Abstract
In 2006 following several years of preliminary study, the American Society of Clinical Oncology (ASCO) launched the Quality Oncology Practice Initiative (QOPI). This cancer‐focused quality initiative evolved considerably over the next decade‐and‐a‐half and is expanding globally. QOPI is undoubtedly the leading standard‐bearer for quality cancer care and contemporary medical oncology practice. The program garners attention and respect among federal programs, private insurers, and medical oncology practices across the nation. The MaineHealth Cancer Care Network (MHCCN) has undergone expansive growth since 2017. The network provides cancer care to more than 70% of the cases in Maine in a largely rural health system in Northern New England. In fall 2020, the MHCCN QOPI project leadership, following collaborative discussions with the ASCO‐QOPI team, elected to proceed with a health system–cancer network‐wide QOPI certification. Key themes emerged over the course of our two‐year journey including: (1) Developing a highly interprofessional team committed to the project; (2) Capitalizing on a single electronic medical record for data transmission to CancerLinQ; (3) Prior experience, especially policy development, in other cancer‐focused accreditation programs across the network; and (4) Building consensus through quarterly stakeholder meetings and awarding Continuing Medical Education (CME) and American Board of Medical Specialists (ABMS) Maintenance of Certification (MOC) credits to oncologists. All participants demonstrated a genuine spirit to work together to achieve certification. We report our successful journey seeking ASCO‐QOPI certification across our network, which to our knowledge is the first‐of‐its‐kind endeavor.
Keywords: accreditation, ASCO‐QOPI, cancer network, oncology, quality improvement
1. INTRODUCTION
In June 2017, MaineHealth (MH), a leading integrated health system in the nation announced a $10 million, 5‐year grant from the Harold Alfond Foundation supporting The Development of an Integrated Patient‐Centered Oncology Service Line for Maine. The award formally launched the MaineHealth Cancer Care Network (MHCCN), which is comprised of nine MH member organizations and two affiliates at MaineGeneral Medical Center–Harold Alfond Center for Cancer Care in Augusta and St. Mary's Regional Medical Center in Lewiston. In 2023, MH was divided into three regions—Southern, Coastal, and Mountain (Figure 1/Panel A). MaineHealth provides healthcare services to 1.1 million people in 11 of 16 counties in south‐central Maine and Carroll County in eastern New Hampshire. Since December 2018, the MHCCN has undergone expansive growth substantiated by rapid evolution of a network‐wide employed medical oncology group. In August 2019, MHCCN was competitively awarded entry into the NCI Community Oncology Research Program. The Portland practice in October 2020, assumed a major teaching role in the Maine Track Program—Tufts University School of Medicine undergraduate medical education for the second‐year hematology‐oncology course (last 3 years taught in Maine); and in July 2023, launched a 3‐year combined Hematology‐Oncology Fellowship focusing on rural cancer care.
FIGURE 1.
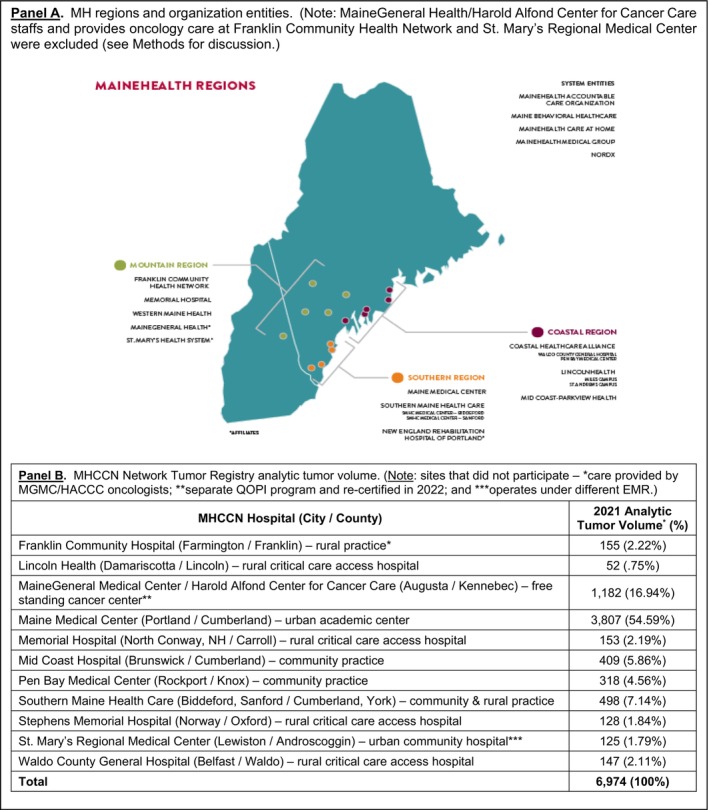
MaineHealth's (MH) three regions with member/affiliate organizations (Panel A); and MaineHealth Cancer Care Network (MHCCN) analytic tumor volume by member site (Panel B).
