Abstract
Anaemia in women of reproductive age (WRA) can be effectively addressed if supported by a better understanding of the spatial variations, magnitude, severity and distribution of anaemia. This study aimed to map the subnational spatial distribution of anaemia (any, moderate and severe forms) among WRA in Ethiopia. We identified and mapped (any, moderate and severe) anaemia hotspots in WRA (n = 14,923) at the subnational level and identified risk factors using multilevel logistic regression. Kulldorff scan statistics were used to identify hotspot regions. Ordinary kringing was used to predict the anaemia prevalence in unmeasured areas. The overall anaemia prevalence increased from 16.6% in 2011 to 23.6% in 2016, a rise that was mostly related to the widening of existing hotspot areas. The primary clusters of (any) anaemia were in Somali and Afar regions. The horn of the Somali region represented a cluster of 330 km where 10% of WRA were severely anaemic. The Oromia–Somali border represented a significant cluster covering 247 km, with 9% severe anaemia. Population‐dense areas with low anaemia prevalence had high absolute number of cases. Women education, taking iron‐folic‐acid tablets during pregnancy and birth‐delivery in health facilities reduced the risk of any anaemia (P < 0.05). The local‐level mapping of anaemia helped identify clusters that require attention but also highlighted the urgent need to study the aetiology of anaemia to improve the effectiveness and safety of interventions. Both relative and absolute anaemia estimates are critical to determine where additional attention is needed.
Keywords: anaemia, effective coverage, inequality, severe anaemia, women
Key messages.
Contrary to the World Health Assembly's target, anaemia increased in Ethiopian women.
Both relative and absolute anaemia estimates are critical to prioritize interventions.
Anaemia distribution shows clustering in a handful of clusters.
1. INTRODUCTION
Anaemia is highly prevalent in low‐and‐middle income countries (LMICs) and disproportionately affects women of reproductive age (WRA) (15–45 years of age) and young children (Chaparro & Suchdev, 2019). Anaemia adversely affects the health and well‐being of WRA, and it compromises cognitive function and reduces productivity (Cook et al., 2017; Scholz et al., 1997). Besides, anaemia is associated with adverse perinatal outcomes and maternal mortality. Consequently, reducing anaemia in WRA by 50% by 2025 was set as one of the six World Health Assembly (WHA) targets and was then endorsed as one of the Sustainable Development Goals (SDGs) target for 2030.
The aetiology of anaemia is complex and includes micronutrient deficiencies, malaria, inadequate water, sanitation and hygiene (WASH), hemoglobinopathies, and chronic diseases (Chaparro & Suchdev, 2019). However, many earlier studies have estimated that about 50% of anaemia in WRA is associated with iron deficiency, and this has led to iron interventions becoming the main and often the only intervention delivered for the prevention and treatment of anaemia (Stevens et al., 2013). Recent studies have contested this assumption and highlighted that the magnitude and cause of anaemia is context dependent, but World Health Organization (WHO) advocates for iron‐folic acid provision for WRA living in settings where the prevalence of anaemia is 20% or higher (Petry et al., 2016; WHO, 2014; Wieringa et al., 2016). This cut‐off of 20% is often used to make decisions at the national level, hence undermining local variations in the magnitude and causes of anaemia.
A subnational estimate of anaemia in WRA is critical to monitor progress, but also target interventions to reach the most vulnerable segments of the population. Anaemia prevention and treatment could be more effective if the spatial variations, magnitude, severity and distribution were characterized. Such local‐level mapping has been generated for WHA targets like stunting, wasting and overweight, but not for anaemia (Kinyoki et al., 2020; Osgood‐Zimmerman et al., 2018). Ethiopia has endorsed the WHA and SDG targets of reducing anaemia in WRA by 50% relative to 2012 baseline figures (FDRE, 2016). However, according to the WHO global targets tracking tool (https://www.who.int/tools/global-targets-tracking-tool), and the recent WHO estimates (WHO, 2021), Ethiopia is off‐track from meeting the anaemia targets. Consequently, effective interventions that prevent and treat anaemia among WRA are urgently needed.
Therefore, the present study aimed to map the spatial distribution of anaemia (any, moderate and severe forms) among WRA in Ethiopia to support the design and implementation of effective anaemia prevention interventions. We, therefore, identified and mapped anaemia hotspots at the subnational level and identified risk factors. Given that Ethiopia has not started a widespread food fortification with nutrients, our results are not confounded by the access and consumption of fortified foods. This study can inform the design and prioritization of interventions aiming to prevent/treat anaemia among WRA and can show within‐country inequalities, and contribute towards achieving nutrition goals of the WHA and the SDGs.
