Abstract
Background and purpose:
Highly metastatic breast cancer is a population of cancer cells that has metastasized to other organs in the body leading to apoptosis resistance. It was reported that MDAMB-231 cells contain lower levels of reactive oxygen species associated with metastatic capability. Curcuma longa (CL) possesses cytotoxic effects in several cancer cells including metastatic breast cancer cells. This study aimed to investigate the effect of CL-inhibited cell migration in highly metastatic breast cancer MDAMB-231 cells.
Experimental approach:
CL was extracted under maceration with methanol. The cytotoxic effect on single and combination treatment of CL was assessed through the MTT assay. Migration analysis was evaluated using scratch wound healing assay, MMP-9 expression by gelatine zymography, Rac-1, and MMP-9 gene expression using Real-Time Quantitative Reverse transcription polymerase chain reaction (qRT-PCR). The apoptosis induction was analyzed through Bax gene expression and Bcl-2 protein expression.
Findings/Results:
We found that CL inhibits the growth of MDAMB-231 cells, induces Bax gene expression, and suppresses Bcl-2 expression in a dose-dependent manner. Moreover, cancer cell migration was suppressed by the presence of CL. qRT-PCR and gelatine zymography assay showed that CL downregulates Rac-1 and MMP-9 gene expression.
Conclusion and implications:
CL could inhibit the growth and migration of highly metastatic breast cancer cells by reducing the Rac-1 gene expression and regulating apoptosis protein expression.
Keywords: Curcuma longa, MDAMB-231, Migration, MMP-9, Rac-1
INTRODUCTION
Metastatic breast cancer usually exhibits a high mortality rate due to its complexity of physiological aberration with drug resistance, relapse, and genetic mutation phenomena (1). Currently, first-line therapy for metastatic breast cancer still relies on doxorubicin chemotherapy (2). However, a previous study reported that doxorubicin can induce cell cancer migration (3,4). Doxorubicin induces the transforming growth factor β1 (TGF-β1) pathway activation and increases the ability of perivascular TIE2+ macrophage to promote cancer cell invasion and metastasis through epithelial-mesenchymal transition activation (4,5). Thus, the search for new therapeutic compounds based on cancer cell biology with special emphasis on molecular factors involved in migration and metastasis is reasonable (6,7). Curcuma longa (CL) has been reported in a wide range of biological activities including anticancer effects in several cancer cells (8,9). However, the anti-metastatic mechanism of CL in single treatment on highly metastatic breast cancer cells remains unclear.
The mechanism of CL in inhibiting the migration of highly metastatic breast cancer cells involving the Ras-related C3 botulinum toxin substrate 1 (Rac-1) pathway has not yet been studied. Therefore, in this study, we examined the effects of CL as a single-treatment chemotherapeutic agent on inhibiting the expression of matrix metalloproteinase-9 (MMP-9) and Rac-1 associated with optimal anti-metastatic effects in highly metastatic MDAMB-231 breast cancer cells.
MATERIALS AND METHODS
Cell cultures
Highly metastatic breast cancer cell line (murine mammary carcinoma cell line from a BALB/cfC3H mouse; MDAMB-231 cells) were provided from American Type Culture Collection (ATCC). The cells were grown as a monolayer in Dulbecco’s modified eagle medium high glucose (DMEM; Catalog #12100046 Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA) enriched with 10% (v/v) fetal bovine serum (FBS) (Catalog #10099142 Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA), 100 IU/mL penicillin-100 μg/mL streptomycin (Catalog #10378016 Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA), and maintained at 37 °C with 5% CO2 in 100% humidified atmosphere.
Extraction procedure
The extractions of CL are explained as follows; the material for this study was in the form of herbs, namely turmeric rhizome simplicia (Curcuma longa); the plants were then cleaned with running water until clean, then each herb was dried in a place that was not exposed to direct sunlight, after which it was chopped and powdered (simplicia powder). The simplicia powder was weighed 300 g and then put into the maceration container. One liter of ethanol extraction was added into the maceration container and left for a few hours, then 1 L of 70% ethanol was added until all the sample powder was submerged, then closed tightly. The maceration container was stored in a place protected from direct sunlight for 5 days while stirring frequently. The mixture was then filtered, and the dregs were soaked again with a new solvent. The extraction process was then carried out 4 times with 2 L of ethanol each time. The liquid extract was collected and then concentrated using a Rotavapor device to obtain a thick extract (9,10).
