Abstract
A range of sleep disorders has the potential to adversely affect cognitive function. This study was undertaken with the objective of investigating the effects of ozone rectal insufflation (O3-RI) on cognitive dysfunction induced by chronic REM sleep deprivation, as well as elucidating possible underlying mechanisms. O3-RI ameliorated cognitive dysfunction in chronic REM sleep deprived mice, improved the neuronal damage in the hippocampus region and decreased neuronal loss. Administration of O3-RI may protect against chronic REM sleep deprivation induced cognitive dysfunction by reversing the abnormal expression of Occludin and leucine-rich repeat and pyrin domain-containing protein 3 inflammasome as well as interleukin-1β in the hippocampus and colon tissues. Moreover, the microbiota diversity and composition of sleep deprivation mice were significantly affected by O3-RI intervention, as evidenced by the reversal of the Firmicutes/Bacteroidetes abundance ratio and the relative abundance of the Bacteroides genus. In particular, the relative abundance of the Bacteroides genus demonstrated a pronounced correlation with cognitive impairment and inflammation. Our findings suggested that O3-RI can improve cognitive dysfunction in sleep deprivation mice, and its mechanisms may be related to regulating gut microbiota and alleviating inflammation and damage in the hippocampus and colon.
Keywords: blood-brain barrier, cognitive impairment, hippocampus, IL-1β, intestinal barrier, intestinal microbiota, NLRP3 inflammasome, Occludin, ozone rectal insufflation, REM sleep deprivation
INTRODUCTION
Sleep deprivation (SD) is a major contemporary public health problem characterized by partial or complete SD, leading to an inability to meet physiological sleep requirements. SD exerts detrimental effects on diverse physiological systems encompassing cardiovascular,1 neurological,2 immunological,3 and endocrine.4 At the same time, extensive research has consistently shown that SD, particularly rapid eye movement (REM) SD, also significantly impairs cognitive function.5,6,7,8 Utilizing electroencephalography and electromyography, the sleep cycle in both humans and rodents is categorized into distinct phases, namely non-REM sleep and REM sleep. Given the profound association between REM sleep and crucial cognitive functions such as learning, memory, and emotional regulation in both humans and animals, REM SD has been extensively employed in animal experimentation and continues to be a prevalent methodology in contemporary research endeavors.
Numerous pharmacological and nonpharmacological interventions, such as benzodiazepines, benzodiazepine-receptor agonists, and cognitive behavioral therapy have been proposed for SD treatments. However, these therapeutic options face limited acceptance due to notable side effects and limited therapeutic efficacy. Transcranial magnetic or electrical brain stimulation, as well as innovative approaches for delivering psychological interventions, are currently available but are in the research phase.9 Hence, it is imperative to develop suitable therapy in this field. Ozone is a potent oxidant with potential therapeutic benefits for chronic diseases and is increasingly being studied for its beneficial effects in improving cognitive dysfunction.10,11,12,13 Although these studies have elucidated some of the mechanisms underlying liquid ozone intraperitoneal injection in cognitive disorders, ozone rectal insufflation (O3-RI) is more prevalent in clinical practice which uses gaseous ozone injected into the rectum to heal and prevent disorders.14 And in the clinical work of our team, O3-RI is a common method of Multimodal sleep treatment for insomnia.
The gut-brain axis encompasses a complex network involving various physiological systems, including the central nervous system, neuroendocrine system, neuroimmune system, hypothalamic-pituitary-adrenal axis, autonomic nervous system comprising both the sympathetic and parasympathetic branches, the enteric nervous system, the vagus nerve, and the gut microbiota. Of particular significance is the pivotal role played by the gut microbiota in mediating communication between the gut and the brain, thereby giving rise to the concept of the microbiota-gut-brain axis. It is well-established that the microbiota-gut-brain axis plays a critical role in maintaining brain homeostasis and is implicated in various neurological and psychiatric disorders. These encompass a diverse spectrum of conditions, including but not limited to SD, Parkinson's disease, Alzheimer's disease, multiple sclerosis, autism spectrum disorder (ASD), and major depressive disorder.15,16,17 Specifically, alterations in gut microbiota composition can induce neurogenic and inflammatory responses within both the enteric and peripheral systems, thereby contributing to neuroinflammation.18 Notably, the nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing protein 3 (NLRP3) inflammasome plays a crucial role in mediating immune and inflammatory reactions within both peripheral and central nervous system diseases.19,20,21,22 In individuals afflicted with conditions such as SD, Alzheimer's disease, Parkinson's disease, and major depressive disorder, changes in gut microbiota can activate NLRP3, thereby initiating an inflammatory cascade that detrimentally affects the integrity of both the gut barrier and the blood-brain barrier (BBB). Consequently, these reactions contribute to central neuroinflammation and the progressive degeneration of neurological tissues.23 This intricate web of interactions has been collectively described as the “microbiota-gut-NLRP3 inflammasome-brain axis”.24
The specific effects of O3-RI on cognitive impairment induced by chronic REM SD have yet to be explored. As a result, the aims of the research are as follows: (1) research into the effects of O3-RI treatment on the cognitive dysfunction of SD, and (2) to find out whether O3-RI can improve SD-induced cognitive function by inhibiting inflammation and regulating gut microbiota.
MATERIALS AND METHODS
Animals and grouping
Laboratory procedures were meticulously conducted in strict adherence to the guidelines set forth by the National Institutes of Health for the Care and Use of Laboratory Animals. We procured specific-pathogen-free male ICR mice aged 7 weeks with a weight range of 32–34 g from Jinan Pengyue Laboratory Animal Breeding Co., Ltd. (Jinan, Shandong, China; animal license No. SCXK (Lu) 2019-0003). These mice were subsequently housed within the facilities of the Experimental Animal Center at the Shandong Second Medical University. Ethical approval for the study was obtained from the Animal Experiment Committee of Shandong Second Medical University (approval date: June 15, 2023, No. 2023SDL259). A total of five mice were accommodated per plastic cage within a room maintained at a temperature of 25°C, and the light intensity of the room was 15–20 lx. The alternation of light and dark was 12/12-hour. The mice were provided with unrestricted food and water. A 1-week acclimatization period was provided before the commencement of the experiment.
Male ICR mice were randomly assigned: control group (Ctrl group, n = 12), SD group (n = 12), low-concentration O3-RI group (O3-RI-L group, n = 12), and high-concentration O3-RI group (O3-RI-H group, n = 12).
REM SD
This research used the modified multiple platforms method to establish models. This method led to a nearly 95% reduction in REM sleep.25,26 Mice were kept in a plastic tank (40 cm × 25 cm × 15 cm) filled with water maintained at a temperature of 25°C. The tank was equipped with 15 small platforms, arranged in three rows to allow mice to freely move between platforms. The water level cannot be as high as the surface of the platform. Following a 1-week adaptation period, mice underwent 20 hours of SD per day for three consecutive weeks (Figure 1). SD was administered from 12:00 p.m. to 8:00 a.m. of the following day.27
Figure 1.