2. METHODS
2.1. Overview and data source
We estimated and mapped the prevalence of any, moderate and severe forms of anaemia in WRA (15–49 years of age), using the latest two rounds of the Ethiopian Demographic and Health Surveys (EDHS 2011–2016) (CSA & ICF, 2011, 2016). Enumeration areas where the haemoglobin concentrations were taken were linked to the geographical coordinates using global positioning system (GPS). The subnational prevalence of any, moderate and severe anaemia was estimated and mapped. Spatial heterogeneity analysis was conducted using Kulldorff scan statistics and hotspot regions were identified (Kulldorff, 1997). The prevalence of any, moderate and severe anaemia was predicted using ordinary kringing for unmeasured areas. Finally, using multilevel logistic regression, factors associated with any and severe forms of anaemia were identified. A mixed‐effect logistic regression model was run, and adjusted odds ratios (AORs) with corresponding 95% confidence intervals (CI) were estimated.
2.2. Outcomes
The outcomes of interest were any, moderate and severe anaemia in WRA (15–49 years of age), which were defined as altitude‐adjusted haemoglobin concentrations of <12.0 g/dl (<11.0 g/dl for pregnant women), 8.0–10.9 g/dl (7.0–10.9 g/dl for pregnant) and <8.0 g/dl (7.0 g/dl for pregnant), respectively (WHO, 2011).
2.3. Statistical analysis
2.3.1. Spatial statistics
Hotspots and coldspots were identified using local G* statistics. Based on 95% CI, hotspots were defined as areas with anaemia prevalence with a z‐score ≥ 2 and cold‐spots as areas with a z‐score ≤ −2 (P < 0.05).
Kulldoruff's scan statistic was used to quantify the spatial distribution of the prevalence of any, moderate, and severe anaemia (Kulldorff, 1997). A purely spatial scan statistic was used to identify areas with higher anaemia cases than would have been predicted if the risk of anaemia was uniformly distributed (Jung et al., 2007). Spatially important higher and lower aggregate concentrations were identified and were represented by circular windows. Finally, confirmatory spatial analysis was run using SATScan and QGIS by applying purely spatial Poisson scan statistics. With the discrete Poisson model, the number of cases in each cluster (enumeration area) was estimated (Jung et al., 2007; Kulldorff & Nagarwalla, 1995; Kutoyants, 2012).
To forecast the prevalence of anaemia from unmeasured areas, spatial interpolation using ordinary kriging was applied using SAGA GIS (Stein, 2012).
2.3.2. Multilevel logistic regression
Multilevel logistic regression was run to assess factors associated with clustering of anaemia prevalence. Variables with P < 0.2 in the univariate analysis were included in the multiple regression. Considering the hierarchical nature of the DHS data, we run a multilevel logistic regression at the individual, household and community levels (Hox et al., 2017). Four models containing social and biologically relevant variables have been constructed. The first model (M0) is a null model without independent variables to measure random variability using the Intra Community Correlation (ICC). The ICC was evaluated to determine whether the difference in cases is mainly within or between households (Diez, 2002). The second model (M1) was adapted to all lower level (individual level) factors; the third model (M2) was used for all higher level factors; and the fourth model (M3) accounted both lower and higher‐level factors. Model goodness‐of‐fit was checked by the Akaike Information Criterion (AIC). Statistical analyses were conducted using Stata v14.
3. RESULTS
Our analyses included a total of 14,923 WRA (15–49 years of age). The anaemia prevalence increased from 16.6% in 2011 to 23.6% in 2016 (Figure 1). The increase (~7%) was proportional for both urban and rural areas. The clusters with high prevalence of any anaemia had increased in number between 2011 and 2016 (Figure 2). Further disaggregating anaemia cases by severity, Figure 3 shows significant clusters of (any) anaemia spread across the eastern part of Ethiopia, with few smaller clusters in the central, southern, and western part of the country. In contrast, clusters of severe anaemia were almost entirely localized in the southern and eastern part of Ethiopia.
Figure 1.
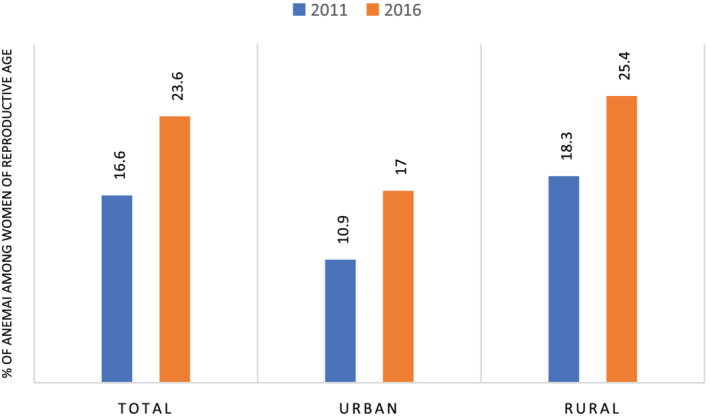
Prevalence of anaemia among women of reproductive age, 2011 and 2016
Figure 2.
