Abstract
Ischemia-reperfusion injury (IRI) is a cause of acute kidney injury in patients after renal transplantation and leads to high morbidity and mortality. Damaged kidney resident cells release cytokines and chemokines, which rapidly recruit leukocytes. Fibronectin (FN-1) contributes to immune cell migration, adhesion and growth in inflamed tissues. CCAAT/enhancer-binding protein delta is responsive to inflammatory cytokines and stresses and plays functional roles in cell motility, extracellular matrix production and immune responses. We found that the expression of CCAAT/enhancer-binding protein delta was increased in renal epithelial cells in IRI mice compared with sham mice. Following IRI, the colocalization of FN-1 with the macrophage marker F4/80 was increased in renal injury model wild-type mice but was significantly attenuated in Cebpd-deficient mice. Inactivation of CEBPD can repress hypoxia-induced FN-1 expression in HK-2 cells. Moreover, the inactivation of CEBPD and FN-1 also reduces macrophage accumulation in HK-2 cells. These findings suggest that the involvement of CEBPD in macrophage accumulation through the activation of FN-1 expression and the inhibition of CEBPD can protect against renal IRI.
Subject terms: Extracellular matrix, Genetic engineering, Apoptosis
Introduction
Ischemia-reperfusion injury (IRI) is a detrimental condition for which physicians must develop strategies to attenuate cell damage and preserve organ function. Renal IRI, which is an unavoidable consequence of renal transplantation due to blood flow occlusion, often leads to acute kidney injury in transplant recipients, thus contributing substantially to morbidity and mortality [1, 2]. Inflammatory responses affect the quality and survival of renal allografts [3]. Although the complexity of the pathophysiology and detailed mechanisms of IRI continue to be recognized, strategies for preventing the consequences of IRI remain challenging. Therefore, the dissection of critical factors related to IRI is important for improving renal transplant outcomes.
IRI results in an inflammatory response during kidney procurement, with underlying factors that involve hypoxia, leukocyte extravasation and cell death. Recent studies have demonstrated that injured renal tubular epithelial cells are responsive to external and internal stresses and contribute to the upregulation of proinflammatory gene expression [4]. In addition, several tissue IRI animal models have been used to study macrophage-epithelial interactions (MEIs) [5–9]. In response to damaged or affected cells, such as epithelial or dendritic cells, macrophage infiltration is responsive to the production of inflammatory mediators and plays a crucial role in both innate and adaptive immune responses in the kidneys following ischemia. However, the details of the regulation and relationships among protein molecules and cellular phenomena in renal MEI during IRI remain less well characterized.
The adhesion of leukocytes to the vascular endothelium is a hallmark of the inflammatory response. The original leukocyte adhesion cascade is initiated through integrin-mediated arrest, selectin-dependent rolling, and chemokine-triggered activation. Leukocyte arrest is instigated by chemokines or similar chemoattractants, leading to the binding of leukocyte integrins to intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) expressed on endothelial cells, thereby facilitating their adhesion [10]. Additionally, several studies have indicated that fibronectin (FN-1), a crucial component of the extracellular matrix (ECM), plays a significant role in the adhesion of various cell types. The synthesis or absence of FN-1 can influence cell adhesion [11–14].
The transcription factor CCAAT/enhancer-binding protein delta (CEBPD) belongs to the CCAAT/enhancer-binding protein family. It plays functional roles in cell differentiation, motility, growth arrest, cell death, metabolism, ECM production and immune responses [15–18]. In addition to highly conserved protein sequences, the critical motifs involved in transcriptional regulation are almost identical between the human CEBPD and mouse Cebpd genes [19]. Inflammation, including macrophage accumulation and activation marked by the release of cytokines, has been implicated in initiating renal IRI. The role of the ECM in modulating immune cell migration into inflamed tissues has been previously neglected. Recent studies have suggested that ECM fragments are upregulated during inflammation and consequently perpetuate inflammatory responses, including the regulation of macrophage adhesion [20–22]. The expression and regulation of ECM components, such as FN-1 and laminin, modulate macrophage adhesion and play important roles in inflammation [23, 24]. A recent study showed that CEBPD contributes to the transcriptional activation of the FN1 gene in glioblastoma under hypoxia [25]. However, whether the regulation of FN1 gene transcription is responsive to CEBPD in epithelial cells and its consequent effect on IRI tissue remain open questions.
Results
Cebpd transcripts are significantly upregulated in kidney transplantation rats and a mouse model of kidney ischemia-reperfusion
Transcriptional activation plays a critical and vital role during the initiation and sequential response of various acute and chronic inflammatory diseases. To explore the involvement of C/EBPs in renal transplantation and injury, the expression of C/EBPs was assessed using the GEO database GSE5104 (n = 12). In contrast to the Cebpa and Cebpg transcripts, the Cebpd transcript was significantly increased in the kidney isografts of the rats after kidney transplantation (Fig. 1A). Moreover, according to the GEO database GSE182793 (n = 12), among the various C/EBP family members, only Cebpd was upregulated in mouse kidneys after IRI (Fig. 1B). Although there are differences between the samples in the database and the experimental model that was used in this study, it is still implied that CEBPD is a significant and common effector in kidney IRI.
Fig. 1. CEBPD is significantly upregulated in a kidney transplant rat model and a kidney ischemia-reperfusion mouse model.
A The relative mRNAs of Cebpd, Cebpa and Cebpg were extracted from the GEO (GSE5104) database. Comparing the before and after kidney transplantation (K. T.), the Cebpd, but not Cebpa and Cebpg, mRNA was highly increased. B The relative mRNAs of the indicated C/EBP family members from the GEO (GSE182793) database. Comparing the control and ischemia-reperfusion groups, the Cebpd, but not the rest of the C/EBP family members, mRNAs were highly increased (Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001).
Cebpd expression is increased in a U-IRI mouse model
To address the involvement and contribution of CEBPD in renal injury, a U-IRI mouse model was established with a midline incision, thus allowing for ischemia-reperfusion of the left kidney and simultaneous right nephrectomy to mimic human kidney transplantation. In U-IRI mice, Cebpd signals were increased and overlapped with those of the epithelial cell marker E-cadherin, which indicated that Cebpd was primarily expressed in renal tubular epithelial cells, especially in necrotic epithelial cells (Fig. 2A, yellow arrow). Compared with other tubular epithelial injury features, including loss of the brush border (epithelial simplification), the expression of Cebpd was associated with the severity of necrosis (loss of nuclei) in epithelial cells. Moreover, the fluorescence intensity of Cebpd was quantified and the results demonstrated that the expression of Cebpd was increased in U-IRI mice compared with sham mice (Fig. 2B). In addition to immunofluorescence, immunohistochemical staining also showed that the expression of Cebpd was increased in U-IRI mice (Fig. 2A & Supplementary Fig. 1). These results imply that epithelial Cebpd plays a potential role in renal IRI.
