Abstract
Compelling evidence now suggests that αβ CD8 cytotoxic T lymphocytes (CTL) have an important role in preventing human immunodeficiency virus (HIV) infection and/or slowing progression to AIDS. Here, we describe an HIV type 1 CTL polyepitope, or polytope, vaccine comprising seven contiguous minimal HLA A2-restricted CD8 CTL epitopes conjoined in a single artificial construct. Epitope-specific CTL lines derived from HIV-infected individuals were able to recognize every epitope within the construct, and HLA A2-transgenic mice immunized with a recombinant virus vaccine coding for the HIV polytope also generated CTL specific for different epitopes. Each epitope in the polytope construct was therefore processed and presented, illustrating the feasibility of the polytope approach for HIV vaccine design. By simultaneously inducing CTL specific for different epitopes, an HIV polytope vaccine might generate activity against multiple challenge isolates and/or preempt the formation of CTL escape mutants.
A considerable body of compelling indirect evidence suggests that cytotoxic T lymphocytes (CTL) have a role in preventing or limiting (i) initial human immunodeficiency virus (HIV) infection (36) and (ii) progression to AIDS (16). Correlations between CTL activity and protection against challenge have been observed in lentivirus models (12, 22) and in studies of HIV-exposed but uninfected individuals (36). The inverse correlation between viral load and CTL levels in HIV patients also implies a significant role for HIV-specific CTL in the control of HIV replication (31). Direct evidence for the importance of CTL was recently obtained from an ovine retrovirus model in which a prophylactic vaccine designed to induce only CTL prevented the establishment of a latent infection (21).
Induction of protective HIV-specific CTL responses is complicated by the presence of multiple HIV variants, any one of which may contain mutations in the target CTL epitopes (16), and/or by CTL escape mutants being rapidly generated following infection (16, 29). An ideal vaccine might induce a sufficient diversity of CTL specificities to ensure CTL-mediated protection against all or most of the potential variants within HIV challenge inocula and perhaps also preempt the generation of CTL escape mutants. Vaccines containing multiple recombinant antigens (10) may be able to induce CTL populations sufficiently diverse to be capable of cross-recognizing multiple isolates (15); however, even if homology sufficient to make such an approach feasible existed, highly variant epitopes may dominate at the expense of relatively conserved, protective subdominant epitopes (30). A CTL epitope-based approach has the advantage of being able to focus immunity toward protective, perhaps less variant, epitopes. Sequences outside the CTL epitope regions, which might adversely affect the immune response (7, 17, 20), can also be avoided. However, an epitope-based approach would be of advantage only if multiple CTL epitopes covering a range of epitopes could be simultaneously codelivered to induce a defined spectrum of CTL specificities. The polyepitope, or polytope, approach represents a strategy whereby multiple contiguous minimal CTL epitopes can be delivered as a single artificial construct (1, 14, 19, 38, 40, 41). Here, we demonstrate the immunogenicity of an HIV polytope vaccine containing multiple contiguous HLA A2-restricted HIV CTL epitopes from a range of HIV antigens. The vaccine construct was recognized by human HIV-specific CTL and raised multiple independent CTL responses in HLA A2-transgenic mice. Thus, apart from offering a considerable reduction in size compared to a recombinant multiantigen construct, the polytope approach represents an attractive strategy for CTL-based HIV vaccine design.
MATERIALS AND METHODS
HIV polytope and other recombinant vaccinia viruses.
The HIV polytope recombinant vaccinia virus (rVV.HIV.pt) was constructed as follows. A synthetic oligonucleotide fragment (Fig. 1) was constructed from three 70-mer and one 72-mer synthetic oligonucleotides by the splicing-by-overlap-extension method and PCR (40, 41). The nucleic acid sequence of the fragment contained (from the 5′ end) a BamHI restriction site, a Kozac sequence, a methionine start codon, sequences corresponding to seven contiguous minimal HLA A2 HIV CTL epitopes (Table 1), and a stop codon and a SalI site at the 3′ end. The amino acid sequences of the CTL epitopes were converted to DNA sequences by using universal codon usage, but inclusion of restriction sites was avoided. Dimers were made of synthetic oligonucleotides 1-2 and 3-4 (0.4 μg of each) in 40-μl reaction mixtures containing standard 1× Pfu PCR buffer, 0.5 mM deoxynucleoside triphosphates, and 1 U of cloned Pfu DNA polymerase (hot start at 94°C) with the following thermal program: 94°C for 10 s, 52°C for 20 s, and 72°C for 20 s for five cycles. At the end of five cycles the PCR program was paused at 72°C and 20-μl aliquots of the two dimer reaction mixtures were mixed and subjected to a further five cycles (94°C for 10 s, 48°C 20 s, and 72°C for 20 s). The reaction mixture was resolved on a 3% agarose gel, the 220-bp fragment was excised, and the agarose was removed by microcentrifugation through filter paper. Two 20-mer oligonucleotide primers (Fig. 1) were used to amplify by PCR the full-length product for 25 cycles at an annealing temperature of 50°C. The full-length gel-purified PCR fragment was cloned into the EcoRV site of pBluescript II KS(−) and checked by sequencing. The insert was then subcloned behind the vaccinia virus p7.5 promoter in the plasmid shuttle vector pPS 7.5 A with BamHI and SalI. Construction of the recombinant vaccinia virus was then performed by marker rescue recombination as described previously (8), by insertion of the fragment into the f region of a thymidine kinase-negative vaccinia virus, followed by plaque purification and selection with methotrexate.
