Abstract
The capacity of the brain to compensate for insults during development depends on the type of cell loss, whereas the consequences of genetic mutations in the same neurons are difficult to predict. We reveal powerful compensation from outside the cerebellum when the excitatory cerebellar output neurons are ablated embryonically and demonstrate that the minimum requirement for these neurons is for motor coordination and not learning and social behaviors. In contrast, loss of the homeobox transcription factors Engrailed1/2 (EN1/2) in the cerebellar excitatory lineage leads to additional deficits in adult learning and spatial working memory, despite half of the excitatory output neurons being intact. Diffusion MRI indicates increased thalamo-cortico-striatal connectivity in En1/2 mutants, showing that the remaining excitatory neurons lacking En1/2 exert adverse effects on extracerebellar circuits regulating motor learning and select non-motor behaviors. Thus, an absence of cerebellar output neurons is less disruptive than having cerebellar genetic mutations.
Introduction
The brain has a large capacity to compensate for neuronal loss due to injury when it occurs during development but not in adulthood. In contrast, germline mutations in genes that regulate neural development that result in hypoplasia can have deficits that range from minor to devastating. It is particularly important to understand the causes of behavior deficits in the context of pediatric cerebellar defects, as the degree of recovery of cerebellar function seems to be differentially influenced by the location and extent of insult or the type of genetic mutation1,2,3,4,5. As the cerebellum is a complex folded structure housing the majority of the neurons in the brain6,7 and many lobules in the cerebellum share functions with another lobule that converges onto similar downstream forebrain targets8, the cerebellum is an important structure to study developmental compensation.
The communication between the cerebellum and the rest of the brain is through the downstream cerebellar nuclei (CN) and they contribute to a wide range of motor and non-motor functions9,10,11,12. The CN comprise three bilaterally symmetrical nuclei and form a topographic circuit between their presynaptic Purkinje cells (PCs) based on their spatial position within lobules and along the mediolateral axis of the cerebellar cortex13,14,15,16,17,18,19. The circuit functions of subregions of the CN have begun to be defined in adult animals by mapping their projections and transiently inhibiting neural activity using targeted viral injections or neuronal ablation9,10,11,12. In addition, many studies have manipulated specific lobules in the cerebellar cortex and inferred which CN are involved in regulating motor coordination, motor learning and/or non-motor behaviors based on circuit topography. An additional consideration for ablation studies is that during development, the excitatory neurons of the CN (eCN) play a pivotal role in supporting the survival of PCs which in turn ensure the proper expansion of other cell types in the cerebellar cortex through secretion of Sonic hedgehog18,20,21. Thus, growth of each lobule is dependent on the eCN targets of their PCs, and removing a subset of eCN embryonically will impact the development of both their downstream forebrain targets and presynaptic PCs and their local microcircuit. Given the crucial role eCN play in generation and circuit function of the cerebellum, it is important to develop tools to enable manipulations of the same eCN during development and in the adult to define the cerebellar-associated behaviors dependent on the neurons and uncover possible developmental compensation.
We used the medial eCN as a test case for comparing the necessity of a CN subregion during development versus in adulthood. We then determined the baseline requirement for having all eCN intact during development and compare the behavior deficits to mouse mutants lacking developmental genes in the eCN. We identified a Cre transgene that targets the medial eCN from embryogenesis onwards. Using this tool we demonstrate that while in adult mice medial eCN activity is required for reversal learning, the brain can compensate for embryonic loss of these neurons alleviating the behavioral deficit. Surprisingly, we find that when all eCN are killed in the embryo there is only a selective impairment in motor coordination behaviors. In contrast, genetic loss of the engrailed (EN1/2) developmental transcription factors in all eCN results in additional deficits in motor learning, acquisition/reversal learning, and spatial working memory, despite only half the eCN dying embryonically. Circuit mapping and diffusion MRI (dMRI) provide evidence for aberrant thalamo-cortical-striatal connectivity as a result of aberrant eCN development.
Results
SepW1-Cre targets the excitatory neurons in the medial cerebellar nucleus
We chose the medial CN as a region to compare adult inhibition and embryonic ablation of a CN subregion as it can be divided into 4–5 subregions based on transcriptomic profiling and circuit mapping14,22. We screened transgenic databases (Gene Expression Nervous System Atlas and Allen Brain Atlas Transgenic Characterization) for a Cre driver that within the CN selectively targets the medial eCN. SepW1-Cre, a Selenow BAC construct23, was found to mediate Cre recombination only in the medial eCN. Immunostaining of cerebellar sections from SepW1-Cre mice carrying a Cre-dependent nuclear tdTomato reporter24 (SepW1-Cre; Ai75D) revealed that within the eCN 92.6% of all tdTomato+ cells are in the medial CN (Fig. 1a–f). Moreover, within the medial CN, 100% of tdTomato+ neurons expressed the eCN marker MEIS2+, and 8% of MEIS2+ neurons were not tdTomato+ (Fig. 1f). Approximately 50% of NeuN+ GCs in the internal granule cell layer (IGL) also expressed tdTomato (Supplementary Fig. 1a,b), as well as all TBR2+ unipolar brush cells (UBCs) (Supplementary Fig. 1a,c and ref25). Outside the cerebellum, tdTomato+ labeling was restricted to the vestibular nucleus, cerebral cortex, hippocampus, a subpopulation of hypothalamic nuclei and the nucleus of Darkschewitsch (see also Allen Brain Atlas Experiment ID: 488246361). Thus, among the CN neurons, the SepW1-Cre transgene selectively labels the eCN of the medial CN. Importantly, Cre is expressed in adult medial eCN, allowing targeting of subpopulations of the neurons in adult mice using viral injections (see below).
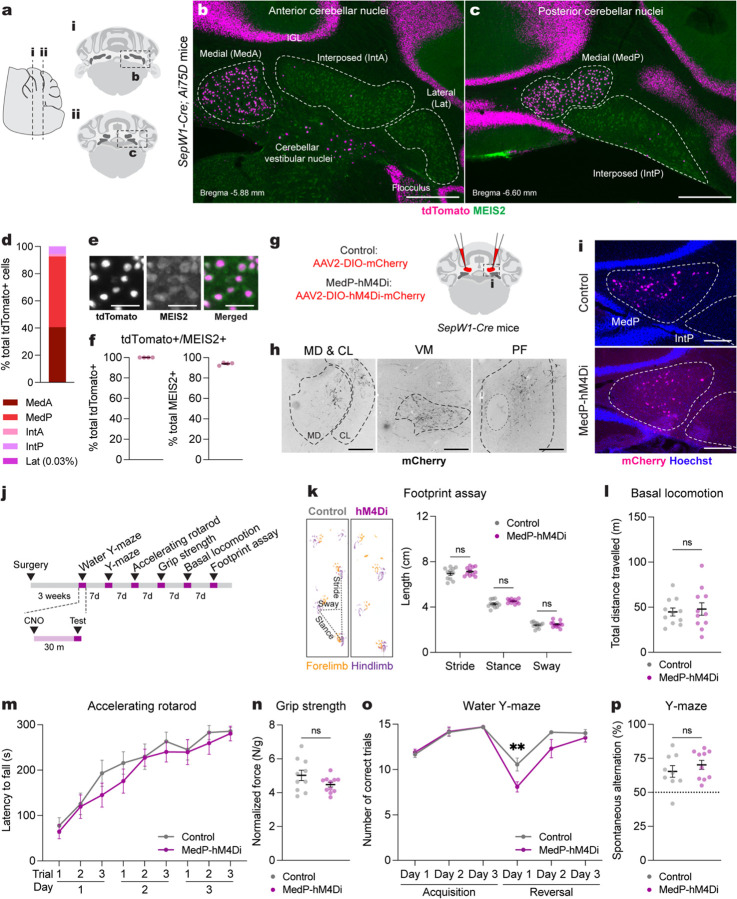
Fig. 1 |. Acute adult chemogenetic inhibition of MedP eCN impairs reversal learning and not motor behaviors.
a, Schematic representation of a lateral sagittal plane of the mouse cerebellum on the left with vertical lines (i and ii) indicating the location of the anterior and posterior coronal schematics shown to right.
b,c, Representative coronal images of tdTomato expression in the anterior CN (b) and posterior (c) CN of SepW1-Cre; Ai75D mice. CN were subdivided into five subregions based on histological distinctions (Paxinos and Franklin, 2007) and MEIS2 immunostaining. Abbreviations: MedA=Anterior medial; MedP=Posterior medial; IntA=Anterior interposed; IntP=Posterior interposed; Lat=Lateral. Scale bars = 500 um.
d, Quantification of tdTomato+ cells on every second coronal section of SepW1-Cre; Ai75D mice (n=4) in the lateral CN (Lat) and subregions of the intermediate (Int) and medial (Med) CN (n=4 mice).
e, Representative image of tdTomato (magenta) and MEIS2 (green) co-expressing eCN in SepW1-Cre; Ai75D mice. Scale bars = 50 um.
f, Quantification of tdTomato+ cells that co-express MEIS2 and the reverse in SepW1-Cre; Ai75D mice (n=4 mice).
g, Schematic of viral injection to express mCherry (control) or hM4Di-mCherry (MedP-hM4Di) in adult MedP eCN. Dashed line indicates region shown in (i).
h, Representative images of MedP eCN mCherry+ axon terminals (black) in four thalamic nuclei of control mice. Fluorescent images were inverted using the look up table in Fiji. Abbreviations: MD=mediodorsal; CL=centrolateral; VM=ventromedial; PF=parafascicular. Scale bars = 250 um.
i, Representative images of viral mCherry expression in MedP eCN in control (top, AAV-DIO-mCherry) and MedP-hM4Di (bottom, AAV-DIO-hM4Di-mCherry) mice. Scale bars = 250 um.
j, Experimental timeline of surgery, CNO injection and behavioral tests.
k, (left) Representative images of footprints from control and MedP-hM4Di mice. (right) quantification of stride, stance, and sway (n=11 per group). Multiple Mann-Whitney U tests for effect of genotype on stride (U = 48, P = 0.4385), stance (U = 34, P = 0.0843) and sway (U = 58, P = 0.8851).
l, Total distance travelled during basal locomotion (n=11 per group; t20 = 0.3910, P = 0.7000).
m, Latency to fall during the accelerating rotarod test (MedP-hM4Di: n=11, control: n=10). Repeated measure two-way ANOVA: main effect of time (F4.750,90.25 = 27.51, P < 0.0001), but not of chemogenetics (F1,19 = 0.9367, P = 0.3453) or interaction (F5,152 = 0.3699, P = 0.9351).
n, Forelimb grip strength normalized to body weight (MedP-hM4Di: n=11, control: n=10; two-tailed unpaired t-test: t19 = 1.677, P = 0.1099).
o, Total number of correct trials during the water Y-maze test (MedP-hM4Di: n=10, control: n=9). Repeated measure two-way ANOVA: main effect of time (F5,85 = 27.65, P < 0.0001) and chemogenetics (F1,17 = 9.855, P = 0.006), but not of interaction (F5,85 = 2.257, P = 0.0559); with post hoc two-tailed t-tests with Šídák correction for effect of chemogenetics on Reversal Day 1 (t102 = 3.386, P = 0.006), and not other comparisons (P ≥ 0.05).
p, Percentage spontaneous alternations in the Y-maze (MedP-hM4Di: n=10, control: n=9; two-tailed unpaired t-test: t17 = 0.9024, P = 0.3794).
ns, not significant: P ≥ 0.05. Data are presented as mean values ± SEM.
Acute inhibition of the adult posterior medial eCN impairs reversal learning and not motor behaviors
Leveraging SepW1-Cre mice, we tested the contribution of the posterior region of the medial eCN (MedP eCN) to adult cerebellar-associated motor coordination, learning and non-motor behaviors. Projections to the MedP CN14,18,26 that are preferentially from vermis lobules 6–8 have been shown to contribute to cognitive flexibility, anxiolytic and stereotyped/repetitive behaviors16,27,28, whereas projections to the anterior medial CN (MedA), which originate from lobules 1–517,18, are associated with motor functions17,29. We therefore tested whether acute chemogenetic inhibition of the adult MedP eCN would impair cognitive flexibility without affecting motor functions. A Cre-dependent inhibitory (hM4Di) Gi-DREADD (AAV2-hSyn-DIO-hM4Di-mCherry; MedP-hM4Di mice) or control vector (AAV2-hSyn-DIO-mCherry; Control mice) was injected bilaterally into the MedP CN of SepW1-Cre/+ littermates of both sexes (Fig. 1g). mCherry and hM4Di-mCherry expression was confirmed to be limited to the MedP (Fig. 1i and Supplementary Fig. 2a,b) and we observed the expected mCherry+ axon terminals in downstream motor and non-motor thalamic nuclei including mediodorsal (MD), centrolateral (CL), ventromedial (VM) and parafascicular (PF) thalamus (Fig. 1h).
The MedP eCN were acutely inhibited during a battery of motor and non-motor behaviors by injecting clozapine N-oxide (CNO, 5 mg/kg) 30 minutes before each behavioral test (Fig. 1j). Motor coordination and balance were tested using the footprint assay and revealed no differences in MedP-hM4Di mice compared to littermate controls injected with CNO (Fig. 1k). The open field assay also showed no differences in total distance travelled (Fig. 1l and Supplementary Fig. 2c) and average velocity (Supplementary Fig. 2d). Motor performance and learning using an accelerating rotarod test and forelimb grip strength also revealed no differences (Fig. 1m,n and Supplementary Fig. 2e). In contrast, when we tested acquisition and reversal learning as a readout of cognitive flexibility using a water Y-maze (WYM) test, we found that MedP-hM4Di mice showed normal acquisition learning for finding the submerged escape platform location, but had significantly impaired reversal learning for a new platform location compared to controls (Fig. 1o). As a test for spatial working memory, spontaneous alternations in a Y-maze revealed no differences between groups (Fig. 1p). Although the MedP-hM4Di mice showed lower total distance travelled (Supplementary Fig. 2f) in the Y-maze after CNO administration, the total number of entries (Supplementary Fig. 2g) were comparable to control mice. Altogether, these results demonstrated that acute inhibition of the MedP eCN in adult mice selectively impairs reversal learning without having a major effect on motor functions.
Generation of mice lacking the MedP eCN by conditional knockout of En1/2
To examine the impact of embryonic loss of MedP eCN on reversal learning we deleted En1/2 in the medial eCN using SepW1-Cre which initiates recombination at embryonic day 14.5 (E14.5; Supplementary Fig. 3a–d), since deletion of En1/2 in all eCN leads to preferential death of the MedP and posterior interposed (IntP) eCN after E14.518. We confirmed that 7-week-old SepW1-En1/2 CKO mice of both sexes (SepW1-Cre; En1flox/flox; En2flox/flox) have a loss of medial eCN by quantifying large NeuN (100–600 um2) neurons as a proxy for eCN18. As predicted, there was a preferential loss of eCN in the posterior medial CN, with little loss in the interposed and lateral CN compared to littermate control mice (En1flox/flox; En2flox/flox)(Fig. 2a,b). As recently show, the loss of medial eCN (MEIS2+ cells) was observed by E17.5 and primarily in the MedP region25. This was confirmed in 7-week-old SepW1-Cre; Ai75D and SepW1-En1/2 CKO; Ai75D mice, with complete loss of tdTomato+ MedP eCN and approximately half of the tdTomato+ MedA eCN remaining in the mutants (Supplementary Fig. 3e). The CN interneurons were also lost in the MedP region as the only NeuN+ cells remaining in the region were displaced mutant UBCs (Supplementary Fig. 3e and ref25).
