Abstract
Glycosphingolipids from human erythrocytes mediate CD4-dependent fusion with cells expressing human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins. To identify the glycosphingolipid(s) which participates in the fusion process, we have analyzed the interaction of HIV-1 gp120 (X4 and R5X4 isolates) with reconstituted membrane microdomains of human erythrocyte glycosphingolipids. We identified globotriaosylceramide (Gb3) and ganglioside GM3 as the main glycosphingolipids recognized by gp120. In the presence of CD4, Gb3 interacted preferentially with the X4 gp120, whereas GM3 interacted exclusively with the R5X4 gp120. These data suggest that glycosphingolipid microdomains are required in CD4-dependent fusion and that Gb3 and/or GM3 may function as alternative entry cofactors for selected HIV-1 isolates.
The entry of human immunodeficiency virus type 1 (HIV-1) into cells requires the sequential interaction of the viral surface envelope glycoprotein gp120 with the CD4 receptor and a coreceptor (or a fusion cofactor) on the cell surface (3). The coreceptors identified so far for HIV and simian immunodeficiency virus include chemokine receptors (mainly CXCR4, CCR5, CCR3, and CCR2b) and a series of orphan receptors, including virus-encoded receptors, all belonging to the family of seven-transmembrane domain receptors (3, 6, 8, 11, 19, 24, 26). Following a primary interaction with CD4, a conformational change in gp120 renders cryptic regions of the viral glycoprotein (including the V3 domain [17]) available for secondary interactions with either CXCR4 or CCR5 (2, 3, 7). Since seven-transmembrane domain receptors are almost flush with the cell membrane, binding of gp120 to the coreceptor is necessary to move the viral spike close to the target membrane (2). Finally, gp120-coreceptor interactions trigger additional conformational changes in the HIV-1 envelope glycoprotein trimer that lead to exposure of the fusion peptide at the N terminus of the transmembrane glycoprotein gp41 (2). Since coreceptors are important determinants of virus tropism, HIV-1 isolates are functionally classified with respect to their ability to use a given coreceptor (1): for instance, viruses using CXCR4 but not CCR5 are referred to as X4, whereas isolates using CCR5 but not CXCR4 are called R5. Dualtropic viruses able to use either CXCR4 or CCR5 are referred to as R5X4.
Most striking is the observation that fusion of either protease- or heat-treated human erythrocyte membranes with murine cells expressing human CD4 renders these cells competent for HIV-1 envelope-mediated membrane fusion (9, 21). These data show that human erythrocyte membranes contain one or more HIV-1 entry cofactors. As a matter of fact, human erythrocytes express the Duffy blood group antigen, which is a promiscuous chemokine receptor (19). Nevertheless, coexpression of Duffy and human CD4 in nonhuman cells failed to support HIV-1-induced fusion (8). Moreover, it has been recently reported that nonhuman cells expressing human CD4 become competent for CD4-dependent HIV-1 fusion following transfer of human erythrocyte glycosphingolipids (22). Taken together, these data suggest that human erythrocyte glycosphingolipids can serve as alternative cofactors in CD4-dependent HIV-1 fusion (7).
To identify these cofactors, we have purified the main glycosphingolipids expressed by human erythrocytes and analyzed their interaction with HIV-1 gp120. The neutral and acidic glycosphingolipids extracted from human erythrocytes were partitioned in accordance with the Folch procedure and resolved by high-performance thin-layer chromatography (5). The neutral glycosphingolipids of the Folch lower phase were composed mainly of four types of lipids: ceramide monohexoside (GlcCer), ceramide dihexoside (LacCer), ceramide trihexoside (Gb3), and tetraosylceramide (Gb4). The acidic fraction of the Folch partition (aqueous upper phase) contained essentially monosialylated gangliosides, including GM1 and GM3. The identification of GlcCer, LacCer, Gb3, Gb4, GM1, and GM3, based on their chromatography mobility with authentic glycosphingolipid standards purified from human erythrocytes, is consistent with previous analysis of glycosphingolipid expression in human erythrocytes (12). All of these glycosphingolipids were purified by preparative high-performance thin-layer chromatography (29).
