Abstract
The global population is aging at an unprecedented rate, resulting in a growing and vulnerable elderly population in need of efficient comprehensive healthcare services that include long-term care and skilled nursing facilities. In this context, severe aspiration pneumonia, a condition that carries substantial morbidity, mortality, and financial burden, especially among elderly patients requiring admission to the intensive care unit, has attracted greater concern. Aspiration pneumonia is defined as a pulmonary infection related to aspiration or dysphagia in etiology. Prior episodes of coughing on food or liquid intake, a history of relevant underlying conditions, abnormalities on videofluoroscopy or water swallowing, and gravity-dependent shadow distribution on chest imaging are among the clues that suggest aspiration. Patients with aspiration pneumonia tend to be elderly, frail, and suffering from more comorbidities than those without this condition. Here, we comprehensively address the epidemiology, clinical characteristics, diagnosis, treatment, prevention, and prognosis of severe aspiration community-acquired pneumonia in the elderly to optimize care of this high-risk demographic, enhance outcomes, and minimize the healthcare costs associated with this illness. Emphasizing preventive measures and effective management strategies is vital in ensuring the well-being of our aging population.
Keywords: Aspiration pneumonia, Aged, Critical illness, Swallowing disorders, Frailty, Antibacterial agents
Introduction
The global demographic landscape is undergoing considerable shifts most marked by an increase in the aging population. This demographic transformation has substantial implications for healthcare systems and society. As life expectancy continues to rise, the elderly are becoming a more prominent and crucial segment of the population. According to recent Census Data,[1] the proportion of people aged 65 and older is rapidly growing, and it is estimated that by 2050, nearly one in six people will be in this age group. With such impending demographic changes, it is essential to understand the unique healthcare challenges that elderly individuals face, particularly in relation to susceptibility to aspiration events and their infectious complications. Aspiration events range from microaspiration (subtle inhalation of minimal amounts of foreign material into the lungs) to macroaspiration (larger, more substantial inhalational events). Aspiration events are particularly relevant to older individuals due to age-related changes in protective mechanisms of the airway and the swallowing process.[2] Aspiration events can lead to inhalation of gastric contents or oral secretions, potentially causing pulmonary complications. Understanding the prevalence and the consequences of such events is crucial for effective healthcare management among this highly vulnerable patient population.
Aspiration events in the elderly frequently lead to severe aspiration pneumonia, a life-threatening condition that often requires intensive care unit (ICU) admission.[3] This review article is concerned with events that lead to aspiration pneumonia in the community setting and thus defines such a condition as severe aspiration community-acquired pneumonia (CAP). As such, aspiration events that occur during hospitalization (48–72 h after hospital admission) and are not related to hospital-acquired or ventilator-associated pneumonia (HAP/VAP) are not discussed in this manuscript. However, as some elderly patients reside in nursing homes or other long-term care facilities, this age group is also included in our review. Such elderly individuals encompass nearly 2% of all the adults over 65 years of age and have increased risk factors due to limited functional status, higher incidence of underlying pulmonary disease, and comorbidities such as stroke, musculoskeletal disorders, and immunosuppression.[4,5]
Severe aspiration CAP is characterized by the presence of aspirated content in the lungs and often leads to severe acute lung injury, acute respiratory distress syndrome (ARDS), and numerous systemic complications.[6] The elderly are particularly vulnerable to such illnesses due to age-related comorbidities and reduced immune function.[7] As such, understanding the epidemiology, clinical characteristics, diagnosis, treatment, and prevention of severe aspiration CAP in the elderly is of critical importance. Here, we explore the clinical challenges related to severe aspiration CAP in the elderly to shed light on potential strategies for improving care and outcomes of the elderly patient population.
Definitions
The definition of severe CAP according to the European Respiratory Society, European Society of Intensive Care Medicine, European Society of Clinical Microbiology, and Infectious Diseases and Asociacion Latinoamericana de Torax (ERS/ESICM/ESCMID/ALAT) refers to patients who require ICU admission due to a potential need for critical organ support.[8,9] Here, we additionally consider the word “aspiration” as referring to the actual presumptive causative mechanisms of the pneumonia. Furthermore, we include in the definition the original stratification of pneumonia severity according to the presence of at least one major criterion or the presence of three or more minor criteria according to the American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) CAP management guidelines.[10] Major criteria include respiratory failure requiring mechanical ventilation or septic shock requiring vasopressors. Minor criteria include an elevated respiratory rate (>30/min), a partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio of ≤250, multilobar infiltrates on chest imaging, confusion, or disorientation on physical exam, uremia (blood urea nitrogen >20 mg/dL), leukopenia (<4000 cells/µL), thrombocytopenia (<100,000/µL), hypothermia (body temperature <36 °C), or hypotension requiring aggressive fluid resuscitation.[10] Some patients with severe aspiration CAP progress to ARDS that is characterized by a diffuse, inflammatory lung injury resulting in poor oxygenation, bilateral pulmonary infiltrates, and an acute nature of onset (<7 days). ARDS is caused by capillary endothelial injury and diffuse alveolar damage, and is defined by a patient's PaO2/FiO2 ratio of <300.[11]
Epidemiology
The epidemiology of severe aspiration CAP depends on diverse factors that warrant defining. The word “severe” relates to critical illness in patients who require ICU admission, either invasive or non-invasive mechanical ventilation, vasopressors, or inotropes. Aspiration events generally occur in the days preceding clinical presentation or during the first 24–48 h of hospitalization in a patient with pneumonia, thus differentiating the pathology from hospital-acquired aspiration that leads to HAP or VAP. It has been estimated that older adults comprise approximately 80% of all aspiration pneumonia cases.[12] Microaspiration (tracer quantity in the order of 0.01–0.20 mL) was reported to occur in 45% of healthy young men during sleep[13]; as such, this phenomenon would be expected to occur at higher rates among older adults.
Nursing home-acquired pneumonia represents the second-most common type of infection among patients living in long-term care facilities in the United States.[4] The incidence of aspiration pneumonia was found to be 30% among patients residing in chronic care facilities admitted to the hospital with a pneumonia diagnosis, in contrast to a 10% incidence among patients with pneumonia who did not.[14]
The prevalence of aspiration pneumonia varies widely. Approximately 7%–19% of all patients who present with pneumonia were reported to have become infected outside the hospital setting.[15,16] However, multiple studies account for the presence of aspiration risk factors, potentially increasing the prevalence estimate to above 14%. Lanspa et al.[17] analyzed data of patients diagnosed with aspiration CAP, identified by ICD-9 code 507. X as either primary or secondary, or described by the treating physician in patient history or discharge summary notes. Aspiration CAP patients were found to have been older (77 years vs. 59 years; P < 0.0001), more likely to have suffered multilobar disease or pleural effusion on chest imaging, and more likely to have suffered from greater disease severity.[17] These patients were more likely to have been admitted to the ICU, have longer hospital stays, suffer from illness of greater severity according to minor criteria set forth by ATS/IDSA recommendations for severe CAP, and have a higher Charlson Comorbidity Index (a scale used to assess the comorbid burden).[17] Additionally, aspiration CAP conferred a twice-higher risk for inpatient mortality after adjusting for age, disease severity, and comorbid conditions. In addition to age, several comorbid conditions are recognized to independently associate with aspiration risk such as chronic liver disease, congestive heart failure, and stroke.[16] Patients at risk for aspiration pneumonia had poorer short-term outcomes (a 30-day mortality seen in 17% as compared to 8% of the patients without aspiration risk factors).[16] Additionally, poor long-term outcomes were also noted in patients with aspiration risk factors including an increased 1-year mortality, increased risk of readmission, and increased risk of recurrent admissions due to pneumonia.[16]
Aspiration pneumonia is a significant healthcare concern among acute cerebrovascular disease patients. Güngen et al.[3] reported the impact of aspiration pneumonia among patients who required neurocritical care due to a cerebrovascular event. The authors found that 16% of the patients suffered aspiration pneumonia during their hospitalization. Patients with aspiration pneumonia were more likely to require ICU admission (37.5%) as compared to patients without aspiration pneumonia (4.7%). Aspiration pneumonia was associated with older age, hypertension, high-risk echocardiographic findings (e.g., thrombus in the left atrium/atrial appendix, left ventricular thrombus, dilated cardiomyopathy, akinetic/hypokinetic left ventricular segment) and a higher modified Rankin Scale Score (used to grade functional ambulation/disability of patients during hospitalization).[3] In addition to patients who suffered cerebrovascular events, those with other neurological conditions including generalized status epilepticus were more likely to suffer aspiration and present with severe aspiration CAP.[18]
Several studies have suggested that aspiration pneumonia is associated with higher mortality in patients requiring ICU admission.[19,20] The mortality rate after first admission for aspiration pneumonia was previously researched by Putot et al.[21]; evidenced mortality rates of 30% at 30 days and 70% at 2 years after hospital admission were reported. In-hospital mortality has been found to occur in nearly one-third of the admissions for aspiration pneumonia.[20] In-hospital mortality was found to have a relationship with both volume and content of the aspiration, with rates found to potentially be as high as 70%.[22,23] Importantly, the mortality rate for severe aspiration CAP in the elderly is likely higher. Recurrence of aspiration pneumonia within 30 days occurred in nearly 30% of the patients, likely due to recurrent aspiration events.[24]
Numerous factors predict mortality among elderly patients admitted to the ICU for aspiration pneumonia. The presence of healthcare-related risk factors, Charlson Comorbidity Index score, Cumulative Illness Rating Scale (used to assess the severity of comorbid conditions across different organ systems) score, assessment of activities of daily living, a high acute physiology and chronic health evaluation (APACHE) score, and a low Glasgow coma scale score correlated with greater mortality in the ICU, worse functional status, and poorer long-term survival.[[25], [26], [27], [28]] The median total cost of hospitalization for aspiration pneumonia in 2012, was reported to be nearly 30,000 dollars in the United States.[29]
Risk Factors
Multiple risk factors have been associated with the risk of aspiration CAP among the elderly, including demographic characteristics, a history of neurological conditions (e.g., stroke, epilepsy/seizures, altered mental states, toxin- or drug-related encephalopathy), medical and non-medical conditions, and chronic interventions (Figure 1).[15] The use of commonly prescribed medications in the elderly such as sedatives, hypnotics, or psychotropic medications can compromise the swallowing reflex and promote muscle relaxation.[30] One large observational, international, multicenter, point-prevalence study identified male sex and previous stroke, dementia, mental illness, low body weight, enteral tube feedings, being bedridden, and admission to hospital from a nursing home as risk factors independently associated with aspiration CAP.[15] Thus, important overlaps among aspiration risk factors in hospitalized CAP patients were suggested.[15] Interestingly, age >65 years was not a recognized risk factor for aspiration CAP. The aforementioned and other risk factors associated with severe aspiration CAP in the elderly will be discussed in following sections.