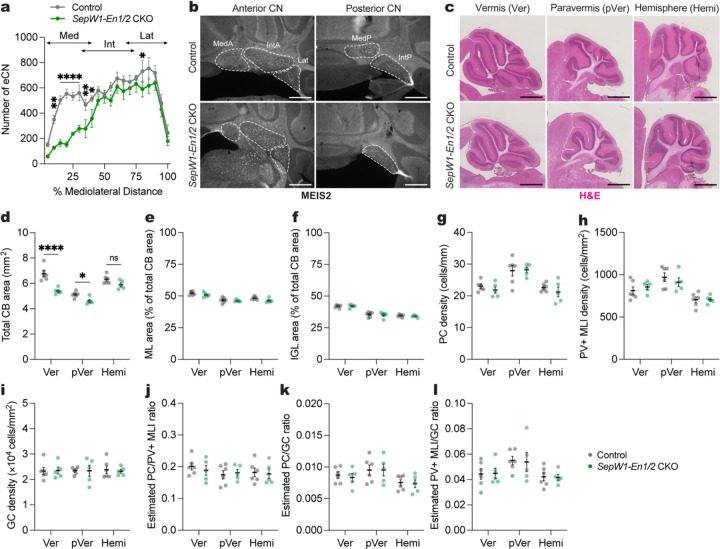
Fig. 2 |. Generation of mice lacking the MedP eCN by conditional knockout of En1/2.
a, Quantification of eCN number (large (100–600 um2) NeuN+ cells) along the medial-lateral axis in adult SepW1-En1/2 CKOs (n=5) and littermate controls (n=6). Ordinary two-way ANOVA: main effect of mediolateral distance (F19,180 = 24.02, P < 0.0001), genotype (F1,180 = 86.54, P < 0.0001), and interaction (F19,180 = 2.449, P < 0.0001); with post hoc two-tailed t-tests with uncorrected Fisher’s LSD for effect of genotype for bin 5–10% (t180 = 3.180, P = 0.0017), bins 10–30% (list of t value for each bin: t180 = 4.858, 5.738, 4.218, 4.028; all P values: P < 0.0001), bin 30–35% (t180 = 2.703, P = 0.0075), bin 35–40% (t180 = 2.238, P = 0.0265), bin 75–80% (t180 = 2.002. P = 0.0468), but not other comparisons (P ≥ 0.05). Abbreviations: Med=medial; Int=interposed; Lat=lateral.
b, Representative coronal images of MEIS2 labeling in the anterior and posterior CN of an adult SepW1-En1/2 CKO and littermate control as indicated. Scale bars = 500 um.
c, Representative images of H&E labeled sagittal sections of vermis (Ver), paravermis (pVer), and hemisphere (Hemi) from a SepW1-En1/2 CKO and littermate control. Scale bars = 1 mm.
d, Quantification of total cerebellar (CB) area of SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6) in the vermis, paravermis and hemispheres. Ordinary two-way ANOVA: main effect of region (F2,27 = 42.06, P < 0.0001) and genotype (F1,27 = 37.13, P < 0.0001), and interaction (F2,27 = 5.825, P = 0.0079); with post hoc two-tailed t-tests with uncorrected Fisher’s LSD for effect of genotype for vermis (t27 = 6.292, P < 0.0001), paravermis (t27 = 2.359, P = 0.0258), and hemisphere (P = 0.0678).
e, Quantification of molecular layer (ML) area as a percent of total CB area in SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,27 = 32.11, P < 0.0001), genotype (F1,27 = 5.236, P = 0.0302), but not of interaction (P = 0.5866).
f, Quantification of internal granule cell layer (IGL) area as a percent of total CB area in SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,27 = 82.04, P < 0.0001), but not of genotype (P = 0.6943) or interaction (P = 0.8641).
g, Quantification of Purkinje cell (PC) density in SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,18 = 18.34, P < 0.0001), but not of genotype (P = 0.4167) or interaction (P = 0.7216).
h, Quantification of PV+ MLI density in SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,27 = 16.66, P < 0.0001), but not of genotype (P = 0.8926) or interaction (P = 0.4617).
i, Quantification of granule cell (GC) density in the IGL of SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: no main effect of region (P = 0.9948), genotype (P = 0.8945) or interaction (P = 0.9502).
j, Quantification of the estimated ratio of the number of PCs to PV+ MLIs in SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: no significant main effect of region (P = 0.3807), genotype (P = 0.7618) or interaction (P = 0.7701).
k, Quantification of the estimated ratio of the number of PCs to GCs in SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F1,27 = 4.769, P = 0.0168), but no main effect of genotype (P = 0.7368) or interaction (P = 0.2521).
l, Quantification of the estimated ratio of the number of PV+ MLIs to GCs in SepW1-En1/2 CKOs (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F1,27 = 4.638, P = 0.0186), but not of genotype (P = 0.9195) or interaction (P = 0.9841).
ns, not significant: P ≥ 0.05. Data are presented as mean values ± SEM.
Given that the eCN support the survival of their presynaptic Purkinje cells (PCs), which in turn support production of GCs and interneurons18, we confirmed that growth of the cerebellum was reduced in the vermis of 6-week-old animals with proportional scaling down of GCs and interneurons to PCs. There was a significant reduction in the vermis (20.3%) and paravermis (10.1%) sagittal area, but not the hemispheres of SepW1-En1/2 CKOs compared to littermate controls (Fig. 2c,d). In addition, the areas of the molecular layer (ML) and the IGL was primarily reduced in the vermis and their proportions were conserved relative to the total cerebellar area in mutants (Fig. 2e,f). The density of Calbindin+ PCs, parvalbumin+ molecular layer interneurons and NeuN+ GCs were also normal throughout the cerebellum (Fig. 2g–l). Thus, loss of MedP eCN leads to a preferential reduction of growth in the vermis maintaining the proportions of neurons.
Mice lacking MedP eCN have normal reversal learning as well as motor behaviors
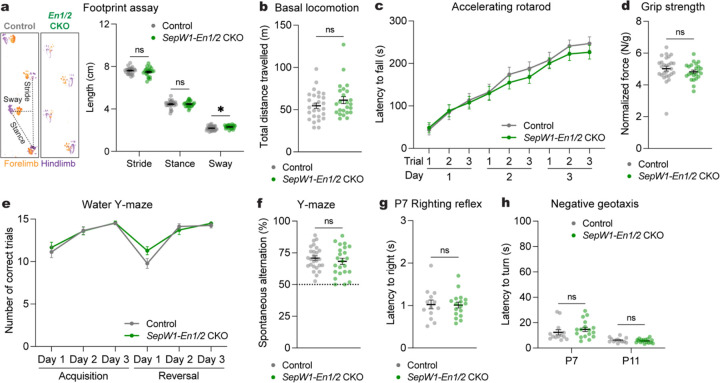
We next evaluated SepW1-En1/2 CKOs and their littermate controls of both sexes in the same behavioral assays as those used in the chemogenetic manipulations of SepW1-Cre mice (Fig. 1). Adult SepW1-En1/2 CKOs showed a very small increase in sway length (Fig. 3a) with no difference in total distance travelled compared to littermate controls (Fig. 3b and Supplementary Fig. 4a,b). Additionally no deficits were observed in motor learning and performance between the genotypes (Fig. 3c,d and Supplementary Fig. 4c). Unlike MedP-hM4Di mice (Fig. 1o), SepW1-En1/2 CKOs showed both normal acquisition and reversal learning compared to littermate controls (Fig. 3e). There were no genotype differences in spatial working memory (Fig. 3f), number of entries or distance travelled in the Y-maze (Supplementary Fig. 4d,e). Motor coordination was also normal in early postnatal pups, using a surface righting reflex assay at P7 (Fig. 3g) and negative geotaxis assay at P7 and P11 (Fig. 3h). Together, these results demonstrate that embryonic loss of the MedP eCN does not have a major impact on motor and non-motor functions.
Fig. 3 |. Mice lacking MedP eCN have normal reversal learning as well as motor behaviors.
a, (left) Representative images of footprints from one SepW1-En1/2 CKO and littermate control. (right) Quantification of stride, stance, and sway (SepW1-En1/2 CKOs: n=27, littermate controls: n=22). Multiple Mann-Whitney U tests showing effect of genotype on sway (U = 188, P = 0.0276), but not stride (U = 234, P = 0.2087) or stance (U = 279, P = 0.7235).
b, Total distance travelled during basal locomotion (SepW1-En1/2 CKOs: n=24, littermate controls: n=27; Mann-Whitney U test: U = 264, P = 0.2640).
c, Latency to fall during the accelerating rotarod test (SepW1-En1/2 CKOs: n=23, littermate controls: n=27). Repeated measure two-way ANOVA: main effect of time (F4.590,220.3 = 69.92, P < 0.0001), but not of genotype (F1,48 = 0.3434, P = 0.5606) or interaction (F8,384 = 0.4280, P < 0.9041).
d, Forelimb grip strength coronal normalized to body weight (SepW1-En1/2 CKOs: n=23, littermate controls: n=27; two-tailed unpaired t-test: t48 = 1.1018, P = 0.6689).
e, Total number of correct trials during the water Y-maze test (SepW1-En1/2 CKOs: n=18, littermate controls: n=23). Repeated measure two-way ANOVA: main effect of time (F3.132,122.1 = 38.86, P < 0.0001), but not of genotype (F1,39 = 0.5638, P = 0.4572) or interaction (F5,195 = 1.492, P = 0.1941).
f, Percentage of spontaneous alternations in the Y-maze (SepW1-En1/2 CKOs: n=23, littermate controls: n=26); two-tailed unpaired t-test: t47 = 0.8600, P = 0.3942).
g, Latency to right onto four paws at P7 (SepW1-En1/2 CKOs: n=17, littermate controls: n=13; two-tailed unpaired t-test: t28 = 0.1171, P = 0.9076).
h, Latency to turn upward on a negative slope at P7 and P11 (SepW1-En1/2 CKOs: n=17, littermate controls: n=13). Multiple Mann-Whitney U tests with Holm-Šídák correction for effect of genotype at P7 (U = 79, P = 0.3562) and at P11 (U = 92.50, P = 0.4634).
ns, not significant: P ≥ 0.05. Data are presented as mean values ± SEM.
Generation of mice in which all embryonic eCN are ablated using Diphtheria toxin
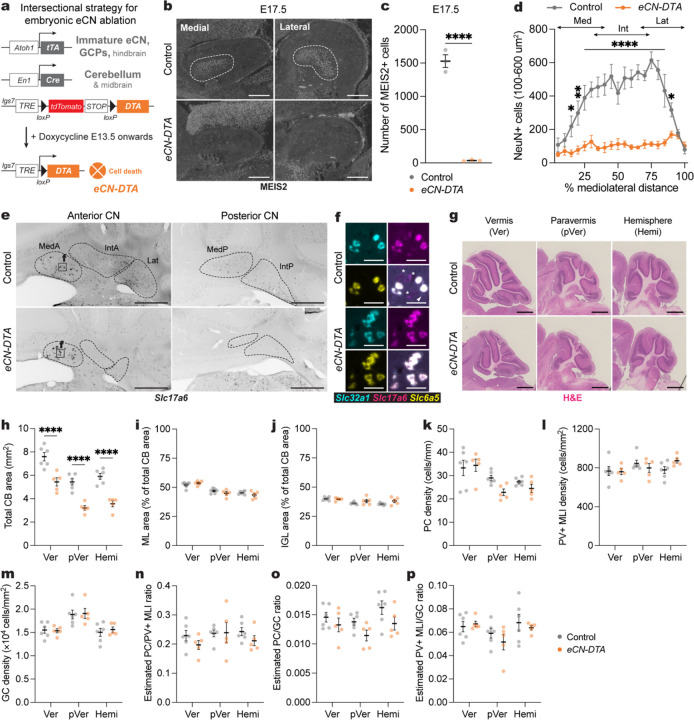
One plausible explanation for the absence of a reversal learning deficit in mice with embryonic loss of the medial eCN compared to adult inhibition of the same neurons could be the sufficiency of the remaining interposed and lateral CN in the SepW1-En1/2 CKOs. We therefore used an intersectional pharmacognetic approach to selectively kill all embryonic eCN soon after they are born30 as a means to determine the baseline requirement for eCN in a range of adult and neonatal behaviors. Mice were engineered to express Diphtheria toxin subunit A (DTA) in the developing eCN by combining an allele that expresses DTA only in cells that express both Cre and tTA (lgs7DRAGON-DTA/+; Atoh1-tTA/+; En1Cre/+ mice) and administering doxycycline from E13.5 onwards (eCN-DTA mice) (Fig. 4a). Since the intersection of Cre and tTA expression is in immature eCN and granule cell precursors (GCPs), by administering doxycycline after E13.5 cell killing is limited to the eCN18. Immunostaining of sagittal sections at E17.5 revealed that 97.6% of the MEIS2+ eCN were missing in eCN-DTA mice compared to littermate controls (lgs7DRAGON-DTA mice with Atoh1-tTA or En1Cre or neither transgene and fed doxycycline)(36±6.8 cells per mutant vs 1529±95.3 per control) (Fig. 4b,c). Quantification of NeuN+ cells in the CN of 7-week-old mice demonstrated a major loss of large CN neurons (100–600 um2) in the eCN-DTA mice (Fig. 4d). RNA in situ hybridization analysis confirmed the loss was mainly due to glutamatergic (Slc17a6) eCN (Fig. 4e). RNAScope triple RNA hybridization analysis (n=3 per genotype) revealed that the rare Slc17a6-expressing cells in eCN-DTA mice also expressed Slc32a1 (GABAergic) and Slc6a5 (glycinergic) markers and were located preferentially in the MedA CN. Furthermore, similar numbers of such triple positive cells were present in controls (Fig. 4f and Supplementary Fig. 5a,b). In the medial CN both the eCN and interneurons were absent in eCN-DTA mice, similar to in SepW1-En1/2 CKOs. We found that the remaining triple positive NeuN+ cells in the MedA CN were not targeted by the Atoh1-tTA transgene (Supplementary Fig. 5c), despite being targeted with Atoh1-Cre (Supplementary Fig. 5d and see refs18,22). Therefore, all remaining NeuN+ cells in the eCN-DTA mice are GABAergic (Slc32a1+) inhibitory CN neurons or triple glutamate-GABA-glycine+ neurons.
Fig. 4 |. Generation of mice in which all embryonic eCN are ablated using Diphtheria toxin.
a, Intersectional approach to pharmacogenetically ablate the embryonic eCN. A doxycycline (Dox)-controlled and recombinase activated gene overexpression allele (DRAGON) for attenuated diphtheria toxin fragment A (DTA) (Igs7DRAGON-DTA) combined with an Atoh1-tTA transgene and En1Cre knock-in allele results in embryonic killing of eCN when Dox is administered starting at E13.5 via expression of DTA. The genotypes of littermate controls are Atoh1-tTA or En1Cre along with the lgs7DRAGON-DTA allele.
b, Representative images of sagittal sections stained for MEIS2 (white) in medial and lateral cerebellum of an E17.5 eCN-DTA and littermate control. Scale bars = 250 um.
c, Quantification of total number of MEIS2+ cells on every 10th sagittal section of E17.5 eCN-DTA mice (n=3) compared to littermate controls (n=3) (two-tailed unpaired t-test: t4 = 15.62, P < 0.0001).
d, Mediolateral distribution of NeuN+ large cells (100–600 um2) in adult eCN-DTA mice (n=5) compared to littermate controls (n=6). Repeated measure two-way ANOVA: main effect of mediolateral distance (F19,180 = 6.669, P < 0.0001), genotype (F1,180 = 359.5, P < 0.0001), and interaction (F19,180 = 5.745, P < 0.0001); with post hoc two-tailed t-tests with uncorrected Fisher’s LSD for effect of genotype show significance for bin 10–15% (t180 = 3.327, P = 0.0163), bin 15–20% (t180 = 4.439, P = 0.0011), bins 20–85% (list of t value for each bin: t180 = 4.349, 4.684, 5.539, 5.676, 6.22, 4.587, 5.939, 6.153, 6.76, 6.181, 7.551, 6.744, 4.676; all P values: P < 0.0001), bin 85–90% (t180 = 2.229, P = 0.027), but not other comparisons (P ≥ 0.05). Abbreviations: Med=medial; Int=interposed; Lat=lateral.
e, Representative images of RNA in situ analysis of coronal sections for Slc17a6 expression in the CN of eCN-DTA mice and littermate controls. Dotted outlines indicate the CN subregions. Images are single channel inverted using lookup table in Fiji. Scale bars = 500 um.
f, Representative images of triple RNA in situ of coronal sections in the medial CN showing some neurons co-express Slc32a1, Slc17a6, and Slc6a5. Arrowhead and asterisk indicate neurons expressing only Slc32a1 or Slc17a6 in controls, respectively. Scale bars = 50 um.
g, Representative images of H&E labeled vermis (Ver), paravermis (pVer), and hemisphere (Hemi) sagittal sections from an eCN-DTA and littermate control. Scale bars = 1 mm.
h, Quantification of total cerebellar (CB) area in eCN-DTA mice (n=5) and littermate controls (n=6) in the vermis, paravermis and hemispheres. Ordinary two-way ANOVA: main effect of region (F2,27 = 32.08, P < 0.0001) and genotype (F1,27 = 89.64, P < 0.0001), but not of interaction (P = 0.9488); with post hoc two-tailed t-tests with uncorrected Fisher’s LSD for effect of genotype for vermis (t27 = 5.260, P < 0.0001), paravermis (t27 = 5.425, P < 0.0001), and hemisphere (t27 = 5.713, P < 0.0001).
i, Quantification of molecular layer (ML) area as a percent of total CB area in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,27 = 37.84, P < 0.0001), but not of genotype (P = 0.3540) or interaction (P = 0.1589).
j, Quantification of IGL area as a percent of total CB area in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,27 = 7.249, P = 0.003), but not of genotype (P = 0.0545) or interaction (P = 0.1911); with post hoc two-tailed t-tests with uncorrected Fisher’s LSD for effect of genotype for hemisphere (t27 = 2.220, P = 0.0350), but not other comparisons (P ≥ 0.05).
k, Quantification of PC density in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,18 = 10.23, P = 0.0011), but not of genotype (P = 0.1660) or interaction (P = 0.2277).
l, Quantification of PV+ MLI density in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: no main effect of region (P = 0.2153), genotype (P = 0.1660) or interaction (P = 0.2277).
m, Quantification of GC density in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of region (F2,18 = 10.08, P = 0.0012), but not of genotype (P = 0.6051) or interaction (P = 0.9220).
n, Quantification of the estimated ratio of the number of PCs to PV+ MLIs in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: no significant main effect of region (P = 0.4484), genotype (P = 0.2061) or interaction (P = 0.5916).
o, Quantification of the estimated ratio of the number of PCs to GCs in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: main effect of genotype (F1,27 = 7.013, P = 0.0134), but not of region (P = 0.0913) or interaction (P = 0.7645).
p, Quantification of the estimated ratio of the number of PV+ MLIs to GCs in eCN-DTA mice (n=5) compared to littermate controls (n=6). Ordinary two-way ANOVA: no significant main effect of region (P = 0.0680), genotype (P = 0.4415) or interaction (P = 0.6184).
ns, not significant: P ≥ 0.05. Data are presented as mean values ± SEM.
