Abstract
Background and Aims
The diagnosis of acute kidney injury (AKI) is of importance among patients with ST segment elevation (STEMI) undergoing primary coronary intervention (PCI). It is often delayed given the need in serial measurements of creatinine or other serum markers. Neutrophil gelatinase‐associated lipocalin (NGAL) is a proven marker for AKI, although its role as an early predictor in this setting was scarcely addressed before and was the aim of our study.
Methods
Prospective observational study including 133 patients with STEMI treated with PCI. Plasma NGAL was drawn immediately before PCI (NGAL‐0) and 24 h after (NGAL‐24). Similar analysis of C‐reactive protein (CRP) was performed for additional comparison.
Results
Mean age was 62 ± 13 years, 78% were men, and 20 (15%) developed AKI after admission. Patients with AKI after admission demonstrated higher levels of NGAL‐0 (164 vs. 95 ng/mL; p < 0.001) and NGAL‐24 (142 vs. 93 ng/mL; p < 0.001). Levels of NGAL‐0 and NGAL‐24 were similar within the AKI and non‐AKI groups. Using ROC curve analysis, NGAL‐0 had best predictive ability for AKI development (AUC 0.841, 95% CI 0.80–0.96), compared with NGAL‐24 (0.783, 95% CI 0.74–0.85), CRP‐0 (0.701, 95% CI 0.58–0.83), and CRP‐24 (0.781, 95% CI 0.66–0.90). The optimal NGAL‐0 cutoff for AKI prediction was 125 ng/mL, with 70% sensitivity, 84% specificity, and 94% negative predictive value.
Conclusions
Among STEMI patients, NGAL measurement upon admission are associated with AKI and may serve as a reliable marker for early AKI detection. Future studies may direct risk stratification using this single test can direct personalized evaluations during the admission, and focused interventions to prevent AKI.
Keywords: ACS, AKI, cardiorenal, NGAL, prediction
1. BACKGROUND
Acute kidney injury (AKI) is well‐known complication in patients with acute coronary syndrome (ACS). 1 , 2 Contrast‐induced nephropathy is considered a major determinant of AKI, and while it mainly relates to the amount of contrast material and to preprocedural renal function, 1 , 2 , 3 recent data points to more complex underlining mechanisms involving cardio‐renal interactions. 4 , 5 , 6 Although current recommendations suggest serum creatinine (sCr) as the gold standard for AKI detection, increased levels are known to be delayed following renal injurious event such as contrast media‐induced injury. 7 , 8 , 9 Neutrophil gelatinase‐associated lipocalin (NGAL) is a 25 kDa protein produced by various cell types. NGAL is released in response to cellular stress and inflammation, particularly by renal tubular epithelial cells, following acute tubular damage. NGAL levels can aid in the early detection of AKI in different clinical settings, including contrast induced AKI (CI‐AKI) (12–21). Currently, there is only limited data on the optimal timing for NGAL testing, its optimal cut‐off values, and its utilization for ruling out AKI. In the present research, we analyzed the predictive ability of early baseline NGAL compared to a 24‐h NGAL test and another commonly used marker (c‐reactive protein). By doing so, we aimed to investigate the diagnostic usefulness of NGAL, measured at separate time points, for the prediction of renal injury among STEMI patients. We hypothesized based on previous data, that if proven effective, a baseline NGAL test could help in risk‐stratification of patients and lead to better AKI prevention, follow‐up, and lowered costs.
2. METHODS
2.1. Patients
A prospective, open‐label, observational trial was performed in the Tel Aviv Sourasky Medical Center. We included patients with STEMI admitted to our intensive care unit. All patients underwent primary PCI and were admitted between 2019 and 2021. The diagnosis of STEMI was based on typical history of chest pain, diagnostic electrocardiographic (ECG) changes, and persistent elevation of serum cardiac biomarkers. 10 Patients with chronic inflammation were excluded as NGAL levels in this subset of patients can be attributed to their baseline condition. These conditions included diagnosed chronic inflammation or malignancy (n = 19). In addition, patients with advanced, endstage renal disease were also excluded (n = 4). No patients were lost to follow‐up. The final study cohort was comprised of 133 patients. Patients with onset of symptoms ≤12 h underwent primary PCI as well as patients with symptoms lasting 12–24 h that presented with ongoing pain upon admission. The contrast medium used in procedures was iodixanol (Visipaque, GE healthcare, Ireland). All patients were intended to receive post‐procedural hydration with 0.9% normal (physiologic) saline at a rate of 1 mL/kg/h for 12 h yet physician discretion was used to modify this rate in any event of overt heart failure.
