Abstract
The endoplasmic reticulum acetylation machinery has emerged as a new branch of the larger endoplasmic reticulum quality control system. It regulates the selection of correctly folded polypeptides as well as reticulophagy-mediated removal of toxic protein aggregates with the former being a particularly important aspect of the proteostatic functions of endoplasmic reticulum acetylation. Essential to this function is the Nε-lysine acetyltransferase activity of acetyltransferase 1 and acetyltransferase 2, which regulates the induction of endoplasmic reticulum–specific autophagy through the acetylation of the autophagy-related protein 9A. Here, we used three mouse models of Charcot–Marie–Tooth disease, peripheral myelin protein 22/Tr-J, C3-peripheral myelin protein 22 and myelin protein zero/ttrr, to study spatial and translational selectivity of endoplasmic reticulum acetyltransferase inhibitors. The results show that inhibition of the endoplasmic reticulum acetyltransferases selectively targets misfolding/pro-aggregating events occurring in the lumen of the organelle. Therefore, they establish acetyltransferase 1 and acetyltransferase 2 as the first proven targets for disease-causing proteotoxic states that initiate within the lumen of the endoplasmic reticulum/secretory pathway.
Keywords: ATase, acetylation, proteostasis, endoplasmic reticulum, Charcot–Marie–Tooth disease
Here, Fernandez-Fuente et al. used three mouse models of Charcot–Marie–Tooth disease, to study spatial and translational selectivity of acetyltransferases inhibitors. The results establish the acetyltransferases as the first proven targets for disease-causing proteotoxic states that initiate within the lumen of the endoplasmic reticulum/secretory pathway.
Graphical Abstract
Graphical Abstract.
Introduction
A fundamental task of the endoplasmic reticulum (ER) is to make proteins that can then engage the secretory pathway to reach their final destination within the cell or be secreted to the extracellular milieu. Quality control mechanisms are in place to ensure that only correctly folded polypeptides can leave the ER. Quality control mechanisms are also in place to remove misfolded/unfolded polypeptides that would otherwise accumulate in the ER and cause proteotoxicity.1,2 By ensuring the continuous and efficient removal of toxic protein aggregates, the ER-specific autophagy (alternatively referred to as reticulophagy, ER-phagy or ER-associated degradation type II) represents a fundamental component of the ER quality control system. Defective removal of toxic protein aggregates through autophagy has been linked to different diseases across lifespan.3-6 As such, it is not surprising that improving normal proteostatic mechanisms represents an active target for biomedical research.
The ER acetylation machinery has emerged as a novel branch of the larger ER quality control system. It regulates the selection of correctly folded polypeptides, but also reticulophagy-mediated removal of toxic protein aggregates.7-16 ER acetylation is ensured by AT-1/SLC33A1, a membrane transporter that allows entry of cytosolic acetyl-Coenzyme A (acetyl-CoA) into the ER lumen, and ATase1/NAT8B and ATase2/NAT8, two ER-based acetyl-CoA:lysine acetyltransferases that use acetyl-CoA to acetylate ER cargo proteins within the ER lumen following their initial tertiary-state folding.7,16-18
The disposal of toxic protein aggregates through reticulophagy is an important aspect of the proteostatic functions of ER acetylation. This process requires ATase1- and ATase2-mediated acetylation of the ER-bound autophagy protein ATG9A, which in turn regulates the recruitment of the autophagy core machinery.13,19 In the mouse, reduced ER acetylation causes increased induction of reticulophagy, while increased ER acetylation has the opposite effect.9,11,12 Genetic disruption or biochemical inhibition of the ATases results in activation of reticulophagy, as well as rescue of disease-associated proteotoxicity.10,12,14,15,20 Disease-causing mutations in genes involved in the regulation of the proteostatic functions of the ER acetylation machinery (i.e. AT-1/SLC33A1, FAM134B and HSPB1) have been associated with different forms of hereditary sensory and autonomic neuropathy/hereditary motor neuropathy (HSAN/HMN) and spastic paraplegias (SPGs).21-26 Finally, HSAN/HMN-causing mutations have been associated with mislocalization of ATG9A and impaired autophagic degradation of pathogenic aggregates.27,28
In terms of translational output for autophagy-based strategies, a fundamental need is to selectively target autophagy to a specific cellular location. Studies conducted in different mouse models of proteotoxicity indicate that increasing reticulophagy through targeted inhibition of the ATases is a valid strategy to rescue disease-causing proteotoxic states of the ER and secretory pathway.10,12,14,15,20 However, these studies were not designed to discriminate between disease-causing events that force a target protein to misfold/aggregate (i.e. a mutation) and disease-causing events that do not (i.e. a gene duplication) or between misfolding events that affect the luminal versus the cytosolic portion of ER-bound membrane proteins. This is a particularly important aspect of ‘personalized medicine’ since different genetic events (i.e. mutation, duplication or deletion) can be the underlying pathogenic mechanism. Sometimes, these different genetic events are associated with the same group of diseases (i.e. HSAN/HMN/SPGs) or even target the same protein (i.e. peripheral myelin protein 22, PMP22).29,30
Here, we used three mouse models of Charcot–Marie–Tooth disease, a peripheral form of neuropathy, to target the above limitations. The effectiveness of targeting ATase activity was tested using neuropathy models that involve duplication or misfolding mutations of two abundant peripheral myelin proteins in Schwann cells of peripheral nerves. The results show that ATase inhibition selectively targets misfolding/pro-aggregating events occurring in the lumen of the ER, thus establishing the ATases as the first proven targets for proteotoxic states that initiate within the lumen of the ER.