Cytotoxic assay
The cytotoxic activity of CL on MDAMB-231 cells was evaluated using MTT assay according to the previous study by Mosman (11) with slight modification. MDAMB-231 (2 × 103 cells/well) were seeded in a 96-well microplate and allowed to settle overnight before being treated with 0.5-30 μg/mL CL (Sigma-Aldrich, St. Louis, MO, USA) for 24 h. The variations are needed to determine the IC50 value, which can be used to determine variations in the concentration of the test solution combination treatments. Untreated cells were considered a negative control. After treatment, 100 μL of MTT (BioVision Inc, SF, USA; 0.5 mg/mL in medium) was added to each well and incubated for a further 4 h at 37 °C with 5% CO2 in a 100% humidified atmosphere. Afterward, the MTT formazan crystal was dissolved using dimethyl sulfoxide (DMSO) and incubated in the dark condition for 4 h. After solubilizing the purple formazan, absorbance was measured using ELISA plate reader (Bio-Rad, California, USA) at a wavelength of 595 nm. Each treatment was carried out in triplicate and cytotoxic activity was recorded as IC50, which is the concentration necessary to reduce the absorbance of treated cells by 50% compared to the untreated cells (12,13,14).
ROS level analysis
For all ROS experiments, MDAMB-231 cells (5 × 103) were collected by centrifugation, washed with phosphate-buffered saline (PBS), and incubated with 2.5 μΜ 2ʹ, 7ʹ-dichlorofluorescein diacetate (DCFDA) in supplemented buffer (10% FBS in PBS) for 30 min in a dark place at 37 °C. Each cell was treated with CL in several concentrations (1/4 IC50 (30 μg/mL), 1/8 IC50 (15 μg/mL), and 1/16 IC50 (7,5 μg/mL) and then incubated for 4 h at 37 °C and CO2 5%. As reported previously, we used doxorubicin as a positive control due to its ability to induce ROS levels. Intracellular ROS was determined by flow cytometry (BD Accuri C6 plus BD Biosciences, USA) (15).
Scratch wound healing assay
The migration rate of CL-treated MDAMB-231 cells was assessed by the scratch wound healing assay. The MDAMB-231 cells density of 10 × 104 cells/well were grown in a 24-well microplate and incubated for 24 h at 37 °C and 5% CO2. After 80% confluent, the medium was discarded and changed to a starvation medium containing 0.5% (v/v) FBS for 24 h. After that, the confluent cells were scraped horizontally with a cell scratcher after 24 h incubation. The debris was removed by washing with PBS and the cells were treated with several concentrations of CL. The cell migration can subsequently be monitored microscopically (×100 magnification), as the cells travel from the intact zones into the scratched region. The migration rate was analyzed using ImageJ (The National Institutes of Health and the Laboratory for Optical and Computational Instrumentation (LOCI), University of Wisconsin, USA), and the percentage of closure after 42 h-treatment with different concentrations of CL was measured and compared to the value obtained at 0 h. An increase in closure percentage suggested cell migration (16).
Gelatine zymography assay
Gelatine zymography was performed to determine the activity of MMPs in the culture supernatants. The MDAMB-231 cells density of 5 × 105 cells/well were seeded in the 6-well microplate and exposed to CL for 24 h at 37 °C and 5% CO2. Culture supernatants were then collected and resolved on 8% sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing conditions. The gel was supplemented with 0.1% gelatine (Sigma-Aldrich, USA). In each gel run equivalent amounts of protein (150 μg) from culture supernatants were used. Gel was soaked for 30 min in 2% Triton®-X 100 (Merck, USA) solution after electrophoresis at room temperature before incubating in 100 mL of the incubation buffer containing 40 mM tris HCl, pH 8, 10 mM CaCl2, 0.02% NaN3 for 24 h at 37 °C. Afterward, the gel was stained by Coomassie Brilliant Blue R-250 solution for 2 h and then de-stained (20% methanol, 10% acetic acid, and 70% water) until bands with a dark blue background appeared clear, indicating gelatinolytic activity of MMP-9. The band intensity was calculated by ImageJ (The National Institutes of Health and the Laboratory for Optical and Computational Instrumentation (LOCI), University of Wisconsin, USA) (17-18).
Rac-1, MMP-9, and Bax gene expression analysis
According to the manufacturer's protocol, the genes’ expressions were conducted using the real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR). Total RNA from the MDAMB-231 cells was extracted with TRIzol (Invitrogen, Shanghai, China). Briefly, first-stranded cDNA was synthesized with 1 ng of total RNA using Super-Script II (Invitrogen, Massachusetts, USA). SYBR No ROX Green I dye (SMOBIO Technology Inc., Hsinchu, Taiwan) was used for reverse transcription in an RT-PCR instrument (PCR Max Eco 48, Staffordshire, UK), and mRNA levels of the Rac-1, MMP-9, and Bax genes were measured using the respective primers according to gene bank. The thermocycler conditions were as follows: initial step at 95 °C for 10 min, followed by 50 cycles at 95 °C for 15 s, and 60 °C for 1 min. The gene expression was recorded as the cycle threshold (Ct). Data were obtained using Eco Software v5.0 (Illumina Inc, San Diego, CA, USA). All reactions were performed in triplicate, and data analysis used the 2−ΔΔCt method (Livak method) (19).
