Abstract
One of the most exceptional adaptations to extreme drought is found in the sister group to tetrapods, the lungfishes (Dipnoi), which can aestivate inside a mucus cocoon for multiple years at reduced metabolic rates with complete cessation of ingestion and excretion. However, the function of the cocoon tissue is not fully understood. Here we developed a new more natural laboratory protocol for inducing aestivation in the West African lungfish, Protopterus annectens, and investigated the structure and function of the cocoon. We used electron microscopy and imaging of live tissue-stains to confirm that the inner and outer layers of the paper-thin cocoon are composed primarily of living cells. However, we also repeatedly observed extensive bacterial and fungal growth covering the cocoon and found no evidence of anti-microbial activity in vitro against E. coli for the cocoon tissue in this species. This classroom discovery-based research, performed during a course-based undergraduate research experience course (CURE), provides a robust laboratory protocol for investigating aestivation and calls into the question the function of this bizarre vertebrate adaptation.
Keywords: Protopterus annectens, aestivation, hibernation, immune function, drought tolerance, adaptation, Dipnoi
Introduction
Evolutionary novelties provide fascinating subjects for engagement with science, tests of evolutionary theory, and case studies for mapping the genetic basis of human diseases (Streelman et al. 2007; Moczek 2008; Shubin et al. 2009; Powder and Albertson 2016; Davis et al. 2019). One set of examples are provided by ephemeral and intromittent aquatic habitats, which have selected for the convergent evolution of aestivation in a diverse group of aquatic and semi-aquatic vertebrates (reviewed in (Glass et al. 2009; Secor and Lignot 2010; Lajus and Alekseev 2019). This includes spadefoot toads (Zamora-Camacho et al. 2019; Calabrese and Pfennig 2023; Chen et al. 2023), African clawed frogs (Childers 2014), turtles (Ligon and Stone 2003), amphiumas (Smith and Secor 2017), and the Australian salamanderfish (Ogston et al. 2016) among many other species known to aestivate. One of the most exceptional examples of aestivation are found in the South American (Lepidosiren paradoxa) and African lungfishes (Protopterus spp.), known to withstand multi-year droughts as adults curled inside a mucus cocoon (Smith 1931; Janssens 1964; Reno et al. 1972). Dipnoi are the sister group to all tetrapods, representing over 400 million years of independent evolutionary history and potentially novel strategies for surviving droughts over this immense timespan (Criswell 2015). As water levels fall, lungfishes remain in their muddy burrows and shed additional mucus to form a thin papery cocoon which was originally thought to be reminiscent of dried leaves (Smith 1931). The only opening in the cocoon is to their mouth for respiration which is maintained through a narrow passage to the surface from their muddy burrow. During this time they cease all feeding and excretion, shift from the production of ammonia to urea, and substantially drop their metabolic and respiratory rates in a state of torpor (Chew et al. 2004; Loong et al. 2008; Hiong et al. 2013; Chew et al. 2015). They can remain in this state for at least several years, losing over 10% of their body mass until the rains return, when they begin normal body movement and foraging activities within a day (Janssens 1964; Fishman et al. 1986; Greenwood 1986; Fishman et al. 1992; Glass et al. 2009; Chew et al. 2015).
It was recently reported that the slender African lungfish (Protopterus dolloi) secretes a cocoon composed of living tissue with antimicrobial properties drawing from large reservoirs of granulocytes in its organs (Heimroth et al. 2021; Salinas et al. 2023). Cocoons were examined after ten days following food restriction and antimicrobial function was inferred from the presence of extracellular protein traps, high levels of beta defensin expression, and potentially new skin toxins (Tacchi et al. 2015; Heimroth et al. 2018, 2021; DeMmon et al. 2022; Casadei and Salinas 2023; Salinas et al. 2023). However, many aspects of cocoon function are still unknown, particularly after longer time periods in aestivation, between inner and outer layers of the cocoon, and in additional lungfish species besides P. dolloi.
Here we first developed a new laboratory protocol that does not involve food restriction for inducing aestivation and cocoon formation in the West African lungfish (P. annectens) and includes the addition of loam-rich wet soil to better recreate the natural conditions surrounding aestivation in this species and avoid unnecessarily restricting food. We confirmed that the paper-thin cocoon tissue of the West African lungfish is composed predominantly of living cells on both the inner and outer surfaces using fluorescent nuclear and cell integrity staining, consistent with its potential role in immune function. However, we found no evidence of antimicrobial activity of cocoon tissue using standard E. coli growth and inhibition assays. Overall, we find the West African lungfish to be a fascinating laboratory model for course-based undergraduate research experience (CURE) courses, during which the aestivation protocol and results reported here were pioneered by undergraduate student coauthors over the past three years.
