Abstract
Depressive and anxiety symptoms are prevalent among patients with various clinical conditions, resulting in diminished emotional well-being and impaired daily functioning. The neural mechanisms underlying these symptoms, particularly across different disorders, remain unclear, limiting the effectiveness of conventional treatments. Therefore, it is crucial to elucidate the neural underpinnings of depressive and anxiety symptoms and investigate novel, effective treatments across clinical conditions. Transcranial direct current stimulation (tDCS) is a neuromodulatory technique that can help understand the neural underpinnings of symptoms and facilitate the development of interventions, addressing the two research gaps at both neural and clinical levels. Thus, this systematic review and meta-analysis aims to evaluate the existing evidence regarding the therapeutic efficacy of tDCS in reducing depressive and anxiety symptoms among individuals with diverse clinical diagnoses. This review evaluated evidence from fifty-six randomized, sham-controlled trials that administered repeated tDCS sessions with a parallel design, applying a three-level meta-analytic model. tDCS targeting the left dorsolateral prefrontal cortex (DLPFC) at 2-mA intensity demonstrates moderate efficacy in alleviating depressive symptoms, identifying the left DLPFC as a transdiagnostic neural mechanism of depressive symptoms across clinical conditions. In comparison, the findings on anxiety symptoms demonstrate greater heterogeneity. tDCS over the left DLPFC is effective in reducing depressive symptoms and shows promising effects in alleviating anxiety symptoms among individuals with diverse diagnoses. These findings enhance our understanding of the neuropsychological basis of depressive and anxiety symptoms, laying the groundwork for the development of more effective tDCS interventions applicable across clinical conditions.
Subject terms: Depression, Human behaviour
Introduction
Depressive and anxiety symptoms are highly prevalent across various clinical conditions and often co-occur, negatively impacting disease prognoses and quality of life [1–3]. The neural underpinnings of these two symptoms across clinical disorders and the co-occurrence of these two symptom categories are unclear [4–6], potentially contributing to the lack of effective treatments [7, 8]. Indeed, a significant portion of patients experiencing these symptoms do not respond well to conventional treatments [9, 10]. Therefore, it is important to understand how these two symptoms can be targeted across clinical conditions.
Neuromodulation therapies, such as Transcranial Magnetic Stimulation (TMS), Transcranial Alternating Current Stimulation (tACS), and Transcranial Direct Current Stimulation (tDCS), offer targeted, non-pharmacological options for modulating brain activity, presenting personalized treatment potentials with reduced side effects for neurological and psychiatric disorders [11]. tDCS can be one approach for furthering the understanding of depressive and anxiety symptoms across conditions and one possible treatment for alleviating these two symptoms across disorders due to its several advantages. It is a non-invasive neuromodulatory technique that modulates neuronal excitability by delivering weak electric current (1–2 mA) to the targeted brain regions [12, 13]. As a substantial proportion of patients have shown poor responses to traditional treatments of psychiatric disorders [3, 14, 15], tDCS is a safe, well-tolerated tool that has been increasingly studied as an alternative or add-on treatment [16–21]. In addition to being a safe treatment option, tDCS can directly target the altered neural activity and functional connectivity of brain regions underlying these symptoms, potentially through mechanisms such as the modulation of neuronal membrane potential, strengthening synaptic connections through long-term potentiation, and enhancing levels of brain-derived neurotrophic factor (BDNF) [22–24]. Thus, tDCS can help establish causal relationships between brain regions and targeted symptoms, thus elucidating the symptoms’ underlying neural underpinnings.
Existing evidence has consistently supported the effectiveness of tDCS interventions for psychiatric disorders. Numerous studies and reviews have provided a high level of evidence demonstrating the therapeutic efficacy of tDCS specifically in Major Depressive Disorder (MDD) [25–29]. According to evidence-based, meta-analytic guidelines [27, 28], repeated tDCS targeting the left dorsolateral prefrontal cortex (DLPFC, F3) has been found to be effective in alleviating depressive symptoms in MDD patients. Furthermore, although the evidence is not as robust, tDCS has also shown promising results in the treatment of anxiety disorders such as Generalized Anxiety Disorder (GAD) and Social Anxiety Disorder (SAD) [20, 30]. These findings highlight tDCS interventions as a safe, cost-effective, and viable treatment option for individuals diagnosed with MDD and anxiety disorders.
However, existing studies and reviews have only focused on evaluating the therapeutic efficacy of tDCS on depressive and anxiety symptoms in patients diagnosed with MDD and anxiety disorders. No review has systematically evaluated evidence on the effects of tDCS on depressive and anxiety symptoms across clinical conditions. As depressive and anxiety symptoms are not limited to MDD and anxiety disorders [31, 32], it is crucial to examine effective treatments for alleviating depressive and anxiety symptoms across heterogeneous clinical populations. This can potentially improve the treatment options and emotional well-being of patients who suffer from these two affective symptoms regardless of their primary diagnoses. Additionally, as these two affective symptoms are often comorbid [33], a better understanding of the shared neural underpinnings of these two symptoms would be necessary for developing more effective treatments. As a result, evaluating evidence on the therapeutic efficacy of tDCS in treating depressive and anxiety symptoms in patients with various clinical diagnoses is essential for addressing this gap in clinical treatments and contributing to a better understanding of the neural underpinnings of these two prevalent affective symptoms.
This review aims to fill the research gap by evaluating evidence from randomized controlled trials (RCTs) that administered repeated (more than one session, without restrictions on the interval between sessions) tDCS sessions with a parallel design. The focus is on evidence with high methodological quality, as RCTs can reduce bias at each study phase and better investigate treatment effects [34]. Repeated stimulation has the potential for cumulative tDCS effects [35] and has demonstrated therapeutic efficacy in clinical populations [36, 37]. Additionally, a parallel design allows for better examination of treatment outcomes by controlling for potential carryover effects [38]. Lastly, conventional tDCS is specifically evaluated as conventional tDCS and high-definition tDCS (HD-tDCS) have distinct protocols and thus can demonstrate neuromodulatory effects through different mechanisms of action [39].
In summary, this systematic review and meta-analysis aim to synthesize and quantitively evaluate the effects of tDCS in alleviating depressive and anxiety symptoms across various clinical conditions. The review focuses on evidence from RCTs that administered repeated, conventional tDCS with a parallel design, with a primary goal of identifying potential transdiagnostic neural underpinnings and exploring effective tDCS interventions for depressive and anxiety symptoms across various clinical populations.
Methods
This systematic review was not pre-registered, and no protocol can be accessed. However, the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were strictly followed [40]. The review objectives, screening criteria and strategy, study selection and data extraction procedures, risk of bias assessment, and anticipated completion date were outlined prior to commencing the review process. Additionally, thorough efforts were made to ensure there was no existing review on the same topic. The PRISMA checklist can be found in supplementary materials.
Inclusion and exclusion criteria
The inclusion criteria for selecting studies were: (a) papers written in English; (b) studies on human subjects; (c) original empirical research on conventional tDCS; (d) randomized, sham-controlled trials with a parallel design; (e) studies with affective outcomes or clinical conditions demonstrating affective symptoms; (f) studies administered more than one tDCS sessions.
Studies were excluded when they satisfied one of the following criteria: (a) review papers; (b) abstracts or conference proceedings; (c) study protocols; (d) studies unrelated to tDCS; (e) studies on HD-tDCS; (f) studies examined tDCS combined with other interventions, treatments, or training; (g) studies with duplicate samples.
Searching strategies
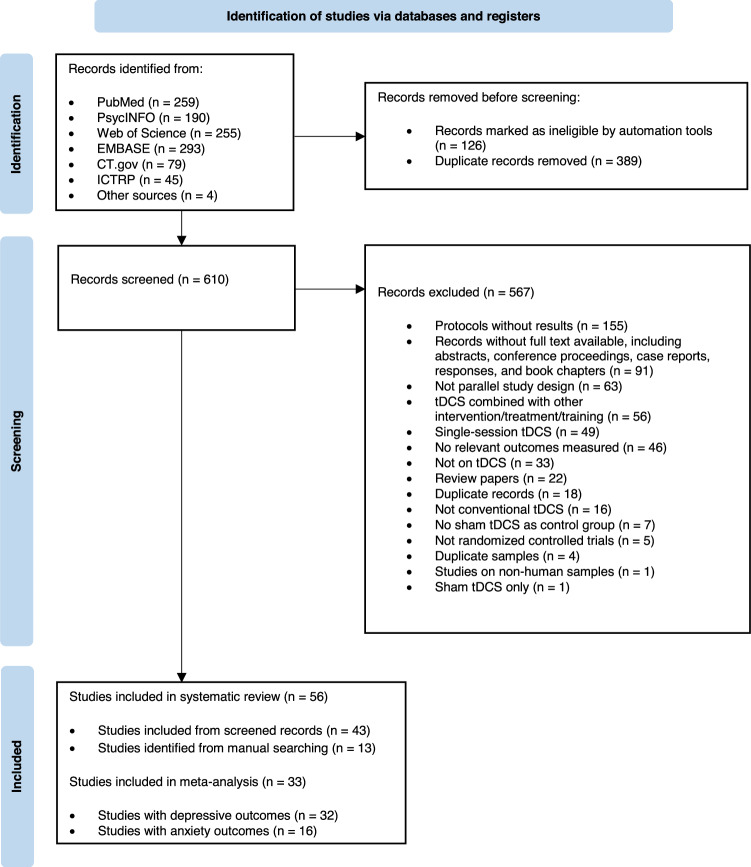
Databases and registers, including PubMed, PsycINFO, Web of Science, EMBASE, ClinicalTrials.gov (CT.gov), and International Clinical Trials Registry Platform (ICTRP), were searched on March 20, 2024. The search string used was: (tDCS OR transcranial direct current stimulation) AND (emotion* OR affective* OR mood*) AND (sham* OR placebo*) AND (random*). This search string was adopted to generate a more comprehensive list of studies examining affective outcomes (including depressive and anxiety symptoms) without overlooking papers that might meet the criteria. Records generated from the search results were combined and imported to EndNote for duplicate removal. Two authors (EZZ, ASYY) independently screened the remaining records based on the inclusion and exclusion criteria by reading their titles, abstracts, and main texts. The review papers identified from the initial records were retrieved for screening of their references. Screening result discrepancies were resolved through consensus discussions (Fig. 1).
Fig. 1.
PRISMA flow diagram of study selection.
Data extraction
Data extraction was performed together by the two authors (EZZ, ASYY). Variables extracted included study and participant characteristics (e.g., participants’ primary diagnoses as indicated in the original studies), tDCS protocol-related information (e.g., montage), outcome measures, and the main results indicating treatment efficacy. A consensus on the accuracy of the extracted information was reached through discussions.
