Abstract
CD46, which serves as a receptor for measles virus (MV; strain Edmonston), is rapidly downregulated from the cell surface after contact with viral particles or infected cells. We show here that the same two CD46 complement control protein (CCP) domains responsible for primary MV attachment mediate its downregulation. Optimal downregulation efficiency was obtained with CD46 recombinants containing CCP domains 1 and 2, whereas CCP 1, alone and duplicated, induced a slight downregulation. Using persistently infected monocytic/promyelocytic U937 cells which release very small amounts of infectious virus, and uninfected HeLa cells as contact partners, we then showed that during contact the formation of CD46-containing patches and caps precedes CD46 internalization. Nevertheless, neither substances inhibiting capping nor the fusion-inhibiting peptide Z-d-Phe-l-Phe-Gly-OH (FIP) blocked CD46 downregulation. Thus, CD46 downregulation can be uncoupled from fusion and subsequent virus uptake. Interestingly, in that system cell-cell contacts lead to a remarkably efficient infection of the target cells which is only partially inhibited by FIP. The finding that the contact of an infected with uninfected cells results in transfer of infectious viral material without significant (complete) fusion of the donor with the recipient cell suggests that microfusion events and/or FIP-independent mechanisms may mediate the transfer of MV infectivity from cell to cell.
Recently, CD46 was identified as a measles virus (MV) receptor on human cells (10, 29). All natural splice variants and recombinant molecules containing at least the first two complement control protein domains (CCPs) of CD46 serve as receptors for certain MV strains (6, 7, 12, 24, 25, 27, 41). CD46 is expressed on almost all human cells except erythrocytes and certain cells in the central nervous system (18, 22, 32). CD46 functions as a cofactor for the cleavage of the C3b and C4b complement components and protects cells from lysis by autologous complement (22, 23).
Both the infection of target cells with certain MV strains and the contact of an uninfected with an MV-infected cell can cause CD46 downregulation from the cell surface (20, 30, 36–38). However, not all MV strains have the same capacity to downregulate CD46. All vaccine strains and several wild-type isolates downregulate CD46, whereas a number of wild-type isolates do not (37, 38). CD46 modulation by certain MV strains renders the cells susceptible to complement lysis, and this may also in vivo cause a rapid clearing of infected cells and contribute to the attenuation of such downregulating MV strains (39). The MV hemagglutinin (H) protein alone is sufficient to induce CD46 downregulation from the cell surface after infection of cells (30, 37) or contact of H-expressing cells with CD46-positive cells (20, 36). Few amino acids in the MV H protein determine the capacity to downregulate CD46. Only two amino acid changes in the H protein of MV-WTF and MV-Ma93 (451 Glu→Val and 481 Asn→Tyr) were sufficient to introduce the capacity to downregulate CD46 into these nondownregulating H proteins (3, 21). Recently, we found that in a human B-cell line (BJAB), a proportion of MV strains not leading to the downregulation of CD46 do use CD46 as receptor whereas others do not (4). Similar observations were made with the monkey cell line B95 (16, 40). These findings led to the suggestion that receptor usage by MV strains and CD46 downregulation may be based on independent mechanisms.
To investigate the mechanism of contact-mediated CD46 downregulation and its association with the receptor-mediated infection of target cells, we used a persistently infected monocytic cell line expressing high levels of the viral glycoproteins at their surface and CD46-positive cells as target cells. Using chimeric molecules, we found that the contact-mediated CD46 downregulation is mediated by CCP domain 1 alone, and better by CCP domains 1 and 2, both of which constitute the MV-binding site. We defined conditions at which associated processes such as CD46 capping, contact-induced microfusions, and cell-cell fusion are uncoupled from CD46 downregulation.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells, CHO cells, and transfected CHO cells expressing natural or chimeric forms of CD46 as described elsewhere (6) (Fig. 1) were grown in minimal essential medium containing 5% fetal calf serum. The monocytic/promyelocytic cell line U937 and the persistently MV-Edmonston (Edm)-infected cell line U937-p were grown in RPMI medium containing 10% fetal calf serum.
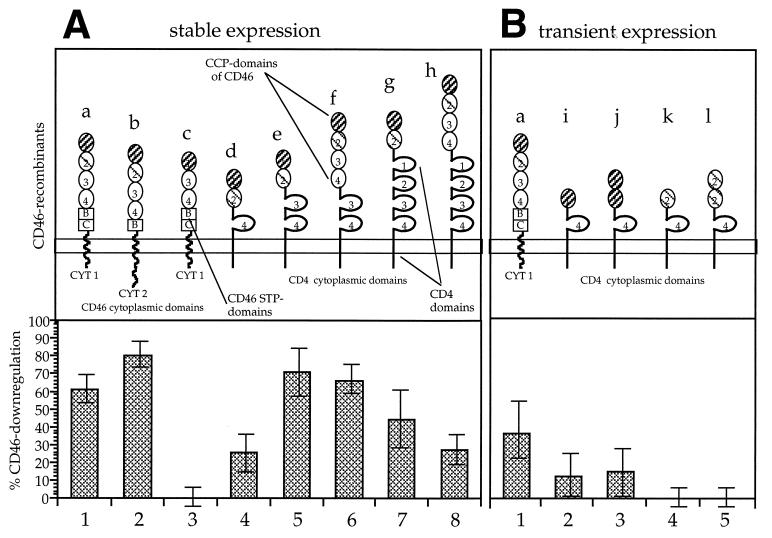
FIG. 1.
