Abstract
Glucose is the main source of energy for the human brain. This paper presents a non-invasive technique to study metabolic changes caused by glucose in human brain cell lines. In this paper we present the spectroscopic characterization of human normal brain (NHA; astrocytes) and human cancer brain (CRL-1718; astrocytoma and U-87 MG; glioblastoma) control cell lines and cell lines upon supplementation with glucose. Based on Raman techniques we have identified biomarkers that can monitor metabolic changes in lipid droplets, mitochondria and nucleus caused by glucose. We have studied the vibrations at 750 cm−1, 1444 cm−1, 1584 cm−1 and 1656 cm−1 as a function of malignancy grade. We have compared the concentration of cytochrome, lipids and proteins in the grade of cancer aggressiveness in normal and cancer human brain cell lines. Chemometric analysis has shown that control normal, control cancer brain cell lines and normal and cancer cell lines after supplementation with glucose can be distinguished based on their unique vibrational properties. PLSDA (Partial Least Squares Discriminant Analysis) and ANOVA tests have confirmed the main role of cytochromes, proteins and lipids in differentiation of control human brain cells and cells upon supplementation with glucose. We have shown that Raman techniques combined with chemometric analysis provide additional insight to monitor the biology of astrocytes, astrocytoma and glioblastoma after glucose supplementation.
Keywords: Brain cancer, Raman spectroscopy, Raman imaging, PLS-DA, Biomarkers, Glucose
Subject terms: Cancer imaging, Raman spectroscopy, Cellular imaging
Introduction
Glucose is a simple sugar (the chemical formula C6H12O6) and the main compound necessary for the functioning of the human organism. Glucose is delivered to organisms from the food that humans eat. The most concentrated glucose sources are starchy foods (bread, rice, pasta and potatoes) as well as dried fruits (prunes and figs), juices, honey and jams1. It is worth emphasizing that glucose plays a crucial role in human body regulation. Glucose is an essential source (“fuel”) of energy and its level is critical for the human body, especially for brain physiology. Glucose is absorbed through the small intestine and then is transported by bloodstream to the brain and other organs2,3.
Metabolic changes and their mechanisms during cancer development in recent years have been the subject of investigation by researchers from all over the world. To precisely monitor these changes modern and noninvasive tools are needed. One of these approaches is Raman spectroscopy which gives very precise, unambiguous results, allowing the detection of even the smallest changes occurring inside a single cell during disease states4–7. Alterations in glucose levels lead to pathological changes and dysfunction in human health8–16. According to the literature the effect of increased glucose consumption contributes to diabetes, proliferation of cancer cells, and cardiovascular disease17–27. In recent years sugars, especially glucose are the class of compounds that aroused the interest of researchers as biomarkers in cancer development28–32. K. L. Stewart et al. have studied the association between sugar and colorectal cancer (CRC). They report that among patients with CRC, sugar intake was associated with inflammation28. The role of glucose in breast cancer has been analyzed by S. Sun et al. They reported that high glucose promoted cancer cell proliferation33. There are also literature reports considering the close relationship between glucose and pancreatic cancer34,35. W-Ch. Liao et al. reported that an increase in glucose in the blood is associated with an increase of pancreatic cancer34. Another paper describes glucose as a factor in brain cancer36. A. M. Bielecka-Wajdman et al. have studied the influence of glucose on glioblastoma cells (T98G) and HROG02, HROG17 supplemented with temozolomide and dexamethasone. Their results suggest that high glucose has an influence on glioblastoma progression and changes cell viability and dispersal36. A review by S. A. Onikani et al. included consideration of the relationship between diabetes and liver cancer37.
In this article, we focus on the role of glucose in human brain cancer. It is well known that the brain is the most important organ that plays a major role in peripheral insulin resistance38 More than 80 years ago Sir Harold Himsworth’s suggested the relationship between insulin resistance and the central nervous system39 For this time researchers are deeply interested in understanding the involvement of glucose in Alzheimer's disease (AD)40–45. Studies show the connection between type 2 diabetes and dementia46. Glucose metabolism in Alzheimer’s disease has also been studied by E. J. Kim et al. Authors have analyzed patients in early and late-onset Alzheimer’s disease. They reported that there are different metabolic patterns in patients with early and late Alzheimer’s disease47. The role of glucose as a marker for Alzheimer’s disease has been studied also by T. C. Hammond et al48. A study conducted by X. Zhanh et al. shows that careful management of cholesterol and glucose can lower the risk for AD49.
Numerous studies reported the relationship between insulin and mitochondrial dysfunction38,50–54 K. Barhwal et al. have studied the role of salidroside in rat brain normoxic and hypoxic conditions. Their results suggest that salidroside positively affects memory acquisition and memory retrieval and augments mitochondrial biogenesis50. E. Beirami et al. demonstrated that the pathway of insulin signaling is useful for the treatment of abnormalities associated with methamphetamine abuse51. The contribution of W. Pratchayasakul et al. showed that a high-fat diet can modify neuronal insulin receptor signaling52. Other studies describe the role of rosiglitazone in neuronal insulin resistance and the impairment of rat brain. The obtained results suggest that rosiglitazone improves peripheral insulin resistance. In addition, they reported that rosiglitazone can improve neuronal insulin resistance in hippocampal regions caused by a fat-enrich diet53.
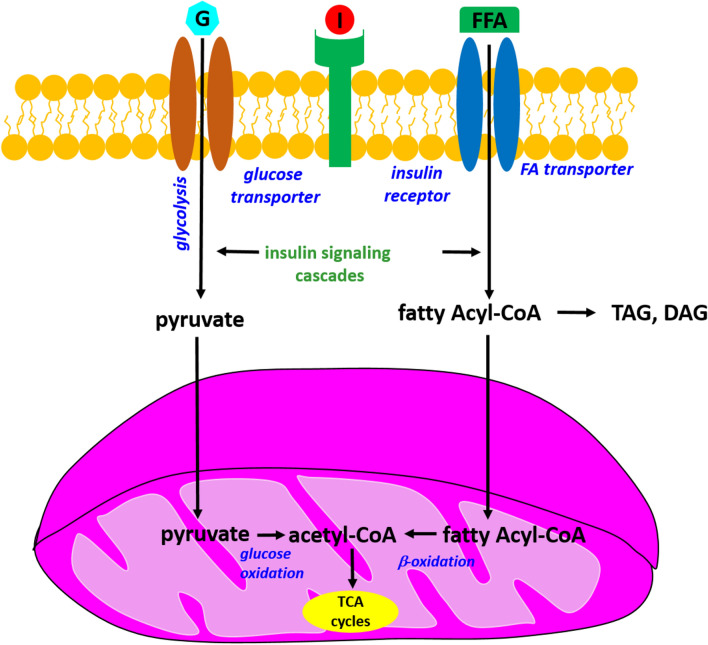
Mitochondria are organelles that generate cellular energy and play a crucial role in insulin signaling. Standardly insulin connects with its receptor and mediates in activation of cellular glucose uptake via glucose transporters. In the next step, glucose is converted to pyruvate during the glycolytic process. In the next phases, pyruvates are converted to acetyl-CoA, which is a substrate of the Krebs cycle, by a glucose oxidation process. Additionally, insulin promotes the uptake of cellular fatty acids (FA) into the cells and then converts them to fatty acyl-CoA. Next, there are two ways to fatty acyl-CoA. In the first process, fatty acyl-CoA can be converted into a lipid product. In another process, acyl-CoA can be directly transported to mitochondria, where induces mitochondrial b-oxidation. As a result of this process, acetyl-CoA is a product of the Krebs cycle. The association between insulin signaling, beta-oxidation and glycolysis is presented in Fig. 138.
