Abstract
Little is known about oxygen utilization during infection by bacterial respiratory pathogens. The classical Bordetella species, including B. pertussis, the causal agent of human whooping cough, and B. bronchiseptica, which infects nearly all mammals, are obligate aerobes that use only oxygen as the terminal electron acceptor for electron transport-coupled oxidative phosphorylation. B. bronchiseptica, which occupies many niches, has eight distinct cytochrome oxidase-encoding loci, while B. pertussis, which evolved from a B. bronchiseptica-like ancestor but now survives exclusively in and between human respiratory tracts, has only three functional cytochrome oxidase-encoding loci: cydAB1, ctaCDFGE1, and cyoABCD1. To test the hypothesis that the three cytochrome oxidases encoded within the B. pertussis genome represent the minimum number and class of cytochrome oxidase required for respiratory infection, we compared B. bronchiseptica strains lacking one or more of the eight possible cytochrome oxidases in vitro and in vivo. No individual cytochrome oxidase was required for growth in ambient air, and all three of the cytochrome oxidases conserved in B. pertussis were sufficient for growth in ambient air and low oxygen. Using a high-dose, large-volume persistence model and a low-dose, small-volume establishment of infection model, we found that B. bronchiseptica producing only the three B. pertussis-conserved cytochrome oxidases was indistinguishable from the wild-type strain for infection. We also determined that CyoABCD1 is sufficient to cause the same level of bacterial burden in mice as the wild-type strain and is thus the primary cytochrome oxidase required for murine infection, and that CydAB1 and CtaCDFGE1 fulfill auxiliary roles or are important for aspects of infection we have not assessed, such as transmission. Our results shed light on the environment at the surface of the ciliated epithelium, respiration requirements for bacteria that colonize the respiratory tract, and the evolution of virulence in bacterial pathogens.
Author summary
Cytochrome oxidases, critical components for aerobic respiration, have been shown to be vital for pathogenesis and tissue tropism in several bacterial species. However, the majority of the research has focused on facultative anaerobes and infections of microoxic to anaerobic host environments, like the gut. We sought to understand the role of cytochrome oxidases during respiratory infection by Bordetella bronchiseptica, an obligate aerobe, performing the first analysis of cytochrome oxidases in an extracellular respiratory pathogen that we know of. By comparing B. bronchiseptica to the closely related B. pertussis, a strictly human-specific pathogen and the causative agent of whooping cough, we found three cytochrome oxidases that are important for growth and survival within the mammalian respiratory tract. We also found that a bo3-type cytochrome oxidase, predicted to have a low affinity for oxygen and therefore best suited to ambient air levels of oxygen, was sufficient for both the establishment of infection and persistence in the respiratory tract in mice. Our findings reveal the importance of low affinity cytochrome oxidases in respiratory pathogens, and emphasize the need to study the physiology of diverse classes of pathogens.
Introduction
To be effective pathogens, bacteria must be able to survive within the host. Even pathogens that survive exclusively within a host must contend with diverse microenvironments, moving from the initial site of infection to colonization sites and eventually to a new host while avoiding or neutralizing host defenses. As bacteria encounter new microenvironments within the host, they must adjust their physiologies to continue to grow. Most of the research on bacterial pathogens has focused on identifying and characterizing virulence factors. However, there is now an increased appreciation for the role that metabolism and physiology play during infection. Without energy, bacteria cannot survive and therefore cannot cause disease.
Electron transport-coupled oxidative phosphorylation pairs the transfer of electrons from donors to acceptors with the transport of protons across a membrane, generating the proton motive force (PMF) used to drive the synthesis of ATP from ADP. This process occurs in a step-wise manner via the electron transport chain (ETC), starting with electron donors like NADPH and ending with a terminal electron acceptor. Aerobic respiration uses oxygen as the terminal electron acceptor, but alternative terminal electron acceptors, like nitrate or nitrite, are used by some bacteria to grow anaerobically. Redox-active protein complexes with heme cofactors called cytochrome oxidases are responsible for the transfer of electrons to oxygen. Cytochrome oxidases are named based on the types of hemes (a, b, c, d, or o) that the proteins bind. bo3-type, aa3-type, and cbb3-type cytochrome oxidases are all heme-copper oxidases (HCOs), so-named for the copper ions that form a bi-metallic center with hemes in their catalytic subunits, while bd-type cytochrome oxidases form a unique class of heme-only cytochrome oxidases found exclusively in bacteria and archaea with no sequence homology to HCOs [1,2]. Cytochrome oxidases can also be categorized by their electron donors. Quinol oxidases receive their electrons directly from reduced quinones, while cytochrome c oxidases receive their electrons from cytochrome c. Cytochrome oxidases can also be grouped according to their affinity for oxygen: either low-affinity (Km for O2 ≅ 200nM) [3], which are best suited for atmospheric levels of oxygen (~21% O2), or high-affinity, which can function in microoxic conditions (Km for O2 = 3–8 nM) [4,5]. Increased affinity for oxygen comes with a trade-off; high-affinity cytochrome oxidases generate less proton motive force per electron transferred than low-affinity cytochrome oxidases [6].
Respiration as a whole, and cytochrome oxidases in particular, have been shown to be required for infection in many pathogenic species, including the gastrointestinal pathogens Escherichia coli [7,8], Vibrio cholerae [9], Listeria monocytogenes [10,11], Shigella flexneri [12,13], and Salmonella enterica Serovar Typhimurium [14–16]; the oral pathogen Aggregatibacter actinomycetemcomitans [17]; the respiratory pathogen Mycobacterium tuberculosis [18,19]; and pathogens that can cause many types of infection including bacteremia and sepsis like Staphylococcus aureus [20] and group B Streptococcus [21,22]. However, the majority of pathogens assessed have been facultative anaerobes, which do not require cytochrome oxidases for survival. Additionally, many of these pathogens colonize microenvironments that are expected to have limited levels of oxygen.
The classical Bordetella species (B. pertussis, B. parapertussisHu, and B. bronchiseptica) are extremely closely related bacteria that colonize the upper and lower respiratory tracts of humans (in the case of B. pertussis and B. parapertussisHu) and other mammals (in the case of B. bronchiseptica). They are obligate aerobes that use only oxygen as their terminal electron acceptor for electron transport-coupled oxidative phosphorylation [23,24]. Therefore, they depend solely on components of the ETC, like cytochrome oxidases, to generate energy. Previous work in B. pertussis has shown that cytochrome c is not required for survival in vitro [25]. Little is known, however, about the role of cytochrome oxidases in the classical Bordetella species.
In this study, we leveraged the evolution of classical bordetellae to investigate which cytochrome oxidases are important for respiration in the mammalian respiratory tract, using B. bronchiseptica as our model organism. B. bronchiseptica, which occupies multiple environmental niches, has the potential to produce eight distinct cytochrome oxidases. By contrast, B. pertussis, which survives in the environment only during transmission between hosts, has undergone significant genome reduction as it evolved from a B. bronchiseptica-like ancestor [24]. Genomic comparisons between B. bronchiseptica and B. pertussis found that B. pertussis strains have only broadly conserved three intact cytochrome oxidase-encoding gene loci. We hypothesized that the three cytochrome oxidases conserved in B. pertussis represent the minimum number and class of cytochrome oxidases required for bordetellae to survive within, and briefly between, mammalian hosts. We genetically manipulated our bacteria to determine the necessity and sufficiency of different cytochrome oxidases, first for growth in vitro under ambient air and different atmospheric conditions, then during infection, using two different mouse models of respiratory infection. Our results demonstrate that B. bronchiseptica is able to survive with as few as one cytochrome oxidase, even during infection and when challenged with low oxygen conditions. These findings also inform our understanding of the murine respiratory tract, indicating that bacteria may have more access to oxygen while attached to the ciliated epithelium than previously supposed.
Results
Bordetella bronchiseptica has eight cytochrome oxidase-encoding gene loci
The B. bronchiseptica RB50 genome has eight cytochrome oxidase-encoding gene loci, annotated computationally based on predicted amino acid homology (Table 1). Three of the loci are predicted to encode high-affinity cytochrome oxidases. ccoNOQP (abbreviated cco) encodes a cbb3-type cytochrome oxidase. In cbb3-type cytochrome oxidases, CcoN contains the active site that facilitates the reduction of molecular oxygen to water and thus functions as the catalytic subunit [26,27]. Two loci encode bd-type cytochrome oxidases (cydAB1 and cydAB3, abbreviated cyd1 and cyd3). The cyd1 operon of B. bronchiseptica also includes a third gene, cydX, which is present in some, but not all, bd-type cytochrome oxidase-encoding loci and may be important for bd-type complex function. The variation across different species in number of subunits, structural arrangement of coordinated hemes, and positioning of the quinol binding site makes it difficult to draw definitive conclusions about bd-type cytochrome oxidases [28]. CydA is the subunit that coordinates the three hemes required by this cytochrome oxidase, making it the catalytic subunit; however, bd-type cytochrome oxidases require both CydA and CydB for catalytic activity, as evidenced by the loss of d-type heme when the genes encoding either subunit are deleted [29,30].
Table 1. Cytochrome oxidase-encoding gene loci in classical Bordetella species.
| Class | Gene names | Bb locus (RB50) | Predicted oxygen affinity | Is the locus intact across Bordetella complexes?1 | |||
|---|---|---|---|---|---|---|---|
| Complex I (n = 6) | Complex II (n = 14) | Complex III (n = 4) | Complex IV (n = 4)3 | ||||
| cbb 3 | ccoNOQP | BB3329-3326 | High | 100% | 0% | 100% | 75% |
| bd | cydAB1 | BB4498-4497 | High | 100% | 100% | 100% | 100% |
| cydAB3 | BB4012-4011 | High | 100% | 50% | 100% | 100% | |
| cioAB | BB1238-1239 | Low2 | 100% | 0% | 100% | 75% | |
| aa 3 | ctaCDFGE1 | BB4831-4827 | Low | 100% | 100% | 100% | 100% |
| ctaD2 | BB4674 | Low | 100% | 7.1% | 100% | 100% | |
| bo 3 | cyoABCD1 | BB1283-1286 | Low | 100% | 92.9% | 100% | 75% |
| cyoABCD2 | BB1310-1307 | Low | 100% | 0% | 100% | 75% | |
Shaded cells represent cytochrome oxidase-encoding loci that are broadly conserved in B. pertussis (Complex II)
1 Further information about strains compared can be found in Table A in S1 Table; details about the differences from RB50 can be found in Table B in S1 Table.
2 cioAB, formerly known as cydAB2, was previously predicted to encode a high-affinity cytochrome oxidase.
3 One strain, the environmental isolate HT200, has gained mutations in 4 cytochrome oxidase-encoding gene loci; the other three strains have maintained all 8 loci
Five of the eight cytochrome oxidase-encoding loci are predicted to encode low-affinity cytochrome oxidases. These include one complete aa3-type cytochrome oxidase (ctaCDFGE1, abbreviated cta1) and one lone aa3-type subunit (ctaD2). The cta1 operon contains two additional genes: a putative membrane protein (BB4829, here named ctaF1) and a putative DUF2970-containing membrane protein (BB4828, here named ctaG1). This operon structure is also found in Achromobacter species, which are closely related to Bordetella. ctaF1 shares no homology to the gene of the same name found in Bacillus species, which encodes part of a caa3-type cytochrome oxidase [31]. CtaD is the catalytic subunit in aa3-type cytochrome oxidases [32]. While CtaD2 cannot function within the electron transport chain on its own, it could be forming heterocomplexes with CtaC1 and CtaE1, as CcoN orphan subunits have been shown to do in Pseudomonas aeruginosa [33]. Two loci encode bo3-type cytochrome oxidases (cyoABCD1 and cyoABCD2, abbreviated cyo1 and cyo2). CyoB is the catalytic subunit for bo3-type cytochrome oxidases [34,35]. Finally, the cioAB locus (abbreviated cio) is predicted to encode a cyanide-insensitive oxidase (CIO). CIOs are a subclass of bd-type cytochrome oxidases that are low-affinity and were first characterized in P. aeruginosa, where CioAB confers resistance to cyanide [36]. CIOs require both subunits to be functional, like other bd-type cytochrome oxidases.
Bordetella pertussis has only three broadly conserved cytochrome oxidase-encoding gene loci
Genomic comparisons of the classical bordetellae have found that the three subspecies can be divided into four complexes [37]. Complex I, which includes RB50, is most similar to the last common ancestor shared by the classical bordetellae and contains B. bronchiseptica strains, primarily those isolated from non-human mammals. Complex II contains B. pertussis and Complex III contains B. parapertussisHu. Complex IV, which phylogenetically lies between Complex I and II, contains B. bronchiseptica strains, primarily those isolated from humans. B. parapertussisHu diverged from Complex I B. bronchiseptica more recently than B. pertussis diverged from Complex IV B. bronchiseptica; therefore, B. parapertussisHu strains share more similarities with B. bronchiseptica strains than B. pertussis strains do [37]. A recent genotyping study identified a novel B. bronchiseptica branch, named lineage II, that is distinct from the classical bordetellae [38]. This branch primarily contains strains that were formerly classed as Complex IV and had been identified by other groups as being divergent from other classical bordetellae [39,40].