FIG. 1.
HIV polytope insert. The first epitope and then every second CTL epitope are underlined.
TABLE 1.
HLA A2-restricted HIV epitopes in the polytopea
| Epitope (positions) | Sequence |
|---|---|
| Nef (157–166) | PLTFGWCYKL |
| Pol (346–354) | VIYQYMDDL |
| Gag (77–85) | SLYNTVATL |
| Nef (180–189) | VLEWRFDSRL |
| Pol (476–484) | ILKEPVHGV |
| gp120 (120–128) | KLTPLCVTL |
| Nef (190–198) | AFHHVAREL |
Recombinant vaccinia virus containing HIV nef and pol expressed p27 Nef, and the reverse transcriptase and integrase of the LAI strain of HIV (Transgene, Strasbourg, France) were made available via the Programme Reactifs de L’ANRS. The control vaccinia virus, rVV.Cont, coded for ovalbumin or the murine polytope (40).
PCR and RT-PCR of rVV.HIV.pt-infected cells.
CV1 cells (2.5 × 106) were infected with rVV.HIV.pt (multiplicity of infection = 5) and stored overnight, and the RNA was extracted and reverse transcribed as described previously (26). A control sample was also prepared without reverse transcriptase (RT). DNA was extracted from a parallel culture of rVV.HIV.pt-infected CV1 cells with a blood kit (Qiagen, Hilden, Germany). PCR was performed in a 20-μl volume containing 1 μl of cDNA or DNA (or an equivalent volume of the control samples) and 0.5 μl each of forward and reverse 20-bp primers (Fig. 1) (20 μM). The reaction mixture was as described previously (26). An initial denaturation at 95°C for 2 min was followed by 30 cycles of PCR (95°C for 10 s, 55°C for 10 s, and 72°C for 50 s) and a 10-min extension at 72°C. PCR products were resolved on a 2% agarose gel. The ≈220-bp fragments were excised, purified (Wizard purification kit; Promega, Madison, Wis.), and sequenced.
Human CTL lines.
Blood was obtained from HIV-infected patients at the Infectious Diseases Unit, Royal Brisbane Hospital. Most of the patients were receiving highly active antiretroviral therapy (HAART) at the Infectious Diseases Unit. HLA A2-positive individuals were identified by fluorescence-activated cell sorter analysis of peripheral blood mononuclear cells (PBMC) with an HLA A2-specific mouse monoclonal antibody derived from the supernatant of the hybridoma line ATCC HBT82, B87.2, followed by fluorescein isothiocyanate-labelled anti-mouse F(ab′)2 (Silenus, Melbourne, Australia). Cells were fixed in fresh 2% paraformaldehyde in phosphate-buffered saline prior to analysis.
PBMC from HLA A2 HIV-infected individuals were prepared by standard Ficoll-Paque gradient separation, and half of the cells were sensitized with the peptides indicated below (see Fig. 3 and Table 2) (Chiron Technologies, Clayton, Australia) (50 μg/ml, 1 h at 37°C, followed by one wash). The sensitized PBMC were added back to the remaining cells in a 24-well plate to 1 × 106 to 2 × 106 cells/ml (effector-to-stimulator ratio, 1:1). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (QIMR), 2 mM glutamine (ICN Biomed. Aust. Pty. Ltd., Seven Hills, Australia), and 100 μg of streptomycin per ml and 100 IU of penicillin per ml (CSL Ltd., Melbourne, Australia). Interleukin-7 (IL-7) (300 IU/ml; Sigma, St. Louis, Mo.) was added on day 0, and IL-2 (10 IU/ml; kindly provided by Cetus Corp., Emeryville, Calif.) was added on day 3. The bulk effectors were used in standard 6-h 51Cr release assays on days 10 to 12, unless stated otherwise. Partial medium changes were performed when required. Some cultures were maintained by weekly restimulations with HLA A2 lymphoblastoid cells that were peptide sensitized (10 μg/ml, 37°C for 1 h, washed, and irradiated [8,000 rads]; responder-to-stimulator ratio ≈ 20:1). Fewer than seven CTL bulk cultures (one for each peptide) were set up when PBMC were limiting, and for these cases, restimulation with SLYNTVATL was always included.
