Abstract
Measles virus (MV) has a tropism restricted to humans and primates and uses the human CD46 molecule as a cellular receptor. MV has been adapted to grow in chicken embryonic fibroblasts (CEF) and gave rise to an attenuated live vaccine. Hallé and Schwarz MV strains were compared in their ability to infect both simian Vero cells and CEF. Whereas both strains infected Vero cells, only the CEF-adapted Schwarz strain was able to efficiently infect CEF. Since the expression of the human MV receptor CD46 rendered the chicken embryonic cell line TCF more permissive to the infection by the Hallé MV strain, the MV entry into CEF appeared to be a limiting step in the absence of prior MV adaptation. CEF lacked reactivity with anti-CD46 antibodies but were found to express another protein allowing MV binding as an alternative receptor to CD46.
Measles virus (MV) was first isolated in 1954 by Enders and Peebles from blood taken from a patient with a typical case of measles (8). This isolate, named Edmonston, was subjected to serial passages in human kidney cells and human amnion cells prior to being successfully transferred into chicken embryos (16) and chicken embryonic fibroblasts (CEF) (14). This adapted virus strain became the progenitor for subsequent measles vaccines (see reference 13 for a review). Understanding the molecular mechanisms of the MV attenuation resulting from a historical empirical process should focus on molecular targets for the rational design of new vaccines against measles.
MV belongs to the Morbillivirus genus, Paramyxoviridae family, and Mononegavirales order. Humans are the only known natural hosts of MV, although the virus can infect and induce disease in some primates. This restricted tropism is thought to reflect the use of the human and simian CD46 molecules as cellular receptors (6, 17). The virus envelope is made of two membrane glycoproteins, the hemagglutinin (H), responsible for binding to the host cell by its direct interaction with the CD46 molecule (see reference 11 for a review), and the fusion protein (F), which mediates fusion of viral and cell membranes and nucleocapsid penetration. The viral synthesis occurs in the cytoplasm, and the infectious particles are released by budding at the cell surface.
Although MV is monotypic, several MV strains can be distinguished by the nature of the host cell used for their isolation and propagation. Wild-type strains are typically isolated on human or simian lymphoblastoid cell lines and are considered pathogenic (23). In contrast, adapted or laboratory strains are isolated on human or simian fibroblastic or epithelial cell lines. Among these adapted strains, some have lost, after serial passage in chicken cells, their in vivo virulence and are known as attenuated strains. During this adaptation, the pressure of the new host cell environment may have the potential to generate phenotypic modification. In order to understand the molecular basis of this new phenotype, we have compared the MV Hallé strain, which is highly related to the Edmonston strain, and the vaccine Schwarz strain for their ability to infect simian Vero cells and CEF.
Kinetics of infection of CEF and Vero cells.
Simian Vero cells and primary CEF were grown in Dulbecco’s modified Eagle’s medium containing 6% heat-inactivated fetal calf serum, 10 mM HEPES, and 2 mM glutamine and supplemented for CEF with 5 × 10−5 M 2-mercaptoethanol, 10% tryptose phosphate broth, 2% heat-inactivated chicken serum, and 50 μg of gentamicin per ml. The cells were infected, at a multiplicity of infection (MOI) of 0.1, with two MV strains, the Hallé laboratory strain (26) propagated in Vero cells, and the Schwarz vaccine strain (kindly provided by Pasteur Mérieux Connaught) maintained by serial passages in CEF. The percentage of infected cells and the amounts of cell-associated virus produced and virus released into the supernatant were determined over time by flow cytometry analysis, after labelling with antihemagglutinin monoclonal antibodies (17), and the 50% tissue culture infective dose (TCID50) method, respectively (Fig. 1).
