Abstract
Purpose
The receptor activator of nuclear factor kappa B (RANK) and its ligand (RANKL) have been shown to promote proliferation of the breast and breast carcinogenesis. The objective of this analysis was to investigate whether tumor-specific RANK and RANKL expression in patients with primary breast cancer is associated with high percentage mammographic density (PMD), which is a known breast cancer risk factor.
Methods
Immunohistochemical staining of RANK and RANKL was performed in tissue microarrays (TMAs) from primary breast cancer samples of the Bavarian Breast Cancer Cases and Controls (BBCC) study. For RANK and RANKL expression, histochemical scores (H scores) with a cut-off value of > 0 vs 0 were established. PMD was measured in the contralateral, non-diseased breast. Linear regression models with PMD as outcome were calculated using common predictors of PMD (age at breast cancer diagnosis, body mass index (BMI) and parity) and RANK and RANKL H scores. Additionally, Spearman rank correlations (ρ) between PMD and RANK and RANKL H score were performed.
Results
In the final cohort of 412 patients, breast cancer-specific RANK and RANKL expression was not associated with PMD (P = 0.68). There was no correlation between PMD and RANK H score (Spearman’s ρ = 0.01, P = 0.87) or RANKL H score (Spearman’s ρ = 0.04, P = 0.41). RANK expression was highest in triple-negative tumors, followed by HER2-positive, luminal B-like and luminal A-like tumors, while no subtype-specific expression of RANKL was found.
Conclusion
Results do not provide evidence for an association of RANK and RANKL expression in primary breast cancer with PMD.
Keywords: Breast cancer, Mammographic density, RANK expression, RANKL expression, Immunohistochemistry
What does this study add to the clinical work?
| The inhibition of the receptor activator of nuclear factor kappa B ligand (RANKL) pathway with denosumab is currently being tested in clinical trials for the primary prevention of breast cancer in women with high breast cancer risk. This study investigated whether the expression of RANK and RANKL in the tumor tissue of patients with primary breast cancer correlates with the well-known breast cancer risk factor mammographic density, and did not find an association. |
Introduction
High mammographic density (MD) has been confirmed to modify breast cancer risk depending on the percentage of MD (PMD) with a two–sixfold increased risk [1, 2]. Besides from familial and genetic factors [3, 4], higher PMD has been linked with the cumulative exposure to growth factors and hormones. This includes a great lifetime number of menstrual cycles by early menarche and late menopause, which is an indicator for cumulative exposure to luteal phase progesterone levels, a low number of parities and life births, adipose body mass index (BMI), combined estrogen-plus-progestin hormone replacement therapy, elevated levels of prolactin, and other factors [1, 3, 5, 6].
PMD reflects the proportion of dense breast tissue comprising epithelial cells, fibroblasts, and connective tissue on a mammogram, whereas adipose tissue is the main component of non-dense breast tissue. Although it has been proposed that stromal architecture and composition of the breast influence epithelial biology and play an initial role in breast carcinogenesis, the molecular mechanisms between PMD and increased breast cancer risk are still not well understood [2, 3].
The receptor activator of nuclear factor kappa B (RANK) and its ligand (RANKL) as well as osteoprotegerin (OPG), functioning as an antagonistic, soluble decoy receptor for RANKL, are expressed by various tissues and cell lines. Besides its role in bone metabolism and osseous metastasis, RANK/RANKL/OPG signaling is also involved in physiological and pathological processes of immune response and proliferation of different tissues including the mammary gland [7–9].
It has been demonstrated that progesterone and prolactin increase the expression of RANKL in the breast and interact with the RANK pathway, inducing lobulo-alveolar differentiation, proliferation, and expansion of mammary epithelial cells. Inhibition of progesterone, RANK or RANKL resulted in less mammary cell proliferation, carcinogenesis, and metastasis in mouse models [7, 9–11]. This has been shown especially in models of BRCA1 mutated breast cancer [7, 9, 12, 13].
The monoclonal antibody against RANKL denosumab has proven efficacy in the prevention and treatment of osteoporosis and bone metastases in breast cancer as well as in other types of cancer [14, 15]. In addition, trials with female BRCA mutation carriers are investigating the effect of denosumab on proliferation of the breast epithelium (BRCA-D, ACTRN12614000694617 [16]) and as a chemopreventive drug against breast cancer (BRCA-P, NCT04711109 [17]).
Because of its association with breast proliferation and mammary tumor development, it has been hypothesized that RANK, RANKL, and OPG expression is linked with PMD. This has been investigated by few studies for serum or plasma expression [18–20], or expression in healthy breast tissue [21], but not for breast cancer-specific expression so far. The aim of the present study was thus to assess the correlation of RANK and RANKL expression in primary breast cancer samples with PMD of the contralateral, healthy breast.
Patients and methods
Patients
The Bavarian Breast Cancer Cases and Controls (BBCC) study is a case–control study investigating molecular and epidemiological breast cancer risk factors as well as prognostic and predictive factors including PMD. Between 2000 and 2007, 1538 patients were included who were at least 18 years old and had a diagnosis of invasive breast cancer. Tissue microarrays (TMAs) were constructed from 894 patients. After exclusion of datasets with ineligible characteristics or missing information, the final study population comprised 412 female patients with unilateral invasive breast cancer. The detailed selection process is provided in Fig. 1.
Fig. 1.
