Abstract
Background and purpose:
Cholestasis is caused by a malfunction of the biliary liver system. Oxidative stress plays an essential role in the progression of cholestasis. This study aimed to investigate the antioxidant and hepatoprotective effects of ethanolic extract of Juniperus excelsa M. Bieb (JE) fruits on hepatic impairment induced by bile duct ligation (BDL) in rats.
Experimental approach:
Forty male Wistar rats were randomly divided into 4 groups; sham control + vehicle (SC), BDL + vehicle (BDL), BDL + JE extract (BDL + JE), and SC + extract (SC + JE). One day after surgery, the animals were treated with vehicle or ethanolic extract of JE (500 mg/kg/day) for 7 days. Finally, the blood was taken for biochemical and oxidative stress analysis. Furthermore, the liver tissue of rats was removed for histological examination.
Findings/Results:
Treatment with the extract of JE decreased the ALP level, whereas it enhanced total protein content compared to the BDL group. Also, JE increased the activity of SOD and GPx, as well as FRAP content compared to the BDL group; while it did not significantly affect the levels of MDA and inflammation markers. However, JE could not improve BDL-induced histopathological alterations in hepatic tissue.
Conclusion and implication:
This study demonstrated that JE may be useful as an adjuvant therapy by attenuating ALP activity, increasing serum total protein and FRAP content, as well as improving the antioxidant enzymes activity of SOD and GPx. However, further research is warranted to explore the other underlying mechanisms of action.
Keywords: Anti-inflammatory, Antioxidant, Bile duct ligation, Juniperus excelsa, Liver, Rat
INTRODUCTION
The liver is the largest organ in the body. It contributes to the glucose balance, bile generation, inactivation and detoxification of several factors, and management of immune reactions (1). The most common liver diseases include jaundice or obstructive cholestasis, liver cirrhosis, hepatitis, fatty liver diseases, and cancers (2). Cholestasis is characterized by dysfunction of bile secretion and/or bile flow after the accumulation of toxic bile acids in hepatocytes (3). Cholestasis may be caused by various drugs, familial cholestatic disturbances, obstructive cholestasis, primary biliary cirrhosis, extrahepatic biliary atresia, oral contraceptive drugs, and sepsis (4).
Bile duct ligation (BDL) is a reliable experimental model for induction of cholestasis (5). The plasma levels of major liver enzymes such as alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) elevated following BDL-induced hepatocyte injury in rats (6).
Oxidative stress plays an essential role in the progression of cholestasis and disrupts the production and secretion of bile salts in the bile ducts (7). The level of oxidative stress can be measured by assessing the production of malondialdehyde (MDA) levels (8). Three major antioxidant enzymes of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) are the first-line defense antioxidant against reactive species in cells (9). Proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) have a critical role in the exacerbation of hepatic fibrosis (10). On the other hand, it has been reported that interleukin 10 (IL-10) leads to a disturbance in bacterial clearance in BDL (11).
Today, herbal medicine is in great demand throughout the world for the remedy of multiple diseases (12). The genus Juniperus is one of the chief categories of the Cupressaceae family (13,14). There are approximately 70 species of Juniperus all over the world (15). It is growing in many countries such as Greece, Bulgaria, Turkey, Syria, Iran, Pakistan, and Oman (16). The habitat of this plant in Iran is mountainous areas, including Alborz and Zagros mountains (17,18).
The main chemical compounds of Juniperus excelsa (JE) leaves are monoterpene, hydrocarbons, flavonoids, flavonoid glycosides, and tannins (19).
The antioxidant function of JE extract has been revealed (20). In addition, the therapeutic effects of Juniperus species in diabetes, intestinal worms, regenerating of wounds, and cure or protection of hepatic disturbances (21) and liver reperfusion injury have been reported (22). To the best of our knowledge, the effects of JE fruits on BDL-induced liver damage have not been evaluated. Therefore, in this study, we aimed to investigate the hepatoprotective effects of ethanolic fruit extract of JE on BDL-induced injuries in rats.
MATERIALS AND METHODS
Plant material and extraction
The fruits of JE were collected from the mountains around the city of Kakan-Kohgivieh and Boyer-Ahmad-Iran (altitude: 1800 m above sea level) and were identified in the herbarium section of the Faculty of Pharmacy of Tehran University of Medical Sciences-Iran (Voucher No. 6859 TEH). After collection, the juniper fruits are cleaned and placed in room air away from direct light for several days to dry, then chopped and prepared. One hundred grams of the dried plant was soaked in 1000 mL of solvent (70% ethanol). The resulting mixture was kept at 37 °C for 48 h and then filtered. The mixture was concentrated in vacuo at -40 °C using a rotary evaporator, and the obtained extract was dried and frozen at -20 °C (23).
Animals
Male Wistar rats weighing 200-250 g were obtained from Shahrekord (Shahrekord, Iran) and acclimatized for one week before the experiment. Animals were housed in an air-conditioned room with 12/12-h light/dark cycles under a controlled temperature (22 ± 2 °C). The rats were housed in cages, fed with a standard rat chow, tap water ad libitum, and fasted overnight before the procedures, but were given free access to water. The study followed the ARRIVE guidelines, and all protocols were done under the Ethics Committee of Yasuj University of Medical Sciences (Ethical code: IR.YUMS.REC.1400.045).
Experimental design study
In this study, 40 rats were randomly assigned to the following four groups (n = 10 each):
Group 1: Sham-control (SC), rats experienced laparotomy without BDL surgery; group 2: rats experienced BDL surgery alone; group 3: JE, rats received an ethanolic extract of JE fruits at 500 mg/kg for seven days through gavage (24); group 4: BDL + JE, rats received an ethanolic extract of JE fruits at 500 mg/kg for seven days through gavage and then underwent BDL surgery.