Immunocytochemistry analysis
The cells (5 × 104 cells/well) were placed on the slide with a coupled bottle (Thermo Fisher, USA) and incubated at 37 °C and 5% CO2 for 24 h. After that, the cells were treated with CL in several concentrations for 24 h. Then, the culture medium was removed, and the cells were washed with PBS. For fixing the cells, the bottle was incubated with 1 mL of 4% formaldehyde for 20 min. Subsequently, the slides were incubated with 10% hydrogen peroxide for 30 min to block endogenous peroxidases. Antigen retrieval was performed in a pressure cooker (ARNO, São Paulo, SP, Brazil) at 95 °C with citrate buffer (pH 6.0) for 30 min. After cooling, the slides were covered with bovine serum albumin solution and incubated for 30 min at 4 °C, overnight with B-cell lymphoma 2 (Bcl-2) antibody (Abcam, USA; 1:500). Thus, the cells were washed with PBS for 15 min and incubated with Starr TREK Universal HRP Detection Kit (Biocare Medi-Cal, Concord, CA, USA) for 20 min, and streptavidin-peroxidase complex for 10 min, followed by washing with PBS for 15 min. Next, 0.5% 3,3-diaminobenzidine tetrachloridine (DAB; Sigma Aldrich, USA) was applied for 2 min. The slides were counterstained with hematoxylin and eosin (H&E) for 40 s.
Statistical analysis
All values represent the mean ± SEM of three independent experiments. For statistical comparisons, a one-way analysis of variance (ANOVA) test followed by LSD post hoc was performed using SPSS version 26. P-values < 0.05) were considered statistically significant. The 50% inhibitory concentration (IC50) was calculated by a nonlinear regression curve with the use of Microsoft Office Excel for Windows.
RESULTS
Cytotoxic activity of CL against MDAMB-231
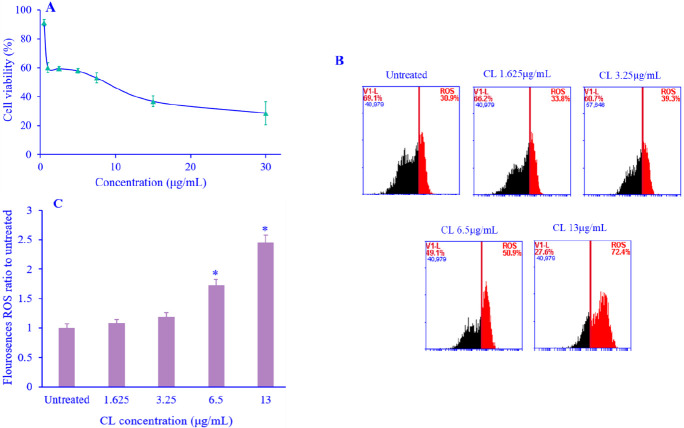
The cytotoxic effect of CL on MDAMB-231 was analyzed using an MTT assay. Various concentrations of CL were used for a 24-h treatment. In the presence of CL, the growth of MDAMB-231 cells was inhibited in a concentration-dependent manner with IC50 of 13 μg/mL value on MDAMB-231 (Fig. 1A).
Fig. 1.
(A) Cytotoxic activity of various concentrations of CL on MDAMB-231 cells after 24 h; (B) induction of ROS level by CL treatment on MDAMB-231 breast cancer cells, the ROS level of cells exposed to CL individually for 24 h was measured by flow cytometry after staining with 2ʹ, 7ʹ-dichlorofluorescein diacetate; (C) the % level of ROS. Data are presented as mean + SEM, N = 3. *P < 0.05 Indicates significant differences in comparison with the untreated cells. CL, Curcuma longa; ROS, reactive oxygen species.
CL elevated ROS generation on MDAMB-231
To evaluate the potential effect of CL on cell viability loss due to CL-triggered ROS generation, ROS analysis under fluorescence of DCFDA through fluorescence-activated cell sorting (FACS) flow analysis was performed (Fig. 1B). CL increased ROS generation significantly in a concentration-dependent m anner (Fig. 1C). These results showed that CL might exert cytotoxic effects through ROS level elevation.
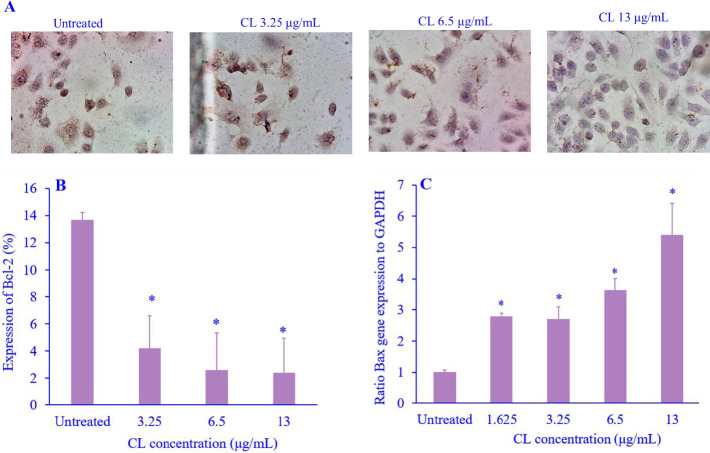
CL-induced cell apoptosis
Bcl-2 and Bax are proteins that have a role in regulating cell apoptosis. In this study, CL significantly decreased the expression of the anti-apoptotic protein Bcl-2 (Fig. 2A and B), however, CL was also able to increase the expression of the pro-apoptotic gene Bax protein in a concentration-dependent manner (Fig. 2C).
