Abstract
The prenatal environment can alter neurodevelopmental and clinical trajectories, markedly increasing risk for psychiatric disorders in childhood and adolescence. To understand if and how fetal exposures to stress and inflammation exacerbate manifestation of genetic risk for complex brain disorders, we report a large-scale context-dependent massively parallel reporter assay (MPRA) in human neurons designed to catalogue genotype x environment (GxE) interactions. Across 240 genome-wide association study (GWAS) loci linked to ten brain traits/disorders, the impact of hydrocortisone, interleukin 6, and interferon alpha on transcriptional activity is empirically evaluated in human induced pluripotent stem cell (hiPSC)-derived glutamatergic neurons. Of ~3,500 candidate regulatory risk elements (CREs), 11% of variants are active at baseline, whereas cue-specific CRE regulatory activity range from a high of 23% (hydrocortisone) to a low of 6% (IL-6). Cue-specific regulatory activity is driven, at least in part, by differences in transcription factor binding activity, the gene targets of which show unique enrichments for brain disorders as well as co-morbid metabolic and immune syndromes. The dynamic nature of genetic regulation informs the influence of environmental factors, reveals a mechanism underlying pleiotropy and variable penetrance, and identifies specific risk variants that confer greater disorder susceptibility after exposure to stress or inflammation. Understanding neurodevelopmental GxE interactions will inform mental health trajectories and uncover novel targets for therapeutic intervention.
Keywords: MPRA, context-specific expression quantitative trait loci, psychiatric genetics, stress and inflammation, fetal brain development
Introduction
Genome-wide association studies (GWAS) identified hundreds of significant loci associated with psychiatric9-12 and neurodegenerative disease risk13,14. The overwhelming majority of these risk loci are comprised of variants that are non-coding, common in the population at large, and thought to confer heightened risk by regulating the expression of one or more target genes (eGenes)1,2,16. Critically, the weighted sum of all GWAS risk alleles falls short of explaining phenotypic variance and does not predict individual outcomes3, suggesting that additional factors may underlie the penetrance and expressivity of genetic risk for complex brain disorders. For example, immune signaling and stress, particularly fetal exposures, are well-established environmental risk factors associated with psychiatric disorders. Although the causal mechanisms involved remain unresolved, maternal immune activation (MIA) induces pro-inflammatory agents and neuropoietic cytokines, circulating levels of which alter the release of glucocorticoids from the adrenal gland4,5. Maternal cytokines and steroid hormones cross the placental barrier and reach the fetal vasculature and brain6,7, where they impact gene expression and neurodevelopment8,9,10 in vivo, effects that are recapitulated in a dose-dependent manner in vitro11,12. Interaction of cytokines in the developing brain with risk-associated regulatory elements may explain, in part, how fetal exposures increase risk for brain disorders in offspring.
Genomic analyses typically yield static predictions of gene expression derived from post-mortem analyses, predicting genetic regulation at baseline. If, however, the regulatory activity of non-coding risk variants is indeed influenced by environmental interactions, context-specific influences of the regulome could shape risk for brain disorders. Consistent with this, genetic loci that explain variation in expression levels of mRNAs (termed expression quantitative trait loci, “eQTL”) differ between tissues13-15, cell-types16-18, and sexes19-21. eQTLs are likewise dynamically regulated (e.g. by immune22,23 and stress24-26 signaling); notably, these changes in regulatory activity also occur in a cell-type specific manner27,28. Nonetheless, to date, nearly all evidence of context-dependent genetic regulation reflects studies of blood cells only. Here we describe the mechanisms by which regulatory sequences result in distinct patterns of neuronal gene expression across stress and inflammatory cues.
To test the hypothesis that environmental effects interact with putative regulatory elements to mediate gene expression and impact risk for psychiatric disorders, we characterized thousands of GWAS loci in neurons en masse. Coupling massively parallel reporter assays (MPRAs)29-32 with human induced pluripotent stem cell (hiPSC) models makes it possible to empirically evaluate the regulation of transcriptional activity33,34,35 in live human neurons30,36. We quantified dynamic psychiatric risk loci activity of 240 GWAS loci associated with ten complex brain psychiatric disorders and traits, totaling ~9,000 candidate regulatory risk elements (CREs), in neurons treated with glucocorticoids and cytokines associated with MIA [synthetic cortisol (hCort)4,12,37, interleukin-6 (IL-6)43-46, and interferon alfa-2b (IFNa-2b)41,42]. We uncovered GxE influences that altered the impact of distinct disorder-specific risk elements across contexts. Moreover, we described how stress- and inflammation-dynamic regulatory effects converge on downstream biology to impact shared risk for neuropsychiatric and neurodevelopmental disorders. Altogether, we modelled dynamic prenatal contributions to psychiatric risk that can precede symptom onset and disorder etiology by decades.
Results
Stress and inflammatory factors have shared and distinct neuronal impacts on cellular function and the transcriptome.
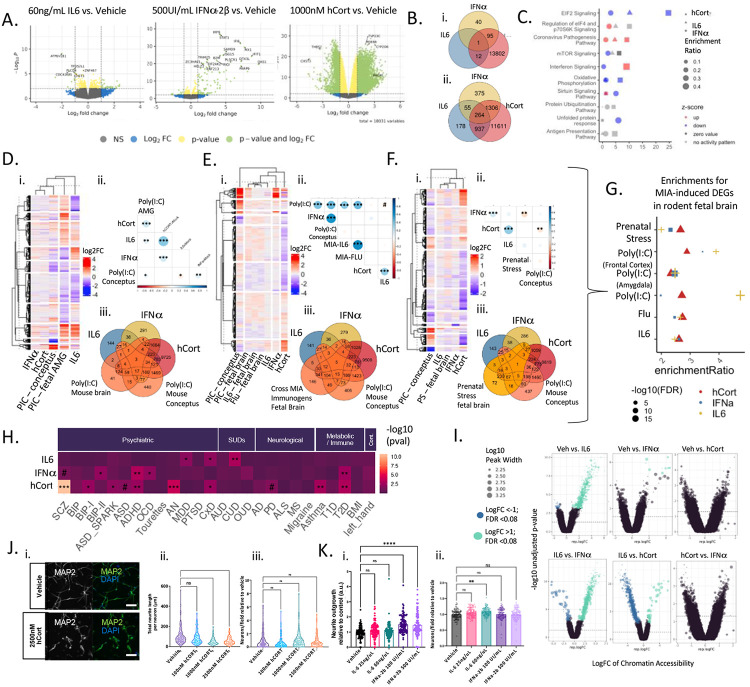
To assess the influence of stress and inflammatory cues on developing human neurons, hiPSC-derived NGN2-induced glutamatergic neurons (iGLUTs)32,33 were acutely (48-hours) treated with hCort (1000nM), IL-6 (60ng/uL), or IFNa-2b (500 UI/mL). hCort significantly reduced neurite outgrowth (D7) (p-value<0.001) and IFNa significantly increased neurite outgrowth (p-value<0.001) (Figure 1j-k). Exposure to hCort, IFNa, and IL6 did not affect neuronal survival (Figure 1j-k) or synaptic puncta density (D24) (SI Figure 4). Differential gene expression analysis revealed that hCort resulted in the greatest transcriptome-wide dysregulation (7332 down- and 5684 up-regulated differentially expressed genes (DEGs)) (FDR<0.05), with more modest effects resulting from IFNa-2b (25 down- and 80 up-regulated) and IL-6 (10 down-regulated genes) (Figure 1A). Reciprocally, chromatin accessibility changes were greatest with IL-6 (675 differentially active regions (DARs); FDR <=0.05), more modest with IFNa (12 DARs; FDR<=0.05) and not significant with hCort (Figure 1D).
Figure 1. Stress and inflammatory factors impacted gene expression, chromatin accessibility, cell morphology, but not survival, in developing neurons.
Differential gene expression analysis in iGLUTs exposed to 1000nM hCort, 60ng/uL IL-6, or 500 UI/mL IFNa-2b (compared to vehicle) revealed unique and shared effects on the transcriptome. (A) 1000nM hCort resulted in transcriptomic-wide dysregulation, while the effects of 60ng/mL IL-6 produced moderate downregulation of few genes, and IFNa-2b resulted in strong upregulation of select immune-related genes. (B) Overlapping (i) significant (FDR<0.05) and (ii) nominally significant (unadjusted p-value<0.05) DEGs by cue. (C) Signed pathway enrichment of cue-specific transcriptomic dysregulation (DEGs; unadjusted p-value<0.05) (Supplemental Data 2). (D-F) Gene expression changes in the mouse amygdala, frontal cortex, and whole brain following Poly(I∣C), IL6, or H1N1-induced MIA significantly correlated with transcriptomic signatures of IL-6 and hCort. (i) Heatmap of log2FC across shared genes. (ii) Pearson’s correlation of log2FC across cue-exposures and MIA-induced mouse brain DEGs (# = FDR<0.08; * = FDR<0.05; ** = FDR<0.01; *** = FDR<0.001). (iii) Overlap of nominal significantly different DEGs and MIA-induced mouse brain DEGs (G) Enrichment of cue-specific dysregulation (DEGs; unadjusted p-value<0.05) for MIA-induced fetal brain DEGs across MIA rodent models. (H) Enrichment of cue-specific transcriptomic dysregulation (DEGs; unadjusted p-value<0.05) for GWAS risk genes across psychiatric, neurological, and immune traits. # = FDR<0.08; * = FDR<0.05; ** = FDR<0.01; *** = FDR<0.001. (I) Differential peak accessibility analysis in iGLUTs exposed to 1000nM hCort, 60ng/uL IL-6, or 500 UI/mL IFNa-2b (compared to vehicle) showed significant decreases in chromatin accessibility following exposure to IL-6. (J) Dose-dependent impact of exposure of hCort, IL-6, and IFNa-2b on early (D7) neurite outgrowth in iGLUTs. (i-iii) Exposure of 1000nM and 2500nM hCort significantly decreased early neurite outgrowth while exposure to (K) (i-iii) 100 UI/mL and 500 UI/mL IFNa-2b increased neurite outgrowth, relative to vehicle control conditions. N = minimum of 2 independent experiments across 2 donor lines with 12 technical replicates per condition and 9 images analyzed per replicate. One way ANOVA with post-hoc Bonferroni multiple comparisons test. * = p<0.05; ** = p<0.01; *** = p<0.001; **** = p<0.0001.
Stress and inflammation DEGs (unadjusted p-value <=0.05) were enriched for GWAS risk genes across complex brain and immune disorders; each exposure revealed unique disease enrichment profiles (SI Tables 1-2). Beyond GWAS, there were shared and unique significant enrichments in genes associated with neurological, neurodegenerative, and neurocognitive disorders across exposures (Supplemental Table 1-2). Shared enrichments were occasionally predicted to have opposing effects—for example, while all cues were enriched for dementia, tauopathy, and Alzheimer’s Disease (AD), hCort exposure decreased activity of these and related pathways [Alzheimer’s disease; Alzheimer’s disease or frontotemporal dementia; Degenerative dementia (FDR<=0.01, Activation z-score=−1); Dementia (FDR<=0.01, Activation z-score=−1.02); Tauopathy (FDR<=0.01, Activation z-score=−0.447)] and uniquely activated context memory (FDR<=0.01, Z=2.178).
Transcriptomic signatures following exposure to hCort, IL-6, and IFNa-2b resembled fetal expression signatures from four rodent models of MIA and prenatal stress: poly(I∣C) (conceptus, whole brain, amygdala, and frontal cortex)43,44,45, IL-6 (whole brain)44, H1N1 Flu virus (whole brain)44, and chronic unpredictable maternal stress (whole brain)46. hCort, IFNa-2b, and IL-6 DEGs were correlated to (Figure 1D-F) and significantly enriched for (Figure 1G) MIA-induced DEGs across immunogens and tissues. Meta-analysis across contexts revealed convergent changes of gene expression in the same direction across all conditions: 258 genes down- and 352 genes were upregulated change (meta-analysis FDR<=0.05) (SI Figure 3B). Pathway analysis47 contextualized the impact of nominally significant DEGs (unadjusted p-value<=0.05); for example, across exposures, DEGs were enriched for mTOR signaling [hCort(−log(p)=2.08, Z=−0.71); IFNa-2B(−log(p)=6.25, Z=0; IL-6(−log(p)=3.7, no activity pattern] and decreased oxidative phosphorylation pathways [hCort(−log(p)=1.32, Z=−3.4); IFNa-2B(−log(p)=6.66, Z=−4.9; IL-6(−log(p)=1.15, −3] (SI Figure 3C, SI Table 2), pathways associated with MIA and linked to neuronal survival and the regulation of synapse formation, growth, and survival.
