Graphical abstract

Keywords: Enteric fungi, Intestinal ischemia–reperfusion injury, Mannan, Antifungal therapy, Pyroptosis
Research highlights
-
•
Enteric fungi diversity was markedly altered in IIR injury.
-
•
Fungal colonization or mannan could alleviate IIR injury by decreasing GSDMD-mediated pyroptosis of IECs.
-
•
The protective role of fungi in IIR injury depends on macrophage involvement.
-
•
Single-fungal strains and mannan both alleviate GSDMD cleavage induced by reducing SAA1 expression of macrophages.
Abstract
Introduction
Prophylactic antifungal therapy has been widely used for critical patients, but it has failed to improve patient prognosis and has become a hot topic. This may be related to disruption of fungal homeostasis, but the mechanism of fungi action is not clear. As a common pathway in critical patients, intestinal ischemia–reperfusion (IIR) injury is fatal and regulated by gut microbiota. However, the exact role of enteric fungi in IIR injury remains unclear.
Objectives
This is a clinical study that aims to provide new perspectives in clarifying the underlying mechanism of IIR injury and propose potential strategies that could be relevant for the prevention and treatment of IIR injury in the near future.
Methods
ITS sequencing was performed to detect the changes in fungi before and after IIR injury. The composition of enteric fungi was altered by pretreatment with single-fungal strains, fluconazole and mannan, respectively. Intestinal morphology and function impairment were evaluated in the IIR injury mouse model. Intestinal epithelial MODE-K cells and macrophage RAW264.7 cells were cultured for in vitro tests.
Results
Fecal fungi diversity revealed the obvious alteration in IIR patients and mice, accompanied by intestinal epithelial barrier dysfunction. Fungal colonization and mannan supplementation could reverse intestinal morphology and function impairment that were exacerbated by fluconazole via inhibiting the expression of SAA1 from macrophages and decreasing pyroptosis of intestinal epithelial cells. Clodronate liposomes were used to deplete the number of macrophages, and it was demonstrated that the protective effect of mannan was dependent on macrophage involvement.
Conclusion
This finding firstly validates that enteric fungi play a crucial role in IIR injury. Preventive antifungal treatment should consider damaging fungal balance. This study provides a novel clue to clarify the role of enteric fungi in maintaining intestinal homeostasis.
Introduction
Intestinal ischemia–reperfusion (IIR) injury is a frequent and fatal condition with manifestations such as increased vascular permeability, mucosal barrier damage, and inflammatory cell infiltration. IIR injury can result in morbidity and mortality in 60%–80% of cases [1], [2], [3], [4]. Typically, mesenteric thromboembolism, necrotizing enterocolitis, and intestine transplantation all cause IIR injury [5], [6], [7]. Recent studies have also reported IIR injury in hemodialysis patients, in which effective blood volume is decreased due to the temporary “accumulation” of blood in the hemodialysis machine, resulting in ischemia–reperfusion injury of the intestinal mucosa [8], [9], [10], [11]. Moreover, some studies suggested that hemodialysis-induced IIR injury is directly related to abdominal pain in chronic kidney disease (CKD) patients [9], [12] and contributes to intestinal epithelial barrier (IEB) dysfunction, gut-derived infection [13], and even multiple organ dysfunction [13], [14], [15], [16]. Currently, it is necessary to further clarify the mechanism underlying IIR injury in different disease contexts, including hemodialysis, and to identify novel effective approaches for the prevention and treatments of IIR injury.
Gut microbes play a crucial role in maintaining intestinal homeostasis [17], [18]). Imbalances in the gut microbiota induced by IIR injury can lead to loss of IEB function, excessive inflammatory responses, and immune dysfunction [19], [20], [21]. Enteric fungi play an indispensable role in the gut [22] and have a stable synergistic, antagonistic, or symbiotic relationship with other gut microbiota [23], [24], stabilizing the gut microenvironment and maintaining intestinal mucosal barrier function [22]. Accumulating evidence suggests that enteric fungi are more unstable than bacteria and induce an immune response more actively in the host during changes in the internal environment [25], [26]. Considering that patients undergoing acute IIR stimulation or repeated hemodialysis treatments have a fragile gut environment [27], it is highly possible that enteric fungi can display marked changes, as revealed in our earlier research on sepsis [28]. However, the exact role and the underlying mechanism of enteric fungi in IIR injury remain unclear.
Our previous studies demonstrated that relieving the intestinal fungi burden promotes Gasdermin D (GSDMD) cleavage and increases the incidence of septic shock [28], suggesting the potential role of GSDMD-mediated pyroptosis under fungi disturbance when facing pathological stimulation. In IIR injury, intestinal epithelial cell (IEC) damage and death are the main mechanisms of intestinal barrier destruction, and inhibition of IEC death reduces IIR injury [29], [30]. Inflammasomes and GSDMD proteins are commonly expressed in IECs, which induce pyroptosis, one of the major mechanisms of IEC death [31], [32]. GSDMD proteins are recently identified executioner of pyroptosis which functions downstream of inflammatory mediator activation [33]. Upon activation, pro-inflammatory caspases are transformed into cleavage caspases, which then cleave GSDMD proteins, leading to pyroptosis [34]. Moreover, knockdown of GSDMD in Caco-2 cells significantly reduced IIR injury via blocking cell pyroptosis [35]. However, the precise role of enteric fungi on GSDMD-mediated pyroptosis in IIR injury or hemodialysis has been poorly reported.
Based on the above considerations, we hypothesized that enteric fungi disturbance is involved in IIR injury. Enteric fungi inhibition of GSDMD cleavage may be an important pathway for the prevention of IIR injury. This study provides new perspectives in clarifying the underlying mechanism of IIR injury and proposes potential strategies that could be relevant for the prevention and treatment of IIR injury in the near future.
Materials and methods
Ethics statement
All procedures involving human subjects were approved by the Ethical Committee of Xinqiao hospital, Army Medical University (approval number 2018–006-02). All experimental protocols involving mice were performed in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Welfare and Ethics Committee of the Army Medical University.
Patient cohorts
Patients with stage 6 CKD undergoing their first hemodialysis were recruited (n = 20) in the Chronic Kidney Disease and Hemodialysis center. Participants were not included if they (1) were aged < 18 or > 75 years; (2) had chronic digestive disorders, gastrointestinal surgery, or confirmed or suspected ischemia/necrosis of the intestine; (3) had used antidiarrheals or laxatives within 1 week, or antibiotics and antifungal drugs within 3 months. Patient’s fecal and blood samples were collected 2 h before and 24 h after the first hemodialysis.
Patient SMA ultrasonic test
The superior mesenteric artery (SMA) morphology and blood flow in patients before and 2 h after the start of hemodialysis were performed by ultrasound test (Mindray Bio-Medical Electronics, China) as previously reported [36], [37]. Sagittal visualization of the abdominal aorta and SMA extending from it was performed using a convex C60x probe with a scan rate of 2–5 MHz. The starting point of the SMA was identified, the direction of movement of the blood vessel and tube wall was observed, the direction of the ultrasound beam was adjusted to be as perpendicular as possible to the tube wall, and the tube’s internal diameter was measured 1.0 cm above the starting point. Blood flow parameters were sampled from 1.0 cm to 2.0 cm. Ultrasonic Doppler detection was performed with a sampling range of 0.2–0.3 cm. The angle between the corrected ultrasound beam and the direction of blood flow was<60°, and the sampling line was parallel to the direction of the jet stream rather than to the wall of the vessel.
ITS1 sequencing
E.Z.N.A.® soil DNA Kit (Omega Bio-Tek, USA) was used to extracted total microbial genomic DNA from patient’s fecal samples. Internal transcribed spacer (ITS) gene was amplified with the primer pairs ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS1R (5′-GTTCAAAGAYTCGATGATTCAC-3′), as previously described [26]. Purified amplicons were pooled in equimolar amounts and subjected to paired-end sequencing on an Illumina Miseq PE300 platform (Illumina, USA), in accordance with the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Mice
All the WT mice (8–10-week-old C57BL/6J) purchased from GemPharmatech (Chengdu, China) were kept at the animal center. All the germ-free C57BL/6J mice were provided by and kept in the Medical Animal Center of Army Medical University. GSDMD-/- C57BL/6J mice were kindly gifted by Dr. Shu Zhu from the University of Science and Technology of China.
IIR model
Mice were anesthetized by intraperitoneal injection of approximately 100–150 µL of 2.5% 2,2,2-Tribromoethanol (Sigma, USA), and abdominal laparotomy was operated to expose the SMA, as previously reported [38]. To induce intestinal ischemia, warm sterile saline (37 °C) was dropped on the target SMA, and the vessels were clamped with atraumatic microvascular clips for 30 min. The ischemia status was confirmed by the change in the color of the intestines from red–pink to wine–red. Then, the clamp was gently released to allow reperfusion for 6 h, the blood samples were collected, and intestine tissues harvested (n = 6).