Figure 1.
Graphic representation of risk-factor overlap in elderly patients with multiple comorbidities.
CHF: Congestive heart failure; COPD: Chronic obstructive pulmonary disease; HIV: Human immunodeficiency virus; PPI: Proton pump inhibitor.
Due to a variety of factors that compromise immunity, the elderly are particularly susceptible to infections. Aging lungs were reported to recruit excessive CD8+ resident memory T cells after viral pneumonia, subsequently driving chronic inflammation, worsening pulmonary fibrosis, and deregulating cytokine production, thereby contributing to a weakened immune response.[31,32] Malnutrition, very prevalent in the elderly population, leads to metabolic changes and impaired immunity.[33,34] Muscle weakness, weakened immunity, altered mental status, poor oral health, and frailty all contribute to the development of malnutrition and are associated with an increased risk of aspiration CAP in the elderly.[33,34] Social and environmental factors similarly play crucial roles, as this patient population tends to suffer higher rates of depression, psychosocial isolation, and dependence on family support. Stress and depression are known to impair the immune system, creating a chronic inflammatory state that favors development of infections.[35] There is a substantially increased risk of infection among the elderly who reside in chronic care facilities. It is estimated that 1.64–3.83 million infections per year affect individuals residing in chronic care facilities in the United States alone.[36,37] Such risk factors predispose to infection among the elderly population (Figure 1).
In cases where aspiration CAP is suspected, no history of aspiration may be apparent. Aspiration is often caused by muscular weakness or sensory nerve damage in the pharynx.[38] Pikus et al.[39] reported that patients diagnosed with silent aspiration were more prone to developing pneumonia compared to those who swallowed normally. It is therefore essential to consider the possibility of prior silent aspiration, especially in the setting of multiple risk factors. Almirall et al.[40] identified a 91.7% prevalence of dysphagia that was accompanied by silent aspiration in half of such patients. These findings emphasize the importance of recognizing and addressing silent aspiration in the comprehensive management of severe aspiration CAP. Advanced age is a significant risk factor for dysphagia, particularly in patients aged ≥70 years.[41] Furthermore, comorbidities have been found to independently correlate with poor outcomes among institutionalized elderly individuals who suffer aspiration pneumonia.[42,43] Patients with a diagnosis of frailty, defined as an identifiable state of increased vulnerability among older adults, were found to suffer higher mortality from an incidence of aspiration pneumonia.[[44], [45], [46]]
Aspiration pneumonia can also result from gastroesophageal disorders including those affecting esophageal motility and gastroesophageal reflux disease. Certain medications can cause dysphagia by causing esophageal injury and increasing abnormal swallowing, either as a result of medication side effects or as complications of drug action. Esophageal injury and abnormal swallowing develop secondary to local irritation and are common among older adults who recline shortly after taking medications orally, especially in the setting of inadequate fluid intake at the time of the ingestion. In such instances, medications can remain in the esophagus for extended periods of time, potentially causing damage and affecting swallowing. Acid-containing products (e.g., erythromycin, tetracycline), aspirin, bisphosphonates (for the treatment of osteoporosis), iron-containing compounds, non-steroidal anti-inflammatory medications, potassium chloride supplements, and vitamin C (ascorbic acid) supplements are among the causative agents of esophageal damage and abnormal swallowing.[[47], [48], [49]] Antiepileptic drugs (e.g., carbamazepine, gabapentin, phenobarbital, phenytoin, valproic acid); benzodiazepines or other anti-anxiety medications (e.g., alprazolam, clonazepam, clorazepate, diazepam, lorazepam); narcotics used for pain management (e.g., codeine, fentanyl [patches], propoxyphene); and skeletal muscle relaxants used to treat muscle spasms (e.g., baclofen, cyclobenzaprine, tizanidine) may cause dysphagia as a side effect. Some of these medications can also cause central sedation and have the potential to decrease coordination among muscles involved in swallowing.[50] Drug-induced dysphagia is commonly seen in elderly patients treated with benztropine mesylate (sometimes indicated to alleviate movement abnormalities caused by psychotropic agents) as well as oxybutynin or tolterodine (sometimes indicated for bladder overactivity). Medications that can cause xerostomia may also influence swallowing and impair proper food movement; this sometimes occurs in the setting of treatment with agents such as angiotensin-converting enzyme inhibitors (ACEI), antiarrhythmics, antiemetics, antihistamines, decongestants, calcium channel blockers, diuretics, selective serotonin reuptake inhibitors, and antipsychotic and neuroleptic medications.[51] Inhaled corticosteroids, usually recommended for the treatment of asthma or chronic obstructive pulmonary disease (COPD), also increase the risk of pneumonia by decreasing pulmonary host defense via stimulation of extracellular nitric oxide release from alveolar macrophages and inhibition of cytokine production.[52]
Dental care is another crucial factor related to aspiration among the elderly. In a population-based cohort study conducted in Korea, higher dental cavity burden and tooth loss were associated with an increased risk of pneumonia.[53] Poor oral hygiene was also found to be associated with higher rates of mortality from aspiration pneumonia among frail, elderly patients.[54] As such, it is critical to maintain proper oral hygiene to decrease the risk of CAP and improve patient outcomes.
Clinical Presentation
Aspiration pneumonia is an infectious pathology caused by the aspiration of oropharyngeal secretions colonized by pathogenic bacteria; aspiration pneumonitis is caused by the aspiration of gastric contents and resultant damage to tissues.[55] Aspiration pneumonitis presents similarly to aspiration pneumonia yet has no infectious etiology, although secondary superinfection can occur. Aspiration pneumonitis is generally self-limited and primarily characterized by more rapid onset of dyspnea, hypoxemia, tachycardia, and diffuse wheezes or crackles on physical examination after witnessed aspiration. Chest imaging is usually abnormal with 16.5% of the patients exhibiting characteristic ARDS findings.[22] The difficulty in differentiating between aspiration pneumonia and pneumonitis can lead to overprescription of antibiotics by healthcare providers; antibiotics should only be initiated when infection is suspected or confirmed based on symptoms and laboratory parameters.[56]
Symptomatology after aspiration is varied and includes chronic cough, exacerbation of pre-existing COPD or asthma, lung parenchymal syndromes (e.g., aspiration pneumonitis, exacerbation of pulmonary fibrosis), and bacterial infections (e.g., aspiration pneumonia and CAP).[57] These, importantly, tend to vary depending on the volume of content aspirated. Aspiration pneumonitis and aspiration pneumonia are two important complications of large-volume aspiration that often abruptly change patient status.[57,58] Mortality was found to be related to the volume and content of the aspirate and is reported to be as high as 70% in the setting of large-volume aspiration.[59]
The clinical presentation of aspiration is diverse, particularly among the elderly (Table 1). Classical symptoms that suggest pneumonia include increased sputum production, fatigue, new onset fever, new onset dyspnea, and hypoxemia.[60] In older adults, the triad of fever, cough, and dyspnea is only found in 56% of the pneumonia cases; these cardinal symptoms are completely absent in 10% of the cases, making diagnosis particularly challenging due to an atypical presentation. In fact, nearly 50% of the elderly patients may not exhibit obvious symptoms such as fever and cough.[61,62] Difficulties with sputum production and collection result from factors such as muscle weakness, coordination difficulty, and inability to adequately expectorate. Additionally, such patients often present with symptoms unrelated to respiratory health, such as changes in overall functional abilities, altered mental status, fatigue, and decreased appetite.[[63], [64], [65]] During physical assessment, abnormal breath sounds (such as rales) may be heard. Hypoxia may also be apparent, which may in turn precipitate ARDS and an increase in respiratory rate.[60] Comorbid conditions such as COPD and chronic heart failure can mimic the initial presentation of pneumonia. Individuals with such pre-existing comorbidities may develop symptoms such as ongoing respiratory distress, persistent cough, and abnormal breathing.[66] Thus, conducting a thorough clinical assessment is critical when evaluating elderly patients who suffer from other chronic underlying conditions.
