Abstract
We derive approximate expressions for pre- and post-steady state regimes of the velocity-substrate-inhibitor spaces of the Michaelis-Menten enzyme kinetic scheme with fully and partial competitive inhibition. Our refinement over the currently available standard quasi steady state approximation (sQSSA) seems to be valid over wide range of enzyme to substrate and enzyme to inhibitor concentration ratios. Further, we show that the enzyme-inhibitor-substrate system can exhibit temporally well-separated two different steady states with respect to both enzyme-substrate and enzyme-inhibitor complexes under certain conditions. We define the ratios fS = vmax/(KMS + e0) and fI = umax/(KMI + e0) as the acceleration factors with respect to the catalytic conversion of substrate and inhibitor into their respective products. Here KMS and KMI are the Michaelis-Menten parameters associated respectively with the binding of substrate and inhibitor with the enzyme, vmax and umax are the respective maximum reaction velocities and e0, s0, and i0 are total enzyme, substrate and inhibitor levels. When (fS/fI) < 1, then enzyme-substrate complex will show multiple steady states and it reaches the full-fledged steady state only after the depletion of enzyme-inhibitor complex. When (fS/fI) > 1, then the enzyme-inhibitor complex will show multiple steady states and it reaches the full-fledged steady state only after the depletion of enzyme-substrate complex. This multi steady-state behavior especially when (fS/fI) ≠ 1 is the root cause of large amount of error in the estimation of various kinetic parameters of fully and partial competitive inhibition schemes using sQSSA. Remarkably, we show that our refined expressions for the reaction velocities over enzyme-substrate-inhibitor space can control this error more significantly than the currently available sQSSA expressions.
1. Introduction
Enzymes catalyze various reactions of biochemical pathways [1–4]. The Michaelis-Menten (MM) kinetics [5,6] is the fundamental mechanistic description of the biological catalysis of enzyme reactions [3,7–9]. In this kinetics scheme, the enzyme reversibly binds its substrate to form the enzyme-substrate complex which subsequently decomposes into free enzyme and product of the substrate. Integral solution to the rate equations associated with the Michaelis-Menten scheme (MMS) is not expressible in terms of elementary functions. Several analytical methods were tried to obtain the approximate solution of MMS in terms of ordinary [10] and singular perturbation series [11–13] and perturbation expansions over slow manifolds [14,15]. In general, the singular perturbation expansions yield a combination of inner and outer solutions which were then combined via proper matching at the boundary layer [11,16–21].
Several steady state approximations were proposed in the light of experimental characterization of a single substrate MM enzyme. The standard quasi steady state approximation (sQSSA) is widely used across several fields of biochemical research to obtain the enzyme kinetic parameters such as vmax and KM from the experimental datasets on reaction velocity versus initial substrate concentrations. This approximation works well when the product formation step is rate-limiting apart from the condition that the substrate concentration is much higher than the enzyme concentration. In general, sQSSA yields expressions which can be directly used by the experimentalists to obtain various enzyme kinetic parameters [22]. Recently, explicit closed form expressions of the integrated rate equation corresponding to sQSSA were obtained in terms of Lambert’s W functions [23–27]. The total QSSA (tQSSA) assumes that the amount of product formed near the steady state is much negligible compared to the total substrate concentration [28,29]. The reverse QSSA (rQSSA) works very well [30,31] when the substrate concentration is much lesser than the enzyme concentration.
Several linearization techniques such as Lineweaver-Burk representation were also proposed [32,33] to obtain the kinetic parameters from the experimental data. Although sQSSA, rQSSA and tQSSA methods work well under in vitro conditions, there are several situations such as single molecule enzyme kinetics [34] and other in vivo experimental conditions where one cannot manipulate the ratio of substrate to enzyme concentrations much. Recent studies on the liver cytochrome P450 3A4 enzyme revealed that tQSSA based methods work very well irrespective of the relative values of KM and the total enzyme concentration [35,36]. It seems that one can accurately obtain the enzyme kinetic parameters using tQSSA based equations [37]. Further, successfulness of various QSSAs in accurately obtaining the kinetic parameters is strongly dependent on the timescale separation between the pre- and post-steady state regimes of MMS [38,39]. Particularly, when the timescale separation between pre- and post-steady states of MMS is high enough, then the sQSSA along with stationary reactant assumption where one replaces the unknown steady state substrate concentration with the total substrate concentration [27] can be used to directly obtain the kinetic parameters.
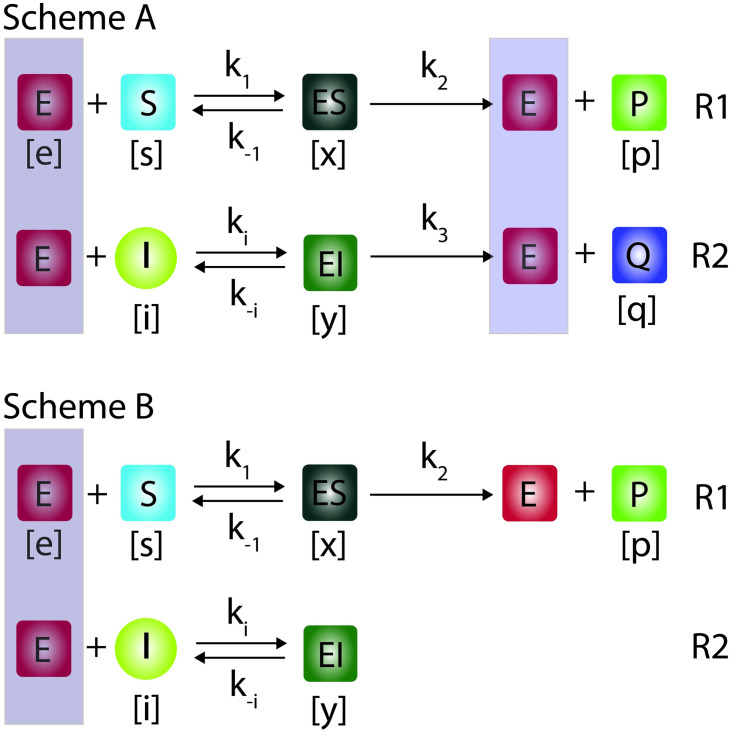
The catalytic properties of an enzyme can be manipulated by an inhibitor. Inhibitors can be competitive or allosteric in nature [2]. Competitive inhibitors (Fig 1) are substrate like molecules which reversibly bind the active site of the same enzyme and hence block further binding of substrate. This in turn deceases the catalytic efficiency of the enzyme over its natural substrate. In a fully competitive inhibition (Scheme A in Fig 1), the inhibitor competes with the substrate to bind the active site of the enzyme and subsequently gets converted into the respective product. In this case, both substrate and inhibitor will be converted into their respective products by the same enzyme. In case of partial competitive inhibition (Scheme B in Fig 1), the reversibly formed enzyme-inhibitor complex will be a dead-end one. Several drugs have been designed to strongly inhibit the pathogenic or metabolic enzymes. Understanding the dynamical behavior of the fully and partial competitive inhibition of MM enzymes is critical to understand the pharmacokinetic and efficiency aspects of such enzyme inhibiting drugs. Variation of vmax and KM of the enzyme with respect to the concentration of an inhibitor decides the efficiency of a given drug molecule in targeting that enzyme. In steady state experiments on the single substrate enzymes, the total substrate concentration will be iterated to obtain the respective substrate conversion velocities. This substrate concentration versus reaction velocity dataset will be then used to obtain the kinetic parameters such as KM and vmax. To obtain the kinetic parameters related to the enzyme inhibition, one needs to conduct a series of velocity versus substrate type steady state experiments at different concentrations of inhibitor. Using this dataset on the steady state substrate, inhibitor versus reaction velocities, one can obtain the kinetic parameters related to the enzyme-substrate-inhibitor system. The enzyme kinetic rate constants can also be directly obtained by non-linear least square fitting of the time dependent progress curve data over the corresponding differential rate equations using the Marquardt-Levenberg algorithm. However, the propagated error levels will be high upon the computation of KM and vmax from these individual rate constants obtained from the nonlinear least square fitting procedures [40].
Fig 1. Fully (Scheme A) and partial (Scheme B) competitive inhibition schemes of Michaelis-Menten type enzyme kinetics.
In fully competitive inhibition, both substrate (S) and inhibitor (I) compete for the same active site of enzyme (E) to bind and form reversible complexes (ES, EI) which subsequently get converted into their respective products (P, Q). Whereas, in partial competitive inhibition, the reversibly formed enzyme-inhibitor (EI) is a dead-end complex. Here (e, s, i, x, y, p, q) are respectively the concentrations of enzyme, substrate, inhibitor, enzyme-substrate, enzyme-inhibitor, product of substrate and product of inhibitor. Further, k1 and ki are the respective forward rate constants, k-1 and k-i are the reverse rate constants and, k2 and k3 are the respective product formation rates.
The successfulness of various steady state approximations in obtaining the kinetic parameters of enzymes from the experimental datasets strongly depends on the occurrence of a common steady state with respect to both substrate and inhibitor binding dynamics in both fully and partial competitive inhibition schemes. Mismatch in the steady state timescales can be resolved by setting higher substrate and inhibitor concentrations than the enzyme concentration. This condition drives the steady state reaction velocities as well as the timescales corresponding to the binding of substrate and inhibitor with the same enzyme close to zero. However, under in vivo conditions, one cannot manipulate the relative concentrations of substrate, inhibitor and enzyme much. All the quasi steady state type approximations will fail when the concentration of the enzyme is comparable with that of the substrate and inhibitor which is generally true under in vivo conditions. In this article, we will address this issue in detail and derive accurate expressions for the steady state reaction velocities when the concentrations of enzyme, substrate and inhibitor are comparable with each other.
2. Theory
The competitive inhibition of Michaelis-Menten enzymes can be via fully or partial mode as depicted in Scheme A and B of Fig 1. In fully competitive inhibition given in Scheme A, both the substrate and inhibitor molecules compete for the same active site of the target enzyme for binding and subsequently get converted into their respective products in a parallel manner. In case of partial competitive inhibition, the reversibly formed enzyme-inhibitor complex will not be converted into any product and it will be a dead-end complex. Particularly, several drug molecules are partial competitive inhibitors [41,42]. Fully competitive inhibition plays important roles in the regulation of metabolic reaction pathways. In the following sections we will analyze various kinetic aspects of fully and partial competitive inhibition schemes in detail. We use the equation numbering as the section number followed by the respective equation number within that section e.g., in the notation Eq. x.y.z.k, x.y.z is the section number and k is the equation number in that section.
2.1. Fully competitive inhibition
The fully competitive inhibition of Michaelis-Menten enzymes as depicted in Scheme A of Fig 1 can be quantitatively described by the following set of differential rate equations.
| [2.1.1] |
| [2.1.2] |
| [2.1.3] |
| [2.1.4] |
| [2.1.5] |
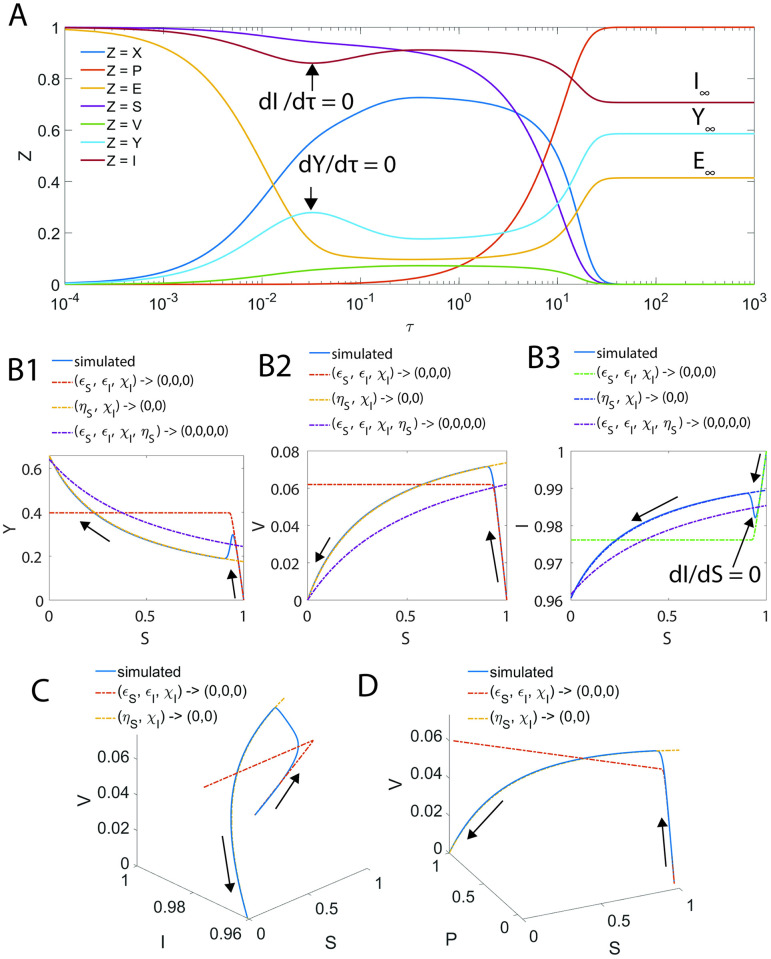
Here . In Eqs 2.1.1–2.1.5, (s, i, e, x, y, p, q) are respectively the concentrations (mol/lit, M) of substrate, inhibitor, enzyme, enzyme-substrate complex, enzyme-inhibitor complex, product of substrate and product of inhibitor. Here k1 and ki are the respective forward bimolecular rate constants (1/M/second), k-1 and k-i (1/second) are the respective reverse unimolecular rate constants, u and v (M/second) are the respective reaction velocities and, k2 and k3 (1/second) are the respective unimolecular product formation rate constants along with the mass conservation laws: e = e0 − x − y; s = s0 − x − p; i = i0 − y − q. The initial conditions are (s, i, e, x, y, p, q, v, u) = (s0, i0, e0, 0, 0, 0, 0, 0, 0) at t = 0. When t → ∞, then the reaction ends at (s, i, e, x, y, p, q, v, u) = (0, 0, e0, 0, 0, s0, i0, 0, 0). The steady states occur when especially under the condition that t < ∞ since when t → ∞. However, the timescale 0 < tCS < ∞ at which can be different from the timescale 0 < tCI < ∞ at which . When there is a mismatch in the steady state timescales i.e., (tCS ≠ tCI), then one cannot obtain a common steady state solution to Eqs 2.1.1–2.1.5 by simultaneously equating all of them to zero. This means that there exist two different steady states with respect to enzyme-substrate and enzyme-inhibitor complexes at two different time points along with four different timescales viz. two different pre-steady state timescales and two different post-steady state timescales. Various definitions and symbols used in the theory section are summarized in Table 1.
Table 1. Summary of variables and parameters used in the theory section.
| Parameters / Variables | Definition | Remarks |
|---|---|---|
| e, s, i, p, q, x, y, v, u | Concentration of enzyme, substrate, inhibitor, product of substrate, product of inhibitor, enzyme-substrate complex, enzyme-inhibitor complex, velocity of substrate-product formation, velocity of inhibitor-product formation. | mol/lit, M |
| e0, s0, i0 | Initial enzyme, substrate and inhibitor concentrations. | M |
| E, S, I, P, Q, X, Y, V, U | are the normalized concentrations of substrate, enzyme, product of substrate, enzyme-inhibitor complex, enzyme-substrate complex, inhibitor and product of inhibitor, velocity of product of substrate formation, and velocity of product of inhibitor formation. | dimensionless |
| EC, SC, IC, PC, QC, XC, YC, VC, UC | Overll steady state values with respect to both X as well as Y. | dimensionless |
| SCP, ICP, PCP, QCP, XCP, YCP, VCP, UCP | Values at the steady state with respect to only X. | dimensionless |
| SCQ, ICQ, PCQ, QCQ, XCQ, YCQ, VCQ, UCQ | Values at the steady state with respect to only Y. | dimensionless |
| k2, k3 | Unimolecular product formation rate constants. | 1/second |
| τ | = k 2 t | dimensionless |
| vmax, umax | vmax = k2 e0, umax = k3 e0 | M/second |
| k1, ki | Bimolecular rate constants associated with the binding of substrate and inhibitor respectively with the same enzyme. | 1 / (M second) |
| k-1, k-i | Dissociation rate constants associated with the enzyme-substrate and enzyme-inhibitor complexes. | 1/second |
| KRS, KRI | KRS = k2 / k1, KRI = k3 / ki | M |
| KDS, KDI | KDS = k-1 / k1, KDI = k-i / ki | M |
| KMS, KMI | KMS = KRS + KDS, KMI = KRI + KDI | M |
| ηS, ηI | ηS = k2 / k1 s0, ηI = k3 / ki i0 | dimensionless |
| εS, εI, εIS | εS = e0 / s0, εI = e0 / i0, εIS = εI / εS = s0 / i0 | dimensionless |
| κS, κI | κS = k-1 / k1s0, κI = k-i / ki i0 | dimensionless |
| χ I | = k2/kii0 defined for the partial competitive inhibition scheme. | dimensionless |
| ρ | = k3 / k2 = umax / vmax | dimensionless |
| σ | = k1 / ki | dimensionless |
| μS, μI | μS = ηS + κS, μI = ηI + κI | dimensionless |
| ϒ | = k-1 / k-i | dimensionless |
| δ | dimensionless | |
| = εI + κI + ηI = εI + μI | dimensionless | |
| = εS + κS + ηS = εS + μS | dimensionless | |
| = εI + κI | dimensionless | |
| αS | = 1 + εS + κS + ηS | dimensionless |
| αI | = 1 + εI + κI + ηI | dimensionless |
| βI | = 1 + εI + κI | dimensionless |
| ϕ S | = ηSεS | dimensionless |
| ϕ I | = ηIεI | dimensionless |
| τ CS |
, steady state timescale corresponding to the enzyme-substrate complex (fully competitive). , steady state timescale corresponding to the enzyme-substrate complex (partial competitive). |
dimensionless Eq 2.6.1.1. |
| τ CI | , steady state timescale corresponding to the enzyme-inhibitor complex (fully competitive) | dimensionless Eq 2.6.1.1. |
| τ CY | , (pesudo) steady state timescale corresponding to enzyme-inhibitor complex (partial competitive). | Dimensionless Eq 2.9.3.2. |
| fS, fI |
are the reaction acceleration factors associated with the conversion of substrate and inhibitor into their products. δ = fS/fI. |
M/second. |
2.2. Scaling and non-dimensionalization
To simplify the system of Eqs 2.1.1–2.1.5, we introduce the following set of scaling transformations.
| [2.2.1] |
We further define the following parameters.
| [2.2.2] |
| [2.2.3] |
| [2.2.4] |
| [2.2.5] |
| [2.2.6] |
Here (S, I, E, X, Y, P, Q) ∈ [0, 1] are the dimensionless time dependent dynamical variables along with the mass conservation laws: E = 1 − X − Y; S = 1 − εSX − P; I = 1 − εIY − Q. With these scaling transformations, one can reduce Eqs 2.1.1–2.1.5 into the following set of equations.
| [2.2.7] |
| [2.2.8] |
| [2.2.9] |
Upon expanding S and I with their definition in the right-hand side of Eqs 2.2.7 and 2.2.8 and rearranging the linear and nonlinear terms, we arrive at the following form.
| [2.2.10] |
| [2.2.11] |
Here αS and αI are defined as in Eq 2.2.6. The coupled first order nonlinear ODEs given in Eqs 2.2.9–2.2.11 completely characterize the dynamics of fully competitive inhibition scheme over (P, Q, X, Y, τ) space. Here the initial conditions are (S, I, E, X, Y, P, Q, V, U) = (1, 1, 1, 0, 0, 0, 0, 0, 0) at τ = 0. When τ → ∞, then the reaction trajectory ends at (S, I, E, X, Y, P, Q, V, U) = (0, 0, 1, 0, 0, 1, 1, 0, 0). The steady state with respect to X occurs at 0 < τCS < ∞ where . Since S and P monotonically varying functions of τ one finds that throughout the reaction timescale except at τ → 0 where and at τ → ∞ where , one implicitly finds at the steady state that . Using the same arguments, one can show that at the steady state with respect to Y at 0 < τCI < ∞. Since I and Q are monotonically varying functions of τ one finds that throughout the entire timescale regime except at τ = 0 where and at τ → ∞ where . Here (τCS, τCI) = k2(tCS, tCI). When τCS = τCI = τC, then we represent the common steady state values of the dynamical variables as (SC, IC, EC, XC, YC, PC, QC, VC, UC).
