Abstract
The viral polymerase of influenza virus, a negative-strand RNA virus, is believed to polyadenylate the mRNAs by stuttering at a stretch of five to seven uridine residues which are located close to the 5′ ends of the viral RNA templates. However, a mechanism of polyadenylation based on a template-independent synthesis of the poly(A) tail has not been excluded. In this report, we present new evidence showing the inherent ability of the viral polymerase to stutter at the poly(U) stretch of a viral RNA template during RNA replication. Variants which possess 1- to 13-nucleotide-long insertions at the poly(U) stretch have been identified. These results support a stuttering mechanism for the polyadenylation of influenza virus mRNAs.
Transcription and replication of the RNA segments of influenza A virus are catalyzed by a virally encoded RNA-dependent RNA polymerase which contains three protein subunits, PB1, PB2, and PA. In addition, the viral nucleoprotein, or NP, which encapsidates the RNA templates, is also required for RNA synthesis. During replication, the viral RNA segments (vRNAs) are copied into cRNAs which, in turn, are templates for the synthesis of new copies of vRNA (5). By contrast, during transcription the vRNAs are copied into mRNAs. The mRNAs are not exact complementary copies of the vRNAs as they contain (i) a short 5′ cap sequence derived from cellular mRNAs and (ii) a poly(A) tail at the 3′ end (9). The cis-acting signals responsible for mRNA polyadenylation have been analyzed previously (3, 10, 11, 17), and it is believed that the addition of the poly(A) tail to the end of the mRNAs is achieved by stuttering of the viral polymerase at a stretch of five to seven uridine residues which are located near the 5′ end of the vRNA template (11, 18). However, to date there is no direct evidence available indicating that such a stuttering mechanism exists in vivo during synthesis of influenza virus mRNAs. In this communication, we present evidence that the polymerase of influenza virus stutters at or near the poly(U) stretch during RNA synthesis in infected cells.
Variants of the NAdD virus containing insertions in the neuraminidase (NA) gene.
Influenza A viruses have eight vRNA segments of negative polarity which contain at least one open reading frame (ORF) flanked by short noncoding regions. These untranslated regions contain the signals responsible for RNA replication, transcription, and polyadenylation and packaging of the RNA segments (12). Previously, we have generated a transfectant influenza virus, NAdD, in which the noncoding sequences at the 3′ and 5′ ends of the vRNA encoding the NA protein were modified by deleting 7 nucleotides (nt) upstream of the stop codon of the NA ORF and 4 nt downstream of the initiation codon of the NA ORF (Fig. 1) (22). The NAdD virus showed reduced replication (approximately 60-fold) of the NA-specific vRNA in infected cells compared with that in wild-type influenza A/WSN/33 virus (WSN) and grew to titers which were 2 logs lower than those of wild-type viruses (22). Five independent NAdD virus plaques were serially passaged 10 times in Madin-Darby bovine kidney (MDBK) cells. This experiment was conducted in order to investigate if compensatory mutations in the NA-specific vRNA would be selected, thus restoring wild-type levels of RNA replication. Liquid supernatants from the 10th passage were used to isolate vRNA and to clone and sequence the 3′ and 5′ noncoding regions of the NA vRNA. Sequences at the 3′ ends of the NA genes of the viruses were amplified by reverse transcription-PCR (RT-PCR) with in vitro 3′-polyadenylated RNA (22) as template and the primers 5′-GCGCAAGCTTCTAGATTTTTTTTTTTTTT-3′ and 5′-GCGCAAGCTTTATTGAGATTATATTTCC-3′, the latter containing nt 115 to 98 of the NA gene of WSN. Direct sequencing of the PCR products derived from the five passaged virus plaques revealed the same sequence at the 3′ noncoding region of the NA gene. This sequence was identical to that of the original NAdD transfectant virus.
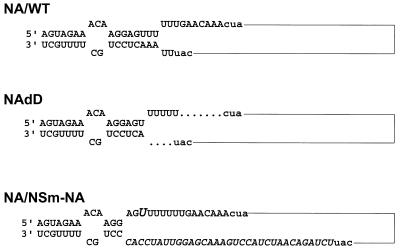
FIG. 1.