Materials and methods
Animals
All the animals used in this study were obtained from the Jackson Laboratory. Strains B6.Cg-Tg(PMP22)C3Fbas/J (#JAX:030052), B6.D2-Pmp22Tr−J/J (#JAX:002504) and B6.Cg-Mpzttrr/GrsrJ (#JAX:010494) were maintained on the C57BL/6J (#JAX:000664) genetic background. B6.Cg-Tg(PMP22)C3Fbas/J and B6.D2-Pmp22Tr−J/J mice were bred as heterozygous while B6.Cg-Mpzttrr/GrsrJ were bred as homozygous.
Mice were housed and fed as described.31,32 The diet with Compound 9 (C9; 1 mg/g) was manufactured by Bio-Serv.12,15,20 Treatment with C9 began at weaning and continued throughout the entire life of the animals. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison (protocol #M005120) and performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Wild-type (WT) littermates were used as controls throughout the study. The age and sex of the animals at time of experimentation are specified in the figure and figure legends. Genotyping from tail DNA was performed at weaning by Transnetyx using real-time polymerase chain reaction.
Behaviour testing
All behavioural assays were conducted at the Waisman Center Behavioral Testing Service (Madison, WI, USA). All mice received a minimum of 30 min acclimation time to the testing room prior to each behaviour assay.
Phenotypic severity score
We used a simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia described previously.33 Blind analysis of the animals was performed for three different tests: ledge, hindlimb clasping and gait. Individual measures are scored on a scale of 0 to 3, with 0 representing an absence of the relevant phenotype and 3 representing the most severe manifestation (Supplementary Table 1).
Open field exploration
The specified protocol was performed according to Rigby et al.31,32 Data were recorded using the Omnitech Fusion system.
Hot plate
Mice were placed on a hot plate (Columbus Instruments, hot plate analgesia metre) to evaluate the reaction time. The reaction time was scored when the animal jumped or licked its paws. A cut-off of 40 s was used to avoid any paw damage. Five reaction times were determined for each mouse with a latency of at least 15 min apart between measurements. The results shown for each animal are the average of the middle three values.
Grip strength
Grip strength was measured using an Ametek Chatillon DFE II (Columbus Instruments, grip strength metre). The mouse was held by the base of the tail above a wire bar connected to the force gauge. The mouse was placed in a position that allowed it to grasp the wire with its forepaws and then pulled away from the bar at a constant speed. The maximum force generated just before the mouse lost its grasp was recorded. This was repeated five times for each animal. The results shown for each animal are the average of the middle three values.
Inverted screen
Mice were removed from the home cage and placed on top of the screen. A gentle shake of the screen was performed to make sure the mouse had gripped the screen. Then, the screen was carefully inverted at 30 cm over the empty cage so that the mouse was upside down on the screen. Time was measured from the inversion moment to record the time latency to fall. This was repeated five times for each animal with a latency of at least 10 min between measurements. The results shown for each animal are the average of the middle three values.
Balance beam
The balance beam apparatus is composed of one smooth, plastic beam 70 cm in length and 3 cm in diameter. The beam is securely suspended 25 cm above the surface. Enclosed safe house is placed at the escape end of the beam, and bedding is added to encourage the mouse to enter. A training session was conducted the day before the test session. The test session consists of five runs per animal, and the results are shown as total successful runs per animal (0–5 score).
Electron microscopy
Following CO2 euthanasia, sciatic nerves were extracted and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer overnight at 4°C. The specified protocol involving fixation, dehydration, embedding and sectioning was performed according to Peng et al. and Pehar et al.9,34 The sectioned samples were viewed at 80 kV on a Philips CM120 transmission electron microscope equipped with AMT BioSprint12 digital camera (AMT Imaging Systems).
Morphometry
Non-overlapping electron micrographs of sciatic nerves were analysed for axon diameter and g-ratio. A minimum of 100 randomly selected fibers were analysed per animal using the g-ratio plug-in of the ImageJ software, which allowed for semi-automated analysis of randomly selected sets of fibres.35 For each nerve, the percentage of non-myelinated axons was calculated by direct count from the electron microscopy micrographs.
Protein extraction and western blotting
Detergent-soluble and detergent-insoluble fractions were generated as described.10 The following primary antibodies were used in this study: anti-DDK #TA50011-100 antibody from OriGene and anti-HAtag #26183 antibody from Invitrogen. Goat anti-mouse IRDye 800CW and 680RD-conjugated secondary antibodies (LI-COR Biosciences, #926-32210, #926-68070) were used for infra-red imaging (LI-COR Odyssey Infrared Imaging System; LI-COR Biosciences).
