Abstract
Hantaan virus, the prototypic member of the Hantavirus genus, causes hemorrhagic fever with renal syndrome in humans. We examined the human memory T-lymphocyte responses of three donors who had previous laboratory-acquired infections with Hantaan virus. We demonstrated virus-specific responses in bulk cultures of peripheral blood mononuclear cells (PBMC) from all donors. Bulk T-cell responses were directed against either Hantaan virus nucleocapsid (N) or G1 protein, and these responses varied between donors. We established both CD4+ and CD8+ N-specific cell lines from two donors and CD4+ G1-specific cell lines from a third donor. All CD8+ cytotoxic T-lymphocyte (CTL) lines recognized one of two epitopes on the nucleocapsid protein: one epitope spanning amino acids 12 to 20 and the other spanning amino acids 421 to 429. The CTL lines specific for amino acids 12 to 20 were restricted by HLA B51, and those specific for amino acids 421 to 429 were restricted by HLA A1. The N-specific CTL lines isolated from these two donors included both Hantaan virus-specific CTLs and hantavirus cross-reactive CTLs. Responses to both epitopes are detectable in short-term bulk cultures of PBMC from one donor, and precursor frequency analysis confirms that CTLs specific for these epitopes are present at relatively high precursor frequencies in the peripheral T-cell pool. These data suggest that infection with Hantaan virus results in the generation of CTL to limited epitopes on the nucleocapsid protein and that infection also results in the generation of cross-reactive T-cell responses to distantly related hantaviruses which cause the distinct hantavirus pulmonary syndrome. This is the first demonstration of human T-lymphocyte responses to Hantaan virus.
Hantaan virus, the prototype member of the Hantavirus genus, causes hemorrhagic fever with renal syndrome (HFRS) in humans. Hantaviruses are carried by rodents and are spread to humans via inhalation of aerosolized virus particles which are shed in the rodent feces and urine (7, 46). Infections with Hantaan virus can range in severity from asymptomatic to a severe, life-threatening illness characterized by fever, hemorrhage, and renal failure. More than 100,000 cases of HFRS are reported yearly, with a mortality rate of between 2 and 10% (16, 27, 42). Hantaan virus, which was first isolated in Korea in 1978, is endemic primarily in Asia and causes a relatively severe form of HFRS (28). Other hantaviruses that also cause HFRS include Dobrava virus (Balkans), Seoul virus (worldwide), and Puumala virus (Scandinavia and Europe). Dobrava virus is also associated with a relatively severe form of HFRS, while Seoul and Puumala viruses cause more mild disease (16, 33). Recent outbreaks of related hantaviruses in the southwestern United States and South America (e.g., Sin Nombre and Andes viruses) have resulted in the isolation of a newly recognized group of hantaviruses that cause a fatal pulmonary syndrome, hantavirus pulmonary syndrome (HPS) (31, 37, 53). HFRS and HPS are distinct in the predominant target organ of virus infection (kidney versus lung) but have important clinical features in common, including fever, thrombocytopenia, and a capillary leak syndrome. These common clinical manifestations suggest that the underlying mechanisms of disease may be similar in the two syndromes.
The pathogenesis of hantavirus infections is not understood. Hantaan virus infects primary human endothelial cells as well as kidney glomerular cells and monocytes in vitro but does not have any direct cytopathic effect on these cells (40, 52). Autopsy samples from individuals with Sin Nombre virus infection show evidence of predominant infection of alveolar microvascular endothelial cells without apparent cytopathic effect (54). It is thus unlikely that direct viral cytotoxicity is the primary cause of pathology in vivo. In many other viral infections, virus-specific cytolytic T-cell responses have been shown to be involved in both clearance of virus and induction of immunopathology (5, 9, 21, 36, 48). This may also be the case in hantavirus infections. Recent studies of Hantaan and related virus infections suggest a role for the cellular immune response in the pathology of HFRS. Evidence supporting a role for the immune response in the pathology of disease includes increases in the number of activated, circulating CD8+ cells in patients with acute HFRS (18), the presence of infiltrating lymphocytes (predominantly CD8+ T cells) in kidney biopsies from patients with acute Puumala virus infections (34, 51), and increases in production of cytokines such as tumor necrosis factor alpha, gamma interferon (IFN-γ), IFN-α, and interleukin-6 (IL-6) in both the kidneys and peripheral blood of patients with acute Hantaan virus infections (23, 29, 51). The presence of activated lymphocytes in the kidneys of infected individuals may result in local or systemic increases in production of cytokines and other inflammatory mediators which may, in turn, contribute to the capillary leak syndrome and kidney damage characteristic of HFRS. Further, a recent study by Mustonen et al. (35) demonstrated a correlation between severe disease caused by Puumala virus infection and HLA type B8, DR3. A similar correlation has been observed between HLA B35 and increased severity of Sin Nombre virus-induced HPS (22). These findings suggest a role for T lymphocytes in exacerbating disease and support a potential role for the cellular immune response in the pathology of both HPS and HFRS.
An understanding of the mechanisms underlying HFRS pathology will be important both in improving the diagnosis and treatment of infected individuals and in the design of vaccine strategies. Little is known about the specific cellular immune responses to hantavirus infections. Our laboratory has previously described three cytolytic T-cell clones isolated from the peripheral blood of patients with hantavirus pulmonary syndrome caused by Sin Nombre virus infection (12). In the present study, we examine the memory T-cell responses of three donors who had previous laboratory-acquired infections with Hantaan virus. We demonstrate virus-specific responses in bulk cultures of peripheral blood mononuclear cells (PBMC) from each of these donors. Bulk T-cell responses were directed against Hantaan virus nucleocapsid (N) and/or G1 proteins, and these responses varied in specificity between individuals. We established both CD8+ and CD4+ N-specific cell lines from two donors and CD4+ G1-specific cell lines from the third donor. All CD8+ cytotoxic T-lymphocyte (CTL) lines isolated recognize one of two epitopes on the nucleocapsid protein; one epitope is located at the N-terminal region of the protein and spans amino acids 12 to 20, and the other spans the C-terminal nine amino acids of the protein (421 to 429). All CTL lines specific for amino acids 12 to 20 were restricted by HLA B51, and those specific for amino acids 421 to 429 were restricted by A1. The memory CTL response detected in these immune individuals included both Hantaan virus-specific T-cell responses and hantavirus cross-reactive responses. This is the first demonstration of Hantaan virus-specific human CD8+ and CD4+ T-cell responses.
MATERIALS AND METHODS
PBMC donors.
Donors were American scientists who became infected with the Hantaan virus strain 76-118 10 to 15 years prior to the study. Infections were subclinical and were confirmed by detection of Hantaan virus-specific immunoglobulin in the serum. The HLA types of these donors are as follows: donor A, A1, A2, B35, B51, Cw4, Cw7, DR2, DR3, DQ2, DQ6, and DRw52; donor B, A2, A11, B39, B60, Cw3, Cw12, DR4, DR9, DQ3, and DRw53; donor C, A1, A11, B8, B51, Cw7, Cw15, DR7, DR11, DQ3, DRw52, and DRw53.
Human PBMC.
PBMC were purified by Ficoll-Hypaque density gradient centrifugation. Buffy coats were recovered, and mononuclear cells were resuspended at 5 × 106 to 8 × 106/ml in RPMI 1640–20% fetal bovine serum (FBS)–10% dimethyl sulfoxide and cryopreserved until needed.