2.2. Laboratory evaluation
We collected blood samples for NGAL measurement in two separate occasions. First, upon admission and before any intervention or contrast exposure (NGAL‐0), and second within 24 h and after planned intervention (NGAL‐24). Samples were drawn in EDTA collection tubes and centrifuged within 10 min. Plasma was stored at −80°C until analysis. NGAL levels were measured using a turbidimetric immunoassay (Bioporto Diagnostics) on a Cobas 502 clinical chemistry analyzer (Roche).
Serial measurements of serum creatinine were taken during each patient hospitalization and at least once every day. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation. 11 Chronic kidney disease (CKD) was categorized as admission eGFR of ≤60 mL/min/1.73 m². AKI was the study's main outcome and was determined using the KDIGO criteria 12 —an increase of more than 0.3 mg/dL in serum creatinine within 48 h or an elevation ≥1.5 times from baseline values that is presumed to occur within 7 days.
Blood samples for determination of high‐sensitive c‐reactive protein (CRP) were drawn in all patients upon admission to the emergency department or at the catheterization laboratory, before primary PCI (CRP‐0). A second sample of high‐sensitive CRP (CRP‐24) was drawn following primary PCI, within 12–24 h from CICU admission (mean 16 ± 4 h).
2.3. Statistics
Patients were stratified according to presence of AKI. All data is summarized and displayed as mean with standard deviation or median with IQR for continuous variables and as number (percentage) of patients in each group for categorical variables. To compare patients with and without AKI, independent sample t‐test or Mann–Whitney U test were used for continuous variables, and Fisher's exact test for categorical variables. Receiver operator characteristic (ROC) curve analysis was performed to identify the optimal cutoff point of NGAL‐0 and NGAL‐24 (at which sensitivity and specificity would be maximal) for the prediction of AKI. Areas under the curve (AUC) were calculated as measures of the accuracy of the tests. Multivariate logistic regression analysis was used to assess NGAL as an independent predictor for AKI, and included baseline characteristics with significant associations with AKI. A two‐tailed p value of <0.05 was defined as significant for all analyses. All analyses were performed with the SPSS 20.0 software (SPSS Inc.).
3. RESULTS
One‐hundred and thirty‐three STEMI patients were included in the final analysis (mean age was 62 with 13 years SD, 78% of them were men), 20 of whom (15%) developed AKI. The median time for AKI diagnosis, based on sCr changes was 36 h following admission (IQR 24–48 h). Baseline characteristics are presented in Table 1 for patients with and without AKI. Patients developing AKI tended to be older, had higher rates of hypertension, and lower baseline eGFR or left‐ventricular ejection fraction. Most importantly, the presence of baseline CKD was prominent, with 70% of patients developing AKI having baseline CKD compared with only 11% in those without CKD. Median (IQR) baseline creatinine in the study cohort was 0.86 (0.72–1.00) and at 24‐h 0.91 (0.79–1.04).
Table 1.
Baseline variables of the study cohort and comparison between patients with and without acute kidney injury (AKI).
| Variable | Total | No AKI n = 113 | AKI n = 20 | p |
|---|---|---|---|---|
| Age, years (mean ± SD) | 65 ± 12 | 64 ± 13 | 71 ± 9 | 0.02 |
| Female sex, n (%) | 23 (17) | 19 (17) | 4 (20) | 0.73 |
| Hyperlipidemia, n (%) | 73 (55) | 61 (54) | 12 (60) | 0.62 |
| Hypertension, n (%) | 71 (53) | 53 (47) | 18 (90) | <0.001 |
| Diabetes, n (%) | 38 (29) | 32 (28) | 6 (30) | 0.88 |
| Smoker, n (%) | 66 (50) | 59 (52) | 7 (35) | 0.16 |
| Chronic kidney disease, n (%) | 26 (19) | 12 (11) | 14 (70) | <0.001 |
| Past myocardial infarction, n (%) | 31 (23) | 27 (24) | 4 (20) | 0.71 |
| Left ventricle EF (mean ± SD) | 46 ± 8 | 47 ± 8 | 43 ± 8 | 0.08 |
| Baseline creatinine | 0.86 (0.72–1.00) | 0.82 (0.69–0.97) | 1.15 (0.94–1.41) | <0.001 |
| 24‐h Creatinine | 0.91 (0.79–1.04) | 0.87 (0.77–0.98) | 1.48 (1.14–1.67) | <0.001 |
Abbreviations: EF, ejection fraction; SD, standard deviation.