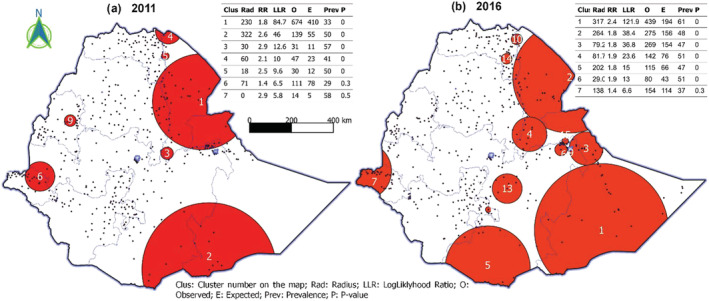
Clusters of any anaemia among women of reproductive age, 2011 and 2016
Figure 3.
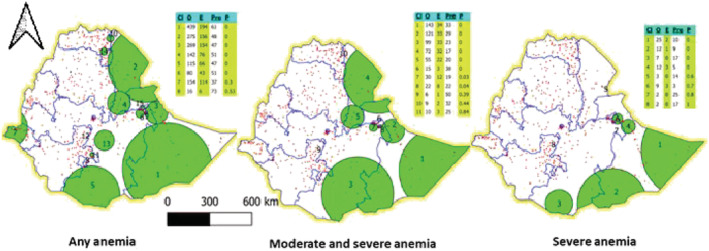
Clusters of anaemia among women of reproductive age by severity, 2016
The primary clusters of (any) anaemia were found in Somali and Afar region. A cluster of 318 km radius was found in the Somali region, where 33% of WRA were anaemic with a relative risk (RR) of 2.4 (P < 0.001; Tables S1 & S2). The second important cluster was 265 km wide and found in the Afar region, where 50% of WRA were anaemic with a RR of 1.8 (P < 0.001). The Somali region was also a primary cluster for severe forms of anaemia. The horn of the Somali region represented a cluster of severe anaemia that spanned 330 km wide and where 10% (RR: 3.5; P < 0.001) of WRA were severely anaemic. The Oromia–Somali region's border also represented a significant cluster covering 247 km, with 9% of WRA with severe anaemia (RR: 5; P < 0.001).
Figure 4a,b presents the predicted anaemia prevalence and case‐load density interpolated from measured areas. Contrasting findings, characterized by higher density of anaemia in low prevalence areas were obtained. Based on prevalence figures, anaemia was a serious public health concern in much of the eastern part of Ethiopia, but the density of anaemia cases was higher in low prevalence areas like the central and western part of Ethiopia. Moderate and severe cases were denser in much of the Great Rift Valley, Harar, part of Oromia, Afar and the Somali region. In contrast, both the density and prevalence figures showed that severe anaemia was a serious problem in large parts of the Somali and Harar regions, and in part of Afar and Oromia region.
Figure 4.
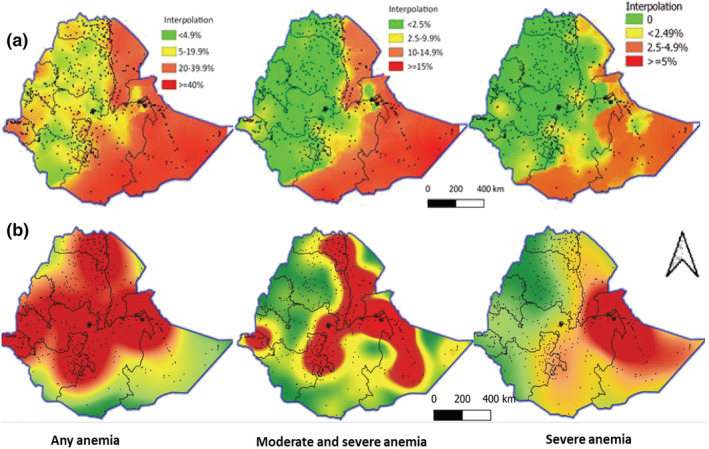
Predicted anaemia prevalence (a) and case‐load density (b) by severity, 2016
Our adjusted multilevel model suggested that there is a strong association between anaemia and parity (birth in the last 5 years). Women who had >2 births in the last 5 years were 2.3 times more likely to have anaemia. Taking IFA tablets during pregnancy reduced the odds of being anaemic. Although being from a female‐headed household increased the odds of anaemia, residing in temperate zones (higher altitude) had lower risk of anaemia after adjusting haemoglobin values for altitude (Tables 1 and 2). Household wealth was inversely associated with anaemia, but WASH was not a significant predictor of anaemia. Regression analyses done separately for areas with anaemia prevalence above and below 20% showed similarities in factors associated with anaemia, except for taking IFA supplementation and low BMI found only associated in higher (>20%) and lower (<20%) prevalence areas, respectively (Table S3).
Table 1.
