New
References
References to studies included in this review
Amato 2013 {published data only}
- Amato A, Radaelli F, Paggi S, Baccarin A, Spinzi G, Terruzzi V. Carbon dioxide insufflation or warm‐water infusion versus standard air insufflation for unsedated colonoscopy: a randomized controlled trial. Diseases of the Colon & Rectum 2013;56(4):511‐8. [PUBMED: 23478620] [DOI] [PubMed] [Google Scholar]
Bayupurnama 2013 {published data only}
- Bayupurnama P, Ratnasari N, Indrarti F, Triwikatmani C, Nurdjanah S, Leung FW. The water method colonoscopy in routine unsedated colonoscopy examinations: a randomized controlled trial in diagnostic cases in Indonesian patients. Journal of Interventional Gastroenterology 2013;3(1):12‐7. [PUBMED: 24147221] [Google Scholar]
Cadoni 2014 {published data only}
- Cadoni S, Gallittu P, Sanna S, Fanari V, Porcedda ML, Erriu M, et al. A two‐center randomized controlled trial of water‐aided colonoscopy versus air insufflation colonoscopy. Endoscopy 2014;46(3):212‐8. [PUBMED: 24218307] [DOI] [PubMed] [Google Scholar]
Falt 2012 {published data only}
- Falt P, Liberda M, Smajstrla V, Kliment M, Bártková A, Tvrdík J, et al. Combination of water immersion and carbon dioxide insufflation for minimal sedation colonoscopy: a prospective, randomized, single‐center trial. European Journal of Gastroenterology and Hepatology 2012;24(8):971‐7. [PUBMED: 22569079] [DOI] [PubMed] [Google Scholar]
Hsieh 2011a {published data only}
- Hsieh YH, Tseng KC, Hsieh JJ, Tseng CW, Hung TH, Leung FW. Feasibility of colonoscopy with water infusion in minimally sedated patients in an Asian Community Setting. Journal of Interventional Gastroenterology 2011;1(4):185‐90. [PUBMED: 22586535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsieh 2013 {published data only}
- Hsieh YH, Leung FW. A randomized, controlled trial comparing air insufflation, water immersion and water exchange during minimally sedated colonoscopy ‐ an interim report. Journal of Interventional Gastroenterology 2013;3(3):96‐9. [PUBMED: 24478927] [DOI] [PubMed] [Google Scholar]
Leung CW 2010 {published data only}
- Leung CW, Kaltenbach T, Soetikno R, Wu KK, Leung FW, Friedland S. Water immersion versus standard colonoscopy insertion technique: randomized trial shows promise for minimal sedation. Endoscopy 2010;42(7):557‐63. [PUBMED: 20593332] [DOI] [PubMed] [Google Scholar]
Leung FW 2010 {published data only}
- Leung FW, Harker JO, Jackson G, Okamoto KE, Behbahani OM, Jamgotchian NJ, et al. A proof‐of‐principle, prospective, randomized, controlled trial demonstrating improved outcomes in scheduled unsedated colonoscopy by the water method. Gastrointestinal Endoscopy 2010;72(4):693‐700. [PUBMED: 20619405] [DOI] [PubMed] [Google Scholar]
Leung J 2011 {published data only}
- Leung J, Mann S, Siao‐Salera R, Ransibrahmanakul K, Lim B, Canete W, et al. A randomized, controlled trial to confirm the beneficial effects of the water method on U.S. veterans undergoing colonoscopy with the option of on‐demand sedation. Gastrointestinal Endoscopy 2011;73(1):103‐10. [PUBMED: 21184876] [DOI] [PubMed] [Google Scholar]
Leung JW 2009 {published data only}
- Leung JW, Mann SK, Siao‐Salera R, Ransibrahmanakul K, Lim B, Cabrera H, et al. A randomized, controlled comparison of warm water infusion in lieu of air insufflation versus air insufflation for aiding colonoscopy insertion in sedated patients undergoing colorectal cancer screening and surveillance. Gastrointestinal Endoscopy 2009;70(3):505‐10. [PUBMED: 19555938] [DOI] [PubMed] [Google Scholar]
Leung JW 2013 {published data only}
- Leung JW, Mann S, Siao‐Salera R, Canete W, Prather D, Leung FW. The established and time‐tested water exchange method in scheduled unsedated colonoscopy significantly enhanced patient‐centered outcomes without prolonging procedural times ‐ A randomized controlled trial. Journal of Interventional Gastroenterology 2013;3(1):7‐11. [PUBMED: 24147220] [Google Scholar]
Luo 2013 {published data only}