In the outer leaflet of the plasma membrane, glycosphingolipids organize into moving platforms, or rafts, on which specific proteins attach within the bilayer (27). This lateral organization probably results from preferential packing of sphingolipids and cholesterol, resulting in the formation of membrane microdomains. To analyze glycosphingolipid-gp120 interactions, a reconstituted monolayer of purified glycosphingolipid was prepared at the air-water interface as a model for a glycosphingolipid membrane microdomain (13, 25). To determine whether the glycosphingolipid organized into a monomolecular film, isotherms (i.e., the variations of surface pressure according to apparent molecular area) were recorded at the air-water interface for each purified glycosphingolipid. The high compressibility of the glycosphingolipids at all film pressures and the absence of discontinuities in their isotherms show that they exist in the liquid-expanded state up to film collapse. In our experiments, glycosphingolipid monolayers were prepared at an initial pressure of 10 mN/m, corresponding to the pressure of a compressible film (18). The interaction of gp120 with the glycosphingolipid patch was detected by measuring the variations of the interfacial pressure with a Langmuir film balance, as previously reported (13, 14).
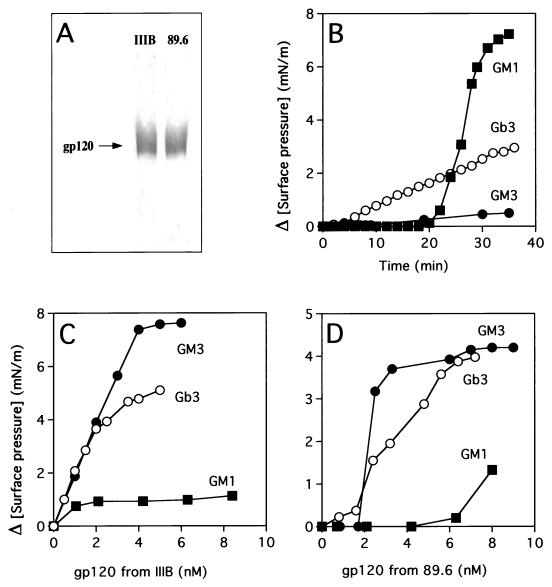
The surface envelope glycoproteins used in this study were the recombinant gp120 (T-cell-line-adapted X4 virus, isolate IIIB) produced in CHO cells (13), gp120 from HIV-1(LAI) (T-cell-line-adapted X4 virus), and gp120 from HIV-1(89.6), a dualtropic primary R5X4 virus (8). HIV-1(LAI) and HIV-1(89.6) were produced in peripheral blood mononuclear cells as described elsewhere (13). The viral glycoproteins were purified by lectin affinity chromatography, as reported previously (13). Each preparation was found to be highly purified as determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (Fig. 1A). The concentration of gp120 was determined by an enzyme-linked immunosorbent assay using rabbit polyclonal anti-gp120 antibodies as described before (13). None of these gp120s affected the surface pressure of monolayers of GlcCer, LacCer, Gb4, or GM1 purified from human erythrocytes. However, GM1 was specifically recognized by cholera toxin (Fig. 1B), in agreement with previous data obtained with monomolecular films of this glycosphingolipid (4). As shown in Fig. 1, a dose-dependent increase of the surface pressure occurred upon addition of gp120 from both isolates IIIB (Fig. 1C) and 89.6 (Fig. 1D) under a monolayer of Gb3 (globotriaosylceramide [Galα1-4Galβ1-4Glc-Cer]) or the ganglioside GM3 (NeuAcα2-3Galβ1-4Glc-Cer). These data demonstrate that the viral glycoproteins interact specifically with the reconstituted membrane microdomains of Gb3 and GM3. Indeed, cholera toxin used as a control protein in these experiments did not interact with GM3 and showed a weak interaction with Gb3 (Fig. 1B).
FIG. 1.