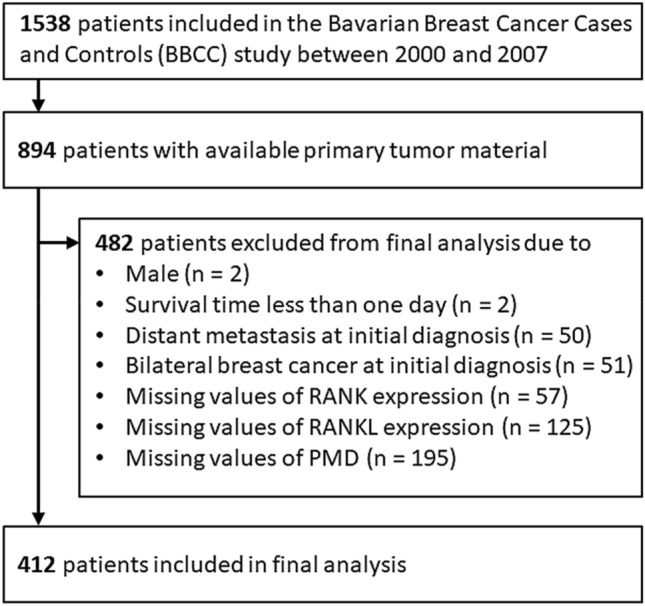
Flowchart of patient selection
Histopathological, epidemiological and follow-up data
Comprehensive data on tumor and patient characteristics as well as follow-up data for a minimum of 10 years after initial diagnosis were documented conforming to the requirements of the German Cancer Society (Deutsche Krebsgesellschaft) and the German Society for Breast Diseases (Deutsche Gesellschaft für Senologie) as part of the certification process [22]. Breast cancer subtypes were defined as previously described [23]. Shortly, HER2 receptor-negative tumors which showed either estrogen receptor (ER) or progesterone receptor (PR) expression (≥ 10%) were classified as luminal A-like for Ki-67 < 15% and grading of 1 or 2 or luminal B-like for Ki-67 ≥ 15% and grading of 2 or 3. HER2 receptor-positive breast cancer was stated in patients with HER2 staining of 3 + as assessed by immunohistochemistry or HER2 gene amplification. Patients with HER2-negative and hormone receptor (HR)-negative or weakly positive (< 10%) breast cancer were considered as triple-negative (TNBC) [23].
Assessment of PMD
For the assessment of PMD, mammograms were eligible if they were taken 1 year before or 3 months after breast cancer diagnosis. PMD was measured on the contralateral breast, which was not affected by breast cancer in cranio-caudal (CC) projection. In this work, full-field digital mammograms and film-based mammograms were examined. Analog film-based mammograms were digitized by a CadPro Advantage® film digitizer (VIDAR Systems Corporation, Herndon, Virginia, USA). Breast area measurements and quantitative computer-based threshold density assessments were performed by two individual, experienced readers with special training in the applied method. Mammograms were analyzed in an independent and arbitrary order, and the readers were unaware of any previous findings. Finally, the mean PMD of the two readers was used for analysis. The MD proportion was evaluated using the Madena software program, version 3.26 (Eye Physics, LLC, Los Alamitos, California, USA). This method has been validated and described before [24], and we used it in several previous works [1, 6, 25–28].
Assessment of RANK and RANKL expression
Tumor specimens were formalin-fixed and paraffin-embedded (FFPE). In a first step, an experienced pathologist marked the tumor areas of interest on a hematoxylin–eosin-stained slide. For the construction of TMAs, cylindric tissue core biopsies (0.8 mm per dot) from multiple sample donor blocks were re-embedded in a second step into a single microarray block at predefined coordinates. Staining of the TMA was performed with anti-human RANK (N-1H8; Amgen, Thousand Oaks, California, USA) or RANKL (M366; Amgen, Thousand Oaks, California, USA) mouse monoclonal antibodies or isotype-matched control mouse IgG, as previously described [29, 30]. For each primary tumor, RANK and RANKL expression was scored according to the semiquantitative histochemical score (H score) [31]. Experienced pathologists conducted the immunohistochemical interpretation blinded to any sample identification. The percentage of RANK and RANKL-positive tumor cells was multiplied by staining intensity, respectively: 0, negative; 1 + , weak; 2 + , moderate; and 3 + , strong. The sum of all calculated tumor cell percentage/intensity product for every TMA dot was defined as H score, ranging from 0–300. As a result, 300 represents 100% of tumor cells having a strong staining intensity.
Statistical analysis
The primary objective of this analysis was to investigate the association between RANK and RANKL tumor expression, quantified as H score, and PMD. For that purpose, we calculated linear regression models with PMD as outcome. The square root of PMD was used to gain normally distributed residuals of the models. First, a basic model with the following predictors was set up: age at diagnosis (continuous), BMI (continuous), and parity (number of children born, categorical: 0, 1, 2, and ≥ 3). Afterwards, RANK (> 0 vs 0) and RANKL (> 0 vs 0) was added to the basic model to obtain a full model. Due to large proportions of zeroes in RANK and RANKL H scores, those variables were dichotomized in either negative or positive to perform further analyses.
The basic and the full model were compared using the F test. A significant result means that RANK or RANKL H score is associated with PMD. As sensitivity analysis we calculated Spearman rank correlations (ρ) between PMD and RANK or RANKL H score and tested their significance.
Subjects with missing values in RANK and RANKL H score and PMD were excluded from analysis. Missing values in other predictors were imputed as described in Salmen et al. [32]. The 15 (3.6%) values for BMI were substituted by the median of non-missing data. For the imputation of the 25 (6.1%) values in parity, we calculated a multinomial logistic regression model with the predictors age, BMI, and PMD.
All of the tests were two-sided, and P < 0.05 was regarded as statistically significant. Calculations were carried out using the R system for statistical computing (version 3.4.0; R Development Core Team, Vienna, Austria, 2017).
Results
Patient and tumor characteristics
Overall, 412 female patients with primary breast cancer were included in the final analysis. Mean age at breast cancer diagnosis was 58.6 (standard deviation, SD 12.7) years, and median BMI was 25.2 (interquartile range, IQR 22.5–28.6) kg/m2. A majority of patients gave birth to two children (40.5%), while a minority was nulliparous (13.6%). Most patients had a pathological tumor size of T1 (n = 198, 48.1%) or T2 (n = 166, 40.3%), had no lymph node involvement (n = 219, 53.2%), and had either luminal A-like (n = 133, 32.3%) or luminal B-like (n = 154, 37.4%) tumors (Table 1).