Table 1.
Physical, imaging, and laboratory examinations in the diagnosis of aspiration pneumonia.
| Items | Details |
|---|---|
| Symptoms | Classical symptoms: |
| • Increased sputum production | |
| • Fatigue | |
| • New onset fever | |
| • New onset dyspnea and hypoxemia | |
| Atypical symptoms (most common in elderly) | |
| • Altered mental status | |
| • Fatigue | |
| • Decreased appetite | |
| Physical exam findings | Decreased oxygen saturation |
| Tachypnea | |
| Crackles | |
| Wheezing | |
| Use of accessory respiratory muscles | |
| Lung imaging modalities | CXR |
| Chest CT | |
| Lung ultrasound | |
| Consider echocardiography | |
| Laboratory testing | CRP |
| Procalcitonin | |
| Legionella urine antigen | |
| Respiratory viral PCR | |
| Streptococcal antigen | |
| Sputum cultures |
CRP: C-reactive protein; CT: Computerized tomography; CXR: Chest X-ray; PCR: Polymerase chain reaction.
Diagnosis
Early diagnosis of aspiration pneumonia in the elderly is important and is linked with improved clinical outcomes. Documentation of aspiration on videofluoroscopy (i.e., videofluoroscopy swallow study or modified barium swallow study) using food or liquids to determine swallowing dynamics is useful in identifying causes of aspiration.[67,68] Direct visualization with video laryngoscopy has become a key diagnostic method in the assessment of patients with suspected aspiration.[69] Imaging techniques are usually the first diagnostic step for patients with aspiration CAP, with chest radiography widely used as an initial screen. Chest radiography is a particularly useful and cost-effective tool in revealing abnormalities that suggest aspiration pneumonia such as alveolar infiltrates. In instances where the diagnosis remains uncertain, computerized tomography (CT) imaging facilitates the detection of more subtle infiltrates or fluid collections, such as abscesses. Infiltrates typically appear as gravity-dependent scattered or lobe-specific patterns in pulmonary regions (on recumbency, the superior segments of the lower and posterior segments of the upper lobes are most frequently affected).[70] In a retrospective study of 900 CAP patients evaluated in the emergency department, the use of chest CT revealed findings positive for illness in 91% of the patients, with 41.5% being newly diagnosed after negative or inconclusive radiograph findings.[71] Pulmonary ultrasound is emerging as a non-invasive point-of-care tool for identifying effusions, consolidation, or pleural abnormalities. Although this modality has a sensitivity and specificity comparable to that of CT imaging, its use depends on specialist experience.[[72], [73], [74]] Heart failure has a high prevalence among elderly patients, and cardiac ultrasonography (echocardiography) is useful in distinguishing between aspiration pneumonia and cardiogenic pulmonary edema.[75,76]
Laboratory testing can also assist in the diagnosis of aspiration pneumonia. Initial laboratory examinations, including complete blood counts and comprehensive metabolic panels, provide valuable information that may suggest infection, such as an elevated white blood cell count. Measuring C-reactive protein (CRP) levels can reveal an ongoing inflammatory response, with elevated levels suggesting an infectious cause.[77,78] Adnet et al.[77] demonstrated the utility of elevated CRP levels in diagnosing bacterial pneumonia resulting from aspiration in patients suffering from drug-induced coma and pulmonary infiltrates, when other indicators such as fever and white blood cell counts had limited sensitivity and specificity in such cases. CRP concentrations >75 mg/L were associated with bacterial infection at a sensitivity of 87%, specificity of 76%, positive predictive value of 78%, and negative predictive value of 87%. CRP levels are elevated in pneumonias caused by Streptococcus pneumoniae, especially those associated with bacteremia, due to generalized inflammation elicited by the presence of bacteria. This was evidenced by higher levels of CRP in the setting of S. pneumoniae infection as compared to CAP due to Mycoplasma pneumoniae or viral etiologies.[77,79]
Procalcitonin has been extensively studied for its role in the inflammatory response. This peptide often assists in distinguishing between bacterial and viral infections, although its utility in differentiating causative etiologies in severely ill patients remains limited. However, existing data support the utility of procalcitonin in guiding the duration of antibiotic therapy in patients treated for pneumonia.[80,81]
Clinical guidelines recommend testing for common pathogens that cause severe CAP such as testing for S. pneumoniae and Legionella urinary antigens.[10,82] Antigen testing for these pathogens from sputum and blood samples is less frequently performed in clinical practice. Comprehensive respiratory polymerase chain reaction (PCR) panel that tests for both pathogens is likely to eliminate the need for single pathogen antigen detection. Sputum samples properly collected and observed under microscopy generally assist the clinician in selecting initial antibiotic management. Laboratory testing of sputum samples, however, is not without limitations, especially when samples are collected from elderly patients. Despite the overall low yield of sputum samples, such cultures have a significant impact on patient management and accurately reflect regional susceptibility patterns.[10] One study that evaluated sputum sample microscopic quality revealed that higher-quality samples exhibited an increased yield in detecting causative pathogens. Both high- and low-quality samples exhibited similar specificity, however, highlighting that sputum sample culture, regardless of quality, aids in diagnosis.[83] Furthermore, elderly individuals typically have greater oropharyngeal colonization with aerobic Gram-negative bacilli; this complicates accurate pathogen identification.[84] In some cases, patients with pneumonia who are admitted to the ICU may benefit from early bronchoscopy and lower respiratory tract secretion collection using a bronchial brush or bronchoalveolar lavage.[85] Bronchoalveolar lavage amylase and pepsin have been suggested for use in the diagnosis of gastric content aspiration. Bronchoalveolar lavage amylase levels of ≥204 U/L were reported to have a 77.1% sensitivity and an 84.2% specificity as a diagnostic index; pepsin levels of ≥7.45 ng/mL were reported to have an 87.2% sensitivity and a 59.9% specificity. Multivariate analysis revealed that such levels were associated with higher odds of ongoing aspiration pneumonia.[86]
Swallowing assessments are also important in the diagnostic process. One non-invasive and cost-effective assessment of dysphagia is a bedside swallowing evaluation.[87] In cases where the diagnosis of aspiration remains uncertain, the utilization of fiberoptic endoscopic evaluation of swallowing can aid in diagnosis. This technique evaluates oropharyngeal and esophageal functions, thus playing a crucial role in assessing the risk of dysphagia and identifying instances of aspiration. This test details swallowing mechanics and is even capable of detecting silent aspiration events that lack obvious clinical indicators.[88] An overview of physical, imaging, and laboratory findings is shown in Table 1.