BDL was induced under general anesthesia by a combination of ketamine HCl (50 mg/kg) and xylazine HCl (10 mg/kg), injected intraperitoneally (25). Briefly, after a midline incision in the abdomen, the common bile duct was ligated and the bile duct was cut between the ligatures (26). One day after surgery, the animals received 0.5 mL of normal saline/kg (27) or JE (500 mg/kg/day) for 7 consecutive days orally. At the end of the protocol, the rats were killed by puncturing the heart under anesthesia and the blood was taken. Furthermore, the liver tissue of rats was removed and divided into two pieces; one piece was administered for preparing homogenate tissue and the other piece was fixed in 10% formalin for hepatic histologic studies.
Biochemical analysis
To assess the serum levels of ALT, AST, ALP, total bilirubin, total proteins, and oxidative stress markers, blood was collected from the heart without the use of anticoagulant and allowed to clot before being centrifuged at 5000 g for 10 min (28). Then, the above-mentioned parameters were measured by commercial assay kits (Pars Azmoon Co. Iran), and read by a biochemical autoanalyzer.
Preparation of liver homogenate tissue
For evaluation of oxidative stress parameters, one piece of liver tissue was quickly removed, weighed, and homogenized with a homogenizer in ice-cold phosphate-buffered saline solution (PBS; 50 mM, pH 7.4). It centrifuged for 10 min at 10000 g at 4 °C and the supernatant was maintained at -20 °C to determine the oxidative stress parameters as described below (29).
Assay of ferric-reducing antioxidant plasma/power
In this method, ferric reducing antioxidant plasma/power (FRAP) was measured based on the ability of plasma or liver tissue to homogenate in the restoration of ferric ion (Fe3+) to ferrous ion (Fe2+) in the presence of tripyridyl-s-triazine (TPTZ) reagent at low pH. Blue resulting color intensity was determined spectrophotometrically at a wavelength of 593 nm (30). The FRAP content was expressed as μmol/L for plasma and μmol/g for tissue.
Assay of total thiol
To assay total thiol (TSH), the tissue homogenate was treated with 5,5'-dithiols-(2-nitrobenzoic acid (DTNB) reagents. Then, it was vortexed gently and the absorbance was determined at 412 nm (31).
Assay of carbonyl protein
The concentration of serum protein carbonyl (PCO) was measured using a spectrophotometric method (32). In summary, serum samples were treated with 2,4dinitrophenylhydrazine (DNPH). After treatment with trichloroacetic acid, the sedimentation was washed with ethanol: ethyl acetate (1:1, v/v) and dissolved in guanidine hydrochloride (29).
Assay of MDA
In this method, a total of 0.5 mL of tissue homogenate or serum was mixed separately with 2 mL of a solution containing thiobarbituric acid (15% w/v), trichloroacetic acid (.375% w/v), and 0.25 N HCl. The content was heated in a water bath for 15 min, then cooled for 10 min in ice water, and then centrifuged for 15 min at 2000 g. The absorbance was read at 535 nm and expressed as μmol/g for tissue and μmol/L for serum (31).
Assay of antioxidant enzyme activity
The activity of antioxidant enzymes of SOD, GPx, and CAT in the homogenate tissue was calorimetrically determined according to the manufacturer’s instructions of commercial kits (Nagpix, Iran) using an ELISA reader (Biotek, Netherlands) and presented as U/mL.
Histopathological analysis
For histopathological evaluation, the liver tissue was removed and fixed in a 10% formalin solution. Then, automatically dehydrated through a graded series of alcohols, and embedded in paraffin. Afterward, cut into 5 μm sections using a microtome, and then stained with hematoxylin and eosin (H&E) (33).
Statistical analysis
The data were analyzed by SPSS 19 and shown as mean ± SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Tukey post hoc analysis test. The P-values < 0.05 were considered statistically significant.
RESULTS
Effect of JE on biochemical parameters in BDL rats
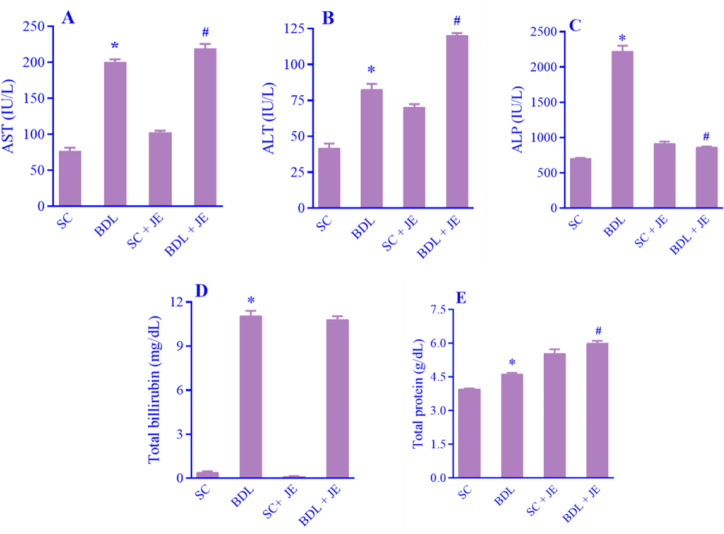
As shown in Fig. 1A-E, following BDL-induced hepatic injury the plasma levels of AST, ALP, ALT, total bilirubin, and total protein markedly increased in the BDL group in comparison with the SC group. Treatment with the ethanolic extract of JE significantly decreased the plasma level ALP, whereas it significantly enhanced the total protein level in rats in comparison to the BDL group. There was no significant alteration in the plasma concentration of total bilirubin relative to the BDL group (P > 0.05).
Fig. 1.