Interaction of HIV-1 gp120 (IIIB and 89.6 isolates) with glycosphingolipid monolayers. (A) The SDS-gel electrophoresis analysis of purified HIV-1 gp120s (from isolates IIIB and 89.6) is shown. Five micrograms of purified proteins was electrophoresed on a 10% polyacrylamide gel in the presence of SDS. The proteins were stained with Coomassie blue. A unique diffuse band typical of a glycoprotein with an apparent molecular mass of 120 kDa was observed in both cases. (B to D) Monolayers of Gb3, GM3, or GM1 purified from human erythrocytes were prepared at an initial surface pressure of 10 mN/m. The data show the variations of surface pressure induced by the addition of various concentrations of HIV-1 gp120 from isolate IIIB (C) or 89.6 (D). The kinetics of interaction between glycosphingolipid monolayers and cholera toxin (1 μg/ml) are also shown (B).
In the plasma membrane of human T lymphocytes, CD4 and GM3 are colocalized in the same detergent-insoluble microdomain (28). Using a reconstituted membrane patch of GM3 at the air-water interface, we recently demonstrated that soluble CD4 interacts with GM3 and that the binding of gp120 to CD4 complexed with GM3 unmasks the V3 domain of gp120, allowing secondary interactions between V3 amino acids and the GM3 patch (14). The soluble form of CD4 used in these experiments is a four-domain CD4 lacking the transmembrane domain of the protein (kindly provided by the Medical Research Council, Oxford, United Kingdom). Therefore, it was important to study how this receptor interacts with reconstituted glycosphingolipid microdomains. The increase in surface pressure upon addition of CD4 beneath a GM3 monolayer is interpreted as an insertion of CD4 between GM3 molecules (14). To rule out the possibility that the surface pressure increase could be due to an impurity in the recombinant preparation, CD4 was depleted with anti-CD4 antibodies (MT-151; Boehringer, Mannheim, Germany) and protein A-Sepharose. The CD4-depleted supernatant no longer reacted with the GM3 monolayer. In contrast, the activity of CD4 was not altered by immunoprecipitation with control anti-HLA antibodies (data not shown). Moreover, when CD4 was preincubated with 3′-sialyllactose (the oligosaccharide corresponding to the sugar moiety of GM3; Oxford GlycoSystems, Abington, United Kingdom), there was no increase in the surface pressure of the GM3 monolayer (Table 1). The specificity of 3′-sialyllactose as an inhibitor of GM3-CD4 association is demonstrated by the incapacity of the oligosaccharide to affect the interaction of cholera toxin with its ganglioside receptor, GM1 (Table 1). Taken together, these data suggest that CD4 binds to GM3 through a specific interaction with its sugar moiety.
TABLE 1.
Effect of 3′-sialyllactose on the interaction between CD4 or cholera toxin and ganglioside monolayersa
| Protein | Maximal surface pressure variation (mN/m) upon interaction withb:
|
|
|---|---|---|
| GM1 | GM3 | |
| Cholera toxin | 8.2 | 0.5 |
| Cholera toxin + 3′-sialyllactose | 8.4 | ND |
| CD4 | 0.6 | 9.2 |
| CD4 + 3′-sialyllactose | ND | 0.4 |
Cholera toxin (1 μg/ml) or soluble CD4 (1 ng/ml) was preincubated for 30 min at room temperature with 3′-sialyllactose (1.85 mg/ml) or with buffer alone. The proteins were then added beneath a monolayer of GM1 or GM3 at an initial surface pressure of 10 to 11 mN/m.
The variations in surface pressure were measured after equilibrium was reached, and the maximal value is indicated. ND, not determined.