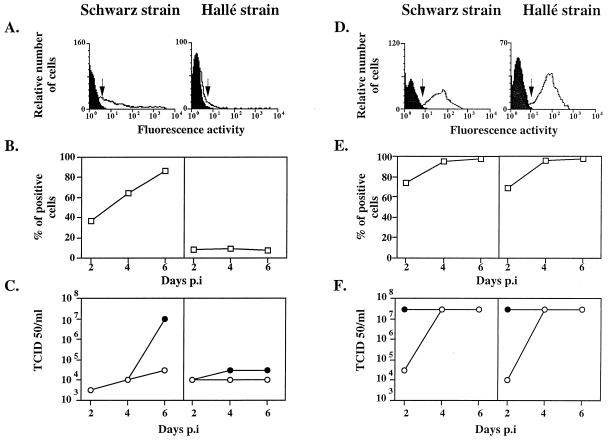
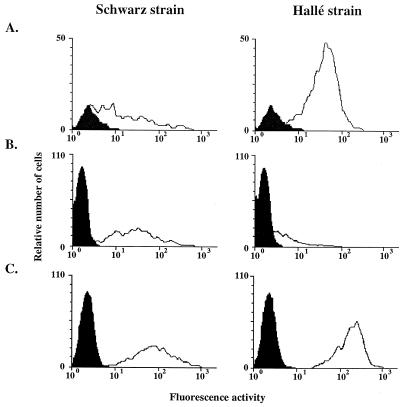
FIG. 1.
Kinetics of infection of CEF and Vero cells. CEF (A to C) and Vero cells (D to F) were infected at an MOI of 0.1 with the Schwarz or Hallé strain of MV. (A and D) Expression of the hemagglutinin H, 4 days p.i., is shown by the shift between the white histogram and the black control histogram. The lower limit, where cells were scored as positive, is indicated by arrows. (B and E) Kinetics of H expression up to 6 days p.i. expressed as the percentages of positive cells. (C and F) Kinetics of infectious particles spontaneously released in the supernatant (open circles) and total production, i.e., cell-associated plus released virus (closed circles).
Vero cells were similarly permissive for both MV strains with more than 95% of cells expressing MV hemagglutinin 4 days postinfection (p.i.) (Fig. 1D and E), and a total virus production of 3 × 107 TCID50/ml on day 4 (Fig. 1F). The kinetics of infection of CEF by the Schwarz strain was slower, with only 65% of cells being infected after 4 days (Fig. 1A and B) and 86% being infected by day 6 (Fig. 1B). Accordingly, the virus progeny was delayed, reaching 107 TCID50/ml on day 6 with little release of virus into the supernatant (Fig. 1C). This suggests that the virus budding process could be inefficient in CEF. Alternatively, the CEF could favor the production of defective interfering particles and reduce the yield of infectious particles, as observed with other host cells (25).
CEF were poorly permissive to the infection by the Hallé strain, with less than 10% of cells being infected on day 4 (Fig. 1A and B) and a low total viral production of 3 × 104 TCID50/ml on day 6 (Fig. 1C). Thus, the potency of the Schwarz MV strain in totally invading the CEF suggests that, during the adaptation process, the envelope glycoproteins have been selected for efficient entry into CEF. MV entry into cells requires a precise dynamic molecular scaffold involving the binding of H to the receptor, an appropriate pairing of H and F, and conformational change in the receptor, H, and/or F protein (2, 4, 5, 10). Therefore, any structural change in the H and/or the F glycoprotein could promote the fusion step with the CEF plasma membranes. Because the hemagglutinin is responsible for the binding step (reference 5 and this study), it is reasonable to suggest that a hemagglutinin mutant has been selected during the serial passage in CEF, as described for influenza A(H1N1) virus (21). Likewise, an F glycoprotein mutant could have been selected. Indeed, Borges et al. have reported that the Schwarz MV strain differs from the Edmonston strain by its inability to lyse simian erythrocytes, a process known to involve the F protein after the H-CD46 interaction (1). Moreover, the Schwarz MV strain formed small plaques after infection of Vero cells, in contrast to the Hallé and Edmonston virus strains, which induced extensive cell-cell fusion (reference 1 and data not shown). A comparison of the primary sequences of H and F proteins from the Hallé and Schwarz strains revealed only five differences (Phe117 to Leu117 and Gly546 to Ser546 for the H protein and Ala166 to Thr166, Arg266 to Gly266, and Ser365 to Tyr365 for the F protein). Among these sequence variations, only the Ser546 of the H glycoprotein has been found to be a common feature of every attenuated vaccine strain grown in chicken cells (22). Although these differences are limited, they may have subtle effects on the three-dimensional structures which could modulate their interactions with their protein partner.
Effect of CD46 expression at the surface of chicken fibroblasts on MV replication.
