Abstract
Objective. Photon-counting micro-computed tomography (micro-CT) is a major advance in small animal preclinical imaging. Small molecule- and nanoparticle-based contrast agents have been widely used to enable the differentiation of liver tumors from surrounding tissues using photon-counting micro-CT. However, there is a notable gap in the application of these market-available agents to the imaging of breast and ovarian tumors using photon-counting micro-CT. Herein, we have used photon-counting micro-CT to determine the effectiveness of these contrast agents in differentiating ovarian and breast tumor xenografts in live, intact mice. Approach. Nude mice carrying different types of breast and ovarian tumor xenografts (AU565, MDA-MB-231 and SKOV-3 human cancer cells) were injected with ISOVUE-370 (a small molecule-based agent) or Exitron Nano 12000 (a nanoparticle-based agent) and subjected to photon-counting micro-CT. To improve tumor visualization using photon-counting micro-CT, we developed a novel color visualization method, which changes color tones to highlight contrast media distribution, offering a robust alternative to traditional material decomposition methods with less computational demand. Main results. Our in vivo experiments confirm the effectiveness of this color visualization approach, showing distinct enhancement characteristics for each contrast agent. Qualitative and quantitative analyses suggest that Exitron Nano 12000 provides superior vasculature enhancement and better quantitative consistency across scans, while ISOVUE-370 delivers a more comprehensive tumor enhancement but with significant variance between scans due to its short blood half-time. Further, a paired t-test on mean and standard deviation values within tumor volumes showed significant differences between the AU565 and SKOV-3 tumor models with the nanoparticle-based contrast agent (p-values < 0.02), attributable to their distinct vascularity, as confirmed by immunohistochemical analysis. Significance. These findings underscore the utility of photon-counting micro-CT in non-invasively assessing the morphology and anatomy of different tumor xenografts, which is crucial for tumor characterization and longitudinal monitoring of tumor progression and response to treatments.
Keywords: computed tomography, preclinical, photon-counting micro-CT, tumor imaging, tumor xenografts, contrast agent
1. Introduction
Murine tumor models play a critical role in oncology research, enabling a wide variety of pre-clinical studies important for the diagnostic and treatment of cancer. In particular, human breast cancer lines have been widely used to generate tumor xenograft models in immunocompromised mice. These cell-line derived xenografts have been used to investigate the biology and morphology of human breast tumors (Souto et al 2022, Boix-Montesinos et al 2021). Here, we have used photon-counting contrast-enhanced micro-computed tomography (micro-CT) to characterize the anatomy and morphology of various types of breast tumor xenografts and their respective microenvironment in live, intact nude mice.
Non-invasive longitudinal studies in tumor mouse models have been performed using widely available tomographic imaging techniques, such as magnetic resonance imaging (MRI) (Sulheim et al 2018, Fiordelisi et al 2019), optical microscopy (Li et al 2020, Smith et al 2022), ultrasound imaging (Sulheim et al 2018) and x-ray computed tomography (CT) (Ashton et al 2015, Cassol et al 2019). Among those modalities, x-ray micro-CT has gained significant traction for several reasons, including its noninvasive 3D imaging capability, isotropic spatial resolution (∼10–100 μm), good balance between resolution and field of view, fast speed, easy processing, and relatively low cost compared to MRI (Clark and Badea 2021). Since tumors generally have a similar density to their surrounding tissues, contrast material is needed for improved tumor visibility when using micro-CT. For example, iodine-based small molecule contrast agents (such as ISOVUE-370) are the most widely used contrast agents in diagnostic CT imaging (Caschera et al 2016), and they are also used in animal studies for assessing perfusion in lung (Xin et al 2023), enhancing breast lymphatic pathways (Suga et al 2003), and monitoring the blood flow through tumors (Badea et al 2006). Fenestra LC is one of the most used contrast agents for liver and spleen micro-CT imaging (Bakan et al 2000, Almajdub et al 2007, Kim et al 2008, Sweeney et al 2019). Nanoparticle (NP)-based bloodstream contrast agents have been used to monitor the development of liver and spleen tumors in a longitudinal manner (Boll et al 2011, Cassol et al 2019, Liu et al 2019), via volume extraction (Cassol et al 2019), and vascularity measurements (Sulheim et al 2018). However, compared with liver/spleen tumors, breast tumor imaging is more challenging, since it relies on positive contrast improvement based on the enhanced permeability and retention (EPR) (Maeda 2001, Wu 2021). In this context, a dedicated contrast agent with long blood half-life is desired for best vasculature tumor imaging (Hainfeld et al 2018, Sulheim et al 2018), and active targeting can be even better for specific tumor imaging (Ramesh et al 2022).
The micro-CT technology for small animal imaging has been rapidly evolving in recent years. Energy-integrating detectors (EIDs) are commonly used for most micro-CT scanners due to their stable performance and low cost. However, EIDs only record grayscale intensity and are often insufficient to differentiate a contrast agent from high density tissues like bones or other distinct contrast agents. Dual-energy micro-CT with dual sources was developed for material discrimination, enabling imaging with two different contrast agents (Badea et al 2011, Clark et al 2013). Unfortunately, EIDs have a limited contrast sensitivity due to their higher weights for high energy photons in the image formation process. Moreover, the overlapping of the two energy spectra from the dual sources further reduces its material characterization capability. Photon-counting detectors (PCDs) have been used for micro-CT to record x-ray photons individually in an energy-discriminative fashion, which allows for the separation of multiple materials with superior sensitivity. Furthermore, PCDs are free of electronic noise in theory and typically considered as quantum noise limited. Given all these benefits, photon-counting CT (PCCT) can achieve similar image quality with much less radiation dose, limiting the radiation risk from multiple scans and facilitating longitudinal studies (Lu et al 2017, Cassol et al 2019). There are several commercially available photon-counting micro-CT systems for small animal imaging on the market, e.g., the MARS scanner (MARS Bioimaging Ltd, Christchurch, New Zealand) supporting simultaneous differentiation of four materials (Anderson et al 2010). In parallel, Siemens recently announced its first Food and Drug Administration (FDA)-cleared PCCT product NAEOTOM Alpha. These technological advances have spurred the development of novel contrast agents for CT molecular imaging, benefiting tumor visualization in numerous studies. Notably, NP contrast agents based on alkaline earth metal and heavy metal (ExiTron Nano and AuroVist) have been developed and are commercially accessible, and they have gained widespread use in small animal liver CT imaging (Boll et al 2011, Boll et al 2013, Rothe et al 2015, Das et al 2016, Mannheim et al 2016, Mahan and Doiron 2018, Cassol et al 2019). Various new NP agents have also been developed in the laboratory for other types of tumor CT imaging (Ashton et al 2018, Hainfeld et al 2018, Badea et al 2019, Ramesh et al 2022). However, there are few studies in which commercially available contrast agents were used for photon-counting micro-CT pre-clinical imaging of breast or ovarian tumors.
To close this gap, we have investigated two commercial contrast agents, ISOVUE-370 (human-compatible iopamidol injections) and Exitron Nano 12000 (alkaline earth metal-based NPs), for breast tumor contrast enhancement in vivo imaging using photon-counting micro-CT. Live nude mice carrying distinct breast and ovarian tumor xenografts were injected with different contrast agents and subjected to photon-counting micro-CT imaging. Tumor xenografts were generated from two breast cancer cell lines, which were selected as representatives of different breast cancer types: AU565 exemplifies HER2 positive breast cancer, which overexpresses epidermal growth factor 2 (HER2) receptor and lacks both estrogen (ER) and progesterone (PR) receptors and MDA-MB-231 illustrates triple-negative breast cancer lacking HER2, ER and PR receptors. SKOV-3 tumor xenografts represent HER2-positive ovarian tumors, which are well-known for their stiffness, reduced vascularity, and increased collagen content. These three tumor xenografts provide different types of tumor origin (breast versus ovarian), receptor expression (HER2+ versus triple negative) and tumor microenvironments (high versus low vascularity and collagen content) to demonstrate breast tumor contrast enhancement in vivo using photon-counting micro-CT.