Cell culture and plasmids
CHO-K1 (Ovary Chinese Hamster, ATCC CCL-61™) cells and mouse embryo fibroblasts were grown in Dulbecco’s modified Eagle’s medium (DMEM; Corning #10-017-CV) supplemented with 10% foetal bovine serum (Corning #35-010-CV) and 1% penicillin/streptomycin/glutamine (Gibco #10378016). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2. Cells were transiently transfected with Lipofectamine 2000 (Invitrogen #11668019) using the following constructs: mCherry-ER-3 plasmid, a gift from Michael Davidson (Addgene plasmid #55041); PMP22 human-tagged ORF clone (OriGene #RC216500); and MPZ human-tagged ORF clone (OriGene #RC202450). Additionally, plasmids for human PMP22Tr-J (T47C, Leu to Pro) and human MPZttrr (GTGC deletion and TGTATGCAATGC duplication) were developed in our laboratory using site-directed mutagenesis (New England Biolabs Q5 Site-Directed Mutagenesis Kit #E0554S). Primers for Tr-J mutation are as follows: 5′-GTCGCGGTGCCGGTGCTGCTG-3′ and 5′-GTGGAGGACGATGATACTCAGCAAC-3′. Primers for MPZ fragment deletion are as follows: 5′-TGTATGCAATGCTGGACCACAGCAGAAGCAC-3′ and 5′-TGGCGTCTGCCGCCCGCG-3′. Primers for MPZ fragment duplication are as follows: 5′-CAATGCTGGACCACAGCAGAAGCA-3′ and 5′-CATACAGCATTGCATACATGGCGTC-3′. Cells were fixed and prepared for imaging (anti-DDK #TA50011-100 antibody from OriGene; ProLong Gold antifade reagent with DAPI #P36931) 48 h after transfection. All cell slides were imaged on a Nikon A1 inverted confocal microscope using the Galvo scan head, NIS-Elements and ImageJ software for quantification.
Statistics and reproducibility
No statistical method was used to determine the necessary sample size for each experiment. The number of experimental replicates, representing the number of mice per genotype, is indicated in the respective legends. Data analysis was performed using GraphPad Prism version 9.5.1.733. Data are expressed as mean ± standard deviation. Comparison of the means was performed using an unpaired t-test for two groups and ordinary one-way or two-way ANOVA for ≥3 groups followed by Tukey–Kramer (comparison between all groups) multiple comparison test. Statistical details are described in the figure legends. Differences were declared statistically significant if P < 0.05. The following statistical significance indicators are used: *P < 0.05, **P < 0.005 and #P < 0.0005.
Results
To determine the ability of ATase inhibitors to discriminate between disease states characterized by ER proteotoxicity and those that are not, we targeted three mouse models of Charcot–Marie–Tooth disease, Pmp22Tr−J, C3-PMP22 and Mpzttrr. Although they all develop a peripheral form of neuropathy that resembles Charcot–Marie–Tooth disease, the genetic and molecular underpinnings are very different. Pmp22Tr−J mice carry a single copy of Pmp22 with a spontaneous L16C mutation that causes mouse Pmp22 to misfold and aggregate in the ER, while C3-PMP22 mice express three copies of WT human PMP22, which does not aggregate in the ER.36-42 Consistently, when expressed in Chinese Hamster Ovary (CHO) cells, the Tr-j/trembler Jackson mutant version of human PMP22 (PMP22Tr-J) displayed a punctate staining that appeared to be fully sequestered in the ER while the WT version (PMP22WT) did not (Supplementary Fig. 1). In contrast to PMP22-based mice, Mpzttrr mice carry a spontaneous mutation of mouse Mpz. Both PMP22 and MPZ are membrane proteins that insert into the ER membrane. However, the Tr-j mutation on PMP22 resides in the lumen of the ER while the ttrr/totterer mutation on MPZ resides on the cytosolic portion of the protein (Supplementary Fig. 2). When expressed in CHO cells, the ttrr mutant version of human MPZ (MPZttrr) appeared mislocalized, as compared with the WT version (MPZWT), but did not display any sequestration within the ER (Supplementary Fig. 1). This finding is consistent with the mislocalization of other C-terminal alterations of MPZ.43 Finally, to separate soluble and aggregated species of both mutants, MPZttrr- and PMP22Tr-J-expressing cells were sequentially lysed with Triton™ X-100 (soluble protein species) and sodium dodecyl sulfate (aggregated protein species), and the mutant proteins were analysed based on their migration profile. In contrast to MPZttrr, PMP22Tr-J was almost exclusively found in the aggregated form (Supplementary Fig. 3). Therefore, Pmp22Tr−J, C3-PMP22 and Mpzttrr mice can be used as proof of concept to establish the selectivity of ATase1/ATase2 inhibitors towards ER-specific proteotoxicity.