B lymphoblastoid cell lines (BLCL).
PBMC (1 × 106 to 4 × 106) were cultured in RPMI 1640 supplemented with penicillin, streptomycin, l-glutamine, HEPES, and 20% FBS in the presence of a 1:3 dilution of Epstein-Barr virus from B95-8 cells (American Type Culture Collection) in 24-well flat-bottom plates (Costar) as previously described (8). Cyclosporine was added at 1 μg/ml to inhibit proliferation of T lymphocytes.
In vitro stimulation of PBMC.
PBMC (2 × 106 to 5 × 106) were resuspended in 1 ml of AIM/V (Gibco)–10% human AB serum in a single well of a 24-well plate (Costar). A gamma-irradiated, inactive Hantaan virus preparation was added at a 1:160 final dilution. Hantaan virus was grown in Vero E6 cells (American Type Culture Collection) and purified as described previously (45). The purified virus was then gamma irradiated (8 × 106 rads) to inactivate the virus. Inactivation of the virus was confirmed by plaque assay. Recombinant human IL-2 (20 U/ml) was added on day 4 to 5 in 1 ml of fresh AIM/V–10% human AB serum, and the medium was replenished every third day thereafter. Bulk culture 51Cr release assays were performed between days 7 and 14 of culture. Donor A PBMC were restimulated on day 7 with inactivated Hantaan virus (1:160) and 2 × 106 to 3 × 106 gamma-irradiated autologous PBMC. Recombinant IL-2 was added to these cultures on day 11, and cultures were tested on day 14. Donor A PBMC were restimulated in order to reduce nonspecific background lysis.
CTL lines.
Hantaan virus-specific CTL lines were established by limiting-dilution plating as described previously (25). In vitro-stimulated PBMC were plated at 1, 3, 10, or 30 cells/well in a 96-well, round-bottom plate (Costar) in 0.2 ml of AIM/V–10% FBS and stimulated with anti-CD3 antibody (12F6) and gamma-irradiated allogeneic PBMC as feeder cells. Medium was replenished every 3 days, and wells were restimulated with 12F6 and gamma-irradiated feeder cells every 14 days. Individual wells were tested for recognition of targets expressing Hantaan virus proteins in a 51Cr release assay, and positive wells were expanded and restimulated as described above. Surface expression of CD4 and CD8 was determined by flow cytometry with fluorescein isothiocyanate-conjugated antibodies (Becton Dickinson).
Hmy cell lines.
Hmy2.C1R (Hmy) is a human plasma cell line that lacks endogenous HLA A and -B antigens (50). Hmy cell lines transfected with B35 or B51 were generated as described previously (15, 38) and were kindly provided by Masafumi Takaguchi (University of Tokyo, Tokyo, Japan).
Recombinant vaccinia viruses.
Recombinant vaccinia viruses expressing Hantaan virus genes were constructed as described previously (44). The recombinant vaccinia viruses used in this study were derived from the WR strain of vaccinia virus and express either individual Hantaan virus genes encoding G1, G2, or nucleocapsid (denoted vac-G1, vac-G2, and vac-N, respectively) or all three genes simultaneously (denoted vac-G1+G2+N).
Synthetic peptides.
Seventy Hantaan nucleocapsid peptides that spanned the entire published sequence of the Hantaan nucleocapsid protein (47) (GenBank accession no. M14626) were synthesized. Peptides were 15 amino acids in length and overlapped by 9 amino acids. N protein peptides from other hantavirus strains were synthesized based on published sequences and included Seoul (1) (accession no. M34881), Sin Nombre (10) (accession no. L37904), Prospect Hill (39) (accession no. M34011), Andes (32) (accession no. AF004660), Puumala (49) (accession no. M32750), New York-1 (17) (accession no. U47135), Dobrava (4) (accession no. L41916), and Convict Creek (43) (accession no. L33683) viruses. Peptides were synthesized at the Protein Chemistry Core Facility at the University of Massachusetts Medical Center with an automated Rainin Symphony peptide synthesizer.
Preparation of target cells. (i) Virus-infected targets.
Autologous or allogeneic (for major histocompatibility complex [MHC] restriction analysis) BLCL were infected with recombinant vaccinia viruses at a multiplicity of infection of 15 for 60 min at 37°C. The cells were then diluted in 1 ml of RPMI 1640–10% FBS for an additional 12 to 16 h. Target cells were then labeled with 0.25 mCi of 51Cr for 60 min at 37°C. Following labeling, the cells were washed three times and resuspended at 2 × 104/ml in RPMI–10% FBS.
(ii) Peptide-pulsed targets.
Uninfected BLCL were 51Cr labeled as described above. Labeled cells were incubated with 25 μg of peptide per ml in 96-well round-bottom plates at 1 × 103 to 2 × 103/well for 30 min at 37°C before addition of effector cells. The peptides remained in the wells for the duration of the assay.
51Cr release assay.
In vitro-stimulated effector cells were added to 2 × 103 51Cr-labeled target cells at various effector cell/target cell (E/T) ratios. For CTL assays with synthetic peptides, T-cell lines or clones were added to 1 × 103 to 2 × 103 51Cr-labeled, peptide-pulsed targets at an E/T ratio of 10:1 (unless otherwise specified). Plates were incubated for 4.5 h at 37°C, supernatants were harvested (Skatron Instruments, Sterling, Va.), and specific lysis was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. All assays were performed in triplicate. All experiments were performed at least twice. Negative controls included target cells infected with wild-type vaccinia virus or unpulsed target cells. Spontaneous lysis was <25% in all assays.
Enzyme-linked Immunospot (ELISPOT) assay for single-cell IFN-γ secretion.
ELISPOT assays were performed as described previously (26). Briefly, 96-well filtration plates (MAIP S 45; Millipore, Bedford, Mass.) were coated with 15 μg of mouse anti-human IFN-γ monoclonal antibody (clone N1B42; Pharmingen, San Diego, Calif.) per ml. PBMC were incubated for 24 h in RPMI–10% FBS with no stimulation and added at 2 × 105 cells/well in RPMI 1640–10% FBS. Peptides were added at 25 μg/ml, and plates were incubated for 18 to 20 h at 37°C. Biotinylated mouse anti-human IFN-γ monoclonal antibody (clone 4S.B3; Pharmingen) was then added and left for 2 h at room temperature, followed by a 1:400 dilution of streptavadin alkaline phosphatase for 45 min. Substrate (3-amino-9-ethyl-carbazole–0.15% H2O2) was added and left for 10 min at room temperature. The precursor frequency was calculated as number of visible spots/total number of cells per well. Experiments were performed in triplicate.
RESULTS
Protein specificity of CTL lines generated from the PBMC of Hantaan virus-immune donors.