Fig. 2. CEBPD expression is increased in a U-IRI mouse model.
A Cebpd expression in U-IRI mice. Tubular epithelial cells were recognized with E-cadherin antibodies (green); CEBPD was recognized with specific CEBPD antibodies (red). The dead epithelial cells are marked with yellow arrows. Images were taken with a fluorescence microscope at a magnification of 200×. (n = 3 for each group). B Cebpd is mainly induced in the kidney epithelial cells of U-IRI mice following the quantification of fluorescence intensity using ImageJ software (Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001).
Cebpd-deficient mice exhibit attenuated U-IRI with reduced inflammatory cytokine release
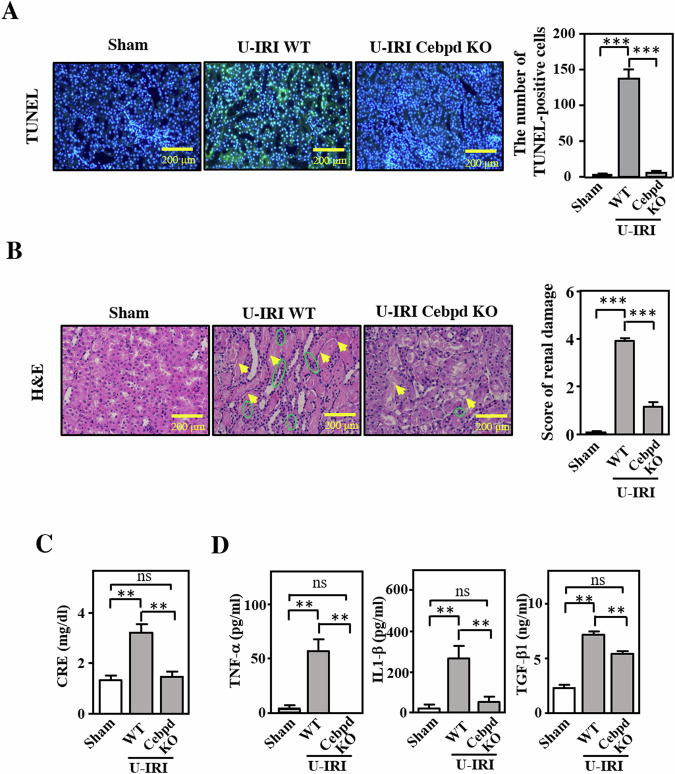
To test and address the contribution of Cebpd to kidney ischemia-reperfusion injury, U-IRI was induced in Cebpd-deficient (Cebpd−/−) and wild-type (WT) mice. The apoptotic effect in the U-IRI kidney was examined using a TUNEL assay. The findings showed a reduction in apoptosis in Cebpd-deficient mice compared to wild-type mice (Fig. 3A). Hematoxylin and eosin (H&E) staining demonstrated that the regions of tissue injury (marked by the yellow arrow) and immune cell infiltration (marked by the green circle) were increased after U-IRI treatment (Fig. 3B). Moreover, as measured via H&E staining, renal damage was ameliorated in Cebpd-deficient mice (Fig. 3B). Moreover, creatinine (CRE) levels were significantly reduced in U-IRI-treated Cebpd-deficient mice (Fig. 3C). As mentioned above, CEBPD has been suggested to upregulate inflammation- and ECM production-related genes, and renal parenchymal cells have the potential to produce various types of proinflammatory cytokines in response to IRI. However, gene regulation in response to CEBPD in epithelial cells remains elusive. Triggered by IRI, many cytokines, including TNF-α, IL-1β and TGF-β1, have been suggested to participate in inflammatory responses [26]. The levels of TNF-α, IL-1β and TGF-β1 in mouse plasma were measured and the results showed that the levels of TNF-α, IL-1β and TGF-β1 were dramatically increased in U-IRI-treated mice but were significantly attenuated in U-IRI-treated Cebpd-deficient mice (Fig. 3D). These results suggest that inactivation of Cebpd/CEBPD can attenuate inflammatory responses in the kidney.
Fig. 3. Cebpd-deficient mice exhibit attenuated IRI with reduced inflammatory cytokine release and FN-1 expression.
A The apoptotic effect on the kidneys of U-IRI mice. A TUNEL assay was performed to measure the apoptosis of kidney cells. The images were taken using fluorescence microscopy. The number of apoptotic cells was measured by counting the number of double -positive TUNEL (green) and DAPI (blue) cells. (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). B Association between kidney injury and immune cell infiltration in U-IRI Cebpd wild-type (WT) and -deficient (Cebpd KO) mice. H&E staining was performed to demonstrate the cell morphology and types in the kidneys of U-IRI mice. The yellow arrow indicates acute tubular injury; the green circle shows the infiltrated immune cells. The renal damage score was quantified via H&E staining and renal histopathology. (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). C The loss of Cebpd attenuates the U-IRI-induced increase in creatinine levels. The creatinine levels in plasma samples harvested from U-IRI-treated Cebpd-WT and -deficient mice (n = 6 for each group) (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). D The levels of inflammatory indicators were attenuated in U-IRI-induced kidney injury. The levels of the inflammatory factors TNF-α, IL-1β and TGF-β1 in U-IRI-treated Cebpd-WT and -deficient mice were measured by ELISA (n = 6 for each group) (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001).