Fig. 2.
The effect of CL on the apoptosis regulation. (A) Immunocytochemistry morphology of Bcl-2 expression; (B) quantification of Bcl-2 expression; and (C) percentage of Rac-1 gene expression level of cell migration-related proteins was measured by quantitative real-time polymerase chain reaction. Data are presented as mean + SEM, N = 3. *P < 0.05 Indicates significant differences in comparison with the untreated cells. CL, Curcuma longa; Bcl-2, B-cell lymphoma 2; Rac-1, Ras-related C3 botulinum toxin substrate 1.
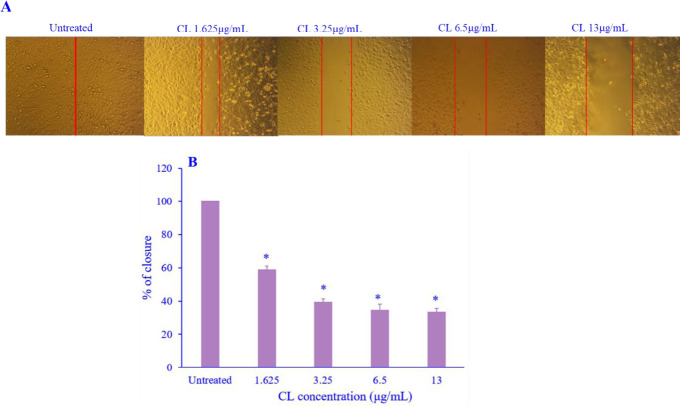
CL inhibited cell migration
The ability of MDAMB-231 cell migration was assessed using a scratch wound healing assay. The wound width of the untreated cells narrowed at 24 h and closed completely throughout 42 h (Fig. 3A and B). Treatment with CL was found to significantly inhibit the migration of MDAMB-231 cells in a concentration-dependent manner after 42 h compared to untreated cells. The results indicated the antimigratory potential of CL against MDA-MB-231 cells.
Fig. 3.
The effect of CL treatment on the migration ability of MDAMB-231 breast cancer cells. (A) Cell migration was assessed via scratch wound healing assay; (B) the migration abilities were quantified by counting the area of closure using ImageJ and the percentage of the treated groups compared to the untreated group. Data are presented as mean + SD, N = 3. *P < 0.05 Indicates significant differences in comparison with the untreated cells. CL, Curcuma longa.
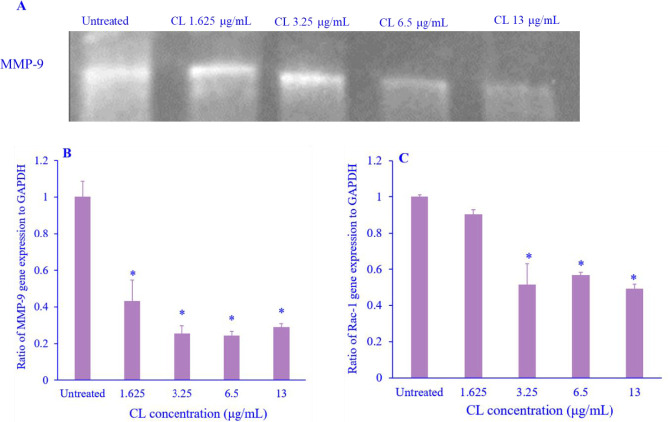
CL inhibited MMP-9 and Rac-1 expression
MMP-9 and Rac 1 are important proteins that regulate the migration process by degrading the matrix. CL significantly decreased the protein and gene expression of MMP-9 (Fig. 4A and B) in a concentration-dependent manner. More importantly, CL also reduced the expression of Rac-1 compared with the untreated cells (Fig. 4C). Overall, CL decreased Rac-1 expression associated with the anti-metastatic activity on highly metastasis breast cancer cells.
Fig. 4.
The activity of MMP-9 and the expression of Rac-1 after CL treatment on MDAMB-231 breast cancer cells. (A) The MMP activity of MMP-9 was visualized as a clear band against the dark blue background of the gelatine substrate in the gel. Molecular weight markers were used to estimate the molecular masses of the pro and active forms of MMP-9. Blots were scanned and intensities of the bands were measured by ImageJ software, normalized with untreated, and plotted; (B) percentage gene expression of MMP-9 activity was measured by qRT-PCR, and (C) Percentage of Rac-1 gene expression level of cell migration-related proteins was measured by qRT-PCR. Data are presented as mean + SEM, N = 3. *P < 0.05 Indicates significant differences in comparison with the untreated cells. CL, Curcuma longa; MMP-9, matrix metalloproteinase-9; Rac-1, Ras-related C3 botulinum toxin substrate 1; qRT-PCR, quantitative real-time polymerase chain reaction.