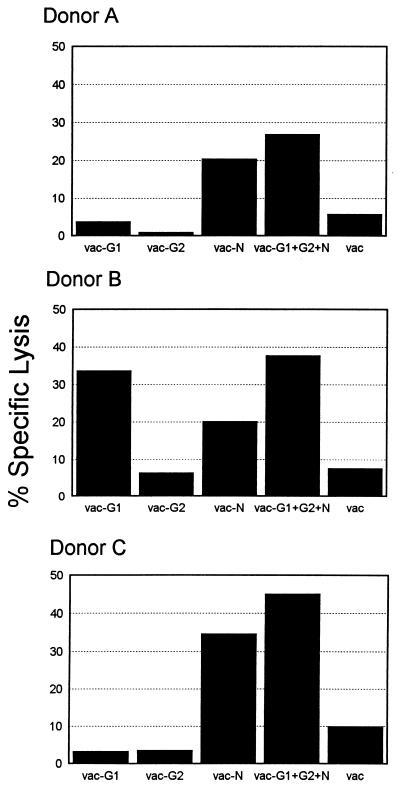
PBMC from three Hantaan virus-immune donors were stimulated in vitro for 7 to 14 days with an inactivated Hantaan virus preparation. These bulk cultures were then tested for specific lysis of autologous BLCL target cells infected with vaccinia virus recombinants expressing one or more Hantaan virus proteins. Stimulated PBMC from donor A recognized target cells expressing the nucleocapsid (N) protein, including those infected with vac-N and vac-G1+G2+N (Fig. 1). Lysis of target cells expressing the N protein was relatively low (18 to 27%) but was consistently higher than killing of targets infected with wild-type vaccinia virus (<7%). PBMC from donor B consistently displayed lysis of targets expressing the G1 glycoprotein (vac-G1 and vac-G1+G2+N) (Fig. 1). Donor B PBMC also displayed transient, low-level recognition of the nucleocapsid protein, although the killing of targets expressing G1 was always higher than that of targets expressing N. PBMC from donor C consistently recognized targets expressing the Hantaan virus N protein (Fig. 1). In summary, the above data show that PBMC from donors A and C recognized targets expressing the nucleocapsid protein while PBMC from donor B lysed targets expressing G1. We were unable to detect responses specific for the G2 glycoprotein in the PBMC from any of these donors.
FIG. 1.
Bulk culture recognition of Hantaan virus proteins. PBMC (2 × 106 to 5 × 106/well) were stimulated for 7 to 14 days with a gamma-irradiated Hantaan virus as described in Materials and Methods. Target cells were autologous BLCL infected with recombinant vaccinia viruses expressing one or more Hantaan virus proteins. Vaccinia recombinants used to infect targets (shown on the x axis) express individual Hantaan virus proteins (vac-G1, -G2, and -N) or a combination of G1, G2, and N (vac-G1+G2+N). Lysis of targets infected with wild-type vaccinia virus served as a negative control. E/T ratio, 80. Data from a representative experiment are shown.
When T-cell lines were cloned from the PBMC of these donors, the CTL lines isolated from each donor were specific for the protein that was consistently recognized in bulk culture. A summary of representative T-cell lines isolated from these donors is shown in Table 1. Both CD8+ and CD4+ T-cell lines specific for the N protein were isolated from PBMC from donors A and C. In contrast, only CD4+ T-cell lines specific for the G1 glycoprotein were detected in the PBMC from donor B. Although low-level recognition of targets expressing the N protein was seen in the bulk PBMC cultures from donor B, we were not able to isolate cell lines that recognize this protein. These data demonstrate that Hantaan virus-specific T-cell responses are readily detectable in the PBMC of these immune individuals and that responses are directed primarily against the viral N protein and the G1 glycoprotein. The bulk culture responses as well as the specificity and phenotype (CD4/CD8) of the virus-specific T-cell lines varied between individuals.
TABLE 1.
Cytotoxicities of CD4+ and CD8+ lines isolated from Hantaan virus-immune donorsa
| Donor | Cell line | Phenotypeb | % Specific lysisc with:
|
|||
|---|---|---|---|---|---|---|
| vac-G1 | vac-G2 | vac-N | vac | |||
| A | 1-C8 | CD8 | −2.2 | —d | 70.3 | 5.5 |
| 3-G7 | CD8 | −2.6 | — | 55 | −1.9 | |
| 10-E2 | CD8 | −0.5 | — | 53.1 | 0.9 | |
| 10-B5 | CD8 | −1.6 | 3.2 | 58.8 | — | |
| 1-G5 | CD4 | −0.4 | — | 66.7 | 1.6 | |
| 1-D6 | CD4 | −3.4 | — | 61.4 | −0.7 | |
| B | 3-E3 | CD4 | 33.5 | −0.3 | −0.4 | −0.1 |
| 10-C11 | CD4 | 30.9 | −2.6 | −2.8 | −2.9 | |
| 10-F4 | CD4 | 25.4 | −2.4 | −1.0 | −0.9 | |
| C | 1A-B7 | CD8 | −2.9 | −7.0 | 87.7 | — |
| 3A-B8 | CD8 | −6.3 | −7.0 | 60.0 | — | |
| 3A-C4 | CD8 | −6.1 | 1.0 | 44.5 | — | |
| 3A-C10 | CD8 | −6.1 | −7.2 | 68.5 | — | |
| 3A-G3 | CD8 | 0.0 | −0.2 | 84.1 | — | |
| 3A-F2 | CD4 | 0.3 | 2.8 | 47.0 | — | |
| 3-E10 | CD4 | −4.6 | — | 40.3 | — | |
| 3-F8 | CD4 | −3.6 | — | 55.6 | — | |
Data from representative cell lines are shown. E/T ratios vary from 20:1 to 50:1.
Cell lines were >95% CD4 or CD8 by flow cytometry.
Boldface indicates significant target cell recognition.
—, Target not tested.
Peptide specificities of CD8+ CTL lines generated from the PBMC of Hantaan virus-immune donors A and C.
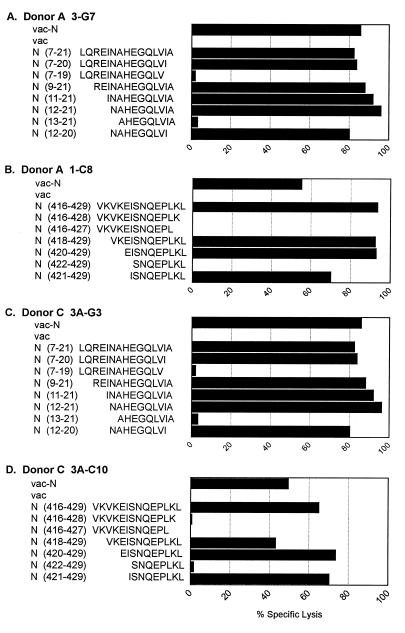
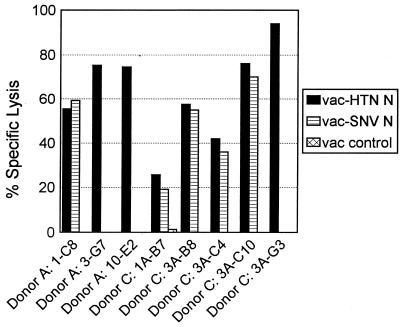
To identify the specific peptide on the N protein recognized by these CD8+ CTL lines, each cell line was tested in a 51Cr release assay against targets pulsed with a series of 70 overlapping peptides spanning the entire Hantaan virus nucleocapsid sequence. The 15-mer peptides recognized by the CD8+ CTL lines of the two donors are shown in Table 2. Interestingly, all N-specific CTL lines isolated from donors A and C recognized one of two 15-mer peptides on the nucleocapsid protein. CTL lines 3-G7 and 10-E2 from donor A and 3A-G3 from donor C recognized targets pulsed with a nucleocapsid peptide which is located at the amino terminus of the protein and spans amino acids 7 to 21 (LQREINAHEGQLVIA). CTL line 1-C8 from donor A and lines 1A-B7, 3A-B8, 3A-C4, and 3A-C10 from donor C recognized targets pulsed with a peptide which is located at the extreme carboxy terminus of the protein and spans amino acids 416 to 429 (VKVKEISNQEPLKL). Subsequent screening of numerous other cell lines resulted in the isolation of additional CTL lines that recognize one or the other of these peptides (data not shown).
