Abstract
The primary objective of this study was to investigate the pharmacokinetics of inhaled argon in young pigs using mechanical ventilation. Also a physiologically based model of argon pharmacokinetics (PBPK) is validated with human data for xenon from the literature and the new data from juvenile pigs. The inherent difficulty in performing pharmacokinetics studies of argon makes the use of the PBPK model especially relevant. The model is used to investigate argon pharmacokinetics for adult and neonate applications. Juvenile pigs (n = 4) were anesthetized, submitted to endotracheal intubation, and mechanical ventilation using a conventional ventilator. Argon inhalation was achieved by switching the animal from the first mechanical ventilator (with air/oxygen) to a second one that was supplied with 75% argon and 25% oxygen from premixed gas cylinders. This administration yielded blood samples that were analyzed using a quadrupole based technique for determining argon concentration. The range of blood:gas partition coefficient corresponding to the average measured Cmax of 190–872 μM is 0.005–0.022. Based on the average curve, the time for 50% reduction was 75 seconds. The PBPK was shown to be in general agreement with the experimental data in pigs. Inhaled argon administration exhibited an on-off nature such that AUC was proportional to administration time. Confidence in the PBPK model and the remarkably robust and stable on-off nature of argon pharmacokinetics, notwithstanding intersubject variability and comorbidity, suggests that inhaled argon could readily be applied to any treatment regime.
Keywords: absorption, distribution, elimination, headspace, mechanical ventilation, metabolism, partition coefficient, perfusion limited model, physiologically based model, quadrupole, xenon
INTRODUCTION
Argon is a noble gas with anti-ischemic and anti-inflammatory properties in several experimental conditions.1,2,3,4 For example, Silachev et al.5 Showed that inhalation of argon-oxygen mixture decreased the volume of brain damage by 2 times and reduced the severity of neurological deficit in a rat model of stroke.
Pharmacokinetics (PK), the disposition of the drug molecule in the body through time, is a scientific discipline that is a core discipline within drug discovery, development and even post-marketing.6 The fundamental measurement to perform in pharmacokinetic studies is blood concentration of the molecule (and its metabolites) of interest. While a physiologically based model of argon PK has appeared in the literature,7 no experimental data has been presented. Thus, this is particularly important for the validation of a theoretical mathematical model of physiologically based PK (PBPK).
The primary goal of this study was to investigate the PK of inhaled argon in anesthetized juvenile pigs using mechanical ventilation. In another paper, we have reported on the development of a technique for measuring argon solubility in blood, using quadrupole mass spectrometry that was employed in this study.8 Herein, we also extend the validation of the PBPK model that has previously appeared7 based on human data for xenon from the literature9 and the new data for pigs. The inherent difficulty in performing PK studies of argon makes the use of the PBPK model especially relevant. The model is used to investigate argon PK for human applications.
MATERIALS AND METHODS
Measurements in pigs
The protocol was approved by the French National Ethical Committee (ComEth n°016, project #17800-2020012017214953) on August 10, 2020. Juvenile pigs, 6–8 weeks old (Elevage Lebeau, Gambais, France) (two females and two males (n = 4)), weighing 12–15 kg (13.65 ± 1.65 kg), were anesthetized using a mixture of zolazepam/tiletamine at 10 mg/kg intramuscular (Zoletil, Virbac AH, Inc., Carros, France) and propofol at 10 mg/kg/h intravenous (Propovet, Richmond Vet Pharma, Buenos Aires, Argentina). They were submitted to endotracheal intubation using a dedicated tube (Rüshelit®) and to mechanical ventilation using a conventional ventilator (T-BIRD®, Soma Technology, Bloomfield, CT, USA). An intravenous administration of methadone (0.3 mg/kg) was delivered for analgesia. The mechanical ventilation parameters were initially set as follows: respiratory rate 20 per minute, tidal volume (TV) 9–10 mL/kg, inspiratory/expiratory time ratio = 1/2, positive end-expiratory pressure = 5 cmH2O (1 cmH2O = 98.0665 Pa), inspired fraction of oxygen = 30%. Tidal volume was adjusted between 9 and 10 mL/kg in order to obtain normal expired CO2 levels. Two catheters were inserted into the femoral and carotid arteries, respectively, for blood pressure evaluation and blood sampling.
Argon inhalation was achieved by switching the animal from the first mechanical ventilator (with air/oxygen) to a second one (AGV02430, VIASYS Respiratory Care, Conshohocken, PA, USA) that was supplied with 75% argon and 25% oxygen from premixed gas cylinders (Air Liquide Santé International, Les loges-en-Josas, France). At the end of the expected duration of exposure to argon, animals were switched back to air/oxygen ventilation using the initial ventilator.