Additionally, we propose a fast and convenient color visualization method to highlight the contrast media distribution without involving computationally intensive material decomposition. This color visualization method takes advantage of human eyes’ keen sensitivity to color change by correlating the spectral attenuation change with the color tone change for qualitative evaluation, and its effectiveness has been validated in our in vivo study. Furthermore, qualitative and quantitative enhancement effects using the two different contrast agents are compared to identify their benefits and/or disadvantages for micro-CT imaging of different types of tumor xenografts. In summary, we have investigated the potential of using photon-counting micro-CT to discriminate tumor xenografts from different origins, and cross-validated the results with immunohistochemical analysis.
2. Material and methods
2.1. Cell culture
All human breast cancer cell lines used in this study were obtained from ATCC. HER2-overexpressing human breast cancer cell line AU565 was cultured in RPMI medium supplemented with 10% fetal bovine serum, 10 mM HEPES and 50 Units ml−1/50 mg ml−1 penicillin and streptomycin solution. Triplenegative (estrogen receptor negative, progesterone receptor negative and HER2 positive) MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 4 mM L-glutamine, 10 mM HEPES, and penicillin/streptomycin (50 Units ml−1/50 mg ml−1) at 37 °C and 5% CO2. HER2-overexpressing human ovarian SKOV-3 cancer cell line was cultured in McCoy’s media, supplemented with 10% fetal bovine serum and 50 Units ml−1/50 μg ml−1 penicillin/streptomycin. All the cell lines were incubated at 37 °C and 5% CO2 and were used within passage ten to prevent phenotype drift and any changes in their phenotypic characteristics. Cells were routinely tested for mycoplasma to avoid contamination. For all reagents used in micro-CT imaging experiments refer to Supplementary table 1.
2.2. Tumor xenografts
For generating tumor xenografts, 10 × 106 AU565, 4 × 106 SKOV-3, and 5 × 106 MDA-MB-231 cells were mixed 1:1 with Cultrex BME and injected into the right and left inguinal mammary fat pad of 4-week-old athymic nude mice (CrTac:NCr-Foxn1nu). The subcutaneous tumors were allowed to grow for 4–5 weeks and were subjected to daily monitoring.
2.3. Experiments using iodine and/or NP contrast agents
To improve tumor visualization with micro-CT imaging, two commercial contrast agents, ISOVUE-370 (iodine-based with K-edge at ∼33 keV) and Exitron Nano 12000 NPs (barium-based with K-edge at ∼37 keV), were injected into mice carrying tumor xenografts. To compare the two contrast agents, our experiments included three imaging sessions: mice injected with only iodine, mice injected with both iodine and NPs, and mice injected with only NPs, as detailed below. In total, 7 athymic nude mice were used in our study, and 16 effective scans have been performed (table 1). At the end of each session, mice were sacrificed, and tumors were excised and fixed in formalin (3–8 ml/tumor in 15 ml tubes) for 2–5 d depending on the tumor.
-
(1)
To perform comparative imaging of different tumors using the iodine contrast agent ISOVUE-370, three athymic nude mice (tagged as AB, AL and AR) were injected subcutaneously in the right inguinal mammary pad with AU565 cells and on the opposite side with MDA-MB-231 cells. All mice had firm protruding tumors on both sides after 4–5 weeks, and sequential retroorbital injections of 120 μl iodine contrast ISOVUE-370 were performed in the anesthetized animals followed by micro-CT imaging. The injection and micro-CT imaging were repeated one week later.
-
(2)
To fairly compare the enhancement effects of different contrast agents, we injected two contrast agents into the same mice. Two athymic nude mice (tagged as JB and JR) were injected subcutaneously in the right inguinal mammary pad with AU565 cells. All mice had firm protruding tumors after 4–5 weeks, and sequential retroorbital injections of 120 μl iodine contrast ISOVUE-370 were performed in the anesthetized animals followed by micro-CT imaging. One week later, 100 ml Exitron Nano 12000 NPs retroorbital injections were performed in the anesthetized mice and 1–2 h later, micro-CT imaging was performed.
-
(3)
To investigate the imaging enhancement effect of the NP contrast agent on different types of tumors, two athymic nude mice (tagged as S1 and S2) were injected in the right inguinal mammary pad with AU565 cells. In parallel, ovarian cancer cells SKOV-3 were injected on the left side. After protruding tumors were detected, retroorbital injections of 100 μl Exitron Nano 12000 NPs were performed in the anesthetized animals, 1–2 h before micro-CT imaging. One week later, Exitron Nano 12 000 NPs injections followed by micro-CT were repeated. In the third week, micro-CT imaging was repeated without any additional injection.
Table 1.
Summary of all 16 effective scans. Each mouse has a unique tag and is coded in the same color within one session. The scan tag denotes in which week the scan was performed. The combination of a mouse tag and a scan tag uniquely identifies each scan, e.g. S1–3 denotes the scan of the mouse S1 in the third week. Note for the follow-up scans S1–3 and S2–3, they were performed without contrast injection, making the time gap from the last injections to the current scans one week. RO = retroorbital; IP = intraperitoneal.
| Session | Mouse tag | Scan tag | Contrast agent | Tumor type | Injection type | Time gap | Scan settings | |
|---|---|---|---|---|---|---|---|---|
| Left | Right | |||||||
| 1st | AL | 1 | Iodine | MDA231 | AU565 | RO | <10 min | |
| AB | 1 | Iodine | MDA231 | AU565 | RO | <10 min | ||
| AL | 2 | Iodine | MDA231 | AU565 | RO | <10 min | 80 kVp, 32 μA, No extra filtration, 720 views, 250 ms | |
| AR | 2 | Iodine | MDA231 | AU565 | RO | <10 min | ||
| AB | 2 | Iodine | MDA231 | AU565 | IP | ∼1 hour | ||
|
| ||||||||
| 2nd | JB | 1 | Iodine | NA | AU565 | RO | <10 min | |
| JR | 1 | Iodine | NA | AU565 | RO | <10 min | ||
| JB | 2 | NPs | NA | AU565 | RO | <10 min | ||
| JR | 2_0 | NPs | NA | AU565 | RO | <10 min | ||
| JR | 2 | NPs | NA | AU565 | RO | ∼1 hour | ||
|
| ||||||||
| 3rd | S1 | 1 | NPs | SKOV-3 | AU565 | RO | ∼1 hour | |
| S2 | 1 | NPs | SKOV-3 | AU565 | RO | ∼2 hour | 80 kVp, 50 μA, 1.96 mm Aluminium filtration, 1440 views, 300 ms | |
| S1 | 2 | NPs | SKOV-3 | AU565 | RO | ∼2 hour | ||
| S2 | 2 | NPs | SKOV-3 | AU565 | RO | ∼1 hour | ||
| S1 | 3 | NPs | SKOV-3 | AU565 | RO | 1 week | ||
| S2 | 3 | NPs | SKOV-3 | AU565 | RO | 1 week | ||
2.4. Immunohistochemistry
All the tumors were extracted, fixed in 10% formalin and paraffin embedded, followed by immunohistochemistry. The 5 μM tumor tissue sections were deparaffinized, rehydrated, and subjected to epitope retrieval using 1 mM EDTA pH 8.0 for 30 min. Vectastain ABC Elite kit protocol was followed for IHC staining and Vectro NovaRED was used as a peroxidase substrate. Methyl Green was used for counterstaining the tumor tissue sections. Hematoxylin and Eosin stain was used for morphological characterization and Masson’s Trichrome staining was performed for staining collagen. The tumor tissue was immunostained using HER2 and CD31 primary antibodies. Brightfield photomicrographs were captured using Olympus BX40 microscope equipped with Infinity 3 camera (Lumenera Inc., Ottawa, ON, Canada).