We next examined the size and number of neurons in the cerebellar lobules. As expected, cerebellar size and neuron numbers were reduced across the entire mediolateral axis in eCN-DTA mice compared to littermate controls while the proportions and densities of each cell type were maintained (Fig. 4h–p) As triple neurotransmitter positive eCN remain in the MedA, we examined the growth of the vermis lobules that preferentially target the MedA. The anterior vermis lobules (1–5) showed a significant reduction in area, but the magnitude was smaller than the reduction in the central vermis (lobules 6–8) (Supplementary Fig. 6). The posterior vermis (lobules 9 and 10) was not significantly reduced (Supplementary Fig. 6), consistent with the PCs projecting outside the cerebellum to the vestibular nuclei31,32. Thus, pharmacogenetic killing of the developing eCN in eCN-DTA mice leads to loss of all Slc17a6 single neurotransmitter positive eCN and reduced cerebellar size with proportional scaling down of cell numbers throughout the cerebellum.
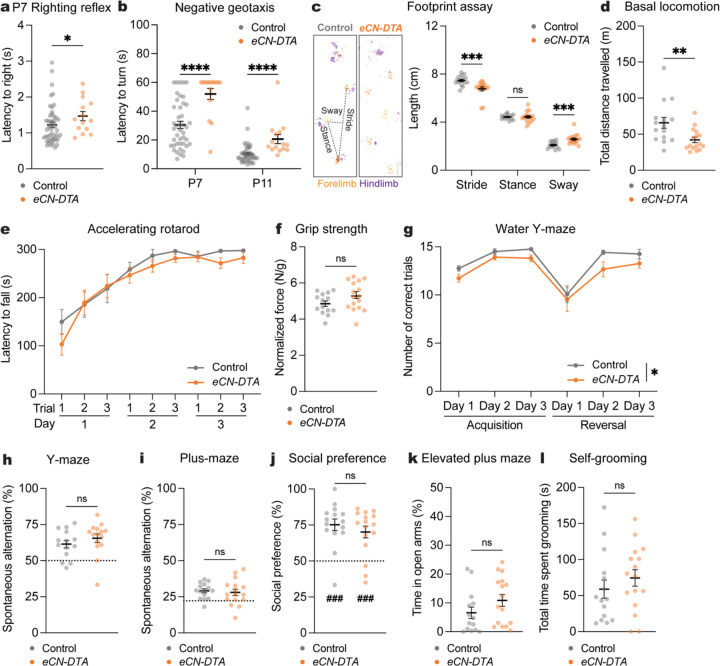
Loss of all eCN impairs motor coordination, but not motor learning and non-motor behaviors.
We repeated the same battery of behaviors in eCN-DTA mice of both sexes as in SepW1-En1/2 CKOs (Fig. 3). eCN-DTA mice of both sexes had motor coordination deficits in negative geotaxis at P7 and P11 and righting reflex at P7 (Fig. 5a,b). Motor coordination continued to be abnormal in adult eCN-DTA mice as they had a significant decrease in stride and increase in sway length (Fig. 5c) and significantly reduced total distance travelled compared to littermate controls (Fig. 5d and Supplementary Fig. 7a), but with no difference in average velocity (Supplementary Fig. 7b). Interestingly, eCN-DTA mice showed normal motor learning (Fig. 5e,f and Supplementary Fig. 7c). eCN-DTA mice showed normal reversal learning, although there was a main effect of geneotype in the WYM (Fig. 5g) with no change in swim speed (Supplementary Fig. 7d). Moreover, there was no genotype difference in spatial working memory (Fig. 5h,i), although the total distance travelled and number of arm entries were decreased in the Y-maze (Supplementary Fig. 7e–h).
Fig. 5 |. Loss of all eCN impairs motor coordination, but not motor learning and non-motor behaviors.
a, Latency to right onto four paws at P7 (eCN-DTA mice: n=15, littermate controls: n=43; Mann-Whitney U test: U = 226, P = 0.0436).
b, Latency to turn upward on a negative slope at P7 and P11 (eCN-DTA mice: n=15, littermate controls: n=43). Multiple Mann-Whitney U tests with Holm-Šídák correction for effect of genotype at P7 (U = 109, P < 0.0001) and at P11 (U = 89, P < 0.0001).
c, (left) Representative images of footprints from an eCN-DTA and littermate control. (right) Quantification of stride, stance, and sway (eCN-DTA mice: n=16, littermate controls: n=15). Multiple Mann-Whitney U tests for effect of genotype on stride (U = 33, P = 0.00028) and sway (U = 32, P = 0.00023), but not stance (U = 113.5, P = 0.8073).
d, Total distance travelled during basal locomotion (eCN-DTA mice: n=16, littermate controls: n=15; two-tailed unpaired t-test: t29 = 2.865, P = 0.0077).
e, Latency to fall in the accelerating rotarod test (eCN-DTA mice: n=15; littermate controls: n=15). Repeated measure two-way ANOVA: main effect of time (F3.426,95.92 = 34.31, P < 0.0001), but not of genotype (P = 0.3873) or interaction (P = 0.6987).
f, Forelimb grip strength normalized to body weight (eCN-DTA mice: n=16, littermate controls: n=15; two-wailed unpaired t-test: t28 = 1.684, P = 0.1033).
g, Total number of correct trials during the water Y-maze test (eCN-DTA mice: n=15, littermate controls: n=12). Repeated measure two-way ANOVA: main effect of time (F1.667,41.67 = 17.92, P < 0.0001) and genotype (F1,25 = 4.898, P = 0.0362), but not of interaction (P = 0.9183); with post hoc two-tailed t-tests with Šídák correction for effect of genotype all being P ≥ 0.05.
h, Percentage of spontaneous alternations in the Y-maze (eCN-DTA mice: n=15, littermate controls: n=14; Mann-Whitney U test: U = 76.50, P = 0.2209). Chance level performance is 50% (dotted line).
i, Percentage of spontaneous alternations in the plus-maze (eCN-DTA mice: n=16, littermate control: n=15; two-tailed unpaired t-test: t29 = 0.4309, P = 0.6698). Chance level performance is 22.2% (dotted line).
j, Social preference (percent time nose spent within novel mouse contact zone) during the three-chamber social approach test (eCN-DTA mice: n=16, littermate controls: n=15; Mann-Whitney U test: U = 102, P = 0.4945). Wilcoxon test against a null hypothesis (50%) in eCN-DTA mice (W = 122, P = 0.0006) and littermate controls (W = 116, P = 0.0002).
k, Percentage of time spent in the open arms of an elevated plus maze (eCN-DTA mice: n=16, littermate controls: n=14; Mann-Whitney U test: U = 74, P = 0.1179).
l, Total time spent self-grooming (eCN-DTA mice: n=16, littermate controls: n=15; Mann-Whitney U test: U = 92, P = 0.2770).
ns, not significant: P ≥ 0.05. Data are presented as mean values ± SEM.
Since all eCN die in the embryo we tested additional cerebellar-associated non-motor behaviors9,11,12. In a more challenging spatial working memory test (plus-maze), eCN-DTA mice showed no deficits (Fig. 5i), although the total distance travelled and number of arm entries were decreased (Supplementary Fig. 7g,h). Compared to littermate controls eCN-DTA mice showed no difference in social preference after normalizing for hypolocomotion (three-chambered social approach assay33,34; Fig. 5j and Supplementary Fig. 7i,j). We also did not find a genotype difference in anxiety-like behavior (elevated plus maze) when normalizing for hypolocomotion (Fig. 5k and Supplementary Fig. 7k,l). Finally, we did not find a genotype difference in total time spent self-grooming (Fig. 5l). Altogether, the behavior analyses reveal that eCN-DTA mice, lacking nearly all eCN, exhibit early motor coordination deficits that persist into adulthood, but motor learning, cognitive, social, and anxiety-like behaviors are largely intact.
Loss of En1/2 in all eCN impairs motor coordination and learning, cognitive flexibility, and spatial working memory
Given a previous report that adult mice lacking excitatory activity in all eCN (Atoh1-Slc17a6 CKO)5 have motor learning deficits that we did not observe following loss of all eCN (eCN-DTA mice), we were prompted to analyze in more detail the behavior of a previously generated En1/2 conditional knockout mice (Atoh1-En1/2 CKOs) that has motor learning deficits18. In Atoh1-En1/2 CKOs all eCN and GCs lack En1/2 and the mice have an overall ~50% loss of eCN during embryogenesis that includes a complete loss in the MedP and IntP eCN (Supplementary Fig. 8a–e and ref18). Further characterization revealed that similar to SepW1-En1/2 CKOs (Supplementary Fig. 3e) there was a significant loss of local interneurons, especially in the MedP and IntP where all neurons are missing (Supplementary Fig. 8b).
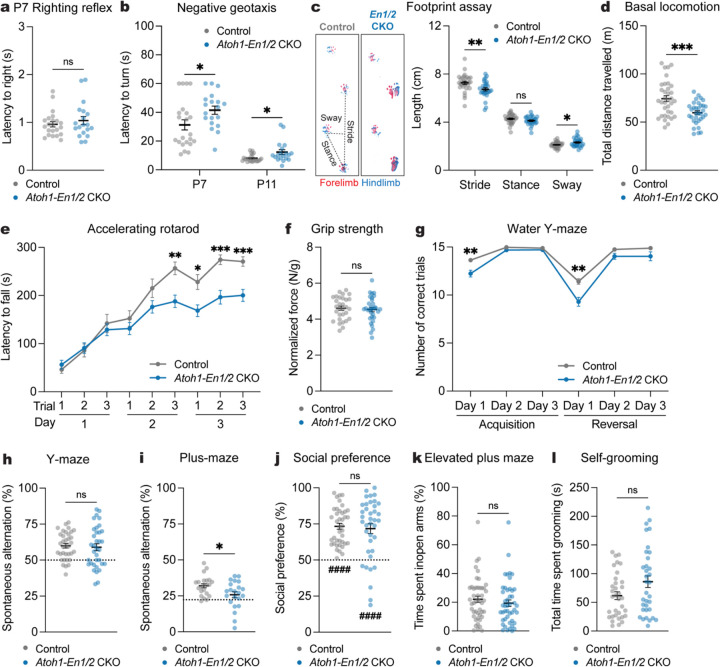
Atoh1-En1/2 CKO mice of both sexes had motor coordination deficits in negative geotaxis at P7 and P11 compared to littermate controls (Fig. 6a), but no genotype difference in righting reflex at P7 (Fig. 6b). Replicating our previous findings18, adult Atoh1-En1/2 CKOs of both sexes had motor coordination (Fig. 6c) and motor learning deficits (Fig. 6e and Supplementary Fig. 9c) and reduced total distance travelled (Fig. 6d and Supplementary Fig. 9a) and slower velocity (Supplementary Fig. 9b) compared to littermate controls. Forelimb grip strength was normal (Fig. 6f). Thus, Atoh1-En1/2 CKOs have motor coordination deficits detected by P7 that persist into adulthood and adult motor learning deficits.
Fig. 6 |. Loss of En1/2 in all eCN impairs motor coordination and learning, cognitive flexibility, and spatial working memory.
a, Latency to right onto four paws at P7 (Atoh1-En1/2 CKOs: n=19, littermate controls: n=22; Mann-Whitney U test: U = 192.5, P = 0.6741).
b, Latency to turn upward on a negative slope at P7 and P11 (Atoh1-En1/2 CKOs: n=19, littermate controls: n=22). Multiple Mann-Whitney U tests with Holm-Šídák correction for effect of genotype at P7 (U = 128, P = 0.0245) and P11 (U = 114.5, P = 0.033).
c, (left) Representative images of footprints from an Atoh1-En1/2 CKO and littermate control. (right) Quantification of stride, stance, and sway (Atoh1-En1/2 CKOs: n=28, littermate controls: n=30). Multiple Mann-Whitney U tests for effect of genotype on stride (U = 237, P = 0.0039) and sway (U = 292.5, P = 0.047), but not stance (U = 312, P = 0.0936).
d, Total distance travelled during basal locomotion (Atoh1-En1/2 CKOs: n=33, littermate controls: n=35; two-tailed unpaired t-test: t66 = 3.931, P = 0.0002).
e, Latency to fall in the accelerating rotarod test (Atoh1-En1/2 CKOs: n=32, littermate controls: n=30). Repeated measure two-way ANOVA: main effect of time (F5.648,338.9 = 90.56, P < 0.0001), genotype (F1,60 = 7.791, P = 0.0070), and interaction (F8,480 = 5.827, P < 0.0001); with post hoc two-tailed t-tests with Šídák correction for effect of genotype on day 2-trial 3 (t59.83 = 3.721, P = 0.0040), day 3-trial 1 (t55.69 = 3.019, P = 0.0338), day 3-trial 2 (t55.46 = 4.502, P = 0.0003), day 3-trial 3 (t57.98 = 4.416, P = 0.0004), and other comparisons (P ≥ 0.05).
f, Forelimb grip strength normalized to body weight (Atoh1-En1/2 CKOs: n=32, littermate controls: n=30; two-tailed unpaired t-test: t60 = 0.4298, P = 0.6689).
g, Total number of correct trials during the water Y-maze test (Atoh1-En1/2 CKOs: n=31, littermate controls: n=35). Repeated measure two-way ANOVA: main effect of time (F3.003,192.2 = 118.4, P < 0.0001), genotype (F1,64 = 21.47, P < 0.0001), and interaction (F5,320 = 5.101, P = 0.0002); with post hoc two-tailed t-tests with Šídák correction for effect of genotype on Acquisition Day 1 (t42.50 = 3.583, P = 0.0052), Reversal Day 1 (t52.14 = 3.821, P = 0.0021), and no other comparisons (P ≥ 0.05).
h, Percentage of spontaneous alternations in the Y-maze (n=35 per genotype; two-tailed unpaired t-test: t68 = 0.3622, P = 0.7183). Chance level performance is 50% (dotted line).
i, Percentage of spontaneous alternations in the plus-maze (n=22 per genotype; two-tailed unpaired t-test: t42 = 2.486, P = 0.0170). Chance level performance is 22.2% (dotted line).
j, Social preference (percent time nose spent within novel mouse contact zone) during the three-chamber social approach test (Atoh1-En1/2 CKOs: n=38, littermate controls: n=40; Mann-Whitney U test: U = 723, P = 0.7167). Wilcoxon test against a null hypothesis (50%) in Atoh1-En1/2 CKOs (W = 605, P < 0.0001) and littermate controls (W = 820, P < 0.0001).
k, Percentage of time spent in the open arms of an elevated plus maze (Atoh1-En1/2 CKOs: n=46, littermate control: n=47; Mann-Whitney U test: U = 923, P = 0.2266).
j, Total time spent self-grooming (n=34 per genotype; Mann-Whitney U test: U = 455, P = 0.1336).
ns, not significant: P ≥ 0.05. Data are presented as mean values ± SEM.