Table 1.
Patient and tumor characteristics
| Characteristic | ||
|---|---|---|
| Age at diagnosis (years) | Mean (SD) | 58.6 (12.7) |
| BMI at diagnosis | Median (IQR) | 25.2 (22.5–28.6) |
| Parity (children born) | 0 | 56 (13.6%) |
| 1 | 125 (30.3%) | |
| 2 | 167 (40.5%) | |
| ≥ 3 | 64 (15.5%) | |
| PMD contralateral | Median (IQR) | 0.37 (0.24–0.53) |
| RANK H score | 0 | 269 (65.3%) |
| > 0 | 143 (34.7%) | |
| RANKL H score | 0 | 369 (89.6%) |
| > 0 | 43 (10.4%) | |
| Tumor stage* | T1 | 198 (48.1%) |
| T2 | 166 (40.3%) | |
| T3 | 21 (5.1%) | |
| T4 | 27 (6.6%) | |
| Lymph node status* | N negative | 219 (53.2%) |
| N positive | 193 (46.8%) | |
| Molecular subtype | Luminal A-like | 133 (32.3%) |
| Luminal B-like | 154 (37.4%) | |
| HER2-positive | 58 (14.1%) | |
| Triple-negative | 67 (16.3%) | |
Mean (standard deviation, SD) or median (interquartile range, IQR), where appropriate, are shown for continuous characteristics and frequency (percentage) for categorical characteristics. *In patients undergoing neoadjuvant chemotherapy, the initial clinical tumor and/or lymph node stage was used if the tumor was pathologically downstaged under neoadjuvant chemotherapy. BMI body mass index, PMD percent mammographic density
RANK and RANKL expression and correlation with PMD
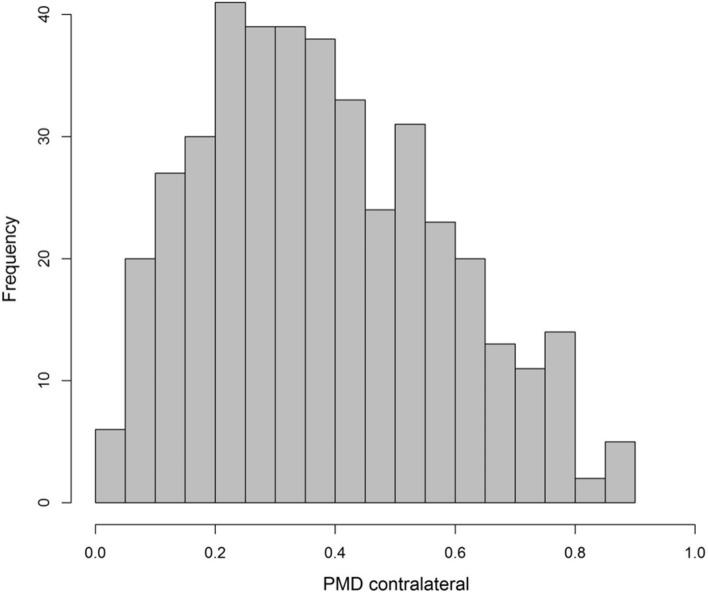
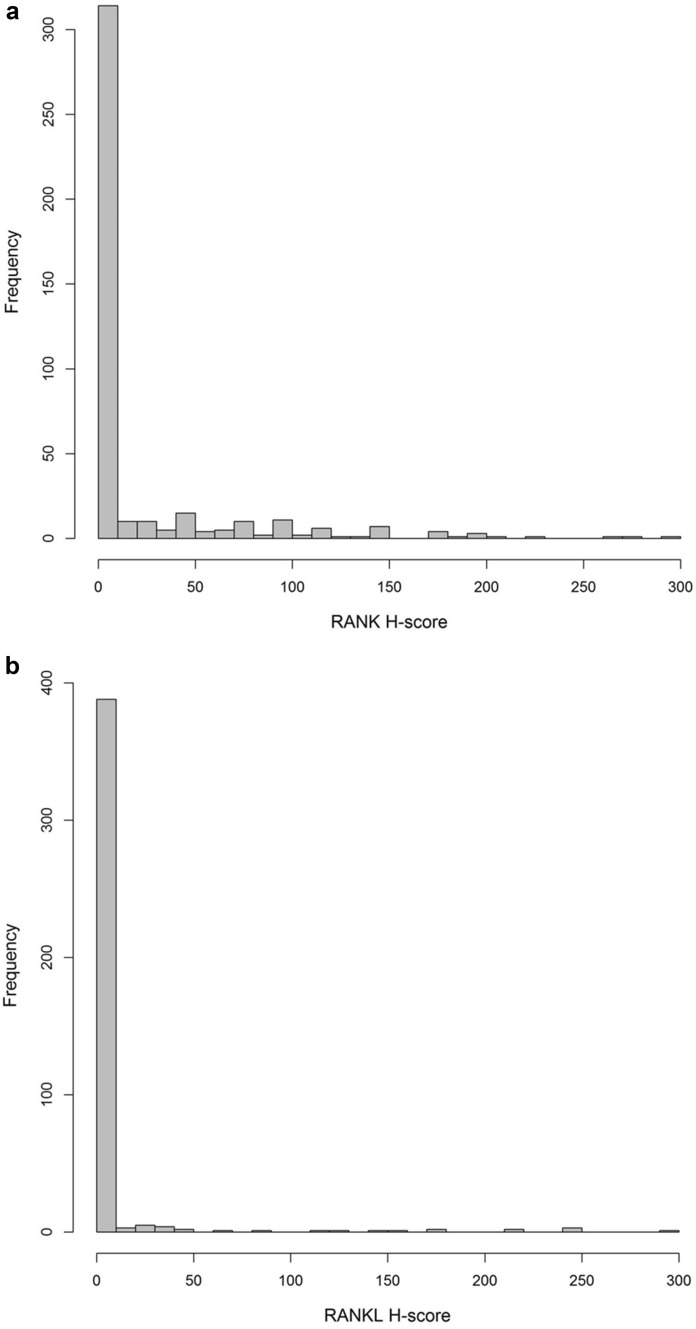
The median PMD was 0.37 (IQR 0.24–0.53) (Table 1). The distribution of PMD is depicted in Fig. 2. In the majority of the cases, the H score for immunohistochemical assessment of RANK and RANKL was 0, while 143 patients (34.7%) showed an H score > 0 for RANK and 43 patients (10.4%) for RANKL (Table 1). The median H score of cases with a positive expression was 50 (IQR 10–100) for RANK and 30 (IQR 8–125) for RANKL. Concerning molecular subtypes, the frequency of positive RANK expression was lowest among patients with luminal A-like breast cancer (19.5%), increasing in those with luminal B-like breast cancer (26.0%), HER2-positive breast cancer (55.2%), and TNBC (67.2%). The median RANK H score among patients with detectable RANK expression increased in the same order across molecular subtypes. No subtype-specific pattern could be seen for RANKL expression (Table 2). The distribution of RANK and RANKL H score is presented in Fig. 3a, b.