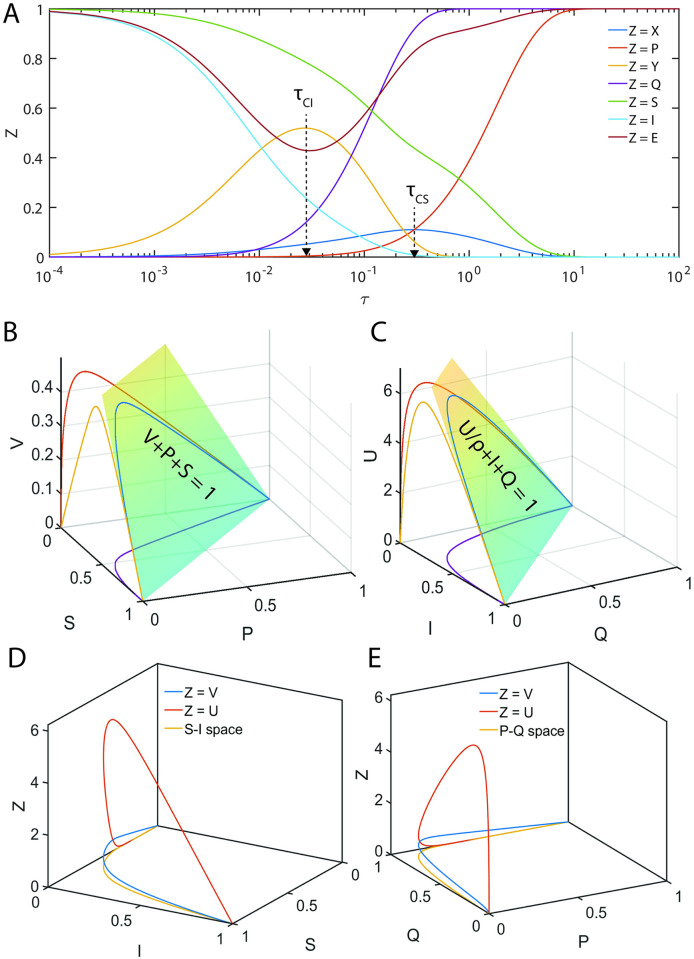
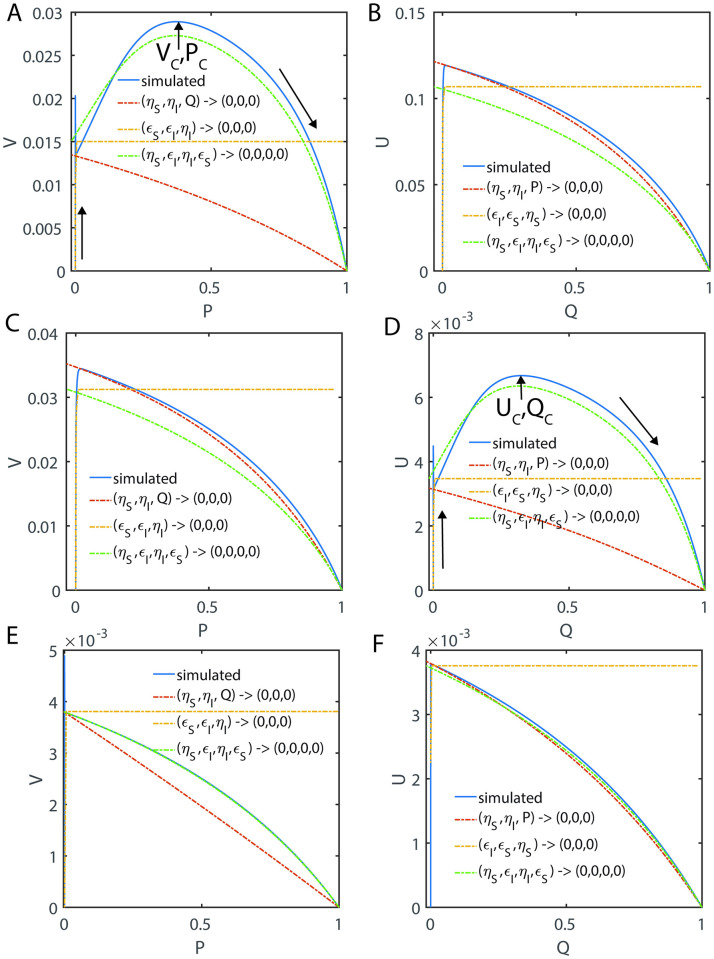
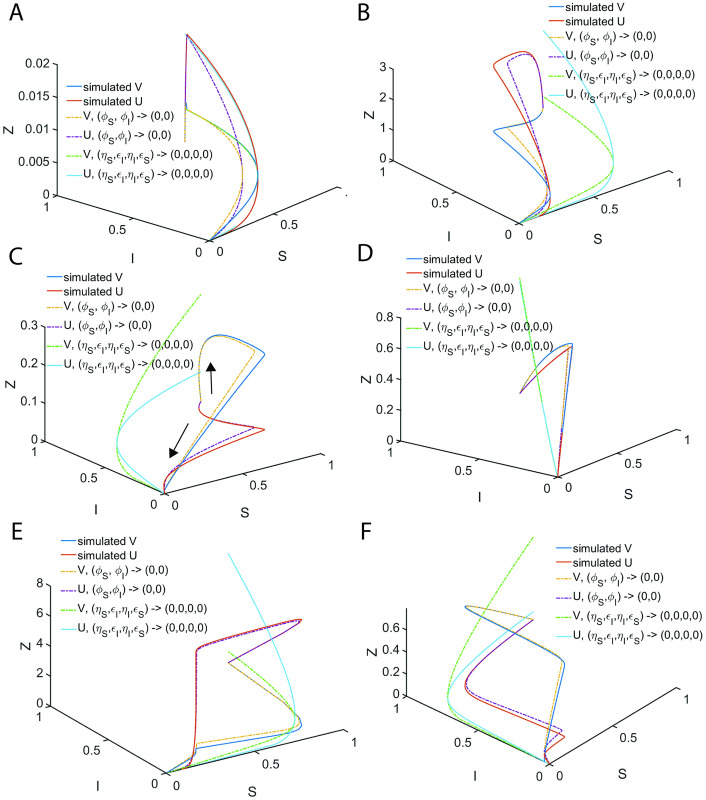
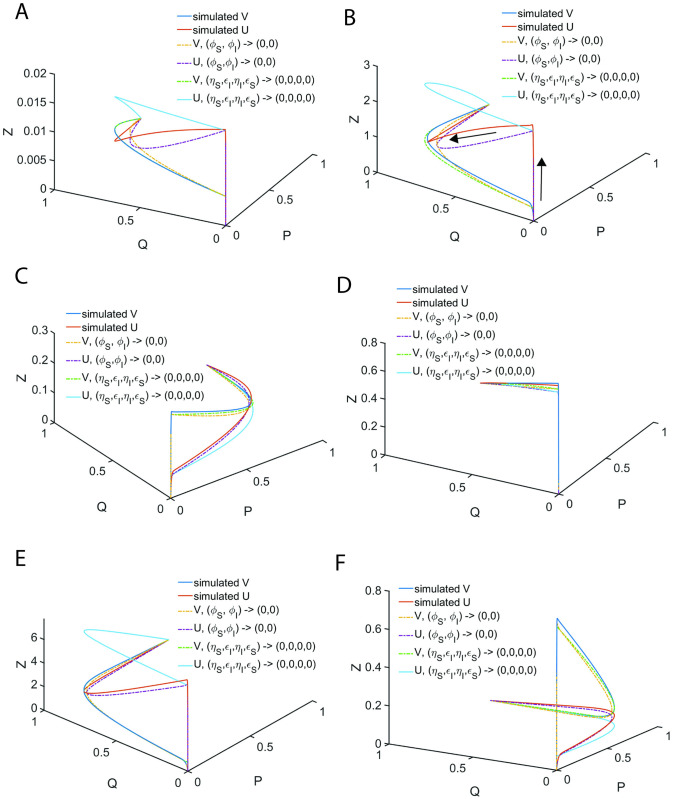
In Eqs 2.2.7–2.2.9, V and U are the dimensionless reaction velocities associated with the substrate and inhibitor conversions into their respective products (P, Q) and the mass conservation laws can be rewritten in the dimensionless velocity-substrate-product spaces as . Further, the transformation rules for the reaction velocities (V, U) are and . Numerically integrated sample trajectories of Eqs 2.2.7–2.2.9 are shown in Fig 2A–2E. Clearly, all the reaction trajectories in the (V, P, S) space fall on the plane V + P + S = 1, and all the reaction trajectories in the (U, Q, I) space fall on the plane as demonstrated in Fig 2B and 2C. Parameters associated with the nonlinear system of Eqs 2.2.7–2.2.9 as defined in Eqs 2.2.2–2.2.6 can be grouped into ordinary and singular ones. Here (εS, κS, εI, κI) are the ordinary perturbation parameters. Further, (ηS, ηI, ρ) are the singular perturbation parameters since they multiply or divide the highest derivative terms. Particularly, (ηS, ηI) decide how fast the system of Eqs 2.2.7–2.2.9 attains the steady state, (κS, κI) decide how fast the enzyme-substrate / inhibitor complexes dissociate and (μS, μI) are the dimensionless Michaelis-Menten type constants which describe the summary of the effects of (ηS, κS, ηI, κI). The relative fastness of the conversion of substrate and inhibitor into their respective products as given in Scheme A of Fig 1 can be characterized by the following critical ratios of the reactions rates.
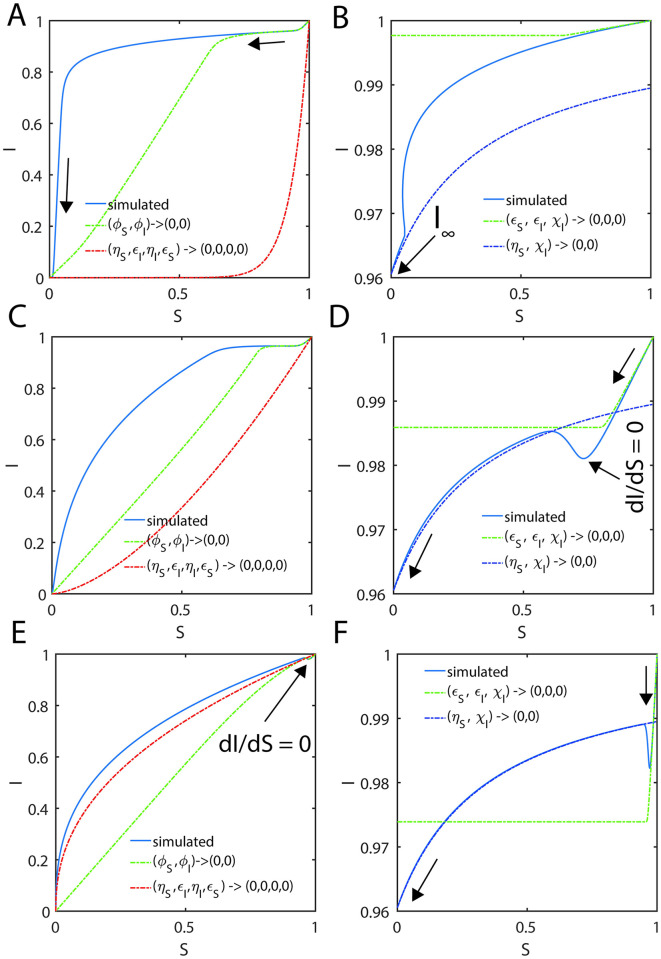
Fig 2. Occurrence of distinct steady state timescales with respect to enzyme-substrate (X) and enzyme-inhibitor (Y) complexes.
Here (S, I, E, X, Y, P, Q) are the dimensionless concentrations of substrate, inhibitor, enzyme, enzyme-substrate, enzyme-inhibitor, product of substrate and product of inhibitor. Trajectories are from numerical integration of Eqs 2.2.7–2.2.9 with the parameters ηS = 0.2, εS = 4.1, κS = 3.1, ρ = 10, ηI = 0.1, εI = 1.2, κI = 0.1 along with the initial conditions (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) at τ = 0. Further, upon fixing ρ, one finds that δ = 0.05, γ = 1.55 and σ = 0.17. Here V = εSX and U = ρεIY are the dimensionless reaction velocities corresponding to the conversion of the substrate and inhibitor into their respective products P and Q. A. The steady states corresponding to the enzyme-inhibitor and enzyme-substrate complexes occur at τCI = 0.03, τCS = 0.31 respectively. We should note that τCI is the time at which and τCS is the time at which . Since τCS ≠ τCI with the current parameter settings, one cannot obtain a common steady state solution to Eqs 2.2.7–2.2.9. B. All the trajectories in the velocity-substrate-product (VPS) space fall within the plane V + P + S = 1. C. All the trajectories in the velocity-inhibitor-product (UQI) space fall within the plane U/ρ + Q + I = 1. D. Sample trajectories in the velocities-inhibitor-substrate (VIS, UIS) and velocities-products spaces (U, P, Q) and (V, P, Q).
| [2.2.12] |
When (σ, γ, ρ) = (1,1,1), then the dynamical aspects of the enzyme-substrate and enzyme-inhibitor complexes will be similar. Here one should note that the parameters (ηS, ηI, εS, εI, ρ, σ) are connected via so that one finds the connection . Similarly, the set of parameters (κS, κI, εS, εI, γ, σ) are connected via so that . In general, the parameters (σ, γ ρ) are connected as follows.
| [2.2.13] |
When the parameters (ηS, ηI, εS, εI, κS, κI) are varied independently, then fixing one parameter in (σ, γ, ρ) eventually fixes the other two parameters. For example, when we fix σ = σf then the corresponding and . The fully competitive enzyme kinetics scheme can exhibit a complex behavior depending on the relative values of the parameters (σ, γ, ρ).
2.3. Variable transformations
Using the substitutions and noting that V = εSX and U = ρεIY, the system of Eqs 2.2.7–2.2.9 can be reduced to the following set of coupled nonlinear second order ODEs in the (P, Q, τ) and first order ODEs in the (V, P, Q) and (U, P, Q) spaces [40].
| [2.3.1] |
| [2.3.2] |
Here the initial conditions are at τ = 0.
| [2.3.3] |
| [2.3.4] |
Here the initial conditions are V = 0; U = 0 at P = 0 and Q = 0. When ρ ≠ 1, ηS ≠ ηI and εS ≠ εI, then the system of Eqs 2.2.7–2.2.9 will have distinct and temporally well separated steady states corresponding to the enzyme-substrate and enzyme-inhibitor complexes. Under such conditions, the system of equations given in Eqs 2.2.7–2.2.9 will not have common steady state solutions both in (V, S, I) and (U, S, I) spaces (as demonstrated in Fig 2A) as given by most of the currently proposed standard QSSAs.
2.4. Standard quasi steady state solutions
Case I: When (ηS, ηI) → (0,0) simultaneously on Eqs 2.2.7 and 2.2.8, then upon noting the fact that V = εSX and U = ρεIY one can obtain the following set of well-known quasi steady state velocity equations in (V, S, I) and (U, S, I) spaces.
| [2.4.1] |
Particularly, these equations approximate the post-steady state dynamics of competitive inhibition scheme A in the (V, S, I) and (U, S, I) spaces. When (εS, εI) → (0,0) along with (ηS, ηI) → (0,0), then one finds that (V, U) ≅ (0,0) along with (P, Q) ≅ (0,0) in the pre-steady state regime. This results in the reactants stationary assumption where we set S ≅ 1 and I ≅ 1 in Eq 2.4.1 and the quasi-steady state velocities become as follows.
| [2.4.2] |
We denote the approximations given in Eq 2.4.2 as V1 and U1. In terms of the original velocity variables (v, u), Eq 2.4.2 can be written as follows.
| [2.4.3] |
Eq 2.4.3 are generally used to obtain the enzyme kinetic parameters such as (KMS, KMI, vmax, umax) from the steady state based fully competitive inhibition experiments via reciprocal plotting methods under the assumptions that (εS, εI) → (0,0) and ρ = 1. Similarly, when the conditions (ηS, ηI) → (0,0) applied on Eqs 2.2.10 and 2.2.11, one can arrive at the following quasi steady state velocities in the (V, P, Q) and (U, P, Q) spaces.
| [2.4.4] |
In these equations, R is the appropriate real root of the cubic equation aR3 + bR2 + cR + d = 0 where the coefficients a, b, c and d are defined as follows.
| [2.4.5] |
| [2.4.6] |
| [2.4.7] |
| [2.4.8] |
Case II: (ηS, ηI, Q) → (0,0,0). When only Q ≅ 0 which can be achieved by setting εI → 0 in the pre-steady state regime along with the conditions that (ηS, ηI) → (0,0), then Eqs 2.2.10 and 2.2.11 can be approximated in the (X, P, S) and (Y, P, S) spaces as follows.
| [2.4.9] |
Upon the substitution of P = 1 − εSX − S in this equation one finds that,
| [2.4.10] |
| [2.4.11] |
Eq 2.4.10 can be derived from Eq 2.4.9, by using the conservation relationship V + P + S = 1 where V = εSX. Upon solving Eqs 2.4.10 and 2.4.11 for (X, Y) and then converting X into V using Eq 2.2.9, one finds the following expressions for the post-steady state reaction velocity in the (V, S) space under the conditions that (ηS, ηI, Q) → (0,0,0).
| [2.4.12] |
Noting that V + P + S = 1, one can express P as function of S, using P = 1 –V–S where V is defined as in Eq 2.4.12. These two equations parametrically express the post-steady state dynamics of the fully competitive inhibition scheme in the (V, P, S) space where S ∈ [0, 1] acts as the parameter. When P ≅ 0 in the pre-steady state regime which can be achieved by setting εS → 0 along with the conditions that (ηS, ηI) → (0,0), then Eqs 2.2.7 and 2.2.8 can be approximated in the (X, Q, I) and (Y, Q, I) spaces as follows.
| [2.4.13] |
| [2.4.14] |
Upon the substitution of Q = 1 − εIY − I in this equation one obtains,
| [2.4.15] |
Eq 2.4.15 can be derived from Eq 2.4.14, by using the conservation relationship U/ρ + Q + I = 1 where U = ρεIY. Upon solving Eqs 2.4.13–2.4.15 for (X, Y) and then converting Y into U, one finds the following expressions for the post-steady state reaction velocity in the (U, I) space under the conditions that (ηS, ηI, P) → (0,0,0).
| [2.4.16] |
Noting that U/ρ + Q + I = 1, one can express Q as function of I, using Q = 1 –U/ρ–I where U is defined as in Eq 2.4.16. These two equations parametrically express the post-steady state dynamics in the (U, Q, I) space where I ∈ [0,1] acts as the parameter.
Case III. When (ηS, ηI, εS, εI) → 0, then one finds that S ≅ 1 − P, I ≅ 1 − Q and (V, U) ≅ (0,0) in the pre-steady state regime from which one can derive the following refined form of sQSSA approximations from Eqs 2.2.7 and 2.2.8. Firstly, by setting (ηS, ηI, εS, εI) → (0,0,0,0) in Eqs 2.2.7 and 2.2.8, one obtains the following set of equations.
| [2.4.17] |
Upon solving this system of equations for (X, Y) and then transforming them into the respective velocities (V, U) using Eq 2.2.9, one obtains the following post-steady state approximations in the (V, P, Q) and (U, P, Q) spaces.
| [2.4.18] |
Similarly, using the substitutions of S ≅ 1 − P and I ≅ 1 − Q, Eq 2.4.18 can be rewritten in the (V, S, I) and (U, S, I) spaces as follows.
| [2.4.19] |
Here and . Eqs 2.4.17–2.4.19 are similar to Eq 2.4.1 where μS and μI are replaced with and . Upon applying the stationary reactant assumptions (S, I) = (1, 1) on Eq 2.4.19 one obtains the refined form of sQSSAs. We will show in the later section that this refined form of sQSSAs can accurately predict the reaction velocities (V, U) over wide range of parameter values. Upon dividing the expression of V by the expression of U in Eqs 2.4.18 and 2.4.19, one can obtain the following differential equation corresponding to the (P, Q) and (S, I) spaces under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0).
| [2.4.20] |
In Eq 2.4.20 which are valid only in the post-steady state regimes, the initial condition in the (P, Q) space will be P = 0 at Q = 0. Similarly, the initial condition in the (S, I) space will be S = 1 at I = 1. We define fS = vmax/(KMS + e0) and fI = umax/(KMI + e0) as the acceleration factors with respect to the conversion dynamics of substrate and inhibitor into their respective products (P, Q). Now let us define the critical control parameter δ as follows.
| [2.4.21] |
Upon solving Eq 2.4.20 with the given initial conditions and using the definition of δ, one obtains the following integral solutions in the (S, I) and (P, Q) spaces [43,44].
| [2.4.22] |
Here δ is the critical parameter which measures the relative speed at which the enzyme-substrate and enzyme-inhibitor complexes attain their steady states. The expression for δ given by Eq 2.4.21 is more refined one compared to those definitions given in Refs. [43,44] and straightforwardly one can show that . The expression for V in Eq 2.4.19 in terms of S and I along with the expression for from Eq 2.4.22 parametrically describe the post steady state dynamics of fully competitive enzyme kinetics in the (V, S, I) space where S ∈ [0,1] acts as the parameter. Similarly, expression for V in terms of P and Q as given in Eq 2.4.18 along with the expression for Q that is given in Eq 2.4.22 parametrically describe the post steady state dynamics in (V, P, Q) space where P ∈ [0,1] acts as the parameter. Upon substituting the expression for Q in terms of P obtained from Eq 2.4.22 into the right-hand side of Eq 2.4.18 and noting that S ≅ (1 − P) and I ≅ (1 − Q) when (εS, εI) → 0, so that and , one can obtain the following approximate differential equations corresponding to the (S, τ) and (I, τ) spaces under the conditions that (ηS, ηI, εS, εI) → 0.
| [2.4.23] |
Eqs 2.4.22 and 2.4.23, can describe the fully competitive enzyme kinetics over the post-steady state regime of (V, S, I) and (U, S, I) spaces strictly under the conditions that (ηS, κS, ρ) = (ηI, κI, 1) apart from (ηS, ηI, εS, εI) → (0,0,0,0). Further, solutions to the variable separated ODEs given in Eq 2.4.23 for the initial conditions (S, I) = (1, 1) at τ = 0 in the (S, τ) and (I, τ) spaces can be implicitly written as follows.
| [2.4.24] |
| [2.4.25] |
When S < 1, then one finds that and and the nonlinear algebraic equation Eq 2.4.24 can be inverted for S under various limiting conditions of δ as follows.
| [2.4.26] |
| [2.4.27] |
| [2.4.28] |
Here W(Z) is the Lambert W function which is the solution of W exp(W) = Z for W [45–47]. Similarly, when I < 1 then one finds that that limδ→0 Iδ → 1 and limδ→∞ Iδ → 0 and the evolution of inhibitor level with time can be derived from Eq 2.4.25 under various values of δ as follows.
| [2.4.29] |
| [2.4.30] |
When δ → ∞, then I → 1 and one finds the following approximate asymptotic expression.
| [2.4.31] |
Expressions similar to Eqs 2.4.23–2.4.31 were proposed earlier to obtain the kinetic parameters from the substrate depletion curves of the fully competitive inhibition scheme [44]. Eqs 2.4.23–2.4.31 are valid only under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0). In such scenarios, the right-hand sides of V and U in Eq 2.4.23 can be expanded around δ ≅ 1 in a Taylor series as follows.
| [2.4.32] |
| [2.4.33] |
Clearly, Eqs 2.4.32 and 2.4.33 reduce to the sQSSA forms given in Eq 2.4.1 only when δ → 1. When δ ≠ 1, then the enzyme-substrate-inhibitor system will exhibit complex dynamics with multiple steady states. This introduces an enormous amount error in sQSSA based parameter estimation from the experimental dataset.