Maine is a small state (population of 1.38 million) and regarded the most rural in the nation based on Rural‐Urban Community Area code criteria. 1 , 2 , 3 It is also the oldest state (median age 44.9 years). 4 The current age‐adjusted cancer incidence and mortality rates exceed US averages of 478.5 v. 439.8 per 100 000 and 163.7 v. 147.3, respectively. 5 This corresponds to the 10th and 11th highest incidence and mortality rate(s) in the nation. 5 In 2019, 9600 cancer cases were diagnosed in Maine. 6 In 2021, the MHCCN reported an analytic tumor volume of 6974 cases (Figure 1/Panel B), which represents 73% of Maine's cancer burden. 7
In 1999, the Institute of Medicine published a report that outlined opportunities and potential strategies to improve evidence‐based cancer care nationally. 8 This report preceded ASCO's efforts to define, measure, and implement a quality‐based cancer program—Quality Oncology Practice Initiative (QOPI). 9 Between 2003 and 2006, ASCO led a pilot program in three distinct phases involving 23 oncology practices that laid the foundation for the formative ASCO‐QOPI certification. 10 , 11 Since 2006, QOPI is now available to practices of any US‐based ASCO member. 10 Refinements include developing standards based on the ASCO/Oncology Nursing Society for safe chemotherapy administration, oral oncolytic drug standards, and on‐site survey(s). 12 , 13 Recently, ASCO‐QOPI standards have been promoted and developed globally. 14
In fall 2020, we embarked on a feasibility discussion with ASCO seeking a health system–rural cancer network‐wide QOPI certification. We hypothesize that pursuit of QOPI certification will have salutary benefits across a rural cancer care network. Illustrative benefits are not limited to establishing quality cancer care across all our practices, once fully operational it will enable network practice leadership to identify quality trends at both the practice and individual provider levels, reduce care variation, and importantly provide documentation of quality cancer care across our network and share with third‐party payers, many of whom identify and/or use QOPI metrics as state‐of‐the‐art for contemporary cancer care. We report our experience spanning more than 2 years seeking accreditation across eight member practices. These included an academic practice, three smaller community practices, and four practices at critical care access hospitals providing cancer care in six rural locations (Figure 1/Panel B) in Northern New England.
2. METHODS
2.1. Background
By 2016, all 11 MHCCN hospitals were integrated into a centralized Network Tumor Registry, which provided an excellent departure point for reporting numerous quality cancer measures. MaineGeneral successfully renewed their QOPI certification in 2022. Their oncologists provide cancer care for patients at Franklin Memorial Hospital in Farmington and accordingly measurement data are not reported from this MH member practice. This arrangement is geographically expedient for rural cancer care in our health system and has been operational for 7 years. The Franklin practice, however, follows all MHCCN (QOPI) policies. MaineGeneral provided valuable mentorship to the MHCCN team over this journey. By January 2021, all MH member oncology practices operated under a single electronic medical record (EMR)—Epic Systems (Verona, WI). This arrangement facilitated the decision to electronically report measurement data to CancerLinQ. 15 , 16 St. Mary's Regional Medical Center utilizes a separate instance of Epic hosted on their premises and managed by their organization. The MHCCN team does not have access to data within St. Mary's Epic instance. Given this, including St. Mary's Epic data would have required a separate Business Associate Agreement (BAA), dedicated IT resources, and an inventory of their workflows and Epic configuration items. We elected to defer including St. Mary's for these reasons. In collaboration with the ASCO‐QOPI administrative team, mutually agreeable accommodations for these three sites were made. With this backdrop, the MHCCN team reviewed published ASCO‐QOPI Standards Manual and QOPI Certification Track 2021 Measures Summary (Table 1). 17 , 18
TABLE 1.
Quality Oncology Practice Initiative certification measures and policies.
| SmartLinQ QOPI® Certification Track 2021 Measures Summary for electronic reporting | ||
|---|---|---|
| Module | Measure | Measure title |
| Core | 2 | Staging documented within 1 month of first office visit. |
| Core | 4a | Pain quantification score during first two encounters. |
| Core | 10 | Chemotherapy intent (curative vs. non‐curative) documented before or within 2 weeks after administration. |
| Core | 21aa | Tobacco use assessment. |
| Core | 25b | Height, weight, and BSA documented prior to curative chemotherapy. |
| Core | N/A | Documentation of current medications in the medical record. |
| Core | QOPI 15 | G‐CSF administered to patients who received chemotherapy for metastatic cancer (lower score—better). |
| Core | QOPI 5 | Chemotherapy administered to patients with metastatic solid tumor with performance status of ECOG 3 or 4; KPS 10‐40; or undocumented. |
| EOL | N/A | Care plan. |
| EOL | N/A | Proportion receiving chemotherapy in the last 14 days of life. |
| SMT | 27 | Corticosteroids and serotonin antagonist prescribed or administered with moderate or high emetic risk chemotherapy. |
| SMT | 28/28a | NKI receptor antagonist and olanzapine prescribed or administered with high emetic risk chemotherapy. |
| BR | 54 | Her‐2/neu testing for breast cancer patients. |
| BR | 59 | Hormonal therapy for breast cancer patients within 1 year of diagnosis. |
| BR | QOPI 11 | Combination chemotherapy received for breast cancer. |
| CRC | 68 | Adjuvant chemotherapy received within 4 months of diagnosis by patients with stage III colon cancer. |
| GynOnc | 94 | Platin and/or taxane administered within 42 days of staging for ovarian, fallopian tube, or peritoneal cancer. |
| Lung | 84 | Percentage of patients with initial AJCC stage IV or distant metastatic lung cancer whose performance status is documented. |
| MM | N/A | Treatment with bisphosphonates. |
| NHL | 78a | Hepatitis B testing prior to rituximab administration for NHL. |
| QOPI policies | ||
|---|---|---|
| Standard | QOPI policy requirement(s) [MH/MHCCN Supporting Policy] | Ref. nos. |
| 1.1 | Clinical staff qualifications of those who order, prepare, document, and administer chemotherapy [MH Validation of Initial Competency and Ongoing Assessment of Oncology Nurses Involved in the Care of Patients Receiving Chemotherapy and Immunotherapy Treatment Policy (Appendix XX. Pharmacy Hazardous Drug Compounding Education and Training Requirements); Advanced Practice Professional (APP) Competencies; Pharmacy Compounding Competencies] | 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 |
| 1.6 | Identifies a process to provide 24/7 triage [MH Medical Oncology Coverage Policy] | ── |
| 2.1 | The process for obtaining informed consent [MH Informed Consent for Chemotherapy/Biotherapy for Adult and Pediatric Patients Policy] | 21, 31, 32 |
| 3.5 | A policy specifying how intrathecal medication is maintained [MH Nursing Verification, Administration, and Safe Handling of Chemotherapy Biotherapy Policy] | 19, 20, 21, 22, 23, 24 |
| 3.10 | Defines extravasation management procedures [MH Extravasation Management Guidelines] | 33, 34, 35, 36 |
| 4.1 | A policy for emergent treatment of patients [MH Management of Hypersensitivity/Infusion Reaction(s)] | 37, 38, 39, 40 |
| 4.2 | Initial oral adherence assessment policy [MH Corporate Chemotherapy/Biotherapy Treatment Planning, Ordering, Administration Policy] | 21, 41 |
| 4.3 | Ongoing Oral Adherence Assessment Policy [MH Corporate Chemotherapy/Biotherapy Treatment Planning, Ordering, Administration Policy] | 21, 41 |
Abbreviations: BR, breast cancer; CRC, colorectal cancer; EOL—end‐of‐life; GynOnc, gynecologic oncology; MM, multiple myeloma; N/A, not applicable; NHL non‐hodgkin lymphoma; SMT, symptom/toxicity management.