Microbiology
Over recent decades, the adoption of culture-free techniques (e.g., multiplex PCR and high-throughput 16S rRNA sequencing) has revolutionized the pathophysiological understanding of aspiration pneumonia.[89,90] The greatest potential benefit of multiplex PCR testing is a rapid (1–24 h) availability of results for a large number of potential viral and bacterial pathogens.[8] In the setting of severe aspiration CAP, such tests remain underutilized, however. Importantly, anaerobic bacteria may no longer be the predominant causative agents of aspiration pneumonia.[91] Such findings have generally shifted management guidelines away from empiric antibiotic administration targeting anaerobes and limited anti-anaerobic therapy for patients suffering pleuro-pulmonary pathologies, in general. Importantly, Gram-positive cocci were reported to represent the commonest pathogens isolated from patients diagnosed with aspiration pneumonia.[91] Improved identification of anaerobic bacteria over recent decades has clarified subtle differences in diagnostic methods, antimicrobial therapies, and host–pathogen interactions. Interestingly, patients with severe aspiration CAP tend to present with lower rates of S. pneumoniae infection.[91]
Microorganisms generally considered pathogenic include Pseudomonas aeruginosa, Haemophilus influenzae, Acinetobacter baumannii, and other Gram-negative bacteria (e.g., Coxiella spp., Proteus spp., Serratia spp., Klebsiella pneumoniae, Escherichia coli, Moraxella catarrhalis, Enterobacter spp.), S. pneumoniae, Staphylococcus aureus, other Gram-positive cocci (e.g., S. pyogenes, Streptococcus spp.), and mixed oral flora (i.e., anaerobic and polymicrobial colonization).[91] Similar to what was observed among HAP/VAP patients, those suffering aspiration pneumonia, and in particular severe aspiration CAP, tend to have higher rates (prevalence ranging 5%–55%) of colonization by Enterobacteriaceae (with smaller proportions of E. coli and Klebsiella spp.) and Pseudomonas spp.[15,92,93] Despite the lack of information regarding multidrug regimens or extended spectrum beta-lactamase resistance, Enterobacteriaceae likely represents a significant cause of severe aspiration CAP that warrants further study.[15] One multicenter point-prevalence study reported that patients with severe aspiration CAP had higher rates of P. aeruginosa and other Gram-negative bacterial infections compared to patients suffering non-severe aspiration CAP.[15]
Treatment
Non-pharmacological measures
Non-invasive mechanical ventilation including use of a high-flow nasal cannula (HFNC) is widely used in the acute care setting for patients suffering acute hypoxemic or hypercapnic respiratory failure. However, there are important concerns regarding the use of such non-invasive treatments in the context of severe aspiration CAP. It has been reported that HFNC with rates above 40 L/min increased the aspiration risk, similar to data reported concerning non-invasive mechanical ventilation. Thus, such techniques should be used with caution and avoided in settings of altered mental status.[[94], [95], [96]] Patients treated with HFNC at flow rates above 20 L/min were reported to exert increased swallowing effort and experience decreased swallowing frequency.[96] Controversially, HFNC was reported to improve the oxygenation status, alleviate respiratory failure, and reduce the necessity of using invasive mechanical ventilation among post-stroke patients who suffered aspiration.[97] Further studies assessing the impact of HFNC on the management of patients with severe aspiration CAP is thus warranted.
In intubated patients who suffered large-volume aspiration, flexible bronchoscopy may be recommended to clear secretions and obtain bronchoalveolar lavage samples for further microbiological studies. One study reported that early bronchoscopy (within 24 h of aspiration) in mechanically ventilated patients with aspiration pneumonitis (either CAP or hospital-acquired) resulted in an overall improvement in respiratory function and a reduced incidence of aspiration pneumonia.[85] However, bronchoscopy use in the setting of severe aspiration CAP has been poorly studied to date and was performed in studies evaluating small sample sizes that excluded non-intubated patients.
Antibiotic therapy
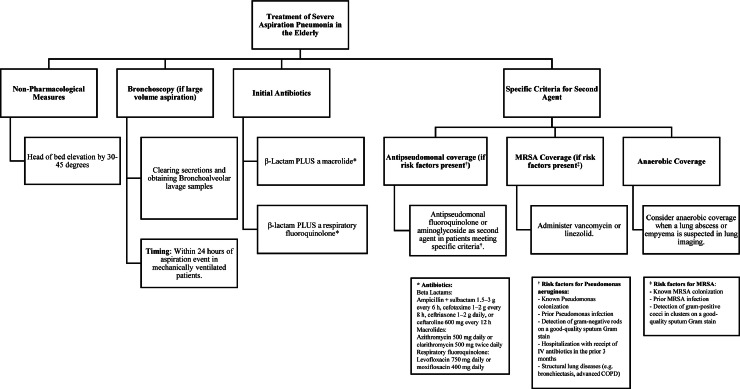
In patients with severe aspiration pneumonia (as defined by the ATS/IDSA CAP severity criteria guidelines), we recommend the initiation of a β-Lactam in addition to a macrolide (e.g., ampicillin-sulbactam 1.5–3 g every 6 h; cefotaxime 1–2 g every 8 h, ceftriaxone 1–2 g daily, or ceftaroline 600 mg every 12 h AND azithromycin 500 mg daily or clarithromycin 500 mg twice daily) OR a β-lactam PLUS a fluroquinolone (levofloxacin 750 mg daily or moxifloxacin 400 mg daily).[10] Although antibiotics should be started as soon as possible, a delay in administering antibiotics to patients with CAP is more common when patients present with an altered mental status or any signs of sepsis.[98] Coverage for resistant pseudomonas and methicillin-resistant Staphylococcus aureus (MRSA) should be considered for patients with relevant risk factors for these infections. The strongest risk factor for P. pneumonia is a prior history of pseudomonas colonization or infection. Additionally, patients found to have evidence of Gram-negative rods on high-quality sputum Gram stains and who were recently hospitalized and treated with intravenous antibiotics within the last 3 months should be considered at high risk for P. pneumonia and managed accordingly, especially in the setting of structural lung pathology (Figure 2).[10,99,100]
Figure 2.
Treatment approach to severe community-acquired aspiration pneumonia.
COPD: Chronic obstructive pulmonary disease; MRSA: Methicillin-resistant Staphylococcus aureus.
According to the ATS/IDSA guidelines, patients meeting specific criteria (Figure 2) should be treated with either an antipseudomonal fluoroquinolone or an aminoglycoside as a second agent for coverage of Gram-negative bacilli. The use of aminoglycosides should be avoided if alternative agents with sufficient Gram-negative activity are available due to possible toxicity and side effects.[10] Risk factors for MRSA pneumonia include a prior history of MRSA colonization or MRSA infections as well as identification of Gram-positive cocci in clusters on high-quality sputum Gram stain.[10] Such patients should additionally receive treatment against MRSA with vancomycin or linezolid. However, if MRSA is not detected, these agents should be discontinued as early as possible; the negative predictive value of nasal MRSA colonization is above 95%.[10]
Empiric anaerobic coverage in aspiration pneumonia patients remains controversial and has changed over the years. A recently published meta-analysis found no benefit in mortality when patients suffering aspiration pneumonia were treated with anti-anaerobic agents.[101] A recent study that evaluated anaerobic coverage in critically ill patients reported an increased risk of adverse clinical outcomes. Patients who received antibiotics with anaerobic coverage had lower VAP-free, infection-free, and overall survival rates compared to patients treated with antibiotics without anti-anaerobic coverage.[102] In a study involving 2606 patients with CAP, 7.4% were found to have aspiration CAP. Another global study reported that anaerobes were present in a very small proportion of severe aspiration CAP patients, with the predominant group of pathogens having been Gram-negative bacteria; Gram-positive bacteria were less common when compared to patients with severe CAP but with risk factors for aspiration.[15] As such, anaerobic bacteria are much less commonly identified in patients with aspiration, even in those presenting with severe disease. Thus, ATS/IDSA guidelines recommend anaerobic coverage only in instances when a lung abscess or empyema are suggested on pulmonary imaging.[10]
The use of systemic corticosteroids remains controversial in the management of patients with severe CAP. Recent studies excluded patients with aspiration pneumonia from analyses due to the limited availability of data concerning potential benefits.[103,104] Sukumaran et al.[105] reported that patients who aspirated gastric contents and were given corticosteroids had prolonged ICU stays. Additionally, patients with aspiration pneumonia treated with corticosteroids were reported to have had higher rates of Gram-negative bacteria infection and no survival benefits.[106] Therefore, corticosteroid use in the setting of severe aspiration CAP is not routinely recommended. Recognition of acute conditions such as COPD or asthma exacerbation may require the administration of corticosteroids to manage bronchospasms, but not to treat inflammation in the setting of aspiration pneumonia.
End-of-life Management
Management of elderly patients with severe aspiration CAP poses clinical challenges, considering that multiple comorbidities significantly worsen prognosis in this patient population. Recognizing whether a patient who presents with severe aspiration CAP is at the end of life is important, as is acknowledging the limitations of pursuing aggressive treatment strategies in the context of providing compassionate care. Early goals of care should be established for patients with severe CAP not suitable for ICU admission, whether due to a deemed futility of such medical care or do-not-resuscitate orders. Several communication tools are available to better determine if a patient is at the end of life or likely to deteriorate due to illness severity. One systematic review and meta-analysis concerning communication tools for end-of-life decision-making revealed that communication tools decreased health care utilization (e.g., use of invasive mechanical ventilation), reduced ICU stay, and decreased health care costs.[107,108] Shared decision-making is a key step in management at the end of life as it involves open communication with patients and their family members. In situations where aggressive treatment does not align with the patient's goals or quality of life, a shift toward palliative and supportive care becomes the focus.[108] The implementation of palliative care in symptom management and comfort greatly enhances the quality of life for patients facing end-of-life decisions. In instances where aggressive treatment yields limited benefits and may cause undue suffering, there are ethical considerations regarding the cessation of life-sustaining treatments. Any decision requires thoughtful and compassionate discussion that aims to align with either the patient's expressed desires or the best interests.[108] In patients with a poor prognosis, transition to hospice care can be an appropriate step. Hospice focuses on enhancing the quality of life by managing pain, providing emotional support, and maintaining dignity in the final stages of life.[108]
Prevention
Various interventions have been reported to be prophylactic against the development of aspiration CAP (Table 2).
