Blindness is one of the most feared complications of diabetes but also one of the most preventable. Diabetes is the commonest cause of blindness in people aged 30 to 69 years. Twenty years after the onset of diabetes, almost all patients with type 1 diabetes and over 60% of patients with type 2 diabetes will have some degree of retinopathy. Even at the time of diagnosis of type 2 diabetes, about a quarter of patients have established background retinopathy. Treatment can now prevent blindness in the majority of cases, so it is essential to identify patients with retinopathy before their vision is affected.
This article is adapted from the 5th edition of the ABC of Diabetes, which is published by BMJ Books (www.bmjbooks.com)
Classification of retinopathy
Diabetic retinopathy is due to microangiopathy affecting the retinal precapillary arterioles, capillaries, and venules. Damage is caused by both microvascular leakage from breakdown of the inner blood-retinal barrier and microvascular occlusion. These two pathological mechanisms can be distinguished from each other by fluorescein angiography.
Background retinopathy
Microaneurysms are small saccular pouches, possibly caused by local distension of capillary walls. They are often the first clinically detectable sign of retinopathy and appear as small red dots, commonly temporal to the macula.
Haemorrhages may occur within the compact middle layers of the retina and appear as “dots” or “blots.” Rarely, haemorrhages occur in the superficial nerve fibre layer, where they appear flame shaped; these are better recognised as related to severe hypertension.
Hard exudates are yellow lipid deposits with relatively discrete margins. They commonly occur at the edges of microvascular leakage and may form a circinate pattern around a leaking microaneurysm. They may coalesce to form extensive sheets of exudate. Vision is affected when hard exudates encroach on the macula.
Retinal oedema is due to microvascular leakage and indicates breakdown of the inner blood-retinal barrier. It appears as greyish areas of retinal thickening. The thickening may look like a petal shaped cyst on the macula, and this can cause severe visual deterioration.
Clinically significant macular oedema requires treatment. It is defined as any one of the following:
Retinal oedema within 500 μm (one third of a disc diameter) of the fovea
Hard exudates within 500 μm of the fovea if associated with adjacent retinal thickening
Retinal oedema that is one disc diameter (1500 μm) or larger, any part of which is within one disc diameter of the fovea.
Twenty per cent of eyes with clinically significant macular oedema will have serious visual loss in two years without treatment compared with 8% of treated eyes.
Preproliferative retinopathy
Retinal ischaemia due to microvascular occlusion may lead to neovascular proliferation. Signs of ischaemia include cotton wool spots, large dark blot haemorrhages, venous beading and looping, and intraretinal microvascular abnormalities. Cotton wool spots appear as white patches with rather feathery margins and represent microinfarcts in the nerve fibre layer; they become clinically important when there are more than five.
Proliferative retinopathy
New vessel formation may occur at the optic disc (NVD) or elsewhere on the retina (NVE). New vessels on the disc are particularly threatening to vision, and if allowed to progress they often lead to vitreous haemorrhage. If untreated, 26% of eyes with “high risk” and neovascular proliferation on the disc will progress to severe visual loss within two years. With laser treatment, this figure is reduced to 11%.
Advanced eye disease
In advanced proliferative diabetic retinopathy, progressive fibrovascular proliferation leads to blindness due to vitreous haemorrhage and traction retinal detachment. Rubeosis iridis and neovascular glaucoma occur when new vessels form on the iris and in the anterior chamber drainage angle, leading to a painful blind eye that occasionally requires enucleation.
Blindness in diabetic patients
Vision threatening retinopathy is usually due mainly to neovascularisation in type 1 diabetes and maculopathy in type 2 diabetes. In North America, 3.6% of patients with type 1 diabetes and 1.6% of patients with type 2 diabetes are legally blind. In England and Wales, about 1000 diabetic patients are registered as blind or partially sighted each year, with diabetic retinopathy being the commonest cause of blindness in the working population.
Vitreous haemorrhage occurs suddenly and painlessly. The blood usually clears over the following weeks, but the underlying proliferative retinopathy causes repeated haemorrhages and progressive visual loss in most cases if it is not treated. Retinal detachment resulting from contracting fibrous bonds sometimes causes blindness.