DISCUSSION
The formation of metastases is a cascade of closely consecutive molecular and morphological changes, including the reconstruction of the cytoskeleton and extracellular matrix (ECM) (20). MMPs particularly MMP-2 and MMP-9 are responsible for the degradation of several ECM components associated with tumor metastasis and invasiveness (21,22). Furthermore, Rac-1 a member of Rho family GTPases specifically involved in metastasis through the production of sheet-like protrusions known as lamellipodia leading to metastasis (23,24,25). The inhibition of Rac1-mediated lamellipodia formation blocks the migration and invasion of cancer cells (26). Interestingly, Rac-1 in the interaction with Bcl-2 induced apoptosis through inhibition of S70pBcl-2 (27). This suggests that the inhibition of Rac-1 and MMPs plays an important role in breast cancer metastasis which can also indirectly induce apoptosis in breast cancer cells. CL has cytotoxic effects on some cancer cells, but has no toxic effects on normal cells, indicating the selectivity of CL towards cancer cells.
A previous study by Al-Amin et al. stated that chloroform fraction from Curcuma caesia contains furanodienone, curcuzederone, (1S, 10S), (4S,5S)-germacrone-1, 4-diepoxide, wenyujinin B, alismoxide, aerugidiol, zedoalactone B, zedoarondiol, isozedoarondiol. The study demonstrated some of the compounds are bioactive compounds and the effect of curcuzederone on MDA-MB-231 cell migration showed significant inhibition in scratch and Transwell migration assays (28).
Another study by Gao et al. reporting the effect of petroleum ether extracts of Curcuma zedoaria on the proliferation of human triple-negative breast cancer cell line MDA-MB-231 showed that MDA-MB-231 cells were inhibited by petroleum ether extracts of Curcuma zedoaria, and the inhibition rate depended on concentrations and time. Petroleum ether extracts of Curcuma zedoaria as well as epirubicin significantly arrest the cell cycle at the G0/G1 phase. The level of mRNA expression of proteins E-cadherin and E-cadherin significantly increased, while SDF-1, CCR7, and CXCR4 mRNA expression decreased after being incubated with petroleum ether extracts of Curcuma zedoaria. The differences were that the protein CXCR4 mRNA expression level was higher than the vehicle. This study stated that MDA-MB-231 cells’ proliferation was inhibited by petroleum ether extracts of Curcuma zedoaria (29).
In the current study, we explored the potency of CL extract against the highly metastatic MDAMB-231 breast cancer cell. Recently, metastasis has still been highlighted as one of the main problems in cancer cure. Therefore, suppression of cancer migration is urgent and necessary. Cytotoxic activity of CL in several cancer cells and a side effect of chemotherapy-induced metastasis through the activation of the TGF-β pathway have been reported in several articles (4,30-32). Regardless of the potent cytotoxic activity of CL, the anti-metastatic mechanism of CL to inhibit migration has not yet been investigated.
The hallmark of the current work is that CL exhibited a strong cytotoxic effect in a concentration-dependent manner with the IC50 value of 13 μg/mL. Interestingly, we also found that CL induced elevation of ROS levels up to 2.5 folds than untreated cells. ROS recently gained much attention for its role in cancer progression. ROS is physiologically generated from metabolism activities, therefore, over-proliferated cells like cancer cells generate higher levels of ROS (33,34). Cancer cells have mechanisms to shift the redox balance to maintain a high level of ROS such as overexpressing ROS metabolic enzymes (34). On the contrary, highly metastatic cancer cells require a low level of ROS to maintain their metastasis ability (33). The low level of ROS is achieved by keeping the ROS production low and/or enhancing the ROS scavenging system (35). Our study revealed that CL significantly enhanced the ROS levels. Similar to a previous study that curcumin induced ROS levels in MCF-7 cells (36,37). Furthermore, we explored the underlying mechanism involved in the cytotoxic effect of CL. We found that CL significantly induced pro-apoptotic protein Bax gene expression and inhibited anti-apoptotic protein Bcl-2 in a concentration-dependent manner compared with the untreated cells. CL was also reported to cause A549 lung cancer and Hela cervix cancer cell death through apoptosis induction (38,39). In addition, apoptosis induction by CL was reported due to its ability to regulate p53 signaling (40). Moreover, CL treatment caused cell accumulation in the G2/M arrest by blocking the transition of cells from S to G2 phases, thereby consequently leading to apoptosis (41,42). These findings indicated that the CL treatment inhibited MDAMB-231 cell growth through the regulation of apoptosis protein and elevated ROS levels. Previous studies reported that the apoptosis phenomenon with the increasing doses of CL led to the G2/M phase cell cycle arrest causing ROS-mediated apoptosis in human gall bladder carcinoma and osteosarcoma MG-63 cells (41,43).