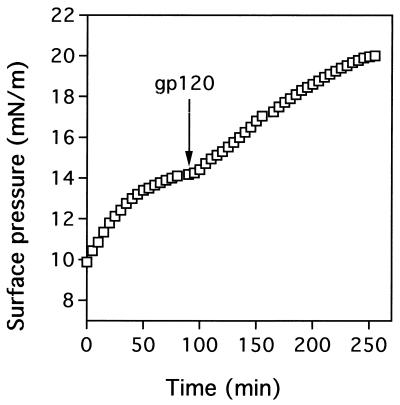
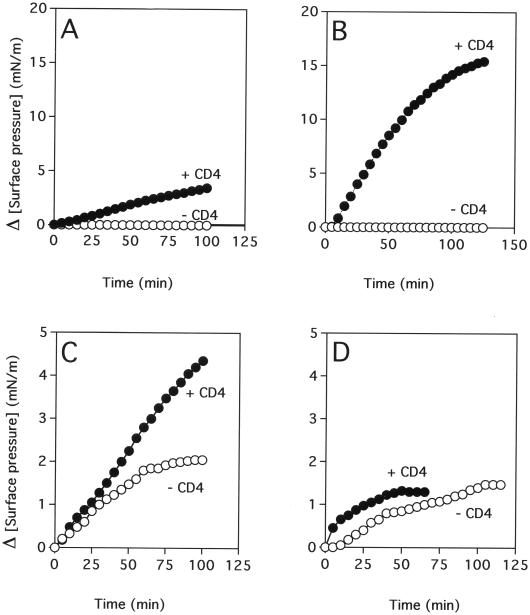
The sequential interaction of CD4 and gp120 with the glycosphingolipid microdomain induces a biphasic increase in the surface pressure. The first response corresponds to the insertion of CD4 in the patch, and the second one corresponds to the CD4-induced penetration of gp120, through its V3 domain, in the glycosphingolipid monolayer. In a typical experiment (Fig. 2), recombinant soluble CD4 (0.5 ng/ml) is added first under a monolayer of glycosphingolipid (Gb3 in this case). After a plateau value in surface pressure is reached, gp120 (from isolate IIIB in this case) is added at a concentration of 1.85 nM and the variations in surface pressure are measured from the time of this second input. Similar experiments were performed with a monolayer of GM3. For comparison, the surface pressure increase induced by gp120 (1.85 nM) in the absence of CD4 is also presented (Fig. 3). As previously reported (14), the penetration of the IIIB gp120 in a GM3 patch is stimulated by CD4 (maximal surface pressure increase of 3.5 mN/m) (Fig. 3A). However, the effect of CD4 is far more pronounced with gp120 from isolate 89.6 (maximal surface pressure increase of 16.0 mN/m in the presence of CD4), which at a concentration of 1.85 nM, does not interact with GM3 in the absence of CD4 (Fig. 3B). In marked contrast with these data, the gp120 from isolate 89.6 shows, at this concentration, a very poor interaction with a reconstituted patch of Gb3 in the absence or presence of CD4 (Fig. 3D). Yet Gb3 interacts with the IIIB gp120 in a CD4-dependent manner (maximal surface pressure increases of 5.8 mN/m in the presence of CD4 and only 2.1 mN/m without CD4) (Fig. 3C).
FIG. 2.
CD4-induced interaction of HIV-1(IIIB) gp120 with a reconstituted microdomain of Gb3. At time zero, recombinant CD4 (0.5 ng/ml) was added beneath a monolayer of Gb3 prepared at an initial surface pressure of 10 mN/m. The increase in surface pressure is due to the interaction of CD4 with the Gb3 monolayer. After the surface pressure reached a plateau value, HIV-1(IIIB) gp120 was added at a concentration of 1.85 nM. Secondary gp120-Gb3 interactions are evidenced by a second phase of surface pressure increase.
FIG. 3.
CD4-induced interaction of HIV-1 gp120 with glycosphingolipid monolayers. Recombinant CD4 (0.5 ng/ml) was added beneath a GM3 (A and B) or Gb3 (C and D) monolayer prepared at an initial surface pressure of 10 mN/m. The insertion of CD4 into the GM3 or Gb3 monolayer induced a mean increase in surface pressure of 3 to 5 mN/m (Fig. 2). After stabilization of the monolayer, gp120 from IIIB (A and C) or 89.6 (B and D) was added at a concentration of 1.85 nM. The data show the variations of surface pressure induced by gp120. For comparison, the surface pressure increase induced by gp120 (1.85 nM) alone at an initial surface pressure of 13 mN/m is indicated.