Murine intestinal epithelial cell line MODE-K cells (BNCC338300, BeNa Culture Collection, China) cells were used to establish the IIR injury model in vitro, according to the oxygen–glucose deprivation/reperfusion (OGD/R) method [35]. Briefly, the MODE-K cells were cultured in a hypoxic growth chamber (95% N2, 5% CO2) with glucose-free medium for 6 h and then moved to a normoxia incubator chamber (95% O2, 5 % CO2) with glucose-containing growth medium for 2 h. Recombinant human SAA1 protein (P00100, Solabio, China) was added to the cell medium at 600 ng/ml in OGD/R to verify an increase in GSDMD cleavage.
Pretreatment programmes
Fluconazole (Sigma, USA) or mannan from Saccharomyces cerevisiae (Sigma, USA) were dissolved in the feeding water at 0.5 mg/mL and 0.1 mg/mL for 2 weeks, respectively. A single Candida tropicalis (ATCC13803) or Saccharomyces cerevisiae (ATCC204991) clone was picked and shaken for 24 h in yeast extract peptone dextrose medium (Solarbio, China) at 30 °C in an incubator. The next day, approximately 108 CFU of single fungal strains were administered by oral gavage to the indicated mice. Clodronate liposomes (Liposoma, China) were administered intraperitoneally 3 days before establishing the IIR model to deplete macrophages from the gut.
Fence quantitative abundance test
For fungal DNA extraction, fecal pellets were weighed and beaded with 250 μL 20% SDS, 500 μL PB buffer (Qiagen, Hilden, Germany), 200 μL 0.1-mm beads (Biospec, USA), and 550 μL phenol: chloroform: IAA (SolarBio, China). The aqueous phase was collected, centrifuged at 8000 rpm for 3 min at room temperature, and purified using Qiagen PCR Purification Kit, according to the manufacturer’s instructions (Qiagen, Germany) [26]. Quantitative PCR of fecal rDNA was conducted on the CFX96 real-time system (Bio-Rad, USA) using GoTaq® SYBR PCR Master Mix (Promega, USA). The fecal rDNA was determined by the Fungi quant method. The plasmid initiation copies number conversion formula (copies/μl) = concentration (ng/μl) * 10-9 * 6.02 * 1023 / (molecular weight * 660). Plasmid containing fecal gene was diluted serially to obtain the standard curves. For all fecal rDNA copy numbers, values were normalized to the fecal weight input. The PCR primer sequences we used are shown in Table S1.
Fungus plate culture
Briefly, 10 mg of fecal formed stool were homogenized in 1 ml sterile phosphate-buffered saline(PBS)and plated on yeast peptone dextrose medium (Beyotime, China) plates complemented with 0.01 mg/ml vancomycin and 0.1 mg/ml gentamicin to culture fungi for 48 h at 37 °C.
Enzyme-linked immunosorbent assay (ELISA)
The special biomarkers for gut function were detected by ELISA kit, according to the manufacturer’s instructions. The ELISA kit used were as follows: Human I-FABP (DFBP20, R&D Systems, USA), Human ZO-1 (EK8725, SAB, USA), Human Citrulline (EK16947, SAB, USA), and Mouse I-FABP (EK1622, Boster Biological Technology, USA).
The FITC-Dextran 4 kDa (FD-4) permeability test
To determine intestinal permeability, mice were given 600 mg/kg FD-4 (Sigma, USA) by oral gavage, 1 h prior to establishment of the IIR injury model. Blood was then harvested after IIR and centrifuged, and then determined using an enzyme-labeling instrument (Thermo Fisher Scientific, USA), with an excitation and emission of 485 nm and 528 nm, respectively.
Ultra-high performance liquid chromatography
Blood samples of mice were centrifuged for 10 min at 4000 rpm. Serum fluconazole was measured using ultra-high performance liquid chromatography as previously described [39].
Serum ALT
Fluconazole toxicity was determined by serum ALT (C009-2–1, Jiancheng, China) and the manufacturer's instructions were followed. Briefly, serum samples were collected from mice and allowed to equilibrate at room temperature for one hour before being subjected to centrifugation at 4000 rpm for 10 min. Following the manufacturer's instructions, the corresponding reagent was introduced into a 96-well plate to ensure complete reactivity of the sample. Subsequently, the OD values of the sample were measured at a wavelength of 510 nm and compared to the standard curve to obtain the corresponding ALT values.
Histopathological examination
Intestine tissues were fixed in 4% paraformaldehyde solution (Sangon Biotech, China). Histological damage in the mucosa stained with hematoxylin and eosin (H&E) was evaluated through quantitative measurement of tissue injury by a blinded observer. The Chiu’s score classification was applied to evaluate the damage in the small intestine samples, as previously described [35], [40].
Serum cytokine levels
The levels of serum cytokines were determined by LEGENDplex™ assay kit (BioLegend, USA), according to the manufacturer’s instructions. Samples were run on Gallios (Beckman Coulter, USA), and data were analyzed online (https://legendplex.qognit.com/workflow).
Western blot assay
Briefly, RIPA lysis buffer (Solarbio, China) supplemented with 1% phenylmethanesulfonyl fluoride (Boster, China) was used to extract the total protein, followed by centrifugation at 12,000 g for 10 min at 4 °C. The protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride membrane. The blots obtained were blocked with 5% skimmed milk and incubated with the anti-occludin antibody (1:1000; ab167161; Abcam, USA), anti-ZO1 tight junction protein antibody (1:1000; ab276131; Abcam, USA), anti-GSDMD antibody (1:1000; ab209845; Abcam, USA), anti-SAA1 antibody (1:1000; ab199030; Abcam, USA) and anti-beta actin antibody (1:1000; ab115777; Abcam, USA) overnight at 4 °C. The bolts were then incubated with horseradish peroxidase-conjugated secondary antibody (1:2000; SA00001-2; Proteintech, China) for 1 h at room temperature. Proteins were detected using the chemiluminescent system and quantified using ImageJ software.
Flow cytometric analysis
The isolation and subsequent analysis of murine lamina propria macrophages from ileal tissue was conducted following previously established methods [41]. The procedure involved the removal of the intestines from deceased mice, followed by the removal of intestinal feces and cutting the tissue into 4–5 cm pieces. The tissue was then incubated with EDTA and dithiothreitol (DTT) in Hanks' balanced salt solution (HBSS) to eliminate epithelial cells. The remaining lamina propria was incubated for 30 min at 37 °C under slow rotation in an incubator with collagenase D (C4-22, Sigma, USA) and DNase I (10104159001, Roche, Switzerland). After obtaining a suspension of single-celled organisms, the cells were separated by centrifugation at 600g at 4 °C and rinsed with FACS buffer. Subsequently, any nonspecific binding of antibodies was prevented by incubating the cells with TruStain FcX™ (CD16/CD32) antibody (101320, BioLegend, USA) for 15 min on ice. The cell surface was then labeled with anti-mouse CD45.2 antibody (109822, BioLegend, USA) and anti-mouse F4/80 antibody (123114, BioLegend, USA). Flow cytometric analysis was conducted using Gallios instruments (Beckman Coulter, USA) and the data were analyzed using FlowJo software (Tree Star Inc.).
Immunohistochemistry staining
Paraffin slides were incubated with the anti-SAA1 antibody (1:1000; ab199030; Abcam, USA) and anti-GSDMD antibody (1:1000; ab219800; Abcam, USA) to analyze the SAA1 and GSDMD protein levels, as previously described. Two independent pathologists blinded to the outcome analyzed the immunoreactivity semi-quantitatively.
Immunofluorescence staining
To identify SAA1-expressing cells, paraffin section samples were generated as above, blocked for 1 h, and incubated overnight at 4 °C with anti-SAA1 antibody (1:1000; ab199030; Abcam, USA) and anti-F4/80 antibody (1:1000; SC-52664; Santa Cruz Biotechnology, USA). Then, the intestine paraffin slide was washed and stained with DAPI (C1005; Beyotime, China) for 20 min, and images were captured using a laser scanning confocal microscope (Olympus, Japan). To clarify the action site of mannan, we covalently attached a Cy3 fluorescent marker to mannan and gave it to the mice by oral gavage. Intestine paraffin slides containing cy3-mannan were then stained with F4/80 and DAPI in accordance with the above method. To demonstrate that mannan can enter macrophages in vitro, RAW264.7 cells were incubated with cy3-mannan and stained with Hoechst (10 µg/ml; Beyotime, China).
Rna-seq analysis
For RNA-seq, total RNA of intestinal tissues was extracted by trizol following the manufacturer’s instruction. RNA isolation, library construction, and sequencing were performed on a BGISEQ-500 (BGI-Shenzhen, China). Data mining and graphic presentation processes were all performed by a BGI custom data mining system called Dr. Tom (https://report.bgi.com).
Cell culture
MODE-K cells were maintained in RPMI 1640 culture fluid with 10% fetal bovine serum (BI & Viva cell, China), penicillin (100 U/mL, Sangon Biotech, China) and ciprofloxacin (10 μg/mL, Sangon Biotech, China). RAW264.7(ATCC TIB-71) cells were maintained in complete DMEM culture fluid with fetal bovine serum, ciprofloxacin, and penicillin as mentioned above.
PCR analyses
Total RNA was extracted using trizol reagent (Takara, Tokyo, Japan), and qPCR was performed using a SYBR Green qPCR kit (Promega, Madison, WI, USA), as previously described [28]. The -ΔΔCT method was utilized to ascertain alterations in gene expression. The primer sequences used showed in Table S1.