2.5. Photon-counting micro-CT scanning
Traditional EIDs convert incoming x-ray photons into visible light with a scintillator and then electric signals using a light-sensing circuit. To suppress light crosstalk during propagation, septa are often used between pixels to improve detection resolution at a cost of reduced fill factor. In contrast, PCDs utilize a direct x-ray photon-to-signal conversion mechanism with semiconductors, enabling a much smaller pixel size without compromising the dose efficiency. Additionally, the signal pulse height of each incident photon is measured while the photon is being counted, allowing us to simultaneously record the number of photons and their individual energy levels at each pixel. By the nature of the photon-counting process, PCDs are theoretically free from electronic noise. This contrasts with EIDs, whose detection limit is determined by electronic noise. These unique features of PCDs bring great advantages for PCCT in medical applications for high-resolution, high-contrast, and/or spectral imaging. On the other hand, compared to human PCCT scanners, photon-counting micro-CT uses a smaller detector pixel around 0.05–0.1 mm for even higher resolution but with a smaller field of view. To address the magnified charge sharing effect with a small pixel, pixel-level charge-summing circuits are often adopted but they have a limited processing speed, which in turn constrains the counting rate of the detector. In our preclinical imaging studies, this compromised counting rate is not an issue since we are mainly interested in the equilibrium states of contrast-enhanced mice. Thus, photon-counting micro-CT is very suitable for high-resolution spectral imaging of small animals with multiple contrast agents of different K-edges.
CT scanning was performed on an adapted commercial small animal photon-counting micro-CT system (MARS16, MARS Bioimaging Ltd, Christchurch, New Zealand) with a custom-designed animal bed for anesthesia. There are 3 CZT-based photon-counting chips stitched together forming a detection area of 42.24 mm 14.08 mm, and each chip consists of 128 x 128 pixels with a pitch of 110 110 μm2. The detector supports 5 thresholds with a charge sharing correction mechanism to form 5 effective energy bins (please refer to Getzin et al 2019, Jorgensen et al 2016 for details). During the scan, the mouse was anesthetized in a prone position with 2% isoflurane delivered via a face-cone on a customized bed for live animal imaging with its physiological status (heart rate and oxygen saturation) monitored using a paw sensor (Kent Scientific Corporation, CT, USA) clamped on its front foot as illustrated in figure 1. Also, the scanner has a video camera enabling the constant monitoring of the mouse in the cabinet during imaging sessions.
Figure 1.
Illustration of the mouse posture and imaging setup. The associated coordinate system for image reconstruction is also displayed.
To minimize the radiation dosage, we have scanned the portion of the mouse-bearing tumors using a cone-beam circular scan protocol. The first imaging run was performed with the x-ray tube operated at 80 kVp, 32 μA without extra filtration to collect 720 projections per rotation with 250 ms exposure per view. To optimize the radiation dose and improve the image quality, the second and third imaging runs were performed with the tube operated at 80 kVp, 50 μA with 1.96 mm aluminum filtration to collect 1440 projections for one rotation with 300 ms exposure time. The other parameters for all the imaging runs were kept the same. Specifically, the source to detector distance and the source to iso-center distance were 267 mm and 222 mm, respectively. X-ray photons were simultaneously counted in the energy bins of and keV. The mouse was laid on the stomach with its belly gently taped to the bed via a piece of medical surgery tape. The tumor regions were placed within the predefined field of view. The images were reconstructed at 90 μm isotropic voxel size with the MARS software. The relationship between the orientations of the reconstruction and the practical posture of the mouse is illustrated in figure 1. Note that the left side of the mouse is displayed on the right of the image.
2.6. Summary of the micro-CT imaging scans
The effective micro-CT scans are summarized in table 1. Each mouse has its unique tag and is coded in the same color within the session. The scan tag indicates which week the scan was performed, which also reflects the tumor development. Each scan could be uniquely identified with the combination of the corresponding mouse and scan tags. Hence, we will use this notation referring to one specific scan for simplicity and clarity, e.g. AL-2 refers to the scan of the mouse AL in the second week, which happened in the first session according to the name tag.
It is worth mentioning that in the first session, we performed intraperitoneal (IP) injection of iodine for the scan AB-2 and observed weak absorption of iodine into bloodstream and some accumulation in the urine system after one hour as shown in Supplementary figure 1. The tumors were barely enhanced compared to the muscle regions as indicated by the violine/box plots of the pixel values within tumors and muscle regions, showing tumor regions do not have iodine accumulation hence posing smaller median attenuation values compared to that of the muscle region. Thus, we have stuck to the retroorbital injection method to deliver iodine into the tumor vasculature. In the second session, to illustrate the contrast enhancement in the bloodstream, the liver region with big vessels and spleen regions were included in the field of view for the scan JR-2_0. JR-2 is a normal tumor scan and is different from JR-2_0.
2.7. Image visualization analysis
Since photon-counting micro-CT provides images in multiple energy bins and different materials have their unique attenuation patterns over these bins, the material composition can be inferred from such spectral measurements for each pixel. Many material decomposition methods were proposed based on this principle, but they often require tedious system calibrations with phantoms of known material concentrations (Li et al 2015, Symons et al 2017, Badea et al 2019, Curtis and Roeder 2019, Wang et al 2021, 2023). Furthermore, intensive computation is needed in a typical material decomposition process. For visualization of contrast-enhanced image features, these methods are widely used in a typical procedure, including material decomposition, material color coding, and a combination of color maps. However, the results may suffer from high noise as the material decomposition is easily influenced by data/image noise while the hard color coding magnifies the error visually. Hence, virtual monochromatic images are often preferred in medical practice since they are relatively insensitive to noise, but they would require an additional computational effort. Since quantitative decomposition is not necessary for many applications such as ours, to avoid the computational complexity and associated issues, here we propose a fast color visualization method to highlight contrast-enhanced features. Taking advantage of the attenuation property changes between energy bins among different materials, we selectively highlight the contrast enhancement by arranging the weighted inter-bin differences into three channels to form an RGB image in the following way.
Instead of directly going to the details, we first present a basic version of our method to better illustrate our idea, i.e. to directly assign the spectral images into three channels to form an RGB image:
where and correspond to images from bins and in keV respectively, and and are the three channels of the synthesized color image. The images from bins and in keV suffer from fluorescence escaping effects and high quantum noise respectively, giving much attenuation errors and degrading image quality, hence they were not used in this study. This basic version has already the ability to differentiate materials through the color tone difference as shown in figure 1. This can be understood as follows. If we treat each pixel from the spectral image as a three-element vector, the concentration change of a given material will only scale the vector length without altering the direction. When we put the vector into the RGB space (decomposing it into orthogonal bases: instead of the material bases in conventional material decomposition), its direction will determine the color tone while its length will define the intensity (or brightness). The same type of materials will share an identical color tone in the RGB space, and similar materials will appear similarly in color tone. Hence, we can differentiate materials based on their color tone. Note that the orthogonal bases ( and ) are complete, making the RGB decomposition unique, which means tissue, bone and iodine/barium have their unique color coding and we cannot match the color tone of any one of the three by blending the color tones of the other two. The process can be described as
where y is the three-element vector of a PCCT image pixel, is the decomposition results. is the RGB material basis matrix and can be related to the material basis matrix composed of bone, tissue, and iodine/barium bases used for conventional material decomposition. Different from material decomposition where material bases are nonorthogonal with volume fraction solved from in the sense of least square with nonnegative constraints, is the identity matrix and always holds which does not introduce any residual error.
However, the basic version does not provide sufficient color differentiation between similar materials due to the similarity between their spectral attenuation curves. To magnify the spectral differences, we heuristically introduce subtractions between channels in our improved version; or more generally, a transform of the channels which can be described as an invertible linear transformation matrix Hence, the RGB representation can be reformulated as
Note that changes the color mapping for better color differentiation between materials, and the above equation can be also understood as
The term in the parenthesis means that we perform a linear transformation of the RGB bases. Since is invertible, we can always convert it back to the original formula:
That is, the transformation is unique. The operation will not alter the material property of vector and the color coding of materials is still unique after the transformation. The functions of are to enhance the color differentiation between materials (by increasing the differences between for bones, tissues, and contrast agents) and to adjust the color mapping scheme.
For example, based on the improved version, empirically we find that the following transformation satisfactorily highlights the iodine and NP contrast agents:
Note that the weights were empirically tuned for our scanning protocol in the second and third imaging sessions, and therefore should be adjusted for other scanning protocols or contrast agents; e.g. a different set of coefficients ] was used for the first imaging session results to make the images acquired under two conditions share similar color tones. It is worth mentioning that the matrix maps one color space to another color space, changing the correspondence between color tones and material types without breaking the mapping uniqueness. In other words, if we know one Calcium pixel has a yellow color, then all yellow pixels will imply the existence of Calcium due to this uniqueness. Color visualization for material calibration phantoms is shown in Supplementary figure 4 to help illustrate this point. Additionally, since the display window is always [0, 1] for a true color RGB image, a multiplicative constant was applied to the resultant images to make them display an adequate intensity for all scans. In this sense, it is the color tone (the ratio between channel values) and relative intensity (brightness) that matter rather than the absolute values. Hence, the display window information is not included in our colorized visualization figures.