Methods
Wild-caught West African lungfish (P. annectens) imported from Nigeria (n = 3) were acquired from U.S. commercial retailers in 2021 and 2022. Adult fish (30 cm in length) were housed in flow-through 400-liter partitioned acrylic tanks at 23–27° C, pH 8, with a 12:12 photoperiod under artificial light following standard husbandry conditions for other freshwater fishes in the lab (Martin 2012; Martin et al. 2019; Palominos et al. 2023). Fish were fed every other day with primarily commercial pellets (New Life Spectrum and Hikari) supplemented with occasional frozen bloodworms (Hikari) or live feeder fish. Fish were housed in the laboratory for at least two months before use in any experiments.
Aestivation protocol
In order to monitor aestivation visually without disturbing the fish, we developed a new experimental procedure to induce aestivation in the lab (see also (Delaney et al. 1974; Ip et al. 2005). Terrestrial loam-rich soil with minimal organic matter was collected from the UC Berkeley campus. Because the lungfish were not sterile, we did not sterilize the soil but took care to avoid collecting soil from aquatic habitats to avoid contamination or potential pathogen exposure. An approximately 2 cm layer of soil covered with tank water to 2 cm depth was placed in a clean 40-liter aquarium under a photoperiod of 12:12. Each lungfish was placed directly into its own tank without any preceding period of starvation at 23–25° C ambient air temperatures, taking care to maintain high levels of humidity with a tight-fitting lid (but not completely airtight) and mud along the bottom of the tank. Over the course of a few days, lungfish progressively suspended movement and secreted an increased amount of mucus from their opercula as water evaporated naturally from each tank, leaving only a layer of mud (Fig. 1). Within approximately 1–2 weeks, depending on the amount of residual water in each tank, the lungfish cocoon dried around each animal alongside the hardened mud after most residual water evaporated. Animals were monitored daily for any signs of desiccation, but no additional food or water was provided during the aestivation period, except a few milliliters of water added to the surrounding hardened mud as needed (approximately weekly) to maintain humidity at 80–100%. One animal responded with an audible ‘barking’ vocalization in response to light touch but did not respond in later aestivation trials (initially reported by (Smith 1931)). This was a vocalization in response to touch and distinct from respiration which could be observed at the opercula for approximately the first week before the cocoon hardened around the lungfish. Initial pilot experimental trials were unsuccessful in inducing aestivation if a deep layer of mud (0.3 m) was provided, which may substantially prolong the time needed for cocoon formation.
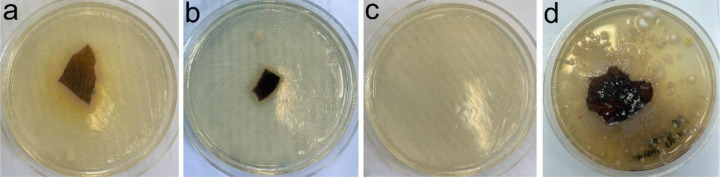
Fig. 1.
West African lungfish in various states of aestivation and recovery. a-b) Newly added to 40-liter terrarium with mud layer. c-f) After multiple weeks and months in aestivation. Note the papery thin cocoon covered in mold and fungal colonies in some areas. g) Removal of cocoon following addition of tank water after approximately one hour. h) One week post recovery after aestivation period of three months, readily feeding on commercial pellet food.
Animals were maintained in a state of aestivation for up to three months with no ill effects as long as humidity levels remained high within each covered tank (Fig. 1). Lungfish could be recovered from their state of aestivation by simply adding dechlorinated water to the aestivation tank to a depth of approximately 2–3 cm. After approximately one hour, the cocoon tissue softened, sloughed off, and the animals began to respond slowly to gentle touch. Cocoon tissue could either be peeled away or left attached. Lungfish were returned to their home tanks with a reduced water level to allow easy access to the water surface for respiration and were provided with a small amount of food. Within 24 hours, animals were eating normally and within a few days had completely recovered typical levels of movement and response to stimuli. Repeated aestivation trials on the same animal were possible with a recovery period of only one week. However, all samples were collected from animals that had recovered from aestivation in laboratory aquaria for at least three months before repeating an aestivation trial.
Sampling cocoon tissue
After at least two weeks in aestivation, the mucus layer hardened into a thin papery shell around each fish (Fig. 1). Samples of this tissue after approximately 1 month in aestivation were removed by carefully puncturing the cocoon with forceps at an angle parallel to the surface of the fish to avoid damaging the inner tissue. The cocoon tissue layer could then be peeled off for imaging, staining, and antimicrobial assays. We noted that cocoon tissue reformed around these sampling areas after approximately one week (darkened central dorsal patches visible in Fig. 1c). Cocoon tissue was always sampled fresh from the aestivating animal before procedures and never preserved before use.