Effects of ethanolic extract of JE (500 mg/kg, 7 consecutive days, gavage) on serum levels of liver enzymes of (A) AST, (B) ALT, (C) ALP, (D) total bilirubin, and (E) total protein following BDL-induced liver damage in rats. Data are expressed as mean ± SEM, n = 10. *P < 0.05 Indicates significant difference in comparison with the SC group; #P < 0.05 versus the BDL group. SC, Sham control; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BDL, bile duct ligation; JE, Juniperus excelsa.
Effect of JE on serum oxidative stress markers in BDL rats
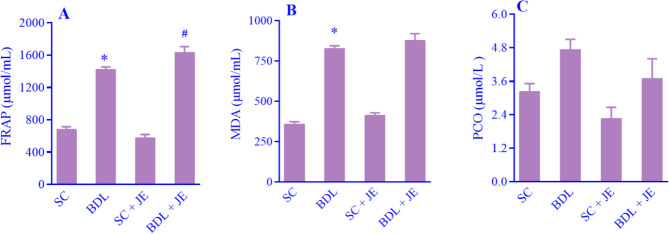
As shown in Fig. 2A-C, following BDL-induced hepatic injury the serum levels of FRAP and MDA significantly increased in the BDL rats when compared with the SC group. Treatment with the ethanolic extract of JE dramatically enhanced the level of FRAP in comparison with the BDL group, while treatment with JE had no significant effect on the serum level of MDA relative to the BDL group (P > 0.05). As indicated, there was no significant change in serum level of PCO (P > 0.05).
Fig. 2.
Effects of ethanolic extract of JE (500 mg/kg, 7 consecutive days, gavage) on the level of (A) FRAP, (B) MDA, and (C) PCO after BDL-induced liver injury in rats. Data are expressed as mean ± SEM, n = 10. *P < 0.05 Indicates significant difference in comparison with the SC group; #P < 0.05 versus the BDL group. SC, Sham control; FRAP, ferric reducing antioxidant plasma; MDA, malondialdehyde; PCO, protein carbonyl; BDL, bile duct ligation; JE, Juniperus excelsa.
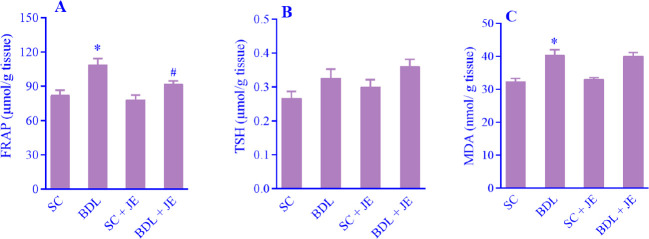
Effect of JE on tissue oxidative stress markers in BDL rats
As indicated in Fig. 3A-C, following BDL surgery in rats the concentrations of FRAP and MDA in liver tissue significantly increased in the BDL rats in comparison with the SC group. Treatment with the ethanolic extract of JE remarkably decreased the level of FRAP in comparison with the BDL group, while pretreatment with JE had no significant effect on the tissue level of MDA relative to the BDL group (P > 0.05). As indicated, there was no significant change in the level of TSH in liver tissue in rats (P > 0.05).
Fig. 3.
Effects of ethanolic extract of JE (500 mg/kg, 7 consecutive days, gavage) on the tissue concentrations of (A) FRAP, (B) TSH, and (C) MDA after BDL-induced liver injury in rats. Data are expressed as mean ± SEM, n = 10. *P < 0.05 Indicates significant difference in comparison with the SC group; #P < 0.05 versus the BDL group. SC, Sham control; FRAP, ferric reducing antioxidant power; TSH, total thiol; MDA, malondialdehyde; BDL, bile duct ligation; JE, Juniperus excelsa.
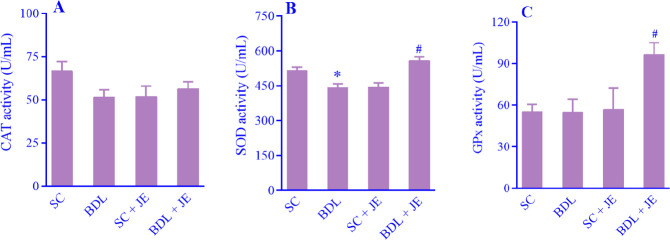
Effect of JE on antioxidant enzyme activity in BDL rats
As shown in Fig. 4A-C, following BDL-induced liver damage in rats the activity of the antioxidant enzyme of SOD significantly decreased in the BDL group when compared with the SC group. Treatment with the ethanolic extract of JE significantly enhanced the activity of SOD in comparison with the BDL group. BDL induction had no significant effect on CAT and GPx in rats (P > 0.05). Treatment with JE significantly increased the activity of GPx in liver tissue as compared with the BDL group.
Fig. 4.
Effects of ethanolic extract of JE (500 mg/kg, 7 consecutive days, gavage) on serum levels of (A) CAT, (B) SOD, and (C) GPx following BDL-induced hepatic damage. Data are expressed as mean ± SEM, n = 10. *P < 0.05 Indicates significant difference in comparison with the SC group; #P < 0.05 versus the BDL group. SC, Sham control; CAT, catalase; SOD, superoxide dismutase; GPx, glutathione peroxidase; BDL, bile duct ligation; JE, Juniperus excelsa.
Effect of JE on cytokine markers in BDL rats
As shown in Fig. 5 BDL-induced liver damage in rats had no significant effect on the levels of cytokine markers such as TNF-α, IL-10, and IL-6 in comparison with the SC group (P > 0.05). Treatment with the ethanolic extract of JE did not significantly affect the above-mentioned parameters when compared to the BDL group (p > 0.05).
Fig. 5.