Case IV: When (ηS, ηI, P, Q) → (0,0,0,0) so that S ≅ (1 − εSX) and I ≅ (1 − εIY) in the pre-steady state regime of Eqs 2.2.7 and 2.2.8, then one can arrive at the total QSSA [48,49]. We will derive explicit expressions for tQSSA in the later sections.
2.4.1. Exact steady state solutions
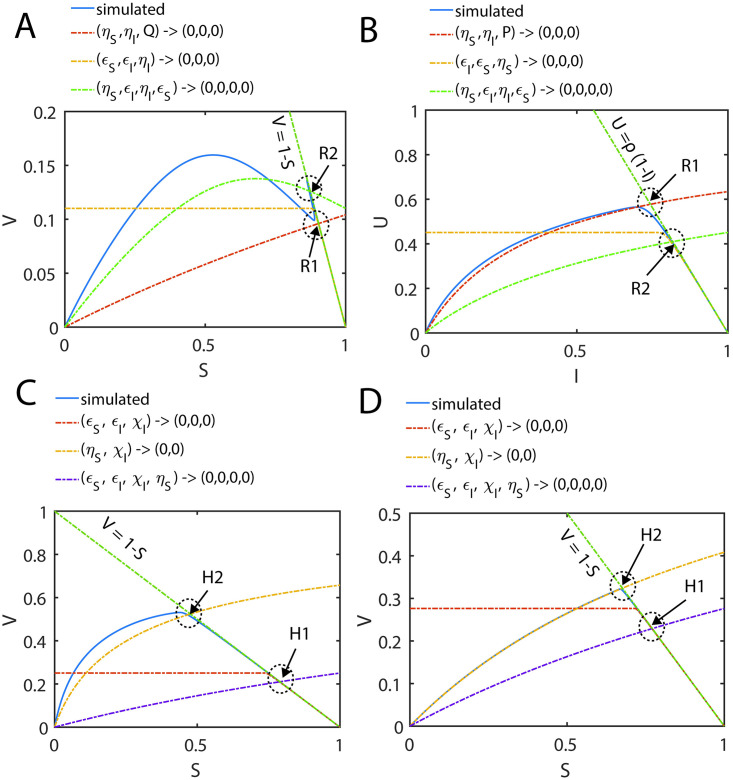
When the steady state timescales associated with the enzyme-substrate and enzyme-inhibitor complexes are different from each other, then Eqs 2.2.7–2.2.9 will not have a common steady state solution with respect to both enzyme-substrate and enzyme-inhibitor complexes. In such scenarios, one can derive exact steady state velocities as follows. Let us assume that the steady state in the (V, P, S) space occurs at τCP at which (V, S, P, U, I, Q) = (VCP, SCP, PCP, UCP, ICP, QCP) and in the (U, Q, I) space it occurs at τCQ at which (V, S, P, U, I, Q) = (VCQ, SCQ, PCQ, UCQ, ICQ, QCQ). Noting the fact that at τCP, and (Fig 3A and 3B) and one can derive the following expression from Eq 2.3.3 by setting and using the conservation laws.
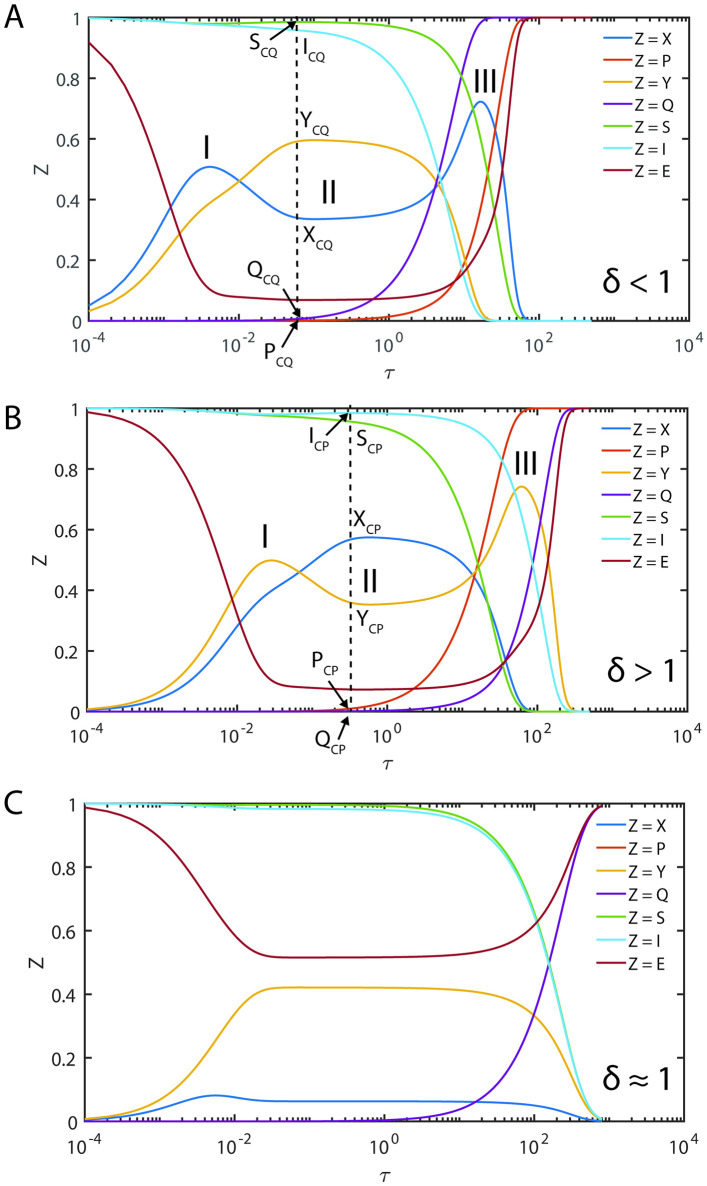
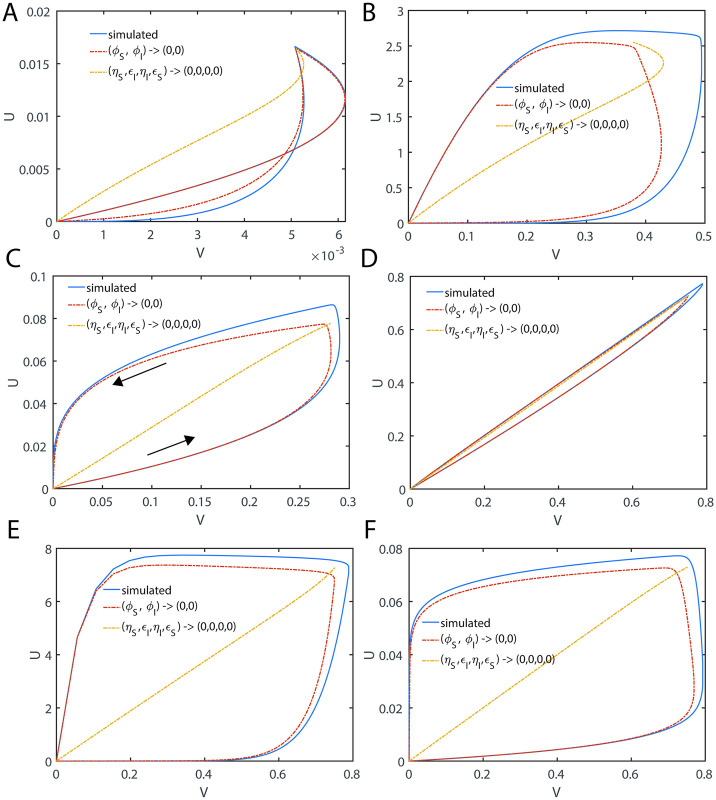
Fig 3. Trajectories of the enzyme kinetics with fully competitive inhibition at different values of δ.
The initial conditions for the simulation of Eqs 2.2.7–2.2.9 are set as (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) at τ = 0. A. Here the settings are ηS = 0.002, εS = 0.04, κS = 0.2, ηI = 0.01, εI = 0.06, κI = 0.1 and ρ = 3.333, σ = 1, δ = 0.1405, ϒ = 3. When δ < 1 and the steady state timescale of the enzyme-substrate complex is lower than the enzyme-inhibitor complex i.e., τCS < τCI, then the evolution of enzyme-substrate complex shows a bimodal type curve with respect to time. Particularly, when σ = 1 and δ > 1 or δ < 1, the temporal evolution of the enzyme-substrate and enzyme-inhibitor complexes show a complex behavior with multiple steady states. Single steady state with respect to Y occurs at (YCQ, QCQ, ICQ) and the corresponding non-steady state values in (V, P, S) space are (VCQ, PCQ, SCQ). B. Here the simulation settings are ηS = 0.02, εS = 0.06, κS = 0.1, ηI = 0.003, εI = 0.04, κI = 0.2 and ρ = 0.225, σ = 1, δ = 9, ϒ = 0.33. When δ > 1 and the steady state timescales of enzyme-substrate complex is higher than the enzyme-inhibitor complex i.e. τCS > τCI, then the evolution of enzyme-inhibitor complex shows a bimodal type curve with respect to time. The single steady state with respect to X occurs at (XCP, SCP, PCP) and the corresponding non-steady state values in the (U, I, Q) space are (UCP, ICP, QCP). C. Here the simulation settings are ηS = 0.02, εS = 0.06, κS = 8.1, ηI = 0.003, εI = 0.04, κI = 1.2 and ρ = 0.225, σ = 1, δ = 1.013, ϒ = 4.5.
| [2.4.1.1] |
Upon solving this equation for VCP, one finds the following expression.
| [2.4.1.2] |
Here (VCP, SCP, PCP) are the steady state values in the (V, P, S) space at τCP. In Eq 2.4.1.2, is the standard Michaelis-Menten type velocity term and is the inhibitor dependent modifying factor. The corresponding non-steady state values in the (U, Q, I) space are (UCP, QCP, ICP). Similarly, one obtains the following steady state equation from Eq 2.3.4 by setting and using the conservation laws of (U, Q, I) space for the enzyme-inhibitor complex at the time point τCQ at which and .
| [2.4.1.3] |
Upon solving this equation for UCQ, one finds the following expression.
| [2.4.1.4] |
Here (UCQ, ICQ, QCQ) are the corresponding steady state values in the (U, Q, I) space with respect to the enzyme-inhibitor complex at τCQ and (SCQ, PCQ, VCQ) are the corresponding non-steady state values in the (V, P, S) space. In Eq 2.4.1.4, is the standard Michaelis-Menten type velocity term and is the substrate dependent modifying factor. However, to find VCP and UCQ which are the exact steady state velocities, one needs to know SCP, UCP, ICQ and VCQ. When the steady state timescales corresponding to the enzyme-substrate and enzyme-inhibitor complexes are the same, then SCP = SCQ, UCP = UCQ, ICQ = ICP and VCQ = VCP as shown in Fig 3C and subsequently Eqs 2.4.1.1–2.4.1.4 reduce to the standard QSSA Eq 2.4.1.
2.4.2. Complexity of the steady states
When δ = 1, then the approximate post-steady state reaction velocities under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) can be given by Eq 2.4.23 which are monotonically increasing (and decreasing) functions of S and I. Approximate steady state velocities (V, U) can be obtained from Eq 2.4.23 by asymptotic extrapolation as (S, I) → (1,1) which is the stationary reactant assumption. When δ ≠ 1, then Eq 2.4.23 will exhibit a turn over type behavior upon increasing (S, I) from (0,0) towards (1,1) with extremum points at which . This dynamical behavior is demonstrated in Fig 3. This means that there are at least two different points at which or within the range (0,0) < (S, I) < (1, 1) depending on the value of δ and sQSSAs given by Eq 2.4.23 are valid only when δ = 1. When δ < 1, then the reaction velocity associated with the enzyme-substrate complex will show two different steady states at which (so that in the (V, S) and (V, P) spaces respectively) as demonstrated in Fig 3A. The velocity expressions given in Eq 2.4.23 corresponding to the stationary reactant assumptions (S, I) → (1,1) approximately represent the first transient steady state in the (V, S) space. The approximate prolonged second steady state corresponding to the enzyme-substrate dynamics can be obtained by solving for S where V is given as in Eq 2.4.23 as follows.
| [2.4.2.1] |
One can obtain PC using the conservation relationship VC + SC + PC = 1. When δ > 1, then the reaction velocity associated with the enzyme-inhibitor complex will show two different steady state regions at which (so that in the (U, I) and (U, Q) spaces respectively) as demonstrated in Fig 3B. The velocity expressions given in Eq 2.4.23 with (S, I) → (1,1) approximately represent the first transient steady state in the (U, I) space. One can obtain the approximate prolonged second steady state velocity corresponding to the enzyme-inhibitor complex UC and the inhibitor concentration IC by solving for I where U is given as in Eq 2.4.23 as follows.
| [2.4.2.2] |
One can obtain QC using the conservation relationship UC/ρ + QC + IC = 1. Here one should note that Eq 2.4.2.1 will not be valid when δ ≥ 1 and Eq 2.4.2.2 will not be valid when δ ≤ 1 since the steady state values can be negative or complex under such conditions. A common steady state can occur only when δ = 1 as demonstrated in Fig 3C and Eq 2.4.23. Remarkably, for the first time in the literature we report this phenomenon and none of the earlier studies on the fully and partial competitive inhibition captured this complex dynamical behavior.
2.5. Solutions under coupled and uncoupled conditions
For the general case, the approximate steady state timescales corresponding to the enzyme-substrate and enzyme-inhibitor complexes can be obtained as follows. Using the scaling transformations and , Eqs 2.3.1 and 2.3.2 can be rewritten in the following form of Murugan equations [10,40] with appropriate initial conditions.
| [2.5.1] |
| [2.5.2] |
| [2.5.3] |
Here ϕS and ϕI are the ordinary perturbation parameters which multiply the nonlinear terms. Eqs 2.5.1 and 2.5.2 along with the initial conditions given in Eq 2.5.3 completely characterize the dynamical aspects of the fully competitive enzyme inhibition scheme. Eqs 2.5.1–2.5.3 are the central equations of this paper from which we will derive several approximations for the pre- and post-steady state regimes under various set of conditions.
2.5.1. Approximate solutions under coupled conditions
When (ϕS, ϕI) → (0,0), then Eqs 2.5.1 and 2.5.2 become coupled linear system of ordinary differential equations as follows.
| [2.5.1.1] |
| [2.5.1.2] |
Eqs 2.5.1–2.5.3 are derived here for the first time in the literature. We denote Eqs 2.5.1–2.5.3 as Murugan type II equations [40] and the φ-approximations given by Eqs 2.5.1.1 and 2.5.1.2 are exactly solvable. Interestingly, these equations can be rewritten in terms of fourth order uncoupled linear ODEs with constant coefficients both in (M, τ) and (N, τ) spaces as follows (see Appendix A in S1 Appendix for details). In (M, τ) space, one can straightforwardly derive the following results.
| [2.5.1.3] |
| [2.5.1.4] |
Upon obtaining the solution for the (M, τ) space, one can straightforwardly obtain the expression corresponding to the (N, τ) space as follows.
| [2.5.1.5] |
The first two initial conditions corresponding to the fourth order uncoupled ODE given by Eq 2.5.1.3 can be written as follows.
| [2.5.1.6] |
Other two initial conditions directly follow from the initial conditions corresponding to N.
| [2.5.1.7] |
| [2.5.1.8] |
Similar to Eq 2.5.1.3, one can also derive the following solution set corresponding to (N, τ) space.
| [2.5.1.9] |
| [2.5.1.10] |
Upon obtaining the solution for the (N, τ) space, one can directly obtain the expression corresponding to the (M, τ) space as follows.
| [2.5.1.11] |
The initial conditions corresponding to the fourth order uncoupled ODEs given by Eq 2.5.1.9 can be written as follows.
| [2.5.1.12] |
| [2.5.1.13] |
| [2.5.1.14] |
Solution to Eqs 2.5.1.1 and 2.5.1.2 can be obtained either by solving Eqs 2.5.1.3–2.5.1.8 or Eqs 2.5.1.9–2.5.1.14. The detailed expressions for the solution are given in Appendix A in S1 Appendix. Upon obtaining solutions in the (M, τ) and (N, τ) spaces, one can revert back to (P, τ) and (Q, τ) spaces using the scaling transformations (P, Q) = εSηS2(M, N) from which one can obtain the parametric expressions for the trajectories in the (V, P, S), (U, I, Q), (V, I, S), (U, I, S), (V, P, Q), (U, P, Q) and (V, U) spaces using appropriate mass conservation relationships where τ acts as the parameter.
2.5.2. Approximate solutions under uncoupled conditions
When along with the conditions of ϕ-approximations as (ϕS, ϕI) → (0,0), then Eqs 2.5.1 and 2.5.2 can be approximated by the following uncoupled set of ODEs.
| [2.5.2.1] |
| [2.5.2.2] |
The conditions will be true when (k3/k1s0, k2/kii0) → (0,0). Here the initial conditions are at τ = 0. Upon reverting these equations back into the (P, Q, τ) space, one obtains the following set of uncoupled ODEs along with the corresponding initial conditions.
| [2.5.2.3] |
| [2.5.2.4] |
Along with the conditions that , the uncoupled Eqs 2.5.2.3 and 2.5.2.4 are valid only (a) when (ϕS, ϕI) → 0 so that there is no competitive inhibition kinetics or (b) the dissociation rate constants of both the enzyme-substrate and enzyme-inhibitor complexes are high enough to uncouple the competitive kinetics i.e., (κS, κI) → (∞,∞). Both these conditions will lead to the approximation E = (1 − X − Y) ≅ 1. We will show later that these conditions decrease the mismatch between the steady state timescales of the enzyme-substrate and enzyme-inhibitor complexes. Upon solving these ODEs with the given initial conditions, we can derive the approximate expressions for the dynamics of the competitive inhibition scheme (P, Q, V, U, S, I) as follows [10,40].
| [2.5.2.5] |
| [2.5.2.6] |
| [2.5.2.7] |
| [2.5.2.8] |
| [2.5.2.9] |
The expressions given in Eqs 2.5.2.5–2.5.2.9 for (V, P, S, τ) space will be valid only when and those expressions given for (Q, U, I, τ) space will be valid only when . Upon solving and for τ in where (V, U) are given as in Eqs 2.5.2.7 and 2.5.2.8, one can obtain the following approximations for the steady state timescales corresponding to substrate and inhibitor conversion dynamics. When , then one finds that,
| [2.5.2.10] |
When , then one finds that,
| [2.5.2.11] |
Here τCS and τCI are the approximate timescales at which the steady states with respect to the enzyme-substrate and enzyme-inhibitor complexes occur under uncoupled conditions. Upon substituting the expression for τCS into the expressions for (V, P, S) given in Eqs 2.5.2.5–2.5.2.9 one can obtain the corresponding steady state values (VC, PC, SC). In the same way, upon substituting the expression for τCI into the expressions for (U, Q, I) one can obtain the corresponding steady state values (UC, QC, IC). Clearly, the condition τCS ≅ τCI is critical for the occurrence of a common steady state with respect to the reaction dynamics of both the enzyme-substrate and enzyme-inhibitor complexes. When τCI > τCS, then the substrate depletion with respect to time will show a typical non-monotonic trend since the inhibitor reverses the enzyme-substrate complex formed before time τCI in the pre-steady state regime. In the same way, when τCI < τCS then the inhibitor depletion will show a non-monotonic behavior since the substrate reverses the enzyme-inhibitor complex formed before time τCS in the pre-steady state regime. These phenomena eventually introduce significant amount of error in various QSSAs. Under uncoupled conditions i.e., when , then one can rewrite the uncoupled approximations given in Eqs 2.5.2.3 and 2.5.2.4 over (V, P) and (U, Q) spaces as follows.
| [2.5.2.12] |
Noting that , one can obtain the ODE corresponding to the (V, S) space as follows.
| [2.5.2.13] |
| [2.5.2.14] |
Noting that , one can obtain the ODE corresponding to the (U, I) space as follows.
| [2.5.2.15] |
Upon considering only the linear, uncoupled portions in the (V, P) space as given in Eq 2.5.2.12, one obtains the following approximations for V and S as functions of P.
| [2.5.2.16] |
Using the conservation laws, one finds the following.
| [2.5.2.17] |
Here RP is the solution of the following nonlinear algebraic equation.
| [2.5.2.18] |
In this equation, . These approximate equations parametrically describe the dynamics of the enzyme catalyzed substrate conversion in the entire regime of (V, P, S) space from (V, P, S) = (0, 0, 1) to (V, P, S) = (0, 1, 0) including the steady states (VC, PC, SC) that occurs at . Here P acts as the parameter. Further, upon solving in Eq 2.5.2.16 one obtains the steady state concentration of the product of substrate as follows.
| [2.5.2.19] |
In this equation, . Upon substituting the expression of PC into the expressions of V and S as given in Eqs 2.5.2.16 and 2.5.2.17, one can obtain the steady state expressions for VC and SC. In the same way, upon considering only the linear, uncoupled portions in the (U, Q) space as given in Eq 2.5.2.14, one obtains the following approximations for U and I as functions of Q.
| [2.5.2.20] |
Using the conservation laws, one finds the following.
| [2.5.2.21] |
Here RQ is the solution of the following nonlinear algebraic equation.
| [2.5.2.22] |
In this equation, . These approximate equations parametrically describe the dynamics of the enzyme catalyzed inhibitor conversion in the entire regime of (U, Q, I) space from (U, Q, I) = (0, 0, 1) to (U, Q, I) = (0, 1, 0) including the steady states (UC, QC, IC) that occurs at . Here Q acts as the parameter. Further, upon solving in Eq 2.5.2.20 one obtains the steady state level of the product of inhibitor as follows.
| [2.5.2.23] |
Here the term b is defined as in Eq 2.5.2.22. Upon substituting the expression of QC for Q into the expressions of U and I as given in Eqs 2.5.2.20 and 2.5.2.21, one can obtain the steady state expressions for UC and IC.