FIG. 3.
Epitope-specific CTL lines derived from PBMC of HIV-infected individuals (H28, H10, H19, H21, and H33) used as effectors against (i) HLA A2+ LCLs sensitized with the indicated peptide (black squares) or not sensitized (white squares) (columns headed “Peptide”), (ii) HLA A2+ LCLs infected with rVV.HIV.pt (black squares) or a control recombinant vaccinia virus (white squares) (columns headed “Polytope”), and (iii) HLA A2+ LCLs infected with rVV.nef or rVV.pol (black squares) or a control recombinant vaccinia virus (white squares) (column headed “Antigen”). The epitope listed on the left of each row was used to restimulate the bulk cultures, which were used to generate the data in that row. Bulk cultures from each individual were separately restimulated with the indicated peptide, split, and used against peptide and polytope and sometimes against whole antigen expressing target cells. A summary of patient data is shown in Table 2. The negative results, for patient H28, are shown to illustrate the specificity of the in vitro restimulation protocol.
TABLE 2.
Summary of PBMC donor characteristics and CTL response(s)a
| Donor | HLA | Age (yr) | CD4 count (106)/liter | RNA copies/μl | Date of HIV infection | HIV stage | CTL response(s) |
|---|---|---|---|---|---|---|---|
| H07 | A2 | 34 | 260 | 1 × 105 | 4/1992 | II | 1/3 (VLE) |
| H08 | A2 | 50 | 200 | <400 | 2/1984 | II | 0/3 |
| H09∗ | A2 | 41 | 380 | 1.3 × 105 | 1985 | II | 0/3 |
| H10 | A2 | 34 | 330 | 8.2 × 103 | 1985 | IV C1 | 7/7b |
| H11 | A2 | 50 | 320 | 2 × 104 | 1989 | IV C1 | 0/3 |
| H17∗ | A2 | 44 | 700 | 1.1 × 104 | 2/1996 | II | 0/3 |
| H18∗ | A2 | 47 | 610 | 1.2 × 105 | 1985 | II | 0/3 |
| H19 | A2 | 35 | 90 | 1.3 × 105 | 12/1993 | IV C1 | 2/7 (PLT, VIY) |
| H21 | A2 | 47 | 440 | <400 | 1986 | II | 2/7 (SLY, KLT) |
| H24 | A2 | 48 | 300 | 580 | 1988 | II | 1/7 (SLY) |
| H27 | A2 | 48 | 440 | 5.3 × 103 | 1/1991 | II | 3/7 (SLY, VIY, ILK) |
| H28 | A2 | 41 | 60 | 1.3 × 105 | 1994 | IV C | 0/7 |
| H30 | A2 | 65 | 250 | <400 | 5/1997 | IV C | 0/7 |
| H33 | A2 | 35 | 510 | 1.5 × 103 | 2/1997 | II | 3/7 (SLY, KLT, AFH) |
| H12 | Not A2 | 37 | 350 | <400 | 1995 | III | 0/3 |
| H16 | Not A2 | 44 | 300 | <400 | 9/1997 | II | 0/3 |
| H22 | Not A2 | 32 | 520 | <400 | 9/1996 | IV C1 | 0/1 |
| HC1 | A2 | 46 | 0 | 0/7 | |||
| HC2 | A2 | 37 | 0 | 0/7 |
CTL response(s) is given as the number of epitopes for which specific CTL activity could be generated per the number of epitopes tested (first three amino acids of the epitope(s) for which a response was seen). Date of HIV infection is given as year or month/year. HIV staging is based on the Centers for Disease Control and Prevention’s surveillance case definition for AIDS. Donors HC1 and HC2 are healthy HIV-seronegative controls. ∗, not on HAART therapy at the time that blood was taken. The HAART treatment unresponsiveness of this cohort was similar to that described previously for patients with a history of extended treatment (34).
Responses to all seven epitopes shown in Fig. 3.
The target cell for the epitope-specific bulk CTL effectors was an Epstein-Barr virus (B95.8)-transformed lymphoblastoid cell line (LCL) from an unrelated homozygous HLA A2 healthy individual (HLA A2+ LCL). HLA A2+ LCLs were either (i) infected (multiplicity of infection, 10) overnight with rVV.HIV.pt, a recombinant vaccinia virus coding for the specified HIV antigen, a control recombinant vaccinia virus expressing ovalbumin, or an unrelated polytope construct (rVV.Cont) (40) prior to 51Cr labelling or (ii) sensitized with peptide (10 μg/ml) at the same time as 51Cr labelling followed by two washes before use in the 51Cr release assays. HLA A2+ LCLs were used as cold target inhibitors at a cold-to-hot ratio of 40:1.
Vaccination and CTL assays with HHD transgenic mice.