One of the interesting aspects of CL is to inhibit migration. In this study, we focused on evaluating the mechanism of CL in inhibiting cell migration that was confirmed through several mechanisms including inducing wound closure, MMP-9 protein expression, and Rac-1 gene expression after cells were treated with CL. The presence of CL significantly reversed the inhibition of wound closure and decreased MMP-9 and Rac-1 gene expression. A previous study has reported inhibited migration and invasion under CL administration by suppressing TGF-β and circulating cancer cells on lung metastatic breast cancer cells (44). MMP-9 activity is associated with cancer occurrence and development, curcumin inhibited MMP-9 activity by regulating the integrin pathway and proteolytic cleavage in human breast cancer cells (4,45).
Our study showed that CL significantly inhibited Rac-1 gene expression. Inhibition of Rac-1 leads to inhibition of migration. These indicated that the anti-metastatic effect of CL may be dependent on Rac-1 and MMP-9 gene expression.
CONCLUSION
To summarize, CL attenuated the metastatic ability of highly metastatic breast cancer cells by reducing MMP-9 and Rac1 expression. Collectively, these findings also indicated that CL as a potentially natural chemotherapeutic agent could be used to reduce cancer migration and prevent cancer recurrence.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contributions
All authors contributed equally to this work.
Acknowledgments
The authors express their great appreciation for the financial support received for this work from the Ministry of Education and Culture Republic of Indonesia (Grant No. PDUPT) Fundamental Research 2022.
REFERENCES
- 1.Institute for Quality and Efficiency in Health Care (IQWiG) Metastatic breast cancer: treatments and possible consequences. 2013. Available at: https://www.ncbi.nlm.nih.gov/books/NBK361017/
- 2.Arrieta Ó, Medina LA, Estrada-Lobato E, Hernández-Pedro N, Villanueva-Rodriguez G, Martínez-Barrera L, et al. First-line chemotherapy with liposomal doxorubicin plus cisplatin for patients with advanced malignant pleural mesothelioma: phase II trial. Br J Cancer. 2012;106(6):1027–1032. doi: 10.1038/bjc.2012.44. DOI: 10.1038/bjc.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amalina N, Nurhayati I, Meiyanto E. Doxorubicin induces lamellipodia formation and cell migration. Indones J Cancer Chemoprevent. 2017;8(2):61–66. DOI : 10.14499/indonesianjcanchemoprev8iss2pp61-67. [Google Scholar]
- 4.Bandyopadhyay A, Wang L, Agyin J, Tang Y, Lin S, Yeh I, et al. Doxorubicin in combination with a small TGFβ inhibitor: a potential novel therapy for metastatic breast cancer in mouse models. PLoS One. 2010;5(4):1–13. doi: 10.1371/journal.pone.0010365. e10365. DOI: 10.1371/journal.pone.0010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian B, et al. Real-time imaging reveals local, transient vascular permeability and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 2015;5(9):932–943. doi: 10.1158/2159-8290.CD-15-0012. DOI: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer:an overview. Cancers (Basel) 2014;6(3):1769–1792. doi: 10.3390/cancers6031769. DOI: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenie RI, Handayani S, Susidarti RA, Udin LZ, Meiyanto E. The cytotoxic and antimigratory activity of Brazilin-doxorubicin on MCF-7/HER2 cells. Adv Pharm Bull. 2018;8(3):507–516. doi: 10.15171/apb.2018.059. DOI: 10.15171/apb.2018.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jambunathan S, Bangarusamy D, Padma P, Sundaravadivelu S. Cytotoxic activity of the methanolic extract of leaves and rhizomes of Curcuma amada Roxb against breast cancer cell lines. Asian Pac J Trop Med. 2014;7(S1):S405–S409. doi: 10.1016/S1995-7645(14)60266-2. DOI: 10.1016/S1995-7645(14)60266-2. [DOI] [PubMed] [Google Scholar]
- 9.Paramita D, Hermansyah D, Paramita D, Amalina N. Regulation of p53 and survivin by Curcuma longa extract to caspase-3 dependent apoptosis in triple negative breast cancer cells. Med Glas (Zenica) 2022;19(2):189–196. doi: 10.17392/1453-22. DOI: 10.17392/1453-22. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Shafy S, Alanazi AD, Gabr HSM, Allam AM, Abou-Zeina HAA, Masoud RA, Soliman DE, Alshahrani MY. Efficacy and safety of ethanolic Curcuma longa extract as a treatment for sand tampan ticks in a rabbit model. Vet World. 2020;13(4):812–20. doi: 10.14202/vetworld.2020.812-820. DOI: 10.14202/vetworld.2020.812-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. DOI: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Mursiti S, Amalina N, Marianti A. Inhibition of breast cancer cell development using citrus maxima extract through increasing levels of reactive oxygen species (ROS) J Phys Conf Ser. 2021;1918(5):1–6. 052005. DOI: 10.1088/1742-6596/1918/5/052005. [Google Scholar]