Statistical analyses
The measurement data were analyzed by GraphPad Prism 8.0 software and presented as the mean ± SD. Patients’ data were compared using wilcoxon matched-pairs signed-rank test for two groups. The statistical significance between two groups of mice was assessed through the two-tailed unpaired Student's t-test, while multiple sets of data were subjected to analysis via the one-way ANOVA with Tukey post hoc test (one-way ANOVA-Tukey). All data were assessed by normality and log-normality tests. The statistics of p-values were provided as *p < 0.05, **p < 0.01, and ***p < 0.001, and ns means no significance.
Results
Characteristics of enteric fungi disturbance during IIR injury in patients and mice
To confirm that IIR injury occurs in patients, we evaluated the variations in the SMA morphology and blood flow in patients during the first hemodialysis. The linear and volumetric parameters of the SMA blood flow decreased after 2 h of hemodialysis (Fig. 1A, S1). In addition, intestinal barrier-related biomarkers of IIR injury were detected by ELISA method 2 h before and 24 h after hemodialysis. A significant increase in I-FABP and ZO-1 levels was observed, but citrulline values decreased between the two time points (Fig. 1B). Research has demonstrated that elevated serum concentrations of I-FABP and ZO-1, coupled with reduced serum levels of citrulline, may serve as a highly sensitive biomarker for disrupted barrier function in humans, thereby facilitating the detection of IIR injury [42], [43], [44]. These results support the claim that IIR injury occurs during hemodialysis, causing intestinal barrier damage.
Fig. 1.
Characteristics of enteric fungi in IIR model. (A) The morphology and blood flow of the superior mesenteric artery (SMA) in IIR injury patients, before and 2 h after the start of hemodialysis by color Doppler ultrasound (n = 10). (B) Serum levels of intestinal barrier-related biomarkers (I-FABP, ZO-1, Citrulline) of IIR injury patients were detected by ELISA (n = 20). Wilcoxon matched-pairs signed-rank test was used to calculate the P-value. (C) Alpha diversity indices of genus level in patients. (D) Principal coordinate analysis (PCoA) using Bray–Curtis metric distance algorithms and unweighted UniFrac distance algorithms of beta diversity in patients. (E) The community of enteric fungi on the order and genus level in patients were analyzed with Wilcoxon signed-rank test to calculate the P-value. (F) LEfSe analysis was performed to explore the different species characteristics from phylum to genus levels in patients (LDA > 4). (G) CCA analysis at the genus level. Positive correlation between two indicators < 90°; Negative correlation between two indicators > 90°; No correlation between two indicators = 90°. (H) Correlation linear graph. The relative abundance of Candida and Saccharomyces was positively correlated with citrulline levels and negatively correlated with I-FABP and ZO-1 levels. (I) Alpha diversity indices at the genus level in mice model. (J) Beta diversity indices at the genus level in mice model (based on PCoA and NMDS). (K) LEfSe analysis was performed to explore the different species characteristics from phylum to genus levels between both groups (LDA > 2). (L) Random forests analysis at the genus level. (M) Correlation Heatmap graph of fungi and intestinal barrier-related biomarkers and tissue cytokines expression. Fig. 1 (A, C–H) (n = 10), Fig. 1 (B) (n = 20), Fig. 1 (I-M) (n = 5) *P < 0.05; **p < 0.01; ***p < 0.001.
Considering the potential important role of enteric fungi in maintaining intestinal barrier homeostasis, we collected fecal samples from IIR injury patients at two time points: before IIR injury (Before) and after IIR injury (After). Then, paired fecal samples were selected to explore the fungal diversity, richness, and composition using ITS sequencing. The core and pan genome analysis were utilized to describe the variation of total and core populations with their sample sizes and to assess the adequacy of the sequenced samples (Figure S2). Different alpha diversity indices including Chao, Shannon and Shannoneven displayed similar tendencies, indicating that the diversities within the microbiome samples varied significantly (Fig. 1C). Similarly, the diversities among microbiome samples (β diversity) showed that two clusters were relatively separated, suggesting a difference in fungal structures between two groups (Fig. 1D). The enteric fungi composition between both groups were showed (Figure S3) and compared by community CIRCOS at the genus level (Figure S4). In addition, both groups were tested at order and genus levels, and Saccharomycetales and Candida were found to be significantly decreased (Fig. 1E). LEfSe analysis was performed to explore the species characteristics from phylum to genus levels (LDA > 4), and the abundance of Saccharomyces was also found to be significantly decreased (Fig. 1F). Further, we assessed the correlation between abundance of fungi and intestinal barrier-related biomarkers of IIR injury. The relative abundance of Candida and Saccharomyces was positively correlated with citrulline levels and negatively correlated with I-FABP and ZO-1 levels (Fig. 1G,H).
In order to ascertain the direct correlation between enteric fungi alteration and IIR injury, while eliminating the influence of other factors during hemodialysis, we established an IIR mouse model to collect pre- and post-injury feces and perform ITS sequencing. Alpha diversity analysis at the genus level showed no difference between the two groups (Fig. 1I), while beta diversity analysis revealed separate clusters for IIR mice contrasted with the pre-injury mice (Fig. 1J). LEfSe analysis showed that Saccharomyces decreased after IIR injury in the mice (LDA > 2) (Fig. 1K). The results of the random forests analysis further confirmed that the fungal composition of IIR mice experienced significant alterations, characterized by a notable decrease in Saccharomyces and a subsequent increase in Filobasidium (Fig. 1L). Further, we investigated the correlation of fungi with intestinal barrier-related biomarkers and tissue cytokines expression. Following IIR injury, there was a significant increase in the levels of I-FABP, KC, IL18, IL6, TNF-α, GCSF, and IL1β (Figure S5), which exhibited a negative correlation with the abundance of Saccharomyces (Fig. 1M).
The colonization of single-fungal strains alleviated IIR injury
The above results revealed a significant decrease in Saccharomyces and Candida (genus taxonomic level) during the IIR process, which was linked to IIR injury. Consequently, we aim to validate the correlation between these fungi and IIR injury through fungal colonization experiments. Notably, Saccharomyces cerevisiae (S. cerevisiae) and Candida tropicalis (C. tropicalis), which are dominant colonizers of the human and mouse gut, belong to the Saccharomyces and Candida genus, respectively [45], [46]. Moreover, S. cerevisiae and C. tropicalis specifically play an irreplaceable role in the maintenance of intestinal homeostasis [47], [48]. In our study, S. cerevisiae and C. tropicalis are prevalent in mouse intestine, which was colonized by oral gavage before the IIR injury model was established (Fig. 2A). After 2 weeks, C. tropicalis and S. cerevisiae increased and did not affect the abundance of other fungi, proving that the colonization was successful (Fig. 2B). Further, The FITC-dextran transepithelial permeability assay was performed before sacrificing the mice. We found that FITC-dextran concentration was significantly lower in the serum of the colonization group (Fig. 2C). We further confirmed by serum I-FABP examination, intestinal tissue H&E staining, and histological scoring that intestinal barrier disruption alleviated, which was in agreement with the functional changes (Fig. 2D, E). Expression of tight junction proteins ZO-1 and occludin was estimated by western blotting (WB) (Fig. 2F). The N-terminus (P30) fragment of GSDMD protein is the pyroptosis effector molecule, and we demonstrated that the P30 GSDMD cleavage increased in the IIR-injured intestine (Fig. 2F). Moreover, There was a consistent downward trend in multiple cytokines, especially those associated with cell pyroptosis (IL1β, IL18) (Fig. 2G). The above results confirmed that increasing S. cerevisiae and C. tropicalis burden can alleviate IIR injury. In addition, we found that the protective effects of the fungi were only fully achieved more than two weeks after colonization (Figure S6).
Fig. 2.
The colonization of single fungal strains alleviated IIR injury. (A) The experimental design of the present study. Briefly, 108 CFU C. tropicalis or S. cerevisiae was colonized in the intestine by oral gavage before the IIR injury model was established. (B)C. tropicalis and S. cerevisiae abundance in mouse stool after single fungal strains colonization. (C) Serum FITC-dextran concentration in C. tropicalis and S. cerevisiae colonization groups. (D) Serum I-FABP concentration in different groups. (E) H&E staining and the Chiu’s score in different groups. Scale bar = 100 μm. (F) The expression of the ZO-1, occludin, and GSDMD proteins in different groups. (G) Cytokine levels of intestine tissue supernatant in different groups. (H) Fecal fungi abundance in germ-free mice treated with S. cerevisiae and C. tropicalis mixtures were identified by fungus plate culture and fence quantitative abundance test. (I) Serum FITC-dextran concentration in GF mice colonized with S. cerevisiae and C. tropicalis mixtures. (J) Serum I-FABP concentration in GF mice colonized with S. cerevisiae and C. trop mixtures. (K) H&E staining and the Chiu’s score in GF mice colonized with S. cerevisiae and C. tropicalis mixtures. Scale bar = 100 μm. The values were presented as the mean ± SD in Fig. 2(B–G) (n = 6), and Fig. 2 (H–K) (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group.