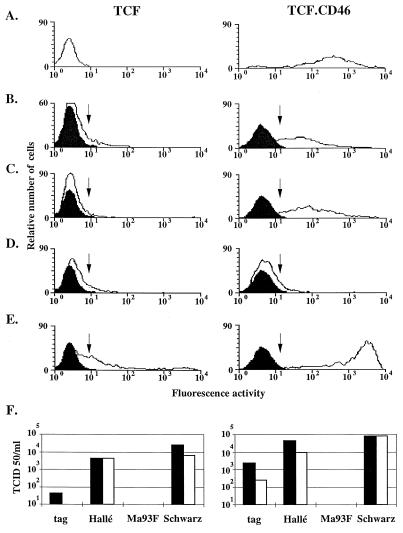
A chicken fibroblast line (TCF) monolayer (kindly provided by Rhône Mérieux) (8 × 105 cells) was transfected with the expression vector Apex-CD46, encoding the C2 isoform of CD46 subcloned downstream of the cytomegalovirus promoter, into the end-filled XbaI site of the Apex vector (9). The TCF cells were found to behave similarly to CEF for MV binding and growth. One TCF clone, stably expressing CD46 (TCF.CD46), was then infected, at an MOI of 1, with four MV strains, tag (derived from Edmonston B) (20), Hallé, Ma93F (15), and Schwarz. TCF.CD46 cells were labelled by the monoclonal anti-CD46 antibody MCI20.6 (Fig. 2A). The TCF.CD46 cells appeared more permissive than the parental TCF cells to infection by both the tag and the Hallé strains with 59 and 79% of TCF.CD46 cells expressing H compared to 11 and 5% of TCF cells, respectively (Fig. 2B and C). The total production of infectious viral particles was also increased by CD46 expression from 45 to 2,500 and 4,400 to 44,000 TCID50/ml for tag and Hallé strains, respectively (Fig. 2F). In agreement with the inability of Ma93F hemagglutinin to down-regulate the expression of CD46 (15), the expression of CD46 did not increase the permissiveness of chicken cells for this MV strain (Fig. 2D). This further indicates that Ma93F MV may use a receptor different from CD46. The low permissivity of chicken fibroblasts for Hallé, tag, and Ma93F MV strains is in agreement with the failure of the virulent Edmonston strain to propagate in CEF (3). Since the expression of CD46 rendered chicken TCF cells more permissive to infection by the Hallé MV strain, MV entry into CEF appeared to be a limiting step in the absence of prior MV adaptation. However, the H expression level was lower than that observed after infection of TCF.CD46 cells with the Schwarz strain, indicating that a virus replication step, downstream from the entry, also has to be subjected to adaptation.
FIG. 2.
Permissivity of TCF and TCF.CD46 cells to MV infection. (A) Expression of CD46 by TCF and TCF.CD46 cells determined by flow cytometry. TCF and TCF.CD46 cells were infected with four different MV strains at an MOI of 1. At 4 days p.i., the expression of H at the cell surface (white histograms) was determined by flow cytometry. The arrow represents the beginning of the gate where cells are considered positive compared to the noninfected cells (black histograms). (B) MV Tag strain derived from Edmonston B strain. (C) MV Hallé strain. (D) MV Ma93F strain. (E) MV Schwarz strain. (F) Production of infectious particles 4 days p.i. by TCF and TCF.CD46 cells. Total infectious particles (black bars) and infectious particles released in the supernatant only (white bars) are represented.
The expression of CD46 by TCF cells also resulted in enhanced cell-cell spreading of the Schwarz strain (99 versus 48% of cells expressing H protein) and greater virus progeny (7.9 × 104 versus 2.5 × 104 TCID50/ml) (Fig. 2E and F). So, despite its adaptation to the CEF, the vaccine Schwarz strain has not lost its ability to interact with CD46 (24). Indeed, the two residues, Val451 and Tyr481, critical for the interaction with CD46 (12, 15) are present in the H protein from the Schwarz strain (22).
An MV binding structure, different from CD46, is expressed on the CEF surface.
The ability of MV to bind to CEF was tested. We first verified by flow cytometry that none of the anti-CD46 antibodies, including MCI20.6, which inhibited the MV binding on CD46 (18), could react with CEF, suggesting the absence of any structure comparable to CD46 (data not shown). The ability of CEF to bind purified MV Schwarz and Hallé compared with the ability of CHO and CHO.CD46 cells was then determined by cytofluorometry as previously described (2) (Fig. 3). MV Schwarz bound to the three cell types, with increased binding to CHO.CD46 cells (mean fluorescence, 121 versus 52 on CHO cells), confirming that the Schwarz strain could interact with CD46 even after adaptation to CEF (Fig. 3, left panels). In contrast, MV Hallé binding was minimal on CHO cells (mean fluorescence, 7) compared to the binding on CEF and CHO.CD46 cells (mean fluorescence, 46 and 179, respectively) (Fig. 3, right panels). Thus, an MV binding structure distinct from CD46, the human receptor for MV (6, 17), and absent from the CHO cell surface is expressed by the CEF. The use of a putative receptor different from CD46 has recently been reported for transformed marmoset and human B cells (12), and its relationship with the MV binding structure on CEF remains to be determined. The differing abilities of MV Schwarz and Hallé strains to bind to CHO cells indicate a difference in the conformation, of their H glycoproteins.