- Luo H, Zhang L, Liu X, Leung FW, Liu Z, Wang X, et al. Water exchange enhanced cecal intubation in potentially difficult colonoscopy. Unsedated patients with prior abdominal or pelvic surgery: a prospective, randomized, controlled trial. Gastrointestinal Endoscopy 2013;77(5):767‐73. [PUBMED: 23394837] [DOI] [PubMed] [Google Scholar]
Pohl 2011 {published data only}
- Pohl J, Messer I, Behrens A, Kaiser G, Mayer G, Ell C. Water infusion for cecal intubation increases patient tolerance, but does not improve intubation of unsedated colonoscopies. Clinical Gastroenterology and Hepatology 2011;9(12):1039‐43.e1. [PUBMED: 21749850] [DOI] [PubMed] [Google Scholar]
Portocarrero 2012 {published data only}
- Portocarrero DJ, Che K, Olafsson S, Walter MH, Jackson CS, Leung FW, et al. A pilot study to assess feasibility of the water method to aid colonoscope insertion in community settings in the United States. Journal of Interventional Gastroenterology 2012;2(1):20‐2. [PUBMED: 22586546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radaelli 2010 {published data only}
- Radaelli F, Paggi S, Amato A, Terruzzi V. Warm water infusion versus air insufflation for unsedated colonoscopy: a randomized, controlled trial. Gastrointestinal Endoscopy 2010;72(4):701‐9. [PUBMED: 20883846] [DOI] [PubMed] [Google Scholar]
Ramirez 2011 {published data only}
- Ramirez FC, Leung FW. A head‐to‐head comparison of the water vs. air method in patients undergoing screening colonoscopy. Journal of Interventional Gastroenterology 2011;1(3):130‐5. [PUBMED: 22163084] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brocchi 2008 {published data only}
- Brocchi E, Pezzilli R, Tomassetti P, Campana D, Morselli‐Labate AM, Corinaldesi R. Warm water or oil‐assisted colonoscopy: toward simpler examinations?. The American Journal of Gastroenterology 2008;103(3):581‐7. [PUBMED: 18076732] [DOI] [PubMed] [Google Scholar]
Hamamoto 2002 {published data only}
- Hamamoto N, Nakanishi Y, Morimoto N, Inoue H, Tatukawa M, Nakata S, et al. A new water instillation method for colonoscopy without sedation as performed by endoscopists‐in‐training. Gastrointestinal Endoscopy 2002;56(6):825‐8. [PUBMED: 12447292] [DOI] [PubMed] [Google Scholar]
Hsieh 2011b {published data only}
- Hsieh YH, Lin HJ, Tseng KC. Limited water infusion decreases pain during minimally sedated colonoscopy. World Journal of Gastroenterology 2011;17(17):2236‐40. [PUBMED: 21633535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ransibrahmanakul 2010 {published data only}
- Ransibrahmanakul K, Leung JW, Mann SK, Siao‐Salera R, Lim BS, Hasyagar C, et al. Comparative effectiveness of water vs. air methods in minimal sedation colonoscopy performed by supervised trainees in the US ‐ randomized controlled trial. The American Journal of Clinical Medicine 2010;7(3):113‐8. [Google Scholar]
Ryu 2012 {published data only}
- Ryu KH, Huh KC, Kang YW, Choi YW, Im EH, Lee TH, et al. An effective instillation method for water‐assisted colonoscopy as performed by in‐training endoscopists in terms of volume and temperature. Digestive Diseases and Sciences 2012;57(1):142‐7. [PUBMED: 21811829] [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang WJ, Gao JX, Qian JY, Qi YR, Sun MH, Han HY, et al. Water injection versus air insufflation for colonoscopy in elderly patients. World Chinese Journal of Digestology 2014;22(4):601‐5. [Google Scholar]
References to studies awaiting assessment
Cadoni 2015 {published data only}
Hsief 2014 {published data only}
Miroslav 2014 {published data only}
Additional references
Annese 2013
- Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, et al. European Crohn's and Colitis Organisation. European evidence based consensus for endoscopy in inflammatory bowel disease. Journal of Crohn's & colitis 2013;7(12):982‐1018. [DOI: 10.1016/j.crohns.2013.09.016; PUBMED: 24184171] [DOI] [PubMed] [Google Scholar]
Baumann 1999
