Abstract
IMPORTANCE:
The opioid crisis is impacting people across the country and deserves attention to be able to curb the rise in opioid-related deaths.
OBJECTIVES:
To evaluate practice patterns in opioid infusion administration and dosing for patients with acute respiratory failure receiving invasive mechanical ventilation.
DESIGN:
Retrospective cohort study.
SETTING AND PARTICIPANTS:
Patients from 21 hospitals in Kaiser Permanente Northern California and 96 hospitals in Philips electronic ICU Research Institute.
MAIN OUTCOMES AND MEASURES:
We assessed whether patients received opioid infusion and the dose of said opioid infusion.
RESULTS:
We identified patients with a diagnosis of acute respiratory failure who were initiated on invasive mechanical ventilation. From each patient, we determined if opioid infusions were administered and, among those who received an opioid infusion, the median daily dose of fentanyl infusion. We used hierarchical regression models to quantify variation in opioid infusion use and the median daily dose of fentanyl equivalents across hospitals. We included 13,140 patients in the KPNC cohort and 52,033 patients in the eRI cohort. A total of 7,023 (53.4%) and 16,311 (31.1%) patients received an opioid infusion in the first 21 days of mechanical ventilation in the KPNC and eRI cohorts, respectively. After accounting for patient- and hospital-level fixed effects, the hospital that a patient was admitted to explained 7% (95% CI, 3–11%) and 39% (95% CI, 28–49%) of the variation in opioid infusion use in the KPNC and eRI cohorts, respectively. Among patients who received an opioid infusion, the median daily fentanyl equivalent dose was 692 µg (interquartile range [IQR], 129–1341 µg) in the KPNC cohort and 200 µg (IQR, 0–1050 µg) in the eRI cohort. Hospital explained 4% (95% CI, 1–7%) and 20% (95% CI, 15–26%) of the variation in median daily fentanyl equivalent dose in the KPNC and eRI cohorts, respectively.
CONCLUSIONS AND RELEVANCE:
In the context of efforts to limit healthcare-associated opioid exposure, our findings highlight the considerable opioid exposure that accompanies mechanical ventilation and suggest potential under and over-treatment with analgesia. Our results facilitate benchmarking of hospitals’ analgesia practices against risk-adjusted averages and can be used to inform usual care control arms of analgesia and sedation clinical trials.
Keywords: acute respiratory failure, analgesia, intensive care unit, mechanical ventilation, opioids
KEY POINTS
Question: What are opioid infusion administration practices in patients with acute respiratory failure receiving invasive mechanical ventilation?
Findings: Using two large retrospective multicenter cohorts (Kaiser Permanente Northern California [KPNC] and electronic ICU Research Institute [eRI]), we found that: 1) almost half of patients undergoing invasive mechanical ventilation received opioid infusions, 2) hospital variation in opioid infusion use and dose was larger in the eRI U.S.-wide cohort than the KPNC integrated health system cohort, and 3) there was little correlation between opioid dose and the dose of other sedatives.
Meaning: Our findings highlight the considerable opioid exposure and variation in exposure that accompanies critical illness for respiratory failure.
Between 1999 and 2020, more than 564,000 Americans died from an overdose involving opioids (1). As a result of the opioid epidemic and prior studies linking routine opioid use in acute care settings to long-term opioid use disorders (2, 3), there has been increased scrutiny of opioid administration across the inpatient setting (3–7). Critical care clinicians, who routinely care for patients with severe opioid overdose, have become keenly aware of the potential for harm from opioids including short-term exposure of opioids in the hospital. However, contemporary critical care practice guideline recommends IV opioids as the first-line agent for pain during acute invasive mechanical ventilation (IMV) for acute respiratory failure (ARF) (a condition affecting over half of patients admitted to the ICU) (8), which emerged from efforts to prevent delirium and its associated complications (9, 10). Given the potentially competing goals of treating pain and preventing both delirium and long-term opioid-associated complications, understanding the current practice of infusing opioid during mechanical ventilation is important for quality improvement and guideline implementation in the critical care setting. For example, evidence of large practice pattern variation could suggest both over- and under-treatment and the need for analgesia/sedation protocols and risk-adjusted average practices could be used to assist hospitals with benchmarking.
Using two (nonintegrated vs. integrated) multicenter databases (Philips electronic ICU Research Institute [eRI] and Kaiser Permanente Northern California [KPNC]) in the United States, we sought to describe the practice patterns in opioid administration and dosing among patients with ARF who required IMV. We hypothesized there would be wide variation among hospitals in the use of IV opioids in this clinical setting, including the potential for wider variation in ICUs across health systems vs. the medical centers within a single integrated health system.
METHODS
Data Sources
We used data from two large electronic health record repositories to identify patients for inclusion in the study: eRI and KPNC. The eRI database contains granular, de-identified data from more than 400 ICUs in the United States that participate in Philips electronic ICU program including greater than 100 million medication administration records. KPNC is an integrated health system serving greater than 4.4 million members in 21 medical centers in Northern California and has granular, longitudinal electronic health record data since implementation of Epic in 2010. All ICUs in KPNC are staffed by critical care board certified physicians. We analyzed these two data sources, harmonizing operational definitions and analytic approaches, to assess variation across regions of the country and within integrated healthcare systems.