Figure 1.
The mechanism of cellular insulin signaling on glucose and fatty acid metabolism; G-Glucose; I-Insulin; FFA-Free fatty acid; TCA cycles-tricarboxylic acid cycle, DAG-Diacylglycerol, TAG-Triacylglycerol.
Despite numerous research, the association between obesity and brain mitochondrial function is still not fully understood. In our previous paper, we discussed the role of glucose, fructose and mannose in the human normal bronchial cell line (BEpiC) and human cancer lung cell line (A549)55–57. Now we decided to extend our analysis to another type of cancer. In this paper, we focus on studying the effect of glucose supplementation on human brain cell lines. We have used Raman spectroscopy and Raman imaging combined with chemometric methods to study astrocytes (NHA), astrocytoma (CRL-1718) and glioblastoma (U-87 MG). The influence of different concentrations of glucose on lipid droplets and mitochondrial metabolism has been discussed.
Materials and methods
Chemical compounds
Glucose (product number G7021) was purchased from MERCK.
Cell culturing
The normal human astrocyte (Clonetics NHA) cell line was purchased from Lonza (Lonza, CC-2565TM) and maintained in Astrocyte Medium Bulletkit Clonetics (AGM BulletKit, Lonza CC-3186TM) and Reagent Pack (Lonza CC-5034) without antibiotics. Human astrocytoma CRL-1718 cell line (CCF-STTG1TM) was purchased from American Type Culture Collection (ATCC) and cultured in RPMI-1640 medium (ATCC, 30-2001TM) with 10% fetal bovine serum (FBS) (ATCC, 30–2020 TM) without antibiotics. Human glioblastoma U-87 MG cell line was purchased from ATCC (ATCC, HTB-14TM) and cultured in an Eagle's Minimum Essential Medium with L-glutamine (ATCC, 30-2003TM) with 10% FBS (ATCC, 30-2020TM) without antibiotics. The cell lines (NHA, CRL-1718 and U-87 MG) used in this study were grown in flat-bottom culture flasks made of polystyrene with a cell growth surface of 75 cm2. Flasks containing cells were stored in a humidified incubator providing environmental conditions at 37 °C, 5% CO2, 95% air. The culture medium was renewed from 2 to 3 times a week. All cells were seeded on CaF2 window (Crystal GmbH, Berlin, Germany) in 35 mm Petri dish at a density of 5 × 104 cells per Petri dish the day before the examination. After 24 h incubation on the CaF2, the standard growth medium was removed, and glucose dissolved in medium in final concentrations 50 mM and 100 mM solution was added for incubation time intervals equal to 24 h. Before Raman measurements, cells were washed twice with phosphate-buffered saline (PBS, Gibco no. 10010023), then fixed with 4% formalin solution (neutrally buffered) for 15 min and kept in PBS during the experiment.
Raman spectroscopy
Raman measurements were prepared by using a confocal Raman microscope (WITec alpha 300 RSA + (Ulm, Germany)) by using a 50 μm core diameter fiber an Ultra High Throughput Spectrometer (UHTS) and a CCD camera (Andor Newton DU970-UVB-353). Measurements were conducted by using a 532 nm excitation laser beam. The water immersion objective with a magnification of 40x (Nikon) and a numerical aperture (NA = 1.0) have been used. All Raman maps and Raman spectra were registered by using an average laser excitation power 10 mW and 0.5 s integration time for the fingerprint region and 0.3 s for the high-frequency region have been used. The obtained results were preprocessed using the WITec Control/Project Four 4.1 package (cosmic rays’ removal (model: filter size: 2, dynamic factor: 10), smoothing (Savitzky and Golay procedure (model: order: 4, derivative: 0) and background corrections). To analyze Raman maps Cluster Analysis (CA) method was used.
CA analysis method allows to combination of Raman spectra into groups called a cluster. Each of the clusters (the Raman spectra) must be as similar as possible in contrast to the Raman spectra belonging to another group. The separation of n observations (x) into k (k ≤ n) clusters S should be done to minimize the variance (Var) according to the formula:
where μi is the mean of experimental points. In our study, the number of clusters was 7. Each cluster is characterized by specific average Raman spectra, which reflect the inhomogeneous distribution of the analyzed sample. More details about the description of the Cluster Analysis method are available in our previous works55,58.
Statistical analysis
The normalization was performed using Origin software (model: divided by norm). The normalization model: divided by norm was done according to the formula:
where:
is the nth V value.
More information of normalization is described in detail in our previous works 56,58,59.
Partial least squares-discriminant analysis (PLS-DA) (mean center model) was performed using Matlab and PLS_Toolbox Version 4.060,61.
One-way ANOVA was conducted in Origin software (p≼0.05, Tukey test).
Results and discussion
We concentrated on determining the differences caused by glucose in lipid droplets and mitochondrial metabolism of human normal brain cells (NHA) and cancer brain cells (CRL-1718 and U-87 MG). Figure 2 presents the molecular formula of glucose (panel A) and Raman spectra of glucose in the fingerprint (panel B) and high-frequency (panel C) region.
Figure 2.
Molecular formula of glucose (panel A) Raman spectra of glucose in the fingerprint region (panel B) and high-frequency region (panel C).