The colocalization of the macrophage marker F4/80 with FN-1 is diminished in the kidneys of Cebpd-deficient mice following U-IRI
Our previous study showed that CEBPD contributes to ECM production in spinal cord injury and pancreatic cancer [27, 28]. FN-1 is an ECM component that induces and promotes macrophage migration and adhesion [13, 29]. To assess whether CEBPD contributes to FN-1 production in kidney epithelial cells, we also examined FN-1 expression in the kidneys of U-IRI mice. Compared with that in U-IRI-treated mice, the expression of FN-1 was significantly attenuated in U-IRI-treated Cebpd-deficient WT mice (Fig. 4A). As shown in Fig. 3B, reduced immune cell infiltration was observed in U-IRI-treated Cebpd-deficient mice. Moreover, an increase in F4/80-positive immune cells was observed in the kidneys of U-IRI-treated WT mice but decreased in those of U-IRI-treated Cebpd-deficient mice (Fig. 4B, C). Loss of macrophage accumulation surrounding the necrotic tubules in response to U-IRI in Cebpd-deficient mice suggests that Cebpd contributes to macrophage accumulation. To further verify whether Cebpd affects macrophage accumulation in renal IRI, an immunofluorescence assay was performed, and the results showed that, compared with that in U-IRI-treated WT mice, decreased FN-1 deposition in the kidneys of Cebpd-deficient mice was observed following U-IRI surgery (Fig. 4B, D). Importantly, colocalized F4/80 and FN-1 signals were mainly observed in renal tubular epithelial cells from U-IRI kidneys, thus suggesting that macrophage accumulation is associated with FN-1 expression in epithelial cells. In addition, neutrophil infiltration can be observed in renal IRI [30]. Our immunofluorescence staining demonstrated that neutrophil accumulation was also diminished in U-IRI-treated Cebpd-deficient mice, but the signals of the neutrophil marker Ly6G did not colocalize with epithelial FN-1 during renal injury (Supplementary Fig. 2). These results suggested that Cebpd-mediated FN-1 expression in epithelial cells specifically affects macrophage, but not neutrophil, accumulation.
Fig. 4. F4/80 and FN-1 colocalization was reduced in the kidneys of Cebpd-deficient mice following U-IRI.
A FN-1 levels are attenuated in the kidneys of Cebpd-deficient mice following U-IRI administration. FN-1 expression was examined via Western blotting and quantified by statistical analysis. (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). B The number of macrophages surrounding epithelial cells was attenuated in the kidneys of U-IRI Cebpd-deficient mice. The macrophages were labeled with F4/80 (red) and FN-1 was detected using a specific anti-FN-1 antibody (green). The images were taken with a fluorescence microscope and magnified at 200×. C The fluorescence intensity of colocalized F4/80 and FN-1 signals in the kidneys of U-IRI mice was quantified by ImageJ software (n = 6 for each group) (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001).
Loss of CEBPD through reduced fibronectin inhibits hypoxia-induced macrophage adhesion in HK-2 cells
To verify whether CEBPD regulates FN-1 in renal epithelial cells, HK-2 cells (a human renal tubular epithelial cell line) were used and subjected to a loss-of-function assay. CEBPD and FN-1 expression was upregulated under hypoxic conditions in HK-2 cells, and the expression of both genes was attenuated in CEBPD knockdown HK-2 cells in response to hypoxia (Fig. 5A, B). In addition, a previous study demonstrated that, compared to M2 macrophages, M1 macrophages showed stronger adhesion activity [31]. Afterward, we evaluated whether macrophage adhesion was modulated by CEBPD activation in HK-2 cells. mCherry THP-1 M1 macrophages, differentiated by PMA, interferon-gamma and lipopolysaccharide [31], were coincubated with parental and CEBPD knockdown HK-2 cells to assess adhesion under hypoxic conditions. Compared to coincubation with parental HK-2 cells, the adhesion activity of THP-1 M1 macrophages was attenuated in CEBPD knockdown HK-2 cells under hypoxic conditions (Fig. 5C). Moreover, following the confirmation of FN-1 knockdown in HK-2 cells (Fig. 5D), the contribution of FN-1 to macrophage adhesion was assessed. The results showed that after coincubation of THP-1 M1 macrophages with CEBPD knockdown HK-2 cells, the adhesion of macrophages was attenuated in FN-1 knockdown HK-2 cells under hypoxic conditions (Fig. 5G). These results suggest that epithelial CEBPD and FN-1 contribute to macrophage adhesion.
Fig. 5. Loss of CEBPD attenuates hypoxia-induced macrophage adhesion to HK-2 cells.
A FN-1 expression is attenuated in CEBPD knockdown HK-2 cells. FN-1 expression was analyzed in shControl (shCtl) and shCEBPD (shCD) HK-2 cells under normoxia (21% O2) or hypoxia (1% O2). (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). B Macrophage adhesion is attenuated in coculture with CEBPD knockdown HK-2 cells. mCherry THP-1 M1 macrophages were coincubated with shCtl or shCEBPD (shCD) HK-2 cells. After washing with PBS, an ELISA reader determined the number of attached fluorescent macrophages. (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). C FN-1 expression is attenuated in FN-1 knockdown HK-2 cells. FN-1 expression was analyzed in shControl (shCtl) and shCEBPD (shCD) HK-2 cells under normoxia (21% O2) or hypoxia (1% O2). (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). D Macrophage adhesion is attenuated in coculture with FN-1 knockdown HK-2 cells. mCherry THP-1 M1 macrophages were coincubated with shCtl or shFN-1 HK-2 cells. After washing with PBS, an ELISA reader determined the number of attached fluorescent macrophages. (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001).
