Abstract
Scabies is a parasitic disease of the skin that disproportionately affects disadvantaged populations. The disease causes considerable morbidity and leads to severe bacterial infection and immune-mediated disease. Scientific advances from the past 5 years suggest that scabies is amenable to population-level control, particularly through mass drug administration. In recognition of these issues, WHO added scabies to the list of neglected tropical diseases in 2017. To develop a global control programme, key operational research questions must now be addressed. Standardised approaches to diagnosis and methods for mapping are required to further understand the burden of disease. The safety of treatments for young children, including with ivermectin and moxidectin, should be investigated. Studies are needed to inform optimum implementation of mass treatment, including the threshold for intervention, target, dosing, and frequency. Frameworks for surveillance, monitoring, and evaluation of control strategies are also necessary.
Introduction
In 2017, scabies was added to the WHO list of neglected tropical diseases (NTDs). Scabies was recommended to be included as a category A NTD, defined as those conditions that fulfil all four specified criteria, and are recom mended for large-scale action in the portfolio of the NTD Department.1 In reaching this recommendation, the WHO NTD Strategic and Technical Advisory Group noted the need for further research to inform control strategy and key issues for programmatic implementation, including ensuring affordable access to oral medications and developing guidelines for their public health use.2 In March, 2018, the WHO NTD Global Working Group on Monitoring and Evaluation discussed scabies for the first time.3 Key recommendations from that meeting included the need to better define the global burden, to integrate control efforts to capitalise on ivermectin-based programmes for other NTDs, and to establish interim guidelines for public health interventions for scabies control. In this rapidly evolving context, we review major advances in the science of control of human scabies from the past 5 years and identify key operational research questions that need to be addressed to develop a global scabies control programme.
The burden of scabies
Scabies is caused by infestation with the microscopic ectoparasite Sarcoptes scabiei var hominis, and leads to severe itch, skin lesions, and more serious complications due to bacterial superinfection (figure 1). Transmission requires skin-to-skin contact, and no non-human reservoir exists. Scabies occurs in all countries, but its distribution is not uniform. In high-income settings, most cases are sporadic, and the predominant public health issue is the management of outbreaks in institutions such as hospitals and residential care facilities for older people. A far greater burden of disease is found in low-income and middle-income countries, where access to effective treatment is often inadequate and population crowding increases the opportunities for transmission. Areas with hot, humid climates have the highest reported prevalence, most prominently island communities in the Pacific region and central America, and Indigenous communities of northern Australia.4–6 In these settings, the community prevalence has been consistently estimated in the range of 20–30%, with a higher prevalence of up to 40–50% in children aged younger than 18 years (figure 2).7,16,17
Figure 1:
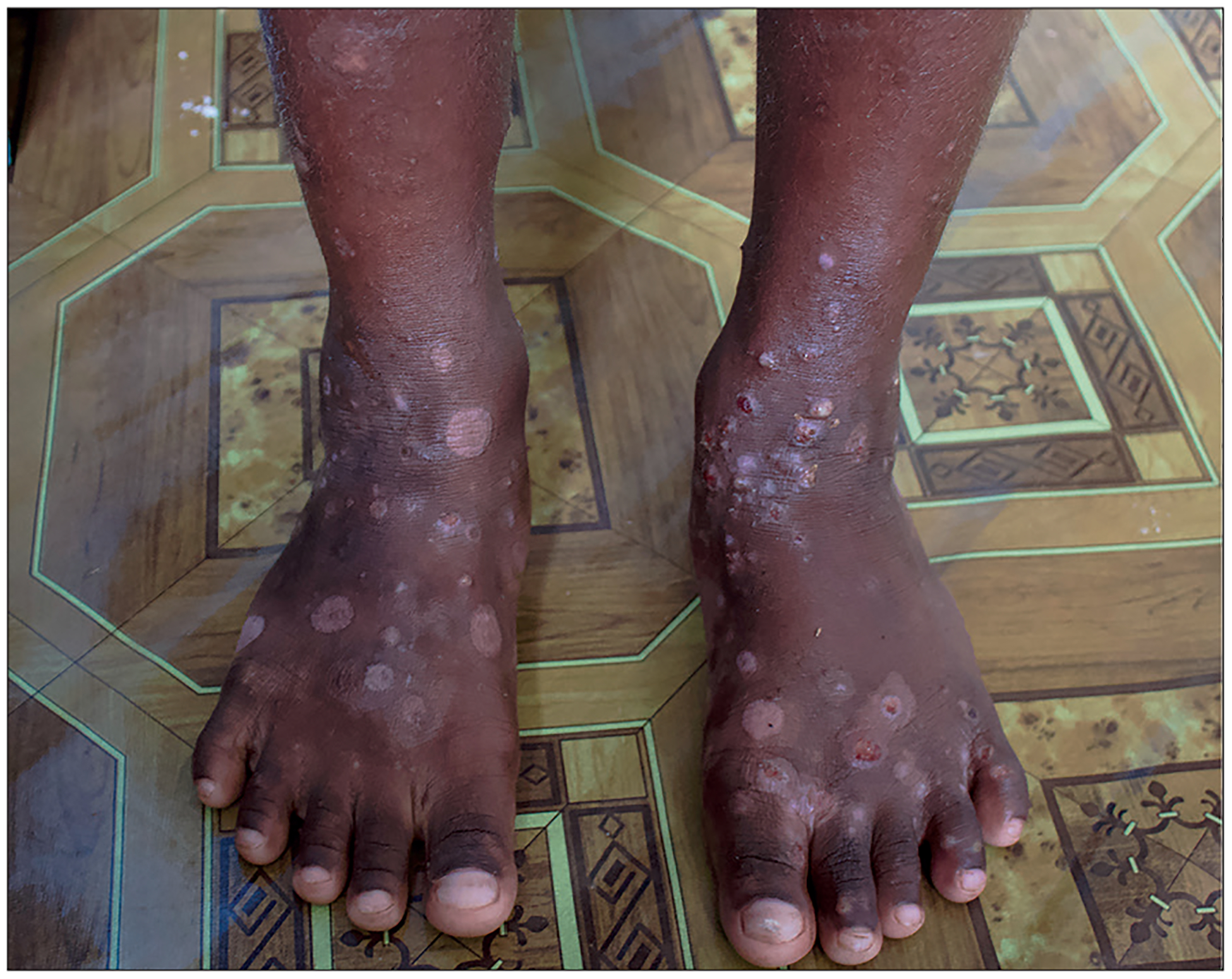
A child’s feet with skin manifestations of scabies infestation and secondary pyoderma
Image credit: Millicent Osti.
Figure 2:
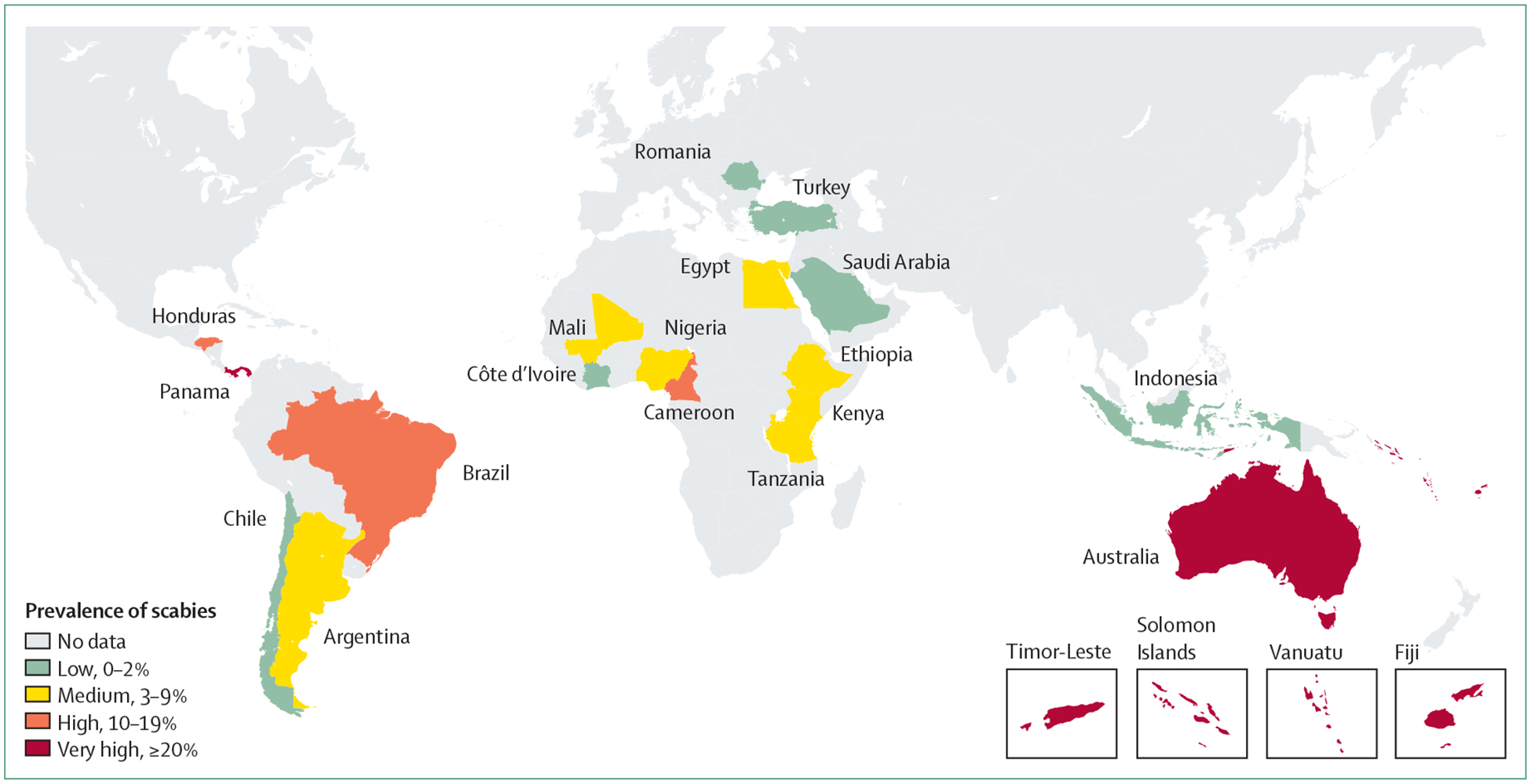
Prevalence of scabies in children and adolescents aged younger than 19 years
Prevalence is shown at the country level, using available data from Romani and colleagues4 and updated with additional references.7–15 Subnational variation exists but is not represented in the map.