B. pertussis and B. parapertussisHu have undergone genome reduction since evolving from a B. bronchiseptica-like ancestor, with genomes approximately 77% and 89% the size of the B. bronchiseptica genome, respectively [24]. Additionally, B. pertussis and B. parapertussisHu have specialized, infecting only humans instead of maintaining the wide mammalian host range and environmental survival capabilities of B. bronchiseptica. We investigated which cytochrome oxidase-encoding genes have been conserved across the classical bordetellae, hypothesizing that the human-adapted B. pertussis and B. parapertussisHu have lost cytochrome oxidases that are not required for survival within, and briefly between, human respiratory tracts. We chose 28 Bordetella strains for comparison, including both laboratory strains and clinical isolates: 6 Complex I, 14 Complex II, 4 Complex III, and 4 Complex IV (2 of which could be classified as lineage II) (Table A in S1 Table). We found that almost all the strains in Complex I, III, and IV have maintained intact copies of all eight cytochrome oxidase-encoding gene loci (Table 1). Only one strain, the environmental isolate HT200, differed, having acquired frameshift mutations leading to premature stop codons in ccoN, cioB, cyoB1, and cyoB2 which most likely would prevent the complexes encoded by each of these cytochrome oxidase-encoded loci (here notated as Cco, Cio, Cyo1, and Cyo2) from functioning in this strain (Table B in S1 Table). This result is intriguing, as it indicates that not all cytochrome oxidases are required to survive in the environment, or at least within the thermal spring in which HT200 was isolated [40]. This strain is also a lineage II strain, and thus phylogenetically distant from the classical Bordetella strains [38] (S1 Fig).
B. pertussis strains have significantly diverged from the other classical bordetellae in terms of their cytochrome oxidase-encoding genes. All 14 examined strains contained premature stop codons within ccoN and cyoA2 (Table B in S1 Table). These mutations would most likely prevent Cco and Cyo2 from functioning. Interestingly, the point mutations that led to the premature stops were identical across all 14 strains, indicating that these mutations were acquired by the ancestral strain that became B. pertussis. Additionally, all 14 strains have lost cio and 13 strains have lost ctaD2. The identical changes across diverse B. pertussis strains indicate that functional copies of Cco, Cio, Cyo2, and CtaD2 were lost during the evolution from the last common ancestor shared by B. pertussis and B. bronchiseptica.
There are mutations in cytochrome oxidase-encoding genes that differ between B. pertussis strains. Strain 18323, a laboratory strain commonly used in the past, had the most differences. 18323 has since been shown by many typing methods to differ from most B. pertussis strains, lacking some genes usually seen in B. pertussis while having copies of genes normally found in B. bronchiseptica and B. parapertussisHu but not B. pertussis [41–44]. These genomic differences are reflected in the phylogenetic tree, where 18323 is the closest B. pertussis strain to Complex IV (S1 Fig). 18323 was the only B. pertussis strain containing ctaD2. 18323 also contained unique mutations, resulting in premature stop codons in ccoP, cydB3, cyoB1, and cyoC1, as well as losing cyoA1 and cyoD1 (Table B in S1 Table). Interestingly, 18323 was not the only B. pertussis strain that lost the ability to make Cyd3. Six of the 13 remaining Complex II strains contain a frameshift mutation in the same position as 18323 but are shifted into a different reading frame. The fact that these 6 strains all have identical mutations indicates that they may have evolved from a common ancestor with this mutation and that this mutation occurred after B. pertussis became its own species.
Despite the differences within B. pertussis strains, a pattern emerges; excluding 18323, all these strains have maintained functional loci encoding Cyd1, Cta1, and Cyo1. Therefore, we hypothesize that these three cytochrome oxidases are necessary and sufficient for Bordetella infection within the mammalian respiratory tract, the environment in which B. pertussis has specialized, and the brief time spent between hosts during transmission.
Cyd1, Cta1, or Cyo1 is required for growth in ambient air
To assess the necessity of each cytochrome oxidase for survival under various conditions, we generated B. bronchiseptica strains with in-frame gene loci deletions using allelic exchange. Starting with our wild-type strain RB50 (WT), we were able to generate strains lacking cco (Δcco), cyd1 (Δcyd1), cyd3 (Δcyd3), cio (Δcio), cta1 (Δcta1), ctaD2 (ΔctaD2), or cyo1 (Δcyo1) (S2 Fig). We were unable to delete the cyo2 locus in its entirety; instead, we deleted cyoABC2 and left cyoD2 intact. The resulting strain lacks a functional complex generated by the proteins encoded by cyo2 and is therefore called Δcyo2 hereafter.
Based on genome comparisons between B. bronchiseptica and B. pertussis, we aimed to generate strains that allowed us to test the necessity and sufficiency of the three cytochrome oxidase-encoding loci broadly conserved in B. pertussis (cyd1, cta1, and cyo1). We tried to make a strain that lacks cyd1, cyo1, and cta1 but were unable to generate it using our normal growth conditions; all co-integrants reverted to wild-type at the last step of the allelic exchange procedure, suggesting that at least one of the Bp-conserved cytochrome oxidases is required for growth of B. bronchiseptica under standard laboratory conditions. We were able to make a strain with only the three conserved cytochrome oxidases by deleting the other five cytochrome oxidase-encoding loci (cyd1+ cta1+ cyo1+, abbreviated Bp-conserved). We then used this strain to generate strains for assessing the necessity of each of the cytochrome oxidases conserved in B. pertussis when only these three are present. We were able to create all three iterations; a strain with only cta1 and cyo1 (cta1+ cyo1+), a strain with only cyd1 and cyo1 (cyd1+ cyo1+), and a strain with only cyd1 and cta1 (cyd1+ cta1+).
To assess the sufficiency of each cytochrome oxidase for growth under various conditions, we generated strains that contain in-frame deletions of seven of the eight cytochrome oxidase-encoding loci. We were able to generate three of the eight possible strains with only one cytochrome oxidase-encoding gene locus: a strain with only cyd1 (cyd1+), a strain with only cta1 (cta1+), and a strain with only cyo1 (cyo1+). We were unable to generate the five other possible strains with only one cytochrome oxidase-encoding gene locus remaining under our normal growth conditions; indeed, we were only able to generate strains lacking six of the eight oxidase-encoding gene loci if one of the two remaining loci was cyd1, cta1, or cyo1 (S2 Fig). This result, combined with the fact that we were unable to generate a strain missing all three cytochrome oxidases conserved in B. pertussis, indicates that Cyd1, Cta1, and Cyo1 are the primary cytochrome oxidases used under normal growth conditions in ambient air and that at least one is required under this condition.
All of the strains we constructed are able to respire
To determine how well our strains were able to respire after losing one or more cytochrome oxidase(s), we grew our strains on medium containing triphenyl tetrazolium chloride (TTC). TTC accepts electrons from the electron transport chain. When TTC is reduced in the presence of cytochrome oxidases in a sufficiently dense culture, it undergoes an irreversible color shift from colorless to red [45]. We examined the color of colony biofilms after 24 hours of growth on media containing TTC. All strains were able to respire, as evidenced by the red color of the colonies (S3A Fig). By contrast, bacteria that are unable to respire do not form red colony biofilms, as seen using an S. aureus strain that is unable to generate menaquinone and thus has a non-functional electron transport chain (S3A Fig, Sa SCV). All strains except cta1+ had a similar level of redness, indicating that all of the other mutant strains had a similar level of respiration as WT. cta1+ was less red overall but had spots with a higher density of red pigment than WT in some replicates (S3B Fig). This color variation could reflect heterogeneity in the mutant population.
cta1+ displays other phenotypes that differ from WT. This strain generated smaller colonies than WT on Bordet-Gengou (BG) blood agar plates, our standard culturing medium, requiring 72 hours to produce visible colonies as opposed to the 48 hours required by WT. Moreover, cta1+ could not grow in our normal liquid culturing medium, Stainer-Scholte (SS) broth, when taken from BG agar. Both media types are optimized for culturing Bordetella. However, BG is an undefined medium, containing potato extract, blood, and glycerol [46], while SS is a defined medium that specifically provides amino acids as a carbon and nitrogen source [47]. We found that colonies of cta1+ from BG agar are able to grow on SS agar, and these colonies can then be grown in SS broth. cta1+ cultures grown in SS broth or on SS agar can grow on BG agar. This phenotype was not due to second-site mutations, as whole-genome sequencing revealed no unique mutations compared to WT, cyd1+, or cyo1+. The reason for this unusual phenotype is not known, and we did not pursue it further.
No cytochrome oxidase is necessary for growth in ambient air, but Cyd1, Cta1, and Cyo1 are each sufficient
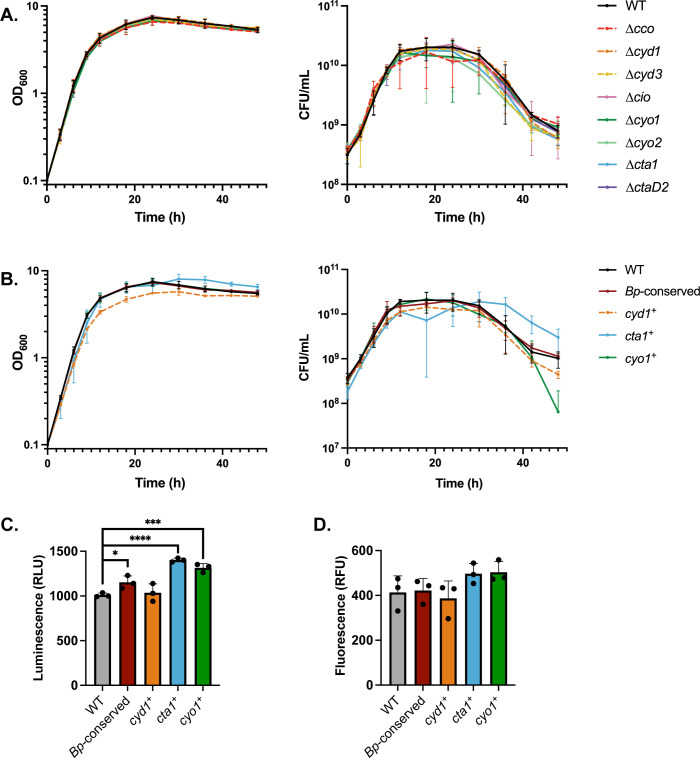
To assess the roles of the different cytochrome oxidases during laboratory growth, we grew strains in SS medium in ambient air conditions and measured growth over time via optical density (OD600), which correlates to the total cells in a sample. WT grew exponentially until 12 hours post-inoculation, then reached stationary phase between 12 and 18 hours, followed by a gradual decrease in OD600 until the end of the experiment (Fig 1A). We also assessed the number of viable bacteria within each sample by quantifying the CFU/mL. Through the first 30 hours of the experiment, OD600 and CFU/mL were consistent. While the OD600 of WT stayed relatively consistent after the culture entered stationary phase, however, the CFU/mL began to decline after 30 hours, indicating that many cells were dying, but not lysing, within the population.
Fig 1. No cytochrome oxidase is necessary for growth in ambient air, but Cyd1, Cta1, and Cyo1 are each sufficient.
(A-B) Growth over time, measured via optical density (left) or CFU/mL (right). Growth of RB50 (WT, black) is compared to strains lacking a single cytochrome oxidase-encoding gene loci (A) or strains with only one of the cytochrome oxidase-encoding gene loci conserved in B. pertussis (cyd1+, cta1+, and cyo1+) or just the three Bp-conserved loci intact (Bp-conserved) (B). Cultures were started at 0.1 OD600, which contains approximately 3.5x108 CFU/mL. Points represent the mean of at least 3 biological replicates, with bars representing the standard deviation. (C) Intracellular ATP levels, as measured through luciferase activity, for the same strains as in B. (D) Proton motive force, as measured through uptake of fluorescently labeled gentamicin, for the same strains as in B. Statistical significance was determined using unpaired Student’s t-test. *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ****, p < 0.0001. Raw data for Fig 1A: https://doi.org/10.15139/S3/F89WZH. Raw data for Fig 1B: https://doi.org/10.15139/S3/NKQ2BK. Raw data for Fig 1C: https://doi.org/10.15139/S3/EURIPE. Raw data for Fig 1D: https://doi.org/10.15139/S3/QSJXPT.