Laboratory analysis is presented in Table 2. Patients with AKI were found to have higher plasma NGAL levels on admission (median NGAL‐0 148 ng/mL [117–202] vs. 95 ng/mL [73–116]; p < 0.001) and at 24 h (median NGAL‐24 147 ng/mL [98–195] vs. 90 ng/mL [72–107]; p < 0.001) as well as higher median CRP‐0 and CRP‐24 levels.
Table 2.
Neutrophil gelatinase‐associated lipocalin (NGAL) and C‐reactive protein (CRP) values between patients with and without acute kidney injury (AKI).
| Variable | No AKI n = 113 | AKI n = 20 | p |
|---|---|---|---|
| NGAL‐0, ng/mL (median, IQR) | 95 (73,116) | 148 (117,202) | <0.001 |
| NGAL‐24, ng/mL (median, IQR) | 90 (72,107) | 147 (98,195) | <0.001 |
| CRP‐0, mg/dL (median, IQR) | 5 (2,10) | 16 (5,28) | 0.004 |
| CRP‐24, mg/dL (median, IQR) | 20 (9,47) | 110 (32,151) | <0.001 |
There were no significant changes between NGAL‐0 and NGAL‐24 within the two groups (Figure 1A), while there were significant changes in levels of CRP‐0 and CRP‐24 within the two groups (Figure 1B).
Figure 1.
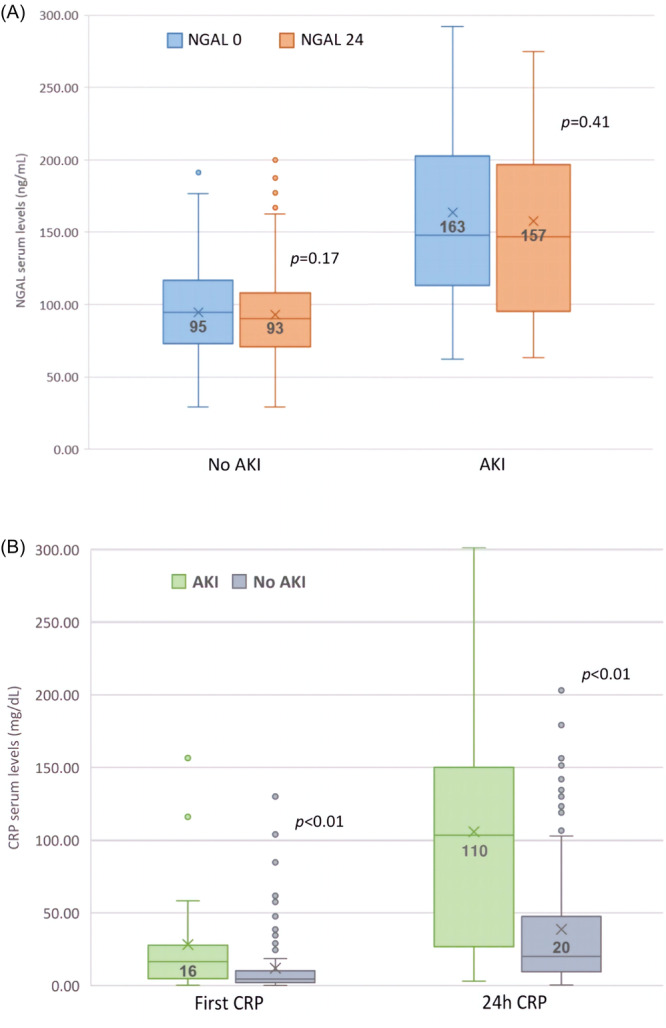
Comparison of neutrophil gelatinase‐associated lipocalin (NGAL) levels in 0 and 24‐h for patients with and without acute kidney injury (AKI). (A) Comparison of CRP levels patients with and without AKI divided for 0 and 24‐h levels (B).