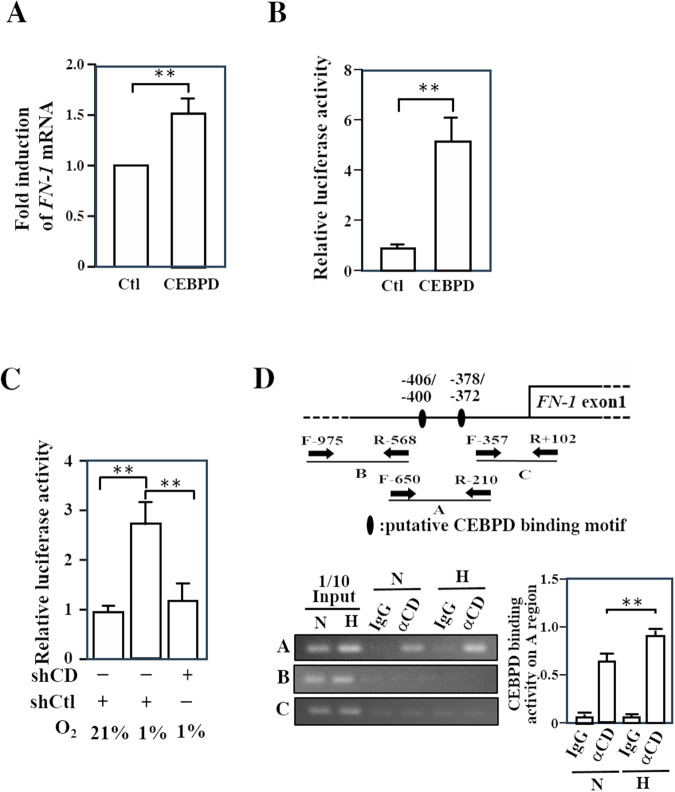
As mentioned above, CEBPD is a transcription factor and FN-1 contributes to an increase in the adhesion of various cells [32–34]. We further tested whether FN-1 is a downstream target of CEBPD in kidney epithelial cells. Firstly, co-transfection of the FN-1 reporter and the CEBPD expression vector in HK-2 cells was performed to test whether FN-1 transcription and reporter activity are responsive to CEBPD activation in epithelial HK-2 cells. The results demonstrated that CEBPD can activate FN-1 transcription and reporter activity in HK-2 cells (Fig. 6A, B). We subsequently tested whether hypoxia could activate FN-1 reporter activity and whether the inactivation of CEBPD could disrupt hypoxia-induced FN-1 reporter activity. The results showed that FN-1 reporter activity was activated under hypoxic conditions and that the inactivation of CEBPD attenuated hypoxia-induced FN-1 reporter activity (Fig. 6C). Moreover, a ChIP assay was conducted to determine whether CEBPD could directly bind to the FN-1 promoter region in HK-2 cells. Two putative CEBPD binding motifs on the FN-1 promoter were predicted using PROMO 3.0 (https://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3; Fig. 6D upper panel). The results of ChIP assay showed that the binding of CEBPD was increased in HK-2 cells under hypoxic conditions (Fig. 6D, lower panel). These results suggested that CEBPD can directly bind to the FN-1 promoter and that CBEPD binding is responsive to hypoxia in HK-2 cells.
Fig. 6. CEBPD directly contributes to FN-1 gene activation in HK-2 cells.
A Exogenous CEBPD activates FN-1 transcription in HK-2 cells. A RT‒qPCR assay was performed with total RNA from control and CEBPD-expressing vector transfectants. (Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001). B Exogenous CEBPD activates the FN-1 reporter in HK-2 cells. A reporter assay was performed with cell lysates from the FN-1 reporter and control or CEBPD-expressing vector transfectants. (Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001). C The inactivation of CEBPD attenuates hypoxia-induced FN-1 reporter activity in HK-2 cells. A reporter assay was performed with cell lysates from FN-1 reporter and lentiviral shcontrol (shCtl)- or shCEBPD (shCD)-infected transfectants. (one-way ANOVA; *P < 0.05, **P < 0.01, ***P < 0.001). D CEBPD (CD) directly binds to the FN-1 gene promoter and this binding is responsive to hypoxia in HK-2 cells. A ChIP-PCR assay was performed with the indicated antibody-pull down formaldehyde-treated HK-2 cell lysates under normoxic (N) or hypoxic (H) conditions (Student’s t test; *P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
IRI in renal transplantation is closely linked to a myriad of complications, including delayed graft function, graft rejection, and chronic graft dysfunction, which is due to its impact on diverse cellular regulatory systems that induce a distinct inflammatory response in the transplanted kidney. This finding underscores the significance of IRI (which is believed to be the foremost nonspecific factor influencing both early and late allograft dysfunction) as a critical concern in kidney transplantation [35–38]. To improve renal graft outcomes, the underlying mechanisms of IRI to the graft are very important for defining strategies to prevent or treat IRI after kidney transplantation. In this study, we found that CEBPD can directly bind to the promoter of the FN-1 gene and consequently contribute to the enhancement of macrophage attachment, which is involved in the pathogenesis of renal IR (Fig. 7).
Fig. 7. A schematic diagram illustrates the proposed model.
In response to IRI, CEBPD can be activated in epithelial cells. CEBPD activation is associated with apoptosis and contributes to the transcriptional activation of the FN-1 gene in epithelial cells. An increase in FN-1 can enhance macrophage adhesion and is involved in inflammatory activation and kidney injury.
The determination of the pathophysiology of renal IRI via a good experimental model that reproducibly induces acute kidney injury (AKI) is critical. Many investigators have utilized bilateral renal pedicle clamping as a renal IRI model to examine the molecular pathophysiology of AKI. To mimic IRI after single kidney transplantation, the contralateral nephrectomy was replaced by a unilateral IRI model [39]. In this U-IRI mouse model, renal damage and elevated creatinine were observed. Moreover, the decline in renal function was inhibited by Cebpd deficiency (Fig. 3B, D).
In previous studies, CEBPD was shown to be upregulated by many extracellular stimuli, such as IL-1β, IL-6, TNF-α, IFN-α, IFN-γ, LPS and TGF-β1 [27, 40–42]. In hypoxia, the activation of CEBPD has been suggested to be associated with HIF-1α activity and cellular function in profibrotic and protumor conditions [25, 43–45]. However, the associations between CEBPD and macrophage attachment and renal fibrosis remain poorly characterized. CEBPD is also reciprocally implicated in the activation of TNF-α, IL-1β and IL-6 [46]. In addition, monocytes/macrophages that appear in kidney injury produce inflammation-associated cytokines, such as IL-1, IL-6 and TGF-β [47, 48]. Consistent with these results, the levels of TNF-α, IL-1β and TGF-β1 in the plasma and the level of macrophage recruitment to the kidney were reduced in Cebpd-deficient mice after U-IRI (Figs. 3B, D and 4B). These findings suggest that reduced disease susceptibility in Cebpd-deficient mice is associated with a reduced inflammatory response. As Cebpd influences the regulation of cytokine-producing inflammatory cells, epithelial Cebpd-mediated inflammatory cell recruitment is expected to play an important role in renal IRI. However, a recent study suggested that TNF-α, IL-1β and TGF-β are also expressed by renal epithelial cells after renal injury [49]. Accordingly, the interactions among renal tubular epithelial cells, inflammatory cells and cytokines in acute kidney injury require further investigation.