Ethics Review
For eRI, this study was designated not Human Subjects Research by Boston University’s Institutional Review Board (IRB) (No. H-38964). For KPNC, which required de-identification of medical record information, the study was approved by the KPNC IRB with a waiver of informed consent (IRBNet project [1616338-1] Evaluating Routine Opioid Use During Acute Respiratory Failure, Approved August 14, 2020). Procedures were followed in accordance with the ethical standards of the KPNC IRB and with the Helsinki Declaration of 1975.
Study Population
We included adult patients (≥ 18 yr) admitted to an ICU between 2012 and 2019 with ARF who received IMV for greater than or equal to 24 hours. This minimum time requirement excludes patients who received IMV for a brief interval, such as for a procedure. We excluded patients with alternative indications for opioids other than sedation related to IMV (patients with diagnoses related to surgery, trauma, burns, and drug overdose; patients with the goal of comfort care within 24 hr of IMV initiation; and patients with admitting diagnoses where the routine use of opioid analgesia during IMV may be restricted to facilitate frequent neurologic assessment, i.e., cardiac arrest). We also excluded patients with tracheostomy on ICU admission or within 72 hours of IMV initiation because these patients represented a chronic respiratory failure population that likely existed before hospitalization. For patients with multiple episodes of IMV in either database, we limited our evaluation of opioid use to the first IMV episode during a hospitalization and chose a random hospitalization if there were multiple encounters. Supplemental Table 1 (http://links.lww.com/CCX/B368) includes details of how study inclusion/exclusion criteria were applied to the eRI and KPNC cohorts.
Outcomes
The two primary outcomes of interest were: 1) the use of opioid (fentanyl, hydromorphone, or morphine) infusions within the first 21 days of IMV among all mechanically ventilated patients meeting inclusion criteria and 2) the median daily dose of fentanyl equivalents (µg) within the first 21 days of IMV among patients who received an opioid infusion. The median daily dose of fentanyl infusion equivalents was calculated as follows: 1) starting with the first hour of IMV, we summed the total dose of fentanyl equivalents administered in each 24-hour period of IMV up to 21 days and 2) calculated the median of daily summed fentanyl dose equivalents over all days of IMV. Doses of hydromorphone and morphine were converted to fentanyl using the following equivalencies: 100 µg/hr of fentanyl = 1.5 mg/hr of hydromorphone = 10 mg/hr of morphine (11). Secondary outcomes were the maximum hourly rate of fentanyl equivalents (µg/hr) during the first 21 days of IMV and the cumulative dose of fentanyl equivalents (µg) during IMV (adjusted for duration of IMV). Opioid doses were calculated from the first hour of IMV until the first occurrence of extubation, tracheostomy, and change to a “comfort measures only” goals of care or after 21 days of IMV. We chose 21 days because this is typically the threshold in which patients transition from acute to chronic critical illness (12). Additional details about the definitions of the outcomes are in Supplemental Table 2 (http://links.lww.com/CCX/B368).
In secondary analyses using the KPNC cohort only, we examined the outcome when it included administration of intermittent opioid “pushes” in addition to continuous infusions (the eRI database does not contain granular dosing information on IV push medications).
Risk Adjustment Variables
We identified patient- and hospital-level variables for inclusion in models for risk- and case-mix-adjusted opioid infusion doses. Patient-level variables included demographics (e.g., age, sex, race, body mass index), measures of acute disease severity (Sequential Organ Failure Assessment [SOFA] scores, number of mechanical ventilation episodes during the encounter), invasive procedures (central line, arterial line, chest tube), comorbidities (unweighted Charlson score, individual Charlson comorbidities), and measures of opioid use (opioid prescription at admission inpatient medication reconciliation in eRI; history of opioid use or abuse by diagnosis code in the 1 yr before hospitalization in KPNC). Hospital-level variables were teaching hospital designation, hospital bed number, and U.S. census region (eRI only). Additional details and the complete description of risk adjustment variables are included in Supplemental Table 2 (http://links.lww.com/CCX/B368).
Missing Data
After data cleaning and variable creation, we determined the proportion of patients missing each variable and the proportion missing at least one covariate. We performed multiple imputation (mitml R package, just another gibbs sampler, Hamburg, Germany) (13, 14) to create 20 new datasets in KPNC and five in eRI (the number of datasets was chosen to maximally use the computing infrastructure available at each site) (15, 16). Each multiply imputed dataset was used to separately model risk-adjusted outcomes rates, and model effect estimates were then pooled.
Statistical Analysis
Modeling Variation in Receipt of Any Opioid Continuous Infusion During IMV
We reported the number of patients who received any opioid continuous infusion and the median number of days (interquartile range [IQR]) that patients received an opioid infusion. We then used hierarchical logistic regression to model the outcome of receiving an opioid continuous infusion, adjusting for the patient- and hospital-level variables as fixed effects and hospital of admission as a random effect (random intercept) to calculate the risk-adjusted rate of opioid continuous infusion overall and by the hospital. Risk-adjustment variables with prevalence less than 5% were excluded from the final models to improve model convergence. From the hierarchical models, we reported the adjusted odds ratio (aOR) for the association between each fixed effect and receipt of opioid continuous infusion, the mode of the conditional distribution of the random effects (i.e., how far each hospital’s average predicted outcome deviates from the overall average in the cohort), and the intraclass correlation coefficient (ICC), the percentage of total variation in outcomes explained by admission hospital after risk adjustment. Larger ICCs suggest more idiosyncratic (i.e., not due to the fixed effect patient- and hospital-level covariates) differences in practice between hospitals. For example, an ICC of 20% would suggest that, after accounting for patient- and hospital-level covariates (fixed effects), the hospital that a patient was admitted to contributes to 20% of the total variation in outcome.