It is important to note that the VIASYS ventilator was not calibrated for argon mixtures. The argon premix was supplied through the oxygen input connection on the ventilator, so to administer 75% argon the ventilator control was set at 100% oxygen. Since the density of the argon mixture differs from that of air/oxygen the flow measurement, and therefore, the volume measurement of the argon was inaccurate. To alleviate this problem, we used pressure-controlled ventilation mode when shifting to the argon exposure. The target inspiratory pressure was set as the peak pressure measured at baseline with air/oxygen using a tidal volume of 9–10 mL/kg. We note that in a numerical analysis of argon mixtures and air in human newborns, the property value differences caused relatively minor differences in ventilation parameters during pressure controlled ventilation.10
Two females and two males (n = 4) were submitted to two different exposures of argon separated by a washout of at least 60 minutes (Figure 1). The first exposure lasted 3 minutes for a unique blood sample for argon concentration evaluation after 2 minutes of inhalation. The second exposure lasted 30 minutes for argon concentration evaluation after 29 minutes of inhalation, as well as 2 minutes after resumption to air/oxygen ventilation. One-milliliter samples were drawn into gas tight syringes (Valco Instruments Co., Houston, TX, USA) that were put on ice and transported about 1 hour for analysis. Considering the time required for transportation and analysis of samples was 2 hours, and that no long term storage method was suitable to fully avoid argon leakage, this protocol was designed to minimize and standardize the time between the blood sampling and analysis. Those conditions are optimal to limit and minimize the impact of argon leakage.
Figure 1.
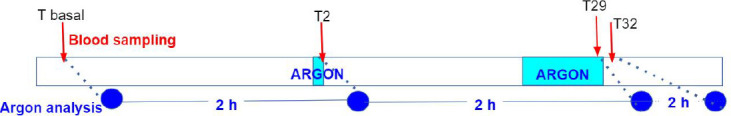
Protocol for gas administration and blood sampling for PK measurements in pigs.
Note: PK: Pharmacokinetics.
Analytical method
The full analytical method suitable for argon PK determination has been described previously.8 Briefly, it is based on a prototype quadrupole mass spectrometer gas analyzer (Stanford Research Systems, Sunnyvale, CA, USA) in which the sample injection has been modified to allow headspace gas analysis from a screwed gas tight syringe containing liquid. As explained in the method validation paper, the output of the quadrupole mass spectrometer is sensitive to argon, but the exact correlation with the amount (moles) of argon is not stable. The integral over time (in units of torr-s) of this value after the blood sample is injected into the system is, therefore, related to the total amount of argon in the 1 mL blood sample.
PBPK model
The description of the model begins with a background discussion of gas PK.
Gas PK
PK is the branch of pharmacology concerned with the movement of drugs within the body. The key phases of drug passage through the body are absorption, distribution, metabolism and elimination.
Drug absorption is generally defined as the rate and extent to which the drug moves from its site of administration to its intended target (site) of action and is a source of variability in drug response. Passage of drug through membranes dividing the absorption site from the blood is a necessary step in absorption.
Administration of a medicinal gas is, with rare exceptions, through the pulmonary route. What should also be considered is the passage of the gas from the source (e.g., a pressurized gas cylinder), through the controlling device and tubing to a patient interface. The controlling device can be simply a pressure regulator and flow valve up to a highly sophisticated mechanical ventilator. The patient interface can be closed to the ambient using an intubation tube or open through a mask or nasal cannula. It is important to recognize that if the interface is open and the gas flow rate is less than the inhalation flow rate of the patient, ambient air will be drawn into the gas stream diluting the dose concentration of the medicinal gas.11
Within the respiratory tract the medicinal gas is diluted by the gas present in the lung. Under normal conditions, we continuously breathe 78% nitrogen (N2) such that the body tissues and fluids are saturated with dissolved N2. Furthermore, the residual volume of the lung contains a large fraction of N2. For normobaric medicinal gas administration at high concentrations, the N2 concentration must be less than in the ambient atmosphere; therefore, N2 will 1) washout of the lung and 2) begin to be released by the body tissues. Processes 1) and 2) occur over two distinct time periods. Nitrogen washout from the lung is well understood because it has been measured as a diagnostic lung test.12 Typically, after 10 breaths (about 1 minute) virtually all of the N2 has left the lung. Because of the low solubility of nitrogen in blood the washout out from body tissues is relatively slow, the time for 50% reduction (T1/2) being several hours.13
Medicinal gas molecules that reach the alveoli can then diffuse across the membrane to the circulating pulmonary blood to complete the absorption of the gas. This process can be affected by 2nd gas effects.14 That is, the differential rate of uptake of gas components can alter their concentration in the alveoli, in turn affecting the resulting blood concentrations. The equilibrium concentration dissolved in the blood compared to the concentration (C) in the gas phase is determined empirically and is known as the blood:gas partition coefficient (PC):
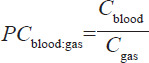
For argon we assume absorption is greatly simplified. Immediately upon opening the gas source, we assume the argon gas concentration of the source is the same as the alveolar concentration. Furthermore, the concentration of argon in the lung blood in contact with the gas in the alveoli is given by the PCblood:gas. There is no effect of the membrane in determining the instantaneous transfer kinetics. Thus, the rate of absorption can be determined by the blood perfusion rate through the lung and the PCblood:gas; this type of transfer is called perfusion limited. Note, that with these assumptions all of the respiratory and lung parameters have negligible influence on the uptake kinetics but are necessary to predict exhaled gas concentration.
Drug distribution is the movement of a drug to and from the blood circulation and the various tissues of the body (for example, fat, muscle, and brain tissue) and the relative proportions of drug in the tissues. Similar to absorption, in principle, the rate of transfer depends on the diffusion rate across the membrane separating the blood and the tissue and the perfusion rate of blood to the tissue. Finally, the concentration in the tissue will depend on its volume.