Multilevel regression analyses identifying individual factors associated with anaemia, 2016 (N = 14,923)
| Lower (individual) level characteristics | Sample (n) | Anaemia prevalence (%) | Any anaemia | |
|---|---|---|---|---|
| COR | AOR | |||
| Birth interval (ref: ≥24 months) | 6801 | 25.7 | 1 | 1 |
| <24 | 1621 | 32 | 1.26 (1.11, 1.44) | 0.96 (0.8, 1.15) |
| Currently pregnant (ref. no) | 13,835 | 23.2 | 1 | 1 |
| Yes | 1088 | 29.1 | 1.24 (1.07, 1.44) | 1.1 (0.9, 1.38) |
| Birth in last 5 years (ref. only 1) | 12,070 | 21.2 | 1 | 1 |
| Two | 2429 | 32.6 | 1.58 (1.42, 1.76) | 1.44 (1.23, 1.68)** |
| Above two | 424 | 43.1 | 2.43 (1.95, 3.03) | 2.32 (1.76, 3.05)** |
| Sex of household head (ref. male) | 11,404 | 24.1 | 1 | 1 |
| Female | 3518 | 22.1 | 0.9 (0.82, 1) | 1.26 (1.04, 1.53)** |
| Age (ref. 40–49) | 2213 | 24.3 | 1 | 1 |
| 30–39 | 4078 | 25.5 | 1.06 (0.93, 1.2) | 1.05 (0.85, 1.3) |
| 20–29 | 5467 | 24.2 | 0.96 (0.8, 1.1) | 0.97 (0.78, 1.21) |
| 15–19 | 3165 | 19.9 | 0.75 (0.65, 0.9) | 0.55 (0.23, 1.29) |
| Education (ref. secondary and above) | 2464 | 15.8 | 1 | 1 |
| Primary | 5244 | 21.8 | 1.32 (1.14, 1.52) | 0.74 (0.5, 1.08) |
| No education | 7215 | 27.8 | 1.82 (1.58, 2.1) | 0.93 (0.63, 1.37) |
| Currently breastfeeding (ref. no) | 10,266 | 21.5 | 1 | 1 |
| Yes | 4657 | 28.3 | 1.33 (1.2, 1.4) | 1.08 (0.93, 1.27) |
| BMI (ref. normal) | 10,609 | 23.2 | 1 | |
| Under weight | 3154 | 26 | 1.1 (0.99, 1.2) | NI |
| Over weight | 1133 | 21.3 | 0.99 (0.8, 1.2) | NI |
| Place of delivery (ref. health facility) | 2326 | 22.5 | 1 | 1 |
| Home | 4890 | 30.4 | 1.27 (1.1, 1.47) | 0.99 (0.82, 1.19) |
| IFA supplementation during pregnancy (ref. no) | 4206 | 31.1 | 1 | 1 |
| Yes | 3108 | 23.6 | 0.79 (0.7, 0.9) | 0.82 (0.7, 0.95)** |
| ANC (ref. >4) | 2331 | 24.8 | 1 | NS |
| <4 | 4983 | 29.4 | 1.1 (0.96, 1.3) | NS |
| Source of drinking water (ref. improved) | 9635 | 21.6 | 1 | 1 |
| Unimproved | 4936 | 28.1 | 1.26 (1.13, 1.4) | 1 (0.85, 1.18) |
| Toilet facility (ref. improved) | 2241 | 20.4 | 1 | 1 |
| Unimproved | 7914 | 22 | 1.05 (0.9, 1.2) | 0.92 (0.7, 1.2) |
| Open defecation | 4414 | 28.7 | 1.3 (1.1, 1.5) | 0.9 (0.67, 1.22) |
| Type of cooking fuel (ref. clean fuel) | 888 | 15.9 | 1 | 1 |
| Polluting fuel | 13,670 | 24.3 | 1.46 (1.17, 1.8) | 1.1 (0.66, 1.83) |
Abbreviations: ANC, ante‐natal care; AOR, adjusted odds ratio; BMI, body mass index; COR, crude odds ratio; NI, not included because P > 0.2 in the unadjusted model; total sample size was 14,923.
Table 2.