Typically, the damaged tubular epithelium releases proinflammatory cytokines and chemokines, thus triggering and intensifying inflammatory responses. Subsequently, inflammatory cells are recruited and cause direct injury to tubular epithelial cells. However, some leukocytes migrate through the endothelium into the interstitial compartment [20]. Additionally, ECM proteins are thought to be endogenous danger signals in inflamed tissue and can stimulate resident immune cells in the absence of microbial infection [50]. I In the current study, we demonstrated that Cebpd was primarily expressed in renal tubular epithelial cells, especially in the necrotic epithelium, and regulated the expression of FN-1 (which is an ECM component) following U-IRI. Furthermore, the colocalization of the macrophage marker F4/80 with FN-1 in renal injuries showed that the overlap was mainly localized in the cytoplasm of renal tubular epithelial cells (Fig. 4). Altered expression of FN-1 is observed in some pathological situations, including inflammation [51, 52]. Moreover, excessive macrophage accumulation can exacerbate inflammatory diseases [20]. Collectively, in renal tubular epithelial cells, CEBPD-induced FN-1 can mediate macrophage accumulation directly to the site of tubular injury and be conducive to renal IRI.
Tissue injury elicits innate immune responses followed by the local production of chemokines, which recruit neutrophils, naïve monocytes and macrophages to sites of inflammation [53]. Both neutrophils and macrophages are effective at eliminating pathogens; however, neutrophils and macrophages found in IRI are likely to extend the early injury phase of tubular epithelial cells. In the present study, CEBPD activation mediated kidney injury after IRI through macrophage and neutrophil accumulation, but the signals of the neutrophil marker Ly6G did not colocalize with FN-1 in renal injury (Supplementary Fig. 2). Therefore, the molecular mechanisms underlying CEBPD-enhanced neutrophil accumulation require further analysis. In injured kidneys, inflammation is induced by resident tubular cells and inflammatory cells, particularly macrophages [42]. Monocytes and macrophages infiltrate tissue within a few days after IRI; however, the direct role of macrophages in mediating early tubular injury is still being investigated. Macrophage infiltration appears in the mouse kidney within 1 h after reperfusion; it then peaks at 24 h and persists for 7 days [20].
Depletion of macrophages prior to IRI was shown to attenuate renal injury in a murine IRI model [54]. A previous study showed that blockade or deficiency of C-C motif chemokine receptor 1, which is an immune cell accumulation regulator, leads to decreased macrophage accumulation in the damaged kidney but cannot alter the degree of renal injury [55]. In this study, we demonstrated that epithelial CEBPD contributes to macrophage accumulation by activating FN-1-mediated cell adhesion (Fig. 5B, D). A previous study demonstrated that under hypoxic conditions, CEBPD can bind to the FN-1 promoter and activate FN-1 expression in cancer cells [25]. Our current results agree with this observation, thus indicating that CEBPD could be a common activator of FN-1 transcriptional activation under hypoxic conditions. In addition, FN-1 deamidation on the Asn-Gly-Arg region can promote monocyte adhesion [56]. Therefore, whether CEBPD can potentially affect the deamination of FN-1 and its consequent effect on FN-1 stability can be tested in the future.
Materials and methods
A uninephrectomized mouse model of ischemia-reperfusion injury (U-IRI)
Twelve- to 14-week-old male C57BL/6J mice were acquired from BioLASCO Taiwan Co. Cebpd−/− mice (on a C57BL/6 background) were generously provided by Sterneck [57], and reproduction was performed at the National Laboratory Animal Center. The abdomens of the male C57BL/6 mice were opened under mixed anesthesia. The left renal pedicle was occluded for 45 min with a microvascular clip, followed by 24 h of reperfusion. During the ischemic period, dissection was continued to release the right kidney’s lateral and posterior renal attachments. The right kidney was retracted inferiorly to permit the remaining upper pole attachment dissection. The right renal hilum was isolated and ligated with 4-O silk, and the right kidney was carefully removed. Adequate restoration of the left renal blood flow was checked before abdominal closure. Before wound closure, warm sterile saline was intraperitoneally administered to each mouse. The experimental mice were sacrificed, and the remaining kidney was removed after 24 h of reperfusion. Kidney tissues and blood samples were taken for analysis. The remnant kidney was harvested and transversally cut at the midline. One-half of the kidneys were fixed in formalin and embedded in paraffin. The other sample was homogenized, and the protein extraction solution was added for Western blotting. Animal procedures followed protocols approved by the Institutional Animal Care and Use Committee of National Cheng Kung University (approval IACUC number: 109121).
Cell culture
The human proximal tubular cell line HK-2 cells were cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (Thermo Fisher Scientific Inc., USA #11320033) supplemented with 10% fetal calf serum. The human monocyte cell line THP-1 cells were maintained in RPMI 1640 Medium (Thermo Fisher Scientific Inc., USA #11875119) supplemented with 10% fetal calf serum.
Cell hypoxia
Cell hypoxia (0.1% O2) was induced with an anaerobic bag (Mitsubishi Gas Chemical Company, Inc., Japan). The experimental HK-2 cells were incubated in 0.1% O2 at 37 °C for 24 h, and phorbol 12-myristate 13-acetate (PMA) (320 nM)/IFN-gamma (20 ng)/LPS (100 ng)-differentiated THP-1 cells were then incubated with HK-2 cells for 10 min. After coculture, the experimental cells were gently washed with PBS to remove the unattached cells. Subsequently, all of the cells were collected in trypsin-EDTA buffer, and multimode microplate readers were used to detect the number of fluorescent THP-1 cells.
Renal histopathology
Renal tissue samples were immersed in 10% formalin for 24 h for fixation and subsequently embedded in paraffin. Histological analysis was performed with hematoxylin staining. The severity of renal damage was scored on hematoxylin and eosin (H&E)-stained sections by grading the percentage of tubule injury that showed loss of the proximal tubule brush border, cell swelling or vacuolization, and cell necrosis as follows: 0 (<1%); 1 (1–10%); 2 (11–20%); 3 (21–40%); 4 (41–60%); 5 (61–75%); and 6 (>75%) [58]. The score ranges of 1–2 represent mild injury, 3–4 represent moderate injury, and 5–6 represent severe injury. Ten random fields of view from the corticomedullary junction, known as the most susceptible area of the kidney to ischemia-reperfusion injury, were observed on each slide section at a magnification of 200×.
Western blot analysis
The tissues were dissected and frozen on dry ice. Tissues or experimental cells were homogenized in lysis buffer (0.05 M Tris-HCl, 0.15 M NaCl, 1 mM EDTA, 1%NP40, 0.25% NaDOC, 1 μg/ml Aprotinin, 1 μg/ml Leupeptin, 100 μg/ml PMSF, 1 mM DTT, 1 mM Na3VO4, 0.5 μM NaF and 0.01% SDS). Sections were incubated with primary antibodies, including anti-CEBPD (GTX115047; GeneTex), anti-GAPDH (GTX100118; GeneTex), anti-FN-1(#15613-1-AP; Proteintech) and anti-α-tubulin (T6199; Sigma), and the signals were visualized using an enhanced chemiluminescence Western blot system.