Fig. 2.
Distribution of percent mammographic density (PMD) contralateral
Table 2.
RANK and RANKL expression across molecular subtypes of breast cancer
| Molecular subtype | RANK H score > 0 | RANKL H score > 0 | ||||||
|---|---|---|---|---|---|---|---|---|
| Number | Percentage | Median* | IQR* | Number | Percentage | Median* | IQR* | |
| Luminal A-like | 26/133 | 19.5% | 15 | 5–50 | 12/133 | 9.0% | 25 | 6–40 |
| Luminal B-like | 40/154 | 26.0% | 30 | 10–80 | 20/154 | 13.0% | 45 | 13.8–165 |
| HER2-positive | 32/58 | 55.2% | 70 | 20–100 | 4/58 | 6.9% | 15 | 5–81.3 |
| Triple-negative | 45/67 | 67.2% | 80 | 30–150 | 7/67 | 10.4% | 30 | 5.5–77.5 |
| All subtypes | 143/412 | 34.7% | 50 | 10–100 | 43/412 | 10.4% | 30 | 8–125 |
Number and percentage of patients with RANK and RANKL H score > 0 is shown across molecular subtypes of breast cancer. *Among the subset of patients with RANK and RANKL H score > 0, the median including interquartile range (IQR) is shown
Fig. 3.
a Distribution of RANK H score. b Distribution of RANKL H score
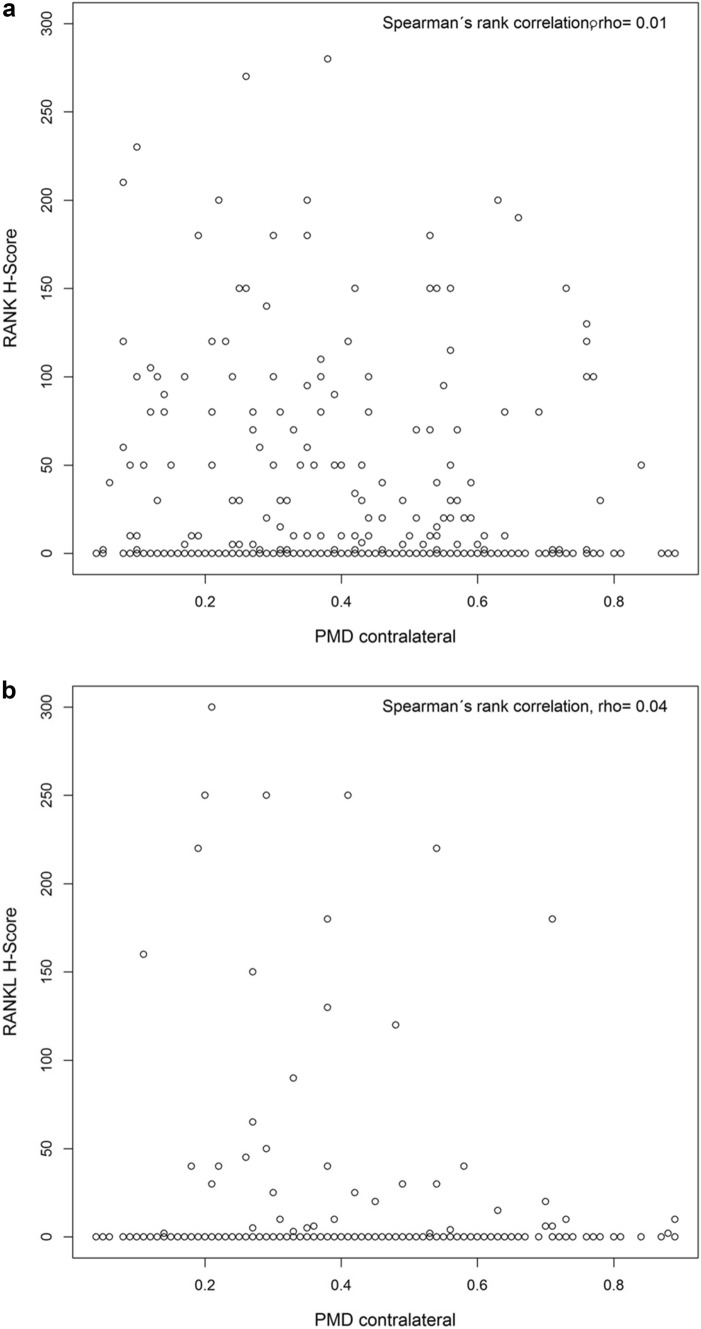
The linear regression analysis did not show an association of PMD with RANK and RANKL expression assessed by H score (F test, P = 0.68). Furthermore, sensitivity analysis revealed no significant correlation between PMD and RANK (Spearman’s ρ = 0.01, P = 0.87) or RANKL H score (Spearman’s ρ = 0.04, P = 0.41). Scatterplots for PMD and RANK or RANKL H score are shown in Fig. 4a, b.