- Baumann UA. Water intubation of the sigmoid colon: water instillation speeds up left‐sided colonoscopy. Endoscopy 1999;31(4):314‐7. [PUBMED: 10376459] [DOI] [PubMed] [Google Scholar]
Baxter 2009
- Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Annals of Internal Medicine 2009;150(1):1‐8. [PUBMED: 19075198] [DOI] [PubMed] [Google Scholar]
Church 2002
- Church JM. Warm water irrigation for dealing with spasm during colonoscopy: simple, inexpensive, and effective. Gastrointestinal Endoscopy 2002;56(5):672‐4. [PUBMED: 12397274] [DOI] [PubMed] [Google Scholar]
Condon 2008
- Condon A, Graff L, Elliot L, Ilnyckyj A. Acceptance of colonoscopy requires more than test tolerance. Canadian Journal of Gastroenterology 2008;22(1):41‐7. [PUBMED: 18209780] [DOI] [PMC free article] [PubMed] [Google Scholar]
Corley 2014
- Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. The New England Journal of Medicine 2014;370(14):1298‐306. [DOI: 10.1056/NEJMoa1309086; PUBMED: 24693890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 2011
- Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta‐analysis. Gastrointestinal Endoscopy 2011;74(4):885‐96. [PUBMED: 21951478] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falchuk 1984
- Falchuk ZM, Griffin PH. A technique to facilitate colonoscopy in areas of severe diverticular disease. The New England Journal of Medicine 1984;310(9):598. [PUBMED: 6694718] [DOI] [PubMed] [Google Scholar]
Falt 2013
- Falt P, Šmajstrla V, Fojtík P, Tvrdík J, Urban O. Cool water vs warm water immersion for minimal sedation colonoscopy: a double‐blind randomized trial. Colorectal Disease 2013;15(10):e612‐7. [PUBMED: 23819909] [DOI] [PubMed] [Google Scholar]
Froehlich 2006
- Froehlich F, Harris JK, Wietlisbach V, Burnand B, Vader JP, Gonvers JJ, EPAGE Study Group. Current sedation and monitoring practice for colonoscopy: an International Observational Study (EPAGE). Endoscopy 2006;38(5):461‐9. [PUBMED: 16767580] [DOI] [PubMed] [Google Scholar]
Harris 2007
- Harris JK, Vader JP, Wietlisbach V, Burnand B, Gonvers JJ, Froehlich F, Epage Study Group. Variations in colonoscopy practice in Europe: a multicentre descriptive study (EPAGE). Scandinavian Journal of Gastroenterology 2007;42(1):126‐34. [PUBMED: 17190772] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hu 2013
- Hu D, Xu Y, Sun Y, Zhu Q. Water infusion versus air insufflation for colonoscopy: a meta‐analysis of randomized controlled trials. Techniques in Coloproctology 2013;17(5):487‐96. [PUBMED: 23652813] [DOI] [PubMed] [Google Scholar]
Häfner 2007
- Häfner M. Conventional colonoscopy: technique, indications, limits. European Journal of Radiology 2007;61(3):409‐14. [PUBMED: 17169521] [DOI] [PubMed] [Google Scholar]
Jun 2013
- Jun WU, Bing HU. Comparative effectiveness of water infusion vs air insufflation in colonoscopy: a meta‐analysis. Colorectal Disease 2013;15(4):404‐9. [PUBMED: 22889295] [DOI] [PubMed] [Google Scholar]
Kelly 1998
- Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain?. Acad Emerg Med 1998;5(11):1086‐1090. [PUBMED: 9835471] [DOI] [PubMed] [Google Scholar]
Kim 2000
- Kim WH, Cho YJ, Park JY, Min PK, Kang JK, Park IS. Factors affecting insertion time and patient discomfort during colonoscopy. Gastrointestinal Endoscopy 2000;52(5):600‐5. [PUBMED: 11060182] [DOI] [PubMed] [Google Scholar]
Ladas 2010
- Ladas SD, Satake Y, Mostafa I, Morse J. Sedation practices for gastrointestinal endoscopy in Europe, North America, Asia, Africa and Australia. Digestion 2010;82(2):74‐6. [PUBMED: 20407247] [DOI] [PubMed] [Google Scholar]
le Clercq 2014
- Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, Ridder RJ, et al. Postcolonoscopy colorectal cancers are preventable: a population‐based study. Gut 2014;63(6):957‐63. [DOI: 10.1136/gutjnl-2013-304880; PUBMED: 23744612] [DOI] [PubMed] [Google Scholar]
Leung FW 2008a
- Leung FW. Methods of reducing discomfort during colonoscopy. Digestive Diseases and Sciences 2008;53(6):1462‐7. [PUBMED: 17999189] [DOI] [PubMed] [Google Scholar]
Leung FW 2008b