- 13.Amalina N, Wahyuni S, Harjito Cytotoxic effects of the synthesized Citrus aurantium peels extract nanoparticles against MDA-MB-231 breast cancer cells. J Phys Conf Ser. 2021;1918(3):1–6. 032006. DOI: 10.1088/1742-6596/1918/3/032006. [Google Scholar]
- 14.Ikawati M, Jenie RI, Utomo RY, Amalina ND, Nur Ilmawati GP, Kawaichi M, et al. Genistein enhances cytotoxic and antimigratory activities of doxorubicin on 4T1 breast cancer cells through cell cycle arrest and ROS generation. J Appl Pharm Sci. 2020;10(10):95–104. DOI: 10.7324/JAPS.2020.1010011. [Google Scholar]
- 15.Jenie RI, Amalina ND, Ilmawati GPN, Utomo RY, Ikawati M, Khumaira A, et al. Cell cycle modulation of CHO-K1 cells under genistein treatment correlates with cells senescence, apoptosis and ROS level but in a dose-dependent manner. Adv Pharm Bull. 2019;9(3):453–461. doi: 10.15171/apb.2019.054. DOI: 10.15171/apb.2019.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riis S, Newman R, Ipek H, Andersen J, Kuninger D, Boucher S, et al. Hypoxia enhances the wound-healing potential of adipose-derived stem cells in a novel human primary keratinocyte-based scratch assay. Int J Mol Med. 2017;39(3):587–594. doi: 10.3892/ijmm.2017.2886. DOI: 10.3892/ijmm.2017.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzery M, Cahyono B, Amalina ND. Citrus sinensis (L) peels extract inhibits metastasis of breast cancer cells by targeting the downregulation matrix metalloproteinases-9. Open Access Maced J Med Sci. 2021;9(B):464–469. DOI: 10.3889/oamjms.2021.6072. [Google Scholar]
- 18.Tajhya R, Patel R, Beeton C. Detection of matrix metalloproteinases by zymography. Methods Mol Biol. 2017;1579:231–244. doi: 10.1007/978-1-4939-6863-3_12. DOI: 10.1007/978-1-4939-6863-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2^X-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3(3):71–85. PMID: 25558171. [PMC free article] [PubMed] [Google Scholar]
- 20.Chan Z, Oentaryo M, Lee C. MMP-mediated modulation of ECM environment during axonal growth and NMJ development. Neurosci Lett. 2020;724:1–13. doi: 10.1016/j.neulet.2020.134822. 134822. DOI: 10.1016/j.neulet.2020.134822. [DOI] [PubMed] [Google Scholar]
- 21.Walker C, Mojares E, Hernández ADR. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19(10):1–31. doi: 10.3390/ijms19103028. 3028. DOI: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paolillo M, Schinelli S. Extracellular matrix alterations in metastatic processes. Int J Mol Sci. 2019;20(19):1–18. doi: 10.3390/ijms20194947. 4947. DOI: 10.33 90/ij ms20194947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. DOI: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 24.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–529. doi: 10.1016/j.tcb.2006.08.006. DOI: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Hernández E, Mota-Peynado A, Dharmawardhane S, Vlaar C. Novel inhibitors of Rac1 in metastatic breast cancer. P R Health Sci J. 2010;29(4):348–356. PMID: 21261173. [PubMed] [Google Scholar]
- 26.Akbar H, Cancelas J, Williams D, Zheng J, Zheng Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Meth Enzymol. 2006;406(20):554–565. doi: 10.1016/S0076-6879(06)06043-5. DOI: 10.1016/S0076-6879(06)06043-5. [DOI] [PubMed] [Google Scholar]
- 27.Chong S, Lai J, Qu J, Hirpara J, Kang J, Swaminathan K, et al. A feedforward relationship between active Rac1 and phosphorylated Bcl-2 is critical for sustaining Bcl-2 phosphorylation and promoting cancer progression. Cancer Lett. 2019;457:151–167. doi: 10.1016/j.canlet.2019.05.009. DOI: 10.1016/j.canlet.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Al-Amin M, Eltayeb NM, Khairuddean M, Salhimi SM. Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231. Nat Prod Res. 2021;35(18):3166–3170. doi: 10.1080/14786419.2019.1690489. DOI: 10.1080/14786419.2019.1690489. [DOI] [PubMed] [Google Scholar]