In the KPNC cohort, we performed an additional analysis examining variation in opioid use including IV “push” doses in addition to continuous infusion in the opioid exposure measurement.
Modeling Variation in Dose of Opioid Continuous Infusion
We limited the eRI and KPNC cohorts to patients who had at least one medication administration record for an opioid continuous infusion, that is, the denominator of patients in this analysis had to have received an opioid continuous infusion. We then used hierarchical linear regression models for three separate outcomes (median daily fentanyl equivalent dose, maximum fentanyl equivalent rate, and cumulative fentanyl equivalent dose) and included the same fixed and random effects as the opioid use models to calculate risk-adjusted outcome estimates overall and by hospital. We reported risk-adjusted outcomes (aORs) for associations between fixed effects and outcomes and the ICC.
Continuous Infusion Sedatives Beside Opioids
To explore relationships between opioid dosing and other sedatives, we calculated the median daily dose equivalents and maximum rates during the first 21 days of IMV for the following medications: lorazepam equivalents (1 mg lorazepam = 2 mg midazolam = 5 mg diazepam), propofol, ketamine, and dexmedetomidine. We then examined correlations between doses of fentanyl equivalents and each of these medications using Spearman correlation.
Alpha was two-sided and set at less than 0.05. R (Version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
RESULTS
Characteristics
We included 13,140 patients (within 21 hospitals) in the KPNC cohort and 52,033 patients (across 148 ICUs within 96 hospitals) in the eRI cohort (Supplemental Figs. 1 and 2, http://links.lww.com/CCX/B368). Characteristics between the two study cohorts were generally similar (Table 1; and Supplemental Table 3, http://links.lww.com/CCX/B368). The median age of patients was 68 in KPNC and 65 in eRI. The median IMV duration (KPNC: 69 hr [IQR, 42–133 hr] and eRI: 89 hr [IQR, 47–172 hr]) was similar between cohorts; few patients received IMV for more than 14 days (KPNC 5.0%; eRI 7.5%). The most common principal discharge diagnosis was sepsis in both cohorts (KPNC: 48.5%, eRI 26.7%). Patients in the eRI cohort had lower median daily SOFA scores (median, 8 [IQR, 6–10] in KPNC vs. median, 3 [IQR, 0–6] in eRI). Very few patients in both cohorts had missing data (1.6% in KPNC and 5.2% in eRI; Supplemental Figs. 3 and 4, http://links.lww.com/CCX/B368).
TABLE 1.
Baseline Characteristics
| Characteristic | Kaiser Permanente Northern California, n = 13,140 | Philips Electronic ICU Research Institute, n = 52,033 |
|---|---|---|
| Age at admission, yr, median (IQR) | 68 (58–77) | 65 (55–75) |
| Male sex, n (%) | 7,083 (53.9) | 27,646 (53.1) |
| Race, n (%) | ||
| Asian | 1,901 (14.5) | 510 (1.0) |
| Black | 1,777 (13.5) | 6,710 (12.9) |
| White | 6,766 (51.5) | 38,217 (73.4) |
| Hispanic | 1,932 (14.7) | 2,273 (4.4) |
| Other/unknown/missing | 764 (5.8) | 4,323 (8.3) |
| Prior opioid usea, n (%) | 318 (2.4) | 937 (1.8) |
| Admission body mass index, kg/m2, median (IQR), missing | 28.1 (23.7–34.3), 0 | 28.1 (23.4–34.4), 512 |
| Central line during MV (until 21 d), n (%) | 10,006 (76.1) | 21,453 (41.2) |
| Arterial line during MV (until 21 d), n (%) | 3,675 (28.0) | 22,643 (43.5) |
| Chest tube during MV (until 21 d), n (%) | 638 (4.9) | 1,412 (2.7) |
| Number of mechanical ventilation episodes during encounter, median (IQR) | 1 (1–1) | 1 (1–1) |
| Charlson score (unweighted) points, median (IQR) | 3 (1–5) | 2 (1–3) |
| SOFA score points in first 24 hr of MV, median (IQR) | 10 (7–12) | 4 (0–7) |
| Median of patients’ daily SOFA score points during MV (up to 21 d), median (IQR) | 8 (6–10) | 3 (0–6) |
| Highest PEEP in first 24 hr of MV, cm H2O, median (IQR), missing | 5 (5–8), 16 | 5 (5–10), 1,992 |
| Median highest daily PEEP during MV (up to 21 d), cm H2O, median (IQR), missing | 5 (5–8), 4 | 5 (5–8), 415 |
| Code status at beginning of hospitalization, n (%) | ||
| Do not resuscitate | 496 (3.8) | 2,561 (4.9) |
| Full code | 12,300 (93.6) | 49,426 (95.0) |
| Partial code | 344 (2.6) | 46 (0.1) |
| Limitation of code status after admission until end of MV (or until 21 d), n (%) | 4,502 (34.3) | 12,705 (27.6) |
| Mechanical ventilation duration, hr, median (IQR) | 69 (42–133) | 89 (47–172) |
| Principal discharge diagnosis group, n (%) | ||
| Sepsis | 6,374 (48.5) | 13,899 (26.7) |
| Respiratory failure | 1,961 (14.9) | 11,139 (21.4) |
| Other | 909 (6.9) | 15,453 (29.7) |
| Hospital number of beds, n (%) | ||
| < 100 | 1,489 (11.3) | 1,701 (3.3) |
| 100–500 | 11,651 (88.7) | 24,509 (47.1) |
| > 500 | 0 (0) | 25,823 (49.6) |
| Teaching hospital, n (%) | 2,314 (17.6) | 10,173 (19.6) |
| Facility location, West, n (%) | 13,140 (100) | 18,656 (35.9) |
| Facility location, Northeast, n (%) | 0 | 4,512 (8.7) |
| Facility location, Midwest, n (%) | 0 | 6,669 (12.8) |
| Facility location, South, n (%) | 0 | 22,196 (42.7) |
IQR = interquartile range, MV = mechanical ventilation, PEEP = positive end-expiratory pressure, SOFA = Sequential Organ Failure Assessment.