FIG. 3.
Ability of CEF to bind MV Hallé and MV Schwarz. CEF (A), CHO cells (B), and CHO.CD46 cells (C) were incubated for 1 h with purified MV Schwarz or Hallé strain (50 μg/ml). The binding of MV was revealed with a monoclonal anti-MV H antibody and analyzed by cytofluorometry. Background binding is represented by the black histogram.
Pronase sensitivity and recovery of MV binding activity after pronase treatment.
CEF were treated with 0.8 mg of pronase per ml for 30 min at 37°C as previously described (18), and MV Hallé binding was tested by flow cytometry. Such treatment reduced the ability of MV to bind to CEF by more than 75%. MV binding was also sensitive to papain and trypsin proteolysis. After pronase stripping and 6 h of regeneration, the CEF recovered 60% of their MV binding activity. In the presence of 10 μg of the protein synthesis inhibitor cycloheximide per ml, this regeneration was impaired and only 20% of the binding activity was recovered (18). The addition of the N-glycosylation inhibitor tunicamycin, which abolished the ability of CD46 to bind MV (reference 18 and this study), had no effect on the regeneration of the MV binding structure of CEF. Thus, the MV binding structure on CEF is an endogenously synthesized protein and does not require N-glycosylation for its interaction with MV.
Characterization of the interaction between the binding structure on CEF and MV.
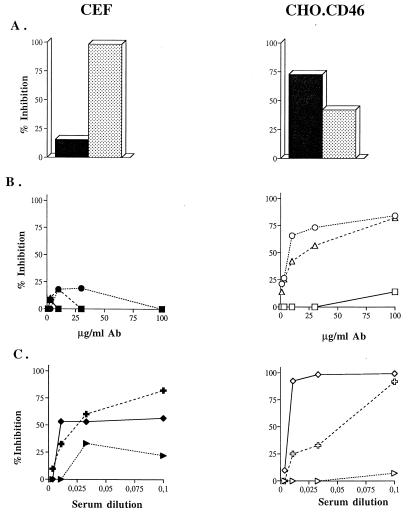
To determine which viral component(s) interact(s) with the putative MV receptor on CEF, experiments involving inhibition of MV binding to CEF and CHO.CD46 cells were performed. Briefly, cells or purified virus were preincubated with the relevant inhibitor for 1 h at 37°C prior to the virus binding assay determination by flow cytometry. Recombinant soluble CD46 (sCD46; 50 μg/ml) and sH (13.75 μg/ml) inhibited MV binding on CHO.CD46 cells by 75 and 40%, respectively (Fig. 4A, right panel). In contrast, inhibition of MV binding on CEF was maximal with sH (100%), compared to 18% inhibition by sCD46 (Fig. 4A, left panel). Moreover, the 48Cl6 and Cl55 monoclonal anti-H antibodies, which inhibited the MV-CD46 interaction by 80% at a final concentration of 100 μg/ml (Fig. 4B, right panel), were inefficient in preventing MV binding to the CEF surface (Fig. 4B, left panel). Finally, several polyclonal anti-H antibodies displayed a different pattern of inhibition of MV binding to CHO.CD46 cells and CEF (Fig. 4C). These results suggest that the MV binding structure interacts with the MV hemagglutinin, but by determinants different from those required for the interaction via CD46. The F protein alone does not seem to be involved in the MV-CEF interaction because none of the anti-F antibodies tested had any inhibitory activity (data not shown).
FIG. 4.
Characterization of MV binding to CEF with inhibitors. CEF or CHO.CD46 cells or purified MV Hallé strain was preincubated with different inhibitors prior to the virus binding assay. Results were expressed as percentages of inhibition of MV binding ability. (A) Inhibitory ability of recombinant soluble CD46 (black columns) or hemagglutinin (stippled columns). (B) Inhibitory ability of monoclonal anti-MV hemagglutinin antibodies: Cl55 (triangles), 48Cl6 (circles), and 19H40 (squares). (C) Inhibitory ability of anti-H polyclonal antibodies: monkey antiserum Bms 94 (crosses), guinea pig antiserum Houx (diamonds), and rabbit antiserum Harry (arrowheads).