Meta-analysis
All analyses were performed using the metafor package [41] within the statistical software environment R. The effects of tDCS on depressive and anxiety symptoms were evaluated separately. Due to some studies including multiple measurements for these symptoms, a three-level structure was applied to the meta-analytic model to account for between-study and within-study heterogeneity and the dependence of effect sizes [42–46].
The endpoint means and standard deviations of depressive and anxiety outcomes were extracted for the active and sham groups. If the outcome data were unavailable, the corresponding authors were contacted systematically. The standardized mean difference (SMD) was then calculated to report the intervention effect, considering the different measurements used in the included studies. To assess potential publication bias, funnel plots, and Egger’s regression test were applied [47, 48]. In cases where significant publication bias was detected, leave-one-out analysis was conducted. Additionally, moderator analyses were conducted to examine the moderating effects of stimulation intensity and duration (coded as dummy variables), in instances of significant heterogeneity.
Risk of bias assessment
The Cochrane Collaboration’s risk-of-bias tool was applied to assess the methodological quality of the studies [49]. Two authors (EZZ, ASYY) independently rated the risk of bias (ROB) in each study in domains including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting by giving a rating of low, unclear, or high ROB. Each study’s overall ROB was given based on the criteria used in a meta-analytic systematic review of tDCS and MDD [50]. Discrepancies in the ratings were resolved through discussions.
Results
Study selection
A total of 1125 records were identified from the initial search (259 from PubMed, 190 from PsycINFO, 255 from Web of Science, 293 from EMBASE, 79 from CT.gov, 45 from ICTRP, and 4 from other sources). After applying filters, 126 records were excluded. After importing the remaining records in EndNote, 389 duplicate records were removed. The remaining 610 records were screened by examining their titles, abstracts, and full texts based on the pre-specified criteria, and 567 records were excluded. A total of 43 studies were included after screening the initial search records. The references of the review papers identified from the initial search were screened, resulting in 13 more studies. In summary, 56 studies were included in the systematic review; 33 of these studies with available data were included in the meta-analysis (Fig. 1).
Study overview
Table 1 provides comprehensive information on sample characteristics, tDCS protocols, and measurements of depressive and anxiety symptoms across the 56 studies published between 2006 and 2024. The combined participant count across these studies was 2349, with sample sizes ranging from 10 to 174. The most frequently implemented tDCS protocol involved targeting the left DLPFC as the anodal stimulation site, employing an intensity of 2 mA, and administering stimulation for 20 min per session. Among the included studies, 43 investigated the effects of tDCS interventions on depressive symptoms, and 24 examined anxiety symptoms.
Table 1.
Overview of included studies in alphabetical order.
| Study | Sample characteristics | tDCS protocols | Symptom measurement | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | N (Total) | % Female | Age (SD) | Montage (anode, cathode) | Intensity (mA) | Duration (min) | Depressive symptoms | Anxiety symptoms | |
| Acler et al. [94] | Post-polio syndrome | 32 | 53% | 61.4 (5.9) | Right and left pre-motor cortex (2 cm ahead C3-C4), Left shoulder | 1.5 | 15 | HDRS | – |
| Ahmadizadeh et al. [68] | PTSD | 40 | 65% | 43.8 (10.6) | F3, F4 | 2 | 20 | BDI-II | BAI |
| Aksu et al. [69] | Panic disorder | 30 | 63% | 37.3 (11.5) | F3, F4 | 2 | 20 | HDRS; BDI | HARS; PDSS |
| Barham et al. [100] | ADHD | 22 | 68% | 22.0 (2.8) | F4, F3 | 2 | 20 | – | – |
| Bennabi et al. [51] | Treatment-resistant MDD | 24 | 75% | 61.8 (16.3) | F3, Fp2 | 2 | 30 | MADRS; HDRS; BDI | STAI |
| Benninger et al. [95] | Parkinson’s disease | 25 | 36% | NR | Symmetrically either over the premotor and motor (electrode center 10 mm anterior to Cz) or prefrontal cortices (forehead above eyebrows), Mastoids | 2 | 20 | BDI | – |
| Blumberger et al. [52] | Treatment-resistant MDD | 24 | 83% | 47.3; Range: 24–62 | F3, F4 | 2 | 20 | MADRS; HDRS; BDI | – |
| Boggio et al. [53] | MDD | 40 | 70% | 49.4 (7.4) | F3, Fp2 | 2 | 20 | HDRS; BDI | – |
| Borckardt et al. [83] | Patients undergoing unilateral primary TKA | 39 | 74% | 67.0 (9.1) | Knee representation of the motor strip, F4 | 2 | 20 | – | – |
| Brunelin et al. [80] | Schizophrenia with verbal hallucinations | 30 | NR | NR | Left DLPFC (Midway FP1/F3), Left TPJ (Midway T3/P3) | 2 | 20 | – | – |
| Chen et al. [65] | MDD | 63 | 71% | NR | F3, Fp2 | 2 |
60 30 |
HDRS | HARS |
| da Silva et al. [91] | Lesch’s type IV alcohol-dependent patients | 13 | 0% | 49.0 (29.6) | F3, Right supradeltoid area | 2 | 20 | HDRS | HARS |
| de Lima et al. [70] | GAD | 30 | 63% | NR | F3, Fp2 | 2 | 20 | BDI | HARS; BAI |
| Doruk et al. [96] | Parkinson’s disease | 18 | 33% | 61 (8); Range: 40–71 |
F3, Fp2 F4, Fp1 |
2 2 |
20 20 |
BDI; HDRS | HARS |
| Dutra et al. [84] | Primary dysmenorrhea | 26 | 100% | NR | F3, Fp2 | 2 | 20 | – | HARS |
| Fitzgerald et al. [78] | Schizophrenia or schizoaffective disorder | 13 | NR | NR |
F3, TP3 F3 and F4, TP3 and TP4 |
2 2 |
20 20 |
CDSS | – |
| Fregni et al. [54] | MDD | 10 | NR | 42.7 (10) | F3, Fp2 | 1 | 20 | HDRS; BDI | – |
| Fregni et al. [55] | MDD | 18 | 61% | 46.4 (9.4) | F3, Fp2 | 1 | 20 | HDRS | – |
| Fregni et al. [85] | Fibromyalgia | 32 | 100% | 53.4 (8.9) |
C3, Fp2 F3, Fp2 |
2 2 |
20 20 |
BDI | VAS - Anxiety |
| Huang et al. [66] | Unipolar and bipolar depression | 70 | 74% | NR |
Fp1, Fp2 F3, F4 |
2 2 |
20 20 |
MADRS; HDRS; QIDS-SR | HARS |
| Jafari et al. [71] | SAD | 45 | 44% | 32.4 (7.0) |
F3, Medial PFC F3, Medial PFC |
1 2 |
20 20 |
BDI-II | LSAS; PSWQ |
| Jeon et al. [74] | Schizophrenia | 54 | 52% | NR | F3, F4 | 2 | 30 | CDSS | – |
| Khedr et al. [86] | Fibromyalgia | 36 | 94% | NR | C3, Right arm | 2 | 20 | HDRS | HARS |
| Klírová et al. [106] | Post-COVID syndrome | 33 | 70% | NR | F3, F4 | 2 | 30 | PHQ-9 | GAD-7 |
| Koops et al. [81] | Medication-resistant auditory hallucinations | 54 | 54% | Range: 23–74 | Left DLPFC (Midway FP1/F3), left TPJ (Midway T3/P3) | 2 | 20 | – | – |
| Leffa et al. [101] | ADHD | 64 | 47% | 38.3 (9.6) | F4, F3 | 2 | 30 | BDI | BAI |
| Lisoni et al. [103] | BPD | 30 | 60% | 40.3 (12.8) | F4, F3 | 2 | 20 | HDRS; BDI | HARS; IDAS – Anxiety subitem |
| Lisoni et al. [82] | Schizophrenia | 50 | 22% | 42.70 (12.11) | F3, Fp2 | 2 | 20 | CDSS | – |
| Liu et al. [97] | Well-controlled temporal lobe epilepsy | 33 | 42% | NR | F3, Fp2 | 2 | 20 | BDI-II; NDDI-E | – |
| Loo et al. [58] | MDD | 40 | 55% | NR | F3, F8 | 1 | 20 | MADRS; HDRS; BDI | – |
| Loo et al. [56] | MDD | 60 | 47% | NR | F3, F8 | 2 | 20 | MADRS | – |
| Loo et al. [57] | MDD; bipolar depression | 84 | 50% | NR | F3, F8 | 2.5 | 30 | MADRS | – |
| Maas et al. [98] | Spinocerebellar ataxia type 3 | 20 | 40% | NR | Cerebellum, right deltoid muscle | 2 | 20 | PHQ-9 | – |
| Mariano et al., 2019 | Chronic low back pain | 21 | 14% | 63.1 (10.5) | Right mastoid process, FC1 | 2 | 20 | PHQ-9 | GAD-7; PASS-20 |
| Molavi et al. [104] | BPD | 32 | 47% | 30.6 (5.4) | F3, F4 | 2 | 20 | BDI | – |
| Mondino et al., 2016 | Schizophrenia | 23 | 35% | NR | Midway F3 and Fp1 (left DLPFC), Midway T3 and P3 (left TPJ) | 2 | 20 | – | – |
| Newstead et al. [72] | Healthy individuals | 21 | 52% | NR | F3, Right cerebellum | 2 | 12 | – | – |
| Palm et al. [76] | Schizophrenia | 20 | 25% | 36.1 (11.4) | F3, Fp2 | 2 | 20 | CDSS | – |
| Pegado et al., 2020 | Primary dysmenorrhea | 20 | 100% | NR | F3, Right supradeltoid area | 2 | 20 | – | HARS |
| Pinto et al. [105] | Primary Sjogren syndrome | 36 | 100% | NR | F4, F3 | 2 | 20 | – | VAS - Anxiety |
| Pinto et al. [99] | Stroke | 60 | 27% | Range: 21–67 | Primary motor cortex (C3/C4) ipsilesional to the side of the stroke, Contralesional primary motor cortex (C3/C4) | Current ramped up across 30 to 60s to reach a target amplitude of between 2 and 3 mA | 30 | HDRS | HARS |
| Quintiliano et al. [89] | Chronic kidney disease patients with chronic pain | 30 | 73% | NR | C3, Fp2 | 2 | 20 | BDI | HARS |
| Salehinejad et al. [59] | MDD | 30 | 57% | NR | F3, F4 | 2 | 20 | HDRS; BDI | – |
| Salehinejad et al. [60] | MDD | 24 | 63% | NR | F3, F4 | 2 | 20 | HDRS; BDI | – |
| Samartin-Veiga et al. [90] | Fibromyalgia | 130 | 100% | NR |
C3, Fp2 F3, Fp2 OIC multi-electrode montage |
2 2 Differed by electrode |
20 20 20 |
HADS | HADS |
| Sampaio-Junior et al. [61] | Bipolar depression | 59 | 68% | 45.9 (12) | F3, F4 | 2 | 30 | MADRS; HDRS | – |
| Shahbabaie et al. [92] | Early-abstinent methamphetamine users | 90 | 0% | 30.8 (6.2) |
F3, Right shoulder F4, Left shoulder F3, Right supraorbital ridge F4, Left supraorbital ridge F3, F4 |
2 2 2 2 2 |
13 13 13 13 13 |
– | – |
| Sharafi et al. [62] | Treatment-resistant MDD | 30 | 53% | 47.2 (12.0) | F3, F4 | 2 | 20 | HDRS | – |
| Silva et al. [73] | Treatment-resistant OCD | 43 | 60% | NR | Left deltoid, SMA (1.5 cm anteriorly to the measured location of Cz) | 2 | 30 | BDI | BAI |
| Smith et al. [79] | Schizophrenia or schizoaffective disorder | 33 | 27% | NR | F3, Fp2 | 2 | 20 | – | – |
| Valiengo et al. [63] | Post-stroke depression | 48 | 50% | NR | F3, F4 | 2 | 30 | MADRS; HDRS | – |
| Valiengo et al. [77] | Schizophrenia | 100 | 20% | 35.5 (9.3) | Left DLPFC (Midway FP1/F3), left TPJ (Midway T3/P3) | 2 | 20 | CDSS | – |
| Verveer et al. [93] | Smokers | 71 | 51% | 22.0 (4.7) | F4, F3 | 2 | 13 | – | – |
| Vigod et al. [64] | MDD | 20 | 100% | 32.3 (4.2) | F3, F4 | 2 | 30 | MADRS; EPDS | STAI |
| Woodham et al. [67] | MDD | 174 | 69% | NR | F3, F4 | 2 | 30 | MADRS; HDRS | HARS |
| Zemestani et al. [102] | ASD | 32 | 28% | 10.2 (1.9) | F3, F4 | 1.5 | 15 | – | – |
Montage: C3, left M1; C4, right M1; F3, left DLPFC; F4, right DLPFC; F8, right orbit; FC1, left dorsal anterior cingulate cortex (dACC); Fp1, left supraorbital area; Fp2, right supraorbital area; OIC, left operculo-insular cortex; SMA, supplementary motor area; TP3/4, temporoparietal area; TPJ, temporoparietal junction.