History of opioid use or abuse by diagnosis code in 1 yr before admission based on International Classification of Diseases, 10th revision codes (Kaiser Permanente Northern California) or opioid prescription at admission inpatient medication reconciliation (Philips electronic ICU Research Institute).
The binary comorbidities and expanded list of principle discharge diagnoses are available in Supplemental Table 3 (http://links.lww.com/CCX/B368).
The eRI sample contained facilities across the continental United States (35.9% in the West, 8.7% in the Northeast, 12.8% in the Midwest, and 42.7% in the South). The majority of hospitals (88.7%) in the KPNC cohort were medium size (100–500 beds), whereas the eRI cohort contained medium and large facilities (49.6% medium size and 49.6% large size, which was > 500 beds). Approximately one-fifth of hospitals in both cohorts were considered teaching hospitals (17.5% in KPNC, 19.6% in eRI).
Variation in Opioid Infusion Use Among Hospitals
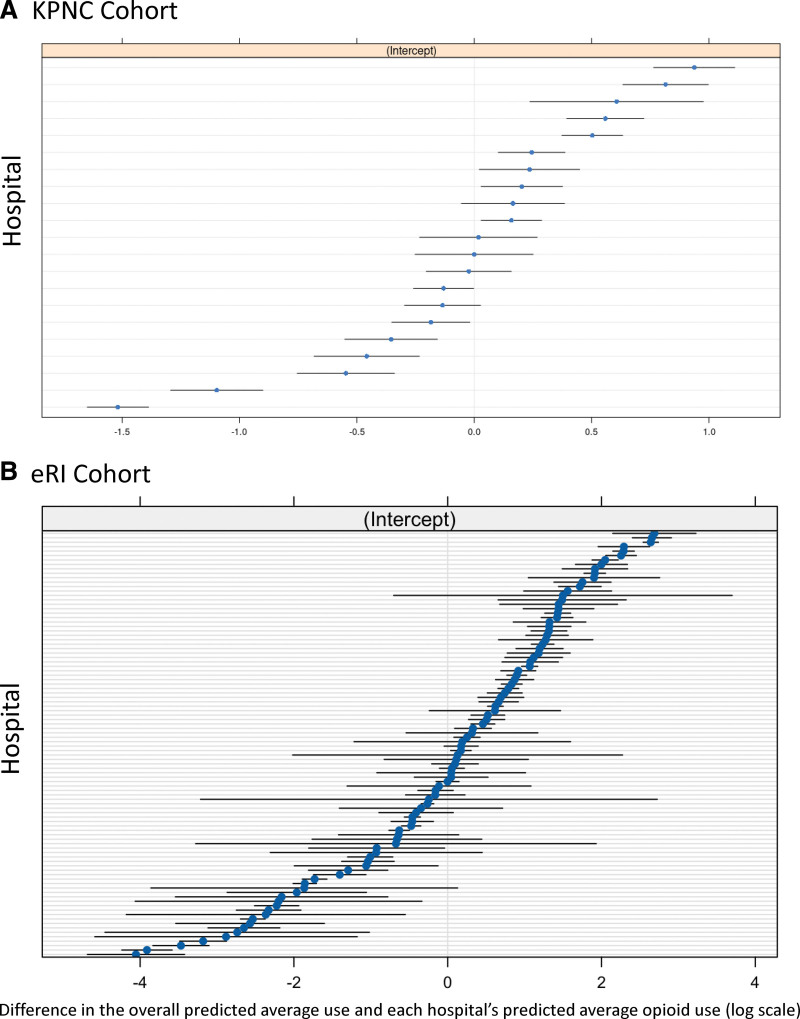
A total of 7,023 (53.4%) and 16,311 (31.1%) patients received an opioid infusion in the first 21 days of IMV in the KPNC and eRI cohorts, respectively. The deviation from the overall cohort average in hospital-level predicted opioid infusion use was larger in the eRI cohort compared with the KPNC cohort (Fig. 1). After accounting for all patient- and hospital-level covariates (i.e., model fixed effects), the hospital that a patient was admitted to explained 7% (95% CI, 3–11%) and 39% (95% CI, 28–49%) of the variation in opioid infusion use in the KPNC and eRI cohorts, respectively. Factors associated with higher odds of opioid infusion included the presence of indwelling lines (arterial line aOR: KPNC, 1.7 [95% CI, 1.55–1.88] and eRI, 1.65 [95% CI, 1.55–1.74]), higher median highest daily positive end-expiratory pressure during IMV (aOR: KPNC, 1.11 [95% CI, 1.07–1.13] and eRI, 1.09 [95% CI, 1.08–1.11]), comorbidities (e.g., cancer aOR: KPNC, 1.36 [95% CI, 1.17–1.58] and eRI, 1.25 [95% CI, 1.16–1.36]), and principal diagnosis (Supplemental Table 4, http://links.lww.com/CCX/B368).
Figure 1.