Pmp22Tr−J mice were treated with an oral formulation (50 mg/kg/day) of C9, a specific inhibitor of ATase1 and ATase2, following the well-established administration protocols.10,12,15,20 Treatment began at weaning (post-natal day 21), a time when the mice already displayed phenotypic deficits. The evolution of the disease phenotype was initially determined with a modified ataxia severity score (Supplementary Table 1), which examines ledge wall, hindlimb clasping and gait functions.33,44,45 As expected, Pmp22Tr−J mice scored very poorly with a combined severity score in the 6–8 range in both sexes while WT remained stable within the 0–2 range (Fig. 1A). C9 treatment reduced the severity score in both sexes (Fig. 1A). Importantly, the improvement remained stable throughout the entire period of treatment and was manifested across all measured functions (Fig. 1A–1C; Supplementary Fig. 4). Next, we evaluated the animals for total distance travel and rear/hind paws standing frequency on the open field test battery. In both cases, C9 treatment produced a consistent and stable improvement, which was evident in both males and females and throughout the entire period of treatment (Fig. 2A and B).
Figure 1.
ATase inhibition rescues the phenotypic severity score of Pmp22Tr−J mice. (A) Phenotypic severity score represented as a sum of ledge, gait and hindlimb clasping (n = 4–20 animals/group; single data points are shown in Supplementary Fig. 4). *P < 0.05, **P < 0.005 and #P < 0.0005 via a two-way ANOVA (genotype × age; F males statistic = 409.8 and F females statistic = 270.4 for genotype factor). Histogram (n = 12 animals/group), #P < 0.0005 via a two-way ANOVA (genotype × age; F statistic = 1256 for genotype factor). (B) Ledge, gait and hindlimb clasp severity score of males (upper panel) and female (lower panel; n = 4–24 animals/group; single data points are shown in Supplementary Fig. 4). *P < 0.05 and **P < 0.005 via a two-way ANOVA (genotype × age; F males statistic = 310.6:169.8:168.7 and F females statistic = 267.4:72.95:170.1 for ledge, gait and hindlimb clasp genotype factor, respectively). (C) Representative image of WT, Pmp22Tr−J and Pmp22Tr−J with C9 treatment animals at 5 months during the hindlimb clasp test. Figure 1 with single point graphs is shown as Supplementary Fig. 4.
Figure 2.
ATase inhibition rescues spontaneous motor activity of Pmp22Tr−J mice. (A) Total distance travelled. Two-month-old males (WT, n = 7; Pmp22Tr−J, n = 11; Pmp22Tr−J treated, n = 7), 2-month-old females (WT, n = 9; Pmp22Tr−J, n = 9; Pmp22Tr−J treated, n = 6), 5-month-old males (WT, n = 6; Pmp22Tr−J, n = 7; Pmp22Tr−J treated, n = 8) and 5-month-old females (n = 6 animals per group). *P < 0.05, **P < 0.005 and #P < 0.0005 via a two-way ANOVA (genotype × age; F males statistic = 56.91 and F females statistic = 16.07 for genotype factor). (B) Rear/hind legs standing. Two-month-old males (WT, n = 7; Pmp22Tr−J, n = 9; Pmp22Tr−J treated, n = 7), 2-month-old females (WT, n = 9; Pmp22Tr−J, n = 9; Pmp22Tr−J treated, n = 6), 5-month-old males (WT, n = 6; Pmp22Tr−J, n = 6; Pmp22Tr−J treated, n = 7) and 5-month-old females (WT, n = 6; Pmp22Tr−J, n = 5; Pmp22Tr−J treated, n = 6). *P < 0.05, **P < 0.005 and #P < 0.0005 via a two-way ANOVA (genotype × age; F males statistic = 72.25 and F females statistic = 18.79 for genotype factor).
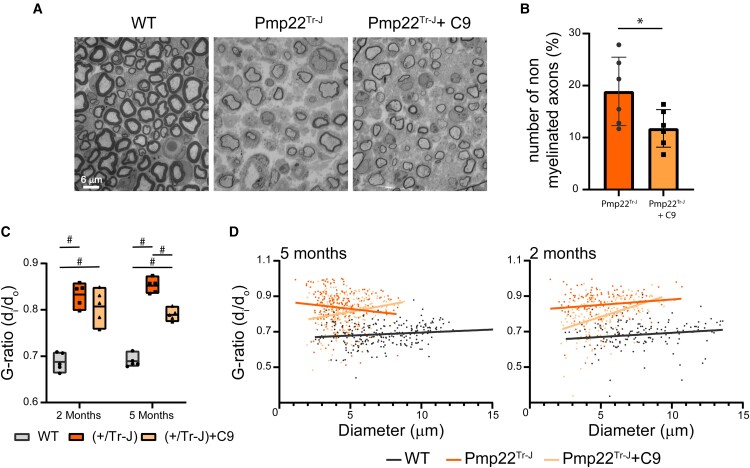
Post-mortem electron micrograph evaluation of the sciatic nerve revealed a marked loss of myelin in Pmp22Tr−J mice (Fig. 3A). This was partially prevented by C9 treatment (Fig. 3A). Importantly, C9 treatment reduced the number of unmyelinated axons by about 40–50% (Fig. 3B) and increased the thickness of myelin sheets around myelinated axons, as manifested by the ratio of inner-to-outer axonal diameter (g-ratio; Fig. 3C and D). Again, the g-ratio rescuing effect remained stable throughout the entire period of treatment. A salient feature of C9 treatment was the restoration of myelin around smaller diameter axons (Fig. 3A; Supplementary Fig. 5).
Figure 3.