To determine if individual cytochrome oxidases are required for normal growth, we grew strains with deletions of individual cytochrome oxidase-encoding loci (i.e. Δcco, Δcyd1, Δcyd3, Δcta1, ΔctaD2, Δcio, Δcyo1, and Δcyo2) and compared their growth to that of WT. All eight strains lacking a single cytochrome oxidase phenocopied WT by both OD600 and CFU/mL (Fig 1A). This result indicates that no single cytochrome oxidase is required for growth under standard laboratory conditions.
To determine if the cytochrome oxidases broadly conserved in B. pertussis are sufficient for growth under the same conditions, we assessed the growth of the Bp-conserved strain (i.e. cyd1+ cta1+ cyo1+). Bp-conserved phenocopied WT over time (Fig 1B), indicating that the combination of Cyd1, Cta1, and Cyo1 is sufficient under these conditions. Given that the combination of the three Bp-conserved cytochrome oxidases was sufficient, we then assessed their individual sufficiency. To do so, we used strains with only a single cytochrome oxidase-encoding locus remaining (i.e. cyd1+, cta1+, and cyo1+). Overall, these strains behaved similarly to WT, reaching stationary phase at the same time and having a similar growth rate (Fig 1B). cyd1+ consistently had a slightly lower but not significantly different OD600 and CFU/mL than WT. After 30 hours, when WT began to decrease in CFU/mL, cta1+ decreased in CFU/mL more slowly than WT, while cyo1+ decreased in CFU/mL more quickly than WT, especially between 36 and 48 hours. These data indicate that each of the cytochrome oxidases conserved in B. pertussis is sufficient for WT levels of growth in liquid media in ambient air.
Each of the three Bp-conserved cytochrome oxidases is sufficient for generating energy under normal culturing conditions
The different cytochrome oxidases are predicted to generate different levels of proton motive force (PMF), and therefore ATP, per oxygen molecule reduced. We first measured the level of intracellular ATP using a luciferase-based assay, comparing levels between WT, Bp-conserved, cyd1+, cta1+, and cyo1+. Lysed cells were mixed with luciferase, which luminesces when ATP is present. The amount of luminescence corresponds to the amount of ATP present. WT and cyd1+ produced similar levels of luminescence, indicating that they produce similar levels of ATP, while Bp-conserved, cta1+, and cyo1+ resulted in higher levels of luminescence than WT, corresponding to increased levels of ATP (Fig 1C). This result indicates that under normal lab culturing conditions, any of the three cytochrome oxidases conserved in B. pertussis are sufficient to generate at least WT-levels of ATP.
To assess the PMF of the different strains, we measured the uptake of gentamicin, as uptake of aminoglycosides like gentamicin requires a PMF [48]. Bacterial cultures were treated with fluorescently-labeled gentamicin, washed, then measured for fluorescence. WT, Bp-conserved, cyd1+, cta1+, and cyo1+ all took up similar levels of gentamicin based on the relative levels of fluorescence (Fig 1D), indicating that these strains have similar levels of PMF.
None of the cytochrome oxidases conserved in B. pertussis are critical for growth in low oxygen or in elevated levels of carbon dioxide
Under normal laboratory conditions, bacteria have access to ambient air, which contains approximately 21% O2. When growing within the mammalian respiratory tract, however, bacteria encounter a different atmospheric environment. Upon inhalation, alveoli inflate and oxygen in the air is exchanged with carbon dioxide (CO2) produced by the TCA cycle; upon exhalation, this new gas mixture, which contains approximately 16% O2 and 5% CO2, is released back into the environment (reviewed in [49]). The classical bordetellae are likely further restricted from access to oxygen since the ciliated epithelial cells to which the bacteria adhere are coated in a layer of secreted mucus designed to protect the respiratory tract from environmental pathogens [50].
To investigate how different cytochrome oxidases contribute to the ability of B. bronchiseptica to grow in the different atmospheric conditions it may encounter in the mammalian respiratory tract, we grew strains within an incubator that allowed us to manipulate the atmospheric concentrations of oxygen, nitrogen, and CO2. We collected endpoint samples to keep the gas concentrations consistent for the duration of the experiment since taking samples required us to open the incubator, thus exposing the cultures to ambient air. Since we hypothesize that the three Bp-conserved cytochrome oxidases are sufficient for growth within the mammalian respiratory tract, we focused on assessing the growth of Bp-conserved, cyd1+, cta1+, and cyo1+ relative to WT.
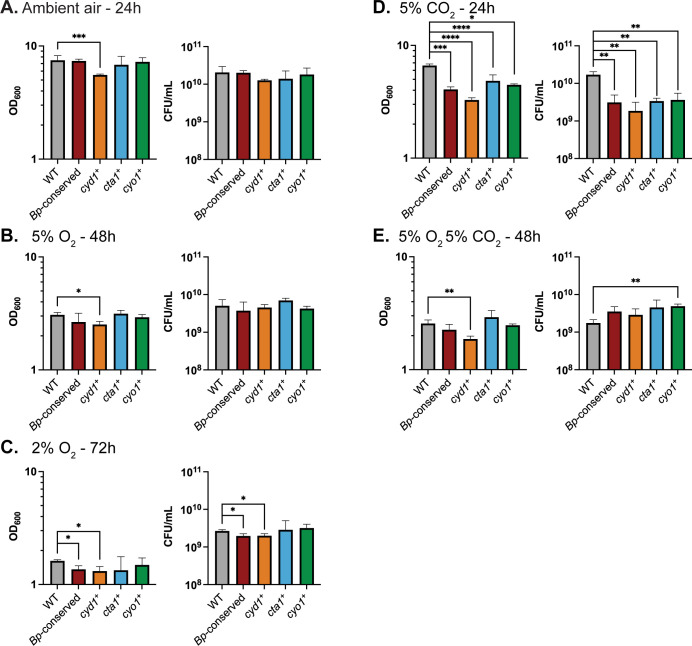
First, we assessed growth in low oxygen. Previous studies in E. coli have shown that atmospheric oxygen concentrations at or below 5% are sufficient to shift expression from low-affinity to high-affinity cytochrome oxidases in liquid cultures [51]. We therefore grew our cultures under 5% O2 with constant agitation to maximize gas exchange. WT grew slower in 5% O2 than in ambient air; cultures grown for 48 hours at 5% O2 contained less than half the bacteria found at 24 hours in cultures grown in ambient air (~21% O2) (Fig 2A vs Fig 2B). Surprisingly, Bp-conserved, cyd1+, cyo1+, and cta1+ were all able to grow to WT levels in 5% O2, with no strain having a significant defect relative to WT in both OD600 and CFU/mL (Fig 2B). Since no cytochrome oxidase was critical for growth at 5% O2, we examined the growth of the bacteria in 2% O2. Under 2% O2, WT struggled to grow; cultures grown for 72 hours at 2% O2 contained approximately 13% of the bacteria found at 24 hours in cultures grown in ambient air (~21% O2) (Fig 2A vs Fig 2C). Despite low oxygen availability, cyo1+ and cta1+ were able to grow to similar levels as WT and Bp-conserved and cyd1+ only had a slight growth defect (Fig 2C). Together, these data indicate that none of these cytochrome oxidases is required for growth under low oxygen conditions. Conversely, these data indicate that Cyd1, Cta1, or Cyo1 is sufficient for WT-levels of growth under low oxygen conditions.
Fig 2. Growth under different atmospheric conditions relevant to the environment of the mammalian respiratory tract.
Growth, measured via optical density (left) or CFU/mL (right), for RB50 (WT, gray), a strain with only the cytochrome oxidase-encoding gene loci conserved in B. pertussis (Bp-conserved, maroon), a strain with only cydAB1 (cyd1+, orange), a strain with only ctaCDFGE1 (cta1+, blue), or a strain with only cyoABCD1 (cyo1+, green). Cultures were started at 0.1 OD600, which contains approximately 3.5x108 CFU/mL. Cultures were grown in either: ambient air for 24 hours (A), 5% O2 for 48 hours (B), 2% O2 for 72 hours (C), 5% CO2 for 24 hours (D), or 5% O2 5% CO2 for 48 hours (E). The data from A are identical to the 24-hour timepoint of Fig 1A and are included for comparison. Bars represent the mean of at least 3 biological replicates, with error bars representing the standard deviation. Statistical significance was determined using unpaired Student’s t-test. *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ****, p < 0.0001. Raw data: https://doi.org/10.15139/S3/UTSSJM.
Since bacteria in the respiratory tract also encounter increased carbon dioxide, we next assessed whether CO2 levels impacted growth rates. WT grew similarly in 5% CO2 as in ambient air (Fig 2A vs Fig 2D). Bp-conserved, cyd1+, cyo1+, and cta1+, however, were all defective relative to WT by both OD600 and CFU/mL (Fig 2D). This result is surprising, as it suggests that the three cytochrome oxidases conserved in B. pertussis are not sufficient for efficient growth in the presence of 5% CO2. Indeed, it suggests that at least one of the cytochrome oxidases deleted in Bp-conserved contributes to growth in 5% CO2.
Given that the respiratory environment can simultaneously contain higher levels of CO2and lower levels of oxygen than ambient air, we tested how well our strains were able to grow in 5% O2 5% CO2. Under this condition, WT grew similarly to how it grew in 5% O2 alone (Fig 2B vs Fig 2E). After 48 hours of growth, Bp-conserved, cyd1+, cyo1+, and cta1+ were all able to grow to WT levels, with no strain having a significant defect relative to WT by both OD600 and CFU/mL (Fig 2E).
No single cytochrome oxidase is required for persistence in the murine respiratory tract
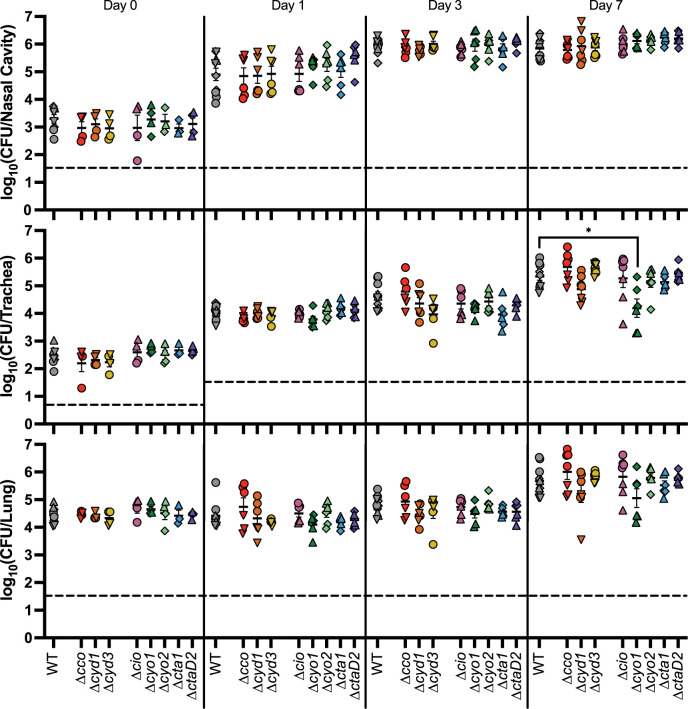
To determine the role of individual cytochrome oxidases during infection, we inoculated mice intranasally with bacteria using our high-dose, large-volume protocol, which allows us to assess how well different strains can persist once introduced in the respiratory tract. We first used strains containing deletions of individual cytochrome oxidase-encoding loci to determine which cytochrome oxidases, if any, are necessary for persistence. We collected the right lung, the trachea, and nasal cavity tissue at 3 hours, 1 day, 3 days, and 7 days post-inoculation and determined the bacterial burden in each organ. Due to the number of strains involved, we could not test all eight strains simultaneously. Instead, we tested them in batches, with each including WT, then compiled the data. All strains caused the same bacterial burden in mice as WT in the nasal cavity, trachea, and lung (Fig 3). These data indicate that no individual cytochrome oxidase is required for persistence in the murine respiratory tract.
Fig 3. No single cytochrome oxidase is required during murine infection.
Bacterial burden over time within the nasal cavity (upper), trachea (middle), and right lung (lower) of mice infected with either RB50 (WT) or strains lacking a single cytochrome oxidase-encoding gene loci. Different symbols represent different batches of inoculations, which always include WT for comparison: circles (Δcco, Δcyd1, Δcyd3, and Δcio), upright triangles (Δcio, Δcyo1, Δcyo2, Δcta1, and ΔctaD2), upside-down triangles (Δcco, Δcyd1, and Δcyd3), and diamonds (Δcyo1, Δcyo2, Δcta1, and ΔctaD2). Each point represents data from one mouse. n = 4 for day 0, n = 6 for all other timepoints, from two independent experiments, for all mutant strains. Each point represents a single mouse. Dashed line represents the limit of detection. Statistical significance was determined using unpaired Student’s t-test; *, p < 0.01. Raw data: https://doi.org/10.15139/S3/POHLJP.