Multilevel regression analyses identifying community factors associated with anaemia, 2016 (N = 14,923)
| Community level characteristics | Sample (n) | Anaemia prevalence (%) | Any anaemia | |
|---|---|---|---|---|
| COR | AOR | |||
| Wealth quintile (ref. richest) | 2519 | 17.4 | 1 | 1 |
| Poorest | 2519 | 34.3 | 2.1 (1.7, 2.5) | 1.75 (1.24, 2.48)** |
| Second | 2717 | 25.3 | 1.6 (1.4, 1.9) | 1.38 (0.99, 1.91) |
| Middle | 2891 | 23.7 | 1.6 (1.3, 1.9) | 1.4 (1.03, 1.92)** |
| Fourth | 2979 | 21.0 | 1.3 (1.1, 1.5) | 1.17 (0.85, 1.6) |
| Residence (ref. urban) | 3169 | 17.0 | 1 | 1 |
| Rural | 11,754 | 25.4 | 1.73 (1.4, 2.1) | 1.5 (0.97, 2.32) |
| Ecology (ref. highland/>2300) | 4177 | 23.2 | 1 | 1 |
| Temperate (1501–2300 masl) | 8691 | 21.5 | 0.91 (0.7, 1.1) | 0.6 (0.46, 0.8)** |
| Lowland (501–1500 masl) | 1886 | 31.6 | 1.9 (1.5, 2.5) | 0.7 (0.47, 1.05) |
| Subtropical (<501 masl) | 168 | 58.2 | 5.2 (3.3, 8.1) | 1.66 (0.73, 3.74) |
| Distance to health facility (ref. not a big problem) | 7367 | 21.7 | 1 | NI |
| Big problem | 7556 | 25.5 | 1.08 (0.98, 1.2) | NI |
| Region (ref. Addis Ababa) | 825 | 16.0 | 1 | 1 |
| Tigray | 1073 | 19.7 | 1.35 (0.95, 1.92) | 1.54 (0.68, 3.53) |
| Afar | 119 | 44.7 | 4.73 (2.84, 7.86) | 2.65 (0.92, 2.68) |
| Amhara | 3645 | 17.2 | 1.07 (0.78, 1.48) | 1.08 (0.49, 2.4) |
| Oromia | 5422 | 27.3 | 2.02 (1.48, 2.77) | 1.59 (0.72, 3.54) |
| Somalia | 417 | 59.5 | 9 (6.14, 13.16) | 5.39 (2.19, 13.2)** |
| Benishangul‐Gumuz | 146 | 19.2 | 1.28 (0.75, 2.19) | 1.28 (0.45, 3.62) |
| SNNPR | 3124 | 22.5 | 1.44 (1.04, 1.98) | 1.24 (0.23, 5.4) |
| Gambela | 42 | 26.1 | 1.93 (0.87, 4.27) | 1.13 (0.23, 5.34) |
| Harari | 32 | 27.7 | 2.09 (0.87, 5) | 3.25 (0.68, 15.4) |
| Dire Dawa | 77 | 30.1 | 2.4 (1.3, 4.4) | 3.85 (1.11, 13.39)** |
Abbreviations: AOR, adjusted odds ratio; COR, crude odds ratio; NI, not included because P > 0.2 in the unadjusted model; the sample size for the analyses was 14,923.
4. DISCUSSION
In contrast to the WHA's ambition of reducing anaemia by 50% (relative to 2012) by 2025, the anaemia prevalence in WRA in Ethiopia has increased between 2011 and 2016. The prevalence of any, moderate and severe forms of anaemia was not equally distributed throughout the country, but instead showed clustering in a handful of hotspot areas. The rise in anaemia prevalence between 2011 and 2016 was for the most part related to the widening of existing hotspot areas, rather than the appearance of new hotspots. Noteworthy is the relatively low prevalence, but high absolute number of anaemia cases among WRA in densely populated areas.
The national prevalence of anaemia suggests that anaemia in WRA was a mild public health concern (<20%) in 2011, but increased to become a moderate public health concern in 2016 (WHO, 2011). However, such national level estimates mask within‐country differences, overestimating the spread and level of public health concern of anaemia in much of the west and central part of Ethiopia, while underestimating hotspot areas like Somali, Afar, Harar and part of Oromia. Our study has also showed that areas with low anaemia prevalence can still have higher anaemia density (caseloads), particularly in densely populated areas. Consequently, if decisions are not supported by subnational relative and absolute anaemia prevalence estimates, applying the WHO recommended cut‐off of 20% as a benchmark to prioritize interventions may not lead to efficient and effective use of available resources (WHO, 2014). Instead, our results highlight the value of subnational anaemia estimates and variation in programme design and policy decision‐making.
Our subnational mapping also triggered a number of questions related to which interventions are needed and how best should they be targeted for maximal impact? In light of limited resources, should interventions focus on increasing coverage to reach the maximum number of anaemia cases, or focus on reaching hotspots with high prevalence, yet with lower density of anaemia cases? This poses a coverage‐equity paradox, where increasing coverage may be more cost‐effective if prioritized to be implemented in more densely populated areas (e.g., west and central Ethiopia) than in more remote, less‐populated, areas (e.g., Harari and Somali). From an equity perspective, and considering the high prevalence of anaemia, more remote and less‐populated areas will still need to be prioritized. This coverage‐equity dilemma is however of a lesser concern if interventions are prioritized to focus only on the treatment of moderate and severe forms of anaemia, as both absolute and relative estimates converged, but also mild anaemia is associated with less serious health consequences than severe anaemia.