Table 2.
Aspiration pneumonia-prevention strategies.
| Prevention strategies |
|---|
| Elevation of head of bed to 30°–45° |
| Vaccinations (e.g., pneumococcal, influenza, SARS-CoV2) |
| Proper oral hygiene (e.g., tooth brushing, regular dental visits) |
| Post-pyloric feeding |
| Early physiotherapy |
| Avoidance of polypharmacy |
| Adequate nutrition |
Prevention of aspiration includes positioning patients in bed with the head elevated at a 30°–45° angle to decrease the risk of recurrent gastric content aspiration.[109] Vaccination is recommended to protect against pneumonia in older adults (e.g., pneumococcal, influenza, severe acute respiratory syndrome Coronavirus 2 vaccines). One study reported that pneumococcal vaccination exhibited a 40% effectiveness rate in preventing pneumococcal CAP.[109] Numerous observational studies have assessed risk of hospitalization due to pneumonia and overall mortality in elderly individuals who received the influenza vaccine when compared to non-vaccinated patients during the influenza season. These studies consistently demonstrated the substantial risk reduction among vaccinated groups, with a remarkable 50% decrease in all-cause mortality and 27%–33% reduction in rates of hospitalization.[110]
Ensuring adequate oral hygiene is important in reducing the incidence of aspiration pneumonia among the more vulnerable elderly individuals. One study revealed that employing mechanical methods for oral hygiene significantly reduces the occurrence of pneumonia in frail older adults.[54] Son et al.[53] found a lower incidence of pneumonia in Korean groups that engaged in frequent tooth-brushing and regular professional dental care. Factors such as dental caries, missing teeth, and the frequency of both tooth brushing and professional dental cleaning were associated with a lower incidence of pneumonia. The cohort with more frequent tooth brushing, specifically two or more times per day, exhibited a significantly lower incidence of pneumonia, while the group brushing three or more times per day exhibited the lowest incidence over a 10.6-year follow-up period (±1.1 years).
Another clinical trial that explored the effectiveness of chlorhexidine 0.12% oral rinse twice daily in conjunction with upright positioning for prevention of pneumonia in nursing home residents was terminated prematurely due to a lack of evidence supporting a beneficial association between interventions and pneumonia prevention.[111] Additionally, evidence from different settings (e.g., HAP/VAP) suggest that oral care with chlorhexidine is associated with higher mortality rates and exerts no benefits on duration of mechanical ventilation and ICU stay; thus, it is not recommended to prevent aspiration pneumonia.[112]
Post-pyloric feeding correlated with a reduced risk of pneumonia in a moderate-quality meta-analysis of 14 trials involving 1109 participants (risk ratio=0.65, 95% confidence interval [CI]: 0.51 to 0.84) when compared to gastric tube feeding. Lower-quality evidence indicated increased nutrient delivery efficiency with post-pyloric feeding (mean difference=7.8%, 95% CI: 1.43 to 14.18).[113] Thus, post-pyloric tubes may be preferable when prevention of aspiration pneumonia and enhanced nutrient delivery are most important. Although enteral feeding is initiated when there is a high risk for aspiration, use of enteral nutrition has been associated with aspiration events. Therefore, modes of providing nutrition and aspiration risk should be carefully assessed concerning the risks and benefits so as to optimize clinical outcomes.[62] The use of early enteral nutrition may be indicated in cases of severe dysphagia and evidence of persistent aspiration.
The use of ACEI was reported to exert a preventive effect on aspiration pneumonia but only in Asian populations. However, a recent systematic review by Tsunoda et al.[114] suggested that ACEI treatment does not significantly prevent aspiration pneumonia and should not be routinely used for this purpose.
Physiotherapy including early mobilization as well as effects of altered mental status are important in patients with severe aspiration CAP. Larsen et al.[115] found that early mobility reduced duration of hospitalization by 1 day, but had no effect on mortality or rates of hospital readmissions.
Delirium in the ICU was found to associate with higher mortality and increased lengths of hospital and ICU stays.[116,117] Diverse approaches including pharmacological and non-pharmacological interventions have been attempted, with the latter being the cornerstone for delirium management in the ICU. A Cochrane analysis performed by Burry et al.[118] found an association between dexmedetomidine and reduced duration of mechanical ventilation and ICU stay. Other pharmacological interventions including statins, ketamine, and antipsychotics had no effect on treating or preventing delirium.[[119], [120], [121]] The Society of Critical Care Medicine recommends against routine use of such medications for the prevention of delirium.[122]
Finally, interventions focused on treatment and prevention of exacerbation of chronic comorbid conditions, minimization of exposure to toxic agents (e.g., smoking, illicit drugs, and alcohol), prevention of polypharmacy, and early nutritional management are critical in optimizing aspiration CAP patient outcomes.
Conclusions
The elderly population is significantly more vulnerable to severe aspiration pneumonia. This life-threatening condition often necessitates ICU admission. Here, aspiration pneumonia is discussed in the context of various age-related vulnerabilities. Severe aspiration pneumonia predominantly affects older adults, accounting for nearly 80% of all the cases. As clinical presentation frequently differs from that of classical pneumonia, the diagnosis of severe aspiration pneumonia in elderly patients requires a detail-oriented approach and the use of multiple modalities to aid in accurate diagnosis and tailor-specific management strategies.
Treatment includes non-pharmacological measures such as early bronchoscopy for large-volume aspiration and antibiotic therapy for broader coverage. Recent studies have shown that anaerobic coverage is not clinically beneficial and may be linked to an increased risk of adverse events and higher mortality rates in this population. These findings highlight the importance of reconsidering traditional antibiotic approaches and utilizing more evidence-based strategies for managing aspiration pneumonia that ultimately focus on improving overall patient outcomes.
Author Contributions
Sebastian Ocrospoma: Writing – review & editing, Writing – original draft. Marcos I. Restrepo: Writing – review & editing, Writing – original draft.
Acknowledgments
Acknowledgments
None.
Funding
This material is the result of work supported by the resources and the use of facilities at the South Texas Veterans Health Care System in San Antonio, TX, USA. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Ethics Statement
Not applicable.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
Not applicable.
Managing Editor: Jingling Bao/Zhiyu Wang
References
- 1.Bureau UC. U.S. older population grew from 2010 to 2020 at fastest rate since 1880 to 1890. CensusGov, n.d. Available from: https://www.census.gov/library/stories/2023/05/2020-census-united-states-older-population-grew.html [Last accessed on 2023 November 7].