- 29.Gao X, Li Q, Li H, Zhang H, Su J, Wang B, et al. Extracts from Curcuma zedoaria inhibit proliferation of human breast cancer cell MDA-MB-231 in vitro. Evid Based Complementary Altern Med. 2014;2014:1–9. doi: 10.1155/2014/730678. 730678. DOI: 10.1155/2014/730678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amalina ND, Salsabila IA, Zulfin UM, Jenie RI, Meiyanto E. In vitro synergistic effect of hesperidin and doxorubicin downregulates epithelial-mesenchymal transition in highly metastatic breast cancer cells. J Egypt Natl Canc Inst. 2023;35(1):6–13. doi: 10.1186/s43046-023-00166-3. DOI: 10.1186/s43046-023-00166-3. [DOI] [PubMed] [Google Scholar]
- 31.Cincin Z, Kiran B, Baran Y, Cakmakoglu B. Hesperidin promotes programmed cell death by downregulation of nongenomic estrogen receptor signalling pathway in endometrial cancer cells. Biomed Pharmacother. 2018;103:336–345. doi: 10.1016/j.biopha.2018.04.020. DOI: 10.1016/j.biopha.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Suzery M, Cahyono B, Amalina ND. Antiproliferative and apoptosis effect of hyptolide from Hyptis pectinata (L) Poit on human breast cancer cells. J Appl Pharm Sci. 2020;10(2):1–6. DOI: 10.7324/JAPS.2020.102001. [Google Scholar]
- 33.Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16(11):1215–1228. doi: 10.1089/ars.2012.4529. DOI: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S, Kim H, Seo Y. Understanding of ROS-inducing strategy in anticancer therapy. Oxid Med Cell Longev. 2019;2019:1–12. doi: 10.1155/2019/5381692. 5381692. DOI: 10.1155/2019/5381692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding S, Li C, Cheng N, Cui X, Xu X, Zhou G. Redox regulation in cancer stem cells. Oxid Med Cell Longev. 2015;2015:1–11. doi: 10.1155/2015/750798. 750798. DOI: 10.1155/2015/750798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prathyusha E, Prabakaran A, Ahmed H, Dethe MR, Agrawal M, Gangipangi V, et al. Investigation of ROS generating capacity of curcumin-loaded liposomes and its in vitro cytotoxicity on MCF-7 cell lines using photodynamic therapy. Photodiagnosis Photodyn Ther. 2022;40:103091. doi: 10.1016/j.pdpdt.2022.103091. DOI: 10.1016/j.pdpdt.2022.103091. [DOI] [PubMed] [Google Scholar]
- 37.Liu JL, Pan YY, Chen O, Luan Y, Xue X, Zhao JJ, et al. Curcumin inhibits MCF-7 cells by modulating the NF-κB signaling pathway. Oncol Lett. 2017;14(5):5581–5584. doi: 10.3892/ol.2017.6860. DOI: 10.3892/ol.2017.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu MF, Huang YH, Chiu LY, Cherng SH, Sheu GT, Yang TY. Curcumin induces apoptosis of chemoresistant lung cancer cells via ROS-regulated p38 MAPK phosphorylation. Int J Mol Sci. 2022;23(15):8248–8256. doi: 10.3390/ijms23158248. DOI: 10.3390/ijms23158248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu C, Yang B, Najafi M. Targeting of cancer cell death mechanisms by curcumin: implications to cancer therapy. Basic Clin Pharmacol Toxicol. 2021;129(6):397–415. doi: 10.1111/bcpt.13648. DOI: 10.1111/bcpt.13648. [DOI] [PubMed] [Google Scholar]
- 40.Li P, Pu S, Lin C, He L, Zhao H, Yang C, et al. Curcumin selectively induces colon cancer cell apoptosis and S cell cycle arrest by regulates Rb/E2F/p53 pathway. J Mol Struct. 2022;1263:1–9. 133180. DOI: 10.1016/j.molstruc.2022.133180. [Google Scholar]
- 41.Hu A, Huang JJ, Zhang JF, Dai WJ, Li RL, Lu ZY, et al. Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway. Oncotarget. 2017;8(31):50747–50760. doi: 10.18632/oncotarget.17096. DOI: 10.18632/oncotarget.17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali NM, Yeap SK, Abu N, Lim KL, Ky H, Pauzi AZM, et al. Synthetic curcumin derivative DK1 possessed G2/M arrest and induced apoptosis through accumulation of intracellular ROS in MCF-7 breast cancer cells. Cancer Cell Int. 2017;17:1–12. doi: 10.1186/s12935-017-0400-3. 30. DOI: 10.1186/s12935-017-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu TY, Tan ZJ, Jiang L, Gu JF, Wu XS, Cao Y, et al. Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int. 2013;13(1):1–9. doi: 10.1186/1475-2867-13-64. 64. DOI: 10.1186/1475-2867-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas S, Guix M, Rinehart C, Dugger T, Chytil A, Moses H, et al. Inhibition of TGF beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117(5):1305–1313. doi: 10.1172/JCI30740. DOI: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartsch J, Staren E, Appert H. Matrix metalloproteinase expression in breast cancer. J Surg Res. 2003;110(2):383–392. doi: 10.1016/s0022-4804(03)00007-6. DOI: 10.1016/s0022-4804(03)00007-6. [DOI] [PubMed] [Google Scholar]