According to the ROC curve analysis (Figure 2), the optimal NGAL‐0 level for AKI prediction was >125 ng/mL (AUC 0.841, 95% CI 0.801–0.961, p < 0.001), with a sensitivity of 70%, specificity of 84%, and negative predictive value (NPV) of 94%. The optimal NGAL‐24 level for prediction was >140 ng/mL (AUC 0.783, 95% CI 0.741–0.846, p < 0.001). NGAL‐24 cutoff of 140 ng/mL had 65% sensitivity, 91% specificity, and NPV of 91% for AKI. The AUC for AKI prediction by CRP‐0 was 0.701 (95% CI 0.576–0.830) and by CRP‐24 was 0.781 (95% CI 0.658–0.895).
Figure 2.
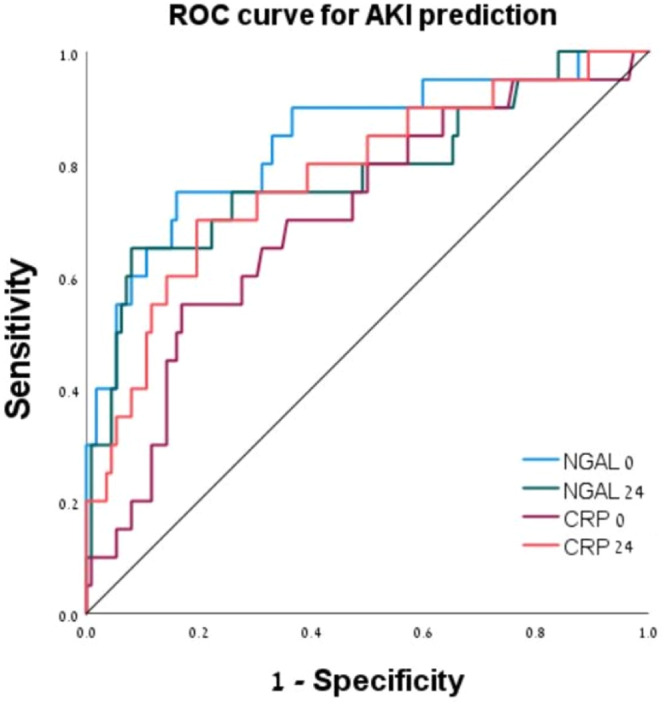
Receiver operating curve (ROC) for the prediction of acute kidney injury (AKI) using different biomarkers.
Next, we wanted to analyze NGAL‐0 as an independent predictor for AKI devolvement. Baseline characteristics that were found associated with AKI in univariate analysis (Table 1) were older age (mean age 71 vs. 64 years, p = 0.02), hypertension (90% vs. 47%. p < 0.001), and CKD (70% vs. 11%, p < 0.001). In a multivariate analysis accounting for these baseline characteristics (Table 3), NGAL‐0 levels remained an independent predictor for AKI development (adjusted OR for each increase of 1 ng/mL −1.04, 95% CI 1.02–1.06, p < 0.001). Additional independent predictors for AKI were CKD (AOR 18.3, 95% CI 3.6–91, p < 0.001) and hypertension (AOR 19.9, 95% CI 2.3–170, p = 0.007).
Table 3.
Univariate and multivariate analysis of predictors for post‐admission acute kidney injury.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | Adjusted OR (95% CI) | p | |
| Age | 1.05 (1.01–1.09) | 0.02 | 1.01 (0.94–1.08) | 0.84 |
| Hypertension | 10.2 (2.3–46) | <0.001 | 19.9 (2.3–170) | 0.007 |
| CKD | 19.7 (6.4–61) | <0.001 | 18.3 (3.6–91) | <0.001 |
| NGAL‐0 | 1.04 (1.02–1.05) | <0.001 | 1.04 (1.02–1.06) | <0.001 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; NGAL, neutrophil gelatinase‐associated lipocalin; OR, odds ratio.
To validate our results and address the association of AKI with older age, we divided the cohort based on age above or equal/less than 65 years (cohort's mean age) and performed a similar ROC analysis as described above. Fifteen (24%) subjects had AKI among those older than 65, while 5 (7%) had AKI in among the rest. In subjects older than 65 years, NGAL‐0 had the largest ROC‐AUC for AKI prediction (Supporting Information S1: Figure S1, AUC 0.864, 95% CI 0.746–0.982). As shown in Supporting Information S1: Figure S2, in subjects aged 65 or younger, NGAL‐0 had the only significant AUC‐ROC (0.775, 95% CI 0.568–0.982, p = 0.04).