Transgenic HHD mice have a transgene comprising the α1 (H) and α2 (H) domains of HLA A2 linked to the α3 transmembrane and cytoplasmic domains of H-2Db (D), with the α1 domain linked to human β2 microglobulin. This transgene was introduced into murine β2 microglobulin and H-2Db double knockout mice; thus, the only major histocompatibility complex (MHC) molecule expressed by the HHD mouse was the modified HLA A2 molecule (32).
For the first experiment, six HHD mice were vaccinated intraperitoneally with 5 × 107 PFU of rVV.HIV.pt. After 3 weeks splenocytes were harvested and pooled, and 5 × 106 splenocytes were restimulated with 1 × 106 lipopolysaccharide blasts per 24-well plate (42). The lipopolysaccharide blasts were sensitized with peptide (10 μg/ml for 1 h at 37°C), irradiated (3,000 rads), and washed twice prior to use. Cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum (QIMR), 2 mM glutamine (Sigma), 5 × 10−5 M β-mercaptoethanol (Sigma), and antibiotics as described above. On day 4, 1 ml of medium containing 5 IU of recombinant human IL-2 (Cetus) per ml was added. On day 6 the cultures were used as effectors in standard 6-h 51Cr release assays against EL4S3-RobHHD target cells (32), which were sensitized with the indicated peptide (10 μg/ml) at the same time as being radiolabelled and were washed twice prior to use.
For the DNA prime boost experiment mice were anesthetized with 100 μl of a solution containing ketamine (10 mg/ml), xylazine (2 mg/ml), and water (4:1:1) and were given 100 μg (50 μg into each quadriceps muscle) of either pJWHIV (n = 3) or a control plasmid, pJW4303 (27) (n = 3) followed after 14 days with an identical booster injection. After another 14 days the mice received rVV.HIV.pt. Three weeks later the splenocytes were restimulated and 51Cr release was performed as described above, except that splenocytes from each animal were restimulated separately. Plasmid preparation was undertaken by using the EndoFree Plasmid Maxi kit (Qiagen). pJWHIV was generated by subcloning the HIV polytope insert from the HIV polytope pBluescript (see above) into pJW4303 (27) with HindIII and EcoRI.
RESULTS
Confirmation of the polytope sequence and transcription of the polytope insert.
rVV.HIV.pt was constructed to contain a synthetic insert (Fig. 1) coding for seven HIV HLA A2 CTL epitopes (Table 1). The epitopes were selected from the list of optimal HLA A2 CTL epitopes described by Brander and Walker (2), excluding the more variant epitopes from env, and they included the relatively conserved gp120 epitope described by Dupuis et al. (9).
Direct sequencing of the insert in rVV.HIV.pt was used to confirm the presence of an uncorrupted polytope insert in the recombinant vaccinia virus. PCR of viral DNA extracted from rVV.HIV.pt-infected cells generated an ≈220-bp fragment (Fig. 2, lane E), the expected size of the insert (Fig. 1). A water control for the PCR of viral DNA is shown in Fig. 2, lane D. Sequencing of the ≈220-bp fragment gave the expected nucleotide sequence, shown in Fig. 1.
FIG. 2.
Agarose gel of HIV polytope PCR products from cDNA (lane B) and viral DNA (lane E) derived from rVV.HIV.pt-infected cells. Lanes A and F, 1-kb markers (Gibco BRL, Gaithersburg, Md.); lane B, RT-PCR of the HIV polytope mRNA; lane C, RT-PCR control without RT; lane D, PCR control with water as template; lane E, PCR of viral DNA. Molecular size markers indicated by arrowheads are (from the top) 394, 344, 298, and 220 bp.
Polytope proteins have been very difficult to detect with antibody probes, possibly due to their lack of structure and resulting poor stability (40). RT-PCR was thus used to show appropriate transcription of HIV polytope mRNA by rVV.HIV.pt-infected cells (Fig. 2, lane B). A control for the RT-PCR without RT is shown in Fig. 2, lane C.
Following infection, rVV.HIV.pt thus transcribed an HIV polytope mRNA coding for seven HIV CTL epitopes.
Epitope-specific CTL lines from HIV patients recognize the HIV polytope construct.
CTL from 14 HLA A2 HIV patients (Table 2) were separately restimulated in vitro with up to seven peptide epitopes (Table 1) to generate epitope-specific bulk CTL cultures. CTL cultures capable of recognizing at least one of the seven epitopes were generated from seven HLA A2 HIV patients (Fig. 3; Table 2). CTL cultures specific for more than one epitope were derived from the PBMC of five patients (Fig. 3; Table 2), with PBMC from H10 generating cultures specific for all seven epitopes (Fig. 3). The lower number of patients responding to SLYNTVATL in this study than in previous studies (3) may reflect the fact that nearly all the patients in this study were on HAART therapy.