Fig. 4.
a Scatterplot of RANK H score and PMD contralateral. b Scatterplot of RANKL H score and PMD contralateral
Discussion
In this retrospectively conducted study of 412 female patients with primary breast cancer, we could not find an association of RANK and RANKL expression, as assessed by immunohistochemistry of FFPE tumor tissue samples, with PMD of the contralateral, non-diseased breast.
In a recent observational study, we linked soluble RANKL and OPG expression to breast volume changes during pregnancy in healthy women, implicating an impact on breast proliferation [33]. Likewise, different in vitro and in vivo studies revealed a progesterone- and prolactin-driven induction of the RANK/RANKL/OPG pathway, triggering the development, growth, and migration of mammary epithelial cells, and leading to tumorigenesis and metastasis [7, 9–13]. An analysis of a subcohort of prospectively observed, initially healthy, postmenopausal women of the UKCTOCS study who developed breast cancer 12–24 months after sample collection, showed that high RANKL and progesterone serum levels led to a 5.3-fold increase of breast cancer risk [34]. Few studies also confirmed an inverse relationship of OPG serum levels with breast cancer risk in cohorts of primarily premenopausal patients with a BRCA1/2 mutation (mean age 42 years) [35, 36] as well as in the general population for primarily postmenopausal women (mean age 61 years) [37], while another investigation did not find an association in general premenopausal women (median age 44 years) [38].
Data on the association of RANK, RANKL, and OPG expression with PMD is limited. An analysis of 365 cancer-free premenopausal women described RANK serum levels to be positively correlated with PMD. The same association was found for RANKL serum levels if progesterone levels were elevated [20]. A second study of 368 postmenopausal women showed that an increase in RANK plasma gene expression led to higher volumetric percent density. Moreover, in patients with very high vs very low PMD, RANKL and surprisingly also OPG plasma gene expression were significantly upregulated, while RANKL and OPG plasma gene expression were not higher in women with heterogeneously dense breasts compared with those with almost entirely fatty breasts [18]. Another report on 43 postmenopausal confirmed higher mean PMD for those with lower serum OPG levels, and no association was identified in 57 premenopausal women [19]. In summary, the few available studies on healthy individuals propose that elevated RANK or RANKL circulating protein levels or plasma gene expression are associated with increased PMD [18, 20], while data concerning the effect of OPG expression on PMD are inconsistent [18, 19].
Data on the breast tissue expression of RANK, RANKL, and OPG are even rarer. One report demonstrated that in 48 healthy, premenopausal women, increasing RANKL gene expression in non-diseased FFPE breast tissue was associated with greater PMD [21]. In this context, it has to be noted that our study is the first one which investigated the expression of RANK and RANKL in breast cancer tissue with regard to PMD of the contralateral, healthy breast. Generally, it has been shown that tissue expression of RANK and RANKL is increased in healthy breast tissue compared with breast cancer tissue [39, 40], that tissue expression of RANKL varies with changing levels of sex hormones during the menstrual cycle [7, 41, 42], and that it is higher in premenopausal than in postmenopausal women [43, 44]. With a mean age of 58.6 years, our study collective represents primarily postmenopausal women, which could contribute to the relatively low tumor expression of RANK and RANKL.
Immunohistochemical staining of TMAs in the current study was performed with the same antibodies as has been reported in previous trials [29, 30, 45]. We detected a positive tumor expression of RANK and RANKL in 34.7% and 10.4% of the patients, respectively, and most of these had a low expression. In line with these results, a study on TMAs of 601 breast cancer patients found a positive expression of RANK in 27% and of RANKL in 6% [30], and another large analysis of TMAs of 2299 breast cancer patients from four independent cohorts (of these 777 patients with ER-negative disease) showed even lower expression of RANK and RANKL in the tumor compartment [46]. In a trial exclusively on TNBC patients, similar expression rates as in our study were identified [47]. Some other breast cancer studies reported higher expression [43, 48–50], partly with greater rates for RANK than for RANKL [30, 46–49]. The differences could be explained by varying specificity of immunohistochemical reagents or methodologies, other scoring systems, and the distribution of patient cohorts, histological subtypes, and clinical stages.
In the current study, tumor expression of RANK and RANKL was quantified as H scores. Since expression was low with 65.3% negative cases for RANK and 89.6% negative cases for RANKL, we performed a dichotomization in either negative or positive expression. The cut-off for RANK H score is different to a previously used cut-off of ≥ 8.5, which was identified as optimal for the prediction of pathological complete response and survival in a group of patients who all underwent neoadjuvant chemotherapy [30]. In contrast to this study, we investigated the association of RANK and RANKL expression with PMD in breast cancer patients of whom 55.1% received neoadjuvant or adjuvant chemotherapy and whose breast cancers had more favorable tumor characteristics.
In our study, triple-negative and HER2-positive tumors had a greater number of RANK-positives and a stronger RANK expression compared with luminal B-like and luminal A-like tumors, while no subtype-specific expression of RANKL was detected. In line with this finding, other studies correlated tumor expression of RANK predominantly with worse prognostic molecular parameters such as ER-negative, HR-negative, triple-negative, or basal-like breast cancer [30, 39, 45–49, 51], higher grading [30, 39, 46, 49, 51], and higher Ki-67 [30, 39, 46, 51]. Tumor expression of RANKL was associated with HR-positive, luminal A-like, or non-basal-like breast cancer [39, 43, 48], lower grading [39, 49], and lower Ki-67 in some studies [39, 50].