2.2. QOPI pre‐certification Phase I—Preliminary work
We initially sought an IRB‐approved research exemption to consider the project quality improvement (Figure 2/Panel A). Thereafter, a BAA and registration to gain access to the Quality Initiative dashboard was executed with ASCO‐QOPI. Our team worked with our Information Technology Department for feasibility assessment for electronic data transfer. This required a project charter and led to another BAA with CancerLinQ. During the onboarding process, CancerLinQ provided the MHCCN team with SQL code to extract data elements they required from Epic. This code targeted Epic's Clarity reporting environment, which is updated nightly with changes from Epic's Hyperspace (front‐end interface). The MHCCN team worked with CancerLinQ to develop code to target the Medical Oncology population from Epic, and to isolate specific Epic configuration items that were needed for measure calculations, including Flowsheets, Note Types, and SmartData Elements. In total, 19 SQL extracts were executed weekly to pull the previous week's data. The MH Integration team established secure delivery to CancerLinQ through the Secure File Transfer Protocol (SFTP). During this period, the team also began to socialize the QOPI initiative across all MHCCN sites and assemble a stakeholder group. Taking advantage of our Network Tumor Registry we embarked upon manual abstracting 2020 QOPI Pre‐Certification measures from up to 10 (randomly selected) charts across representative tumor types including breast, colorectal, and non‐small cell lung cancer cases. This was undertaken at all eight participating member sites to determine baseline assessment, identify gaps, and to help with electronic data transfer (see Table 2). Following review of the manually abstracted data our QOPI team convened once or twice a month via Zoom.
FIGURE 2.
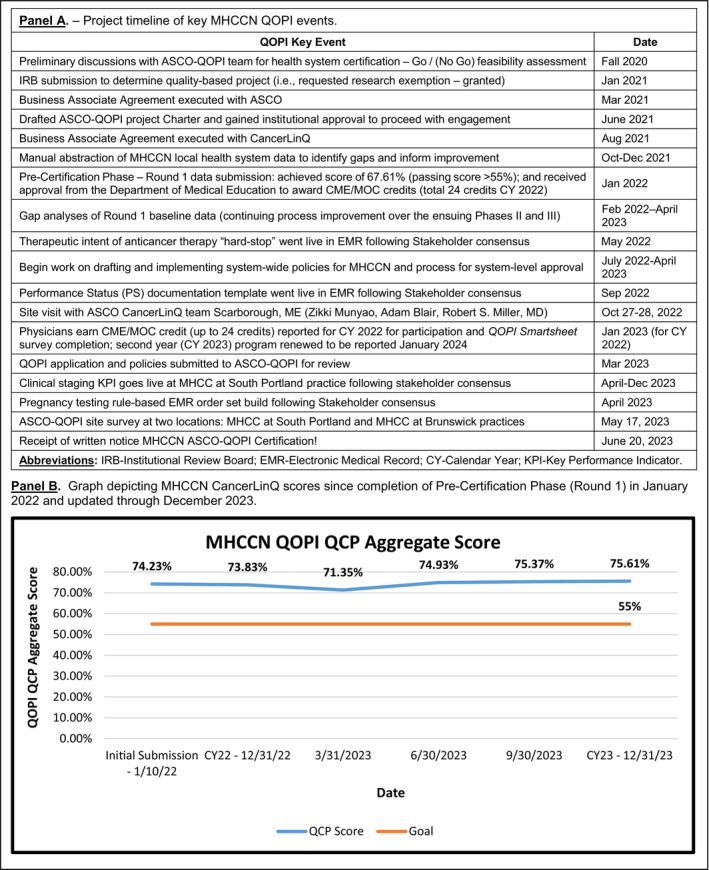
Project timeline (Panel A) and graph depicting MaineHealth Cancer Care Network (MHCCN) CancerLinQ scores over course of project (Panel B).
TABLE 2.