The peptide-restimulated bulk cultures from the remaining seven HIV patients failed to generate significant peptide-specific activity; however, only three peptides could be tested for five of these patients (Table 2). An example of the data derived from seven negative bulk cultures of the PBMC of one such individual, H28, is shown to illustrate the specificity of the in vitro restimulation protocol (Fig. 3). Two HLA A2 HIV-seronegative controls (HC1 and HC2) and three HIV-seropositive non-HLA A2 individuals (H12, H16, and H22) (Table 2) gave results essentially similar to those for H28 (data not shown), further demonstrating the specificity of the peptide restimulation protocol. Failure to generate epitope-specific CTL lines does not mean that the individuals did not have CTL specific for the corresponding antigen. Most HIV patients have CTL responses to a least one, and usually multiple, antigens (36). Detection of such CTL would require the use of antigen or HIV restimulation protocols, rather than the peptide restimulation used in this study.
The CTL effectors, which showed lysis against peptide-sensitized target cells (Fig. 3), also lysed target cells infected with rVV.HIV.pt (Fig. 3), illustrating that each epitope in the HIV polytope was individually processed and presented. Furthermore, in cases where sufficient bulk effectors were available, the CTL lines were also shown to be able to lyse LCLs infected with recombinant vaccinia virus coding for the whole antigen from which each respective epitope was derived (Fig. 3).
HHD mice vaccinated with the HIV polytope generated CTL specific for multiple epitopes.
To determine whether the HIV polytope construct was capable of raising CTL responses in vivo, HHD transgenic mice were vaccinated with rVV.HIV.pt, and the splenocytes were restimulated in vitro and used to kill peptide-sensitized target cells (Fig. 4A). CTL responses to SLYNTVATL, ILKEPVHGV, KLTPLCVTL, and AFHHVAREL were generated. CTL responses to the remaining epitopes could not be generated in these mice by rVV.HIV.pt immunization (Fig. 4A and data not shown). The data illustrated that the HIV polytope vaccine was able to induce in vivo CTL responses to multiple HLA A2 HIV CTL epitopes.
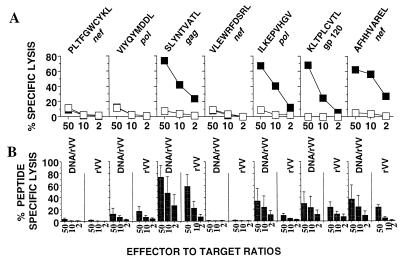
FIG. 4.
Data from HHD mice immunized with HIV polytope vaccines. (A) Mice were immunized with rVV.HIV.pt, and pooled splenocytes were restimulated in vitro with each of the indicated peptides and used as effectors against target cells sensitized with the same peptide (black squares) or not sensitized (white squares). (B) Mice were immunized with two injections, either of DNA vaccine coding for the HIV polytope followed by rVV.HIV.pt (DNA/rVV) or of a control DNA plasmid followed by rVV.HIV.pt (rVV). Splenocyte populations from each mouse were individually restimulated with each peptide and used as effectors against target cells sensitized with peptide and not sensitized. (Thus, the first six bars represent restimulation and lysis with PLTFGWCYKL.) Results were calculated as follows: percent lysis of target cells sensitized with peptide − percent lysis of target cells not sensitized (± standard error). DNA vaccination alone produced only weak CTL responses ranging from 5 to 10% (data not shown).
To determine whether polytope CTL responses could be enhanced by using strategies combining DNA priming and boosters with recombinant vaccinia virus, as described previously for whole-antigen-based vaccines (24, 35, 37), mice were immunized with a DNA vaccine coding for the HIV polytope and were then given a booster with rVV.HIV.pt. No significant improvement in the responses to epitopes which failed to generate a response following rVV.HIV.pt immunization (Fig. 4A) was observed following prime boost vaccination (Fig. 4B). The responses to SLYNTVATL, ILKEPVHGV, KLTPLCVTL, and AFHHVAREL were, however, significantly enhanced (an average of 2.4-fold at an effector-to-target ratio of 10:1, P = 0.008) by prime boost strategies (Fig. 4B). CTL responses to polytope vaccines can therefore also be enhanced by DNA prime-plus-poxvirus boost strategies.
DISCUSSION
Here, we demonstrate the feasibility of delivering multiple HLA A2 HIV CTL epitopes with a polytope vaccine construct. Each epitope in the polytope construct was recognized by CTL lines from HIV patients, and the polytope vaccine induced multiple epitope-specific responses in HHD transgenic mice. The DNA prime-plus-virus vector boost strategy (24, 35, 37) also improved CTL responses when applied to polytope vaccines.