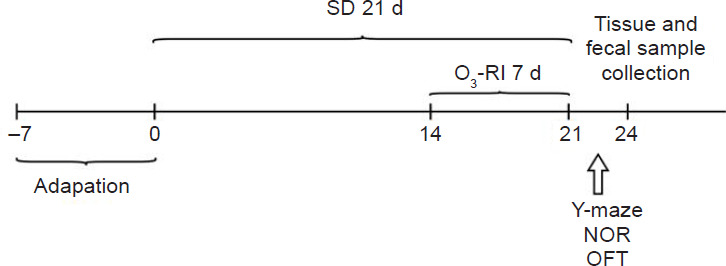
Experimental design.
Note: NOR: New object recognition; O3-RI: ozone rectal insufflation; OFT: open field test; SD: sleep deprivation.
O3-RI treatment
The gaseous ozone in this study was created by an ozone generator (OZOMED Basic; Kastner-Praxisbedarf-GmbH, Rastatt, Germany), and the applied dose was 1.1 mg/kg. Considering the toxicity associated with high concentrations of ozone and the intestinal tolerance of mice, we used safe and appropriate gaseous ozone concentrations (10 or 20 μg/mL) previously reported in the literature for this experimental study.28 On day 15, mice were immobilized, and an antioxidant cannula (0.1 mm × 5 cm) was carefully inserted into the colorectum of each mouse to a depth of 2–3 cm. To prevent gas leakage, the anus of the mice was gently manually sealed for a period of 3 to 5 minutes following treatment. The O3-RI treatment was performed for 5 minutes each time and once a day for 7 consecutive days. The Ctrl and SD groups underwent immobilization but did not receive O3-RI treatment.
Y-maze test
Measurement of spontaneous alternation
This test was utilized to evaluate short-term working memory. The device (Shanghai Xinsoft Information Technology Co., Ltd., Shanghai, China) comprised three arms (30 cm × 5 cm × 15 cm), with an angle of 120° between them. The central region of the maze formed a triangular shape. Each mouse was placed from the same arm, the starting arm, and alternated autonomously for 5 minutes continuously. During this period, the number of arm entries and the order of arm exploration were meticulously recorded. The initial two arm entries were excluded from the analysis. Starting from the third arm entry, any subsequent entry into an arm distinct from the previous two was considered a correct arm entry.29
Measurement of spatial memory
The experimental procedure encompassed two distinct phases: training and testing. During the training phase, mice were only given a 5-minute opportunity to explore two of the arms. Before testing, the mice were placed in their holding cages for 1 hour. In the testing phase, the mice were positioned in the same arm and subsequently granted to explore all three arms for a 5-minute exploration period. And the duration of time spent in the novel arm and the number of entries made into the novel arm were meticulously recorded. The mice exhibiting proficient spatial memory would exhibit a higher frequency of entries into the previously unexplored (novel) arm. Conversely, the mice with impaired spatial memory would display no specific tendency.30
Novel object recognition test
First day, each mouse was acclimatized in the test apparatus (Shanghai Xinsoft Information Technology Co., Ltd.) for 5 minutes. On the second day, the mice were kept in the apparatus with their backs facing the two identical objects and then granted the freedom to explore the area for a period of 5 minutes. One hour later, the mice were placed in the test apparatus in the same way, wherein one object remained familiar, while the other was replaced by a novel object. The mice were also afforded 5 minutes of exploration time to investigate both objects. The time spent exploring the familiar object (TF) and the time spent exploring the novel object (TN) were recorded and subsequently analyzed. The recognition index (RI, %) = [TN / (TN + TF)] × 100.31
Open field test
The open field test was employed to assess the anxiety levels of the mice. The test took place within an open field apparatus constructed from black frosted acrylic plates (dimensions: 50 cm × 50 cm × 30 cm). A period of 5 minutes was allocated for the assessment of mouse behavior, during which their activities were recorded using an overhead video camera. Upon completion of each trial, the apparatus was thoroughly cleaned to remove any feces or urine, disinfected with a 75% ethanol solution, and dried using gauze. Subsequent data analysis was conducted using the SuperMaze software (Xinruan Information Technology Co., Ltd., Shanghai, China), allowing for the evaluation of parameters such as traveling distance, traveling speed, and the time spent in the open field.32
Tissue and fecal sample collection
After behavioral tests, and before sacrifice, the mice were transferred into empty cages and waited for natural defecation. During defecation, the feces were collected in time to avoid contamination of the feces by the mice. The samples were placed into an individual 1.5 mL aseptic centrifuge tube. Subsequently, the collected fecal samples were promptly stored at a temperature of −80°C to ensure their preservation for subsequent microbiome analysis. Notably, all feces were collected during the same time period to maintain consistency. Following the fecal sample collection, the mice were sacrificed while they were deeply anesthetized, and deep anesthesia was induced in the mice using sodium pentobarbital (150 mg/kg, intraperitoneal, Shanghai New Asia Pharmaceutical Co., Shanghai, China). Tissues from the hippocampus and colon were swiftly excised and immediately stored at a temperature of −80°C for future utilization in western blotting experiments. Simultaneously, tissues from the brain and colon were harvested and subjected to fixation, embedding, and sectioning procedures for histopathological and immunofluorescence staining analyses.
Hematoxylin-eosin staining
The procedure involved staining the hippocampus sections with hematoxylin (Solarbio, Beijing, China) and then rinsing the sections quickly in distilled water. Then the sections were differentiated in a mixed acidic solution. After rinsed into distilled water, the sections were stained with eosin. Following dehydration using increasing concentrations of alcohol, the sections were mounted with neutral gum. Additionally, hematoxylin-eosin staining was conducted on colon tissue sections that had been embedded in paraffin to examine morphological and histopathological changes. Observations and photomicrographs were captured using a light microscope (Nikon, Tokyo, Japan).
Nissl staining
The brain tissues underwent overnight dehydration by immersion in a 30% sucrose solution. Subsequently, tissue sections measuring 20 μm were obtained using a cryostat for Nissl staining (Solarbio). Following this, the tissue was subjected to staining with a 0.1% cresyl violet solution in an oven at 37°C for 5–10 minutes. After a 3-minute dehydration step with 70% alcohol, the tissue underwent a differentiation process. Sequential dehydration was then performed using alcohol at various concentrations, followed by two rounds of permeabilization with xylene (5 minutes each time). Finally, the tissue was sealed with neutral resin and subjected to photomicrography.
Immunofluorescence staining
The tissues were fixed for 4 hours and then were dehydrated for 72 hours. After tissues were transversely sectioned, the sections were then blocked and subsequently incubated with the corresponding primary antibody overnight at 4°C. The primary antibody used in this study was anti-Occludin (rabbit, 1:1000, Servicebio, Wuhan, China, Cat# GB111401, RRID: AB_2938979). The following day, washing the sections in Tris-buffered saline Tween-20 followed by incubation with FITC-conjugated goat anti-rabbit IgG antibody (1:200, Servicebio, Cat# GB22303, RRID: AB_2904189) for 1 hour at room temperature. The images were viewed and captured with the aid of Nikon eclipse E100 microscope. Throughout all image acquisition sessions, consistent microscope settings were maintained for quantitative analysis of fluorescence intensity.