To further demonstrate that enteric fungi can affect the host directly rather than modulate other strains within the body, germ-free (GF) mice treated as previously mentioned were used to establish the IIR model. Fecal fungi abundance in germ-free mice treated with S. cerevisiae and C. tropicalis mixtures was identified by fungus plate culture and fence quantitative abundance test (Fig. 2H). The results confirmed the absence of fungi in the gut of GF mice, and that the colonization was successful. Importantly, the IEB function and morphology were examined according to the above methods, and we found that fungi colonization in GF mice also alleviated IIR injury (Fig. 2I–K, S7). This proves that S. cerevisiae and C. tropicalis mitigate IIR injury by acting directly on the host without interference from other bacteria or other microorganisms in the gut. Therefore, we can conclude that increasing S. cerevisiae and C. tropicalis can alleviate IIR injury.
Prophylactic antifungal therapy aggravates IIR injury
To investigate whether the reduction of S. cerevisiae and C. tropicalis could worsen IIR injury. Mice were pretreated with the antifungal drug fluconazole at 0.5 mg/mL for 2 weeks in their drinking water and the IIR injury procedure was subsequently performed (Fig. 3A). Quantitative analysis of fungal abundance to evaluate fungal clearance (Fig. 3B) and ultra-high performance liquid chromatography to detect serum fluconazole concentration in vivo were performed (Fig. 3C, S8). In addition, serum alanine aminotransferase (ALT) test was used to determine the toxic effects of fluconazole, which indicated that fluconazole was not medically toxic to worsen the damage (Fig. 3D). Intestinal function and morphology were measured using the previously described method, and the damage to the IEB was independently worsened by fungal clearance (Fig. 3E–G). Furthermore, GSDMD cleavage and cytokines expression of intestinal tissue were rised at varying degrees (Fig. 3H, I). These results suggest that the clearance of S. cerevisiae and C. tropicalis aggravates GSDMD cleavage and promotes cytokine release.
Fig. 3.
Prophylactic antifungal therapy aggravated IIR injury in mice. (A) The experimental design. Mice were pretreated with fluconazole for 2 weeks, and the IIR model procedure was subsequently performed (ischemia 30 min, reperfusion 6 h). (B) Quantitative fungal abundance (ITS, C. tropicalis and S. cerevisiae) was determined by qPCR of fungal rDNA and expressed as copy numbers (copies/g). (C) Serum fluconazole concentration in mice detected by ultra-high performance liquid chromatography. (D) Serum alanine aminotransferase (ALT) level. (E) Intestinal permeability test determined by FITC-dextran transepithelial permeability assay. (F) Serum I-FABP concentration detected by ELISA. (G) The histopathological damage of IIR injury was estimated by H&E staining and the Chiu’s score. Scale bar = 100 μm. (H) Cytokine levels of intestine tissue supernatant were determined using the LEGENDplex™ assays. (I) The ZO-1, occludin, and GSDMD protein expressions were analyzed by WB. (J) Serum FITC-dextran concentration in GF mice. (K) Serum I-FABP concentration in GF mice. (L) H&E staining and the Chiu’s score in GF mice. Scale bar = 100 μm. The values were presented as the mean ± SD in Fig. 3(B–I) (n = 6) and Fig. 3 (J–L) (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group.
To verify that fungal clearance directly affects the host, not fluconazole itself, we treated GF mice with fluconazole using the method mentioned above. By examining gut function and morphology, we found that fluconazole itself did not worsen IIR injury. Serum levels of FITC-dextran and I-FABP were not significantly altered after fluconazole treatment (Fig. 3J, K). H&E staining showed no significant difference between both groups (Fig. 3L). The proteins level of ZO-1, occludin, and GSDMD P30 also showed no difference (Figure S7). These results confirmed that the fluconazole pretreatment in GF mice due to the absence of S. cerevisiae and C. tropicalis did not aggravate IIR injury by fungal clearance. Therefore, we suggest that reduced the burden of S. cerevisiae and C. tropicalis may aggravate IIR injury, and that GSDMD-mediated pyroptosis is involved in this injury mechanism.
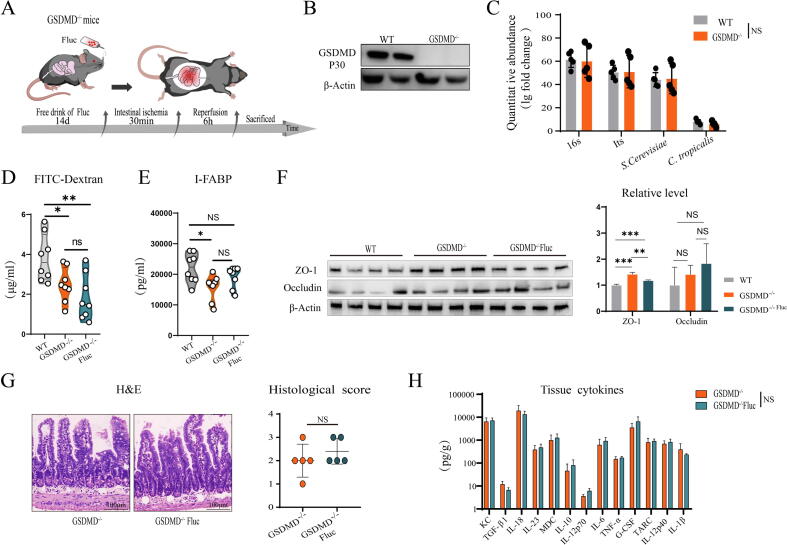
Enteric fungi lose protective effect in GSDMD-/- mice
We previously found that enteric fungi protected against sepsis primarily through inhibition of GSDMD cleavage [28], and since enteric fungal clearance exacerbated GSDMD cleavage and the S. cerevisiae and C. tropicalis colonization could alleviate GSDMD cleavage in the IIR injury model, we sought to investigate whether enteric fungi protected mice from IIR injury mainly by inhibiting GSDMD cleavage. Thus, GSDMD-/- mice were used to test our hypothesis by preprocessing with fluconazole to clear enteric fungi, as mentioned earlier (Fig. 4A), and WB confirmed that GSDMD disappeared in the GSDMD-/- mice (Fig. 4B). To verify whether the fungal diversity in GSDMD-/- mice feces was consistent with that of the WT group, quantitative analysis of fungal abundance was performed, and no differences in fungal burden were observed in the intestine of GSDMD-/- mice contrasted with the caged WT controls (Fig. 4C). Furthermore, to confirm whether fungal clearance in GSDMD-/- mice can alter IEB morphology and function, we determined the serum concentration of FITC-dextran and I-FABP, intestinal mucosal injury score, and epithelial tight junction protein expression, and the results confirmed fungal burden reduction in GSDMD-/- mice did not worsen barrier damage (Fig. 4D–G). Moreover, the cytokine levels of intestinal tissue supernatant also showed no difference (Fig. 4H). In addition, there was a significant decrease in IIR injury in GSDMD-/- mice when compared to caged WT controls. Therefore, we conclude that fungal burden reduction of S. cerevisiae and C. tropicalis in GSDMD-/- mice cannot aggravates IIR injury, and that these fungi alleviate IIR injury by inhibiting GSDMD cleavage.
Fig. 4.
Enteric fungal clearance did not exacerbate GSDMD cleavage in GSDMD-/- mice. (A) The experimental design of the present study. GSDMD -/- mice were pretreated with fluconazole for 2 weeks, and the IIR model procedure was subsequently performed (ischemia 30 min, reperfusion 6 h). (B) GSDMD protein expression in GSDMD-/- mice. (C) Quantitative microbiological abundance (16S, ITS, C. tropicalis, and S. cerevisiae) of GSDMD-/- mice compared with the WT mice. (D) Serum FITC-dextran concentration in GSDMD-/- mice. (E) Serum I-FABP concentration in GSDMD-/- mice. (F) The expressions of ZO-1 and occludin protein in GSDMD-/- mice. (G) H&E staining and the Chiu’s score in GSDMD-/- mice. Scale bar = 100 μm. (H) The levels of cytokines in the supernatant of GSDMD-/- mice intestine tissue. The values were presented as the mean ± SD in Fig. 4 (B) (n = 2) and Fig. 4 (C–H) (n = 5–8). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group. WT, wild-type, GSDMD-/-, GSDMD-knockout mice.
Mannan can replicate the protective effects of S.cerevisiae and C. tropicalis on IIR injury
Given the observed remission effect of both S. cerevisiae and C. tropicalis on IIR injury, we investigated whether these fungi share a common active substance. Notably, S. cerevisiae and C. tropicalis are two types of yeast that share a common structural component, namely mannan, which is a major constituent of the yeast cell wall and periplasmic space [49], [50]. Moreover, Numerous studies have shown that mannan alone provides immune protection [22], [49]. We previously found that mannan could inhibit GSDMD cleavage [28]. To confirm whether mannan is the key component of S. cerevisiae and C. tropicalis that protects against IIR injury, C57/B6 mice were pretreated with mannan (0.1 mg/mL) for 2 weeks before the IIR injury model, through their drinking water (Fig. 5A). Interestingly, by comparing intestinal morphology and function, the IEB structure and function was preserved, consistent with the role of single-fungal strain colonization (Fig. 5B–F), as well as the level of GSDMD cleavage and cytokines expression (Fig. 5D, F). This result confirms that the role of S. cerevisiae and C. tropicalis in IIR injury may be linked to mannan. Meanwhile, GF mice were also pretreated with mannan, and the evaluation of IIR injury was performed. The result showed the same protective effect of GF mice in IIR injury (Fig. 5G–I, S7).