Taken together, these data confirm that reconstituted glycosphingolipid microdomains are recognized by gp120s from various HIV-1 isolates (13). CD4 interacts with GM3 and Gb3 and induces glycosphingolipid-dependent interactions with gp120 from selected HIV-1 isolates. In the case of HIV-1(IIIB) gp120, these secondary interactions with GM3 are specifically abrogated with 3′-sialyllactose and monoclonal antibodies against the V3 loop, suggesting that charged V3 amino acid residues interact with the oligosaccharide moiety of the glycosphingolipid (reference 14 and data not shown). In the presence of CD4, gp120 from IIIB (a T-cell-line-adapted X4 isolate) interacts preferentially with Gb3, whereas gp120 from 89.6 (a dualtropic primary R5X4 isolate) interacts exclusively with GM3. Therefore, HIV-1 isolates might select glycosphingolipids of their choice in addition to the chemokine receptors to promote fusion. It should be noted that the results obtained with recombinant gp120 (from isolate IIIB) were fully confirmed by the results with gp120 purified from HIV-1(LAI)-infected peripheral blood mononuclear cells (data not shown). Thus, the ability of gp120 from X4 viruses to interact with Gb3 is not restricted to recombinant proteins produced in CHO cells.
These data, which support the concept that human erythrocyte membrane glycosphingolipids can function as fusion cofactors for HIV-1 entry, are consistent with the recent characterization of human erythrocyte Gb3 as a functional fusion cofactor for an X4 isolate (23). Interestingly, this glycosphingolipid is also the main receptor for various bacterial toxins, including Escherichia coli verocytotoxin (20). Our data also suggest that GM3 may function as an alternative fusion cofactor for selected HIV-1 isolates, especially macrophage-tropic or dualtropic primary isolates such as 89.6.
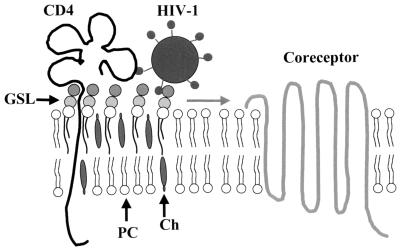
We are aware that these experiments suggest that human erythrocyte glycosphingolipids GM3 and Gb3 may function as surrogate fusion cofactors only in cells expressing CD4 but lacking the HIV-1 coreceptor activity. However, the recent observation that inhibitors of glycosphingolipid biosynthesis affect HIV-1 infection through cell surface masking of CD4 suggests a role for glycosphingolipids in the fusion process between HIV-1 and CD4+ lymphocytes and/or macrophages (30). GM3 and Gb3 are highly expressed in human macrophages but are also present in CD4+ lymphocytes and T-cell lines (5, 10). These glycosphingolipids have a common structural feature, i.e., a free hydroxyl group in position 4 of a terminal galactose residue. Based on the inhibitory effect of 3′-siallylactose on the GM3-CD4 interaction (Table 1), one can hypothesize that CD4 binds to this structural determinant borne by the oligosaccharide part of both GM3 and Gb3. In the model shown in Fig. 4, the glycosphingolipid interacts with domain 4 of CD4, which is plausible given its proximity to the plasma membrane. Glycosphingolipids recognized by both CD4 and gp120 may induce the formation of a trimolecular complex, CD4-glycosphingolipid-gp120 (14). The role of the glycosphingolipid in this multimolecular organization could be to facilitate the migration of the CD4-gp120 complex to an appropriate coreceptor (e.g., CCR5 or CXCR4), since CD4 and these coreceptors are not physically associated in the absence of HIV-1 (16). By moving freely in the external leaflet of the plasma membrane, the glycosphingolipid patch may behave as a raft dragging the CD4 receptor and taking aboard the viral particle (Fig. 4). The binding of the virion to the raft is stabilized by secondary interactions between the polar heads of glycosphingolipid molecules and the V3 loop of gp120 (5, 14, 15). The raft may then float on the cell surface until it finds an adequate coreceptor which can displace the glycosphingolipid-V3 loop interactions to its own benefit, resulting in the initiation of the fusion process. In the absence of any available coreceptor, the glycosphingolipid may eventually allow the conformational change of gp41, as may be the case for human erythrocyte glycosphingolipids transferred into murine cells expressing human CD4 (22, 23).