Schematic representation of NA gene-specific influenza virus RNAs. The wild-type sequence of the noncoding regions of the NA gene of WSN (NA/WT) is shown on the top. The sequence of the noncoding regions of the NAdD RNA is shown in the middle. The dots indicate the deleted nucleotides of the NA gene of the transfectant virus. The rescued NA/NSm-NA RNA is shown at the bottom. Altered sequences are in italics. For convenience, the 5′- and 3′-terminal sequences are shown in a panhandle configuration (11). Fork and/or corkscrew configurations are also possible (2, 8). The start and stop codons of the NA genes are indicated in lowercase. The lines represent the coding sequence of the NA gene.
The 5′ ends of the NA genes present in the supernatants of the five sequentially passaged NAdD virus plaques were sequenced by direct RNA sequencing with a primer complementary to nt 1280 to 1299 of the WSN NA gene. However, the sequence ladders were not clearly readable past nt 1394. This finding indicates the presence in the supernatants of a heterogeneous population of NA vRNAs containing different sequences at their 5′ ends. We then subjected to RT-PCR the 5′ ends of the NA genes present in the supernatants with, as primers, 5′-GTGGCAATAACTAATCGGTCA-3′ (complementary to nt 1151 to 1171 of the WSN NA gene) and 5′-ATGCTCTAGAAGCTTAGTAGAAACAAGG-3′ (containing the last conserved 13 nt at the 5′ end of every influenza A virus vRNA). PCR products were cloned into a pUC19-derived plasmid with SpeI and HindIII restriction sites. One or two clones derived from each of the five samples were sequenced. Sequences at the 5′ noncoding regions of the NA cDNAs are listed in Table 1. Only one sequence of nine analyzed clones was identical to that of the original NAdD virus. The other eight sequences contained 2- to 13-nt insertions at the poly(U) stretch. These findings suggest that the influenza virus RNA polymerase stutters in this region before resuming replication, resulting in short insertions in the newly synthesized RNA.
TABLE 1.
5′ noncoding sequences of the NA genes from virus-containing supernatants after 10 passages of the NAdD virusa
| Supernatant | cDNA | 5′ noncoding sequence of the NA vRNAb |
|---|---|---|
| S1-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUA3′ |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUU(U)13CUA 3′ | |
| S2-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUCUA3′c |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUA3′ | |
| S3-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUUCUA3′ |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUUCUA3′ | |
| S4-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUCUUCUCUA3′ |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUCUCUA3′ | |
| S5-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUCUCUA3′ |
Five independent virus plaques (S1, S2, S3, S4, and S5) were passaged 10 times in MDBK cells.
The first 13 nt which are conserved among influenza virus genes are derived from the primer. Insertions are shown in boldface; the stop codon of the NA ORF is underlined.
This sequence is identical to that of the original NAdD virus.
Isolation of viable NAdD variants.
In order to obtain clonal populations of infectious viruses from the analyzed supernatants, viruses from the supernatants after 10 liquid passages of five independent NAdD plaques (S1-10, S2-10, S3-10, S4-10, and S5-10 samples) were plaque purified in MDBK cells overlaid with agar. Two plaques from each sample were used for liquid infections of MDBK cells, and the 5′ ends of the NA genes present in the obtained samples were subjected to RT-PCR and sequenced as described above. The results are shown in Table 2. Of the 10 clones, only one sequence was identical to that of the 5′ end of the original NAdD virus. The other sequences contained the following insertions at the poly(U) stretch of the 5′ end of the NA vRNA: U (one sequence), UU (three sequences), UUCUUU (one sequence), UCUUCU (one sequence), and UUCUUCU (three sequences). The last three insertions seem to be originated by polymerase stuttering at a region of the template which includes not only the poly(U) stretch but also the adjacent C residue. These results suggest that viral polymerase stuttering may involve a template realignment mechanism similar to that proposed for RNA editing in paramyxoviruses (6).
TABLE 2.