In other resource-poor settings where baseline prevalence is lower, change in environmental or sociopolitical conditions can be associated with scabies epidemics.18 An example is the outbreak in the Amhara region in Ethiopia that has been in progress since 2015, and has been estimated to affect more than 1 million people.19,20 Circumstances associated with substantial population crowding frequently lead to high transmission, and outbreaks are common within schools, prisons, and camps for refugees and internally displaced people.21
Scabies is one of the world’s most common illnesses. The 2016 Global Burden of Disease (GBD) Study estimated the global point prevalence of scabies to be around 147 million, with 455 million annual incident cases.22 Further GBD analyses estimated that scabies caused approximately 3·8 million disability-adjusted life-years (DALYs), ranking scabies as one of the most important NTDs.23 Scabies causes an age-adjusted morbidity burden similar to Haemophilus influenzae, type B meningitis, and acute lymphoid leukaemia.24 These GBD analyses are modelled on the small number of published reports on the prevalence of scabies. Of note, the disability weighting only considers the skin changes and itch directly caused by infestation.24 The estimated burden would be far greater if the morbidity and mortality caused by the complications of scabies were included (figure 3). Scabies infestation causes a considerable proportion of bacterial skin infection (pyoderma) in many resource-poor settings, most commonly manifesting as infected sores (impetigo).7,16,25,26 For example, Aboriginal children in Australia were 12 times more likely to develop impetigo when infected with the scabies mite than where no infestation was evident,26 and studies from Pacific island nations have estimated the attributable risk of scabies as a cause of impetigo to be 41–93%.7,16,27 Scabetic lesions and traumatic scratching create breaches in the skin barrier that are a portal for bacterial entry. Scabies mite components such as serpins inhibit innate immune pathways including neutrophil function and directly promote the growth of Staphylococcus aureus and Streptococcus pyogenes.28–31 Skin infection due to these bacteria can lead to severe soft tissue infections and invasive disease. Infection with S pyogenes can also lead to immune-mediated complications including poststreptococcal glomerulonephritis and possibly acute rheumatic fever, which in turn contribute to chronic kidney disease and rheumatic heart disease respectively (figure 3).32,33 Quantification of the burden of these serious health consequences attributable to scabies will give a more accurate estimate of the global burden of scabies and the potential benefit of scabies control (panel 1).
Figure 3:
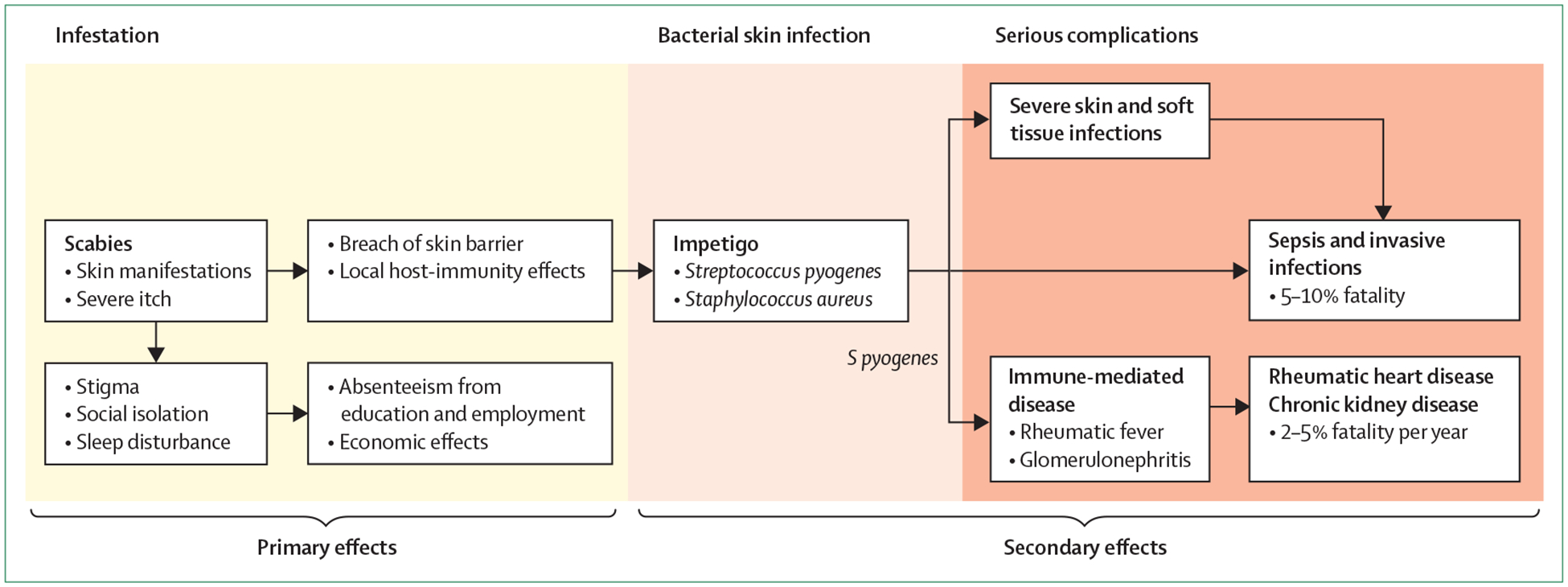
Primary and secondary effects of scabies infestation
Panel 1: Key research questions.
Diagnosis
What is the accuracy and reproducibility of diagnosis using the 2018 International Alliance for the Control of Scabies criteria?
Can a basic skin examination protocol by non-expert health workers provide acceptable accuracy for estimating prevalence?
Can accurate skin-sample or blood tests be developed for scabies diagnosis?
Epidemiology and mapping
What is the global burden of scabies? Which countries have the highest burdens of scabies?
What is the size of the at-risk population living in highly endemic settings?
Are there identifiable risk factors for areas of high prevalence?
What is the correlation between prevalence in school-attending children and the community?
Transmission and complications
What are the transmission dynamics of scabies?
To what extent does crusted scabies drive transmission in highly endemic settings?
What is the association between scabies and impetigo outside of the Pacific region?
Social and economic
What is the social burden of scabies as perceived and understood by affected communities?
What is the acceptability of mass drug administration (MDA) for scabies for affected communities?
What approaches can effectively engage communities to support scabies control initiatives?
Treatment
Can ivermectin or other oral medications be safely administered to children less than 90 cm in height, weighing less than 15 kg, or aged younger than 5 years?
Are novel topical medications as effective, better tolerated, or more affordable than permethrin?
What is the effectiveness of moxidectin for individual treatment and MDA for scabies?
MDA for scabies
What is the optimal dose and dosing strategy of ivermectin for MDA?
What is the optimal interval between rounds of MDA?
What is the effect of MDA for scabies on the burden of impetigo, severe bacterial soft-tissue and systemic infections, glomerulonephritis, and rheumatic heart disease?
Under what conditions would MDA for scabies be cost-effective?
How can ivermectin-based MDA for scabies be safely and effectively integrated or coadministered with MDA for other neglected tropical diseases (NTDs)?
Control strategy
In what circumstances (threshold of scabies prevalence or other factors) should MDA and intensified case management strategies be used?
If MDA is not being used, how can intensified case management be implemented?
What are the effects of ivermectin-based MDA programmes for other NTDs on scabies? What happens to scabies prevalence when these programmes cease?
What is the feasibility and effect of adding permethrin treatment of young children to existing ivermectin-based MDA programmes for other NTDs?