For argon, the fundamental simplifying assumption of perfusion limited transfer is applied for distribution to all tissues.
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. From the lung, unlike from the gut, the drug enters directly into the arterial blood distribution without a metabolic passage through the liver.
For the inert gas argon, the fundamental assumption of no metabolism is applied.
Drug elimination is the removal of drugs from the body. They may be eliminated after being chemically altered (metabolized), or they may be eliminated intact. Most drugs, particularly water-soluble drugs and their metabolites, are eliminated by the kidneys in urine.
For argon, the fundamental assumption of elimination is that it is only via the pulmonary route, analogous and quantitatively at the same rate as absorption.
Because absorption, distribution, and elimination are based only on physical (PCs) and physiological (compartment volumes, perfusion rates, etc.), with no complex biochemistry associated with metabolism, we conclude from a conceptual standpoint, that argon PK are assumed to be very simple and can be readily extrapolated between species and compared to other inert gases.
Model implementation
The PBPK model used to analyze argon administration has previously been used to study the chronic administration of gases,7 preclinical experiments in small animals,15 and denitrogenation kinetics.13 The PBPK model uses as a basis the one Lockwood16 proposed for inhaled anesthesia. The model is described by the schematic shown in Figure 2 and the data listed in Table 1. Readers should access reference 7 for a detailed description of the model and an initial validation.
Figure 2.
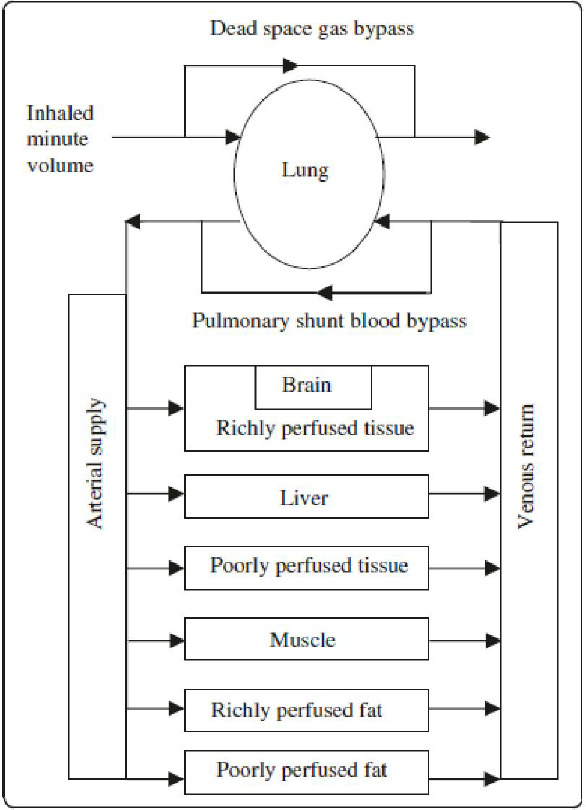
Schematic of the pharmacokinetic model, compartments and gas species flows.
Note: The model does not consider the lung tissue per se (except as part of the richly perfused tissue compartment), but the gas volume within it. Reprinted from Katz et al.7
Table 1.
Partition coefficients and physiological parameters used for the pig and human PBPK model
| Human |
||||
|---|---|---|---|---|
| Parameter | Pig | Male | Female | Neonate |
| Body mass (kg) | 13.65 | 70 | 60 | 3.3 |
| Cardiac output (L/min) | 1.19 | 6 | 4.9 | 0.63 |
| Perfusion per compartment (as a fraction of cardiac output) | ||||
| Fat (richly perfused) | 0.1747 | 0.04 | 0.0589 | 0.05 |
| Fat (poorly perfused) | NA | 0.01 | 0.0147 | NA |
| Liver | 0.3052 | 0.26 | 0.26 | 0.25 |
| Richly perfused tissue | 0.1829 | 0.3469 | 0.3469 | 0.3775 |
| Poorly perfused tissue | 0.0553 | 0.01 | 0.01 | NA |
| Muscle (+skin) | 0.2523 | 0.24 | 0.2164 | 0.0625 |
| Brain | 0.0296 | 0.0931 | 0.0931 | 0.26 |
| Volumes (fraction of body weight) | ||||
| Fat (richly perfused) | 0.3 | 0.09 | 0.1325 | 0.161 |
| Fat (poorly perfused) | NA | 0.09 | 0.1325 | NA |
| Liver | 0.0294 | 0.06 | 0.06 | 0.0381 |
| Richly perfused tissue | 0.1397 | 0.0624 | 0.0624 | 0.3089 |
| Poorly perfused tissue | 0.1269 | 0.24 | 0.24 | NA |
| Muscle (+skin) | 0.4 | 0.44 | 0.355 | 0.377 |
| Brain | 0.004 | 0.0176 | 0.0176 | 0.115 |
| Argon partition coefficients | ||||
| Blood:gas | 0.03 | 0.03 | 0.03 | 0.03 |
| Fat:blood | 4.162 | 4.162 | 4.162 | 4.162 |
| Liver:blood | 0.754 | 0.754 | 0.754 | 0.754 |
| Richly perfused tissue:blood | 1.028 | 1.028 | 1.028 | 1.028 |
| Poorly perfused tissue:blood | 0.999 | 0.999 | 0.999 | 0.999 |
| Muscle:blood | 0.72 | 0.72 | 0.72 | 0.72 |
| Brain:blood | 0.675 | 0.675 | 0.675 | 0.675 |
Note: NA is not applicable because the compartment is not included in the model.
