Abstract
Introduction
Lobar torsion is a rare, challenging diagnosis that requires a high index of suspicion and prompt investigation and management. Detection and urgent fixation or lung resection are critical to avoid catastrophic sequelae of lung necrosis, bronchopleural fistulae, and death.
Report
A case of lobar torsion following open repair of a type B aortic dissection and thoraco-abdominal aortic aneurysm in a patient with Marfan syndrome is presented. After a non-specific constellation of symptoms, the diagnosis was confirmed with computed tomography and bronchoscopic findings and the patient underwent detorsion and plication of a torted, yet viable, left upper lobe on post-operative day 6. The patient is currently being followed with serial imaging to follow a necrotic consolidation of the left upper lobe.
Discussion
This was a case of lobar torsion in a patient with Marfan syndrome and the degree of connective tissue disease may have predisposed the patient to this rare surgical complication. The case presents a challenging dilemma due to the risks associated with exposing a synthetic aortic graft to a potentially infected space if lobar resection or a pneumonectomy was performed.
Keywords: Aortic dissection, Lobar ischaemia, Lobar torsion, Marfan syndrome, Thoracoabdominal aneurysm
Highlights
-
•
This report involves the multidisciplinary diagnostic and surgical approach to a case of post-operative lobar torsion following a thoraco-abdominal aortic aneurysm repair in a patient with Marfan syndrome.
-
•
Faced with the dilemma of exposing a synthetic aortic graft to a potentially infected space, a decision was made to proceed with lung detorsion and plication rather than lobar resection or total pneumonectomy.
-
•
Despite radiological evidence of lung involution and fibrosis, the patient is clinically doing well and not requiring long term antimicrobial therapy.
-
•
This is the first reported case of lobar torsion in a patient with Marfan syndrome. It is speculated that the presence of abnormal connective tissue may have potentiated this complication.
Introduction
Lobar torsion is a life threatening condition that results from rotation of the bronchovascular pedicle, causing airway, vascular, and lymphatic occlusion of the involved lobes.1 The incidence of post-operative lobar torsion varies from 0.089–0.3% in the current literature and is mainly reported as a complication of thoracic surgery (62.4%) with the right middle lobe being the most affected site after ipsilateral lobectomy.1, 2, 3 Lobar torsion has also been described to occur spontaneously (29.4%) and after blunt trauma (8.3%).3
Lobar torsion can be a difficult diagnosis to confirm since patients often develop non-specific symptoms such as fever, chest discomfort, dyspnoea, cough, and infrequently haemoptysis, mimicking presentations of infection and atelectasis.3,4 For individuals with suspected lobar torsion, contrast enhanced thoracic computed tomography (CT) angiography and bronchoscopy are the suggested initial diagnostic investigations.3, 4, 5, 6
Unrecognised lobar torsion or delayed surgical intervention carry a high risk of death secondary to lung gangrene and overwhelming sepsis.4,6 Devastating sequelae of delayed or untreated lobar torsion include recurrent infections, chronic pulmonary oedema, and thrombo-embolic complications.4,7
Report
This is the case of a 35 year old patient with Marfan syndrome who was followed for a type B3,11aortic dissection and thoraco-abdominal aortic aneurysm that progressed despite optimal blood pressure control. The patient's past medical history included a 50 mm aortic root aneurysm and mitral valve regurgitation treated with valve sparing aortic root reconstruction with mitral valve repair three years previously, and remote scoliosis surgery.
Given the progression of dissection and aneurysm, the patient underwent open repair via a left thoracotomy approach; the proximal descending thoracic aorta was repaired with a 28 mm Dacron tube graft (Gelsoft Plus, Vascutek, Terumo Aortic, Scotland) under cardiopulmonary bypass and deep hypothermia. A bifurcated aortobi-iliac graft (Gelsoft Plus, Vascutek, Terumo Aortic, Inchinnan, UK) was used to repair the thoraco-abdominal aneurysm. Chest tubes were inserted on the left side. The patient was transferred to the intensive care unit (ICU) for post-operative care.
In the immediate post-operative period, the patient's first X-ray showed a residual left sided apical pneumothorax as well as bilateral opacities predominating on the left (Fig. 1). On the second and third post-operative days, the opacities on the left side persisted, despite successful weaning of mechanical ventilation and of supplementary oxygen. Prior to extubation, the ICU team performed a bedside bronchoscopy to investigate the mismatch in the pulmonary imaging and the clinical evolution of the patient. Apart from some clear bronchial secretions, the bronchoscopic findings were unremarkable, but no detailed report was provided.
Figure 1.
Plain chest X-ray. Post-operative day 1 showing near complete white out of the left lung.