Immunofluorescence analysis
Tissue sections were cut from frozen kidney blocks onto precoated slides. The slides were subjected to a 1-hour treatment with a protein blocker. Antigen retrieval was performed by heating the slides to 121 °C in 10 mM citrate buffer (pH 6) for 20 min, followed by washing with phosphate-buffered saline. Subsequently, the slides were incubated with specific antibodies targeting CEBPD (sc‐365546; Santa Cruz Biotechnology), F4/80 (sc-377009; Santa Cruz Biotechnology), FN-1 (#15613-1-AP; Proteintech), and E-cadherin (GTX100443; GeneTex), each antibody was diluted at a ratio of 1:200. The slides were treated with secondary antibodies conjugated with Alexa 488 and 594 (diluted at a ratio of 1:200) and then incubated for 1 h at room temperature. The images were observed with an Olympus fluorescence microscope (BX51) and quantified via densitometry with ImageJ. The quantification method involved capturing images of 10 randomly selected regions from each slide and quantifying them using ImageJ. Statistical analysis was performed by comparing the values of other groups with the mean value of the Sham group as the reference.
ELISA of chemokines in plasma samples
Concentrations of the chemokines IL-1β, TNF-α and TGF-β1 were measured in mouse plasma samples using R&D ELISA kits according to the manufacturer’s protocol (IL-1β #DY401-05, TNF-α #DY410-05, TGF-β1 #DY1679-05). For renal injury, the creatinine (CRE) concentration was measured from the plasma samples of sacrificed experimental mice after one day of reperfusion.
Short hairpin RNA (shRNA) assay
Lentiviruses were generated by transfecting Phoenix cells with the indicated shRNA expression vectors and pMD2.G and psPAX2 plasmids. After viral infection efficiency was confirmed, HK-2 and THP-1 cells were infected with shCEBPD and mCherry lentiviruses, respectively, for 48 h. The lentiviral expression vectors were purchased from the National RNAi Core Facility at the Genomic Research Center of the Institute of Molecular Biology, Academia Sinica, Taiwan. The sequences of the indicated target genes were as follows: shCDBPD, 5′-CCGGGCTGTCGGCTGAGAACGAGAACTCGAGTTCTCGTTCTCAGCCGACAGCTTTTT-3′; shControl, 5′-CCGGAGTTCAGTTACGATATCATGTCTCGAGACATTCGCGAGTAACTGAAC TTTTTT -3′.
Apoptosis assay
Cell apoptosis was analyzed according to the instructions of the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit (# E-CK-A331, Elabscience company, USA). Fluorescence images were taken by fluorescence microscopy (BX51, Olympus). Capture ten images at 100X magnification and count the number of apoptotic cells in each image.
Reporter plasmids and luciferase assay
The promoter region of the FN-1 gene was obtained from the genomic DNA of HK-2 cells via PCR with the following specific primers: forward, 5′-CGACGCGTCGCGTGGGAAAGGACACGAAGA-3′ and reverse 5′-CCGCTCGAGCGGGCCACCAAGTTTGCTTCCCTTC-3′. The amplified DNA from the −976~+233 region of the FN1 gene locus was further subcloned and inserted into the pGL3-basic vector. For the luciferase assay, the FN-1 reporter vector was cotransfected with pCDNA3 with or without HA/CEBPD cDNA in HK-2 cells or transfected into HK-2 cells infected with lentivirus containing shCEBPD or shControl sequences using the transfection reagent TransIT-2020 (Mirus) for 18 h, or it was further incubated under hypoxic or normoxic conditions for another 24 h.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) was performed as described by Wang et al. [59] Briefly, following exposure to normoxia (21% O2) or hypoxia (1% O2), various treatments were used. Experimental HK-2 cells, including those treated with lentivirus containing shCtl or shCEBPD (shCD), were fixed with 1% formaldehyde. The chromatin was cross-linked and subsequently prepared and sonicated to an average size of 500 bp. The DNA fragments were subjected to immunoprecipitation overnight at 4 °C using antibodies specific for CEBPD or control rabbit immunoglobulin G (IgG). The immunoprecipitated chromatin was amplified using primers targeting specific regions after cross-linking reversal. The sequences of the paired primers are as follows: region A (5′-AAGAAGTCCGAACAGGGAGC-3′ and 5′-AAAGAGATGCTGATGGCCCG-3′), region B (5′-CGTGGGAAAGGACACGAAGA-3′ and 5′-ATCCCGCTCCCTTTCTTTGG-3′) and region C (5′-CCCCTTCGCTTCACACAAGT-3′ and 5′-AAGGGATGCAGAGGACCAGA-3′). After PCR, the amplified products were analyzed via agarose gel electrophoresis.
Statistical analysis
All experiments were performed with a minimum of three independent replicates. The statistical analysis was performed using a two-tailed unpaired Student’s t test and one-way ANOVA, with a significance threshold set at p < 0.05. The statistical analysis was conducted using GraphPad Prism software. The values plotted are depicted as the means with standard error of the means (SEMs).
Supplementary information
Acknowledgements
We thank the Laboratory Animal Center, College of Medicine, National Cheng Kung University, and Taiwan Animal Consortium. We also acknowledge Dr. Ming-Yuan Hong, who provided the HK-2 cells for the experiment.
Author contributions
Shen-Shin Chang and Chao-Chun Cheng contributed equally to this work. Shen-Shin Chang, Chao Chun Cheng and Ju-Ming Wang conceived and designed experiments, performed experiments, analyzed the data and wrote the manuscript. Shen-Shin Chang and Chao-Chun Cheng performed the animal experiments. Ying-Ren Chen and Feng-Wei Chen assisted with the pathology report and analyzed the data. Shen-Shin Chang, Chao-Chun Cheng, Ya-Min Cheng, and Ju-Ming Wang reviewed the data and edited the manuscript.
Funding
This study was supported financially by the Kuo general hospital research grant 113-02, NSTC 111-2327-B-006-009, NSTC 109-2320-B-006-026-MY3, NSTC 111-2320-B-006-015, NSTC 112-2327-B-006-009 and NSTC 112-2320-B-006-008.