What, if any, environmental measures should be recommended?
Diagnosis
Both the mapping of scabies and population-level control are hampered by the absence of a reliable, reproducible, and standardised approach to diagnosis. Two systematic reviews of diagnostic methods found inconsistency in approaches to scabies diagnosis.34,35 Microscopy of skin scrapings to visualise mites and eggs is highly specific but insensitive and operator dependent, and therefore generally not useful for field settings. To address this gap, an international panel of experts, convened by the International Alliance for the Control of Scabies (IACS), used a Delphi consensus method to develop the 2018 IACS Criteria for Scabies Diagnosis in research and epidemiological settings.36 These criteria enable diagnosis and reporting in three bands of diagnostic certainty: confirmed, clinical, and suspected scabies. A diagnosis of confirmed scabies requires identification of the mite on microscopy or non-invasive visualisation techniques such as videomicrosopy and dermoscopy.37 Clinical and suspected scabies categories rely on features of clinical history and examination. As such, the criteria can be adapted for use in a variety of settings, including field surveys, and might help to standardise reporting and the conduct of scabies research. Validation of these criteria in diverse environments is now required, followed by development of standardised training methods and materials.
Although the current focus should be on development of diagnostics for mapping and surveillance, in future, objective diagnostic tests might be needed, particularly if areas of low endemicity transition to a target of disease elimination. A point-of-care diagnostic test would be ideal for public health use. Direct skin-based testing for infestation using molecular techniques, including PCR38,39 and loop-mediated isothermal amplification40 have been proposed, but none are currently ready for programmatic use. Development of ELISA-based tests to detect antibodies against scabies antigens41 has been hindered by cross-reactivity between antigens from scabies and house dust mites.42,43 If a specific antibody test for scabies were developed, for it to be diagnostic, further evaluation would need to ensure that measured antibody responses were directly associated with ongoing infestation and not simply previous exposure.
In addition to molecular diagnostics, non-invasive mite visualisation methods should be further evaluated. These high-magnification methods allow direct, in-vivo visualisation of the mite (and with some methods, determination of mite survival or demise after treatment) without extraction of the mite by skin scraping.44,45 Although these devices are expensive and require specialised training, more simple and affordable video-microscopes also permit accurate visualisation of the mite in vivo, and low-cost dermatoscopes have been developed.46,47 Further investigation and standardisation of outcome measures by use of these methods would help in assessing the efficacy of new treatments, and possibly validation of mapping and confirmation of outbreaks.48
Epidemiology and mapping
Despite an expansion of research on scabies in the past decade, the development of a global strategy has been constrained by the scarcity of prevalence data, including from those countries suspected to be at risk on the basis of routinely reported clinical data or geographical or socioeconomic characteristics. For example, a worldwide systematic review of published scabies prevalence estimates found 48 studies, of variable quality, with over-representation of countries in the Pacific region and large areas of the world having no published prevalence data.4 Experience from other NTDs suggests that the knowledge of disease burden gained through mapping is crucial for stakeholder engagement, translational research, and successful scale-up of control programmes.49
Given the logistic challenge and cost of detailed mapping for NTDs,50 development of a simple, low-cost, rapid assessment tool is a priority to assist policy makers in initially showing whether scabies is likely to be a public health problem in a given context (panel 2). Standardised survey methods to map scabies prevalence would then be required for a more detailed estimate of disease burden and its variation within and between regions. Survey methods could use the 2018 IACS Diagnostic criteria,36 if these criteria are found to be valid and reproducible. It might be possible to train non-expert examiners in a basic, brief skin examination, which could then be correlated with more detailed diagnostic methods.51 A similar approach has been used for trachoma, in which a rapid assessment can be followed by a standardised mapping survey.52–55 Survey design will need to consider the most appropriate populations and settings in which to do mapping, including ease of access, spatial and age distributions of disease, and community acceptability. For example, mapping of children attending school could be expected to provide a representation of the groups with the highest burden in a community. This approach might be efficient, practicable, and amenable to integration with mapping for other NTDs and other health and education programmes.56,57 Formal comparison of school-based versus community-based sampling would help to show how well school prevalence correlates with community prevalence.58,59
Panel 2: Key programmatic actions.
Epidemiology
Develop a protocol and training package for scabies mapping
Map scabies prevalence using standardised methods, focusing on low-income and middle-income countries
Do integrated surveys with other neglected tropical diseases (NTDs) and health programmes
Develop surveillance strategies for potential outbreaks in high-risk settings
Understand high-burden groups within high-income settings
Population-level control
Develop interim guidance for public health control of scabies, including thresholds for starting and stopping mass drug administration, number and frequency of rounds, drugs, and doses
Develop interim guidance on control of scabies outbreaks in institutions and communities
Develop a monitoring and evaluation strategy for effect of interventions on scabies, its complications and other conditions
Monitor for resistance
Strategy
Define a global control strategy with targets
Facilitate affordable and reliable access to oral and topical medications for treatment
List ivermectin on WHO Model Lists of Essential Medicines for scabies indication
Integrate scabies control with programmes for other NTDs and other health programmes
Develop proposals for a future World Health Assembly resolution
Beyond national-level prevalence data, understanding is poor regarding the epidemiology of specific high-burden areas or populations within otherwise high-income or low-prevalence settings. Recognised examples include dis advantaged Indigenous populations within Australia, New Zealand, and Canada,26,60–62 imprisoned63,64 and homeless populations,65 and groups seeking asylum within Europe.66–68 Scabies is a disease of poverty and inequity and subpopulations with higher burdens would probably be found in many otherwise high-income settings.69
Institutional scabies outbreaks result in considerable morbidity, stigma, and health-care cost in high-income settings. These outbreaks are typically challenging to identify and manage, leading to lengthy delays in confirmation and control, and substantial cost.70–73 Changes in social demography, particularly ageing, mean that these outbreaks might become more common, and the development of appropriate public health strategies is therefore warranted. A deeper appreciation of the features of institutional outbreaks in high-income settings could influence control of scabies elsewhere.
Transmission and complications
Despite scabies being an ancient disease, our understanding of the drivers of transmission remains poor. Understanding transmission dynamics is important for investigating the comparative effects of different control strategies, including through mathematical modelling.74,75 The observed cycles of scabies prevalence in some temperate settings (leading to the misnomer the 7-year itch) previously raised the possibility of herd immunity. However, in many tropical settings, recurrent infestations are common and present with more rapid onset of symptoms than in the initial infection.43 Very high population prevalence is sustained in some of these settings, suggesting that variations across geographic regions and time are more likely to be explained by factors other than personal or herd immunity.76,77
Of particular importance to transmission is the role of individuals with the rare clinical variant crusted scabies (previously known as Norwegian scabies), usually in association with immunosuppression (disease-related or drug-related) or neurological illness. People with crusted scabies can carry thousands to millions of mites and are highly infectious, thereby acting as core transmitters within some communities.78 Being unable to identify and manage these individuals might undermine the success of control programmes.79 Understanding is incomplete as to why prevalence of crusted scabies is high in northern Australia despite the absence of identifiable causes of immunosuppression, but relatively few such cases have been described in other high-prevalence settings.16,78,80,81
The pathogenic links between scabies, impetigo, the infectious complications of S aureus and S pyogenes, and the immune-mediated complications of S pyogenes need further investigation. If these high-morbidity and high-mortality conditions can be more definitively linked with scabies, and can be shown to be prevented effectively through scabies control, the rationale to invest in scabies control will be more compelling for governments, potential donors, and other stakeholders. The relevant associations have been considered most thoroughly in the Pacific region,61,82–84 where impetigo is very common, but scarce data from other regions suggest that endemic scabies occurs in those environments with lower prevalence of impetigo.4 This pattern needs to be properly quantified and the reasons for any variation, if genuinely present, further explored. Impetigo caused by S pyogenes is a major cause of acute glomerulonephritis,85 which in turn contributes to the high burden of chronic kidney disease in low-income settings.86 Scabies has been associated with chronic kidney disease in both epidemiological studies and case reports.87–89 Rheumatic heart disease is estimated to cause over 300 000 deaths per year, with a global distribution that overlaps with areas that are highly endemic for scabies.90 A global resolution on rheumatic fever and rheumatic heart disease was adopted by the World Health Assembly in 2018.91 Primary prevention of streptococcal skin infection through scabies control could potentially be an important component of preventing these diseases.61
Social and economic issues
As a disease that affects the skin, scabies is a potent cause of stigma and reduced quality of life.9,92,93 Increasingly, the chronic disfigurement caused by scabies and other NTDs is understood to adversely affect mental health,56,94 although this aspect has not been factored into current GBD estimates of DALYs. Understanding the conception of scabies, itch, and impetigo within various cultures will help define what scabies as a public health problem means to the most affected communities. This research will also help to build partnerships with communities to develop control interventions, and to maximise participation in them. Scabies is often incorrectly attributed to poor hygiene, which can lead to stigma, shame, and reduced health seeking. However, hygiene and handwashing do not affect the mite or transmission,95 and highly effective control has been shown without any measures addressing hygiene or environment (see section on population-level control),96 suggesting that associations with situations of poverty and disadvantage are probably due to poor access to health care and treatment, or the effects of overcrowding.21,97 The economic burden of scabies in some areas is thought to be substantial, particularly due to the infestation leading to absence from employment and education, and the direct costs of accessing health care and repeated treatments (figure 3).98 Economic studies of the costs of scabies infestation and complications are needed to define and advocate for the most cost-effective control strategies.