Immunohistochemical staining
Paraffin sections of brain tissue were cut into 5 μm thick sections, dewaxed with xylene, and dehydrated with gradients of 100%, 95%, 85%, and 80% ethanol. The antigen was extracted in citric acid buffer (pH 6.0). Subsequently, the sections were rinsed 3 times and then incubated with normal goat serum. The sections were incubated with rabbit anti-NOD-like receptor protein 3 (NLRP3; rabbit, 1:400, Servicebio, Cat# GB114320, RRID: AB_3073726), apoptosis-associated speck-like protein containing CARD (ASC; rabbit, 1:2000, Servicebio, Cat# GB113966, RRID: AB_3083744), and Caspase-1 (rabbit, 1:1000, Servicebio, Cat# GB11383, RRID: AB_3073727) at 4°C overnight. And HRP-conjugated goat anti-rabbit IgG (1:500, Servicebio, Cat# GB23303, RRID: AB_2811189) was added and incubated for 50 minutes at room temperature following washed with phosphate buffered saline. The sections were observed using a microscope and quantification was carried out using ImageJ analysis software (version 1.43, National Institutes of Health, Bethesda, MD, USA).33
Western blotting
Tissues were ground, lysed, and centrifuged. The supernatant was extracted, and the total protein concentration in the hippocampus and intestine was determined using the BCA kit (Solarbio). The protein was heated to denature, electrophoresed after loading, transferred to the membrane, blocked with skim milk, incubated the corresponding primary antibodies against NLRP3 (1:1000), interleukin-1β (IL-1β; rabbit, 1:1000, Servicebio, Cat# GB11113, RRID: AB_3073728), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; rabbit, 1:3000, Affinity Biosciences, Scoresby, Australia, Cat# AF7021, RRID: AB_2839421) at 4°C overnight, and then incubated with HRP-conjugated goat anti-rabbit IgG (1:1000) for 2 hours at room temperature after Tris-buffered saline Tween-20 washing. Finally, ImageJ was utilized to analyze the bands to obtain data.
16S rRNA sequencing
Total genomic DNA was prepared before library construction and sequencing. The qualified samples were purified and subjected to the next round of polymerase chain reaction amplification, and the final amplicons were quantified using the appropriate test kits. The amplified region was the region corresponding to the identification of microbiota diversity: 16S rRNA gene V3–V4 region, and the samples were sequenced using the Illumina Novaseq 6000 platform (Illumina, San Diego, CA, USA). After pre-processing, sequences with ≥ 97% similarity were clustered and merged by vsearch software. Sequences were clustered and merged into one operational taxonomic unit (OTU). The sequences with the highest richness were selected as representatives, and the representative sequences were compared with SILVA data libraries (http://www.arb-silva.de), labeled and classified for subsequent analysis.34
Statistical analysis
Data was firstly tested for normality before differences among the four groups were assessed using one-way analysis of variance, followed by Tukey's multiple comparison test. The study was statistically analyzed using R software and SPSS 26.0 (IBM Corporation, Armonk, NY, USA) and plotted using GraphPad Prism (version 10.0.0 for Windows, GraphPad Software, Boston, MA, USA, www.graphpad.com). P < 0.05 was considered significant.
RESULTS
Effects of O3-RI on improving cognition dysfunction in SD mice
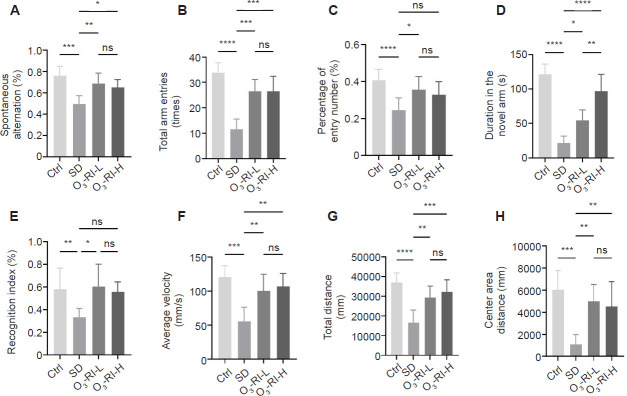
The impact of O3-RI on the cognitive function of SD mice was assessed using the Y-maze. Compared to the Ctrl group, the spontaneous alternation rate, the total arm entries, the entries into the novel arm and the time spent exploring the novel arm were significantly reduced in the SD group. In contrast, after the O3-RI treatment, a significant increase in the four testing indicators was observed (Figure 2A–D).
Figure 2.
O3-RI improves the cognition dysfunction in SD mice.
Note: (A–D) Y-maze test. (A) The spontaneous alternation rate. (B) The total arm entries. (C) The number of entries into the novel. (D) The time spent in the novel arms. (E) The recognition index in Novel object recognition test. (F–H) Open field test. (F) Average velocity. (G) Total distance. (H) Center area distance. Data are expressed as mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way analysis of variance, followed by the Tukey's multiple comparison test). Ctrl: Control; ns: no significance; O3-RI: ozone rectal insufflation; O3-RI-H: high-concentration O3-RI; O3-RI-L: low-concentration O3-RI; SD: sleep deprivation.
Cognitive functions were also assessed using the novel object recognition test. The exploration time for novel objects in the SD group was significantly reduced compared with the three groups (Figure 2E). Furthermore, anxiety levels were evaluated in the open field test. The average velocity, the total distance and the center area distance were markedly decreased in the SD group. On the contrary, the mice from the O3-RI-L and O3-RI-H groups showed an improvement trend by increasing the three testing indicators (Figure 2F–H).
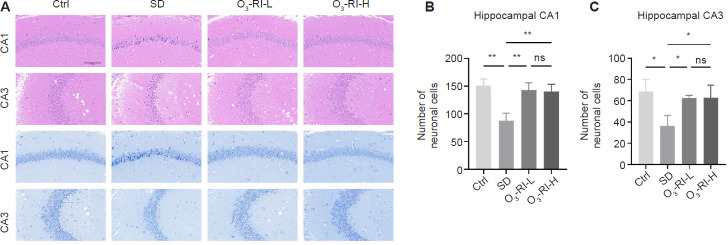
Effects of O3-RI on hippocampal pathology in SD mice
Pathological examination of the hippocampus was conducted using hematoxylin-eosin and Nissl staining. Hematoxylin-eosin staining showed that in the Ctrl group, the hippocampal neurons of mice exhibited orderly arrangement, intact cell structure, and visible nuclei. Conversely, the SD group displayed neuronal damage characterized by irregular cell shape, condensed cytoplasm and nuclei. And administration of O3-RI mitigated the extent of damage induced by SD (Figure 3A). Nissl staining showed a notable reduction in neuronal density and number within the hippocampal CA1 and CA3 regions in the SD group. However, O3-RI-L increased the number and density of pyramidal neurons in all the mentioned regions compared with the SD group. Similarly, the O3-RI-H treatment also raised pyramidal neuron counts in SD mice (Figure 3B and C).
Figure 3.
O3-RI improves the hippocampal pathology in SD mice.
Note: (A) The images of hematoxylin and eosin (upper two rows) and Nissl (lower two rows) staining of hippocampal CA1 and CA3 regions. Compared with the Ctrl group, the neurons in the SD group were necrotic and damaged, which was mainly manifested by the shrinkage and condensation of the nucleus, the enhancement of basophilia, and the number of neurons was also significantly reduced, which was gradually improved in the two O3-RI treatment groups. Scale bars: 100 μm. (B) Quantity of neurons in the hippocampal CA1 (B) and CA3 (C) regions using Nissl staining. Data are expressed as mean ± SD (n = 6). *P < 0.05, **P < 0.01 (one-way analysis of variance, followed by Tukey's multiple comparison test). Ctrl: Control; ns: no significance; O3-RI: ozone rectal insufflation; O3-RI-H: high-concentration O3-RI; O3-RI-L: low-concentration O3-RI; SD: sleep deprivation.