FIG. 4.
Plasma membrane glycosphingolipid microdomains as preferential sites of formation of the HIV-1 fusion complex. In the plasma membrane of CD4+ cells, CD4 is present in glycosphingolipid-enriched microdomains but is not associated with HIV-1 coreceptors. Once bound to CD4, the viral particle is conveyed to an appropriate coreceptor by the glycosphingolipid raft, which moves freely in the external leaflet of the plasma membrane. Ch, cholesterol; GSL, glycosphingolipid; PC, phosphatidylcholine.
Acknowledgments
This work was supported by SIDACTION funds from the Fondation pour la Recherche Médicale (SIDACTION grant to J.F. and fellowship to D.H.).
We are grateful to the Medical Research Council for the generous gift of soluble CD4 and recombinant gp120-producing cells. We also thank S. Ivaldi for setting up the Langmuir film balance apparatus and C. Tamalet for providing constant support and stimulating discussions throughout this study.
REFERENCES
- 1.Berger E A, Doms R W, Fenyo E M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 2.Binley J, Moore J P. The viral mousetrap. Nature. 1997;387:346–348. doi: 10.1038/387346a0. [DOI] [PubMed] [Google Scholar]
- 3.Clapham P R, Weiss R A. Spoilt for choice of coreceptors. Nature. 1997;388:230–231. doi: 10.1038/40758. [DOI] [PubMed] [Google Scholar]
- 4.Cumar F A, Maggio B, Caputto R. Ganglioside-cholera toxin interactions: a binding and lipid monolayer study. Mol Cell Biochem. 1982;46:155–160. doi: 10.1007/BF00239664. [DOI] [PubMed] [Google Scholar]
- 5.Delézay O, Hammache D, Fantini J, Yahi N. SPC3, a V3 loop-derived synthetic peptide inhibitor of HIV-1 infection, binds to cell surface glycosphingolipids. Biochemistry. 1996;35:15663–15671. doi: 10.1021/bi961205g. [DOI] [PubMed] [Google Scholar]
- 6.Deng H K, Choe S, Ellmeier W, Liu R, Unumatz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of C-C chemokine receptor 5 as the major coreceptor for entry of macrophage-tropic human immunodeficiency virus type 1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrov D S. How do viruses enter cells? The HIV coreceptors teach us a lesson of complexity. Cell. 1997;91:721–730. doi: 10.1016/s0092-8674(00)80460-2. [DOI] [PubMed] [Google Scholar]
- 8.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 9.Dragic T, Picard L, Alizon M. Proteinase-resistant factors in human erythrocyte membranes mediate CD4-dependent fusion with cells expressing human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1995;69:1013–1018. doi: 10.1128/jvi.69.2.1013-1018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantini J, Tamalet C, Hammache D, Tourrès C, Duclos N, Yahi N. HIV-1-induced perturbations of glycosphingolipid metabolism are cell-specific and can be detected at early stages of HIV-1 infection. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;19:221–229. doi: 10.1097/00042560-199811010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry co-factor: functional cDNA cloning of a seven-transmembrane G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 12.Hakomori S, Kannagi R. Carbohydrate antigens in higher animals. In: Weir D M, editor. Handbook of experimental immunology. 1. Immunochemistry. Oxford, United Kingdom: Blackwell Scientific Publications; 1986. pp. 9.1–9.36. [Google Scholar]