ATase inhibition improves myelin morphology in the Pmp22Tr−J mice. (A) Electron micrographs of the sciatic nerves at 5 months of Pmp22Tr−J, Pmp22Tr−J treated and WT littermates. (B) Percentage of non-myelinated axons (n = 6 animals per group; total axons per animal > 110). *P < 0.05. t = 2.32 via mean comparison using unpaired Student’s t-test. (C) G-ratio value representation of sciatic nerves (n = 5 animals/group; total axons per animal > 25). #P < 0.0005 via a two-way ANOVA comparison (genotype × age; F statistic = 248.2 for genotype factor; F statistic = 1.19 for age factor). (D) G-ratio distribution according to axon diameter (n = 5 animals per group; total axons per animal > 25).
In contrast to Pmp22Tr−J mice, ATase inhibition of C3-PMP22 or Mpzttrr mice did not elicit phenotypic improvement (Figs. 4 and 5). The Charcot–Marie–Tooth disease–like phenotype of Mpzttrr mice developed earlier than C3-PMP22 mice and was much more severe, with marked defects across different motor-based paradigms. The different phenotypic severity was manifested at the histological level with Mpzttrr mice displaying a drastic loss of neurons and myelin (Figs. 4 and 5). Importantly, no rescuing effect was observed following treatment with C9 (Figs. 4 and 5).
Figure 4.
Phenotypic assessment of C3-PMP22 mice. (A) Phenotypic severity score represented as a sum of ledge, gait and hindlimb clasping at different ages (n = 7/group). **P < 0.005 and #P < 0.0005 via a two-way ANOVA comparison (genotype × age; F statistic = 110.2 for genotype factor; F statistic = 33.99 for age factor). (B) Open field assay. Total distance travelled. Two months old (n = 8/group). Non-significant P > 0.05 via a one-way ANOVA comparison (F statistic = 0.7895). (C) Open field assay. Rears. Two months old (n = 8/group). Non-significant P > 0.05 via a one-way ANOVA comparison (F statistic = 0.6302). (D) Hot plate time to reaction. Two months old (n = 8/group). Non-significant P > 0.05 via a one-way ANOVA comparison (F statistic = 0.3002). (E) Grip strength normalized to body weight. Two months old (n = 8/group). Non-significant P > 0.05 via a one-way ANOVA comparison (F statistic = 3.169). (F) Time to fall from the inverted screen. Two months old (WT, n = 6; C3-PMP22, n = 7; C3-PMP22 treated, n = 7). #P < 0.0005 via a one-way ANOVA (F statistic = 35.89). (G) Electron micrographs of the sciatic nerves at 5 months of C3-PMP22, C3-PMP22 treated and WT littermates. (H) G-ratio value representation of sciatic nerves (n = 5 animals/group; total axons per animal > 35). *P < 0.05 via a one-way ANOVA comparison (F statistic = 5.2).
Figure 5.
Phenotypic assessment of Mpzttrr mice. (A) Phenotypic severity score represented as a sum of ledge, gait and hindlimb clasp at different ages (WT, n = 7; Mpzttrr, n = 7; Mpzttrr treated, n = 6). #P < 0.0005 via a two-way ANOVA comparison (genotype × age; F statistic = 769.4 for genotype factor; F statistic = 2.2 for age factor). (B) Open field assay. Total distance travelled. Two months old (n = 8 animals/group). *P < 0.05 and **P < 0.005 via a one-way ANOVA comparison (F statistic = 6.56). (C) Open field assay. Rears. Two months old (n = 8 animals/group). #P < 0.0005 via a one-way ANOVA comparison (F statistic = 20.51). (D) Hot plate time to reaction. Two months old (n = 8 animals/group). #P < 0.0005 via mean one-way ANOVA comparison (F statistic = 17.45). (E) Grip strength normalized to body weight. Two months old (n = 8 animals/group). #P < 0.0005 via a one-way ANOVA comparison (F statistic = 28.41). (F) Time to fall from the inverted screen. Two months old (WT, n = 6; Mpzttrr, n = 7; Mpzttrr treated, n = 9). #P < 0.0005 via a one-way ANOVA (F statistic = 68.21). (G) Number of complete walks through the balance beam (n = 10 animals/group). #P < 0.0005 via a one-way ANOVA (F statistic = 106.8). (H) Electron micrographs of the sciatic nerves at 5 months of Mpzttrr, Mpzttrr treated and WT littermates. (I) G-ratio value representation of sciatic nerves (n = 5 animals/group; total axons per animal > 35. *P < 0.05 and **P < 0.005 via a one-way ANOVA comparison (F statistic = 9.82).
In conclusion, the inhibition of the ATases by C9 partially rescued both the behavioural and pathological features associated with the Charcot–Marie–Tooth disease–like phenotype of Pmp22Tr−J mice but was not able to modify the progression of the disease in C3-PMP22 or Mpzttrr mice.