Scanning electron microscopy
Fresh cocoon tissue samples were prepared for electron microscopy imaging using standard protocols by the Electron Microscope Lab at UC Berkeley. Samples were fixed in 2% glutaraldehyde in 0.1M sodium cacodylate buffer for 1–2 hours, rinsed in 0.1M sodium cacodylate buffer, and then post-fixed in 1% osmium tetroxide in 0.1M sodium cacodylate buffer. After three rinses with 0.1M sodium cacodylate buffer, samples were dehydrated in a stepped series of ethanol washes. Following critical point drying, each sample was cut into at least 2 parts and placed upwards and downwards onto the SEM stub, resulting in images taken from both the outer and inner surface of the cocoon. Samples were mounted onto stubs by using conductive carbon tape or silver paint and imaged on a Zeiss Crossbeam 550 FIB-SEM.
Cocoon tissue staining
Fresh cocoon tissue sampled from two aestivating lungfish was double-stained with propidium iodide (Thermo Fisher Scientific) to detect newly dead cells from the damage to the integrity of the cell membrane and DAPI (Sigma-Aldrich) to label cell nuclei. Stains were conducted at two different timepoints after initiating aestivation trials: one month post and three months post aestivation. Briefly, the dissected pieces of the cocoon were mounted on positively charged (X) Poly-L lysine (Sigma-Aldrich) coated slides and stained for 15 min covered from light with a staining solution containing propidium iodide and DAPI, respectively. We stained and imaged both the inside and outside of the cocoon, sampled from the dorsal medial surface of the fish.
Antimicrobial assays
Fresh cocoon tissue was sampled from two aestivating lungfish at two different timepoints after initiating aestivation trials: two weeks post and six weeks post. E. coli (OP50 strain originally obtained from the Caenorhabditis Genome Center (Brenner 1974)) was prepared by inoculating a 1L flask of liquid Luria Broth (LB; 10g Tryptone, 5g Yeast Extract, 5g NaCl, to 1L H2O, autoclaved) from a single streaked colony, and then incubated at 25°C overnight to saturation, then concentrated tenfold via centrifugation, and lastly stored at 4°C for up to a week prior to use. Either 24 hours before exposure to tissue to test for bactericidal compounds or a few hours before tissue exposure to test for bacterial growth inhibitors, 500 μL of 10X concentrated OP50 was inoculated and sterilely spread on LB agar plates (LB + 15g agar). Dorsal sections of cocoon tissue were placed on each plate (Fig. 4) and incubated at room temperature to carefully monitor bacterial growth over the following seven days. We incubated at lower than optimal temperatures to carefully track bacterial growth progress over multiple days. We tested a total of 16 plates at both inoculation conditions. We also exposed a set of control plates that were not inoculated with E. coli (n = 4). In all cases, we examined plates for evidence of bactericidal or growth inhibition around the cocoon tissues after 1, 3, and 7 days post-exposure.
Fig. 4.
Antimicrobial assays using LB plates inoculated with E. coli. a-b) Lungfish tissue removed after two months of aestivation shows no evidence of antimicrobial activity against E. coli growth rings surrounding tissue relative to normal E. coli growth in c) control plate. d) LB plate without E. coli inoculation shows extensive microbial growth surrounding cocoon tissue.
Results
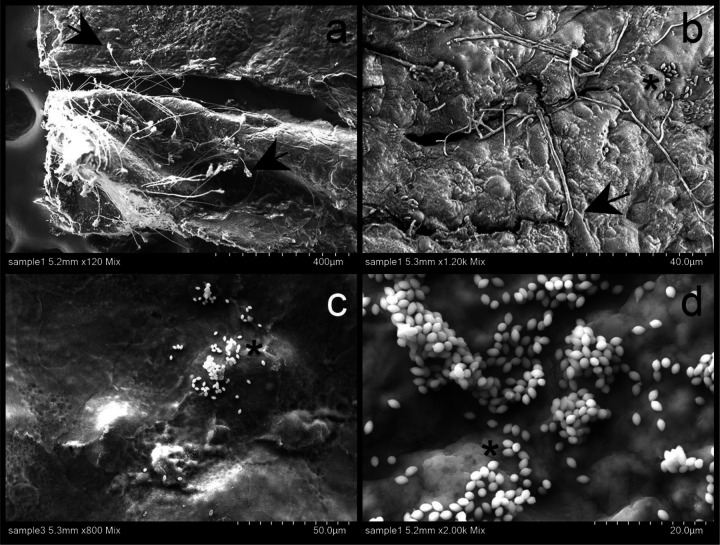
We successfully pioneered a new and efficient laboratory protocol for inducing aestivation in the West African lungfish. We confirmed that repeated aestivation trails and repeated sampling of tissue from the same region were possible. In all aestivating animals (n = 3) across three sets of trials over three years (2021, 2022, and 2023), we observed extensive growth of mold on the surface of cocoons and in the surrounding mud, usually within two to three weeks after initiating aestivation. This growth did not appear to impact the subsequent health of the animal but does call into question the anti-microbial properties of the cocoon tissue during early stages of aestivation. However, no mold growth was observed on the inner surface of the fish after removing cocoon tissue, so the cocoon may still be providing a barrier to microbial entry. Scanning electron micrographs confirmed the uneven, semi-porous structure of the outer surface of the cocoon (Fig. 2), which was also covered with bacteria, fungal spores, and fungal hyphae (Fig. 3).