Depressive Symptom Measurement: BDI, Beck Depression Inventory; CDSS, Calgary Depression Scale for Schizophrenia; EPDS, Edinburgh Postnatal Depression Scale; HADS, Hospital Anxiety and Depression Scale; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; NDDI-E, Neurological Disorders Depression Inventory for Epilepsy; PHQ-9, Patient Health Questionnaire; QIDS-SR, Quick Inventory of Depressive Symptomatology Self Report.
Anxiety Symptom Measurement: BAI, Beck Anxiety Inventory; GAD-7, Generalized Anxiety Disorder Scale; HARS, Hamilton Anxiety Rating Scale; IDAS, Irritability-Depression-Anxiety Scale; LSAS, Liebowitz Social Anxiety Scale; PASS, Pain Anxiety Symptoms Scale; PDSS, Panic Disorder Severity Scale; PSWQ, Penn State Worry Questionnaire; STAI, State-Trait Anxiety Inventory; VAS, Visual Analog Scale.
ADHD attention deficit hyperactivity disorder, ASD autism spectrum disorder, BPD borderline personality disorder, GAD generalized anxiety disorder, MDD major depressive disorder, OCD obsessive-compulsive disorder, PTSD posttraumatic stress disorder, SAD social anxiety disorder, TKA total knee arthroplasty, NR not reported.
Participant diagnoses varied in the studies. Seventeen studies focused on depression [51–67]. Four studies focused on anxiety disorders [68–71]. One study included healthy individuals [72]. Other diagnoses addressed in the studies encompassed Obsessive-Compulsive Disorder (OCD) [73], Schizophrenia or Schizoaffective Disorder [74–82], pain conditions [83–90], substance use [91–93], neurological disorders [94–99], Attention Deficit Hyperactivity Disorder (ADHD) [100, 101], Autism Spectrum Disorder (ASD) [102], Borderline Personality Disorder (BPD) [103, 104], Primary Sjogren Syndrome [105], and post-COVID syndrome [106].
Subsequent sections present the effects of tDCS on depressive symptoms, anxiety symptoms, and other affective outcomes. The results regarding tDCS effects are presented in terms of the comparison between the active and sham groups before and after interventions.
tDCS effects on depressive symptoms
Overview
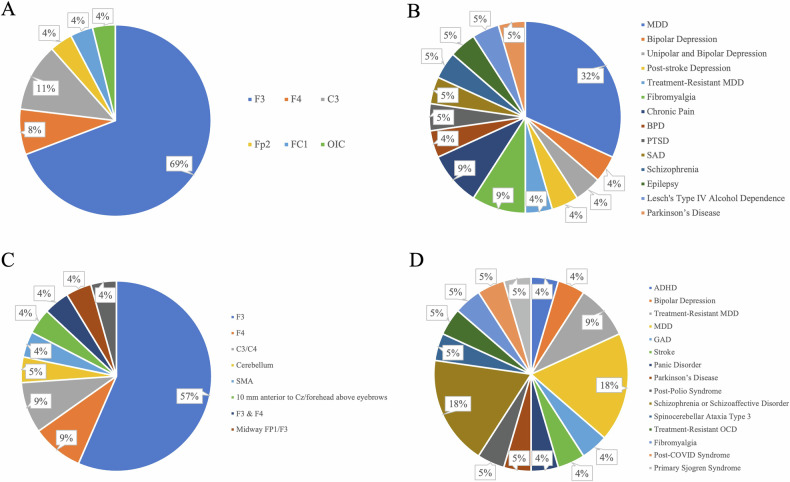
Twenty-two studies showed positive effects of tDCS on depressive symptoms across various clinical conditions (Table S1; Fig. 2B). The left DLPFC was the target region for 18 of these studies (Table S1; Fig. 2A). Anodal stimulation over the left primary motor cortex (M1; C3) and the right DLPFC (F4) also showed positive effects. Twenty studies utilized an intensity of 2 mA. The stimulation duration consisted of a single session lasting either 20 or 30 min.
Fig. 2. Breakdown of target regions and clinical conditions in studies with depressive outcomes.
A Target regions in studies with depressive symptom alleviation; B Clinical conditions in studies with depressive symptom alleviation; C Target regions in studies without depressive symptom alleviation; D Clinical conditions in studies without depressive symptom alleviation. (Target region abbreviation: C3, left M1; C4, right M1; F3, left DLPFC; F4, right DLPFC; FC1, left dorsal anterior cingulate cortex (dACC); Fp1, left supraorbital area; Fp2, right supraorbital area; OIC, left operculo-insular cortex; SMA, supplementary motor area. Condition abbreviations: ADHD attention deficit hyperactivity disorder, BPD borderline personality disorder, GAD generalized anxiety disorder, MDD major depressive disorder, OCD obsessive-compulsive disorder, PTSD posttraumatic stress disorder, SAD social anxiety disorder).
In contrast to studies that demonstrated positive effects of tDCS, the 21 studies that did not show positive effects exhibited greater heterogeneity in terms of the clinical diagnoses, stimulation protocols, sham conditions, and depressive symptom measurement (Table S1; Fig. 2C, D). The applied current intensity ranged from 1 to 2.5 mA, with 2 mA being the most frequently used intensity (17 studies). The duration of a single stimulation session ranged from 15 to 30 min.
Meta-analysis results: all studies
The multivariate meta-analysis model included 32 studies, encompassing 67 outcomes reflecting levels of depressive symptoms (Table 2; Fig. S1).
Table 2.
Meta-analysis results on depressive and anxiety symptoms.
| Studies | tDCS intervention effects | Heterogeneity | Egger’s regression test | Moderator:intensity | Moderator:duration | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | t | p | SE | CI | Within-study variance | Between-study variance | t | p | F | p | F | p | |
|
Studies with depressive outcomes All studies (study n = 32, outcome n = 67) | |||||||||||||
| –0.355 | –4.049 | <0.001*** | 0.088 | [–0.530, –0.180] | Insignificant | Significant | –1.523 | 0.133 | 2.095 | 0.092 | 1.146 | 0.337 | |
|
Studies with depressive outcomes F3 studies (study n = 23, outcome n = 50) | |||||||||||||
| –0.422 | –4.393 | <0.001*** | 0.096 | [–0.616, –0.229] | Insignificant | Significant | –1.756 | 0.085 | 3.696 | 0.032* | 0.827 | 0.444 | |
|
Studies with anxiety outcomes All studies (study n = 16, outcome n = 27) | |||||||||||||
| –0.398 | –2.049 | 0.051 | 0.194 | [–0.796, 0.001] | Insignificant | Significant | –2.602 | 0.015* | 0.483 | 0.698 | 2.767 | 0.083 | |
|
Studies with anxiety outcomes F3 studies (study n = 9, outcome n = 16) | |||||||||||||
| –0.449 | –1.428 | 0.174 | 0.314 | [–1.119, 0.221] | Insignificant | Significant | –1.806 | 0.093 | 1.801 | 0.201 | 1.287 | 0.309 | |
*p < 0.05, **p < 0.01, ***p < 0.001.
The overall effect of active tDCS interventions on depressive symptoms was significant (estimate = –0.355, t(66) = –4.049, p < 0.001), with a standard error of 0.088. This indicated that the active interventions were associated with significantly lower levels of depressive symptoms compared to the sham intervention. The confidence interval ranged from –0.53 to –0.18.
The within-study variance was not significant (p > 0.05), whereas the between-study variance was significant (p < 0.001). Results from the funnel plot and Egger’s regression test demonstrated no evidence of publication bias (t(65) = –1.523, p = 0.133; Fig. S2).
Moderator analysis showed that the overall effect of tDCS interventions on depressive symptoms was not moderated by either stimulation intensity (F(4, 62) = 2.095, p = 0.092) or duration (F(3, 63) = 1.146, p = 0.337). However, compared to an intensity of 1 mA, an intensity of 2 mA had a significant alleviating effect (estimate = –0.694, p = 0.048), suggesting a notable impact of this intensity level in reducing depressive symptoms.
Meta-analysis results: studies targeted F3
The multivariate meta-analysis model was performed on a subgroup of studies that targeted the left DLPFC to further examine the role of this region. This analysis included 23 studies and 50 outcomes (Table 2; Fig. S3).
The overall effect of active tDCS interventions over the left DLPFC on depressive symptoms was significant (estimate = –0.422, t(49) = –4.393, p < 0.001), with a standard error of 0.096. This indicated that the active interventions targeting this region were associated with significantly lower levels of depressive symptoms compared to the sham intervention. The confidence interval ranged from –0.616 to –0.229.