One of the strengths of our work is the inclusion of all women with incident breast cancer from clinical routine work regardless of any other criteria, reducing the risk of bias in the selection of patients and of treatment effects on PMD. Patients were recruited from a tertiary referral center in a university hospital and not from a population-based screening facility, which generally detects earlier tumor stages. The semiautomated quantification of MD, with two experienced, independent readers for all images and a mean value for PMD being used, has been validated as a robust method in different previous studies [1, 6, 24–28]. A limitation is the retrospective nature of the analysis with the potential of missing data. Several cases had to be excluded because of incomplete values in variables of interest such as PMD, RANK, and RANKL H score or due to technical issues of TMA evaluation (e.g., inadequate tumor tissue recognizable or tumor core washed off).
Conclusion
Although a limited number of studies has described an association between RANK, RANKL, and OPG expression in serum, plasma, or healthy breast tissue with PMD, our study does not show a correlation between tumor-specific RANK and RANKL expression with PMD in patients with primary breast cancer. Since RANK/RANKL/OPG signaling appears to play a role in the development of breast cancer and since RANKL inhibition may be a novel chemoprevention strategy in women at an increased breast cancer risk, this pathway will remain under investigation of present and future trials.
Abbreviations
- BMI
Body mass index
- ER
Estrogen receptor
- FFPE
Formalin-fixed and paraffin-embedded
- HER2
Human epidermal growth factor receptor 2
- HR
Hormone receptor
- IQR
Interquartile range
- MD
Mammographic density
- OPG
Osteoprotegerin
- PMD
Percent mammographic density
- PR
Progesterone receptor
- RANK
Receptor activator of nuclear factor kappa B
- RANKL
Receptor activator of nuclear factor kappa B ligand
- TMA
Tissue microarray
- TNBC
Triple-negative breast cancer
Author contributions
JE and PAF contributed substantially to the conception and supervision of the study and to the interpretation of data. CCH, FH, and KH performed measurements of mammographic density. AH and RE were involved in construction of TMAs and pathological assessment. MFP and WCD were involved in expression analysis of TMAs. LH performed statistical analyses. MW drafted the manuscript and revised it critically for important intellectual content. AH, ASB, CCH, FH, HH, JE, KH, MFP, MR, MW, MWB, PAF, PG, RE, RSW, and WCD contributed to the acquisition of data. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Research for the present study was supported by a grant from the Bavaria California Technology Center (www.bacatec.de), a grant from the Breast Cancer Research Foundation (BCRF-22–132 to MFP), and a grant from the California Breast Cancer Research Program (BCRP 12IB-0155 to MFP). PAF was funded by a grant from the Dr. Mildred Scheel Foundation of German Cancer Aid (Deutsche Krebshilfe e.V.).
Data availability
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
AH has had an advisory role and has received honoraria from Abbvie, Agilent, AstraZeneca, BMS, Boehringer Ingelheim, Cepheid, Diaceutics, Ipsen, Jansen-Cilag, Lilly, MSD, Pfizer, Qiagen, and Roche. RE has received honoraria from AstraZeneca, BioNTech, Diaceutics, Eisai, MEDAC, Mindpeak, Novartis, Pfizer, Roche, and Veracyte (PROCURE). The institution of AH and RE conducts research for AstraZeneca, Biocartis, BioNTech, Cepheid, Gilead, Janssen-Cilag, Mindpeak, MSD, NanoString Technologies, Novartis, palleos healthcare, Owkin, Roche, and ZytoVision. CCH has received honoraria from AstraZeneca, Daiichi Sankyo, Eisai, Gilead, MSD, Novartis, Pfizer, and Roche. JE has received honoraria from Eisai, Novartis, and Pfizer. PAF has received honoraria from Agendia, AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Gilead, Hexal, Lilly, MacroGenics, Merck Sharp and Dohme, Novartis, Pfizer, Pierre Fabre, Roche, Seattle Genetics, and received grant support from BioNTech and Cepheid. PG has received honoraria from AstraZeneca, MSD, and Novartis. MFP has received consulting fees from AstraZeneca, Curio Science, Eli Lilly & Company, Lilly USA, LLC, Merck & Co, Novartis Pharmaceuticals Corporation, and Zymeworks Inc, honoraria for speaking engagements from Physicians’ Education Resource®, LLC (PER®) and Medscape, and he reports institutional support from TORL BioTherapeutics LLC. WCD is a member of the EpimAb Biotherapeutics scientific advisory board and holds patents related to the Receptor Activator of Nuclear Factor-κ B Ligand (RANKL) pathway. The other authors declare no conflict of interest.