Stress and inflammatory factors dynamically and specifically impacted allelic shifts in neuronal transcriptional activity.
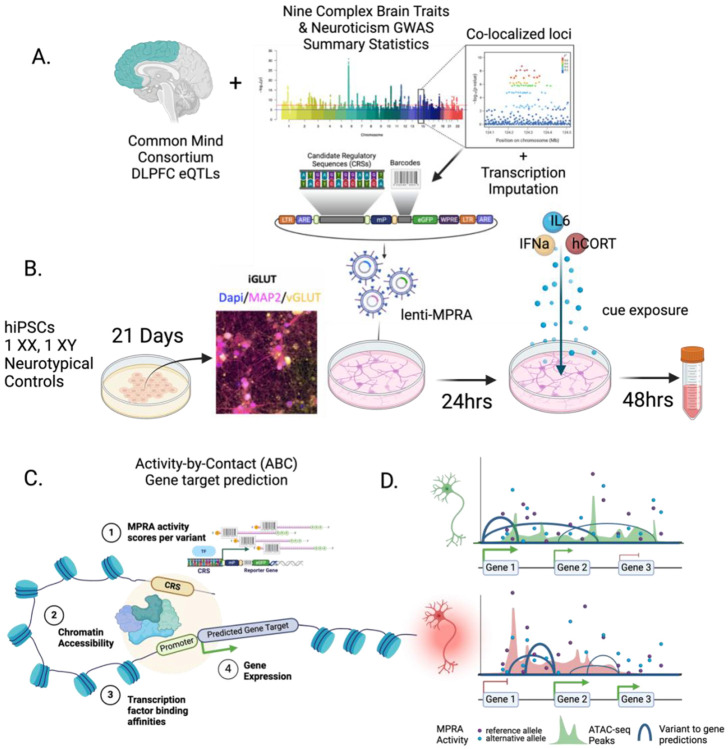
We designed a Lenti-MPRA31 library through statistical fine-mapping of ten psychiatric GWAS: Alzheimer’s Disease (AD)48, Attention Deficit hyper-Activity Disorder (ADHD)49, Anorexia Nervosa (AN)50, Autism spectrum disorder (ASD)51, Bipolar Disorder (BIP)52, Major Depressive Disorder (MDD)53, Obsessive Compulsive Disorder (OCD)54, Post traumatic stress disorder (PTSD)55, and Schizophrenia (SCZ)56, as well as quantitative measurement of the personality trait, neuroticism (NEU-P)57 (SI Table 1) using two complimentary methods incorporating dorsolateral prefrontal cortex (DLPFC)8 expression quantitative trait loci (eQTLs): Bayesian co-localization (coloc258,59) and transcriptomic imputation (S-PrediXcan60,61) (SI Figure 1-2; SI Table 1-3). In total, from 240 GWAS loci, our Lenti-MPRA library included ~9,000 SNPs, represented as ~4,500 biallelic candidate regulatory elements (CREs).
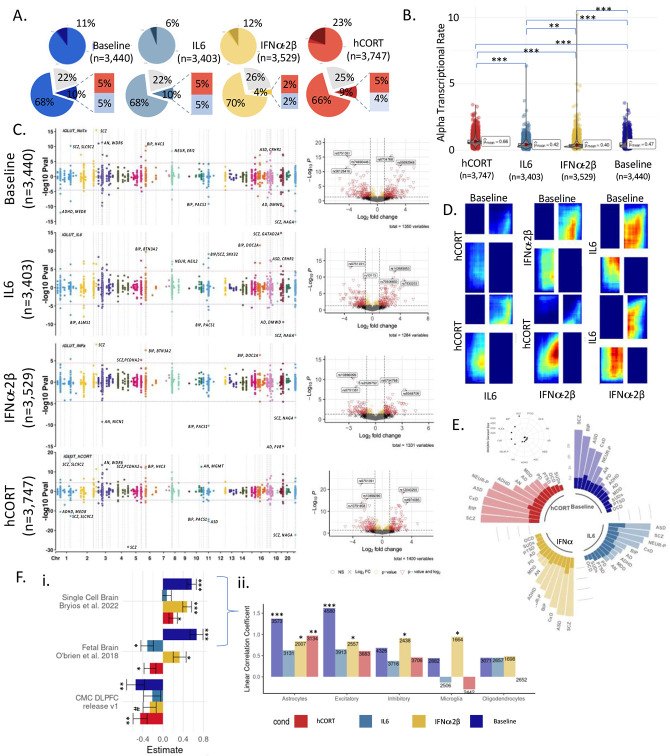
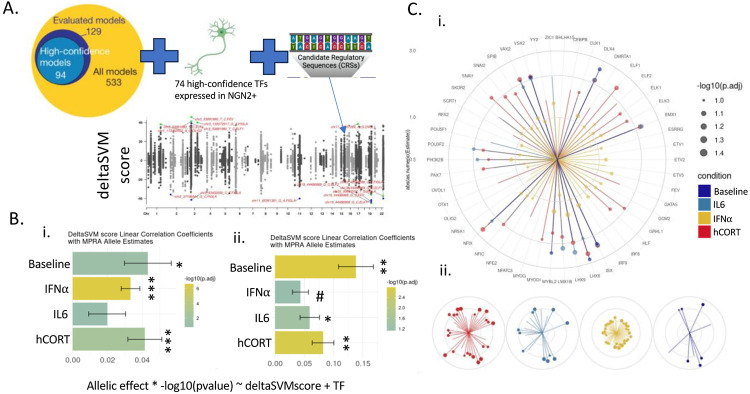
Glutamatergic neuron promoter and enhancer regions62 and gene expression63 are highly enriched across multiple psychiatric and behavioral traits. MPRAs were performed in iGLUTs induced from two control donors, with two biological replicates each, exposed to acute (48 hours) hCort (1000nM), IL-6 (100ng/mL), IFNa-2b (500 UI/mL), or matched vehicles, 24 hours following Lenti-MPRA transduction. Paired MPRAs in iGLUT neurons with or without exposure resolved the context-specific regulatory activity of 3400-3747 variants. Across MPRAs, activity between replicates and donors was highly correlated (SI Figure 5-6). At baseline, 11% of all captured CRE (N=363) were significantly (FDR<0.1) transcriptionally active compared to scramble sequences (Figure 2A), comparable to previous eQTL-based MPRAs64. Mean alpha transcriptional activity varied between cues [Fwelch (3,7805.15) =517.79, p<0.001, nObs=14,119], with hCort most increasing overall transcriptional activity (23% of CRE; N=850) (Games-Howell Pairwise comparison of hCort to baseline, Holm-adjusted p-value=2.43e-11) (Figure 2B), and IL-6 and baseline being most similar (rank-rank hypergeometric overlap (RRHO) across all CRE) (Figure 2D). The majority of top significant CRS were unique to each cue exposure (Figure 2C; SI Fig 7). Moreover, active CRE showed cue-specific enrichments for psychiatric disorder GWAS risk loci, despite each having the same proportion of tested CRS by prioritization GWAS (Figure 2E; SI Figure 6).
Figure 2. Inflammatory and stress factors altered allele-specific regulatory activity of risk-CREs and were uniquely enriched for psychiatric disorders.
(A) ~3440 variants were captured and tested at baseline, with an average transcriptional activity of 11%. The average percent of significantly active candidate regulatory elements (CRE) varied by cue exposure. Top circle represents the proportion of significantly active CRE (FDR<=0.1) across conditions (dark blue=baseline, teal=IL-6, golden yellow=IFNa-2B, red=hCort). (B) Exposure to different cues resulted in significantly different levels of mean transcriptional activity across all CRE tested, with hCort resulting in the largest increase in mean-transcriptional rate. (C) Top allelic shifts by disorder, locus, and SNP showed dynamic cue-specific effects on genetic regulation. Manhattan plots of differential allelic shifts (ref vs. alt) across cue-exposures with loci labeled by PrediXcan/Coloc2 associated disorder eGenes. Each point represents an individual tested SNP at a given locus with chromosome location on the x-axis and the signed −log10 p-value of differential transcriptional activity between the reference and alternative alleles on the y-axis. Volcano plots demonstrate allelic shifts (ref vs. alt) across cue-exposures labeled by top SNPs. (D) Rank-rank hypergeometric test of transcriptional activity across cues: IL6 exposure is most highly correlated with activity at baseline, while hCORT exposure is the least correlated . (E) Significant CRE show cue-biased enrichments for GWAS risk SNPs across psychiatric disorders, scaled and ordered by −log10 (FDR). (F) (i) Enhancer activity of regulatory elements tested in iGLUTs are significantly positively correlated with brain single cell and the fetal postmortem brain eQTL effects, but significantly negatively correlated adult DLPFC eQTL effects. (ii) Linear coefficients of single cell eQTLs with MPRA activity by cell-type shows that, at baseline, activity of CREs significantly recapitulates eQTL effects in excitatory neurons, while IFNa and hCORT exposure recapitulate effects in astrocytes, and IFNa exposure uniquely recapitulates these effects in microglia. Bar chart of linear correlation coefficients of eQTL betas [abs(MPRA allelic shift) ~ abs(eQTL Beta) + log distance to the transcriptional start site + Gene/Transcript + CellType]. (FDR<=0.08#, FDR<0.05*, FDR<0.01**, FDR<0.001***).
A single variant change frequently influenced dynamic regulatory activity, with significant allelic shifts between baseline and IL-6 (10% CRE), hCort (9%), or IFNa-2b (4%) (Figure 2A;). Most of these allelic shifts were context-specific (Figure 2, SI Figure 7). These allelic-shifts recapitulated eQTL effects, with allelic shifts significantly positively associated (FDR<0.05) with single-cell brain eQTLs16 (baseline, IFNa, hCort) and fetal brain eQTLs65 (baseline, IFNa). Notably, allelic shifts across all conditions were negatively associated with adult DLPFC eQTLs, significantly so at baseline and following hCort exposure (Figure 2Fi-ii).
CTCF binding negatively influenced transcriptional activity in a cue-specific manner.
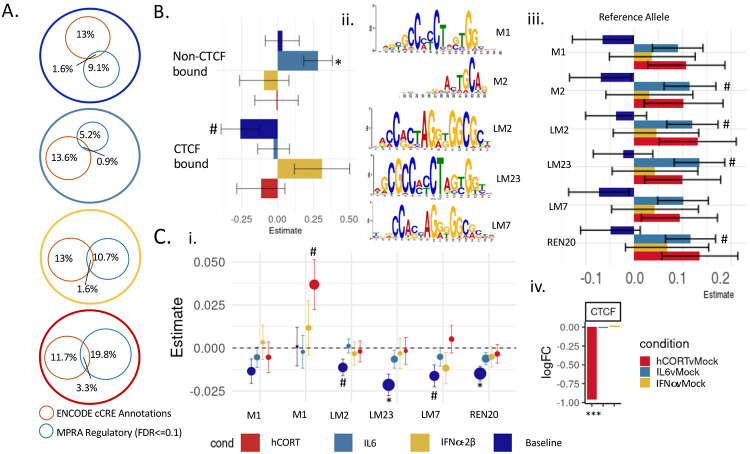
The majority of active CRE identified by MPRA were novel and not previously annotated (Figure 3A). Baseline MPRA CRE activity was inversely associated with binding of CCCTC-binding factor (CTCF), with active CREs disproportionately overlapping non-CTCF bound enhancers (SI Figure 9A), and non-CTCF-bound sequences having significantly higher average activity of than CTCF-bound regions (SI Figure 9B). There was likewise a nominally significant negative relationship with annotated CTCF binding sites and transcriptional activity (linear correlation coefficient = −0.25, unadjusted p-value<=0.05) (Figure 3B). Quantitative CTCF motif binding scores across highly conserved motifs [FDR<0.05 (LM23, REN20); unadjusted P<0.05 (LM2, LM7)] revealed negative associations with transcriptional activity at baseline (Figure 3Bi-ii).