- 2.Cichero J.A.Y. Age-related changes to eating and swallowing impact frailty: aspiration, choking risk, modified food texture and autonomy of choice. Geriatrics. 2018;3:69. doi: 10.3390/geriatrics3040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Güngen A.C., Aydemir Y., Güngen B.D., Yazar E.E., Yağız O., Aras Y.G., et al. Effects of aspiration pneumonia on the intensive care requirements and in-hospital mortality of hospitalised patients with acute cerebrovascular disease. Arch Med Sci. 2017;13:1062–1068. doi: 10.5114/aoms.2016.61011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Solh A.A., Niederman M.S., Drinka P. Nursing home-acquired pneumonia: a review of risk factors and therapeutic approaches. Curr Med Res Opin. 2010;26:2707–2714. doi: 10.1185/03007995.2010.530154. [DOI] [PubMed] [Google Scholar]
- 5.Stamm D.R., Katta S., Stankewicz H.A. StatPearls Publishing; Treasure Island, FL: 2023. Nursing home–acquired pneumonia. [PubMed] [Google Scholar]
- 6.Wind J., Versteegt J., Twisk J., van der Werf T.S., Bindels A.J., Spijkstra J.J., et al. Epidemiology of acute lung injury and acute respiratory distress syndrome in The Netherlands: a survey. Respir Med. 2007;101:2091–2098. doi: 10.1016/j.rmed.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Weyand C.M., Goronzy J.J. Aging of the immune system. mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13:S422–S428. doi: 10.1513/AnnalsATS.201602-095AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Loeches I., Torres A., Nagavci B., Aliberti S., Antonelli M., Bassetti M., et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 2023;49:615–632. doi: 10.1007/s00134-023-07033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G., Calfee C.S., Camporota L., Poole D., Amato M.B.P., Antonelli M., et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49:727–759. doi: 10.1007/s00134-023-07050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond M., Peniston H.L., Sanghavi D.K., Mahapatra S. StatPearls Publishing; Treasure Island, FL: 2023. Acute respiratory distress syndrome. [PubMed] [Google Scholar]
- 12.Teramoto S., Fukuchi Y., Sasaki H., Sato K., Sekizawa K., Matsuse T., et al. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc. 2008;56:577–579. doi: 10.1111/j.1532-5415.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson K., Eggli D.F., Maxwell S.L. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266–1272. doi: 10.1378/chest.111.5.1266. [DOI] [PubMed] [Google Scholar]
- 14.Reza Shariatzadeh M., Huang J.Q., Marrie T.J. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54:296–302. doi: 10.1111/j.1532-5415.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- 15.Marin-Corral J., Pascual-Guardia S., Amati F., Aliberti S., Masclans J.R., Soni N., et al. Aspiration risk factors, microbiology, and empiric antibiotics for patients hospitalized with community-acquired pneumonia. Chest. 2021;159:58–72. doi: 10.1016/j.chest.2020.06.079. [DOI] [PubMed] [Google Scholar]
- 16.Taylor J.K., Fleming G.B., Singanayagam A., Hill A.T., Chalmers J.D. Risk factors for aspiration in community-acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126:995–1001. doi: 10.1016/j.amjmed.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Lanspa M.J., Jones B.E., Brown S.M., Dean N.C. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8:83–90. doi: 10.1002/jhm.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tortuyaux R., Wallet F., Derambure P., Nseir S. Bacterial aspiration pneumonia in generalized convulsive status epilepticus: incidence, associated factors and outcome. J Clin Med. 2022;11:6673. doi: 10.3390/jcm11226673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leroy O., Vandenbussche C., Coffinier C., Bosquet C., Georges H., Guery B., et al. Community-acquired aspiration pneumonia in intensive care units. Am J Respir Crit Care Med. 1997;156:1922–1929. doi: 10.1164/ajrccm.156.6.9702069. [DOI] [PubMed] [Google Scholar]
- 20.Shin D., Lebovic G., Lin R.J. In-hospital mortality for aspiration pneumonia in a tertiary teaching hospital: a retrospective cohort review from 2008 to 2018. J Otolaryngol Head Neck Surg. 2023;52:23. doi: 10.1186/s40463-022-00617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putot A., Putot S., Manckoundia P. Long-term survival after aspiration pneumonia in older inpatients: a comparative study. J Am Med Dir Assoc. 2023;24:1088–1091. doi: 10.1016/j.jamda.2023.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Mandell L.A., Niederman M.S. Aspiration pneumonia. N Engl J Med. 2019;380:651–663. doi: 10.1056/NEJMra1714562. [DOI] [PubMed] [Google Scholar]
- 23.Neill S., Dean N. Aspiration pneumonia and pneumonitis: a spectrum of infectious/noninfectious diseases affecting the lung. Curr Opin Infect Dis. 2019;32:152–157. doi: 10.1097/QCO.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi S., Yatera K., Kato T., Chojin Y., Fujino Y., Akata K., et al. Impact of the number of aspiration risk factors on mortality and recurrence in community-onset pneumonia. Clin Interv Aging. 2017;12:2087–2094. doi: 10.2147/CIA.S150499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Çiyiltepe F., Özgültekin A. The predictors of early mortality in geriatric patients who hospitalized to the intensive care unit with aspiration pneumonia. J Contemp Med. 2022;12:27–32. doi: 10.16899/jcm.985283. [DOI] [Google Scholar]
- 26.Nascè A., Malézieux-Picard A., Hakiza L., Fassier T., Zekry D., Stirnemann J., et al. How do geriatric scores predict 1-year mortality in elderly patients with suspected pneumonia? Geriatrics. 2021;6:112. doi: 10.3390/geriatrics6040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hickling K.G., Howard R. A retrospective survey of treatment and mortality in aspiration pneumonia. Intensive Care Med. 1988;14:617–622. doi: 10.1007/BF00256765. [DOI] [PubMed] [Google Scholar]
- 28.Ma H.M., Tang W.H., Woo J. Predictors of in-hospital mortality of older patients admitted for community-acquired pneumonia. Age Ageing. 2011;40:736–741. doi: 10.1093/ageing/afr087. [DOI] [PubMed] [Google Scholar]
- 29.Wu C.-P., Chen Y.-W., Wang M.-J., Pinelis E. National trends in admission for aspiration pneumonia in the United States, 2002-2012. Ann Am Thorac Soc. 2017;14:874–879. doi: 10.1513/AnnalsATS.201611-867OC. [DOI] [PubMed] [Google Scholar]
- 30.Poorjavad M., Derakhshandeh F., Etemadifar M., Soleymani B., Minagar A., Maghzi A.-H. Oropharyngeal dysphagia in multiple sclerosis. Mult Scler. 2010;16:362–365. doi: 10.1177/1352458509358089. [DOI] [PubMed] [Google Scholar]
- 31.Crooke S.N., Ovsyannikova I.G., Poland G.A., Kennedy R.B. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goplen N.P., Wu Y., Son Y.M., Li C., Wang Z., Cheon I.S., et al. Tissue-resident CD8+ T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci Immunol. 2020;5:eabc4557. doi: 10.1126/sciimmunol.abc4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan Y., Yao Q., Liu Y., Jia T., Zhang J., Jiang E. Underlying causes and co-existence of malnutrition and infections: an exceedingly common death risk in cancer. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.814095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chebib N., Cuvelier C., Malézieux-Picard A., Parent T., Roux X., Fassier T., et al. Pneumonia prevention in the elderly patients: the other sides. Aging Clin Exp Res. 2021;33:1091–1100. doi: 10.1007/s40520-020-01490-7. [DOI] [PubMed] [Google Scholar]
- 35.Cañas-González B., Fernández-Nistal A., Ramírez J.M., Martínez-Fernández V. Influence of stress and depression on the immune system in patients evaluated in an anti-aging unit. Front Psychol. 2020;11:1844. doi: 10.3389/fpsyg.2020.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchida M., Pogorzelska-Maziarz M., Smith P.W., Larson E. Infection prevention in long-term care: a systematic review of randomized and non-randomized trials. J Am Geriatr Soc. 2013;61:602–614. doi: 10.1111/jgs.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strausbaugh L.J., Joseph C.L. The burden of infection in long-term care. Infect Control Hosp Epidemiol. 2000;21:674–679. doi: 10.1086/501712. [DOI] [PubMed] [Google Scholar]
- 38.Sue Eisenstadt E. Dysphagia and aspiration pneumonia in older adults. J Am Acad Nurse Pract. 2010;22:17–22. doi: 10.1111/j.1745-7599.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- 39.Pikus L., Levine M.S., Yang Y.X., Rubesin S.E., Katzka D.A., Laufer I., et al. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol. 2003;180:1613–1616. doi: 10.2214/ajr.180.6.1801613. [DOI] [PubMed] [Google Scholar]
- 40.Almirall J., Rofes L., Serra-Prat M., Icart R., Palomera E., Arreola V., et al. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J. 2013;41:923–928. doi: 10.1183/09031936.00019012. [DOI] [PubMed] [Google Scholar]
- 41.Wirth R., Dziewas R., Beck A.M., Clavé P., Hamdy S., Heppner H.J., et al. Oropharyngeal dysphagia in older persons – from pathophysiology to adequate intervention: a review and summary of an international expert meeting. Clin Interv Aging. 2016;11:189–208. doi: 10.2147/CIA.S97481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Solh A.A., Pietrantoni C., Bhat A., Aquilina A.T., Okada M., Grover V., et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650–1654. doi: 10.1164/rccm.200212-1543OC. [DOI] [PubMed] [Google Scholar]