Lockwood models the wash-in of gas into the lung as a fully mixed chamber.16 Using the respiratory parameters used in his model as a basis this would overestimate wash-in time compared to nitrogen washout experiments. In the current model we underestimate wash-in time. This fact must be taken into account in interpreting the kinetics results. As a consequence, respiratory parameters such as minute ventilation and alveolar ventilation are not relevant (and are not included in Table 1) unless exhaled concentration needs to be calculated.
The physiological data for humans in Lockwood were extrapolated to pigs based on data from several references.16,17,18,19 The body mass and cardiac output for the pig model reflect the juvenile pigs used for the experimental measurements. The resulting parameters are compiled in Table 1. Intersubject variability can be assessed by using scaling of compartment volumes, and cardiac output and their distributions based on size, gender and age. For example, the percentage of fat as a function of body mass and sex is on average 18% for men and 26.5% for women. It was assumed that the relative amounts of highly and poorly perfused fat and their perfusion were the same as the adult male human model with the residual excess assigned to the muscle compartment.16 Adaptations for arterial blood volume20 and cardiac output19,21 were made based on correlations found in the literature. The human neonate physiological data are based on Haddad et al.22 for compartment volumes and Price et al.23 for perfusion functional data.
The specific PC for each gas in each compartment for each species is perhaps the most important physical parameter. These data are difficult to determine, thus requiring extrapolation from correlations from known data for other gases; for example, compartment:blood PCs were determined from known fat:blood values using the linear correlations for organs described by Fiserova-Bergerova and Diaz.24 Argon solubility in the form of Ostwald solubility coefficients are available for human blood, ranging from 0.03725 to 0.026,26 and for olive oil, 0.154. Note that in Table 1, we use 0.030 compared to 0.037 in the previous paper.7 The solubility coefficient is equivalent to the PC if one of the compartments is in the gas phase; in our model this is the case for the lung compartment. Furthermore, due to the scarcity of data, solubility in olive oil is used in lieu of fat. The relevant fat:blood PC for argon is found by the ratio of (olive oil:gas)/(blood:gas), or 4.162.
RESULTS
The integral outputs from each pig experiment at each time point are given in Table 2 and plotted in Figure 3. Also shown in Figure 3 are the average values that are provided in Table 3. PK parameters of interest are the maximum concentration (Cmax) and the time to increase or decrease concentration by 1/2 (T1/2). As discussed in the previous paper,8 a measurement of the moles of argon in the sample was not stable enough to have a consistent calibration. The calibration of integral value to argon moles yields a Cmax measured in the range of 190–872 μM. The key physics/physiological parameter necessary to quantify argon solubility in blood is the blood:gas PC that determines the Cmax. The blood:gas PC for argon in pigs is not available in the literature. The range of PC corresponding to the measured Cmax of 190–872 μM is 0.005–0.022. Based on the average curve, T1/2 =75 seconds based on linear interpolation between the 29 and 32 minute samples.
Table 2.
Integral data output for the experiments in 4 pigs
| Time (min) | pig n°1 | pig n°2 | pig n°3 | pig n°4 |
|---|---|---|---|---|
| 0 | 1.01E-05 | 8.22E-06 | 1.13E-05 | 8.38E-06 |
| 2 | 2.40E-05 | 3.89E-05 | 2.45E-05 | 3.67E-05 |
| 29 | 2.70E-05 | 3.41E-05 | 3.62E-05 | 5.23E-05 |
| 32 | 1.30E-05 | 1.03E-05 | 1.66E-05 | 1.05E-05 |
Note: Gas administration began at 0 and ended at 30 minutes. The 0-time point basal sample and the 2-minute sample were actually taken 4 and 2 hours, respectively before the start of gas administration to reduce the storage time of the samples.
Figure 3.
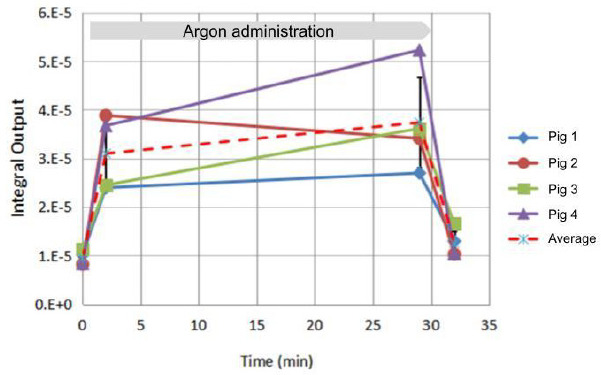
Integral data output for each pig and the average.
Note: Error bars are the standard deviation. Gas administration began at 0 and ended at 30 minutes. The 0-time point basal sample and the 2-minute sample were actually taken 4 and 2 hours, respectively before the start of gas administration to reduce the storage time of the samples.