Treatment
Although various effective, topical preparations exist for treatment of individual cases, for multiple reasons these agents are poorly suited to population-level control interventions. Reasons include the prolonged duration of application required and local irritation, resulting in inadequate adherence.99 Permethrin 5% cream is the most effective topical treatment,100 but is expensive and unavailable in most countries.21 Topical treatments that are available in some low-income and middle-income countries include benzyl benzoate and sulphur ointments, but these are less well tolerated. Both options commonly cause skin irritation and stinging after application, and sulphur ointments are messy, malodourous, and require repeat treatments.21 The effectiveness and safety profile of novel topical agents, including tea tree oil, for individual case management should be further explored.101–108
Oral medicines have clear advantages for treatment of asymptomatic individuals, and particularly for treatment of whole populations through mass drug administration (MDA), where oral medications are more likely to be accepted and ingestion can be directly observed. Oral ivermectin is highly effective against scabies. As ivermectin does not kill the scabies eggs, a second dose is usually recommended after 7–14 days to kill newly hatched mites. A recent Cochrane review did not find any difference in efficacy when one dose was compared with two doses of ivermectin, or between oral ivermectin and topical permethrin.109 Although the strength of the review conclusions was limited by the quality of included studies, available evidence suggests that a single dose of ivermectin has some efficacy, and that two doses are likely to be similarly effective to permethrin for individual treatment. Extensive knowledge exists on the feasibility and safety of using ivermectin for MDA for other NTDs such as lymphatic filariasis and onchocerciasis.110
An oral agent with a longer duration of activity in the skin, and which could persist for sufficient time to kill newly hatched mites, would obviate the need for a second dose and represent a major advance. Slow-release formulations of ivermectin have been developed, providing potential therapeutic effect for up to 6 months.111 These treatments warrant further investigation for scabies, although if the dosage form is large, administration to children might be challenging. Moxidectin, a macrocyclic lactone antiparasitic agent related to ivermectin, has a half-life of up to 43 days, with prolonged activity in the skin.112,113 The drug is effective against Sarcoptes infestations in animals, and in a preclinical trial using a porcine model of scabies, single-dose moxidectin was shown to be superior to two doses of ivermectin on the basis of the primary outcome measure of mite score.114 In 2018, moxidectin was approved by the US Food and Drug Administration (FDA) for treatment of onchocerciasis in individuals aged 12 years and older.115 Clinical trials of moxidectin for scabies (eg, NCT03905265) have now commenced, with a plan to develop palatable products for children.116
Research into scabies treatments also needs to consider safety, including treatment of pregnant and breastfeeding women and small children, groups that carry a disproportionate burden of scabies and are responsible for much of its transmission. Administration of topical treatment to these groups during ivermectin-based MDA is a major cost and logistical issue. Due to the inadequacy of existing safety data, use of ivermectin in MDA programmes for other NTDs has been restricted to individuals weighing over 15 kg or taller than 90 cm (or aged 5 years or older in some settings). However, several studies have reported no serious adverse outcomes from clinical and inadvertent public health use in young children.117,118 Further investigation of the safety of ivermectin in young children, and collection of prospective data on the safety of moxidectin, are priorities. Ivermectin is considered a pregnancy category C drug by the FDA, but this determination is based on animal studies that used doses far in excess of those recommended for people.119 Studies comparing inadvertent treatment in pregnant women to controls have not shown any concerning safety signal.120–123 In other NTD programmes, ivermectin is offered when the risk of disease is considered higher than the theoretical risk to the fetus.124 In France, ivermectin is a recommended second-line treatment for scabies during pregnancy.125 Ivermectin is excreted in very low concentrations in human milk, and is generally regarded as safe in lactating women after the infant is 7 days old. Ivermectin can cause serious adverse events in individuals with high blood counts of Loa loa (a parasite found in central Africa) microfilariae. Development of new diagnostic technologies such as the LoaScope (University of California Berkeley, Berkeley, CA, USA) might enable safe delivery of ivermectin MDA in these areas,126 but further investigation of implementation is needed.127
To capitalise on existing MDA platforms for other NTDs, the safety of coadministration of ivermectin with other medications needs to be established. In addition to the long-standing practice of coadministration with albendazole, large-scale studies have now shown the safety of coadministration with azithromycin, opening the possibility of integrating control of scabies with control of yaws or trachoma.128–130 Similarly, early analysis of a multinational safety cohort study of combination therapy of ivermectin, diethylcarbamazine, and albendazole (IDA) for lymphatic filariasis did not reveal safety concerns, and administration of this combination of drugs has now been recommended for specific epidemiological contexts within WHO guidelines.131–134
Monitoring for development of resistance of mites to acaricides will also be important. Isolated cases of resistance to ivermectin have been reported in patients with crusted scabies who received repeated and prolonged treatment.135–137 Annual MDA might be less likely to promote the development of resistance than repeated individual treatments, but insufficient research has been done on this subject.138 The proposed use of ivermectin MDA for malaria control119,139 could also promote resistance, particularly if a strategy of multiple doses each month is used.140 However, the risk of resistance seems to be greater for intestinal helminths than ectoparasites, based on evidence from livestock, in which resistance to ivermectin among intestinal parasites is now widespread.141,142 Increasing use of topical treatments for other conditions in humans, such as permethrin for head lice and topical ivermectin for rosacea, might also promote resistance in scabies mites.143
Detailed treatment guidelines for scabies have been developed in several high-income countries and regions, but recommendations vary.144–146 Standardised, evidence-based treatment guidelines for resource-poor settings, including individual case management and management of outbreaks in institutions or closed communities, would be valuable. Further investigation and development of guidelines for the appropriate treatment for individuals with crusted scabies in these settings would also facilitate the success of control interventions.81,147–149
Population-level control
The strategy of individual case management has not appreciably reduced the transmission of scabies in high-prevalence settings.6,150 Data from studies using MDA have led to renewed interest in the potential of MDA to contribute to sustained population-level control.151,152 Programmes of mass treatment combined with additional screening and case management in Panama and Australia (using permethrin)6,17,153 and the Solomon Islands (using ivermectin)154 considerably reduced scabies prevalence. Indirect evidence from Zanzibar, Tanzania, suggested a reduction in consultations and prescriptions for scabies following annual MDA of ivermectin and albendazole for lymphatic filariasis,155,156 but a study from a lower-prevalence setting in mainland Tanzania did not show a sustained effect.157 However, in lymphatic filariasis control programmes, children aged younger than 5 years of age are not given ivermectin (or any other agent active against scabies), which might explain ongoing transmission in this context. The only controlled trial of community treatment published to date was done in small, relatively isolated island populations in Fiji, where a single round of ivermectin-based MDA reduced the prevalence of scabies by 94%, which exceeded that of the permethrin-based MDA (62% reduction) and screen-and-refer (49% reduction) groups.158 These effects have now been shown to be sustained up to 24 months after intervention.159 Ivermectin-based MDA also resulted in a reduction of impetigo prevalence of 67% without adjunctive mass antibiotic treatment. Further studies in the Solomon Islands using ivermectin-based MDA in a much larger population reported reductions in scabies prevalence of around 90%, and in impetigo prevalence of around 75%.14,130 Conversely, ivermectin-based MDA for scabies in northern Australia was not associated with a sustained reduction in prevalence.79 The different results observed in the Australian study might have been because of increased interactions with surrounding untreated communities, lower baseline prevalence (4% compared with 32–42% in the Fiji study),158 or transmission from untreated individuals with crusted scabies.
These studies set the scene for further investigation of the role of programmatic MDA in scabies control. Priority future studies include investigation of whether a single dose of ivermectin (as opposed to two doses, 7–14 days apart) is sufficiently effective as MDA for scabies, and investigation of ivermectin-based MDA in other settings, including non-island populations where population mobility might be greater. While existing MDA data suggest effectiveness of 10–40% in populations with high or very high prevalence,12,130,158 the relative roles of MDA and case management in lower-prevalence (eg, <10%) populations have not yet been adequately explored. Investigating the effect of MDA for scabies on complications such as skin and soft tissue infection, sepsis, and autoimmune sequelae will be important, but will require a large sample size and substantial investment in infrastructure for active surveillance. Studies of moxidectin-based MDA should also be prioritised if initial individually randomised clinical trials show efficacy.