In terms of non-motor behaviors, Atoh1-En1/2 CKOs showed both acquisition (day 1) and reversal learning deficits in the WYM compared to littermate controls (Fig. 6g), which was not due to a difference in swim speed (Supplementary Fig. 9d). There was no genotype difference in spatial working memory (Fig. 6h) despite reduced total distance travelled and arm entries (Supplementary Fig. 9e,f). However Atoh1-En1/2 CKOs showed impaired spatial working memory in the plus-maze (Fig. 6i) with no difference in total distance travelled or arm entries (Supplementary Fig. 9g,h). Atoh1-En1/2 CKOs showed no difference in social preference when normalizing for hypolocomotion compared to littermate control mice (Fig. 6j and Supplementary Fig. 9i,j). Also, genotype differences were not found in anxiety-like behavior (Fig. 6k and Supplementary Fig. 9k,l) and self-grooming (Fig. 6l). Thus, Atoh1-En1/2 CKOs have impaired adult motor learning, acquisition/reversal learning and spatial working memory, in addition to the motor coordination deficits as seen in eCN-DTA mice.
Atoh1-En1/2 CKOs have reduced but not ectopic cerebellothalamic projections
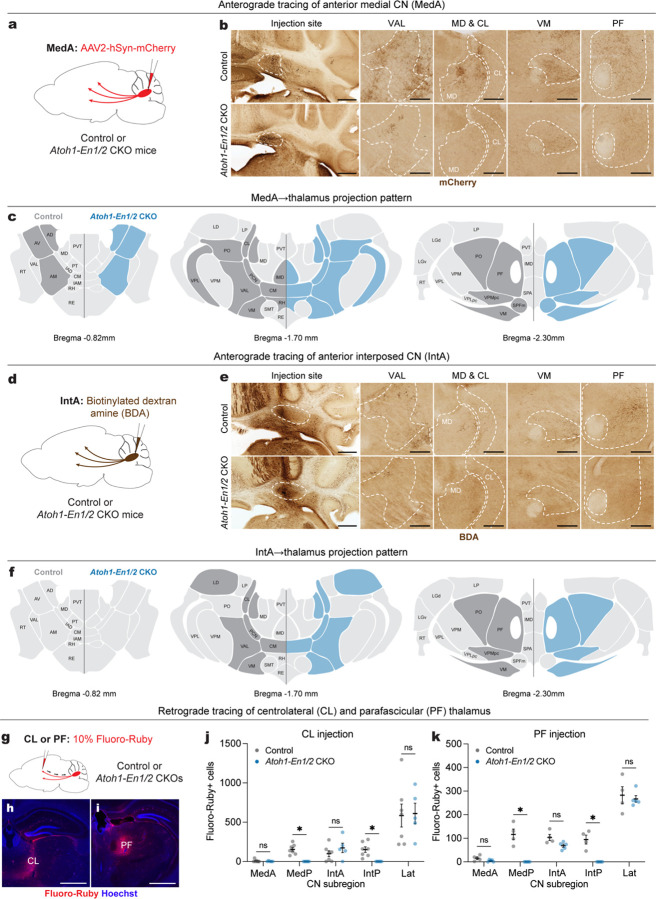
One possible explanation for the differing behavioral outcomes in Atoh1-En1/2 CKOs compared to eCN-DTA mice is that the remaining eCN in Atoh1-En1/2 CKOs exhibit aberrant projections to the thalamus, a primary target region involved in behaviors studied. To address this question, we mapped the projections to the thalamus of the remaining eCN in adult Atoh1-En1/2 CKO and littermate controls. We first examined whether the remaining MedA and IntA CN make ectopic projections by injecting an AAV2-hSyn-mCherry virus or biotinylated dextran amine (BDA) into the MedA or IntA, respectively (n=3 per genotype; Fig. 7a,d). Given the reduced neuron number in the MedA and IntA CN, Atoh1-En1/2 CKOs showed reduced mCherry+ or BDA+ axon terminals in all thalamic nuclei compared to littermate controls (Fig. 7b,e), but importantly no obvious ectopic projections were observed compared to littermate controls (Fig. 7c,f). We next performed retrograde tracing from the intralaminar thalamic nuclei (ILM), which regulate motor learning and cognitive flexibility35,36,37,38. ILM receive strong projections from the MedP, interposed and lateral CN, but not MedA22, allowing us to examine whether inputs to ILM from the remaining MedA (and other nuclei) in Atoh1-En1/2 CKOs are altered. We injected 10% Fluoro-Ruby preferentially into the centrolateral (CL) or parafascicular (PF) thalamus, two nuclei within the ILM, of adult Atoh1-En1/2 CKOs and littermate controls (Fig. 7g–i). As expected, Atoh1-En1/2 CKOs showed no Fluoro-Ruby+ cells in the region of the MedP and IntP CN. Furthermore, no significant differences in the total number of Fluoro-Ruby+ cells in the MedA, IntA, and lateral CN were detected compared to littermate controls for CL injections (Fig. 7j) and PF injections (Fig. 7k). Thus, Atoh1-En1/2 CKOs have reduced cerebellothalamic projections, but the remaining CN do not make ectopic projections.
Fig. 7 |. Atoh1-En1/2 CKOs have reduced cerebellothalamic projections, but no ectopic cerebellothalamic projections.
a, Schematic of anterograde tracing of MedA CN cells in adult Atoh1-En1/2 CKOs and littermate controls.
b, Representative images of coronal sections showing injection site and mCherry+ axon terminals (brown) in various thalamic regions from an Atoh1-En1/2 CKO and littermate control. Scale bar: injection site = 1 mm and mCherry images = 250 um.
c, Summary of mCherry+ axon terminals observed in thalamic nuclei of Atoh1-En1/2 CKOs (blue) versus littermate controls (dark grey) on three representative coronal planes adapted from Allen Brain Atlas. Blue indicates reduced density.
d, Schematic of anterograde tracing of anterior interposed CN (IntA) cells in adult Atoh1-En1/2 CKOs and littermate controls.
e, Representative images of coronal sections showing injection site and biotinylated dextran amine (BDA)+ axon terminals (brown) in various thalamic regions from an Atoh1-En1/2 CKO and littermate control. Scale bar: injection site = 1 mm and BDA images = 250 um.
f, Summary of BDA+ axon terminals observed in thalamic nuclei of Atoh1-En1/2 CKOs (blue) and littermate controls (dark grey) versus on three representative coronal planes adapted from Allen Brain Atlas. Blue indicates reduced density.
g, (top) Schematic of retrograde tracing in adult Atoh1-En1/2 CKOs and littermate controls.
h,i, Representative images of the injection site of Fluoro-Ruby (red) and Hoechst (blue) in centrolateral thalamus (CL, h) and parafascicular thalamus (PF, i). Scale bars = 1 mm.
j, Quantification of Fluoro-Ruby+ cells in CN subregions that are retrogradely labeled from CL injection. Multiple Mann-Whitney U tests with Holm-Šídák correction for effect of genotype on MedP (U = 0, P = 0.0126) and IntP (U = 0, P = 0.0126), but not on MedA (U = 15.5, P = 0.9400), IntA (U = 11, P = 0.6882), and Lat (U = 15, P = 0.9400).
k, Quantification of Fluoro-Ruby+ cells in CN subregions that are retrogradely labeled from PF injection. Multiple Mann-Whitney U tests with Holm-Šídák correction for effect of genotype on MedP (U = 0, P = 0.0390) and IntP (U = 0, P = 0.0390), but not on MedA (U = 3.5, P = 0.2516), IntA (U = 1, P = 0.0922), and Lat (U = 10, P > 0.9999).
Abbreviations: AD=Anterodorsal nucleus; AM=Anteromedial nucleus; AV=Anteroventral nucleus of thalamus; CL=Central lateral nucleus; CM=Central medial nucleus; IAD=Interanterodorsal nucleus; IAM=Interanteromedial nucleus; IMD=Intermediodorsal nucleus; LD=Lateral dorsal nucleus of thalamus; LGv=Ventral part of the lateral geniculate complex; LP=Lateral posterior nucleus; MD=Mediodorsal nucleus of thalamus; PCN=Paracentral nucleus; PF=Parafascicular nucleus; PO=Posterior complex; PT=Parataenial nucleus; PVT=Paraventricular nucleus; RE=Nucleus of reuniens; RH=Rhomboid nucleus; RT=Reticular nucleus; SMT=Submedial nucleus; SPFm=Subparafascicular nucleus, magnocellular part; VAL=Ventral anterior-lateral complex; VM=Ventral medial nucleus; LGd=Dorsal part of the lateral geniculate complex; VPM=Ventral posteromedial nucleus; VPL=Ventral posterolateral nucleus; SPA=Subparafascicular area; VPMpc=Ventral posteromedial nucleus, parvicellular part; VPLpc=Ventral posterolateral nucleus, parvicellular part. ns, not significant: P ≥ 0.05. Data are presented as mean values ± SEM.
Diffusion MRI shows Atoh1-En1/2 CKOs have connectivity changes outside the cerebellum that are distinct from eCN-DTA mice
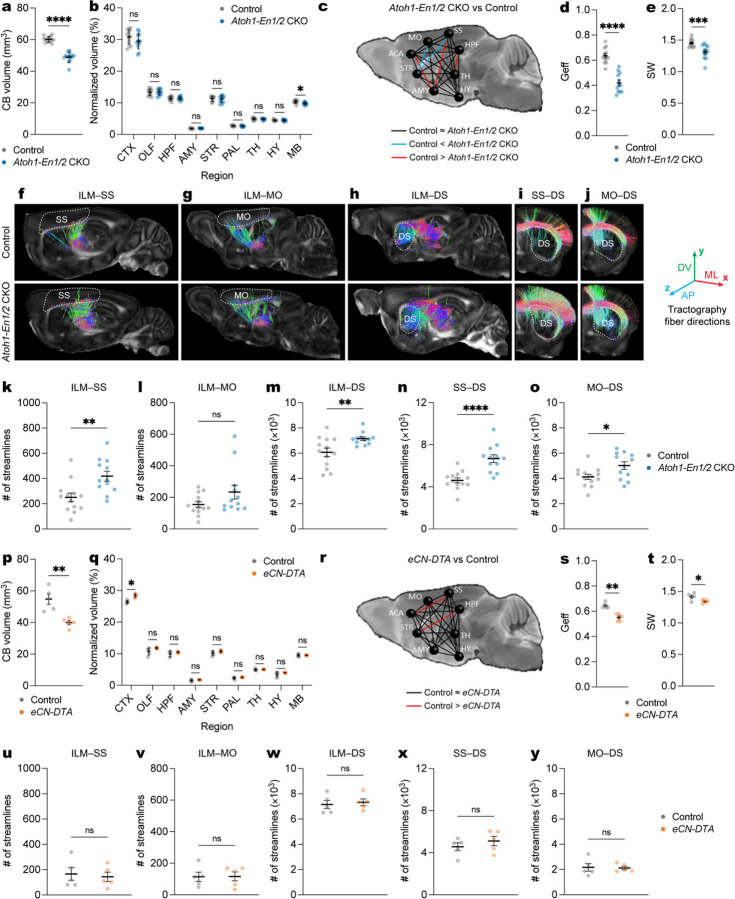
Since the remaining eCN circuits in Atoh1-En1/2 CKOs appear intact, we used high resolution ex vivo dMRI39,40 to examine whether there are alterations in regional volume, connectivity, and network properties outside of the cerebellum in adult Atoh1-En1/2 CKO of both sexes as well as eCN-DTA mice for comparison. We first examined regional volume in 10 distinct brain regions (Supplementary Fig. 10a) and as expected a large reduction in the volume of the cerebellum was detected in Atoh1-En1/2 CKO compared to littermate controls (Fig. 8a). No changes in regional volumes were seen except for a small but significant reduction in the midbrain (Fig. 8b), when normalizing for an overall smaller brain in mutants (Supplementary Fig. 10b,c). Network analysis using eight brain regions as nodes revealed a significant reduction in global efficiency and small-worldness in Atoh1-En1/2 CKOs compared to controls (Fig. 8c–e and Supplementary Fig. 10d–f). Examining the number of streamlines between the thalamus and CN revealed the expected significant reduction in Atoh1-En1/2 CKOs compared to controls (Supplementary Fig. 10g). We further examined the number of streamlines between the ILM and three of its primary downstream targets; the primary somatosensory cortex (SS), primary motor cortex (MO), and dorsal striatum (DS). Interestingly, we observed a significant increase in the number of streamlines in ILM-SS, ILM-MO, and ILM-DS circuits in Atoh1-En1/2 CKOs compared to littermate controls (Fig. 8f–h,k–m and Supplementary Fig. 10h–j). Furthermore, the number of streamlines of the SS-DS and MO-DS circuits were significantly increased in Atoh1-En1/2 CKOs compared to littermate controls (Fig. 8i,j,n,o and Supplementary Fig. 10k,l).
Fig 8 |. Diffusion MRI shows Atoh1-En1/2 CKOs have connectivity changes outside the cerebellum that are distinct from eCN-DTA mice.
a, Quantification of cerebellar (CB) volume in Atoh1-En1/2 CKOs (n=12) compared to littermate controls (n=13) (two-tailed unpaired t-test: t23 = 11.15, P < 0.0001).
b, Quantification of regional volumes normalized to forebrain plus midbrain combined volume in Atoh1-En1/2 CKOs (n=12) compared to littermate controls (n=13). Two-tailed unpaired t-tests to test for effect of genotype on MB (t23 = 2.834, P = 0.0094) and other comparisons P ≥ 0.05.
c, Schematic representation of global connectivity in Atoh1-En1/2 CKOs compared to littermate controls. Black lines indicate no significant difference, red lines indicate reduced connectivity in Atoh1-En1/2 CKOs and blue lines indicate increased connectivity in Atoh1-En1/2 CKOs compared to littermate controls (two-tailed unpaired t-tests with Welch’s correction).
d, Quantification of global efficiency (Geff; Atoh1-En1/2 CKOs: n=12, littermate controls: n=13; two-tailed unpaired t-test: t23 = 7.876, P < 0.0001).
e, Quantification of small worldness (SW; Atoh1-En1/2 CKOs: n=12, littermate controls: n=13; two-tailed unpaired t-test: t23 = 3.913, P = 0.0007).
f-j, Representative images of ILM-SS (f), ILM-MO (g), ILM-DS (h), SS-DS (i), MO-DS (j) tractographies in right hemisphere of one Atoh1-En1/2 CKO and littermate control. Target regions are outlined in dotted lines. The color of streamlines indicates their orientations, as indicated by the colored arrows on the right. Abbreviations: AP=anteroposterior; ML=mediolateral; DV=dorsoventral.
k-o, Quantification of average (left plus right hemispheres) of ILM-SS tractography (k, two-tailed unpaired t-test: t23 = 3.225, P = 0.0038), ILM-MO tractography (I, two-tailed unpaired t-test: t23 = 1.701, P = 0.1024), ILM-DS tractography (m, two-tailed unpaired t-test: t23 = 2.902, P = 0.0080), SS-DS tractography (n, two-tailed unpaired t-test: t23 = 4.813, P < 0.0001), and MO-DS tractography (o, two-tailed unpaired t-test: t23 = 2.515, P = 0.0194) in Atoh1-En1/2 CKO compared to littermate controls (Atoh1-En1/2 CKOs: n=12, littermate controls: n=13).
p, Quantification of cerebellar (CB) volume in eCN-DTA mice compared to littermate controls (n=5 per genotype; two-tailed unpaired t-test: t8 = 4.295, P = 0.0026).
q, Quantification of regional volume normalized to forebrain plus midbrain combined volume in eCN-DTA mice (n=5) compared to littermate controls (n=5). Two-tailed unpaired t-tests to test for effect of genotype on CTX (t8 = 5.876, P = 0.0004) and other comparisons P ≥ 0.05.
r, Schematic representation of global connectivity for eCN-DTA mice compared to littermate controls. Black lines indicate no significant difference and red lines indicate reduced connectivity in eCN-DTA mice compared to littermate controls (two-tailed unpaired t-tests with Welch’s correction).
s, Quantification of global efficiency (Geff; n=5 per genotype; two-tailed unpaired t-test: t8 = 2.535, P = 0.035).
t, Quantification of small worldness (SW; n=5 per genotype; two-tailed unpaired t-test: t8 = 1.591, P = 0.1503).
u-y, Quantification of average (left and right hemispheres) ILM-SS tractography (u, two-tailed unpaired t-test: t8 = 0.3742, P = 0.7180), ILM-SS tractography (v, two-tailed unpaired t-test: t8 = 0.0516, P = 0.9601), ILM-SS tractography (w, two-tailed unpaired t-test: t8 = 0.4177, P = 0.6871), ILM-SS tractography (x, two-tailed unpaired t-test: t8 = 0.9447, P = 0.3725), and ILM-SS tractography (y, two-tailed unpaired t-test: t8 = 0.1414, P = 0.8911) in eCN-DTA mice compared to littermate controls (n=5 per genotype).