Downregulation of CD46 from the surface of CHO cells expressing CD46 isoforms and chimeric CD46-CD4 proteins after contact with persistently infected U937-p cells. Schematic representations of the CD46 isoforms and CD46-CD4 chimeric molecules are shown for stably (A) and transiently (B) expressing cells. Molecules a and b are naturally expressed CD46 isoforms. Recombinant c is missing CCP domain 2 (cross-hatched thin line), which is important for the interaction with MV H. STP domains B and C, the transmembrane domain, and the cytoplasmic domains CYT1 and CYT2 of CD46 were replaced by corresponding domains of CD4 in recombinants d to l. Surface expression of the CD46 forms was detected with antibody 13/42, directed against the first CD46 domain (cross-hatched dark line), in the case of stable transfectants or with a polyclonal serum against CD46 in the case of the transient transfectants. The percentages of downregulation (n = 3) were calculated from the differences in the mean fluorescence intensities of cells contacted at 37°C and kept at 4°C and are given in the corresponding lower panels for stable expression (A, lanes 1 to 8) and transient expression (B, lanes 1 to 5).
Transfection and transient expression of recombinant CD46 variants.
For expression of recombinant CD46 proteins (Fig. 1B, constructs i to l; described in reference 6 as I/4, I+I/4, II/4, and II+II/4) and pT7CD46 expressing a natural form of CD46 (isoform a [Fig. 1B]), wells of a 12-well plate were seeded with 5 × 104 CHO cells. After 16 h, cells were infected with VV-T7 (recombinant vaccinia virus expressing T7 RNA polymerase; multiplicity of infection [MOI] = 1). After 1 h at 37°C, the cells were transfected with 1.5 μg of plasmid DNA expressing the CD46 recombinants under the control of the T7 promoter and 5 μl of Superfect (Qiagen). After 48 h of incubation, cells were detached from the surface and incubated with U937-p cells for 4 h at 37°C or, for control, at 4°C as described below for the contact-mediated CD46 downregulation assay. The fluorescence intensity of the recombinants was determined with a polyclonal rabbit anti-CD46 serum by flow cytometry.
Antibodies and inhibitors.
The monoclonal antibodies against CD46 (13/42), against MV H (L77), and against MV nucleocapsid (N) protein (F227) were generated and cultivated in our laboratory and were purified over protein G columns. Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated rabbit anti-mouse immunoglobulin was purchased from Dako. The polyclonal rabbit anti-CD46 serum was a generous gift of G. Yeh, Cytomed Inc., Cambridge, Mass. The fusion-inhibiting peptide Z-d-Phe-l-Phe-Gly-OH (FIP) (31, 34) was purchased from Bachem (Bubendorf, Switzerland). The inhibitors of capping and formation of the cytoskeleton colchicine, cytochalasins B and D, and vinblastine sulfate were purchased from Sigma and used as described elsewhere (5, 19).
Contact-mediated CD46 downregulation assay.
U937-p cells (105), on which the endogenous CD46 is constantly downregulated and which express high levels of MV H and F (fusion) proteins, were mixed at 37°C with monolayers of 105 uninfected cells (HeLa or CHO) and incubated for 4 h at 37°C. As control, U937-p cells were chilled on ice, mixed with uninfected cells, and incubated for 4 h at 4°C. Afterwards, cells were fixed with paraformaldehyde and processed for flow cytometric staining.
Immunofluorescence and flow cytometry.
For immunohistochemistry, cells were grown on glass coverslips in plastic dishes. For flow cytometry and microscopy, the same dilutions of monoclonal antibodies were used (10 μg/ml). The second antibodies were either FITC- or PE-conjugated rabbit anti-mouse immunoglobulin (1:100; Dako). For flow cytometry, cells (2 × 105/tube) were harvested and washed with FACS (fluorescence-activated cell sorting) buffer (calcium- and magnesium-free phosphate-buffered saline [PBS] containing 0.2% bovine serum albumin and 0.01 M NaN3). After incubation with the first antibody diluted in FACS buffer on ice for 45 min, cells were washed two times with FACS buffer, incubated for 45 min with the second antibody, washed two times, and analyzed with a FACScan flow cytometer (Becton Dickinson).
Biotinylation and precipitation of surface proteins and Western blotting.
HeLa cell monolayers were biotinylated with N-hydroxysuccinamide–biotin (Pierce) by incubation with 0.5 mg of biotin per ml in PBS for 1 h at 4°C and then washed three times with PBS to remove unbound biotin. After the contact with and washing off of the U937-p cells, the separated cells (106) were suspended in 300 μl of PBS; an equal volume of RIPA-Det (150 mM NaCl, 10 mM Tris, 0.1% sodium dodecyl sulfate [SDS], 1% sodium deoxycholate, 1% Triton X-100) was added, and the mixture was incubated for 30 min at 4°C. The nuclei were removed by centrifugation; CD46 was precipitated with 3 μg of monoclonal antibody 13/42 and protein G beads (Pharmacia). Precipitated proteins were dissolved in 200 μl of sample buffer (80 mM Tris [pH 6.8], 2% SDS, 5% glycerol, 3.3% β-mercaptoethanol, 0.02% bromophenol blue), separated on 10% polyacrylamide gels containing SDS, and blotted on polyvinylidene difluoride membranes (Millipore) with semidry blotting chambers. Filters were blocked with 5% dry milk powder in Tris-buffered saline (10 mM Tris [pH 7.2], 0.9% NaCl, 0.5% Tween 20) overnight, incubated with streptavidin-peroxidase (Dianova) for 1 h, washed and rinsed in a peroxidase-sensitive enhanced chemiluminescence (ECL) solution (Amersham), and exposed to X-ray films.
RESULTS
The two external CD46 domains mediated CD46 downregulation.
To investigate which domains are necessary for contact-mediated CD46 downregulation, we used a panel of eight CHO cell clones stably expressing recombinant forms of CD46 (Fig. 1A) and, for transient expression of CD46 recombinants, transfected CHO cells (Fig. 1B). The generation of the recombinants and their capacities to mediate MV binding and fusion are described in reference 6. Recombinants a and b are naturally occurring CD46 isoforms; in recombinant c the second MV-binding domain is missing; in recombinants d to l the serine-, threonine-, proline-rich (STP) domains, transmembrane, and cytoplasmic domains (CYT1 and CYT2) of CD46 were substituted by domains of CD4. The contact-mediated downregulation was induced by mixing the cells with the same number of U937-p cells. The transfected CHO cells were mixed at 37°C with the U937-p cells for 4 h or, as controls, were chilled on ice, mixed, and incubated for 4 h at 4°C. The difference in mean fluorescence intensities of the resulting CD46 signals between cells incubated at 4 and 37°C is a measure of the downregulation of the CD46 recombinant and is presented as a percentage of the reduction of the CD46 signal (Fig. 1A, lanes 1 to 8; Fig. 1B, lanes 1 to 5).