Fig. 5.
Mannan replicated the protective effects of enteric fungi on IIR injury. (A) Mice were pretreated with mannan for 2 weeks, and the IIR model procedure was subsequently performed (ischemia 30 min, reperfusion 6 h). (B) Serum FITC-dextran concentration in mice pretreated with mannan. (C) Serum I-FABP concentration. (D) Cytokine levels of intestine tissue supernatant were determined LEGENDplex™ assays. (E) H&E staining and the Chiu’s score. Scale bar = 100 μm. (F) The ZO-1, occludin, and GSDMD protein expressions were analyzed by WB. (G) Serum FITC-dextran concentration in GF mice pretreated with mannan. (H) Serum I-FABP concentration in GF mice pretreated with mannan. (I) H&E staining and the Chiu’s score in GF mice pretreated with mannan. The values were presented as the mean ± SD in Fig. 5(B–I) (n = 5–6). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group.
The protective role of mannan in IIR injury depends on macrophage involvement
To further investigate the interaction between mannan and the host, we covalently attached a Cyanine3 (Cy3) fluorescent marker to mannan (Fig. 6A) and found that mannan was co-localized only with macrophages marked with F4/80 in mice after oral gavage (Fig. 6B). This result shows that mannan is recognized by macrophages as it enters the gut and acts accordingly. In vitro, Cy3-mannan was added to RAW264.7 cells, which showed that mannan entered RAW264.7 cells after 6 h of incubation (Fig. 6B). Flow cytometry results showed mannan could reduce the number of macrophages (F4/80+CD45.2+) in the gut. To further prove that mannan’s protective effects are related to macrophages, clodronate liposomes were administered intraperitoneally 3 days before establishing the IIR model, to deplete macrophages from the gut (Fig. 6C). The results indicated that clodronate liposomes reduced the number of macrophages in the gut. Comparing the morphology and function of the gut, we found that following macrophage clearance, the protective effect of mannan diminished and showed no statistical difference (Fig. 6D-G). Meanwhile, the level of GSDMD cleavage and cytokines expression caused by IIR injury was also alleviative, and there was no statistic difference (Fig. 6G-H). These results demonstrated that mannan plays a protective role via macrophages.
Fig. 6.
Mannan’s protective role in IIR injury depends on macrophage involvement. (A) Molecular structure diagram of mannan attached to the cy3 fluorescent marker. (B) Co-localization of mannan and macrophages in the ileum, detected by immunofluorescence staining. Scale bar = 50 μm and 10 μm, respectively. In vitro, Cy3-mannan was added to RAW264.7 cells, which showed that mannan entered RAW264.7 cells after 6 h of incubation. (C) The number of ileum F4/80+CD45.2+ macrophages in different groups were analyzed by flow cytometry in mice. (D) Serum FITC-dextran concentration in mice. (E) Serum I-FABP concentration. (F) H&E staining and the Chiu’s score. Scale bar = 100 μm. (G) The expressions of ZO-1, occludin, and GSDMD protein were analyzed by western blotting. (H) mRNA expression of GSDMD, IL1β, IL18, and SAA1 in ileum tissue after establishing the IIR mouse model. The values were presented as the mean ± SD in Fig. 6 (B-H) (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group.
Single-fungal strains and mannan both alleviate GSDMD cleavage induced by reducing SAA1 expression of macrophages
To further explore the key pathways through which S. cerevisiae and C. tropicalis can alleviate IIR injury via macrophages, we performed RNA-Seq with intestinal tissue collected from mice treated with single-fungal strains or mannan before the IIR injury model was established. We found a significant decrease in serum amyloid a1 (SAA1) levels in both RNA-Seq results and independently validated the mRNA expression using qPCR (Fig. 7A, B). SAA1 is an acute-phase protein that is well proved to be closely associated with acute inflammation and aggravates the development of several inflammatory diseases [51], [52]. To detect the distribution of SAA1 protein expression in the intestine, immunohistochemistry assay was performed, which showed that SAA1 was mainly expressed in intestinal endothelial cells, while there was significantly less SAA1 expression in the pretreated mice (Fig. 7C). Additionally, the expression of protein SAA1 decreased in intestinal macrophages marked with F4/80, which was detected by immunofluorescence staining (Fig. 7D). To confirm whether mannan could decrease SAA1 in vitro, RAW264.7 cells were treated with mannan (1 mg/mL) for 6 h, and then move to a medium containing 1 µg/mL lipopolysaccharides (LPS) and incubate 2 h, which was used to increase SAA1 basal expression. We found SAA1 expression decrease in both mRNA and protein levels (Fig. 7E, F). This result is consistent with our findings that macrophages participate in the protective role of mannan, demonstrating that mannan play a protective role by reducing the secretion of macrophage SAA1.
Fig. 7.
Single fungal strains and mannan both alleviated GSDMD cleavage induced by reducing SAA1 expression. (A) Volcano plot of differentially expressed genes in intestinal tissue treated with C. trop detected by RNA-Seq. The x-axis of the volcano plot shows the log2 transformed gene expression fold change. The y-axis shows the negative log10 transformed adjusted q-values of the differential expression. The upregulated genes are represented by red dots. The downregulated genes are represented by blue dots. SAA1 mRNA expression was detected by qPCR. (B) Volcano plot of differentially expressed genes in the mannan group detected by RNA-Seq. The upregulated genes are represented by red dots. The downregulated genes are represented by green dots. SAA1 mRNA expression in the mannan group was detected by qPCR. (C) SAA1 protein expression in different groups was detected by immunohistochemistry assay. Scale bar = 20 μm. (D) Co-localization of SAA1 protein and macrophages marked with F4/80. Scale bar = 50 μm and 20 μm, respectively. (E) SAA1 mRNA expression in RAW264.7 cells was detected by qPCR. RAW264.7 cells were pretreated with mannan(1 mg/mL) for 6 h, and then move to the medium containing LPS(1 µg/mL) to incubate 2 h. (F) SAA1 protein expression in RAW264.7 macrophage was detected by WB. (G) GSDMD protein expression in MODE-K cell treated with active SAA1 protein in the OGD/R model. (H) mRNA expression of Occludin, ZO1, GSDMD, NLRP3, IL1β and IL18 in MODE-K cell treatment with SAA1 detected by qPCR in the OGD/R model. The values were presented as the mean ± SD in Fig. 7 (A-H) (n = 3–6). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Our previous work has fully proven that mannan can decrease GSDMD-mediated pyroptosis during IIR injury. Accumulating evidence demonstrates that the SAA1 is closely involved in GSDMD-mediated pyroptosis [53], [54], [55]. In addition, we found GSDMD expression mainly in IECs during IIR injury by immunohistochemistry assay (Figure S9). Therefore, we used mouse intestinal epithelial cells (MODE-K) treated with SAA1 protein (600 ng/ml) in an oxygen-glucose deprivation/reoxygenation (OGD/R) model, which further demonstrated that SAA1 directly aggravates GSDMD cleavage in vitro (Fig. 7G). In addition, tight junction proteins also showed significant damage due to the SAA1 protein stimulation (Fig. 7G, H). Moreover, we measured the mRNA expression of GSDMD, NLRP3, IL1β, IL18, Caspase-1 and Caspase-11 after treatment with SAA1 in MODE-K cells using qPCR (Fig. 7H, S10). These results suggest that the aggravation of GSDMD cleavage may be due to the upregulation of GSDMD and NLRP3 expression. In summary, enteric fungi alleviated IIR injury damage by reducing the expression of SAA1 in macrophages, thereby alleviating GSDMD cleavage in IECs.
Discussion
This study revealed that enteric fungi disturbance occurred in IIR patients, which exacerbated IIR injury in a mouse model. Fungal diversity analysis showed the potential importance of Candida and Saccharomyces when facing IIR injury, indicated by the significant decrease of their relative abundance. Furthermore, aggravation of IIR injury with commensal fungi clearance was efficiently relieved by mono-colonization with either C. tropicalis and S. cerevisiae in a mouse model, as demonstrated by inhibition of GSDMD cleavage, increased tight junction barrier function, decreased mucosal damage, and epithelial permeability, as well as lower release of inflammatory cytokines. Interestingly, mannan, one of the major components of the C. tropicalis and S. cerevisiae, displayed similar protective effects against IIR injury. Importantly, the present study revealed that the acute phase protein SAA1 is closely involved in the mechanism of intestinal epithelial GSDMD cleavage, which was further confirmed in in vitro experiments. Our study demonstrated that C. tropicalis and S. cerevisiae could protect against IIR injury mainly by inhibiting GSDMD cleavage through the SAA1 pathway.