Fig. 2.
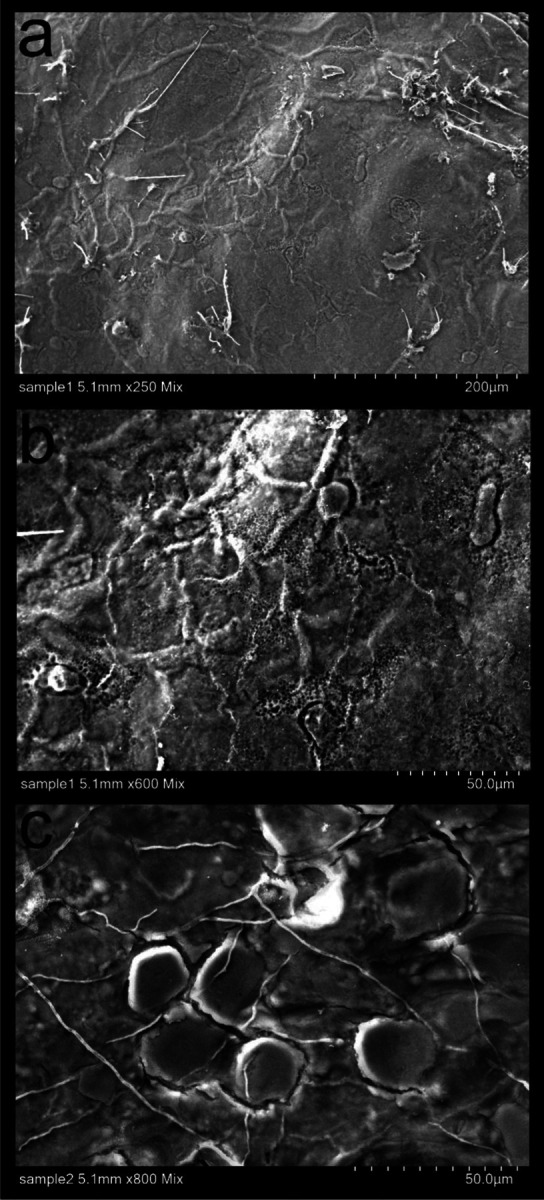
SEM images of the outer surface of lungfish cocoons from two different aestivating animals. a-c) Note the cocoon filaments and in some cases porous texture of the surface of the cocoon.
Fig. 3.
SEM images of the outer surface of lungfish cocoon from two aestivating individuals. a-b) Fungal hyphae. c-d) Aggregation of possible yeast. Arrows pointing to hyphae and asterisks to spores.
Antimicrobial assays using E. coli inoculated LB plates provided no evidence of bactericidal or bacterial growth inhibition of cocoon tissue samples from multiple animals at two different timepoints during aestivation trials in 2023. In all cases, a ‘halo’ of reduced growth was not observed surrounding tissue samples placed in the center of each plate, whether exposed to tissue 24-hours post-inoculation or a few hours after inoculation (Fig. 4). Instead, increased bacterial growth was observed around each sample over several days post exposure. To test if this was E. coli growth or another microbe, we also examined sterile LB plates exposed to cocoon tissue and observed the same increased level of microbial growth around each sample, potentially due to the bacterial communities already present on the lungfish cocoon.