Table 3.
Averages of the integral data output for each pig experiment
| Time (min) | Raw data | Normalized data |
|---|---|---|
| 0 | 9.52E-06±1.29334E-06 | 2.55E-01±3.46E-02 |
| 2 | 3.10E-05±6.81246E-06 | 8.29E-01±1.82E-01 |
| 29 | 3.74E-05±9.2593E-06 | 1.00E+00±2.48E-01 |
| 32 | 1.26E-05±2.52924E-06 | 3.36E-01±6.76E-02 |
Note: The data were normalized by the maximum value at 29 minutes. Gas administration began at 0 and ended at 30 minutes. The 0-time point basal sample and the 2-minute sample were actually taken 4 and 2 hours, respectively before the start of gas administration to reduce the storage time of the samples (see Figure 1).
Model validation with xenon
Validation is an important step in the use of any model. Unfortunately, in vivo measurements of noble gas concentration are difficult and therefore, quite rare. In a previous paper a comparison with Lockwood16 showed xenon uptake in humans was equivalent indicating system parameters, equations, and solution techniques do not have major errors. A second validation comparison was made from in vivo measurements of xenon concentration in arterial and mixed venous blood using gas chromatography-mass spectrometry of the headspace gas over samples taken during the wash-in of xenon into eight pigs.27 For this comparison, compartment volumes were estimated because the weight of each pig was not available.
More recently, data on the elimination of xenon in humans after xenon anesthesia has been published.9 A comparison of the PBPK model and these data appear in Figure 4. The only physiological variable change from those given in Table 1 for the adult male is the average body mass of the subjects (66 kg). PCs for xenon are given in the previous paper.6 The PBPK simulation (Figure 4) is within the variability and uncertainty of the experiment.
Figure 4.
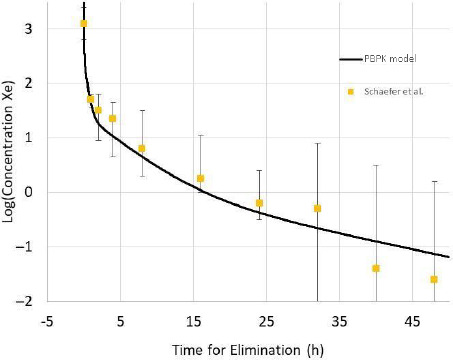
Log concentration of xenon during elimination estimated from Figure 3 in Schaefer et al.9
Note: The solid line is the PBPK model results with the physiological variables adjusted for 66 kg body weight.
Model validation with argon in pigs
We have presented PK data with the administration of 75% argon to juvenile pigs. The results in Figure 3 are in the form of the integral output of the prototype quadrupole-based technique.8 As discussed there, a direct measurement of the moles of argon in the sample was not stable enough to have a consistent calibration. Thus, to compare the results of the PBPK model to the experimental data each data set was normalized to the maximum values at 29 minutes. Figure 5 shows this comparison of the average (n = 4) PK experimental curve compared to the PBPK model. The time to increase or decrease concentration by 1/2 (T1/2) is the key PK parameter to consider. For the experiment T1/2exp = 75 seconds versus T1/2mod = 30 seconds for the model. The model estimates a faster time because gas washout of the lung is not considered. On the other hand, the experimental estimate is slower because a linear decrease is assumed based on a 2-point linear approximation compared to the theoretical exponential decrease. Thus, we can assume that both the experiment and the model agree that T1/2 for the pig is about 1 minute.
Figure 5.
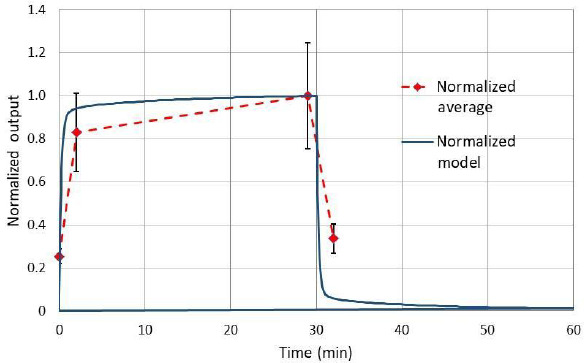
Comparison of experimental integral output and the PBPK model results of concentration each normalized to the maximum values at 29 minutes.
PBPK analysis in humans
The PBPK model for inert gas administration is here used to analyze the administration of 60% argon during 60 minutes to an adult male (70 kg). Time of administration of 30 and 120 minutes was also simulated. Intersubject variation was investigated by adjusting the male model by ±20 kg of body mass, and a female model that redistributed body mass (60 kg) from muscle to fat (men = 18.0%, women = 26.5% with proportional cardiac output) and reduced total cardiac output (men = 6.0 L/min, women = 4.9 L/min). The effects due to comorbidities on argon PK are most likely those related to impaired respiration. Specific embodiments of comorbidity effects are pulmonary shunt fraction, ventilation/perfusion mismatch, and pulmonary fibrosis. To illustrate the effect of respiratory impairment the PBPK model was used to simulate the adult male with a 50% pulmonary shunt. An obese case was modeled by increasing the total fat fraction to 50% (equally distributed between rich and poor perfused) and reducing the total fat blood flow by 28% for the 90 kg male with all the other PBPK parameters remaining identical.28 Finally, the same 60 minute administration was applied to the neonate model.