Implementation research assessing integration of scabies control with that of other NTDs, particularly those affecting the skin, could assess whether multiple health interventions can be delivered cost-efficiently.56,160 Initial studies could focus on understanding the effect of ivermectin-based MDA for onchocerciasis and lymphatic filariasis on the prevalence of scabies in areas that are scaling up treatment, and the effect of cessation of MDA in areas that are scaling down treatment. Concern exists that when ivermectin use in these programmes is rolled back in some countries, a resurgence of scabies infestation could occur, which might be a major unintended consequence for communities. An additional research priority will be to investigate the feasibility and effect of adding topical scabies treatment of young children to existing ivermectin-based NTD programmes. The possibility of developing a scabies vaccine has been considered, although this approach is still at an early stage of development.43,161,162
Developing a global strategy
Despite some deficiencies in the evidence for scabies control strategies, preliminary recommendations are needed for countries wishing to commence control measures, alongside pursuit of the research agenda described here. Initial guidance is required on the threshold of scabies prevalence above which MDA could be recommended and other health-system-related, geographical, sociopolitical, or pragmatic factors that might affect this decision. In lower-prevalence settings, intensified case management strategies might be more appropriate. If MDA is to be used on a large scale, then guidance will be required on the control target, number and frequency of MDA rounds, and monitoring and surveillance plan, including post-MDA surveillance, drawing on the lessons from other NTDs.163 This initial guidance should draw on the existing evidence base, and—acknowledging the limitations of current data—expert opinion and modelling studies, with plans made for guidance to be refined as further evidence becomes available. If sustained control can be shown, then the possibility of elimination (interruption of transmission) at a national or regional level might be appropriate to consider.
Once a mapping strategy is developed as part of a global control strategy, it will be possible to estimate the size of at-risk populations requiring ivermectin and permethrin within individual countries and, with less certainty, at regional and global levels. Individual countries would be able to develop control plans as part of broader NTD strategies, taking into consideration the relative burden of scabies and other health issues.
Advocating for access to affordable, quality-assured medications will be essential. Ivermectin has been generously donated for onchocerciasis and lymphatic filariasis in Africa and Latin America through the Mectizan Donation Program. This donation has enabled ivermectin use to be expanded to countries where onchocerciasis is not endemic, as part of the IDA treatment strategy for lymphatic filariasis elimination.164 However, whether such a programme could be extended to include scabies control is unclear. Although a donation programme would maximise the likelihood of achieving control, low-cost, high-quality generic ivermectin might also be affordable in some settings. In addition to advocacy for donations, manufacturers of generic ivermectin could be supported to apply for WHO prequalification.3 No precedent exists for a donation programme for permethrin, and considerable amounts of the drug would be required for treatment of individuals for whom ivermectin is contraindicated. The licence holders of moxidectin, Medicines Development for Global Health, have committed to providing moxidectin at an affordable price for scabies control in low-income and middle-income countries, if efficacy is shown.116
Conclusions
Scabies is a common illness and major health issue affecting communities in many resource-poor settings, warranting public health intervention. Strong evidence suggests that ivermectin-based MDA strategies can be highly effective in reducing the burden of scabies and impetigo in some settings. Future research priorities include further defining the burden of disease, development of standardised approaches to diagnosis and population burden estimation, novel diagnostics, and treatments, and evaluation of large-scale community control strategies. Interim guidance on scabies control is now required.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed for articles published between Jan 1, 1990, and March 31, 2019, using the terms “scabies” and “Sarcoptes scabiei”. Reference lists of identified manuscripts were reviewed to identify additional relevant material. No language restrictions were imposed.
Acknowledgments
DaE, LR, JMK, and ACS were supported by Australian National Health and Medical Research Council Fellowships. ACS was also supported by the National Heart Foundation of Australia. MM was supported by the UK National Institute of Health Research. We acknowledge the contributions of Patrick Lammie and Aya Yajima for the ideas contained in this manuscript. Karly Cini assisted with preparation of figure 2. PTC and AWS are employees of WHO. DLM is an employee of the Centers for Disease Control and Prevention (CDC).
Footnotes
Declaration of interests
OC reports personal fees from Codexial and Zambon. The other authors declare no competing interests.
The findings and opinions expressed in this Review are those of the authors and do not necessarily represent the views, decisions, or policies of WHO, CDC, or other institutions with which the authors are affiliated.
References
- 1.WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases. Recommendations for the adoption of additional diseases as neglected tropical diseases. 2016. http://www.who.int/neglected_diseases/diseases/Adoption_additional_NTDs.pdf (accessed May 12, 2018).
- 2.WHO. Report of the tenth meeting of the WHO Strategic and Technical Advisory Group for Neglected Tropical Diseases. 2017. http://www.who.int/neglected_diseases/NTD_STAG_report_2017.pdf?ua=1 (accessed March 17, 2018).
- 3.WHO. 9th NTD-STAG Global Working Group Meeting on Monitoring and Evaluation of Neglected Tropical Diseases. Geneva: World Health Organization, 2018. [Google Scholar]
- 4.Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis 2015; 15: 960–67. [DOI] [PubMed] [Google Scholar]
- 5.Bowen AC, Mahe A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One 2015; 10: e0136789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taplin D, Porcelain SL, Meinking TL, et al. Community control of scabies: a model based on use of permethrin cream. Lancet 1991; 337: 1016–18. [DOI] [PubMed] [Google Scholar]
- 7.Mason DS, Marks M, Sokana O, et al. The prevalence of scabies and impetigo in the solomon islands: a population-based survey. PLoS Negl Trop Dis 2016; 10: e0004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korte LM, Bowen AC, Draper ADK, et al. Scabies and impetigo in Timor-Leste: a school screening study in two districts. PLoS Negl Trop Dis 2018; 12: e0006400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker SL, Lebas E, De Sario V, et al. The prevalence and association with health-related quality of life of tungiasis and scabies in schoolchildren in southern Ethiopia. PLoS Negl Trop Dis 2017; 11: e0005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yotsu RR, Kouadio K, Vagamon B, et al. Skin disease prevalence study in schoolchildren in rural Cote d’Ivoire: implications for integration of neglected skin diseases (skin NTDs). PLoS Negl Trop Dis 2018; 12: e0006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouotou EA, Nansseu JR, Kouawa MK, Zoung-Kanyi Bissek AC. Prevalence and drivers of human scabies among children and adolescents living and studying in Cameroonian boarding schools. Parasit Vectors 2016; 9: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romani L, Marks M, Sokana O, et al. Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial. Lancet Infect Dis 2019; 19: 510–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ugbomoiko US, Oyedeji SA, Babamale OA, Heukelbach J. Scabies in resource-poor communities in Nasarawa State, Nigeria: epidemiology, clinical features and factors associated with infestation. Trop Med Infect Dis 2018; 3: E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin D, Wiegand R, Goodhew B, Lammie P, Mkocha H, Kasubi M. Impact of ivermectin mass drug administration for lymphatic filariasis on scabies in eight villages in Kongwa District, Tanzania. Am J Trop Med Hyg 2018; 99: 937–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegab DS, Kato AM, Kabbash IA, Dabish GM. Scabies among primary schoolchildren in Egypt: sociomedical environmental study in Kafr El-Sheikh administrative area. Clin Cosmet Investig Dermatol 2015; 8: 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis 2015; 9: e0003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carapetis JR, Connors C, Yarmirr D, Krause V, Currie BJ. Success of a scabies control program in an Australian Aboriginal community. Pediatr Infect Dis J 1997; 16: 494–99. [DOI] [PubMed] [Google Scholar]
- 18.Dayrit JF, Bintanjoyo L, Andersen LK, Davis MDP. Impact of climate change on dermatological conditions related to flooding: update from the International Society of Dermatology Climate Change Committee. Int J Dermatol 2018; 57: 901–10. [DOI] [PubMed] [Google Scholar]
- 19.WHO Africa. Ethiopia—scabies outbreak response in Amhara regional state. 2017. https://www.afro.who.int/news/ethiopia-scabies-outbreak-response-amhara-regional-state (accessed July 6, 2018).