In stably transfected cells (Fig. 1A), the natural forms of CD46 (a and b) led to 61 and 80% downregulation, whereas the CD46 molecule missing the second CCP domain (c) did not induce CD46 modulation. The other chimeric forms of CD46 led to downregulation of between 26 and 71%, with the strongest downregulation observed with the mutant molecule containing CCP domains 1 and 2 and CCP domains 3 and 4 of CD4 (e [Fig. 1A, lane 5). CD46 recombinants containing CCP domain 1 or 2, alone or duplicated, and fused to domain 4 and the transmembrane part of CD4 were analyzed in a transient expression system (Fig. 1B). These constructs (i to l) and a plasmid coding for isoform BC/CYT1 of CD46 (a) were transfected into CHO cells, and their expression was driven by the T7 polymerase provided by vaccinia virus (VV-T7). Expression of the constructs was measured with a polyclonal anti-CD46 serum. The cells were tested for downregulation in the same assay system with persistently infected U937-p cells. The control, CD46 isoform a, was downregulated by approximately 36% (Fig. 1B, lane 1), recombinants i and j expressing CCP domain 1 were slightly (approximately 10 and 12%, respectively) downregulated (lanes 2 and 3), and recombinants k and l were not downregulated (lanes 4 and 5). Since none of these recombinants supported binding of the MV glycoproteins (6), the slight downregulation detected with CCP domain 1 constructs was a surprising finding. Thus, CCP domains 1 and 2 were necessary to mediate optimal contact-mediated CD46 downregulation, whereas the CCP domains 3 and the STP, transmembrane, and cytoplasmic domains could be substituted by domains of CD4.
CD46 downregulation is unrelated to patching and capping of CD46.
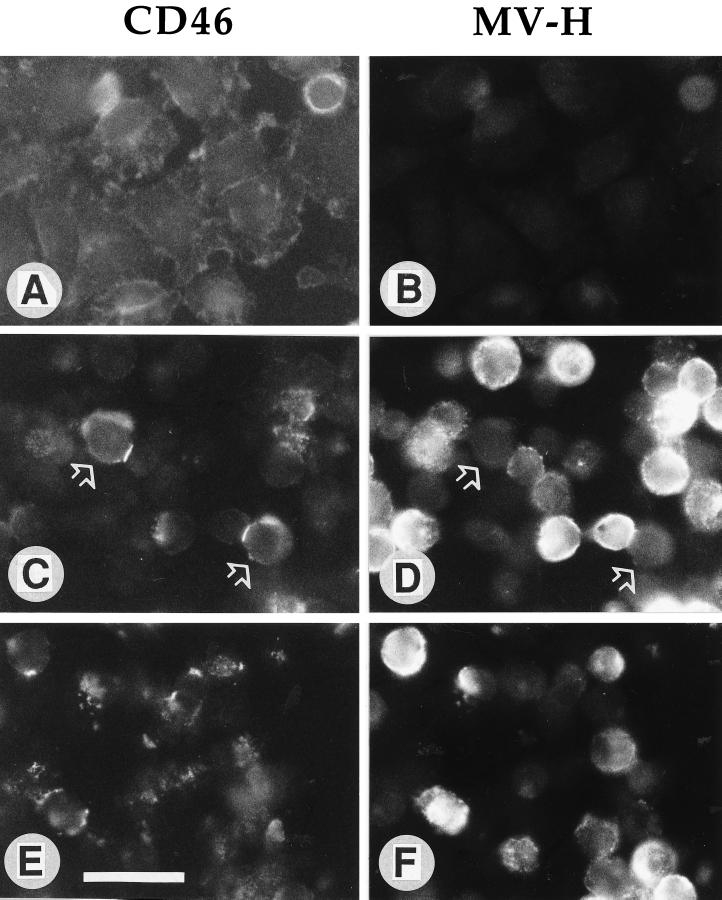
After a 30-min contact of persistently infected U937 cells with uninfected HeLa cells, we observed the formation of CD46-containing patches and caps followed by a reduction of the CD46 signal within 3 h of incubation at 37°C (Fig. 2). In the double-staining experiment, capping was observed at the interfaces between CD46-positive HeLa cells (Fig. 2C, arrows) and MV H-positive U937-p cells (Fig. 2D). These data show that CD46 downregulation is associated with the formation of patches and caps. To investigate if a causal relationship between this mechanism and CD46 downregulation exists, we treated the uninfected cells prior to contact with the persistently infected cells with inhibitors affecting capping and the cytoskeletal organization. Colchicine (0.01 M), cytochalasin B (50 μM), cytochalasin D (10 μM), and vinblastine sulfate (0.1 mM) inhibited the formation of caps; however, they did not block the CD46 downregulation (not shown).
FIG. 2.
Contact of HeLa cells with persistently infected U937-p cells induces patching, capping, and downregulation of CD46. A monolayer of HeLa cells (A and B) was overlaid with U937-p cells for 30 min (C and D) and 2 h (E and F). The cells were fixed and then double stained with CD46 antibodies and FITC-conjugated second antibodies (A, C, and E) and with MV H antibodies and PE-conjugated second antibodies (B, D, and F). CD46 caps on the contact sites of two MV H-negative cells with MV H-positive cells are labeled with arrows in panels C and D. The bar in panel E represents 10 μm.