Discussion
ATase1 and ATase2 are type II ER-resident membrane proteins with the catalytic domain facing the lumen of the organelle.18 They use acetyl-CoA, imported into the ER lumen by AT-1/SLC33A1, to acetylate ER cargo and resident proteins.17 Genetic disruption of either Atase1 or Atase2 in the mouse stimulates reticulophagy.14 Biochemical inhibition of the Atases in the mouse also stimulates reticulophagy.10,15,20 Finally, reduced import of acetyl-CoA into the ER lumen stimulates reticulophagy by limiting the catalytic activity of the ATases.9,10,17,46 Both cell- and animal-based studies indicate that the regulation of reticulophagy downstream of the ATases depends on the acetylation status of ATG9A. Specifically, acetylated ATG9A prevents reticulophagy while non-acetylated ATG9A stimulates reticulophagy.10,12-14,19 Importantly, the acetylation of ATG9A occurs in the lumen of the ER.10,12-14,19 In essence, the ER acetylation machinery appears to be spatially positioned to act as a novel branch of the ER quality control system to help disposing of protein aggregates that form within the ER lumen.
Inhibition of the ATases was able to rescue the progeria-like phenotype of AT-1 sTg and SLC13A5 sTg, two mouse models of ER hyperacetylation.12,15,20 Inhibition of the ATases was also able to resolve the Alzheimer’s disease–like phenotype of APP695/swe and APP695/swe/PS1-dE9 mice.10,20 Importantly, ATase inhibition did not resolve the disease phenotype of mHttQ160 mice, a model of Huntington disease, or hSOD1G93A mice, a model of amyotrophic lateral sclerosis.10 APP is a type I membrane protein that inserts into the ER to engage the secretory pathway, while Htt and SOD1 are cytosolic proteins and do not engage the secretory pathway. Furthermore, the proteotoxic aggregates of the A53T mutant version of α-synuclein, which is associated with an autosomal dominant form of Parkinson’s disease, were successfully degraded by reduced ER acetylation only when α-synuclein was forced to insert into the ER lumen by adding a signal peptide to its N-terminus.10 In essence, ATase inhibitors appear to be selective for ER/secretory pathway proteotoxicity.
This study adds an additional layer of evidence by showing that misfolding states that occur in the lumen of the ER, and that are associated with aberrant aggregation of the misfolded polypeptide in the ER, can be targeted by ATase inhibitors, while misfolding states that occur elsewhere, or that do not yield protein aggregates within the ER, cannot. In essence, work conducted with different mouse models of human diseases has established the ATases as the first proven targets for proteotoxic states that initiate within the lumen of the ER (see present study and Peng et al., Rigby et al., Fernandez-Fuente et al. and Murie et al.10,12,14,15,20). This selectivity provides encouraging hopes for many hereditary diseases caused by mutations that force the protein to misfold and aggregate within the ER and secretory pathway, such as Charcot–Marie–Tooth disease, Pelizaeus–Merzbacher disease and cystic fibrosis. It also provides a way to limit or completely avoid unwanted effects caused by non-selective global activation of autophagy. This selectivity, however, also requires a personalized form of medicine with careful selection of patients, particularly for those diseases, such as hereditary forms of neuropathy, where different genetic events (i.e. mutation, duplication or deletion) can be the underlying pathogenic mechanism. Using Charcot–Marie–Tooth disease as a test case, our data would indicate that ATase inhibition may not be effective in patients with PMP22 duplication (classified as CMT1A) but may be effective in patients with PMP22 mutations (classified as CMT1E). Although treatment of mice with the Mpzttrr mutation was ineffective, there are a variety of dominant MPZ mutations (classified as CMT1B) that are associated with the misfolding of the ER luminal portion of the protein, ER retention and even ER aggregation together with activation of the unfolded protein response.47,48 These forms of Charcot–Marie–Tooth disease may be responsive to ATase inhibition and stimulation of reticulophagy.
Supplementary Material
Contributor Information
Gonzalo Fernandez-Fuente, Department of Medicine, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI 53705, USA; Waisman Center, University of Wisconsin-Madison, Madison, WI 53705, USA.
Mark A Farrugia, Department of Medicine, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI 53705, USA; Waisman Center, University of Wisconsin-Madison, Madison, WI 53705, USA.
Yajing Peng, Department of Medicine, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI 53705, USA; Waisman Center, University of Wisconsin-Madison, Madison, WI 53705, USA.
Andrew Schneider, Waisman Center, University of Wisconsin-Madison, Madison, WI 53705, USA.
John Svaren, Waisman Center, University of Wisconsin-Madison, Madison, WI 53705, USA; Department of Comparative Biosciences, School of Veterinary Medicine, University of Wisconsin-Madison, Madison, WI 53706, USA.
Luigi Puglielli, Department of Medicine, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI 53705, USA; Waisman Center, University of Wisconsin-Madison, Madison, WI 53705, USA; Geriatric Research Education Clinical Center, Veterans Affairs Medical Center, Madison, WI 53705, USA.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
The Puglielli laboratory is supported by the National Institute of Neurological Disorders and Stroke (R01NS094154), the National Institute of General Medical Sciences (R01GM148487), the National Institute on Aging (R01AG078794) and the Charcot-Marie-Tooth Association. The Svaren laboratory is supported by the National Institute of Neurological Disorders and Stroke (R01NS130566) and the Charcot-Marie-Tooth Association. This research also benefitted from a core grant to the Waisman Center from the National Institute of Child Health and Human Development (U54 HD105353).