Maculopathy—Macular disease has three causes in diabetic patients: exudative maculopathy, retinal oedema, and ischaemia. Deterioration of vision in these situations is often insidious. Deterioration can be prevented to some extent by appropriate laser treatment, but once vision has been lost it cannot be restored. Ischaemic maculopathy due to loss of perifoveal capillaries may cause severe visual loss and is difficult to treat.
Cataract—Lens opacities or cataract develop earlier in diabetic patients and often progress more rapidly.
Primary open angle glaucoma has an increased prevalence in diabetic patients compared with the general population.
Prevention of blindness
Physicians must actively seek retinopathy in diabetic patients as laser photocoagulation can often prevent blindness if the condition is detected early enough. The indications for laser treatment are:
New vessels on disc or elsewhere in retina; advanced pre-proliferative changes
Clinically significant macular oedema (see above)
Encroachment of hard exudates on the fovea.
Chronic vitreous haemorrhage that precludes a view of the retina can be treated by vitrectomy and endolaser. Tractional retinal detachment can be managed by vitrectomy. Restoration of visual acuity can be impressive, but it is dependent on the underlying condition of the retina.
Clinical examination of eyes and screening
All diabetic patients older than 12 years should have their visual acuity tested and retina examined annually, or more often if advancing changes are observed. Vision threatening retinopathy rarely occurs in type 1 diabetes in the first five years after diagnosis or before puberty. However, more than a quarter of patients with type 2 diabetes have been found to have retinopathy at diagnosis, and screening should start immediately.
Visual acuity should be checked annually, or more often if appreciable retinopathy is present or if it has changed unexpectedly. This should be done with patients wearing their spectacles or through a “pinhole.”
Retinal examination—All diabetic patients should have routine fundal examination by funduscopy or retinal photography, and preferably both. The pupils should be dilated and the fundus examined in a darkened room. Tropicamide 1% eye drops are recommended as they act for only two to three hours. There is no reason to avoid pupillary dilatation in patients being treated for chronic open angle glaucoma, although those being treated for closed angle glaucoma must not have pupillary dilatation.
Patients with background retinopathy should be examined every six to 12 months (or more often if there is any change of visual acuity) and referred to an ophthalmologist when indicated. Pregnant patients require more frequent follow up as retinopathy can progress rapidly during pregnancy.
Screening methods
Conventional examination with an ophthalmoscope in a darkened room and the pupil dilated is a minimum requirement. Observers must be well trained, but even consultant ophthalmologists do not achieve the required 80% sensitivity.
Indications for referral to an ophthalmologist
Reduced visual acuity from any cause
Proliferative or preproliferative changes
Clinically significant macular oedema
Hard exudates near the macula
Any form of progressing or extensive diabetic retinopathy especially when the lesions are near the macula
Retinal photography is done through dilated pupils. Digital photography is now the preferred method as the images can be stored electronically, making them easily available for consultation, review, and teaching. Conventional colour photographs also provide good images, but the quality of Polaroid photographs is less than ideal.
It would be ideal to provide both conventional funduscopic and photographic screening, and there is some evidence that the combined screening procedure reduces the failure rate.
Blind diabetic patients
Patients with visual acuity of less than 3/60 in their better eye or gross field defects can register as blind. This gives them some financial help and social service support. Patients with a visual acuity of less than 6/60 in their better eye are eligible for registration as partially sighted. They must be registered by an ophthalmologist using the BD8 form. Printing in braille is valuable, but many diabetic patients have impaired fine sensation in their fingertips, making it difficult for them to read it. Insulin pens, in which palpable clicks correspond to units of insulin, are valuable for blind patients.
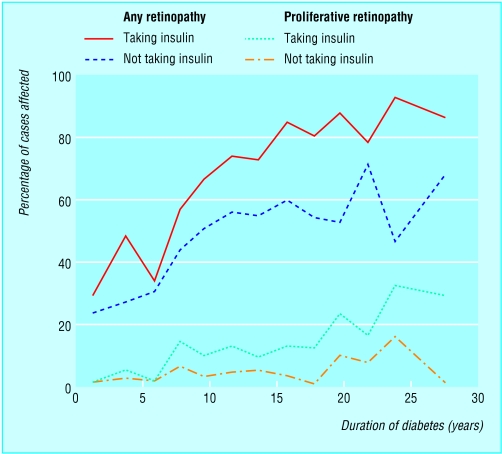
Figure.