Opioid use by hospital. Shown are the difference between the overall predicted average use of opioid infusions on the log scale (centered at 0) for the cohort and each hospital’s predicted average use of opioid infusions (blue dots) and 95% CIs (black band) for Kaiser Permanente Northern California (KPNC) (A) and Philips electronic ICU Research Institute (eRI) (B).
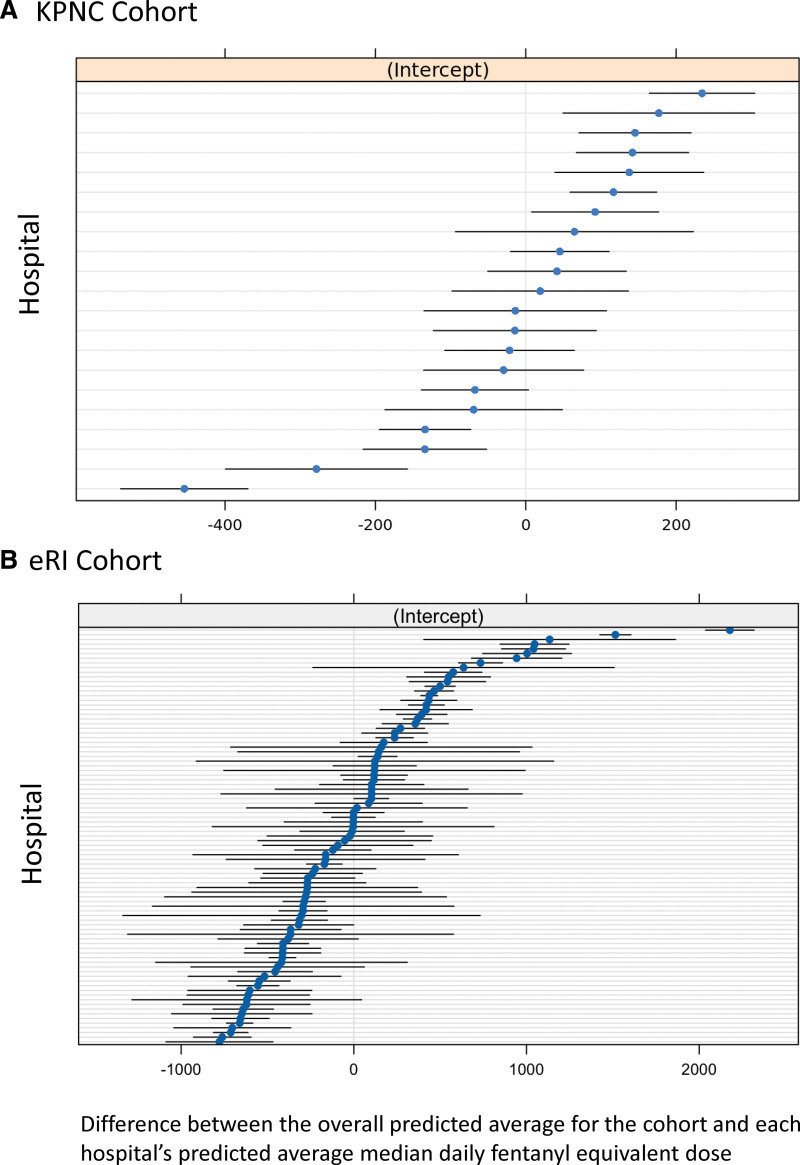
Variation in Opioid Infusion Dose Among Hospitals
Among patients who received an opioid infusion, the overall median daily fentanyl equivalent dose was 692 µg (IQR, 129–1341 µg) in the KPNC cohort and 200 µg (IQR, 0–1050 µg) in the eRI cohort and the median days of opioid infusion was 3 (IQR, 2–5) in both cohorts (Supplemental Fig. 5, http://links.lww.com/CCX/B368). Similar to the outcome of opioid infusion use, there was larger deviation from the overall cohort average of hospital-level predicted median daily fentanyl equivalent dose in eRI compared with KPNC (Fig. 2). After accounting for fixed effects, hospital explained 4% (95% CI, 1–7%) and 20% (95% CI, 15–26%) of the variation in median daily fentanyl equivalent dose in the KPNC and eRI cohorts, respectively. Similarly to the analysis of opioid use, factors associated with higher median daily fentanyl equivalent doses included use of arterial lines (KPNC, 137.31 µg [95% CI, 91.08–183.53 µg] and eRI, 98.85 µg [95% CI, 54.96–142.74 µg] compared with no arterial line) and median highest daily positive end-expiratory pressure during IMV (KPNC, 40.05 [95% CI, 29.62–50.47] and eRI, 62.87 µg per 1 cm H2O increase in pressure [95% CI, 54.54–71.20 µg per 1 cm H2O increase in pressure]). Factors associated with lower median daily fentanyl equivalent doses included increasing age (KPNC, –11.22 µg [95% CI, –17.73 to –9.71 µg] and eRI, –12.83 µg per 1-yr increase in age [95% CI, –14.34 to –11.33 µg per 1-yr increase in age]) and increasing Charlson score (KPNC, –41.67 [95% CI, –78.87 to –4.46] and eRI, –41.73 µg per 1-point increase [95% CI, –78.15 to –5.31 µg per 1-point increase]) (Supplemental Table 5, http://links.lww.com/CCX/B368).
Figure 2.