Despite the well-known predisposing factors for acute IIR injury, such as mesenteric thromboembolism and intestine transplantation, hemodialysis is often easily neglected as a potential risk factor for IIR injury, especially among older adults with CKD [11]. The long-term disease course, slow development, complicated and weak conditions, and all the above-mentioned factors of CKD suggest the great negative implications of IIR injury induced by repeated hemodialysis. Some studies demonstrated that risk factors for IIR injury during hemodialysis are generally considered in patients with underlying conditions such as abnormal blood pressure, vascular diseases, or peptic ulcers [10], [11], [56]. Unlike the unpredictability of surgical IIR injury, IIR in hemodialysis treatment is considered as a “planned,” predictable, and temporary ischemia–reperfusion phenomenon caused by blood pooling in the hemodialysis machines [8], [57]. Therefore, it is worth exploring the underlying mechanisms, based on which novel preventive strategies can be employed against hemodialysis-associated injury in CKD patients.
Numerous studies have highlighted the crucial role of enteric microbes, including fungi, in maintaining intestinal homeostasis and protecting the intestinal barrier function [22], [58]. However, fewer studies have investigated the role of gut microbes, particularly enteric fungi, in IIR injury, especially under hemodialysis conditions. Although fungi are often studied as infectious diseases, they are common inhabitants of the gastrointestinal tract of healthy mammals [59]. In particular, numerous studies have shown that fungal disorders can contribute to the occurrence of clinical diseases such as colitis, alcoholic liver disease, and even allergic respiratory diseases [59], [60], [61]. Indeed, a great change in fungal composition was observed after IIR injury in the present study, supporting our hypothesis that enteric fungal disturbance is a contributing factor for IIR injury progression. Reducing the fungal burden in a mouse IIR injury model exacerbated IIR injury, but fungal colonization can obviously reverse mucosal damage, further confirming the indispensable role of enteric fungi in intestinal protective mechanisms under IIR injury threat. In particular, the rapid change in enteric fungi in hemodialysis patients and the mouse IIR model shows that enteric fungi are very sensitive and vulnerable to acute changes in intestinal blood supply, which is further confirmed with the subsequent IIR investigations on mice.
GF mice, which lack enteric microorganisms, represent a definitive assay system for studying the influence of microbiota members on host gut colonization [62], [63]. In order to eliminate the influence of gut bacteria and other microorganisms, GF mice were subjected to single-fungal strain colonization. The findings indicate that fungal colonization in GF mice ameliorated IIR injury, thereby demonstrating the direct impact of fungi on the host without any interference from other gut bacteria or fungi. Nevertheless, further investigation is required to establish the relationship between changes in gut fungi and gut bacteria in the context of IIR injury. Currently, numerous studies are being conducted on the interplay between intestinal fungi and bacteria, with the aim of investigating their relationship. We are endeavoring to investigate the correlation between them.
As demonstrated in the present study, both Candida and Saccharomyces display the obvious sensitivity under IIR stimulation. C. tropicalis and S. cerevisiae, which belong to the genus Candida and Saccharomyces, respectively, are the most common and abundant fungi in the gut [64]. Consistently, recent studies also demonstrated the pivotal role of these two fungi in maintaining intestinal homeostasis. S. cerevisiae was decreased in the feces and mucosa of inflammatory bowel disease (IBD) patients [22], [65]. S. cerevisiae activated intestinal macrophage phagocytosis and inhibited colony disorders in inflammatory conditions [66]. C. albicans or S. cerevisiae were identified by CX3CR1+ macrophages to promote intestinal homeostasis [48] and protect against virus-induced lung inflammation in mice [47]. We also found early that colonization by C. tropicalis strains protected mice from sepsis by inhibiting GSDMD cleavage [28]. Therefore, it seems highly possible that both C. albicans and S. cerevisiae are worthy of further exploration regarding the underlying intestinal protective mechanisms against various pathological stimuli.
Numerous studies have shown that the cell wall component of enteric fungi play a crucial but complex role in host immunity, infection control, inflammatory disease, and even tumor progression [22]. Mannan is the major component of the cell wall of yeast. In S. cerevisiae, mannan comprises over 35% of the cell wall component and is associated with proteins present in the cell wall, forming mannoprotein complexes that play an important role in maintaining the stability of the fungus’ structure and thus ensuring its gut content [67]. Mannan from C. albicans can clear circulating atherosclerotic lipoproteins (LDL) by stimulating macrophages via mannose receptors to exhibit immunomodulatory activity [68]. Mannan from S. cerevisiae can activate systemic protection against DSS-induced intestinal inflammation [47]. Our previous studies have also indicated that that mannan supplementation significantly improve the intestinal damage caused by sepsis [28]. Therefore, the role of mannan was investigated in our study to clarify the protective immune mechanism of fungi.
Similar to fungal colonization, pretreatment with mannan significantly relieved the mucosal damage and barrier disruption induced by IIR injury, further confirming the important protective role of mannan. However, it remains unclear how the fungi, especially S. cerevisiae, through mannan are involved in the defense mechanism against pathological stimuli such as IIR injury and sepsis. Our results confirmed the inhibitory effect of mannan on GSDMD cleavage and raised the possibility about the novel effect of mannan against GDSMD-mediated pyroptosis. To verify which type of cells fungi interact with during IIR injury, mannan marked with Cy3 fluorescent marker was administered by oral gavage to the mice. Interestingly, mannan was traced in co-localized macrophages, which was further confirmed via in vitro macrophage experiments, suggesting the important role of macrophages in the modulation of GSDMD cleavage of enteric fungi. Previous studies have revealed the close relationship between mannan and macrophages. Mannan exerts hypolipidemic effects by stimulating macrophages via mannose receptors [69]. Intestinal CX3CR1+ resident macrophages expressing c-type lectin receptors, such as dectin-1, dectin-2, and mincle, recognize and ingest intestinal yeast and filamentous fungi, and then activate Th17 immunity [22], [48], [70]. These studies emphasize that macrophages play a crucial role in fungi recognition during IIR injury, similar to the situation of intestinal bacteria. Furthermore, mannan reduces gastrointestinal infection in susceptible animals by blocking the mechanisms by which pathogenic Gram-negative bacteria attach to and invade the gut [67]. Moreover, mannan has a good scavenging effect on superoxide anions and hydroxyl radicals, indicating that it has potential antioxidant activity [71]. IIR with oxidative stress injury may be significantly regulated. More researches are needed to further explore the classification of macrophages and the underlying molecular mechanisms of the interaction between mannan and macrophages as well as other potential mechanisms.
We previously reported that enteric fungi protected against sepsis by depressing GSDMD cleavage [28]. Since enteric fungal clearance exacerbated GSDMD cleavage in the IIR injury model in this study, we investigated whether enteric fungi also act similarly by inhibiting GSDMD cleavage. According to the results of the fungal clearance and single strain colonization, it is highly possible that GSDMD-mediated pyroptosis is one of the key events accompanying enteric fungi disturbance under acute IIR injury. Consistent with other reports, the present study supported the finding that GSDMD protein is mainly expressed in IECs and closely involved in the progression of IIR injury [31], [35]. GSDMD-mediated epithelial cell death has also been shown to significantly regulate inflammatory intestinal disease [72], [73]. However, the following experiments should also be performed in GSDMDf/f Villin-Cre mice to further clarify the underlying mechanisms of GSDMD cleavage in intestinal epithelial cells.
A few studies have shown that SAA1 is able to activate several important molecules in the GSDMD-mediated pyroptosis pathway. SAA1 significantly upregulates NLRP3 expression in macrophages through the P2X7R receptor, mediates the activation of inflammasomes and caspase-1, and then promotes IL-1β secretion [53], [54], [55]. Moreover, SAA1 has been shown to be associated with gut microbial ecology and inflammation in recent years [74], [75]. SAA1 regulates bacterial growth and the pro-inflammatory response in vitro in colitis [76]. Interestingly, SAA1 binds to the membrane of C. albicans during fungal infection and induces C. albicans death [77], indicating the anti-fungal effect of SAA1. Our studies are the first to report the potential association between SAA1 and enteric fungi under IIR stimulation. Single fungal strains or mannan can block GSDMD cleavage accompanied with the significant decrease of SAA1 protein expression in macrophages. In other studies, macrophages in inflammatory tissue induced SAA1/2 expression in multiple chronic inflammatory diseases [51], [78]. However, it has also been reported that SAA1 inhibits LPS-induced inflammation and tissue damage by promoting LPS clearance [79]. This verifies that SAA1 could play a complicated role in the various inflammatory backgrounds.
The present study has several limitations. While gut fungi may cause different degrees of IIR damage in patients depending on their individual level, gut microbes are a complex system, and synergies between different strains can influence the gut. Therefore, there is no explicit evidence that a particular fungal strain is the most salutary. In this research, we only explored the effects of C. albicans and S. cerevisiae on IIR injury, not other strains. Moreover, decreased expression of SAA1 induced by transplanted fungi has not been demonstrated to have a direct fungal effect in vivo. SAA1-/- mice are needed to confirm that it relieves GSDMD cleavage and reduces IIR injury. Moreover, we do not have a clear understanding of the cellular receptors through which fungi or mannan interact with macrophages, which could lead to further research. In addition, our initial patient-specific study was performed in a limited participants numbers and needs to be acknowledged in a larger cohort.
Conclusions
Our results suggest that enteric fungi disturbance occurs after IIR injury. S. cerevisiae and C. tropicalis have the potential to mitigate IIR injury via mannan by inhibiting the expression of SAA1 in macrophages and reducing GSDMD cleavage in IECs. This study reveals a novel mechanism of IIR injury in hemodialysis and suggests that maintaining intestinal fungal homeostasis could be a new therapy to prevent IIR injury.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to thank all participants who were willing to participate in this study. The authors would also like to thank all the medical and experimental staff who provided technical support and assistance. This study was supported by National Natural Science Foundation of China (to WX) Grant No. 82270585. Army Medical University project (CX2019JS212 to WX/ 2021XJS25 to WX).