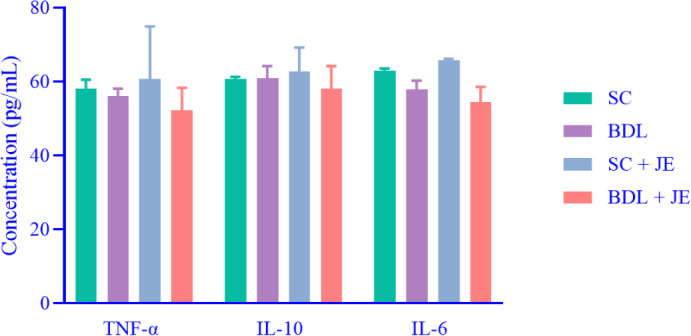
Effect of JE on cytokine markers including TNF-α IL-10, and IL-6 in bile-duct-ligated rats. Data are expressed as mean ± SEM, n = 10. TNF-α Tumor necrosis factor-alpha; IL, interleukin; SC, sham control; JE, Juniperus excelsa.
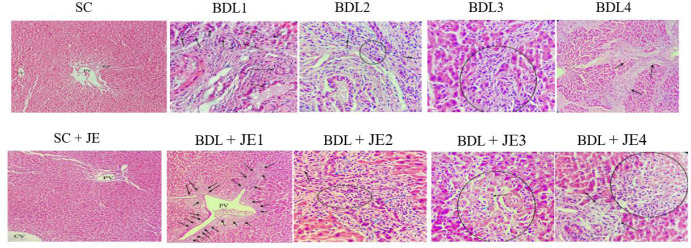
Effect of JE on histopathological changes in BDL rats
As indicated in Fig. 6, our experimental model of BDL-induced hepatic cholestasis led to remarkable histopathological changes in the BDL group such as bile duct proliferation, moderate portal inflammation, mild/moderate piecemeal necrosis (inflammation extending into the adjacent parenchyma) in some portal area and fibrosis of some portal tract. While the views of SC and SC + JE groups were relatively normal hepatic lobule and portal triad structure. The view of the BDL + JE group was severe BD proliferation (ductular reaction) in some portal areas, severe BD proliferation associated with moderate portal inflammation in some portal areas, focal inflammation with hepatocyte necrosis in hepatic tissue, and piecemeal necrosis portal tract. Therefore, JE fruits could not effectively improve BDL-induced histopathological alterations in hepatic tissue.
Fig. 6.
Effects of ethanolic extract of JE on histopathological changes of BDL-induced cholestasis (hematoxylin and eosin staining) in rats. SC group view: relatively normal hepatic lobule and portal triad structure. BDL group view: severe BD proliferation (BDL1, black arrow, 200×), moderate portal inflammation (BDL2, circle/arrow, 400×), mild/moderate piecemeal necrosis (BDL3, circle, 400×), and fibrosis of some portal tract (BDL4, arrow, 200×). JE group view: relatively normal hepatic lobule and portal triad structure (SC + JE, 200×). BDL + JE group view: severe BD proliferation (BDL + JE1, arrow, 200×), severe BD proliferation associated with moderate portal inflammation (BDL + JE2, blue/black arrows and circle, 400×), focal inflammation (BDL + JE3, blue arrow) with hepatocyte necrosis (BDL + JE3, black arrow/circle. 400×) and mild piecemeal necrosis portal tract (BDL + JE4, arrow/circle, 400×). SC, Sham control; PV, portal vein; BD, bile duct; CV, central vein; BDL, bile duct ligation; JE, Juniperus excels.
DISCUSSION
The present study demonstrated that BDL-induced cholestasis plays a pivotal role in liver damage by enhancing liver function tests, oxidative stress markers, and significant histopathological changes. Furthermore, we showed that the ethanolic extract of JE has hepatoprotective properties against BDL-induced cholestasis in rats through attenuating ALP level, increasing the SOD activity and the FRAP level, as well as fairly alleviating the histopathological alterations in hepatic tissue.
The BDL experimental model has been used extensively to investigate cholestatic liver disease and is associated with oxidative stress, inflammation, and fibrosis (34). Serum levels of liver enzymes ALT, AST, and ALP are measured to track liver damage so that their increased levels can be attributed to liver cell dysfunction (35). Several studies showed a remarkable elevation in the serum levels of the above mentioned biomarkers associated with significant histopathological changes after BDL surgery (6,36). For instance, one study showed that histopathological changes were associated with an elevation in serum level of ALP during BDL surgery (37). This elevation may be related to the accumulation of bile salts that leads to cell membrane injury and eventually releases the ALP enzyme into the blood (38). In the current study, we observed a significant increase in serum levels of ALT, AST, and ALP following BDL-induced liver damage.
Data from the present investigation showed that treatment with ethanolic extract of JE has a partially protective effect on BDL-induced hepatic injury through attenuating serum levels of ALP when compared to the BDL group. In this regard, it is reported that J. phoenicea berries attenuated the level of plasma ALP after CCl4-induced liver injury (39). On the other hand, it has been demonstrated that ethyl acetate fraction of J. communis leaves had a significantly decreased effect on the increased serum level of ALP and, in contrast, this agent had a significantly decreased effect on the enhanced serum levels of AST and ALT following acetaminophen-induced liver injury (27). Moreover, it has been reported that the administration of ethanolic fruit extract of Solanum xanthocarum associated with J. communis on hepatotoxicity induced by co-administration of paracetamol and azithromycin not only returned the biochemical parameters to near-normal levels but also improved the histopathological changes in the liver tissue of rats (40). Earlier reports showed that hyperbilirubinemia is associated with cholestasis-induced liver injury (41,42). Inconsistent, our data showed that bilirubin as an important and valid biochemical index of bile flow disruption increased following BDL-induced liver damage and treatment with ethanolic extract of JE had no remarkable effect on the level of total bilirubin versus the BDL group.