- 13.Hammache D, Piéroni G, Yahi N, Delézay O, Koch N, Lafont H, Tamalet C, Fantini J. Specific interaction of HIV-1 and HIV-2 surface envelope glycoproteins with monolayers of galactosylceramide and ganglioside GM3. J Biol Chem. 1998;273:7967–7971. doi: 10.1074/jbc.273.14.7967. [DOI] [PubMed] [Google Scholar]
- 14.Hammache D, Yahi N, Piéroni G, Ariasi F, Tamalet C, Fantini J. Sequential interaction of CD4 and HIV-1 gp120 with a reconstituted membrane patch of ganglioside GM3: implications for the role of glycolipids as potential HIV-1 fusion cofactors. Biochem Biophys Res Commun. 1998;246:117–122. doi: 10.1006/bbrc.1998.8531. [DOI] [PubMed] [Google Scholar]
- 15.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones P L, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273:404–409. doi: 10.1074/jbc.273.1.404. [DOI] [PubMed] [Google Scholar]
- 17.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maggio B, Cumar F A, Caputto R. Surface behaviour of gangliosides and related glycosphingolipids. Biochem J. 1978;171:559–565. doi: 10.1042/bj1710559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxton W A, Dragic T, Koup R A, Moore J P. The β-chemokines, HIV type 1 second receptors, and exposed uninfected persons. AIDS Res Hum Retroviruses. 1996;12:1203–1207. doi: 10.1089/aid.1996.12.1203. [DOI] [PubMed] [Google Scholar]
- 20.Pellizzari A, Pang H, Lingwood C A. Binding of verocytotoxin 1 to its receptor is influenced by differences in receptor fatty acid content. Biochemistry. 1992;31:1363–1370. doi: 10.1021/bi00120a011. [DOI] [PubMed] [Google Scholar]
- 21.Puri A, Morris S J, Jones P, Ryan M, Blumenthal R. Heat-resistant factors in human erythrocyte membranes mediate CD4-dependent fusion with cells expressing HIV-1 envelope glycoproteins. Virology. 1996;219:262–267. doi: 10.1006/viro.1996.0244. [DOI] [PubMed] [Google Scholar]
- 22.Puri A, Hug P, Munoz-Barroso I, Blumenthal R. Human erythrocyte glycolipids promote HIV-1 envelope glycoprotein-mediated fusion of CD4+ cells. Biochem Biophys Res Commun. 1998;242:219–225. doi: 10.1006/bbrc.1997.7941. [DOI] [PubMed] [Google Scholar]
- 23.Puri A, Hug P, Jernigan K, Barchi J, Kim H-Y, Hamilton J, Wiels J, Murray G J, Brady R O, Blumenthal R. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1998;95:14435–14440. doi: 10.1073/pnas.95.24.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Serizawa T, Okhata Y. Binding of influenza A virus to monosialoganglioside (GM3) reconstituted in glucosylceramide and sphingomyelin membranes. Biochim Biophys Acta. 1996;1285:14–20. doi: 10.1016/s0005-2736(96)00138-1. [DOI] [PubMed] [Google Scholar]
- 26.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 28.Sorice M, Parolini I, Sansolini T, Garofalo T, Dolo V, Sargiacomo M, Tai T, Peschle C, Torrisi M R, Pavan A. Evidence for the existence of ganglioside-enriched plasma membrane domains in human peripheral lymphocytes. J Lipid Res. 1997;38:969–980. [PubMed] [Google Scholar]
- 29.Spitalnik P F, Danley J M, Burger S R, Spitalnik S L. The glycosphingolipid composition of the human hepatoma cell line, Hep-G2. Arch Biochem Biophys. 1989;273:578–591. doi: 10.1016/0003-9861(89)90518-3. [DOI] [PubMed] [Google Scholar]
- 30.Tamma S L, Sundaram S K, Lev M, Coico R F. Inhibition of sphingolipid synthesis down-modulates CD4 expression by peripheral blood T lymphocytes and T lymphoma cells. Biochem Biophys Res Commun. 1996;220:916–921. doi: 10.1006/bbrc.1996.0506. [DOI] [PubMed] [Google Scholar]