TABLE 2.
Nucleocapsid peptide recognition of CD8+ CTL lines
| Donor | CTL line | Amino acids |
|---|---|---|
| A | 1-C8 | 416–429 |
| 3-G7 | 7–21 | |
| 10-E2 | 7–21 | |
| B | 1A-B7 | 416–429 |
| 3A-B8 | 416–429 | |
| 3A-C4 | 416–429 | |
| 3A-C10 | 416–429 | |
| 3A-G3 | 7–21 |
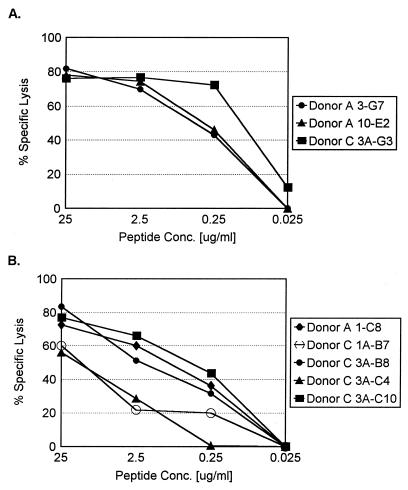
Once the nucleocapsid peptides (15-mers) recognized by the CTL lines were identified, sequential N- and C-terminal truncations of the peptides were synthesized and tested in similar 51Cr release assays. The patterns of peptide recognition of representative clones from each donor are shown in Fig. 2 and 3. The clones specific for nucleocapsid peptide 7-21 (donor A, 3-G7 and 10-E2; donor C, 3A-G3) were all found to recognize a minimal 9-mer peptide spanning amino acids 12 to 20 (NAHEGQLVI). A representative CTL line from each donor is shown in Fig. 2A and C. These cell lines required comparable peptide concentrations for target cell recognition (Fig. 3A). The CTL lines specific for the nucleocapsid peptide 416-429 (donor A, 1-C8; donor C, 1A-B7, 3A-B8, 3A-C4, and 3A-C10) were all found to recognize a minimal 9-amino-acid epitope at the extreme carboxy terminus of the protein spanning amino acids 421 to 429 (ISNQEPLKL). Data from representative CTL lines from both donors are shown in Fig. 2B and D. These CTL lines required similar peptide concentrations for target cell recognition, with the exception of CTL lines 1A-B7 and 3A-C4, which required a slightly higher peptide concentration for optimal target recognition (Fig. 3B).
FIG. 2.
Localization of CTL epitopes on Hantaan virus nucleocapsid protein recognized by representative cell lines isolated from donors A and C. Target cells were autologous BLCL infected with either a recombinant vaccinia virus expressing Hantaan virus N protein or wild-type vaccinia virus or pulsed with the indicated peptide at 25 μg/ml. E/T ratio, 10.
FIG. 3.
Peptide dose-response curves for N-protein-specific cell lines isolated from donors A and C. Autologous BLCL were pulsed with an optimal 9-mer peptide epitope recognized by the T-cell lines at the indicated concentrations (Conc.). (A) Cell lines from donors A and C specific for N protein amino acids 12 to 20. (B) Cell lines specific for N protein amino acids 421 to 429. Data from a representative experiment are shown.
MHC restriction of nucleocapsid-specific CD8+ CTL lines.
HLA restriction of the CD8+ CTL lines was determined by testing each CTL line against a panel of partially HLA-matched allogeneic BLCL infected with a vaccinia virus recombinant expressing the Hantaan virus N protein. To ensure that lysis of the allogeneic targets was not due to recognition of an allogeneic or vaccinia determinant, each target cell line was also infected with wild-type vaccinia virus and tested in the same assay. In all assays, specific lysis of targets infected with wild-type vaccinia was negative (data not shown). The results of the HLA restriction analysis of representative CTL lines isolated from donors A and C are shown in Fig. 4.
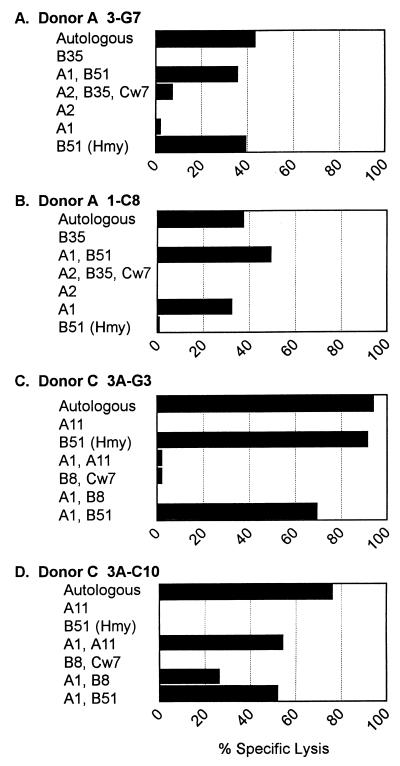
FIG. 4.
MHC restriction of T-cell lines from donors A and C. T-cell lines were tested in a CTL assay against a panel of partially HLA-matched allogeneic target cells infected with a recombinant vaccinia virus expressing the Hantaan virus N protein. The HLA alleles shared by the donor and the allogeneic target cell are shown on the y axis. Data from a representative experiment are shown. E/T ratio, 10.
All of the CD8+ CTL lines generated from donor A recognized and lysed allogeneic targets expressing both HLA A1 and B51 (Fig. 4A and B and data not shown). CTL lines 3-G7 and 10-E2, which recognize N(12-20), also recognize an Hmy-2C1R cell line expressing only HLA B51, indicating that these CTL lines are restricted by HLA B51 (Fig. 4A and data not shown). CTL line 1-C8, which recognizes N(421-429), did not recognize the Hmy-B51 target cells. Since this cell line also recognizes targets expressing both A1 and B51 or A1 alone, this cell line is restricted by HLA A1 (Fig. 4B).
Donor C CTL line 3A-G3, which is specific for N(12-20), recognizes only targets that express B51, including the Hmy-2C1R-B51 cell line (Fig. 4C), thus definitively demonstrating that CTL line 3A-G3 is restricted by B51. CTL lines 1A-B7, 3A-B8, 3A-C4, and 3A-C10 from donor C, which are all specific for N(421-429), recognized allogeneic targets expressing both A1 and A11, A1 and B8, and A1 and B51 (Fig. 4D and data not shown) but did not recognize other targets expressing A11, B8, or B51 alone, indicating that these cell lines are all restricted by HLA A1.
In summary, CTL lines from both donors that recognize the N-terminal nucleocapsid epitope [N(12-20)] are restricted by HLA B51, while CTL lines that recognize the C-terminal nucleocapsid epitope [N(421-429)] are restricted by HLA A1.