CRediT authorship contribution statement
Y. C., B. H., X.G., G.D. contributed equally to this work; Experimental Design and Manuscript Writing: Y. C., W. X.; Study supervision: W. X., W. C., J. W.; Clinical investigation and sample collection: X.G., Q.Z.; Experiment organization and implementation: Y. C., B. H., G.D., H. X.; Experimental proponents: B. S., X.T; Germ free Mice Maintenance: X. J., Data analysis: S. C., Q. T. All authors have read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.09.008.
Contributor Information
Jian Wang, Email: wj_xqhospital@163.com.
Wei Chen, Email: chongqingchenwei@126.com.
Weidong Xiao, Email: xiaoweidong@tmmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References:
- 1.Moon C.M., Zheng J.H., Min J.J., Jeong Y.Y., Heo S.H., Shin S.S. In vivo bioluminescence imaging for targeting acute hypoxic/ischemic small intestine with engineered salmonella typhimurium. Molecular therapy Methods & clinical development. 2020;18:484–492. doi: 10.1016/j.omtm.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoots I.G., Koffeman G.I., Legemate D.A., Levi M., van Gulik T.M. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91(1):17–27. doi: 10.1002/bjs.4459. [DOI] [PubMed] [Google Scholar]
- 3.Chassin C., Hempel C., Stockinger S., Dupont A., Kübler J.F., Wedemeyer J., et al. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med. 2012;4(12):1308–1319. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L., Yao J., Li Z., Zu G., Feng D., Li Y., et al. miR-381-3p knockdown improves intestinal epithelial proliferation and barrier function after intestinal ischemia/reperfusion injury by targeting nurr1. Cell Death Dis. 2018;9(3):411. doi: 10.1038/s41419-018-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guneli E., Cavdar Z., Islekel H., Sarioglu S., Erbayraktar S., Kiray M., et al. Erythropoietin protects the intestine against ischemia/ reperfusion injury in rats. Mol Med. 2007;13(9–10):509–517. doi: 10.2119/2007-00032.Guneli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenaerts K., Ceulemans L.J., Hundscheid I.H., Grootjans J., Dejong C.H., Olde Damink S.W. New insights in intestinal ischemia-reperfusion injury: implications for intestinal transplantation. Curr Opin Organ Transplant. 2013;18(3):298–303. doi: 10.1097/MOT.0b013e32835ef1eb. [DOI] [PubMed] [Google Scholar]
- 7.Oltean M. Intestinal transplantation: an overview of the recent experimental studies. Curr Opin Organ Transplant. 2021;26(2):240–244. doi: 10.1097/MOT.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 8.Ritz E. Intestinal-renal syndrome: mirage or reality? Blood Purif. 2011;31(1–3):70–76. doi: 10.1159/000321848. [DOI] [PubMed] [Google Scholar]
- 9.Giménez Francés C., Tamayo Rodríguez M.E., Albarracín M.-B. Non-oclusive mesenteric ischemia as a complication of dialysis. Rev Esp Enferm Dig. 2021;113(10):731–732. doi: 10.17235/reed.2021.7897/2021. [DOI] [PubMed] [Google Scholar]
- 10.Rossi U.G., Petrocelli F., Seitun S., Ferro C. Nonocclusive mesenteric ischemia in a dialysis patient with extensive vascular calcification. Am J Kidney Dis. 2012;60(5):843–846. doi: 10.1053/j.ajkd.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Li S.Y., Chen Y.T., Chen T.J., Tsai L.W., Yang W.C., Chen T.W. Mesenteric ischemia in patients with end-stage renal disease: a nationwide longitudinal study. Am J Nephrol. 2012;35(6):491–497. doi: 10.1159/000338451. [DOI] [PubMed] [Google Scholar]
- 12.Taylor J., Mandzhieva B., Shobar R. Diagnosis of acute mesenteric ischemia in a patient with end-stage renal disease with normal serum lactate. Cureus. 2020;12(1):e6708. doi: 10.7759/cureus.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi K., Wang F., Jiang H., Liu H., Wei M., Wang Z., et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci. 2014;59(9):2109–2117. doi: 10.1007/s10620-014-3202-7. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim M, Behairy M, El-Ashry M, Mostafa AE. Cardiovascular risk of circulating endotoxin level in prevalent hemodialysis patients. The Egyptian heart journal : (EHJ) : official bulletin of the Egyptian Society of Cardiology. 2018;70(1):27–33. [DOI] [PMC free article] [PubMed]
- 15.Wolfgram D.F. Intradialytic cerebral hypoperfusion as mechanism for cognitive impairment in patients on hemodialysis. J Am Soc Nephrol. 2019;30(11):2052–2058. doi: 10.1681/ASN.2019050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krack A., Sharma R., Figulla H.R., Anker S.D. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26(22):2368–2374. doi: 10.1093/eurheartj/ehi389. [DOI] [PubMed] [Google Scholar]
- 17.Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 18.Lewis J.D., Abreu M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology. 2017;152(2):398–414 e6. doi: 10.1053/j.gastro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Chen J., Wang Y., Shi Y., Liu Y., Wu C., Luo Y. Association of gut microbiota with intestinal ischemia/reperfusion injury. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.962782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadatani Y., Watanabe T., Shimada S., Otani K., Tanigawa T., Fujiwara Y. Microbiome and intestinal ischemia/reperfusion injury. J Clin Biochem Nutr. 2018;63(1):26–32. doi: 10.3164/jcbn.17-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belkaid Y., Harrison O.J. Homeostatic immunity and the microbiota. Immunity. 2017;46(4):562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X.V., Leonardi I., Iliev I.D. Gut mycobiota in immunity and inflammatory disease. Immunity. 2019;50(6):1365–1379. doi: 10.1016/j.immuni.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapitan M., Niemiec M.J., Steimle A., Frick J.S., Jacobsen I.D. Fungi as part of the microbiota and interactions with intestinal bacteria. Curr Top Microbiol Immunol. 2019;422:265–301. doi: 10.1007/82_2018_117. [DOI] [PubMed] [Google Scholar]
- 24.Peleg A.Y., Hogan D.A., Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol. 2010;8(5):340–349. doi: 10.1038/nrmicro2313. [DOI] [PubMed] [Google Scholar]
- 25.Fiers W.D., Gao I.H., Iliev I.D. Gut mycobiota under scrutiny: fungal symbionts or environmental transients? Curr Opin Microbiol. 2019;50:79–86. doi: 10.1016/j.mib.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolling T., Zhai B., Gjonbalaj M., Tosini N., Yasuma-Mitobe K., Fontana E., et al. Haematopoietic cell transplantation outcomes are linked to intestinal mycobiota dynamics and an expansion of Candida parapsilosis complex species. Nat Microbiol. 2021;6(12):1505–1515. doi: 10.1038/s41564-021-00989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simões-Silva L., Araujo R., Pestana M., Soares-Silva I., Sampaio-Maia B. The microbiome in chronic kidney disease patients undergoing hemodialysis and peritoneal dialysis. Pharmacol Res. 2018;130:143–151. doi: 10.1016/j.phrs.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Sheng B., Chen Y., Sun L., Xu P., Han B., Li X., et al. Antifungal treatment aggravates sepsis through the elimination of intestinal fungi. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/2796700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patankar J.V., Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17(9):543–556. doi: 10.1038/s41575-020-0326-4. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian S., Geng H., Tan X.D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng li xue bao : [Acta physiologica Sinica] 2020;72(3):308–324. [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C., Yang D., Wang B., Wu C., Wu Y., Li S., et al. Gasdermin D in macrophages restrains colitis by controlling cGAS-mediated inflammation. Sci Adv. 2020;6(21):eaaz6717. doi: 10.1126/sciadv.aaz6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulek K., Zhao J., Liao Y., Rana N., Corridoni D., Antanaviciute A., et al. Epithelial-derived gasdermin D mediates nonlytic IL-1beta release during experimental colitis. J Clin Invest. 2020;130(8):4218–4234. doi: 10.1172/JCI138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H., et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia Y., Cui R., Wang C., Feng Y., Li Z., Tong Y., et al. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minvaleev R.S., Bogdanov R.R., Kuznetsov A.A., Bahner D.P., Levitov A.B. Yogic agnisara increases blood flow in the superior mesenteric artery. J Bodyw Mov Ther. 2022;31:97–101. doi: 10.1016/j.jbmt.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y., He H., Wang X., Cui N., Zhou X., Long Y., et al. Resistance index of the superior mesenteric artery: Correlation with lactate concentration and kinetics prediction after cardiac surgery. Front Med. 2021:8. doi: 10.3389/fmed.2021.762376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubernatorova E.O., Perez-Chanona E., Koroleva E.P., Jobin C., Tumanov A.V. Murine model of intestinal ischemia-reperfusion injury. Journal of visualized experiments : JoVE. 2016;111 doi: 10.3791/53881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kami M., Sawada Y., Mori S., Hirate J., Kojima N., Kanda Y., et al. Serum levels of fluconazole in patients after cytotoxic chemotherapy for hematological malignancy. Am J Hematol. 2001;66(2):85–91. doi: 10.1002/1096-8652(200102)66:2<85::AID-AJH1022>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 40.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal, Archives of surgery (Chicago, Ill : 1960). 1970;101(4):478–83. [DOI] [PubMed]