2.8. Manual tumor annotation
The tumors were manually segmented with the software LabelMe to outline the boundaries slice by slice in either the axial or coronal view. Due to the insufficient contrast between the tumor and background, the boundaries were not always clear, producing more zigzag shapes in other views than the annotation view. As the tumor boundary should have similar smoothness in all views, directional Gaussian filtration was applied on each volume mask. Thresholding to reduce uncertainty was then implemented by leveraging the information from adjacent slices via the voting mechanism introduced by the Gaussian convolution kernel. Finally, volume masks were obtained for all the tumors presented in the micro-CT scans. It is worth mentioning that due to the limited field of view along the axial direction ( direction), tumors were often partially covered by a circular scan not permitting adequate quantitative tumor volume measurements.
3. Results and discussion
3.1. Color visualization
Representative images from scans with the two contrast agents are displayed in figure 2 using our visualization method described in section 2.8. From the left to right columns in figure 2, the images (a)–(d), (e)–(h), and (i)–(l) correspond to scans of JR-1, JB-1, and JB-2, respectively. The first row displays the axial cross-section views, while the other rows are the images from coronal views at different depths, showing the kidney, gut, and skin regions. Red boxes (axial) and circles (coronal) are used to highlight the contrast agent localized around the kidney regions, where the major differences in the enhancement effects between iodine and NP agents are revealed. Red circles in (j) and arrows in (k)–(l) point to the NP-enhanced small vessels inside the abdomen and those on the skin, whereas red arrows in (c)–(d) and (g)–(h) indicate iodine-labeled undefined vessel-like structures. The black arrow in (g) indicates calcium dots inside the digestive tract.
Figure 2.
Comparison of contrast enhancement using iodine versus NP agents. (a)–(d), (e)–(h), and (i)–(l) are from photon-counting micro-CT scans of JR-1, JB-1 and JB-2 (table 1), respectively. The first row shows axial images, while the remaining rows show the images from coronal views at different depths, displaying the kidney, gut, and skin regions. Yellow and brown colors represent bone and soft tissue respectively, while green color indicates the concentration of the contrast agent at the tissue location. (e) and (f) have a wider display window (doubled) than the others to avoid saturation of the high-contrast details. Red boxes highlight the kidney in axial images, whereas red circles indicate the kidney vasculature in coronal views in (b), (f) and (j). NP-enhanced small vessels inside the belly and those on the skin as pointed by the red arrows in (k)–(l), whereas red arrows in (c)–(d) and (g)–(h) indicate iodine labeled undefined vessel-shape structures. The black arrow in (g) indicates calcifications.
From the color tone, we can easily discriminate between different materials, e.g. the high intensity calcification dots in the stomach appear bright yellow in (figure 2(g), black arrows) while the vessel cross-sections in the kidney appear bright green in (figures 2(k)–(l), red arrows and (j), red circle). This analysis would be a challenge if the images were presented in grayscale with a single channel image or a single virtual monochromatic image, since they are of similar intensity levels, as shown figure 3(f), where the bright dots inside the tumor caused by NPs are similar to the bony dots above the spine. The contrast agents concentrated in the kidney or vessels were successfully highlighted in green to distinguish them from the background tissues displayed in brownish and high-density bones in yellow. Moreover, the relative concentration map of the contrast agent can be inferred from the green intensity levels, as reflected in the kidney region in figures 2(b), (f) and (j) compared to the soft tissue regions in (c-d), (g-h) and (k-l). Therefore, a qualitative material decomposition effect has been achieved using our visualization method. Since the human eye is more sensitive to color change than to grayscale intensity change, our method provides improved visualization and more information compared to the virtual monochromatic imaging method.
Figure 3.
Contrast enhancement with the iodine and NP agents through visualization of exemplary slices from the axial and coronal views as shown in (a) and (b) respectively, and the corresponding gray-scale images from individual channels as shown in (d)–(f) for iodine, and (g)–(i) for NP, with display windows indicated in the right top corner of each image. The box plots of the pixel values (linear attenuation coefficient (LAC) value in unit of per cm) within tumors (AU565, MDA-MB-231, SKOV-3) and background tissues (muscle) are also displayed for each channel (CH 1–3) with the grayscale images. The contrast-to-noise ratio (CNR) with the tumor as the signal and muscle as the background for each channel is tabulated in (c) together with the signal-to-noise ratio (SNR) calculated from the muscle regions. Right-tailed Wilcoxon rank sum tests were also performed to check the statistical significance of the median value difference between tumor and muscle ROIs in each channel corresponding to (d)–(i). The alternative hypothesis was accepted for all tumors that the median value of the tumor ROI is significantly greater than that of the muscle ROI with p-values smaller than 0.01, except for the iodine enhanced imaging in channel 1, where the p-values are 0.6165 and 0.9289 for AU565 and MDA-MB-231 respectively, and the null hypothesis was accepted and the statistical significance cannot be established in either cases (see Supplementary table 2 for more details on statistical analysis).
3.2. Qualitative comparison between iodine and NP contrast agents
Representative results of iodine enhanced images from two scans (JR-1 and JB-1) and NP enhanced images from one scan (JB-2) are shown in figure 2 for comparison. The distinct appearances of the contrast-enhanced regions demonstrate that NPs are more suitable for vessel enhanced imaging, as shown in figures 2(b) and (f) versus (j), red circles. Looking at the kidney regions marked with the red boxes or circles, the iodine contrast agent was mainly accumulated in the renal cortex, renal medulla, and renal pelvis, as well as the ureter, as shown in figures 2(a)–(b) and (e)–(f). Interestingly, the renal artery and renal vein appear unenhanced, such as in figures 2(e)–(f). In contrast, injected NPs visualize the renal arteries and veins in a brighter green color, while the renal cortex, renal medulla and pelvis show a much darker green tone, as shown in figures 2(i)–(j). These differences in contrast enhancement agree well with the results shown in (Hainfeld et al 2018) and appear to be caused by the short half-time of the iopamidol (iodine contrast medium in Isovue-370) as well as by the ability of renal filtration to clear most of the iodine contrast agent from the blood during the 20 min scan post injection period, due to its low molecular weight. NPs that are greater than 5 nm in size do not undergo glomerular filtration, and hence they can stay longer in the blood pool (Hainfeld et al 2018). Based on a study with the same Exitron 12000 NPs contrast agent described in Cassol et al (2019), high metal load particles are expected to be taken up by macrophages in the liver and spleen, boosting the tissue contrast at those regions within the initial 2 h post-injection period. Similar results have been observed in our experiments as shown in Supplementary figure 2. The liver and spleen tissue contrast increases continually till reaching a maximum level at 24 h post-injection, with a gradual decay of tissue contrast occurring over the following 4–6 weeks post-injection. Consistently, with the results shown in Cassol et al (2019), we detect most of the NPs in the bloodstream for at least the initial several hours, allowing for the clear visualization of the big arteries and veins in figures 2(i)–(j).
Due to NP’s longer clearing-out time from the blood pool, we can detect the NP enhanced small vessels inside the belly and those on the skin as pointed by the red arrows in figures 2(k)–(l). However, in the iodine enhanced images, the dimmer greenish appearance all over the tissues suggests a diffused pattern for iodine within the soft tissues, as shown in figures 2(c)–(d) and (g)–(h). Moreover, iodine enhanced images do not display clearly identifiable vessels inside the body as shown in NP enhanced images (figures 2(k)–(l), arrows). Although some enhanced line structures very close to the skin can be seen in figures 2(c)–(d) and (g), red arrows, it is difficult to determine whether these structures are indeed vessels. The enhanced line between the animal skin and the bed in figure 2(h) could be urine that was excreted after iodine injection and left on the skin. Additionally, the short clearing-out time of iopamidol makes it more challenging to generate consistent image quality. As illustrated in figure 2, the results of JR-1 and JB-1 gave significantly different appearances, which can be attributed to a few minutes difference in the time gaps between the injection and the scanning sessions as well as the different metabolic levels between individual mice. These small operational differences or inter-mice variance could be magnified in iodine enhanced images because of iodine’s short half-time as a contrast agent and relatively long scanning time. In contrast, the longer half-time NPs do not appear to be subjected to this image quality variability. Qualitative comparison between iodine and NP enhanced images indicates that the NP contrast agent displays improved vessel visualization. In summary, the NP contrast agent provides a high contrast enhancement in big arterials and veins, improves the visibility of small vessels, and allows for consistent image quality between scans.