Potential competition and/or immunodominance phenomena (30, 45) did not appear to interfere significantly with simultaneous presentation of, and priming by, the multiple HLA A2 epitopes within the polytope construct. Factors intrinsic to the epitope, such as MHC binding affinity, can determine the immunodominance of an epitope (45). For instance, the immunodominant SLYNTVATL (3) binds well to HLA A2 (44), whereas the subdominant AFHHVAREL (3) binds poorly to HLA A2 (23). However, the subdominance of an epitope can often be ascribed to inefficient proteolytic liberation of the epitope from the full-length protein (45). Such processing constraints are less likely to operate for polytope proteins, since these proteins appear to be rapidly degraded (40, 41) and the epitopes in the polytope are not flanked by poorly cleaved glycine or proline residues (13, 38, 41). The subdominance of AFHHVAREL may in part also reflect inefficient processing, since it appears to be codominant when presented in a polytope construct (Fig. 4). An ability to mitigate against dominance effects and generate multiple codominant responses may emerge as an important attribute of polytope vaccines.
A controversy over the HLA A2 restriction of AFHHVAREL was recently reported, based on the inability of this epitope to bind to HLA A2 efficiently in in vitro binding assays (4, 23). Two HIV-infected HLA A2 individuals recognized the AFHHVAREL epitope in this study, and rVV.HIV.pt vaccination induced AFHHVAREL-specific CTL in HHD mice, supporting the original contention that this epitope is restricted by HLA A2 (18). The relatively conserved gp120 CTL epitope, KLTPLCVTL, was recognized by three patients, suggesting that this also is a commonly recognized HLA A2-restricted epitope (9).
The HHD mouse system clearly represents an ideal model for preclinical and quality control testing of vaccines designed to induce HLA A2-restricted CTL responses in humans. The inability of HLA A2-transgenic mice to respond to some epitopes has been reported previously (44) and may reflect a limited T-cell receptor (TCR) repertoire educated on the HLA A2 transgene in these animals (32). Murine TAP proteins are more selective than their human equivalents (28), and other murine proteins involved in processing may also be inefficient at delivering some peptides for HLA binding (5, 33). Such factors may result in inefficient processing of certain polytope epitopes but may also limit the diversity of self-epitopes loaded onto HLA A2 in the thymus in HHD mice. The latter would reduce the diversity of the peripheral TCR repertoire educated on HLA A2 (11). Other factors are clearly also involved, since deletion of murine MHC expression in HHD mice appears to increase the TCR repertoire over that found in A2Kb transgenic mice (32), which retain murine MHC (10a, 44).
A prophylactic HIV polytope vaccine might ultimately contain a series of epitopes covering the diversity of HLA alleles in any target population and might also contain a number of common epitope variants. In a therapeutic setting, a cocktail of four single HLA polytope vaccines might be used to cover the four HLA alleles of any given individual patient. Several human delivery modalities vectors might be envisaged for HIV polytope constructs, perhaps in conjunction with prime boost and/or cytokine codelivery strategies (25, 41). These vectors include DNA-based vaccination (41, 43), avipoxvirus (6, 24), and/or modified vaccinia virus Ankara (13, 19, 37, 39).
ACKNOWLEDGMENTS
This work was supported by the Australian Commonwealth AIDS Research Grants Program, the Australian Centre for International & Tropical Health & Nutrition, and the ANRS, Paris, France.
We thank D. Harrich and E. Gowans (Australian National Centre in HIV Virology Research, Sir Albert Sakzewski Virus Research Centre, Brisbane, Australia) for access to their PC3 facility and S. Rowland-Jones (Institute of Molecular Medicine, Nuffield Department of Medicine, Oxford, United Kingdom) and G. Haas (Department of Molecular Biology, Max-Planck-Institut fuer Infektionsbiologie, Berlin, Germany) for help with the selection of epitopes.
REFERENCES
- 1.An L-L, Whitton J L. A multivalent minigene vaccine, containing B-cell, cytotoxic T-lymphocyte, and Th epitopes from several microbes, induces appropriate responses in vivo and confers protection against more than one pathogen. J Virol. 1997;71:2292–2302. doi: 10.1128/jvi.71.3.2292-2302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brander, C., and B. D. Walker. 1998, revision date. Systematic identification of optimal HIV-1 CTL epitopes. [Online.] http://hiv-web.lanl.gov/immunology/articles/Brander.97.html. [20 March 1999, last date accessed.]