The results of the simulations are provided in the form of arterial blood argon concentration curves shown in Figure 6. PK parameters of Cmax and T1/2 are essentially identical for the healthy cases of males (including the obese case) and the female. T1/2 is slightly longer for neonates (about 36 seconds vs. 10 seconds). The area under the curve (AUC) is directly proportional to the time of administration.
Figure 6.
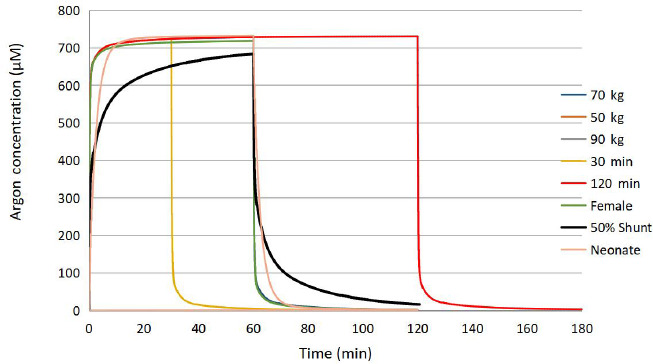
Arterial blood concentration for several scenarios of argon administration.
Note: The healthy adult simulations of 60 minutes exposure are virtually superimposed on each other.
DISCUSSION
Measurements in pigs
Argon administration in mechanically ventilated juvenile pigs has yielded blood samples that were analyzed using a quadrupole based technique to determine argon concentration.8
Solubility of argon in blood as indicated in the blood:gas PC were estimated to be in the range 0.005–0.022 for young pigs. In comparison, Ostwald solubility coefficients, equivalent to the blood:gas PC, are available for human blood ranging from 0.03725 to 0.026.27 For other mammals, Langø et al. lists solubility coefficients for rabbits and dogs as 0.0305 and 0.0257, respectively.29 Thus, the solubility coefficient determined in pigs appears to be low. One factor that probably contributed to this underestimate is leakage of argon from the syringes while waiting for analysis. Indeed, the difficulty in performing in vivo experiments with argon administration, sampling, and measurement cannot be over emphasized.8
In terms of kinetics, consider the parameter T1/2. The “on-off” nature of argon kinetics is evident with T1/2 = 75 seconds. A linear decrease in concentration is assumed between time points 29 and 32 minutes; however, the behavior is expected to be exponential.7 Thus, the T1/2 estimate is probably high (i.e., in reality the kinetics are faster).
The expected ratio of Cmax to Cbasal is 75/0.93 = 80, based on 75% argon administration and 0.93% ambient argon. It is noted above that the Cmax measurement is probably low due to leakage of argon from the sample before analysis. The measurement of basal values is problematic because of pollution from the ambient argon to the sample in transport or during the quadrupole analysis. The concentration of argon in ambient air is about 33 times greater than expected in the basal blood sample (based on PC = 0.030). Furthermore, if there were residual argon in the quadrupole system it would increase the basal measurement.
The key limitation in this study is the lack of commercial equipment dedicated to argon medicinal use. The measure of Cmax, and therefore PC, is compromised by the instability of argon sensitivity of the prototype quadrupole based technique. Furthermore, the limited number of time points due to the duration of each analysis makes the measurement of T1/2 an overestimate. A ventilator designed and calibrated for argon administration does not exist. As such, the ability to perform any argon PK experiments is extremely difficult. Thus, in spite of these important limitations and their impact on limiting the accuracy and extent of the overall study, we believe this first attempt at argon PK is of fundamental interest to all potential developers of the medicinal uses of argon.
PBPK analysis
Based on fundamental physics and physiological principles of argon gas administration a PBPK model has been developed and shown to be in general agreement with data in the literature for xenon in humans and to new experimental data for argon in juvenile pigs. Inhaled argon administration exhibited an on-off nature such that AUC was proportional to administration time. Confidence in the PBPK model and the remarkably robust and stable on-off nature of argon PK, notwithstanding intersubject variability and comorbidity, suggests that inhaled argon could readily be applied to any treatment regime.
There are two key outcomes: 1) the fast uptake and washout (a square wave pattern) of the arterial blood concentration, and 2) the insignificance of intersubject variation to the PK. Regarding point 1), the AUC assuming a square wave is only 0.17% different from the model results for the standard adult male case. Furthermore, consistent with this observation, AUC is directly proportional to the time of administration. Note, as discussed for the pig experiments, the model result of about T1/2 = 10 seconds is underestimated because time for lung clearance (about 1 minute) is not considered. Cmax is largely governed by the blood:gas PC and the concentration of argon in the gas administered. It is for this reason that Cmax will always be essentially the same for all patients (with small differences due to the time for administration and the uptake rate due to physiological differences). Because of its high perfusion rate, the brain PK is very much the same as arterial blood. It is notable that xenon, another uncharged atom larger than argon, readily crosses the blood-brain barrier and has rapid onset of effect.30 In a similar manner, for argon the PBPK model predicts T1/2 is about 100 seconds for the brain. An indication of the robust and stable nature of argon PK is that with this massive insult of 50% shunt, the Cmax and AUC only moderately decrease by about 5% and 6%, respectively. It is true that T1/2 does increase by about 270% but not enough to change the qualitative on-off nature of argon PK. In fact, any gas exchange impairment that would degrade argon PK would have a much more serious and primary effect on oxygen and carbon dioxide gas exchange such that argon administration would likely be a secondary clinical priority.