Frequency of retinopathy (any degree) and proliferative retinopathy by duration of diabetes in people with diabetes diagnosed at age 30 or under, according to insulin treatment
Figure.
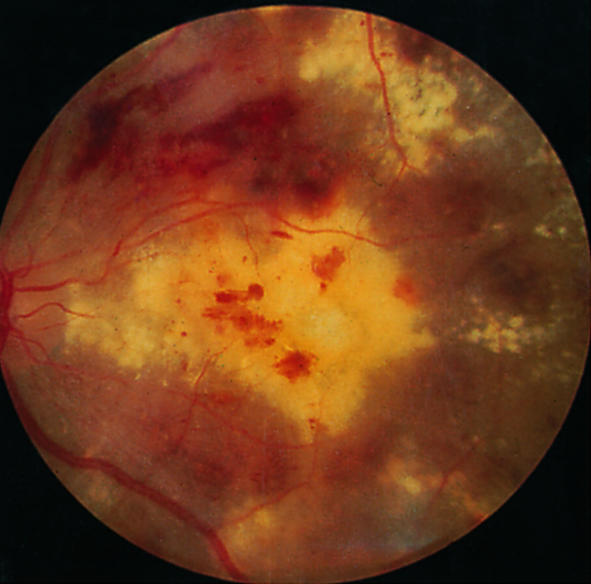
Exudative maculopathy
Figure.
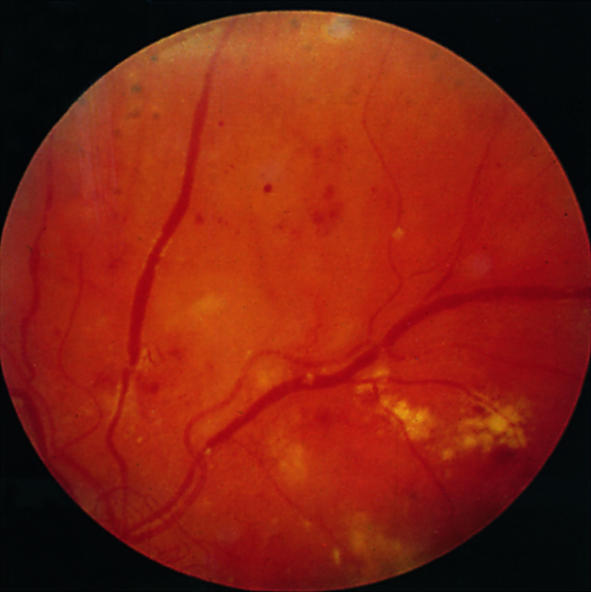
Preproliferative retinopathy with venous bleeding, cotton wool spots, and some hard exudates
Figure.
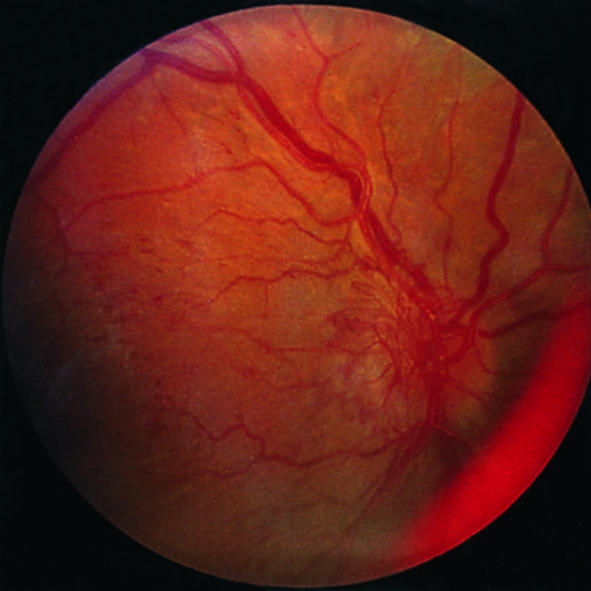
Disc new vessels
Figure.