A previous study indicated that total protein was significantly reduced in the acute phase (week 1) of BDL induction (43). In contrast, our data showed that total protein significantly increased beyond BDL induction (after 7 days) and treatment with ethanolic extract of JE dramatically increased the serum total protein in BDL rats. In this regard, it is reported that stimulation of protein synthesis is considered the hepatoprotective mechanism that promotes the regeneration activity of hepatocytes (44).
Oxidative stress plays an important role in cholestatic hepatic damage (45). Oxidative stress results from an imbalance between the generation of reactive oxygen species (ROS) and antioxidant systems (46) and is associated with increasing the levels of ROS and lipid peroxidation. Peroxidation of lipids leads to remarkable changes in the membrane structure and cell death (40). Numerous studies have shown that the level of MDA, a major lipid peroxidation indicator, increased in the serum and liver tissue of cholestatic rats(47,48). Its elevated levels could reflect the degrees of lipid peroxidation injury in hepatocytes.
In the current study, MDA remarkably increased in both serum and liver tissue in BDL rats and treatment with JE at the dose of 500 mg/kg had no significant effect on serum and liver tissue of MDA.
PCO is a well-known form of oxidative injury to proteins, serving as an initial marker for assessing free-radical-mediated protein oxidation (49,50). Earlier literature indicated that oxidative damage to proteins takes place in cholestatic hepatic injury models of BDL and PCO levels increased following BDL surgery (29,51). Data from this study showed that there was no statistically significant difference among the studied groups. Therefore, treatment with JE 500 mg/kg did not significantly impact the serum level of PCO biomarkers.
TSH is a sensitive biomarker of oxidative stress and plays an important role in antioxidant systems (51). Preceding investigations showed that the level of TSH in liver tissue decreased following BDL-induced hepatic damage (51). In the current study, no statistical difference was found among all groups.
Endogenous antioxidants enzymes such as SOD, CAT, and GPx, or non-enzymatic compounds like uric acid, bilirubin, albumin, and metallothioneins (52) play a key role in protecting the liver against various toxicants (53). Several studies have been reported that the antioxidants mitigate BDL-induced liver injury (29,54). There is much evidence that herbal medicine including Origanum majorana (55), and Nasturtium officinale (29) has hepatoprotective effects through antioxidative effects against BDL-induced cholestasis. The antioxidant activity of JE essential oils and extracts was determined (56). Furthermore, it is reported that the antioxidant activity of JE extracts is partly mediated by FRAP (57). In the current study, we showed that JE at 500 mg/kg significantly increased the activity of SOD and GPx, as well as the serum level of FRAP, while did not significantly affect the activity of CAT in comparison with the BDL rats.
Photomicrographs of H&E-stained liver sections of our study showed severe bile duct proliferation, moderate portal inflammation, mild/moderate piecemeal necrosis, and fibrosis of some portal tracts in BDL rats. Inconsistent, other studies showed a significant histopathological change such as portal vein congestion with bile duct proliferation with edematous of the epithelial lining and inflammation, moderate portal and peri-portal inflammation, bile stasis, and apoptotic hepatocytes in BDL rats (6). In this study, no gross histopathological alterations were found in rats treated with ethanolic extract of JE in comparison with BDL operation.
CONCLUSION
Our results clarified that JE has therapeutic effects on BDL-induced liver injury by attenuating ALP activity, increasing total protein and FRAP levels, as well as raising the antioxidant enzyme activity of SOD and GPx.
Collectively, the current study demonstrated that JE may be useful as adjuvant therapy in cholestasis by increasing antioxidant activity and hepatoprotective effects.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contributions
Gh. Akbari, M. Eftekhari, and A.H. Doustimotlagh were involved in the conception and design, S. Nouripoor, N. Shakerinasab, M.R. Abasi, M. Azizi, and M. Salahi analyzed and interpreted the data; M. Gharaghani, S. Nouripoor, and N. Shakerinasab prepared the draft of the paper. The article was critically revised for intellectual content by M. Eftekhari, M. Gharaghani, F. Karimi, D. Razmjoue, and A.H. Doustimotlagh. The finalized article was read and approved by all authors.
Acknowledgments
This work was financially supported by Yasuj University of Medical Sciences, Yasuj, Iran through Grant No. 990221.