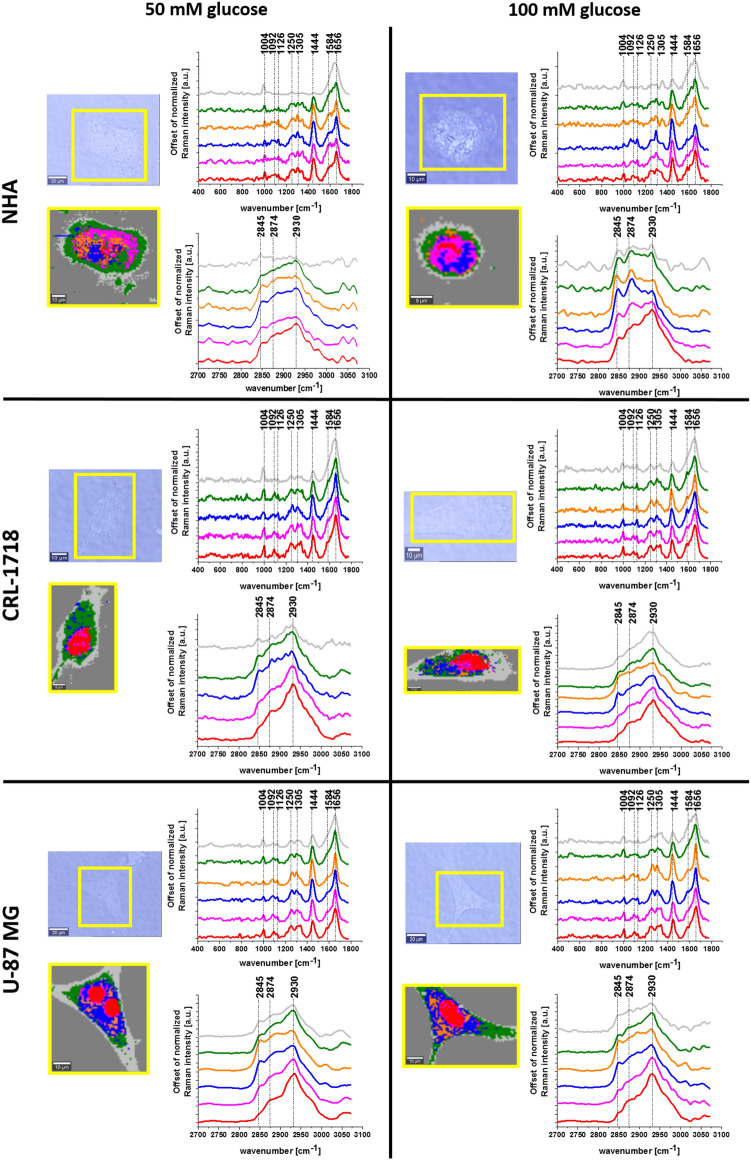
The main goal of this study was to monitor biochemical changes caused by glucose supplementation in human normal and cancer brain cells based on the vibrational features and chemometric methods. Therefore, to realize this task we start our analysis by using Raman spectroscopy methods. We have used Raman spectroscopy and Raman imaging for the biochemical characterization of human normal and cancer cell lines. Figure 3 shows microscopy images, Raman images performed by using the Cluster Analysis (CA) method and Raman spectra characteristic for organelles of astrocyte (NHA cell line), astrocytoma (CRL-1718 cell line) and glioblastoma (U-87 MG cell line) upon supplementation with 50 mM glucose and 100 mM glucose. Areas defined by CA represent nucleus (red color), mitochondria (magenta color), lipid droplet (blue color), endoplasmic reticulum (orange color), cytoplasm (green color), cell membrane (light gray) and extracellular area (dark gray color).
Figure 3.
The microscope images, Raman images and Raman spectra of cell organelles (nucleus (red), mitochondria (magenta), oleic lipid droplets (orange), lipid droplets/endoplasmic reticulum (blue), cytoplasm (green) and cell membrane (grey)) of a typical normal human astrocyte (NHA), astrocytoma (CRL-1718) and glioblastoma (U-87 MG) cells supplemented with 50 mM glucose and 100 mM glucose.
Results presented in Fig. 3 confirm that by using Raman spectroscopy and Raman imaging it is possible to achieve not only information about the localization of organelles but also biochemical information of each type of human brain cell: normal, mildly malignant and malignant. Detailed analysis of Raman spectra provides us an information about the main chemical compounds: nucleic acids, lipids, cytochromes and proteins. A peak at 1004 cm-1 is characteristic for phenylalanine, while a peak at 1092 cm-1 corresponds to symmetric v(PO2-), v(C–C) and v(C-N) of the DNA, phospholipids, glycogen and collagen. The peak vibration characteristic for cytochrome c and b is observed at 1126 cm-1. The peak at 1250 cm-1 corresponds to guanine, cytosine (NH2). The band at 1305 cm-1 is typical for CH2 deformation (lipid), adenine, cytosine. The peak at 1444 cm-1 corresponds to CH2 deformation of lipids and proteins. Band characteristic for cytochrome c is observed at 1584 cm-1. The band at 1656 cm-1 is associated with amide I. In the high-frequency region, one can see peaks characteristic for CH2 symmetric stretch of lipids (2845 cm-1), CH3 asymmetric stretch of lipids and proteins (2874 cm-1) and CH2 asymmetric stretch of proteins (2930 cm-1)56,62,63.
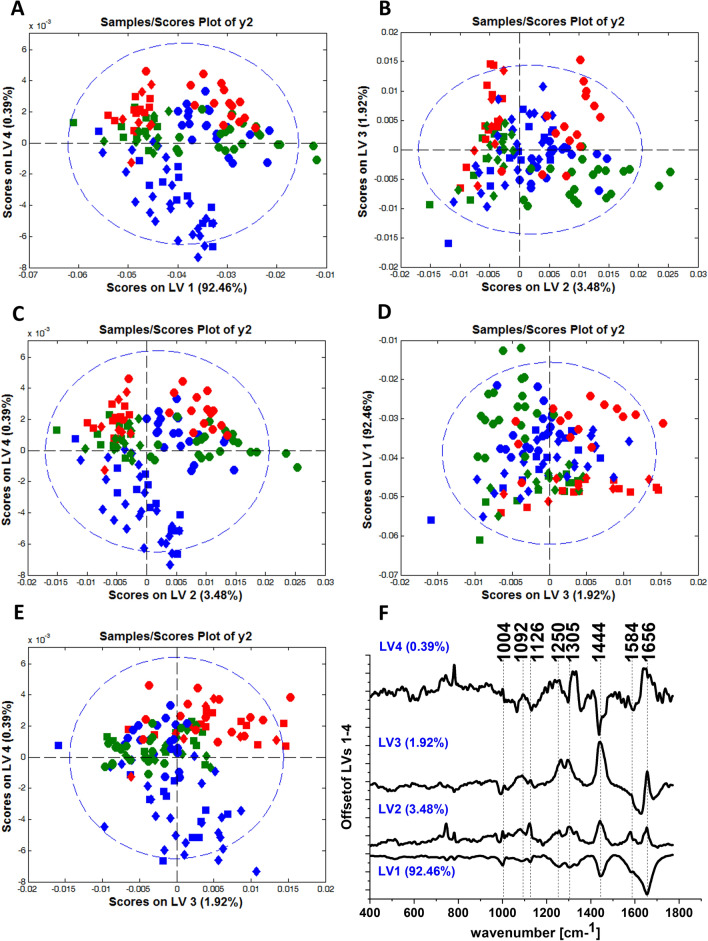
To better visualize the changes in brain cell lines caused by glucose we extended our analysis of chemometric methods. We have used PLS-DA analysis to show differences between control normal, control cancer brain cell lines and normal and cancer cell lines after supplementation with glucose. Figure 4 shows the scores plots and loadings plots obtained from PLS-DA analysis for NHA, CRL-1718 and U-87 MG cell lines. Raman spectra characteristic for astrocytes are marked by blue color, for astrocytoma by green color and glioblastoma by red color.
Figure 4.
PLS-DA score plots (model: normalization) LV4 vs. LV1 (panel A), LV3 vs. LV2 (panel B), LV4 vs. LV2 (panel C), LV1 vs. LV3 (panel D), LV4 vs. LV3 (panel E) for Raman spectra obtained from Cluster Analysis for NHA (blue color), CRL-1718 (green color) and U-87 MG (red color) control cell line and cell line supplemented with 50 mM glucose and 100 mM glucose: control cells (circle), cells supplemented with 50 mM glucose (square), cells supplemented with 100 mM glucose (diamond); the loadings plots of LV1, LV2, LV3 and LV4 versus wavenumber [cm-1] obtained from PLS-DA analysis (panel F).