3.3. Contrast agent effectiveness
To evaluate the effectiveness of the contrast agents, we analyzed the contrast enhancement of the tumor regions against the nearby background tissues, as shown in figure 3. A typical axial slice from the scan AL-1 (AL mouse, the 1st scan) and one coronal slice from the scan S1–2 (S1 mouse, the 2nd scan) are displayed in figures 3(a)–(b), respectively. The small regions of interest (ROI) highlighted with the red/green/orange and blue circles within the tumor and non-tumor muscle tissues were selected for analysis as the tumor signal or the non-tumor background, respectively. Specifically, two metrics that are widely used in medical CT imaging evaluation (Gramer et al 2012), i.e. contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR), were used to quantify the contrast enhancement and the image noise levels. The CNR values were calculated by normalizing the difference in mean pixel values between the tumor ROI and the non-tumor background ROI with the standard deviation of the non-tumor background. The SNR values were calculated from only the muscle ROIs by dividing the mean value by the standard deviation. The CNR and SNR values for each channel are summarized in figure 3(c). The SNR of the images is between 18 and 25 indicating reasonable image quality for analysis.
3.3.1. Iodine enhancement of tumor xenografts
Figures 3(d)–(f), correspond to the iodine-enhanced image in (a), showing the gray-scale images from individual channels and corresponding box plots of the pixel values (linear attenuation coefficient, in unit of per cm) within tumors and background tissues. The display window of each gray-scale image is indicated at the right top corner. The AU565 and MDA-MB-231 tumor ROIs are indicated by red and orange circles in (a) and asterisks in (d)–(f), respectively, whereas background muscle ROIs are indicated by blue circles (a-b) and asterisks (d-i). In (e) and (f) images, the red and orange asterisks appear significantly brighter than the background muscle tissue regions indicated by blue asterisk, while in (d) they appear similarly in brightness. The trends are evident in the respective box plots comparing the intensity distributions of those regions in three channels. The trend of channel 1 (CH 1) is different from that in the other two channels, which agrees well with channel 1 only covering the energy range right below the K-edge of iodine where the enhancement effect of Iodine is not as strong as those in channels 2 and 3 that cover the energy range above the K-edge. Our results show that more iodine agent is perfused to the tumors via vasculature and diffused to the whole tumor volume resulting in an overall increased mean value in channels 2 and 3 compared to the muscle region with strong statistical significance (p-values smaller than 0.01 for tests on median value difference between tumor and muscle ROIs for CH2 and CH3, see Supplementary table 2), as shown in figures 3(e)–(f); this can be interpreted as due to the angiogenesis and the EPR effect of tumors. Checking the quantitative values in figure 3(c), tumor visualization is enhanced as shown by the CNR values in channels 2 and 3 (CNR > 1.0) being substantially greater than that in channel 1 (CNR ∼ 0), indicating the effectiveness of the iodine contrast agent.
3.3.2. NP enhancement of tumor xenografts
Figures 3(g)–(i) corresponds to the NP-enhanced image in (b), which displays tumors with different appearances as compared to (d)–(f). For example, after iodine enhancement, AU565 tumor (red asterisks) showed a relatively uniform increased intensity across the whole tumor region (e)–(f), whereas after NP injection, intensity levels across other AU565 tumors were enhanced non-uniformly (g)–(i). In AU565 tumors shown in (g)–(i) (red asterisks), large bright spots can be detected within the tumor, most probably since the NPs cannot diffuse across the vessels easily due to their large size. Alternatively, the slice in (b) could be close to the surface of the AU565 tumor, a region that is richer in vessels than the center of the tumor. Brighter dots are also observed at the boundary of SKOV-3 tumors (green asterisks) than inside, while in the muscle region (blue asterisks) we did not observe such vessel aggregation pattern. Overall, NP-enhanced tumor regions are brighter than the muscle regions in all three channels, which is also supported by the box plots shown in (g)–(i) with strong statistical significance (p-values smaller than 0.01 for tests on median value difference between tumor and muscle ROIs, see Supplementary table 2). The NP agent appears to mainly improve the vessel contrast as NPs can hardly diffuse away from the vessels due to their large size (110 nm as indicated by the manufacturer’s specification datasheet (Miltenyi Biotec GmbH 2022)).
The NP-mediated mean value elevation and CNR trends among channels are different from those displayed by iodine enhancement because of the different spectral attenuation properties of NP versus iodine contrast agents. The SNR values in three channels for NP-enhancement are between 22.7 and 24.8, which are significantly higher than the values found for iodine-enhancement (SNR = 18–20), as shown in figure 3(c). Such difference may be the result of different imaging settings, i.e. the combined effects of the increased number of views and additional 1.96 mm aluminum filtration (see Supplementary figure 3). The positive signs of all CNR values suggest the improvement of the tumor visibility in all channels, but the values for NP enhancement are smaller than those for iodine enhancement. A possible explanation for these results is that tumor vasculature only contributes partially towards an improved tumor volume visualization and therefore to increased CNR values. Alternatively, the position of the selected ROI for analysis could affect the number of vessels included. It is also worth pointing out that the images in (g)–(i) (NPs) might visually demonstrate a more pronounced difference between tumor and non-tumor muscle ROIs compared to panels (d)–(f) (iodine), which seems to contradict the CNR values in (c). This discrepancy between visual perception and quantitative measurement can be attributed to two main factors. Firstly, the NP images are presented in a narrower window, resulting in a brighter appearance of the enhancement; Secondly, while the CNR measures all pixels within the tumor, the NP agent only enhances the vessel pixels, which appear brighter, but the surrounding non-vessel pixels could be darker compared to the iodine-enhanced images, thus lowering the overall CNR score. Overall, these results suggest that instead of improving the whole tumor discrimination, the NP agent mainly enhances the vessel contrast, therefore being beneficial to the vasculature imaging of tumors.
3.4. Tumor discrimination
Given the respiratory motion of the mouse and the resolution limit of the scanner, we are not able to directly visualize the detailed vasculature inside the tumor or perform analysis on a vascular segmentation within the tumor. Nevertheless, inspired by radiomics for medical CT, we propose to use statistical analysis of tumor pixels to reflect the vasculature information obtained from different tumors. Specifically, we have calculated the mean values and standard deviation values across the tumor volume. The mean values reflect the average amount of contrast medium in the tumor, while the standard deviation indicates the heterogeneity of the contrast medium distribution within the tumor. From a biological perspective, the former stands for the average perfusion of the tumor, while the latter measures the heterogeneity of the tumor angiogenesis, reflecting the distribution of effective vessels. Higher perfusion rates could lead to more contrast agent influx and higher mean values. For the diffusible contrast agent, iopamidol, higher standard deviation values could be due to the defective perfusion of tumor tissues. For example, well-perfused tumors will be uniformly enhanced resulting in a smaller standard deviation in contrast to poorly-perfused tumors which will still have unenhanced portions due to the absence of vessels resulting in a larger standard deviation. In contrast, for the non-diffusible NP contrast agent, higher standard deviation values would indicate richer vessels (or better perfusion) since most tumor pixels would have remained unenhanced and only the vessel pixels would show increased intensity, causing the nonuniformity.