- 3.Brander C, Hartman K E, Trocha A K, Jones N G, Johnson R P, Korber B, Wentworth P, Buchbinder S P, Wolinsky S, Walker B D, Kalams S A. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. J Clin Investig. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brander C, Walker B D, Korber B. Questionable HLA-A2 restriction of two HIV-1 Nef-derived CTL epitopes listed in the HIV Molecular Immunology Database. AIDS Res Hum Retroviruses. 1998;14:923–924. [Google Scholar]
- 5.Braud V M, McMichael A J, Cerundolo V. Differential processing of influenza nucleoprotein in human and mouse cells. Eur J Immunol. 1998;28:625–635. doi: 10.1002/(SICI)1521-4141(199802)28:02<625::AID-IMMU625>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Schwartz D, Siliciano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J L, Tartaglia J, Duliege A-M, Sinangil F, Paoletti E, et al. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. NIAID AIDS Vaccine Evaluation Group. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 7.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 8.Coupar B E, Andrew M E, Boyle D B. A general method for the construction of recombinant vaccinia viruses expressing multiple foreign genes. Gene. 1988;68:1–10. doi: 10.1016/0378-1119(88)90593-8. [DOI] [PubMed] [Google Scholar]
- 9.Dupuis M, Kundu S K, Merigan T C. Characterization of HLA-A 0201-restricted cytotoxic T cell epitopes in conserved regions of the HIV type 1 gp160 protein. J Immunol. 1995;155:2232–2239. [PubMed] [Google Scholar]
- 10.Ferrari G, Berend C, Ottinger J, Dodge R, Bartlett J, Toso J, Moody D, Tartaglia J, Cox W I, Paoletti E, Weinhold K J. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood. 1997;90:2406–2416. [PubMed] [Google Scholar]
- 10a.Firat, H., et al. Submitted for publication.
- 11.Fukui Y, Hashimoto O, Inayoshi A, Gyotoku T, Sano T, Koga T, Gushima T, Sasazuki T. Highly restricted T cell repertoire shaped by a single major histocompatibility complex-peptide ligand in the presence of a single rearranged T cell receptor beta chain. J Exp Med. 1998;188:897–907. doi: 10.1084/jem.188.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, et al. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 13.Gardner J, Sutter G, Michaelis J, Mateo L, Suhrbier A. 12th World AIDS Conference, 1 Basic Science. Bologna, Italy: Moduzzi Editore; 1998. A cytotoxic T cell polyepitope based vaccine against HIV delivered by modified vaccinia Ankara; pp. 295–298. [Google Scholar]
- 14.Gilbert S C, Plebanski M, Harris S J, Allsopp C E, Thomas R, Layton G T, Hill A V. A protein particle vaccine containing multiple malaria epitopes. Nat Biotechnol. 1997;15:1280–1284. doi: 10.1038/nbt1197-1280. [DOI] [PubMed] [Google Scholar]
- 15.Gotch F. Cross-clade T cell recognition of HIV.1. Curr Opin Immunol. 1998;10:388–392. doi: 10.1016/s0952-7915(98)80109-x. [DOI] [PubMed] [Google Scholar]
- 16.Goulder P, Price D, Nowak M, Rowland-Jones S, Phillips R, McMichael A. Co-evolution of human immunodeficiency virus and cytotoxic T-lymphocyte responses. Immunol Rev. 1997;159:17–29. doi: 10.1111/j.1600-065x.1997.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 17.Gratton S, Julius M, Sekaly R P. Ick-independent inhibition of T cell antigen response by the HIV gp120. J Immunol. 1998;161:3551–3556. [PubMed] [Google Scholar]
- 18.Haas G, Plikat U, Debre P, Lucchiari M, Katlama C, Dudoit Y, Bonduelle O, Bauer M, Ihlenfeldt H G, Jung G, Maier B, Meyerhans A, Autran B. Dynamics of viral variants in HIV-1 Nef and specific cytotoxic T lymphocytes in vivo. J Immunol. 1996;157:4212–4221. [PubMed] [Google Scholar]
- 19.Hanke T, Blanchard T J, Schneider J, Ogg G S, Tan R, Becker M, Gilbert S C, Hill A V, Smith G L, McMichael A. Immunogenicities of intravenous and intramuscular administrations of modified vaccinia virus Ankara-based multi-CTL epitope vaccine for human immunodeficiency virus type 1 in mice. J Gen Virol. 1998;79:83–90. doi: 10.1099/0022-1317-79-1-83. [DOI] [PubMed] [Google Scholar]
- 20.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O’Brien W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 21.Hislop A D, Good M F, Mateo L, Gardner J, Gatei M H, Daniel R C W, Meyers B V, Lavin M F, Suhrbier A. Vaccine-induced cytotoxic T lymphocytes protect against retroviral challenge. Nat Med. 1998;4:1193–1196. doi: 10.1038/2690. [DOI] [PubMed] [Google Scholar]
- 22.Hosie M J, Flynn J N, Rigby M A, Cannon C, Dunsford T, Mackay N A, Argyle D, Willett B J, Miyazawa T, Onions D E, Jarrett O, Neil J C. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J Virol. 1998;72:7310–7319. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunziker I P, Cerny A, Pichler W J. Who is right? Or, how to judge the disagreement about HLA restriction of Nef peptides. AIDS Res Hum Retroviruses. 1998;14:921–924. doi: 10.1089/aid.1998.14.921. [DOI] [PubMed] [Google Scholar]