- 20.Enbiale W, Ayalew A. Investigation of a scabies outbreak in drought-affected areas in Ethiopia. Trop Med Infect Dis 2018; 3: E114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect 2012; 18: 313–23. [DOI] [PubMed] [Google Scholar]
- 22.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1260–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karimkhani C, Colombara DV, Drucker AM, et al. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis 2017; 17: 1247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J 2016; 35: 374–78. [DOI] [PubMed] [Google Scholar]
- 26.Aung PTZ, Cuningham W, Hwang K, et al. Scabies and risk of skin sores in remote Australian Aboriginal communities: a self-controlled case series study. PLoS Negl Trop Dis 2018; 12: e0006668–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornley S, Sundborn G, Arbuckle M, Loring B, Heather M, Reynolds E. Is impetigo a missed opportunity for scabies treatment? N Z Med J 2018; 131: 78–81. [PubMed] [Google Scholar]
- 28.Swe PM, Reynolds SL, Fischer K. Parasitic scabies mites and associated bacteria joining forces against host complement defence. Parasite Immunol 2014; 36: 585–93. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds SL, Pike RN, Mika A, et al. Scabies mite inactive serine proteases are potent inhibitors of the human complement lectin pathway. PLoS Negl Trop Dis 2014; 8: e2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swe PM, Fischer K. A scabies mite serpin interferes with complement-mediated neutrophil functions and promotes staphylococcal growth. PLoS Negl Trop Dis 2014; 8: e2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swe PM, Zakrzewski M, Kelly A, Krause L, Fischer K. Scabies mites alter the skin microbiome and promote growth of opportunistic pathogens in a porcine model. PLoS Negl Trop Dis 2014; 8: e2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelman D, Kiang K, Chosidow O, et al. Toward the global control of human scabies: introducing the International Alliance for the Control of Scabies. PLoS Negl Trop Dis 2013; 7: e2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks T, Smeesters PR, Steer AC. Streptococcal skin infection and rheumatic heart disease. Curr Opin Infect Dis 2012; 25: 145–53. [DOI] [PubMed] [Google Scholar]
- 34.Leung V, Miller M. Detection of scabies: a systematic review of diagnostic methods. Can J Infect Dis Med Microbiol 2011; 22: 143–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson MJ, Engelman D, Gholam K, Fuller LC, Steer AC. Systematic review of the diagnosis of scabies in therapeutic trials. Clin Exp Dermatol 2017; 42: 481–87. [DOI] [PubMed] [Google Scholar]
- 36.Engelman D, Fuller LC, Steer AC. Consensus criteria for the diagnosis of scabies: a Delphi study of international experts. PLoS Negl Trop Dis 2018; 12: e0006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micali G, Lacarrubba F, Verzi AE, Chosidow O, Schwartz RA. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis 2016; 10: e0004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahm JE, Kim CW, Kim SS. The efficacy of a nested polymerase chain reaction in detecting the cytochrome c oxidase subunit 1 gene of Sarcoptes scabiei var hominis for diagnosing scabies. Br J Dermatol 2018; 179: 889–95. [DOI] [PubMed] [Google Scholar]
- 39.Angelone-Alasaad S, Molinar Min A, Pasquetti M, et al. Universal conventional and real-time PCR diagnosis tools for Sarcoptes scabiei. Parasit Vectors 2015; 8: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraser TA, Carver S, Martin AM, Mounsey K, Polkinghorne A, Jelocnik M. A Sarcoptes scabiei specific isothermal amplification assay for detection of this important ectoparasite of wombats and other animals. Peer J 2018; 6: e5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Huang X, Dong X, et al. Serodiagnostic potential of alpha-enolase from Sarcoptes scabiei and its possible role in host-mite interactions. Front Microbiol 2018; 9: 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arlian LG, Feldmeier H, Morgan MS. The potential for a blood test for scabies. PLoS Negl Trop Dis 2015; 9: e0004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors 2017; 10: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slutsky JB, Rabinovitz H, Grichnik JM, Marghoob AA. Reflectance confocal microscopic features of dermatophytes, scabies, and demodex. Arch Dermatol 2011; 147: 1008. [DOI] [PubMed] [Google Scholar]
- 45.Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for quantification of Sarcoptes scabiei in Norwegian scabies. J Eur Acad Dermatol Venereol 2013; 27: e176–78. [DOI] [PubMed] [Google Scholar]
- 46.Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol 2016; 30: 1573–77. [DOI] [PubMed] [Google Scholar]
- 47.Micali G, Lacarrubba F, Verzi AE, Nasca MR. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis 2015; 60: 327–29. [DOI] [PubMed] [Google Scholar]
- 48.Miller H, Trujillo-Trujillo J, Feldmeier H. In situ diagnosis of scabies using a handheld digital microscope in resource-poor settings-a proof-of-principle study in the Amazon lowland of Colombia. Trop Med Infect Dis 2018; 3: E116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courtright P, Rotondo LA, MacArthur C, et al. Strengthening the links between mapping, planning and global engagement for disease elimination: lessons learnt from trachoma. Br J Ophthalmol 2018; 102: 1324–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trotignon G, Jones E, Engels T, et al. The cost of mapping trachoma: data from the Global Trachoma Mapping Project. PLoS Negl Trop Dis 2017; 11: e0006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marks M, Engelman D, Romani L, et al. Exploration of a simplified clinical examination for scabies to support public health decision-making. PLoS Negl Trop Dis 2018; 12: e0006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Negrel AD, Mariotti SP. Trachoma rapid assessment: rationale and basic principles. Community Eye Health 1999; 12: 51–53. [PMC free article] [PubMed] [Google Scholar]
- 53.Ngondi J, Reacher M, Matthews F, Brayne C, Emerson P. Trachoma survey methods: a literature review. Bull World Health Organ 2009; 87: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solomon AW, Pavluck AL, Courtright P, et al. The global trachoma mapping project: methodology of a 34-country population-based study. Ophthalmic Epidemiol 2015; 22: 214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solomon AW, Willis R, Pavluck AL, et al. Quality assurance and quality control in the Global Trachoma Mapping Project. Am J Trop Med Hyg 2018; 99: 858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engelman D, Fuller LC, Solomon AW, et al. Opportunities for integrated control of neglected tropical diseases that affect the skin. Trends Parasitol 2016; 32: 843–54. [DOI] [PubMed] [Google Scholar]
- 57.Finn TP, Stewart BT, Reid HL, et al. Integrated rapid mapping of neglected tropical diseases in three States of South Sudan: survey findings and treatment needs. PLoS One 2012; 7: e52789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JL, Sturrock HJ, Olives C, Solomon AW, Brooker SJ. Comparing the performance of cluster random sampling and integrated threshold mapping for targeting trachoma control, using computer simulation. PLoS Negl Trop Dis 2013; 7: e2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheehan JP, Gebresillasie S, Shiferaw A, et al. School-based versus community-based sampling for trachoma surveillance. Am J Trop Med Hyg 2018; 99: 150–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clucas DB, Carville KS, Connors C, Currie BJ, Carapetis JR, Andrews RM. Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bull World Health Organ 2008; 86: 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thornley S, Marshall R, Jarrett P, Sundborn G, Reynolds E, Schofield G. Scabies is strongly associated with acute rheumatic fever in a cohort study of Auckland children. J Paediatr Child Health 2018; 54: 625–32. [DOI] [PubMed] [Google Scholar]
- 62.Banerji A. Scabies. Paediatr Child Health 2015; 20: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kouotou EA, Nansseu JRN, Sangare A, et al. Burden of human scabies in sub-Saharan African prisons: evidence from the west region of Cameroon. Australas J Dermatol 2018; 59: e6–10. [DOI] [PubMed] [Google Scholar]
- 64.Ribeiro Fde A, Taciro E, Guerra MR, Eckley CA. Oral ivermectin for the treatment and prophylaxis of scabies in prison. J Dermatolog Treat 2005; 16: 138–41. [DOI] [PubMed] [Google Scholar]
- 65.Arnaud A, Chosidow O, Detrez MA, et al. Prevalences of scabies and pediculosis corporis among homeless people in the Paris region: results from two randomized cross-sectional surveys (HYTPEAC study). Br J Dermatol 2016; 174: 104–12. [DOI] [PubMed] [Google Scholar]
- 66.Beeres DT, Ravensbergen SJ, Heidema A, et al. Efficacy of ivermectin mass-drug administration to control scabies in asylum seekers in the Netherlands: a retrospective cohort study between January 2014 – March 2016. PLoS Negl Trop Dis 2018; 12: e0006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodman LF, Jensen GW, Galante JM, Farmer DL, Tache S. A cross-sectional investigation of the health needs of asylum seekers in a refugee clinic in Germany. BMC Fam Pract 2018; 19: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bloch-Infanger C, Battig V, Kremo J, et al. Increasing prevalence of infectious diseases in asylum seekers at a tertiary care hospital in Switzerland. PLoS One 2017; 12: e0179537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hotez PJ. NTDs V.2·0: “blue marble health”--neglected tropical disease control and elimination in a shifting health policy landscape. PLoS Negl Trop Dis 2013; 7: e2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engelman D, Steer AC. Diagnosis, treatment, and control of scabies: can we do better? Lancet Infect Dis 2018; 18: 822–23. [DOI] [PubMed] [Google Scholar]