- 41.Weigmann B., Tubbe I., Seidel D., Nicolaev A., Becker C., Neurath M.F. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2(10):2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 42.Treskes N., Persoon A.M., van Zanten A.R.H. Diagnostic accuracy of novel serological biomarkers to detect acute mesenteric ischemia: a systematic review and meta-analysis. Intern Emerg Med. 2017;12(6):821–836. doi: 10.1007/s11739-017-1668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin J., Wang S., Qiu Y., Jiang E., Du G., Wang W., et al. Screening for and combining serum intestinal barrier-related biomarkers to predict the disease severity of AECOPD. Annals of palliative medicine. 2021;10(2):1548–1559. doi: 10.21037/apm-20-1060. [DOI] [PubMed] [Google Scholar]
- 44.Sapone A., de Magistris L., Pietzak M., Clemente M.G., Tripathi A., Cucca F., et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55(5):1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 45.Underhill D.M., Iliev I.D. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14(6):405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamprinaki D., Beasy G., Zhekova A., Wittmann A., James S., Dicks J., et al. LC3-Associated phagocytosis is required for dendritic cell inflammatory cytokine response to gut commensal yeast saccharomyces cerevisiae. Front Immunol. 2017;8:1397. doi: 10.3389/fimmu.2017.01397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang T.T., Shao T.Y., Ang W.X.G., Kinder J.M., Turner L.H., Pham G., et al. Commensal fungi recapitulate the protective benefits of intestinal bacteria. Cell Host Microbe. 2017;22(6) doi: 10.1016/j.chom.2017.10.013. 809-16.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leonardi I., Li X., Semon A., Li D., Doron I., Putzel G., et al. CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi. Science. 2018;359(6372):232–236. doi: 10.1126/science.aao1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faustino M., Durão J., Pereira C.F., Pintado M.E., Carvalho A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae - A sustainable source of functional ingredients. Carbohydr Polym. 2021;272 doi: 10.1016/j.carbpol.2021.118467. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-de-Lomas J, Morales C, Grau MA, Mir A. Detection of Candida sp. mannan antigen by indirect ELISA-inhibition. In vitro crossed reactivity among mannans obtained from C. albicans A, C. albicans B, and C. tropicalis. Mycopathologia. 1988;102(3):175–8. [DOI] [PubMed]
- 51.Lee J.Y., Hall J.A., Kroehling L., Wu L., Najar T., Nguyen H.H., et al. Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell. 2020;180(1):79–91.e16. doi: 10.1016/j.cell.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meek R.L., Benditt E.P. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986;164(6):2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niemi K., Teirilä L., Lappalainen J., Rajamäki K., Baumann M.H., Öörni K., et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186(11):6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 54.Shridas P., De Beer M.C., Webb N.R. High-density lipoprotein inhibits serum amyloid A-mediated reactive oxygen species generation and NLRP3 inflammasome activation. J Biol Chem. 2018;293(34):13257–13269. doi: 10.1074/jbc.RA118.002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Facci L., Barbierato M., Zusso M., Skaper S.D., Giusti P. Serum amyloid A primes microglia for ATP-dependent interleukin-1β release. J Neuroinflammation. 2018;15(1):164. doi: 10.1186/s12974-018-1205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu C.C., Hsu H.J., Wu I.W., Lee C.C., Tsai C.J., Chou C.C., et al. Factors associated with mortality from non-occlusive mesenteric ischemia in dialysis patients. Ren Fail. 2009;31(9):802–806. doi: 10.3109/08860220903180624. [DOI] [PubMed] [Google Scholar]
- 57.Zingerman B., Ori Y., Korzets A., Herman-Edelstein M., Lev N., Rozen-Zvi B., et al. Occlusive mesenteric ischemia in chronic dialysis patients. The Israel Medical Association journal : IMAJ. 2021;23(9):590–594. [PubMed] [Google Scholar]
- 58.Leonardi I., Gao I.H., Lin W.Y., Allen M., Li X.V., Fiers W.D., et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell. 2022;185(5) doi: 10.1016/j.cell.2022.01.017. 831-46.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iliev I.D., Funari V.A., Taylor K.D., Nguyen Q., Reyes C.N., Strom S.P., et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336(6086):1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wheeler M.L., Limon J.J., Bar A.S., Leal C.A., Gargus M., Tang J., et al. Immunological consequences of intestinal fungal dysbiosis. Cell Host Microbe. 2016;19(6):865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang A.M., Inamine T., Hochrath K., Chen P., Wang L., Llorente C., et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127(7):2829–2841. doi: 10.1172/JCI90562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhattarai Y., Kashyap P.C. Germ-free mice model for studying host-microbial interactions. Methods in molecular biology (Clifton, NJ) 2016;1438:123–135. doi: 10.1007/978-1-4939-3661-8_8. [DOI] [PubMed] [Google Scholar]
- 63.Kamareddine L., Najjar H., Sohail M.U., Abdulkader H., Al-Asmakh M. The microbiota and gut-related disorders: insights from animal models. Cells. 2020;9(11) doi: 10.3390/cells9112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hallen-Adams H.E., Suhr M.J. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8(3):352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sokol H., Leducq V., Aschard H., Pham H.P., Jegou S., Landman C., et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shruthi B., Deepa N., Somashekaraiah R., Adithi G., Divyashree S., Sreenivasa M.Y. Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol Rep (Amst) 2022;34:e00716. doi: 10.1016/j.btre.2022.e00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faustino M., Durao J., Pereira C.F., Pintado M.E., Carvalho A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae - A sustainable source of functional ingredients. Carbohydr Polym. 2021;272 doi: 10.1016/j.carbpol.2021.118467. [DOI] [PubMed] [Google Scholar]
- 68.Korcová J., Machová E., Filip J., Bystrický S. Biophysical properties of carboxymethyl derivatives of mannan and dextran. Carbohydr Polym. 2015;134:6–11. doi: 10.1016/j.carbpol.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Korolenko T.A., Johnston T.P., Machova E., Bgatova N.P., Lykov A.P., Goncharova N.V., et al. Hypolipidemic effect of mannans from C. albicans serotypes a and B in acute hyperlipidemia in mice. Int J Biol Macromol. 2018;107(Pt B):2385–2394. doi: 10.1016/j.ijbiomac.2017.10.111. [DOI] [PubMed] [Google Scholar]
- 70.Shao T.Y., Ang W.X.G., Jiang T.T., Huang F.S., Andersen H., Kinder J.M., et al. Commensal candida albicans positively calibrates systemic Th17 immunological responses. Cell Host Microbe. 2019;25(3) doi: 10.1016/j.chom.2019.02.004. 404-17.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y., Huang G., Lv M. Extraction, characterization and antioxidant activities of mannan from yeast cell wall. Int J Biol Macromol. 2018;118(Pt A):952–956. doi: 10.1016/j.ijbiomac.2018.06.145. [DOI] [PubMed] [Google Scholar]
- 72.Schwarzer R., Jiao H., Wachsmuth L., Tresch A., Pasparakis M. FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity. 2020;52(6) doi: 10.1016/j.immuni.2020.04.002. 978 93.e6. [DOI] [PubMed] [Google Scholar]
- 73.Yu Y.Q., Gamez-Belmonte R., Patankar J.V., Liebing E., Becker C. The Role of Programmed Necrosis in Colorectal Cancer. Cancers (Basel) 2022;14(17) doi: 10.3390/cancers14174295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lloyd-Price J., Arze C., Ananthakrishnan A.N., Schirmer M., Avila-Pacheco J., Poon T.W., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J.Y., Hall J.A., Kroehling L., Wu L., Najar T., Nguyen H.H., et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell. 2020;183(7):2036–2039. doi: 10.1016/j.cell.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eckhardt E.R., Witta J., Zhong J., Arsenescu R., Arsenescu V., Wang Y., et al. Intestinal epithelial serum amyloid A modulates bacterial growth in vitro and pro-inflammatory responses in mouse experimental colitis. BMC Gastroenterol. 2010;10:133. doi: 10.1186/1471-230X-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong J., Wu J., Ikeh M., Tao L., Zhang Y., Bing J., et al. Antifungal Activity of Mammalian Serum Amyloid A1 against Candida albicans. Antimicrob Agents Chemother. 2019;64(1) doi: 10.1128/AAC.01975-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan Z.Y., Zhang X.X., Wu Y.J., Zeng Z.P., She W.M., Chen S.Y., et al. Serum amyloid A levels in patients with liver diseases. World J Gastroenterol. 2019;25(43):6440–6450. doi: 10.3748/wjg.v25.i43.6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng N., Liang Y., Du X., Ye R.D. Serum amyloid A promotes LPS clearance and suppresses LPS-induced inflammation and tissue injury. EMBO Rep. 2018;19(10) doi: 10.15252/embr.201745517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.