Figure 3. CTCF binding negatively influenced transcriptional activity in a cue-specific manner.
Baseline activity of CTCF-bound sequences was significantly reduced related to non-CTCF-bound regions (SI Figure 6). (A) Percent overlap between MPRA CRE with known ENCODE cCRE annotations (orange circle) and significantly active CRE (FDR<0.1) (blue circle). Active MPRA CRE were not enriched for ENCODE cCREs, with only 1-3% previously annotated as regulatory elements. The proportion of active MPRA CRE overlapping with known proximal or distal enhancer- or promoter-like sequences shifted with exposure to stress and inflammatory factors. (B) Linear correlation coefficients for CTCF-binding showed significant negative correlations of CTCF binding enhancer-like regions at baseline, and a significantly positive association of non-CTCF bound enhancer-like regions with IL-6-responsive activity (MPRA Median Z-score ~ CTCFe + nonCTCF + nonoverlap) (C) Six highly conserved core CTCF binding motifs best match active CRE sequences (Supplemental Data 1). (i) At baseline, motif scores for two (LM7, LM2) were nominally and two (LM23, REN30) were significantly negatively associated with transcriptional activity. A separate motif (M1) was nominally positively associated with transcriptional activity after hCort exposure. (ii) Motif logos of five highly conserved CTCF binding motifs tested in (i). (iii). Linear coefficients for allele effects. (iv) CTCF is significantly downregulated following exposure to 1000nM in iGLUTs. (MPRA median z-score ~ CTCF motif score + orientation + allele + distance from center SNP + motif; estimates for orientation are reported in SI Figure 8). (FDR<=0.08#, FDR<0.05*, FDR<0.01**, FDR<0.001***).
Conversely, stress and inflammatory MPRA CRE activity was more likely to be CTCF-independent and/or mediated by CTCF interactions distinct from those identified at baseline. There was a significant positive association between a lack of CTCF-binding and transcriptional activity following IL-6 exposure (linear correlation coefficient = 0.3, FDR<=0.05) (Figure 3B), although the proportion of active CRE overlapping with non-CTCF-bound enhancers still exceeded CTCF-bound enhancer-like sequences (SI Figure 9A). CTCF expression was significantly downregulated following hCort, and a unique CTCF motif (M1) was nominally positively associated with hCort-responsive transcriptional activity (Figure 3Ci-ii).
While CTCF binding may partially mediate cue-specific genetically regulated expression of brain disorder risk loci, only a subset of the CRE overlapping with annotated CTCF binding regions genome-wide (n=~220-250) were significantly active by MPRA (n=18-60). Thus, we next sought to resolve additional transcriptional regulators mediating dynamic regulation of gene expression.
Risk variants disrupted transcription factor binding to underly GxE specific activity.
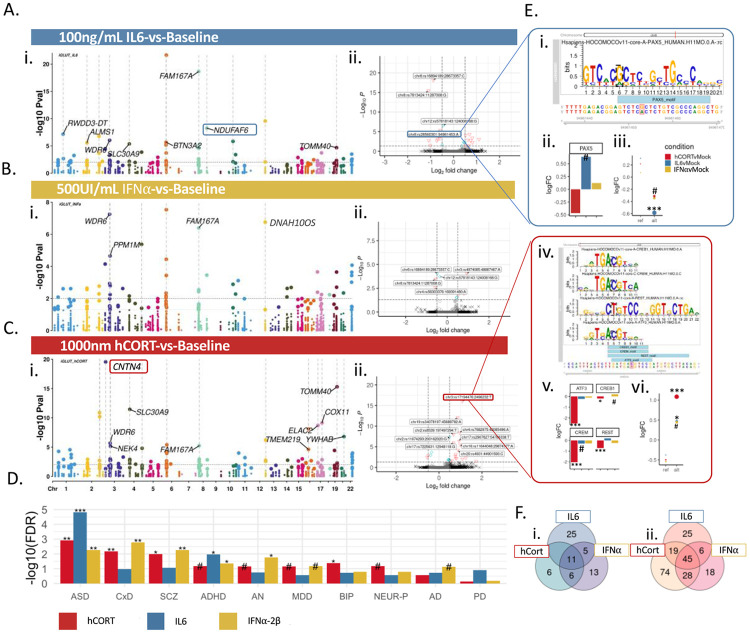
Direct comparative analysis of transcriptional activity revealed cue-by-variant specific effects (Figure 4A-C); many CREs showed disproportionately increased activity relative to baseline (hCort: 166 nominally significant CRE (p<0.05) showed increased activity, 25 decreased activity; IL-6: 95 increased, 41 decreased; IFNa-2b: 97 increased, 37 decreased). Altogether, 45 CREs were consistently up-regulated and 11 down-regulated across all cues (Figure 4F). The top significant GWAS-risk enrichments of differentially active allele-specific CREs differed by exposure, with non-specific psychiatric enrichments for hCort (ASD FDR=1.2e-03; ratio=15.5; CxD FDR=6.8-03, ratio=1.75; SCZ FDR= 1.04e-02, ratio=1.3) and IFNa-2b (ASD FDR=5.4e-03; ratio=13.7; CxD FDR=1.7e-03, ratio=1.98; SCZ FDR=5.4e-03,ratio=1.4) and more specific ASD/ADHD enrichments strongest for IL-6 (ASD FDR=1.49e-05; enrichment ratio=24.5; ADHD FDR=1.07e-02; ratio=7.4) (Figure 4D).
Figure 4. Cue-specific differences in active CREs were driven by transcription factor binding affinities.
Stress and inflammatory factors dynamically and specifically impacted allelic shifts in transcriptional activity of CREs in iGLUTs. (A-C) Manhattan plots of differentially active CREs between each cue-exposure and baseline labeled by PrediXcan associated disorder and eGenes. Each point represents an individual tested SNP at a given locus with chromosomal location on the x-axis and the −log10 p-value of differential transcriptional activity between cue-exposure and baseline on the y-axis. (D) Differentially active CRE enrichments across GWAS psychiatric disorder and neurodegenerative disease risk SNPs. For each cue exposure, different disorders showed top enrichments (FDR<=0.08#, FDR<0.05*, FDR<0.01**, FDR<0.001***). (E) Motif-binding was significantly impacted by variant shifts at SNPs with cue-specific responses. Example of two SNPs with cue-by-variant specific effects with variant-specific TF binding affinities: (i) MotifBreaker image of the regulatory sequence centered at rs28560301 in the AD-associated NDUFAF6 locus, showing PAX5, which has decreased predicted binding affinity with the risk allele. (ii) Cue-specific differential gene expression; PAX5 expression is increased following IL-6 exposure. (iii) Cue-specific difference in CRE activity compared to baseline of represented SNP; rs28560301 risk allele is significantly decreased follow IL-6 exposure compared to baseline. (iv) Transcriptional activators (CREB, CREM, ATF3) have increased binding affinities for the reference allele, while REST, a master transcriptional silencer, has increased binding to the risk allele. (v) CREB, CREM, ATF3, and REST were significantly downregulated following hCort exposure. (vi) SCZ-associated alternative allele (rs1719446) were significantly more active following hCort exposure. (F) Venn diagram of overlapping nominally significant (p-value<=0.05) CRE with increased activity (i) or decreased activity (ii) by condition.
Given the dysregulation of transcription factor (TF) expression following stress and inflammatory exposures (SI Figures 8,11), we speculated that TFs mediate cue-specific genetic regulation of gene expression. Motif enrichment analysis indeed uncovered shared and unique TF binding motifs for significantly active CRE across exposures, many of which were TFs differentially expressed between exposures (SI Figure 8). To identify the top TFs most likely to have variant specific binding across CREs, allele-specific TF binding affinities centered at the variant of interest were resolved using local differential enrichment for known motifs (CentriMo66) (SI Figure 8). MXI1, POU3F2, and AHR binding motifs were uniquely differentially enriched in IL-6, IFNa-2b, and hCort respectively; ARNT and EPAS were differentially enriched across all cues. Many enriched TFs were associated with complex brain disorders, infection, and autoimmune disease67 and tended to be important for neurodevelopment and/or neuron-specific (SI Figure 8). We further explored cue-by-variant specific regulation for top differentially active regulatory elements individually (MotifBreaker68), identifying multiple CREs were binding affinity of TFs with cue-dynamic expression differed between the alternative and reference alleles. (Figure 4E).
MPRA TF affinity binding patterns with variant-specific transcriptional activity were predicted using differential TF binding affinity prediction scores (deltaSVM69-71) (Figure 4Bi; Figure 5A;T SI Figure 8), revealing a significant association of cue-specific allelic shifts (Figure 5Bii), with distinct TFs across exposures (Figure 5C). Top TFs were enriched for neurogenesis (FDR=8.06e-3), forebrain development (FDR=3.37e-2), and neural crest differentiation (FDR=8.58e-4) and many were previously linked to risk across complex brain disorders. For example, CUX1, ESRRG, HLF, SCRT1 are primary targets of has-miR-137 (FDR<0.05) – a key miRNA regulating expression of SCZ risk genes. LHX6 and SPIB, and ELK1 and RFX2, are targets of primary non-synonymous SCZ and ASD rare variants, respectively and YY2 has been previously implicated in the regulation of PTSD risk.
Figure 5. Variant-specific changes in transcription factor binding affinities predicted cue-specific allelic shifts in transcriptional activity.
(A) DeltaSVM quantifies the effect of single nucleotide sequence changes on transcription factor binding activity. Using 74 high-confidence TFs (Yan et al. 202169) expressed in iGLUTs, DeltaSVM scores were calculated for each CRE. Manhattan plot of deltaSVM scores for each CRE comparing reference to alternative allele. (B) DeltaSVM scores significantly positively correlated with allele-specific activity quantified in the MPRA. DeltaSVM score linear correlation coefficients with MPRA allelic shifts (i) before and (ii) after restricting to regions with predicted allelic preference by deltaSVM (Allelic effect * −log10(pvalue) ~ deltaSVMscore + TF model). (C) Specific TF binding affinities model predicted allele-specific activity by exposure. (i) TF linear correlation coefficients by condition. Circular segments represent the absolute value of TF-factor specific linear correlation coefficients, the size of the dot represents the −log10 FDR of the estimate. Colors indicate cue-specific activity (dark blue=Baseline, teal = IL-6, golden yellow=IFNa-2b, dark red=hCort). (ii) TF binding affinities explain, in part, cue specific changes in transcriptional activity. (FDR<=0.08#, FDR<0.05*, FDR<0.01**, FDR<0.001***).
Gene targets of context-specific regulatory elements showed distinct associations with brain disorders.
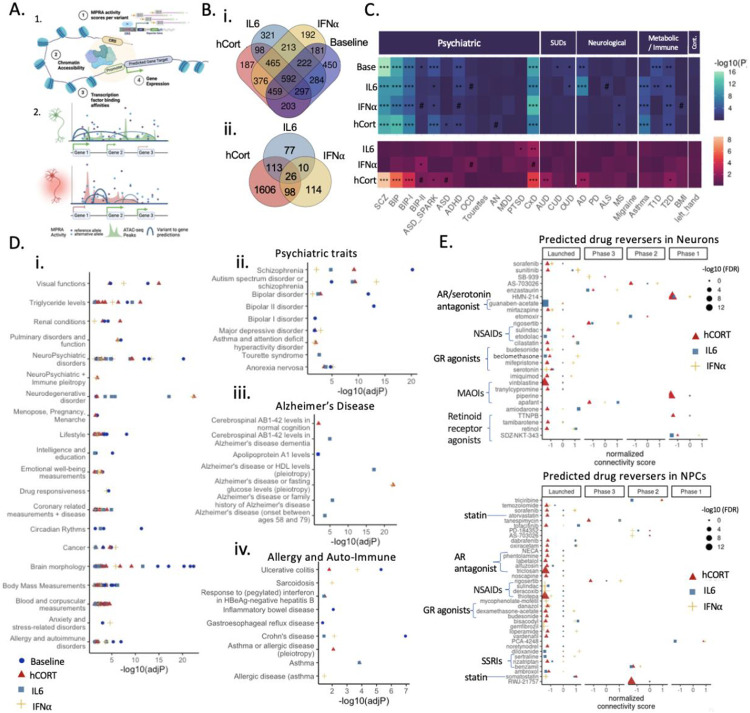
Target genes directly regulated by cue-specific genetic regulation were predicted using an adapted activity-by-contact (ABC79) model that incorporates TF binding affinities (STARE72,73), thereby integrating dynamic MPRA and cue-specific chromatin peak calls in matched iGLUTs. Shared and unique cue-specific gene targets were identified across contexts (Figure 6A-B), revealing significant overlap between enhancer-to-gene mapping (ABC) and the original eGene predictions (S-PrediXcan and Coloc2) (Table 1). Across all MPRA CREs, 5157 unique ABC genes with high interaction scores (ABC score >=0.8) were identified, 95 of which were amongst the 168 eGenes predicted by S-PrediXcan/Coloc2 that were also expressed in iGLUTs (hypergeometric test for enrichment, p-vale=1.5e-16) (Table 1). ABC genes and eGenes were found in cue-responsive DEGs; 45 DEGs were both (hypergeometric test; p-value=1.9e-8) (Table 1). Whereas FURIN, associated with BIP and SCZ, or AP2A2, associated with AD, were both predicted targets of active MPRA CREs at baseline only, IRF2BP1, TOMM40, and EML2 were specifically predicted to be targets of IL-6-responsive CREs. Gene targets unique to cue-specific regulatory elements (ABC genes of active MPRAs CRE with differential activity between one or more cues and baseline; FDR<=0.1) revealed unique gene ontology (GO) enrichments for proteasome complex pathways, clathrin coating, and the nuclear speck (hCort) and apical dendrite, G-protein alpha-subunit binding, and mitochondrial function (IL-6) and ganglion development and cytoplasmic translation (IFNa-2b) (SI Figure 12).