- 71.Cassell JA, Middleton J, Nalabanda A, et al. Scabies outbreaks in ten care homes for elderly people: a prospective study of clinical features, epidemiology, and treatment outcomes. Lancet Infect Dis 2018; 18: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White LC, Lanza S, Middleton J, et al. The management of scabies outbreaks in residential care facilities for the elderly in England: a review of current health protection guidelines. Epidemiol Infect 2016; 144: 3121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mounsey KE, Murray HC, King M, Oprescu F. Retrospective analysis of institutional scabies outbreaks from 1984 to 2013: lessons learned and moving forward. Epidemiol Infect 2016; 144: 2462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lydeamore MJ, Campbell PT, Cuningham W, et al. Calculation of the age of the first infection for skin sores and scabies in five remote communities in northern Australia. Epidemiol Infect 2018; 146: 1194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lydeamore MJ, Campbell PT, Regan DG, et al. A biological model of scabies infection dynamics and treatment informs mass drug administration strategies to increase the likelihood of elimination. Math Biosci 2019; 309: 163–73. [DOI] [PubMed] [Google Scholar]
- 76.Mimouni D, Gdalevich M, Mimouni FB, Haviv J, Ashkenazi I. The epidemiologic trends of scabies among Israeli soldiers: a 28-year follow-up. Int J Dermatol 1998; 37: 586–87. [DOI] [PubMed] [Google Scholar]
- 77.Savin JA. Scabies in Edinburgh from 1815 to 2000. J R Soc Med 2005; 98: 124–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roberts LJ, Huffam SE, Walton SF, Currie BJ. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect 2005; 50: 375–81. [DOI] [PubMed] [Google Scholar]
- 79.Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote australian aboriginal community. PLoS Negl Trop Dis 2015; 9: e0004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walton SF, Beroukas D, Roberts-Thomson P, Currie BJ. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol 2008; 158: 1247–55. [DOI] [PubMed] [Google Scholar]
- 81.Lokuge B, Kopczynski A, Woltmann A, et al. Crusted scabies in remote Australia, a new way forward: lessons and outcomes from the East Arnhem Scabies Control Program. Med J Aust 2014; 200: 644–48. [DOI] [PubMed] [Google Scholar]
- 82.McDonald M, Currie BJ, Carapetis JR. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect Dis 2004; 4: 240–45. [DOI] [PubMed] [Google Scholar]
- 83.Engelman D, Hofer A, Davis JS, et al. Invasive Staphylococcus aureus Infections in children in tropical northern Australia. JPIDS 2014; 3: 304–11. [DOI] [PubMed] [Google Scholar]
- 84.Jenney A, Holt D, Ritika R, et al. The clinical and molecular epidemiology of Staphylococcus aureus infections in Fiji. BMC Infect Dis 2014; 14: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eison TM, Ault BH, Jones DP, Chesney RW, Wyatt RJ. Post-streptococcal acute glomerulonephritis in children: clinical features and pathogenesis. Pediatr Nephrol 2011; 26: 165–80. [DOI] [PubMed] [Google Scholar]
- 86.Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int 2012; 81: 1026–32. [DOI] [PubMed] [Google Scholar]
- 87.Chung SD, Wang KH, Huang CC, Lin HC. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol 2014; 28: 286–92. [DOI] [PubMed] [Google Scholar]
- 88.Wang D, Li L, Wei L, Liu Y, Sun S. Acute postinfectious glomerulonephritis associated with scabies in the elderly: a case report. Parasitol Int 2017; 66: 802–05. [DOI] [PubMed] [Google Scholar]
- 89.Svartman M, Finklea JF, Earle DP, Potter EV, Poon-King T. Epidemic scabies and acute glomerulonephritis in Trinidad. Lancet 1972; 1: 249–51. [DOI] [PubMed] [Google Scholar]
- 90.Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017; 377: 713–22. [DOI] [PubMed] [Google Scholar]
- 91.WHO. 71st World Health Assembly adopts resolution calling for greater action on rheumatic heart disease. May 25, 2018. http://www.who.int/ncds/management/rheumatic-heart-disease-resolution/en/ (accessed Sept 10, 2018).
- 92.Jackson A, Heukelbach J, Filho AF, Junior Ede B, Feldmeier H. Clinical features and associated morbidity of scabies in a rural community in Alagoas, Brazil. Trop Med Int Health 2007; 12: 493–502. [DOI] [PubMed] [Google Scholar]
- 93.Worth C, Heukelbach J, Fengler G, Walter B, Lisenfeld O, Feldmeier H. Impaired quality of life in adults and children with scabies from an impoverished community in Brazil. Int J Dermatol 2012; 51: 275–82. [DOI] [PubMed] [Google Scholar]
- 94.Litt E, Baker MC, Molyneux D. Neglected tropical diseases and mental health: a perspective on comorbidity. Trends Parasitol 2012; 28: 195–201. [DOI] [PubMed] [Google Scholar]
- 95.Cinotti E, Perrot JL, Labeille B, et al. Inefficacy of alcohol-based hand rub on mites in a patient with hyperkeratotic scabies. Clin Exp Dermatol 2015; 40: 177–81. [DOI] [PubMed] [Google Scholar]
- 96.Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med 2016; 374: 1690. [DOI] [PubMed] [Google Scholar]
- 97.Middleton J, Cassell JA, Jones CI, Lanza S, Head MG, Walker SL. Scabies control: the forgotten role of personal hygiene—authors’ reply. Lancet Infect Dis 2018; 18: 1068–69. [DOI] [PubMed] [Google Scholar]
- 98.Hay RJ, Estrada Castanon R, Alarcon Hernandez H, et al. Wastage of family income on skin disease in Mexico. BMJ 1994; 309: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.La Vincente S, Kearns T, Connors C, Cameron S, Carapetis J, Andrews R. Community management of endemic scabies in remote Aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis 2009; 3: e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnstone P, Strong M. Scabies. BMJ Clin Evid 2014; 2014: 1707. [PMC free article] [PubMed] [Google Scholar]
- 101.Gopinath H, Aishwarya M, Karthikeyan K. Tackling scabies: novel agents for a neglected disease. Int J Dermatol 2018; 57: 1293–98. [DOI] [PubMed] [Google Scholar]
- 102.Thomas J, Christenson JK, Walker E, Baby KE, Peterson GM. Scabies—an ancient itch that is still rampant today. J Clin Pharm Ther 2017; 42: 793–99. [DOI] [PubMed] [Google Scholar]
- 103.Walton SF, McKinnon M, Pizzutto S, Dougall A, Williams E, Currie BJ. Acaricidal activity of Melaleuca alternifolia (tea tree) oil: in vitro sensitivity of Sarcoptes scabiei var hominis to terpinen-4-ol. Arch Dermatol 2004; 140: 563–66. [DOI] [PubMed] [Google Scholar]
- 104.Pasay C, Mounsey K, Stevenson G, et al. Acaricidal activity of eugenol based compounds against scabies mites. PLoS One 2010; 5: e12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas J, Carson CF, Peterson GM, et al. Therapeutic potential of tea tree oil for scabies. Am J Trop Med Hyg 2016; 94: 258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thomas J, Davey R, Peterson GM, et al. Treatment of scabies using a tea tree oil-based gel formulation in Australian Aboriginal children: protocol for a randomised controlled trial. BMJ Open 2018; 8: e018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fang F, Candy K, Melloul E, et al. In vitro activity of ten essential oils against Sarcoptes scabiei. Parasit Vectors 2016; 9: 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rutherford T, Nixon R, Tam M, Tate B. Allergy to tea tree oil: retrospective review of 41 cases with positive patch tests over 4·5 years. Australas J Dermatol 2007; 48: 83–87. [DOI] [PubMed] [Google Scholar]
- 109.Rosumeck S, Nast A, Dressler C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst Rev 2018; 4: CD012994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thylefors B, Alleman MM, Twum-Danso NA. Operational lessons from 20 years of the Mectizan Donation Program for the control of onchocerciasis. Trop Med Int Health 2008; 13: 689–96. [DOI] [PubMed] [Google Scholar]
- 111.Chaccour C, Barrio A, Gil Royo AG, et al. Screening for an ivermectin slow-release formulation suitable for malaria vector control. Malar J 2015; 14: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mounsey KE, Bernigaud C, Chosidow O, McCarthy JS. Prospects for moxidectin as a new oral treatment for human scabies. PLoS Negl Trop Dis 2016; 10: e0004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cotreau MM, Warren S, Ryan JL, et al. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol 2003; 43: 1108–15. [DOI] [PubMed] [Google Scholar]
- 114.Bernigaud C, Fang F, Fischer K, et al. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl Trop Dis 2016; 10: e0005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olliaro PL, Kuesel AC, Halleux CM, Sullivan M, Reeder JC. Creative use of the priority review voucher by public and not-for-profit actors delivers the first new FDA-approved treatment for river blindness in 20 years. PLoS Negl Trop Dis 2018; 12: e0006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Medicines Development for Global Health. Development programs. 2018. https://www.medicinesdevelopment.com/development-programs.htm (accessed Aug 3, 2018).