Ethical approval
This study was approved by the ethics committee of the Faculty of Medicine at Friedrich-Alexander-Universität Erlangen-Nürnberg (reference numbers 2700 and 297_17 BC). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rauh C, Hack CC, Haberle L, et al. Percent mammographic density and dense area as risk factors for breast cancer. Geburtshilfe Frauenheilkd. 2012;72(8):727–733. doi: 10.1055/s-0032-1315129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd NF, Martin LJ, Bronskill M, et al. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst. 2010;102(16):1224–1237. doi: 10.1093/jnci/djq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin LJ, Boyd NF. Mammographic density Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008 doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H, Fan S, Stone J, et al. Genome-wide and transcriptome-wide association studies of mammographic density phenotypes reveal novel loci. Breast Cancer Res. 2022;24(1):27. doi: 10.1186/s13058-022-01524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne C, Ursin G, Martin CF, et al. Mammographic density change with estrogen and progestin therapy and breast cancer risk. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hack CC, Emons J, Jud SM, et al. Association between mammographic density and pregnancies relative to age and BMI: a breast cancer case-only analysis. Breast Cancer Res Treat. 2017;166(3):701–708. doi: 10.1007/s10549-017-4446-7. [DOI] [PubMed] [Google Scholar]
- 7.Infante M, Fabi A, Cognetti F, et al. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J Exp Clin Cancer Res. 2019;38(1):12. doi: 10.1186/s13046-018-1001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ney JT, Fehm T, Juhasz-Boess I, et al. RANK, RANKL and OPG expression in breast cancer–influence on osseous metastasis. Geburtshilfe Frauenheilkd. 2012;72(5):385–391. doi: 10.1055/s-0031-1298276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono T, Hayashi M, Sasaki F, et al. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2. doi: 10.1186/s41232-019-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Suarez E, Jacob AP, Jones J, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 11.Schramek D, Leibbrandt A, Sigl V, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468(7320):98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigl V, Owusu-Boaitey K, Joshi PA, et al. RANKL/RANK control Brca1 mutation-driven mammary tumors. Cell Res. 2016;26(7):761–774. doi: 10.1038/cr.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan E, Vaillant F, Branstetter D, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med. 2016;22(8):933–939. doi: 10.1038/nm.4118. [DOI] [PubMed] [Google Scholar]
- 14.Cadieux B, Coleman R, Jafarinasabian P, et al. Experience with denosumab (XGEVA(R)) for prevention of skeletal-related events in the 10 years after approval. J Bone Oncol. 2022;33:100416. doi: 10.1016/j.jbo.2022.100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Untch M, Banys-Paluchowski M, Brucker SY, Budach W, Denkert C, Ditsch N, Fasching PA, Haidinger R, Heil J, Jackisch C, Janni W, Kolberg H-C, Krug D, Loibl S, Lüftner D, van Mackelenbergh M, Radosa JC, Reimer T, Welslau M, Würstlein R, Harbeck N, Huober J. Treatment of early breast cancer: the 18th St. gallen international breast cancer consensus conference against the background of current German treatment recommendations. Geburtshilfe Frauenheilkd. 2023;83(9):1102–1116. doi: 10.1055/a-2121-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindeman GJ, Nolan E, Pal B, et al. Abstract P3–11–05 RANK ligand is a target for breast cancer prevention in BRCA1 mutation carriers. Cancer Res. 2016 doi: 10.1038/nm.4118. [DOI] [PubMed] [Google Scholar]
- 17.Bhulani N, Wood M, Tsai T, et al. A phase 3 study to determine the breast cancer risk reducing effect of denosumab in women carrying a germline BRCA1 mutation (BRCA-P Study) J Clin Oncol. 2022 doi: 10.1200/JCO.2022.40.16_suppl.TPS10616. [DOI] [Google Scholar]
- 18.Mintz R, Wang M, Xu S, et al. Hormone and receptor activator of NF-kappaB (RANK) pathway gene expression in plasma and mammographic breast density in postmenopausal women. Breast Cancer Res. 2022;24(1):28. doi: 10.1186/s13058-022-01522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran O, Zaman T, Eisen A, et al. Serum osteoprotegerin levels and mammographic density among high-risk women. Cancer Causes Control. 2018;29(6):507–517. doi: 10.1007/s10552-018-1035-y. [DOI] [PubMed] [Google Scholar]
- 20.Toriola AT, Appleton CM, Zong X, et al. Circulating Receptor Activator of Nuclear Factor-κB (RANK), RANK ligand (RANKL), and mammographic density in premenopausal women. Cancer Prev Res (Phila) 2018;11(12):789–796. doi: 10.1158/1940-6207.CAPR-18-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toriola AT, Dang HX, Hagemann IS, et al. Increased breast tissue receptor activator of nuclear factor-κB ligand (RANKL) gene expression is associated with higher mammographic density in premenopausal women. Oncotarget. 2017;8(43):73787–73792. doi: 10.18632/oncotarget.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wöckel A., Festl J., Stüber T., et al. 2018. Interdisciplinary Screening, Diagnosis, Therapy and Follow-up of Breast Cancer Guideline of the DGGG and the DKG (S3-Level, AWMF Registry Number 032/045OL, December 2017) with Recommendations for the Screening, Diagnosis and Therapy of Breast Cancer. Geburtshilfe Frauenheilkd. 10.1055/a-0646-4522 [DOI] [PMC free article] [PubMed]
- 23.Wunderle M, Gass P, Häberle L, et al. BRCA mutations and their influence on pathological complete response and prognosis in a clinical cohort of neoadjuvantly treated breast cancer patients. Breast Cancer Res Treat. 2018;171(1):85–94. doi: 10.1007/s10549-018-4797-8. [DOI] [PubMed] [Google Scholar]
- 24.Ursin G, Astrahan MA, Salane M, et al. The detection of changes in mammographic densities. Cancer Epidemiol Biomarkers Prev. 1998;7(1):43–47. [PubMed] [Google Scholar]
- 25.Heusinger K, Jud SM, Haberle L, et al. Association of mammographic density with hormone receptors in invasive breast cancers: results from a case-only study. Int J Cancer. 2012;131(11):2643–2649. doi: 10.1002/ijc.27515. [DOI] [PubMed] [Google Scholar]