The cytochrome oxidases conserved in B. pertussis are sufficient for persistence during murine infection
We next assessed the role of the three cytochrome oxidases conserved in B. pertussis for persistence during infection. We infected mice with either Bp-conserved or WT, and determined the bacterial burden in the nasal cavity, trachea, and lung over the course of infection. The Bp-conserved strain was statistically indistinguishable from WT in all tissues at all time points tested (Fig 4), indicating that the three cytochrome oxidases conserved in B. pertussis are sufficient for persistence in mice.
Fig 4. The three cytochrome oxidases conserved in B. pertussis are sufficient for persistence in the murine respiratory tract.
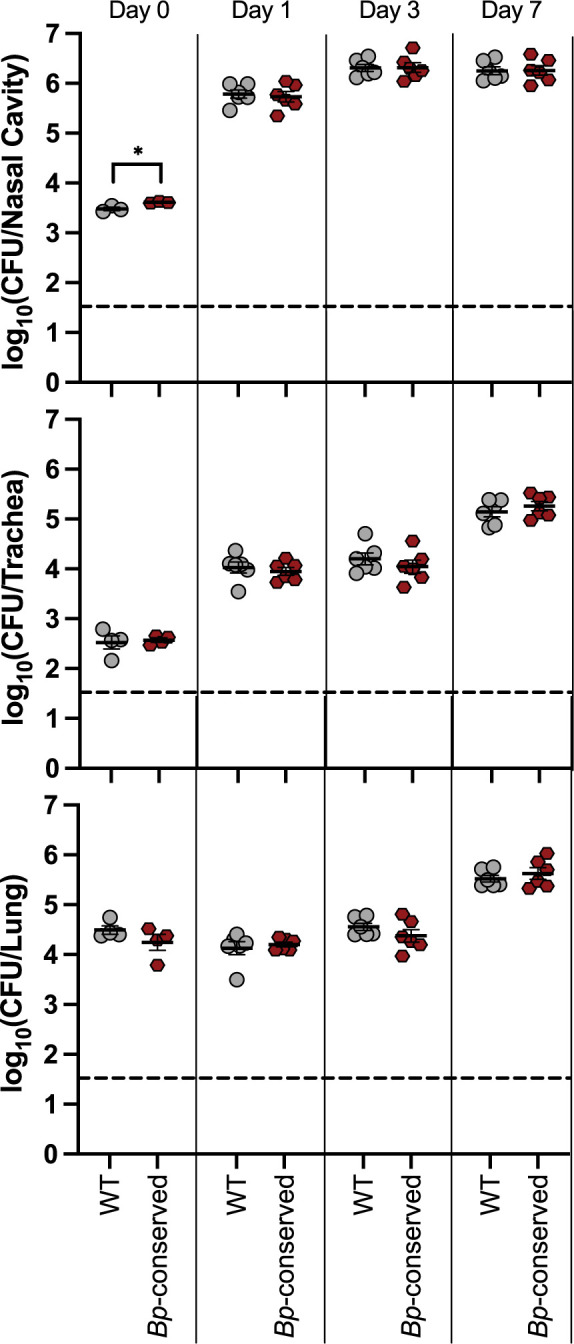
Bacterial burden over time within the nasal cavity (upper), trachea (middle), and right lung (lower) of mice infected with either wild-type bacteria (WT, grey circles) or a strain with only the cytochrome oxidase-encoding gene loci conserved in B. pertussis (Bp-conserved, maroon hexagon). n = 4 for day 0, n = 6 for all other timepoints, with each point representing a single mouse. Samples were collected from two independent experiments. Dashed line represents the limit of detection. Statistical significance was determined using unpaired Student’s t-test; *, p < 0.05. Raw data: https://doi.org/10.15139/S3/ZO4B8O.
Cyo1 is sufficient for persistence during infection, while strains with only Cyd1 or Cta1 are defective for persistence relative to WT
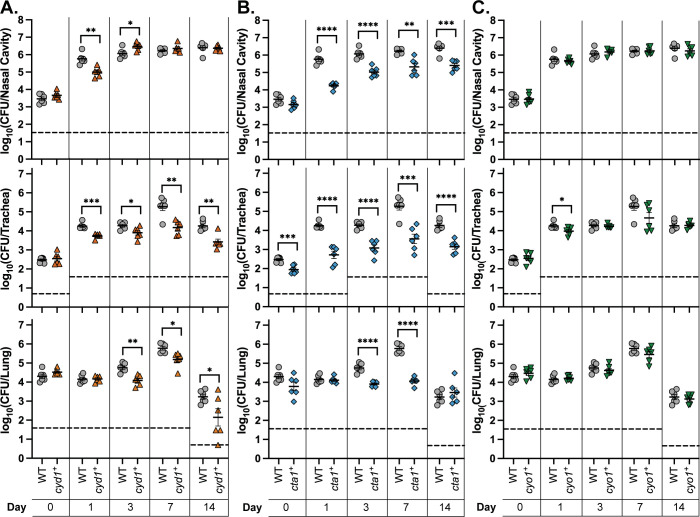
The three cytochrome oxidases conserved in B. pertussis are predicted to have different characteristics (Table 1). Cyd1 is predicted to be a high-affinity bd-type ubiquinol oxidase. Cta1 is predicted to be a low-affinity heme-copper oxidase (HCO) cytochrome c oxidase. Cyo1 is predicted to be a low-affinity HCO ubiquinol oxidase. We hypothesize that each of these cytochrome oxidases fulfills a different function during infection due to their different biochemical properties. To test this hypothesis, we infected mice using strains with only a single cytochrome oxidase-encoding gene locus remaining (i.e. cyd1+, cta1+, and cyo1+). cyd1+ had a slight defect relative to WT in the nasal cavity, causing a lower bacterial burden at day 1 that was able to reach WT levels by day 3 (Fig 5A, top). cyd1+ was consistently defective in the lower respiratory tract relative to WT, causing a significantly lower bacterial burden in the trachea and the lung out to 14 days (Fig 5A, middle and bottom). cta1+ was defective relative to WT in all three sampled tissues (Fig 5B). However, this strain was not cleared from the lung faster than WT, as seen by the statistically similar burdens recovered on day 14 when the burden of WT decreased relative to earlier time points (Fig 5B, bottom). Most surprisingly, cyo1+ was recovered at levels similar to WT in all three sampled tissues across the course of infection (Fig 5C). These data indicate that Cyo1 is sufficient for WT-levels of growth in this model of mouse infection, despite being predicted to be optimized for ambient air levels of oxygen, while Cyd1 and Cta1 are not sufficient. Additionally, all three strains are able to persist to some degree throughout the respiratory tract once introduced, indicating that any of these cytochrome oxidases is sufficient for survival within a mouse.
Fig 5. Cyo1 is sufficient for WT-levels of persistence within the mammalian respiratory tract, while Cyd1 and Cta1 are not.
Bacterial burden over time within the nasal cavity (upper), trachea (middle), and right lung (lower) of mice infected with wild-type bacteria (WT, grey circles), a strain with only cydAB1 (cyd1+, orange upright triangles), a strain with only ctaCDFGE1 (cta1+, blue diamonds), or a strain with only cyoABCD1 (cyo1+, green upside-down triangles). These strains were tested in the same experiment but separated into separate graphs for analysis; thus, the mutant strains are compared to the same WT data. n = 4 for day 0, n = 6 for all other timepoints, with each point representing a single mouse. Samples were collected from two independent experiments. Dashed line represents the limit of detection. Statistical significance was determined using unpaired Student’s t-test; *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ****, p < 0.0001. Raw data: https://doi.org/10.15139/S3/60DN0G.
Given that Cyo1 is sufficient in this mouse model, we tested if Cyo1 is also necessary within the three B. pertussis-conserved cytochrome oxidases. We infected mice with a strain containing only the other two B. pertussis-conserved cytochrome oxidase encoding loci (cyd1+ cta1+), which has comparable growth to WT in ambient air (S4A Fig). cyd1+ cta1+ phenocopied cyd1+ throughout infection and was thus defective relative to WT in the lower respiratory tract (S4B Fig). cyd1+ cta1+ also caused a significantly higher bacterial burden than cta1+ in most tissues across the course of infection. This result indicates that Cyd1 and Cta1 together cannot compensate for the loss of Cyo1; therefore, Cyo1 is necessary for infection in this mouse model when only the cytochrome oxidases conserved within B. pertussis are present.
The Bp-conserved strain is indistinguishable from WT in its ability to establish respiratory colonization
We and others have used high-dose, large-volume and low-dose, small-volume mouse protocols to investigate respiratory tract persistence and establishment of infection, respectively. In our lab, we have previously used the low-dose, small-volume protocol only in rats, where we determined that doses of 20 CFU are sufficient for infection [52]. To adapt this approach to mice, we performed preliminary experiments using WT to determine a viable volume and dose for intranasal inoculation in wild-type mice. We found that 4μL was sufficient to introduce bacteria to the nasal cavity but not the trachea, as we were able to recover CFU from the nasal cavity but not the trachea at this volume on the same day as we inoculated the mice (S5A Fig). We also found that 100 CFU per inoculum was sufficient to ensure robust colonization of the nasal cavity, as well as consistently recoverable burden from the trachea by day 7 post-inoculation (S5A Fig). In this model, bacteria were not consistently recovered from the lungs.
We next assessed whether the three Bp-conserved cytochrome oxidases are sufficient for infection. Bp-conserved phenocopied WT in the high-dose, large-volume persistence model (Fig 4), demonstrating that the three cytochrome oxidases found in B. pertussis are sufficient for persistence. We performed a preliminary experiment to determine if these three cytochrome oxidases are also sufficient for establishing infection. Mice infected with Bp-conserved had a similar bacterial burden in the nasal cavity 3 days post-inoculation as mice infected with WT, indicating that the combination of Cyd1, Cyo1, and Cta1 is sufficient for establishing infection (S5B Fig).
Cyo1 is sufficient for efficient establishment of infection, while Cyd1 and Cta1 are not
To assess the individual roles of Cyd1, Cyo1, and Cta1 during the establishment of infection, we first tested whether any of these three cytochrome oxidases is required. We used the derivatives of Bp-conserved that have two remaining cytochrome oxidases (i.e. cta1+ cyo1+, cyd1+ cyo1+, and cyd1+ cta1+). These strains phenocopied WT growth in ambient air (S4A and S6A Figs). We infected mice with these strains using our low-dose, small-volume protocol and determined the nasal cavity bacterial burdens 3 and 7 days post-inoculation. We found that mice infected with the strains with two cytochrome oxidases had similar bacterial burdens to mice infected with Bp-conserved (S6B Fig). One strain, cyd1+ cta1+, trended towards causing a lower bacterial burden than Bp-conserved, but this difference was not significant. Based on these data, no single cytochrome oxidase is required to establish infection.
To determine if any of the three Bp-conserved cytochrome oxidases is sufficient to establish infection, we infected mice with the strains with only a single cytochrome oxidase-encoding locus remaining (i.e. cyd1+, cta1+, and cyo1+) and determined the bacterial burden as above. Overall, the trends in the low-dose, small-volume experiments mirrored those seen in the high-dose, large-volume experiments. Mice infected with cyd1+ had a lower bacterial burden 3 days post-inoculation than mice infected with Bp-conserved but reached the same burden by day 7 (Fig 6). Mice infected with cta1+ had a significantly lower bacterial burden than mice infected with Bp-conserved at both day 3 and 7 (Fig 6). Despite causing less than one percent of the bacterial burden of Bp-conserved strain at day 7 in mice, cta1+ nonetheless was able to establish infection and persist for the duration of the experiment. Mice infected with cyo1+ had the same bacterial burden as mice infected with Bp-conserved at both timepoints (Fig 6). Together, these data indicate that while all three cytochrome oxidases are sufficient to enable the establishment of infection, as seen by recoverable bacteria following infection with all three strains, only Cyo1 is sufficient to achieve the level of colonization reached when all three cytochrome oxidases conserved in B. pertussis are present.
Fig 6. Cyo1 is sufficient for the efficient establishment of colonization, while Cyd1 and Cta1 are not.