The within-study variance was not significant (p > 0.05), whereas the between-study variance was significant (p < 0.001). Results from the funnel plot and Egger’s regression test demonstrated no evidence of publication bias (t(48) = –1.756, p = 0.085; Fig. S4).
Moderator analysis showed that the overall effect of active tDCS interventions targeting the left DLPFC in reducing depressive symptoms was moderated by stimulation intensity (F(2, 47) = 3.696, p = 0.032) but not duration (F(2, 47) = 0.827, p = 0.444). Specifically, compared to an intensity of 1 mA, an intensity of 2 mA demonstrated a significant alleviating effect (estimate = –0.704, p = 0.037), suggesting the critical role of this intensity level in alleviating depressive symptoms, specifically in tDCS interventions targeting the left DLPFC.
tDCS effects on anxiety symptoms
Overview
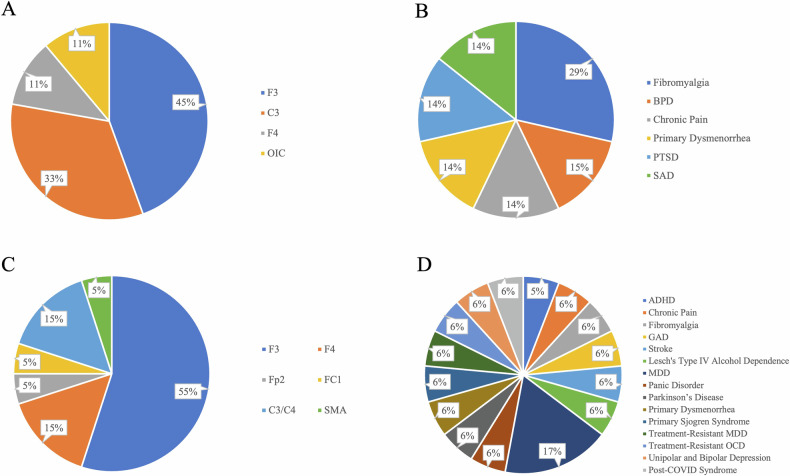
Seven studies reported positive effects of tDCS on anxiety symptoms across clinical conditions (Table S2; Fig. 3A, B). Among these studies, four included patients with pain conditions. Four studies targeted the left DLPFC as the target region, three targeted the left M1, and one targeted the right DLPFC. All studies applied 2 mA except for one study that used 1 mA for patients with SAD [52]. Each session lasted 20 min in all studies.
Fig. 3. Breakdown of target regions and clinical conditions in studies with anxiety outcomes.
A Target regions in studies with anxiety symptom alleviation; B Clinical conditions in studies with anxiety symptom alleviation; C Target regions in studies without anxiety symptom alleviation; D Clinical conditions in studies without anxiety symptom alleviation. (Target region abbreviation: C3, left M1; C4, right M1; F3, left DLPFC; F4, right DLPFC; FC1, left dorsal anterior cingulate cortex (dACC); Fp2, right supraorbital area; OIC, left operculo-insular cortex; SMA, supplementary motor area. Condition abbreviations: ADHD attention deficit hyperactivity disorder, BPD borderline personality disorder, GAD generalized anxiety disorder, MDD major depressive disorder, OCD obsessive-compulsive disorder, PTSD posttraumatic stress disorder, SAD social anxiety disorder).
Seventeen studies did not find positive effects of tDCS on anxiety symptoms (Fig. 3C, D). Among these, eleven studies stimulated the left DLPFC. Two studies employed cathodal tDCS [54, 67], which exerts inhibitory effects on the target regions. Most of these studies utilized a current intensity of 2 mA, with single sessions lasting either 20, 30, or 60 min.
Meta-analysis results: all studies
The multivariate meta-analysis model included 16 studies, encompassing 27 outcomes indicating levels of anxiety symptoms (Table 2; Fig. S5).
The overall effect of tDCS on anxiety symptoms was marginally significant (estimate = –0.398, t(26) = –2.049, p = 0.051), with a standard error of 0.194. The confidence interval ranged from –0.796 to 0.001.
The within-study variance was not significant (p > 0.05), whereas the between-study variance was significant (p < 0.001). Results from the funnel plot and Egger’s regression test demonstrated evidence of publication bias (t(25) = –2.602, p = 0.015; Fig. S6). As a result, the leave-out-out analysis was performed to assess the changes in the pooled estimate when studies were omitted one at a time (Fig. S7).
Moderator analysis revealed that the overall effect of tDCS interventions on anxiety symptoms was not moderated by either stimulation intensity (F(3, 23) = 0.483, p = 0.698) or duration (F(2, 24) = 2.767, p = 0.083). Notably, compared to a stimulation duration of 60 min, a duration of 20 min had a significant alleviating effect (estimate = –1.184, p = 0.039), suggesting a significant effect of this duration in reducing anxiety symptoms.
Meta-analysis results: studies targeted F3
The multivariate meta-analysis model was performed on a subgroup of studies that targeted the left DLPFC. This analysis included 9 studies and 16 outcomes (Table 2; Fig. S8).
The overall effect of tDCS over F3 on anxiety symptoms was insignificant (estimate = –0.449, t(15) = –1.428, p = 0.174), with a standard error of 0.314. This indicated that the active interventions targeting F3 and the sham interventions were associated with similar levels of anxiety symptoms. The confidence interval ranged from –1.119 to –0.221.
The within-study variance was not significant (p > 0.05), whereas the between-study variance was significant (p < 0.001). Results from the funnel plot and Egger regression demonstrated no evidence of publication bias (t(14) = –1.806, p = 0.093; Fig. S9).
Moderator analysis showed that the overall effect of tDCS interventions over F3 on anxiety symptoms was not moderated by stimulation intensity (F(1, 14) = 1.801, p = 0.201) or duration (F(2, 13) = 1.287, p = 0.309).
tDCS effects on other affective outcomes
Table S3 presents the effects of tDCS interventions on various affective outcomes. Notably, emotional functioning was examined in eight studies, with positive effects observed for anodal tDCS over the left DLPFC, specifically on emotion regulation, executive functions related to emotional control, stress tolerance, and attentional bias towards threat-related stimuli. However, no effects were found on measures of emotional functioning without control-related factors.
Risk of bias assessment
Figure S10 visualizes the ROB for each domain of each study. Figure S11 presents an overview of the ROB for all studies. The figures were generated using the Risk-of-bias VISualization [107]. Out of the 56 studies, 29 were judged to have a low ROB, and 27 were judged to have an unclear ROB. No study was judged to have a high ROB. Overall, the studies demonstrated high methodological quality.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis investigating the effects of tDCS interventions on depressive and anxiety symptoms across various clinical conditions. The findings demonstrated that tDCS interventions effectively alleviated depressive symptoms and showed promise in reducing anxiety symptoms across diverse populations. Notably, tDCS interventions targeting the left DLPFC with a 2-mA intensity significantly reduced depressive symptoms, underscoring the left DLPFC as a transdiagnostic neural mechanism underlying depressive symptoms, not only in MDD but also in other conditions. Additionally, tDCS interventions with a 20-min duration were associated with alleviated anxiety symptoms. At the clinical level, these findings inform the development of more personalized interventions based on patients’ comorbidity patterns and diagnostic profiles, thus enhancing treatment efficacy. Overall, these findings represent a crucial advancement in understanding the neuropsychological basis of depressive and anxiety symptoms and inform the development of effective tDCS interventions targeting these symptoms across diverse clinical conditions.
tDCS alleviated depressive symptoms
Neural level
The findings regarding the effects of tDCS on depressive symptoms highlighted the left DLPFC as an important transdiagnostic mechanism underlying depressive symptoms across clinical conditions. This adds to the extant evidence by showing that repeated anodal tDCS over the left DLPFC alleviated depressive symptoms not only in patients diagnosed with MDD but also in patients with other clinical diagnoses (Fig. 2B).
Neuroimaging evidence has supported structural and functional differences in the left DLPFC in patients with depressive symptoms compared to healthy controls [108–111]. The left DLPFC is implicated in the altered emotion regulation in MDD [112]. Abnormal activity in the left DLPFC can lead to an impaired ability to regulate negative emotions or maintain positive emotions, resulting in persistent, more intense negative emotions [110]. Consistently, previous research employing combined tDCS and neuroimaging techniques has shed light on the mechanisms of action underlying the efficacy of tDCS interventions in reducing depressive symptoms. The treatment’s efficacy was found to be achieved through the modulation of DLPFC-involved networks, which includes inducing widespread perfusion changes, generating magnetic fields, and increasing cerebral blood flow at the left DLPFC [23, 113–115]. In addition, tDCS interventions targeting the left DLPFC demonstrated anti-depressant effects by inducing neurostructural changes at the target region [116]. These associations between neuroplasticity-related processes at the left DLPFC and the anti-depressant effect align with the findings regarding altered neurotrophic factors (such as BDNF) in MDD patients [23, 117]. The findings from this meta-analysis not only corroborate these previous studies, emphasizing the critical role of the left DLPFC in MDD but also extend the research by highlighting its importance in patients with diverse clinical diagnoses. This represents a significant step toward elucidating the neural underpinnings of depressive symptoms in various patient populations.
However, the current findings do not provide evidence of the specific neural changes associated with the alleviation of depressive symptoms by tDCS over the left DLPFC. Neuroimaging evidence has suggested activity alterations in both cortical and subcortical regions in MDD patients [118]. Based on existing neuropsychological models related to depressive symptoms, a decreased activity in frontal regions and increased activity in limbic regions may underline the altered emotion regulation in patients with depressive symptoms [119]. It has also been suggested that the imbalance between frontal and limbic regions leads to depressive symptoms [120]. Some evidence puts more emphasis on the frontal regions by suggesting a disrupted functional balance within the DLPFC in MDD patients, for example, hypoactivity in the left DLPFC and hyperactivity in the right DLPFC [121].
Although these pieces of evidence all corroborate the therapeutic efficacy of tDCS over the left DLPFC on depressive symptoms, the root causes of depressive symptoms, especially across disorders, still require examination. tDCS interventions could alleviate depressive symptoms by altering the activity of the frontal regions to exert greater control over the limbic regions, or by altering the connectivity between the frontal and limbic regions. Combining tDCS with neuroimaging techniques in future RCTs would be particularly beneficial to gain a deeper understanding of the core neural mechanisms underlying depressive symptoms in MDD and across other disorders.
Clinical level
The current findings demonstrate significant antidepressant effects of tDCS applied over the left DLPFC with a 2-mA intensity, providing evidence to optimize tDCS protocols not only for MDD but also across various diagnoses. Existing findings regarding the optimal intensity and duration for tDCS interventions have been inconsistent. Earlier evidence has shown that a longer stimulation duration (more than 10 min as compared to a few seconds) and a higher intensity (for example, 1 mA) could lead to longer after-effects [122, 123]. On the other hand, it has been argued that these after-effects depend more on the timing of the stimulation rather than the duration [124]. The findings of this review suggest that, compared to duration, intensity plays a more significant role in the tDCS effects on depressive symptoms, providing valuable insights into the design of treatment protocols.