Abbreviations: CTX=cerebral cortex; OLF=olfactory bulb; HPF=hippocampal formation; AMY=amygdala; STR=striatum; PAL=pallidum; TH=thalamus; HY=hypothalamus; MB=midbrain; HB=hindbrain; CB=cerebellum; ILM=intralaminar nuclei; SS=primary somatosensory cortex; MO=primary motor cortex; DS=dorsal striatum. ns, not significant: P ≥ 0.05. Data are presented
as mean values ± SD for a, j and mean value ± SEM for d,e,k–o,s–y.
In eCN-DTA mice, in addition to the expected reduction in cerebellum volume (Fig. 8p), there was a small but significant increase in the cerebral cortex but no other changes in the rest of the brain (Fig. 8q) when normalized to a smaller overall brain compared to littermate controls (Supplementary Fig. 10m–o). There was a significant decrease in global efficiency, but no changes in small-worldness in mutants (Fig. 8r–t and Supplementary Fig. 10p,q). Examining the number of streamlines between the thalamus and CN confirmed a significant reduction in eCN-DTA mice (Supplementary Fig. 10r). There were no genotype differences in the thalamo-corticostriatal connectivity that were detected in Atoh1-En1/2 CKOs (Fig. 8u–y and Supplementary Fig. 10s–w). Thus, unlike eCN-DTA mice, Atoh1-En1/2 CKOs show extracerebellar changes involving an aberrant increase in connectivity of ILM-cortico-striatal circuits.
Discussion
Our study applied a novel combination of cell-type-specific genetic manipulations, chemogenetics, dMRI and mouse behavior tests to uncover the requirements during development of the eCN as a whole or the MedP in regulating motor and non-motor functions. We revealed that the main requirement for the eCN if they are all removed in the embryo is for postnatal and adult motor coordination. Furthermore, leveraging an En1/2 CKO model we demonstrate that seemingly intact eCN that lack critical developmental transcription factors can have major adverse effects on cerebellar and extracerebellar circuits regulating adult motor learning and non-motor behaviors.
We identified a genetic tool (SepW1-Cre) to manipulate the medial eCN from E14.5 onwards, adding to existing tools to manipulate all eCN or several subregions38,41,42,43,44,45,46,47. By comparing developmental loss (SepW1-En1/2 CKOs) to adult inhibition of MedP eCN (MedP-hM4Di) we show the neurons are critical for adult reversal learning (WYM) but dispensable if they die embryonically. The MedP-hM4Di results are in line with impaired reversal learning after indirectly inhibiting27 or directly activating16,28 PCs that preferentially target the MedP eCN (lobule 6–8). Also, inhibition of the adult MedP eCN to ventrolateral periaqueductal gray48 or MD49 circuits impairs fear extinction, another form of cognitive flexibility. Interestingly, manipulation of other regions like Crus I neurons that target the lateral CN also show reversal learning deficits27,28. Furthermore, inhibiting lobule 6 versus Crus I PCs differentially alters c-Fos staining of recruited forebrain regions during reversal learning28, likely reflecting distinct downstream pathways16,22,50. Therefore, in SepW1-En1/2 CKOs it is possible that the intact lateral eCN modify their activity to carry out normal reversal learning.
On the contrary, we discovered that when all eCN are ablated embryonically, adult eCN-DTA mice show normal reversal learning, indicating that reversal learning must be regulated by extracerebellar brain regions in these mutants. Indeed, regional specific manipulation of the intralaminar thalamus36, dorsal striatum51, and cerebral cortex52 are sufficient to impair reversal learning. Therefore, one possibility is that during development the corticostriatal or thalamostriatal circuits are altered to confer reversal learning without cerebellar input. Similarly, extracerebellar circuits can modulate social preference53, spatial working memory54, anxiety-like12,55, and stereotyped/repetitive56 behaviors independent of the CN, which may explain why eCN-DTA mice can perform these behaviors.
As expected for a cerebellar-specific developmental perturbation, eCN-DTA mice show impaired neonatal and adult motor coordination5,57,58,59,60. However, unlike when all eCN activity is inhibited5 motor learning is not impaired in eCN-DTA mice. In addition to the eCN58, the GABAergic CN interneurons play a role in motor learning through their projections to the inferior olive61. Therefore, the remaining GABAergic CN interneurons in eCN-DTA mutants might contribute to motor learning, as well as extracerebellar circuits62 providing compensation. Curiously, these possible sources for compensation in eCN-DTA mutants also are expected to apply to mice lacking neurotransmission in all adult eCN5. Since Atoh1Cre was used to generate Atoh1-Slc17a6 CKO5 mice rather than our intersectional approach that targets eCN, one possibility is that one or more of the cell types outside the cerebellum that express Atoh1Cre63 are responsible for the motor leaning deficits in adult Atoh1-Slc17a6 CKO.
We found that although only ~50% of eCN are lost in Atoh1-En1/2 CKOs, adult mutants exhibit impaired motor learning, acquisition/reversal learning, and spatial working memory, which is not seen in eCN-DTA mice. Of likely relevance, the remaining eCN in Atoh1-En1/2 CKOs lack the EN1/2 transcription factors, key regulators of cerebellar development18,64,65,66,67,68,69,70,71,72,73. The behavioral deficits in Atoh1-En1/2 CKOs compared to eCN-DTA mice provides direct evidence that dysfunctional eCN circuits due to genetic mutations can have worse outcomes than losing the neurons embryonically. We propose that in Atoh1-En1/2 CKOs, the remaining eCN while targeting the correct thalamic nuclei are dysfunctional due to altered gene expression. In line with these conclusions, removal of the cerebellum or vermis neonatally in genetically dystonic rats74,75 or weaver mutants76,77 significantly rescues the adult motor coordination deficits seen in both mutants. Circuits that comprise the ILM-cortico-striatal circuitry have been implicated in motor learning36,37,78, cognitive flexibility37,79,80, and spatial working memory81. The excessive connectivity seen in Atoh1-En1/2 CKOs using dMRI might thus be caused by the remaining En1/2-lacking eCN and contribute to their behavioral deficits.
In conclusion, our study highlights the importance of developing relevant models for directly comparing developmental versus adult loss of neurons and the contribution of dysfunctional neurons to understanding behavioral defects and possible compensation (Supplementary Fig. 11). Moreover, our findings offer the potential to be leveraged for the development of therapeutic avenues for patients with pediatric cerebellar injuries.
Methods
Animals.
All animal care and procedures were performed according to the Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine Institutional Animal Care and Use Committee guidelines. Mice were kept in a 12-h/12-h light/dark cycle and in temperature- and humidity-controlled rooms and had ad libitum access to standard laboratory mouse chow and water. All transgenic mouse lines were maintained on a mixed genetic background containing 129, C57BL/6J, and Swiss Webster. For behavior analysis, males and females were analyzed separately, but as there were no sex differences all final analyses combined the two sexes. Estrous cycle was not evaluated for females. The following mouse lines were used in the study: Atoh1-Cre (JAX #011104)82, tetO-Cre (JAX #006234)83, R26LSL-nls-tdTomato (Ai75D, JAX #025106)24, 129S1/SvImJ (JAX #002448), Swiss Webster mice (Taconic Biosciences catalog #SW), En1flox (JAX #007918)71, En2flox (JAX #008872)64, Atoh1-tTA18, En1Cre (JAX #007916)84, lgs7TRE-lox-tdTomato-STOP-lox-DTA*G128D (or lgs7DRAGON-DTA, JAX #034778)30, and SepW1-Cre (MMRRC #036190-UCD)23. Details of all mouse strains used in the study are listed in Table 1 and primers used for genotyping are listed in Table 2.
Table. 1 |.
Key resources and sources.
| REAGENT OR RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-NeuN (A60) | Millipore Sigma | Catalog No: MAB377 |
| Guinea pig polyclonal anti-NeuN | Millipore Sigma | Catalog No: ABN90 |
| Rabbit anti-Calbindin D-28k | Swant Inc | Catalog No: CD38 |
| Guinea pig polyclonal anti-Parvalbumin | Synaptic Systems | Catalog No: 195 044 |
| Chicken polyclonal anti-RFP | Rockland Immunochemicals | Catalog No: 600-901-379 |
| Mouse monoclonal anti-MEIS2 (H-10) | Santa Cruz Biotechnology | Catalog No: sc-515470 |
| Rabbit polyclonal anti-TBR2 | Abcam | Catalog No: ab23345 |
| Rabbit anti-p27 | BD Biosciences | Catalog No: 610241 |
| Goat Alexa Fluor 555 anti-chicken IgY (H+L) | Invitrogen | Catalog No: A21437 |
| Donkey Alexa Fluor 488 anti-rabbit IgG (H+L) | Invitrogen | Catalog No: A21206 |
| Donkey Alexa Fluor 647 anti-rabbit IgG (H+L) | Invitrogen | Catalog No: A31573 |
| Donkey Alexa Fluor 488 anti-mouse IgG (H+L) | Invitrogen | Catalog No: A21202 |
| Donkey Alexa Fluor 647 anti-mouse IgG (H+L) | Invitrogen | Catalog No: A31571 |
| Donkey Alexa Fluor 647 anti-guinea pig IgG (H+L) | Invitrogen | Catalog No: A21450 |
| Goat anti-mouse IgG antibody (H+L), Biotinylated | Vector Laboratories | Catalog No: BA-9200 |
| Goat anti-chicken IgG antibody (H+L), Biotinylated | Vector Laboratories | Catalog No: BA-9010 |
| Virus strains | ||
| AAV2-hSyn-mCherry (titer: 2.6 × 1013 GC/mL) | Addgene | Catalog No: 114472-AAV2 (Lot No: v53550) |
| AAV2-hSyn-DIO-mCherry (titer: 2.1 × 1013 GC/mL) | Addgene | Catalog No: 50459-AAV2 (Lot No: v122065) |
| AAV2-hSyn-DIO-hM4Di-mCherry (titer: 2.3 × 1013 GC/mL) | Addgene | Catalog No: 44362-AAV2 (Lot No: v117556) |
| Chemicals | ||
| 32% paraformaldehyde | Electron Microscopy Sciences | Catalog No: 15714 |
| Hematoxylin 2 | Richard-Allan Scientific | Catalog No: 7231 |
| Eosin-Y | Richard-Allan Scientific | Catalog No: 7111 |
| Bluing reagent | Richard-Allan Scientific | Catalog No: 7301 |
| Clarifier 2 | Richard-Allan Scientific | Catalog No: 7402 |
| Gadodiamide | Millipore Sigma | Catalog No: 131410-48-5 |
| Hoechst 33258, Pentahydrate (bis-Benzimide) | Invitrogen | Catalog No: H3569 |
| Dextran, Tetramethylrhodamine, 10,000 MW, Lysine Fixable (Fluoro-Ruby) | Thermo-Fisher Scientific | Catalog No: D1817 |
| Dextran, Biotin, 10,000 MW, Lysine Fixable (BDA-10K) | Thermo-Fisher Scientific | Catalog No: D1956 |
| Doxycycline hyclate | Sigma Aldrich | Catalog No: D9891 |
| VECTASTAIN Elite ABC-HRP Kit, Peroxidase (Standard) | Vector Laboratories | Catalog No: PK-6100 |
| 3,3’-Diaminobenzidine tetrahydrochloride (DAB) | Sigma Aldrich | Catalog No: D5905 |
| Fluoro-Gel | Electron Microscopy Sciences | Catalog No: 17985-10 |
| Fluoromount-G | ThermoFisher Scientific | Catalog No: 00-4958-02 |
| Heparin sodium salt | Sigma Aldrich | Catalog No: H3393–50KU |
| UltraPure DNase/RNase-Free Distilled Water | ThermoFisher Scientific | Catalog No: 10977023 |
| Phosphate-Buffered Saline (10X) pH 7.4, RNase-free | ThermoFisher Scientific | Catalog No: AM9624 |
| Clozapine N-oxide | Enzo Life Sciences | Catalog No: BML-NS105-0025 |
| Critical commercial assays | ||
| RNAscope Multiplex Fluorescent Detection Kit v2 | Advanced Cell Diagnostics | Catalog No: 323110 |
| RNAscope Target Retrieval Reagents | Advanced Cell Diagnostics | Catalog No: 322000 |
| RNAscope Protease III | Advanced Cell Diagnostics | Catalog No: 322337 |
| RNAscope LS Multiplex TSA Buffer Pack | Advanced Cell Diagnostics | Catalog No: 322810 |
| Mm-Slc32a1-C1 | Advanced Cell Diagnostics | Catalog No: 319191 |
| Mm-Slc17a6-E2-C2 | Advanced Cell Diagnostics | Catalog No: 428871-C2 |
| Mm-Slc6a5-C3 | Advanced Cell Diagnostics | Catalog No: 409741-C3 |
| TSA Vivid fluorophore 520 | Advanced Cell Diagnostics | Catalog No: 323271 |
| TSA Vivid fluorophore 570 | Advanced Cell Diagnostics | Catalog No: 323272 |
| TSA Vivid fluorophore 650 | Advanced Cell Diagnostics | Catalog No: 323273 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Swiss Webster | Taconic Biosciences | Stock No: SW |
| Mouse: Atoh1-Cre: B6.Cg-Tg(Atoh1-Cre)1Bfri/J | The Jackson Laboratory; Materi et al., 2005 | Stock No: 011104 |
| Mouse: SepW1-Cre: B6.FVB(Cg)-Tg(Selenow-cre)NP39Gsat/Mmucd | Mutant Mouse Resource & Research Centers (MMRRC); Gerfen, Paletzki & Heintz, 2013 | 037622-UCD (mice generously provided by N. De Marco Lab, Weill Cornell Medicine) |
| Mouse: tetO-Cre: B6.Cg-Tg(TetOcre)1Jaw/J | The Jackson Laboratory | Stock No: 006234 |
| Mouse: 129S1/SvImJ | The Jackson Laboratory | Stock No: 002448 |
| Mouse: En1Cre: En1tm2(cre)Wrst/J | The Jackson Laboratory; Kimmel et al. 2000 | Stock No: 007916 |
| Mouse: Atoh1-tTA | Willett et al., 2019 | N/A |
| Mouse: lgs7TRE-lox-tdTomato-STOP-lox-DTA*G128D (Cre- and tTA- dependent DTA; lgs7DRAGON-DTA in manuscript; DRAGON = Doxycycline-controlled and Recombinase Activated Gene OverexpressioN): Igs7tm2(tetO-tdTomato, - DTA*G128D)Rdiez/AljJ | The Jackson Laboratory; Ahmadzadeh et al., 2020 | Stock No: 034778 |
| Mouse: Ai75D (Cre-dependent nuclear tdTomato reporter, Ai75 in manuscript): B6.Cg-Gt(ROSA)26Sortm75.1(CAG-tdTomato*)Hze/J | The Jackson Laboratory; Daigle et al., 2018 | Stock No: 025106 |
| Mouse: En1flox: En1tm8.1Alj/J | The Jackson Laboratory; Sgaier et al., 2007 | Stock No: 007918 |
| Mouse: En2flox: En2tm6Alj/J | The Jackson Laboratory; Cheng et al., 2010 | Stock No: 008872 |
| Mouse: En1flox/flox; En2flox/flox (littermate control in manuscript) | Willett et al., 2019 | N/A (generated with 007918 and 008872 in-house) |
| Mouse: Atoh1-Cre/+; En1flox/flox; En2flox/flox (Atoh1-En1/2 CKOs in manuscript) | Willett et al., 2019 | N/A (generated with 007918, 008872, and 011104 in-house) |
| Mouse: SepW1-Cre/+; En1flox/flox; En2flox/flox (SepW1-En1/2 CKOs in manuscript) | This manuscript | N/A (generated with 007918, 008872 in-house) |
| Mouse: Atoh1-tTA/+; En1Cre/+; lgs7DRAGON-DTA/+ (eCN-DTA in manuscript) | This manuscript; Ahmadzadeh et al., 2020 | N/A (generated with Atoh1-tTA, 007916, and 034778 in-house) |
| Software | ||
| Fiji | ImageJ/Fiji | https://fiji.sc/ |
| Prism8 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| ANY-maze | Stoelting Co | https://www.anymaze.co.uk/index.htm |
| BORIS | University of Torino | https://www.boris.unito.it |
| AMIRA | ThermoFisher Scientific | |
| DTIStudio | Jiang et al., 2006 | https://mristudios.org |
| MRtrix | Wu and Zhang (2016) | https://www.mrtrix.org/ |
| MATLAB R2022b | MathWorks | https://www.mathworks.com |
Table. 2 |.