Cross-reactivity of Hantaan virus CTL lines against other hantavirus N proteins.
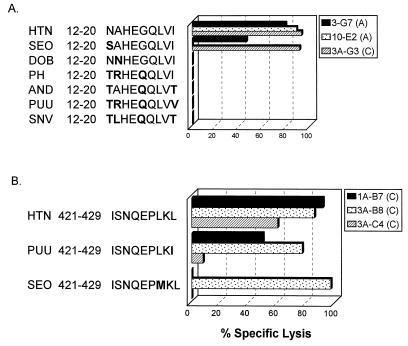
Little is known about cross-reactive immunity between different hantaviruses. The hantavirus N proteins have an overall sequence identity of 50%, although specific regions of the protein are more highly conserved (e.g., the C-terminal 100 amino acids are 85% identical). Closely related hantaviruses, such as Hantaan and Seoul viruses, have nucleocapsid proteins that are 82% identical. The high level of sequence identity suggests that hantavirus infection may result in the development of immune responses that are cross-reactive among numerous hantaviruses. To test whether the CTL lines were cross-reactive with other hantaviruses, we tested the CTL lines for recognition of targets infected with a recombinant vaccinia virus expressing the Sin Nombre virus N protein. The CTL lines were also tested against targets pulsed with corresponding 9-mer peptide epitopes from various other hantaviruses which were synthesized based on published sequences.
The CD8+ CTL lines that recognize the N-terminal nucleocapsid epitope (donor A, 3-G7 and 10-E2; donor C, 3A-G3) do not recognize target cells infected with a recombinant vaccinia virus expressing the Sin Nombre virus N protein or targets pulsed with 9-mer peptides representing the corresponding epitope from various other hantaviruses, including Sin Nombre, Dobrava, Prospect Hill, Andes, and Puumala viruses (Fig. 5 and 6A). This epitope is variable among the different hantaviruses, differing by up to four amino acids (Fig. 6A). The Dobrava virus peptide varies by only one amino acid (A2→N) from the Hantaan virus epitope but is not recognized by any of the CTL lines (Fig. 6). The Seoul virus peptide also varies by only one amino acid from the Hantaan virus epitope (N1→S) and is recognized by CTL lines 3-G7 and 3A-G3 but not by 10-E2 (Fig. 6A).
FIG. 5.
Recognition of Sin Nombre virus nucleocapsid protein by Hantaan virus N protein-specific T-cell lines from donors A and C. T-cell lines were tested in a CTL assay against autologous BLCL infected with recombinant vaccinia viruses expressing Hantaan virus N protein or Sin Nombre virus N protein or infected with a wild-type vaccinia virus control. E/T ratio, 10.
FIG. 6.
Cross-reactivity of CTL lines against epitopes from other hantavirus N proteins. CTL lines were tested against autologous BLCL targets pulsed with the Hantaan virus epitope or the corresponding epitope from other hantavirus N proteins (25 μg/ml). Amino acids that differ from the corresponding amino acid in the Hantaan virus epitope sequence are shown in boldface. E/T ratio, 10. SEO, Seoul virus; DOB, Dobrava virus; PH, Prospect Hill virus; AND, Andes virus; PUU, Puumala virus; SNV, Sin Nombre virus. (A) CTL lines specific for N protein amino acids 12 to 20. (B) CTL lines specific for N protein amino acids 421 to 429. Cell lines 1-C8 (donor A) and 3A-C10 (donor C) showed a pattern of peptide recognition identical to that of cell line 3A-B8 (donor C).
The CTL lines specific for the C-terminal nucleocapsid epitope 421-429 (donor A, 1-C8; donor C, 1A-B7, 3A-B8, 3A-C4, and 3A-C10) all recognized targets infected with a vaccinia virus recombinant expressing the Sin Nombre virus nucleocapsid protein (Fig. 5). It is not surprising that these CTL lines are cross-reactive with the Sin Nombre virus nucleocapsid, since the epitope is identical in the two viruses (ISNQEPLKL). This epitope is also identical in other hantaviruses, including Andes, NY-1, Dobrava, and Convict Creek viruses. This C-terminal epitope in Puumala, Prospect Hill, and Bayou viruses differs by one amino acid, with a conservative Leu-to-Ile change at position 9. Four of the five CTL lines isolated recognized this epitope (donor A, 1-C8; donor C, 1A-B7, 3A-B8, and 3A-C10) (Fig. 6B and data not shown). The epitope in Seoul virus also differs from the Hantaan virus epitope by one amino acid, with a Leu-to-Met change at position 7. Three of the five CTL lines lysed targets pulsed with this peptide epitope, with cell lines 1A-B7 and 3A-C4 failing to recognize and lyse these targets (Fig. 6B and data not shown).
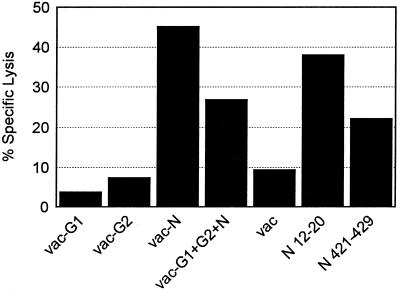
Recognition of nucleocapsid epitopes by T cells in 7-day bulk cultures.
The Hantaan virus-specific clones isolated from donors A and C are all specific for one of two epitopes on the nucleocapsid protein. In order to address the in vivo significance of these epitopes, we tested recognition of these epitopes in short-term bulk cultures from donor C. Seven-day bulk cultures of donor C PBMC demonstrated significant recognition of both peptide epitopes, with the N-terminal epitope (amino acids 12 to 20) being recognized at levels comparable to that of the whole N protein (vac-N) (Fig. 7). Precursor frequency analysis confirms that T cells recognizing these two epitopes are present at relatively high frequencies in the peripheral T-cell pool. Single cell IFN-γ secretion analysis (ELISPOT assay) of donor C PBMC indicates that T cells specific for N(12-20) have a frequency ranging from 1 per 7,326 to 1 per 8,968 PBMC, and T cells specific for N(421-429) have a frequency ranging from 1 per 18,181 to 1 per 19,417 PBMC.
FIG. 7.
Recognition of Hantaan virus proteins and CTL epitopes in short-term bulk culture of PBMC from donor C. PBMC (2 × 106 to 5 × 106) were stimulated in vitro with a gamma-irradiated Hantaan virus for 7 days. Bulk culture cells were tested on day 7 against autologous BLCL targets either infected with recombinant vaccinia viruses expressing Hantaan virus proteins (vac-G1, -G2, -N, and -G1+G2+N) or pulsed with the indicated peptides at 25 μg/ml. E/T ratio, 80.
DISCUSSION
In this study, we have analyzed virus-specific memory T-cell responses in three donors who had previous laboratory-acquired infections with Hantaan virus, the prototype hantavirus. In bulk culture, PBMC from two donors (donors A and C) recognized only targets expressing nucleocapsid protein, while PBMC from the third donor (donor B) recognized targets expressing the G1 glycoprotein. The detection of CD8+ T-cell responses in two of three donors following stimulation with an inactivated virus suggests that the viral proteins were able to access the class I MHC peptide presentation pathway following endocytosis of the inactive virus. Priming of CD8+ CTL by inactivated virus has also been demonstrated in other virus systems, including influenza and Sendai viruses (6, 30). This is the first demonstration of specific human T-cell responses to Hantaan virus.