The patient was extubated successfully and transferred out of the intensive care unit without supplementary oxygen requirements. Within 24 hours, the patient had his first episode of non-massive haemoptysis and noted increased work of breathing associated with pleuritic left sided chest pain. He remained afebrile, haemodynamically stable, and was saturating at 100% on room air. On physical examination, he had poor air entry over the left lung field. His laboratory work showed leucocytosis (white blood cells 21 × 109/L) and elevated C-reactive protein (385 mg/L).
The patient underwent an urgent thoracic CT angiogram, which showed a tapered obliteration of the proximal left upper pulmonary artery, obliterated left upper bronchus, and poorly enhancing left upper and lingular lobe consolidated atelectasis (Fig. 2). There was a patent left lower pulmonary artery; however, the left lower lobe bronchus was not well visualised. There was also enhancing left lower lobe consolidated atelectasis.
Figure 2.
Computed tomography angiography (CTA) of the chest. Post-operative day 5. (A) Coronal cross section: tapered obliteration (arrow) of the proximal left upper pulmonary artery with swirl sign at the left apex. There is obliteration of the left upper lobe and lingular lobe bronchi. Loss of normal parenchymal enhancement with consolidated atelectasis of the left upper and lingular lung lobes with cavitations consistent with pulmonary infarction. (B) Axial cross section. (C) 3D reconstruction.
A diagnosis of left upper lobar torsion was suspected. This was confirmed by repeat bronchoscopy where the bifurcation to the left upper and lower lobes were not visualised.
On post-operative day 6, the patient underwent a thoracotomy via the previous incision in the lateral decubitus position. The left upper lobe appeared torted and congested while the lower lobe was ventilating well. The patient was placed on full cardiopulmonary bypass to alleviate the risk of manipulating the lung off bypass and to allow operating in a less congested thoracic cavity without a filled heart. Ventilation was maintained with double lumen intubation, and the left upper lobe was detorted manually and began to ventilate well.
The left upper lobe appeared significantly congested; however, there were no signs of necrosis, infection, or abscess. Given the viable appearance and perfusion of the lung, it was left intact and was plicated at three points to avoid further torsion events. To reduce the risk of future graft infections of the thoracic aortic graft, a serratus flap was mobilised and brought down onto the descending thoracic aorta.
The patient self-extubated on post-operative day 7 and was re-intubated on post-operative day 11 for persistent hypercapnic respiratory failure secondary to multifocal pneumonia and presumed left upper lobe infarction. Bronchoscopy done on post-operative day 17 due to ongoing haemoptysis identified diffuse oedema of the left lung with friable mucosa. The patient was eventually extubated on post-operative day 19 and recovered. The patient continued to receive antibiotics during his admission and was discharged with a course of oral antibiotics with a total duration of antibiotic therapy of approximately eight weeks.
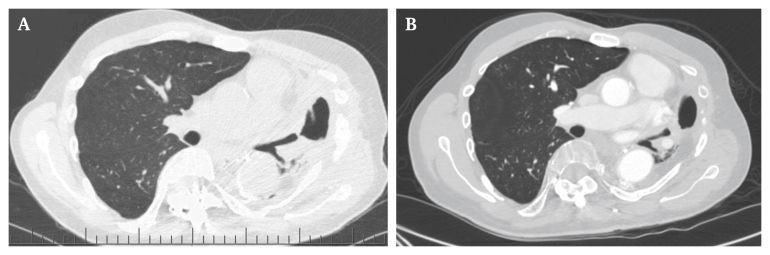
During scheduled follow up eight weeks post-operatively, the patient had imaging findings consistent with progression of left upper lobe necrotic consolidation with a residual gradually improving productive cough with brown sputum (Fig. 3A). At six months, the patient was doing well clinically with mild respiratory symptoms, such as cough and bronchiectasis, and stable repeat imaging (Fig. 3B). The main symptom was dyspnoea on exertion; however, he was quite functional without major limitations in daily activities. There have not been any episodes of pneumonia following discharge from hospital. The patient will continue to be followed as an outpatient at six month intervals.
Figure 3.
Follow-up imaging. (A) Outpatient computed tomography angiography (CTA) eight weeks post-operatively: Left upper lobe necrotic consolidation. Patent central airways with necrotic consolidation and cavitation involving the left upper lobe and lingula associated with pleural thickening in the left lung apex. (B) Outpatient CTA six months post-operatively. Stable imaging with no significant changes from the previous scan.
Discussion
Marfan syndrome is a rare genetic connective tissue disorder with a mainly autosomal dominant mode of inheritance with various complex systemic manifestations.8 While the most likely direct cause of lung torsion in this case was iatrogenic during the index thoracotomy, it is speculated that the presence of abnormal connective tissue may have potentiated this complication.