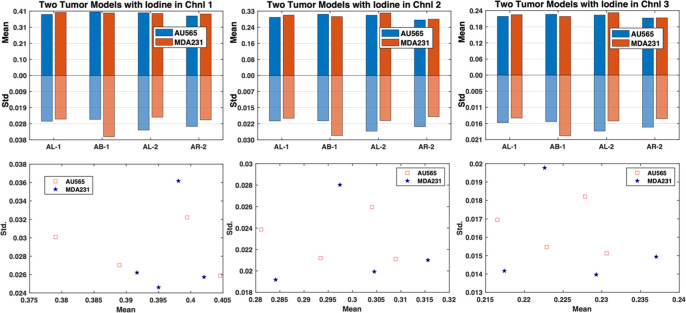
3.4.1. Iodine enhancement of AU565 versus MDA-MB-231 tumor xenografts
The mean and standard deviation statistics extracted from the first imaging session (table 1) are shown in figure 4. The mean values of the two tumors followed closely in all three channels, while AU565 always gave larger standard deviation values except for the scan AB-1. If we treat every tumor as an independent variable and plot their properties with the mean as the coordinate and the standard deviation as the coordinate, it is hard to separate them within the three channels. This could be caused by several reasons. First, the short clearing-out time of iopamidol could have disrupted inter-tumor relationships due to complicated combination results of inconsistencies from injection-to-imaging time intervals, metabolic level variance between mice, and different tumor characteristics. Second, the two tumor models have quite similar perfusion and vasculature characteristics and are difficult to differentiate from each other based on tumor vasculature and perfusion properties.
Figure 4.
The mean and standard deviation on the AU565 and MDA-MB-231 (MDA231) tumor models from the first imaging session (AL-1, AB-1, AL-2, AR-2) using the iodine contrast agent. Top row: the bar graph comparing the mean and standard deviation of the two tumor models; bottom row: the scatter point plot of the tumors with the mean value as the x-axis and the standard deviation as the y-axis.
3.4.2. Nanoparticle enhancement of AU565 versus SKOV-3 tumor xenografts
The mean and standard deviation data from the third imaging session are shown in figure 5. Different from the results extracted from the iodine imaging session, the results from the NP enhancement present consistent trends. AU565 tumors display significantly larger standard deviations and slightly greater mean values than those of SKOV-3 tumors for almost every scan and across all channels. Based on our hypothesis about the biological meaning of the mean value and the standard deviation, the results suggest that the AU565 tumors show increased perfusion and more effective vascularity than those of SKOV-3 tumors. The two tumors can be easily separated linearly in the space spanned by channels 1 and 2 when using the scatter points of the coordinates of the tumors. This suggests that the combination of mean and standard deviation values could be a metric to discriminate between AU565 and SKOV3 tumor models with the aid of the NP contrast agent.
Figure 5.
The mean and standard deviation values on the AU565 and SKOV-3 tumor models from the third imaging session (S1–1, S2–1, S1–2, S2–2, S1–3, S2–3) after the NP contrast enhancement. Top row: the bar graph comparing the mean and standard deviation of the two tumor models; bottom row: the scatter plot of the tumors with the mean value as the x-axis and the standard deviation value as the y-axis.
To test the statistical significance of this observation, we conducted a paired t-test to see whether there was a significant difference between the mean values of the two tumors with the NP enhancement, as well as the difference between the standard deviation values of the two tumors. Since each mouse bears both tumors which were scanned simultaneously and the scan for each mouse is independent, the paired t-test was used to avoid the influence of individual differences among the mice. One-sample Kolmogorov–Smirnov test (Massey 1951) was first used to check the normality of the paired mean value differences () using the MATLAB (The MathWorks, Natick, Massachusetts) built-in function kstest. The normality test results suggest that we cannot reject the null hypothesis that the data came from a normal distribution, and the p-values for the mean value differences in three channels are 0.8871, 0.5762, 0.4807 and all significantly greater than the 5% significance level. Similar results for the standard deviation value differences were obtained with the p-values in three channels being 0.6623, 0.3178, 0.5763 and all greater than the 5% significance level (Supplementary table 3). Therefore, we should accept the null hypothesis and consider that they followed normal distributions.
The paired t-test was performed as follows. Let us denote where and are the paired observations of two random variables with mean expectations being and and follows a normal distribution The test hypotheses are defined as:
The test statistic is where The p-value is calculated as
In our case and the p-values for mean value difference check are 0.0107, 0.0053, 0.0078 in the three channels, and similarly the p-values for standard deviation are 0.0024,0.0029 and 0.0181 in the respective channels (Supplementary table 4). All are significantly smaller than 0.05, allowing the rejection of the null hypothesis at the significance level of 0.05. Thus, statistically significant differences have been established between the two tumors in terms of mean and standard deviation values with the NP contrast enhancement, indicating that AU565 and SKOV-3 tumor models can be discriminated with the aid of the NP contrast agent using micro-CT imaging, probably due to their distinct vascularity properties.
3.5. Immunohistochemical validation
The three representative human tumor models used in this study are MDA-MB-231, representing triple-negative breast tumors, and AU565 and SKOV-3 representing HER2 positive tumors from breast and ovarian cancer cells, respectively. In figure 6, we show the immunohistochemical analysis of MDA-MB-231 and AU565 tumor sections from the first imaging session (mouse tag: AB, AR, and AL). Qualitatively, there is no dramatic difference in the CD31 immunostaining indicating that the vasculature is similarly disrupted in both MDA-MB-231 and AU565 tumors. These results are consistent with the inability of iodine contrast agent to differentiate each tumor based on their tumor vasculature and perfusion properties using micro-CT imaging.
Figure 6.
Hematoxylin and eosin (H&E) (i) and anti-CD31 (ii) staining of AU565 (top row; (a), (c) and (e)) and MDA-MB-231 (MDA231; bottom row; (b), (d) and (f)) tumor xenografts extracted in the first imaging session. (a)–(b) correspond to mouse tag AB, (c)–(d) mouse tag AR and (e)–(f) mouse tag AL (table 1). Each tumor section was labeled by H&E staining (i), and immunostained with anti-CD31 (ii) for labeling the tumor vasculature. (a), (b) and (e) show lymph node infiltration. All the images are displayed on the same scale, and the scale bar in (a) is 200 μm.
The immunohistochemical analysis of AU565 and SKOV-3 HER2 positive tumor sections from the third imaging session (mouse tag: S1 and S2) is shown in figure 7. Whereas AU565 represents a non-aggressive, relatively well-perfused tumor model, SKOV-3 is an example of an aggressive, collagen-rich, poorly-perfused tumor model (Smith et al 2022). Importantly, AU565, and SKOV3 HER2 positive xenografts display contrasting tumor morphologies. Although they express HER2 at similar levels, their tumor vasculature is strikingly different, with SKOV3 displaying smaller and compressed vessels, in contrast to AU565. Moreover, the collagen fiber density in SKOV3 tumors is significantly higher than that in AU565 tumors, which show increased stroma instead (figure 7). These results correlate with the ability of NP contrast agent to discriminate AU565 from SKOV-3, probably due to their distinct vascularity properties.
Figure 7.
Hematoxylin and eosin (H&E) (i), Masson’s Trichrome (ii), anti-HER2 (iii), anti-CD31 staining of AU565 (a) and (c) and SKOV-3 (b) and f) tumor xenografts extracted in the third imaging session. (ai)–(biv) correspond to mouse tag S1, (ci)–(div) mouse tag S2 (table 1). Each tumor section was labeled by H&E staining (i), Masson’s Trichrome (ii) for collagen labeling and immunostained with anti-HER2 (iii) or anti-CD31 (iv) for labeling the tumor vasculature. All the images are displayed on the same scale, and the scale bar in (a) is 100 μm.