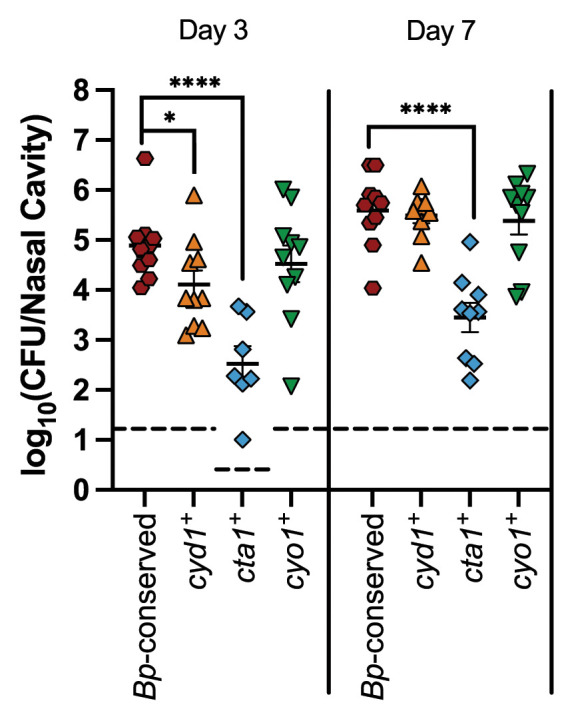
Bacterial burden over time within the nasal cavity of mice infected with a strain with only the cytochrome oxidase-encoding gene loci conserved in B. pertussis (Bp-conserved, maroon hexagon), a strain with only cydAB1 (cyd1+, orange upright triangles), a strain with only ctaCDFE1 (cta1+, blue diamonds), or a strain with only cyoABCD1 (cyo1+, green upside-down triangles). Samples from 10 mice across two independent experiments were collected at each timepoint for each strain. However, due to the natural microbiota of the nasal cavity, B. bronchiseptica could not always be enumerated due to contamination. Therefore, n = 7 for cta1+ day 3, n = 9 for cyd1+ day 7 and cta1+ day 7. Each point represents a single mouse. Dashed line represents the limit of detection. Statistical significance was determined using unpaired Student’s t-test; *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ****, p < 0.0001. Raw data: https://doi.org/10.15139/S3/VO1E3R.
Regulation of cytochrome oxidase-encoding genes broadly follows the predicted affinity of the cytochrome oxidases they encode
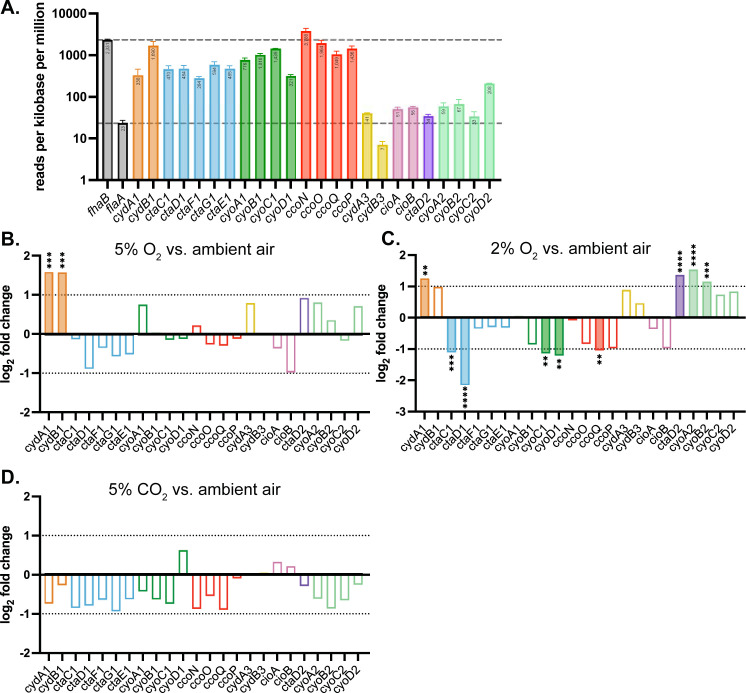
Given that we were unable to construct a strain that lacks all three of the cytochrome oxidase-encoding gene loci conserved in B. pertussis (S2 Fig), we investigated how different cytochrome oxidase-encoding genes are regulated, hypothesizing that some loci may not be expressed under laboratory growth conditions. We diluted overnight cultures to the same density, grew them for 4 hours in either ambient air, 5% O2, 2% O2, or 5% CO2, extracted RNA, and sent it for sequence analysis.
First, we examined the level of transcripts in ambient air to investigate the expression of cytochrome oxidase-encoding genes under our normal growth conditions. Raw normalized read counts mapped to each gene were converted to counts per million per kilobase of the open reading frame for comparison. All eight cytochrome oxidase-encoding gene loci had detectable transcripts (Fig 7A). However, when compared to fhaB and flaA, two well-characterized and highly-regulated genes, we found that transcript levels for most of the non-conserved cytochrome oxidase-encoding gene loci (cyd3, cio, ctaD2, and cyo2) were similar to that of flaA, the protein product of which cannot be detected under these growth conditions [53]. Therefore, transcription of cyd3, cio, ctaD2, and cyo2 may be insufficient to produce enough functional enzyme complexes to support respiration. By contrast, transcript abundance for ccoN was the highest of all of the loci, with high expression of the rest of the cco locus as well, yet we were unable to make a strain with only cco. This result suggests that there was a lack of sufficient translation, complex assembly, or cofactors required for Cco. The three B. pertussis-conserved cytochrome oxidase-encoding loci had high levels of expression comparable to that of fhaB, which is highly expressed under these conditions.
Fig 7. Regulation of cytochrome oxidase genes under different atmospheric conditions, measured using RNA sequencing.
(A) Transcript abundance, measured by counts per million per kilobase, for cytochrome oxidase-encoding genes from B. bronchiseptica grown in ambient air. fhaB and flaA, two highly-regulated and well-characterized genes, are included for comparison. flaA transcription level (lower line) is used as a reference, as flaA is negatively regulated under this growth condition and no protein product can be detected. fhaB (upper line) is highly expressed under this growth condition and is thus used as a reference for a highly transcribed gene. Numbers in the bars represent the mean number of transcripts normalized to gene length. (B-D) Comparative transcription, measured by fold change in transcript abundance, comparing 5% O2 (B), 2% O2 (C), or 5% CO2 (D) to ambient air. Genes with log2 fold changes greater than 1 or less than -1 have filled-in bars. Differential expression analysis was performed using edgeR’s glmQLFTest [73]; significance was adjusted using the Benjamini–Hochberg procedure. *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ****, p < 0.0001. Raw data: GEO repository GSE268598
We next examined the fold change in transcript abundance in bacteria grown in our conditions of interest (5% O2, 2% O2, and 5% CO2) and ambient air. In 5% O2, cydA1 and cydB1 had ~3 times more transcripts than in ambient air (Fig 7B and Table A in S2 Table). This result is consistent with the prediction that Cyd1 functions as a high-affinity cytochrome oxidase and is therefore adapted for use in low oxygen environments. No other cytochrome oxidase-encoding gene had a significant difference in transcript abundance in 5% O2 versus ambient air.
In 2% O2, differences in transcript abundance were greater (Fig 7C and Table B in S2 Table). cydA1 had significantly more transcripts in 2% O2 than in ambient air but fewer transcripts than in 5% O2. Genes within the cyo1 locus (cyoC1 and cyoD1) had significantly fewer transcripts in 2% O2 relative to ambient air, which matches the prediction that these genes encode components of a low-affinity cytochrome oxidase. ctaC1 and ctaD1 have less expression in 2% O2 relative to ambient air, with ctaD1 having a larger relative decrease in transcripts. Interestingly, ctaD2 had higher expression in 2% O2 relative to ambient air, with ~2.6 times more transcripts in 2% O2 than in ambient air. Since the genes encoding other components of Cta1 did not significantly decrease in expression in 2% O2, CtaD2 could be interacting with CtaC1 and CtaE1 (and potentially CtaF and CtaG1) to form a heterocomplex (i.e. CtaC1D2F1G1E1). Unexpectedly, ccoQ had significantly lower levels of transcripts in 2% O2 relative to ambient air, despite being predicted to encode part of a high-affinity cytochrome oxidase, and cyoC2 and cyoD2 had significantly higher levels of transcripts, despite being predicted to encode parts of a low-affinity cytochrome oxidase.
In 5% CO2, no gene had significant changes in expression relative to growth in ambient air (Fig 7D and Table C in S2 Table). This was surprising, as 5% CO2 was the only growth condition tested that resulted in a large growth defect in Bp-conserved relative to WT (Fig 2), leading us to hypothesize that a non-Bp-conserved cytochrome oxidase that is not required for growth in ambient air must be important for growth in 5% CO2. While this hypothesis could still be true, these results indicate that differences in growth in ambient air and 5% CO2 are not due to differences in cytochrome oxidase-encoding gene expression.
Discussion
Our goal was to understand the importance of cytochrome oxidases for bacterial pathogens that infect the mammalian respiratory tract. B. bronchiseptica contains eight cytochrome oxidase-encoding gene loci, while B. pertussis, which is strictly a human pathogen that survives only briefly in the environment during transmission between hosts, contains only three cytochrome oxidase-encoding gene loci that are broadly conserved across strains. Perhaps unsurprisingly, the three B. pertussis-conserved cytochrome oxidases were sufficient for B. bronchiseptica to both establish infection and persist in the respiratory tracts of mice. Somewhat surprisingly, however, B. bronchiseptica producing only a single low-affinity cytochrome oxidase (encoded by cyoABCD1, abbreviated Cyo1) was indistinguishable from WT in both infection models.
While it is well known that we inhale ambient air containing ~21% O2 and low levels of CO2 and exhale air containing ~16% O2 and ~5% CO2, the levels of oxygen within specific microenvironments in the respiratory tract are unknown. The classical bordetellae bind to the surface of ciliated epithelial cells lining the respiratory tract, which do not continue into the smallest bronchioles or the alveoli. The ciliated epithelium is covered in a layer of mucus secreted by neighboring goblet cells and tethered mucins produced by the ciliated cells themselves. These viscous layers protect the respiratory tract from environmental pathogens but impede the diffusion of molecules, including oxygen, to the epithelium. We therefore anticipated that a high-affinity cytochrome oxidase would be important in this environment, and hence were surprised that cyo1+, a strain producing only a predicted low-affinity cytochrome oxidase, was able to infect mice with the same efficiency as WT. If the Cyo1 enzyme is, in fact, low-affinity as predicted, then this result indicates that the surface of the ciliated epithelium has access to more oxygen than previously thought.
The strain producing only Cyd1, by contrast, was attenuated relative to WT in both animal models. The level of attenuation depended on the site of the respiratory tract examined. In the nasal cavity, the cyd1+ strain caused lower bacterial burdens than WT early in infection, but was able to reach WT-levels later in infection, indicating a growth delay in this environment. In the lower respiratory tract, however, the cyd1+ strain was consistently defective relative to WT throughout the course of infection. If the surface of the ciliated epithelium is sufficiently oxygenated, as the results with the cyo1+ strain suggest, this result could reflect the lower efficiency of high-affinity cytochrome oxidases, which pump fewer protons, and therefore generate less proton motive force, per electron transferred [6].
bd-type cytochrome oxidases have been linked to virulence in other pathogens not only for their oxygen affinity but also for their unique resiliency in the face of immune system attacks. bd-type cytochrome oxidases are more tolerant of oxidative and nitrosative stress than heme-copper oxidases and have been shown to detoxify hydrogen peroxide and peroxynitrite produced by neutrophils and macrophages [54]. However, Cyo1 was sufficient to enable WT-levels of bacterial burden out to 14 days post-inoculation, indicating that Cyd1 is not critical for overcoming the host immune response in our high-dose murine infection model. Given the conservation of cyd1 in B. pertussis, however, the protective properties of bd-type cytochrome oxidases may play a larger role during infection of other mammals, including humans.
Cta1 was the least effective during infection, with the strain producing only this cytochrome oxidase being recovered at lower levels than WT in all tissues at all timepoints in both models of infection. Cta1 could, however, play a role that was not assessed by our models. Indeed, previous microarray data indicated that ctaC1, ctaD1, ctaG1, and ctaE1 are repressed by BvgAS, the two-component system that positively regulates all known protein virulence factors in Bordetella [55]. Given that the Bvg+ mode is both necessary and sufficient for infection, Cta1 may play a role outside the host during transmission [56].
Importantly, the mouse respiratory tract is not identical to the respiratory tract of humans; it is more similar to the human distal airways, both in terms of surface area and mucus composition. In humans, proximal airways are coated in mucus primarily made of MUC5AC mucins and distal airways have mucus made of MUC5B, whereas in mice, Muc5b is constitutively produced throughout the airway and almost no Muc5ac is produced [57]. The organization of the mucus is also different, with humans and other large mammals producing long thick bundles of mucins such that the proximal airway is coated in a layer of mucus, while in mice (and most likely, the distal airways of humans), the mucus is unevenly distributed, forming a patchy covering [58,59]. This patchy covering creates areas with thinner mucus layers, which would make oxygen diffusion easier. Therefore, the bacteria attached to the mouse ciliated epithelium may have access to more oxygen than they would on the human ciliated epithelium.