Thirteen common Quality Oncology Practice Initiative measures that we initially recorded as part of our preparatory manual abstraction and carried forward during our electronic transfer.
| QOPI measure | Manual abstraction | First electronic submission CY2021 | Last electronic submission CY2023 |
|---|---|---|---|
| 9/8/2021 | 1/10/22 | 12/31/23 | |
| Chemotherapy intent documented | 98% | 71.4% | 91.5% |
| Current medications documented | 100% | 98.1% | 97.9% |
| Height, weight, BSA documented | 100% | 99.1% | 97.2% |
| Smoking status/tobacco use documented | 100% | 99.9% | 100% |
| Her‐2/Neu testing (breast only) | 100% | 75.1% | 66.7% |
| Corticosteroid and serotonin antagonist administered for moderate/high emetic risk chemotherapy | 100% | 97.5% | 94.1% |
| Adjuvant chemotherapy received within 4 months for stage III colon cancer | 100% | 78.9% | 69.2% |
| Advanced care plan discussed/documented | 97% | 61% | 39.2% |
| Pain intensity quantified by second office visit | 53% | 90.5% | 85.5% |
| Staging documented within 1 month of first office visit | 91% | 41.8% | 44.5% |
| G‐CSF administered to patients who receive chemotherapy for stage IV cancer | 12% a | 23% a | 9% a |
| Chemotherapy administered to patients with metastatic solid tumor with performance status of ECOG 3; KPS 10–40; or undocumented | 6.3% a | 100% a | 35.2% a |
| Percentage of patients with initial AJCC stage IV or distant metastatic lung cancer whose performance status is documented | 100% | 27.3% | 81.2% |
Note: The table reports status over time of each metric, while Figure 2, Panel B graphs composite CancerLinQ scores over course of project.
Denotes lower score is better.
2.3. Phases II and III—Electronic data transfer to CancerLinQ
CancerLinQ did not supply Application Programming Interface(s) (APIs) for MHCCN to programmatically extract scores from their environment. MHCCN worked with CancerLinQ to establish the functionality to manually export measure data from their dashboard in a Comma Separated Values (CSV) file. The measure data included numerator, denominator, and actionable patient counts. MHCCN established an Excel template to be able to calculate their QCP score based on CancerLinQ's scoring algorithm, which weighs each measure based on the denominator value. MHCCN also calculated the lowest/highest possible scores for each measure and total based on the number of actionable patients. This method was used to generate the QCP score(s) for the graph in Figure 2/Panel B. In October 2022, a valued component of this phase was a CancerLinQ site visit convened in Scarborough. We shared progress on data reporting and updated CancerLinQ applications for our practices.
2.4. Stakeholder meetings
In December 2021, the QOPI leadership team assembled our first stakeholder group meeting. The group is comprised of all MHCCN oncology providers, pharmacists, select Advance Practice Professionals (APPs), and practice managers, and our informatics team is devoted to the project. These forums were convened quarterly, chaired by our leadership team with an agenda distributed in advance, and minutes kept. Physicians were required to complete a post‐meeting survey. A monthly electronic newsletter was distributed that reported progress to date.
2.5. Continuing Medical Education/Maintenance of Certification credit for oncologists
Upon receipt of the SmartLinQ QOPI Certification Registry Submission Report (Pre‐Certification Phase) in January 2022, we submitted an application to the Department of Medical Education (DME) to offer Continuing Medical Education (CME) and corresponding Maintenance of Certification (MOC) credits to our physicians. We submitted a formal institutional application (Quality Improvement Intake Form) since MH is a sponsoring program of the American Board of Medical Specialists (ABMS) Portfolio Program, included our results for each of the 20 QOPI measures, and defended our processes for quality improvement over the ensuing year. Our proposal, titled FY22 ASCO‐QOPI Clinical Transformation, was accepted to award a total of 24 credits annually with successful completion of the work. On a monthly basis, 1.5 CME/MOC (subtotal 18) credits were awarded to both acknowledge and reward provider effort to record EMR data in discrete searchable fields. An additional 1.5 CME/MOC credits (subtotal six) were awarded for participation and survey completion following the quarterly stakeholders meeting. These surveys invited feedback on ways forward and developed provider consensus on strategies to build into the EMR to facilitate data capture; thus participants actively contributed to Plan‐Do‐Study‐Act cycles. Participation was monitored and recorded quarterly with credits reported to the DME and the American Boards of Internal Medicine and Neurology and Psychiatry (participating neuro‐oncologist). Data were reported to the ABMS for calendar year 2022 in February 2023 and awarded to all participating physicians. To receive credit physicians were required to submit their unique ABMS and individual National Provider Identification (NPI) numbers.
2.6. Phase IV – Preparation for QOPI certification and policies development
Project leadership and analyst teams were regularly monitoring progress. Analysts conducted manual reviews of QOPI measure scores and data at the patient/provider level to ensure CancerLinQ accurately portrayed MH performance.
We embarked on formal QOPI policies development during summer 2022 (Table 1). Some policies were already available and periodically updated by the MHCCN Clinical Oncology Practice (COP) Committee (established 2017). This interprofessional committee includes all sites, meets monthly, and charged with developing chemotherapy orders and clinical guidelines (e.g., antiemetic guidance among others). Historically, policies emanating from the COP Committee were primarily for Maine Medical Center but provided to other sites for reference and/or to incorporate in local health system policies. With MH reorganization and our QOPI initiative, an approach to adopt system‐level policies was undertaken. Drafts were developed by the QOPI leadership team and subsequently sent to COP Committee for initial approval; from there nursing policies were routed to the MH Nursing Council and all others to the Clinical Leadership Council chaired by the MH Chief Nursing and Medical Officers respectively for final approval. Once approved all policies were loaded on MCN Policy Manager, an enterprise policy management software (ellucid, MCN Healthcare, Denver, CO). The software icon is located on all desktops for ready reference across the MHCCN.
2.7. Phase V—Survey preparation
Leading up to survey the leadership team and stakeholder group convened several ad hoc meetings to review final preparations. We worked closely with the ASCO‐QOPI surveyor conveying policies, answering queries, assisting with organizing the on‐site review, and selecting two sites for survey.
3. RESULTS
3.1. QOPI pre‐certification Phase I
In January 2022, we achieved an initial Round 1 passing score of 67.61% (target ≥55%) required for the QOPI Pre‐Certification Track. With cumulative reporting of CancerLinq data, our original January 2022 submission score is now 74.23%, which improved to 75.61% as of December 2023 (see Figure 2, Panel B). Our manual abstraction observed nearly 90‐100% compliance on the majority of measures (Table 2). While several measures were documented in the EMR, they were not in discrete “searchable” fields for electronic transfer. Six QOPI measures/standards are worthy of comment (Table 1).