From the scatter plot of PLS-DA analysis presented in Fig. 4A, one can see the spectral separation between control brain cells (circles) and brain cells supplemented with glucose (squares and diamonds). It is visible from Fig. 4 that the first LV1 has a contribution of 92.46%, LV2 the contribution of 3.48%, LV3 the contribution of 1.92% and LV4 the contribution to variance, respectively. Raman spectra characteristic for control cells (circles) are grouped above latent variable LV4. Moreover, Raman spectra characteristic for cancer cells after supplementation with glucose (green squares, green diamonds, and red squares, red diamonds) are grouped above LV4, while spectra for normal cells after supplementation with glucose (blue square and blue diamonds) are grouped below LV4. In the scatter plot LV3 vs. LV2 (panel B) one can see that spectra for control cells (circles) are grouped along LV2 ( +), while most of the spectra characteristic for cells after supplementation with glucose (squares and diamonds) in LV2 (-). From Fig. 4 panel C, it is visible that the Raman spectra characteristic for control cells (circles) are grouped in the upper of LV4 and along LV2 ( +). Detailed analysis of Fig. 4 panel D reveals that the most of Raman spectra characteristic for the control U-87 MG cell line (red circles) are grouped along LV3 ( +), while spectra for the control NHA cell line (blue circle) and CRL-1718 cell line (green circle) are grouped along LV3 (-). In the scatter plot of PLS-DA analysis presented in Fig. 4 panel E one can see Raman spectra characteristic for control cells for all brain cell lines (circles) are grouped above latent variable LV4. There is visible separation between Raman spectra characteristic for the NHA cell line after supplementation with glucose (blue squares and blue diamonds), which are grouped under LV4 and Raman spectra characteristic for both cancer cell lines after supplementation with glucose (CRL-1718: green squares and green diamonds; U-87 MG: red squares and red diamonds), which are grouped above LV4.
A similar separation of spectra of control normal, control cancer brain cell lines and normal and cancer cell lines after supplementation with glucose is shown in a loading plot of PLS-DA analysis (Fig. 4 panel E). Analysis of the loading plot of PLS-DA provides a spectral profile characterizing NHA, CRL-1718 and U-87 MG cells. Raman bands, which are positively correlated with the LV2 loading plot, characterize control cells. Moreover, Raman bands, which are negatively correlated with the LV4 loading plot, characterize normal cells after supplementation with glucose and positively correlated bands in the LV4 loading plot characterize control brain cells and cancer brain cells after supplementation with glucose.
Detailed analysis of LV1 loading plots (Fig. 4E) shows that all bands have a negative tendency. One can see from the LV2 loading plot that for all bands the tendency is the opposite (negative correlation). In the LV3 loading plot, the band at 1004 cm−1 has a negative correlation, while the bands at 1092 cm−1, 1126 cm-1, 1250 cm-1, 1305 cm-1, 1444 cm-1 and 1656 cm-1 have a positive correlation. Analyzing the LV4 loading plot one can see that the band at 1656 cm−1, has a positive tendency, while the remaining bands have a negative tendency.
To verify the accuracy of the test we calculated the values of sensitivity and specificity. (Table 1).
Table 1.
The values of sensitivity and specificity obtained for PLS-DA analysis.
| Control cells | Sensitivity | Specificity |
|---|---|---|
| NHA | 100.0 | 68.5 |
| CRL-1718 | 95.0 | 89.2 |
| U-87 MG | 80.0 | 82.8 |
| Cells supplemented with 50 mM glucose | Sensitivity | Specificity |
|---|---|---|
| NHA | 91.7 | 82.4 |
| CRL-1718 | 100.0 | 66.9 |
| U-87 MG | 91.7 | 83.2 |
| Cells supplemented with 100 mM glucose | Sensitivity | Specificity |
|---|---|---|
| NHA | 78.3 | 89.8 |
| CRL-1718 | 100.0 | 63.0 |
| U-87 MG | 85.7 | 70.2 |
The high values of specificity and sensitivity suggest that the PLS-DA model successfully classified Raman spectra of control normal, control cancer brain cell lines and normal and cancer cell lines after supplementation with glucose. First, we focus on the analysis of the values of sensitivity and specificity obtained for control cells. The sensitivity for the control NHA cell line is equal 100.0%, while the specificity is equal 68.5%. For CRL-1718 control cells the sensitivity is equal 95.0%, while the specificity is equal 89.2%. The sensitivity for the most malignant cancer control cells is equal 80.0%, while the specificity equals 82.8%. Secondly, let us focus on the analysis of the values for sensitivity and specificity obtained for cell lines supplemented with glucose. The sensitivities for NHA cell line are equal 91.7% for 50 mM glucose and 78.3 for 100 mM glucose. For CRL-1718 cell line the sensitivities are equal 100.0% for both types of supplementation with glucose. For U-87 MG cell line the sensitivities are equal 91.7% for 50 mM glucose and 85.7 for 100 mM of glucose. The specificity for NHA cell line is equal 82.4% and 89.8% for 50 mM and 100 mM glucose, respectively. For astrocytoma the specificity equals 66.9% (50 mM glucose) and 63.0% (100 mM glucose). For glioblastoma specificity equals 83.2% (50 mM glucose) and 70.2% (100 mM glucose).
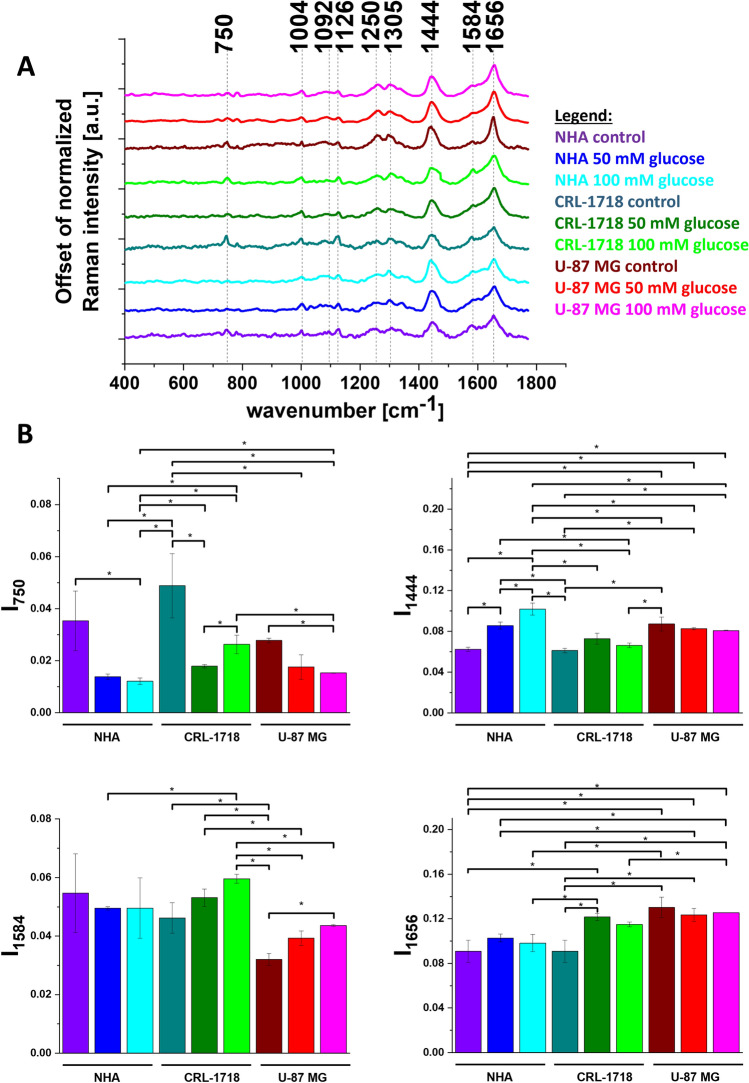
Loading plots from PLS-DA analysis presented in Fig. 4E showed that the main differences between control normal, control cancer brain cell lines and normal and cancer cell lines after supplementation with glucose are visible in the Raman bands at 750 cm-1, 1444 cm-1, 1584 cm-1 and 1656 cm-1 assigned to cytochrome c, lipid, cytochrome c and proteins, respectively.