REFERENCE
- 1.Devi SS. Structure and function of hepatic parenchymal cells. Comprehensive Toxicology. Elsevier. 2018;2:10–28. DOI: 10.1016/B978-0-12-801238-3.64335-0. [Google Scholar]
- 2.Williams R. Global challenges in liver disease. Hepatology. 2006;44(3):521–526. doi: 10.1002/hep.21347. DOI: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 3.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139(5):1481–1496. doi: 10.1053/j.gastro.2010.09.004. DOI: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford AE, Pratt DS. Cholestasis and cholestatic syndromes. Curr Opin Gastroenterol. 2006;22(3):209–214. doi: 10.1097/01.mog.0000218956.63311.47. DOI: 10.1097/01.mog.0000218956.63311.47. [DOI] [PubMed] [Google Scholar]
- 5.Pereira F, Facincani I, Jorgetti V, Ramalho LNZ, Volpon JB, Dos Reis LM, et al. Etiopathogenesis of hepatic osteodystrophy in Wistar rats with cholestatic liver disease. Calcif Tissue Int. 2009;85(1):75–83. doi: 10.1007/s00223-009-9249-3. DOI: 10.1007/s00223-009-9249-3. [DOI] [PubMed] [Google Scholar]
- 6.Nassef NA, Abu-Shadi E, El Agaty SEI, Abdel Hamid G. Quercetin mitigates liver injury in a rat model of liver cholestasis. Bull. Egypt. Soc. Physiol. 2020;40(1):84–95. DOI: 10.21608/besps.2019.14512.1026. [Google Scholar]
- 7.Chatterjee S, Richert L, Augustijns P, Annaert P. Hepatocyte-based in vitro model for assessment of drug-induced cholestasis. Toxicol Appl Pharmacol. 2014;274(1):124–136. doi: 10.1016/j.taap.2013.10.032. DOI: 10.1016/j.taap.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Cherian DA, Peter T, Narayanan A, Madhavan SS, Achammada S, Vynat GP. Malondialdehyde as a marker of oxidative stress in periodontitis patients. J Pharm Bioallied Sci. 2019;11(Suppl 2):S297–S300. doi: 10.4103/JPBS.JPBS_17_19. DOI: 10.4103/JPBS.JPBS_17_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018;54(4):287–293. DOI: 10.1016/j.ajme.2017.09.001. [Google Scholar]
- 10.Bahçecioğlu İH, Yalniz M, Ataseven H, Bülbüller N, Keçeci M, Demirdağ K, et al. TNF-α and leptin in experimental liver fibrosis models induced by carbon tetrachloride and by common bile duct ligation. Cell Biochem Funct. 2004;22(6):359–363. doi: 10.1002/cbf.1114. DOI: 10.1002/cbf.1114. [DOI] [PubMed] [Google Scholar]
- 11.Abe T, Arai T, Ogawa A, Hiromatsu T, Masuda A, Matsuguchi T, et al. Kupffer cell-derived interleukin 10 is responsible for impaired bacterial clearance in bile duct-ligated mice. Hepatology. 2004;40(2):414–423. doi: 10.1002/hep.20301. DOI: 10.1002/hep.20301. [DOI] [PubMed] [Google Scholar]
- 12.Djordjevic SM. El-Shemy H, editor. From medicinal plant raw material to herbal remedies. Aromatic and medicinal plants: back to nature. 2017. pp. 269–288. InTech. Available from: http://dx.doi.org/10.5772/636962017 . DOI: 10.5772/66618.
- 13.Azzimonti B, Cochis A, Beyrouthy MEI, Iriti M, Uberti F, Sorrentino R, et al. Essential oil from berries of Lebanese Juniperus excelsa M. bieb displays similar antibacterial activity to chlorhexidine but higher cytocompatibility with human oral primary cells. Molecules. 2015;20(5):9344–9357. doi: 10.3390/molecules20059344. DOI: 10.3390/molecules20059344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stankov S, Fidan H, Petkova Z, Stoyanova M, Petkova N, Stoyanova A, et al. Comparative study on the phytochemical composition and antioxidant activity of Grecian juniper (Juniperus excelsa M. Bieb) unripe and ripe galbuli. Plants. 2020;9(9):1–18. doi: 10.3390/plants9091207. 1207. DOI: 10.3390/plants9091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Göze I, Göze ÖF, Yelkovan I, Çetinus Ş, Saygin H, Ercan N. The review of certain in vivo antioxidant effects on essential oils of origanum minutiflorum O Schwarz-Ph Davis, Juniperus excelsa bieb subsp excelsa and histopathologic Changes. Rev Bras Cienc Avic. 2017;19:333–338. DOI: 10.1590/1806-9061-2016-0452. [Google Scholar]
- 16.Khan M, Khan AU, Rehman NUR-, Zafar MA, Hazrat A, Gilani AH. Cardiovascular effects of Juniperus excelsa are mediated through multiple pathways. Clin Exp Hypertens. 2012;34(3):209–216. doi: 10.3109/10641963.2011.631651. DOI: 10.3109/10641963.2011.631651. [DOI] [PubMed] [Google Scholar]
- 17.Pirani A, Moazzeni H, Mirinejad S, Naghibi F, Mosaddegh M. Ethnobotany of Juniperus excelsa M. Bieb.(Cupressaceae) in Iran. Ethnobot Res Appl. 2011;9:335–341. DOI: 10.17348/ERA.9.0.335-341. [Google Scholar]
- 18.Noroozi J, Akhani H, Breckle SW. Biodiversity and phytogeography of the alpine flora of Iran. Biodivers Conserv. 2008;17:493–521. DOI: 10.1007/s10531-007-9246-7. [Google Scholar]
- 19.Weli AM, AL-Hinai JRK, Al-Mjrafi JMA, Alnaaimi JRS, Hossain MA, Saeed S, et al. Effect of different polarities leaves crude extracts of Omani Juniperus excels on antioxidant, antimicrobial and cytotoxic activities and their biochemical screening. Asian Pac J Reprod. 2014;3(3):218–223. DOI: 10.1016/S2305-0500(14)60029-4. [Google Scholar]
- 20.Atas AD, Goze I, Alim A, Cetinus SA, Durmus N, Vural N, et al. Chemical composition, antioxidant, antimicrobial and antispasmodic activities of the essential oil of Juniperus excelsa subsp. excelsa. J Essent Oil Bear Pl. 2012;15(3):476–483. DOI: 10.1080/0972060X.2012.10644075. [Google Scholar]