Staging documentation (Core 2) and Performance Status (PS) were nearly uniformly entered in EMR office notes, though not in searchable fields. Our lung cancer PS score (NSCL84) was our lowest at 5.19 (4/77). Chemotherapy intent, curative versus non‐curative (Core 10), while a defined and required element in our informed consent form for treatment, which is scanned into the EMR, is also not searchable. Use of G‐CSF (QOPI15) in patients receiving chemotherapy for metastatic cancer (lower score is better) at 20.95 (44/210) was judged to be slightly higher than it should be. As we approached survey an admitted gap was to develop a consistent approach for pregnancy testing in women of childbearing potential (QOPI Standard 1.2.4), who are about to receive chemotherapy. These issues were addressed at the stakeholder meetings. When using CancerLinQ for automated abstraction, a passing QCP score of ≥55% is required.
Antiemetic therapy for high‐risk antineoplastic agents proved challenging. Our Round 1 score (SMT28/28a) was among the lowest at 12.34 (10/81). This measure requires use of an NK1 receptor antagonist and olanzapine. We reviewed our guidelines and discussed this with our providers and pharmacy teams. First, this was not judged a clinical problem from our patient experience and interdisciplinary discussion among providers, pharmacists, and chemotherapy nurses. Manual abstraction revealed that neither drug was administered nor one of the two agents in the majority of instances. We elected not to aggressively pursue this measure and shared this recommendation with the stakeholder group. Rationale was based on an unsubstantiated problem for our patients, would entail new purchasing arrangements, and potential for polypharmacy and introducing new drug interactions and side effects. Our current practice administers a combination of palonosetron (5‐HT3 antagonist), fosaprepitant (NK1 antagonist), and dexamethasone in this setting with excellent results. We reached out to ASCO‐QOPI to share our concerns about this measure and learned this has been previously raised by other practices. On balance, we thought it prudent to defer managing up on this particular measure.
3.2. Phases II/III
The largest barrier to technical implementation proved to be a lack of standardization in documentation across facilities and providers, in addition to limited structured data (e.g., cancer staging). For instance, due to Epic build incompleteness, a range of PS scores were not being transferred into the reporting environment. This significantly contributed to score reductions.
3.3. Stakeholder meetings and CME/MOC credits
Participation by providers, other healthcare team members, and our QOPI leadership was robust. Physicians valued participation as well as their input on addressing ways forward. The meetings were free of commercial bias or other interests. We successfully applied for continuation of this activity for 2023 and 2024.
The Stakeholder meetings provided an interprofessional forum to discuss improvement approaches. A fundamental principle when making revisions to EMR workflows was to develop strategies that were efficient, reduced redundancies, and limited the number of “clicks” to help physicians. At the outset, G‐CSF utilization was reviewed in patients receiving chemotherapy for metastatic disease and it was deemed best to permit clinical discretion for use based on acceptable guidelines (e.g., age, comorbidities, myelotoxic risk of regimen among others) rather than prescribe this. The group was also apprised of foregoing major modifications in our approach to antiemetic support for patients receiving highly emetogenic chemotherapy. These recommendations were valued. Accordingly, following physician consensus (post‐meeting survey responses) and working closely with our informatics team, we built a chemotherapy intent “hard‐stop” in our electronic order sets in May 2022; built a PS documentation template in Office Notes in September 2022; and in April 2023 piloted a tumor staging strategy in our South Portland practice to go‐live across the network if efficient. Finally, the group agreed to modify all of our chemotherapy order sets framed in a “rules‐based” manner for women of childbearing potential defined as between 18 and 50 years of age and excluding patients having undergone tubal ligation, hysterectomy, and/or salpingo‐oophorectomy to have pregnancy testing prior to chemotherapy. While this requires more time to build it was the preferred approach and more expedient to avoid a best‐practice advisory in the EMR.
3.4. Phases IV/V—Preparation for QOPI certification and survey
Extensive experience with numerous MHCCN member organization on‐site surveys [e.g., Commission on Cancer (CoC), National Accreditation Programs for Breast Cancer and Rectal Cancer, American College of Radiology among others] provided a solid foundation to prepare for the ASCO‐QOPI survey. Our surveyor was a strong advocate and mentor for our leadership team and was actively engaged in advance of our survey.
3.5. Immediate quality improvement outcome—Cancer staging
An illustrative example of the impact of our QOPI accreditation immediately followed our pilot staging quality improvement workflow process that is now going live across our network. We are now able to track this work at the individual practice or provider level. Effective October 1, 2023, our Epic TNM staging key performance indicator (KPI) will deploy across all medical oncology sites. The goal is to stage ≥80% of new cancer patients within 31 days post‐consult using the Epic TNM staging form on the problem list. If staging is not completed within 31 days or at all, providers must communicate a reason to the KPI champion and Pareto charts have been created for missed staging reasons. All data are collected and analyzed over time to help us succeed with this goal. Currently, cancer care network practices at Sanford, Brunswick, and South Portland have this KPI active at their sites. In January 2023, Sanford had a staging rate of 25% and averaged 40.8% over the year through December 2023. The corresponding staging rates at Brunswick and South Portland, our largest practices, had initial staging rates of 50% and 65% in January 2023, and end of December 2023 achieved averages of 46.8% and 62.3%, respectively.
4. DISCUSSION
Prior to embarking on this effort, our quality program was largely driven by data reporting to the Commission on Cancer/American College of Surgeons accredited Network Tumor Registry. While cancer is a reportable disease and our cancer registry is robust, the data elements largely surround diagnosis, staging, first course of treatment (including surgery, radiation, and/or systemic therapy), and survival. QOPI focuses on these elements in addition to continuous treatment and follow‐up of cancer patients over the arc of the cancer care continuum devoted to contemporary medical oncology practice. The majority of QOPI measures are not captured in our tumor registry database and provides another reason to participate in this program.