2.6. Approximate pre-steady state solutions
Using the scaling transformation P = εSηS2M one can rewrite the set of coupled nonlinear ODEs corresponding to the fully competitive inhibition scheme given in Eqs 2.5.1 and 2.2.11 in the (M, Y, Q, τ) space as follows.
| [2.6.1] |
Noting that , one finds from Eq 2.2.11 that,
| [2.6.2] |
Here the initial conditions are at τ = 0. Using these equations one can derive the pre-steady state expressions associated with the enzyme-substrate complex under various conditions as follows.
Case I: When (εI, ηI, εS) → (0,0,0), then one finds that I = (1 − εIY − Q) ≅ 1 − εIY since Q ≅ 0 in the pre-steady state regime. Under such conditions one can arrive at the approximation for Y from Eq 2.6.2 as . Upon substituting this expression for Y in Eq 2.6.1 and using the variable transformation , one finally arrives at the following approximate ODEs corresponding to the pre-steady state regimes in the (M, τ) and (F, M) spaces.
| [2.6.3] |
| [2.6.4] |
Case II: When (εI, ηI, ϕS) → (0,0,0) in Eqs 2.6.1 and 2.6.2, then following the same arguments as in Eqs 2.6.3 and 2.6.4, one finds the following refined approximations in the (M, τ) and (F, M) spaces.
| [2.6.5] |
| [2.6.6] |
We will discuss the solutions of Eqs 2.6.5 and 2.6.6 in the later section in detail. Eq 2.6.3 is a linear second order ODE with constant coefficients that is exactly solvable. Upon solving the nonlinear ODE given in Eq 2.6.4 along with the initial condition, one arrives at the following approximate integral solution under the conditions that (εS, ηI, εI) → 0 in the (F, M) space.
| [2.6.7] |
Upon solving Eqs 2.6.3 and 2.6.4 with the given initial conditions as in Eq 2.6.7 and then reverting back to (V, P), (V, S) and (V, τ) spaces using the transformations and using the conservation relationships, we arrive at the following approximate solutions under the conditions that (εS, ηI, εI) → (0,0,0). In the (V, τ) space one finds the following result.
| [2.6.8] |
In the (V, P) space the approximate solution becomes as follows.
| [2.6.9] |
Upon solving Eq 2.6.4 implicitly in the (F, M) space and then reverting back to (V, P) space using the transformation and substituting P = 1 –V–S before the inversion of (Appendix B in S1 Appendix) the hitherto obtained implicit expression in terms of Lambert W function, one obtains the following pre-steady state solution in the (V, S) space under the conditions that (εS, ηI, εI) → (0,0,0).
| [2.6.10] |
The parameters a and b in Eq 2.6.10 are defined as follows.
| [2.6.11] |
By expanding Eq 2.6.10 in a Taylor series around S = 1, one finds that V ≅ 1 − S + Ο((S − 1)2) which means that P ≅ 0 in the pre-steady state regime. It is also interesting to note that all the trajectories in the (V, S) space will be confined inside the triangle defined by the lines V ≅ 1 − S, V = 0 and S = 0. When P or τ becomes sufficiently large, then Eqs 2.6.8 and 2.6.9 asymptotically converge to the following limiting value that is close to the steady state reaction velocity under the conditions that (εS, ηI, εI) → (0,0,0). We denote this approximation as V2.
| [2.6.12] |
We will show in the later sections that this approximation works very well in predicting the steady state reaction velocities over wide ranges of parameters. One can also arrive at Eq 2.6.12 under the conditions that (εS, ηI, εI, ηS) → (0,0,0,0) similar to the refined sQSSA expression given in Eqs 2.4.19. In terms of original variables, this equation can be written as follows.
| [2.6.13] |
Similar to Eqs 2.6.1 and 2.6.2, using the transformation , one can rewrite Eqs 2.5.2 and 2.2.7 as the following coupled system of ODEs.
| [2.6.14] |
Noting that , one finds from Eq 2.2.10 that,
| [2.6.15] |
Here the initial conditions are ; N = 0; X = 0 at τ = 0. Using these equations one can derive the pre-steady state expressions associated with the enzyme-inhibitor complex under various conditions.
Case III: When (εS, ηS, εI) → (0,0,0), then S = (1 − εS X − P) ≅ 1 − εS X and P ≅ 0 in the pre-steady state regime and one finds from Eq 2.6.15 that . Upon substituting this expression of X into Eq 2.6.14, setting (εS, ηS, εI) → 0 and using the transformation in Eqs 2.6.14 and 2.6.15 one can derive the following approximations in the (N, τ) and (G, N) spaces.
| [2.6.16] |
| [2.6.17] |
Case IV: When (εS, ηS, ϕI) → (0,0,0), then following the same arguments with respect to Eqs 2.6.5 and 2.6.6, one finds the following approximations in the (N, τ) and (G, N) spaces.
| [2.6.18] |
| [2.6.19] |
We will discuss the solutions to Eqs 2.6.18 and 2.6.19 in the later section in detail. Eq 2.6.16 is a second order linear ODE with constant coefficients that is exactly solvable. Upon solving the nonlinear ODE given in Eq 2.6.17 along with the initial condition, one can arrive at the following integral solution in the pre-steady state regime in the (G, N) space.
| [2.6.20] |
Upon solving Eqs 2.6.16 and 2.6.17 with the given initial conditions and then reverting back to the (U, Q), (U, I) and (U, τ) spaces using the transformations one finds the following approximate solutions to Eqs 2.6.16 and 2.6.17 under the conditions that (εS, ηI, εI) → (0,0,0). In (U, τ) space one finds the following result.
| [2.6.21] |
In (U, Q) space the approximate solution becomes as follows.
| [2.6.22] |
Upon solving Eq 2.6.17 implicitly in (G, N) space and then reverting back to (U, Q) space using the transformations and substituting Q = 1 –U/ρ–I before the inversion in terms of Lambert W function, one obtains the following pre-steady state solution in the (U, I) space under the conditions that (εS, ηI, εI) → (0,0,0).
| [2.6.23] |
The terms g and h in Eq 2.6.23 are defined as follows.
| [2.6.24] |
Upon expanding the right-hand side of Eq 2.6.23 in a Taylor series around I = 1, one finds that U ≅ ρ(1 − I) + Ο((I − 1)2) which means that Q ≅ 0 in the pre-steady state regime where Eqs 2.6.20–2.6.24 are valid. When Q or τ becomes sufficiently large, then Eqs 2.6.21 asymptotically converges to the following limiting value that is close to the steady state value of U under the conditions that (εS, ηI, εI) → (0,0,0). We denote this approximation as U2. We will show in the later sections that this approximation works very well over wide ranges of parameter values.
| [2.6.25] |
In terms of original variables, this equation can be written as follows.
| [2.6.26] |
This equation is similar to the refined sQSSA given in Eq 2.4.19 that was derived under the conditions that (εI, ηI, εS, ηS) → (0,0,0,0).
2.6.1. Steady state timescales
From the pre-steady state velocity expressions given in Eqs 2.6.8 and 2.6.21, one can find the following approximate steady state timescales associated with the substrate and inhibitor conversion dynamics under coupled conditions.
| [2.6.1.1] |
In terms of original variables, Eq 2.6.1.1 can be written as follows.
| [2.6.1.2] |
Eq 2.6.1.2 are similar to the equations derived in Ref. [44] (see Eqs 34–35 in this reference) for the steady state timescales. However, the numerator terms were set to unity for ρ = 1 and also e0 was not added with (KMS, KMI) in their expressions. When e0 → ∞, then (tCS, tCI) → (0,0) is a reasonable observation from Eq 2.6.1.2. Further, those approximate expressions suggested in Ref. [44] for tCS and tCI predicted that when (i0, s0) → (∞, ∞), then (tCS, tCI) → (0,0). However, when i0 increases, then the probability of binding of substrate with the enzyme will decrease. As a result, when s0 is fixed, then tCS will increase asymptotically towards a limiting value as i0 → ∞. Similarly, when s0 increases, then the probability of binding of inhibitor with the enzyme will decrease towards a minimum. As a result, when i0 is fixed, then tCI will increase asymptotically towards a limiting value as s0 → ∞. In this context, Eq 2.6.1.2 correctly predict the following limiting behaviors of the steady state timescales.
| [2.6.1.3] |
| [2.6.1.4] |
Here one should note that s0 → 0 or i0 → ∞ will have similar limiting behavior on tCS. This is reasonable since setting i0 → ∞ will eventually decreases the binding probability of substrate with the enzyme to a minimum. Similarly, one also finds the following limiting behaviors of tCI.
| [2.6.1.5] |
| [2.6.1.6] |
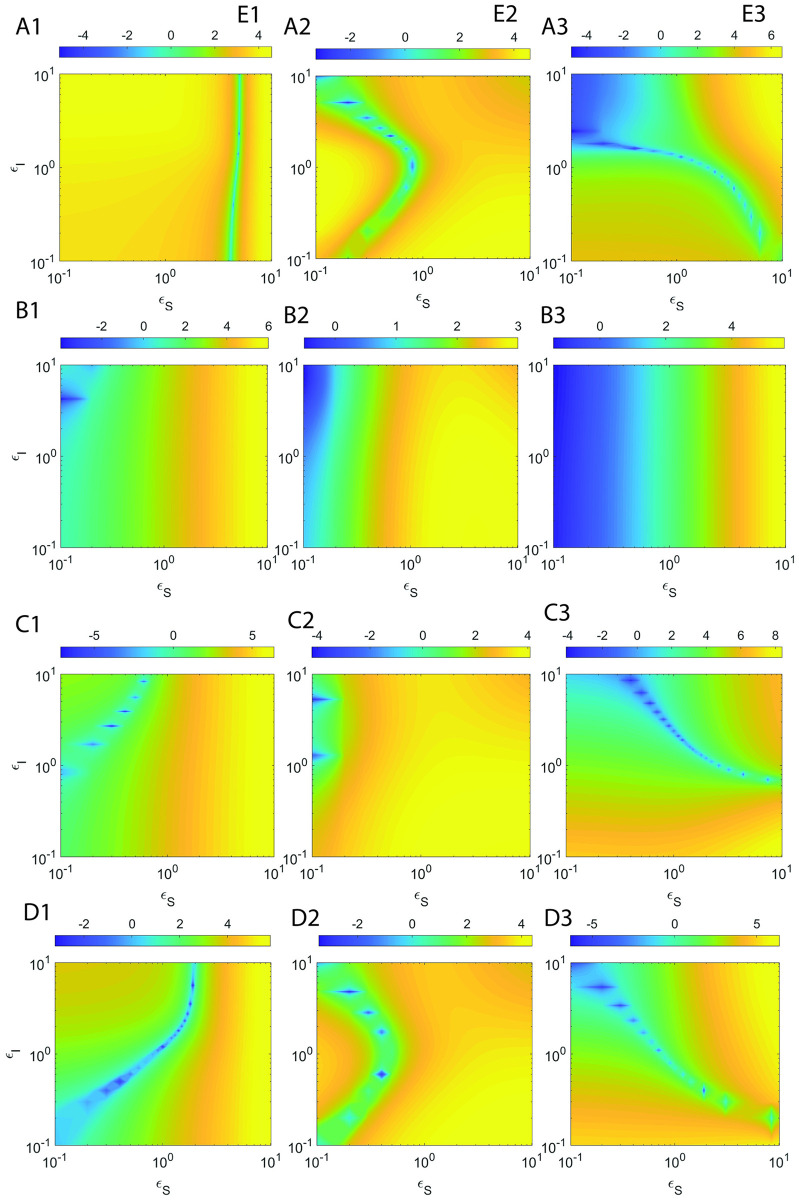
Here one should note that i0 → 0 or s0 → ∞ will have similar limiting behavior on tCI since setting i0 → ∞ will eventually decreases the binding probability of inhibitor with the enzyme. Eqs 2.6.1.3–2.6.1.6 should be interpreted only in the asymptotic sense since setting (s0, i0) = (0, 0) will eventually shuts down the respective substrate or inhibitor catalytic channel. Eq 2.6.1.1 clearly suggest that a common steady state between enzyme-substrate and enzyme-inhibitor complexes can occur only when αI ηS ≅ αS ηI or explicitly when the ratio . When ηS ≅ ηI, then the condition ψ ≅ 1 can be achieved by simultaneously setting large values for any one of the parameters (εS, κS) in the numerator part and any one of the parameters (εI, κI) from the denominator part so that their ratio ψ tends towards one. For example, one can consider setting (εI, κS) → ∞ or a combination (εS, κI) → ∞ and so on. Under such conditions, the error in the estimation of the kinetic parameters using sQSSAs will be at minimum. In general, the condition for the minimal error in sQSSA can also be derived from ψ ≅ 1 in the following form.
| [2.6.1.7] |
Upon considering the connection between the parameters (ρ, γ, σ) as given in Eq 2.2.13 and noting that (ρ, σ) ≅ (1,1) for most of the substrate-inhibitor pairs, the required conditional Eq 2.6.1.7 can be rewritten upon setting ρσ = 1 as follows.
| [2.6.1.8] |
In terms of original variables this conditional equation Eq 2.6.1.8 can be written as follows.
| [2.6.1.9] |
In most of the in vitro quasi-steady state experiments, one sets larger values for (s0, i0) than (e0, KMS, KMI) so that the left-hand side of Eq 2.6.1.9 tends towards unity which is essential (but not sufficient) condition to minimize the error of such QSSAs as suggested [43,44] by most of the earlier studies in a slightly different form as ρ ≅ 1, σ ≅ 1 and εIS ≅ 1. Further, Eqs 2.6.1.8 and 2.6.1.9 will work only when the condition ρσ = 1 is true.
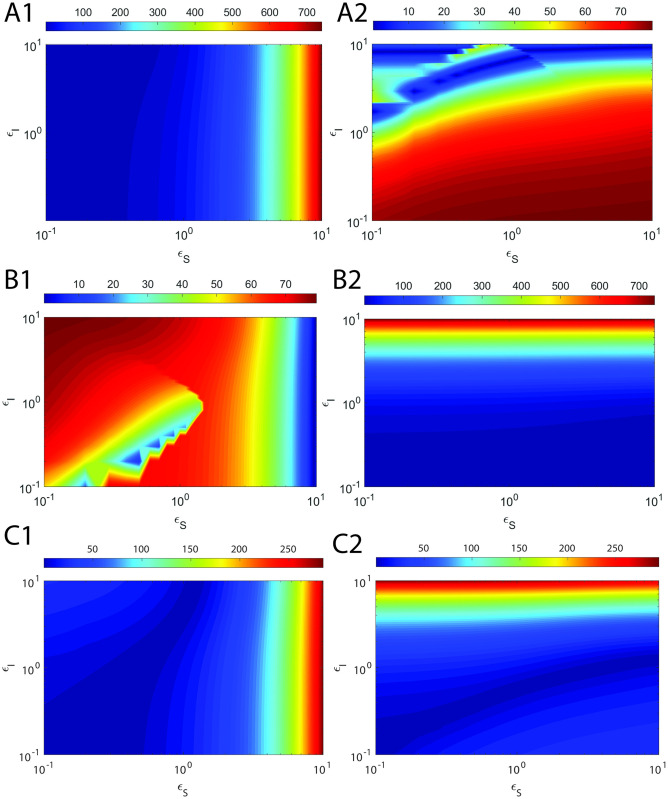
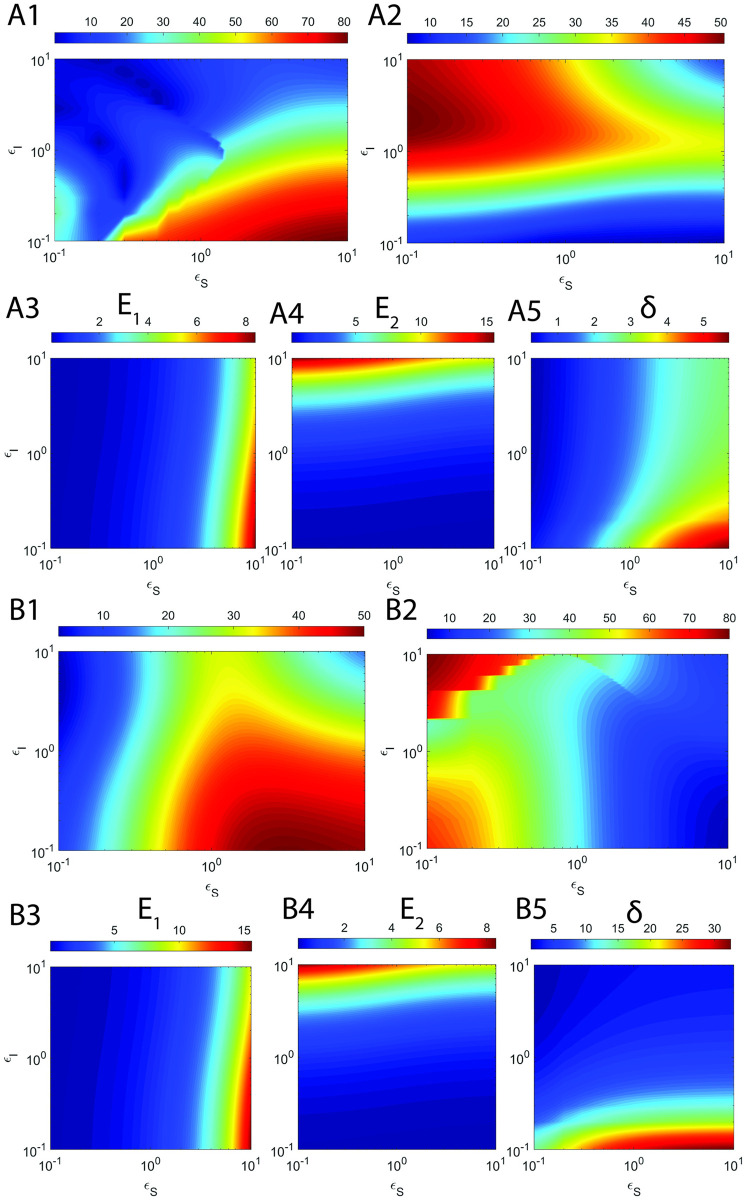
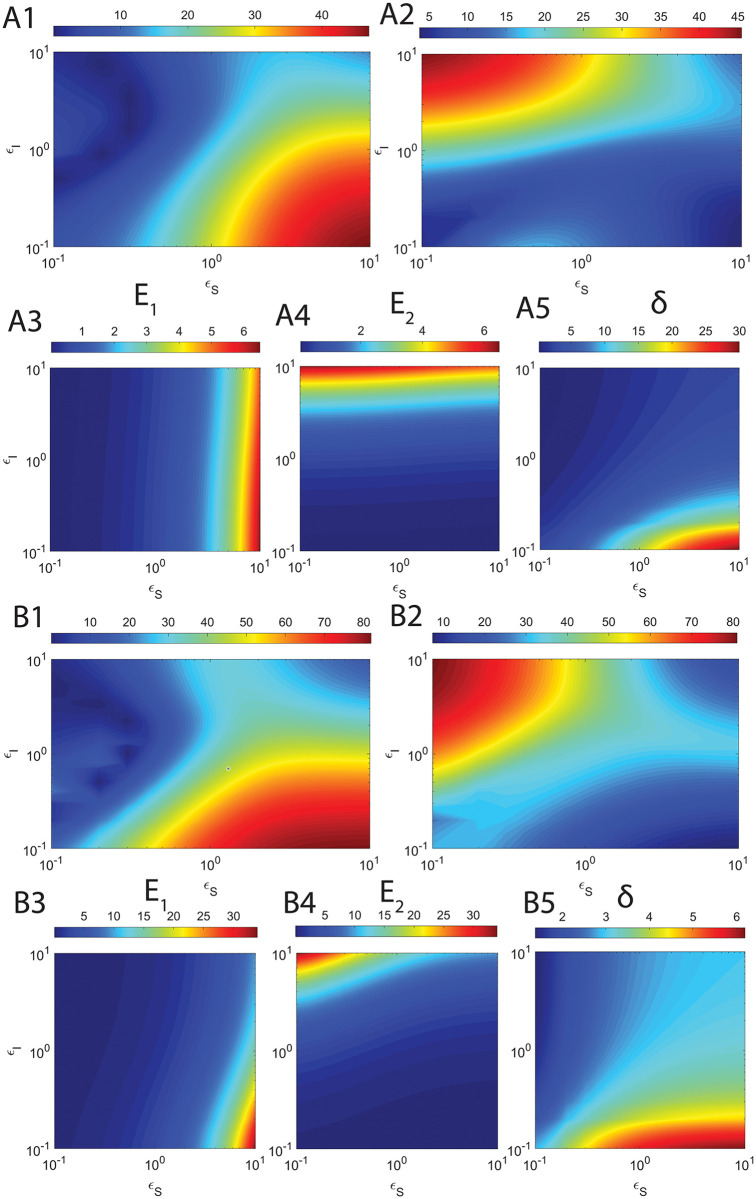
2.7. Minimization of error in sQSSA
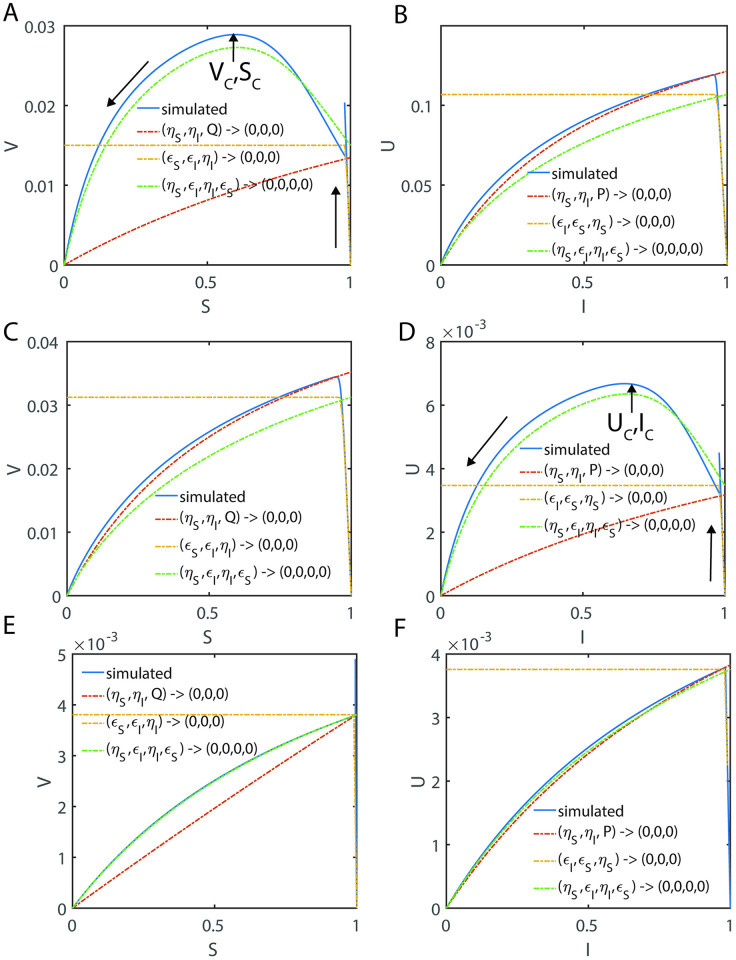
The essential conditions required to minimize the error in various sQSSAs of fully competitive inhibition scheme with stationary reactants assumption can be obtained as follows. The sQSSAs (Eq 2.4.19) describe only the post steady state regime in the (V, S, I) and (U, S, I) spaces and approximate the entire pre-steady state regime with the asymptotic velocities corresponding to (S, I) → (1,1). To extract the enzyme kinetic parameter, one generally uses Eq 2.4.19 with stationary reactant assumptions (S, I) = (1, 1) that does not account for the pre-steady state regime. From the pre-steady state approximations given in Eqs 2.6.8 and 2.6.21 and the refined sQSSAs given in Eq 2.4.19 with (S, I) → (1,1), one can define the overall error associated with the asymptotic approximation of pre-steady state regime by sQSSAs as follows [40].