Figure 6. Gene targets of context-specific regulatory elements showed distinct associations with brain disorders and medications.
(A) Workflow of activity-by-contact enhancer-gene interaction scoring (made with BioRender). (1.) By integrating cell type and cue-specific MPRA enhancer activity, cell and cue-specific chromatin accessibility, and transcription factor binding affinities we (2.) predict cell-type and cue-specific genetically regulated genes (ABC genes) (B) Predicted ABC gene targets of active CREs across cues. Venn diagrams of overlapping ABC genes mapped to (i) active significantly enhancers across cue-exposures (FDR<0.1) and to (ii) differentially active CRE (nominal p-value <=0.05) by cue compared to baseline. (C) MAGMA enrichment analysis across psychiatric, substance use (SUD), neurological, and metabolic/immune disorders revealed unique association of cue specific genetically regulated genes with disorder risk. (D) GWAS catalogue over representation analysis (i) identified unique and shared trait enrichment across cues, including enrichments for psychiatric (ii), neurodegenerative (iii), and allergy/autoimmune enrichments (iv) (Supplemental Data 2). (E) Cue-specific ABC target genes resolved shared and unique drug reversers. (FDR<=0.08#, FDR<0.05*, FDR<0.01**, FDR<0.001***).
Cue-specific ABC gene targets were uniquely enriched for risk genes associated with complex brain disorders and common comorbid metabolic and immune syndromes (MAGMA74). For example, IFNa-2b-specific gene targets highlighted BIP sub-type II, whereas hCort was broadly linked to SCZ, BIP, and BIP sub-type I risk genes, and IL-6 to PTSD risk genes (Figure 6C). Likewise, shared and unique enrichments for neuropsychiatric, neurodegenerative, and emotional well-being, as well as allergy and autoimmune disorder-related GWAS risk genes, were revealed via over representation analysis across GWAS catalogue gene sets (FUMA75) (Figure 6D). ABC genes across all contexts were significantly enriched for SCZ, ASD, and AN, whereas IL-6 ABC genes were uniquely enriched in numerous measurements relating to Alzheimer’s disease.
Drugs predicted to reverse cue-specific signatures in neural cells (defined as the z-scored ABC activity score of reference versus alternative alleles, using cMAP query tool76) and then filtered based on clinical trial phase included targets of glucocorticoid, androgen, and serotonin receptors, as well as drugs regulating inflammatory signaling (statins and non-steroidal anti-inflammatory drugs (NSAIDs)) (Figure 6E). Cue-specific signatures were reversed by drugs modulating serotonin – IFNa-specific signatures were reversed by treatment with serotonin itself while monoamine oxidase inhibiters (MOAIs) and the serotonin reuptake inhibitors (SSRIs) sertraline reversed hCort-specific and IL6-specifc transcriptomic signatures respectively. This highlights the importance of context in predicting targets of risk-associate genetic regulation and for precision pharmacology. Notably, FDA approved statins reversed the hCort-specific (atorvastatin) and INFa-specific (somatostatin) signatures, which is notable given that patients with SCZ, mood disorders, and AD often have reduced CSF (cerebral spinal fluid) and brain (DLPFC, hippocampus) somatostatin levels77. Somatostatin-positive interneurons are associated with cognitive deficits in schizophrenia78, where adjunctive therapy with statins improved symptoms and cognition in SCZ patients79,80.
Discussion
This work empirically annotated the functional impact of thousands of variants across hundreds of brain disorder GWAS loci, demonstrating that many variants could only be resolved under cue-responsive conditions. Treatment of human neurons with stress and inflammatory cues associated with prenatal exposures altered genetic regulation of expression of genes linked to a psychiatric and neurodegenerative conditions. From ~3,400 common-risk variants associated with ten complex brain disorders and traits, 10-11% were active at baseline and following IFNa exposure, while IL6 reduced overall transcriptional activity (6%) and hCort increased activity (23%). Across cues, MPRA validated risk-associated eQTLs and identifying novel regulatory elements—only 15% of tested CREs overlapped with annotated cCREs81,82; just 1-3% of annotated cCREs showed activity in our neuronal MPRAs, with active CRE not significantly enriched for annotated ENCODE cCREs81,82. This lack of overlap was observed in other studies and highlighted the importance of cell-type specificity64. The proportion of proximal or distal enhancer-like sequences in active MPRA CREs varied between contexts, suggesting a role for CCCTC-binding factor (CTCF) in cue-specific regulation; CTCF binds to DNA, forms chromatin loops, and regulates gene expression by promoting distal enhancer-dependent gene activation83. While CTCF binding was modestly negatively correlated with cue-specific activity at baseline, many TFs were strongly enriched for dynamic transcriptional regulation, with predicted potential downstream effects on neurodevelopment, synaptic plasticity, and immune response processes. As a simple example, expression of PAX5, a transcriptional activator and important regulator of neurodevelopment84,85 associated with multiple psychiatric traits84,86,67, showed upregulated expression following IL-6 exposure, with IL-6-responsive CREs likewise enriched for the Pax5 motif. At the AD-associated NDUFAF6 locus, PAX5 has decreased predicted binding affinity with the risk allele (rs28560301); IL6-exposure increased PAX5 expression and significantly decreased risk allele activity (Figure 4Ei-iii). More complex co-regulation occurs at the CNTN4 locus, where the SCZ-associated alternative allele (rs1719446) was significantly more active following hCort exposure: several transcriptional activators (CREB, CREM, ATF3) with increased binding affinities for the reference allele were significantly downregulated following hCort exposure; reciprocally, REST, a master transcriptional silencer, had increased binding to the risk allele and was also downregulated following hCort. Altogether, hCort-specific activity of this CRE may be mediated by both decreased expression of transcriptional activators and increased expression of transcriptional repressors, which showed differential allele-specific binding (Figure 4Eiv-vi). We posit that differential TF binding to risk-associated variants underlies dynamic GxE interactions, with cue-specific enrichments for psychiatric risk.
In modelling the GxE impact of maternal stress and inflammation in utero, we selected three MIA and psychiatric-risk associated immune molecules (IL643-46, IFNa-2b41,42, and synthetic cortisol4,12,37) capable of transfer across blood brain barrier (BBB)6,7 and modulation of the hypothalamic-pituitary-adrenal axis (HPA) signaling. The influence of pro-inflammatory environmental factors on brain-related regulatory elements may explain, in part, the biological mechanisms through which immune signaling contributes to increased susceptibility for complex brain disorders. Specifically, cytokine and glucocorticoid signaling can interact with genetic risk to mediate the genetically regulated gene expression22,23,25,87,88, potentially contributing to the increased relative risk associated with stress and inflammation. Physiological and behavioral outcomes in rodent MIA models are sensitive to immunogen-type, exposure duration, and developmental timepoint89. Moreover, indirect mechanisms (e.g., changes to the microbiome) may facilitate immune activation and lead to alterations in brain-wide connectivity90-92. Therefore, the complexity and variability of human prenatal stress and rodent MIA are impossible to fully reproduce in a controlled in vitro model. Future MPRA incorporating longitudinal, repeated dosing, and recovery windows following exposure will be crucial for understanding how type, duration, and timing of immune activation during fetal development contributes to risk.
Developmental patterns of TF expression and enhancer activity may in part underly critical periods of heighted susceptibility to environmental stressors. Previous MPRAs identified time-point specific enhancers regulating neurodevelopment30,36. Likewise, temporally-dynamic and age-specific eQTLs highlight the importance of neurodevelopment and aging on genetically regulated gene expression93-95. Enhancer activity of regulatory elements tested in iGLUTs are significantly positively correlated with eQTL effect sizes from single cell data16 and the fetal postmortem65 brain, but, negatively correlated with adult DLPFC eQTLs96. Indeed, although stronger positive associations in fetal datasets relative to adult brain is not surprising, the reversal of the association supports the need to increase age-eQTL analyses. Age-eQTLs16,95,97 modify the functional impact of genetic risk throughout life, particularly given the variable age of onset of symptom presentation across psychiatric disorders98. Previous studies have identified time-point specific enhancers regulating development of neuronal progenitor cells using MPRAs . It is likely that shifting developmental patterns of TF expression mediates temporal specific enhancer activity, contributing to critical periods of heighted susceptibility to environmental stressors. Thus, further exploration of the impact of shifting patterns of TF expression on age-eQTLs across neurodevelopment, brain maturation, and aging will inform mapping of brain-related GWAS and will be crucial to improving precision medicine.
Notable technical limitations reduce the broad generalizability of our GxE analyses. First, reflecting the present state of GWAS, the variants tested were identified in exclusively European-ancestry data and excluded the MHC locus. Moreover, even some of the most recent publicly available GWAS remain underpowered, overall biasing the MPRA library towards specific disorders (e.g., Eating Disorders, PTSD and OCD had very few variants included). Given this bias, our finding that IL-6-responsive ABC genes were uniquely enriched for PTSD despite the low proportion of tested variants is particularly surprising (Figure 6B). Second, our library design prioritized brain eQTL from the adult DLPFC, whereas enhancer activity was quantified in hiPSC-neurons that more resemble fetal-like glutamatergic neurons. That our results showed higher concordance with fetal brain, and a lack of concordance to adult DLPFC (Figure 2), suggests that the degree or direction of eGene regulation may be altered by age and environment, in line with previous literature94,95,97. Third, this may be the first neuronal MPRA conducted in multiple donors; while enhancer activity was highly correlated between donors at baseline (SI Figure 6), inter-donor variability in cue-specific transcriptional activity was less so, particularly for IFNa-2b response. We caution that donor genotype and polygenic context may influence functional genomics100,101. Fourth, as MPRA-validated regulatory elements were tested independent from their endogenous context, there was an inherent loss of information regarding chromatin accessibility at the endogenous location102. Here, we address this through the integration of matched cue-specific ATAC-sequencing, RNA-sequencing, and TF binding affinities to predict cell-type and cue-specific gene targets of enhancer activity72,73. These models require additional validation through cue and cell-type specific crisprQTL103 or prime-editing. Fifth, technical limitations in MPRAs restricted tested CREs to relatively short DNA fragments flanking prioritized variants, potentially omitting crucial portions of larger regulatory regions, a limitation addressed by recent MPRA adaptations (e.g., tiling MPRAs and technical advances in oligo synthesis for library design)79,91,92. Finally, drug repurposing databases need to expanded across dose ranges, exposure times, drugs, and cell-types76,104,105 to improve reproducibility (i.e., concordance between cMAP releases 1 and 2 is low105, and both cMAP releases were similarly discordant with a third drug compendium105). Future studies of cue-specific GxE effects across larger libraries of variants, additional cell-types9,12,35 (particularly brain-specific immune cells), contexts, doses, and timepoints, and ultimately within more physiologically relevant brain organoids10,106,107 via emerging single cell MPRA methods108, and across an expanded number of donors via village-in-a-dish109-111, will be crucial for further dissection of how immune activation during fetal development contributes to brain disorder risk.