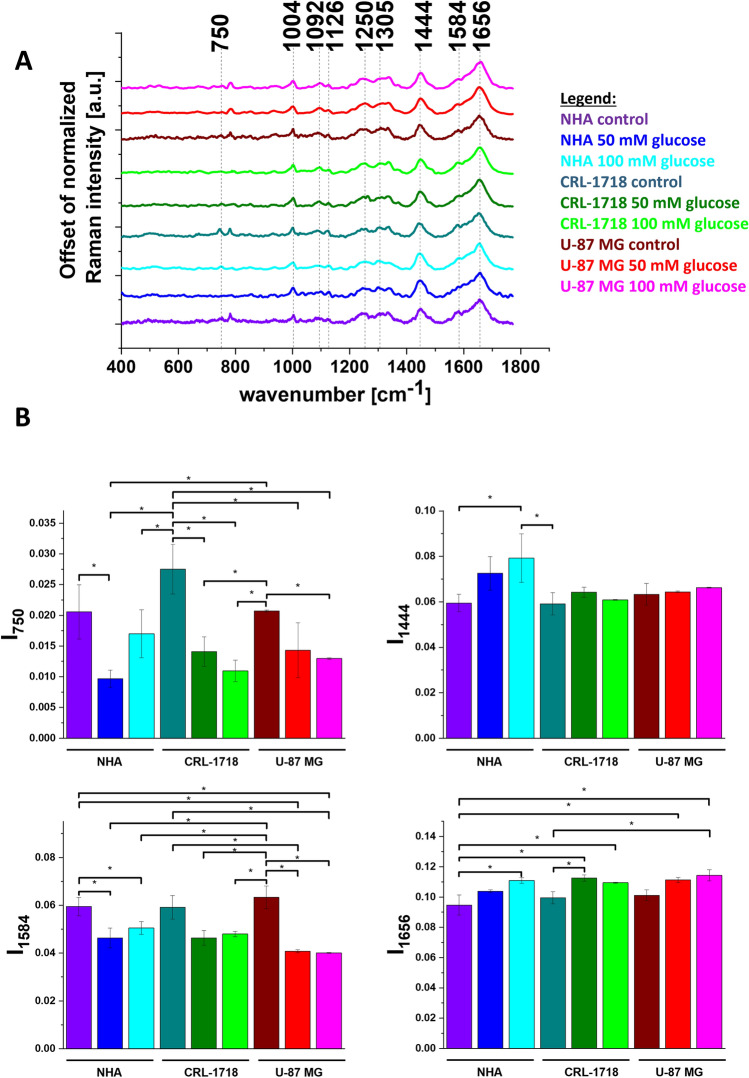
To confirm the differences in biochemical composition of NHA, CRL-1718 and U-87 MG cell line identified by a chemometric method we compared the averaged Raman spectra characteristic for lipid droplets for control NHA, CRL-1718 and U-87 MG control cell line and cell line supplemented with 50 mM and 100 mM of glucose. To better visualize the changes caused by glucose in lipid droplets we prepared the histograms of Raman band intensity. Results are presented in Fig. 5. The violet bar represents control NHA cells, the blue bar represents NHA cells supplemented with 50 mM of glucose, the turquoise bar represents NHA cells supplemented with 100 mM of glucose, the dark cyan bar represents control CRL-1718 cells, the olive bar represents CRL-1718 cells supplemented with 50 mM of glucose, the green bar represents CRL-1718 cells supplemented with 100 mM of glucose, the wine bar represents control U-87 MG cells, the red bar represents U-87 MG cells supplemented with 50 mM of glucose and magenta bar represents U-87 MG cells supplemented with 100 mM of glucose. We have prepared the same analysis for mitochondria and nucleus. Results are presented in Figs. 6, 7.
Figure 5.
Average Raman spectra (panel A) and normalized Raman intensity characteristic for lipid droplets for Raman bands at: 750 cm−1, 1444 cm−1, 1584 cm-1, 1656 cm-1 for NHA, CRL-1718 and U-87 MG cell line without supplementation and supplemented with 50 mM glucose and 100 mM glucose (panel B); * means statistically significant results; p-value≼ 0,05.
Figure 6.
Average Raman spectra (panel A) and normalized Raman intensity characteristic for mitochondria for Raman bands at: 750 cm−1, 1444 cm−1, 1584 cm-1, 1656 cm-1 for NHA, CRL-1718 and U-87 MG cell line without supplementation and supplemented with 50 mM glucose and 100 mM glucose (panel B); * means statistically significant results; p-value≼ 0,05.
Figure 7.
Average Raman spectra (panel A) and normalized Raman intensity characteristic for nucleus for Raman bands at: 750 cm−1, 1444 cm−1, 1584 cm-1, 1656 cm-1 for NHA, CRL-1718 and U-87 MG cell line without supplementation and supplemented with 50 mM glucose and 100 mM glucose (panel B); * means statistically significant results; p-value≼ 0,05.
It is well known that lipid droplets in the brain are responsible for protecting neuronal stem cells from oxidative stress64. Understanding the role of sugars in brain lipid droplet metabolism is crucial for developing clinical strategies65,66. To better visualize the different causes of glucose for normal and cancer brain cell lines we prepared a bar chart for Raman vibration characteristics for cytochromes, proteins and lipids. In Fig. 5 we present the Raman intensity for peaks at 750 cm-1, 1444 cm-1, 1584 cm-1 and 1656 cm-1. In view of the results presented in Fig. 5, it is evident that the band at 750 cm-1 characteristic for cytochrome c and b correlates with brain tumor aggressiveness. The Raman signal intensity of the band at 750 cm−1 in control cell lines increases for CRL-1718 cell line and then decreases for the most aggressive cell line (U-87 MG). Moreover, the intensity of the band at 750 cm-1 for all control cells is higher in comparison to after supplementation with glucose. The intensities of the Raman biomarker at 1584 cm-1 for CRL-1718 and U-87 MG control cell lines are lower in comparison to cells upon supplementation with glucose. As we reported previously this band can be used as a “redox state Raman marker” to balance between cancer oxidative phosphorylation and apoptosis55. For Raman bands at 1444 cm-1 characterizing CH2 deformation of lipids and proteins the signal increases with cancer aggressiveness. Our results are in agreement with those previously reported67. After supplementation with glucose for NHA cell line the signal increases in comparison to control cells. The intensity of the Raman biomarker at 1656 cm−1 corresponding to a concentration of proteins in lipid droplets increases with brain tumor aggressiveness. It suggests that lipid droplets may have other mechanisms of synthesis and can participate in other functions in normal and pathological conditions68.