The clear differences in protein recognition among Hantaan virus-immune donors suggests that the nature and composition of the immune response against the virus vary between individuals, possibly due to differences in HLA haplotypes. In one donor, CTL responses were directed primarily against the G1 glycoprotein. The CD4+, G1-specific CTL lines isolated from this donor did not recognize autologous targets infected with a recombinant vaccinia virus expressing the Sin Nombre virus G1 protein (data not shown). This is not surprising, since the hantavirus glycoproteins are less conserved than the nucleocapsid proteins. In two of three donors, CTL responses were directed primarily against the N protein. Interestingly, our laboratory has previously isolated N-specific CTL from donors with hantavirus pulmonary syndrome caused by Sin Nombre virus infection (12). We did not detect CTL responses specific for the G2 glycoprotein in any of the donors. We were unable to examine CTL recognition of the viral polymerase (L protein), because we do not have a vaccinia virus recombinant expressing this gene product.
It is possible that memory responses against other viral proteins or nucleocapsid peptides are present in these individuals but are not prevalent enough to detect following short-term culture. It is also possible that the use of inactivated virus led to preferential stimulation of T cells specific for the two epitopes described. Both epitopes are located at the termini of the nucleocapsid protein, potentially allowing them to be more readily degraded and presented on MHC class I molecules than more internal peptides. However, the facts that both epitopes described here are detectable in short-term bulk cultures and that T-cell precursors specific for these epitopes have high precursor frequencies in PBMC from donor C suggest that these CTL lines are prevalent in the peripheral T-cell pool and are thus likely to play a role in the in vivo immune response. In addition, subsequent attempts to identify other CTL lines resulted in the repeated isolation of CTL lines specific for the two epitopes described here.
The three CTL lines specific for amino acids 12 to 20 (NAHEGQLVI) are all restricted by HLA B51 and are either Hantaan virus specific or recognize only the very similar Seoul virus epitope. The consensus motif for peptides binding to HLA B51 consists of anchor residues at positions 2 (A, P, or G) and 9 (L, V, or I) (41). This Hantaan virus N peptide fits the consensus B51 peptide binding motif with an alanine (A) at position 2 and an isoleucine (I) at position 9. It is not surprising that the clones specific for this epitope fail to recognize the corresponding regions of other hantaviruses, since this region of the nucleocapsid protein is quite variable among different viruses, with many of the amino acid differences occurring at the anchor positions for HLA binding (Fig. 6A). The corresponding epitope from Dobrava virus differs from the Hantaan virus epitope by only one amino acid. However, the amino acid change in the Dobrava virus epitope is located at anchor position 2 (A13→N). It is interesting that the HLA B51 is very common among populations in Asia, where Hantaan virus is endemic, being present in 8 to 12% of the population (19).
The CTL lines that recognize the C-terminal nine amino acids of the nucleocapsid protein (421 to 429: ISNQEPLKL) are restricted by HLA A1 and are cross-reactive against several different hantavirus sequences. Interestingly, this peptide does not fit the consensus HLA A1 binding motif in which an acidic residue (D or E) is preferred at position three and a tyrosine (Y) is highly preferred at anchor position 9 (11, 41). This epitope is identical among various hantaviruses, including Sin Nombre, Andes, NY-1, Dobrava, and Convict Creek viruses, and differs from the Puumala, Prospect Hill, and Bayou virus epitope by a single conservative change at position 9 (L429→I). The corresponding epitope from Seoul virus also differs by one amino acid, with a substitution at position 7 (L427→M). The sequence conservation at this epitope and the recognition of target cells expressing Sin Nombre virus N protein by CTL suggest that infection with Hantaan virus induces some cross-reactive T-cell responses. This cross-reactive epitope is particularly interesting in that it is identical not only among closely related viruses that cause HFRS (e.g., Hantaan and Dobrava viruses) but also among viruses that are more distantly related and cause distinct syndromes (e.g., Hantaan and Sin Nombre viruses). The restricting allele for this CTL epitope, HLA A1, is very common in North American Caucasian populations (17 to 19%) but is relatively rare among Asian populations (0.5 to 5%) (19).
There is no existing evidence to indicate whether a person who has been infected with one type of hantavirus may be reinfected with the same or a different type of hantavirus. The data presented here demonstrate that infection with Hantaan virus results in the development of both Hantaan virus-specific and hantavirus cross-reactive T-cell responses. Similarly, the Sin Nombre virus-specific CTL lines established in our laboratory included both Sin Nombre virus-specific and cross-reactive lines (12). This pattern is also similar to that seen in individuals who received a live, attenuated dengue virus immunization, in which some T-cell responses are dengue virus serotype specific and some are serotype cross-reactive and/or cross-reactive with other flaviviruses (13, 14, 24). The existence of cross-reactive responses suggests that infection with one hantavirus may confer a limited degree of protection against illness caused by other types of hantavirus, even those more distantly related to the original infecting strain. Murine studies have demonstrated that immunization with one type of hantavirus elicits protective CTL responses that are cross-reactive with other hantaviruses (2, 3). This is the first demonstration that cross-reactive CTL responses are also generated in humans following infection with Hantaan virus. In addition to providing protection against subsequent infection, it is also possible that activation of cross-reactive memory T cells may be deleterious in the event of reinfection by contributing to excessive activation of the immune response and resulting immunopathology. Kidney damage during acute HFRS may be caused, in part, by T-cell-mediated immunopathology. T lymphocytes (primarily CD8+) have been detected infiltrating the kidneys of patients with acute HFRS (34, 51). However, no work has been done to assess the specificity or effector functions of these cells. Human immunodeficiency virus-specific CD8+ cytolytic T lymphocytes have been isolated from human immunodeficiency virus-positive patients with neurologic disorders. It has been suggested that these cytotoxic T lymphocytes may contribute to the neurologic pathology seen in these patients (48).
The two epitopes identified in this study were recognized by short-term PBMC cultures that were stimulated with an inactivated Hantaan virus preparation and were shown to have high precursor frequencies in one donor. The precursor frequencies of CTL specific for these epitopes are similar to those detected for several immunodominant epitopes identified from influenza virus (20, 26). This indicates that T cells specific for these epitopes are present in relatively high numbers in the peripheral T-cell pool of these Hantaan virus-immune donors and are thus likely to play a role in vivo. It will be interesting to determine whether other immune individuals who share HLA alleles A1 and/or B51 have a memory T-cell response dominated by cells specific for these epitopes. Also, studies analyzing PBMC from naturally infected individuals should be carried out to determine if the immune response to natural infection is focused on a single viral protein and/or a few dominant epitopes, as these limited studies in individuals who had laboratory-acquired infections suggest.
In conclusion, we have demonstrated CD4+ and CD8+ hantavirus-specific CTL responses in three donors who had previous laboratory-acquired infections with Hantaan virus. The identification of specific T-cell responses in hantavirus infection is essential to both the understanding of the mechanisms involved in recovery from and pathology of infection and the design of vaccine strategies.
ACKNOWLEDGMENTS
We thank Alan Rothman for critical review of the manuscript, Julie Jameson for assistance with ELISPOT assays, and Jurand Janus for propagation of recombinant vaccinia viruses. We also thank the scientists who generously donated blood for this study.
This work was supported by NIH grants AI39780 and AI07349.