- 24.Kent S J, Zhao A, Best S J, Chandler J D, Boyle D B, Ramshaw I A. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leong K H, Ramsay A J, Boyle D B, Ramshaw I A. Selective induction of immune responses by cytokines coexpressed in recombinant fowlpox virus. J Virol. 1994;68:8125–8130. doi: 10.1128/jvi.68.12.8125-8130.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linn M L, Mateo L, Gardner J, Suhrbier A. Alphavirus-specific cytotoxic T lymphocytes recognize a cross-reactive epitope from the capsid protein and can eliminate virus from persistently infected macrophages. J Virol. 1998;72:5146–5153. doi: 10.1128/jvi.72.6.5146-5153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momburg F, Roelse J, Howard J C, Butcher G W, Hammerling G J, Neefjes J J. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature. 1994;367:648–651. doi: 10.1038/367648a0. [DOI] [PubMed] [Google Scholar]
- 29.Mortara L, Letourneur F, Gras-Masse H, Venet A, Guillet J-G, Bourgault-Villada I. Selection of virus variants and emergence of virus escape mutants after immunization with an epitope vaccine. J Virol. 1998;72:1403–1410. doi: 10.1128/jvi.72.2.1403-1410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowak M A, May R M, Phillips R E, Rowland-Jones S, Lalloo D G, McAdam S, Klenerman P, Koeppe B, Sigmund K, Bangham C R, McMichael A J. Antigenic oscillations and shifting immunodominance in HIV-1 infections. Nature. 1995;375:606–611. doi: 10.1038/375606a0. [DOI] [PubMed] [Google Scholar]
- 31.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 32.Pascolo S, Bervas N, Ure J M, Smith A G, Lemonnier F A, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from β2 microglobulin (β2m) HLA-A2.1 monochain transgenic H-2Db β2m double knockout mice. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peh C A, Burrows S R, Barnden M, Khanna R, Cresswell P, Moss D J, McCluskey J. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity. 1998;8:531–542. doi: 10.1016/s1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- 34.Piketty C, Castiel P, Belec L, Batisse D, Si Mohamed A, Gilquin J, Gonzalez-Canali G, Jayle D, Karmochkine M, Weiss L, Aboulker J P, Kazatchkine M D. Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. AIDS. 1998;12:745–750. doi: 10.1097/00002030-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Ramsay A J, Leong K H, Ramshaw I A. DNA vaccination against virus infection and enhancement of antiviral immunity following consecutive immunization with DNA and viral vectors. Immunol Cell Biol. 1997;75:382–388. doi: 10.1038/icb.1997.60. [DOI] [PubMed] [Google Scholar]
- 36.Rowland-Jones S, Tan R, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 37.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 38.Suhrbier A. Multi-epitope DNA vaccines. Immunol Cell Biol. 1997;75:402–408. doi: 10.1038/icb.1997.63. [DOI] [PubMed] [Google Scholar]
- 39.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson S A, Elliott S L, Sherritt M A, Sproat K W, Coupar B E, Scalzo A A, Forbes C A, Ladhams A M, Mo X Y, Tripp R A, Doherty P C, Moss D J, Suhrbier A. Recombinant polyepitope vaccines for the delivery of multiple CD8 cytotoxic T cell epitopes. J Immunol. 1996;157:822–826. [PubMed] [Google Scholar]
- 41.Thomson S A, Sherritt M A, Medveczky J, Elliott S L, Moss D J, Fernando G J, Brown L E, Suhrbier A. Delivery of multiple CD8 cytotoxic T cell epitopes by DNA vaccination. J Immunol. 1998;160:1717–1723. [PubMed] [Google Scholar]
- 42.Vitiello A, Sette A, Yuan L, Farness P, Southwood S, Sidney J, Chesnut R W, Grey H M, Livingston B. Comparison of cytotoxic T lymphocyte responses induced by peptide or DNA immunization: implications on immunogenicity and immunodominance. Eur J Immunol. 1997;27:671–678. doi: 10.1002/eji.1830270315. [DOI] [PubMed] [Google Scholar]
- 43.Wang R, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith M, Ng J, Weiss W R, Sedegah M, de Taisne C A, Norman J, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 44.Wentworth P A, Vitiello A, Sidney J, Keogh E, Chesnut R W, Grey H, Sette A. Differences and similarities in the A2.1-restricted cytotoxic T cell repertoire in humans and human leukocyte antigen-transgenic mice. Eur J Immunol. 1996;26:97–101. doi: 10.1002/eji.1830260115. [DOI] [PubMed] [Google Scholar]
- 45.Yewdell, J. W., and J. R. Bennink. Immunodominance in MHC class I-restricted T lymphocyte responses. Annu. Rev. Immunol., in press. [DOI] [PubMed]