The MV binding structure on CEF is relatively inefficient in mediating fusion.
MV infection resulted in the formation of multinucleated cells, syncytia, because of the fusion between infected cells expressing H and F and cells expressing an MV receptor. A quantitative test based on the transactivation of the reporter gene lacZ was used to investigate the ability of H and F proteins from the Hallé strain to induce the fusion with the chicken fibroblast cell lines TCF and TCF.CD46 (19). Briefly, a first fusion partner was transfected with a plasmid DNA containing the T7 promoter linked to the lacZ gene and infected with the recombinant vaccinia virus encoding MV H and F (MOI of 10) (7). This partner was then cocultured with another cell partner infected with the recombinant vaccinia virus encoding the T7 polymerase (MOI of 10). The fusion was monitored by reporter β-galactosidase gene activation by using a colorimetric assay. When CEF were used as both fusion partners, significant but low β-galactosidase activity (0.109 ± 0.029 optical density units above background) was observed, indicating the limited ability of Hallé-derived H and F glycoproteins to induce fusion with the CEF membrane. As a control, a coculture of TCF cells expressing H and F with TCF.CD46 cells resulted in significantly higher β-galactosidase activity (0.489 ± 0.116 optical density units above background). Thus, the putative receptor expressed on CEF has a poor ability to mediate the fusion driven by the MV Hallé envelope glycoproteins, and this may explain the low infectivity of the Hallé strain in these cells and, at least partly, its limited cell-cell spreading. Such a poor fusion efficiency of the CEF putative receptor also correlates with the slow kinetics of the propagation of the Schwarz MV strain in TCF cells and CEF compared to that observed on TCF.CD46 cells (data not shown).
In conclusion, CEF express at their surface a putative MV receptor, which is a cellular protein different from CD46. This protein interacts with the H protein, but by determinants distinct from those implicated in H interaction with CD46. During the adaptation to the chicken cell, the MV glycoproteins have been selected so as to favor the virus entry into these cells and, particularly, the fusion step. Determining whether this particular structure is responsible, at least partly, for the in vivo attenuation phenotype of the MV vaccine grown in chicken fibroblasts may be rewarding if this represents a general principle for attenuation. The identification of the chicken cell-encoded alternative receptor presently under investigation would be a useful tool to explore this question.
Acknowledgments
We thank D. Christiansen for his help in writing the manuscript, F. Wild and A. Osterhaus for providing us with monoclonal and polyclonal antibodies and recombinant vaccinia viruses, Pasteur Mérieux Connaught for the Schwarz MV strain, M. Billeter for the tag MV strain, R. Fernandez-Munoz for the Ma93F MV strain, Rhône Mérieux for the TCF.3B cell line, B. Loveland for sCD46, and O. Nussbaum for the plasmid pGNT7βGal.
C. Escoffier was supported by the Ministère de l’Education Nationale et de la Recherche. The work was supported in part by a grant from the Ministère de l’Education Nationale et de la Recherche et de la Technologie (grant PRFMMIP).