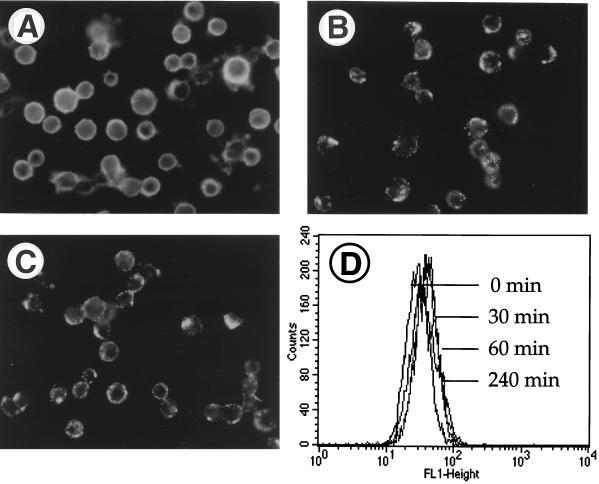
To investigate whether antibodies against CD46 can induce the capping process and/or the downregulation of CD46, we treated HeLa cells with the monoclonal anti-CD46 antibody 13/42 for various times. Interestingly, the antibodies against CD46 induced patching and capping but not the downregulation of CD46 from the cell surface (Fig. 3). The CD46-specific fluorescence is evenly distributed on cells incubated on ice with the antibody (Fig. 3A). After incubation at 37°C for 30, 60, and 240 min, caps were formed (Fig. 3B and C), but the average CD46-specific fluorescence intensity per cell was not reduced (Fig. 3D). Thus, CD46 capping does not necessarily lead to disappearance of CD46 from the cell surface.
FIG. 3.
Capping of CD46 by anti-CD46 antibodies in the absence of modulation. HeLa cells were detached from the plastic dish prior to incubation with anti-CD46 antibody 13/42 for 30 min on ice (A), 30 min at 37°C (B), and 60 min at 37°C (C) and analyzed in a fluorescence microscope to visualize the formation of caps. In addition, CD46 expression on aliquots of the cells was quantified by flow cytometry (D). The average CD46 signals on the differentially treated cell populations after 0 min (=control on ice), and 30, 60, and 240 min at 37°C were similar.
CD46 internalization after contact-mediated downregulation.
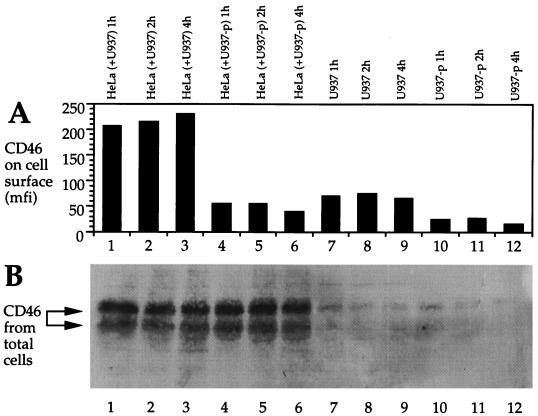
The fate of CD46 after a short contact to the MV glycoproteins on an infected cell is not known. CD46 might be internalized by the contacted target cell (HeLa), be released from the target cell into the supernatant, or bind to the surface of the MV H-expressing cells (U937-p). To investigate this, we labeled the surface proteins of HeLa cells with biotin, brought these cells into contact with U937-p cells (or uninfected U937 cells as a control), separated the cell populations, and analyzed the separated cell populations and the supernatant. The presence of CD46 was quantified on the cell surface by FACS (Fig. 4A) and in total cell lysates by Western blotting (Fig. 4B). On the cell surface, large amounts of CD46 were found only on HeLa cells which were cocultivated as a control with uninfected U937 cells (Fig. 4A, lanes 1 to 3). In contrast, HeLa cells which were mixed with the infected U937-p cells had an approximately fivefold-reduced signal for surface CD46 (lanes 4 to 6). Uninfected U937 cells separated from the HeLa cells expressed little CD46 at their surface (lanes 7 to 9), and U937-p cells expressed even lower amounts (lanes 10 to 12). The expression of biotinylated CD46 in the corresponding total cell lysates was then analyzed by Western blotting. Biotinylated CD46 was present in similar amounts in all HeLa cell populations irrespective of having had contact with uninfected or infected U937 cells (Fig. 4B, lanes 1 to 6). Very low levels of biotinylated CD46 were detected after contact in U937-p and U937 cells (lanes 7 to 12). This result indicates that very low numbers of HeLa cells were contaminating these populations after the separation. In addition, no CD46 was detected in the concentrated supernatant of contacted cells by Western blotting (not shown). These findings strongly support the assumption that contact-mediated downregulation leads to the internalization of CD46 by the contacted cells and not to its degradation.
FIG. 4.
CD46 expression on the surface and in total lysates of contacted cells. HeLa cells were brought into contact with uninfected U937 cells (control) and with persistently infected U937-p cells for 1, 2, and 4 h. Afterwards, the cell types were separated and analyzed separately. (A) CD46 expression (mean fluorescence intensity [mfi]) was measured on the cell surface by flow cytometry on HeLa cells, which were mixed with uninfected U937 cells as a control for indicated times prior to removal of the cells (lanes 1 to 3), on HeLa cells, which were mixed with U937-p cells for indicated times prior to removal of the cells (lanes 4 to 6), and on the uninfected (lanes 7 to 9) and infected U937 cells (lanes 10 to 12), which were washed off the HeLa cell monolayer. CD46 in total cell lysates was analyzed by Western blotting (B) in the same cell populations as used for panel A. For the detection of CD46 in cell lysates, surface CD46 on HeLa cells was labeled with biotin before cells were brought into contact with U937-p cells. Total cell lysates were immunoprecipitated with anti-CD46 antibodies, separated on SDS–10% polyacrylamide gels, and blotted. Biotinylated molecules (CD46) were detected by streptavidin-peroxidase and an ECL reaction.
Uptake of viral material by target cells after contact with persistently infected cells leads to infection.