To our knowledge, the QOPI accreditation journey reported herein is the first‐ever successfully executed across a health system in an essentially rural cancer care network. Several themes emerged from the start. Foremost, this endeavor involved a highly interactive and engaged interprofessional leadership team of oncologists, nurses, pharmacists, informatics analysts, and quality improvement experts over the course of our journey. This approach is of paramount importance. The team was committed to the project, demanded discipline, and met routinely to monitor progress over the two‐year course seeking QOPI certification. Secondly, strong institutional and informatics support to realize this goal was vital. Without the electronic linkages in our health system, the collective effort of single site‐by‐site, manual abstraction of data across a healthcare system is highly impractical. The difference between achieving a pre‐certification score of >75% for manual abstraction versus ≥55% for electronic transfer must be carefully considered. While a lower benchmark for the latter, the time and effort required at the outset for manual abstraction is comparable to expended effort to track and report data electronically. Now that we have operationalized CancerLinQ electronic data reporting enormous efficiencies in time and effort are realized for each successor accreditation period. We feel this is a justifiable and worthwhile approach that spanned more than 2 years. Attention to identifying EMR searchable fields became readily apparent and the need for a strong informatics team to accommodate and build the changes that are required cannot be understated. This was undoubtedly a much more cost‐effective manner in which to proceed and once built, tees up future activities and engagements. Thirdly, prior extensive experience across a variety of cancer‐focused CoC accreditations (especially for tumor registration, breast and rectal cancer) and American College of Radiology for radiation oncology practice proved valuable. Our robust Network Tumor Registry provided strong leadership for this undertaking and readily identified gaps and helped frame ways forward in the EMR. Finally, creating a Stakeholder group and offering CME/MOC credits was prescient and valued by physicians. This venue provided opportunities to define more optimal workflows, especially electronic, and allow physician‐driven input on clinical decision‐making. Offering professional educational and certification credits placed value on physicians' time and participation.
There are opportunities to provide better care to patients and improve documentation. Cancer staging is an illustrative example. Clinical staging presents challenges across the nation and improvements in our EMR documentation were needed, despite frequent documentation in individual office visit notes. 42 Access to traceable staging data in the EMR facilitates participation in treatment pathways given the complexities and expense of contemporary cancer treatment, and referral of advanced stage patients for palliative care. Our care teams appreciated the need to develop strategies to protect women of childbearing potential and the adopted approach will clearly benefit patients and providers. The efficiencies we are building into our EMR and the power of the CancerLinQ platform will be realized by our practices in the coming years. 43 This is nicely illustrated by our recent progress on the Epic TNM staging initiative.
The origins of healthcare quality improvement date back nearly 60 years ago. 44 , 45 , 46
Contemporary cancer care is complex and exorbitantly expensive with numerous alternative payment models and value‐based care initiatives. Accordingly, quality measurement in oncology is rapidly advancing. 47 Current published observations acknowledge considerable stress in healthcare quality improvement. 48 , 49 , 50 What is clear—physicians genuinely want what is best for their patients, sensitivity to federal and private reimbursement approaches are increasingly tethered to quality initiatives, and strategies that embrace physician input and clinical discretion are likely to be more successful. 50 Given this, ASCO‐QOPI is a physician‐driven, peer‐reviewed process that our organization embraced and for this reason set upon this journey. The MHCCN is better for this achievement.
5. CONCLUSIONS
We report a successful two‐year journey to seek ASCO‐QOPI certification in a rural cancer network. Keys to our success included a robust interprofessional team devoted to the work, a single EMR across our health system and participation by our informatics team, extensive experience with other cancer‐focused accreditations, and willingness to embrace and value physician input and clinical discretion capitalizing upon an innovative approach with our Stakeholder group. In the end, this effort was simply the “right‐thing‐to‐do” for our patients, their families, and our providers. By sharing our approach we are hopeful this can guide other rural health system cancer care practices and networks.
AUTHOR CONTRIBUTIONS
Evelyn Taylor, Hilary Perrey, Brett Cropp, Joy Moody, Michael Bianchi, Mark Parker, Amit Sanyal, and Scot Remick: Conception and design. Hilary Perrey, Meaghan Bumpus, and James Reich: Administrative support. Brett Cropp, Shannon Lessard, Lauren Couture, Jeanette Pretorius Jonathan Angus, Megan Duperreault, Amanda Snow, Dorothy Wang, Hilary Perrey, and Evelyn Taylor: Collection and assembly of data. Manuscript: All authors were accountable for all aspects of the work and participated in writing and final approval of the manuscript.
FUNDING INFORMATION
This work was supported in part by the Harold Alfond Foundation, Portland, ME.
CONFLICT OF INTEREST STATEMENT
None of the authors of this manuscript have any conflicts to disclose.
ACKNOWLEDGMENTS
The authors wish to acknowledge the partnership of all MHCCN medical oncologists and other members of the QOPI Stakeholders group including oncology nurses, Advance Practice Professionals, pharmacists, and informatics team for embracing and participating in this collective effort for the past two years for the benefit of our patients and their families. We wish to acknowledge Maureen van Benthuysen, former Chief Administrative Officer, for her support and contributions at the outset of this journey as well as Debbie Bowden, Administrative Director for Oncology Services at the Harold Alfond Center for Cancer Care in Augusta, over the course of the project. We are indebted to Laura Stanley, Medical Education Program Coordinator II, in the MMC Educational Services and Academic Affairs Office, for assisting with coordinating our Continuing Medical Education (CME) program and reporting Maintenance of Certification (MOC) credits to the American Board of Medical Specialists—Internal Medicine and Neurology and Psychiatry for our physicians including a neuro‐oncologist for the later. The QOPI leadership team valued the ASCO‐QOPI team site visit with Zikki Munyao, Adam Blair, and Robert Miller, MD on October 27–28, 2022, which provided further insights into the CancerLinQ platform. Lastly, we wish to acknowledge the efforts of Anna Vioral Gavin, PhD, MEd, RN, OCN, BMTCN, Director Oncology Practice and Professional Development, Allegheny Health Network Cancer Institute, Pittsburgh, PA. Dr. Vioral Gavin served not only as our QOPI Certification Site Surveyor but additionally as a valued mentor in this accreditation engagement. She visited our MHCC at South Portland (Maine Medical Center) and MHCC at Brunswick (Mid Coast Hospital) medical oncology practices on May 17, 2023. Her insights were highly valued.