Specifically, the current meta-analytic result supports that a 2-mA intensity should be strongly considered when designing tDCS protocols to reduce depressive symptoms. Although this finding was derived from participants with various clinical conditions and should be applicable across these conditions, the roles of patients’ individual characteristics and the different phenotypes of patients’ primary diagnoses need further exploration to enhance treatment planning in clinical settings. For example, the patterns observed regarding stimulation parameters suggest that a more intense tDCS protocol may be necessary to demonstrate positive effects in treatment-resistant patients. Among studies that examined depressive symptoms in treatment-resistant MDD, only Sharafi et al. [62] who administered tDCS of a current density of 0.1 mA/cm2 twice daily showed positive effects. Previous research has revealed that treatment-resistant and treatment-sensitive patients with MDD can be differentiated by the hypoconnectivity within the cognitive control network, which involves the DLPFC [125]. This could be the potential reason why depressive symptoms were less likely to be reduced by tDCS in treatment-resistant MDD patients and why a more intense protocol may be required. These findings underscore the significance of comprehending the subtypes of MDD psychopathology and the underlying neural differences to tailor more effective tDCS protocols based on individual characteristics. Additionally, it is crucial to investigate the biopsychosocial predictors of optimal tDCS effects for different clinical populations.
Furthermore, the findings suggest that tDCS may be a potential adjunctive treatment option for depressive symptoms in patients with other primary diagnoses. For instance, individuals diagnosed with schizophrenia may experience secondary depressive symptoms alongside their primary symptoms. If these patients are already receiving medications for their primary symptoms, they might respond more favorably to tDCS compared to traditional pharmacological treatments of depressive symptoms. However, the interaction between pharmacological treatments and tDCS is complex. Some medications, for example, benzodiazepines, have been found to reduce tDCS effects, while anti-depressants have been found to enhance tDCS effects in MDD patients [126]. Thus, for clinical practice, it would be crucial to first investigate the combined effects of tDCS and medications in various patient populations, and then explore the use of tDCS as an add-on treatment for addressing secondary depressive symptoms in these patients. Such research has the potential to enhance treatment outcomes by offering more efficient, safer, and personalized approaches to improve the emotional well-being of diverse patient groups.
tDCS alleviated anxiety symptoms
Neural level
The findings regarding the effects of tDCS on anxiety symptoms suggested that tDCS is a promising tool for alleviating anxiety symptoms in patients with various clinical conditions, as the overall intervention effect was marginally significant. Although the meta-analytic result did not support that stimulating the left DLPFC drove the overall effect on anxiety symptoms, among the included studies that effectively reduced anxiety symptoms, the majority targeted the left DLPFC (Fig. 3A). Previous studies have demonstrated that the hypoactivation of the left DLPFC and hyperactivation of the right DLPFC may contribute to functional abnormalities associated with anxiety symptoms. Anodal tDCS over the left DLPFC has the potential to address this interhemispheric imbalance and subsequently alleviate anxiety symptoms. This imbalance has also been found to be implicated in depressive symptoms as discussed earlier [121]. The finding that active stimulation of the left DLPFC alleviates both symptom categories is consistent with their frequent comorbidity. The involvement of the left DLPFC as a transdiagnostic neural mechanism for both depressive and anxiety symptoms supports existing neuroimaging findings, which suggest that common prefrontal alterations may underlie the clinical similarities between MDD and anxiety disorders [127]. The current findings provide novel evidence that the left DLPFC plays a critical role in both depressive and anxiety symptoms, not only in MDD and anxiety disorders but also across other disorders.
Furthermore, tDCS over the right DLPFC has been shown to reduce anxiety symptoms in patients with BPD [103]. This finding is consistent with the results of a meta-analysis that indicated a reduction in anxiety symptoms by targeting the right DLPFC [128]. However, one study in this review [96], examining tDCS effects on both the left and right DLPFC, found no positive effects on anxiety symptoms in Parkinson’s disease patients. This inconsistency might be explained by differences in focus and methodology: targeted exclusively anxiety disorders and included both TMS and tDCS, whereas this review focused on tDCS and encompassed various diagnoses. This discrepancy highlights the need to further examine the role of the right DLPFC in reducing anxiety symptoms across various patient populations by applying diverse neuromodulatory techniques.
In addition to the DLPFC, studies that have shown positive effects on anxiety symptoms often involved stimulation of the left M1. This observation could be attributed to the fact that many of these studies focused on patients with pain conditions. Previous guidelines on tDCS have recommended anodal tDCS over the left M1 as an effective montage for reducing pain severity associated with pain conditions [27, 28]. It has been speculated that stimulating the M1 could activate neural circuits involving structures associated with the emotional component of pain processing, such as the DLPFC, thereby facilitating the inhibitory control of pain [28, 129]. It is possible that tDCS over the M1 alleviated anxiety symptoms by modulating DLPFC activity. The reduction in anxiety symptoms may be a consequence of the decreased pain severity. Further examination of the neuropsychological changes associated with tDCS over this region is essential for developing effective interventions for patients with comorbid pain and anxiety symptoms.
Compared to depressive symptoms, the findings regarding the neural underpinnings of the impact of tDCS on anxiety symptoms are less consistent. A systematic review of neuroimaging evidence has demonstrated structural or functional alterations in the frontal cortex and limbic regions in both MDD and anxiety disorders [5]. The current review’s findings, indicating that tDCS over the left DLPFC produced greater therapeutic efficacy on depressive symptoms than on anxiety symptoms, offer a novel insight suggesting that alterations in the left DLPFC region may be a more fundamental cause of depressive symptoms than anxiety symptoms. Additionally, anxiety symptoms may exhibit more distinct phenotypes across clinical conditions, making it less likely for tDCS over a specific region to generate an overall therapeutic effect on anxiety symptoms assessed by general scales. Modeling tDCS-induced electric fields in conjunction with neuroimaging techniques would be particularly valuable in understanding the changes in functional connectivity associated with the therapeutic effects of tDCS over different brain regions implicated in anxiety symptoms.
Clinical level
In comparison to the findings concerning depressive symptoms, tDCS interventions have shown less consistent therapeutic effects on anxiety symptoms. The overall effect was marginally significant, and neither intensity nor duration was found to be significant moderators. This finding aligns with the existing evidence-based guidelines [27, 28], which currently do not provide any recommendations for effective tDCS protocols in anxiety disorders. This disparity may be attributed to the fact that there have been fewer RCTs investigating the effects of tDCS in anxiety disorders, in contrast to those conducted for MDD. Additionally, anxiety symptoms are often less comprehensively assessed across different disorders and have less clearly defined neural underpinnings when compared to depressive symptoms. This emphasizes the necessity for larger-scale RCTs to explore the effects of tDCS on anxiety symptoms, as well as the importance of evaluating and treating anxiety symptoms to enhance the emotional well-being and quality of life in heterogeneous patient groups instead of restricting to anxiety disorders. Moreover, it is possible that studies that examined the effects of tDCS on anxiety symptoms adopted the tDCS montages that have shown positive effects on depressive symptoms due to limited evidence of effective protocols specifically on anxiety symptoms. Future trials should consider administering tDCS over other cortical regions implicated in anxiety symptoms, such as the medial PFC [130, 131], to investigate tDCS protocols with potentially greater therapeutic efficacy.
Left DLPFC as a transdiagnostic mechanism
The findings from the current review provide support for the left DLPFC as a transdiagnostic neural mechanism underlying depressive symptoms across various clinical conditions. In addition, tDCS over the left DLPFC has also shown positive effects on other affective outcomes. Notably, tDCS over the left DLPFC specifically improved affective outcomes associated with cognitive control functions. However, no beneficial effects of anodal tDCS over the left DLPFC were observed on emotional recognition, expression, or positive and negative affect. These consistent patterns of results across diverse clinical samples reinforce the distinct role of the left DLPFC in top-down cognitive control functions [132, 133]. Repeated stimulation of this region may alter cognitive control functions, leading to enhanced control of emotional processes and altered attentional bias towards negative stimuli. These improved affective outcomes could potentially lead to the reduction of depressive symptoms. Nevertheless, it is also possible that the improved affective outcomes are downstream effects resulting from the improvement in depressive symptoms. Cognitive control functions may serve as a transdiagnostic mechanism underlying depressive symptoms across different disorders, while further investigation is needed to elucidate the relationships between improved affective outcomes and depressive symptoms, as well as the associated neuropsychological changes.
tDCS as a transdiagnostic intervention
Although tDCS is not an FDA-approved treatment for depression or anxiety, this review found that tDCS is effective in alleviating depressive and anxiety symptoms across clinical conditions, highlighting tDCS as a transdiagnostic intervention for targeting these two symptom categories in various patient populations and the significance of further examining the treatment efficacy of tDCS in future clinical trials.
Considering the frequent co-occurrence of depressive and anxiety symptoms regardless of primary clinical diagnoses, the left DLPFC should be given priority as the target region when designing tDCS interventions to alleviate these symptoms. Moreover, in individuals at risk of developing these symptoms or comorbidity, the left DLPFC should be considered as the target region for preventive interventions. However, although the role of 2-mA-intensity tDCS interventions targeting the left DLPFC in alleviating depressive symptoms has been highlighted, the current findings still lack the specificity needed for clinicians to design more effective treatment plans. Future studies should focus on examining more specific tDCS protocols, such as current density, that demonstrate the highest therapeutic efficacy for addressing these two symptom categories across diverse patient populations. Additionally, future trials can investigate the most effective tDCS protocols for patients exhibiting different comorbidity patterns of depressive and anxiety symptoms, enabling the development of more individualized treatment plans, either as an adjuvant or stand-alone approach.
The current findings also hold significant clinical implications for patients experiencing depressive or anxiety symptoms as secondary symptoms. In cases where patients have various clinical conditions, depressive and anxiety symptoms may not receive adequate attention in their treatment plans for the primary diagnoses. tDCS intervention can serve as an effective add-on treatment to target and alleviate these affective symptoms, complementing the treatment of patients’ primary diagnoses. Exploring effective tDCS protocols for patients with different conditions to address depressive and anxiety symptoms can be particularly valuable, as it offers clinicians and patients more flexible treatment options and the potential for more effective and safer tools to enhance emotional well-being across diverse patient populations.
Limitations and future directions
There are several limitations to consider when evaluating this current review. First, this review only included studies that examined tDCS as a stand-alone intervention, as the primary goal was to evaluate tDCS as the sole intervention for depressive and anxiety symptoms. As existing studies have reported improved clinical benefits when tDCS was combined with other treatments or interventions [126, 134, 135], future reviews should systematically investigate the augmenting effects of tDCS combined with traditional treatments to address these symptoms across conditions. This would be especially beneficial for treatment-resistant patients and those with lower socio-economic status, as tDCS is a cost-effective alternative to other brain stimulation tools and conventional treatments.