With broad relevance across complex traits and diseases, we demonstrated that it is critical to experimentally resolve dynamic genetic regulation across exposures in a neuronal context. Downstream target genes of cue-specific CREs were uniquely enriched for complex brain disorders and common comorbid metabolic and immune syndromes. FDA-approved medications were predicted to reverse many GxE regulatory interactions, representing potential novel therapeutic interventions for high-risk individuals immediately following environmental exposures, some of which are already established as safe to take during pregnancy112,113. Thus, the clinical impact of resolving GxE interactions include preventative measures (improved maternal care, public policy to alleviate life stress, early life intervention for high-risk individuals) and care of current patients (drug repurposing, patient stratification by immune status, personalized prescription). For example, cognitive impairment in SCZ has been linked to increased inflammatory cytokines and to imbalances in cortisol114. We identified hundreds of GWAS variants that confer greater susceptibility to complex brain disorders following developmental exposure to stress and inflammation, mapped risk-associated genes, and predicted novel points of therapeutic intervention, altogether informing the influence of GxE interactions on mental health outcomes.
Methods
Selection of predicted cis-expression quantitative trait loci (cis-eQTLs):
Variants selected for inclusion in the MPRA library were prioritized from nine GWAS (AD, ADHD, AN, ASD, BIP, MDD, PTSD, SCZ, and NEU) using Bayesian co-localization (coloc258,59) and transcriptomic imputation (S-PrediXcan60,61) (SI Figure 1). For the former, overlapping significant GWAS loci and Dorsolateral Pre-frontal Cortex (DLPFC)8 eQTLs from the Common Mind Consortium (CMC) were tested for co-localization using coloc259. The most probable causal eQTLs from colocalized loci (PPH4>=0.5) were selected, along with all SNPs in high LD (r2>=0.9). For the latter, all SNPs within the predictor models of Bonferroni-corrected significant trait-associated CMC DLPFC S-PrediXcan genes (p<~4.64x10−6 (0.05/10786)) and all SNPs in high LD (r2 >= 0.9) with them were selected.
50 positive and 50 negative controls were selected from SNPs (i) producing the 50 greatest and 50 least transcriptional shifts based on a previous SCZ and AD MPRA115 and (ii) present in the CMC DLPFC dataset. Additional negative controls were selected from significant BIP GWAS loci that (i) did not colocalize (PPH4 < 0.1) and (ii) were not significant CMC DLPFC eQTLs. While our methods for identification were expression-based and greatly differed from the MPRA used to select the controls, four positive controls were also identified by our methods, most notably rs4702 the top causal SNPs for FURIN expression. After accounting for SNPs identified through multiple strategies, and removal of SNPs with sequences containing restriction digest sites, we synthesized a library of ~4,500 SNPs (~10,000 variants) will Oligonucleotides were synthesized by Agilent and cloned into the lentiMPRA vector81. We performed a power analysis to determine size limitations of our MPRA; with 10000 SNPs, 50 barcodes per SNP, activity standard deviation of 1 (typical range = 0.3-2), and 3 biological replicates, the power of a t-test to detect differential variant shifts of 0.75 or greater (≥ 5 times as much mRNA per input DNA) at a Bonferroni corrected alpha=0.05 level is 100%10.
Lenti-MPRA library preparation and viral titration:
The MPRA library was generated according to published lenti-MPRA protocols with slight modifications31. Briefly, 200 base pair oligonucleotides flanking each prioritized SNP were synthesized by Agilent to create an MPRA library of ~9,500 neuropsychiatric associated variants. The Agilent oligo pool was PCR amplified and a minimal promoter and spacer sequence added downstream of the CRE. Amplified fragments were purified and amplified again for 15 cycles to add a random 15bp sequence to serve as a unique barcode. Barcoded fragments were inserted in the SbfI/AgeI site of the pLS-SceI vector (AddGene #13772) and then transformed into 10-beta competent cells (NEB, C3020) via electroporation. Bacterial colonies were grown overnight on Ampicillin-positive plates and midi-prepped for plasmid collection. The quality of the purified plasmid was evaluated by Sanger Sequencing of 16 colonies at random. CRE-barcode associations were identified by sequencing of the purified plasmid (MiSeq; paired-end; 15milion reads). 2nd-generation lentiviral packaging of the purified plasmid was performed by the viral core at Boston’s Children Hospital. To determine MOI and approximation of appropriate viral volume we infected day 14 iGLUTs (0, 1, 2, 4, 8, 10, 16, 32, 64 μL) with control lentivirus (pLS-SV40-mP-EGFP; AddGene #137724) and harvested for 48hrs later. Following DNA isolation, we performed qPCR to calculate the MOI based the relative ratios of genomic DNA to inserted viral DNA (after subtracting background noise caused by residual backbone DNA).
NGN2-glutamatergic neuron induction of clonalized hiPSC lines32,33.
Clonal hiPSCs from two neurotypical donors of European ancestry with average schizophrenia PRS and no history of psychiatric diagnoses (#3182 (XX) and #2607 (XY)) were generated by lentiviral transduction with pLV-TetO-hNGN2-eGFP-Neo and lentiviral FUW-M2rtTA (Addgene #20342), followed by antibiotic selection and clonal expansion. Stably selected clones were validated to ensure robust cell survival, expression of fluorescent tags, and transgene expression. hiPSCs were maintained in StemFlex™ Medium (ThermoFisher #A3349401) and passaged with EDTA (Life Technologies #15575-020).
On day 1, medium was switched to non-viral induction medium (DMEM/F12 (Thermofisher, #10565018), 1% N-2 (Thermofisher, #17502048), 2% B-27-RA (Thermofisher, #12587010)) and doxycycline (dox) was added to each well at a final concentration of 1 μg/mL. At day 2, transduced hiPSCs were treated with 500 μg/mL G418 (Thermofisher, #10131035). At day 4, medium was replaced including 1 μg/mL dox and 4 μM cytosine arabinoside (Ara-C) to reduce the proliferation of non-neuronal cells. On day 5, young neurons were dissociated with Accutase Cell Detachment Solution (Innovative Cell Technologies, # AT-104), counted and seeded at a density of 1x106 per well of a Matrigel-coated 12-well plate. Medium was switched to Brainphys neuron medium (Brainphys (STEMCELL, # 05790), 1% N-2, 2% B27-RA, 1 μg/mL Natural Mouse Laminin (Thermofisher, # 23017015), 10 ng/mL BDNF (R&D, #248), 10 ng/mL GDNF (R&D, #212), 500 μg/mL Dibutyryl cyclic-AMP (Sigma, #D0627), 200 nM L-ascorbic acid (Sigma, # A4403)). For seeding, 10 μM Thiazovivin (Millipore, #S1459), 500 μg/mL G418 and 4 μM Ara-C and 1 μg/mLdox were added. At day 6, medium was replaced with Brainphys neuron medium with 4 μM Ara-C and 1 μg/mL dox. Subsequently, 50% of the medium was replaced with fresh neuronal medium (lacking dox and Ara-C) once every other day until the neurons were harvested at d21.
Lentiviral infections of iGLUTs and DNA/RNA barcode sequencing.
Day 21 iGLUTs were spinfected (1krcf for 1 hr @37C, slow accel, slow deceleration) with lenti-MPRA library, (based on titrations of the control virus from the Gordon et al. 2020 lentiMPRA Nature Protocol Supplement). 24 hours after spinfections, full media was replaced to remove un-integrated virus. At 48 hours post-infection, cells were treated with context cues or basal media. The number of cells required pre-replicate was calculated according to the Gordon et al. 2020 protocol. On average, 6mil cells were seeded per replicate. 72hrs post-lentiviral-infection, and 48 hrs post-exposure to stress or inflammatory compounds neuronal cells were washed three times and harvested using AllPrep DNA/RNA mini kit (Qiagen) and the libraries prepped as previously described. The libraries were sequenced as paired end reads on a NextSeq 2x50 on S2 flow cell (3.3-4.1 B reads/cell) by the New York Genome Center.
| Compound | Solvent | Supplier | Product # | Conc. |
|---|---|---|---|---|
| Human-recombinant IL-6 | UltraPure H2O | Sigma/Aldrich | GF338 | 60 ng/mL |
| Human-recombinant INFa2-b | UltraPure H2O | Mount Sinai Pharmacy | NDC 0085-4350-01 | 500 IU/mL |
| Hydrocortisone | 17.1 μM EtOH | Sigma/Aldrich | H0888 | 1000nM |
Phenotyping
NGN2-glutamatergic neuron induction of hiPSC-derived NPCs for phenotypic assays32,33.
hiPSCs-derived NPCs were dissociated with Accutase Cell Detachment Solution (Innovative Cell Technologies, #AT-104), counted and transduced with rtTA (Addgene 20342) and NGN2 (Addgene 99378) lentiviruses in StemFlex media containing 10 μM Thiazovivin (Millipore, #S1459). They were subsequently seeded at 1x106 cells/well in the prepared 6-well plate. On day 1, medium was switched to non-viral induction medium (DMEM/F12 (Thermofisher, #10565018), 1% N-2 (Thermofisher, #17502048), 2% B-27-RA (Thermofisher, #12587010)) and doxycycline (dox) was added to each well at a final concentration of 1 μg/mL. At day 2, transduced hiPSCs were treated with 500 μg/mL G418 (Thermofisher, #10131035). At day 4, medium was replaced including 1 μg/mL dox and 4 μM cytosine arabinoside (Ara-C) to reduce the proliferation of non-neuronal cells. On day 5, young neurons were dissociated with Accutase Cell Detachment Solution (Innovative Cell Technologies, #AT-104), counted and seeded at a density of 1x106 per well of a Matrigel-coated 12-well plate. Medium was switched to Brainphys neuron medium (Brainphys (STEMCELL, # 05790), 1% N-2, 2% B27-RA, 1 μg/mL Natural Mouse Laminin (Thermofisher, # 23017015), 10 ng/mL BDNF (R&D, #248), 10 ng/mL GDNF (R&D, #212), 500 μg/mL Dibutyryl cyclic-AMP (Sigma, #D0627), 200 nM L-ascorbic acid (Sigma, # A4403)). For seeding, 10 μM Thiazovivin (Millipore, #S1459), 500 μg/mL G418 and 4 μM Ara-C and 1 μg/mLdox were added. At day 6, medium was replaced with Brainphys neuron medium with 4 μM Ara-C and 1 μg/mL dox. Subsequently, 50% of the medium was replaced with fresh neuronal medium (lacking dox and Ara-C) once every other day until the neurons were harvested at d21.
Neurite analysis:
Day 7 iGLUTs were seeded as 1.5x104 cells/well in a 96-well plate coated with 4x Matrigel at day 3 followed by half medium changes until the neurons were fixed at day 7. At day 5, cells were treated for 48hrs with either hCort (1000nM), IL-6 (60 ng/μL), INFa-2b (500 UI/mL), or matched vehicles. Following cue exposure, cultures were fixed using 4% formaldehyde/sucrose in PBS with Ca2+ and Mg2+ for 10 minutes at room temperature (RT). Fixed cultures were washed twice in PBS and permeabilized and blocked using 0.1% Triton/2% Normal Donkey Serum (NDS) in PBS for two hours. Cultures were then incubated with primary antibody solution (1:1000 MAP2 anti chicken (Abcam, ab5392) in PBS with 2% NDS) overnight at 4°C. Cultures were then washed 3x with PBS and incubated with secondary antibody solution (1:500 donkey anti chicken Alexa 647 (Life technologies, A10042) in PBS with 2% NDS) for 1 hour at RT. Cultures were washed a further 3x with PBS with the second wash containing 1 μg/ml DAPI. Fixed cultures were then imaged on a CellInsight CX7 HCS Platform with a 20x objective (0.4 NA) and neurite tracing analysis performed using the neurite tracing module in the Thermo Scientific HCS Studio 4.0 Cell Analysis Software. 12 wells were imaged per condition across a minimum 2 independent cell lines, with 9 images acquired per well for neurite tracing analysis. A one-way ANOVA with a post hoc Bonferroni multiple comparisons test was performed on data for neurite length per neuron using Graphpad Prism.