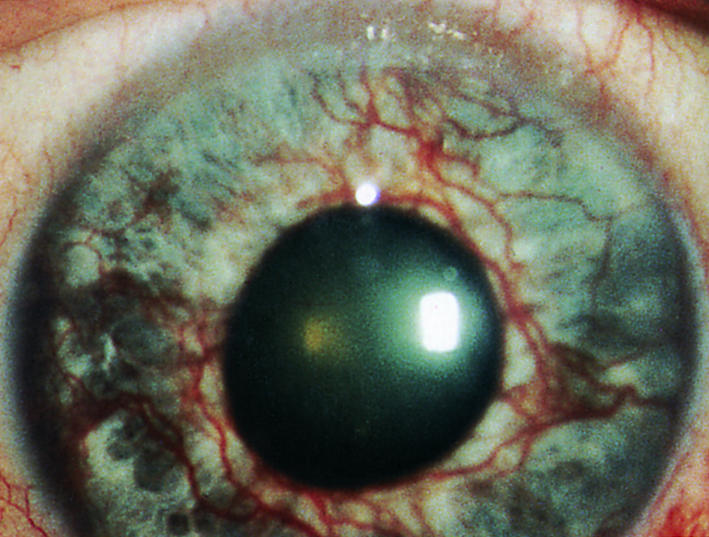
Rubeosis iridis
Figure.
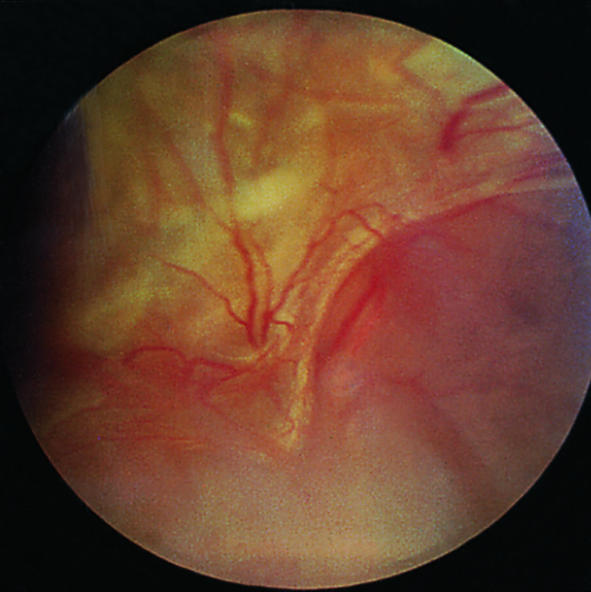
Advanced eye disease—retinitis proliferans
Figure.
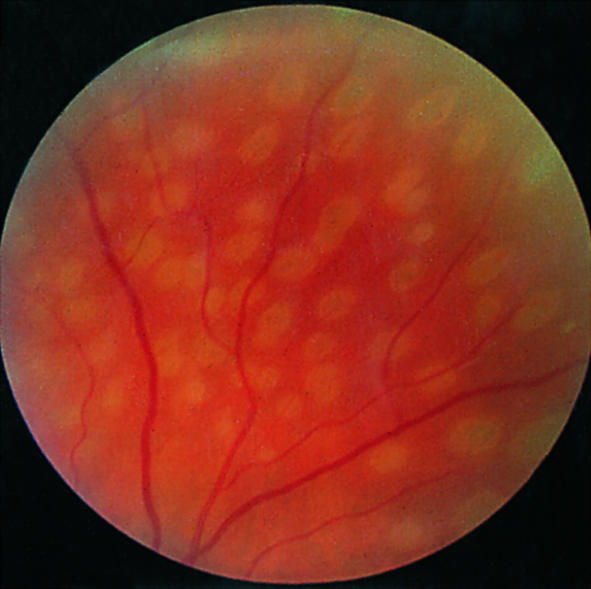
Recent argon laser photocoagulation
Figure.