Second, this review did not conduct a quantitative analysis of the differential impacts of the timing of clinical assessment, current density, electrode size, and various sham conditions on the treatment effects. This was primarily because the current review aimed to examine the neural underpinnings of the effects of tDCS on depressive and anxiety symptoms and to inform intervention design focused on stimulation intensity and duration. However, these other factors could impact the treatment effects and are important aspects to consider in designing treatment protocols. Future studies are encouraged to quantitatively analyze these parameters to further optimize intervention design for clinical application.
Third, this review focused solely on conventional tDCS. While HD-tDCS provides more precise targeting, it was not examined due to differing stimulation protocols. Conventional tDCS, extensively studied for various applications, aligns well with our research question regarding transdiagnostic therapeutic purposes. However, our findings—that the left DLPFC is a transdiagnostic target and that intensity is crucial for treatment efficacy—could guide future HD-tDCS studies in targeting this region more precisely, potentially enhancing outcomes through more individualized interventions.
Lastly, although the PRISMA guidelines were meticulously adhered to, this review was not pre-registered.
Conclusion
tDCS interventions targeting the left DLPFC consistently alleviate depressive symptoms and show promising effects on anxiety symptoms across various clinical conditions. This supports the role of the left DLPFC as a transdiagnostic neural mechanism underlying these symptoms, extending beyond specific disorders. The findings also highlight the critical role of stimulation intensity, specifically in the effects of tDCS on the left DLPFC, in reducing depressive symptoms. Clinically, this review demonstrates that tDCS is a viable transdiagnostic intervention applicable across clinical conditions for reducing depressive and anxiety symptoms. Tailoring interventions based on patients’ psychological and diagnostic profiles is crucial for enhancing treatment effectiveness.
Supplementary information
Supplementary Materials: Tables and Figures
Supplementary Materials: The PRISMA Checklist
Author contributions
Conceptualization, Nichol ML Wong and Tatia MC Lee; methodology, Nichol ML Wong and Esther Zhiwei Zheng; formal analysis, Nichol ML Wong and Esther Zhiwei Zheng; data curation, Esther Zhiwei Zheng and Angela SY Yang; writing - original draft preparation, Esther Zhiwei Zheng; writing - review and editing, Nichol ML Wong and Tatia MC Lee; supervision, Nichol ML Wong and Tatia MC Lee; funding acquisition, Tatia MC Lee. All authors contributed to and have approved the final manuscript.
Funding
This work was supported by the Hong Kong Research Grant Council General Research Fund [17600522].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Esther Zhiwei Zheng, Nichol M. L. Wong.
Contributor Information
Nichol M. L. Wong, Email: nmlwong@eduhk.hk
Tatia M. C. Lee, Email: tmclee@hku.hk
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-024-03003-w.
References
- 1.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110:585–99. doi: 10.1037/0021-843X.110.4.585. [DOI] [PubMed] [Google Scholar]
- 2.Johansson R, Carlbring P, Heedman Å, Paxling B, Andersson G. Depression, anxiety and their comorbidity in the Swedish general population: point prevalence and the effect on health-related quality of life. PeerJ. 2013;1:e98. doi: 10.7717/peerj.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smits JA, Minhajuddin A, Thase ME, Jarrett RB. Outcomes of acute phase cognitive therapy in outpatients with anxious versus nonanxious depression. Psychother Psychosom. 2012;81:153–60. doi: 10.1159/000334909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kircanski K, LeMoult J, Ordaz S, Gotlib IH. Investigating the nature of co-occurring depression and anxiety: comparing diagnostic and dimensional research approaches. J Affect Disord. 2017;216:123–35. doi: 10.1016/j.jad.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sindermann L, Redlich R, Opel N, Böhnlein J, Dannlowski U, Leehr EJ. Systematic transdiagnostic review of magnetic-resonance imaging results: depression, anxiety disorders and their co-occurrence. J Psychiatr Res. 2021;142:226–39. doi: 10.1016/j.jpsychires.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 6.van Tol M-J. Mood related insights: functional and structural MRI studies in depression and anxiety disorders. Leiden University; 2011.
- 7.Shafran R, Wroe A, Nagra S, Pissaridou E, Coughtrey A. Cognitive behaviour treatment of co-occurring depression and generalised anxiety in routine clinical practice. PLoS ONE. 2018;13:e0201226. doi: 10.1371/journal.pone.0201226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wüsthoff LE, Waal H, Gråwe RW. The effectiveness of integrated treatment in patients with substance use disorders co-occurring with anxiety and/or depression-a group randomized trial. BMC Psychiatry. 2014;14:67. doi: 10.1186/1471-244X-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bystritsky A. Treatment-resistant anxiety disorders. Mol Psychiatry. 2006;11:805–14. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- 10.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 11.Davidson B, Bhattacharya A, Sarica C, Darmani G, Raies N, Chen R, et al. Neuromodulation techniques – From non-invasive brain stimulation to deep brain stimulation. Neurotherapeutics. 2024;21:e00330. doi: 10.1016/j.neurot.2024.e00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–48. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul Basic Transl Clin Res Neuromodulation. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Papakostas GI, Perlis RH, Scalia MJ, Petersen TJ, Fava M. A meta-analysis of early sustained response rates between antidepressants and placebo for the treatment of major depressive disorder. J Clin Psychopharmacol. 2006;26:56–60. doi: 10.1097/01.jcp.0000195042.62724.76. [DOI] [PubMed] [Google Scholar]
- 15.Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2006;2006:Cd002795. doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul Basic Transl Clin Res Neuromodulation. 2016;9:641–61. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Palm U, Hasan A, Strube W, Padberg F. tDCS for the treatment of depression: a comprehensive review. Eur Arch Psychiatry Clin Neurosci. 2016;266:681–94. doi: 10.1007/s00406-016-0674-9. [DOI] [PubMed] [Google Scholar]
- 19.Garcia S, Nalven M, Ault A, Eskenazi MA. tDCS as a treatment for anxiety and related cognitive deficits. Int J Psychophysiol. 2020;158:172–7. doi: 10.1016/j.ijpsycho.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Vergallito A, Gallucci A, Pisoni A, Punzi M, Caselli G, Ruggiero GM, et al. Effectiveness of noninvasive brain stimulation in the treatment of anxiety disorders: a meta-analysis of sham or behaviour-controlled studies. J Psychiatry Neurosci. 2021;46:E592. doi: 10.1503/jpn.210050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W-L, Cai D-B, Sun C-H, Yin F, Goerigk S, Brunoni AR, et al. Adjunctive tDCS for treatment-refractory auditory hallucinations in schizophrenia: a meta-analysis of randomized, double-blinded, sham-controlled studies. Asian J Psychiatry. 2022;73:103100. doi: 10.1016/j.ajp.2022.103100. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier SJ, Cicchetti F. Cellular and molecular mechanisms of action of transcranial direct current stimulation: evidence from in vitro and in vivo models. Int J Neuropsychopharmacol. 2014;18:pyu047. doi: 10.1093/ijnp/pyu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jog MV, Wang DJJ, Narr KL. A review of transcranial direct current stimulation (tDCS) for the individualized treatment of depressive symptoms. Pers Med Psychiatry. 2019;17-18:17–22. doi: 10.1016/j.pmip.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podda MV, Cocco S, Mastrodonato A, Fusco S, Leone L, Barbati SA, et al. Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci Rep. 2016;6:22180. doi: 10.1038/srep22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumpf U, Palm U, Eder J, Ezim H, Stadler M, Burkhardt G, et al. TDCS at home for depressive disorders: an updated systematic review and lessons learned from a prematurely terminated randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci. 2023;273:1403–20. doi: 10.1007/s00406-023-01620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiozawa P, Fregni F, Benseñor IM, Lotufo PA, Berlim MT, Daskalakis JZ, et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol. 2014;17:1443–52. doi: 10.1017/S1461145714000418. [DOI] [PubMed] [Google Scholar]
- 27.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2020;24:256–313. doi: 10.1093/ijnp/pyaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2017;128:56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 29.Razza LB, Palumbo P, Moffa AH, Carvalho AF, Solmi M, Loo CK, et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. 2020;37:594–608. doi: 10.1002/da.23004. [DOI] [PubMed] [Google Scholar]
- 30.Lee HJ, Stein MB. Update on treatments for anxiety-related disorders. Curr Opin Psychiatry. 2023;36:140–5. doi: 10.1097/YCO.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 31.Choi KW, Kim YK, Jeon HJ. Comorbid anxiety and depression: clinical and conceptual consideration and transdiagnostic treatment. Adv Exp Med Biol. 2020;1191:219–35. doi: 10.1007/978-981-32-9705-0_14. [DOI] [PubMed] [Google Scholar]
- 32.Tiller JW. Depression and anxiety. Med J Aust. 2013;199:S28–31. doi: 10.5694/mja12.10628. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Wangqing P, Baima Y, Wang S, Shen Z, Zhou J, et al. Comorbid depressive and anxiety symptoms and their correlates among 93,078 multiethnic adults in Southwest China. Front Public Health. 2021;9:783687. doi: 10.3389/fpubh.2021.783687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hariton E, Locascio JJ. Randomised controlled trials – the gold standard for effectiveness research. BJOG Int J Obstetr Gynaecol. 2018;125:1716–1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodham R, Rimmer RM, Mutz J, Fu CHY. Is tDCS a potential first line treatment for major depression? Int Rev Psychiatry. 2021;33:250–65. doi: 10.1080/09540261.2021.1879030. [DOI] [PubMed] [Google Scholar]
- 36.Mutz J, Edgcumbe DR, Brunoni AR, Fu CHY. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev. 2018;92:291–303. doi: 10.1016/j.neubiorev.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Lam CLM, Peng X, Zhang D, Zhang C, Huang R, et al. Efficacy and acceptability of transcranial direct current stimulation for treating depression: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev. 2021;126:481–90. doi: 10.1016/j.neubiorev.2021.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Krogh HB, Storebø OJ, Faltinsen E, Todorovac A, Ydedahl-Jensen E, Magnusson FL, et al. Methodological advantages and disadvantages of parallel and crossover randomised clinical trials on methylphenidate for attention deficit hyperactivity disorder: a systematic review and meta-analyses. BMJ Open. 2019;9:e026478. doi: 10.1136/bmjopen-2018-026478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parlikar R, Vanteemar SS, Shivakumar V, Narayanaswamy CJ, Rao PN, Ganesan V. High definition transcranial direct current stimulation (HD-tDCS): a systematic review on the treatment of neuropsychiatric disorders. Asian J Psychiatr. 2021;56:102542. doi: 10.1016/j.ajp.2020.102542. [DOI] [PubMed] [Google Scholar]
- 40.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD,, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 42.Cheung MWL. Modeling dependent effect sizes with three-level meta-analyses: a structural equation modeling approach. Psychol methods. 2014;19:211–29. doi: 10.1037/a0032968. [DOI] [PubMed] [Google Scholar]
- 43.Hox J, Hutchison D. Multilevel analysis: techniques and applications. vol. 452003, pp 117-118 (Lawrence Erlbaum Associates 2002).