For prevention and treatment interventions to be effective and equitable, determining the aetiology of anaemia is critical. However, in many LMICs including Ethiopia, little is known about the aetiology of anaemia (Baye, 2019). Nutritional deficiencies including those of iron, folate, vitamin A and B12 may contribute, to a varying extent, to the anaemia prevalence in Ethiopia (EPHI, 2016). For example, both Afar and Somali regions have high prevalence of anaemia, but according to the Ethiopian National Micronutrient Survey (ENMS), iron deficiency (assessed by inflammation‐adjusted serum ferritin and serum transferrin concentrations) was relatively high in Somali (25%), but not in Afar (<10%) (EPHI, 2016). Besides, deficiencies of folate and vitamin B12 were found prevalent to a varying extent (EPHI, 2016). Such geographical differences in the contributors of anaemia remind us of the need for routine assessments of micronutrient status prior to implementing micronutrient interventions.
On the other hand, inflammation can lead to anaemia and may also coexist with iron deficiency in low income settings, where nutritional deficiencies and infections are common (Shaw & Friedman, 2011). From an evolutionary perspective, hypoferremia and increased production and activation of leukocytes serve as a host‐defense mechanism that is activated when facing infection or inflammatory events (Ganz, 2019). The resulting reduction in plasma iron concentration and transferrin saturation prevents the generation of non‐transferrin bound iron, a form of iron known to stimulate pathogenic microorganisms (Seyoum et al., 2021; Stefanova et al., 2017). Thus, indiscriminate iron supplementation to treat anaemia triggered by inflammation can override this host‐defense mechanism and lead to increases in the concentration of non‐transferrin bound iron, which has been linked to increased morbidity and mortality (Baye, 2019; Hurrell, 2010). Consequently, in settings where infections are widespread, addressing the primary cause of anaemia through infection treatment may be more effective than iron interventions (Ganz, 2019). This is of particular importance given the relatively high levels of inflammation in Dire Dawa and Somali, also identified as anaemia hotspots in this study (Challa et al., 2021). More importantly, given the protection conferred by mild and moderate iron deficiency, interventions may need to prioritize the treatment of moderate and severe forms over any anaemia (Wander et al., 2009).
Chronic exposure to inflammation/infections as seen in malaria‐endemic settings have been directly linked with anaemia, but have also been linked to more long‐term consequences like genetic polymorphisms (i.e., hemoglobinopathies) that can also cause anaemia (Goheen et al., 2017). Unfortunately, hemoglobinopathies have been rarely assessed, but are expected to be high in Africa (Modell & Darlison, 2008). The identified hotspot areas of anaemia are also malaria‐endemic areas, suggesting that the potential contribution of haemoglobin genetic disorders to the anaemia prevalence cannot be overruled, but evidence on this remains in‐existent. As iron‐based interventions remain the main anaemia prevention and treatment strategy, their effectiveness and possible adverse effects in the presence of hemoglobinopathies need to be evaluated (Coates et al., 2016; Wieringa et al., 2016).
On the other hand, underlying factors like birth interval, pregnancy and female‐headed households were associated with higher risk of anaemia. This is not surprising given the higher nutrient demands during pregnancy, which can be further exacerbated by multiple pregnancies, economic vulnerabilities, food insecurity and poverty (Chaparro & Suchdev, 2019; Wirth et al., 2017). Not surprisingly, taking IFA tablets during pregnancy reduced the risk of any anaemia (Oh et al., 2020), a finding that can also be partly a reflection of higher women (health) literacy and access to health care (Sendeku et al., 2020).
The present study has a number of limitations that need to be considered. First, although our study is based on nationally and regionally representative data from the last two rounds of DHS, the study remains cross‐sectional and thus does not allow causal inferences to be made. Although diet quality, nutrient intake and infections like malaria can be key determinants, they are not captured in the DHS and thus were not reflected in our multilevel regression models.
Notwithstanding the above limitations, this study provides a unique spatio‐temporal analysis of anaemia among WRA. To our knowledge, this is the first study, to provide subnational, local‐level, maps by anaemia prevalence by severity and density of caseloads. Such granular analyses can inform anaemia prevention and treatment interventions to be more precise, effective and locally adapted. Our study has identified a number of clusters of anaemia that require attention, but also highlighted that low prevalence areas can still have high absolute number of cases, particularly in populated areas, suggesting that both relative and absolute estimates are critical to determine where additional attention is needed. Our estimates of any, moderate and severe anaemia allow policymakers to prioritize interventions against resources available, but also calls for an urgent assessment of the aetiology of anaemia to increase effectiveness, safety and equity of interventions.
CONFLICT OF INTEREST
None.
CONTRIBUTIONS
KB, SC and AL conceived the study with inputs from BA and JB; BA and KB prepared and analysed the data; KB and BA wrote the paper with inputs from AL, SC and JB. All authors read and approved the final manuscript.