Synapse analyses:
Commercially available primary human astrocytes (pHAs, Sciencell, #1800; isolated from fetal female brain) were seeded on D3 at 1.7x104 cells per well on a 4x Matrigel-coated 96 W plate in neuronal media supplemented with 2% fetal bovine serum (FBS). iGLUTs were seeded over the astrocyte monolayer as 1.5x105 cells/well at day 5 post induction. Half changes of neuronal media were performed twice a week until fixation. At day 13, iGLUTs were treated with 200 nM Ara-C to reduce the proliferation of non-neuronal cells in the culture. At day 18, Ara-C was completely withdrawn by full medium change followed by half medium changes until the neurons were fixed at day 21. At day 21, cells were treated for 48hrs with hCort (1000nM), IL-6 (60 ng/μL), IFNa-2b (500 IU/mL), or matched vehicles. Following exposure, cultures were fixed and immune-stained as described previously, with an additional antibody stain for Synapsin1 (primary antibody: 1:500 Synapsin1 anti mouse (Synaptic Systems, 106 011); secondary antibody: donkey anti mouse Alexa 568 (Life technologies A10037)). Stained cultures were imaged and analyzed as above using the synaptogenesis module in the Thermo Scientific HCS Studio 4.0 Cell Analysis Software to determine SYN1+ puncta number, area, and intensity per neurite length in each image. 20 wells were imaged per condition across a minimum of 2 independent cell lines, with 9 images acquired per well for synaptic puncta analysis. A one-way ANOVA with a post hoc Bonferroni multiple comparisons test was performed on data for puncta number per neurite length using Graphpad Prism.
| Antibody | Species | Supplier | Product # | Dilution |
|---|---|---|---|---|
| MAP2 | Ck | Abcam | ab5392 | 1:500 |
| SYNAPSIN1 | Ms | Synaptic Systems | 106 011 | 1:500 |
| Alexa 568 anti-Mouse | Ms | Life technologies | A10037 | 1:500 |
| Alexa 647 anti-Chicken | Ck | Life technologies | A10042 | 1:500 |
Multiple Electrode array (MEA):
Commercially available primary human astrocytes (pHAs, Sciencell, #1800; isolated from fetal female brain) were seeded on D3 at 1.7x104 cells per well on a 4x Matrigel-coated 48 W MEA plate (catalog no. M768-tMEA-48W; Axion Biosystems) in neuronal media supplemented with 2% fetal bovine serum (FBS). At D5, iGLUTs were detached, spun down and seeded on the pHA cultures at 1.5x105 cells per well. Half changes of neuronal media supplemented with 2% FBS were performed twice a week until day 42. At day 13, co-cultures were treated with 200 nM Ara-C to reduce the proliferation of non-neuronal cells in the culture. At Day 18, Ara-C was completely withdrawn by full medium change. At day 26, cells were treated for 48hrs with hCort (1000nM), IL-6 (60 μg/mL), IFNa-2b (500 IU/mL), or matched vehicles. Following exposure, electrical activity of iGLUTs was recorded at 37°C using the Axion Maestro MEA reader (Axion Biosystems). Recording was performed via AxiS 2.4. Batch mode/statistic compiler tool was run following the final recording. Quantitative analysis of the recording was exported as Microsoft excel sheet. Data from 6-12 biological replicates were analyzed using GraphPad PRISM 6 software or R.
Context-cue Specific RNAseq:
RNA Sequencing libraries were prepared using the Kapa Total RNA library prep kit. Paired-end sequencing reads (100bp) were generated on a NovaSeq platform. Raw reads were aligned to hg19 using STAR aligner100 (v2.5.2a) and gene-level expression were quantified by featureCounts101 (v1.6.3) based on Ensemble GRCh37.70 annotation model. Genes with over 10 counts per million (CPM) in at least four samples were retained. After filtering, the raw read counts were normalized by the voom102 function in limma and differential expression was computed by the moderated t-test implemented in limma103. Differential gene expression analysis was performed between each cue and paired vehicle. Bayes shrinkage (limma::eBayes) estimated modified t- and p- values and identified differentially expressed genes (DEGs) based on an FDR <= 0.05 (limma::TopTable)104. GO/pathways were evaluated using Gene-set Enrichment Analysis (GSEA)105. In these analyses, the t-test statistics from the differential expression contrast were used to rank genes in the GSEA using the R package ClusterProfiler106. Permutations (up to 100,000 times) were used to assess the GSEA enrichment P value.
Meta-analysis of gene expression across contexts.
We performed a meta-analysis and Cochran’s heterogeneity Q-test (METAL40) using the p-values and direction of effects (t-statistic), weighted according to sample size across all sets of perturbations (Target vs. Scramble DEGs). Genes were defined as convergent if they (1) had the same direction of effect across cue exposure (2) were Bonferroni significant in our meta-analysis (Bonferroni adjusted p-value <= 0.05), and (3) had a heterogeneity p-value = >0.05 (SI Figure 1D).
Context-cue Specific ATAC-Seq:
Mature neurons were washed with 500uL of PBS (-Ca/-Mg)-0.5mM EDTA per well of a 12-well plate. Then, 300uL dissociation solution (0.042 U/μL papain suspension (Worthington-Biochem LS003126) in HBSS (Thermofisher #14025076)-10mM HEPES (Thermofisher #J61275AE)-0.5mM EDTA (Life Technologies #15575-020), pre-activated at 37C for 5 minutes) supplemented with 0.017U/μL DNase (Thermofisher #EN0521) and 1x Chroman I was added to each well before incubating the plate at 37C for 10 minutes, shaking at 125rpm. 600uL deactivating solution (DMEM-FBS-Chroman I) was then added to each well, and cells were dissociated into single cells by pipetting gently. For each condition, cells from 4 wells of a 12-well-plate were combined into a single 15mL conical tube for higher yield. After spinning at 600g for 5 minutes at room temperature, cells were resuspended in 310uL of DMEM (Thermofisher #10566-016)-10% FBS. Then, the cell suspension was filtered through a 37um reversible strainer and frozen in DMEM-10% FBS-10% DMSO.
ATAC sequencing library prep and sequencing were performed by the Yale Sequencing Core. The adaptor sequence for pair-end sequencing was removed using trim_galore116 and sequencing quality measure by FastQC117 and MutliQC118. Data was aligned with Bowtie2119 against hg38 reference genome including rare SNVs. Mitochondrial reads were removed, sam files sorted and indexed, and converted to compressed BAM files with samtools120 and ATAC peaks called using Genrich121. Differential peak activity analysis was performed with edgeR122,123 and csaw124.
Processing of MPRA sequencing data.
Barcode-CRE association was performed as previously described using the association utility of MPRAflow v2.3.531 (run as:). We demultiplexed the indexed DNA and RNA libraries and generated fastq files with bcl2fastq v2.20 and used the count utility of MPRAflow 2.3.5 with the --mpranalyze flag included (run as: nextflow run count.nf -w – experiment-file –dir –outdir –labels –design –bc-length 14 –umi-length 16) to compute the activity score for each element and produce count files formatted for analysis with MPRAnalyze125. We filtered variants using a minimum threshold of 10 observed barcodes per variant and used a generalized linear model to quantify CRE with significantly greater transcriptional activity compared to scramble controls using MPRAnalyze::analyzeQuantification and MPRAAnalyze::testEmpirical functions. We performed comparative analysis on the normalized counts to identify differences in transcriptional activity between cue exposures and vehicle using MPRAnalyze::analyzeComaprative and MPRAnalyze::LRT function. Variants with an FDR adjusted p-value<=0.1 were considered significantly differentially active.
Comparison of regulatory activity across replicates, donors, and cue exposures.
Transcriptional activity, measured as the normalized log2 DNA and RNA counts per CRE, across conditions is strongly correlated between replicates (Pearson’s rho-correlation 0.98-1.00; SI Figure 4) and the log2 normalized RNA/DNA ratios are strongly correlated between donors (rho=0.50-0.71) (SI Figure 5a). Across conditions 3,440-3747 of CRE were captured (minimum requirement of 10 barcodes each) and the number of barcodes per unique CRE were highly correlated between CRE shared across all conditions (rho 0.968-0.999; nCRE=3139). There was no significant difference in mean number of barcodes per insert or the proportion of CRE by prioritization method or disorder association across the conditions (SI Figure 5b-e).
Analysis of allelic shifts in MPRA activity and comparison to eQTL datasets.
For CRE where both the alternative and reference allele were captured with a minimum of 10 barcodes, we tested for significant shifts in transcriptional activity.-To calculate significant allelic shifts in activity, we tested each allelic pair in a generalized linear model (log2(RNA) ~ log2(DNA) + replicate + barcode + n_bc) as in the saturation mutagenesis analysis from MPRAflow31. Variant differences with an FDR adjusted p-value<=0.05 were considered significantly differentially active. We then tested the correlation between MPRA allelic shifts and post-mortem single cell16, fetal brain65, and the CMC adult DLPFC eQTL betas96 (filtering for significant MPRA activity but including non-significant eQTLs) using a generalized linear model [Abs(MPRA allelic shift) ~ abs(eQTL Beta) + log_Dis_TSS + Gene + CellType]. Linear correlation coefficients with an FDR adjusted p-value of <= 0.05 were considered significant, and coefficients with unadjusted p-values <=0.05 were considered nominally significant.
Functional enrichment of significantly active MPRA variants.
Functional enrichment WebGestalt (WEB-based Gene SeT AnaLysis Toolkit)82. Over-representation analysis (ORA) was performed on all significantly active variants at baseline and after cue-exposures and all significantly differentially active variants for each cue compared to baseline against a list of common variant target genes pulled from PGC-GWAS summary statistics using MAGMA74.
Overlap of active CRE with cCRE ENCODE annotations.
We assessed overlap of active CRE in these MPRA with previously annotated enhancer-like sequences across the human genome (hg38) from the cCRE Encode Registry81,82. To further explore the relationship between CTCF binding and condition-specific transcriptional activity, we scanned each 200bp CRE sequence to identify and score best matches of six highly conserved core CTCF binding motifs (CTCFBSDB2.0)126,127. We assessed the impact of CTCF binding affinities across these motifs on transcriptional activity using a generalized linear model (MPRA median z-score ~ motif binding score + orientation + variant + binding motif distance from SNP). Linear correlation estimates with an FDR adjusted p-value <0.05 were considered significant.
TF motif enrichment analysis
We performed TF binding enrichment across all significantly active CRE for each condition using the MEME Suite Simple Enrichment Analysis (SEA)128 with the Human HOCOMOCO V11 reference. We performed a differential enrichment analysis comparing motif binding in significant CRE following exposure to hCort, IL-6, and INFα with Baseline by setting the control sequences as those significant at baseline. Results were filtered based on expression data of in DIV24 iGLUTs described above.
DeltaSVM and allele-specific binding affinity scoring with MotifBreaker.
We calculated a deltaSVM score69,70 which represent predicted changes in transcriptional activity due to changes in TF binding affinity, across all sequence tested in the MPRA using high-confidence 94 high-confidence SVM models created in from HI-SELEX experiment testing 270 human TFs and 95,886 noncoding variants in the human genome69,70. Of these 94 TFs, 74 are expressed in iGLUTs and used for downstream analyses. To identify allelic effects on TF binding for CRE that were specifically differentially active after an exposure compared to baseline, we used the motifbreakR package68 and filtered for strong allelic effects of TFs expressed in DIV24 iGLUTs.
Activity-by-Contact prediction of context-specific regulatory element target genes.
To predict target genes of condition-specific enhancer activity, we scored enhancer-gene interaction using STARE73. STARE combines an adapted Activity-By-Contact (ABC)72 interaction modeling with TF binding affinities in regions to summarize these affinities at the gene level. For each condition, we used the median z-score of each CRE tested in the MPRA to represent “Activity”, and the condition-specific ATAC-sequencing peaks to represent “Contact” in the model.
Over-representation analysis and biological theme comparison of ABC genes.
To identify pathway enrichments unique to context-specific regulatory activity, we performed biological theme comparison using ClusterProfiler129. And gene set enrichment for GWAS catalogue risk genes using the GENE2FUNC query tool of FUMA GWAS75.