Effects of O3-RI on gut microbiota diversity in SD mice
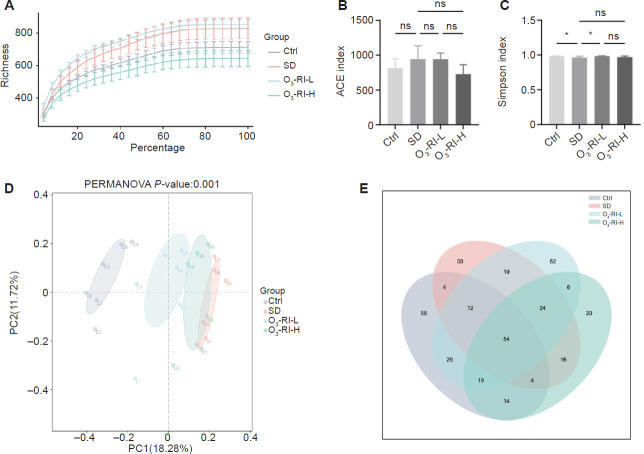
The curve dilution analysis
Dilution curves can be used to indicate whether the amount of sequencing data in a sample is sufficient. As depicted in Figure 4A, as the number of sequences increased, each group showed a gradual trend of plateauing in the number of species. The findings revealed that the sequencing depth met the required standards and was capable of accurately reflecting the majority of microbial information.
Figure 4.
O3-RI affects the gut microbiota diversity in SD mice.
Note: (A) Rarefaction curves. (B) Simpson index. (C) ACE index. Data are expressed as mean ± SD (n = 6). *P < 0.05 (one-way analysis of variance, followed by Tukey's multiple comparison test). (D) Principal coordinates analysis analysis. (E) Venn of similarity and overlap of operational taxonomic unit number in each group. ACE: Abundance-based coverage estimator; Ctrl: control; ns: no significance; O3-RI: ozone rectal insufflation; O3-RI-H: high-concentration O3-RI; O3-RI-L: low-concentration O3-RI; SD: sleep deprivation.
The α- and β-diversity analysis
The abundance-based coverage estimator index indicated no significant difference among these groups (Figure 4B). However, in terms of the Simpson index, a difference was found between the SD group and the Ctrl group. After O3-RI, an increase in the Simpson index was observed (Figure 4C). To assess β-diversity, which reflects differences in microbial community composition among groups, principal coordinates analysis was conducted. The bray-Curtis distances were utilized for principal coordinates analysis to compare the microbiota's overall structures among groups. As depicted in Figure 4D, the two principal component scores accounted for 18.28% and 11.72% of the total variations, respectively. The principal coordinate analysis showed a distinct clustering of gut bacteria between the SD group and the Ctrl group. Notably, O3-RI resulted in significant alterations to the intestinal microbiota.
The OTUs number analysis
To gain deeper insights into specific variations, the shared and unique OTUs of groups were shown (Figure 4E). While most OTUs (n = 54) were shared across all tested groups, distinct OTUs were observed in the SD and O3-RI groups. Specifically, the analysis revealed the presence of 33 OTUs in SD group that were not present in the Ctrl group, and the two different concentrations of O3-RI groups had 52 and 20 OTUs, respectively.
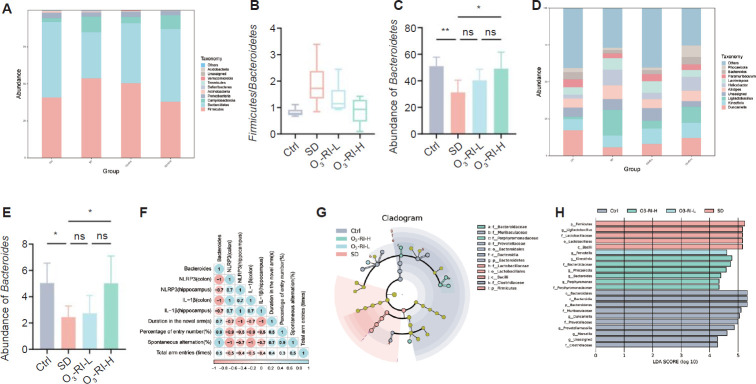
The gut microbiota composition analysis
Microbiota communities were analyzed for their composition at two taxonomic levels. At the phylum level, a significant decrease in the Bacteroidetes abundance and an increase in the Firmicutes abundance was observed in the SD group. In contrast, the administration of O3-RI at different concentrations resulted in the reversal of the phenomenon (Figure 5A–C). At the genus level, the top 10 abundant genera were listed (Figure 5D). Among the changes depicted in Figure 5E, SD decreased the relative abundance of Bacteroides. This change was reversed by O3-RI. Furthermore, the Bacteroides abundance was negatively correlated with the expression of NLRP3 and IL-1β in the hippocampus and colon through Spearman correlation analysis. On the contrary, a positive correlation between the Bacteroides abundance and four metrics in the Y-test was found (Figure 5F).
Figure 5.
Effect of O3-RI on the gut microbiota composition in SD mice.
Note: (A) Gut microbiota composition at the phylum level. (B) The Firmicutes/Bacteroidetes ratio. (C) The abundances of Bacteroidetes (phylum level). (D) Gut microbiota composition at the genus level. (E) The abundances of Bacteroides (genus level). Data are expressed as mean ± SD (n = 6). *P < 0.05, **P < 0.01 (one-way analysis of variance, followed by Tukey's multiple comparison test). (F) Spearman analysis. (G) Cladogram for taxonomy. (H) LDA scores of taxa (score > 2). Ctrl: Control; LDA: linear discriminant analysis; ns: no significance; O3-RI: ozone rectal insufflation; O3-RI-H: high-concentration O3-RI; O3-RI-L: low-concentration O3-RI; SD: sleep deprivation.
The linear discriminant analysis
LEfSe analysis was utilized at all levels to calculate species that differed significantly in abundance among groups. The dendrogram (Figure 5G) showed that compared with the Ctrl group, SD and O3-RI groups exhibited significant changes in gut microbiota in different taxa, and the linear discriminant analysis score histogram (Figure 5H) clearly showed the changed microbiota in each group. Among the 22 biomarkers, p_Firmicutes, g_Ligilactobacillus, f_Lactobacillaceae, o_Lactobacillales, and c_Bacilli were specific in the SD group. Therefore, these microbiota changes may indicate an association with cognitive dysfunction in SD mice. After O3-RI, changes of the g_Prevotella, g_Kineothrix, f_Bacteroidaceae, g_Phocaeicola, g_Bacteroides, g_Porphyromonas, f_Porphyromonadaceae occurred (LDA score > 2), thus, these microbiota could be used as biomarkers of O3-RI to improve cognitive ability in SD mice and as indicators of improved SD-induced cognitive ability by changing gut microbiota.