Supporting information
Figure S1: Cold and hot‐spots of any, moderate and severe anemia
Table S1 Clusters of any anemia among women of reproductive age, 2011 and 2016
Table S2 Clusters of anemia among women of reproductive age by severity, 2016
Table S3 Multi‐level regression analyses identifying individual factors associated with anemia in high (> 20%) and low (< 20%) prevalence areas, 2016
ACKNOWLEDGMENTS
This study was funded by UNICEF Ethiopia.
Hailu, B. A. , Laillou, A. , Chitekwe, S. , Beyene, J. , & Baye, K. (2024). Subnational mapping for targeting anaemia prevention in women of reproductive age in Ethiopia: A coverage‐equity paradox. Maternal & Child Nutrition, 20(S5), e13277. 10.1111/mcn.13277
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the https://dhsprogram.com/data/.
REFERENCES
- Baye, K. (2019). Maximising benefits and minimising adverse effects of micronutrient interventions in low‐and middle‐income countries. The Proceedings of the Nutrition Society, 78, 1–7. 10.1017/S0029665119000557 [DOI] [PubMed] [Google Scholar]
- Challa, F. , Gelibo, T. , Getahun, T. , Sileshi, M. , Geto, Z. , Bekele, A. , Getachew, T. , Defar, A. , Teklie, H. , Nagasa, B. , Girma, F. , Seifu, D. , Tebeje, S. , Teferra, S. , Wolde, M. , Carobene, A. , & Abate, E. (2021). Distribution and determinants of serum high‐sensitivity C‐reactive protein in Ethiopian population. Clinica Chimica Acta, 517, 99–107. 10.1016/j.cca.2021.02.013 [DOI] [PubMed] [Google Scholar]
- Chaparro, C. M. , & Suchdev, P. S. (2019). Anemia epidemiology, pathophysiology, and etiology in low‐and middle‐income countries. Annals of the New York Academy of Sciences, 1450(1), 15–31. 10.1111/nyas.14092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates, T. D. , Carson, S. , Wood, J. C. , & Berdoukas, V. (2016). Management of iron overload in hemoglobinopathies: What is the appropriate target iron level? Annals of the New York Academy of Sciences, 1368(1), 95–106. 10.1111/nyas.13060 [DOI] [PubMed] [Google Scholar]
- Cook, R. L. , O'Dwyer, N. J. , Parker, H. M. , Donges, C. E. , Cheng, H. L. , Steinbeck, K. S. , Cox, E. P. , Franklin, J. L. , Garg, M. L. , Rooney, K. B. , & O'Connor, H. T. (2017). Iron deficiency anemia, not iron deficiency, is associated with reduced attention in healthy young women. Nutrients, 9(11), 1216. 10.3390/nu9111216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSA & ICF . (2011). Ethiopian demographic and health survey. Addis Ababa, Ethiopia and Rockville, Maryland, USA: CSA and ICF. [Google Scholar]
- CSA & ICF . (2016). Ethiopian demographic and health survey. Addis Ababa, Ethiopia and Rockville, Maryland, USA: CSA and ICF. [Google Scholar]
- Diez, R. (2002). A glossary for multilevel analysis. Journal of Epidemiology and Community Health, 56(8), 588–594. 10.1136/jech.56.8.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHI . (2016). Ethiopian national micronutrient survey. Ethiopian Public Health Institute. [Google Scholar]
- FDRE . (2016). National Nutrition Programme (NNP II) 2016–2020. Federal Democratic Republic of Ethiopia. [Google Scholar]
- Ganz, T. (2019). Anemia of inflammation. New England Journal of Medicine, 381(12), 1148–1157. 10.1056/NEJMra1804281 [DOI] [PubMed] [Google Scholar]
- Goheen, M. M. , Campino, S. , & Cerami, C. (2017). The role of the red blood cell in host defence against falciparum malaria: An expanding repertoire of evolutionary alterations. British Journal of Haematology, 179(4), 543–556. 10.1111/bjh.14886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hox, J. J. , Moerbeek, M. , & Van de Schoot, R. (2017). Multilevel analysis: Techniques and applications. Routledge. 10.4324/9781315650982 [DOI] [Google Scholar]
- Hurrell, R. (2010). Iron and malaria: Absorption, efficacy and safety. International Journal for Vitamin and Nutrition Research, 80(4), 279–292. 10.1024/0300-9831/a000035 [DOI] [PubMed] [Google Scholar]
- Jung, I. , Kulldorff, M. , & Klassen, A. C. (2007). A spatial scan statistic for ordinal data. Statistics in Medicine, 26(7), 1594–1607. 10.1002/sim.2607 [DOI] [PubMed] [Google Scholar]
- Kinyoki, D. K. , Ross, J. M. , Lazzar‐Atwood, A. , Yadollahpour, A. , & LBD Double Burden of Malnutrition Collaborators . (2020). Mapping local patterns of childhood overweight and wasting in low‐and middle‐income countries between 2000 and 2017. Nature Medicine, 26(5), 750–759. 10.1038/s41591-020-0807-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulldorff, M. (1997). A spatial scan statistic. Communications in Statistics‐Theory and Methods, 26(6), 1481–1496. 10.1080/03610929708831995 [DOI] [Google Scholar]