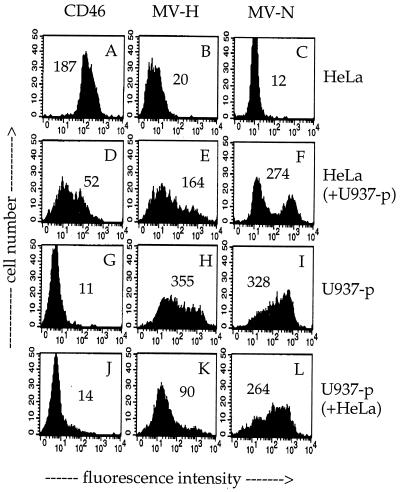
We made an interesting observation concerning the fate of viral proteins after the contact. A monolayer of HeLa cells was overlaid with washed U937-p cells (no virus in the supernatant) for 3 h at 37°C; subsequently the U937-p cells were removed by extensive washing, and levels of CD46, MV H, and MV N expression were separately analyzed on the contacted HeLa and U937-p cells by flow cytometry (Fig. 5). While CD46 was downregulated from the surface of the contacted HeLa cells (Fig. 5D), we found high amounts of MV H (Fig. 5E) and N (Fig. 5F) in the contacted HeLa cells. Inversely, the U937-p cells lost MV H and N during the contact (Fig. 5K and L; compare with Fig. 5H and I, respectively). In contrast to the HeLa cells, which took up viral material, U937-p cells did not take up CD46 from HeLa cells (Fig. 5J).
FIG. 5.
Contact-mediated CD46 downregulation and transfer of viral proteins to HeLa cells. A HeLa cell monolayer was overlaid with persistently MV-Edm-infected U937-p cells for 3 h (cell ratio of 1:1). After contact, U937-p cells were removed by intensive washing with PBS from the HeLa cell monolayer, and the contacted HeLa (D to F) and U937-p (J to L) cells were separately analyzed by flow cytometry. Control cells were untreated HeLa (A to C) and U937-p (G to I) cells. The signals for the surface expression of CD46 (A, D, G, and J) and MV H (B, E, H, and K) and the expression of MV N after permeabilization (C, F, I, and L) are shown. Numbers in the panels represent mean fluorescence intensities.
During the contact, the HeLa cells were productively infected by the U937-p cells. The contact-mediated infection was very fast and efficient, leading to the infection of virtually all HeLa cells after 24 h, as detected by immunofluorescence (not shown). This efficient infection of the HeLa cells within 1 h of contact to U937-p cells cannot be due to virus released into the supernatant, since within 1 h only small amounts of infectious virus particles are released from U937-p cells (maximally 102 PFU/ml/105 cells, or MOI of 0.001). These results indicated that the contact between the infected and uninfected cells leads to a transfer of viral material to the uninfected target cell, which is efficiently infected. Within this 1 h of contact, we did not observe complete cell-cell fusions in the cultures. The fact that after the contact U937-p cells can be washed off, and that this population of cells considerably lost MV H and -N, as observed by flow cytometry, indicates that the transfer of viral material left the persistently infected U937-p cells intact.
FIP inhibits fusion and uptake of viral proteins but not CD46 downregulation.
FIP does not inhibit CD46 modulation from the surface of Jurkat cells after contact with MV H-expressing transfected L cells in the absence of the viral F protein (19). However, it is not clear whether during the contact of a functional H-F complex with CD46 the downregulation is influenced by fusion. Therefore, we analyzed the influence of FIP in the presence of a functional H-F complex on the contact-mediated CD46 downregulation, using persistently infected U937-p and HeLa cells. In addition, we determined whether the inhibition of fusion impairs the transfer of the viral proteins H and N into uninfected cells.
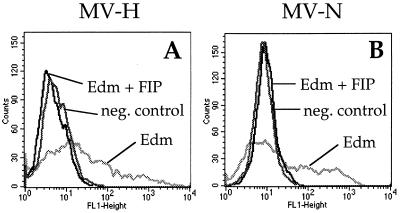
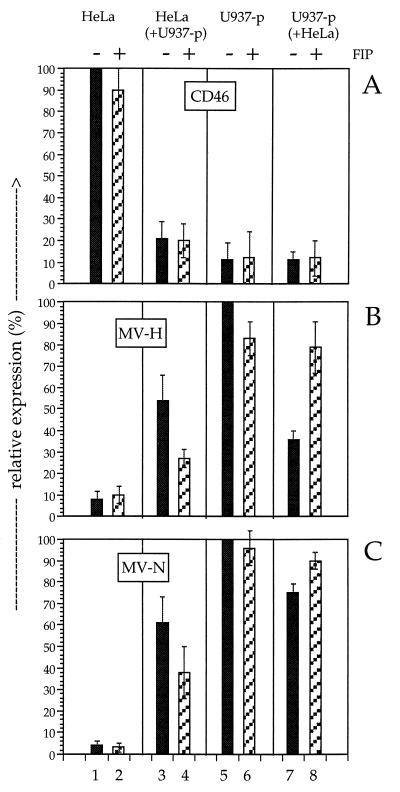
The activity of FIP was controlled by analysis of its capacity to inhibit the infection of HeLa cells with MV-Edm (MOI = 1) for 48 h. In the presence of 200 mM FIP the infection was completely blocked, whereas in the absence of FIP the HeLa cells were successfully infected (Fig. 6). The influence of FIP on contact-mediated CD46 downregulation was measured in an experiment similar to that shown in Fig. 5. HeLa and U937-p cells were brought into contact for 3 h in the absence and presence of 200 mM FIP. Subsequently, the two cell populations were separated, and the surface expression of CD46 and MV H and intracellular expression of MV-N were analyzed by flow cytometry. The mean fluorescence intensities of the obtained signals are presented in Fig. 7. The presence of FIP had no influence on the downregulation of CD46 (Fig. 7A, lanes 1 to 4). In contrast, the uptake by HeLa cells of MV H and N was inhibited by approximately 50%. Interestingly, the transfer of viral material was not completely inhibited in the presence of 200 mM FIP, although similar concentrations of FIP blocked the infection of cells with cell-free MV preparations by more than 90%. The reduction of the H and N transfer into HeLa cells in the presence of FIP demonstrates that the transfer requires fusion. However, fusion was not required for the CD46 downregulation.