Primers and PCR conditions used for genotyping.
| Allele | Primer sequence (5’ to 3’) |
|---|---|
| Atoh1-Cre, En1 Cre | GATATCTCACGTACTGACGGTGACCAGAGTCATCCTTAGC |
| SepW1-Cre | ACTTGGTTTGCTCTGACTCGTGAGGCGGCAAACGGACAGAAGCATT |
| Atoh1-tTA | GTACTGGCACGTGAAGAACAAGCGGCTACTTGATGCTCCTGATCCTCC |
| lgs7 DRAGON-WT | CCCAACGGTCACTTACTTCCCACACCTTTAATCCCGATGC |
| lgs7 DRAGON-DTA | CCCAACGGTCACTTACTTCCGGTAACCGCGGCATAAAAC |
| En1 lox | GCTTGTTTGGTTTCCGAGTCGGGCAGAGTAAGCCTTGAGA |
| En2 lox | GAAGGTCTCAAGTTTTAGCCGGTAGCCCCCCTTCCTCCTACATAGTTGGCAGTG |
| Ai75D | CTGTTCCTGTACGGCATGGGGCATTAAAGCAGCGTATCC |
For allele specific primers, refer to allele reference.
Embryonic ablation of excitatory cerebellar nuclei (eCN) was achieved by crossing Atoh1-tTA/+; En1Cre/+ mice with lgs7DRAGON-DTA/DRAGON-DTA mice to generate Atoh1-tTA/+; En1Cre/+; lgs7DRAGON-DTA/+ mice (eCN-DTA). Noon of the day that a vaginal plug was discovered was designated as embryonic day 0.5 (E0.5). Doxycycline hyclate (Sigma D9891) was diluted in drinking water (0.02 mg/mL) and provided at E13.5 (when neurogenesis of eCN is complete) until postnatal day 28 (P28) for behavioral studies or otherwise until the end of the experiment and replaced with fresh doxycycline every 3–4 days. To determine if the Atoh1-tTA transgene targets the Slc6a5-expressing anterior medial eCN, Atoh1-tTA/+; tetO-Cre/+; R26LSL-nls-tdTomato/+ mice were generated and administered doxycycline from E13.5 onwards. Atoh1-En1/2 CKOs and Atoh1-En1/2 CKO; Ai75D mice were generated by crossing Atoh1-Cre/+; En1flox/flox; En2flox/flox males with En1flox/flox; En2flox/flox or En1flox/flox; En2flox/flox; R26LSL-nls-tdTomato/LSL-nls-tdTomato females, respectively. Non-littermate control Atoh1-Cre; Ai75D mice were generated by crossing Atoh1-Cre/+ males with R26LSL-nls-tdTomato/LSL-nls-tdTomato females. SepW1-En1/2 CKO and SepW1-En1/2 CKO; Ai75D mice were similarly generated. SepW1-Cre; Ai75D mice were generated by crossing SepW1-Cre/+ or SepW1-Cre/Cre males with R26LSL-nls-tdTomato/LSL-nls-tdTomato females. For all experiments and analyses, investigators were blinded to the genotypes and experimental conditions.
Behavioral assays.
All adult behavioral tests were conducted in a behavioral suite at Weill Cornell Medicine (WCM) and early postnatal behavioral tests were conducted in an animal room at Memorial Sloan Kettering Cancer Center (MSKCC). Six-week-old mice were transferred from MSKCC to WCM two weeks prior to the start of the first behavioral test. Adult mice were acclimated to the animal suite for 1 hour the day before testing. Before conducting each behavioral test, mice were brought to the behavioral suite and left undisturbed for at least 1 hour before testing. The order of each behavior test and age of animals (in brackets) for eCN-DTA and Atoh1-En1/2 CKO mice (and their littermate controls) was as follows: basal locomotor activity33 (6 weeks), three-chambered social approach33,34 (7 weeks), elevated plus maze33 (8 weeks), self-grooming27 (9 weeks), accelerating rotarod18, grip strength18 and footprint assay18 (11 weeks), spontaneous alternation in dry Y-maze33,85 (12 weeks), water Y-maze16,59,86 (13 weeks). Plus-maze87 was tested with different groups of mice at 15 weeks (see details below). MedP-hM4Di mice (and control) and SepW1-En1/2 CKOs (and their littermate controls) followed the same order and age of animals as eCN-DTA and Atoh1-En1/2 CKOs, but were only tested for basal locomotor activity, accelerating rotarod, grip strength, footprint assay, spontaneous alternation, and water Y-maze. For SepW1-En1/2 CKO, Atoh1-En1/2 CKO, and eCN-DTA mice, negative geotaxis88 (at P7 and P11) and surface righting reflex88 (P7) were tested. For MedP-hM4Di and control mice, clozapine N-oxide dissolved in 0.9% saline (5 mg/kg, CNO, Enzo Life Sciences) was injected intraperitoneally 30 minutes before the start of each behavioral testing. For accelerating rotarod and water Y-maze, CNO was injected every day of behavioral testing. All behavioral experiments were recorded with a Logitech C920 HD Pro Webcam (30 fps) and analyzed with ANY-maze (Stoelting Co) or hand scored using BORIS software89.
Negative geotaxis.
Mice were tested for negative geotaxis reflex at P7 and P11 as previously described88. Mice were placed head down on a negative incline (−35°) platform that was covered with a sterile poly-lined drape. Time until mice turned 180° in either direction was measured using a stopwatch. The test was suspended if mice did not turn within 60 seconds (s) or fell down the slope (considered failed trials). Failed trials were assigned a 60 s latency. All mice were tested three trials per test day.
Surface righting reflex.
Mice were tested for surface righting reflex at P7 as previously described88. Mice were placed in the supine position on a flat surface with a sterile poly-lined drape. The time taken for each mouse to turn onto their four paws was measured. All mice were tested for three trials per test day.
Basal locomotor activity.
Mice were placed in a polycarbonate test chamber (27.3 cm × 27.3 cm) equipped with three infrared beam arrays. Horizontal locomotor activity was monitored by computer-assisted activity monitoring software (Med Associates). For each test session, animals were placed in the chamber and recorded for 1 hour without interruption with no incandescent lighting33. Locomotor activity was measured as total distance traveled in centimeters.
Three-chamber social approach.
Mice were tested with a modified version of the three-chamber social approach and social novelty assays as described previously33,34. All testing was conducted in the three-chamber apparatus (Ugo Basile sociability apparatus, Stoelting Co) in a room with 30 lux lighting at the center of each chamber (1~2 lux difference across chambers) and a ceiling-mounted camera for ANY-maze tracking. Two days before testing, age- and sex-matched 129S1/SvImJ mice were habituated to the wire cup (see ref34 for details). After 1-hour habituation to the testing room, two 129S1/SvImJ mice were placed individually under a wire cup (3.8 cm bottom diameter, rust-proof/rust-resistant, noncorrosive, steel wire pencil cups) in the left or right chamber of the apparatus. The entrance to each chamber was blocked. 129S1/SvImJ mice were observed for 10 min for behaviors that are potentially disruptive, such as bar-biting, excessive self-grooming, circling, or clinging to the side bars with all four paws. Only 129S1/SvImJ mice showing docile behavior were used as novel mice in the social approach and novelty testing.
On the day of testing, mutant or control mice were placed in the center of an empty apparatus to freely explore all three chambers for 10 min. During the 10 min habituation, empty wire cups were placed in both left and right chamber. Mice were briefly taken out of the apparatus and a novel object (orange rectangle block) and a novel mouse were placed under each wire cup. The location of the novel mouse was randomly assigned across each subject mouse. Test mice were placed back into the center chamber and allowed to freely explore for 5 min. Test mice were kept in a separate cage until all animals from its original home cage were tested. Time spent in each chamber and time spent in the contact zone (nose within a 2 cm radius around the wire cups) were calculated by automated detection.
Elevated plus maze.
Mice were placed in the center of an elevated plus maze (L × W × H = 50 cm × 5 cm × 50 cm and 38 cm above the floor) for 10 min with 15 lux lighting in the open arms and 5 lux lighting in the closed arms33. A ceiling-mounted camera for ANY-maze tracking was used to measure time spent in and entries into each arm.
Self-grooming.
Mice were placed in a clean cage for 10 min to habituate to the test arena with 25–30 lux lighting27. After habituation, mouse activity was recorded for 10 min. Time spent grooming was hand scored by an experimenter blind to the genotype.
Accelerating rotarod and grip strength.
Mice were tested using an accelerating rotarod protocol as previously described18 with 30 lux lighting. Mice were put on a rotarod (47650, Ugo Basile) rotating at 10 rpm until all mice (3~5 mice tested simultaneously) were facing forward for at least 5 s. The rod was then accelerated from 10 rpm to 40 rpm over 5 min. Mice were tested for 3 trials per day across 3 consecutive days. Animals rested for 10 min in their home cage between each trial. Latency to fall (seconds) was recorded for each animal. If a subject mouse held onto the rod and rotated 3 consecutive times, the latency at which the mouse first started to rotate with the rod was recorded as the latency to fall and the mouse was returned to their home cage. If a subject mouse repeatedly fell within 10–15 s after the start of each trial, the mouse was excluded from the final analysis. If mice did not fall throughout the entire trial, 300 s was assigned as latency to fall. The following day after the accelerating rotarod test, forelimb grip strength was measured using a horizontal grip bar (1027SM Grip Strength meter with single sensor, Columbus Instruments). Mice were allowed to hold the triangle grip bar while being gently pulled away by the base of their tail with their body parallel to the bench. The average of 5 measurements was normalized to the mouse’s body weight (force/gram). Animals rested 5 min in their home cage between each measurement.
Footprint assay.
Mice were tested using a footprint assay as previously described with 30 lux lighting18. After forepaws and hindpaws were painted with different nontoxic acrylic paint colors (red/blue or orange/purple, Crayola), mice were allowed to walk through a plexiglass tunnel (L × W × H = 50 cm × 10 cm × 15 cm) lined with a strip of paper. A dark box was placed at the end of the plexiglass tunnel. Each mouse was tested three times, and three gait parameters (stride, sway, and stance) were measured from each run and averaged.
Y-maze.
Mice were tested using a Y-shaped maze with equal length arms (L × W × H = 33.0 cm × 7.6 cm × 38.1 cm) as described previously33,85 with 20 lux lighting at the bottom of the center of the arena. Mice were placed in the center of the arena and allowed to freely explore the arena for 5 min. A ceiling-mounted camera for ANY-maze tracking was used to record the distance travelled in the maze. The sequence of arm entries and number of entries to each arm was manually scored. The percentage of spontaneous alternation was calculated as previous described33,85.
Water Y-maze.
Mice were tested using an adapted version of the water Y-maze test as described previously16,59,86 with no incandescent lighting (red light for experimenter). The same Y-shape maze used in the dry Y-maze test above was filled with room temperature water colored with nontoxic white paint to be opaque. After dry Y-maze testing and ~1 week prior to testing, mice were placed at the base of one of the Y-maze arms (filled with paint but no hidden platform) and allowed to freely swim for 3 minutes. Average velocity was measured using ANY-maze tracking software. On day 1, mice were placed in the base of one arm of the Y-maze and allowed to swim for 60 s without a platform present to further habituate the mice to the arena. On the next three days (2–4), a white platform was submerged about 1 cm below the surface of the water at the end of one of the arms of the Y-maze. The location of the platform was randomly assigned to either the left or right arm relative to the starting arm (base arm). Mice were placed in the base of the Y-maze and latency to find the platform was recorded. The number of correct trials were recorded only when the first arm entered was the arm with the platform and the mouse found the platform without entering another arm. Once the mouse found the platform, the mouse was allowed to sit on it for 15 s and removed from the Y-maze and placed under a heat lamp in an empty cage. If the mouse did not find the platform within 35 s, it was placed onto the platform for 20 s. All mice were run for the same trial before repeating the process for all 15 trials. On average 10–15 mice were run during one session. This procedure was repeated with each animal for 15 trials per day for 3 consecutive days. For days 5–7, the location of the platform was switched to the opposite arm from which the mouse was initially trained on days 2–4. The same procedure as days 2–4 were repeated. Each day, at the end of the set of 15 trials, the mouse was toweled dry and placed under a heat lamp for 5 min before returning to its home cage. The correct choices (when the first arm entered was the correct arm and the mouse found the platform) and latency to find platform were hand scored using a stopwatch during each trial.
Plus-maze.
Mice were tested using a plus-shape maze with equal length arms (L × W × H = 30.48 × 5.08 × 15.24 cm) as described previously87 with incandescent lighting. Mice were placed in one arm of the maze designated the base of the arena and allowed to freely explore the arena for 12 min each day for a total of 4 days (day 1 was considered habituation to the arena). A ceiling-mounted camera for ANY-maze tracking was used to record the distance travelled in the maze. The sequence of arm entries and number of entries into each arm was scored using a custom MATLAB script. The percentage spontaneous alternation was calculated as previous described87 and the last 3 days of the behavioral testing was averaged for final analysis. As previously described, the estimated chance level performance for the plus maze test is 22.2% spontaneous alternations87. For Atoh1-En1/2 CKOs, mice were tested in two different locations: Weill Cornell Medicine (New York, NY) and University of Tennessee Health Science Center (Memphis, TN). Data from both institutions were pooled as the results were consistent. For eCN-DTA mice, all mice were tested at Weill Cornell Medicine (New York, NY).
Stereotaxic Injections.
Mice were anesthetized with 2.5% isoflurane and 80% O2 and head-fixed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) equipped with digital manipulator arms (Stoelting Co, Wood Dale, IL). A nose cone was used to deliver isoflurane to maintain anesthesia. Mice were given a subcutaneous injection of meloxicam (2 mg/kg) and 0.1 mL of 0.25% Marcaine around the incision site. After exposing the skill, craniotomies were made with an electric drill (Stoelting CO, Wood Dale, IL) with a ball bur attached. A Neuros 7000 series 1 μL Hamilton syringe with a 33-gauge needle (Reno, NV) connected to a remote automated microinfusion pump (KD Scientific, Holliston, MA) was used for delivery of tracers or viral constructs at a rate of 100 or 50 nL/min, respectively. Details of dye, viruses, and stereotaxic coordinates are provided in Table 3. Following infusion, the needle was left in place for 5 min and then slowly manually retracted. Mice were placed on a heat pad for at least 30 min post-surgery before being returned to their home cage. Mice were monitored for three days post-surgery to ensure recovery. Brains were collected and imaged (see below) to confirm viral expression and injection placements and no misplacements were found.
Table. 3 |.
Details of dye and viruses used in stereotaxic surgeries.
| Tracer/virus | Serotype | Company | Catalog no. | Concentr ation/titer | Stereotaxi c coordinate (distance from Bregma) | Injection volume |
|---|---|---|---|---|---|---|
| Dextran, Tetramethylrhodamine, 10,000 MW, Lysine Fixable (Fluoro-Ruby) | n/a | Thermo-Fisher Scientific | D1817 | 10% | CL: AP, −1.20 mm; ML, 0.69 mm; DV, −3.8 mm PF: AP, −2.1 mm; ML, 0.6 mm; DV, −3.5 mm | CL: 300 nL PF: 100 nL |
| Dextran, Biotin, 10,000 MW, Lysine Fixable (BDA-10K) | n/a | Thermo-Fisher Scientific | D1956 | 10% | IntA: AP, −6.24 mm; ML, 1.10 mm; DV, −3.80 mm | 300 nL |
| pAAV-hSyn-mCherry | AAV2 | Addgene | 114472-AAV2 (Lot No: v53550) | 2.6 × 1013 GC/mL | MedA: AP, −6.24 mm; ML, 0.85 mm; DV, −3.70 | 200 nL |
| pAAV-hSyn-DIO-mCherry | AAV2 | Addgene | 50459-AAV2 (Lot No: v122065) | 2.1 × 1013 GC/mL | MedP: AP, −6.7 mm; ML, ±0.9 mm; DV, −3.20 | 300 nl |
| pAAV-hSyn-DIO-hM4Di-mCherry | AAV2 | Addgene | 44362-AAV2 (Lot No: v117556) | 2.3 × 1013 GC/mL | MedP: AP, −6.7 mm; ML, ±0.9 mm; DV, −3.20 | 300 nl |
Tissue preparation.