4. DISCUSSION
In this study, we aimed to evaluate the predictive ability of baseline NGAL for the occurrence of AKI, compared with follow‐up NGAL measurement and similar serial CRP tests. We demonstrated several important findings: First, among STEMI patients, NGAL levels before primary PCI may be utilized to predict the risk of AKI. We found high baseline NGAL levels measured before contrast media exposure. This finding suggests that there hemodynamic compromise plays an important role in STEMI patients by provoking renal function deterioration highlighting the complexity of cardio‐renal interactions. Second, NGAL levels at admission may serve as a reliable marker for ruling out AKI having a NPV of 94% for NGAL‐0 levels <120 ng/mL. Multivariate regression suggests an independent association between NGAL and AKI.
Previous reports showed an association between Elevated NGAL and adverse renal outcomes. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 In many of these reports, however, NGAL levels were determined at a single point, and as such the dynamics of NGAL before and following PCI could have not been assessed. A recent report suggested that NGAL can also be a marker for inflammation in STEMI patients with normal renal function yet elevated plasma NGAL. However, when eGFR is reduced (e.g., in CKD patients), plasma NGAL is more reflective for renal function. 23 This cohort included patients with chronic renal impairment and severe hemodynamic instability manifested as cardiogenic shock, both are known to be associated with increased NGAL expression due to chronic renal damage 9 or acute inflammatory response, 15 in this cases NGAL levels may not truly reflect acute tubular damage.
Traditionally, contrast‐induced nephropathy is considered to play a risk factor in the development of AKI following PCI. 1 , 2 , 3 However, recent reports suggest that AKI in STEMI patients can be attributed to a complex and multifactorial pathway in which contrast media exposure has only a small role. 4 , 5 , 6 In the present cohort, while both NGAL‐0 and NGAL‐24 were significantly higher among patients having AKI, we detected no significant change between NGAL‐0 and NGAL‐24 within the two groups. Furthermore, NGAL‐0 performed better both for the prediction of AKI as well a ruling out AKI occurrence.
We previously demonstrated that elevated NGAL levels before primary PCI suggesting renal tubular damage are common among STEMI patients. This finding may be attributed to hemodynamic changes in the acute stage of STEMI, related to the sudden decrease in left ventricular ejection fraction and cardiac output. The lack of change between the sequential measurements may suggest unique pathway of NGAL in myocardial infarction, possibly representing both acute inflammatory and early renal injury with a shift of the dominant element between two exams. Indeed, we observed a significant rise in peak CRP levels in patients with AKI, pointing to the possible strong element of inflammation in these patients.
Lindberg et al. were the first to describe an interaction between NGAL and CRP, especially in STEMI patients, their findings were later reproduced by others. 24 , 25 , 26 In those reports, high NGAL and high CRP combination was associated with higher rated of mortality in compare with low NGAL and low CRP. In the present cohort, we attempted to reinfornce the potential usefulness of NGAL over CRP regarding the prediction of AKI. We demonstrated that in STEMI patients, NGAL conferred better and independent prognostic information over CRP for the prediction of AKI in line with previous studies.
In this study we found, AKI was associated with higher peak CRP and troponin levels. We hypothesize that among this STEMI subpopulation CRP may be more the mere marker or an epiphenomenon, it may also directly contribute to the inflammatory state and ongoing damage involved. Our findings may have important clinical implications. In current guidelines and diagnostic criteria, the deterioration of renal function in manifested as elevated creatinine. However, this rise in serum creatinine can be a late marker for renal injury as the kidneys have relatively high functional reserves. In fact, 50% of renal mass needs to be damaged to provoke significant rise in serum Scr. Therefore, the increase in sCr lags behind the initial insult by 24–48 h. In view of this limitation, utilization of biomarkers for identifying patients at risk for future AKI progression might be of value for the clinician. Application of early NGAL measurement can aid in risk stratification for further deterioration, especially in patients with high NGAL‐0 and NGAL‐24 levels. Furthermore, the high NPV of NGAL‐0 for ruling out future AKI may indicate more liberal administration of medications and possibly less strict monitoring of sCr throughout hospitalization.
In our cohort, 70% of patients developing AKI had baseline CKD. We have previously demonstrated that CKD patients may demonstrate elevated baseline NGAL levels reflecting chronic renal impairment. Our current results show that NGAL is independently associated with AKI as shown by multivariate regression.