Now let us concentrate on the Raman intensities I750, I1444, I1584 and I1656 in mitochondria as a function of brain cancer aggressiveness. Detailed inspection of Fig. 6 reveals several spectacular distinctions in mitochondria between the normal and cancer brain cells. Our results show that the intensity of the Raman biomarker at 750 cm-1 upon glucose supplementation is lower than for the control cells. Moreover, the intensity of the peak at 750 cm-1 after glucose supplementation increases for CRL-1718 cells and then decreases for U-87 MG. That tendency is in agreement with cell lines without incubation with glucose presented in our earlier reports67. The correlation between lipids and cancer aggressive (reflected by the Raman intensity of the bands at 1444 cm-1) decreases for the mildly malignant cell line and then increases for the most cancer-aggressive cell line. Moreover the Raman signal at 1444 cm-1 increases after glucose supplementation for NHA and CRL-1718 cell line. It can be associated with increased hepatic lipid production and the possibility of easily crossing the blood–brain barrier38. The correlation between cytochrome c and cancer aggressiveness is characterized by a signal at 1584 cm-1. One can see that the intensity of the Raman biomarker at 1584 cm-1 is the lowest for the most aggressive cell line (U-87 MG). This result is consistent with our previous considerations for cell lines without supplementation with glucose67. This observation can be associated with determining mitochondrial damage. It is well known that in normal conditions cytochrome c is in mitochondria. When cytochrome c is released into the cytoplasm the non-inflammatory process of apoptosis is generated67. To track the amount of proteins in normal and cancer brain cell lines supplemented with glucose we have analyzed the intensity of the band at 1656 cm-1. One can see that the intensity of the Raman biomarker at 1656 cm−1 in NHA and CRL-1718 cell lines increases after glucose supplementation. For the glioblastoma cells, supplementation with glucose practically does not change the intensity of the Raman peaks at 1656 cm-1.
Comparing the Raman intensity at 750 cm-1 for control cells and cells supplemented with glucose in nucleus (Fig. 7) one can see that the intensity is higher for control cells for all analyzed cell lines. Moreover the Raman band at 750 cm-1 increases for control mildly malignant cells and then decreases for the most aggressive cell line. For the band at 1584 cm-1 one can see that this band after glucose supplementation in comparison to control cells decreased for all analyzed cell lines. The intensity of the Raman biomarker at 1444 cm-1 increased after glucose supplementation for NHA cell line. For astrocytoma and glioblastoma cell lines this band does not change statistically significantly after supplementation with glucose. The intensity of the Raman biomarker at 1656 cm−1 has a lower intensity for control cells than after glucose supplementation for all analyzed cell lines. Moreover, comparing the results for NHA and U-87 MG cell lines one can see that the intensity for the band at 1656 cm−1 is higher for 100 mM concentrations of glucose than for 50 mM.
Our results presented in Figs. 5, 6 and 7 confirm the importance of cytochrome c, lipids and proteins in brain cancer progression. In this manuscript, we have shown that glucose spectacularly modifies lipid droplets, mitochondrial functional activity and nucleus in human normal brain cells and human cancer brain cells. Simple sugars promote inflammation in the body and the production of some factors that stimulate cancer growth. Cancer cells, in their chaotic rate of division, are different from healthy cells. They are characterized by an "excessive appetite" for glucose, and this effect is known as the Warburg effect. This means that cancer cells prefer to convert glucose into energy inefficiently, even in the presence of oxygen, leading to the production of lactic acid. A team from Washington University School of Medicine in St. Louis, in experiments on mice, discovered, for example, that increased blood glucose levels quickly increase the concentration of beta-amyloid in the brain69.
Our results suggest that diabetes or other disorders that make it difficult to control sugar levels may have a harmful effect on brain function and intensify neurological problems and be one of the causes of brain cancer.
Conclusions
In this work, we present a comprehensive spectroscopic and chemometric analysis of the biochemical phenotype of control brain cell lines. The role of glucose in regulating cell metabolism has also been discussed. We have analyzed the metabolic response for glucose in normal astrocytes (NHA), astrocytoma (CRL-1718), and glioblastoma (U87-MG) cell lines. The biochemical modifications observed for brain cell lines supplemented with glucose enabled tracking changes in cellular metabolism. We have shown that glucose spectacularly modifies lipid droplets, mitochondrial functional activity and nucleus in human normal brain cells and human cancer brain cells by combining Raman spectroscopy with chemometric methods.
The PLS-DA chemometric analysis confirmed that the presence of the band characteristic for cytochrome c, lipids and proteins can be used as markers to distinguish the grade of human brain cell line supplemented with glucose.
Based on vibrational features we have shown that peaks at 750 cm-1, 1444 cm-1, 1584 cm-1 and 1656 cm-1 can be considered as Raman biomarkers to monitor the chemical composition of the lipid droplets, mitochondria and nucleus after glucose supplementation. Considering the associations between cytochromes, lipids, proteins and brain cancer aggressiveness we have shown that these compounds regulate biochemical modifications in lipid droplets, mitochondria and nucleus during brain cancer development.
Presented results suggest that Raman spectroscopy combined with chemometric methods, in the future, could be an alternative technique for monitoring the role of glucose during brain cancer development.
Acknowledgements
This work was supported by the National Science Centre of Poland (Narodowe Centrum Nauki, UMO-2021/43/B/ST4/01547).
Author contributions
Conceptualization: M.K.; Funding acquisition: H.A. Investigation: M.K., J.S.; Methodology: M.K., K.B-M., J.S. Sample preparation: K.B-M., J.S. Writing – original draft: M.K.; Manuscript editing: M.K.; Manuscript reviewing: M.K., K.B-M., J.S., H.A. All authors reviewed and provided feedback on the manuscripts. All authors have read and agreed to the published version of the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Monika Kopec, Email: monika.kopec@p.lodz.pl.
Halina Abramczyk, Email: halina.abramczyk@p.lodz.pl.