| [2.7.1] |
| [2.7.2] |
Here τCS and τCI are defined as in Eq 2.6.1.1, HS and HI are the overall errors in the refined sQSSAs on enzyme-substrate and enzyme-inhibitor complexes respectively. Upon solving for εS and for εI after substitution of the approximate expressions for τCS and τCI from Eq 2.6.1.1, and expanding the terms αS, αI, and with their original definitions, one obtains the following expressions for εS,max and εI,max at which the errors due to sQSSAs attain maxima.
| [2.7.3] |
| [2.7.4] |
Clearly, the following generalized conditions i.e., εS ≪ εS,max and εI ≪ εI,max are essential to minimize the error in sQSSAs (refined sQSSA given in Eq 2.4.19 with (S, I) → (1,1)) associated with the enzyme-substrate and enzyme-inhibitor dynamics since (ηS, ηI, εS, εI) → (0,0,0,0) are the preconditions for the validity of sQSSA. One can write these sufficient conditions (we denote them as E1 and E2) explicitly as follows.
| [2.7.5] |
| [2.7.6] |
Clearly, E1 will be true upon setting εS → 0 and E2 will be true upon setting εI → 0. These conditions eventually drive the pre-steady state timescales towards zero leading to less error in sQSSA based parameter estimation. E1 is essential to minimize the error in substrate conversion velocity and E2 is essential to minimize the error in inhibitor conversion velocity. We will show later that there are strong correlations between Eqs 2.7.5 and 2.7.6 and the corresponding observed overall errors in the estimation of (V, U) using the refined form of sQSSAs.
2.8. Approximate time dependent solutions
From Eqs 2.6.5 and 2.6.6 one can derive the refined expressions for the reaction velocity V and product P under the conditions that (εI, ηI, ϕS) → (0,0,0) as follows.
| [2.8.1] |
| [2.8.2] |
Here the terms a, b and c are defined as follows.
| [2.8.3] |
From Eqs 2.6.18 and 2.6.19 one can derive the refined expressions for the reaction velocity U and product Q under the conditions that (εI, ηS, ϕS) → (0,0,0) as follows.
| [2.8.4] |
| [2.8.5] |
Here the terms r, g and d are defined as follows.
| [2.8.6] |
Clearly, one can conclude from Eqs 2.8.1 and 2.8.4 that there exist four different timescales viz. two in the pre-steady state regime and two in the post steady state regimes corresponding to enzyme-substrate and enzyme-inhibitor conversions. We denote them as (τS1, τS2, τI1, τI2). From Eqs 2.8.1 and 2.8.4 one can define these timescales as follows.
| [2.8.7] |
The terms a, b, c, r, g and d are defined as in Eqs 2.8.3 and 2.8.6. Here τS1 and τS2 are the pre-steady state and post-steady state timescales corresponding to enzyme-substrate dynamics and τI1 and τI2 are the pre-steady state and post steady state timescales associated with the enzyme-inhibitor dynamics. The errors in various QSSAs will decrease when the timescale separation ratios tend towards zero.
| [2.8.8] |
Eqs 2.8.1–2.8.7 can approximately describe the dynamics of fully competitive enzyme kinetics scheme over the entire (V, U) space in the parametric form when the conditions associated with Eqs 2.8.1 and 2.8.4 are true.
2.9. Partial competitive inhibition
The differential rate equations corresponding to the Michaelis-Menten type partial competitive inhibition Scheme B can be written as follows.
| [2.9.1] |
| [2.9.2] |
| [2.9.3] |
| [2.9.4] |
Here and the dynamical variables (s, e, x, y, i, p) denote respectively the concentrations (M) of substrate, enzyme, enzyme-substrate complex, enzyme-inhibitor complex and free inhibitor. Further k1 and ki are the respective bimolecular forward rate constants (1/M/s) and k-1 and k-i are the respective reverse rate constants (1/s) and k2 is the product formation rate constant (1/s). Here the initial conditions are (s, e, x, y, i, p) = (s0, e0, 0, 0, i0, 0) at t = 0. The mass conservation laws are e = e0 − x − y; s = s0 − x − p; i = i0 − y. When t → ∞, then the reaction trajectory ends at (s, e, x, y, i, p) = (0, e∞, 0, y∞, i∞, s0) where (e∞, y∞, i∞) are the equilibrium concentrations of free enzyme, enzyme-inhibitor complex and free inhibitor. The steady state of the enzyme-substrate complex occurs at the time point 0 < tCS < ∞ when and the steady state of the enzyme-inhibitor complex occurs at the time point 0 < tCY < ∞ when where one also finds from Eq 2.9.3 that . Similar to the scaling transformations used in Eqs 2.2.7–2.2.9, Eqs 2.9.1–2.9.4 can also be reduced to the following set of coupled dimensionless equations.
| [2.9.5] |
| [2.9.6] |
| [2.9.7] |
| [2.9.8] |
Here (S, E, X, Y, I, P) ∈ [0,1]. The mass conservation relations in the dimensionless form can be written as I = (1 − εI Y), E + X + Y = 1 and V + P + S = 1. Other dimensionless parameters are defined similar to the case of fully competitive inhibition scheme as given in Eqs 2.2.2–2.2.6. Here (χI, ηS) are singular perturbation parameters and (εI, εS, κS, κI) are ordinary perturbation parameters. The initial conditions in the dimensionless space are (S, E, X, Y, I, P) = (1, 1, 0, 0, 1, 0) at τ = 0. When τ → ∞, then the reaction ends at (S, E, X, Y, I, P) = (0, E∞, 0, E∞, I∞, 1) where the terms (E∞, Y∞, I∞) are the final equilibrium concentrations of the free enzyme, enzyme-inhibitor complex and free inhibitor. When (χI, ηS) → (0,0), then Eqs 2.9.5 and 2.9.6 can be equated to zero and solved for (X, Y). Under these conditions, upon converting X into the velocity using V = εS X as given in Eq 2.5.7 one obtains the following sQSSA results.
| [2.9.9] |
Upon applying the stationary reactants assumptions (S, I) → (1,1) under the condition that (ηS, εS, εI, χI) → (0,0,0,0), one finally obtains the following steady state expressions.
| [2.9.10] |
The reaction velocity V in Eq 2.9.10 can be written in terms of the original variables as follows.
| [2.9.11] |
Eq 2.9.11 is generally used to estimate the kinetic parameters from the experimental datasets obtained from partial competitive inhibition experiments using double reciprocal plotting methods where the observed linearly increases with i0. Upon substituting the conservation law I = (1 − εI Y) in Eq 2.9.9 for Y and subsequently solving the resulting quadratic equation for Y, one obtains the following post-steady state approximations in the (V, S) and (Y, S) spaces under the conditions that (χI, ηS) → (0,0).
| [2.9.12] |
By setting I = (1 − εI Y) (where Y is expressed as a function of S as given in Eq 2.9.12) in the expression of V in Eq 2.9.9, one obtains the post steady state approximation in the (V, S) space. Using the mass conservation laws, one can directly obtain the post-steady state approximation for P from P = 1 − V − S. Post-steady state approximation in the (I, S) space can be expressed in a parametric form where S ∈ [0,1] acts as the parameter. One can obtain the exact equilibrium values I∞ and Y∞ by setting S → 0 in Eq 2.9.12 as follows.
| [2.9.13] |
Here we have defined βI = 1 + εI + κI. In Eq 2.9.13, one finds from the mass conservation law that I∞ = 1 − εI Y∞.
2.9.1. Variable transformations
Using the transformation along with the other scaling transformations given as in Eqs 2.2.7–2.2.9 and 2.9.5–2.9.7 can be reduced to the following set of coupled nonlinear second order ODEs in the (P, Y, τ) space.
| [2.9.1.1] |
| [2.9.1.2] |
Here the initial conditions are ; P = 0; Y = 0 at τ = 0. Eqs 2.9.1.1 and 2.9.1.2 can be transformed into the (V, Y, P) and (V, Y, S) spaces using the substitution and associated mass conservation laws as follows.
| [2.9.1.3] |
Particularly, upon substituting P = 1 − V − S in this equation one finds that,
| [2.9.1.4] |
Upon the substitution of in Eq 2.9.1.2 one finds that,
| [2.9.1.5] |
In this equation is defined as follows.
| [2.9.1.6] |
Here the initial conditions are V = 0; P = 0; Y = 0 at τ = 0. Eqs 2.9.1.1–2.9.1.6 completely characterize the partial competitive inhibition scheme in the (V, Y, P) and (V, Y, S) spaces from which we will derive following approximations under various conditions.
2.9.2. Post-steady state approximations
Case I: When (ηS, χI, εS, εI) → 0, then using X = V/ƐS, Eqs 2.9.1.3–2.9.1.5 can be approximated in the (X, Y, P) space as follows.
| [2.9.2.1] |
Upon solving Eqs 2.9.2.1 for (X, Y) and noting that S ≅ 1 − P, I ≅ 1 in such conditions, we obtain the following results similar to Eq 2.4.1 related to the fully competitive inhibition scheme in the (V, S, I) and (Y, S, I) spaces.
| [2.9.2.2] |
Here we have defined . The expression for V in Eq 2.9.2.2 is similar to the one in Eq 2.9.9 where μS and κI are replaced with and and I = 1 in the definition of Y. The post steady state approximation in the (S, I) space can be expressed in a parametric form using I = 1 − εI Y. Here Y is given in terms of S as in Eq 2.9.2.2 where S ∈ [0,1] acts as the parameter. Upon applying the transformation in Eqs 2.9.1.1 and 2.9.1.2, one finally arrives at the following set of coupled nonlinear second order ODEs in the (M, Y, τ) space.
| [2.9.2.3] |
| [2.9.2.4] |
Here the initial conditions are ; M = 0; Y = 0 at τ = 0. Eqs 2.9.2.3 and 2.9.2.4 are the central equations corresponding to the Michaelis-Menten type enzyme kinetics with partial competitive inhibition. Using Eqs 2.9.2.3 and 2.9.2.4, one can derive several approximations under various limiting conditions as follows.
Case II: When (χI, εI, ϕS) → 0, then from Eq 2.9.2.4 one finds that which results in the approximation . Upon substituting this approximation for Y in Eqs 2.9.2.3, and setting ϕS = ηS εS → 0, one arrives at the following second order linear ODE corresponding to the (M, τ) space.
| [2.9.2.5] |
Upon solving this linear ODE for M with respect to the given initial conditions and then reverting back to the velocity V using the transformation , one can obtain the following expression in the (V, τ) space under the conditions that (εI, χI, ϕS) → (0,0,0).
| [2.9.2.6] |
The terms a, b and c in Eq 2.9.2.6 are defined as follows.
| [2.9.2.7] |
Upon solving dV⁄dτ = 0 for τ in Eq 2.9.2.6, one can obtain the following expression for the steady state timescale associated with the enzyme-substrate complex.
| [2.9.2.8] |
In this equation, b is defined as follows.
| [2.9.2.9] |
2.9.3. Pre-steady state approximations
Case III: When ηS → 0, then one obtains the uncoupled equation in (Y, τ) space from Eq 2.9.2.4 as . The integral solution of this first order nonlinear ODE with the initial condition [Y]τ = 0 = 0 can be written as follows.
| [2.9.3.1] |
In this equation . Eq 2.9.3.1 suggests the following the timescale associated with the enzyme-inhibitor complex to attain the steady state under the conditions that ηS → 0.
| [2.9.3.2] |
Clearly, Eqs 2.9.1.3–2.9.1.5 can have common steady states only when τCY = τCS. We will show in the later sections that when τCY > τCS, then the substrate depletion with respect to time will show a typical non-monotonic trend since the inhibitor reverses the enzyme-substrate complex which is formed before the timescale τCY in the pre-steady state regime with respect to the enzyme-substrate complex. This phenomenon introduces significant amount of error in sQSSA. Using the transformation , Eqs 2.9.2.3 and 2.9.2.4 can be rewritten in the (F, Y, M, τ) space with the initial conditions F = 0 at M = 0 and Y = 0 at τ = 0 as follows.
| [2.9.3.3] |
| [2.9.3.4] |
Case IV: When the conditions (εS, χI, εI) → 0 are true, then one can derive the approximate differential rate equations governing the pre-steady state dynamics in the (F, M) and (F, Y) spaces from Eqs 2.9.3.3 and 2.9.3.4 as follows.
| [2.9.3.5] |
The initial condition corresponding to Eq 2.9.3.5 is F = 0 at M = 0. Upon substituting the expression of Y into the differential equation corresponding to the (F, Y, M) space given in Eq 2.9.3.5, one finally obtains the following approximation.
| [2.9.3.6] |
Upon solving Eq 2.9.3.6 with the given initial condition in the (F, M) space, and then reverting back to (V, P) space using the transformations , one finally obtains the following expression for the pre-steady state regime in the (V, P) space under the conditions that (εS, χI, εI) → 0.
| [2.9.3.7] |
To obtain the expression for the (V, S) space one needs to first solve Eq 2.9.3.6 implicitly in the (V, P) space. Then replace P in this implicit solution with the conservation law P = 1 –V–S leading the implicit solution in the (V, S) space that can be inverted for V as a function of S in terms of Lambert W function (see Appendix B in S1 Appendix) as follows.
| [2.9.3.8] |
In this equation, the terms a and b are defined as,
| [2.9.3.9] |
By expanding the right-hand side of Eq 2.9.3.8 in a Taylor series around S = 1, one finds that V ≅ 1 −S + Ο((S − 1)2) which means that P ≅ 0 in the pre-steady state regime. One can directly translate the (V, S) space approximation given in Eq 2.9.3.8 into the pre-steady state of (Y, S) space under the conditions that (εS, χI, εI) → 0 using Eq 2.9.3.5 that results in . But there is a mismatch in the required initial condition for Y in this expression i.e., Y = 0 at S = 1. Particularly, Eq 2.9.3.5 sets the initial condition for Y as at S = 1 (at which V = 0 and therefore F = 0) that is inconsistent since Y ≠ 0 at τ = 0 or S = 1. Detailed numerical analysis of the (Y, S) space trajectories suggests an approximate expression as for the pre-steady state regime under the conditions that (εS, χI, εI) → (0,0,0). Explicitly, one can write this approximation derived from numerical analysis as follows.
| [2.9.3.10] |
Here a and b are defined as in Eq 2.9.3.9. By expanding Eq 2.9.3.10 in a Taylor series around S = 1, one finds that . Using Eq 2.9.3.10 and the conservation law I = (1 − εI Y), one can express I as a function of S in the pre-steady state regime of (I, S) space under the condition that (εS, χI, εI) → 0. Similarly, in (F, τ) space the differential equation Eq 2.9.3.6 can be written as follows.
| [2.9.3.11] |
Upon solving Eq 2.9.3.11 with the given initial condition and then reverting back to (V, τ) space using the transformation , one obtains the following integral solution corresponding to the reaction velocity in the pre-steady state regime.
| [2.9.3.12] |
This equation at τ → ∞ along with Eq 2.9.3.2 suggest the following expressions for the steady state timescale associated with the enzyme-substrate and enzyme inhibitor complexes under the conditions that (εI, χI, εS) → (0,0,0).
| [2.9.3.13] |
Clearly, common steady states between enzyme-substrate and enzyme-inhibitor complexes can occur only when the ratio in Eq 2.9.3.13 becomes as ξ ≅ 1 at which the error associated with various QSSAs will be at minimum. Upon solving the minimum error condition ξ ≅ 1 for βI, one obtains the following two possible roots.
| [2.9.3.14] |
When ηI → 0, then from Eqs 2.9.3.13 and 2.9.3.14 one finds the following expression for ξ.
| [2.9.3.15] |
When ηS ≅ 2χI, then Eq 2.9.3.15 suggests that the condition ξ ≅ 1 can be achieved by simultaneously settings sufficiently large values for any one of the parameters in the numerator (εI, κI) and any one of the parameters in the denominator (εS, χI, κS). When P, S and τ become sufficiently large, then Eqs 2.9.3.7 and 2.9.3.12 asymptotically converge to the following limiting value that is close to the steady state reaction velocity V associated with the partial competitive enzyme kinetics described in Scheme B of Fig 1 in the limit (εS, χI, κS) → (0,0,0). We denote this approximation as V3. We will show in the later sections that this approximation works very well in predicting the steady state reaction velocity of the partial competitive inhibition Scheme B over wide ranges of parameters.
| [2.9.3.16] |
In terms of original variables, Eq 2.9.3.16 can be written as follows.
| [2.9.3.17] |
2.9.4. Error in the sQSSA of the partial competitive inhibition scheme
Similar to Eqs 2.7.1 and 2.7.2, the overall error in the refined form of sQSSA with stationary reactant assumption S ≅ 1 that is given in Eq 2.9.2.2 can be computed using the pre-steady state approximation given in Eq 2.9.3.12 as follows [40].
| [2.9.4.1] |
Here τCS is defined as in Eq 2.9.3.13. Upon solving for εS, one obtains the following expression for εS,max at which the error in the standard QSSA attains maximum. This means that the sufficient condition to minimize such error will be given as follows.
| [2.9.4.2] |
Inequality in Eq 2.9.4.2 (we denote this by E3) can be explicitly written in the following form.
| [2.9.4.3] |
Case IV: When (ηS, χI) → 0, then Eqs 2.9.1.3 and 2.9.1.4 reduce to the following form in the (V, Y, P) and (V, Y, S) spaces.
| [2.9.4.4] |
Upon using the mass conservation law P = 1 − V − S in Eq 2.9.4.4, one obtains the following.
| [2.9.4.5] |
| [2.9.4.6] |
Upon solving Eqs 2.9.4.4–2.9.4.6 for (V, Y), we can obtain the following expressions for V, P, Y and I in terms of S under the conditions that (ηS, χI) → (0,0).
| [2.9.4.7] |
In this equation . We will show later that Eq 2.9.4.7 can predict the post-steady state reaction velocity much better than Eq 2.9.2.2 in the (V, S) space. Noting that V + P + S = 1, one can derive the approximate expression for P in terms of S under the conditions that (ηS, χI) → (0,0) as follows.
| [2.9.4.8] |
Eqs 2.9.4.7 and 2.9.4.8 can be used to generate trajectories in the post-steady state of (V, P), (V, P, S) and (P, S) spaces in the parametric form where S ∈ [0,1] acts as the parameter. Upon solving Eqs 2.9.4.4–2.9.4.6 for Y, the post-steady state approximation in the (Y, S) space can be written as follows.
| [2.9.4.9] |
This equation is more refined one than Eq 2.9.12. Noting that I = (1 − εI Y), one can obtain the following approximate expression for the inhibitor concentration in terms of S corresponding to the post-steady state regime in (I, S) space.
| [2.9.4.10] |
In Eqs 2.9.4.8–2.9.4.10, h is defined as in Eq 2.9.4.7. Solutions obtained under the conditions that (ηS, χI) → (0,0) can approximate the original trajectory in the (V, S) space very well only in the post-steady state regime. By setting S → 0 in Eqs 2.9.4.9 and 2.9.4.10 representing τ → ∞, one obtains the exact equilibrium values of (Y, I) similar to Eq 2.9.12 as follows.
| [2.9.4.11] |
In terms of original variables, the steady state velocity approximation corresponding to the stationary reactant assumption S → 1 can be written from Eq 2.9.4.7 as follows.
| [2.9.4.12] |
Upon inserting the experimental values of e0 and i0 into this equation one can directly extract the values of KMS, KDI and vmax from the data on velocity v versus total substrate concentration s0 using non-linear least square fitting methods.