The utilization of early NGAL measurement for ruling out (rather than prediction) AKI has not been assessed before. Our findings may be nevertheless hypothesis generating for the future utilization of NGAL in patients undergoing elective PCI. For example, among patients with baseline CKD, up to 50% will develop contrast‐associated nephropathy following PCI. In these patients, early identification of those at risk on one hand may aid in early measurements for AKI prevention (i.e., pre‐PCI hydration and contrast volume limitation) on one hand and more liberal approach (regarding contrast volume and early discharge) in those with low AKI risk. In patients undergoing urgent PCI early NGAL can be used to direct early initiation of Renin‐Angiotensin blockers following PCI with less concern regarding concomitant AKI.
Our study bares some limitations. This was a single‐center cohort with a relatively small size, this fact combined with a low occurrence rate of AKI does not allow for a correlation of high NGAL levels with AKI. To enhance the role of NGAL, the population size needs to be increased. Plasma NGAL levels were used solely without urinary NGAL which can have added another aspect for this study. We used sCr and eGFR as surrogate markers of kidney function, acknowledging that these markers have limitations when used in acute hospitalized patients with STEMI. Finally, our analysis was based on using ELISA batch, thus analysis was performed after completing the sample numbers. For future research, in order for early AKI prevention, exams and results must be available early (i.e., before the complication has occurred) thus a rapid check (i.e., ProNephro AKI check) should be used.
5. CONCLUSION
Among STEMI patients, NGAL levels upon admission may serve as a reliable marker for ruling out AKI, having NPV of 94% for NGAL level ≤ 120 ng/mL. This single biomarker can therefore direct personalized evaluations during the admission, and focused interventions to prevent AKI.
AUTHOR CONTRIBUTIONS
Nils Erik Magnusson: Conceptualization; writing—original draft. Shir Frydman: Data curation; writing—review and editing. Ophir Freund: Data curation; Formal analysis. Lior Zornizki: Methodology; writing—review and editing. Shmuel Banai: Supervision; validation; writing—review and editing. Yacov Shacham: Conceptualization; writing—original draft. All authors have read and approved the final version of the manuscript, had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
CONFLICT OF INTEREST STATEMENT
Nils Erik Magnusson is an advisor for Bioporto Diagnostics yet Bioporto did not fund any part of this study. All other authors have no competing interests to declare and no relevant financial relationships.
ETHICS STATEMENT
This study was approved by the institutional review board (TLV‐16‐0224) and performed in adherence with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all subjects involved in the study.
TRANSPARENCY STATEMENT
The lead author Shir Frydman affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Magnusson NE, Frydman S, Freund O, Zornizki L, Banai S, Shacham Y. Early neutrophil gelatinase‐associated lipocalin (NGAL) measurement could rule out future acute kidney injury in patients with acute coronary syndrome—Prospective observational study. Health Sci Rep. 2024;7:e2229. 10.1002/hsr2.2229
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. James MT, Ghali WA, Knudtson ML, et al. Alberta Provincial Project for outcome assessment in coronary heart disease I. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123(4):409‐416. 10.1161/CIRCULATIONAHA.110.970160 [DOI] [PubMed] [Google Scholar]
- 2. Gurm HS, Dixon SR, Smith DE, et al. Renal function‐based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2011;58(9):907‐914. 10.1016/j.jacc.2011.05.023 [DOI] [PubMed] [Google Scholar]
- 3. Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast‐induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33(16):2007‐2015. 10.1093/eurheartj/ehr494 [DOI] [PubMed] [Google Scholar]
- 4. Shacham Y, Leshem‐Rubinow E, Gal‐Oz A, et al. Relation of time to coronary reperfusion and the development of acute kidney injury after ST‐segment elevation myocardial infarction. Am J Cardiol. 2014;114(8):1131‐1135. 10.1016/j.amjcard.2014.07.032 [DOI] [PubMed] [Google Scholar]
- 5. Shacham Y, Leshem‐Rubinow E, Gal‐Oz A, et al. Acute cardio‐renal syndrome as a cause for renal deterioration among myocardial infarction patients treated with primary percutaneous intervention. Can J Cardiol. 2015;31(10):1240‐1244. 10.1016/j.cjca.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 6. Shacham Y, Steinvil A, Arbel Y. Acute kidney injury among ST elevation myocardial infarction patients treated by primary percutaneous coronary intervention: a multifactorial entity. J Nephrol. 2016;29(2):169‐174. 10.1007/s40620-015-0255-4 [DOI] [PubMed] [Google Scholar]