5′ noncoding sequences of the NA genes of plaque-purified viruses derived from passage 10 supernatantsa of the NAdD virus
| Supernatant | Plaque | 5′ noncoding sequence of the NA vRNAb |
|---|---|---|
| S1-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUA3′ |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUA3′ | |
| S2-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUCUA3′c |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUCUA3′ | |
| S3-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUA3′ |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUUCUA3′ | |
| S4-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUCUUCUCUA3′ |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUCUCUA3′ | |
| S5-10 | 1 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUCUCUA3′ |
| 2 | 5′ AGUAGAAACAAGGAGUUUUUUUUCUUCUCUA3′ |
See text for passaging history.
The first 13 nt which are conserved among influenza virus genes are derived from the primer. Insertions are shown in boldface; the stop codon of the NA ORF is underlined.
This sequence is identical to that of the original NAdD virus.
Isolation of variants of NA/NSm transfectant virus.
We have obtained further evidence for viral polymerase stuttering at the poly(U) stretch of the vRNA template by using a different transfectant influenza virus. The NA gene of the NA/NSm-NA virus is derived from an engineered WSN NA gene in which the the 3′ noncoding sequence was modified (Fig. 1). In this engineered NA gene, positions 13, 14, and 15 at the 3′ end were changed to those present in the wild-type NS gene. In addition, we replaced the noncoding nucleotide after position 15 with a novel sequence of 30 nt. One single virus plaque was obtained from the tissue culture medium after ribonucleoprotein transfection of the NA/NSm-NA gene (1). After three plaque-to-plaque passages of the NA/NSm-NA transfectant virus, one single plaque was used for infecting four 35-mm-diameter dishes of MDBK cells. The resulting virus preparation was used for further analysis. The identity of the NA/NSm-NA transfectant virus was confirmed by sequencing the 3′ end of its NA gene. The sequence was identical to that of the transfected gene. However, RNA sequencing of the 5′ end of the NA gene of the NA/NSm-NA virus revealed the incorporation of one more U residue in the poly(U) stretch (Fig. 1). The NA/NSm-NA virus has a phenotype similar to that of the NAdD virus in MDBK cells (22). Specifically, the levels of NA-specific vRNA are reduced approximately fourfold in viruses and in infected cells compared to those of wild-type virus (4a).
Four samples of the NA/NSm-NA seed virus were independently passaged 10 times at 10−2 dilutions in MDBK cells, and two samples were also passaged 10 times in 10-day-old embryonated eggs. Viruses were then plaque purified, and the 3′ and 5′ noncoding regions of the NA genes of passaged viruses (TC2, TC3, TC5, TC6, E1, and E2) were sequenced. No changes were detected at the 3′ ends of the NA genes. However, the sequence of the 5′ ends of the NA genes of the passaged viruses revealed several mutations (Table 3). In two cases (TC2 and TC6), a G residue was inserted at position 16 at the 5′ end. In two other cases (TC3 and TC5), two residues of the poly(U) stretch were mutated to a G residue. In one case (E2), the U16 was mutated to a G and the A14 was deleted. The E1 virus had an insertion of a triplet (GUU) at positions 23 to 25 at the 5′ end. Some of the observed changes, and even the presence of an extra U residue in the original NA/NSm-NA virus, can be explained by stuttering of the viral polymerase at or near the poly(U) stretch of the vRNA template. In contrast to the NAdD variants, several NA/NSm-NA variants have mutations at the 5′ end of the poly(U) stretch. It is possible that the sequence context of the poly(U) stretch favors the generation of different mutations. It should be noted that, in the case of RNA editing in paramyxoviruses, the sequence context also determines the stuttering pattern during RNA synthesis (7).
TABLE 3.