- Leung FW. Water‐related techniques for performance of colonoscopy. Digestive Diseases and Sciences 2008;53(11):2847‐50. [PUBMED: 18461455] [DOI] [PubMed] [Google Scholar]
Leung FW 2011
- Leung FW, Leung JW, Mann SK, Friedland S, Ramirez FC, Olafsson S. DDW 2011 cutting edge colonoscopy techniques ‐ state of the art lecture master class ‐ warm water infusion/CO(2) insufflation for colonoscopy. Journal of Interventional Gastroenterology 2011;1(2):78‐82. [PUBMED: 21776430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung FW 2012
- Leung FW, Amato A, Ell C, Friedland S, Harker JO, Hsieh YH, et al. Water‐aided colonoscopy: a systematic review. Gastrointestinal Endoscopy 2012;76(3):657‐66. [PUBMED: 22898423] [DOI] [PubMed] [Google Scholar]
Leung FW 2013
- Leung FW, Hu B, Wu J. Comparative effectiveness of water immersion and water exchange versus air insufflation for colonoscopy. Journal of Interventional Gastroenterology 2013;3(3):100‐3. [PUBMED: 24478928] [Google Scholar]
Lieberman 2012
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi‐Society Task Force on Colorectal Cancer. Gastroenterology 2012;143(3):844‐57. [DOI: 10.1053/j.gastro.2012.06.001; PUBMED: 22763141] [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin S, Zhu W, Xiao K, Su P, Liu Y, Chen P, et al. Water intubation method can reduce patients' pain and sedation rate in colonoscopy: a meta‐analysis. Digestive Endoscopy 2013;25(3):231‐40. [PUBMED: 23368955] [DOI] [PubMed] [Google Scholar]
Rabenstein 2012
- Rabenstein T, Radaelli F, Zolk O. Warm water infusion colonoscopy: a review and meta‐analysis. Endoscopy 2012;44(10):940‐51. [PUBMED: 22987214] [DOI] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3.. Copenhagen: The Cochrane Collaboration, 2014.
Rex 2015
- Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, et al. Quality indicators for colonoscopy. Gastrointestinal Endoscopy 2015;81(1):31‐53. [DOI: 10.1016/j.gie.2014.07.058; PUBMED: 25480100] [DOI] [PubMed] [Google Scholar]
Rondonotti 2014
- Rondonotti E, Zolk O, Amato A, Paggi S, Baccarin A, Spinzi G, et al. The impact of hyoscine‐N‐butylbromide on adenoma detection during colonoscopy: meta‐analysis of randomized, controlled studies. Gastrointestinal Endoscopy 2014;80(6):1103‐12.e2. [DOI: 10.1016/j.gie.2014.05.319; PUBMED: 25053528] [DOI] [PubMed] [Google Scholar]
Seip 2010
- Seip B, Bretthauer M, Dahler S, Friestad J, Huppertz‐Hauss G, Høie O, et al. Patient satisfaction with on‐demand sedation for outpatient colonoscopy. Endoscopy 2010;42(8):639‐46. [PUBMED: 20669075] [DOI] [PubMed] [Google Scholar]
Singh 2010
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010;139(4):1128‐37. [PUBMED: 20600026] [DOI] [PubMed] [Google Scholar]
Smith 2015
- Smith RA, Manassaram‐Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians 2015;65(1):30‐54. [DOI: 10.3322/caac.21261; PUBMED: 25581023] [DOI] [PubMed] [Google Scholar]
U.S. Preventive Services Task Force 2008
- U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2008;149(9):627‐37. [PUBMED: 18838716] [DOI] [PubMed] [Google Scholar]
Ussui 2013
- Ussui VM, Silva AL, Borges LV, Silva JG, Zeitune JM, Hashimoto CL. What are the most important factors regarding acceptance to the colonoscopy?: study of related tolerance parameters. Arquivos de Gastroenterologia 2013;50(1):23‐30. [PUBMED: 23657303] [DOI] [PubMed] [Google Scholar]
van Rijn 2006
- Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. American Journal of Gastroenterology 2006;101(2):343‐50. [PUBMED: 16454841] [DOI] [PubMed] [Google Scholar]
Whitlock 2008
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2008;149(9):638‐58. [PUBMED: 18838718] [DOI] [PubMed] [Google Scholar]
Winawer 1993
- Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. The New England Journal of Medicine 1993;329(27):1977‐81. [PUBMED: 8247072] [DOI] [PubMed] [Google Scholar]
Wolfe 2007
- Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti‐tumor necrosis factor therapy. J Rheumatol 2007;34(8):1674‐1683. [PUBMED: 17611989] [PubMed] [Google Scholar]