- 7. Maisel AS, Wettersten N, van Veldhuisen DJ, et al. Neutrophil gelatinase‐associated lipocalin for acute kidney injury during acute heart failure hospitalizations: The AKINESIS Study. J Am Coll Cardiol. 2016;68(13):1420‐1431. [DOI] [PubMed] [Google Scholar]
- 8. Yndestad A, Landro L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase‐associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229‐1236. [DOI] [PubMed] [Google Scholar]
- 9. Sivalingam Z, Larsen SB, Grove EL, Hvas AM, Kristensen SD, Magnusson NE. Neutrophil gelatinase‐ associated lipocalin as a risk marker in cardiovascular disease. Clin Chem Lab Med. 2017;56(2018):5‐18. [DOI] [PubMed] [Google Scholar]
- 10. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2012;61(4):e78‐e140. [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skalsky K, Levi A, Bental T, et al. The definition of “acute kidney injury” following percutaneous coronary intervention and cardiovascular outcomes. Am J Cardiol. 2021;156:39‐43. 10.1016/j.amjcard.2021.06.033 [DOI] [PubMed] [Google Scholar]
- 13. Haase‐Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase‐associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51(Pt 3):335‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helanova K, Spinar J, Parenica J. Diagnostic and prognostic utility of neutrophil gelatinase‐associated lipocalin (NGAL) in patients with cardiovascular diseases—review. Kidney Blood Press Res. 2014;39:623‐629. [DOI] [PubMed] [Google Scholar]
- 15. Kafkas N, Demponeras C, Zoubouloglou F, Spanou L, Babalis D, Makris K. Serum levels of gelatinase associated lipocalin as indicator of the inflammatory status in coronary artery disease. Int J Inflammation. 2012;2012:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khatami M., Sabbagh M., Nikravan N., et al. The role of neutrophil‐gelatinase‐associated lipocalin in early diagnosis of contrast nephropathy. Indian J Nephrol. 2015;25:292‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shang W, Wang Z. The update of NGAL in acute kidney injury. Curr Protein Pept Sci. 2017;18(12):1211‐1217. [DOI] [PubMed] [Google Scholar]
- 18. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase‐Fielitz A. Accuracy of neutrophil gelatinase‐associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta‐analysis. Am J Kidney Dis. 2009;54:1012‐1024. [DOI] [PubMed] [Google Scholar]
- 19. Corbacıoglu SK, Cevik Y, Akinci E, et al. Value of plasma neutrophil gelatinase‐associated lipocalin (NGAL) in distinguishing between acute kidney injury (AKI) and chronic kidney disease (CKD). Turk J Emerg Med. 2017;17:85‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H, Yu Z, Gan L, Peng L, Zhou Q. Serum NGAL and FGF23 may have certain value in early diagnosis of CIN. Ren Fail. 2018;40:547‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rysz J, Gluba‐Brzózka A, Franczyk B, Jabłonowski Z, Ciałkowska‐Rysz A. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int J Mol Sci. 2017;18(8):1‐17. 10.3390/ijms18081702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abella V, Scotece M, Conde J, et al. The potential of lipocalin‐2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers. 2016;20(8):565‐571. 10.3109/1354750X.2015.1123354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Milwidsky A, Ziv‐Baran T, Letourneau‐Shesaf S, et al. CRP velocity and short‐term mortality in ST segment elevation myocardial infarction. Biomarkers. 2017;22:383‐386. [DOI] [PubMed] [Google Scholar]
- 24. Lindberg S, Jensen JS, Hoffmann S, et al. Plasma neutrophil gelatinase‐associated lipocalin reflects both inflammation and kidney function in patients with myocardial infarction. Cardio Renal Medicine. 2016;6:180‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindberg S, Pedersen SH, Mogelvang R, et al. Prognostic utility of neutrophil gelatinase‐associated lipocalin in predicting mortality and cardiovascular events in patients with ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2012;60:339‐345. [DOI] [PubMed] [Google Scholar]
- 26. Helanova K, Littnerova S, Kubena P, et al. Prognostic impact of neutrophil gelatinase‐associated lipocalin and B‐type natriuretic in patients with ST‐elevation myocardial infarction treated by primary PCI: a prospective observational cohort study. BMJ Open. 2015;5(10):e006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