Competing interests
The authors declare the following competing interests: L.P. is a consultant for Belharra Therapeutics. The remaining authors have no competing interests to disclose.
Data availability
Source data for the graphs and charts are available as Supplementary material, and any remaining information can be obtained from the corresponding author upon reasonable request.
References
- 1. Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: Brothers in arms. Mol Cell. 2010; 40(2):238–252. [DOI] [PubMed] [Google Scholar]
- 2. Trombetta ES, Parodi AJ. Quality control and protein folding in the secretory pathway. Annu Rev Cell Dev Biol. 2003; 19:649–676. [DOI] [PubMed] [Google Scholar]
- 3. Frake RA, Ricketts T, Menzies FM, Rubinsztein DC. Autophagy and neurodegeneration. J Clin Invest. 2015; 125(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levine B, Packer M, Codogno P. Development of autophagy inducers in clinical medicine. J Clin Invest. 2015; 125(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013; 19(8):983–997. [DOI] [PubMed] [Google Scholar]
- 6. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008; 451(7182):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrugia MA, Puglielli L. Nepsilon-lysine acetylation in the endoplasmic reticulum—A novel cellular mechanism that regulates proteostasis and autophagy. J Cell Sci. 2018; 131(22):jcs221747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dieterich IA, Cui Y, Braun MM, et al. Acetyl-CoA flux from the cytosol to the ER regulates engagement and quality of the secretory pathway. Sci Rep. 2021; 11(1):2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng Y, Li M, Clarkson BD, et al. Deficient import of acetyl-CoA into the ER lumen causes neurodegeneration and propensity to infections, inflammation, and cancer. J Neurosci. 2014; 34(20):6772–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peng Y, Kim MJ, Hullinger R, et al. Improved proteostasis in the secretory pathway rescues Alzheimer's disease in the mouse. Brain. 2016; 139(Pt 3):937–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hullinger R, Li M, Wang J, et al. Increased expression of AT-1/SLC33A1 causes an autistic-like phenotype in mice by affecting dendritic branching and spine formation. J Exp Med. 2016; 213(7):1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peng Y, Shapiro SL, Banduseela VC, et al. Increased transport of acetyl-CoA into the endoplasmic reticulum causes a progeria-like phenotype. Aging Cell. 2018;17(5):e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheehan BK, Orefice NS, Peng Y, Shapiro SL, Puglielli L. ATG9A regulates proteostasis through reticulophagy receptors FAM134B and SEC62 and folding chaperones CALR and HSPB1. iScience. 2021; 24(4):102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rigby MJ, Lawton AJ, Kaur G, et al. Endoplasmic reticulum acetyltransferases Atase1 and Atase2 differentially regulate reticulophagy, macroautophagy and cellular acetyl-CoA metabolism. Commun Biol. 2021; 4(1):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandez-Fuente G, Overmyer KA, Lawton AJ, et al. The citrate transporters SLC13A5 and SLC25A1 elicit different metabolic responses and phenotypes in the mouse. Commun Biol. 2023; 6(1):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandez-Fuente G, Rigby MJ, Puglielli L. Intracellular citrate/acetyl-CoA flux and endoplasmic reticulum acetylation: Connectivity is the answer. Mol Metab. 2023; 67:101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jonas MC, Pehar M, Puglielli L. AT-1 is the ER membrane acetyl-CoA transporter and is essential for cell viability. J Cell Sci. 2010; 123(Pt 19):3378–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ko MH, Puglielli L. Two endoplasmic reticulum (ER)/ER Golgi intermediate compartment-based lysine acetyltransferases post-translationally regulate BACE1 levels. J Biol Chem. 2009; 284(4):2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pehar M, Jonas MC, Hare TM, Puglielli L. SLC33A1/AT-1 protein regulates the induction of autophagy downstream of IRE1/XBP1 pathway. J Biol Chem. 2012; 287(35):29921–29930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murie M, Peng Y, Rigby MJ, et al. ATase inhibition rescues age-associated proteotoxicity of the secretory pathway. Commun Biol. 2022; 5(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurth I, Pamminger T, Hennings JC, et al. Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nat Genet. 2009; 41(11):1179–1181. [DOI] [PubMed] [Google Scholar]
- 22. Murphy SM, Davidson GL, Brandner S, Houlden H, Reilly MM. Mutation in FAM134B causing severe hereditary sensory neuropathy. J Neurol Neurosurg Psychiatry. 2012; 83(1):119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Almeida-Souza L, Goethals S, de Winter V, et al. Increased monomerization of mutant HSPB1 leads to protein hyperactivity in Charcot-Marie-Tooth neuropathy. J Biol Chem. 2010; 285(17):12778–12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Capponi S, Geroldi A, Fossa P, et al. HSPB1 and HSPB8 in inherited neuropathies: Study of an Italian cohort of dHMN and CMT2 patients. J Peripher Nerv Syst. 2011; 16(4):287–294. [DOI] [PubMed] [Google Scholar]
- 25. Evgrafov OV, Mersiyanova I, Irobi J, et al. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet. 2004; 36(6):602–606. [DOI] [PubMed] [Google Scholar]