Noncoding sequences at the 5′ ends of the NA genes of plaque-purified variants obtained from the NA/NSm-NA transfectant virus after passage 10
| Virus | 5′ noncoding sequence of the NA vRNAa |
|---|---|
| NA/NSm-NA | 5′ AGUAGAAACAAGGAGUUUUUUUGAACAAACUA3′ |
| TC2b | 5′ AGUAGAAACAAGGAGGUUUUUUUGAACAAACUA3′ |
| TC3 | 5′ AGUAGAAACAAGGAGG·UUUUUGAACAAACUA3′ |
| TC5 | 5′ AGUAGAAACAAGGAGG·UUUUUGAACAAACUA3′ |
| TC6 | 5′ AGUAGAAACAAGGAGGUUUUUUUGAACAAACUA3′ |
| E1b | 5′ AGUAGAAACAAGGAGUUUUUUUGUUGAACAAACUA3′ |
| E2 | 5′ AGUAGAAACAAGG·GGUUUUUUGAACAAACUA3′ |
The first 13 nt which are conserved among influenza virus genes are derived from the primer. The stop codon of the NA ORF is underlined. Insertions are shown in boldface. Nucleotide mutations are shown in italics. Nucleotide deletions are represented by dots.
TC and E designate virus obtained after 10 passages in tissue culture (MDBK cells) and embryonated eggs, respectively.
Variants of the NAdD virus generated during multicycle replication.
We then analyzed whether different variants of the NA segment of NAdD virus could be detected after one single passage in MDBK cells with a low multiplicity of infection. MDBK cells were infected at a multiplicity of infection of 0.01 with NAdD virus or with wild-type WSN virus. Two days postinfection, cells were harvested and washed with phosphate-buffered saline, and total RNA was extracted with an RNAzol isolation kit (Tel-Test, Inc., Friendswood, Tex.). The RNA was subjected to RT-PCR as described above, in order to amplify the 5′ ends of the NA-specific RNA segments. As a control for potential errors during the RT-PCR, we used RNA synthesized in vitro by T3 RNA polymerase from plasmid pT3NAdD, containing the cDNA of the NA segment of NAdD virus. PCR products were cloned into pUC19 with SpeI and HindIII restriction sites, and several clones were used for sequencing reactions with dideoxyribosylthymine termination mix and a primer annealing downstream from the polylinker of pUC19. This experiment allowed us to determine the presence of insertions or deletions in the 5′ noncoding region of the NA segments. No changes were seen in 25 clones derived from T3 RNA and in 28 clones derived from WSN virus-infected cells. By contrast, the analysis of 20 clones derived from NAdD virus-infected cells revealed the insertion of two uridine residues at the poly(U) stretch in 14 clones, the insertion of seven uridine residues in one clone, and no changes in the remaining five clones.
Discussion.
A poly(U) stretch near the 5′ end of the vRNAs of influenza virus is required for the polyadenylation of the synthesized mRNAs (10). Interestingly, polyadenylation has not been observed before during cRNA synthesis. It is possible that the use of a capped primer for the initiation of RNA synthesis during transcription determines the subsequent polyadenylation of the newly synthesized RNA. Nevertheless, the influenza virus RNA polymerase must choose to ignore the polyadenylation signal during replication in order to synthesize a correct cRNA. Our sequencing data suggest that, in some instances, the viral polymerase makes a wrong choice and stutters at the poly(U) stretch of the vRNA during replication. It is possible that this happens more frequently than previously thought, since in most cases such polymerase errors will result in a nonfunctional cRNA template that will not be amplified. We propose that the insertions described in Tables 1 and 2 are due to the inherent ability of the influenza virus polymerase to stutter at the poly(U) stretch of the vRNAs, supporting a stuttering mechanism for the polyadenylation of the mRNAs of influenza virus.
It is not understood why the modified NA genes of NAdD and NA/NSm-NA viruses have reduced levels of replication. Most likely, the cis-acting signals and/or structures responsible for the replication of the NA vRNA (2, 4, 13–15, 19–22) have been altered by the engineered modifications. Passaging of these two viruses resulted in an accumulation of mutations at the 5′ end of the NA gene. Why is it that this frequent insertion of nucleotides at the poly(U) stretch has not been observed previously? We suggest that the reduced replication levels of the NA genes of the NAdD and NA/NSm-NA viruses allow the identification of other genes (viruses) with a reduced ability to replicate. In the presence of the wild-type NA, this “optimal” gene will easily outcompete mutants with insertions which would be replicated to lower levels. Alternatively, we cannot exclude the possibility that some of the novel mutant viruses have a selective advantage over the original (mutant) viruses. However, we did not find a reversion to wild-type levels of replication of the NA gene in these mutants (data not shown). In addition, all mutant viruses grew to titers in MDBK cells which were similar to those of the parental virus (approximately 2 logs less than that of wild-type WSN virus).