Data availability
All data associated with this study are presented in the paper.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Animal experiments were performed according to the guidelines of Council of Agriculture, Taiwan, and were approved by the Institutional Animal Care and Use Committee, National Cheng Kung University (IACUC number 109121).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shen-Shin Chang, Chao-Chun Cheng.
Contributor Information
Ya-Min Cheng, Email: chengym@mail.ncku.edu.tw.
Ju-Ming Wang, Email: yumingw@mail.ncku.edu.tw.
Supplementary information
The online version contains supplementary material available at 10.1038/s41420-024-02082-4.
References
- 1.Nieuwenhuijs-Moeke GJ, Pischke SE, Berger SP, Sanders JSF, Pol RA, Struys MMRF, et al. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J Clin Med. 2020;9:253. doi: 10.3390/jcm9010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87:859–64. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 3.Vazquez MA, Jeyarajah DR, Kielar ML, Lu CY. Long-term outcomes of renal transplantation: a result of the original endowment of the donor kidney and the inflammatory response to both alloantigens and injury. Curr Opin Nephrol Hypertens. 2000;9:643–8. doi: 10.1097/00041552-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Khan AM, Maderdrut JL, Simon EE, Batuman V. The effect of PACAP38 on MyD88-mediated signal transduction in ischemia-/hypoxia-induced acute kidney injury. Am J Nephrol. 2010;32:522–32. doi: 10.1159/000321491. [DOI] [PubMed] [Google Scholar]
- 5.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–6. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, et al. Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med. 2011;183:1380–90. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- 7.Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1191–1198. doi: 10.1152/ajplung.00055.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bissonnette EY, Lauzon-Joset JF, Debley JS, Ziegler SF. Cross-Talk Between Alveolar Macrophages and Lung Epithelial Cells is Essential to Maintain Lung Homeostasis. Front Immunol. 2020;11:583042. doi: 10.3389/fimmu.2020.583042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao F, Kobzik L. Lung macrophage-epithelial cell interactions amplify particle-mediated cytokine release. Am J Resp Cell Mol. 2002;26:499–505. doi: 10.1165/ajrcmb.26.4.4749. [DOI] [PubMed] [Google Scholar]
- 10.Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–17. doi: 10.1016/S0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 11.Sumarokova M, Iturri J, Toca-Herrera JL. Adhesion, unfolding forces, and molecular elasticity of fibronectin coatings: An atomic force microscopy study. Microsc Res Tech. 2018;81:38–45. doi: 10.1002/jemt.22954. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama SK, Yamada KM, Hayashi M. The structure of fibronectin and its role in cellular adhesion. J Supramol Struct Cell Biochem. 1981;16:345–8. doi: 10.1002/jsscb.1981.380160405. [DOI] [PubMed] [Google Scholar]
- 13.Kao WJ, Lee D, Schense JC, Hubbell JA. Fibronectin modulates macrophage adhesion and FBGC formation: the role of RGD, PHSRN, and PRRARV domains. J Biomed Mater Res. 2001;55:79–88. doi: 10.1002/1097-4636(200104)55:1<79::AID-JBM110>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Souza ST, Agra LC, Santos CE, Barreto E, Hickmann JM, Fonseca EJ, et al. Macrophage adhesion on fibronectin evokes an increase in the elastic property of the cell membrane and cytoskeleton: an atomic force microscopy study. Eur Biophys J. 2014;43:573–9. doi: 10.1007/s00249-014-0988-3. [DOI] [PubMed] [Google Scholar]
- 15.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–36. doi: 10.1128/MCB.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–43. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardinaux JR, Magistretti PJ. Vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and noradrenaline induce the transcription factors CCAAT/enhancer binding protein (C/EBP)-beta and C/EBP delta in mouse cortical astrocytes: involvement in cAMP-regulated glycogen metabolism. J Neurosci. 1996;16:919–29. doi: 10.1523/JNEUROSCI.16-03-00919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zannetti C, Bonnay F, Takeshita F, Parroche P, Menetrier-Caux C, Tommasino M, et al. C/EBP{delta} and STAT-1 are required for TLR8 transcriptional activity. J Biol Chem. 2010;285:34773–80. doi: 10.1074/jbc.M110.133884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JM, Tseng JT, Chang WC. Induction of human NF-IL6beta by epidermal growth factor is mediated through the p38 signaling pathway and cAMP response element-binding protein activation in A431 cells. Mol Biol Cell. 2005;16:3365–76. doi: 10.1091/mbc.e05-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transpl. 2008;23:842–52. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74:1526–37. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huleihel L, Dziki JL, Bartolacci JG, Rausch T, Scarritt ME, Cramer MC, et al. Macrophage phenotype in response to ECM bioscaffolds. Semin Immunol. 2017;29:2–13. doi: 10.1016/j.smim.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt DR, Kao WJ. The interrelated role of fibronectin and interleukin-1 in biomaterial-modulated macrophage function. Biomaterials. 2007;28:371–82. doi: 10.1016/j.biomaterials.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Mao XG, Xue XY, Lv R, Ji A, Shi TY, Chen XY, et al. CEBPD is a master transcriptional factor for hypoxia regulated proteins in glioblastoma and augments hypoxia induced invasion through extracellular matrix-integrin mediated EGFR/PI3K pathway. Cell Death Dis. 2023;14:269. doi: 10.1038/s41419-023-05788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feitoza CQ, Goncalves GM, Semedo P, Cenedeze MA, Pinheiro HS, Beraldo FC, et al. Inhibition of COX 1 and 2 prior to renal ischemia/reperfusion injury decreases the development of fibrosis. Mol Med. 2008;14:724–30. doi: 10.2119/2008-00064.Feitoza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YT, Chen FW, Chang TH, Wang TW, Hsu TP, Chi JY, et al. Hepatoma-derived growth factor supports the antiapoptosis and profibrosis of pancreatic stellate cells. Cancer Lett. 2019;457:180–90. doi: 10.1016/j.canlet.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Wang SM, Hsu JC, Ko CY, Wu HE, Hsiao YW, Wang JM. Astrocytic Cebpd Regulates Pentraxin 3 Expression to Promote Fibrotic Scar Formation After Spinal Cord Injury. Mol Neurobiol. 