Perrey HM, Taylor E, Cropp BF, et al. Seeking American Society of Clinical Oncology‐Quality Oncology Practice Initiative (ASCO‐QOPI) certification in a northern New England rural health system and cancer care network. Learn Health Sys. 2024;8(3):e10415. doi: 10.1002/lrh2.10415
Hilary Perrey, Evelyn Taylor, and Brett Cropp contributed equally to this work as first authors.
Contributor Information
Amit Sanyal, Email: amit.sanyal@mainehealth.org.
Scot C. Remick, Email: scot.remick@mainehealth.org.
REFERENCES
- 1. United States Census Bureau . Quick Facts–Maine. https://www.census.gov/quickfacts/ME. Accessed July 17, 2023.
- 2. US Census Bureau–Story Maps . https://www.storymaps.geo.census.gov. Accessed July 17, 2023.
- 3. Wickenham M. Census: Maine most rural state in 2010 as urban centers grow nationwide. Bangor Daily News, March 26, 2012 (Bangor, ME). https://www.bangordaily news.com. Accessed June 30, 2023.
- 4. Infoplease . Oldest States. https://www.infoplease.com/us/states/oldest-states. Accessed July 17, 2023.
- 5. National Cancer Institute . State Cancer Profiles. https://www.statecancer profiles.cancer.gov. Accessed July 17, 2023.
- 6. 2022 Maine Cancer Snapshot . A Report of the Maine Cancer Registry. https://www11.maine.gov/dhhs/mecdc/public-health-systems/data-research/vitalrecords/mcr/reports/documents/2022. Accessed July 17, 2023.
- 7. MaineHealth Cancer Care Network (MHCCN) . Analytic Tumor Volume. Scarborough, ME: MHCCN Network Tumor Registry; 2021. [Google Scholar]
- 8. Hewitt M, Simone J. National Cancer Policy Board, Institute of Medicine: Ensuring Quality Cancer Care. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 9. Neuss MN, Desch CE, McNiff KK, et al. A process for measuring the quality of cancer care: the quality oncology practice initiative. J Clin Oncol. 2005;23:6233‐6239. [DOI] [PubMed] [Google Scholar]
- 10. Jacobson JO, Neuss MN, McNiff KK, et al. Improvement in oncology practice performance through voluntary participation in the quality oncology practice initiative. J Clin Oncol. 2008;26:1893‐1898. [DOI] [PubMed] [Google Scholar]
- 11. Blayney DW, McNiff K, Eisenberg PD, et al. Development and future of the American Society of Clinical Oncology's quality oncology practice initiative. J Clin Oncol. 2014;32:3907‐3913. [DOI] [PubMed] [Google Scholar]
- 12. Gilmore TR, Schulmeister L, Jacobson JO. Quality oncology practice initiative certification program: measuring implementation of chemotherapy administration safety standards in the outpatient oncology setting. J Oncol Pract. 2013;9(2s):14s‐18s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayes DJ, Moore JE. Improving direct pharmacist counseling rates for oral oncolytic medications at an outpatient oncology clinic. J Oncol Pharmacy Pract. 2021;27:1861‐1868. [DOI] [PubMed] [Google Scholar]
- 14. Blayney DW, Albdelhafeez N, Jazieh AR, et al. International perspective on the pursuit of quality in cancer care: global application of QOPI and QOPI certification. JCO Global Oncol. 2020;6:697‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller RS. CancerLinQ update. J Oncol Pract. 2016;12:835‐837. [DOI] [PubMed] [Google Scholar]
- 16. Potter D, Brothers R, Kolacevski A, et al. Development of CancerLinQ, a health information learning platform from multiple electronic health record systems to support improved quality of care. JCO Clin Cancer Inform. 2020;4:929‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ASCO QOPI® Certification Program . Standards Manual. Required Processes and Documentation to Meet Certification Standards and Elements. 2018.
- 18. American Society of Clinical Oncology: QOPI® Certification Track . Measures Summary. 2021. https://practice.asco.org/quality-improvement/quality-programs/quality-oncology-practice-initiative/qopi-related-measures. Updated September 12 2020. Accessed July 31, 2023.
- 19. NIOSH . List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings. 2016. https://www.cdc.gov/niosh/docs/2016-161/pdfs/2016-161.pdf. Accessed July 31, 2023.