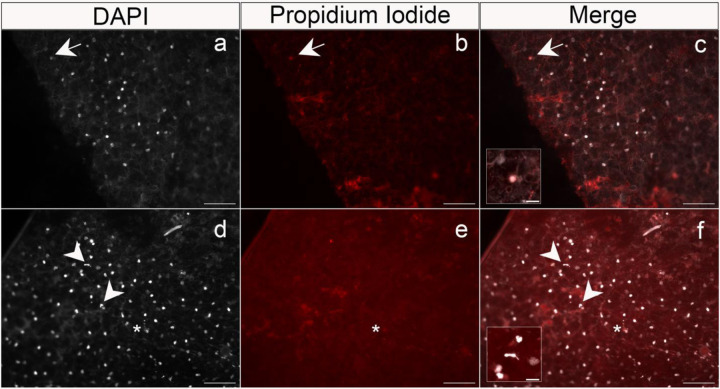
Nonetheless, double-staining of both the wet inside layer and dry outside layer of the cocoon from multiple animals at two timepoints during aestivation indicated that most of the cells on the inner and outer layer of the lungfish cocoon are alive, consistent with previous results in a different species of lungfish, P. dolloi (Heimroth et al. 2021). Propidium iodide staining indicated that dead cells within the cocoon tissue occurred relatively infrequently on both inner and outer layers (Fig. 5, arrows, Fig. 5c, inset). In contrast, DAPI cell nuclei staining indicated dense and regular spacing of cells across both the inner and outer cocoon sections examined (Fig. 5); however, besides the regularly-spaced nuclei, we also observed several neutrophil-like shaped nuclei (Fig. 5d–f, arrowheads, Fig. 5f, inset) in the outer region of the cocoon of one of the aestivating P. annectens. This is also in accordance with the reported infiltration of granulocytic immune cells from the aestivating lungfish to the cocoon (Heimroth et al. 2021), alongside what looks like neutrophilic extracellular traps (Fig. 5d–f, asterisks)
Fig. 5.
Double-staining of the inner region from two lungfish cocoon tissue samples with DAPI (left column) and propidium iodine (PI, middle column). Images were taken on a Axio Imager M2 fluorescent microscope. Merged images are shown in c) and f). Tissues were sampled fresh from two different P. annectens aestivating individuals for two weeks. a) Note the greatly increased number of DAPI-stained nuclei present in the left column relative to only a b) single PI positive nuclei (arrows), pointing out a unique cell in the later stages of cell death among all DAPI-positive PI-negative nuclei (c, inset). d-f) Arrowheads indicate neutrophil-like shaped nuclei, one of them magnified in f). Scale: 100 μm for all pictures, and 20μm for the insets. Asterisks indicate possible neutrophil extracellular traps.
Discussion
We developed a new, efficient and more natural laboratory protocol for inducing aestivation in the West African lungfish P. annectens through an undergraduate course-based undergraduate research experience (CURE) class Ichthyology: an introduction to the scientific process through the study of fishes. This format has resulted in many successful research projects in which students form their own hypotheses and test their ideas during the lab period over a semester, resulting in both student-led publications (Zeng and Martin 2017; Davis et al. 2019; St John et al. 2020; Tan et al. 2023) and contributions to larger research projects in the lab (St. John and Martin 2019; St John et al. 2019; St. John et al. 2020, 2021; Richards et al. 2021; Galvez et al. 2022). We discovered that both the inner wet and outer dry layer of the mucus cocoon is composed predominantly of living cells (Fig. 4), consistent with earlier work showing that the cocoon tissue is alive in a different species of African lungfish (Heimroth et al. 2021). Moreover, we also pioneered a working protocol to stain and quantify live and dead cells on aestivating lungfish cocoon tissue samples.
We observed an alarming amount of mold and microbial growth on this living cocoon over repetitive aestivation trials (Figs. 1–2) in a non-sterile environment, similar to conditions in the wild during aestivation. Nonetheless, animal health does not appear to be affected by microbial growth on the outer layer of the cocoon which may prevent microbes from reaching the inner cocoon layer next to the aestivating lungfish’s skin (Fig. 1d). Recovery time may be needed between induced aestivation trials for the lungfish to be able to produce a functional healthy cocoon that will protect them from the outer desiccating and unsterile environment.
We found no evidence of antimicrobial activity against E. coli growth or bactericidal activity by the cocoon tissue itself. However, additional assays against a wider range of microbial taxa in both liquid media and plates are needed to determine whether this tissue plays a role in immune function. It is also unclear whether recruitment from granulocyte reservoirs in forming the cocoon tissue necessarily leads to immune function by this tissue over the full span of aestivation in natural conditions (Ip et al. 2005; Heimroth et al. 2021).
In favor of the immune function hypothesis for the lungfish cocoon, we found neutrophil-like shaped nuclei and what looks like neutrophil extracellular traps in the inner wet region of P. annectens cocoons (Fig. 5); however, we did not find that the cocoon itself was mainly composed of granulocytes or other innate immune cells. Instead, the cocoon shows a high degree of cell nuclei organization suggesting that it is a complex living organized tissue which harbors a plethora of cell types that have different organizational requirements. Overall, our results highlight the still unknown function of an organized external living tissue in Dipnoi.