Most of the observed mutations consisted of short insertions close to the poly(U) stretch of the NA vRNA, and they were most likely generated by a stuttering viral polymerase. We postulate that the viral polymerase approaching this site has a high propensity to stutter during the synthesis of the cRNAs (and not only the mRNAs). Although we cannot exclude the possibility that extra nucleotides are added during vRNA synthesis, we favor the idea that the “actual” stuttering occurs during cRNA synthesis. Stuttering of the viral polymerase at other U-rich stretches located inside the ORFs of the influenza virus RNAs is avoided because the position of the poly(U) stretch with respect to the panhandle-fork structure appears to be a critical requirement (10).
Some of the mutated NA genes might not result in the formation of viable virus. For example, two of the obtained NA sequences revealed poly(U) stretches of 13 and 19 U residues. Poly(U) stretches of more than eight U residues inserted into a model RNA have previously been shown to be responsible for the loss of transcriptional activity (10). It is also possible that in some cases the polymerase starts to stutter during replication at the poly(U) stretch and then falls off, resulting in the synthesis of a nonfunctional cRNA template. Our method of analysis would not have detected the presence of these molecules. A higher replication error frequency of the NA vRNAs of the NAdD and NA/NSm-NA viruses might contribute in part to the lower levels of replication of these genes.
Our results suggest that, at least in the case of the NAdD and NA/NSm-NA transfectant viruses, the viral RNA polymerase can stutter during replication of cRNA molecules. Insertions in the cRNA occur opposite the poly(U) stretch of the NA-specific vRNA template. Particularly revealing are the cases when nucleotides close to the poly(U) stretch are also copied more than once during replication, which argues for template-directed synthesis. The inherent ability of the polymerase to stutter at the poly(U) stretch favors the idea that the poly(A)s of the influenza virus mRNAs are also synthesized by polymerase stuttering and not by template-independent addition of A residues. Our hypothesis is based on the assumption that transcription and replication of the influenza virus RNAs are related processes. A stuttering mechanism of polyadenylation for mRNAs is in agreement with a current model of transcription of influenza virus genes which proposes that the RNA polymerase of influenza virus, bound to the 5′ end of a vRNA template, acts in cis to polyadenylate the mRNA (16).
Acknowledgments
This work was partially supported by grants from the National Institutes of Health to A.G.-S. and P.P.
ADDENDUM IN PROOF
In the April issue of this journal, Poon et al. (L. L. M. Poon, D. C. Pritlove, E. Fodor, and G. G. Brownlee, J. Virol. 73:3473–3476, 1999) published an article that arrives at similar conclusions, based on a different experimental approach.
REFERENCES
- 1.Enami M, Palese P. High-efficiency formation of influenza virus transfectants. J Virol. 1991;65:2711–2713. doi: 10.1128/jvi.65.5.2711-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flick R, Neumann G, Hoffmann E, Neumeier E, Hobom G. Promoter elements in the influenza vRNA terminal structure. RNA. 1996;2:1046–1057. [PMC free article] [PubMed] [Google Scholar]
- 3.Fodor E, Palese P, Brownlee G G, García-Sastre A. Attenuation of influenza A virus mRNA levels by promoter mutations. J Virol. 1998;72:6283–6290. doi: 10.1128/jvi.72.8.6283-6290.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fodor E, Pritlove D C, Brownlee G G. Characterization of the RNA-fork model of virion RNA in the initiation of transcription in influenza A virus. J Virol. 1995;69:4012–4019. doi: 10.1128/jvi.69.7.4012-4019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.García-Sastre, A., and H. A. Lee. Unpublished results.