- 44.Assink M, Wibbelink CJM. Fitting three-level meta-analytic models in R: a step-by-step tutorial. Tutor Quant Methods Psychol. 2016;12:154–74. doi: 10.20982/tqmp.12.3.p154. [DOI] [Google Scholar]
- 45.Van den Noortgate W, López-López JA, Marín-Martínez F, Sánchez-Meca J. Three-level meta-analysis of dependent effect sizes. Behav Res Methods. 2013;45:576–94. doi: 10.3758/s13428-012-0261-6. [DOI] [PubMed] [Google Scholar]
- 46.Van den Noortgate W, López-López JA, Marín-Martínez F, Sánchez-Meca J. Meta-analysis of multiple outcomes: a multilevel approach. Behav Res Methods. 2015;47:1274–94. doi: 10.3758/s13428-014-0527-2. [DOI] [PubMed] [Google Scholar]
- 47.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 49.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CHY, Young AH. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 2019;364:l1079. doi: 10.1136/bmj.l1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennabi D, Nicolier M, Monnin J, Tio G, Pazart L, Vandel P, et al. Pilot study of feasibility of the effect of treatment with tDCS in patients suffering from treatment-resistant depression treated with escitalopram. Clin Neurophysiol. 2015;126:1185–9. doi: 10.1016/j.clinph.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 52.Blumberger D, Tran L, Fitzgerald P, Hoy K, Daskalakis Z. A randomized double-blind sham-controlled study of transcranial direct current stimulation for treatment-resistant major depression. Front Psychiatry. 2012;3:74. doi: 10.3389/fpsyt.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11:249–54. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fregni F, Boggio PS, Nitsche MA, Marcolin MA, Rigonatti SP, Pascual-Leone A. Treatment of major depression with transcranial direct current stimulation. Bipolar Disord. 2006;8:203–4. doi: 10.1111/j.1399-5618.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 55.Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A. Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety. 2006;23:482–4. doi: 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- 56.Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry. 2012;200:52–59. doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- 57.Loo CK, Husain MM, McDonald WM, Aaronson S, O’Reardon JP, Alonzo A, et al. International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimul Basic Transl Clin Res Neuromodulation. 2018;11:125–33. doi: 10.1016/j.brs.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Loo CK, Sachdev P, Martin D, Pigot M, Alonzo A, Malhi GS, et al. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int J Neuropsychopharmacol. 2010;13:61–69. doi: 10.1017/S1461145709990411. [DOI] [PubMed] [Google Scholar]
- 59.Salehinejad M, Rostami R, Ghanavati E. Transcranial direct current stimulation of dorsolateral prefrontal cortex of major depression: improving visual working memory, reducing depressive symptoms. NeuroRegulation. 2015;2:37–49. doi: 10.15540/nr.2.1.37. [DOI] [Google Scholar]
- 60.Salehinejad MA, Ghanavai E, Rostami R, Nejati V. Cognitive control dysfunction in emotion dysregulation and psychopathology of major depression (MD): evidence from transcranial brain stimulation of the dorsolateral prefrontal cortex (DLPFC) J Affect Disord. 2017;210:241–8. doi: 10.1016/j.jad.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 61.Sampaio-Junior B, Tortella G, Borrione L, Moffa AH, Machado-Vieira R, Cretaz E, et al. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:158–66. doi: 10.1001/jamapsychiatry.2017.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharafi E, Taghva A, Arbabi M, Dadarkhah A, Ghaderi J. Transcranial direct current stimulation for treatment-resistant major depression: a double-blind randomized sham-controlled trial. Clin EEG Neurosci. 2019;50:375–82. doi: 10.1177/1550059419863209. [DOI] [PubMed] [Google Scholar]
- 63.Valiengo LCL, Goulart AC, de Oliveira JF, Benseñor IM, Lotufo PA, Brunoni AR. Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. J Neurol Neurosurg Psychiatry. 2017;88:170–5. doi: 10.1136/jnnp-2016-314075. [DOI] [PubMed] [Google Scholar]
- 64.Vigod SN, Murphy KE, Dennis C-L, Oberlander TF, Ray JG, Daskalakis ZJ, et al. Transcranial direct current stimulation (tDCS) for depression in pregnancy: a pilot randomized controlled trial. Brain Stimul Basic Transl Clin Res Neuromodulation. 2019;12:1475–83. doi: 10.1016/j.brs.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Wu C, Lyu D, Wang F, Huang Q, Yang W, et al. Comparison of 60-minute vs 30-minute transcranial direct current stimulation (tDCS) in major depressive disorder: effects on depression suicidal ideation and anxiety. Psychiatry Res. 2023;330:115556. doi: 10.1016/j.psychres.2023.115556. [DOI] [PubMed] [Google Scholar]
- 66.Huang H, Chen Y, Kong S, Zhang M, Wu C, Lyu D, et al. Targeting right orbitofrontal cortex (OFC) with transcranial direct current stimulation (tDCS) can improve cognitive executive function in a major depressive episode, but not depressive mood: a double-blind randomized controlled pilot trial. J Psychiatr Res. 2023;168:108–17. doi: 10.1016/j.jpsychires.2023.10.016. [DOI] [Google Scholar]
- 67.Woodham RD, Selvaraj S, Lajmi N, Hobday H, Sheehan G, Ghazi-Noori A-R, et al. Home-based transcranial direct current stimulation RCT in major depression. 2023: https://www.medrxiv.org/content/10.1101/2023.11.27.23299059v1.
- 68.Ahmadizadeh MJ, Rezaei M, Fitzgerald PB. Transcranial direct current stimulation (tDCS) for post-traumatic stress disorder (PTSD): a randomized, double-blinded, controlled trial. Brain Res Bull. 2019;153:273–8. doi: 10.1016/j.brainresbull.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Aksu S, Soyata AZ, Mursalova Z, Eskicioğlu G, Tükel R. Transcranial direct current stimulation does not improve clinical and neurophysiological outcomes in panic disorder: a randomized sham-controlled trial. Psychiatry Clin Neurosci. 2022;76:384–92. doi: 10.1111/pcn.13378. [DOI] [PubMed] [Google Scholar]
- 70.de Lima AL, Braga FMA, da Costa RMM, Gomes EP, Brunoni AR, Pegado R. Transcranial direct current stimulation for the treatment of generalized anxiety disorder: a randomized clinical trial. J Affect Disord. 2019;259:31–37. doi: 10.1016/j.jad.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 71.Jafari E, Alizadehgoradel J, Pourmohseni Koluri F, Nikoozadehkordmirza E, Refahi M, Taherifard M, et al. Intensified electrical stimulation targeting lateral and medial prefrontal cortices for the treatment of social anxiety disorder: a randomized, double-blind, parallel-group, dose-comparison study. Brain Stimul Basic Transl Clin Res Neuromodulation. 2021;14:974–86. doi: 10.1016/j.brs.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Newstead S, Young H, Benton D, Jiga-Boy G, Andrade Sienz ML, Clement RM, et al. Acute and repetitive fronto-cerebellar tDCS stimulation improves mood in non-depressed participants. Exp Brain Res. 2018;236:83–97. doi: 10.1007/s00221-017-5109-y. [DOI] [PubMed] [Google Scholar]
- 73.Silva RdMFD, Brunoni AR, Goerigk S, Batistuzzo MC, Costa DLdC, Diniz JB, et al. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for obsessive-compulsive disorder: a randomized, sham-controlled trial. Neuropsychopharmacology. 2021;46:1028–34. doi: 10.1038/s41386-020-00928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeon D-W, Jung D-U, Kim S-J, Shim J-C, Moon J-J, Seo Y-S, et al. Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: a double-blind 12-week study. Schizophr Res. 2018;197:378–85. doi: 10.1016/j.schres.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Mondino M, Jardri R, Suaud-Chagny M-F, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull. 2016;42:318–26. doi: 10.1093/schbul/sbv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palm U, Keeser D, Hasan A, Kupka MJ, Blautzik J, Sarubin N, et al. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr Bull. 2016;42:1253–61. doi: 10.1093/schbul/sbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valiengo LdCL, Goerigk S, Gordon PC, Padberg F, Serpa MH, Koebe S, et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77:121–9. doi: 10.1001/jamapsychiatry.2019.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fitzgerald PB, McQueen S, Daskalakis ZJ, Hoy KE. A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul Basic Transl Clin Res Neuromodulation. 2014;7:813–6. doi: 10.1016/j.brs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 79.Smith RC, Boules S, Mattiuz S, Youssef M, Tobe RH, Sershen H, et al. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr Res. 2015;168:260–6. doi: 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny M-F, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169:719–24. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]
- 81.Koops S, Blom JD, Bouachmir O, Slot MI, Neggers B, Sommer IE. Treating auditory hallucinations with transcranial direct current stimulation in a double-blind, randomized trial. Schizophr Res. 2018;201:329–36. doi: 10.1016/j.schres.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 82.Lisoni J, Nibbio G, Baldacci G, Zucchetti A, Cicale A, Zardini D, et al. Improving depressive symptoms in patients with schizophrenia using bilateral bipolar-nonbalanced prefrontal tDCS: results from a double-blind sham-controlled trial. J Affect Disord. 2024;349:165–75. doi: 10.1016/j.jad.2024.01.050. [DOI] [PubMed] [Google Scholar]
- 83.Borckardt JJ, Reeves ST, Robinson SM, May JT, Epperson TI, Gunselman RJ, et al. Transcranial direct current stimulation (tDCS) reduces postsurgical opioid consumption in total knee arthroplasty (TKA) Clin J Pain. 2013;29:925–8. doi: 10.1097/AJP.0b013e31827e32be. [DOI] [PubMed] [Google Scholar]
- 84.Dutra L, Pegado R, Silva LK, da Silva Dantas H, Câmara HA, Silva-Filho EM, et al. Modulating anxiety and functional capacity with anodal tDCS over the left dorsolateral prefrontal cortex in primary dysmenorrhea. Int J Womens Health. 2020;12:243–51. doi: 10.2147/IJWH.S226501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fregni, Gimenes F, Valle R, Ferreira MJL AC, Rocha RR, Natalle L, et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006;54:3988–98. doi: 10.1002/art.22195. [DOI] [PubMed] [Google Scholar]
- 86.Khedr EM, Omran EAH, Ismail NM, El-Hammady DH, Goma SH, Kotb H, et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimul Basic Transl Clin Res Neuromodulation. 2017;10:893–901. doi: 10.1016/j.brs.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 87.Mariano TY, Burgess FW, Bowker M, Kirschner J, van’t Wout-Frank M, Jones RN, et al. Transcranial direct current stimulation for affective symptoms and functioning in chronic low back pain: a pilot double-blinded, randomized, placebo-controlled trial. Pain Med. 2019;20:1166–77. doi: 10.1093/pm/pny188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pegado R, Silva LK, da Silva Dantas H, Andrade Câmara H, Andrade Mescouto K, Silva-Filho EM, et al. Effects of transcranial direct current stimulation for treatment of primary dysmenorrhea: preliminary results of a randomized sham-controlled trial. Pain Med. 2020;21:3615–23. doi: 10.1093/pm/pnz202. [DOI] [PubMed] [Google Scholar]