Alternatively, the cocoon may function to regulate metabolism and air exchange with the aestivating lungfish, which largely ceases to breathe and stops all ingestion and excretion of waste during this period (Chew et al. 2004, 2015; Ip et al. 2005). Furthermore, it remains unknown how the living cells within the cocoon are nourished or supplied with oxygen through passive diffusion or some sort of additional circulatory network.
Ultimately, no other aestivating vertebrate animals are known to secrete a living cellular matrix during aestivation to our knowledge (Glass et al. 2009; Secor and Lignot 2010; Lajus and Alekseev 2019). However, given that this was reported only recently in lungfish, likely few if any other studies have investigated the mucus layers surrounding other aestivating vertebrate groups which were previously assumed to be dried, desiccated mucus or interlocked layers of proteins (Glass et al. 2009; Secor and Lignot 2010; Storey and Storey 2012). The investigation of extraordinary evolutionary novelties can often lead in unexpected directions and is increasingly urgent during this period of rapid biodiversity loss during the Anthropocene (Moyle and Leidy 1992; Darwall and Freyhof 2016; Johnson et al. 2017).
Acknowledgements
We thank the University of California, Berkeley, NSF CAREER 1749764, NIH NIDCR 5R01DE027052-02, the Berkeley Collegium Fund, and a Berkeley Discovery-based learning grant for funding to CHM. We also thank Danielle Jorgens at the Electron Microscope Lab at UC Berkeley for quickly processing our samples in collaboration with undergraduate researchers. All animal care protocols (AUP-2021-02-14062-1 and AUP-2021-07-14515) were approved by the University of California, Berkeley Animal Care and Use committees.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Data Accessibility
Data will be deposited to Dryad digital repository.
References
- Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese G. M., and Pfennig K. S.. 2023. Climate Change Alters Sexual Signaling in a Desert-Adapted Frog. Am. Nat. 201:91–105. [DOI] [PubMed] [Google Scholar]
- Casadei E., and Salinas I.. 2023. Fighting pathogens in two battlefields: Antimicrobial defenses in the African lungfish. PLoS Pathog. 19:e1011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Pfennig D. W., and Pfennig K. S.. 2023. A maladaptive parental effect: offspring survival decreases with maternal over-condition in an amphibian. Biol. J. Linn. Soc. Lond. academic.oup.com. [Google Scholar]
- Chew S. F., Chan N. K. Y., Loong A. M., Hiong K. C., Tam W. L., and Ip Y. K.. 2004. Nitrogen metabolism in the African lungfish (Protopterus dolloi) aestivating in a mucus cocoon on land. J. Exp. Biol. 207:777–786. The Company of Biologists. [DOI] [PubMed] [Google Scholar]
- Chew S. F., Ching B., Chng Y. R., Ong J. L. Y., Hiong K. C., Chen X. L., Ip Y. K., Zaccone G., Dabrowski K., Hedrick M. S., and Others. 2015. Aestivation in African lungfishes: physiology, biochemistry and molecular biology. Phylogeny, anatomy and physiology of ancient fishes 81–132. CRC Press; Boca Raton, FL. [Google Scholar]
- Childers C. 2014. Regulation of skeletal Muscle Glycolysis During Dehydration in the Aestivating African Clawed Frog, Xenopus Laevis. repository.library.carleton.ca.
- Criswell K. E. 2015. The comparative osteology and phylogenetic relationships of African and South American lungfishes (Sarcopterygii: Dipnoi). Zool. J. Linn. Soc. [Google Scholar]
- Darwall W. R. T., and Freyhof J.. 2016. Lost fishes, who is counting? The extent of the threat to freshwater fish biodiversity. Conservation of freshwater fishes 1–36. Cambridge University Press; Cambridge. [Google Scholar]
- Davis A. L., Babb M. H., Lowe M. C., and Yeh A. T.. 2019. Testing Darwin’s hypothesis about the wonderful venus flytrap: marginal spikes form a “horrid prison” for moderate-sized insect prey. The American. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney R. G., Lahiri S., and Fishman A. P.. 1974. Aestivation of the African lungfish Protopterus aethiopicus: cardiovascular and respiratory functions. J. Exp. Biol. 61:111–128. [DOI] [PubMed] [Google Scholar]
- DeMmon D. M., Benedicenti O., Casadei E., and Salinas I.. 2022. The diversity of beta defensins in lungfish (Dipnoi). J. Immunol. 208:59.16–59.16. The American Association of Immunologists. [Google Scholar]
- Fishman A. P., Galante R. J., Winokur A., and Pack A. I.. 1992. Estivation in the African Lungfish. Proc. Am. Philos. Soc. 136:61–72. American Philosophical Society. [Google Scholar]