The BBB and intestinal barrier damage and inflammation in SD mice
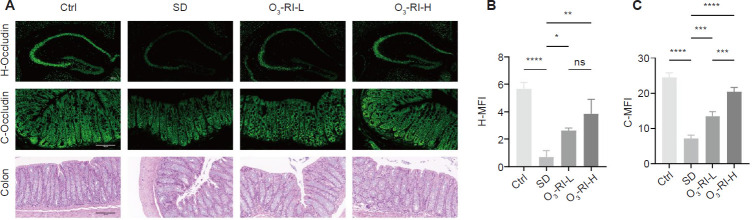
Histological staining and immunofluorescence which were used to examine the tight junction protein (Occludin) expression in the hippocampus and colon were conducted to evaluate the integrity of the BBB and the intestinal barrier. Hematoxylin-eosin staining revealed that the Ctrl group exhibited an intact intestinal mucosa structure with clear outlines of intestinal villi and normal crypt structures. Conversely, the SD group displayed swollen villi with disordered arrangements and reduced glandular structures in the colon (Figure 6A). However, O3-RI reversed these pathological changes. Furthermore, immunofluorescence analysis demonstrated significantly lower Occludin expression in the hippocampus and colon of the SD group compared with the Ctrl group (Figure 6A–C). Notably, O3-RI effectively restored the expression of Occludin.
Figure 6.
Effects of O3-RI on the blood-brain and intestinal barrier structure in SD mice.
Note: (A) Occludin (green) immunofluorescence staining in mouse hippocampus (upper) and intestinal tissue (middle), hematoxylin and eosin staining in intestinal tissue (lower). Compared with the Ctrl group, the expression levels of Occludin in the hippocampus and colon in the SD group decreased, and the colon showed villous swelling and disordered arrangement, which were gradually improved in the two O3-RI treatment groups. Scale bars: 100 μm. (B, C) Occludin expression in the hippocampus and colon of mice. Data are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way analysis of variance, followed by the Tukey's multiple comparison test). C-MFI: Colon-mean fluorescence intensity; Ctrl: control; H-MFI: hippocampus-mean fluorescence intensity; ns: no significance; O3-RI: ozone rectal insufflation; O3-RI-H: high-concentration O3-RI; O3-RI-L: low-concentration O3-RI; SD: sleep deprivation.
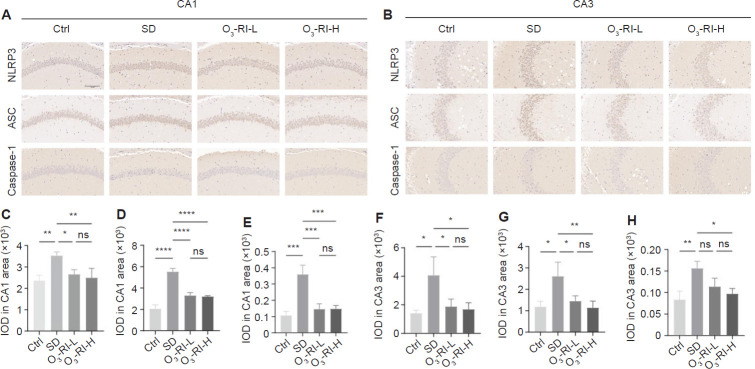
Immunohistochemical staining was used to assess inflammation by observing changes in the NLRP3 inflammasome expression in the CA1 and CA3 regions (Figure 7). SD significantly increased the expression of NLRP3 inflammasome compared with the Ctrl group, O3-RI treatment reversed this phenomenon. To further validate the situation, we also employed Western blotting to measure the expression levels of the NLRP3 and IL-1β in the hippocampus and colon. The trends were consistent with IHC results (Figure 8).
Figure 7.
Effects of O3-RI on NLRP3 inflammasome in hippocampal CA1 and CA3 regions of SD mice using immunohistochemical staining.
Note: (A, B) Representative microphotographs of NLRP3 inflammasome in hippocampal CA1 (A) and CA3 (B) areas. Compared with the Ctrl group, the expressions of NLRP3, ASC and Caspase-1 in the hippocampus CA1 and CA3 regions of mice in the SD group were significantly increased, and this abnormal phenomenon was gradually improved in the two O3-RI treatment groups. Scale bar: 100 μm. (C–E) NLRP3, ASC and Caspase-1 immunopositivity in hippocampal CA1 area. (F–H) NLRP3, ASC and Caspase-1 expression in hippocampal CA3 area. Data are expressed as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way analysis of variance, followed by the Tukey's multiple comparison test). Ctrl: Control; IOD: integrated option density; ns: no significance; O3-RI: ozone rectal insufflation; O3-RI-H: high-concentration O3-RI; O3-RI-L: low-concentration O3-RI; SD: sleep deprivation.
Figure 8.
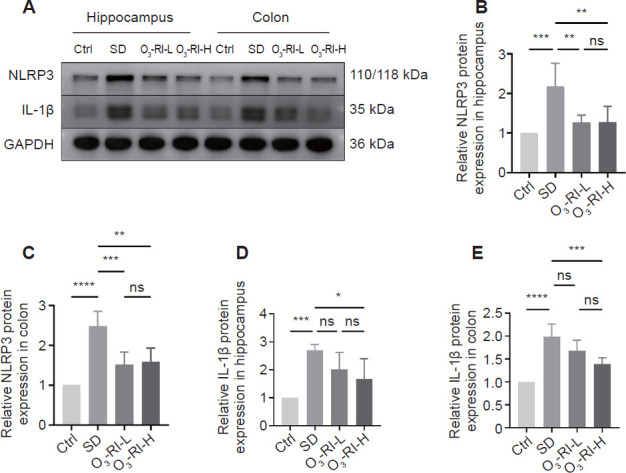
O3-RI prevents SD-induced upregulation of NLRP3 and IL-1β in hippocampus and colon.
Note: (A) Western blotting results. (B–E) Quantitative analysis of NLRP3 and IL-1β expression in hippocampus and colon, which was normalized by control group. Data are expressed as mean ± SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way analysis of variance, followed by the Tukey's multiple comparison test). Ctrl: Control; IL: interleukin; ns: no significance; O3-RI: ozone rectal insufflation; O3-RI-H: high-concentration O3-RI; O3-RI-L: low-concentration O3-RI; SD: sleep deprivation.
DISCUSSION
In this research, we have undertaken an in-depth exploration into the potential protective effects of O3-RI in ameliorating cognitive dysfunction induced by SD. Our study has provided direct evidence substantiating the beneficial impact of O3-RI on the restoration of cognitive function in SD mice. Furthermore, following O3-RI treatment, we documented an array of positive outcomes. These encompassed the restoration of neuronal structural integrity and the preservation of neuronal cell numbers. In addition, O3-RI demonstrated a capacity to mitigate damage to the BBB and the intestinal barrier. Also, our findings revealed a substantial downregulation in the expression levels of the NLRP3 inflammasome and the pro-inflammatory cytokine IL-1β following O3-RI. Moreover, our investigation unveiled an intriguing facet of O3-RI treatment, which pertains to its ability to regulate the gut microbiota. These alterations were discernible both in terms of increased diversity and changes in microbial composition. And we found a correlation between these changes in the gut microbiota and the amelioration of cognitive performance. In sum, our results suggest that O3-RI holds promise as a therapeutic avenue for the enhancement of cognitive function impaired by SD, and this effect is achieved through its anti-inflammatory actions and the regulation of the gut microbiota. In the subsequent sections, we delve into discussing how these results expand our understanding of the amelioration of SD-induced cognition impairments by O3-RI and exploring the underlying mechanistic insights that underpin this phenomenon.