It is perhaps unsurprising that B. bronchiseptica can grow in low oxygen given its many cytochrome oxidase-encoding loci, three of which are predicted to encode high-affinity cytochrome oxidases. WT was able to grow in both 5% and 2% O2 (albeit at a far slower rate than in ambient air). It was surprising to us, however, that the Bp-conserved, cyd1+, cta1+, and cyo1+ strains were all able to grow similarly under low oxygen conditions. Indeed, the cta1+ and cyo1+ strains, which encode only low-affinity cytochrome oxidases, grew as well as WT in 2% O2. This result indicates that all of the B. pertussis-conserved cytochrome oxidases all function equally well under low oxygen in laboratory conditions, despite their predicted affinities. Additionally, B. bronchiseptica is resilient when challenged with low oxygen. It is able to persist in static PBS, where oxygen diffusion is limited, with viable CFU being recovered even 24 weeks post-inoculation [60]. In this aspect, B. bronchiseptica is comparable to M. tuberculosis, another obligate aerobe that can persist in low oxygen conditions for long periods of time [61].
Our analysis primarily focused on the three cytochrome oxidase-encoding loci conserved in B. pertussis. However, we also examined the expression of the other five loci to see if they played a role in any of our experimental conditions. We found that cyd3, cio, ctaD2, and cyo2 had similar levels of transcripts per million per kilobase as flaA, a BvgAS-repressed gene whose protein product is not detected in our growth condition (SS medium) [53]. We were also unable to generate strains with only cco, cyd3, cio, ctaD2, or cyo2 intact; at least one of the three B. pertussis-conserved loci had to be intact to generate a strain. Although the specific environments that B. bronchiseptica occupies outside the mammalian respiratory tract are unknown, our results suggest these environments are complex and varied, requiring different cytochrome oxidases than those utilized in lab culture.
In our RNAseq analyses, we only examined expression in wild-type B. bronchiseptica and therefore did not assess the effect that altering cell homeostasis by deleting cytochrome oxidase-encoding genes could have on global gene expression. In the cyd1+, cta1+, and cyo1+ strains, it is likely that the remaining cytochrome oxidase-encoding locus is more highly expressed to compensate for the loss of the other two loci, which are normally expressed in our growth conditions. Expression changes could also explain the unusual plate-based growth phenotypes we observed for the cta1+ strain, which could not be explained by secondary mutations. Upregulation of the remaining cytochrome oxidase-encoding loci in the strains with only a single locus could be masking synergy that occurs when the cytochrome oxidases are present at their endogenous levels.
Two additional trends emerged from the RNAseq. First, many highly BvgAS-activated genes had lower expression in low oxygen than in ambient air, including bvgA, bvgS, fhaB, and cyaA (Tables A and B in S2 Table). By contrast, the common reference genes rpoD and recA were not differentially expressed in low oxygen as compared to ambient air, indicating that the differences seen for BvgAS-activated genes are not due to global changes in gene expression. In vitro studies using purified solubilized BvgS have shown that BvgS activity is sensitive to the redox state of ubiquinone through its PAS domain [62]. In vivo studies also support that BvgS is sensitive to redox potential via its PAS domain [63]. Thus, decreased expression of BvgAS-activated genes could be directly related to decreased respiration affecting BvgS activity in low oxygen environments.
Second, we found that expression of the ptx genes (ptxABCDE), which encode pertussis toxin, was increased in B. bronchiseptica in low oxygen (Tables A and B in S2 Table). This result is exciting, because ptx gene expression has never been detected in B. bronchiseptica in vitro or in vivo, and only a weak luminescent signal was detected when the B. bronchiseptica ptx promoter was used to drive the expression of luciferase-encoding genes [64]. Pertussis toxin is an important virulence factor during human infection, and in B. pertussis, the ptx genes are activated by BvgAS (reviewed in [65]). In Complex I B. bronchiseptica strains, from which the other classical bordetellae evolved, the ptx promoter region is not BvgAS-regulated but the ptx genes (as well as the ptl genes that encode the export system for pertussis toxin) are present and intact [66,67], suggesting that in B. bronchiseptica, pertussis toxin plays a different role than contributing to mammalian infection. By contrast, many Complex IV B. bronchiseptica strains have lost their ptx genes [37]. These strains also don’t survive as well as Complex I strains when grown in environmental conditions, instead being more adapted to the human hosts from which they were isolated. These results combined, together with the fact that the substrate for pertussis toxin is a eukaryotic G protein, suggest that pertussis toxin was used by ancestral B. bronchiseptica strains to manipulate a non-mammalian eukaryotic host or predator in the environment. As strains evolved to infect humans and lost their ability to survive in the environment, those that became B. pertussis acquired mutations in the ptx promoter that allowed for activation by BvgAS (and presumably not the regulator(s) that control ptx expression in B. bronchiseptica). Our findings suggest that one feature of the environment in which the ptx genes are expressed in B. bronchiseptica is low levels of accessible oxygen.
Previous genomic and experimental comparisons between the classical bordetellae have focused on virulence-associated genes like fhaB, cyaA, and the ptx genes. By comparing genes encoding proteins important for bacterial physiology, like cytochrome oxidases, we can also learn about which bacterial processes and pathways are important for survival within the host. Comparing B. pertussis and B. bronchiseptica led to our hypothesis that the three cytochrome-oxidase encoding gene loci that are conserved in B. pertussis would be sufficient during infection, which was supported by our experimental findings. Conversely, we can also learn about what is important for surviving in the environment. HT200, a B. bronchiseptica environmental isolate, differs from the other examined B. bronchiseptica strains in that HT200 does not have intact copies of all eight cytochrome-oxidase encoding gene loci. In particular, this strain has premature stop codons in both cyoB1 and cyoB2, meaning it likely has no functional bo3-type cytochrome oxidases (Table B in S1 Table). This result, especially in contrast with the broad conservation of cyo1 within B. pertussis strains, indicates that while Cyo1 is important for survival within mammalian hosts, it is not important for survival within aqueous environments. This result also supports the hypothesis that the many different cytochrome oxidases encoded within the B. bronchiseptica genome all contribute to the ability of this species to be a generalist, surviving in many different environments. As strains specialize towards life within specific environments, the bacteria no longer require all of their cytochrome oxidases and the genes that are no longer required can accrue mutations.
While the role of cytochrome oxidases has been investigated in other pathogens, including V. cholerae, M. tuberculosis, and S. aureus, the majority of species studied have been facultative anaerobes [9,18–20]. Additionally, most either infect regions like the gut that are expected to have low levels of oxygen. In these pathogens, individual high-affinity cytochrome oxidases are critical for normal infection. In the respiratory pathogen and obligate aerobe B. bronchiseptica, this is not the case. Not only is no individual cytochrome oxidase required for infection, but a predicted low-affinity cytochrome oxidase is sufficient in mice for both the establishment of infection and persistence once bacteria are introduced. Therefore, targeting bd-type cytochrome oxidases, which is being explored as a treatment for M. tuberculosis among others, would not be effective in B. bronchiseptica [54]. This result emphasizes the need to study not only virulence factors but also the basic bacterial physiology to effectively combat bacterial pathogens. By expanding our understanding of the cytochrome oxidases of other pneumonia-causing bacteria, like Neisseria meningitidis and Haemophilus influenzae, we may find that low-affinity cytochrome oxidases are better drug targets to clear infections within the respiratory tract.
Materials and methods
Ethics statement
All animal studies followed the guidelines in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. Our protocols were approved by the University of North Carolina Institutional Animal Care and Use Committee (Protocol ID: 22–140). All animals were anesthetized for inoculations, monitored daily, and euthanized properly and humanely. All efforts were made to minimize suffering.
Bacterial strains, plasmids, and growth conditions
B. bronchiseptica strains were grown on Bordet-Gengou (BG) agar plates supplemented with 6% defibrinated sheep blood (Hemostat, catalog no. DSB1) or in Stainer-Scholte (SS) broth supplemented with SS supplement at 37°C ([47], updated in [68]). One strain (cta1+) had growth defects on BG agar plates and was therefore plated on SS agar plates when possible. As needed, media were supplemented with streptomycin (Sm, 20 μg/mL), gentamicin (Gm, 30 μg/mL), or sucrose (15% w/v). E. coli strains were grown in Lysogeny Broth (LB) or on LB agar plates supplemented with diaminopimelic acid (DAP, 300 μg/mL), ampicillin (Ap, 100 μg/mL), or gentamicin (Gm, 30 μg/mL) as needed. The S. aureus SCV strain was grown in LB or on tryptic soy agar (TSA) plates (Millipore 22091). All cultures were started from individual colonies from a clonal population when possible; the S. aureus SCV was cultured from 10 individual colonies from a clonal population.
Construction of plasmids and strains
The specific deletion mutations created in each cytochrome oxidase-encoding locus is described in Table 2. The strains and plasmids used in this study are listed in Tables 3 and 4, respectively. In-frame deletions were generated using allelic exchange via derivatives of the pEG7S vector. We used the DH5α E. coli strain for plasmid construction and propagation. We used the RHO3 E. coli strain for transforming B. bronchiseptica. Any mutations made within B. bronchiseptica strains were confirmed by PCR and/or whole-genome sequencing. The order in which deletions were generated in strains with deletions in multiple loci is described in S2 Fig.
Table 2. Mutations generated in this study.
| Deletion number | Deletion Name | Description |
|---|---|---|
| 1 | ΔccoNOQP | Deletion of 3188bp: from nucleotide 7 of ccoN (BB3329) to nucleotide 2084 of ccoP (BB3326) |
| 2 | ΔcydAB1 | Deletion of 2772bp: from nucleotide 9 of cydA1 (BB4498) to nucleotide 1147 of cydB1 (BB4497) |
| 3 | ΔcydAB3 | Deletion of 2373bp: from 41 nucleotides upstream of cydA3 (BB4012) to nucleotide 994 of cydB3 (BB4011) |
| 4 | ΔcioAB | Deletion of 2380bp: from nucleotide 9 of cioA (BB1238) to nucleotide 1000 of cioB (BB1239) |
| 5 | ΔcyoABCD1 | Deletion of 3842bp: from nucleotide 11 of cyoA1 (BB1283) to nucleotide 339 of cyoD1 (BB1286) |
| 6 | ΔcyoABCD2 | Deletion of 3641bp: from nucleotide 6 of cyoA2 (BB1310) to nucleotide 632 of cyoC2 (BB1308); cyoD2 is left intact |
| 7 | ΔctaCDFE1 | Deletion of 4165bp: from nucleotide 9 of ctaC1 (BB4831) to nucleotide 493 of ctaE1 (BB4827) |
| 8 | ΔctaD2 | Deletion of 1578bp: from nucleotide 10 to nucleotide 1587 of ctaD2 (BB4674) |
Table 3. Plasmids used in this study.
| Plasmid name | Description | Source |
|---|---|---|
| pEG7S | Suicide allelic exchange plasmid for B. bronchiseptica used to generate in-frame deletions; confers sucrose sensitivity through sacB. GmR ApR | [52] |
| pEG7S-ΔccoNOQP | pEG7S containing ~500bp upstream and downstream of mutation 1 | This study |
| pMAB19 | pEG7S containing ~500bp upstream and downstream of mutation 2 | This study |
| pEG7S-ΔcydAB3 | pEG7S containing ~500bp upstream and downstream of mutation 3 | This study |
| pEG7S-ΔcioAB | pEG7S containing ~500bp upstream and downstream of mutation 4 | This study |
| pEG7S-Δcyo1 | pEG7S containing ~500bp upstream and downstream of mutation 5 | This study |
| pEG7S-Δcyo2 | pEG7S containing ~500bp upstream and downstream of mutation 6 | This study |
| pEG7S-Δcta1 | pEG7S containing ~500bp upstream and downstream of mutation 7 | This study |
| pEG7S-ΔctaD2 | pEG7S containing ~500bp upstream and downstream of mutation 8 | This study |
Table 4. Strains used in this study.