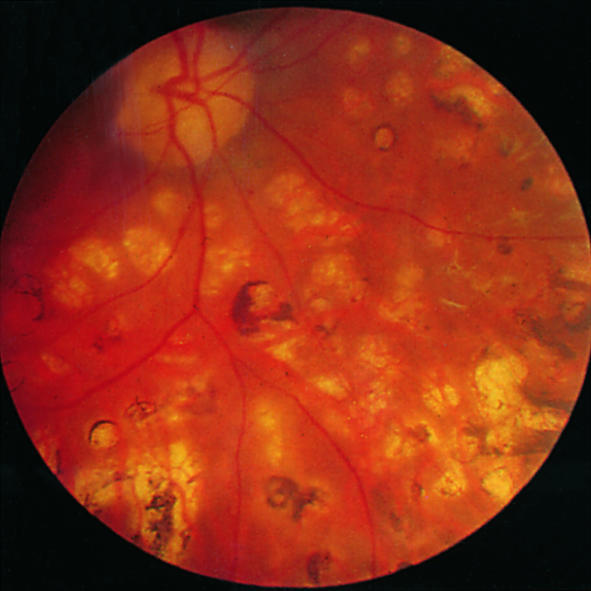
Longstanding photocoagulation scars
Figure.
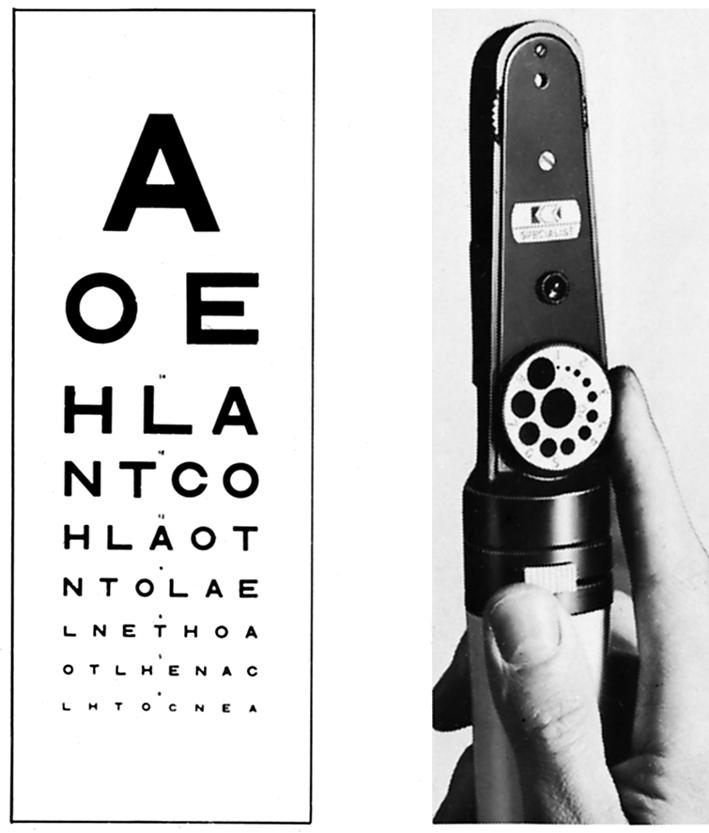
Snellen chart for testing visual acuity and funduscope for examining the retina
Figure.
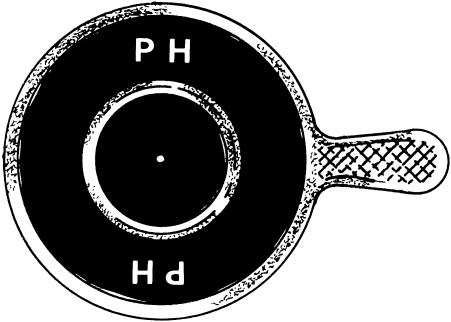
Pinhole
Figure.
Digital camera used for retinal photography
Acknowledgments
The figure showing frequency of retinopathy is adapted from Pickup JC, Williams G, eds. Textbook of diabetes. 2nd ed. Oxford: Blackwell Scientific Publications, 1997.
Footnotes
Peter J Watkins is honorary consultant physician, King's Diabetes Centre, King's College Hospital, London (peter.watkins1@virgin.net).