Drug prioritization based on perturbation signature reversal in LiNCs Neuronal Cell Lines:
To identify drugs that could reverse cue-specific GReX predicted by the ABC model, we used the Query tool from The Broad Institute’s Connectivity Map (Cmap) Server76. Briefly, the tool computes weighted enrichment scores (WTCS) between the query set and each signature in the Cmap LINCs gene expression data (dose, time, drug, cell-line), normalizes the WRCS by dividing by signed mean w/in each perturbation (NCS), and computes FDR as fraction of “null signatures” (DMSO) where the absolute NCS exceeds reference signature127. We prioritized drugs that reversed signatures specifically in neuronal cells (either neurons (NEU) of neural progenitor cells (NPCs) with NCS <= −1.00, FDR<=0.05) and filtered for drugs that are currently launched or in clinical trial according to the Broad Institute Repurposing Data Portal.
Supplementary Material
Supplemental Table 1. GWAS studies used to prioritize cross-psych MPRA library.
Supplemental Table 2. Number of prioritized SNPs by colocalization (Coloc2) across GWAS.
Supplemental Table 3. Number of prioritized SNPs by S-PrediXcan across GWAS.
Supplemental Table 4. Number of captured CRE with sufficient barcodes across MPRA.
Supplemental Figure 1. Selection of predicted cis-expression quantitative trait loci (cis-eQTLs).
Supplemental Figure 2. Design of cross-disorder MPRA library.
Supplemental Figure 3. Stress and inflammatory factors associated with MIA significantly dysregulated the transcriptomic in developing iGLUTs.
Supplemental Figure 4. Stress and inflammatory factors altered neurite outgrowth in hiPSC-derived iGLUTs.
Supplemental Figure 5. Correlation between MPRA experimental replicates.
Supplemental Figure 6. Correlation between MPRA donors and contexts.
Supplemental Figure 7. Stress and inflammatory factors uniquely impacted allelic-specific transcriptional activity of CRE in iGLUTs.
Supplemental Figure 8. Transcription factor binding motifs were differentially enriched in active CRE based on context.
Supplemental Figure 9. CTCF binding, but not chromatic accessibility measures, influenced cue-specific MPRA allelic shifts.
Supplemental Figure 10. Predicted TF binding SNP regulatory effects correlated significantly with MPRA allelic shifts.
Supplemental Figure 11. Expression of TFs predicted to regulate cue-specific MPRA activity.
Supplemental Figure 12. Cue-specific impacts on biological pathways.
Figure 0. Schematic.
Made with BioRender
FUNDING SOURCES
This work was supported by F31MH130122 (K.G.R), R01MH109897 (K.J.B.), R56MH101454 (K.J.B., L.H.), R01MH123155 (K.J.B.) and R01ES033630 (L.H., K.J.B.), R01MH124839 (LMH), R01MH106056 (K.J.B) U01DA047880 (K.J.B), R01DA048279 (K.J.B), DOD TP220451 (K.J.B., L.H.), and by the State of Connecticut, Department of Mental Health and Addiction Services. This publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut.
Footnotes
STATEMENT OF ETHICS
Ethical approval was not required because the hiPSC lines, lacking association with any identifying information and widely accessible from a public repository, are thus not considered to be human subjects research. Post-mortem DLPFC data are similarly lacking identifiable information and are not considered human subjects research.
CONFLICT OF INTEREST STATEMENT
K.J.B is a scientific advisor to Rumi Scientific Inc. and Neuro Pharmaka Inc. All other authors declare no conflicts of interest
DATA AND CODE AVAILABILITY
All source donor hiPSCs have been deposited at the Rutgers University Cell and DNA Repository (study 160; http://www.nimhstemcells.org/).
Full sequencing data, processed data, and accompanying code reported in this paper will be made available through GEO and Synapse upon publication.
REFERENCES
- 1.Edwards S. L., Beesley J., French J. D. & Dunning M. Beyond GWASs: Illuminating the dark road from association to function. American Journal of Human Genetics vol. 93 779–797 Preprint at 10.1016/j.ajhg.2013.10.012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda R. K. & Karch F. Gene expression in time and space: Additive vs hierarchical organization of cis-regulatory regions. Current Opinion in Genetics and Development 21, 187–193 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Young H., Cote A. & Huckins L. M. Chapter 14 - Integration with systems biology approaches and -omics data to characterize risk variation. in Psychiatric Genomics (eds. Tsermpini E. E., Alda M. & Patrinos G. P.) 289–315 (Academic Press, 2022). doi: 10.1016/B978-0-12-819602-1.00017-6. [DOI] [Google Scholar]
- 4.Bellavance M.-A. & Rivest S. The HPA – Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SILVERMAN M. N., PEARCE B. D., BIRON C. A. & MILLER A. H. Immune Modulation of the Hypothalamic-Pituitary-Adrenal (HPA) Axis during Viral Infection. Viral Immunol 18, 41–78 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barabási B. et al. Role of interleukin-6 and interleukin-10 in morphological and functional changes of the blood–brain barrier in hypertriglyceridemia. Fluids and Barriers of the CNS 20, 15 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fioravanti J. et al. The Fusion Protein of IFN-α and Apolipoprotein A-I Crosses the Blood–Brain Barrier by a Saturable Transport Mechanism. The Journal of Immunology 188, 3988–3992 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Wu W. L., Hsiao E. Y., Yan Z., Mazmanian S. K. & Patterson P. H. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain, Behavior, and Immunity 62, 11–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarieva K. et al. Pluripotent stem cell-derived neural progenitor cells can be used to model effects of IL-6 on human neurodevelopment. Dis Model Mech 16, dmm050306 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarieva K. et al. Human brain organoid model of maternal immune activation identifies radial glia cells as selectively vulnerable. Mol Psychiatry 1–13 (2023) doi: 10.1038/s41380-023-01997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng L.-S. et al. Mechanisms for Interferon-α-Induced Depression and Neural Stem Cell Dysfunction. Stem Cell Reports 3, 73–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seah C. et al. Modeling gene × environment interactions in PTSD using human neurons reveals diagnosis-specific glucocorticoid-induced gene expression. Nat Neurosci 25, 1434–1445 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonsdale J. et al. The Genotype-Tissue Expression (GTEx) project. Nature Genetics 45, 580–585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguet F. et al. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryois J. et al. Cell-type-specific cis-eQTLs in eight human brain cell types identify novel risk genes for psychiatric and neurological disorders. Nat Neurosci 25, 1104–1112 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Donovan M. K. R., D’Antonio-Chronowska A., D’Antonio M. & Frazer K. A. Cellular deconvolution of GTEx tissues powers discovery of disease and cell-type associated regulatory variants. Nature Communications 11, 955 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aygün N. et al. Inferring cell-type-specific causal gene regulatory networks during human neurogenesis. Genome Biology 24, 130 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimas A. S. et al. Sex-biased genetic effects on gene regulation in humans. Genome Research 22, 2368–2375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore S. R. et al. Sex differences in the genetic regulation of the blood transcriptome response to glucocorticoid receptor activation. Transl Psychiatry 11, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliva M. et al. The impact of sex on gene expression across human tissues. Science 369, eaba3066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alasoo K. et al. Shared genetic effects on chromatin and gene expression indicate a role for enhancer priming in immune response. Nat Genet 50, 424–431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davenport E. E. et al. Discovering in vivo cytokine-eQTL interactions from a lupus clinical trial. Genome Biology 19, 168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maranville J. C. et al. Interactions between Glucocorticoid Treatment and Cis-RegulatoryB Polymorphisms Contribute to Cellular Response Phenotypes. PLoS Genet 7, e1002162 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arloth J. et al. Genetic Differences in the Immediate Transcriptome Response to Stress Predict Risk-Related Brain Function and Psychiatric Disorders. Neuron 86, 1189–1202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arloth J. et al. DeepWAS: Multivariate genotype-phenotype associations by directly integrating regulatory information using deep learning. PLOS Computational Biology 16, e1007616 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yazar S. et al. Single-cell eQTL mapping identifies cell type–specific genetic control of autoimmune disease. Science 376, eabf3041 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K. et al. Splicing QTL analysis focusing on coding sequences reveals mechanisms for disease susceptibility loci. Nat Commun 13, 4659 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue F. et al. A systematic comparison reveals substantial differences in chromosomal versus episomal encoding of enhancer activity. Genome Research 27, 38–52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue F., Kreimer A., Ashuach T., Ahituv N. & Yosef N. Identification and Massively Parallel Characterization of Regulatory Elements Driving Neural Induction. Cell Stem Cell 25, 713–727.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon M. G. et al. lentiMPRA and MPRAflow for high-throughput functional characterization of gene regulatory elements. Nature Protocols 15, 2387–2412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulvey B., Lagunas T. & Dougherty J. D. Massively Parallel Reporter Assays: Defining Functional Psychiatric Genetic Variants across Biological Contexts. Biological Psychiatry 0, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulvey B., Selmanovic D. & Dougherty J. D. Sex Significantly Impacts the Function of Major Depression–Linked Variants In Vivo. Biological Psychiatry 94, 466–478 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulvey B. & Dougherty J. D. Transcriptional-Regulatory Convergence Across Functional MDD Risk Variants Identified by Massively Parallel Reporter Assays. 2021.03.05.434177 Preprint at 10.1101/2021.03.05.434177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rummel C. K. et al. Massively parallel functional dissection of schizophrenia-associated noncoding genetic variants. Cell 186, 5165–5182.e33 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Kreimer A. et al. Massively parallel reporter perturbation assays uncover temporal regulatory architecture during neural differentiation. Nat Commun 13, 1504 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daskalakis N. P., Cohen H., Cai G., Buxbaum J. D. & Yehuda R. Expression profiling associates blood and brain glucocorticoid receptor signaling with trauma-related individual differences in both sexes. Proceedings of the National Academy of Sciences of the United States of America 111, 13529–13534 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith S. E. P., Li J., Garbett K., Mirnics K. & Patterson P. H. Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience 27, 10695–10702 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armario A., Hernández J., Bluethmann H. & Hidalgo J. IL-6 deficiency leads to increased emotionality in mice: Evidence in transgenic mice carrying a null mutation for IL-6. Journal of Neuroimmunology 92, 160–169 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Borovcanin M. M. et al. Interleukin-6 in Schizophrenia-Is There a Therapeutic Relevance? Front Psychiatry 8, 221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Yehuda H. et al. Maternal Type-I interferon signaling adversely affects the microglia and the behavior of the offspring accompanied by increased sensitivity to stress. Molecular Psychiatry 25, 1050–1067 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaefer M. et al. Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C. J Hepatol 42, 793–798 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Baines K. J. et al. Maternal Immune Activation Alters Fetal Brain Development and Enhances Proliferation of Neural Precursor Cells in Rats. Front Immunol 11, 1145 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garbett K. A., Hsiao E. Y., Kálmán S., Patterson P. H. & Mirnics K. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry 2, e98–e98 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laighneach A., Desbonnet L., Kelly J. P., Donohoe G. & Morris D. W. Meta-Analysis of Brain Gene Expression Data from Mouse Model Studies of Maternal Immune Activation Using Poly(I:C). Genes (Basel) 12, 1363 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong Y. et al. Transcriptomic profiling of the developing brain revealed cell-type and brain-region specificity in a mouse model of prenatal stress. BMC Genomics 24, 86 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Causal analysis approaches in Ingenuity Pathway Analysis ∣ Bioinformatics ∣ Oxford Academic. doi: 10.1093/bioinformatics/btt703. https://academic.oup.com/bioinformatics/article/30/4/523/202720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marioni R. E. et al. Correction: GWAS on family history of Alzheimer’s disease. Transl Psychiatry 9, 161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demontis D. et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics 51, 63–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson H. J. et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nature Genetics 51, 1207–1214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grove J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nature Genetics 51, 431–444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullins N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet 53, 817–829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nature Neuroscience 22, 343–352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnold P. D. et al. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Molecular Psychiatry 23, 1181–1188 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huckins L. M. et al. Analysis of Genetically Regulated Gene Expression Identifies a Prefrontal PTSD Gene, SNRNP35, Specific to Military Cohorts. (2020) doi: 10.1016/j.celrep.2020.107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trubetskoy V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lo M. T. et al. Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nature Genetics 49, 152–156 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pickrell J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nature Genetics 48, 709–717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giambartolomei C. et al. A Bayesian Framework for Multiple Trait Colocalization from Summary Association Statistics. A Bayesian framework for multiple trait colocalization from summary association statistics 155481 (2017) doi: 10.1101/155481. [DOI] [Google Scholar]