FIG. 6.
Inhibition of the infection of HeLa cells with MV-Edm by FIP. HeLa cells were infected with MV-Edm (MOI = 1) in the absence or presence of 200 mM FIP (as indicated), and the expression of MV H (A) and N (B) was determined by flow cytometry after 48 h.
FIG. 7.
Relative expression of CD46, MV H, and MV N after contact between uninfected HeLa cells and persistently infected U937-p cells in the absence and presence of FIP. Cells were brought into contact for 3 h at 37°C and separated by intensive washing; levels of expression of CD46 (A), MV H (B), and MV N (C) were determined by flow cytometry. FIP was absent (lanes 1, 3, 5, and 7) or present at 200 mM (lanes 2, 4, 6, and 8) in the cultures. The relative levels of expression of CD46, MV H, and MV N of HeLa cells (lanes 1 and 2), HeLa cells after contact to U937-p cells (lanes 3 and 4), U937-p cells (lanes 5 and 6), and U937-p cells after contact with HeLa cells (lanes 7 and 8) are shown. The mean fluorescence intensity of the strongest signals of each type of detected molecule was set to 100, and reduced intensities were expressed as a percentage of this value. The data for two experiments ± errors are given.
DISCUSSION
By various methods, the binding site for MV H (strain Edm or Hallé) to CD46 was mapped to two distinct regions of CCP domains 1 and 2 (7, 15, 24, 25–27). To analyze MV receptor CD46 downregulation we used a panel of CD46 mutants previously characterized for other functions related to viral entry, namely, virus particle attachment and membrane fusion (6). Using stably transfected CHO cells, we found that the presence of the complete MV-binding site on CCP domains 1 and 2 of CD46 is optimal for the contact-mediated downregulation. Interestingly, after transient expression a certain level of downregulation was noted even in constructs with one or two copies of CCP domain 1 but without CCP domain 2. Although the percentage of downregulation in experiments with the constructs i to l was low, the effects were reproducibly obtained. Differences in the results obtained with recombinant c and recombinants i and j, all of which contain CCP domain 1, suggest that the molecular context in which CCP domain 1 is situated had an influence on its capacity for downregulation. Since no binding of MV particles to CCP domain 1 alone was monitored previously (6), we conclude that the interactions between virus and CD46 resulting in virus attachment or in the induction of downregulation may not be identical. Recently, it was found that the H proteins of rinderpest virus and peste des petits ruminants virus, closely related morbilliviruses, also induce the downregulation of CD46 from the surface of Vero and B95a cells, although CD46 did not appear to be the cellular receptor for these viruses (11). This finding suggests that a low-affinity or -avidity interaction of the H proteins of these viruses with CD46 (CCP domain 1) may induce its downregulation without leading to the subsequent uptake of viral particles by the cell. The interaction of the H proteins of various morbilliviruses with CD46 might serve other unknown functions in the pathogenesis of these viruses.
Recently, we found that a number of MV strains, mainly wild-type strains, do not induce CD46 downregulation. Furthermore, there are a number of MV strains which do not downregulate CD46 but do use CD46 as a receptor (4). These MV strains interact with CD46 without inducing an event, possibly a conformational change, resulting in CD46 downregulation. On the basis of these findings and the uncoupling of fusion and CD46 downregulation, one could speculate that such nondownregulating strains might use only one of the two binding sites of MV H to CD46, possibly on CCP domain 2. On the other hand, not all MV strains use CD46 as a receptor (4, 16, 40). This finding indicates that CD46 downregulation and the receptor usage by different morbilliviruses are clearly distinct processes.
We have shown here that CD46 downregulation occurs also in the absence of CCP domains 3 and 4, the STP domains, the transmembrane domain, and the cytoplasmic tail of CD46. It is interesting to note that in another system, CV-1 cells persistently infected with MV-Biken, the amino acid motif Tyr-X-X-Leu in the cytoplasmic domain of CD46 was found to be essential for downregulation (13, 42). On the other hand, also glycosyl-phosphatidylinositol-anchored isoforms of CD46 are efficiently downregulated (40). Since in our system domains besides CCP domains 1 and 2 can be substituted by CD4 domains, these cannot provide a CD46-specific mechanism for modulation. The Tyr-X-X-Leu motif is not present in the cytoplasmic part of CD4. However, since CD4 is downregulated by interaction with human immunodeficiency virus (HIV) gp120 and other HIV proteins, it is possible that these CD4 domains provide certain functions also for the CD46 downregulation. This might especially be the case for the short constructs i and k (Fig. 1B) which were downregulated in the transient expression assay.
The phenomenon of receptor downregulation after MV infection or contact was detected with a panel of antibodies against various domains of CD46 (13, 20, 30, 36–42) and therefore seems not to be simply masking of the antigen by viral glycoproteins. In contrast to the attachment of the viral glycoprotein complex, which occurs at 4°C and more efficiently also at 37°C (6), CD46 downregulation is a strictly temperature-dependent process. No downregulation was observed at 4°C. CD46 patching and capping, two temperature-dependent processes, were also observed in association with contact-mediated CD46 downregulation. However, capping inhibitors did not block downregulation, indicating that capping is not a prerequisite for downregulation. In contrast to the interaction of the viral glycoproteins with their receptor, antibodies inducing CD46 capping did not reduce the CD46 signal intensity per cell. Thus, capping alone is also not sufficient to induce the modulation of CD46. These data indicate that modulation and capping occur via independent mechanisms.
We detected surface-biotinylated CD46 in total cell lysates in same amounts in control cells and after contact-mediated downregulation. Since CD46 was not detected in the supernatant or on the cell surface and was not degraded, we conclude that it was internalized. Internalization of CD46 has also been suggested for the CD46 downregulation following infection of cells (30). CD46 internalization is not blocked by inhibitors of endocytosis (20, 36). This process is obviously mechanistically different from CD4 receptor downregulation induced by the infection of cells with HIV. Three gene products of HIV interact with CD4: the viral envelope protein gp160, which retains CD4 in the endoplasmic reticulum of the infected cell by blocking its maturation and transport; the viral Nef protein, which induces CD4 internalization, resulting in its degradation in lysosomes; and the Vpu protein, which induces CD4 degradation in the endoplasmic reticulum (1, 9, 14, 17, 33).