For all histological analyses, mice were anesthetized with isoflurane and then perfused transcardially with room temperature (RT) phosphate buffered saline (PBS) with heparin (0.02 mg/mL) and then ice-cold 4% paraformaldehyde (PFA, Electron Microscopy Sciences, catalog no: 15714). Brains were dissected and post-fixed in 4% PFA overnight at 4°C and cryopreserved in 30% sucrose in PBS for ~2 days at 4°C. Brains were embedded in Tissue-Tek OCT compound (Sakura Finetek). Coronal serial cryosectioning was performed at either 40 um (6 series) or 100 um (3 series) and collected in 0.01M phosphate buffer (PB) with 0.02% sodium azide and kept at 4°C until further processing. For long-term storage, sections were transferred to 24-well plates with cryoprotectant (30% sucrose and 30% ethylene glycol in 0.1M PB) and stored at −20°C. Sagittal serial cryosectioning was performed at either 14 um (10 series) or 30 um (6 series) and collected on charged glass slides (Fisherbrand ColorFrost Plus) and stored at −20°C until further processing. Details of reagents are listed in Table 1.
For single molecular fluorescence in situ hybridization, tissue was prepared as previously described90. Mice were anesthetized with isoflurane and then perfused transcardially with RT 0.9% saline with 10 units/mL heparin (Sigma Aldrich, H3393–50KU) followed by RT 4% PFA in 0.2M phosphate buffer (PB). Brains were dissected and post-fixed in the same fixative overnight at RT. Brains were cryopreserved by incubating in 15% sucrose in 0.2M PB overnight followed by an overnight incubation in 30% sucrose in 0.2M PB. Brains were embedded in Tissue-Tek OCT compound and stored at −80°C until further processing. Coronal serial cryosectioning was performed at 30 um and sections were stored in cryoprotectant solution at −20°C until further processing. Details of reagents are listed in Table 1.
Hematoxylin and eosin (H&E) staining.
All reagents for H&E staining were obtained from Richard-Allan Scientific: hematoxylin 2 solution (catalog no: 7231), eosin-Y (catalog no: 7111), bluing reagent (catalog no: 7301), clarifier 2 (catalog no: 7402). Slides were first washed in PBS for 5 min and incubated in hematoxylin 2 solution for 3 min. Slides were rinsed in deionized water (diH2O) and immersed in staining reagents for 1 min each (diH2O, bluing reagent, diH2O, clarifier 2, diH2O). After dehydration and defatting (dH2O-70% ethanol-95%–95%−100%−100%-xylene-xylene-xylene, 1 min each) slides were mounted with a coverslip and DPX mounting medium (Electron Microscopy Sciences). Details of reagents are listed in Table 1.
Immunofluorescence.
For slide-mounted sections, slides were washed three times in PBS for 5 min. If necessary, antigen retrieval was then performed by immersing slides in sodium citrate buffer (10 mM sodium citrate with 0.05% Tween-20, pH 6.0) at 95°C for 20 min followed by washing with PBS. Slides were then incubated in blocking buffer (5% BSA in 0.4% Triton-X100 in PBS (PBST)) for 1 hour at 4°C. Primary antibody solution in blocking buffer was then applied overnight at 4°C. Primary antibody information and dilutions are listed in Tables 1 and 4. Slides were then washed with RT 0.1% PBST three times and incubated in secondary antibody solution in blocking buffer (1:500) for 1 hour at RT. Slides then were incubated in Hoechst (Invitrogen, catalog no: H3569 diluted 1:1000 in PBS) for 10 min and washed with PBS three times and cover-slipped with Fluoro-Gel (Electron Microscopy Sciences, catalog no: 17985-10).
Table. 4 |.
Details of antibodies used for immunofluorescence.
| Antibody (clone) | Species | Company | Catalog no. | IHC | Antigen retrieval | Temperature | Time |
|---|---|---|---|---|---|---|---|
| Anti-NeuN (A60) | Mouse mono | Millipore Sigma | MAB377 | 1:1000 | 20 m | 4°C | o/n |
| Anti-NeuN | Guinea pig poly | Millipore Sigma | ABN90 | 1:1000 | 20 m | 4°C | o/n |
| Anti-Calbindin D-28k | Rabbit | Swant Inc | CD38 | 1:1000 | 20 m | 4°C | o/n |
| Anti-Parvalbumin | Guinea pig poly | Synaptic Systems | 195 044 | 1:1000 | 20 m | 4°C | o/n |
| Anti-RFP | Chicken poly | Rockland Immunochemicals | 600-901-379 | 1:1000 (no antigen retrieval) 1:500 (with antigen retrieval) | No 20 m | 4°C | o/n or 48 h* |
| Anti-MEIS2 (H-10) | Mouse mono | Santa Cruz Biotechnology | sc-515470 | 1:1000 | 20 m | 4°C | o/n or 48 h* |
| Anti-TBR2 | Rabbit poly | Abcam | ab23345 | 1:500 | 20 m | 4°C | o/n |
| Anti-p27 | Mouse | BD Biosciences | 610241 | 1:500 | 20 m | 4°C | o/n |
| Alexa Fluor 555 anti-chicken IgY (H+L) | Goat | Invitrogen | A21437 | 1:500 | n/a | RT | 1 h |
| Alexa Fluor 488 anti-rabbit IgG (H+L) | Donkey | Invitrogen | A21206 | 1:500 | n/a | RT | 1 h |
| Alexa Fluor 647 anti-rabbit IgG (H+L) | Donkey | Invitrogen | A31573 | 1:500 | n/a | RT | 1 h or 2 h* |
| Alexa Fluor 488 anti-mouse IgG (H+L) | Donkey | Invitrogen | A21202 | 1:500 | n/a | RT | 1 h |
| Alexa Fluor 647 anti-mouse IgG (H+L) | Donkey | Invitrogen | A31571 | 1:500 | n/a | RT | 1 h or 2 h* |
| Alexa Fluor 647 anti-guinea pig IgG (H+L) | Goat | Invitrogen | A21450 | 1:500 | n/a | RT | 1 h |
| Anti-mouse IgG antibody (H+L), Biotinylated | Goat | Vector Laboratories | BA-9200 | 1:500 | n/a | RT | 1 h |
| Anti-chicken IgG antibody (H+L), Biotinylated | Goat | Vector Laboratories | BA-9010 | 1:500 | n/a | RT | 1 h |
Applicable for free-floating immunofluorescence or immunohistochemistry
For free-floating sections, sections were first washed three times in PBS for 10 min. Sections then were incubated in blocking buffer (10% normal donkey serum in 0.5% PBST, Sigma-Aldrich, catalog no: D9663–10ML) for 1 hour at RT before incubating in primary antibody solution (2% normal donkey serum in 0.4% PBST) for 24–48 hours at 4°C. Slides were washed in PBS three times and incubated in secondary antibody solution (1:500 in 2% normal donkey serum in 0.4% PBST) for 2 hours at RT. Slides then were incubated in Hoechst (1:1000 in PBS) for 10 min and washed with PBS three times. Sections were mounted on glass slides and after sections had fully dried, cover-slipped with Fluoro-Gel (Electron Microscopy Sciences).
Immunohistochemistry.
All steps were performed at RT, unless specified. Cryosectioned tissue mounted on slides were washed three times in PBS for 5 min (hereafter PBS washes). Antigen retrieval was achieved by immersing sections in sodium citrate buffer at 95°C for 40 min and washing in PBS. Slides were then treated with 50% methanol (in deionized H2O) with 0.03% H2O2 for 1 hour and washed with PBS. Primary antibody solution in blocking buffer (5% BSA in 0.4% PBST) was applied overnight at 4°C. After washing in PBS, a biotinylated secondary antibody in blocking buffer (1:500) was applied for 1 hour, followed by PBS washes. Vectastain Elite ABC HRP solution (Vector Labs, Burlingame, CA, USA; PK-6100) in blocking buffer (1:500 for A and B) was applied for 1 hour, followed by PBS washes. Sections were washed in 0.175 M sodium acetate (in ddH2O) and incubated in either standard DAB (0.02% 3,3’-Diaminobenzidine tetrahydrochloride (Sigma-Aldrich, D5905), 2.5% H2O2 in 0.175 M sodium acetate) or Nickel-enhanced DAB (Ni-DAB) solution (addition of 2.5% NiSO4 in standard DAB solution) for 20 min. DAB or Ni-DAB reaction was stopped by washing sections in 0.175 sodium acetated followed by PBS washes. Sections were mounted on glass slides and dried overnight. After dehydration and defatting (dH2O-70% ethanol-95%–95%-100%-100%-xylene-xylene-xylene, 1 min each) slides were mounted with a coverslip using DPX mounting medium (Electron Microscopy Sciences).
Single molecule fluorescence in situ hybridization.
Tissue was treated according to the RNAscope Multiplex Fluorescent Assay v2 manufacturer’s instructions and reagents from Advanced Cell Diagnostics (Hayward, CA). Details of reagents are listed in Table 1. Sections were rinsed twice (5 min each) in RNAase and DNAase free PBS before mounting onto Superfrost Plus coated slides (VWR, catalog no: 48311–703) in RNase- and DNase-free PBS. Slides were dried overnight and heated for 1 hour at 60°C. Slides were pretreated with target retrieval buffer for 10 min at 95–100°C, then treated with Protease III for 30 min at 40°C, followed by a probe incubation for two hours at 40°C. The probes used were: Mm-Slc32a1-C1, Mm-Slc17a6-C2 and Mm-Slc6a5-C3. After probe incubation, slides underwent three amplification steps, followed by developing horseradish peroxidase signal fluorescence with TSA-based fluorophores. Hoechst 33258 was used as a counterstain and slides were dried for 15 min before cover-slipping with Fluoro-Gel.
Double labeling by RNA in situ hybridization and protein immunofluorescence.
Sagittal sections (14 um) were first processed for in situ hybridization of Slc6a5 as described previously91. Briefly, probes were in vitro transcribed from PCR-amplified templates prepared from cDNA synthesized from an adult cerebellum lysate. The forward (5’-GTATCCCACGAGATGGATTGTT-3’) and reverse (5’- CCATACAGAACACCCTCACTCA-3’) primers used for PCR amplification were based on the Allen Brain Atlas. Primers were flanked in the 5’ with SP6 (antisense) and 3’ with T7 (sense) promoters. After visualizing the probe, slides were incubated in 4% PFA overnight at 4°C. Following PBS washes, slides were incubated in sodium citrate buffer (10 mM sodium citrate with 0.05% Tween-20, pH 6.0) at 95°C for 40 min. Slides were placed in blocking buffer for one hour and then incubated in primary antibody solution (anti-RFP at 1:500 in blocking buffer) overnight at 4°C. Slides were washed with 0.1% PBST three times and incubated in secondary antibody solution in blocking buffer (1:500) for 1 hour at RT. Slides were then incubated in Hoechst (1:1000 in PBS, Invitrogen, catalog no: H3569) for 10 min and washed with PBS three times and cover-slipped with Fluoromount-G (ThermoFisher Scientific, catalog no: 00-4958-02).
Microscopy, image processing, and analyses.
All images were acquired with a DM6000 Leica fluorescent microscope (Leica Camera, Wetzlar, Germany) or NanoZoomer Digital Pathology (Hamamatsu Photonics, Shizuoka, Japan). Images were processed or analyzed using Fiji92 or Photoshop (Adobe Inc., San Jose, CA, USA). Cell counts for littermate controls, SepW1-En1/2 CKO, Atoh1-En1/2 CKO; Ai75D, Atoh1-Cre; Ai75D, and eCN-DTA mice were manually obtained using the Cell Counter plugin for Fiji or semi-automated using a custom script using the Analyze Particle plugin for Fiji as previously described18.
The CN subregions were delineated for quantifying the recombination efficiency in SepW1-Cre; Ai75D mice using MEIS2 staining and the mouse brain atlas93. 10–14 brain slices that were 40-um apart were analyzed. The percentage of tdTomato+ cells across the CN were calculated for each animal (total n=4 mice) and averaged. The same slides were used to calculate the percentage of tdTomato+ cells in the medial CN that were co-labeled for MEIS2 (total n=4 mice) and averaged.
Brain sample preparation for ex vivo dMRI.
Mice were transcardially perfused with RT PBS containing heparin and then 4°C 4% PFA. Brains were kept inside the skull, but skin, eyeballs and muscle surrounding was removed and then kept overnight in 4% PFA at 4°C. Samples were then stored in PBS with 0.02% sodium azide until imaging. One week before imaging, samples were equilibrated with Gadodiamide (0.2 mM) in PBS at 4°C and scanned by an experimenter blind to the sex and genotype.
High resolution ex vivo dMRI.
Imaging of the brains was conducted as previously described39,40. High resolution ex vivo dMRI datasets were acquired on a horizontal 7T MR scanner (Bruker Biospin, Billerica, MA, USA) using a 72-mm conventional circularly polarized birdcage radiofrequency resonator (Bruker Biospin, Ettlingen, Germany) for homogeneous transmission in conjunction with a four-channel receive-only phased array CryoProbe (CRP, Bruker Biospin, Ettlingen, Germany) and a modified 3D diffusion-weighted gradient-and-spin-echo (DW-GRASE) sequence94 (TE/TR: 35 ms/ 400 ms, b-value: 5000 s/mm2, no. of diffusion directions: 60, resolution: 0.1 mm3 isotropic, acquisition time: 10.6h).
dMRI data processing.
dMRI rawdata was processed using a pipeline demonstrated in our earlier studies39,40. In brief, the following steps were followed for each subject: 1) removal of signals from non-brain tissue in the dMRI datasets using AMIRA (ThermoFisher Scientific, https://www.thermofisher.com), 2) Correction for specimen displacements using rigid alignment implemented in DTIStudio95, 3) calculation of diffusion tensor, fractional anisotropy, and fiber orientation and distribution maps Using MRtrix96 (https://www.mrtrix.org/), 4) regional volumetric analysis, and 5) streamline tractography between the selected pairs of brain regions or nodes.
Volumetric analysis.
For regional brain volumetric analysis, each subject was mapped to a high-resolution mouse brain atlas39. Whole brain volume or 10 regional volumes (cortex – CTX, olfactory area – OLF, hippocampus – HPF, amygdala – AMY, striatum – STR, pallidum – PAL, thalamus – TH, hypothalamus – HY, midbrain – MB, and cerebellum – CB) were calculated.
Streamline tractography and structural connectome generation.
Structural connectivity between nodes and the structural connectome were constructed as previously reported39,40,97. In short, eight nodes (anterior cingulate cortex – ACA, motor cortex – MO, somatosensory cortex – SS, HPF, STR, AMY, TH, and HY) were included to construct the node-to-node SC maps using streamline tractography as implemented in MRtrix by seeding (n = 50,000) within the seed region with minimum streamline length of 3 mm, FOD cut-off 0.05, step size 0.025, and angle 45°. Excluding the intra-regional connectivity, in total 28 connections per subject were generated, which were subsequently used to construct the subject-specific structural connectome40.
Global network analysis.
Brain global network properties, global efficiency (Geff) and small-worldness (SW), were evaluated as previously described97 using principles of graph theory98 via GRETNA software99. Geff represents the efficiency of distant information transfer in a network and was defined as the inverse of the average characteristic path length between all nodes in the network. Brain network with short average path length between nodes and high degree of interconnectedness in local networks is considered to have SW properties. SW was computed by normalizing the network with respect to 1000 simulated random networks with equal distribution of edge weight and node strength as reported previously100.
Statistics.
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported previously18,33,59. At least three different litters were used for all experiments (cell counting and behavior). No litter-related effects were seen in behavioral studies. Experimenters were blind to mouse genotypes during behavior analysis and quantifications. All data were subject to the Shapiro-Wilk test and Q-Q plots were generated to evaluate normality. If one or more dependent variable(s) failed the normality test, a non-parametric test was used. For behavioral experiments, outliers were defined if at least one dependent variable was more than mean+2×SD or less than mean-2×SD. Some additional criteria for outliers are indicated for each behavioral test (see above). A given outlier was not consistent across experiments. All statistical analyses were carried out with GraphPad Prism 8 software. Comparisons between two groups were analyzed using parametric test (unpaired t-test) or non-parametric test (Mann-Whitney test). The Holm-Šídák method was used for multiple unpaired t-test comparisons. Wilcoxon signed-rank test (null hypothesis = 50%) was used to test normal social preference in the three-chambered social approach test. Ordinary two-way ANOVA was used to compare means across two or more dependent variables. Repeated measures two-way ANOVA with Šídák’s multiple comparisons test were used for basal locomotion, accelerating rotarod, and water Y-maze. A three-way ANOVA was used to examine the effects of sex and genotype on behavior, but as there were no sex differences (data not shown), final analyses included both males and females. Data are presented as mean ± SEM or mean ± SD as indicated in each figure legend. Mean differences were considered significant if P < 0.05.