- 43.Klapdor B., Ewig S., Schaberg T., Rohde G., Pletz M.W., Schütte H., et al. Presentation, etiology and outcome of pneumonia in younger nursing home residents. J Infect. 2012;65:32–38. doi: 10.1016/j.jinf.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Sternberg S.A., Wershof Schwartz A., Karunananthan S., Bergman H., Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 45.Rofes L., Arreola V., Romea M., Palomera E., Almirall J., Cabré M., et al. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil. 2010;22:851–858. doi: 10.1111/j.1365-2982.2010.01521.x. e230. [DOI] [PubMed] [Google Scholar]
- 46.van der Maarel-Wierink C.D., Vanobbergen J.N.O., Bronkhorst E.M., Schols J.M.G.A., de Baat C. Meta-analysis of dysphagia and aspiration pneumonia in frail elders. J Dent Res. 2011;90:1398–1404. doi: 10.1177/0022034511422909. [DOI] [PubMed] [Google Scholar]
- 47.Stoschus B., Allescher H.D. Drug-induced dysphagia. Dysphagia. 1993;8:154–159. doi: 10.1007/BF02266997. [DOI] [PubMed] [Google Scholar]
- 48.Saleem F., Sharma A. StatPearls Publishing; Treasure Island, FL: 2023. Drug-induced esophagitis. [PubMed] [Google Scholar]
- 49.Balzer K. Drug-induced dysphagia. Int J MS Care. 2000;2:40–50. doi: 10.7224/1537-2073-2.1.40. [DOI] [Google Scholar]
- 50.Hårdemark Cedborg A.I., Sundman E., Bodén K., Hedström H.W., Kuylenstierna R., Ekberg O., et al. Effects of morphine and midazolam on pharyngeal function, airway protection, and coordination of breathing and swallowing in healthy adults. Anesthesiology. 2015;122:1253–1267. doi: 10.1097/ALN.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 51.Shetty S.R., Bhowmick S., Castelino R., Babu S. Drug induced xerostomia in elderly individuals: an institutional study. Contemp Clin Dent. 2012;3:173–175. doi: 10.4103/0976-237X.96821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson C.M., Morrison R.L., D'Souza A., Teng X.S., Happel K.I. Inhaled fluticasone propionate impairs pulmonary clearance of Klebsiella pneumoniae in mice. Respir Res. 2012;13:40. doi: 10.1186/1465-9921-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Son M., Jo S., Lee J.S., Lee D.H. Association between oral health and incidence of pneumonia: a population-based cohort study from Korea. Sci Rep. 2020;10:9576. doi: 10.1038/s41598-020-66312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Müller F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J Dent Res. 2015;94:14S–16S. doi: 10.1177/0022034514552494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marik P.E. Pulmonary aspiration syndromes. Curr Opin Pulm Med. 2011;17:148–154. doi: 10.1097/MCP.0b013e32834397d6. [DOI] [PubMed] [Google Scholar]
- 56.Dragan V., Wei Y., Elligsen M., Kiss A., Walker S.A.N., Leis J.A. Prophylactic antimicrobial therapy for acute aspiration pneumonitis. Clin Infect Dis. 2018;67:513–518. doi: 10.1093/cid/ciy120. [DOI] [PubMed] [Google Scholar]
- 57.Son Y.G., Shin J., Ryu H.G. Pneumonitis and pneumonia after aspiration. J Dent Anesth Pain Med. 2017;17:1–12. doi: 10.17245/jdapm.2017.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Košutova P., Mikolka P. Aspiration syndromes and associated lung injury: incidence, pathophysiology and management. Physiol Res. 2021;70:S567–S583. doi: 10.33549//physiolres.934767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeLegge M.H. Aspiration pneumonia: incidence, mortality, and at-risk populations. JPEN J Parenter Enteral Nutr. 2002;26:S19–S24. doi: 10.1177/014860710202600604. discussion S24–25. [DOI] [PubMed] [Google Scholar]
- 60.Sanivarapu R.R., Gibson J. StatPearls Publishing; Treasure Island, FL: 2023. Aspiration pneumonia. [PubMed] [Google Scholar]
- 61.Metlay J.P., Schulz R., Li Y.H., Singer D.E., Marrie T.J., Coley C.M., et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med. 1997;157:1453–1459. doi: 10.1001/archinte.157.13.1453. [DOI] [PubMed] [Google Scholar]
- 62.Putot A., Prendki V. New horizons in sepsis management in older patients. Age Ageing. 2023;52:afad016. doi: 10.1093/ageing/afad016. [DOI] [PubMed] [Google Scholar]
- 63.Bellelli G., Guerini F., Cerri A.P., Trabucchi M. A sudden decline in mobility status as an early sign of acute infection in elderly patients: evidence from three case reports. Aging Clin Exp Res. 2012;24:281–284. doi: 10.1007/BF03325259. [DOI] [PubMed] [Google Scholar]
- 64.Chong C.P., Street P.R. Pneumonia in the elderly: a review of the epidemiology, pathogenesis, microbiology, and clinical features. South Med J. 2008;101:1141–1145. doi: 10.1097/SMJ.0b013e318181d5b5. quiz 1132, 1179. [DOI] [PubMed] [Google Scholar]
- 65.Fernández-Sabé N., Carratalà J., Rosón B., Dorca J., Verdaguer R., Manresa F., et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine. 2003;82:159–169. doi: 10.1097/00005792-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Frese T., Sobeck C., Herrmann K., Sandholzer H. Dyspnea as the reason for encounter in general practice. J Clin Med Res. 2011;3:239–246. doi: 10.4021/jocmr642w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin-Harris B., Jones B. The videofluorographic swallowing study. Phys Med Rehabil Clin N Am. 2008;19:769–785. doi: 10.1016/j.pmr.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin-Harris B., Canon C.L., Bonilha H.S., Murray J., Davidson K., Lefton-Greif M.A. Best practices in modified barium swallow studies. Am J Speech Lang Pathol. 2020;29:1078–1093. doi: 10.1044/2020_AJSLP-19-00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langmore S.E., Schatz K., Olson N. Endoscopic and videofluoroscopic evaluations of swallowing and aspiration. Ann Otol Rhinol Laryngol. 1991;100:678–681. doi: 10.1177/000348949110000815. [DOI] [PubMed] [Google Scholar]
- 70.Komiya K., Ishii H., Kadota J. Healthcare-associated pneumonia and aspiration pneumonia. Aging Dis. 2014;6:27–37. doi: 10.14336/AD.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibrahim D., Bizri A.R., El Amine M.A., Halabi Z. Chest computed tomography and chest X-ray in the diagnosis of community-acquired pneumonia: a retrospective observational study. J Int Med Res. 2021;49 doi: 10.1177/03000605211039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reissig A., Copetti R., Mathis G., Mempel C., Schuler A., Zechner P., et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: a prospective, multicenter, diagnostic accuracy study. Chest. 2012;142:965–972. doi: 10.1378/chest.12-0364. [DOI] [PubMed] [Google Scholar]
- 73.Lichtenstein D., Goldstein I., Mourgeon E., Cluzel P., Grenier P., Rouby J.J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Chavez M.A., Shams N., Ellington L.E., Naithani N., Gilman R.H., Steinhoff M.C., et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hacquin A., Putot S., Barben J., Chagué F., Zeller M., Cottin Y., et al. Bedside chest ultrasound to distinguish heart failure from pneumonia-related dyspnoea in older COVID-19 patients. ESC Heart Fail. 2020;7:4424–4428. doi: 10.1002/ehf2.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price S., Platz E., Cullen L., Tavazzi G., Christ M., Cowie M.R., et al. Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol. 2017;14:427–440. doi: 10.1038/nrcardio.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adnet F., Borron S.W., Vicaut E., Giraudeaux V., Lapostolle F., Bekka R., et al. Value of C-reactive protein in the detection of bacterial contamination at the time of presentation in drug-induced aspiration pneumonia. Chest. 1997;112:466–471. doi: 10.1378/chest.112.2.466. [DOI] [PubMed] [Google Scholar]
- 78.Hansson L.O., Hedlund J.U., Ortqvist A.B. Sequential changes of inflammatory and nutritional markers in patients with community-acquired pneumonia. Scand J Clin Lab Invest. 1997;57:111–118. doi: 10.1080/00365519709056378. [DOI] [PubMed] [Google Scholar]
- 79.Ortqvist A., Hedlund J., Wretlind B., Carlström A., Kalin M. Diagnostic and prognostic value of interleukin-6 and C-reactive protein in community-acquired pneumonia. Scand J Infect Dis. 1995;27:457–462. doi: 10.3109/00365549509047046. [DOI] [PubMed] [Google Scholar]
- 80.Khilnani G.C., Tiwari P., Zirpe K.G., Chaudhry D., Govil D., Dixit S., et al. Guidelines for the use of procalcitonin for rational use of antibiotics. Indian J Crit Care Med. 2022;26:S77–S94. doi: 10.5005/jp-journals-10071-24326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Samsudin I., Vasikaran S.D. Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 2017;38(2):59–68. [PMC free article] [PubMed] [Google Scholar]
- 82.Costantini E., Allara E., Patrucco F., Faggiano F., Hamid F., Balbo P.E. Adherence to guidelines for hospitalized community-acquired pneumonia over time and its impact on health outcomes and mortality. Intern Emerg Med. 2016;11:929–940. doi: 10.1007/s11739-016-1445-3. [DOI] [PubMed] [Google Scholar]
- 83.Saukkoriipi A., Palmu A.A., Jokinen J. Culture of all sputum samples irrespective of quality adds value to the diagnosis of pneumococcal community-acquired pneumonia in the elderly. Eur J Clin Microbiol Infect Dis. 2019;38:1249–1254. doi: 10.1007/s10096-019-03536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henig O., Kaye K.S. Bacterial pneumonia in older adults. Infect Dis Clin North Am. 2017;31:689–713. doi: 10.1016/j.idc.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Megahed M.M., El-Menshawy A.M., Ibrahim A.M. Use of early bronchoscopy in mechanically ventilated patients with aspiration pneumonitis. Indian J Crit Care Med. 2021;25:146–152. doi: 10.5005/jp-journals-10071-23718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suzuki T., Saitou M., Utano Y., Utano K., Niitsuma K. Bronchoalveolar lavage (BAL) amylase and pepsin levels as potential biomarkers of aspiration pneumonia. Pulmonology. 2023;29:392–398. doi: 10.1016/j.pulmoe.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Hassan H.E., Aboloyoun A.I. The value of bedside tests in dysphagia evaluation. Egypt J Ear Nose Throat Allied Sci. 2014;15:197–203. doi: 10.1016/j.ejenta.2014.07.007. [DOI] [Google Scholar]