References
- 1.Wolever TM, Miller JB. Sugars and blood glucose control. Am J Clin Nutr. 1995;62(1 Suppl):212S-221S; discussion 221S-227S. 10.1093/ajcn/62.1.212S [DOI] [PubMed]
- 2.Ritter S. Monitoring and Maintenance of Brain Glucose Supply: Importance of Hindbrain Catecholamine Neurons in This Multifaceted Task. Published online 2017. http://europepmc.org/books/NBK453140 [PubMed]
- 3.Hantzidiamantis PJ, Lappin SL. Physiology, Glucose. StatPearls Publishing, Treasure Island (FL); 2023. http://europepmc.org/books/NBK545201 [PubMed]
- 4.Cutshaw G, Uthaman S, Hassan N, Kothadiya S, Wen X, Bardhan R. The emerging role of raman spectroscopy as an omics approach for metabolic profiling and biomarker detection toward precision medicine. Chem Rev. 2023;123(13):8297–8346. doi: 10.1021/acs.chemrev.2c00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill IE, Boyd M, Milligan K, et al. Understanding radiation response and cell cycle variation in brain tumour cells using Raman spectroscopy. Analyst. 2023;148(11):2594–2608. doi: 10.1039/d3an00121k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milligan K, Deng X, Shreeves P, et al. Raman spectroscopy and group and basis-restricted non negative matrix factorisation identifies radiation induced metabolic changes in human cancer cells. Sci Rep. 2021;11(1):1–11. doi: 10.1038/s41598-021-83343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng X, Ali-Adeeb R, Andrews JL, et al. Monitor ionizing radiation-induced cellular responses with raman spectroscopy, non-negative matrix factorization, and non-negative least squares. Appl. Spectrosc. 2020;74(6):701–711. doi: 10.1177/0003702820906221. [DOI] [PubMed] [Google Scholar]
- 8.Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed. Pharmacother. 2018;107:306–328. doi: 10.1016/j.biopha.2018.07.157. [DOI] [PubMed] [Google Scholar]
- 9.Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc. Diabetol. 2002;1(1):1. doi: 10.1186/1475-2840-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park C, Pagnini F, Langer E. Glucose metabolism responds to perceived sugar intake more than actual sugar intake. Sci. Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-72501-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit. Rev. Clin. Lab Sci. 2016;53(1):52–67. doi: 10.3109/10408363.2015.1084990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappe CL, Peters B, Dommisch H, Woelber JP, Pivovarova-Ramich O. Effects of reducing free sugars on 24-hour glucose profiles and glycemic variability in subjects without diabetes. Front. Nutrit. 2023;2(10):1213661. doi: 10.3389/fnut.2023.1213661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gannon MC, Nuttall FQ. Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutr. Metab. 2006;3:1–8. doi: 10.1186/1743-7075-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieri S, Muti P, Claudia A, et al. Prospective study on the role of glucose metabolism in breast cancer occurrence. Int. J. cancer. 2012;130(4):921–929. doi: 10.1002/ijc.26071. [DOI] [PubMed] [Google Scholar]
- 15.Raza U, Asif MR, Bin RA, Sheikh A. Hyperlipidemia and hyper glycaemia in Breast Cancer Patients is related to disease stage. Pakistan J. Med. Sci. 2018;34(1):209–214. doi: 10.12669/pjms.341.14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contiero P, Berrino F, Tagliabue G, et al. Fasting blood glucose and long-term prognosis of non-metastatic breast cancer: a cohort study. Breast Cancer Res. Treat. 2013;138(3):951–959. doi: 10.1007/s10549-013-2519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aranceta Bartrina J, Pérez RC. Association between sucrose intake and cancer: a review of the evidence. Nutr. Hosp. 2013;28(Suppl 4):95–105. doi: 10.3305/nh.2013.28.sup4.6802. [DOI] [PubMed] [Google Scholar]
- 18.Malik VS, Popkin BM, Bray GA, Després JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riise HKR, Igland J, Sulo G, et al. Casual blood glucose and subsequent cardiovascular disease and all-cause mortality among 159 731 participants in Cohort of Norway (CONOR) BMJ Open Diabetes Res. Care. 2021 doi: 10.1136/bmjdrc-2020-001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittig R, Coy JF. The role of glucose metabolism and glucose-associated signalling in cancer. Perspect Medicin Chem. 2007;1:11773910700100006. doi: 10.1177/1177391X0700100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieri S, Muti P, Claudia A, et al. Prospective study on the role of glucose metabolism in breast cancer occurrence. Int J. Cancer. 2012;130(4):921–929. doi: 10.1002/ijc.26071. [DOI] [PubMed] [Google Scholar]
- 22.Poursaitidis I, Lamb RF. Metabolism in pancreatic cancer. Pancreat Cancer. Published online 2018:1379–1400. 10.1007/978-1-4939-7193-0_68
- 23.Kellenberger LD, Bruin JE, Greenaway J, Campbell NE, Moorehead RA, Holloway AC, Petrik J. The role of dysregulated glucose metabolism in epithelial ovarian cancer. J. Oncol. 2010;2010(1):514310. doi: 10.1155/2010/514310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cutruzzolà F, Giardina G, Marani M, et al. Glucose metabolism in the progression of prostate cancer. Front. Physiol. 2017 doi: 10.3389/fphys.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai XJ, Zhang JY, Zhang AB, Zhou X, Zhang HY, Li TJ. Emerging role of high glucose levels in cancer progression and therapy. Chin. J. Dent. Res. 2022;25(1):11–20. doi: 10.3290/j.cjdr.b2752695. [DOI] [PubMed] [Google Scholar]
- 26.Zhan Y-S, Feng L, Tang S-H, et al. Glucose metabolism disorders in cancer patients in a Chinese population. Med. Oncol. 2010;27(2):177–184. doi: 10.1007/s12032-009-9189-9. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Chen Y-J, Chang L-J. Fasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012;76(2):242–247. doi: 10.1016/j.lungcan.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Stewart KL, Gigic B, Himbert C, et al. Association of sugar intake with inflammation- and angiogenesis-related biomarkers in newly diagnosed colorectal cancer patients. Nutr Cancer. 2022;74(5):1636–1643. doi: 10.1080/01635581.2021.1957133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCullough ML, Hodge RA, Campbell PT, Guinter MA, Patel AV. Sugar- and artificially-sweetened beverages and cancer mortality in a large US prospective cohort. Cancer Epidemiol. Biomark. Prev a Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2022;31(10):1907–1918. doi: 10.1158/1055-9965.EPI-22-0392. [DOI] [PubMed] [Google Scholar]
- 30.Debras C, Chazelas E, Srour B, et al. Total and added sugar intakes, sugar types, and cancer risk: Results from the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2020;112(5):1267–1279. doi: 10.1093/ajcn/nqaa246. [DOI] [PubMed] [Google Scholar]
- 31.Laguna JC, Alegret M, Cofán M, et al. Simple sugar intake and cancer incidence, cancer mortality and all-cause mortality: a cohort study from the PREDIMED trial. Clin. Nutr. 2021;40(10):5269–5277. doi: 10.1016/j.clnu.2021.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Epner M, Yang P, Wagner RW, Cohen L. Understanding the link between sugar and cancer: an examination of the preclinical and clinical evidence. Cancers (Basel) 2022 doi: 10.3390/cancers14246042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun S, Sun Y, Rong X, Bai L. 2019. High glucose promotes breast cancer proliferation and metastasis by impairing angiotensinogen expression. Biosci. Rep. [DOI] [PMC free article] [PubMed]