REFERENCES
- 1.Borges M B, Mann G F, Freire M D S. Biological characterization of clones derived from the Edmonston strain of measles virus in comparison with Schwarz and CAM-70 vaccine strains. Mem Inst Oswaldo Cruz. 1996;91:507–513. doi: 10.1590/s0074-02761996000400020. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buynak E B, Peck H M, Ceamer A A, Goldner H, Hilleman M R. Differentiation of virulent from avirulent measles strains. Am J Dis Child. 1962;103:460–473. [Google Scholar]
- 4.Cattaneo R, Rose J K. Cell fusion by the envelope glycoproteins of persistent measles viruses which caused lethal human brain disease. J Virol. 1993;67:1493–1502. doi: 10.1128/jvi.67.3.1493-1502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devaux P, Loveland B, Christiansen D, Milland J, Gerlier D. Interactions between the ectodomains of haemagglutinin and CD46 as a primary step in measles virus entry. J Gen Virol. 1996;77:1477–1481. doi: 10.1099/0022-1317-77-7-1477. [DOI] [PubMed] [Google Scholar]
- 6.Dörig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 7.Drillien R, Spehner D, Kirn A, Giraudon P, Buckland R, Wild T F, Lecocq J P. Protection of mice from fatal measles encephalitis by vaccination with vaccinia virus recombinants encoding either the hemagglutinin or the fusion protein. Proc Natl Acad Sci USA. 1988;85:1252–1256. doi: 10.1073/pnas.85.4.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enders J F, Peebles T C. Propagation in tissue cultures of cytopathic agents from patients with measles virus. Proc Soc Exp Biol Med. 1954;86:277–286. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- 9.Evans M J, Hartman S L, Wolff D W, Rollins S A, Squinto S P. Rapid expression of an anti-human C5 chimeric Fab utilizing a vector that replicates in COS and 293 cells. J Immunol Methods. 1995;184:123–138. doi: 10.1016/0022-1759(95)00093-p. [DOI] [PubMed] [Google Scholar]
- 10.Gerlier D, Devaux P. CD46 un premier récepteur du virus de la rougeole. Virologie. 1997;1:321–330. [Google Scholar]
- 11.Gerlier D, Varior-Krishnan G, Devaux P. CD46-mediated measles virus entry: a first key to host-range specificity. Trends Microbiol. 1995;3:338–345. doi: 10.1016/s0966-842x(00)88972-6. [DOI] [PubMed] [Google Scholar]
- 12.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz S L. The history of measles virus and the development and utilization of measles virus vaccines. In: Plotkin S A, Fantini B, editors. Vaccinia, vaccination, vaccinology. Paris, France: Elsevier Editions Scientifiques; 1996. pp. 265–270. [Google Scholar]
- 14.Katz S L, Milovanovic M F, Enders J F. Propagation of measles virus in cultures of chick embryo cells. Proc Soc Exp Biol Med. 1958;97:23–29. doi: 10.3181/00379727-97-23637. [DOI] [PubMed] [Google Scholar]
- 15.Lecouturier V, Fayolle J, Caballero M, Carabana J, Celma M L, Fernandez-Munoz R, Wild T F, Buckland R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milanovic M V, Enders J F, Mitus A. Cultivation of measles virus in human amnion cells and developing chick embryo. Proc Soc Exp Biol Med. 1957;95:120–127. doi: 10.3181/00379727-95-23140. [DOI] [PubMed] [Google Scholar]
- 17.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naniche D, Wild T F, Rabourdin-Combe C, Gerlier D. A monoclonal antibody recognizes a human cell surface glycoprotein involved in measles virus binding. J Gen Virol. 1992;73:2617–2624. doi: 10.1099/0022-1317-73-10-2617. [DOI] [PubMed] [Google Scholar]
- 19.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter M A. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson J S, Bootman J S, Newman R, Oxford J S, Daniels R S, Webster R G, Schild G C. Structural changes in the haemagglutinin which accompany egg adaptation of an influenza A(H1/N1) virus. Virology. 1987;160:31–37. doi: 10.1016/0042-6822(87)90040-7. [DOI] [PubMed] [Google Scholar]
- 22.Rota J S, Wang Z D, Rota P A, Bellini W J. Comparison of sequences of the H, F and N coding genes of measles virus vaccine strains. Virus Res. 1994;31:317–330. doi: 10.1016/0168-1702(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 23.Takeda M, Kato A, Kobune F, Sakata H, Li Y, Shioda T, Sakai Y, Asakawa M, Nagai Y. Measles virus attenuation associated with transcriptional impediment and a few amino acid changes in the polymerase and accessory proteins. J Virol. 1998;72:8690–8696. doi: 10.1128/jvi.72.11.8690-8696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trescol-Biémont M C, Leonov S, Rabourdin-Combe C, Gerlier D. Quantification of measles virus by a virus receptor-dependent and haemagglutinin-specific T cell stimulation assay. J Immunol Methods. 1995;187:253–258. doi: 10.1016/0022-1759(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 25.Whistler T, Bellini W J, Rota P A. Generation of defective interfering particles by two vaccine strains of measles virus. Virology. 1996;220:480–484. doi: 10.1006/viro.1996.0335. [DOI] [PubMed] [Google Scholar]
- 26.Wild T F, Dugre R. Establishment and characterization of a subacute sclerosing panencephalitis (measles) virus persistent infection in BGM cells. J Gen Virol. 1978;39:113–124. doi: 10.1099/0022-1317-39-1-113. [DOI] [PubMed] [Google Scholar]