| Strain | Description | Source |
|---|---|---|
| B. bronchiseptica | ||
| RB50 (WT) | Wild-type B. bronchiseptica strain isolated from nares of a naturally infected rabbit; SmR | [56] |
| Δcco | RB50 containing mutation 1; SmR | This study |
| Δcyd1 | RB50 containing mutation 2; SmR | This study |
| Δcyd3 | RB50 containing mutation 3; SmR | This study |
| Δcio | RB50 containing mutation 4; SmR | This study |
| Δcyo1 | RB50 containing mutation 5; SmR | This study |
| Δcyo2 | RB50 containing mutation 6; SmR | This study |
| Δcta1 | RB50 containing mutation 7; SmR | This study |
| ΔctaD2 | RB50 containing mutation 8; SmR | This study |
| Bp-conserved | RB50 containing mutations 1, 3, 4, 6, and 8; SmR | This study |
| cyo1+ cta1+ | RB50 containing mutations 1–4, 6, and 8; SmR | This study |
| cyd1+ cta1+ | RB50 containing mutations 1, 3–6, and 8; SmR | This study |
| cyd1+ cyo1+ | RB50 containing mutations 1, 3, 4, and 6–8; SmR | This study |
| cyd1 + | RB50 containing mutations 1 and 3–8; SmR | This study |
| cyo1 + | RB50 containing mutations 1–4 and 6–8; SmR | This study |
| cta1 + | RB50 containing mutations 1–6 and 8; SmR | This study |
| E. coli | ||
| DH5α | Molecular cloning strain derived from K-12 | Gibco-BRL |
| RHO3 | Conjugation strain; Δasd ΔaphA, DAP auxotroph | [69] |
| S. aureus | ||
| SCV | USA300 LAC-derived respiration deficient strain from the Nebraska Transposon Mutant Library; transposon insertion in menD (SAUSA300_0946) | [70] |
Genomic comparisons
28 Bordetella strains were selected based on previously published genomic comparisons: six B. bronchiseptica Complex I strains, four B. bronchiseptica Complex IV strains, four B. parapertussisHu strains, and fourteen B. pertussis strains. Additional information on the strains investigated can be found in Table A in S1 Table. The nucleotide sequences of RB50 cytochrome oxidase-encoding loci were used as references to search for corresponding loci in the genomes of the other strains using BLAST. Genomes were determined to lack an intact locus if: 1) no locus corresponding to the RB50 cytochrome oxidase-encoding locus was found via BLAST; 2) a premature stop codon was introduced in any gene in the locus that encoded an essential cytochrome oxidase subunit; 3) a frameshift mutation occurred that did not introduce a premature stop codon but would prevent production of a functional protein in any gene in the locus that encoded an essential cytochrome oxidase subunit. Percent amino acid homology was determined by translating open reading frames using Geneious 11.1.5 and doing pairwise MUSCLE alignments using version 3.8.425 implemented through Geneious.
TTC reduction assay
Cultures of B. bronchiseptica were grown overnight at 37°C in SS media supplemented with SS supplement and Sm. A culture of the S. aureus small colony variant (SCV) strain, which is unable to respire, was included as a negative control. Each culture was normalized to 1 OD600/mL. Five microliters were spotted onto TSA plates containing 0.001% (w/v) TTC (2,3,5-triphenyl-tetrazolium chloride) (Sigma-Aldrich T8877) and incubated in the dark for 24 hours at 37°C. Plates were photographed to capture the irreversible color shift from colorless to red that occurs following TTC reduction.
Intracellular ATP measurements
Cultures of B. bronchiseptica were grown overnight at 37°C in SS media supplemented with SS supplement and Sm. These cultures were diluted to 1.0 x 108 CFU per 100 μL. These diluted samples were then assessed using the BacTiter-Glo Microbial Cell Viability Assay (Promega G8230), which uses luminescence generated by luciferase as a proxy for ATP levels. Luminescence was measured using a BioTek Synergy H1 Plate Reader.
Gentamicin uptake assay
Cultures of B. bronchiseptica were grown overnight at 37°C in SS media supplemented with SS supplement and Sm, then washed with Dulbecco PBS (DPBS). Each culture was normalized to 1 OD600/mL in SS, then treated with 50 μM gentamicin conjugated with Texas Red-succinimidyl ester (Thermo Fisher T6134) for 2 hours at 37°C. After treatment, cultures were washed with DPBS. The level of gentamicin uptake by the bacteria was measured via Texas Red fluorescence using a BioTek Synergy H1 Plate Reader.
Liquid culture growth assays
Cultures of B. bronchiseptica were grown for 18 hours at 37°C in SS media supplemented with SS supplement and Sm. Each culture was normalized to 0.1 OD600/mL and grown on an orbital shaker (VWR 89032–096) at 225 rpm at 37°C. Samples were collected at 0, 3, 6, 9, 12, 18, 24, 36, and 48 hours after the start of the experiments. We measured the OD600 of these samples and determined the CFU/mL by serially diluting the samples and plating on BG blood agar plates. The number of colonies were enumerated after at least 48 hours growth at 37°C, with cta1+ requiring at least 72 hours growth at 37°C.
Growth under different atmospheric conditions
Cultures for outgrowth were prepared in the same manner as for growth curves. Samples were then grown on an orbital shaker at 225 rpm at 37°C within a trigas incubator connected to N2 and CO2 gas sources (HERACELL VIOS 160i; Fisher Scientific 13998258). To reduce O2 levels, pure N2 was introduced. We took endpoint samples to ensure that the experimental conditions remained constant throughout and were not disrupted by the reintroduction of atmospheric oxygen at each sampling point. Samples were collected at 24 hours when grown in 5% CO2, 48 hours when grown in 5% O2 and in 5% O2 5% CO2, and 72 hours when grown in 2% O2. We measured the OD600 of these samples and determined the CFU/mL by serially diluting the samples and plating on BG blood agar plates. The number of colonies were enumerated after at least 48 hours growth at 37°C, with cta1+ requiring at least 72 hours growth at 37°C.
Bacterial infection of the mouse respiratory tract using the persistence model
Six-week-old female BALB/c mice from Charles River Laboratories (catalog no. BALB/cAnNCrl) were inoculated intranasally with 7.5 × 104 CFU B. bronchiseptica in 50 μL of DPBS. Samples were collected at three hours, one day, three days, seven days, and fourteen days post-infection. At each indicated time point, right lung lobes, trachea, and nasal cavity tissue were harvested from each mouse and the tissues were homogenized in DPBS using a mini-beadbeater with 0.1 mm zirconia beads (Biospec catalog no. 11079110zx). The number of CFU was determined by plating dilutions of tissue homogenates on BG Sm blood agar and enumerating the number of colonies per tissue after at least 48 hours growth at 37°C, with cta1+ requiring at least 72 hours growth at 37°C.
Bacterial colonization of the mouse respiratory tract
Six-week-old female BALB/c mice from Charles River Laboratories (catalog no. BALB/cAnNCrl) were inoculated intranasally with 100 CFU B. bronchiseptica in 4 μL of Dulbecco PBS (DPBS), divided equally between the two nares. Samples were collected at three days and seven days post-infection. At each indicated time point, nasal cavity tissue was harvested and homogenized in DPBS using a mini-beadbeater with 0.1 mm zirconia beads (Biospec catalog no. 11079110zx). The number of CFU was determined by plating dilutions of tissue homogenates on BG Sm blood agar and enumerating the number of colonies per tissue after at least 48 hours growth at 37°C, with cta1+ requiring at least 72 hours growth at 37°C.
RNA isolation and sequencing
Cultures of B. bronchiseptica were grown overnight at 37°C in SS media supplemented with SS supplement and Sm. Each culture was normalized to 1 OD600/mL and grown on an orbital shaker at 225 rpm at 37°C for 4 hours under different atmospheric conditions as above. Three biological replicates were generated for each condition. Bacteria were pelleted, resuspended in RNAlater (Invitrogen AM7020), then stored at -80°C until extraction. RNA was isolated using Trizol/chloroform extraction, followed by centrifugation in Phasemaker Tubes (Invitrogen 12183555). The aqueous phase was precipitated in isopropanol, washed in ethanol, and solubilized in RNase-free water. Samples were then cleaned using RNAeasy column purification (Qiagen 74104). RNA sequencing was performed by SeqCenter (Pittsburgh, PA; 12M Paired End Reads rRNA depletion sequencing package). Library preparation was performed using Illumina’s Stranded Total RNA Prep Ligation with Ribo-Zero Plus kit and 10bp unique dual indices. Sequencing was performed using a NovaSeq X Plus.
RNA analysis
RNA analysis was performed by SeqCenter (Pittsburgh, PA; intermediate RNA analysis package). Read mapping was performed using HISAT2 [71] and read quantification was performed using featureCounts [72]. Read counts were loaded into R, then normalized using edgeR’s Trimmed Mean of M values (TMM) algorithm [73]. These values were then converted to counts per million. Differential expression analysis between our different conditions (5% O2 vs ambient air, 2% O2 vs ambient air, and 5% CO2 vs ambient air) was performed using edgeR’s glmQLFTest.
Supporting information
(XLSX)
Table B: Fold change differences in transcript abundance of all genes of RB50 grown either in 2% O2 or ambient air. Table C: Fold change differences in transcript abundance of all genes of RB50 grown either in 5% CO2 or ambient air.
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
Thank you to the members of the Cotter lab for their support and the discussions that aided our investigation. Thanks to the labs of Brain Conlon and Sarah Rowe for providing us with the S. aureus SCV strain and assisting with the gentamicin uptake assay.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by grants from the NIH to PAC (R01 AI153160, R01 AI129541, R21 AI177818). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hemp J, Robinson DE, Ganesan KB, Martinez TJ, Kelleher NL, Gennis RB. Evolutionary migration of a post-translationally modified active-site residue in the proton-pumping heme-copper oxygen reductases. Biochemistry. 2006;45: 15405–15410. doi: 10.1021/bi062026u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. The cytochrome bd respiratory oxygen reductases. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2011;1807: 1398–1413. doi: 10.1016/j.bbabio.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice CW, Hempfling WP. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. Journal of Bacteriology. 1978;134: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preisig O, Zufferey R, Thöny-Meyer L, Appleby CA, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. Journal of Bacteriology. 1996;178: 1532–1538. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’mello R, Hill S, Poole RK. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 1996;142: 755–763. doi: 10.1099/00221287-142-4-755 [DOI] [PubMed] [Google Scholar]
- 6.Han H, Hemp J, Pace LA, Ouyang H, Ganesan K, Roh JH, et al. Adaptation of aerobic respiration to low O2 environments. Proceedings of the National Academy of Sciences. 2011;108: 14109–14114. doi: 10.1073/pnas.1018958108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones SA, Chowdhury FZ, Fabich AJ, Anderson A, Schreiner DM, House AL, et al. Respiration of Escherichia coli in the mouse intestine. Infection and Immunity. 2007;75: 4891–4899. doi: 10.1128/iai.00484-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd M, Achard MES, Idris A, Totsika M, Phan M-D, Peters KM, et al. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Scientific Reports. 2016;6: 35285. doi: 10.1038/srep35285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Alst AJ, Demey LM, DiRita VJ. Vibrio cholerae requires oxidative respiration through the bd-I and cbb3 oxidases for intestinal proliferation. PLOS Pathogens. 2022;18: e1010102. doi: 10.1371/journal.ppat.1010102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbett D, Goldrick M, Fernandes VE, Davidge K, Poole RK, Andrew PW, et al. Listeria monocytogenes has both cytochrome bd-type and cytochrome aa3-type terminal oxidases, which allow growth at different oxygen levels, and both are important in infection. Infection and Immunity. 2017;85: e00354–17. doi: 10.1128/IAI.00354-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera-Lugo R, Deng D, Anaya-Sanchez A, Tejedor-Sanz S, Tang E, Reyes Ruiz VM, et al. Listeria monocytogenes requires cellular respiration for NAD+ regeneration and pathogenesis. eLife. 2022;11: e75424. doi: 10.7554/eLife.75424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Way SS, Sallustio S, Magliozzo RS, Goldberg MB. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. Journal of Bacteriology. 1999;181: 1229–1237. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinevez J-Y, Arena ET, Anderson M, Nigro G, Injarabian L, André A, et al. Shigella-mediated oxygen depletion is essential for intestinal mucosa colonization. Nature Microbiology. 2019;4: 2001–2009. doi: 10.1038/s41564-019-0525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivera-Chávez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, et al. Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host & Microbe. 2016;19: 443–454. doi: 10.1016/j.chom.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craig M, Sadik AY, Golubeva YA, Tidhar A, Slauch JM. Twin-arginine translocation system (tat) mutants of Salmonella are attenuated due to envelope defects, not respiratory defects. Molecular Microbiology. 2013;89: 887–902. doi: 10.1111/mmi.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones-Carson J, Husain M, Liu L, Orlicky DJ, Vázquez-Torres A. Cytochrome bd-dependent bioenergetics and antinitrosative defenses in Salmonella pathogenesis. mBio. 2016;7: doi: 10.1128/mbio.02052-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewin GR, Stacy A, Michie KL, Lamont RJ, Whiteley M. Large-scale identification of pathogen essential genes during coinfection with sympatric and allopatric microbes. Proceedings of the National Academy of Sciences. 2019;116: 19685–19694. doi: 10.1073/pnas.1907619116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Y, Jaecklein E, Mackenzie JS, Papavinasasundaram K, Olive AJ, Chen X, et al. Host immunity increases Mycobacterium tuberculosis reliance on cytochrome bd oxidase. PLOS Pathogens. 2021;17: e1008911. doi: 10.1371/journal.ppat.1008911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, et al. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proceedings of the National Academy of Sciences. 2005;102: 15629–15634. doi: 10.1073/pnas.0507850102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer ND, Reniere ML, Cassat JE, Zhang Y, Hirsch AO, Indriati Hood M, et al. Two heme-dependent terminal oxidases power Staphylococcus aureus organ-specific colonization of the vertebrate host. mBio. 2013;4: doi: 10.1128/mbio.00241-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Poyart C, Trieu-Cuot P, Lamberet G, Gruss A, Gaudu P. Respiration metabolism of Group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Molecular Microbiology. 2005;56: 525–534. doi: 10.1111/j.1365-2958.2005.04555.x [DOI] [PubMed] [Google Scholar]