The initial argon concentration in all compartments was zero for the simulations consistent with the experimental data in pigs, and many potential applications where the patient is first administered medicinal air and oxygen before argon administration begins. If argon administration begins directly there would be an insignificant decrease of washin time and does not change washout T1/2. However, argon, like xenon, is a prohibited substance by the World Anti-Doping Agency.31 Considering the relatively high ambient concentration of argon and the kinetics explained in this paper, anti-doping testing for argon is virtually impossible.32
A limitation of this development program is the lack of argon PK data, particularly in humans; however, the experimental program in pigs provides corroborating data for kinetics (T1/2), but a somewhat different Cmax (79% to 4% underestimate compared to the blood:gas PC = 0.03 used in the model). Nonetheless, uncertainty in Cmax, does not pose any risk for patients, as there is no potential of overdose for normobaric, premixed gas cylinders. That is, Cmax can be achieved with a dedicated gas administration system. Thus, even if the PBPK model has some uncertainty, the overall impact of these uncertainties on a clinical application will be negligible.
In conclusion, in this paper we have presented for the first time in vivo measurement of argon PK in juvenile pigs. This data was used to validate a PBPK model that was used to analyze argon PK in humans. The key overall finding is that the PK will have only insignificant effects on the clinical administration of argon mixture. Therefore, the robust and stable PK of inhaled argon is favorable to accurate drug administration (if proper control of the inhaled concentration is maintained) allowing for the extension to other indications and patient groups.
Funding Statement
Funding: This project was funded by Air Liquide Santé International.
Footnotes
Conflicts of interest
None.
Data availability statement
All relevant data are within the paper.
REFERENCES
- 1.Zafonte RD, Wang L, Arbelaez CA, Dennison R, Teng YD. Medical gas therapy for tissue, organ, and CNS protection: a systematic review of effects, mechanisms, and challenges. Adv Sci (Weinh) 2022;9:e2104136. doi: 10.1002/advs.202104136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheid S, Lejarre A, Wollborn J, Buerkle H, Goebel U, Ulbrich F. Argon preconditioning protects neuronal cells with a Toll-like receptor-mediated effect. Neural Regen Res. 2023;18:1371–1377. doi: 10.4103/1673-5374.355978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Liu W, Bi M, Xu J, Yang H, Zhang Y. Noble gases therapy in cardiocerebrovascular diseases: the novel stars? Front Cardiovasc Med. 2022;9:802783. doi: 10.3389/fcvm.2022.802783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider FI, Krieg SM, Lindauer U, Stoffel M, Ryang YM. Neuroprotective effects of the inert gas argon on experimental traumatic brain injury in vivo with the controlled cortical impact model in mice. Biology (Basel) 2022;11:158. doi: 10.3390/biology11020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silachev DN, Boeva EA, Yakupova EI, et al. Positive neuroprotective effect of argon inhalation after photochemically induced ischemic stroke model in rats. Bull Exp Biol Med. 2023;176:143–149. doi: 10.1007/s10517-024-05984-6. [DOI] [PubMed] [Google Scholar]
- 6.Baillie TA. Metabolism and toxicity of drugs. Two decades of progress in industrial drug metabolism. Chem Res Toxicol. 2008;21:129–137. doi: 10.1021/tx7002273. [DOI] [PubMed] [Google Scholar]
- 7.Katz I, Murdock J, Palgen M, Pype J, Caillibotte G. Pharmacokinetic analysis of the chronic administration of the inert gases Xe and Ar using a physiological based model. Med Gas Res. 2015;5:8. doi: 10.1186/s13618-015-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemaire J, Heninger M, Louarn E, et al. Argon pharmacokinetics: a solubility measurement technique. Med Gas Res. 2023;13:208–211. doi: 10.4103/2045-9912.351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaefer MS, Piper T, Geyer H, et al. Xenon elimination kinetics following brief exposure. Drug Test Anal. 2017;9:666–670. doi: 10.1002/dta.2001. [DOI] [PubMed] [Google Scholar]
- 10.Katz I, Milet A, Chalopin M, Farjot G. Numerical analysis of mechanical ventilation using high concentration medical gas mixtures in newborns. Med Gas Res. 2019;9:213–220. doi: 10.4103/2045-9912.273959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz I, Chen J, Duong K, et al. Dose variability of supplemental oxygen therapy with open patient interfaces based on in vitro measurements using a physiologically realistic upper airway model. Respir Res. 2019;20:149. doi: 10.1186/s12931-019-1104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George SC, Hlastala MP. Airway gas exchange and exhaled biomarkers. Compr Physiol. 2011;1:1837–1859. doi: 10.1002/cphy.c090013. [DOI] [PubMed] [Google Scholar]