Median daily fentanyl equivalent dose by hospital. A, Kaiser Permanente Northern California (KPNC). B, Philips electronic ICU Research Institute (eRI). Hospitals are ranked according to median daily fentanyl equivalent dose. The units shown are the difference between each hospital’s predicted median daily fentanyl equivalent (blue dots) from the average hospital’s median daily fentanyl equivalent (centered at 0 µg). The 95% CIs are black bands.
Secondary outcomes showed similar patterns to the primary outcomes. The overall median maximum hourly fentanyl rate was 100 µg in both cohorts (KPNC: IQR, 50–150 µg and eRI: IQR, 50–175 µg). The overall median cumulative fentanyl dose was similar (2575 µg [IQR, 1100–6100 µg] in the KPNC cohort and 2225 µg [IQR, 675–6870 µg] in the eRI cohort). Variation in secondary outcomes due to admission hospital was low in the KPNC cohort (median maximal hourly rate ICC, 4% [95% CI, 1–7%] and median cumulative dose ICC, 3% [95% CI, 1–5%]) and high in the eRI cohort (median maximal hourly rate ICC, 13% [95% CI, 9–17%] and median cumulative dose ICC, 21% [95% CI, 13–23%]). Factors associated with secondary outcomes are shown in Supplemental Tables 6 and 7 (http://links.lww.com/CCX/B368).
Results from complete case analyses were similar to the imputed data analyses (Supplemental Tables 8 and 9, http://links.lww.com/CCX/B368).
Variation in Opioid Dose in KPNC When Both Drip and Push Administrations Are Counted
Among those who received an opioid in the KPNC cohort only (n = 11,250), 1,240 (9.4%) received opioid infusions alone, 5,783 (44.0%) received opioid infusions and opioid “pushes,” and 4,227 (32.2%) received opioid pushes alone. In the complete case analysis, after adjusting for fixed effects, hospital explained 3.5% (95% CI, 1.1–5.3%) in opioid infusion use. The overall median daily fentanyl equivalent dose was 200 µg (IQR, 30–988 µg). After accounting for fixed effects, hospital explained 5.4% (95% CI, 2.4–9.4%) of the variation in median daily fentanyl equivalent dose.
Correlation Between Median Daily Fentanyl Equivalents and Other Sedative Medications
In the KPNC cohort, the median daily fentanyl equivalent dose was moderately correlated with median daily propofol dose (Spearman = 0.30; p < 0.001) and weakly correlated with median daily lorazepam equivalent dose (Spearman = 0.09; p < 0.001), dexmedetomidine dose (Spearman = 0.15; p < 0.001), and ketamine dose (Spearman = 0.05; p < 0.001) (Supplemental Fig. 6, http://links.lww.com/CCX/B368). In the eRI cohort, the median daily fentanyl equivalent dose was weakly correlated with the median daily propofol dose (Spearman = 0.11; p < 0.001), lorazepam equivalent dose (Spearman = 0.20; p < 0.001), and ketamine dose (Spearman = 0.07; p < 0.001) and had a weak negative correlation with median daily dexmedetomidine dose (Spearman = –0.06; p < 0.001) (Supplemental Fig. 6, http://links.lww.com/CCX/B368).
DISCUSSION
Despite guidelines recommending opioids as initial treatments for ventilator-associated pain, little evidence exists describing contemporary ICU opioid prescribing practices. We evaluated IV opioid use during IMV for patients with ARF across two large multicenter healthcare settings (96 hospitals across the United States and 21 hospitals in the KPNC system). We showed that 31–53% of patients undergoing IMV for ARF received a continuous infusion of opioids with little correlation between opioid dose and dose of other sedatives. Hospital-level variation was substantially greater for opioid use and dose across the eRI sites than the KPNC integrated health system. In the context of efforts to limit healthcare-associated opioid exposure, our findings highlight: 1) the considerable opioid exposure that accompanies critical illness for respiratory failure, 2) potential over- and under-treatment with analgesia and sedation, and 3) wide practice variation that suggests the need for analgesia protocols to reduce unwarranted practice variation.
While prior multicenter studies have not evaluated opioid use in the U.S. adult population requiring IMV, our findings align with studies that have recorded doses of fentanyl equivalents used during IMV. Six randomized trials investigating sedation strategies during IMV showed fentanyl equivalent doses of 300–1000 µg/d (17), which is generally aligned with doses observed in our study. Another single-center study of patients mechanically ventilated for greater than 14 days showed average fentanyl doses of 580 µg/d (18). However, a single-center study of 100 mechanically ventilated patients identified higher rates of use of IV opioids (86%) and doses (2000–3000 µg/d fentanyl equivalents) (19). Thus, the combined evidence confirms that high doses of opioids are routinely administered during the care of patients with ARF. The risk-adjusted averages from our large multicenter cohorts may also have value to hospital systems for benchmarking and may be useful to inform usual care arms of analgesia and sedation clinical trials.