- 88.Hafner G., Neuhuber A., Hirtenfelder S., Schmedler B., Eckel H.E. Fiberoptic endoscopic evaluation of swallowing in intensive care unit patients. Eur Arch Otorhinolaryngol. 2008;265:441–446. doi: 10.1007/s00405-007-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Falguera M., Ruiz-González A., Schoenenberger J.A., Touzón C., Gázquez I., Galindo C., et al. Prospective, randomised study to compare empirical treatment versus targeted treatment on the basis of the urine antigen results in hospitalised patients with community-acquired pneumonia. Thorax. 2010;65:101–106. doi: 10.1136/thx.2009.118588. [DOI] [PubMed] [Google Scholar]
- 90.Abu Sitta E., Hubbard N., Suleyman G. Comparison of multiplex polymerase chain reaction (PCR) and routine culture for the detection of respiratory pathogens in pneumonia patients. Open Forum Infect Dis. 2019;6:S297. doi: 10.1093/ofid/ofz360.711. [DOI] [Google Scholar]
- 91.Vallianou N.G., Skourtis A., Kounatidis D., Margellou E., Panagopoulos F., Geladari E., et al. The role of the respiratory microbiome in the pathogenesis of aspiration pneumonia: implications for diagnosis and potential therapeutic choices. Antibiotics. 2023;12:140. doi: 10.3390/antibiotics12010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suzuki J., Ikeda R., Kato K., Kakuta R., Kobayashi Y., Ohkoshi A., et al. Characteristics of aspiration pneumonia patients in acute care hospitals: a multicenter, retrospective survey in Northern Japan. PLoS One. 2021;16 doi: 10.1371/journal.pone.0254261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramatla T., Mafokwane T., Lekota K., Monyama M., Khasapane G., Serage N., et al. “One Health” perspective on prevalence of co-existing extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae: a comprehensive systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2023;22:88. doi: 10.1186/s12941-023-00638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oczkowski S., Ergan B., Bos L., Chatwin M., Ferrer M., Gregoretti C., et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Euro Respir J. 2022;59(4) doi: 10.1183/13993003.01574-2021. [DOI] [PubMed] [Google Scholar]
- 95.Jahagirdar D., Picheca L. Canadian Agency for Drugs and Technologies in Health; Ottawa, ON: 2019. Heated humidified high flow oxygen for respiratory support: a review of clinical effectiveness, cost-effectiveness, and guidelines. [PubMed] [Google Scholar]
- 96.Arizono S., Oomagari M., Tawara Y., Yanagita Y., Machiguchi H., Yokomura K., et al. Effects of different high-flow nasal cannula flow rates on swallowing function. Clin Biomech. 2021;89 doi: 10.1016/j.clinbiomech.2021.105477. [DOI] [PubMed] [Google Scholar]
- 97.Xing D., Chen Y.-H., Wang L-T, Yu B., Ran Z.-B., Chen L. Evaluation of the therapeutic effect of high-flow nasal cannula oxygen therapy on patients with aspiration pneumonia accompanied by respiratory failure in the post-stroke sequelae stage. BMC Pulm Med. 2021;21:17. doi: 10.1186/s12890-020-01359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waterer G.W., Kessler L.A., Wunderink R.G. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest. 2006;130:11–15. doi: 10.1378/chest.130.1.11. [DOI] [PubMed] [Google Scholar]
- 99.Restrepo M.I., Anzueto A. Guidelines for the diagnoses and treatment of adult lower respiratory tract infections: a true “European cooperative effort.”. Eur Respir J. 2005;26:979–981. doi: 10.1183/09031936.05.00102105. [DOI] [PubMed] [Google Scholar]
- 100.Restrepo M.I., Babu B.L., Reyes L.F., Chalmers J.D., Soni N.J., Sibila O., et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2) doi: 10.1183/13993003.01190-2017. [DOI] [PubMed] [Google Scholar]
- 101.Yoshimatsu Y., Aga M., Komiya K., Haranaga S., Numata Y., Miki M., et al. The clinical significance of anaerobic coverage in the antibiotic treatment of aspiration pneumonia: a systematic review and meta-analysis. J Clin Med. 2023;12:1992. doi: 10.3390/jcm12051992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chanderraj R., Baker J.M., Kay S.G., Brown C.A., Hinkle K.J., Fergle D.J., et al. In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes. Eur Respir J. 2023;61 doi: 10.1183/13993003.00910-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dequin P.F., Meziani F., Quenot J.P., Kamel T., Ricard J.D., Badie J., et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388:1931–1941. doi: 10.1056/NEJMoa2215145. [DOI] [PubMed] [Google Scholar]
- 104.Meduri G.U., Shih M.C., Bridges L., Martin T.J., El-Solh A., Seam N., et al. Low-dose methylprednisolone treatment in critically ill patients with severe community-acquired pneumonia. Intensive Care Med. 2022;48:1009–1023. doi: 10.1007/s00134-022-06684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sukumaran M., Granada M.J., Berger H.W., Lee M., Reilly T.A. Evaluation of corticosteroid treatment in aspiration of gastric contents: a controlled clinical trial. Mt Sinai J Med. 1980;47(4):335–340. [PubMed] [Google Scholar]
- 106.Wolfe J.E., Bone R.C., Ruth W.E. Effects of corticosteroids in the treatment of patients with gastric aspiration. Am J Med. 1977;63:719–722. doi: 10.1016/0002-9343(77)90157-7. [DOI] [PubMed] [Google Scholar]
- 107.Oczkowski S.J.W., Chung H.-O., Hanvey L., Mbuagbaw L., You J.J. Communication tools for end-of-life decision-making in the intensive care unit: a systematic review and meta-analysis. Crit Care. 2016;20:97. doi: 10.1186/s13054-016-1264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ito K., George N., Wilson J., Bowman J., Aaronson E., Ouchi K. Primary palliative care recommendations for critical care clinicians. J Intensive Care. 2022;10:20. doi: 10.1186/s40560-022-00612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orozco-Levi M., Torres A., Ferrer M., Piera C., el-Ebiary M., de la Bellacasa J.P., et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med. 1995;152:1387–1390. doi: 10.1164/ajrccm.152.4.7551400. [DOI] [PubMed] [Google Scholar]
- 110.Trucchi C., Paganino C., Orsi A., De Florentiis D., Ansaldi F. Influenza vaccination in the elderly: why are the overall benefits still hotly debated? J Prev Med Hyg. 2015;56(1):E37–E43. [PMC free article] [PubMed] [Google Scholar]
- 111.Juthani-Mehta M., Van Ness P.H., McGloin J., Argraves S., Chen S., Charpentier P., et al. A cluster-randomized controlled trial of a multicomponent intervention protocol for pneumonia prevention among nursing home elders. Clin Infect Dis. 2015;60:849–857. doi: 10.1093/cid/ciu935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klompas M., Branson R., Cawcutt K., Crist M., Eichenwald E.C., Greene L.R., et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2022;43:687–713. doi: 10.1017/ice.2022.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alkhawaja S., Martin C., Butler R.J., Gwadry-Sridhar F. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev. 2015;2015 doi: 10.1002/14651858.CD008875.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsunoda H., Okami Y., Honda Y., Shiroshita A., Kataoka Y., Tsujimoto Y., et al. Effectiveness of angiotensin converting enzyme inhibitors in preventing pneumonia: a systematic review and meta-analysis. J Gen Fam Med. 2022;23:217–227. doi: 10.1002/jgf2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larsen T., Lee A., Brooks D., Michieli S., Robson M., Veens J., et al. Effect of early mobility as a physiotherapy treatment for pneumonia: a systematic review and meta-analysis. Physiother Can. 2019;71:82–89. doi: 10.3138/ptc.2017-51.ep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ely E.W., Gautam S., Margolin R., Francis J., May L., Speroff T., et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ely E.W., Shintani A., Truman B., Speroff T., Gordon S.M., Harrell F.E., Jr., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 118.Burry L., Hutton B., Williamson D.R., Mehta S., Adhikari N.K., Cheng W., et al. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev. 2019;9 doi: 10.1002/14651858.CD011749.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Avidan M.S., Maybrier H.R., Abdallah A.B., Jacobsohn E., Vlisides P.E., Pryor K.O., et al. Intraoperative ketamine does not affect postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet. 2017;390:267–275. doi: 10.1016/S0140-6736(17)31467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Needham D.M., Colantuoni E., Dinglas V.D., Hough C.L., Wozniak A.W., Jackson J.C., et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4:203–212. doi: 10.1016/S2213-2600(16)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Girard T.D., Exline M.C., Carson S.S., Hough C.L., Rock P., Gong M.N., et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Devlin J.W., Skrobik Y., Gélinas C., Needham D.M., Slooter A.J.C., Pandharipande P.P., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.