REFERENCES
- 1.Arikawa J, Lapenotiere H F, Iacono-Connors L, Wang M, Schmaljohn C S. Coding properties of the S and the M genome segments of Sapporo rat virus: comparison to other causative agents of hemorrhagic fever with renal syndrome. Virology. 1990;176:114–125. doi: 10.1016/0042-6822(90)90236-k. [DOI] [PubMed] [Google Scholar]
- 2.Asada H, Balachandra K, Tamura M, Kondo K, Yamanishi K. Cross-reactive immunity among different serotypes of virus causing haemorrhagic fever with renal syndrome. J Gen Virol. 1989;70:819–825. doi: 10.1099/0022-1317-70-4-819. [DOI] [PubMed] [Google Scholar]
- 3.Asada H, Tamura M, Kondo K, Dohi Y, Yamanishi K. Cell-mediated immunity to virus causing haemorrhagic fever with renal syndrome: generation of cytotoxic T lymphocytes. J Gen Virol. 1988;69:2179–2188. doi: 10.1099/0022-1317-69-9-2179. [DOI] [PubMed] [Google Scholar]
- 4.Avsic-Zupanc T, Toney A, Anderson K, Chu Y-K, Schmaljohn C S. Genetic and antigenic properties of Dobrava virus: a unique member of the Hantavirus genus, family Bunyaviridae. J Gen Virol. 1995;76:2801–2808. doi: 10.1099/0022-1317-76-11-2801. [DOI] [PubMed] [Google Scholar]
- 5.Baenziger J, Hengartner H, Zinkernagal R M, Cole G A. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. J Immunol. 1986;16:387–393. doi: 10.1002/eji.1830160413. [DOI] [PubMed] [Google Scholar]
- 6.Bender A, Bui L K, Feldman M A V, Larsson M, Bhardwaj N. Inactivated influenza virus, when presented on dendritic cells, elicits human CD8+ cytolytic T cell responses. J Exp Med. 1995;182:1663–1671. doi: 10.1084/jem.182.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brummer-Korvenkontio M, Vaheri A, vonBonsdorff C H, Vuorimies J, Manni T, Penttinen K, Oker-Blom N, Lahdevirta J. Nephropathica epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980;141:131–134. doi: 10.1093/infdis/141.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Bukowski J F, Kurane I, Lai C J, Bray M, Falgout B, Ennis F A. Dengue virus-specific cross-reactive CD8+ human cytotoxic T lymphocytes. J Virol. 1989;63:5086–5091. doi: 10.1128/jvi.63.12.5086-5091.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon M J, Openshaw P J M, Askonas B A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chizhikov V E, Spiropoulou C F, Morzunov S P, Monroe M C, Peters C J, Nichol S T. Complete genetic characterization and analysis of isolation of Sin Nombre virus. J Virol. 1995;69:8132–8136. doi: 10.1128/jvi.69.12.8132-8136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiBrino M, Parker K C, Shiloach J, Turner R V, Tsuchida T, Garfield M, Biddison W E, Coligan J E. Endogenous peptides with distinct amino acid anchor residue motifs bind to HLA-A1 and HLA-B8. J Immunol. 1994;152:620–631. [PubMed] [Google Scholar]
- 12.Ennis F A, Cruz J, Spiropolou C F, Waite D, Peters C J, Nichol S T, Kariwa H, Koster F T. Hantavirus pulmonary syndrome: CD8+ and CD4+ cytotoxic T lymphocytes to epitopes on sin nombre virus nucleocapsid protein isolated during acute illness. Virology. 1998;238:380–390. doi: 10.1006/viro.1997.8827. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon S J, Zeng W, Kurane I, Ennis F A. Identification of two epitopes on the dengue 4 virus capsid protein recognized by a serotype-specific and a panel of serotype-cross-reactive human CD4+ cytotoxic T lymphocyte clones. J Virol. 1996;70:141–147. doi: 10.1128/jvi.70.1.141-147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green S, Kurane I, Elelman R, Tacket C O, Eckles K H, Vaughn D W, Hoke C H, Ennis F A. Dengue virus-specific human CD4+ T-lymphocyte responses in a recipient of an experimental live-attenuated dengue virus type 1 vaccine: bulk culture proliferation, clonal analysis, and precursor frequency determination. J Virol. 1993;67:5962–5967. doi: 10.1128/jvi.67.10.5962-5967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi H, Ennis P D, Ariga H, Salter R D, Parham P, Kano K, Takiguchi M. HLA-B51 and HLA-Bw52 differ only by two amino acids which are in the helical region of the α1 domain. J Immunol. 1989;142:306–311. [PubMed] [Google Scholar]
- 16.Hjelle B L, Jenison S A, Goade D E, Green W B, Fedderson R M, Scott A A. Hantaviruses: clinical, microbiologic, and epidemiologic aspects. Crit Rev Clin Lab Sci. 1995;32:469–508. doi: 10.3109/10408369509082592. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Campbell W P, Means R, Ackman D M. Hantavirus S RNA sequence from a fatal case of HPS in New York. J Med Virol. 1996;50:5–8. doi: 10.1002/(SICI)1096-9071(199609)50:1<5::AID-JMV2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Jin B, Wang M, Li E, Sun C. Hemorrhagic fever with renal syndrome: relationship between pathogenesis and cellular immunity. J Infect Dis. 1994;169:868–870. doi: 10.1093/infdis/169.4.868. [DOI] [PubMed] [Google Scholar]
- 19.Imanishi T, Akaza T, Kimura A, Tokunaga K, Gojobori T. Twelfth International Histocompatibility Workshop and Conference. Paris, France: Medical and Scientific International Publisher; 1997. Allele and haplotype frequencies for HLA and complement loci in various ethnic groups; p. 1065. [Google Scholar]
- 20.Jameson J, Cruz J, Ennis F A. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J Virol. 1998;72:8682–8689. doi: 10.1128/jvi.72.11.8682-8689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klavinskis L S, Tishon A, Oldstone M B A. Efficiency and effectiveness of cloned virus-specific cytotoxic T lymphocytes in vivo. J Immunol. 1989;143:2013–2016. [PubMed] [Google Scholar]
- 22.Koster F T, Williams T M, Griffith B B, Goade D E, Hjelle B L. Fourth International Conference on HFRS and Hantaviruses. Atlanta, Ga: American Society for Tropical Medicine and Hygiene; 1998. Genetic associations with hantavirus pulmonary syndrome due to sin nombre virus; p. 179. [Google Scholar]
- 23.Krakauer T, Leduc J W, Morrill J C, Anderson A O, Krakauer H. Serum levels of alpha and gamma interferons in hemorrhagic fever with renal syndrome. Viral Immunol. 1994;7:97–101. doi: 10.1089/vim.1994.7.97. [DOI] [PubMed] [Google Scholar]