In the course of this investigation, our research unveiled direct evidence demonstrating that mice subjected to SD for 21 consecutive days exhibited a spectrum of abnormal behaviors, prominently characterized by cognitive dysfunction and anxiety disorders. However, upon the administration of O3-RI, a remarkable transformation in their aberrant behaviors was observed. To validate the efficacy of O3-RI in reversing the pathological changes in the hippocampus, we conducted meticulous histological examinations, including hematoxylin-eosin and Nissl staining. These analyses revealed that O3-RI exerted a profound ameliorating effect on both the abnormal morphological characteristics and the number of neurons within the hippocampal region of SD mice. The confluence of these findings, which encompassed both hippocampal morphological restoration and the evident improvements in behavioral indices, underscores the therapeutic potential of O3-RI in mitigating the hippocampal damage induced by SD. Importantly, these restorative effects contribute substantively to the amelioration of the abnormal behaviors precipitated by SD. Notably, the beneficial effects of O3-RI closely parallel the outcomes documented by Yan and her colleagues in their prior investigation,13 which examined the effects of an alternate form of ozone therapy on cognitive dysfunction arising from SD. It is pertinent to emphasize that O3-RI, through its indirect absorption of ozone reaction products in the intestinal milieu into the bloodstream, operates with a more moderated and socially acceptable impact. Consequently, this study represents an inaugural endeavor to systematically explore the potential of O3-RI in addressing cognitive dysfunction stemming from REM SD.
A clinical investigation has previously demonstrated the noteworthy and positive effects of ozone therapy in patients afflicted with intestinal dysbiosis.35 Furthermore, multiple research studies have underscored the profound impact of SD on both the structural and functional aspects of gut microbiota. It is well established that dysbiosis within the gut microbiota can act as an intermediary link between SD and subsequent peripheral and central inflammatory responses, ultimately culminating in cognitive impairment.16,36,37 In light of these considerations, the current research endeavor was undertaken to scrutinize the composition of the gut microbiota within each experimental group. Our comprehensive analysis has revealed a significant reduction in the Simpson index of gut microbiota α-diversity in SD mice when compared to the Ctrl group. This finding is consonant with prior foundational research, which consistently demonstrated that SD mice manifest diminished α-diversity within their gut microbiota when contrasted with a cohort of healthy control subjects.38,39 Conversely, an augmentation in microbial diversity was discerned in the O3-RI group. Furthermore, the results of our β-diversity analysis provided additional support for the idea that SD has a discernible impact on shaping the unique microbial community within mice, substantiating the notion that SD significantly influences gut microbial diversity. Crucially, it is noteworthy that the community of the gut microbiota in mice subjected to O3-RI treatment gradually approached that of the Ctrl mice. This observation strongly suggests a beneficial influence exerted by O3-RI in mitigating the dysbiosis within the gut brought about by SD, thereby further accentuating the potential therapeutic promise of O3-RI in ameliorating cognitive impairment resulting from SD.
In this investigation, two concentrations of gaseous ozone were employed for therapeutic intervention. However, the outcomes showed no disparities in both cognitive-behavioral and pathological results, such phenomenon indicated that the therapeutic efficacy of O3-RI may be disproportionate to its concentration level. Furthermore, upon scrutinizing the results derived from the analysis of intestinal microbiota, we discerned that the lower concentration of O3-RI exhibited a superior impact on the diversity of the intestinal microbiota when compared to higher concentrations of O3-RI. This observation suggests that the lower concentration of O3-RI could potentially influence cognitive function by modulating the diversity of the intestinal microbiota. Notably, there exists limited foundational research pertaining to this specific aspect, warranting further in-depth investigation into the underlying mechanisms. On the contrary, the administration of a higher concentration of O3-RI led to more pronounced alterations in the composition of the intestinal microbiota, and below we focus on the composition of the intestinal microbiota to further explore the relevant mechanisms.
At the phylum level, our analysis unveiled a relative elevation in the abundance of Firmicutes and a significant reduction in the abundance of Bacteroidetes in mice subjected to SD. It is noteworthy that Firmicutes and Bacteroidetes constitute the predominant phyla within the intestinal microbiota landscape.40 The imbalance in the relative proportions of Firmicutes and Bacteroidetes has been well-documented in an array of degenerative brain disorders, post-surgical cognitive decline, cerebrovascular events, high blood pressure, and various clinical conditions.41,42 For instance, in states characterized by cognitive impairment, the alterations in gut microbiota composition frequently manifest as an increase in Firmicutes abundance coupled with a concurrent decrease in Bacteroidetes abundance. In conditions such as ASD, a marked reduction in Bacteroidetes abundance along with a significant rise in the Firmicutes/Bacteroidetes ratio has been consistently reported. Furthermore, scientific investigations have established a strong association between an elevated Firmicutes/Bacteroidetes ratio and conditions such as inflammatory intestinal disease and obesity.43,44,45 This association suggests that the mice exposed to SD may potentially manifest chronic intestinal inflammation and metabolic disorders. Notably, our study revealed a finding – O3-RI effectively reversed the Firmicutes/Bacteroidetes ratio, returning it to levels more akin to those observed in the Ctrl group. It is particularly noteworthy that this restorative effect was more pronounced in the O3-RI-H group when compared to the O3-RI-L group.
Furthermore, at the genus level, Bacteroides exhibited significantly decreased abundance in the SD group. The functions of Bacteroides can be divided into those that act in the gut and those that act in the brain. In the gut, Bacteroides are vital in reducing inflammation and intestinal damage.46,47 In the realm of brain function research, numerous clinical and preclinical investigations have convincingly demonstrated the close association between changes in gut Bacteroides and a broad spectrum of neuropsychiatric conditions. These conditions encompass attention-deficit/hyperactivity disorder,48 multiple sclerosis,49 ASD,50 and Alzheimer's disease.51 Moreover, cognitive dysfunction has been established as a transdiagnostic feature of neuropsychiatric disorders, and it has been demonstrated to be linked to the dysregulation of gut Bacteroides.52,53,54 Additionally, the presence of a Bacteroides-dominated gut microbiome can actively modulate cognition during infancy, as it takes part in crucial neurodevelopmental processes.55,56 Notably, Bacteroides can affect several functional pathways, including metabolism of several amino acids, and biosynthesis of unsaturated fatty acids, all of which have an impact on cognition.57,58,59,60 Of course, we also found that O3-RI had a significant effect on other genera, such as Prevotella, Kineothrix, Phocaeicola, and Porphyromonas. Several studies have reported a diminished abundance of Prevotella in children diagnosed with ASD. In addition, reduced levels of Prevotella in infants have been correlated with behavioral issues manifesting at the age of two.61 Patients afflicted with multiple sclerosis exhibit a severe lack of Prevotella within their intestinal microbiota. Intriguingly, a separate investigation demonstrated that treating transgenic mice with a formulation containing Prevotella resulted in a discernible reduction in the pro-inflammatory response associated with these diseases.62 The Kineothrix is well-established for its glycolytic function to butyrate production, a substance known for its capacity to enhance memory and cognitive function. This effect is mediated through the regulation of the microbial-brain-gut axis, thereby mitigating neuroinflammation.63 With regard to Phocaeicola, studies have elucidated that preparations containing this strain, such as CCFM1183, exhibit efficacy in safeguarding against weight loss, ameliorating colon length reduction, and repairing colonic mucosal damage induced by dextran sulfate sodium salt-induced colitis.64 Porphyromonas, primarily featured in periodontal microbiota studies, is infrequently documented in research pertaining to intestinal microbiota. All of the above microbiota have direct or indirect effects on inflammation and cognition, but the detailed mechanism of their role in cognitive impairment caused by SD need more experiments to support research and provide a more solid foundation for the clinical transformation of gut microbiota.