- Fishman A. P., Pack A. I., Delaney R. G., and Galante R. J.. 1986. Estivation inProtopterus. J. Morphol. 190:237–248. Wiley. [Google Scholar]
- Galvez J. R., St John M. E., McLean K., Touokong C. D., Gonwouo L. N., and Martin C. H.. 2022. Trophic specialization on unique resources despite limited niche divergence in a celebrated example of sympatric speciation. Ecol. Freshw. Fish 31:675–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M. L., Amin-Naves J., and da Silva G. S. F.. 2009. Aestivation in Amphibians, Reptiles, and Lungfish. Pp. 179–189 in Glass M. L. and Wood S. C., eds. Cardio-Respiratory Control in Vertebrates: Comparative and Evolutionary Aspects. Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- Greenwood P. H. 1986. The natural history of African lungfishes. J. Morphol. 190:163–179. Wiley. [Google Scholar]
- Heimroth R. D., Casadei E., Benedicenti O., Amemiya C. T., Muñoz P., and Salinas I.. 2021. The lungfish cocoon is a living tissue with antimicrobial functions. Science Advances 7:eabj0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimroth R. D., Casadei E., and Salinas I.. 2018. Effects of Experimental Terrestrialization on the Skin Mucus Proteome of African Lungfish (Protopterus dolloi). Front. Immunol. 9:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiong K. C., Ip Y. K., Wong W. P., and Chew S. F.. 2013. Differential gene expression in the brain of the African lungfish, Protopterus annectens, after six days or six months of aestivation in air. PLoS One 8:e71205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip Y. K., Yeo P. J., Loong A. M., Hiong K. C., Wong W. P., and Chew S. F.. 2005. The interplay of increased urea synthesis and reduced ammonia production in the African lungfish Protopterus aethiopicus during 46 days of aestivation in a mucus cocoon. J. Exp. Zool. A Comp. Exp. Biol. 303:1054–1065. Wiley. [DOI] [PubMed] [Google Scholar]
- Janssens P. A. 1964. THE METABOLISM OF THE AESTIVATING AFRICAN LUNGFISH. Comp. Biochem. Physiol. 11:105–117. Elsevier. [DOI] [PubMed] [Google Scholar]
- Johnson C. N., Balmford A., Brook B. W., Buettel J. C., Galetti M., Guangchun L., and Wilmshurst J. M.. 2017. Biodiversity losses and conservation responses in the Anthropocene. Science 356:270–275. [DOI] [PubMed] [Google Scholar]
- Lajus D. L., and Alekseev V. R.. 2019. Fish: Diapause, Dormancy, Aestivation, and Delay in Gonad Development. Pp. 53–69 in Alekseev V. R. and Pinel-Alloul B., eds. Dormancy in Aquatic Organisms. Theory, Human Use and Modeling. Springer International Publishing, Cham. [Google Scholar]
- Ligon D. B., and Stone P. A.. 2003. Radiotelemetry Reveals Terrestrial Estivation in Sonoran Mud Turtles (Kinosternon sonoriense). hpet 37:750–754. Society for the Study of Amphibians and Reptiles. [Google Scholar]
- Loong A. M., Ang S. F., Wong W. P., Pörtner H. O., Bock C., Wittig R., Bridges C. R., Chew S. F., and Ip Y. K.. 2008. Effects of hypoxia on the energy status and nitrogen metabolism of African lungfish during aestivation in a mucus cocoon. J. Comp. Physiol. B 178:853–865. Springer. [DOI] [PubMed] [Google Scholar]
- Martin C. H. 2012. Weak disruptive selection and incomplete phenotypic divergence in two classic examples of sympatric speciation: cameroon crater lake cichlids. Am. Nat. 180:E90–E109. [DOI] [PubMed] [Google Scholar]
- Martin C. H., McGirr J. A., Richards E. J., and St John M. E.. 2019. How to Investigate the Origins of Novelty: Insights Gained from Genetic, Behavioral, and Fitness Perspectives. Integr Org Biol 1:obz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczek A. P. 2008. On the origins of novelty in development and evolution. Bioessays 30:432–447. Wiley. [DOI] [PubMed] [Google Scholar]
- Moyle P. B., and Leidy R. A.. 1992. Loss of Biodiversity in Aquatic Ecosystems: Evidence from Fish Faunas. Pp. 127–169 in Fiedler P. L. and Jain S. K., eds. Conservation Biology: The Theory and Practice of Nature Conservation Preservation and Management. Springer US, Boston, MA. [Google Scholar]
- Ogston G., Beatty S. J., Morgan D. L., Pusey B. J., and Lymbery A. J.. 2016. Living on burrowed time: Aestivating fishes in south-western Australia face extinction due to climate change. Biol. Conserv. 195:235–244. Elsevier. [Google Scholar]