4. Conclusion
In this work, we have investigated two types of commercial contrast agents, ISOVUE-370 (small molecule-based) and Exitron Nano 12000 (NP-based), for the imaging of breast (AU565 and MDA-MB-231) and ovarian tumor xenografts (SKOV-3), for tumor contrast enhancement in live animal imaging using photon-counting micro-CT. Our proposed novel color visualization method highlights the contrast media distribution through color tone change, achieving a fast qualitative material decomposition effect that is robust to noise and easy for human to perceive. With this visualization method, two contrast agents clearly demonstrated distinct enhancement characteristics due to their large difference in cross-vessel permeability. Our qualitative comparison and quantitative analysis suggest that the nanoparticulate contrast agent provides better vasculature enhancement for each scan and better stability for quantitative analysis among scans, while the iodine contrast agent gives better whole tumor enhancement but with a big variance between scans due to its short half time in blood, which means a high sensitivity to timing and individual differences among mice. Additionally, statistically significant differences in terms of mean and standard deviation values within tumors indicate that AU565 and SKOV-3 tumor models can be discriminated with the aid of the NP contrast agent using photon-counting micro-CT (p-values < 0.02), due to their distinct vascularity properties revealed by immunohistochemical analysis. These results demonstrate the usefulness of photon-counting micro-CT in evaluating the morphology and anatomy of distinct types of tumor xenografts non-invasively, which has great potential in characterizing tumors and longitudinal monitoring of their development and response to treatment. On the other hand, our current results are based on the scanner’s reconstruction, suffering resolution loss from mouse respiratory motion and slight geometry errors. Our next steps include upgrading the reconstruction methods to improve the resolution and SNR for direct vasculature analysis and improved tumor characterization by incorporating the latest techniques for motion compensation (Capostagno et al 2021, Li et al 2022a, 2022b), spectral distortion correction (Li et al 2020, Taguchi et al 2022), and deep learning noise reduction and few-view reconstruction (Niu et al 2023, Li et al 2024).
Acknowledgments
This project is funded by the National Institute of Health R01 CA237267 (GW, XI and MB), R01 CA233888 (GW), R01 CA271371 (XI and MB), and R01 CA250636 (XI and MB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank the support of the Barroso, Intes and Wang labs.
Ethical statement.
All animal procedures were conducted in accordance with the governing protocol approved by the Institutional Animal Care and Use Committee at both Albany Medical College (23–09001) and Rensselaer Polytechnic Institute (WANG-001–21). The animal facility at Albany Medical College has been accredited by the American Association for Accreditation for Laboratory Animals Care International.
Conflict of interest.
The authors report no conflict of interest.
Author contribution.
MB, XI and GW provided critical inputs for establishing the methodology and protocols. MB and ML designed and supervised the study. ML and MB drafted and finalized the manuscript. ML and XG performed micro-CT imaging and analyzed the results. AR generated and analyzed the tumor xenografts, together with AV. AM helped with the animal imaging experiments. All authors have read, significantly revised, and approved the final manuscript.
Contributor Information
Ge Wang, Email: wangg6@rpi.edu.
Margarida Barroso, Email: barrosm@amc.edu.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary information files). Data will be available from 1 May 2024.
References
- Almajdub M, Nejjari M, Poncet G, Magnier L, Chereul E, Roche C, Janier M. In vivo high-resolution x-ray microtomography for liver and spleen tumor assessment in mice. Contrast Media Mol. 2007;2:88–93. doi: 10.1002/cmmi.130. [DOI] [PubMed] [Google Scholar]
- Anderson N G, et al. Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur. Radiol. 2010;20:2126–34. doi: 10.1007/s00330-010-1768-9. [DOI] [PubMed] [Google Scholar]
- Ashton J R, Castle K D, Qi Y, Kirsch D G, West J L, Badea C T. Dual-energy CT imaging of tumor liposome delivery after gold nanoparticle-augmented radiation therapy. Theranostics. 2018;8:1782–97. doi: 10.7150/thno.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton J R, West J L, Badea C T. In vivo small animal micro-CT using nanoparticle contrast agents. Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00256. http://journal.frontiersin.org/Article/10.3389/fphar.2015.00256/abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea C T, Clark D P, Holbrook M, Srivastava M, Mowery Y, Ghaghada K B. Functional imaging of tumor vasculature using iodine and gadolinium-based nanoparticle contrast agents: a comparison of spectral micro-CT using energy integrating and photon counting detectors. Phys. Med. Biol. 2019;64:065007. doi: 10.1088/1361-6560/ab03e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea C T, Hedlund L W, De Lin M, Boslego Mackel J F, Johnson G A. Tumor imaging in small animals with a combined micro-CT/micro-DSA system using iodinated conventional and blood pool contrast agents. Contrast Media Mol. Imaging. 2006;1:153–64. doi: 10.1002/cmmi.103. [DOI] [PubMed] [Google Scholar]
- Badea C T, Johnston S M, Qi Y, Ghaghada K, Johnson G A. Dual-energy micro-CT imaging for differentiation of iodine- and gold-based nanoparticles. In: Pelc N J, et al., editors. SPIE Medical Imaging; Lake Buena Vista; 2011. p. p 79611X. [DOI] [Google Scholar]
- Bakan D A, Weichert J P, Longino M A, Counsell R E. Polyiodinated triglyceride lipid emulsions for use as hepatoselective contrast agents in CT: effects of physicochemical properties on biodistribution and imaging profiles. Invest. Radiol. 2000;35:158–69. doi: 10.1097/00004424-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Boix-Montesinos P, Soriano-Teruel P M, Armiñán A, Orzáez M, Vicent M J. The past, present, and future of breast cancer models for nanomedicine development. Adv. Drug Deliv. Rev. 2021;173:306–30. doi: 10.1016/j.addr.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll H, Figueiredo G, Fiebig T, Nittka S, Doyon F, Kerl H U, Nölte I, Förster A, Kramer M, Brockmann M A. Comparison of fenestra LC, ExiTron nano 6000, and ExiTron nano 12000 for micro-CT imaging of liver and spleen in mice. Acad. Radiol. 2013;20:1137–43. doi: 10.1016/j.acra.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Boll H, Nittka S, Doyon F, Neumaier M, Marx A, Kramer M, Groden C, Brockmann M A. Micro-CT based experimental liver imaging using a nanoparticulate contrast agent: a longitudinal study in mice. PLoS One. 2011;6:e25692. doi: 10.1371/journal.pone.0025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capostagno S, Sisniega A, Stayman J W, Ehtiati T, Weiss C R, Siewerdsen J H. Deformable motion compensation for interventional cone-beam CT. Phys. Med. Biol. 2021;66:055010. doi: 10.1088/1361-6560/abb16e. [DOI] [PubMed] [Google Scholar]
- Caschera L, Lazzara A, Piergallini L, Ricci D, Tuscano B, Vanzulli A. Contrast agents in diagnostic imaging: present and future. Pharmacol. Res. 2016;110:65–75. doi: 10.1016/j.phrs.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Cassol F, et al. Tracking dynamics of spontaneous tumors in mice using photon-counting computed tomography. iScience. 2019;21:68–83. doi: 10.1016/j.isci.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D P, Badea C T. Advances in micro-CT imaging of small animals. Phys. Med. 2021;88:175–92. doi: 10.1016/j.ejmp.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D P, Ghaghada K, Moding E J, Kirsch D G, Badea C T. In vivo characterization of tumor vasculature using iodine and gold nanoparticles and dual energy micro-CT. Phys. Med. Biol. 2013;58:1683–704. doi: 10.1088/0031-9155/58/6/1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis T E, Roeder R K. Quantification of multiple mixed contrast and tissue compositions using photon-counting spectral computed tomography. J. Med. Imaging. 2019;6:013501. doi: 10.1117/1.JMI.6.1.013501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N M, et al. In vivo quantitative microcomputed tomographic analysis of vasculature and organs in a normal and diseased mouse model. PLoS One. 2016;11:e0150085. doi: 10.1371/journal.pone.0150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiordelisi M F, Auletta L, Meomartino L, Basso L, Fatone G, Salvatore M, Mancini M, Greco A. Preclinical molecular imaging for precision medicine in breast cancer mouse models. Contrast Media Mol. Imaging. 2019;2019:1–15. doi: 10.1155/2019/8946729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getzin M, Li M, Rundle D, Butler A P, Wang G. Non-uniformity correction for MARS photon-counting detectors. In: Matej S, Metzler S D, editors. The 15th Int. Meeting on Fully Three-dimensional Image Reconstruction in Radiology and Nuclear Medicine; SPIE; 2019. 110722W. 11072 (28 May 2019) [DOI] [Google Scholar]
- Gramer B M, Muenzel D, Leber V, Von Thaden A-K, Feussner H, Schneider A, Vembar M, Soni N, Rummeny E J, Huber A M. Impact of iterative reconstruction on CNR and SNR in dynamic myocardial perfusion imaging in an animal model. Eur. Radiol. 2012;22:2654–61. doi: 10.1007/s00330-012-2525-z. [DOI] [PubMed] [Google Scholar]
- Hainfeld J F, Ridwan S M, Stanishevskiy Y, Smilowitz N R, Davis J, Smilowitz H M. Small, long blood half-life iodine nanoparticle for vascular and tumor imaging. Sci. Rep. 2018;8:13803. doi: 10.1038/s41598-018-31940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S M, Vercnocke A J, Rundle D S, Butler P H, McCollough C H, Ritman E L. Evaluation of a photon counting Medipix3RX CZT spectral x-ray detector. In: Grim G P, et al., editors. SPIE Optical Engineering + Applications; 2016. 99690J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H W, Cai Q-Y, Jun H Y, Chon K S, Park S H, Byun S J, Lee M S, Oh J M, Kim H S, Yoon K-H. Micro-CT imaging with a hepatocyte-selective contrast agent for detecting liver metastasis in living mice. Acad. Radiol. 2008;15:1282–90. doi: 10.1016/j.acra.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Li M, Shan H, Pryshchep S, Lopez M M, Wang G. Deep adversarial network for super stimulated emission depletion imaging. J. Nanophoton. 2020;14:016009. doi: 10.1117/1.JNP.14.016009. [DOI] [Google Scholar]
- Li M, Bohacova J, Uher J, Cong W, Rubinstein J, Wang G. Motion correction for robot-based x-ray photon-counting CT at ultrahigh resolution. In: Müller B, Wang G, editors. Developments in X-ray Tomography XIV; 14 October 2022. 122420Y. [DOI] [Google Scholar]
- Li M, Lowe C, Butler A, Butler P, Wang G. Motion correction via locally linear embedding for helical photon-counting CT. In: Stayman J W, editor. 7th Int. Conf. on Image Formation in X-Ray Computed Tomography (ICIFXCT 2022); SPIE; 2022. 123042I. [DOI] [Google Scholar]
- Li M, Niu C, Wang G, Amma M R, Chapagain K M, Gabrielson S, et al. Deep few-view high-resolution photon-counting extremity CT at halved dose for a clinical trial. 2024 arXiv: 2403.12331 .