It has been demonstrated that the activation of NLRP3 within the gut, induced by alterations in the gut microbiota composition, plays a pivotal role in compromising the integrity of the intestinal barrier. This event is subsequently followed by an elevation in peripheral lipopolysaccharides levels and heightened inflammatory cytokine production. These events collectively contribute to BBB impairment, ultimately culminating in the activation of NLRP3 within the brain parenchyma, thereby impairing cognitive function.37 The same study showed that knocking out the NLRP3 gene in mice could restore the structural integrity of the gut barrier and BBB, inhibit the immune inflammatory response in the brain, and reverse cognitive impairment caused by SD. Consistent with this phenomenon, our investigation revealed a conspicuous up-regulation of NLRP3 and IL-1β expression, coupled with a notable down-regulation of Occludin expression within the hippocampal and colonic tissues of mice subjected to SD when compared to the Ctrl group. Furthermore, our study sought to establish a related relationship between alterations in gut microbiota, cognitive performance, and the expression of inflammation within both the colon and hippocampus. We employed a battery of test metrics including Y-maze assessments and measurements of colonic and hippocampal NLRP3 and IL-1β expression. In particular, we found a negative association between the abundance of Bacteroides within several differential microbiota genera and the expression of inflammation within the hippocampus and colon. Conversely, Bacteroides abundance exhibited a positive correlation with the four metrics employed in the Y-maze test. At present, a number of studies have verified that the gut microbiota mediates the process of cognitive impairment caused by SD through fecal microbiota transplantation experiments.37,38,65 However, due to the existence of different mice breeds and different SD patterns, the structure and composition of intestinal microbiota are not quite different, and in our study, we found unique changes in the structure and composition of intestinal microbiota in REM SD, with certain microbiota genera correlated with the expression of some inflammatory factors such as NLRP3 inflammasome and IL-1β, and O3-RI can reverse these changes in the process of improving cognition. These findings underscore the possibly potential role of O3-RI in attenuating NLRP3 activation and ultimately ameliorating cognitive dysfunction through its capacity to modulate specific intestinal microbiota in mice subjected to SD.
Nevertheless, these limitations should be noted: Firstly, the number of mice included in the investigation of 16S rRNA amplicon sequencing data was limited. Secondly, how O3-RI restored Occludin expression and inhibited inflammation was not explored due to the complex and diverse reaction products of O3-RI in the intestine. Thirdly, more inflammatory factors or proteins need to be tested in the future to more comprehensively assess the impact of inflammation on cognitive function. Finally, further exploration in the field of gut microbiota is limited, and further in-depth research, such as fecal microbiota transplantation, is needed.
In conclusion, O3-RI could be an efficient intervention to improve Chronic REM SD-induced cognitive dysfunction by regulating the gut microbiota, restoring the integrity of the BBB and intestinal barrier, and decreasing inflammatory responses.
Funding Statement
Funding: This study was supported by the National Natural Science Foundation of China, No. 82072086.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Data availability statement
All relevant data are within the paper.
REFERENCES
- 1.Liu H, Chen A. Roles of sleep deprivation in cardiovascular dysfunctions. Life Sci. 2019;219:231–237. doi: 10.1016/j.lfs.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Parhizkar S, Gent G, Chen Y, et al. Sleep deprivation exacerbates microglial reactivity and Aβ deposition in a TREM2-dependent manner in mice. Sci Transl Med. 2023;15:eade6285. doi: 10.1126/scitranslmed.ade6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99:1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depner CM, Stothard ER, Wright KP., Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. 2014;14:507. doi: 10.1007/s11892-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause AJ, Simon EB, Mander BA, et al. The sleep-deprived human brain. Nat Rev Neurosci. 2017;18:404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cousins JN, Sasmita K, Chee MWL. Memory encoding is impaired after multiple nights of partial sleep restriction. J Sleep Res. 2018;27:138–145. doi: 10.1111/jsr.12578. [DOI] [PubMed] [Google Scholar]
- 7.Kim SM, Zhang S, Park J, et al. REM sleep deprivation impairs learning and memory by decreasing brain O-GlcNAc cycling in mouse. Neurotherapeutics. 2021;18:2504–2517. doi: 10.1007/s13311-021-01094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2008;9:517–526. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14:547–558. doi: 10.1016/S1474-4422(15)00021-6. [DOI] [PubMed] [Google Scholar]
- 10.El-Mehi AE, Faried MA. Controlled ozone therapy modulates the neurodegenerative changes in the frontal cortex of the aged albino rat. Ann Anat. 2020;227:151428. doi: 10.1016/j.aanat.2019.151428. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Feng X, Ren H, Huang H, Wang Y, Yu S. Low-dose ozone therapy improves sleep quality in patients with insomnia and coronary heart disease by elevating serum BDNF and GABA. Bull Exp Biol Med. 2021;170:493–498. doi: 10.1007/s10517-021-05095-6. [DOI] [PubMed] [Google Scholar]
- 12.Lin SY, Ma J, An JX, et al. Ozone inhibits APP/Aβ production and improves cognition in an APP/PS1 transgenic mouse model. Neuroscience. 2019;418:110–121. doi: 10.1016/j.neuroscience.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Yan YN, Williams JP, Niu K, et al. Intraperitoneal ozone injection prevents REM sleep deprivation - induced spatial learning and memory deficits by suppressing the expression of Sema3A in the hippocampus in rats. Iran J Basic Med Sci. 2022;25:980–988. doi: 10.22038/IJBMS.2022.63994.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bocci V, Zanardi I, Borrelli E, Travagli V. Reliable and effective oxygen-ozone therapy at a crossroads with ozonated saline infusion and ozone rectal insufflation. J Pharm Pharmacol. 2012;64:482–489. doi: 10.1111/j.2042-7158.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 15.Socała K, Doboszewska U, Szopa A, et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021;172:105840. doi: 10.1016/j.phrs.2021.105840. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Wang Z, Lu T, et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med Rev. 2022;65:101691. doi: 10.1016/j.smrv.2022.101691. [DOI] [PubMed] [Google Scholar]
- 17.Góralczyk-Bińkowska A, Szmajda-Krygier D, Kozłowska E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int J Mol Sci. 2022;23:11245. doi: 10.3390/ijms231911245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wekerle H. Brain autoimmunity and intestinal microbiota: 100 trillion game changers. Trends Immunol. 2017;38:483–497. doi: 10.1016/j.it.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19:610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 20.Gordon R, Albornoz EA, Christie DC, et al. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018;10:eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao J, Qiao Y, Xie R, et al. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. J Psychiatr Res. 2021;137:147–157. doi: 10.1016/j.jpsychires.2021.02.057. [DOI] [PubMed] [Google Scholar]