2023;60:2200–8. doi: 10.1007/s12035-023-03207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Digiacomo G, Tusa I, Bacci M, Cipolleschi MG, Dello Sbarba P, Rovida E. Fibronectin induces macrophage migration through a SFK-FAK/CSF-1R pathway. Cell Adhes Migr. 2017;11:327–37. doi: 10.1080/19336918.2016.1221566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kezic A, Stajic N, Thaiss F. Innate Immune Response in Kidney Ischemia/Reperfusion Injury: Potential Target for Therapy. J Immunol Res. 2017;2017:6305439. doi: 10.1155/2017/6305439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui K, Ardell CL, Podolnikova NP, Yakubenko VP. Distinct Migratory Properties of M1, M2, and Resident Macrophages Are Regulated by alpha(D)beta(2) and alpha(M)beta(2) Integrin-Mediated Adhesion. Front Immunol. 2018;9:2650. doi: 10.3389/fimmu.2018.02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeki N, Nishino S, Shimizu T, Ogawa K. EphA2 promotes cell adhesion and spreading of monocyte and monocyte/macrophage cell lines on integrin ligand-coated surfaces. Cell Adh Migr. 2015;9:469–82. doi: 10.1080/19336918.2015.1107693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Hachem N, Habel N, Naiken T, Bzioueche H, Cheli Y, Beranger GE, et al. Uncovering and deciphering the pro-invasive role of HACE1 in melanoma cells. Cell Death Differ. 2018;25:2010–22. doi: 10.1038/s41418-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Q, Xue YX, Liu J, Xi Z, Li Z, Liu YH. Fibronectin Promotes the Malignancy of Glioma Stem-Like Cells Via Modulation of Cell Adhesion, Differentiation, Proliferation and Chemoresistance. Front Mol Neurosci. 2018;11:130. doi: 10.3389/fnmol.2018.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J Transpl. 2015;5:52–67. doi: 10.5500/wjt.v5.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norman DJ, Illingworth DR, Munson J, Hosenpud J. Myolysis and acute renal failure in a heart-transplant recipient receiving lovastatin. N. Engl J Med. 1988;318:46–47. doi: 10.1056/NEJM198801073180110. [DOI] [PubMed] [Google Scholar]
- 37.Mehrotra A, Rose C, Pannu N, Gill J, Tonelli M, Gill JS. Incidence and consequences of acute kidney injury in kidney transplant recipients. Am J Kidney Dis. 2012;59:558–65. doi: 10.1053/j.ajkd.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 38.Womer KL, Vella JP, Sayegh MH. Chronic allograft dysfunction: mechanisms and new approaches to therapy. Semin Nephrol. 2000;20:126–47. [PubMed] [Google Scholar]
- 39.Hesketh, EE, Czopek, A, Clay, M, Borthwick, G, Ferenbach, D, Kluth, D, et al. Renal ischaemia reperfusion injury: a mouse model of injury and regeneration. J Vis Exp. 2014;88:51816. [DOI] [PMC free article] [PubMed]
- 40.Chuang CH, Wang WJ, Li CF, Ko CY, Chou YH, Chuu CP, et al. The combination of the prodrugs perforin-CEBPD and perforin-granzyme B efficiently enhances the activation of caspase signaling and kills prostate cancer. Cell Death Dis. 2014;5:e1220. doi: 10.1038/cddis.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko CY, Chang WC, Wang JM. Biological roles of CCAAT/Enhancer-binding protein delta during inflammation. J Biomed Sci. 2015;22:6. doi: 10.1186/s12929-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi J, Tanaka T, Eto N, Nangaku M. Inflammation and hypoxia linked to renal injury by CCAAT/enhancer-binding protein delta. Kidney Int. 2015;88:262–75. doi: 10.1038/ki.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Priya Dharshini LC, Vishnupriya S, Sakthivel KM, Rasmi RR. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell Signal. 2020;72:109670. doi: 10.1016/j.cellsig.2020.109670. [DOI] [PubMed] [Google Scholar]
- 44.Balamurugan K, Mendoza-Villanueva D, Sharan S, Summers GH, Dobrolecki LE, Lewis MT, et al. C/EBPdelta links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene. 2019;38:3765–80. doi: 10.1038/s41388-018-0516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Yang S, Yan M, Guan N, Li J, Xie Q, et al. Interstitial HIF1A induces an estimated glomerular filtration rate decline through potentiating renal fibrosis in diabetic nephropathy. Life Sci. 2020;241:117109. doi: 10.1016/j.lfs.2019.117109. [DOI] [PubMed] [Google Scholar]
- 46.Takata Y, Kitami Y, Yang ZH, Nakamura M, Okura T, Hiwada K. Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res. 2002;91:427–33. doi: 10.1161/01.RES.0000031271.20771.4F. [DOI] [PubMed] [Google Scholar]
- 47.Saat TC, van den Akker EK, IJzermans JN, Dor FJ, de Bruin RW. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med. 2016;14:20. doi: 10.1186/s12967-016-0767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol. 2010;30:268–77. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Black LM, Lever JM, Agarwal A. Renal Inflammation and Fibrosis: A Double-edged Sword. J Histochem Cytochem. 2019;67:663–81. doi: 10.1369/0022155419852932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–23. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 51.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/S0065-230X(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 52.To WS, Midwood KS. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenes Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huen SC, Cantley LG. Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol. 2015;30:199–209. doi: 10.1007/s00467-013-2726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–26. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bignon A, Gaudin F, Hemon P, Tharinger H, Mayol K, Walzer T, et al. CCR1 inhibition ameliorates the progression of lupus nephritis in NZB/W mice. J Immunol. 2014;192:886–96. doi: 10.4049/jimmunol.1300123. [DOI] [PubMed] [Google Scholar]
- 56.Dutta B, Park JE, Kumar S, Hao PL, Gallart-Palau X, Serra A, et al. Monocyte adhesion to atherosclerotic matrix proteins is enhanced by Asn-Gly-Arg deamidation. Sci Rep. 2017;7:5765. doi: 10.1038/s41598-017-06202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan EW, Li CC, Wu WJ, Huang CN, Li WM, Ke HL, et al. FGF7 Over Expression is an Independent Prognosticator in Patients with Urothelial Carcinoma of the Upper Urinary Tract and Bladder. J Urol. 2015;194:223–9. doi: 10.1016/j.juro.2015.01.073. [DOI] [PubMed] [Google Scholar]
- 58.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–63. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chi JY, Hsiao YW, Liu HL, Fan XJ, Wan XB, Liu TL, et al. Fibroblast CEBPD/SDF4 axis in response to chemotherapy-induced angiogenesis through CXCR4. Cell Death Discov. 2021;7:94. doi: 10.1038/s41420-021-00478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are presented in the paper.