- 117.Wilkins AL, Steer AC, Cranswick N, Gwee A. Is it safe to use ivermectin in children less than five years of age and weighing less than 15 kg? Arch Dis Child 2018; 103: 514–19. [DOI] [PubMed] [Google Scholar]
- 118.Becourt C, Marguet C, Balguerie X, Joly P. Treatment of scabies with oral ivermectin in 15 infants: a retrospective study on tolerance and efficacy. Br J Dermatol 2013; 169: 931–33. [DOI] [PubMed] [Google Scholar]
- 119.Chaccour C, Hammann F, Rabinovich NR. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar J 2017; 16: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ndyomugyenyi R, Kabatereine N, Olsen A, Magnussen P. Efficacy of ivermectin and albendazole alone and in combination for treatment of soil-transmitted helminths in pregnancy and adverse events: a randomized open label controlled intervention trial in Masindi district, western Uganda. Am J Trop Med Hyg 2008; 79: 856–63. [PubMed] [Google Scholar]
- 121.Pacqué M, Muñoz B, Poetschke G, Foose J, Greene BM, Taylor HR. Pregnancy outcome after inadvertent ivermectin treatment during community-based distribution. Lancet 1990; 336: 1486–89. [DOI] [PubMed] [Google Scholar]
- 122.Gyapong JO, Chinbuah MA, Gyapong M. Inadvertent exposure of pregnant women to ivermectin and albendazole during mass drug administration for lymphatic filariasis. Trop Med Int Health 2003; 8: 1093–101. [DOI] [PubMed] [Google Scholar]
- 123.Chippaux JP, Gardon-Wendel N, Gardon J, Ernould JC. Absence of any adverse effect of inadvertent ivermectin treatment during pregnancy. Trans R Soc Trop Med Hyg 1993; 87: 318. [DOI] [PubMed] [Google Scholar]
- 124.Brown KR. Changes in the use profile of Mectizan: 1987–1997. Ann Trop Med Parasitol 1998; 92 (suppl 1): S61–64. [DOI] [PubMed] [Google Scholar]
- 125.Centre de Référence sur les Agents Tératogènes. Scabies in pregnancy. July 6, 2018. https://lecrat.fr/spip.php?page=article&id_article=585 (accessed Oct 30, 2018; in French).
- 126.Kamgno J, Pion SD, Chesnais CB, et al. A test-and-not-treat strategy for onchocerciasis in Loa loa-endemic areas. N Engl J Med 2017; 377: 2044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Richards FO Jr. Mass administration of ivermectin in areas where Loa loa is endemic. N Engl J Med 2017; 377: 2088–90. [DOI] [PubMed] [Google Scholar]
- 128.Romani L, Marks M, Sokana O, et al. Feasibility and safety of mass drug coadministration with azithromycin and ivermectin for the control of neglected tropical diseases: a single-arm intervention trial. Lancet Glob Health 2018; 6: e1132–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Coulibaly YI, Dicko I, Keita M, et al. A cluster randomized study of the safety of integrated treatment of trachoma and lymphatic filariasis in children and adults in Sikasso, Mali. PLoS Negl Trop Dis 2013; 7: e2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marks M, Toloka H, Baker C, et al. Randomised trial of community treatment with azithromycin and ivermectin mass drug administration for control of scabies and impetigo. Clin Infect Dis 2019; 68: 927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fischer PU, King CL, Jacobson JA, Weil GJ. Potential value of triple drug therapy with ivermectin, diethylcarbamazine, and albendazole (IDA) to accelerate elimination of lymphatic filariasis and onchocerciasis in Africa. PLoS Negl Trop Dis 2017; 11: e0005163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thomsen EK, Sanuku N, Baea M, et al. Efficacy, safety, and pharmacokinetics of coadministered diethylcarbamazine, albendazole, and ivermectin for treatment of bancroftian filariasis. Clin Infect Dis 2016; 62: 334–41. [DOI] [PubMed] [Google Scholar]
- 133.WHO. Alternative mass drug administration regimens to eliminate lymphatic filariasis. Geneva: World Health Organization, 2017. [PubMed] [Google Scholar]
- 134.King CL, Suamani J, Sanuku N, et al. A trial of a triple-drug treatment for lymphatic filariasis. N Engl J Med 2018; 379: 1801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11: e0005920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Currie BJ, Harumal P, McKinnon M, Walton SF. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin Infect Dis 2004; 39: e8–12. [DOI] [PubMed] [Google Scholar]
- 137.Mounsey KE, Holt DC, McCarthy JS, Currie BJ, Walton SF. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol 2009; 145: 840–41. [DOI] [PubMed] [Google Scholar]
- 138.Webster JP, Molyneux DH, Hotez PJ, Fenwick A. The contribution of mass drug administration to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rabinovich NR. Ivermectin: repurposing an old drug to complement malaria vector control. Lancet Infect Dis 2018; 18: 584–85. [DOI] [PubMed] [Google Scholar]
- 140.Smit MR, Ochomo EO, Aljayyoussi G, et al. Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2018; 18: 615–26. [DOI] [PubMed] [Google Scholar]
- 141.Prichard RK. Ivermectin resistance and overview of the Consortium for Anthelmintic Resistance SNPs. Expert Opin Drug Discov 2007; 2 (suppl 1): S41–52. [DOI] [PubMed] [Google Scholar]
- 142.Mounsey KE, Holt DC, McCarthy J, Currie BJ, Walton SF. Scabies: molecular perspectives and therapeutic implications in the face of emerging drug resistance. Future Microbiol 2008; 3: 57–66. [DOI] [PubMed] [Google Scholar]
- 143.McNair CM. Ectoparasites of medical and veterinary importance: drug resistance and the need for alternative control methods. J Pharm Pharmacol 2015; 67: 351–63. [DOI] [PubMed] [Google Scholar]
- 144.Executive Committee for the Diagnosis and Treatment of Scabies. Guideline for the diagnosis and treatment of scabies in Japan (third edition). J Dermatol 2017; 44: 991–1014. [DOI] [PubMed] [Google Scholar]
- 145.Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermaol Venereol 2017; 31: 1248–53. [DOI] [PubMed] [Google Scholar]
- 146.Sunderkotter C, Feldmeier H, Folster-Holst R, et al. S1 guidelines on the diagnosis and treatment of scabies—short version. J Dtsch Dermatol Ges 2016; 14: 1155–67. [DOI] [PubMed] [Google Scholar]
- 147.Quilty S, Kaye TS, Currie BJ. Crusted scabies in northern and central Australia—now is the time for eradication. Med J Aust 2017; 206: 96. [DOI] [PubMed] [Google Scholar]
- 148.Davis JS, McGloughlin S, Tong SY, Walton SF, Currie BJ. A novel clinical grading scale to guide the management of crusted scabies. PLoS Negl Trop Dis 2013; 7: e2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chosidow O, Bernigaud C, Do-Pham G. High-dose ivermectin in malaria and other parasitic diseases: a new step in the development of a neglected drug. Parasite 2018; 25: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taplin D, Arrue C, Walker J, Roth W, Rivera A. Eradication of scabies with a single treatment schedule. J Am Acad Dermatol 1983; 9: 546–50. [DOI] [PubMed] [Google Scholar]
- 151.Engelman D, Steer AC. Control strategies for scabies. Trop Med Infect Dis 2018; 3: E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thean LJ, Engelman D, Kaldor J, Steer AC. Scabies: new opportunities for management and population control. Pediatr Infect Dis J 2019; 38: 211–13. [DOI] [PubMed] [Google Scholar]
- 153.Andrews RM, Kearns T, Connors C, et al. A regional initiative to reduce skin infections amongst Aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Negl Trop Dis 2009; 3: e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lawrence G, Leafasia J, Sheridan J, et al. Control of scabies, skin sores and haematuria in children in the Solomon Islands: another role for ivermectin. Bull World Health Organ 2005; 83: 34–42. [PMC free article] [PubMed] [Google Scholar]
- 155.Engelman D, Martin DL, Hay RJ, et al. Opportunities to investigate the effects of ivermectin mass drug administration on scabies. Parasit Vectors 2013; 6: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mohammed KA, Deb RM, Stanton MC, Molyneux DH. Soil transmitted helminths and scabies in Zanzibar, Tanzania following mass drug administration for lymphatic filariasis— a rapid assessment methodology to assess impact. Parasit Vectors 2012; 5: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martin D, Wiegand R, Goodhew B, Lammie P, Mkocha H, Kasubi M. Impact of ivermectin mass drug administration for lymphatic filariasis on scabies in eight villages in Kongwa District, Tanzania. Am J Trop Med Hyg 2018; 99: 937–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med 2015; 373: 2305–13. [DOI] [PubMed] [Google Scholar]
- 159.Romani L, Kaldor J, Steer A. Sustained reduction of scabies two years after mass drug administration. N Engl J Med (in press). [Google Scholar]
- 160.Mitja O, Marks M, Bertran L, et al. Integrated control and management of neglected tropical skin diseases. PLoS Negl Trop Dis 2017; 11: e0005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu X, Walton S, Mounsey K. Vaccine against scabies: necessity and possibility. Parasitology 2014; 141: 725–32. [DOI] [PubMed] [Google Scholar]
- 162.Casais R, Granda V, Balseiro A, et al. Vaccination of rabbits with immunodominant antigens from Sarcoptes scabiei induced high levels of humoral responses and pro-inflammatory cytokines but confers limited protection. Parasit Vectors 2016; 9: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chu BK, Deming M, Biritwum NK, et al. Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl Trop Dis 2013; 7: e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mectizan Donation Program. Guide for Donations of Mectizan to accelerate the elimination of lymphatic filariasis in countries where onchocerciasis is not co-endemic. 2017. https://mectizan.org/news-resources/mec-guide-for-donations-of-mectizan-in-ida-countries/ (accessed Oct 8, 2018).