- 89.Quintiliano A, Bikson M, Oehmen T, Pegado R, Kirsztajn GM. Transcranial direct current stimulation (tDCS): pain management in end-stage renal disease - report of an early randomized controlled trial. J Pain Symptom Manag. 2022;64:234–243.e231. doi: 10.1016/j.jpainsymman.2022.05.018. [DOI] [PubMed] [Google Scholar]
- 90.Samartin-Veiga N, Pidal-Miranda M, González-Villar AJ, Bradley C, Garcia-Larrea L, O’Brien AT, et al. Transcranial direct current stimulation of 3 cortical targets is no more effective than placebo as treatment for fibromyalgia: a double-blind sham-controlled clinical trial. PAIN. 2022;163:e850–e861. doi: 10.1097/j.pain.0000000000002493. [DOI] [PubMed] [Google Scholar]
- 91.da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, et al. Behavioral effects of transcranial Direct Current Stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris. 2013;107:493–502. doi: 10.1016/j.jphysparis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 92.Shahbabaie A, Hatami J, Farhoudian A, Ekhtiari H, Khatibi A, Nitsche MA. Optimizing electrode montages of transcranial direct current stimulation for attentional bias modification in early abstinent methamphetamine users. Front Pharmacol. 2018;9:907. doi: 10.3389/fphar.2018.00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verveer I, Remmerswaal D, Jongerling J, Van Der Veen FM, Franken IHA. No effect of repetitive tDCS on daily smoking behaviour in light smokers: a placebo controlled EMA study. PLoS ONE. 2020;15:e0233414. doi: 10.1371/journal.pone.0233414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Acler M, Bocci T, Valenti D, Turri M, Priori A, Bertolasi L. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor Neurol Neurosci. 2013;31:661–8. doi: 10.3233/RNN-130321. [DOI] [PubMed] [Google Scholar]
- 95.Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, et al. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:1105–11. doi: 10.1136/jnnp.2009.202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doruk D, Gray Z, Bravo GL, Pascual-Leone A, Fregni F. Effects of tDCS on executive function in Parkinson’s disease. Neurosci Lett. 2014;582:27–31. doi: 10.1016/j.neulet.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 97.Liu A, Bryant A, Jefferson A, Friedman D, Minhas P, Barnard S, et al. Exploring the efficacy of a 5-day course of transcranial direct current stimulation (TDCS) on depression and memory function in patients with well-controlled temporal lobe epilepsy. Epilepsy Behav. 2016;55:11–20. doi: 10.1016/j.yebeh.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 98.Maas RPPWM, Teerenstra S, Toni I, Klockgether T, Schutter DJLG, van de Warrenburg BPC. Cerebellar transcranial direct current stimulation in spinocerebellar ataxia type 3: a randomized, double-blind, sham-controlled trial. Neurotherapeutics. 2022;19:1259–72. doi: 10.1007/s13311-022-01231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pinto EF, Gupta A, Kulkarni GB, Andrade C. A randomized, double-blind, sham-controlled study of transcranial direct current stimulation as an augmentation intervention for the attenuation of motor deficits in patients with stroke. J ECT. 2021;37:281–90. doi: 10.1097/YCT.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 100.Barham H, Büyükgök D, Aksu S, Soyata AZ, Bulut G, Eskicioğlu G, et al. Evidence for modulation of planning and working memory capacities by transcranial direct current stimulation in a sample of adults with attention deficit hyperactivity disorder. Neurosci Lett. 2022;790:136883. doi: 10.1016/j.neulet.2022.136883. [DOI] [PubMed] [Google Scholar]
- 101.Leffa DT, Grevet EH, Bau CHD, Schneider M, Ferrazza CP, da Silva RF, et al. Transcranial direct current stimulation vs sham for the treatment of inattention in adults with attention-deficit/hyperactivity disorder: the TUNED randomized clinical trial. JAMA Psychiatry. 2022;79:847–56. doi: 10.1001/jamapsychiatry.2022.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zemestani M, Hoseinpanahi O, Salehinejad MA, Nitsche MA. The impact of prefrontal transcranial direct current stimulation (tDCS) on theory of mind, emotion regulation and emotional-behavioral functions in children with autism disorder: A randomized, sham-controlled, and parallel-group study. Autism Res. 2022;15:1985–2003. doi: 10.1002/aur.2803. [DOI] [PubMed] [Google Scholar]
- 103.Lisoni J, Miotto P, Barlati S, Calza S, Crescini A, Deste G, et al. Change in core symptoms of borderline personality disorder by tDCS: a pilot study. Psychiatry Res. 2020;291:113261. doi: 10.1016/j.psychres.2020.113261. [DOI] [PubMed] [Google Scholar]
- 104.Molavi P, Aziziaram S, Basharpoor S, Atadokht A, Nitsche MA, Salehinejad MA. Repeated transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93–102. doi: 10.1016/j.jad.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 105.Pinto A, Piva SR, Vieira A, Gomes S, Rocha AP, Tavares DRB, et al. Transcranial direct current stimulation for fatigue in patients with Sjogren’s syndrome: a randomized, double-blind pilot study. Brain Stimul. 2021;14:141–51. doi: 10.1016/j.brs.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 106.Klírová M, Adamová A, Biačková N, Laskov O, Renková V, Stuchlíková Z, et al. Transcranial direct current stimulation (tDCS) in the treatment of neuropsychiatric symptoms of long COVID. Sci Rep. 2024;14:2193–2193. doi: 10.1038/s41598-024-52763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 108.Arnone D, Job D, Selvaraj S, Abe O, Amico F, Cheng Y, et al. Computational meta-analysis of statistical parametric maps in major depression. Hum Brain Mapp. 2016;37:1393–404. doi: 10.1002/hbm.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–76. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 110.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel H-J, et al. Cortical inefficiency in patients with unipolar depression: an event-related fMRI study with the Stroop task. Biol Psychiatry. 2006;59:958–65. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 112.Li S, Chen J, Gao K, Xu F, Zhang D. Excitatory brain stimulation over the left dorsolateral prefrontal cortex enhances voluntary distraction in depressed patients. Psychol Med. 2023;53:6646–55. doi: 10.1017/S0033291723000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jog MS, Kim E, Anderson C, Kubicki A, Kayathi R, Jann K, et al. In-vivo imaging of targeting and modulation of depression-relevant circuitry by transcranial direct current stimulation: a randomized clinical trial. Transl Psychiatry. 2021;11:138–138. doi: 10.1038/s41398-021-01264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leaver AM, Gonzalez S, Vasavada M, Kubicki A, Jog M, Wang DJJ, et al. Modulation of brain networks during MR-compatible transcranial direct current stimulation. NeuroImage. 2022;250:118874–118874. doi: 10.1016/j.neuroimage.2022.118874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, et al. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci. 2013;33:11425–31. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jog MA, Anderson C, Kubicki A, Boucher M, Leaver A, Hellemann G, et al. Transcranial direct current stimulation (tDCS) in depression induces structural plasticity. Sci Rep. 2023;13:2841–2841. doi: 10.1038/s41598-023-29792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36:2589–602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:736–736. doi: 10.1002/hbm.20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 121.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/S0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 122.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–901. doi: 10.1212/WNL.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 124.Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul Basic Transl Clin Res Neuromodulation. 2013;6:424–32. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 125.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brunoni AR, Ferrucci R, Bortolomasi M, Scelzo E, Boggio PS, Fregni F, et al. Interactions between transcranial direct current stimulation (tDCS) and pharmacological interventions in the major depressive episode: findings from a naturalistic study. Eur Psychiatry. 2013;28:356–61. doi: 10.1016/j.eurpsy.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 127.Maggioni E, Delvecchio G, Grottaroli M, Garzitto M, Piccin S, Bonivento C, et al. Common and different neural markers in major depression and anxiety disorders: a pilot structural magnetic resonance imaging study. Psychiatry Res Neuroimaging. 2019;290:42–50. doi: 10.1016/j.pscychresns.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 128.Gay F, Singier A, Aouizerate B, Salvo F, Bienvenu TCM. Neuromodulation treatments of pathological anxiety in anxiety disorders, stressor-related disorders, and major depressive disorder: a dimensional systematic review and meta-analysis. Front Psychiatry. 2022;13:910897. doi: 10.3389/fpsyt.2022.910897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saldanha JS, Zortea M, Deliberali CB, Nitsche MA, Kuo M-F, Torres ILdS, et al. Impact of age on tDCS effects on pain threshold and working memory: results of a proof of concept cross-over randomized controlled study. Front Aging Neurosci. 2020;12:189. doi: 10.3389/fnagi.2020.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–66. doi: 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Papasideris M, Ayaz H, Hall PA. Medial prefrontal brain activity correlates with emerging symptoms of anxiety and depression in late adolescence: a fNIRS study. Dev Psychobiol. 2021;63:e22199. doi: 10.1002/dev.22199. [DOI] [PubMed] [Google Scholar]
- 132.Mondino M, Thiffault F, Fecteau S. Does non-invasive brain stimulation applied over the dorsolateral prefrontal cortex non-specifically influence mood and emotional processing in healthy individuals? Front Cell Neurosci. 2015;9:399. doi: 10.3389/fncel.2015.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nejati V, Majdi R, Salehinejad MA, Nitsche MA. The role of dorsolateral and ventromedial prefrontal cortex in the processing of emotional dimensions. Sci Rep. 2021;11:1971. doi: 10.1038/s41598-021-81454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brunoni AR, Júnior RF, Kemp AH, Lotufo PA, Benseñor IM, Fregni F. Differential improvement in depressive symptoms for tDCS alone and combined with pharmacotherapy: an exploratory analysis from the sertraline vs. electrical current therapy for treating depression clinical study. Int J Neuropsychopharmacol. 2014;17:53–61. doi: 10.1017/S1461145713001065. [DOI] [PubMed] [Google Scholar]
- 135.Li C, Chen Y, Tu S, Lin J, Lin Y, Xu S, et al. Dual-tDCS combined with sensorimotor training promotes upper limb function in subacute stroke patients: a randomized, double-blinded, sham-controlled study. CNS Neurosci Ther. 2023;30:e14530–e14530. doi: 10.1111/cns.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials: Tables and Figures
Supplementary Materials: The PRISMA Checklist