Interestingly, the patient was not hypoxic at the time of the lung torsion diagnosis.
Hypoxaemia may result from intrapulmonary shunting, alveolar hyperventilation, and ventilation/perfusion (V/Q) mismatch. Since the left upper lobe was completely rotated (180°) causing total vascular obstruction, the lobe was not perfused, and no shunting occurred to generate hypoxaemia. Compression of the pulmonary veins is believed to have caused vascular congestion and resulting haemoptysis.
Despite detorsion taking place six days after the index thoracotomy, the left upper lobe appeared congested but visibly viable, with no apparent signs of necrosis. The CT angiography study showed complete occlusion of the left upper pulmonary artery.
An intra-operative concern was judging whether the left upper lobe would be viable or spared. This presented a dilemma of deciding whether to proceed with detorsion versus an upper lobectomy or committing the patient to a pneumonectomy.
Although lobectomy and detorsion in viable lungs have shown no difference in survival, and given the prolonged ischaemia time, an argument could have been made to proceed with a lobectomy.3 However, given the extensive aortic surgery and dissection, the patient probably had no remaining bronchial circulation as it would most likely have been interrupted in the process of the aneurysm repair. Additionally, there were concerns regarding bronchial stump perfusion and subsequent stump healing, as well as the risk of creating an infected post-pneumonectomy adjacent to a long, synthetic aortic tube graft.
Close multidisciplinary involvement from multiple teams including respiratory medicine, radiology, thoracic surgery, and the ICU was necessary to arrive at a diagnosis and surgical detorsion. While the patient had a prolonged post-operative inpatient stay, he is currently doing well and is not receiving antimicrobial therapy.
Even though the left lung has mostly involuted and fibrosed due to the prolonged ischaemia time (Fig. 3), this has occurred in a manner that has not compromised the aortic graft and did not expose the patient to the problems relating to a post-pneumonectomy space.
In conclusion, lobar torsion is a rare, potentially fatal post-operative complication. While it is a diagnostic challenge, surgeons should be aware of lobar torsion as a differential diagnosis and the symptoms that warrant urgent investigation and surgical management.
While most reported cases of lobar torsion involve resection, a case is presented with an interesting dilemma due to extensive dissection of the bronchial circulation and the presence of an aortic graft.3,7 After weighing the risks involved, detorsion and plication were carried out with a favourable post-operative outcome.
Conflict of interest statement and funding statement
None.
References
- 1.Cable D.G., Deschamps C., Allen M.S., Miller D.L., Nichols F.C., Trastek V.F., et al. Lobar torsion after pulmonary resection: presentation and outcome. J Thorac Cardiovasc Surg. 2001;122:1091–1093. doi: 10.1067/mtc.2001.117839. [DOI] [PubMed] [Google Scholar]
- 2.Larsson S., Lepore V., Dernevik L., Nilsson F., Selin K. Torsion of a lung lobe: diagnosis and treatment. Thorac Cardiovasc Surg. 1988;36:281–283. doi: 10.1055/s-2007-1020097. [DOI] [PubMed] [Google Scholar]
- 3.Dai J., Xie D., Wang H., He W., Zhou Y., Hernández-Arenas L.A., et al. Predictors of survival in lung torsion: a systematic review and pooled analysis. J Thorac Cardiovasc Surg. 2016;152:737–745. doi: 10.1016/j.jtcvs.2016.03.077. [DOI] [PubMed] [Google Scholar]
- 4.Taira N., Kawasaki H., Takahara S., Furugen T., Atsumi E., Ichi T., et al. Postoperative lung torsion with retained viability: the presentation and surgical indications. Heart Lung Circ. 2018;27:849–852. doi: 10.1016/j.hlc.2017.06.733. [DOI] [PubMed] [Google Scholar]
- 5.Hammer M.M., Madan R. Clinical and imaging features in lung torsion and description of a novel imaging sign. Emerg Radiol. 2018;25:121–127. doi: 10.1007/s10140-017-1563-x. [DOI] [PubMed] [Google Scholar]
- 6.Childs L., Ellis S., Francies O. Pulmonary lobar torsion: a rare complication following pulmonary resection, but one not to miss. BJR Case Rep. 2017;3 doi: 10.1259/bjrcr.20160010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alassar A., Marchbank A. Left lower lobe torsion following upper lobectomy-prompt recognition and treatment improve survival. J Surg Case Rep. 2014;2014:rju078. doi: 10.1093/jscr/rju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judge D.P., Dietz H.C. Marfan's syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]