2.9.5. Steady state substrate and inhibitor levels
Similar to earlier studies [40], one can approximate the steady state substrate concentration by finding the intersection point between the pre- and post-steady state approximations in the (V, S) and (U, I) spaces. In case of partial competitive inhibition under the conditions that (ηS, χI, εS, εI) → 0, the steady state substrate level SC can be obtained by finding the intersection point between the pre-steady state approximation given by Eq 2.9.3.8 and the post steady state approximation given by Eq 2.9.2.2 in the (V, S) space as follows.
| [2.9.5.1] |
In this equation, left hand side is the post-steady state approximation and the right-hand side is the pre-steady state approximation, SC is the intersection point that approximates the steady state substrate concentration and, a and b are defined as in Eq 2.9.3.9. Upon expanding the right-hand side of Eq 2.9.5.1 in a Taylor series around SC = 1 and ignoring the second and higher order terms one finds the following equation for the intersection point between pre- and post-steady state solution in the (V, S) space.
| [2.9.5.2] |
Upon solving this quadratic equation for SC one obtains the following approximation for the steady state substrate level.
| [2.9.5.3] |
By substituting this expression of SC into the post-steady state approximation given in Eq 2.9.2.2, one can obtain the following expression for the steady state velocity VC.
| [2.9.5.4] |
Eqs 2.9.5.1–2.9.5.4 are valid only under the conditions that (ηS, χI, εS, εI) → 0. One can also substitute the approximate value of SC obtained from Eq 2.9.5.3, into Eq 2.9.4.7 to obtain a refined steady state velocity as follows.
| [2.9.5.5] |
In this equation where SC is defined as in Eq 2.9.5.3. More accurate value of SC can be obtained from the intersection point of the post-steady state velocity given by Eq 2.9.4.7 and the pre-steady state velocity given by Eq 2.9.3.8. Particularly, this intersection point is the real root of the following cubic equation that is valid under the conditions that (ηS, χI) → (0,0).
| [2.9.5.6] |
In this equation Eq 2.9.3.8 is approximated as V ≅ 1 − S. In case of fully competitive inhibition scheme, when δ ≠ 1 then the second prolonged steady state levels of (S, I) are given by Eqs 2.4.2.1 and 2.4.2.2. The approximate first transient steady state values can be obtained from the intersections of the pre- and post-steady state solutions given by Eq 2.4.23, Eqs 2.6.10 and 2.6.23. Upon expanding the pre-steady state solutions given in Eq 2.6.10 for (V, S) and Eq 2.6.23 for (U, I) space in a Taylor series around (S, I) = (1,1) and ignoring second and higher order terms, one finds the following equations for the intersection points (SC, IC) of the pre- and post-steady state approximations in the (V, S) and (U, I) spaces.
| [2.9.5.7] |
These equations are valid under the conditions that (ηS, ηI, εS, εI) → 0 irrespective of the values of δ. When δ < 1, then the first steady state value SC = Z in the (V, S) space can be obtained by solving the following equation for Z.
| [2.9.5.8] |
When δ > 1, then the primary steady state value IC = R in the (U, I) space can be obtained by solving the following equation for R.
| [2.9.5.9] |
When δ = 1, then explicit expressions for the steady state substrate level can derived by solving the quadratic equation arising from Eq 2.9.5.8 and choosing the appropriate root as follows.
| [2.9.5.10] |
When δ = 1, then explicit expressions for the steady state inhibitor level can derived by solving the quadratic equation arising from Eq 2.9.5.9 and choosing the appropriate root as follows.
| [2.9.5.11] |
Upon substituting these SC and IC obtained from Eqs 2.9.5.8 and 2.9.5.9 in Eq 2.4.23 for S and I, one can obtain accurate values of the steady state reaction velocities (VC, UC) as follows.
| [2.9.5.12] |
Similar to Eq 2.9.5.6, accurate values of SC and IC can be obtained by finding the intersection points between the post-steady state expressions given in Eqs 2.4.12 and 2.4.16 and pre-steady state approximations V ≅ 1 − S and U ≅ ρ(1 − I). When (ηS, ηI, Q) → (0,0,0), then using Eq 2.4.12, SC can be given by the real root of following equation.
| [2.9.5.13] |
Here . When (ηS, ηI, P) → (0,0,0), then using Eq 2.4.16 the steady state inhibitor concentration IC can be the given by the real solution of the following equation.
| [2.9.5.14] |
Here . Although Eqs 2.9.5.13 and 2.9.5.14 can accurately predict the steady state levels of substrate SC and inhibitor IC, one needs to perform more computations than Eqs 2.9.5.10–2.9.5.12.
2.10. Total QSSA of fully competitive inhibition scheme
When the conditions (ηS, ηI, P, Q) → (0,0,0,0) are true so that in the pre-steady state regime of the fully competitive inhibition scheme described by Eqs 2.2.7–2.2.9, then one can arrive at the total QSSA (tQSSA) [48,49]. In this situation, Eqs 2.2.7–2.2.9 can be approximated as follows.
| [2.10.1] |
| [2.10.2] |
Upon solving Eqs 2.10.1 and 2.10.2 one obtains the following tQSSA expressions for V and U.
| [2.10.3] |
Here R is the appropriate real solution of the following cubic equation.
| [2.10.4] |
In this cubic equation, the coefficients a, b and c are defined as follows.
| [2.10.5] |
| [2.10.6] |
| [2.10.7] |
Here μS = κS + ηS and μI = κI + ηI. The real positive root of the cubic equation Eq 2.10.4 can be written as follows.
| [2.10.8] |
The term H in Eq 2.10.8 is defined as follows.
| [2.10.9] |
Here Z is defined as follows.
| [2.10.10] |
In Eqs 2.10.8–2.10.10, a, b and c are defined as in Eqs 2.10.5–2.10.7. In case of partial competitive inhibition, Eq 2.10.2 becomes as (1 − εI Y)(1 − X − Y) − κI Y ≅ 0 in the limit (ηS, ηI, P) → 0 and therefore the solution set given in Eqs 2.10.3 and 2.10.4 needs to be modified accordingly.
3. Simulation methods
We use the following Euler iterative scheme to numerically integrate the set of nonlinear rate equations given in Eqs 2.2.7–2.2.9 corresponding to the fully competitive inhibition Scheme A.
| [3.1] |
| [3.2] |
| [3.3] |
| [3.4] |
Here the initial conditions are set as (X0, Y0, P0, Q0) = (0, 0, 0, 0) at τ = 0. The trajectories of (S, I, E) can be computed from the trajectories of (X, Y, P, Q) using the mass conservation equations E = 1 –X–Y, S = 1 –εS X–P and I = 1 –εI Y–Q. We use the following Euler iterative scheme to numerically integrate the nonlinear rate equations given in Eqs 2.9.5–2.9.7 corresponding to the partial competitive inhibition Scheme B.
| [3.5] |
| [3.6] |
| [3.7] |
Here the initial conditions are set as (X0, Y0, P0) = (0, 0, 0) at τ = 0. From the trajectories of (X, Y, P) the trajectories of (S, I, E) can be computed using the mass conservation equations E = 1 –X–Y, S = 1 –εSX–P and I = 1 –εIY. We further set Δτ < 10−5 so that the dynamics with respect to the shortest timescale can be captured. We use the following scheme to numerically integrate the coupled nonlinear ODEs given in Eqs 2.5.1 and 2.5.2 under various parameter settings.
| [3.8] |
| [3.9] |
| [3.10] |
| [3.11] |
Here the initial conditions are set as (F0, M0, G0, N0) = (0, 0, 0, 0) at τ = 0. Using the transformations (P, V) = εSηS2 (M, F) and , one can revert back to the original dynamical variables V, U, P and Q. Data in (S, I) space can be obtained using the mass conservation laws S = 1 –V–P and I = 1 –U/ρ–Q. The time at which the steady state occurs was numerically computed from the integral trajectories by looking at the time point at which the first derivatives of (X, Y) with respect to τ change the sign. The trajectories corresponding to the ϕ-approximations can also be directly computed by setting (ϕS, ϕI) → (0,0) in Eqs 3.8–3.11 apart from using the complicated integral solutions given in Appendix A in S1 Appendix.
4. Results and discussion
Competitive inhibition of the Michaelis-Menten enzymes plays critical roles in designing drug molecules against the nodal enzymes of various pathogenic organisms. The relative efficiency of an inhibitor type drug depends on the parameters KMS, vmax, umax and KMI. Estimation of these parameters of a given enzyme-substrate-inhibitor system from the experimental data is critical for the screening of various inhibitor type drug molecules against a given enzyme of pathogen both under in vitro as well as in vivo conditions. Almost all the current experimental methods use the expression derived from sQSSA with stationary reactant assumption i.e., approximation under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) (we denote these conditions as C1). Under these conditions along with the stationary reactants assumption that (S, I) ≅ (1,1) (condition C2), one can approximate the steady state reaction velocities of the fully competitive inhibition scheme as as given in Eq 2.4.3 and for the partial competitive inhibition scheme as given in Eq 2.9.11. Reciprocal plots of the dataset on total substrate s0 and inhibitor i0 concentrations versus steady state reaction velocities (v, u) combined with linear least square fitting procedures will eventually reveal the required kinetic parameter values. Clearly, the overall error associated with this procedure can be minimized only when the inequality conditions E1 and E2 given by Eqs 2.7.5 and 2.7.6, C1 and C2 are true. These mean that Eq 2.4.3 will be valid only when conditions C1, C2, E1, and E2 are all true. In the same way, the inequality condition E3 given by Eq 2.9.4.3 should be true to minimize the error in the sQSSA based estimation of kinetic parameters of the partial competitive inhibition scheme. In this context, we have obtained here several approximate solutions to fully as well as partial competitive inhibition schemes over both pre- and post-steady state regimes under various conditions. These phase-space approximations are summarized in Tables 2 and 3 along with the respective conditions of validity.
Table 2. Phase-space approximations of fully competitive inhibition scheme.
| Conditions | Approximations | Remarks |
|---|---|---|
| (ηS, ηI, εS, εI) → (0,0,0,0) |
. In (V, P, S) space S ∈ [0,1] acts as parameter and P = 1 − V − S. In (U, Q, I) space I ∈ [0,1] acts as parameter and Q = 1 − U/ρ − I. Here I ≅ S1/δ in the (I, S) space and in the (Q, P) space. |
Post steady state regime of (V, S) and (U, I) spaces. Eqs 2.4.22 and 2.4.23. |
| (ηS, ηI, εS, εI) → (0,0,0,0) |
. |
Steady state (secondary) value of (V, P, S) and (U, Q, I) spaces. Eqs 2.4.2.1 and 2.4.2.2. |
| (ηS, ηI, Q) → (0,0,0) |
. In (V, P, S) space S ∈ [0,1] acts as parameter and P = 1 − V − S. |
Post-steady state regime of the (V, S) space. Eq 2.4.12. |
| (ηS, ηI, P) → (0,0,0) |
. In (U, Q, I) space, I ∈ [0,1] acts as the parameter and Q = 1 − U/ρ − I. |
Post-steady state regime of the (U, I) space. Eq 2.4.16. |
| (εS, ηI, εI) → (0,0,0) |
. In (V, P, S) space S ∈ [0,1] acts as parameter and P = 1 − V − S. . |
Pre-steady state regime of the (V, S) space. Eq 2.6.10 |
| (εI, ηS, εS) → (0,0,0) |
. The terms g, R and h are defined as follows. . In (U, Q, I) space I ∈ [0,1] acts as parameter and Q = 1 − U/ρ − I. |
Pre-steady state regime of the (U, I) space. Eq 2.6.23. |
| (ηS, ηI, εS, εI) → (0,0,0,0) |
. In (V, S, I) space, S ∈ [0,1] acts as parameter and I ≅ S1/δ. In (U, S, I) space, I ∈ [0,1] acts as parameter and S ≅ Iδ. |
Post steady state regime in the (V, S, I) and (U, S, I) spaces. Eq 2.4.19. |
| (ηS, ηI, εS, εI) → (0,0,0,0) |
. In (V, P, Q) space, P ∈ [0,1] acts as parameter and . In (U, P, Q) space, Q ∈ [0,1] acts as parameter and P ≅ 1 − (1 − Q)δ. |
Post steady state regime in the (V, P, Q) and (U, P, Q) spaces. Eq 2.4.18. |
Table 3. Phase-space approximations of the partial competitive inhibition scheme.
| Conditions | Approximations | Remarks |
|---|---|---|
| (εI, χI, ϕS) → (0,0,0) |
. . |
Entire pre- and post-steady state regimes of (V, τ) space. Eq 2.9.26. |
| (ηS, χI) → (0,0) | . | Post steady state regime in the (V, S, I), (Y, S, I) spaces. Eq 2.9.9. |
| (ηS, χI, εS, εI) → (0,0,0,0) |
. In (I, S) space, the approximation can be expressed in parametric form where I = 1 − εIY and Y is expressed in terms of S ∈ [0,1]. |
Post-steady state regime in (V, S) and (Y, S) and (I, S) spaces. Eq 2.9.2.2. |
| ηS → 0 |
. . |
Pre-steady state regime of (Y, τ) space. Eq 2.9.3.1 |
| (εS, χI, εI) → (0,0,0) |
. Here . In (V, P, S) space, P ∈ [0,1] acts as parameter and S = 1 –V–P. |
Pre-steady state regime of (V, P) space. Eq 2.9.3.7. |
| (εS, χI, εI) → (0,0,0) |
. R = (a − 1) and the terms a, b and c are defined as follows. . In (V, P, S) space, S ∈ [0,1] acts as parameter and P = 1 –V–S. |
Pre-steady state regime of (V, S) space. Eq 2.9.3.8. |
| (εS, χI, εI) → (0,0,0) |
. . |
Pre-steady state regime of (V, τ) space. Eq 2.9.3.12. |
| (εS, χI, εI) → (0,0,0) |
. . R = (a − 1). In (Y, S, I) and (I, S) spaces, S ∈ [0,1] acts as the parameter and I = 1 − εIY. |
Pre-steady state regime in the (Y, S) space. Eq 2.9.3.10. |
| (ηS, χI) → (0,0) |
. . In (V, P, S) space, S ∈ [0,1] acts as parameter and P = 1 –V–S. |
Post-steady state regime of (V, S) space. Eq 2.9.4.7. |
| (ηS, χI) → (0,0) |
. In (Y, S, I) and (I, S) spaces, S ∈ [0,1] acts as parameter and I = 1 − εIY. |
Post-steady state regime of (Y, S) space. Eq 2.9.4.9. |
| (ηS, χI, εS, εI) → 0 |
|
Steady state values of (V, P, S). Eqs 2.9.5.3 and 2.9.5.4. |
4.1. Refined expressions for sQSSA
Upon observing the asymptotic behavior of the pre-steady state regime approximations in the (V, S) and (V, P) spaces, we obtained refined sQSSA expressions with stationary reactant assumption i.e., as given in Eqs 2.6.13 and 2.6.26 for the fully competitive inhibition scheme and as given in Eq 2.9.4.17 for the partial competitive inhibition scheme. These approximations seem to be applicable even when the condition C1 is violated. Particularly, these refined expressions can approximate the steady state reaction velocities even at high values of (εS, εI). Upon using of these refined expressions, one can significantly reduce the error in the estimation of various enzyme kinetic parameters from the experimental datasets.
4.2. Error in the sQSSA of fully competitive inhibition scheme
The conditions given in C1 can be set to a required value under in vitro scenarios by manipulating the relative concentrations of the enzyme, substrate and inhibitor. Remarkably, Eqs 2.4.2.1 and 2.4.2.2 which deals with the critical parameter δ clearly reveals the validity of the stationary reactant assumptions associated with the condition C2. From Eq 2.4.2.1 one can show that the prolonged secondary steady state substrate level becomes as SC ≅ 1 when δ < 1 and δ → 0. This means that the enzyme-substrate complex will exhibit multiple steady states when δ < 1 and δ → 0. The deviation from the sQSSA of enzyme-substrate complex that represents the primary steady state increases with respect to decrease in δ and subsequently the error in the substrate conversion velocity will be negatively correlated with δ. Whereas, sQSSA works very well for the enzyme-inhibitor complex with single steady state when δ < 1 and δ → 0 (Fig 4A and 4B). Particularly, the approximation given in Eq 2.4.16 under the conditions (ηS, ηI, P) → (0,0,0) works very well in the (U, I) space as shown in Fig 4B. On the other hand, from Eq 2.4.1.2 one can show that the prolonged secondary steady state inhibitor level becomes as IC ≅ 1 when δ > 1 and δ → ∞. This means that the enzyme-inhibitor complex will exhibit multiple steady states when δ > 1 and δ → ∞. The deviation from the sQSSA of enzyme-inhibitor complex that represents the primary steady state increases with respect to increase in δ and subsequently the error in the substrate conversion velocity will be positively correlated with δ. Whereas, sQSSA works very well for enzyme-substrate complex with single steady state when δ > 1 and δ → ∞ (Fig 3C). Particularly, the approximation given in Eq 2.4.12 under the conditions.
Fig 4. Pre-steady state and post-steady state approximations of the enzyme kinetics with fully competitive inhibition in the velocity-substrate (V, S), velocity-inhibitor spaces (U, I) at different values of δ.
The phase-space trajectories start with V = 0 at S = 1 and U = 0 at I = 1, and end at V = 0 at S = 0 and U = 0 at I = 0 with maxima at the steady state. We considered the approximations of (V, U) under the conditions (ηS, ηI, εS, εI) → (0,0,0,0) which is the sQSSA in both (V, S) and (U, I) spaces (Eq 2.4.23, refined forms of sQSSA), (ηS, ηI, Q) → (0,0,0) (Eq 2.4.12) and (ηI, εS, εI) → (0,0,0) (Eq 2.6.10) corresponding to the post and pre-steady state regimes in the (V, S) space, (ηS, εS, εI) → (0,0,0) (Eq 2.6.23) and (ηS, ηI, P) → (0,0,0) (Eq 2.4.16) corresponding to the pre and post steady state regimes of the (U, I) space. Initial conditions for the numerical simulation of Eqs 2.2.7–2.2.9 are set as (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) at τ = 0. A-B. The simulation settings are ηS = 0.002, εS = 0.04, κS = 0.2, ηI = 0.01, εI = 0.06, κI = 0.1 and ρ = 3.333, σ = 1, δ = 0.1405, ϒ = 3. C-D. The simulation settings are ηS = 0.02, εS = 0.06, κS = 0.1, ηI = 0.003, εI = 0.04, κI = 0.2 and ρ = 0.225, σ = 1, δ = 9, ϒ = 0.33. E-F. Here the settings are ηS = 0.02, εS = 0.06, κS = 8.1, ηI = 0.003, εI = 0.04, κI = 1.2 and ρ = 0.225, σ = 1, δ = 1.013, ϒ = 4.5.
(ηS, ηI, Q) → (0,0,0) works very well in the (V, S) space as shown in Fig 4C.
When σ ≅ 1 and δ < 0 or δ > 0, then the steady states of enzyme-substrate-inhibitor system can exhibit a complex behavior as demonstrated in the Section 2.4.2. This means that the widely used sQSSA equations along with the stationary reactant assumption as given in Eq 2.4.3 will be valid only when δ ≅ 1. When δ < 0 or δ > 0, then there is a possibility of multiple steady states and Eq 2.4.3 can capture only the transient first occurring steady state point. One needs to use the velocity equations given in Eqs 2.4.2.1 and 2.4.2.2 to capture the actual second and prolonged steady state point. This phenomenon is demonstrated in Figs 3 and 4. When σ ≅ 1 and δ < 1, then the temporal evolution of the enzyme-substrate complex level will show two different time points (denoted as phases I and II in Fig 3A) at which the trajectory attains maxima where with a local minimum (denoted as phase III in Fig 3A) in between these two maxima.
Similarly, when σ ≅ 1 and δ > 1, then the temporal evolution of the enzyme-inhibitor complex will show up two different time points at which the trajectory attains maximum where with a local minimum (Fig 3B) in between these two maxima points. Common steady states corresponding to the enzyme-substrate and enzyme inhibitor complexes can occur only when δ ≅ 1 as demonstrated in Fig 3C. One can interpret these results as follows. In most of the experimental scenarios, binding of substrate or inhibitor with the respective enzyme will be a diffusion-controlled bimolecular collision process. As a result, one can assume that k1 ≅ ki since the size of the substrate and inhibitor are similar which means that σ ≅ 1. However, the rate of dissociation and conversion into the respective products will depend on the specific bonding and non-bonding interactions at the protein-ligand interfaces of enzyme-substrate and enzyme-inhibitor complexes. In this context, represents the cumulative effects of relative speed of binding, dissociation and conversion into the respective products of enzyme-inhibitor and enzyme-substrate complexes on the overall enzyme catalysis.