- 21.Moein MR, Ghasemi Y, Moein S, Nejati M. Analysis of antimicrobial, antifungal and antioxidant activities of Juniperus excelsa M. B subsp. polycarpos (K. Koch) Takhtajan essential oil. Pharmacognosy Res. 2010;2(3):128–131. doi: 10.4103/0974-8490.65505. DOI: 10.4103/0974-8490.65505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones SM, Zhong Z, Enomoto N, Schemmer P, Thurman RG. Dietary Juniper berry oil minimizes hepatic reperfusion injury in the rat. Hepatology. 1998;28(4):1042–1050. doi: 10.1002/hep.510280419. DOI: 10.1002/hep.510280419. [DOI] [PubMed] [Google Scholar]
- 23.Mehrabi S, Askarpour E, Mehrabi F, Jannesar R. Effects of hydrophilic extract of Nasturtium officinale on prevention of ethylene glycol induced renal stone in male Wistar rats. J Nephropathol. 2016;5(4):123–127. doi: 10.15171/jnp.2016.23. DOI: 10.15171/jnp.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan M, Khan AU, Gilani AH. Pharmacological explanation for the medicinal use of Juniperus excelsa in hyperactive gastrointestinal and respiratory disorders. J Nat Med. 2012;66(2):292–301. doi: 10.1007/s11418-011-0605-z. DOI: 10.1007/s11418-011-0605-z. [DOI] [PubMed] [Google Scholar]
- 25.Doustimotlagh AH, Dehpour AR, Etemad Moghadam S, Alaeddini M, KheirandishY, Golestani A. Nitrergic and opioidergic systems affect radiographic density and histomorphometric indices in bile-duct-ligated cirrhotic rats. Histol Histopathol. 2017;32(7):743–749. doi: 10.14670/HH-11-836. DOI: 10.14670/HH-11-836. [DOI] [PubMed] [Google Scholar]
- 26.Uchinami H, Seki E, Brenner DA, D Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44(2):420–429. doi: 10.1002/hep.21268. DOI: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- 27.Ved A, Gupta A, Rawat AKS. Antioxidant and hepatoprotective potential of phenol-rich fraction of Juniperus communis Linn. leaves. Pharmacogn Mag. 2017;13(49):108–113. doi: 10.4103/0973-1296.197648. DOI: 10.4103/0973-1296.197648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganie SA, Dar TA, Zargar B, Hamid R, Zargar O, Dar PA, et al. Antioxidant and hepatoprotective effects of Crataegus songarica methanol extract. J Environ Pathol Toxicol Oncol. 2014;33(2):131–143. doi: 10.1615/jenvironpatholtoxicoloncol.2014010606. DOI: 10.1615/jenvironpatholtoxicoloncol.2014010606. [DOI] [PubMed] [Google Scholar]
- 29.Sadeghi H, Azarmehr N, Razmkhah F, Sadeghi H, Danaei N, Omidifar N, et al. The hydroalcoholic extract of watercress attenuates protein oxidation, oxidative stress, and liver damage after bile duct ligation in rats. J Cell Biochem. 2019;120(9):14875–14884. doi: 10.1002/jcb.28749. DOI: 10.1002/jcb.28749. [DOI] [PubMed] [Google Scholar]
- 30.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. DOI: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 31.Rabani MR, Azarmehr N, Moslemi Z, Sadeghi H, Amini-Khoei H, Doustimotlagh AH. Protective effects of hydroalcoholic extract of Stachys pilifera on paracetamol-induced nephrotoxicity in female rats. Res Pharm Sci. 2021;16(6):643–650. doi: 10.4103/1735-5362.327510. DOI: 10.4103/1735-5362.327510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadeghi H, Jahanbazi F, Sadeghi H, Omidifar N, Alipoor B, Panohi Kokhdan E, et al. Metformin attenuates oxidative stress and liver damage after bile duct ligation in rats. Res Pharm Sci. 2019;14(2):122–129. doi: 10.4103/1735-5362.253359. DOI: 10.4103/1735-5362.253359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbari G, Mard SA, Dianat M, Mansouri E. The hepatoprotective and microRNAs downregulatory effects of crocin following hepatic ischemia-reperfusion injury in rats. Oxid Med Cell Longev. 2017;2017:1–11. doi: 10.1155/2017/1702967. 1702967. DOI: 10.1155/2017/1702967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheen JM, Chen YC, Tain YL, Huang LT. Increased circulatory asymmetric dimethylarginine and multiple organ failure: bile duct ligation in rat as a model. Int J Mol Sci. 2014;15(3):3989–4006. doi: 10.3390/ijms15033989. DOI: 10.3390/ijms15033989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao JY, Li HW, Liu FG, Li YC, Tian S, Cao LH, et al. Effects of Portulaca oleracea extract on acute alcoholic liver injury of rats. Molecules. 2019;24(16):1–14. doi: 10.3390/molecules24162887. 2887. DOI: 10.3390/molecules24162887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moslemi Z, Bahrami M, Hosseini E, Mansourian M, Daneshyar Z, Eftekhari M, et al. Portulaca oleracea methanolic extract attenuate bile duct ligation-induced acute liver injury through hepatoprotective and anti-inflammatory effects. Heliyon. 2021;7(7):1–8. doi: 10.1016/j.heliyon.2021.e07604. e07604. DOI: 10.1016/j.heliyon.2021.e07604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldo G, Kretzmann NA, Tieppo J, Filho GP, Cruz CU, Meurer L, et al. Bone marrow cells reduce collagen deposition in the rat model of common bile duct ligation. J Cell Sci Ther. 2011;2(4):1–6. DOI: 10.4172/2157-7013.1000112. [Google Scholar]
- 38.Noble H, Whitley E, Norton S, Thompson M. A study of preoperative factors associated with a poor outcome following laparoscopic bile duct exploration. Surg Endosc. 2011;25(1):130–139. doi: 10.1007/s00464-010-1146-3. DOI: 10.1007/s00464-010-1146-3. [DOI] [PubMed] [Google Scholar]