Another striking observation related to MV entry is that a short contact between persistently MV-infected cells and HeLa cells results in the efficient transfer not only of the viral envelope proteins but also of ribonucleocapsids, which leads to infection. Since persistently infected cells release minimal amounts of virus in the supernatant, the observation that the majority of HeLa cells absorbed infectious virus upon contact with the infected cells suggests that infectivity may be stripped from persistently infected cells upon contact. Indeed it was observed that MV-infected cells accumulate considerable amounts of virus-like particles at the plasmalemma (35). Since in our system infectivity is transferred efficiently without significant fusion of donor and recipient cells, the membranes of both cells must reseal after transfer. We therefore postulate that microfusion events at the cell-cell contact sites may mediate virus release and subsequent entry. These events may be initiated by the interaction of the viral glycoprotein complex with the receptor and subsequent insertion of part of the F protein in the target cell membrane. The process may result in the sequestration of membranes from the persistently infected cell, similar to what occurs during the release of viral particles. Interestingly, FIP inhibited the virus-cell fusion almost completely, whereas the transfer of viral material after cell-cell contact was only partially reduced. This observation suggests that FIP-independent mechanisms, like pore formation, could also contribute to the transfer of viral material after cell-cell contact.
Contact-mediated infection has another characteristic: material transfer is unidirectional; that is, virus receptor is not taken up by cells releasing infectivity. This fact is also consistent transfer of infectivity being similar to that occurring during standard MV infection. That cell-mediated infectivity transfer appears to occur without release of significant amounts of infectious particles in the supernatant may suggest that most released virus immediately absorbs to acceptor cells. It is interesting to note that there are indications that propagation of MV infectivity in human infections may occur principally by cell contacts in certain tissues (2, 8, 28, 32).
ACKNOWLEDGMENTS
We thank K. Pech for technical assistance and the Deutsche Forschungsgemeinschaft, the Schweizerische Nationalfonds, and the World Health Organization for financial support.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Allen I V, McQuaid S, McMahon J, Kirk J, McConnel R. The significance of measles virus antigen and genome distribution in the CNS in SSPE for mechanisms of viral spread and demyelination. J Neuropathol Exp Neurol. 1996;55:471–480. doi: 10.1097/00005072-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bartz R, Brinckmann U, Dunster L, Rima B, ter Meulen V, Schneider-Schaulies J. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology. 1996;224:334–337. doi: 10.1006/viro.1996.0538. [DOI] [PubMed] [Google Scholar]
- 4.Bartz R, Firsching R, Rima B, ter Meulen V, Schneider-Schaulies J. Differential receptor usage by measles virus strains. J Gen Virol. 1998;79:1015–1025. doi: 10.1099/0022-1317-79-5-1015. [DOI] [PubMed] [Google Scholar]
- 5.Borrow P, Oldstone M B. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- 6.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchholz C J, Koller D, Devaux P, Mumenthaler C, Schneider-Schaulies J, Braun W, Gerlier D, Cattaneo R. Mapping of the primary binding site of measles virus to its receptor CD46. J Biol Chem. 1997;272:22072–22079. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 8.Cathomen T, Mrkic B, Spehner D, Drillien R, Naef R, Pavlovic J, Aguzzi A, Billeter M, Cattaneo R. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 1998;17:3899–3908. doi: 10.1093/emboj/17.14.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalgleish A G, Beverley P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS virus. Nature. 1984;312:763–768. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 10.Dörig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 11.Galbraith S E, Tiwari A, Baron M D, Lund B T, Barrett T, Cosby S L. Morbillivirus downregulation of CD46. J Virol. 1998;72:10292–10297. doi: 10.1128/jvi.72.12.10292-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlier D, Loveland B, Varior-Krishnan G, Thorley B, McKenzie I F C, Rabourdin-Combe C. Measles virus receptor properties are shared by several CD46 isoforms differing in extracellular regions and cytoplasmic tails. J Gen Virol. 1994;75:2163–2171. doi: 10.1099/0022-1317-75-9-2163. [DOI] [PubMed] [Google Scholar]
- 13.Hirano A, Yant S, Iwata K, Korte-Sarfaty J, Seya T, Nagasawa S, Wong T C. Human cell receptor CD46 is down regulated through recognition of a membrane-proximal region of the cytoplasmic domain in persistent measles virus infection. J Virol. 1996;70:6929–6936. doi: 10.1128/jvi.70.10.6929-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoxie J A, Alpers J D, Rackowski J L, Huebner K, Haggarty B S, Cedarbaum A J, Reed J C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986;234:1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- 15.Hsu E C, Dörig R, Sarangi F, Marcil A, Iorio C, Richardson C D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;71:6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabbar M A, Nayak D P. Intracellular interaction of human immunodeficiency virus type 1 (ARV-2) envelope glycoprotein gp160 with CD4 blocks the movement and maturation of CD4 to the plasma membrane. J Virol. 1990;64:6297–6304. doi: 10.1128/jvi.64.12.6297-6304.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone R W, Russel S M, Loveland B E, McKenzie I F C. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993;30:1231–1241. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 19.Joseph B S, Oldstone M B. Antibody-induced redistribution of measles virus antigens on the cell surface. J Immunol. 1974;113:1205–1209. [PubMed] [Google Scholar]