- 13.Katz I, Murdock J, Palgen M, Farjot G. A physiologically based model for denitrogenation kinetics. Med Gas Res. 2017;7:256–259. doi: 10.4103/2045-9912.222449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendrickx J, Peyton P, Carette R, De Wolf A. Inhaled anaesthetics and nitrous oxide: complexities overlooked: things may not be what they seem. Eur J Anaesthesiol. 2016;33:611–619. doi: 10.1097/EJA.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 15.Katz I, Palgen M, Murdock J, Martin AR, Farjot G, Caillibotte G. Gas transport during in vitro and in vivo preclinical testing of inert gas therapies. Med Gas Res. 2016;6:14–19. doi: 10.4103/2045-9912.179342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockwood G. Theoretical context-sensitive elimination times for inhalation anaesthetics. Br J Anaesth. 2010;104:648–655. doi: 10.1093/bja/aeq051. [DOI] [PubMed] [Google Scholar]
- 17.Filser JG, Schmidbauer R, Rampf F, Baur CM, Pütz C, Csanády GA. Toxicokinetics of inhaled propylene in mouse, rat, and human. Toxicol Appl Pharmacol. 2000;169:40–51. doi: 10.1006/taap.2000.9027. [DOI] [PubMed] [Google Scholar]
- 18.Csanády GA, Filser JG. A physiological toxicokinetic model for inhaled propylene oxide in rat and human with special emphasis on the nose. Toxicol Sci. 2007;95:37–62. doi: 10.1093/toxsci/kfl140. [DOI] [PubMed] [Google Scholar]
- 19.Fiserova-Bergerova V. Extrapolation of physiological parameters for physiologically based simulation models. Toxicol Lett. 1995;79:77–86. doi: 10.1016/0378-4274(95)03359-s. [DOI] [PubMed] [Google Scholar]
- 20.Sackner MA, Atkins N, Goldberg J, Segel N, Zarzecki S, Wanner A. Pulmonary arterial blood volume and tissue volume in man and dog. Circ Res. 1974;34:761–769. doi: 10.1161/01.res.34.6.761. [DOI] [PubMed] [Google Scholar]
- 21.Collis T, Devereux RB, Roman MJ, et al. Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation. 2001;103:820–825. doi: 10.1161/01.cir.103.6.820. [DOI] [PubMed] [Google Scholar]
- 22.Haddad S, Restieri C, Krishnan K. Characterization of age-related changes in body weight and organ weights from birth to adolescence in humans. J Toxicol Environ Health A. 2001;64:453–464. doi: 10.1080/152873901753215911. [DOI] [PubMed] [Google Scholar]
- 23.Price K, Haddad S, Krishnan K. Physiological modeling of age-specific changes in the pharmacokinetics of organic chemicals in children. J Toxicol Environ Health A. 2003;66:417–433. doi: 10.1080/15287390306450. [DOI] [PubMed] [Google Scholar]
- 24.Fiserova-Bergerova V, Diaz ML. Determination and prediction of tissue-gas partition coefficients. Int Arch Occup Environ Health. 1986;58:75–87. doi: 10.1007/BF00378543. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal MS, Nickles RJ. Selected noble-gas partition coefficients. Phys Med Biol. 1985;30:945–950. doi: 10.1088/0031-9155/30/9/006. [DOI] [PubMed] [Google Scholar]
- 26.Tomonaga Y, Brennwald MS, Livingstone DM, Tomonaga G, Kipfer R. Determination of natural in vivo noble-gas concentrations in human blood. PLoS One. 2014;9:e96972. doi: 10.1371/journal.pone.0096972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nalos M, Wachter U, Pittner A, Georgieff M, Radermacher P, Froeba G. Arterial and mixed venous xenon blood concentrations in pigs during wash-in of inhalational anaesthesia. Br J Anaesth. 2001;87:497–498. doi: 10.1093/bja/87.3.497. [DOI] [PubMed] [Google Scholar]
- 28.Levitt DG, Schnider TW. Human physiologically based pharmacokinetic model for propofol. BMC Anesthesiol. 2005;5:4. doi: 10.1186/1471-2253-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang⊘ T, M⊘rland T, Brubakk AO. Diffusion coefficients and solubility coefficients for gases in biological fluids and tissues: a review. Undersea Hyperb Med. 1996;23:247–272. [PubMed] [Google Scholar]
- 30.Ntalouka MP, Arnaoutoglou E, Tzimas P. Postoperative cognitive disorders: an update. Hippokratia. 2018;22:147–154. [PMC free article] [PubMed] [Google Scholar]
- 31.World Anti-Doping Agency. The world anti-doping code international standard. Prohibited list January 2017. https://www.wada-ama.org/sites/default/files/resources/files/2016-09-29_-_wada_prohibited_list_2017_eng_final.pdf. [Accessed March 30; , 2021 ]. https://www.wada-ama.org/sites/default/files/resources/files/2016-09-29_-_wada_prohibited_list_2017_eng_final.pdf
- 32.World Anti-Doping Agency. The world anti-doping code international standard. Prohibited list 2024. https://www.wada-ama.org/sites/default/files/2023-09/2024list_en_final_22_september_2023.pdf. [Accessed March 1; , 2024 ]. https://www.wada-ama.org/sites/default/files/2023-09/2024list_en_final_22_september_2023.pdf