- 22.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang D, Wang Z, Chen Y, Guo Q, Dong Y. Interactions between gut microbes and NLRP3 inflammasome in the gut-brain axis. Comput Struct Biotechnol J. 2023;21:2215–2227. doi: 10.1016/j.csbj.2023.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrini C, Antonioli L, Calderone V, Colucci R, Fornai M, Blandizzi C. Microbiota-gut-brain axis in health and disease: Is NLRP3 inflammasome at the crossroads of microbiota-gut-brain communications? Prog Neurobiol. 2020;191:101806. doi: 10.1016/j.pneurobio.2020.101806. [DOI] [PubMed] [Google Scholar]
- 25.Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Qiu H, Zhong R, Liu H, Zhang F, Li S, Le W. Chronic sleep deprivation exacerbates learning-memory disability and Alzheimer’s disease-like pathologies in AβPP(swe)/PS1(ΔE9) mice. J Alzheimers Dis. 2016;50:669–685. doi: 10.3233/JAD-150774. [DOI] [PubMed] [Google Scholar]
- 28.Mallok A, Vaillant JD, Soto MT, et al. Ozone protective effects against PTZ-induced generalized seizures are mediated by reestablishment of cellular redox balance and A1 adenosine receptors. Neurol Res. 2015;37:204–210. doi: 10.1179/1743132814Y.0000000445. [DOI] [PubMed] [Google Scholar]
- 29.Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 2019;1916:105–111. doi: 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Zhu H, Guo Y, Du X, Qin C. Gut microbiota regulate cognitive deficits and amyloid deposition in a model of Alzheimer’s disease. J Neurochem. 2020;155:448–461. doi: 10.1111/jnc.15031. [DOI] [PubMed] [Google Scholar]
- 31.Leger M, Quiedeville A, Bouet V, et al. Object recognition test in mice. Nat Protoc. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 32.Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019;1916:99–103. doi: 10.1007/978-1-4939-8994-2_9. [DOI] [PubMed] [Google Scholar]
- 33.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loprete F, Vaiano F. The use of ozonated water and rectal insufflation in patients with intestinal dysbiosis. Ozone Ther. 2017;2 [Google Scholar]
- 36.Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Zhao N, Chen QG, Chen X, et al. Intestinal dysbiosis mediates cognitive impairment via the intestine and brain NLRP3 inflammasome activation in chronic sleep deprivation. Brain Behav Immun. 2023;108:98–117. doi: 10.1016/j.bbi.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Chen WH, Li SX, et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol Psychiatry. 2021;26:6277–6292. doi: 10.1038/s41380-021-01113-1. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Yuan K, Ji YB, et al. Alterations of the gut microbiota in response to total sleep deprivation and recovery sleep in rats. Nat Sci Sleep. 2022;14:121–133. doi: 10.2147/NSS.S334985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ngo ST, Restuadi R, McCrae AF, et al. Progression and survival of patients with motor neuron disease relative to their fecal microbiota. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21:549–562. doi: 10.1080/21678421.2020.1772825. [DOI] [PubMed] [Google Scholar]
- 43.Abenavoli L, Scarpellini E, Colica C, et al. Gut microbiota and obesity: a role for probiotics. Nutrients. 2019;11:2690. doi: 10.3390/nu11112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5–14. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown EM, Ke X, Hitchcock D, et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe. 2019;25:668–680.e7. doi: 10.1016/j.chom.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng J, Hu J, Geng F, Nie S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci Hum Wellness. 2022;11:1101–1110. [Google Scholar]
- 48.Wang LJ, Yang CY, Chou WJ, et al. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2020;29:287–297. doi: 10.1007/s00787-019-01352-2. [DOI] [PubMed] [Google Scholar]
- 49.Mirza A, Forbes JD, Zhu F, et al. The multiple sclerosis gut microbiota: a systematic review. Mult Scler Relat Disord. 2020;37:101427. doi: 10.1016/j.msard.2019.101427. [DOI] [PubMed] [Google Scholar]
- 50.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang X, Fu Y, Cao WT, et al. Gut microbiome, cognitive function and brain structure: a multi-omics integration analysis. Transl Neurodegener. 2022;11:49. doi: 10.1186/s40035-022-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saji N, Murotani K, Hisada T, et al. The relationship between the gut microbiome and mild cognitive impairment in patients without dementia: a cross-sectional study conducted in Japan. Sci Rep. 2019;9:19227. doi: 10.1038/s41598-019-55851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo XX, Yang C, Zhan GF, et al. Whole brain radiotherapy induces cognitive dysfunction in mice: key role of gut microbiota. Psychopharmacology (Berl) 2020;237:2089–2101. doi: 10.1007/s00213-020-05520-0. [DOI] [PubMed] [Google Scholar]
- 54.Liu P, Jia XZ, Chen Y, et al. Gut microbiota interacts with intrinsic brain activity of patients with amnestic mild cognitive impairment. CNS Neurosci Ther. 2021;27:163–173. doi: 10.1111/cns.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlson AL, Xia K, Azcarate-Peril MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry. 2018;83:148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamana SK, Tun HM, Konya T, et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes. 2021;13:1–17. doi: 10.1080/19490976.2021.1930875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashe K, Kelso W, Farrand S, et al. Psychiatric and cognitive aspects of Phenylketonuria: the limitations of diet and promise of new treatments. Front Psychiatry. 2019;10:561. doi: 10.3389/fpsyt.2019.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 59.Arnoldussen IA, Zerbi V, Wiesmann M, et al. Early intake of long-chain polyunsaturated fatty acids preserves brain structure and function in diet-induced obesity. J Nutr Biochem. 2016;30:177–188. doi: 10.1016/j.jnutbio.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Wyse AT, Netto CA. Behavioral and neurochemical effects of proline. Metab Brain Dis. 2011;26:159–172. doi: 10.1007/s11011-011-9246-x. [DOI] [PubMed] [Google Scholar]
- 61.Kang DW, Park JG, Ilhan ZE, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shahi SK, Freedman SN, Murra AC, et al. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front Immunol. 2019;10:462. doi: 10.3389/fimmu.2019.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei H, Yu C, Zhang C, et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed Pharmacother. 2023;160:114308. doi: 10.1016/j.biopha.2023.114308. [DOI] [PubMed] [Google Scholar]
- 64.Sun Z, Jiang X, Wang B, Tian F, Zhang H, Yu L. Novel Phocaeicola strain ameliorates dextran sulfate sodium-induced colitis in mice. Curr Microbiol. 2022;79:393. doi: 10.1007/s00284-022-03054-6. [DOI] [PubMed] [Google Scholar]
- 65.Li N, Tan S, Wang Y, et al. Akkermansia muciniphila supplementation prevents cognitive impairment in sleep-deprived mice by modulating microglial engulfment of synapses. Gut Microbes. 2023;15:2252764. doi: 10.1080/19490976.2023.2252764. [DOI] [PMC free article] [PubMed] [Google Scholar]