- Li M, Rundle D S, Wang G. X-ray photon-counting data correction through deep learning. In: Kachelrieß M, editor. 6th Int. Conf. Imag. Form. X-ray Comput. Tomo.; Online meeting, Regensburg, Germany: August 2020. pp. 426–29. arXiv: 2007.03119 . [Google Scholar]
- Li Z, Leng S, Yu L, Yu Z, McCollough C H. Image-based material decomposition with a general volume constraint for photon-counting. In: Hoeschen C, et al., editors. Medical Imaging 2015: Physics of Medical Imaging; 2015. 94120T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-N, Morin J, Dokmanovich M, Bluette C T, Goldstein R, Manickam B, Bagi C M. Nanoparticle contrast-enhanced micro-CT: a preclinical tool for the 3D imaging of liver and spleen in longitudinal mouse studies. J. Pharmacol. Toxicol. Methods. 2019;96:67–77. doi: 10.1016/j.vascn.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Lu G, Marsh S, Damet J, Carbonez P, Laban J, Bateman C, Butler A, Butler P. Dosimetry in MARS spectral CT: TOPAS Monte Carlo simulations and ion chamber measurements. Australas. Phys. Eng. Sci. Med. 2017;40:297–303. doi: 10.1007/s13246-017-0532-8. [DOI] [PubMed] [Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/S0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Mahan M M, Doiron A L. Gold nanoparticles as x-ray, CT, and multimodal imaging contrast agents: formulation, targeting, and methodology. J. Nanomater. 2018;2018:1–15. doi: 10.1155/2018/5837276. [DOI] [Google Scholar]
- Mannheim J G, Schlichthaerle T, Kuebler L, Quintanilla-Martinez L, Kohlhofer U, Kneilling M, Pichler B J. Comparison of small animal CT contrast agents. Contrast Media Mol. 2016;11:272–84. doi: 10.1002/cmmi.1689. [DOI] [PubMed] [Google Scholar]
- Massey F J. The Kolmogorov–Smirnov test for goodness of fit. J. Am. Stat. Assoc. 1951;46:68–78. doi: 10.1080/01621459.1951.10500769. [DOI] [Google Scholar]
- Miltenyi Biotec GmbH 2022. Miltenyi Biotec GmbH ExiTron nano 12000—CT contrast ageng for pre-clinical imaging. https://miltenyibiotec.com/upload/assets/IM0001898.PDF .
- Niu C, Li M, Fan F, Wu W, Guo X, Lyu Q, Wang G. Noise suppression with similarity-based self-supervised deep learning. IEEE Trans. Med. Imaging. 2023;42:1590–602. doi: 10.1109/TMI.2022.3231428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh K, Truong A, Wang Y, Rusckowski M, Gkikas M. Ligand-specific nano-contrast agents promote enhanced breast cancer CT detection at 0.5 mg Au. Int. J. Mol. Sci. 2022;23:9926. doi: 10.3390/ijms23179926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe J H, et al. Time course of contrast enhancement by micro-CT with dedicated contrast agents in normal mice and mice with hepatocellular carcinoma. Acad. Radiol. 2015;22:169–78. doi: 10.1016/j.acra.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Smith J T, et al. In vitro and in vivo NIR fluorescence lifetime imaging with a time-gated SPAD camera. Optica. 2022;9:532–44. doi: 10.1364/OPTICA.454790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto E P, Dobrolecki L E, Villanueva H, Sikora A G, Lewis M T. In vivo modeling of human breast cancer using cell line and patient-derived xenografts. J. Mammary Gland Biol. Neoplasia. 2022;27:211–30. doi: 10.1007/s10911-022-09520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga K, Ogasawara N, Yuan Y, Okada M, Matsunaga N, Tangoku A. Visualization of breast lymphatic pathways with an indirect computed tomography lymphography using a nonionic monometric contrast medium iopamidol: preliminary results. Invest. Radiol. 2003;38:73–84. doi: 10.1097/00004424-200302000-00002. [DOI] [PubMed] [Google Scholar]
- Sulheim E, et al. Multi-modal characterization of vasculature and nanoparticle accumulation in five tumor xenograft models. J. Controlled Release. 2018;279:292–305. doi: 10.1016/j.jconrel.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney N, Marchant S, Martinez J D. Intraperitoneal injections as an alternative method for micro-CT contrast enhanced detection of murine liver tumors. BioTechniques. 2019;66:214–7. doi: 10.2144/btn-2018-0162. [DOI] [PubMed] [Google Scholar]
- Symons R, Krauss B, Sahbaee P, Cork T E, Lakshmanan M N, Bluemke D A, Pourmorteza A. Photon-counting CT for simultaneous imaging of multiple contrast agents in the abdomen: an in vivo study. Med. Phys. 2017;44:5120–7. doi: 10.1002/mp.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K, Polster C, Segars W P, Aygun N, Stierstorfer K. Model-based pulse pileup and charge sharing compensation for photon counting detectors: a simulation study. Med. Phys. 2022;49:5038–51. doi: 10.1002/mp.15779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yu H, Xi Y, Gong C, Wu W, Liu F. Spectral-Image Decomposition With Energy-Fusion Sensing for Spectral CT Reconstruction. IEEE Transactions on Instrumentation and Measurement. 2021;70:1–11. doi: 10.1109/tim.2021.3078555. [DOI] [Google Scholar]
- Wang S, Wu W, Cai A, Xu Y, Vardhanabhuti V, Liu F, Yu H. Image-spectral decomposition extended-learning assisted by sparsity for multi-energy computed tomography reconstruction. Quantitative Imaging in Medicine and Surgery. 2023;13:610–30. doi: 10.21037/qims-22-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J. Personalized Med. 2021;11:771. doi: 10.3390/jpm11080771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y, et al. Improving pulmonary perfusion assessment by dynamic contrast-enhanced computed tomography in an experimental lung injury model. J. Appl. Physiol. 2023;134:1496–507. doi: 10.1152/japplphysiol.00159.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Miltenyi Biotec GmbH 2022. Miltenyi Biotec GmbH ExiTron nano 12000—CT contrast ageng for pre-clinical imaging. https://miltenyibiotec.com/upload/assets/IM0001898.PDF .
Data Availability Statement
All data that support the findings of this study are included within the article (and any supplementary information files). Data will be available from 1 May 2024.