The considerable practice pattern variation for opioids across different ICUs outside of the integrated KPNC healthcare system is not surprising in the context of prior ICU clinician surveys. A survey of 30 ICUs across British Columbia identified that the most common institutional approach to sedation was individualized by clinician, rather than institutional protocols (20). Additionally, a survey of European ICUs showed that the sedation practices had the most variation among measured ICU practice, with 25% of ICUs lacking protocols for sedation management (21). Although we did not have information regarding the presence of sedation protocols among the eRI participating sites, KPNC hospitals shared a common order set across sites that includes decision logic related to analgesia. The low practice variation for use of analgesics at KPNC as compared with eRI hospitals suggests that idiosyncratic ICU practice variation potentially resulting in high dosages of IV opioids may be mitigated by standardized protocols for baseline care and the integrated structure of KPNC such as homogenous electronic health records. We also identified lower median daily equivalent doses in the eRI vs. KP cohorts but similar IQRs, maximum infusion rates and opioid durations. The differences in median daily equivalent dose between cohorts is explained by numerous “0s” in the eRI cohort (Supplemental Fig. 5, http://links.lww.com/CCX/B368) that are a result of patients receiving slightly shorter duration and lower dose infusions that, when calculating median daily equivalents, results in 0 µg, rather than the mostly nonzero, low values observed in the KP cohort. The large variation in opioid use and dose in the nonintegrated eRI cohort may also suggest both under- and over-treatment with analgesia and sedation medications and may warrant standardized protocols to reduce unwarranted practice pattern variation.
The lack of strong correlation between doses of opioids and doses of commonly used sedative agents used for each patient suggests that sedatives are titrated separately during mechanical ventilation. We did not observe that high doses of opioids correlated with low doses of other sedatives, suggesting that the opioid-centered analgesic-sedation approach recommended by guidelines has not been routinely adopted (9). However, factors correlated with higher opioid doses included ICU procedures that may be associated with pain or discomfort. Again, these findings suggest a predominance of a combined analgesic-sedation approach driven by individual practices. Studies examining the comparative effectiveness of different sedation strategies, including opioid predominant strategies, should be conducted in the critical care setting (22–24).
Our study has notable strengths and limitations. Strengths include granular electronic health record practice data across greater than 90 hospitals across the United States and type of healthcare delivery system. Limitations include lack of reliable data regarding depth of sedation targets and scores, enteral opioid use and dose, pain and agitation measures, delirium scores, and missingness across some data fields. In addition, we used a last-value carried forward approach (for a 3-hr interval) in instances where a patient’s final opioid infusion rate was not 0. It is possible that this approach led to over- or underestimated opioid infusion doses. However, we were able to do chart review-level granularity in the KPNC data to confirm that this assumption made sense, using nursing notes and medication flow charts for when sedation was being held for spontaneous awakening trials and/or neurologic examinations. The 8-year inclusion period for our study overlapped with release of the ICU Liberation Bundle (25). It is unclear how hospital-level adoption of the bundle contributed to variation in opioid use and dose. We were unable to quantify nonmedication-based analgesic strategies and nonopioid medications (e.g., ketorolac, acetaminophen). Future studies should seek to understand associations between these alternative pain control strategies and the need and dose of opioid analgesics. We did not include ICU type, the specialty of care providers, U.S. census region, teaching status, or cultural expressions of pain in models. Thus, it is possible that these unmeasured variables could explain some of the idiosyncratic variation in practice attributed to hospital of admission. We did not capture differences in opioids based on day vs. night shifts. It is possible that opioids were used differently during the night to facilitate sleep. Future studies should seek to compare opioid dosing strategies between day and night ICU shifts. Although IMV for ARF is extremely common in ICUs, it is unclear if our results generalize to patients with other indications for endotracheal intubation (e.g., surgery, airway control). We included opioid data for up to 21 days of IMV, encompassing both acute critical illness and chronic critical illness (> 14 d). However, only a small portion of our included cohort received IMV for greater than 14 days. Thus, our results largely reflect opioid use practices during acute critical illness.
In conclusion, we characterized opioid use for patients with ARF—a diagnosis that affects more than half of patients admitted to ICUs (8)—who received mechanical ventilation across the U.S. opioids were used in up to half of patients with wide variation in use and opioid dose by ICU and type of healthcare system. Our findings benchmark national practice and provide motivation for future studies comparing short- and long-term outcomes—including post-ICU opioid use of high- vs. low-dose opioid strategies during mechanical ventilation.
ACKNOWLEDGMENTS
We acknowledge the Strategic Programming Group at Kaiser Permanente Northern California Division of Research, especially David Schlessinger, and the programmers of Philips who generated the cohorts used in the article.
Supplementary Material
Footnotes
Drs. Myers and Bosch are co-first authors.
"Dr. Campbell has received support managed through her institution" from the Industry Post Marketing Requirement Consortium, a consortium of companies working together to conduct postmarketing studies required by the Food and Drug Administration that assess risks related to opioid analgesic use. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Some of the data included in this study were presented in abstract form at the American Thoracic Society Conference, San Diego, CA, May 2024 and the Society for Critical Care Medicine Congress Meeting, Phoenix, AZ, January 2024.
This study was supported by the National Institute of Health/National Heart, Lung, and Blood Institute grant number R01HL151607.
Drs. Walkey, Bosch, Myers, and Liu were involved in conceptualization. Ms Soltesz, Dr. Schwager, Dr. Salvati, and Dr. Jafarzadeh were involved in data curation. Dr. Jafarzadeh and Ms Soltesz were involved in formal analysis. Drs. Walkey and Liu were involved in funding acquisition. Drs. Walkey, Bosch, Liu, and Myers were involved in investigation. Drs. Walkey, Liu, Bosch, Myers, and Stevens were involved in methodology. Ms Daly was involved in project administration. Drs. Liu and Walkey were involved in resources. Dr. Jafarzadeh was involved in software. Drs. Walkey, Liu, Bosch, and Myers were involved in supervision. Drs. Walkey, Liu, Bosch, and Myers were involved in validation. Drs. Bosch, Myers, Walkey, Liu, Campbell, and Wunsch were involved in visualization. Drs. Bosch, Myers, Walkey, and Liu were involved in writing—original draft. All authors were involved in writing—review & editing.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Nicholas A. Bosch, Email: nabosch@bu.edu.