Supplementary Material
Acknowledgements
We thank all members of the Joyner, Rajadhyaksha, Heck, and Zhang laboratories over the past five years for insightful discussions and technical support. We thank Alexander Walsh, Narmin Mekawy, Diego Scala Chavez, and Dr. Arlene Martinez-Rivera for their excellent support from the Rajadhyaksha lab. We thank Drs. Kristen Pleil (Weill Cornell Medicine), Peter Tsai (UT Southwestern), and Jacqueline Crawley (UC Davis) for their advice and input on data interpretation and technical support for DREADDs and behavioral testing. We thank Dr. Choong Heon Lee for his help with diffusion MRI scanning and post-processing. This work was supported by grants from the NIH to Alexandra L. Joyner (R37MH085726 and R01NS092096), Andrew S. Lee (NICHD T32HD060600), Anjali M. Rajadhyaksha (R01MH118934, R01MH125006, R01DA029122, R01DA050454), Natalia V. De Marco Garcia (2R01MH110553, 1R01MH125006, 1R01NS116137), Detlef H. Heck (R01MH112143), and Jiangyang Zhang (R01NS102904). Alexandra L. Joyner is also supported by an NCI Cancer Center Support Grant (CCSG, P30 CA08748) and the Cycle for Survival. Andrew S. Lee was supported by a Weill Cornell Medicine Clinical & Translational Science Center Predoctoral Training Award (TL1TR002386) from the National Center for Advancing Translational Sciences. Alina Gubanova was supported by a Fordham College at Lincoln Center Dean’s Undergraduate Research and Creative Practice Grant. Anjana Krishnamurthy was supported by a Dorris J. Hutchison Predoctoral Fellowship. Anjali M Rajadhyaksha was supported by the Weill Cornell Autism Research Program.
Footnotes
Competing interests
All authors declare to have no actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work.
Code Availability
All data reported in this study and the analysis codes used are available upon request.
Data availability
Any additional information and requests for reagents and resources should be directed to and will be satisfied by the lead contact ALJ.
References
- 1.Albazron FM, et al. Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology 93, e1561–e1571 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korah MP, et al. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys 77, 106–112 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Robertson PL, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children’s Oncology Group. J Neurosurg 105, 444–451 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Stoodley CJ, Limperopoulos C. Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin Fetal Neonatal Med 21, 356–364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Heijden ME, et al. Glutamatergic cerebellar neurons differentially contribute to the acquisition of motor and social behaviors. Nat Commun 14, 2771 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herculano-Houzel S. Coordinated scaling of cortical and cerebellar numbers of neurons. Front Neuroanat 4, 12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci U S A 103, 12138–12143 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prati JMP-S A.; Gianlorenco A. C. L. The cerebellum and its connections to other brain structures involved in motor and non-motor functions: a comprehensive review. Behav Brain Res, (2024). [DOI] [PubMed] [Google Scholar]
- 9.Kebschull JM, et al. Cerebellum Lecture: the Cerebellar Nuclei-Core of the Cerebellum. Cerebellum, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novello M, Bosman LWJ, De Zeeuw CI. A Systematic Review of Direct Outputs from the Cerebellum to the Brainstem and Diencephalon in Mammals. Cerebellum 23, 210–239 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph S, et al. Cognitive-Affective Functions of the Cerebellum. J Neurosci 43, 7554–7564 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XY, et al. A role for the cerebellum in motor-triggered alleviation of anxiety. Neuron, (2024). [DOI] [PubMed] [Google Scholar]
- 13.Darmohray DM, Jacobs JR, Marques HG, Carey MR. Spatial and Temporal Locomotor Learning in Mouse Cerebellum. Neuron 102, 217–231 e214 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Fujita H, Kodama T, du Lac S. Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Judd EN, Lewis SM, Person AL. Diverse inhibitory projections from the cerebellar interposed nucleus. Elife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly E, et al. Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits. Nat Neurosci 23, 1102–1110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XX, et al. BOD1 regulates the cerebellar IV/V lobe-fastigial nucleus circuit associated with motor coordination. Signal Transduct Target Ther 7, 170 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett RT, et al. Cerebellar nuclei excitatory neurons regulate developmental scaling of presynaptic Purkinje cell number and organ growth. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugihara I, Fujita H, Na J, Quy PN, Li BY, Ikeda D. Projection of reconstructed single Purkinje cell axons in relation to the cortical and nuclear aldolase C compartments of the rat cerebellum. J Comp Neurol 512, 282–304 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Corrales JD, Blaess S, Mahoney EM, Joyner AL. The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development 133, 1811–1821 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Fleming JT, et al. The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Dev Cell 27, 278–292 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kebschull JM, et al. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 370, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daigle TL, et al. A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 174, 465–480 e422 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnamurthy A, et al. Engrailed transcription factors direct excitatory cerebellar neuron diversity and survival. bioRxiv, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruver KMJ J. W.; Fields E.; Song S.; Sjöström P. J.; Watt A. J. Structured connectivity in the output of the cerebellar cortex. bioRxiv, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badura A, et al. Normal cognitive and social development require posterior cerebellar activity. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verpeut JL, et al. Cerebellar contributions to a brainwide network for flexible behavior in mice. Commun Biol 6, 605 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Yu SY, Ren Z, De Zeeuw CI, Gao Z. A FN-MdV pathway and its role in cerebellar multimodular control of sensorimotor behavior. Nat Commun 11, 6050 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadzadeh E, et al. A collection of genetic mouse lines and related tools for inducible and reversible intersectional mis-expression. Development 147, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leto K, et al. Consensus Paper: Cerebellar Development. Cerebellum 15, 789–828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietrichs E. Cerebellar cortical and nuclear afferents from the feline locus coeruleus complex. Neuroscience 27, 77–91 (1988). [DOI] [PubMed] [Google Scholar]
- 33.Kabir ZD, et al. Rescue of impaired sociability and anxiety-like behavior in adult cacna1c-deficient mice by pharmacologically targeting eIF2alpha. Mol Psychiatry 22, 1096–1109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci Chapter 8, Unit 8 26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci 30, 14390–14398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato S, et al. Action Selection and Flexible Switching Controlled by the Intralaminar Thalamic Neurons. Cell Rep 22, 2370–2382 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Melief EJ, et al. Loss of glutamate signaling from the thalamus to dorsal striatum impairs motor function and slows the execution of learned behaviors. NPJ Parkinsons Dis 4, 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao L, Bornmann C, Hatstatt-Burkle L, Scheiffele P. Regulation of striatal cells and goal-directed behavior by cerebellar outputs. Nat Commun 9, 3133 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arefin TM, et al. Towards reliable reconstruction of the mouse brain corticothalamic connectivity using diffusion MRI. Neuroimage 273, 120111 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arefin TM, Lee CH, White JD, Zhang J, Kaffman A. Macroscopic Structural and Connectome Mapping of the Mouse Brain Using Diffusion Magnetic Resonance Imaging. Bio Protoc 11, e4221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asemi-Rad A, Ghiyamihoor F, Consalez GG, Marzban H. Ablation of Projection Glutamatergic Neurons in the Lateral Cerebellar Nuclei Alters Motor Coordination in Vglut2-Cre+ Mice. Cerebellum, (2023). [DOI] [PubMed] [Google Scholar]
- 42.Carlson ES, et al. Catecholaminergic Innervation of the Lateral Nucleus of the Cerebellum Modulates Cognitive Behaviors. J Neurosci 41, 3512–3530 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumas DB, Gornati SV, Adolfs Y, Shimogori T, Pasterkamp RJ, Hoebeek FE. Anatomical Development of the Cerebellothalamic Tract in Embryonic Mice. Cells 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houck BD, Person AL. Cerebellar Premotor Output Neurons Collateralize to Innervate the Cerebellar Cortex. J Comp Neurol 523, 2254–2271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locke TM, et al. Dopamine D1 Receptor-Positive Neurons in the Lateral Nucleus of the Cerebellum Contribute to Cognitive Behavior. Biol Psychiatry 84, 401–412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Low AYT, et al. Reverse-translational identification of a cerebellar satiation network. Nature 600, 269–273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Low AYT, et al. Precision of Discrete and Rhythmic Forelimb Movements Requires a Distinct Neuronal Subpopulation in the Interposed Anterior Nucleus. Cell Rep 22, 2322–2333 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Frontera JL, et al. Bidirectional control of fear memories by cerebellar neurons projecting to the ventrolateral periaqueductal grey. Nat Commun 11, 5207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frontera JL, et al. The cerebellum regulates fear extinction through thalamo-prefrontal cortex interactions in male mice. Nat Commun 14, 1508 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pisano TJ, et al. Homologous organization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep 36, 109721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, et al. Striatal glutamate delta-1 receptor regulates behavioral flexibility and thalamostriatal connectivity. Neurobiol Dis 137, 104746 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinh MA, et al. The eIF2alpha kinase PERK limits the expression of hippocampal metabotropic glutamate receptor-dependent long-term depression. Learn Mem 21, 298–304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal Cortex and Social Cognition in Mouse and Man. Front Psychol 6, 1805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parnaudeau S, Bolkan SS, Kellendonk C. The Mediodorsal Thalamus: An Essential Partner of the Prefrontal Cortex for Cognition. Biol Psychiatry 83, 648–656 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci 18, 1394–1404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H, Lim CS, Kaang BK. Neuronal mechanisms and circuits underlying repetitive behaviors in mouse models of autism spectrum disorder. Behav Brain Funct 12, 3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lalonde R, Strazielle C. Behavioral effects of neonatal lesions on the cerebellar system. Int J Dev Neurosci 43, 58–65 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Manto M, et al. Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 11, 457–487 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai PT, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647–651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Heijden ME, et al. Maturation of Purkinje cell firing properties relies on neurogenesis of excitatory neurons. Elife 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prekop HT, et al. Sox14 Is Required for a Specific Subset of Cerebello-Olivary Projections. J Neurosci 38, 9539–9550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang B, et al. Striatal direct pathway neurons play leading roles in accelerating rotarod motor skill learning. iScience 25, 104245 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose MF, Ahmad KA, Thaller C, Zoghbi HY. Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Acad Sci U S A 106, 22462–22467 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng Y, et al. The Engrailed homeobox genes determine the different foliation patterns in the vermis and hemispheres of the mammalian cerebellum. Development 137, 519–529 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joyner AL. Engrailed, Wnt and Pax genes regulate midbrain--hindbrain development. Trends Genet 12, 15–20 (1996). [DOI] [PubMed] [Google Scholar]
- 66.Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science 251, 1239–1243 (1991). [DOI] [PubMed] [Google Scholar]
- 67.Kuemerle B, Zanjani H, Joyner A, Herrup K. Pattern deformities and cell loss in Engrailed-2 mutant mice suggest two separate patterning events during cerebellar development. J Neurosci 17, 7881–7889 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Millen KJ, Hui CC, Joyner AL. A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development 121, 3935–3945 (1995). [DOI] [PubMed] [Google Scholar]
- 69.Millen KJ, Wurst W, Herrup K, Joyner AL. Abnormal embryonic cerebellar development and patterning of postnatal foliation in two mouse Engrailed-2 mutants. Development 120, 695–706 (1994). [DOI] [PubMed] [Google Scholar]
- 70.Orvis GD, et al. The engrailed homeobox genes are required in multiple cell lineages to coordinate sequential formation of fissures and growth of the cerebellum. Dev Biol 367, 25–39 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sgaier SK, et al. Genetic subdivision of the tectum and cerebellum into functionally related regions based on differential sensitivity to engrailed proteins. Development 134, 2325–2335 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sillitoe RV, Stephen D, Lao Z, Joyner AL. Engrailed homeobox genes determine the organization of Purkinje cell sagittal stripe gene expression in the adult cerebellum. J Neurosci 28, 12150–12162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development 120, 2065–2075 (1994). [DOI] [PubMed] [Google Scholar]
- 74.LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol 120, 302–310 (1993). [DOI] [PubMed] [Google Scholar]
- 75.LeDoux MS, Lorden JF, Meinzen-Derr J. Selective elimination of cerebellar output in the genetically dystonic rat. Brain Res 697, 91–103 (1995). [DOI] [PubMed] [Google Scholar]
- 76.Grusser C, Grusser-Cornehls U. Improvement in motor performance of Weaver mutant mice following lesions of the cerebellum. Behav Brain Res 97, 189–194 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Grusser-Cornehls U, Grusser C, Baurle J. Vermectomy enhances parvalbumin expression and improves motor performance in weaver mutant mice: an animal model for cerebellar ataxia. Neuroscience 91, 315–326 (1999). [DOI] [PubMed] [Google Scholar]
- 78.Badreddine N, et al. Spatiotemporal reorganization of corticostriatal networks encodes motor skill learning. Cell Rep 39, 110623 (2022). [DOI] [PubMed] [Google Scholar]
- 79.Augustin SM, Loewinger GC, O’Neal TJ, Kravitz AV, Lovinger DM. Dopamine D2 receptor signaling on iMSNs is required for initiation and vigor of learned actions. Neuropsychopharmacology 45, 2087–2097 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Augustin SM, Gracias AL, Luo G, Anumola RC, Lovinger DM. Striatonigral direct pathway 2-arachidonoylglycerol contributes to ethanol effects on synaptic transmission and behavior. Neuropsychopharmacology 48, 1941–1951 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bailey KR, Mair RG. Lesions of specific and nonspecific thalamic nuclei affect prefrontal cortex-dependent aspects of spatial working memory. Behav Neurosci 119, 410–419 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Matei V, et al. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn 234, 633–650 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A 99, 10482–10487 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kimmel RA, Turnbull DH, Blanquet V, Wurst W, Loomis CA, Joyner AL. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev 14, 1377–1389 (2000). [PMC free article] [PubMed] [Google Scholar]
- 85.Bavley CC, et al. Rescue of Learning and Memory Deficits in the Human Nonsyndromic Intellectual Disability Cereblon Knock-Out Mouse Model by Targeting the AMP-Activated Protein Kinase-mTORC1 Translational Pathway. J Neurosci 38, 2780–2795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai PT, et al. Sensitive Periods for Cerebellar-Mediated Autistic-like Behaviors. Cell Rep 25, 357–367 e354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, McAfee SS, Van Der Heijden ME, Dhamala M, Sillitoe RV, Heck DH. Causal Evidence for a Role of Cerebellar Lobulus Simplex in Prefrontal-Hippocampal Interaction in Spatial Working Memory Decision-Making. Cerebellum 21, 762–775 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Der Heijden ME, Gill JS, Rey Hipolito AG, Salazar Leon LE, Sillitoe RV. Quantification of Behavioral Deficits in Developing Mice With Dystonic Behaviors. Dystonia 1, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330 (2016). [Google Scholar]
- 90.Johnson MA, et al. Chronic stress differentially alters mRNA expression of opioid peptides and receptors in the dorsal hippocampus of female and male rats. J Comp Neurol 529, 2636–2657 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blaess S, et al. Temporal-spatial changes in Sonic Hedgehog expression and signaling reveal different potentials of ventral mesencephalic progenitors to populate distinct ventral midbrain nuclei. Neural Dev 6, 29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 6th edn. Academic Press/Elsevier; (2007). [Google Scholar]
- 94.Aggarwal M, Mori S, Shimogori T, Blackshaw S, Zhang J. Three-dimensional diffusion tensor microimaging for anatomical characterization of the mouse brain. Magn Reson Med 64, 249–261 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81, 106–116 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Wu D, Zhang J. In vivo mapping of macroscopic neuronal projections in the mouse hippocampus using high-resolution diffusion MRI. Neuroimage 125, 84–93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.White JD, et al. Early life stress causes sex-specific changes in adult fronto-limbic connectivity that differentially drive learning. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10, 186–198 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci 9, 386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubinov M, Sporns O. Weight-conserving characterization of complex functional brain networks. Neuroimage 56, 2068–2079 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information and requests for reagents and resources should be directed to and will be satisfied by the lead contact ALJ.