Let us define the ratios and as the acceleration factors with respect to the conversion dynamics of substrate and inhibitor into their respective products. When , then the speed of conversion of the enzyme-inhibitor complex Y into the product Q will be faster than the conversion speed of enzyme-substrate complex X into the respective product P. The rapid turn-over of the enzyme-inhibitor complex will eventually causes dissociation of already formed enzyme-substrate complex. As a result, when δ < 1 and σ ≅ 1, then the enzyme-inhibitor complex will show a single steady state and the enzyme-substrate complex will show temporally well-separated two steady states viz. transient primary and prolonged secondary one. This secondary full-fledged steady state can occur only after the depletion of enzyme-inhibitor complex as shown in Fig 3A. On the other hand, when and σ ≅ 1, then the rapid turn-over of the enzyme-substrate complex will eventually causes dissociation of the already formed enzyme-inhibitor complex. As a result, the enzyme-substrate complex will show a single steady state and the enzyme-inhibitor complex will show temporally well-separated two steady states viz. transient primary and prolonged secondary one. This secondary full-fledged steady state can occur only after the depletion of enzyme-substrate complex as shown in Fig 3B. In term of original variables, from which one obtains the limiting condition as . This is a reasonable observation since the fully competitive inhibition scheme will be uncoupled under such limiting condition.
The phase-space behavior of those trajectories described in Fig 3 under the conditions that σ ≅ 1 and δ < 1 or δ > 1 over (V, S) and (U, I) spaces are shown in Fig 4 and over (V, P) and (U, Q) spaces are shown in Fig 5. When δ < 1, then Figs 4A, 4B, 5A and 5B suggest that the first occurring steady state point in the evolution of enzyme-substrate complex will be a transient one and it will be observed as a spike in the (V, S) phase-space plot. As a result, we consider the second occurring prolonged steady state as the original steady state with respect to the enzyme-substrate and enzyme-inhibitor complexes. Clearly, the expression obtained from standard QSSA with the reactants stationary assumption as given by Eq 2.4.3 can be used to obtain the steady state velocities only when σ ≅ 1 and δ ≅ 1. When δ < 1, then using the standard QSSA one can obtain only the steady state velocity of the enzyme-inhibitor complex as shown in Figs 4B and 5B. Similarly, when δ > 1, then only the steady state velocity associated with enzyme-substrate complex can be obtained as shown in Figs 4C and 5C. When δ ≅ 1, then one can obtain the steady state velocities associated with both the enzyme-substrate and enzyme-inhibitor complexes as demonstrated in Figs 4E, 4F, 5E and 5F.
Fig 5. Pre-steady state and post-steady state approximations of the enzyme kinetics with fully competitive inhibition in the velocity-product of substrate (V, P), velocity-product of inhibitor spaces (U, Q) at different values of δ.
The phase-space trajectories start at P = 0 and Q = 0, and end at P = 1 and Q = 1 with maxima at the steady state. We considered the approximations of (V, U) under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) which is the refined standard QSSA in both (V, P) and (U, Q) spaces (Eq 2.4.18), (ηS, ηI, Q) → (0,0,0) (Eq 2.4.12) and (ηI, εS, εI) → (0,0,0) (Eq 2.6.10) corresponding to the post and pre-steady state regimes in the (V, P) space, (ηS, εS, εI) → (0,0,0) (Eq 2.6.23) and (ηS, ηI, P) → (0,0,0) (Eq 2.4.16) corresponding to the pre- and post-steady state regimes of the (U, Q) space. Using the mass conservation laws V + P + S = 1 and U/ρ + Q + I = 1, V and P can be expressed in terms of S in a parametric form and U and Q can be expressed in in terms of I in a parametric form. Initial conditions for the simulation of Eqs 2.2.7–2.2.9 are (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) at τ = 0. A-B. Simulation settings are ηS = 0.002, εS = 0.04, κS = 0.2, ηI = 0.01, εI = 0.06, κI = 0.1 and ρ = 3.333, σ = 1, δ = 0.1405, ϒ = 3. C-D. Here the simulation settings are ηS = 0.02, εS = 0.06, κS = 0.1, ηI = 0.003, εI = 0.04, κI = 0.2 and ρ = 0.225, σ = 1, δ = 9, ϒ = 0.33. E-F. Here the simulation settings are ηS = 0.02, εS = 0.06, κS = 8.1, ηI = 0.003, εI = 0.04, κI = 1.2 and ρ = 0.225, σ = 1, δ = 1.013, ϒ = 4.5.
Irrespective of the values of δ, the expressions corresponding to the pre-steady state dynamics in the (V, S) space under the conditions that (ηI, εS, εI) → (0,0,0) (Eqs 2.6.10 and 2.6.11) and in the (U, I) space under the conditions that (ηI, εS, εI) → (0,0,0) (Eqs 2.6.23 and 2.6.24) can approximate the simulated trajectory very well as demonstrated in Figs 4A–4F and 5A–5F. Interestingly, when δ < 1, then the approximation under the conditions that (ηS, ηI, P) → (0,0,0) as given by Eq 2.4.16 accurately predicts the post-steady state reaction velocity associated with the enzyme-inhibitor complex in the (U, I) space as shown in Figs 4B and 5B. Similarly, when δ > 1, then the approximation under the conditions that (ηS, ηI, Q) → (0,0,0) as given in Eq 2.4.12 can accurately predicts the post-steady state reaction velocity associated with the enzyme-substrate complex in the (V, S) space as shown in Figs 4C and 5C.
4.3. Minimization of error in sQSSA over (V, S) and (U, I) spaces
The overall error associated with the standard QSSA with stationary reactants assumption of the fully competitive enzyme kinetics used in the literature over (V, S) and (U, I) spaces at different values of (εS, εI) and other parameters are shown in Fig 6. Similarly, the error characteristics of the refined form of standard QSSA are shown in Figs 7 and 8. Fig 6 clearly demonstrate the poor performance of sQSSA given in Eqs 2.4.2 and 2.4.3 which are widely used in the literature to obtain the kinetic parameters of fully competitive inhibition systems especially when (κS, κI) ≪ (1,1). We summarize the following essential conditions for the validity of the sQSSA given in Eqs 2.4.2 and 2.4.3 and the refined form of sQSSA given in Eq 2.4.19 with stationary reactant assumptions (S, I) = (1,1). Apparently, these conditions are mandatory to minimize the error in various sQSSAs.
Fig 6. Error associated with the sQSSA with stationary reactant assumption of the fully competitive enzyme kinetics over the velocity-substrate (V, S), velocity-inhibitor spaces (U, I) at different values of εS, εI.
Here δ will vary with respect to each iteration. We considered the error in the approximations of the reaction velocities (V, U) under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) and stationary reactant assumption as defined in Eq 2.4.2. The error was computed as error (%) = 100 |steady state velocities from simulation–approximated velocities| / steady state velocities from simulation. Here the simulation settings are ηS = 0.02, ηI = 0.01 and σ = 1. With these settings, upon fixing σ one finds that and as defined in Eq 2.4.22. A1, B1, C1. Error % in the standard QSSA of V. A2, B2, C2. Error % in QSSA of U. A1-2. κS = 0.1, κI = 1. B1-2. κS = 1, κI = 0.1. C1-2. κS = 1, κI = 1.
Fig 7. Error associated with the refined form of sQSSA with stationary reactant assumption of the fully competitive enzyme kinetics in the velocity-substrate (V, S), velocity-inhibitor spaces (U, I) at different values of εS, εI under the conditions that κS ≠ κI.
Here E1, E2 and δ will vary with respect to each iteration. We considered the error in the approximations of the reaction velocities (V, U) under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) various limiting conditions as defined in Eq 2.4.19 with (S, I) = (1, 1). The error was computed as error (%) = 100 |steady state velocities from simulation–approximated velocities| / steady state velocities from simulation. Here the simulation settings for A1-5 are ηS = 0.02, κS = 1, ηI = 0.01, κI = 0.1 and σ = 1. Simulation settings for B1-5 are ηS = 0.02, κS = 0.1, ηI = 0.01, κI = 1 and σ = 1. With these settings, upon fixing σ one finds that and as defined in Eq 2.4.22 along with the inequality conditions E1, and E2 as defined in Eqs 2.7.5 and 2.7.6. A1, B1. Error % in the QSSA of V. A2, B2. Error % in the QSSA of U. A3, B3. E1 (Eq 2.7.5). A4, B4. E2 (Eq 2.7.6). A5, B5. δ as defined in Eq 2.4.22.
Fig 8. Error associated with the refined form of sQSSA with stationary reactant assumption of the fully competitive enzyme kinetics in the velocity-substrate (V, S), velocity-inhibitor spaces (U, I) at different values of εS, εI under the condition that κS = κI.
Here E1, E2 and δ will vary with respect to each iteration. We considered the error in the approximations given in Eq 2.4.19 with (S, I) = (1, 1). The error was computed as error (%) = 100 |steady state velocities from simulation–approximated velocities| / steady state velocities from simulation. Here the simulation settings for A1-5 are ηS = 0.02, κS = κI = 1, ηI = 0.01 and σ = 1. Similar simulation settings for B1-5 with κS = κI = 0.1. With these settings, upon fixing the value of σ one finds that and as defined in Eq 2.4.22 along with the inequality conditions E1, and E2 as defined in Eqs 2.7.5 and 2.7.6. A1, B1. Error % in QSSA of V. A2, B2. Error % in QSSA of U. A3, B3. E1 (Eq 2.7.5). A4, B4. E2 (Eq 2.7.6). A5, B5. δ as defined in Eq 2.4.22.
| [4.3.1] |
| [4.3.2] |
| [4.3.3] |
| [4.3.4] |
The condition C1 ensure the occurrence of similar steady state timescales associated with the enzyme-substrate and enzyme-inhibitor complexes. C2 is the stationary reactant assumption that is required to approximate the unknown steady state substrate and inhibitor levels. C3 and C4 (following from Eqs 2.7.5 and 2.7.6) are required to minimize the deviations occurring in the pre-steady state regime. Clearly, C3 will be true when εS = (e0/s0) → 0 (or μS = (KMS/s0) → ∞) and C4 will be true when εI → 0 (or μI = (KMI/i0) → ∞). This means that sQSSA of substrate conversion velocity will work well when εS → 0 irrespective of εI and the sQSSA of inhibitor conversion velocity will work well when εI → 0 irrespective of εS. C5 is required to avoid the complex multiple steady state dynamics of enzyme-substrate and enzyme-inhibitor complexes. The error levels of the refined standard QSSA given in Eq 2.4.19 are demonstrated in Figs 7 and 8. Fig 7 show the error levels under the conditions that κS ≠ κI and Fig 7 demonstrate the error levels when κS = κI. Figs 7 and 8 clearly show the error control capability of the refined QSSA expressions given by Eq 2.4.19 in estimating the steady state reaction velocities over Eqs 2.4.2 and 2.4.3. Remarkably, there is a strong correlation between the error levels in the estimated steady state reaction velocities and δ. The error level associated with the steady state velocity of the enzyme-substrate complex is positively correlated with δ. On the other hand, the error levels associated with the steady state velocity of the enzyme-inhibitor complex seem to be negatively correlated with δ. These means that to obtain the accurate estimate of the steady state velocity of enzyme-substrate complex using Eq 2.4.3, one needs to set δ > 1. To obtain the accurate estimate of the steady state velocity of the enzyme-inhibitor complex, one needs to set δ < 1. On the overall basis, we find that the conditions C1, C5 are critical to minimize the error in the refined standard QSSA.
4.4. The φ-approximations
The performance of φ-approximations described by the set of coupled linear ODEs given by Eqs 2.5.1.1 and 2.5.1.2 over the (V, I, S) and (U, I, S) spaces is demonstrated along with the sQSSA trajectories in Fig 9. These parametric solutions were generated with τ as the parameter as given in the integral solutions of Eqs 2.5.1.1 and 2.5.1.2 in Appendix A in S1 Appendix. Clearly, the φ-approximations accurately fit both the pre- and post-steady state regimes especially at large values of (εS, εI). Whereas, the sQSSAs work only in the post steady state regime. Behavior of φ-approximations in the (V, P, Q) and (U, P, Q) spaces are demonstrated in Fig 10 and the performance of φ-approximations in the (V, U) space at various parameter values are demonstrated in Fig 11.
Fig 9. Approximate solutions in the velocity-substrate-inhibitor spaces (V, I, S) and (U, I, S).
We considered the approximations of the reaction velocities (Z = V and U) under the conditions (ηS, ηI, εS, εI) → (0,0,0,0) which is the refined form of standard QSSA as given in Eqs 2.4.19 and 2.6.12 (for the relationship between S and I) and under the conditions (ϕI, ϕS) → (0,0) as given by the solutions of the coupled approximate linear ODEs Eqs 2.5.1.1 and 2.5.1.2 as given in Appendix A in S1 Appendix in a parametric form where τ acts as the parameter. Here the common initial conditions for the numerical simulation of Eqs 2.2.7–2.2.9 are (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) at τ = 0 and other simulation settings are ηS = 0.06, κS = 8.1, ηI = 0.03, κI = 1.2. σ = 1 for (A-D), σ = 0.1 for E and σ = 10 for F. A. εS = 0.08, εI = 0.04, ρ = 1, δ = 0.3083, ϒ = 3.375. B. εS = 13.8, εI = 0.4, ρ = 17.25, δ = 0.1485, ϒ = 0.1957. C. εS = 3.8, εI = 20.4, ρ = 0.093, δ = 3.617, ϒ = 36.24. D. εS = 33.8, εI = 20.4, ρ = 0.8284, δ = 1.031, ϒ = 4.074. E. εS = 33.8, εI = 20.4, ρ = 8.284, δ = 0.1031, ϒ = 0.4074. F. εS = 33.8, εI = 20.4, ρ = 0.0828, δ = 10.31, ϒ = 40.74.
Fig 10. The φ-approximations of the fully competitive enzyme kinetics in the velocity-products spaces (V, P, Q) and (U, P, Q) at different values of δ.
The phase-space trajectories start at P = 0 and Q = 0, and end at P = 1 and Q = 1 with maxima at the steady state. We considered the φ-approximations of (V, U) which are the solutions of Eqs 2.5.1.1 and 2.5.1.2 as given in Appendix A in S1 Appendix in a parametric form where τ act as the parameter and standard QSSA solutions under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) in a parametric form where S acts as the parameter as given in Eqs 2.4.19 and 2.6.12. Common initial conditions for the numerical simulation of Eqs 2.2.7–2.2.9 are (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) at τ = 0 and other simulation settings are ηS = 0.06, κS = 8.1, ηI = 0.03, κI = 1.2. σ = 1 for (A-D), σ = 0.1 for E and σ = 10 for F. A. εS = 0.08, εI = 0.04, ρ = 1, δ = 0.3083, ϒ = 3.375. B. εS = 13.8, εI = 0.4, ρ = 17.25, δ = 0.1485, ϒ = 0.1957. C. εS = 3.8, εI = 20.4, ρ = 0.093, δ = 3.617, ϒ = 36.24. D. εS = 33.8, εI = 20.4, ρ = 0.8284, δ = 1.031, ϒ = 4.074. E. εS = 33.8, εI = 20.4, ρ = 8.284, δ = 0.1031, ϒ = 0.4074. F. εS = 33.8, εI = 20.4, ρ = 0.0828, δ = 10.31, ϒ = 40.74.
Fig 11. The φ-approximations of the fully competitive enzyme kinetics in the velocity spaces (V, U) at different values of δ.
We considered the φ-approximations which are the solutions of Eqs 2.5.1.1 and 2.5.1.2 as given in Appendix A in S1 Appendix in a parametric form where τ act as the parameter and standard QSSA solutions for (V, U) under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) in a parametric form where S acts as the parameter as given in Eqs 2.4.19 and 2.4.22. The trajectory in the (V, U) space starts at (V, U) = (0, 0) and ends at (V, U) = (0, 0). Arrow in C indicates the direction of the trajectory evolution. Common initial conditions for the numerical simulation of Eqs 2.2.7–2.2.9 are (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) at τ = 0 and other simulation settings are ηS = 0.06, κS = 8.1, ηI = 0.03, κI = 1.2. σ = 1 for (A-D), σ = 0.1 for E and σ = 10 for F. A. εS = 0.08, εI = 0.04, ρ = 1, δ = 0.3083, ϒ = 3.375. B. εS = 13.8, εI = 0.4, ρ = 17.25, δ = 0.1485, ϒ = 0.1957. C. εS = 3.8, εI = 20.4, ρ = 0.093, δ = 3.617, ϒ = 36.24. D. εS = 33.8, εI = 20.4, ρ = 0.8284, δ = 1.031, ϒ = 4.074. E. εS = 33.8, εI = 20.4, ρ = 8.284, δ = 0.1031, ϒ = 0.4074. F. εS = 33.8, εI = 20.4, ρ = 0.0828, δ = 10.31, ϒ = 40.74.
4.5. Partial competitive inhibition
Most of the inhibitory drug molecules and the respective experimental systems are partial competitive ones following the Scheme B of Fig 1. These inhibitor molecules competitively bind the active site of the target enzyme against the natural substrate and form reversible dead-end complexes. Upon complete depletion of the substrate level, enzyme-inhibitor complex attains the equilibrium state as demonstrated in Fig 12A. Similar to the fully competitive inhibition scheme, depending on the steady state timescales of the enzyme-substrate and enzyme-inhibitor complexes, partial completive inhibition scheme can also exhibit a complex behavior as demonstrated in Fig 12A. Sample trajectories over (V, S), (Y, S), (I, S), (V, I, S) and (V, P, S) spaces are shown in Fig 12B–12D along with the pre- and post-steady state approximations under the conditions that (εS, χI, εI) → (0,0,0) and (ηS, χI) → (0,0) respectively. When (ηS, χI) → (0,0), then one can approximate the reaction velocity associated with the enzyme-substrate compelx as in the (V, S) space and the level of enzyme-inhibitor compelx as in the (Y, S) space as shown in Eq 2.9.2.2. In the derivation of these equations, we have applied stationary reactant assumption on the inhibitor as I ≅ 1. Upon applying the stationary reactant assumption on the substrate as S ≅ 1, one finally arives at under the conditions that (ηS, χI, εS, εI) → (0,0,0,0). In terms of the original variables, this refined equation for the partial competitive inhbition scheme can be writen as that is given in Eq 2.9.3.17. Remarkably, under the conditions that (ηS, χI) → (0,0) the post-steady state (V, S) space approximation given by Eq 2.9.4.7 seems to be more accurate than the approximation given by Eq 2.9.2.2.
Fig 12. Pre- and post-steady state approximations of the enzyme kinetics with partial competitive inhibition.
Here the simulation settings are ηS = 0.02, εS = 0.1, κS = 0.1, χI = 0.03, εI = 0.06, κI = 0.5. Common initial conditions for the numerical simulation of Eqs 2.9.5–2.9.7 are (S, I, E, X, Y, P) = (1,1,1,0,0,0) at τ = 0. Post-steady state approximations were generated under the conditions that (ηS, χI) → 0 and the pre-steady state approximations were computed under the conditions that (εS, χI, εI) → (0,0,0). A. Simulation trajectories of (S, I, E, X, Y, P). Clearly, (I, E, Y) ends at the equilibrium states (I∞, E∞, Y∞) upon complete depletion of the substrate. When the steady state timescales of X and Y are different, then Y will exhibit a steady state where . B1-3, C and D. Simulated trajectories along with the in the pre- and post-steady state approximations. Post steady state approximations under the conditions that (ηS, χI, εS, εI) → (0,0,0,0) were generated using Eq 2.9.2.2. B1. (Y, S) space trajectory and approximations are computed using Eqs 2.9.3.10 and 2.9.4.9 for the pre and post-steady state regimes respectively. B2. (V, S) space trajectory with approximations using Eqs 2.9.3.8, 2.9.4.7 and 2.9.2.2 corresponding to the pre and post steady state regimes. B3. (S, I) space trajectory and approximations using the mass conservation law I = 1 − εIY (Eqs 2.9.3.10 and 2.9.4.9 with S ∈ [0,1] as the parameter). When , then one finds that , representing a local minimum in the (I, S) space. C. (V, S, I) space approximations (Table 3 for parametric representations). D. (V, P, S) space approximations (Table 3).
In sQSSA expressions which are generally used in the literature, the term e0 will not be added up to KMS and KDI. Similar to Eqs 2.6.13 and 2.6.26, Eq 2.9.3.17 will be valid over wide range of parameters (εS, εI) as demonstrated in Fig 12B–12D. As in Eqs 4.3.1–5, the error in the steady state reaction velocity Eqs 2.9.3.17 can be minimized using the following conditions.
| [4.5.1] |
| [4.5.2] |
| [4.5.3] |
Here the condition G1 is required for the occurrence of common steady states with respect to both the enzyme-substrate and enzyme-inhibitor complexes. The condition G2 is the stationary reactant assumption that is required to approximate the unknown steady state levels of (S, I). The condition G3 ensures the occurrence of minimal error in the pre-steady state regime. Clearly, the condition G3 will be true when εS = (e0/s0) → 0 (or μS = (KMS/s0) → ∞) which means that the sQSSA of the substrate dynamics will work well when εS → 0 irrespective of εI. The overall error in various steady state approximations of the partial competitive inhibition scheme at different parameter settings are demonstrated in Fig 13. We considered the sQSSA given by Eq 2.9.10, refined form of sQSSA given by Eq 2.9.3.16 and the error in Eq 2.9.4.7 with S = 1. Results clearly suggest that the approximations given by Eqs 2.9.10 and 2.9.3.16 works well when (κI, κI) ≫ (1,1). When (κI, κI) ≪ (1,1), then the approximation given by Eq 2.9.4.7 predicts the steady state velocity well.