- 26.Haberle L, Fasching PA, Brehm B, et al. Mammographic density is the main correlate of tumors detected on ultrasound but not on mammography. Int J Cancer. 2016;139(9):1967–1974. doi: 10.1002/ijc.30261. [DOI] [PubMed] [Google Scholar]
- 27.Hack CC, Stoll MJ, Jud SM, et al. Correlation of mammographic density and serum calcium levels in patients with primary breast cancer. Cancer Med. 2017;6(6):1473–1481. doi: 10.1002/cam4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heindl F, Fasching PA, Hein A, et al. Mammographic density and prognosis in primary breast cancer patients. Breast. 2021;59:51–57. doi: 10.1016/j.breast.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood CE, Branstetter D, Jacob AP, et al. Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res. 2013;15(4):R62. doi: 10.1186/bcr3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfitzner BM, Branstetter D, Loibl S, et al. RANK expression as a prognostic and predictive marker in breast cancer. Breast Cancer Res Treat. 2014;145(2):307–315. doi: 10.1007/s10549-014-2955-1. [DOI] [PubMed] [Google Scholar]
- 31.McCarty K.S., Jr., Miller L.S., Cox E.B. et al., 1985. Estrogen receptor analyses Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 109 (8) 716 721 [PubMed]
- 32.Salmen J, Neugebauer J, Fasching PA, et al. Pooled analysis of the prognostic relevance of progesterone receptor status in five German cohort studies. Breast Cancer Res Treat. 2014;148(1):143–151. doi: 10.1007/s10549-014-3130-4. [DOI] [PubMed] [Google Scholar]
- 33.Wunderle M, Ruebner M, Haberle L, et al. RANKL and OPG and their influence on breast volume changes during pregnancy in healthy women. Sci Rep. 2020;10(1):5171. doi: 10.1038/s41598-020-62070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiechl S, Schramek D, Widschwendter M, et al. Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer. Oncotarget. 2017;8(3):3811–3825. doi: 10.18632/oncotarget.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oden L, Akbari M, Zaman T, et al. Plasma osteoprotegerin and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Oncotarget. 2016;7(52):86687–86694. doi: 10.18632/oncotarget.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Widschwendter M, Burnell M, Fraser L, et al. Osteoprotegerin (OPG), the endogenous inhibitor of receptor activator of NF-κB Ligand (RANKL), is dysregulated in BRCA mutation carriers. EBioMedicine. 2015;2(10):1331–1339. doi: 10.1016/j.ebiom.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vik A, Brodin EE, Mathiesen EB, et al. Serum osteoprotegerin and future risk of cancer and cancer-related mortality in the general population: the Tromsø study. Eur J Epidemiol. 2015;30(3):219–230. doi: 10.1007/s10654-014-9975-3. [DOI] [PubMed] [Google Scholar]
- 38.Kotsopoulos J, McGee EE, Lozano-Esparza S, et al. Premenopausal plasma osteoprotegerin and breast cancer risk: a case-control analysis nested within the nurses' health study II. Cancer Epidemiol Biomarkers Prev. 2020;29(6):1264–1270. doi: 10.1158/1055-9965.EPI-19-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azim HA, Jr, Peccatori FA, Brohee S, et al. RANK-ligand (RANKL) expression in young breast cancer patients and during pregnancy. Breast Cancer Res. 2015;17:24. doi: 10.1186/s13058-015-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen S, Ye L, Sanders AJ, et al. Expression profile of receptor activator of nuclear-κB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast cancer. Anticancer Res. 2013;33(1):199–206. [PubMed] [Google Scholar]
- 41.Hu H, Wang J, Gupta A, et al. RANKL expression in normal and malignant breast tissue responds to progesterone and is up-regulated during the luteal phase. Breast Cancer Res Treat. 2014;146(3):515–523. doi: 10.1007/s10549-014-3049-9. [DOI] [PubMed] [Google Scholar]
- 42.Haynes BP, Viale G, Galimberti V, et al. Differences in expression of proliferation-associated genes and RANKL across the menstrual cycle in estrogen receptor-positive primary breast cancer. Breast Cancer Res Treat. 2014;148(2):327–335. doi: 10.1007/s10549-014-3181-6. [DOI] [PubMed] [Google Scholar]
- 43.Timotheadou E, Kalogeras KT, Koliou GA, et al. Evaluation of the Prognostic Value of RANK, OPG, and RANKL mRNA expression in early breast cancer patients treated with anthracycline-based adjuvant chemotherapy. Transl Oncol. 2017;10(4):589–598. doi: 10.1016/j.tranon.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azim HA, Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18(5):1341–1351. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 45.Behrens A, Wurmthaler L, Heindl F, et al. RANK and RANKL Expression in Tumors of Patients with Early Breast Cancer. Geburtshilfe Frauenheilkd. 2024;84(1):77–85. doi: 10.1055/a-2192-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ciscar M, Trinidad EM, Perez-Chacon G, et al. RANK is a poor prognosis marker and a therapeutic target in ER-negative postmenopausal breast cancer. EMBO Mol Med. 2023;15(4):e16715. doi: 10.15252/emmm.202216715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reyes ME, Fujii T, Branstetter D, et al. Poor prognosis of patients with triple-negative breast cancer can be stratified by RANK and RANKL dual expression. Breast Cancer Res Treat. 2017;164(1):57–67. doi: 10.1007/s10549-017-4233-5. [DOI] [PubMed] [Google Scholar]
- 48.Vidula N, Yau C, Li J, et al. Receptor activator of nuclear factor kappa B (RANK) expression in primary breast cancer correlates with recurrence-free survival and development of bone metastases in I-SPY1 (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2017;165(1):129–138. doi: 10.1007/s10549-017-4318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santini D, Schiavon G, Vincenzi B, et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS ONE. 2011;6(4):e19234. doi: 10.1371/journal.pone.0019234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park HS, Lee A, Chae BJ, et al. Expression of receptor activator of nuclear factor kappa-B as a poor prognostic marker in breast cancer. J Surg Oncol. 2014;110(7):807–812. doi: 10.1002/jso.23737. [DOI] [PubMed] [Google Scholar]
- 51.Link T, Blohmer JU, Schmitt WD, et al. RANK expression as an independent predictor for response to neoadjuvant chemotherapy in luminal-like breast cancer: a translational insight from the geparX trial. Clin Cancer Res. 2023;29(22):4606–4612. doi: 10.1158/1078-0432.CCR-23-1801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.