- Palominos M. F., Muhl V., Richards E. J., Miller C. T., and Martin C. H.. 2023. Jaw size variation is associated with a novel craniofacial function for galanin receptor 2 in an adaptive radiation of pupfishes. bioRxiv, doi: 10.1101/2023.06.02.543513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powder K. E., and Albertson R. C.. 2016. Cichlid fishes as a model to understand normal and clinical craniofacial variation. Dev. Biol. 415:338–346. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno H. W., Gehlbach F. R., and Turner R. A.. 1972. Skin and Aestivational Cocoon of the Aquatic Amphibian, Siren intermedia Le Conte. Copeia 1972:625–631. [American Society of Ichthyologists and Herpetologists (ASIH), Allen Press]. [Google Scholar]
- Richards E. J., McGirr J. A., Wang J. R., St John M. E., Poelstra J. W., Solano M. J., O’Connell D. C., Turner B. J., and Martin C. H.. 2021. A vertebrate adaptive radiation is assembled from an ancient and disjunct spatiotemporal landscape. Proc. Natl. Acad. Sci. U. S. A. 118. National Acad Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas I., Posavi M., and Benedicenti O.. 2023. Discovery of a toxin for skin immune defense in African lungfish. J. Immunol. 210:61.20–61.20. The American Association of Immunologists.36445376 [Google Scholar]
- Secor S. M., and Lignot J.-H.. 2010. Morphological Plasticity of Vertebrate Aestivation. Pp. 183–208 in Navas C. Arturo and Carvalho J. E., eds. Aestivation: Molecular and Physiological Aspects. Springer Berlin Heidelberg, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- Shubin N., Tabin C., and Carroll S.. 2009. Deep homology and the origins of evolutionary novelty. Nature 457:818–823. [DOI] [PubMed] [Google Scholar]
- Smith H. W. 1931. Observations on the African Lung-Fish, Protopterus Aethiopicus, and on Evolution from Water to Land Environments. Ecology 12:164–181. Ecological Society of America. [Google Scholar]
- Smith M. E., and Secor S. M.. 2017. Physiological Responses to Fasting and Estivation for the Three-Toed Amphiuma (Amphiuma tridactylum). Physiol. Biochem. Zool. 90:240–256. [DOI] [PubMed] [Google Scholar]
- St John M. E., Dixon K. E., and Martin C. H.. 2020. Oral shelling within an adaptive radiation of pupfishes: Testing the adaptive function of a novel nasal protrusion and behavioural preference. J. Fish Biol. 97:163–171. Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John M. E., Holzman R., and Martin C. H.. 2020. Rapid adaptive evolution of scale-eating kinematics to a novel ecological niche. J. Exp. Biol. 223:jeb217570. The Company of Biologists Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John M. E., and Martin C. H.. 2019. Scale-eating specialists evolved adaptive feeding kinematics within a microendemic radiation of San Salvador Island pupfishes.
- St John M. E., McGirr J. A., and Martin C. H.. 2019. The behavioral origins of novelty: did increased aggression lead to scale-eating in pupfishes? Behav. Ecol. 30:557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John M. E., Richards E. J., Dunker J. C., and Romero S.. 2021. Parallel genetic changes underlie integrated craniofacial traits in an adaptive radiation of trophic specialist pupfishes. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey K. B., and Storey J. M.. 2012. Aestivation: signaling and hypometabolism. J. Exp. Biol. 215:1425–1433. [DOI] [PubMed] [Google Scholar]
- Streelman J. T., Peichel C. L., and Parichy D. M.. 2007. Developmental Genetics of Adaptation in Fishes: The Case for Novelty. Annu. Rev. Ecol. Evol. Syst. 38:655–681. Annual Reviews. [Google Scholar]
- Tacchi L., Larragoite E. T., Muñoz P., Amemiya C. T., and Salinas I.. 2015. African lungfish reveal the evolutionary origins of organized mucosal lymphoid tissue in vertebrates. Curr. Biol. 25:2417–2424. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A., St John M., Chau D., Clair C., Chan H., Holzman R., and Martin C. H.. 2023. Multiple performance peaks for scale-biting in an adaptive radiation of pupfishes. bioRxiv, doi: 10.1101/2023.12.22.573139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora-Camacho F. J., Medina-Gálvez L., and Zambrano-Fernández S.. 2019. The roles of sex and morphology in burrowing depth of Iberian spadefoot toads in different biotic and abiotic environments. J. Zool. 309:224–230. Wiley. [Google Scholar]
- Zeng Y., and Martin C. H.. 2017. Oxford Nanopore sequencing in a research-based undergraduate course. BioRxiv. biorxiv.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be deposited to Dryad digital repository.