Fig 13. Error associated with various steady state approximations along with the stationary reactant assumption corresponding to the enzyme kinetics with partial competitive inhibition in the velocity-substrate (V, S) space at different values of εS, εI.
Here E1 (A1, B1, C1 and D1, error in sQSSA given by Eq 2.9.10), E2 (A2, B2, C2 and D2, error in the refined form of sQSSA given in Eq 2.9.3.16) and E3 (A3, B3, C3 and D3, error in Eq 2.9.5.5) are logarithm of percentage errors. The computed error (%) = 100 |steady state velocities from simulation–approximated velocities| / steady state velocities from simulation. A1-3. ηS = 0.02, κS = 0.001, κI = 0.005, ηI = 0.01. B1-3. ηS = 0.02, κS = 1, κI = 5, ηI = 0.01. C1-3. ηS = 0.02, κS = 0.1, κI = 0.5, ηI = 0.01. D1-3. ηS = 0.02, κS = 0.1, κI = 0.1, ηI = 0.01.
4.6. Substrate-inhibitor (S, I) space dynamics
Remarkably, when the timescales associated with the steady states of enzyme-substrate (X) and enzyme-inhibitor (Y) complexes are not the same, then one can show that there exists a regime in the (I, S) space at which (Fig 14). Since I = 1 − εIY and S = 1 − εSX − P for the partial competitive inhibition scheme, one finds that which will be zero at the steady state of Y that occurs at the time point τCY where . Since the steady state timescale of X is different from the steady state timescale of Y, at τCY. One should note that both under the conditions τ = 0 and τ → ∞ where Y approaches its equilibrium value. Building up of the product P will be a monotonically increasing function of time so that over the entire timescale regime except at τ = 0 and τ → ∞ (Figs 12A, 12B3, 14C and 14D). Further, when τ → ∞ which means that when τ → ∞, then in case of partial competitive inhibition.
Fig 14. Pre- and post-steady state approximations in the inhibitor-substrate space for the enzyme kinetics with fully and partial competitive inhibition.
Common initial conditions for the simulation of fully competitive inhibition Eqs 2.2.7–2.2.9 are (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) and for partial competitive inhibition Eqs 2.9.5–2.9.7 are (S, I, E, X, Y, P) = (1,1,1,0,0,0) at τ = 0. For the fully competitive inhibition scheme (A, C, E where trajectories start at (I, S) = (1,1) and end at (I, S) = (0,0)), approximations were computed using Eq 2.4.22 which are valid under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) and the φ-approximations which are the solutions of Eqs 2.5.1.1 and 2.5.1.2 as given in Appendix A in S1 Appendix in a parametric form with τ as the parameter. For the partial competitive inhibition scheme (B, D, F where trajectories start at (I, S) = (1,1) and end at (I,S) = (I∞, 0)), post-steady state approximations were generated under the conditions that (ηS, χI) → 0 and the pre-steady state approximations were computed for (εS, χI, εI) → (0,0,0) using the conservation law I = 1 − εIY (using Eqs 2.9.3.10 and 2.9.4.9 respectively with S ∈ [0,1] as the parameter). Common simulation settings are ηS = 0.02, κS = 0.1, ηI = 0.03, χI = 0.03, κI = 0.5, σ = 1. A-B. εS = 5.5, εI = 0.06, ρ = 137.5, δ = 0.07, γ = 0.022. C-D. εS = 0.5, εI = 0.06, ρ = 12.5, δ = 0.634, γ = 0.024. E-F.εS = 0.05, εI = 0.06, ρ = 1.5, δ = 2.314, γ = 0.24.
In case of fully competitive inhibition scheme, one finds that which follows from I = 1 − εIY − Q. Here the product levels (P, Q) are monotonically increasing functions of time so that throughout the entire timescale regime except at τ = 0 and τ → ∞. Unlike the partial competitive inhibition, in case of fully competitive inhibition scheme at τ = 0 and at τ → ∞. The conditions and will be true when τ → ∞. This means that when τ → ∞, then or undefined. When there is a significant mismatch in the steady state timescales, then one can still observe a time point at which and leading to in the (I, S) space of the fully competitive inhibition scheme. Contrasting from the partial competition, the time at which may not be equal to the steady state timescale corresponding to the enzyme-inhibitor complex. Various ways to approximate the steady state substrate and inhibitor levels described in section 2.9.5 are demonstrated in Fig 15. Results clearly suggest that the pre-steady states in the (V, S) and (U, I) spaces can be well approximated by V ≅ 1 − S and U ≅ ρ(1 − I) and Eqs 2.9.5.13 and 2.9.5.14 can predict the steady state substrate level SC and inhibitor level IC very well. All the trajectories in the (V, S) space will be confined by the triangle formed by the lines V = 1 − S, V = 0 and S = 0. Similarly, all the trajectories in the (U, I) space will be confined by the triangle formed by the lines U = ρ(1 − I), U = 0 and I = 0.
Fig 15. Approximation of the steady state substrate and inhibitor levels corresponding to fully (A, B) and partial competitive (C, D) schemes.
Common initial conditions for the simulation of fully competitive inhibition Eqs 2.2.7–2.2.9 are (S, I, E, X, Y, P, Q) = (1,1,1,0,0,0,0) and for partial competitive inhibition Eqs 2.9.5–2.9.7 are (S, I, E, X, Y, P) = (1,1,1,0,0,0) at τ = 0. A-D clearly show that (P, Q) ≅ (0,0) in the pre-steady state regime so that V ≅ 1 − S and U ≅ ρ(1 − I). These lines intersect the post-steady state approximations near the original steady state. In case of fully competitive inhibition, we considered the intersection (R1, R2) between the post steady state approximations under the conditions that (ηS, ηI, εS, εI) → (0,0,0,0) (Eq 2.4.23), (ηS, ηI, Q) → (0,0,0) (Eq 2.4.16), (ηS, ηI, P) → (0,0,0) (Eq 2.4.12) and the pre-steady state approximations under the conditions that (ηS, εS, εI) → (0,0,0) (Eq 2.6.10) and (ηI, εS, εI) → (0,0,0) (Eq 2.6.23) along with V ≅ 1 − S and U ≅ ρ(1 − I). In case of partial competitive inhibition, we considered the intersections (H1, H2) between the post-steady state approximations under the conditions that (ηS, χI, εS, εI) → (0,0,0,0) (Eq 2.9.2.2) and (ηS, χI) → (0,0) (Eq 2.9.4.7) and the pre-steady state approximations under the conditions that (χI, εS, εI) → (0,0,0,0) (Eq 2.9.3.8) along with V ≅ 1 − S. The settings are as follows. A-B. ηS = 0.02, κS = 1.1, κI = 0.2, ηI = 0.03, εS = 0.6, εS = 0.4, σ = 1, ρ = 2.25, δ = 0.244, ϒ = 3.67. C. ηS = 0.01, κS = 0.01, κI = 0.05, ηI = 0.02, εS = 0.9, εS = 0.5. D. ηS = 0.01, κS = 1, κI = 5, ηI = 0.02, εS = 0.9, εS = 0.5.
5. Conclusion
Fully and the partial competitive inhibition of the Michaelis-Menten enzyme kinetics play critical role in designing drug molecules against the nodal enzymes of various harmful pathogens. Designing of such drug molecules involves screening of various substrate like small molecules which can act as potential inhibitors of the target enzymes. Estimation of various kinetic parameters associated with the competitive inhibition is essential for such comparative studies and evaluation of various potential drug candidates. The currently available standard quasi steady state approximation with stationary reactant assumption is applicable only in the post-steady state regime of the velocity-substrate-inhibitor space and it is significantly limited by the vast number of conditions of validity. Particularly, this approximation will not work when the concentration of the enzyme is equal to or higher than the substrate.
In this context, we have derived several approximations under various conditions of validity over both pre- and post-steady state regimes of the velocity-substrate-inhibitor spaces of fully and partial competitive inhibition schemes. Our detailed analysis yielded refined expressions over the currently available standard quasi steady state approximation with stationary reactants assumption. We have shown that these refined expressions are valid for wide ranges of enzyme to substrate and inhibitor ratios. Further, we have shown for the first time in the literature that the enzyme-inhibitor-substrate system can exhibit temporally well separated two different steady states with respect to both enzyme-substrate and enzyme-inhibitor complexes under certain conditions. When the total substrate and inhibitor levels are higher than the enzyme level, then one can define which plays critical role in controlling the phase-space dynamics associated with the relative speed of binding, dissociation and conversion into the products of the enzyme-inhibitor and enzyme-substrate complexes of the fully competitive enzyme inhibition scheme.
The ratios fS = vmax/(KMS + e0) and fS = umax/(KMS + e0) are the acceleration factors with respect to the conversion dynamics of substrate and inhibitor into their respective products. When , then the speed of conversion of the enzyme-inhibitor complex into the product will be faster than the speed of conversion of the enzyme-substrate complex into the respective product. As a result, the enzyme-substrate complex will exhibit multiple steady states and eventually can reach the full-fledged steady state value only after the depletion of enzyme-inhibitor complex. On the other hand, when δ > 1, then the enzyme-inhibitor complex will exhibit multiple steady states and eventually can reach the full-fledged steady state value only after the depletion of enzyme-substrate complex.
This complicated behavior of enzyme-substrate-inhibitor system especially when δ ≠ 1 poses enormous difficulties in generating consistent experimental datasets on the steady state velocities versus substrate and inhibitor concentrations and also introduces large amount of error in the estimation of various kinetic parameters from these datasets both in the cases of fully and partial competitive inhibitions. Remarkably, our refined expressions for the reaction velocities over enzyme-substrate-inhibitor space can control this error more significantly than the currently available standard QSSA velocity expressions.
Supporting information
(DOCX)
Data Availability
All data are included in the manuscript files.
Funding Statement
The funding was provided by Science and Engineering Research Board (Grants No. CRG/2019/001208 and No. MTR/2019/00002. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alberts B. Molecular biology of the cell. New York: Garland Science; 2002. [Google Scholar]
- 2.Stryer L. Biochemistry. New York: W.H. Freeman; 1988.
- 3.Voet D, Voet JG. Biochemistry. New York: J. Wiley & Sons; 1995. [Google Scholar]
- 4.Fersht A. Enzyme Structure and Mechanism: W.H. Freeman; 1985.
- 5.Michaelis L, Menten ML. {Die kinetik der invertinwirkung}. Biochem Z. 1913;49(333–369):352. [Google Scholar]
- 6.Briggs GE, Haldane JBS. A Note on the Kinetics of Enzyme Action. Biochemical Journal. 1925;19(2):338–9. doi: 10.1042/bj0190338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson KA, Goody RS. The Original Michaelis Constant: Translation of the 1913 Michaelis-Menten Paper. Biochemistry. 2011;50(39):8264–9. doi: 10.1021/bi201284u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornish-Bowden A. One hundred years of Michaelis–Menten kinetics. Perspectives in Science. 2015;4:3–9. 10.1016/j.pisc.2014.12.002 [DOI] [Google Scholar]
- 9.Deichmann U, Schuster S, Mazat J-P, Cornish-Bowden A. Commemorating the 1913 Michaelis–Menten paper Die Kinetik der Invertinwirkung: three perspectives. FEBS Journal. 2014;281(2):435–63. doi: 10.1111/febs.12598 [DOI] [PubMed] [Google Scholar]
- 10.Murugan R. Solution to Michaelis-Menten enzyme kinetic equation via undetermined gauge functions: Resolving the nonlinearity of Lineweaver-Burk plot. Journal of Chemical Physics. 2002;117(9):4178–83. doi: 10.1063/1.1496459 [DOI] [Google Scholar]
- 11.Murray JD. Mathematical biology. New York: Springer; 2002. [Google Scholar]
- 12.Bersani AM, Borri A, Milanesi A, Vellucci P. Tihonov theory and center manifolds for inhibitory mechanisms in enzyme kinetics. Communications in Applied and Industrial Mathematics. 2017;8(1):81–102. doi: 10.1515/caim-2017-0005 [DOI] [Google Scholar]
- 13.Bersani AM, Borri A, Milanesi A, Tomassetti G, Vellucci P. A study case for the analysis of asymptotic expansions beyond the tQSSA for inhibitory mechanisms in enzyme kinetics. Communications in Applied and Industrial Mathematics. 2019;10(1):162–81. doi: 10.1515/caim-2019-0019 [DOI] [Google Scholar]
- 14.Fraser SJ. Slow manifold for a bimolecular association mechanism. J Chem Phys. 2004;120(7):3075–85. doi: 10.1063/1.1634555 . [DOI] [PubMed] [Google Scholar]
- 15.Roussel MR, Fraser SJ. Invariant manifold methods for metabolic model reduction. Chaos. 2001;11(1):196–206. doi: 10.1063/1.1349891 . [DOI] [PubMed] [Google Scholar]
- 16.Dingee JW, Anton AB. A new perturbation solution to the Michaelis-Menten problem. AIChE Journal. 2008;54(5):1344–57. doi: 10.1002/aic.11461 [DOI] [Google Scholar]
- 17.Vogt D. A simplified perturbation solution of Michaelis-Menten kinetics equations in a “total” framework. Journal of Mathematical Chemistry. 2013;51(9):2257–63. doi: 10.1007/s10910-013-0221-0 [DOI] [Google Scholar]
- 18.Dell’Acqua G, Bersani AM. A perturbation solution of Michaelis–Menten kinetics in a “total” framework. Journal of Mathematical Chemistry. 2012;50(5):1136–48. doi: 10.1007/s10910-011-9957-6 [DOI] [Google Scholar]
- 19.Seshadri MS, Fritzsch G. The time evolution of sequential enzyme reactions: a singular perturbation approach. J Theor Biol. 1981;93(1):197–205. doi: 10.1016/0022-5193(81)90064-3 . [DOI] [PubMed] [Google Scholar]
- 20.Segel LA, Slemrod M. The Quasi-Steady-State Assumption: A Case Study in Perturbation. SIAM Review. 1989;31(3):446–77. doi: 10.1137/1031091 [DOI] [Google Scholar]
- 21.Dell’Acqua G, Bersani AM. On the appropriate use of asymptotic expansions in enzyme kinetics. Journal of Mathematical Chemistry. 2014;52(10):2475–81. doi: 10.1007/s10910-014-0403-4 [DOI] [Google Scholar]
- 22.Golicnik M. The integrated Michaelis-Menten rate equation: deja vu or vu jade? J Enzyme Inhib Med Chem. 2013;28(4):879–93. doi: 10.3109/14756366.2012.688039 . [DOI] [PubMed] [Google Scholar]
- 23.Golicnik M. Explicit reformulations of time-dependent solution for a Michaelis-Menten enzyme reaction model. Anal Biochem. 2010;406(1):94–6. doi: 10.1016/j.ab.2010.06.041 . [DOI] [PubMed] [Google Scholar]
- 24.Golicnik M. Explicit reformulations of the Lambert W-omega function for calculations of the solutions to one-compartment pharmacokinetic models with Michaelis-Menten elimination kinetics. Eur J Drug Metab Pharmacokinet. 2011;36(3):121–7. doi: 10.1007/s13318-011-0040-2 . [DOI] [PubMed] [Google Scholar]
- 25.Golicnik M. Explicit analytic approximations for time-dependent solutions of the generalized integrated Michaelis-Menten equation. Anal Biochem. 2011;411(2):303–5. doi: 10.1016/j.ab.2011.01.016 . [DOI] [PubMed] [Google Scholar]
- 26.Schnell S, Mendoza C. Closed Form Solution for Time-dependent Enzyme Kinetics. Journal of Theoretical Biology. 1997;187(2):207–12. 10.1006/jtbi.1997.0425 [DOI] [Google Scholar]
- 27.Stroberg W, Schnell S. On the estimation errors of KM and V from time-course experiments using the Michaelis-Menten equation. Biophys Chem. 2016;219:17–27. doi: 10.1016/j.bpc.2016.09.004 . [DOI] [PubMed] [Google Scholar]
- 28.Kargi F. Generalized rate equation for single-substrate enzyme catalyzed reactions. Biochem Biophys Res Commun. 2009;382(1):157–9. doi: 10.1016/j.bbrc.2009.02.155 . [DOI] [PubMed] [Google Scholar]
- 29.Bajzer Z, Strehler EE. About and beyond the Henri-Michaelis-Menten rate equation for single-substrate enzyme kinetics. Biochem Biophys Res Commun. 2012;417(3):982–5. doi: 10.1016/j.bbrc.2011.12.051 . [DOI] [PubMed] [Google Scholar]
- 30.Tzafriri AR. Michaelis-Menten kinetics at high enzyme concentrations. Bull Math Biol. 2003;65(6):1111–29. doi: 10.1016/S0092-8240(03)00059-4 . [DOI] [PubMed] [Google Scholar]
- 31.Rami Tzafriri A, Edelman ER. Quasi-steady-state kinetics at enzyme and substrate concentrations in excess of the Michaelis-Menten constant. J Theor Biol. 2007;245(4):737–48. doi: 10.1016/j.jtbi.2006.12.005 . [DOI] [PubMed] [Google Scholar]
- 32.Atkins GL, Nimmo IA. A comparison of seven methods for fitting the Michaelis-Menten equation. Biochem J. 1975;149(3):775–7. doi: 10.1042/bj1490775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lineweaver H, Burk D. The Determination of Enzyme Dissociation Constants. Journal of the American Chemical Society. 1934;56(3):658–66. doi: 10.1021/ja01318a036 [DOI] [Google Scholar]
- 34.Grima R, Walter NG, Schnell S. Single-molecule enzymology a la Michaelis-Menten. FEBS J. 2014;281(2):518–30. doi: 10.1111/febs.12663 . [DOI] [PubMed] [Google Scholar]
- 35.Vu NT, Song YM, Tran QT, Yun HY, Kim SK, Chae JW, Kim JK. Beyond the Michaelis-Menten: Accurate Prediction of Drug Interactions Through Cytochrome P450 3A4 Induction. Clin Pharmacol Ther. 2023;113(5):1048–57. Epub 20230111. doi: 10.1002/cpt.2824 . [DOI] [PubMed] [Google Scholar]
- 36.Back H-m Yun H-y, Kim SK, Kim JK. Beyond the Michaelis-Menten: Accurate Prediction of In Vivo Hepatic Clearance for Drugs With Low KM. Clinical and Translational Science. 2020;13(6):1199–207. doi: 10.1111/cts.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi B, Rempala GA, Kim JK. Beyond the Michaelis-Menten equation: Accurate and efficient estimation of enzyme kinetic parameters. Sci Rep. 2017;7(1):17018. Epub 20171205. doi: 10.1038/s41598-017-17072-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borghans JM, De Boer RJ, Segel LA. Extending the quasi-steady state approximation by changing variables. Bulletin of Mathematical Biology. 1996;58(1):43–63. doi: 10.1007/BF02458281 [DOI] [PubMed] [Google Scholar]
- 39.Li B, Shen Y, Li B. Quasi-Steady-State Laws in Enzyme Kinetics. The Journal of Physical Chemistry A. 2008;112(11):2311–21. doi: 10.1021/jp077597q [DOI] [PubMed] [Google Scholar]
- 40.Murugan R. Theory on the rate equation of Michaelis-Menten type single-substrate enzyme catalyzed reactions. Journal of Mathematical Chemistry. 2018;56(2):508–56. doi: 10.1007/s10910-017-0791-3 [DOI] [Google Scholar]
- 41.Federico R, Angelini R, Ercolini L, Venturini G, Mattevi A, Ascenzi P. Competitive inhibition of swine kidney copper amine oxidase by drugs: amiloride, clonidine, and gabexate mesylate. Biochem Biophys Res Commun. 1997;240(1):150–2. doi: 10.1006/bbrc.1997.7630 . [DOI] [PubMed] [Google Scholar]
- 42.Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology. 2009;150(2):1025–32. Epub 20081009. doi: 10.1210/en.2008-0188 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubinow SI, Lebowitz JL. Time-dependent Michaelis-Menten kinetics for an enzyme-substrate-inhibitor system. J Am Chem Soc. 1970;92(13):3888–93. doi: 10.1021/ja00716a009 . [DOI] [PubMed] [Google Scholar]
- 44.Schnell S, Mendoza C. Time-dependent closed form solutions for fully competitive enzyme reactions. Bull Math Biol. 2000;62(2):321–36. doi: 10.1006/bulm.1999.0156 . [DOI] [PubMed] [Google Scholar]
- 45.Corless RM, Gonnet GH, Hare DEG, Jeffrey DJ, Knuth DE. On the LambertW function. Advances in Computational Mathematics. 1996;5(1):329–59. doi: 10.1007/BF02124750 [DOI] [Google Scholar]
- 46.Abramowitz M, Stegun IA. Handbook of mathematical functions, with formulas, graphs, and mathematical tables. New York: Dover Publications; 1965. [Google Scholar]
- 47.Olver FWJ, National Institute of S, Technology. NIST handbook of mathematical functions. Cambridge: Cambridge University Press Cambridge; 2010. [Google Scholar]
- 48.Pedersena MG, Bersani AM, Bersani E. The total quasi-steady-state approximation for fully competitive enzyme reactions. Bull Math Biol. 2007;69(1):433–57. Epub 2006/07/20. doi: 10.1007/s11538-006-9136-2 . [DOI] [PubMed] [Google Scholar]
- 49.Tzafriri AR, Edelman ER. The total quasi-steady-state approximation is valid for reversible enzyme kinetics. J Theor Biol. 2004;226(3):303–13. Epub 2003/12/04. doi: 10.1016/j.jtbi.2003.09.006 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All data are included in the manuscript files.