- 60.Gamazon E. R. et al. A gene-based association method for mapping traits using reference transcriptome data. Nature Genetics 47, 1091–1098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbeira A. N. et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nature Communications 9, 1–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nott A. et al. Brain cell type–specific enhancer–promoter interactome maps and disease-risk association. Science 366, 1134–1139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agarwal D. et al. A single-cell atlas of the human substantia nigra reveals cell-specific pathways associated with neurological disorders. Nat Commun 11, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tewhey R. et al. Direct identification of hundreds of expression-modulating variants using a multiplexed reporter assay. Cell 165, 1519–1529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien H. E. et al. Expression quantitative trait loci in the developing human brain and their enrichment in neuropsychiatric disorders. Genome Biol 19, 194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailey T. L. & Machanick P. Inferring direct DNA binding from ChIP-seq. Nucleic Acids Research 40, e128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stelzer G. et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Current Protocols in Bioinformatics 54, 1.30.1–1.30.33 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Coetzee S. G., Coetzee G. A. & Hazelett D. J. motifbreakR: an R/Bioconductor package for predicting variant effects at transcription factor binding sites. Bioinformatics 31, 3847–3849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan J. et al. Systematic analysis of binding of transcription factors to noncoding variants. Nature 591, 147–151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yin Y. et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 356, eaaj2239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee D. et al. A method to predict the impact of regulatory variants from DNA sequence. Nat Genet 47, 955–961 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fulco C. P. et al. Activity-by-contact model of enhancer–promoter regulation from thousands of CRISPR perturbations. Nature Genetics 51, 1664–1669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hecker D., Behjati Ardakani F., Karollus A., Gagneur J. & Schulz M. H. The adapted Activity-By-Contact model for enhancer–gene assignment and its application to single-cell data. Bioinformatics 39, btad062 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Leeuw C. A., Mooij J. M., Heskes T. & Posthuma D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLOS Computational Biology 11, e1004219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe K., Taskesen E., van Bochoven A. & Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun 8, 1826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Subramanian A. et al. A Next Generation Connectivity Map: L1000 platform and the first 1,000,000 profiles. Cell 171, 1437–1452.e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin L.-C. & Sibille E. Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front. Pharmacol. 4, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Derveer A. B. et al. A Role for Somatostatin-Positive Interneurons in Neuro-Oscillatory and Information Processing Deficits in Schizophrenia. Schizophr Bull 47, 1385–1398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sommer I. E. et al. Simvastatin Augmentation for Patients With Early-Phase Schizophrenia-Spectrum Disorders: A Double-Blind, Randomized Placebo-Controlled Trial. Schizophrenia Bulletin 47, 1108–1115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen H. et al. Adjunctive therapy with statins in schizophrenia patients: A meta-analysis and implications. Psychiatry Res 262, 84–93 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Kagda M. S. et al. Data navigation on the ENCODE portal. Preprint at http://arxiv.org/abs/2305.00006 (2023). [Google Scholar]
- 82.Luo Y. et al. New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res 48, D882–D889 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim S., Yu N.-K. & Kaang B.-K. CTCF as a multifunctional protein in genome regulation and gene expression. Exp Mol Med 47, e166–e166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gofin Y. et al. Delineation of a novel neurodevelopmental syndrome associated with PAX5 haploinsufficiency. Hum Mutat 43, 461–470 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohtsuka N. et al. GABAergic neurons regulate lateral ventricular development via transcription factor Pax5. genesis 51, 234–245 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacArthur J. et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Research 45, D896–D901 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cruceanu C. et al. Cell-Type-Specific Impact of Glucocorticoid Receptor Activation on the Developing Brain: A Cerebral Organoid Study. Am J Psychiatry 179, 375–387 (2022). [DOI] [PubMed] [Google Scholar]
- 88.Resztak J. A. et al. Genetic control of the dynamic transcriptional response to immune stimuli and glucocorticoids at single-cell resolution. Genome Res 33, 839–856 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hall M. B., Willis D. E., Rodriguez E. L. & Schwarz J. M. Maternal immune activation as an epidemiological risk factor for neurodevelopmental disorders: Considerations of timing, severity, individual differences, and sex in human and rodent studies. Frontiers in Neuroscience 17, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Estes M. L. & McAllister A. K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 353, 772–777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trifonova E. A., Mustafin Z. S., Lashin S. A. & Kochetov A. V. Abnormal mTOR Activity in Pediatric Autoimmune Neuropsychiatric and MIA-Associated Autism Spectrum Disorders. Int J Mol Sci 23, 967 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosario F. J. et al. Mechanistic Target of Rapamycin Complex 2 Regulation of the Primary Human Trophoblast Cell Transcriptome. Front Cell Dev Biol 9, 670980 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yao C. et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun 9, 3268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bryois J. et al. Time-dependent genetic effects on gene expression implicate aging processes. Genome Res. 27, 545–552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamamoto R. et al. Tissue-specific impacts of aging and genetics on gene expression patterns in humans. Nat Commun 13, 5803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoffman G. E. et al. CommonMind Consortium provides transcriptomic and epigenomic data for Schizophrenia and Bipolar Disorder. Sci Data 6, 180 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao C. et al. Sex- and age-interacting eQTLs in human complex diseases. Hum Mol Genet 23, 1947–1956 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Solmi M. et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry 27, 281–295 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inoue F., Kreimer A., Ashuach T., Ahituv N. & Yosef N. Identification and Massively Parallel Characterization of Regulatory Elements Driving Neural Induction. Cell Stem Cell 25, 713–727.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Webber C. Epistasis in Neuropsychiatric Disorders. Trends in Genetics 33, 256–265 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Andreasen N. C. et al. Statistical Epistasis and Progressive Brain Change in Schizophrenia: An Approach for Examining the Relationships Between Multiple Genes. Mol Psychiatry 17, 1093–1102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Townsley K. G., Brennand K. J. & Huckins, Laura. M. Massively parallel techniques for cataloguing the regulome of the human brain. Nat Neurosci 23, 1509–1521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gasperini M. et al. crisprQTL mapping as a genome-wide association framework for cellular genetic screens. 314344 (2018) doi: 10.1101/314344. [DOI] [Google Scholar]
- 104.Musa A., Tripathi S., Kandhavelu M., Dehmer M. & Emmert-Streib F. Harnessing the biological complexity of Big Data from LINCS gene expression signatures. PLoS One 13, e0201937 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lim N. & Pavlidis P. Evaluation of connectivity map shows limited reproducibility in drug repositioning. Sci Rep 11, 17624 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Benson C. A. et al. Immune Factor, TNFα, Disrupts Human Brain Organoid Development Similar to Schizophrenia—Schizophrenia Increases Developmental Vulnerability to TNFα. Frontiers in Cellular Neuroscience 14, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swingler M., Donadoni M., Bellizzi A., Cakir S. & Sariyer I. K. iPSC-derived three-dimensional brain organoid models and neurotropic viral infections. J. Neurovirol. 29, 121–134 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao S. et al. A single-cell massively parallel reporter assay detects cell-type-specific gene regulation. Nat Genet 55, 346–354 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neavin D. R. et al. A village in a dish model system for population-scale hiPSC studies. Nat Commun 14, 3240 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mitchell J. M. et al. Mapping genetic effects on cellular phenotypes with ‘cell villages’. bioRxiv 2020.06.29.174383 (2020) doi: 10.1101/2020.06.29.174383. [DOI] [Google Scholar]
- 111.Wells M. F. et al. Natural variation in gene expression and viral susceptibility revealed by neural progenitor cell villages. Cell Stem Cell 30, 312–332.e13 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.SMITH D. D. & COSTANTINE M. M. The role of statins in the prevention of preeclampsia. Am J Obstet Gynecol 226, S1171–S1181 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meijerink L. et al. Statins in pre-eclampsia or fetal growth restriction: A systematic review and meta-analysis on maternal blood pressure and fetal growth across species. BJOG: An International Journal of Obstetrics & Gynaecology 130, 577–585 (2023). [DOI] [PubMed] [Google Scholar]
- 114.Martínez A. L., Brea J., Rico S., de los Frailes M. T. & Loza M. I. Cognitive Deficit in Schizophrenia: From Etiology to Novel Treatments. Int J Mol Sci 22, 9905 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Myint L. et al. Testing the Regulatory Consequences of 1,049 Schizophrenia Associated Variants With a Massively Parallel Reporter Assay. bioRxiv 447557 (2018) doi: 10.1101/447557. [DOI] [Google Scholar]
- 116.GitHub - FelixKrueger/TrimGalore: A wrapper around Cutadapt and FastQC to consistently apply adapter and quality trimming to FastQ files, with extra functionality for RRBS data. https://github.com/FelixKrueger/TrimGalore. [Google Scholar]
- 117.Babraham Bioinformatics - FastQC A Quality Control tool for High Throughput Sequence Data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. [Google Scholar]
- 118.Ewels P., Magnusson M., Lundin S. & Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Langmead B. & Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.GitHub - jsh58/Genrich: Detecting sites of genomic enrichment. https://github.com/jsh58/Genrich. [Google Scholar]
- 122.Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Y., Chen L., Lun A. T. L., Baldoni P. L. & Smyth G. K. edgeR 4.0: powerful differential analysis of sequencing data with expanded functionality and improved support for small counts and larger datasets. 2024.01.21.576131 Preprint at 10.1101/2024.01.21.576131 (2024). [DOI] [Google Scholar]
- 124.Lun A. T. L. & Smyth G. K. csaw: a Bioconductor package for differential binding analysis of ChIP-seq data using sliding windows. Nucleic Acids Res 44, e45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ashuach T. et al. MPRAnalyze: statistical framework for massively parallel reporter assays. Genome Biology 20, 183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bao L., Zhou M. & Cui Y. CTCFBSDB: a CTCF-binding site database for characterization of vertebrate genomic insulators. Nucleic Acids Research 36, D83–D87 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ziebarth J. D., Bhattacharya A. & Cui Y. CTCFBSDB 2.0: a database for CTCF-binding sites and genome organization. Nucleic Acids Research 41, D188–D194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bailey T. L. & Grant C. E. SEA: Simple Enrichment Analysis of motifs. 2021.08.23.457422 Preprint at 10.1101/2021.08.23.457422 (2021). [DOI] [Google Scholar]
- 129.Wu T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. The Innovation 2, 100141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pickrell J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 48, 709–717 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giambartolomei C. et al. A Bayesian framework for multiple trait colocalization from summary association statistics. Bioinformatics 34, 2538–2545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gamazon E. R. et al. A gene-based association method for mapping traits using reference transcriptome data. Nature Genetics 47, 1091–1098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barbeira A. N. et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nature Communications 9, 1–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. GWAS studies used to prioritize cross-psych MPRA library.
Supplemental Table 2. Number of prioritized SNPs by colocalization (Coloc2) across GWAS.
Supplemental Table 3. Number of prioritized SNPs by S-PrediXcan across GWAS.
Supplemental Table 4. Number of captured CRE with sufficient barcodes across MPRA.
Supplemental Figure 1. Selection of predicted cis-expression quantitative trait loci (cis-eQTLs).
Supplemental Figure 2. Design of cross-disorder MPRA library.
Supplemental Figure 3. Stress and inflammatory factors associated with MIA significantly dysregulated the transcriptomic in developing iGLUTs.
Supplemental Figure 4. Stress and inflammatory factors altered neurite outgrowth in hiPSC-derived iGLUTs.
Supplemental Figure 5. Correlation between MPRA experimental replicates.
Supplemental Figure 6. Correlation between MPRA donors and contexts.
Supplemental Figure 7. Stress and inflammatory factors uniquely impacted allelic-specific transcriptional activity of CRE in iGLUTs.
Supplemental Figure 8. Transcription factor binding motifs were differentially enriched in active CRE based on context.
Supplemental Figure 9. CTCF binding, but not chromatic accessibility measures, influenced cue-specific MPRA allelic shifts.
Supplemental Figure 10. Predicted TF binding SNP regulatory effects correlated significantly with MPRA allelic shifts.
Supplemental Figure 11. Expression of TFs predicted to regulate cue-specific MPRA activity.
Supplemental Figure 12. Cue-specific impacts on biological pathways.