- 26. Lin P, Li J, Liu Q, et al. A missense mutation in SLC33A1, which encodes the acetyl-CoA transporter, causes autosomal-dominant spastic paraplegia (SPG42). Am J Hum Genet. 2008; 83(6):752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Pace R, Skirzewski M, Damme M, et al. Altered distribution of ATG9A and accumulation of axonal aggregates in neurons from a mouse model of AP-4 deficiency syndrome. PLoS Genet. 2018; 14(4):e1007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behne R, Teinert J, Wimmer M, et al. Adaptor protein complex 4 deficiency: A paradigm of childhood-onset hereditary spastic paraplegia caused by defective protein trafficking. Hum Mol Genet. 2020; 29(2):320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Paassen BW, van der Kooi AJ, van Spaendonck-Zwarts KY, Verhamme C, Baas F, de Visser M. PMP22 related neuropathies: Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsies. Orphanet J Rare Dis. 2014; 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pisciotta C, Shy ME. Hereditary neuropathy. Handb Clin Neurol. 2023; 195:609–617. [DOI] [PubMed] [Google Scholar]
- 31. Rigby MJ, Orefice NS, Lawton AJ, et al. Increased expression of SLC25A1/CIC causes an autistic-like phenotype with altered neuron morphology. Brain. 2022;145(2):500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rigby MJ, Orefice NS, Lawton AJ, et al. SLC13A5/sodium-citrate co-transporter overexpression causes disrupted white matter integrity and an autistic-like phenotype. Brain Commun. 2022; 4(1):fcac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guyenet SJ, Furrer SA, Damian VM, Baughan TD, La Spada AR, Garden GA. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp. 2010;(39):1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pehar M, O'Riordan KJ, Burns-Cusato M, et al. Altered longevity-assurance activity of p53:p44 in the mouse causes memory loss, neurodegeneration and premature death. Aging Cell. 2010; 9(2):174–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goebbels S, Oltrogge JH, Kemper R, et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010; 30(26):8953–8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henry EW, Cowen JS, Sidman RL. Comparison of trembler and trembler-J mouse phenotypes: Varying severity of peripheral hypomyelination. J Neuropathol Exp Neurol. 1983; 42(6):688–706. [DOI] [PubMed] [Google Scholar]
- 37. Suter U, Moskow JJ, Welcher AA, et al. A leucine-to-proline mutation in the putative first transmembrane domain of the 22-kDa peripheral myelin protein in the trembler-J mouse. Proc Natl Acad Sci U S A. 1992; 89(10):4382–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suter U, Welcher AA, Ozcelik T, et al. Trembler mouse carries a point mutation in a myelin gene. Nature. 1992; 356(6366):241–244. [DOI] [PubMed] [Google Scholar]
- 39. Colby J, Nicholson R, Dickson KM, et al. PMP22 carrying the trembler or trembler-J mutation is intracellularly retained in myelinating Schwann cells. Neurobiol Dis. 2000; 7(6 Pt B):561–573. [DOI] [PubMed] [Google Scholar]
- 40. Dickson KM, Bergeron JJ, Shames I, et al. Association of calnexin with mutant peripheral myelin protein-22 ex vivo: A basis for “gain-of-function” ER diseases. Proc Natl Acad Sci U S A. 2002; 99(15):9852–9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verhamme C, King RH, ten Asbroek AL, et al. Myelin and axon pathology in a long-term study of PMP22-overexpressing mice. J Neuropathol Exp Neurol. 2011; 70(5):386–398. [DOI] [PubMed] [Google Scholar]
- 42. Marinko JT, Carter BD, Sanders CR. Direct relationship between increased expression and mistrafficking of the Charcot-Marie-Tooth-associated protein PMP22. J Biol Chem. 2020; 295(34):11963–11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fratta P, Ornaghi F, Dati G, et al. A nonsense mutation in myelin protein zero causes congenital hypomyelination neuropathy through altered P0 membrane targeting and gain of abnormal function. Hum Mol Genet. 2019; 28(1):124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castillo-Mariqueo L, Gimenez-Llort L. Clasping, ledge-score coordination and early gait impairments as primary behavioural markers of functional impairment in Alzheimer's disease. Behav Brain Res. 2022; 435:114054. [DOI] [PubMed] [Google Scholar]
- 45. Li QF, Dong Y, Yang L, et al. Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3. Mol Neurodegener. 2019;14(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rigby MJ, Ding Y, Farrugia MA, et al. The endoplasmic reticulum acetyltransferases ATase1/NAT8B and ATase2/NAT8 are differentially regulated to adjust engagement of the secretory pathway. J Neurochem. 2020; 154(4):404–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ptak CP, Peterson TA, Hopkins JB, Ahern CA, Shy ME, Piper RC. Homomeric interactions of the MPZ Ig domain and their relation to Charcot-Marie-Tooth disease. Brain. 2023; 146(12):5110–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bai Y, Wu X, Brennan KM, et al. Myelin protein zero mutations and the unfolded protein response in Charcot Marie Tooth disease type 1B. Ann Clin Transl Neurol. 2018; 5(4):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data for the graphs and charts are available as Supplementary material, and any remaining information can be obtained from the corresponding author upon reasonable request.