- 20. Occupational Safety and Health Administration . Controlling occupational exposure to hazardous drugs. 2016. https://www.osha.gov/SLTC/hazardousdrugs/controlling_occex_hazardousdrugs.html#into. Accessed July 31, 2023. [DOI] [PubMed]
- 21. Neuss MN, Gilmore TR, Belderson KM, et al. Updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards, including standards for pediatric oncology. J Oncol Pract. 2016;12:1262‐1271. doi: 10.1200/JOP.2016.017905 [DOI] [PubMed] [Google Scholar]
- 22. Olsen MM, LeFebvre KB, Brassil KJ, eds. Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice. Pittsburgh, PA: Oncology Nursing Society; 2019. https://www.ons.org. Accessed July 31, 2023. [Google Scholar]
- 23. Oncology Nursing Society . Chemotherapy Immunotherapy Course 2022. Pittsburgh, PA: Oncology Nursing Society; 2022. https://www.ons.org. Accessed July 31, 2023. [Google Scholar]
- 24. Polovich M. Safe Handling of Hazardous Drugs. 3rd ed. Pittsburgh, PA: Oncology Nursing Society; 2018. [Google Scholar]
- 25. Oncology Nursing Society . Oncology Nurse Practitioner Competencies 2019. Pittsburgh, PA: Oncology Nursing Society; 2019. https://www.ons.org. Accessed July 31, 2023. [Google Scholar]
- 26. Coombs LA, Noonan K, Barber FD, et al. Oncology nurse practitioner competencies: defining best practices in the oncology setting. Clin J Oncol Nurs. 2020;24:296‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crandell BC, Bates JS, Grgic T. Start using a checklist, PRONTO: recommendation for a standard review process for chemotherapy orders. J Oncol Pharm Pract. 2018;24:609‐616. [DOI] [PubMed] [Google Scholar]
- 28. United States Pharmacopeia (USP) 797 . Pharmaceutical Compounding–Sterile Preparations. 2022. doi: 10.31003/USPNF_M99925_06_01 [DOI]
- 29. United States Pharmacopeia (USP) 800 . Hazardous Drugs–Handling in Healthcare Settings. 2022. doi: 10.31003/USPNF_M7808_07_01 [DOI]
- 30. United States Pharmacopeia (USP) 797 . Pharmaceutical Compounding–Sterile Preparations. 2023. doi: 10.31003/USPNF_M99925_07_01 [DOI]
- 31. Storm C, Casillas J, Grunwald H, Howard DS, McNiff K, Neuss MM. Informed consent for chemotherapy: ASCO members resource. J Oncol Pract. 2008;4:289‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MaineHealth Informed Consent Policy . Portland, ME: MaineHealth. 2022.
- 33. Goolsby TV, Lombardo FA. Extravasation of chemotherapeutic agents: prevention and treatment. Semin Oncol. 2006;33:139‐143. [DOI] [PubMed] [Google Scholar]
- 34. Jordan K, Behlendorf T, Mueller F, Schmoll H‐J. Anthracycline extravasation injuries: management with dexrazoxane. Ther Clin Risk Manage. 2009;5:361‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schulmeister L. Extravasation management. Semin Oncol Nurs. 2007;23:184‐190. [DOI] [PubMed] [Google Scholar]
- 36. Schulmeister L. Extravasation management: clinical update. Semin Oncol Nurs. 2011;27:82‐90. [DOI] [PubMed] [Google Scholar]
- 37. Burke MJ, Zalewska‐Szewczyk B. Hypersensitivity reactions to asparaginase therapy in acute lymphoblastic leukemia: immunology and clinical consequences. Fut Oncol. 2022;8:1285‐1299. [DOI] [PubMed] [Google Scholar]
- 38. Pagani M, Bavbek S, Alvarez‐Cuesta E, et al. Hypersensitivity reactions to chemotherapy: an EAACI position paper. Allergy. 2022;77:388‐403. [DOI] [PubMed] [Google Scholar]
- 39. Rosello S, Blasco I, Garcia Fabregat L, Cervantes A, Jordan K. On behalf of the ESMO guidelines committee. Management of infusion reactions to systemic anticancer therapy: ESMO clinical practice guidelines. Ann Oncol. 2018;28(Suppl 4):iv100‐iv118. [DOI] [PubMed] [Google Scholar]
- 40. Sicherer SH, Simons ER. Epinephrine for first‐aid management of anaphylaxis. Pediatrics. 2017;139:e1‐e9. doi: 10.1542/peds.2016-4006 [DOI] [PubMed] [Google Scholar]
- 41. Goldspiel B, Hoffman JM, Griffith NL, et al. ASHP guidelines on preventing medication errors with chemotherapy and biotherapy. Am J Health‐Syst Pharm. 2015;72:e6‐e35. [DOI] [PubMed] [Google Scholar]
- 42. Lee JH, Mohamed T, Ramsey C, et al. A hospital‐wide intervention to improve compliance with TNM cancer staging documentation. J Natl Compr Canc Netw. 2022;20:351‐360. [DOI] [PubMed] [Google Scholar]
- 43. Sledge GW Jr, Miller RS, Hauser R. CancerLinQ and the future of cancer care. Am Soc Clin Oncol Ed Book. 2013;430‐434:430‐434. [DOI] [PubMed] [Google Scholar]
- 44. Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966;44(Suppl):166‐206. [PubMed] [Google Scholar]
- 45. Berwick DM. Continuous improvement as an ideal in health care. N Engl J Med. 1989;320:53‐56. [DOI] [PubMed] [Google Scholar]
- 46. Blumenthal D. The origins of the quality‐of‐care debate. N Engl J Med. 1996;335:1146‐1149. [DOI] [PubMed] [Google Scholar]
- 47. Nardi EA, McCanney J, Winckworth‐Prejsnar K, et al. Redefining quality measurement in cancer care. J Natl Compr Canc Netw. 2018;16:473‐478. [DOI] [PubMed] [Google Scholar]
- 48. Rosenbaum L. Reassessing quality assessment — the flawed system for fixing a flawed system. N Engl J Med. 2022;386:1663‐1667. [DOI] [PubMed] [Google Scholar]
- 49. Rosenbaum L. Metric myopia — trading away our clinical judgment. N Engl J Med. 2022;386:1759‐1763. [DOI] [PubMed] [Google Scholar]
- 50. Rosenbaum L. Peers, professionalism, and improvement – reframing the quality question. N Engl J Med. 2022;386:1850‐1854. [DOI] [PubMed] [Google Scholar]