- 39.Laouar A, Klibet F, Bourogaa E, Benamara A, Boumendjel A, Chefrour A, et al. Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCE induced hepatic damage in rats. Asian Pac J Trop Med. 2017;10(3):263–269. doi: 10.1016/j.apjtm.2017.03.005. DOI: 10.1016/j.apjtm.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Singh H, Prakash A, Kalia AN, Majeed ABA. Synergistic hepatoprotective potential of ethanolic extract of Solanum xanthocarpum and Juniperus communis against paracetamol and azithromycin induced liver injury in rats. J Tradit Complement Med. 2016;6(4):370–376. doi: 10.1016/j.jtcme.2015.07.005. DOI: 10.1016/jjtcme.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zajić S, Damnjanović Z, Stojanović M, Višnjić M, Denčić S, Ilić D, et al. Follow-up of biochemical parameters in patients with extrahepathic cholestasis. Acta Medica Medianae. 2008;47(1):5–12. [Google Scholar]
- 42.Jahantab MB, Safaripour AA, Hassanzadeh S, Yavari Barhaghtalab MJ. Demographic, chemical, and helicobacter pylori positivity assessment in different types of gallstones and the bile in a random sample of cholecystectomied iranian patients with cholelithiasis. Can J Gastroenterol Hepatol. 2021:1–8. doi: 10.1155/2021/3351352. 3351352. DOI: 10.1155/2021/3351352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei D, Liao S, Wang J, Yang M, Kong L. Cholestatic liver injury model of bile duct ligation and the protection of Huang-Lian-Jie-Du decoction by NMR metabolomic profiling. RSC Adv. 2015;5(81):66200–66211. DOI: 10.1039/C5RA12224D. [Google Scholar]
- 44.Yang L, Wang CZ, Ye JZ, Li HT. Hepatoprotective effects of polyprenols from Ginkgo biloba L. leaves on CCl4-induced hepatotoxicity in rats. Fitoterapia. 2011;82(6):834–840. doi: 10.1016/j.fitote.2011.04.009. DOI: 10.1016/j.fitote.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30(2):195–204. doi: 10.1055/s-0030-1253228. DOI: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- 46.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52(1):1–8. doi: 10.2337/diabetes.52.1.1. DOI: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Zhao SS, Li NR, Zhao WL, Liu H, Ge MX, Zhang YX, et al. D-chiro-inositol effectively attenuates cholestasis in bile duct ligated rats by improving bile acid secretion and attenuating oxidative stress. Acta Pharmacol Sin. 2018;39(2):213–221. doi: 10.1038/aps.2017.98. DOI: 10.1038/aps.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aktas C, Kanter M, Erboga M, Mete R, Oran M. Melatonin attenuates oxidative stress, liver damage and hepatocyte apoptosis after bile-duct ligation in rats. Toxicol Ind Health. 2014;30(9):835–844. doi: 10.1177/0748233712464811. DOI: 10.1177/0748233712464811. [DOI] [PubMed] [Google Scholar]
- 49.Alou-El-Makarem MM, Moustafa MM, Fahmy MAA, Abdel-Hamed AM, El-fayomy KN, Darwish M. Evaluation of carbonylated proteins in hepatitis c virus patients. Mod Chem Appl. 2014;2(2):1–5. DOI: 10.4172/2329-6798.1000130. [Google Scholar]
- 50.Fedorova M, Bollineni RC, Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev. 2014;33(2):79–97. doi: 10.1002/mas.21381. DOI: 10.1002/mas.21381. [DOI] [PubMed] [Google Scholar]
- 51.Terzioglu D, Uslu L, Simsek G, Atukeren P, Erman H, Gelisgen R, et al. The effects of hyperbaric oxygen treatment on total antioxidant capacity and prolidase activity after bile duct ligation in rats. J Invest Surg. 2017;30(6):376–382. doi: 10.1080/08941939.2016.1257666. DOI: 10.1080/08941939.2016.1257666. [DOI] [PubMed] [Google Scholar]
- 52.Pisoschi AM, Negulescu GP. Methods for total antioxidant activity determination: a review. Biochem Anal Biochem. 2011;1(1):1–10. DOI: 10.4172/2161-1009.1000106. [Google Scholar]
- 53.Chiu H, Gardner CR, Dambach DM, Brittingham JA, Durham SK, Laskin JD, et al. Role of p55 tumor necrosis factor receptor 1 in acetaminophen-induced antioxidant defense. Am J Physiol Gastrointest Liver Physiol. 2003;285(5):G959–G966. doi: 10.1152/ajpgi.00219.2003. DOI: 10.1152/ajpgi.00219.2003. [DOI] [PubMed] [Google Scholar]
- 54.Dulundu E, Ozel Y, Topaloglu U, Toklu H, Ercan F, Gedik N, et al. Grape seed extract reduces oxidative stress and fibrosis in experimental biliary obstruction. J Gastroenterol Hepatol. 2007;22(6):885–892. doi: 10.1111/j.1440-1746.2007.04875.x. DOI: 10.1111/j.1440-1746.2007.04875.x. [DOI] [PubMed] [Google Scholar]
- 55.Gheitasi I, Motaghi N, Sadeghi H, Sadeghi H, Moslemi Z, Eftekhari M, et al. Antioxidant and anti-inflammatory effects of Origanum majorana L. methanolic extract on bile duct ligation in male rats. Evid Based Complement Alternat Med. 2021;2021:1–10. doi: 10.1155/2021/9927196. DOI: 10.1155/2021/9927196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moein S, Moein M. Antioxidant activities and phenolic content of Juniperus excelsa extract. Iran J Pharm Sci. 2010;6(2):133–140. DOI: 10.22037/ijps.v6.41260. [Google Scholar]
- 57.Bakkour Y, Achi NEI, Tabcheh M, El-Nakat H, Omar FEI. Chemical composition and antioxidant activities of the essential oils from green and ripe berries of Juniperus excelsa growing in Lebanon. Int J Pharm Life Sci. 2013;4(2):2362–2367. [Google Scholar]