- 34.Liao W-C, Tu Y-K, Wu M-S, Lin J-T, Wang H-P, Chien K-L. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ. 2015;350:g7371. doi: 10.1136/bmj.g7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283(19):2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 36.Bielecka-Wajdman AM, Ludyga T, Smyk D, et al. Glucose influences the response of glioblastoma cells to temozolomide and dexamethasone. Cancer Control. 2022;29:10732748221075468. doi: 10.1177/10732748221075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onikanni SA, Lawal B, Bakare OS, et al. Cancer of the liver and its relationship with diabetes mellitus. Technol Cancer Res. Treat. 2022;21:15330338221119744. doi: 10.1177/15330338221119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sripetchwandee J, Chattipakorn N, Chattipakorn SC. Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia. Front. Endocrinol. (Lausanne) 2018;9:1–16. doi: 10.3389/fendo.2018.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Himsworth HP. The syndrome of diabetes mellitus and its causes. Lancet. 1949;253(6551):465–473. doi: 10.1016/S0140-6736(49)90797-7. [DOI] [PubMed] [Google Scholar]
- 40.Kumar V, Kim S-H, Bishayee K. Dysfunctional glucose metabolism in Alzheimer’s disease onset and potential pharmacological interventions. Int. J. Mol. Sci. 2022 doi: 10.3390/ijms23179540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Chen C, Chen H, et al. Brain glucose activated mri contrast agent for early diagnosis of Alzheimer’s disease. Anal. Chem. 2022;94(46):16213–16221. doi: 10.1021/acs.analchem.2c03765. [DOI] [PubMed] [Google Scholar]
- 42.Dewanjee S, Chakraborty P, Bhattacharya H, et al. Altered glucose metabolism in Alzheimer’s disease: role of mitochondrial dysfunction and oxidative stress. Free Radic Biol Med. 2022;193:134–157. doi: 10.1016/j.freeradbiomed.2022.09.032. [DOI] [PubMed] [Google Scholar]
- 43.Duran-Aniotz C, Hetz C. Glucose metabolism: a sweet relief of ALZHEIMER’S disease. Curr Biol. 2016;26(17):R806–R809. doi: 10.1016/j.cub.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 44.Yoon JH, Hwang JH, Son SU, et al. How can insulin resistance cause alzheimer’s disease? Int. J. Mol. Sci. 2023 doi: 10.3390/ijms24043506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williamson R, McNeilly A, Sutherland C. Insulin resistance in the brain: an old-age or new-age problem? Biochem. Pharmacol. 2012;84(6):737–745. doi: 10.1016/j.bcp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Huang C-C, Chung C-M, Leu H-B, et al. Diabetes mellitus and the risk of Alzheimer’s disease: a nationwide population-based study. PLoS ONE. 2014;9(1):e87095. doi: 10.1371/journal.pone.0087095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EJ, Cho SS, Jeong Y, et al. Glucose metabolism in early onset versus late onset Alzheimer’s disease: an SPM analysis of 120 patients. Brain. 2005;128(8):1790–1801. doi: 10.1093/brain/awh539. [DOI] [PubMed] [Google Scholar]
- 48.Hammond TC, Lin A-L. Glucose metabolism is a better marker for predicting clinical alzheimer’s disease than amyloid or tau. J. Cell Immunol. 2022;4(1):15–18. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Tong T, Chang A, et al. Midlife lipid and glucose levels are associated with Alzheimer’s disease. Alzheimer’s Dement. 2023;19(1):181–193. doi: 10.1002/alz.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barhwal K, Das SK, Kumar A, Hota SK, Srivastava RB. Insulin receptor A and Sirtuin 1 synergistically improve learning and spatial memory following chronic salidroside treatment during hypoxia. J. Neurochem. 2015;135(2):332–346. doi: 10.1111/jnc.13225. [DOI] [PubMed] [Google Scholar]
- 51.Beirami E, Oryan S, Seyedhosseini Tamijani SM, Ahmadiani A, Dargahi L. Intranasal insulin treatment restores cognitive deficits and insulin signaling impairment induced by repeated methamphetamine exposure. J. Cell Biochem. 2018;119(2):2345–2355. doi: 10.1002/jcb.26398. [DOI] [PubMed] [Google Scholar]
- 52.Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88(13–14):619–627. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2012;153(1):329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- 54.Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91(11–12):409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Kopeć M, Beton K, Jarczewska KAH. Hyperglycemia and cancer in human lung carcinoma by means of Raman spectroscopy and imaging. Sci. Rep. 2022;12:18561. doi: 10.1038/s41598-022-21483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopec M, Beton-Mysur K. The role of glucose and fructose on lipid droplet metabolism in human normal bronchial and cancer lung cells by Raman spectroscopy. Chem. Phys. Lipids. 2023;2023(259):105375. doi: 10.1016/j.chemphyslip.2023.105375. [DOI] [PubMed] [Google Scholar]
- 57.Kopeć M, Beton-Mysur K, Abramczyk H. Biochemical changes in lipid and protein metabolism caused by mannose-Raman spectroscopy studies. Analyst. 2024 doi: 10.1039/d4an00128a. [DOI] [PubMed] [Google Scholar]
- 58.Halina A, Beata Brozek-Pluska MK. Double face of cytochrome c in cancers by Raman imaging. Sci. Rep. 2022 doi: 10.1038/s41598-022-04803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beton-Mysur K, Brożek-Płuska B. A new modality for cholesterol impact tracking in colon cancer development - Raman imaging, fluorescence and AFM studies combined with chemometric analysis. Anal. Methods. 2023;15(39):5199–5217. doi: 10.1039/d3ay01040f. [DOI] [PubMed] [Google Scholar]
- 60.Kopec M, Beton-Mysur K, Abramczyk H. Raman imaging and chemometric methods in human normal bronchial and cancer lung cells: Raman biomarkers of lipid reprogramming. Chem. Phys. Lipids. 2023;257(July):105339. doi: 10.1016/j.chemphyslip.2023.105339. [DOI] [PubMed] [Google Scholar]
- 61.Brozek-Pluska B. Statistics assisted analysis of Raman spectra and imaging of human colon cell lines – Label free, spectroscopic diagnostics of colorectal cancer. J. Mol. Struct. 2020;1218:128524. doi: 10.1016/j.molstruc.2020.128524. [DOI] [Google Scholar]
- 62.Movasaghi Z, Rehman S, Rehman IU. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007;42(5):493–541. doi: 10.1080/05704920701551530. [DOI] [Google Scholar]
- 63.Sciences N, Chemistry A, Centre I, Kingdom U. BBA - M olecular Cell Research Raman microscopy reveals how cell inflammation activates glucose and lipid metabolism A lek sa n d ra B o re k -D o ro s z a , A n n a P ie c za ra b , c , J a go d a O rle a n s k a a , c , K r z y s z to f B r z o z o w s . 2023;(August).
- 64.Ralhan I, Chang C-L, Lippincott-Schwartz J, Ioannou MS. Lipid droplets in the nervous system. J. Cell Biol. 2021 doi: 10.1083/jcb.202102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao X, Zhang S, Sanders AR, Duan J. Brain lipids and lipid droplet dysregulation in alzheimer’s disease and neuropsychiatric disorders. Complex Psych. 2023;9(1–4):154–171. doi: 10.1159/000535131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seyfried TN, Kiebish MA, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Metabolic management of brain cancer. Biochim. Biophys. Acta - Bioenerg. 2011;1807(6):577–594. doi: 10.1016/j.bbabio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Abramczyk H, Surmacki JM, Brozek-Pluska B, Kopec M. Revision of Commonly Accepted Warburg Mechanism of Cancer Development : Redox-Sensitive Mitochondrial Cytochromes in Breast and Brain Cancers by Raman Imaging. Cancers (Basel). Published online 2021. [DOI] [PMC free article] [PubMed]
- 68.Xu S, Zhang X, Liu P. Lipid droplet proteins and metabolic diseases. Biochim. Biophys. Acta - Mol Basis Dis. 2018;1864(5):1968–1983. doi: 10.1016/j.bbadis.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 69.DiNicolantonio JJ, O’Keefe JH, Wilson WL. Sugar addiction: is it real? A narrative review. Br. J. Sports Med. 2018;52(14):910–913. doi: 10.1136/bjsports-2017-097971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sun S, Sun Y, Rong X, Bai L. 2019. High glucose promotes breast cancer proliferation and metastasis by impairing angiotensinogen expression. Biosci. Rep. [DOI] [PMC free article] [PubMed]
Data Availability Statement
No datasets were generated or analysed during the current study.