- 20.Krantic S, Gimenez C, Rabourdin-Combe C. Cell-to-cell contact via measles virus haemagglutinin-CD46 interaction triggers CD46 downregulation. J Gen Virol. 1995;76:2793–2800. doi: 10.1099/0022-1317-76-11-2793. [DOI] [PubMed] [Google Scholar]
- 21.Lecouturier V, Fayolle J, Caballero M, Carabana J, Celma M L, Fernandez-Munoz R, Wild T F, Buckland R. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J Virol. 1996;70:4200–4204. doi: 10.1128/jvi.70.7.4200-4204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liszewski M K, Post T W, Atkinson J P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 23.Loveland B E, Johnstone R W, Russell S M, Thorley B R, McKenzie I F C. Different membrane cofactor protein (CD46) isoforms protect transfected cells against antibody and complement mediated lysis. Transplant Immunol. 1993;1:101–108. doi: 10.1016/0966-3274(93)90002-p. [DOI] [PubMed] [Google Scholar]
- 24.Maisner A, Schneider-Schaulies J, Liszewski M K, Atkinson J P, Herrler G. Binding of measles virus to membrane cofactor protein (CD46): importance of disulfide bonds and N-glycans for the receptor determinant. J Virol. 1994;68:6299–6304. doi: 10.1128/jvi.68.10.6299-6304.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manchester M, Valsamakis A, Kaufman R, Liszewski M K, Alvarez J, Atkinson J P, Lublin D M, Oldstone M B A. Measles virus and C3 binding sites are distinct on membrane cofactor protein (CD46) Proc Natl Acad Sci USA. 1995;92:2303–2307. doi: 10.1073/pnas.92.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manchester M, Gairin J E, Patterson J B, Alvarez J, Liszewski M K, Eto D S, Atkinson J P, Oldstone M B A. Measles virus recognizes its receptor, CD46, via two distinct binding domains within SCR 1-2. Virology. 1997;233:174–184. doi: 10.1006/viro.1997.8581. [DOI] [PubMed] [Google Scholar]
- 27.Manchester M, Lisszewski M K, Atkinson J P, Oldstone M B A. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McQuaid S, Campbell S, Wallace I J, Kirk J, Cosby S L. Measles virus infection and replication in undifferentiated and differentiated human neuronal cells in culture. J Virol. 1998;72:5245–5250. doi: 10.1128/jvi.72.6.5245-5250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naniche D, Wild T F, Rabourdin-Combe C, Gerlier D. Measles virus haemagglutinin induces down-regulation of gp57/67, a molecule involved in virus binding. J Gen Virol. 1993;74:1073–1079. doi: 10.1099/0022-1317-74-6-1073. [DOI] [PubMed] [Google Scholar]
- 31.Norrby E. The effect of a carboxy tripeptide on the bilogical activities of measles virus. Virology. 1971;44:599–608. doi: 10.1016/0042-6822(71)90374-6. [DOI] [PubMed] [Google Scholar]
- 32.Ogata A, Czub S, Ogata S, Cosby S L, McQuaid S, Budka H, ter Meulen V, Schneider-Schaulies J. Absence of measles virus receptor (CD46) in lesions of subacute sclerosing panencephalitis (SSPE) brains. Acta Neuropathol. 1997;94:444–449. doi: 10.1007/s004010050731. [DOI] [PubMed] [Google Scholar]
- 33.Raja N U, Vincent M J, Jabbar M A. Vpu-mediated proteolysis of gp160/CD4 chimeric envelope glycoproteins in the endoplasmic reticulum: requirement of both the anchor and cytoplasmic domains of CD4. Virology. 1994;204:357–366. doi: 10.1006/viro.1994.1540. [DOI] [PubMed] [Google Scholar]
- 34.Richardson C D, Choppin P W. Oligopeptides that specifically inhibit membrane fusion by paramyxovirus: studies on the site of action. Virology. 1983;131:518–532. doi: 10.1016/0042-6822(83)90517-2. [DOI] [PubMed] [Google Scholar]
- 35.Roser K, Bohn W, Mannweiler K. Morphogenesis of measles virus on C6 rat glioma cells. J Neuroimmunol. 1988;20:173–176. doi: 10.1016/0165-5728(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 36.Schneider-Schaulies J, Schnorr J-J, Schlender J, Dunster L M, Schneider-Schaulies S, ter Meulen V. Receptor (CD46) modulation and complement-mediated lysis of uninfected cells after contact with measles virus-infected cells. J Virol. 1996;70:255–263. doi: 10.1128/jvi.70.1.255-263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider-Schaulies J, Schnorr J-J, Brinckmann U, Dunster L M, Baczko K, Liebert U G, Schneider-Schaulies S, ter Meulen V. Receptor usage and differential downregulation of CD46 by measles virus wild type and vaccine strains. Proc Natl Acad Sci USA. 1995;92:3943–3947. doi: 10.1073/pnas.92.9.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider-Schaulies J, Dunster L M, Kobune F, Rima B, ter Meulen V. Differential downregulation of CD46 by measles virus strains. J Virol. 1995;69:7257–7259. doi: 10.1128/jvi.69.11.7257-7259.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnorr J-J, Dunster L M, Nanan R, Schneider-Schaulies J, Schneider-Schaulies S, ter Meulen V. Measles virus induced downregulation of CD46 is associated with enhanced sensitivity to complement mediated lysis of infected cells. Eur J Immunol. 1995;25:976–984. doi: 10.1002/eji.1830250418. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka K, Xie M, Yanagi Y. The hemagglutinin of recent measles virus isolates induces cell fusion in a marmoset cell line, but not in other CD46-positive human and monkey cell lines, when expressed together with the F protein. Arch Virol. 1998;143:213–225. doi: 10.1007/s007050050281. [DOI] [PubMed] [Google Scholar]
- 41.Varior-Krishnan G, Trescol-Biemont M-C, Naniche D, Rabourdin-Combe C, Gerlier D. Glycosyl-phosphatidylinositol-anchored and transmembrane forms of CD46 display similar measles virus receptor properties: virus binding, fusion, and replication; down-regulation by hemagglutinin; and virus uptake and endocytosis for antigen presentation by major histocompatibility complex class II molecules. J Virol. 1994;68:7891–7899. doi: 10.1128/jvi.68.12.7891-7899.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yant S, Hirano A, Wong T C. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J Virol. 1997;71:766–770. doi: 10.1128/jvi.71.1.766-770.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]