- 5.García-Sastre A, Palese P. Genetic manipulation of negative-strand RNA virus genomes. Annu Rev Microbiol. 1993;47:765–790. doi: 10.1146/annurev.mi.47.100193.004001. [DOI] [PubMed] [Google Scholar]
- 6.Hausmann S, Garcin D, Morel A S, Kolakofsky D. Two nucleotides immediately upstream of the essential A6G3 slippery sequence modulate the pattern of G insertions during Sendai virus mRNA editing. J Virol. 1999;73:343–351. doi: 10.1128/jvi.73.1.343-351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacques J P, Hausmann S, Kolakofsky D. Paramyxovirus mRNA editing leads to G deletions as well as insertions. EMBO J. 1994;13:5496–5503. doi: 10.1002/j.1460-2075.1994.tb06884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H J, Fodor E, Brownlee G G, Seong B L. Mutational analysis of the RNA-fork model of the influenza A virus vRNA promoter in vivo. J Gen Virol. 1997;78:353–357. doi: 10.1099/0022-1317-78-2-353. [DOI] [PubMed] [Google Scholar]
- 9.Lamb R A, Krug R M. Orthomyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 2nd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1353–1395. [Google Scholar]
- 10.Li X, Palese P. Characterization of the polyadenylation signal of influenza virus RNA. J Virol. 1994;68:1245–1249. doi: 10.1128/jvi.68.2.1245-1249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo G X, Luytjes W, Enami M, Palese P. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J Virol. 1991;65:2861–2867. doi: 10.1128/jvi.65.6.2861-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luytjes W, Krystal M, Enami M, Parvin J D, Palese P. Amplification, expression, and packaging of a foreign gene by influenza virus. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- 13.Neumann G, Hobom G. Mutational analysis of influenza virus promoter elements in vivo. J Gen Virol. 1995;76:1709–1717. doi: 10.1099/0022-1317-76-7-1709. [DOI] [PubMed] [Google Scholar]
- 14.Parvin J D, Palese P, Honda A, Ishihama A, Krystal M. Promoter analysis of influenza virus RNA polymerase. J Virol. 1989;63:5142–5152. doi: 10.1128/jvi.63.12.5142-5152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccone M E, Fernandez-Sesma A, Palese P. Mutational analysis of the influenza virus vRNA promoter. Virus Res. 1993;28:99–112. doi: 10.1016/0168-1702(93)90129-b. [DOI] [PubMed] [Google Scholar]
- 16.Poon L L, Pritlove D C, Sharps J, Brownlee G G. The RNA polymerase of influenza virus, bound to the 5′ end of virion RNA, acts in cis to polyadenylate mRNA. J Virol. 1998;72:8214–8219. doi: 10.1128/jvi.72.10.8214-8219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritlove D C, Poon L L, Fodor E, Sharps J, Brownlee G G. Polyadenylation of influenza virus mRNA transcribed in vitro from model virion RNA templates: requirement for 5′ conserved sequences. J Virol. 1998;72:1280–1286. doi: 10.1128/jvi.72.2.1280-1286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson J S, Schubert M, Lazzarini R A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981;38:157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seong B L, Brownlee G G. A new method for reconstituting influenza polymerase and RNA in vitro: a study of the promoter elements for cRNA and vRNA synthesis in vitro and viral rescue in vivo. Virology. 1992;186:247–260. doi: 10.1016/0042-6822(92)90079-5. [DOI] [PubMed] [Google Scholar]
- 20.Tiley L S, Hagen M, Matthews J T, Krystal M. Sequence-specific binding of the influenza virus RNA polymerase to sequences located at the 5′ ends of the viral RNAs. J Virol. 1994;68:5108–5116. doi: 10.1128/jvi.68.8.5108-5116.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanaka K, Ogasawara N, Yoshikawa H, Ishihama A, Nagata K. In vivo analysis of the promoter structure of the influenza virus RNA genome using a transfection system with an engineered RNA. Proc Natl Acad Sci USA. 1991;88:5369–5373. doi: 10.1073/pnas.88.12.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H, Palese P, García-Sastre A. Nonconserved nucleotides at the 3′ and 5′ ends of an influenza A virus RNA play an important role in viral RNA replication. Virology. 1996;217:242–251. doi: 10.1006/viro.1996.0111. [DOI] [PubMed] [Google Scholar]