Lauren Soltesz, Email: Lauren.Soltesz@kp.org.
Kathleen A. Daly, Email: kathleen.daly@kp.org.
Cynthia I. Campbell, Email: Cynthia.I.Campbell@kp.org.
Emma Schwager, Email: emma@schwager.us.com.
Emmanuele Salvati, Email: Emmanuele.Salvati@philips.com.
Jennifer P. Stevens, Email: jpsteven@bidmc.harvard.
Hannah Wunsch, Email: Hannah.Wunsch@sunnybrook.ca.
Justin M. Rucci, Email: Justin.Rucci@bmc.org.
S. Reza Jafarzadeh, Email: srjafarz@bu.edu.
Vincent X. Liu, Email: Vincent.X.Liu@kp.org.
Allan J. Walkey, Email: Allan.walkey1@umassmed.edu.
REFERENCES
- 1.Centers for Disease Control and Prevention: Understanding the Opioid Overdose Epidemic. 2024. Available at: https://www.cdc.gov/opioids/basics/epidemic.html. Accessed June 12, 2023 [Google Scholar]
- 2.Barnett ML, Olenski AR, Jena AB: Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376:663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brummett CM, Waljee JF, Goesling J, et al. : New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicero TJ, Ellis MS, Surratt HL, et al. : The changing face of heroin use in the United States: A retrospective analysis of the past 50 years. JAMA Psychiatry 2014; 71:821–826 [DOI] [PubMed] [Google Scholar]
- 5.Lankenau SE, Teti M, Silva K, et al. : Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy 2012; 23:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jena AB, Goldman D, Karaca-Mandic P: Hospital prescribing of opioids to Medicare beneficiaries. JAMA Intern Med 2016; 176:990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SH, Stoicea N, Soghomonyan S, et al. : Intraoperative use of remifentanil and opioid induced hyperalgesia/acute opioid tolerance: Systematic review. Front Pharmacol 2014; 5:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JL, Akça S, De Mendonça A, et al. ; SOFA Working Group. Sequntial organ failure assessment: The epidemiology of acute respiratory failure in critically ill patients(*). Chest 2002; 121:1602–1609 [DOI] [PubMed] [Google Scholar]
- 9.Barr J, Fraser GL, Puntillo K, et al. ; American College of Critical Care Medicine: Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41:263–306 [DOI] [PubMed] [Google Scholar]
- 10.Devlin JW, Skrobik Y, Gelinas C, et al. : Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 11.UptoDate: Approximate Dose Conversions for Commonly Used Opioids. Available at: https://www.uptodate.com/contents/image?imageKey=PALC%2F111216&topicKey=PALC%2F86302&source=see_link. Accessed June 12, 2023 [Google Scholar]
- 12.Nelson JE, Cox CE, Hope AA, et al. : Chronic critical illness. Am J Respir Crit Care Med 2010; 182:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011; 30:377–399 [DOI] [PubMed] [Google Scholar]
- 14.R project: mitml: Tools for Multiple Imputation in Multilevel Modeling. 2023. Available at: https://cran.r-project.org/web/packages/mitml/index.html. Accessed June 14, 2023 [Google Scholar]
- 15.Boston University: Shared Computing Cluster. 2013. Available at: https://www.bu.edu/tech/support/research/computing-resources/scc/. Accessed October 1, 2023 [Google Scholar]
- 16.Kaiser Permanente Division of Research: About Division of Research. 2023. Available at: https://divisionofresearch.kaiserpermanente.org/about/. Accessed December 11, 2023 [Google Scholar]
- 17.Burry L, Rose L, McCullagh IJ, et al. : Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation. Cochrane Database Syst Rev 2014; 2014:CD009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karir V, Hough CL, Daniel S, et al. : Sedation practices in a cohort of critically ill patients receiving prolonged mechanical ventilation. Minerva Anestesiol 2012; 78:801–809 [PubMed] [Google Scholar]
- 19.John K, Cape K, Goodman L, et al. : Impact of the injectable opioid drug shortage on analgesia and sedation management in the medical intensive care unit: A retrospective cohort study. Hosp Pharm 2022; 57:160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu JA, Shergill M, Dhingra V, et al. : Variation in the management of pain, agitation, and delirium in intensive care units in British Columbia. Am J Crit Care 2020; 29:122–129 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen YL, Perrodeau E, Guidet B, et al. ; REVA network: Mechanical ventilation and clinical practice heterogeneity in intensive care units: A multicenter case-vignette study. Ann Intensive Care 2014; 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beloeil H, Laviolle B, Menard C, et al. ; SFAR research network: POFA trial study protocol: A multicentre, double-blind, randomised, controlled clinical trial comparing opioid-free versus opioid anaesthesia on postoperative opioid-related adverse events after major or intermediate non-cardiac surgery. BMJ Open 2018; 8:e020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavand’homme P, Estebe JP: Opioid-free anesthesia: A different regard to anesthesia practice. Curr Opin Anaesthesiol 2018; 31:556–561 [DOI] [PubMed] [Google Scholar]
- 24.Forget P: Opioid-free anaesthesia. Why and how? A contextual analysis. Anaesth Crit Care Pain Med 2019; 38:169–172 [DOI] [PubMed] [Google Scholar]
- 25.Ely EW: The ABCDEF bundle: Science and philosophy of how ICU liberation serves patients and families. Crit Care Med 2017; 45:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.