- 22.Joubert L, Dagieu J-B, Fernandez A, Derré-Bobillot A, Borezée-Durant E, Fleurot I, et al. Visualization of the role of host heme on the virulence of the heme auxotroph Streptococcus agalactiae. Scientific Reports. 2017;7: 40435. doi: 10.1038/srep40435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilgore PE, Salim AM, Zervos MJ, Schmitt H-J. Pertussis: Microbiology, disease, treatment, and prevention. Clinical Microbiology Reviews. 2016;29: 449–485. doi: 10.1128/CMR.00083-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nature Genetics. 2003;35: 32–40. doi: 10.1038/ng1227 [DOI] [PubMed] [Google Scholar]
- 25.Kranz RG, Beckett CS, Goldman BS. Genomic analyses of bacterial respiratory and cytochrome c assembly systems: Bordetella as a model for the System II cytochrome c biogenesis pathway. Research in Microbiology. 2002;153: 1–6. doi: 10.1016/S0923-2508(01)01278-5 [DOI] [PubMed] [Google Scholar]
- 26.Pereira MM, Santana M, Teixeira M. A novel scenario for the evolution of haem–copper oxygen reductases. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2001;1505: 185–208. doi: 10.1016/s0005-2728(01)00169-4 [DOI] [PubMed] [Google Scholar]
- 27.Sharma V, Wikström M, Kaila VRI. Redox-coupled proton transfer in the active site of cytochrome cbb3. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2010;1797: 1512–1520. doi: 10.1016/j.bbabio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 28.Friedrich T, Wohlwend D, Borisov VB. Recent advances in structural studies of cytochrome bd and its potential application as a drug target. International Journal of Molecular Sciences. 2022;23: 3166. doi: 10.3390/ijms23063166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kana BD, Weinstein EA, Avarbock D, Dawes SS, Rubin H, Mizrahi V. Characterization of the cydAB-encoded cytochrome bd oxidase from Mycobacterium smegmatis. J Bacteriol. 2001;183: 7076–7086. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arutyunyan AM, Borisov VB, Novoderezhkin VI, Ghaim J, Zhang J, Gennis RB, et al. Strong excitonic interactions in the oxygen-reducing site of bd-type oxidase: the Fe-to-Fe Distance between Hemes d and b595 is 10Å. Biochemistry. 2008;47: 1752–1759. doi: 10.1021/bi701884g [DOI] [PubMed] [Google Scholar]
- 31.Saraste M, Metso T, Nakari T, Jalli T, Lauraeus M, Van Der Oost J. The Bacillus subtilis cytochrome-c oxidase. European Journal of Biochemistry. 1991;195: 517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x [DOI] [PubMed] [Google Scholar]
- 32.Müller M, Schläpfer B, Azzi A. Cytochrome c oxidase from Paracoccus denitrificans: both hemes are located in subunit I. Proceedings of the National Academy of Sciences. 1988;85: 6647–6651. doi: 10.1073/pnas.85.18.6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirai T, Osamura T, Ishii M, Arai H. Expression of multiple cbb3 cytochrome c oxidase isoforms by combinations of multiple isosubunits in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences. 2016;113: 12815–12819. doi: 10.1073/pnas.1613308113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namslauer A, Brzezinski P. Structural elements involved in electron-coupled proton transfer in cytochrome c oxidase. FEBS Letters. 2004;567: 103–110. doi: 10.1016/j.febslet.2004.04.027 [DOI] [PubMed] [Google Scholar]
- 35.Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, et al. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat Struct Mol Biol. 2000;7: 910–917. doi: 10.1038/82824 [DOI] [PubMed] [Google Scholar]
- 36.Cunningham L, Pitt M, Williams HD. The cioAB genes from Pseudomonas aeruginosa code for a novel cyanide-insensitive terminal oxidase related to the cytochrome bd quinol oxidases. Molecular Microbiology. 1997;24: 579–591. doi: 10.1046/j.1365-2958.1997.3561728.x [DOI] [PubMed] [Google Scholar]
- 37.Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, Mooi FR. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathogens. 2005;1: e45. doi: 10.1371/journal.ppat.0010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bridel S, Bouchez V, Brancotte B, Hauck S, Armatys N, Landier A, et al. A comprehensive resource for Bordetella genomic epidemiology and biodiversity studies. Nature Communications. 2022;13: 3807. doi: 10.1038/s41467-022-31517-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weigand MR, Peng Y, Batra D, Burroughs M, Davis JK, Knipe K, et al. Conserved patterns of symmetric inversion in the genome evolution of Bordetella respiratory pathogens. mSystems. 2019;4: e00702–19. doi: 10.1128/mSystems.00702-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badhai J, Das SK. Genomic plasticity and antibody response of Bordetella bronchiseptica strain HT200, a natural variant from a thermal spring. FEMS Microbiology Letters. 2021;368: fnab035. doi: 10.1093/femsle/fnab035 [DOI] [PubMed] [Google Scholar]
- 41.Brinig MM, Cummings CA, Sanden GN, Stefanelli P, Lawrence A, Relman DA. Significant gene order and expression differences in Bordetella pertussis despite limited gene content variation. Journal of Bacteriology. 2006;188: 2375–2382. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middendorf B, Gross R. Representational difference analysis identifies a strain-specific LPS biosynthesis locus in Bordetella spp. Molecular & general genetics: MGG. 1999;262: 189–198. doi: 10.1007/s004380051074 [DOI] [PubMed] [Google Scholar]
- 43.Musser JM, Hewlett EL, Peppler MS, Selander RK. Genetic diversity and relationships in populations of Bordetella spp. Journal of Bacteriology. 1986;166: 230–237. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber C, Boursaux-Eude C, Coralie G, Caro V, Guiso N. Polymorphism of Bordetella pertussis isolates circulating for the last 10 years in France, where a single effective whole-cell vaccine has been used for more than 30 years. Journal of Clinical Microbiology. 2001;39: 4396–4403. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rich PR, Mischis LA, Purton S, Wiskich JT. The sites of interaction of triphenyltetrazolium chloride with mitochondrial respiratory chains. FEMS Microbiology Letters. 2001;202: 181–187. doi: 10.1111/j.1574-6968.2001.tb10801.x [DOI] [PubMed] [Google Scholar]
- 46.Kendrick P, Eldering G. Cough plate examinations for B. pertussis. Am J Public Health Nations Health. 1934;24: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. Journal of general microbiology. 1970. doi: 10.1099/00221287-63-2-211 [DOI] [PubMed] [Google Scholar]
- 48.Mates SM, Eisenberg ES, Mandel LJ, Patel L, Kaback HR, Miller MH. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1982;79: 6693–6697. doi: 10.1073/pnas.79.21.6693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pleil JD, Wallace MAG, Davis MD, Matty CM. The physics of human breathing: flow, timing, volume, and pressure parameters for normal, on-demand, and ventilator respiration. Journal of Breath Research. 2021;15: 042002. doi: 10.1088/1752-7163/ac2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuomanen EI, Hendley JO. Adherence of Bordetella pertussis to human respiratory epithelial cells. The Journal of Infectious Diseases. 1983;148: 125–130. doi: 10.1093/infdis/148.1.125 [DOI] [PubMed] [Google Scholar]
- 51.Tseng CP, Albrecht J, Gunsalus RP. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. Journal of Bacteriology. 1996;178: 1094–1098. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akerley BJ, Cotter PA, Miller JF. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80: 611–620. doi: 10.1016/0092-8674(95)90515-4 [DOI] [PubMed] [Google Scholar]
- 53.Akerley BJ, Monack DM, Falkow S, Miller JF. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol. 1992;174: 980–990. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borisov VB, Siletsky SA, Paiardini A, Hoogewijs D, Forte E, Giuffrè A, et al. Bacterial oxidases of the cytochrome bd family: Redox enzymes of unique structure, function, and utility as drug targets. Antioxidants & Redox Signaling. 2021;34: 1280–1318. doi: 10.1089/ars.2020.8039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cummings CA, Bootsma HJ, Relman DA, Miller JF. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. Journal of Bacteriology. 2006;188: 1775–1785. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infection and Immunity. 1994;62: 3381–3390. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fahy JV, Dickey BF. Airway mucus function and dysfunction. The New England Journal of Medicine. 2010;363: 2233–2247. doi: 10.1056/NEJMra0910061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ermund A, Meiss LN, Rodriguez-Pineiro AM, Bähr A, Nilsson HE, Trillo-Muyo S, et al. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochemical and Biophysical Research Communications. 2017;492: 331–337. doi: 10.1016/j.bbrc.2017.08.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fakih D, Rodriguez-Piñeiro AM, Trillo-Muyo S, Evans CM, Ermund A, Hansson GC. Normal murine respiratory tract has its mucus concentrated in clouds based on the Muc5b mucin. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2020;318: L1270–L1279. doi: 10.1152/ajplung.00485.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Porter JF, Parton R, Wardlaw AC. Growth and survival of Bordetella bronchiseptica in natural waters and in buffered saline without added nutrients. Applied and Environmental Microbiology. 1991;57: 1202–1206. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wayne LG, Lin KY. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infection and Immunity. 1982;37: 1042–1049. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bock A, Gross R. The unorthodox histidine kinases BvgS and EvgS are responsive to the oxidation status of a quinone electron carrier. European Journal of Biochemistry. 2002;269: 3479–3484. doi: 10.1046/j.1432-1033.2002.03029.x [DOI] [PubMed] [Google Scholar]
- 63.Sobran MA, Cotter PA. The BvgS PAS domain, an independent sensory perception module in the Bordetella bronchiseptica BvgAS phosphorelay. Journal of Bacteriology. 2019;201. doi: 10.1128/JB.00286-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Q, Gray MC, Hewlett E, Stibitz S. Four single-basepair mutations in the ptx promoter of Bordetella bronchiseptica are sufficient to activate the expression of pertussis toxin. Sci Rep. 2021;11: 9373. doi: 10.1038/s41598-021-88852-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Locht C, Coutte L, Mielcarek N. The ins and outs of pertussis toxin. The FEBS Journal. 2011;278: 4668–4682. doi: 10.1111/j.1742-4658.2011.08237.x [DOI] [PubMed] [Google Scholar]
- 66.Park J, Zhang Y, Buboltz AM, Zhang X, Schuster SC, Ahuja U, et al. Comparative genomics of the classical Bordetella subspecies: the evolution and exchange of virulence-associated diversity amongst closely related pathogens. BMC Genomics. 2012;13: 545. doi: 10.1186/1471-2164-13-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hausman SZ, Cherry JD, Heininger U, Wirsing von König CH, Burns DL. Analysis of proteins encoded by the ptx and ptl genes of Bordetella bronchiseptica and Bordetella parapertussis. Infection and Immunity. 1996;64: 4020–4026. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hulbert RR, Cotter PA. Laboratory maintenance of Bordetella pertussis. Current Protocols in Microbiology. 2009;15: 4B.1.1-4B.1.9. doi: 10.1002/9780471729259.mc04b01s15 [DOI] [PubMed] [Google Scholar]
- 69.López CM, Rholl DA, Trunck LA, Schweizer HP. Versatile dual-technology system for markerless allele replacement in Burkholderia pseudomallei. Appl Environ Microbiol. 2009;75: 6496–6503. doi: 10.1128/AEM.01669-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio. 2013;4: e00537–00512. doi: 10.1128/mBio.00537-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37: 907–915. doi: 10.1038/s41587-019-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30: 923–930. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 73.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26: 139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Table B: Fold change differences in transcript abundance of all genes of RB50 grown either in 2% O2 or ambient air. Table C: Fold change differences in transcript abundance of all genes of RB50 grown either in 5% CO2 or ambient air.
(XLSX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.