- 24.Kurane I, Brinton M A, Samson A L, Ennis F A. Dengue virus-specific, human CD4+ CD8− cytotoxic T cell clones: multiple patterns of virus cross-reactivity recognized by NS3-specific T-cell clones. J Virol. 1991;65:1823–1828. doi: 10.1128/jvi.65.4.1823-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurane I, Meager A, Ennis F A. Dengue virus-specific human T cell clones: serotype cross-reactive proliferation, interferon-γ production and cytotoxic activity. J Exp Med. 1989;170:763–775. doi: 10.1084/jem.170.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H W. Hemorrhagic fever with renal syndrome in Korea. Rev Infect Dis. 1989;2:S864–S876. [PubMed] [Google Scholar]
- 28.Lee H W, Lee P-W, Johnson K M. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- 29.Linderholm M, Ahlm C, Settergren B, Waage A, Tarnvik A. Elevated plasma levels of tumor necrosis factor (TNF)-a, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J Infect Dis. 1996;173:38–43. doi: 10.1093/infdis/173.1.38. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Zhou X, Orvell C, Lederer E, Ljunggren H-G, Jondal M. Heat-inactivated Sendai virus can enter multiple MHC class I processing pathways and generate cytotoxic T lymphocyte responses in vivo. J Immunol. 1995;154:3147–3155. [PubMed] [Google Scholar]
- 31.Lopez N, Padula P, Rossi C, Lazaro M E, Franze-Fernandez M T. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology. 1996;220:223–226. doi: 10.1006/viro.1996.0305. [DOI] [PubMed] [Google Scholar]
- 32.Lopez N, Padula P, Rossi C, Miguel S, Edelstein A, Ramirez E, Franze-Fernandez M T. Genetic characterization and phylogeny of Andes virus and variants from Argentina and Chile. Virus Res. 1997;50:77–84. doi: 10.1016/s0168-1702(97)00053-1. [DOI] [PubMed] [Google Scholar]
- 33.Mertz G J, Hjelle B L, Bryan R T. Hantavirus infection. Adv Intern Med. 1997;42:369–421. [PubMed] [Google Scholar]
- 34.Mustonen J, Helin H, Pietila K, Brummer-Korvenkontio M, Hedman K, Vaheri A, Pasternack A. Renal biopsy findings and clinicopathologic correlations in nephropathica epidemica. Clin Nephrol. 1994;41:121–126. [PubMed] [Google Scholar]
- 35.Mustonen J, Partanen J, Kanerva M, Pietila K, Vapalahti O, Pasternack A, Vaheri A. Genetic susceptibility to severe course of nephropathica epidemica caused by puumala hantavirus. Kidney Int. 1996;49:217–221. doi: 10.1038/ki.1996.29. [DOI] [PubMed] [Google Scholar]
- 36.Newell C K, Martin S, Sendele D, Mercadal C M, Rouse B T. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichol S T, Spiropolou C F, Morzunov S, Rollin P E, Ksiazek T G, Feldmann H, Sanchez A, Childs J, Zaki S, Peters C J. Genetic identification of a novel hantavirus associated with an outbreak of acute respiratory illness in the southwestern United States. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- 38.Ooba T, Hayashi H, Karaki S, Tanabe M, Kano K, Takiguchi M. The structure of HLA-B35 suggests that it is derived from HLA-Bw58 by two genetic mechanisms. Immunogenetics. 1989;30:76–80. doi: 10.1007/BF02421534. [DOI] [PubMed] [Google Scholar]
- 39.Parrington M A, Kang C Y. Nucleotide sequence analysis of the S genome segment of Prospect Hill virus: comparison with the prototype hantavirus. Virology. 1990;175:167–175. doi: 10.1016/0042-6822(90)90197-y. [DOI] [PubMed] [Google Scholar]
- 40.Pensiero M N, Sharefkin J B, Dieffenbach C W, Hay J. Hantaan virus infection of human endothelial cells. J Virol. 1992;66:5929–5936. doi: 10.1128/jvi.66.10.5929-5936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rammensee H-G, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 42.Ruo S L, Li Y L, Tong Z, Ma Q R, Liu Z L, Tang Y W, Ye K L, Xu Z Y, McCormick J B, Fisher-Hoch S P. Retrospective and prospective studies of hemorrhagic fever with renal syndrome in rural China. J Infect Dis. 1994;170:527–534. doi: 10.1093/infdis/170.3.527. [DOI] [PubMed] [Google Scholar]
- 43.Schmaljohn A L, Li D, Negley D L, Bressler D S, Turell M J, Korch G W, Ascher M S, Schmaljohn C S. Isolation and initial characterization of a newfound hantavirus from California. Virology. 1995;206:963–972. doi: 10.1006/viro.1995.1019. [DOI] [PubMed] [Google Scholar]
- 44.Schmaljohn C S, Chu Y-K, Schmaljohn A L, Dalrymple J M. Antigenic subunits of hantaan virus expressed by baculovirus and vaccinia virus recombinants. J Virol. 1990;64:3162–3170. doi: 10.1128/jvi.64.7.3162-3170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmaljohn C S, Hasty S E, Harrison S A, Dalrymple J M. Characterization of Hantaan virions, the prototype virus of hemorrhagic fever with renal syndrome. J Infect Dis. 1983;148:1005–1012. doi: 10.1093/infdis/148.6.1005. [DOI] [PubMed] [Google Scholar]
- 46.Schmaljohn C S, Hjelle B L. Hantavirus: a global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmaljohn C S, Jennings G B, Hay J, Dalrymple J M. Coding strategy of the S genome segment of Hantaan virus. Virology. 1986;155:633–643. doi: 10.1016/0042-6822(86)90223-0. [DOI] [PubMed] [Google Scholar]
- 48.Sethi K K, Naher H, Stroehmann I. Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-specific and HLA-restricted cytotoxic T-cell clones. Nature. 1988;335:178–181. doi: 10.1038/335178a0. [DOI] [PubMed] [Google Scholar]
- 49.Stohwasser R, Giebel L B, Zoller L, Bautz E K F, Darai G. Molecular characterization of the RNA S segment of nephropathica epidemica virus strain Hallnas B1. Virology. 1990;174:79–86. doi: 10.1016/0042-6822(90)90056-w. [DOI] [PubMed] [Google Scholar]
- 50.Storkus W J, Howell D N, Salter R D, Dawson J R, Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987;138:1657–1659. [PubMed] [Google Scholar]
- 51.Temonen M, Mustonen J, Helin H, Pasternack A, Vaheri A, Holthofer H. Cytokines, adhesion molecules, and cellular infiltration in nephropathica epidemica kidneys: an immunohistochemical study. Clin Immunol Immunopathol. 1996;78:47–55. doi: 10.1006/clin.1996.0007. [DOI] [PubMed] [Google Scholar]
- 52.Temonen M, Vapalahti O, Holthofer H, Brummer-Korvenkontio M, Vaheri A, Lankinen H. Susceptibility of human cells to puumala virus infection. J Gen Virol. 1993;74:515–518. doi: 10.1099/0022-1317-74-3-515. [DOI] [PubMed] [Google Scholar]
- 53.Williams R J, Bryan R T, Mills J N, Palma R E, Vera I, De Velasquez F, Baez E, Schmidt W E, Figueroa R E, Peters C J, Zaki S R, Khan A S, Ksiazek T G. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am J Trop Med Hyg. 1997;57:274–282. doi: 10.4269/ajtmh.1997.57.274. [DOI] [PubMed] [Google Scholar]
- 54.Zaki S R, Greer P W, Coffield L M, Goldsmith C S, Nolte K B, Foucar K, Fedderson R M, Zumwalt R E, Miller G L, Khan A S, Rollin P E, Ksiazek T G, Nichol S T, Mahy B W J, Peters C J. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]