- Kulldorff, M. , & Nagarwalla, N. (1995). Spatial disease clusters: Detection and inference. Statistics in Medicine, 14(8), 799–810. 10.1002/sim.4780140809 [DOI] [PubMed] [Google Scholar]
- Kutoyants, Y. A. (2012). Statistical inference for spatial Poisson processes (Vol. 134). [Google Scholar]
- Modell, B. , & Darlison, M. (2008). Global epidemiology of haemoglobin disorders and derived service indicators. Bulletin of the World Health Organization, 86(6) PubMed (18568278), 480–487. 10.2471/blt.06.036673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, C. , Keats, E. C. , & Bhutta, Z. A. (2020). Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low‐and middle‐income countries: A systematic review and meta‐analysis. Nutrients, 12(2), 491. 10.3390/nu12020491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood‐Zimmerman, A. , Millear, A. I. , Stubbs, R. W. , Shields, C. , Pickering, B. V. , Earl, L. , Graetz, N. , Kinyoki, D. K. , Ray, S. E. , Bhatt, S. , Browne, A. J. , Burstein, R. , Cameron, E. , Casey, D. C. , Deshpande, A. , Fullman, N. , Gething, P. W. , Gibson, H. S. , Henry, N. J. , … Hay, S. I. (2018). Mapping child growth failure in Africa between 2000 and 2015. Nature, 555(7694), 41–47. 10.1038/nature25760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry, N. , Olofin, I. , Hurrell, R. F. , Boy, E. , Wirth, J. P. , Moursi, M. , Donahue Angel, M. , & Rohner, F. (2016). The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: A systematic analysis of national surveys. Nutrients, 8(11), 693. 10.3390/nu8110693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz, B. D. , Gross, R. , Schultink, W. , & Sastroamidjojo, S. (1997). Anaemia is associated with reduced productivity of women workers even in less‐physically‐strenuous tasks. British Journal of Nutrition, 77(1), 47–57. 10.1017/S0007114500002877 [DOI] [PubMed] [Google Scholar]
- Sendeku, F. W. , Azeze, G. G. , & Fenta, S. L. (2020). Adherence to iron‐folic acid supplementation among pregnant women in Ethiopia: A systematic review and meta‐analysis. BMC Pregnancy and Childbirth, 20(1), 1–9. 10.1186/s12884-020-2835-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyoum, Y. , Baye, K. , & Humblot, C. (2021). Iron homeostasis in host and gut bacteria—A complex interrelationship. Gut Microbes, 13(1), 1–19. 10.1080/19490976.2021.1874855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, J. G. , & Friedman, J. F. (2011). Iron deficiency anemia: Focus on infectious diseases in lesser developed countries. Anemia. 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova, D. , Raychev, A. , Arezes, J. , Ruchala, P. , Gabayan, V. , Skurnik, M. , Dillon, B. J. , Horwitz, M. A. , Ganz, T. , Bulut, Y. , & Nemeth, E. , (2017). Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non–transferrin‐bound iron. Blood, 130(3), 245–257. 10.1182/blood-2017-03-772715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, M. L. (2012). Interpolation of spatial data: Some theory for kriging. Springer Science & Business Media. [Google Scholar]
- Stevens, G. A. , Finucane, M. M. , De‐Regil, L. M. , Paciorek, C. J. , Flaxman, S. R. , Branca, F. , Peña‐Rosas, J. P. , Bhutta, Z. A. , Ezzati, M. , Nutrition Impact Model Study Group . (2013). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non‐pregnant women for 1995–2011: A systematic analysis of population‐representative data. The Lancet Global Health, 1(1), e16–e25. 10.1016/S2214-109X(13)70001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander, K. , Shell‐Duncan, B. , & McDade, T. W. (2009). Evaluation of iron deficiency as a nutritional adaptation to infectious disease: An evolutionary medicine perspective. American Journal of Human Biology, 21(2), 172–179. 10.1002/ajhb.20839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2014). WHA global nutrition targets 2025: Anaemia policy brief. World Health Organization. [Google Scholar]
- WHO . (2021). WHO global anaemia estimates (2021st ed.). World Health Organization. [Google Scholar]
- Wieringa, F. T. , Dahl, M. , Chamnan, C. , Poirot, E. , Kuong, K. , Sophonneary, P. , Sinuon, M. , Greuffeille, V. , Hong, R. , Berger, J. , Dijkhuizen, M. A. , & Laillou, A. (2016). The high prevalence of anemia in Cambodian children and women cannot be satisfactorily explained by nutritional deficiencies or hemoglobin disorders. Nutrients, 8(6), 348. 10.3390/nu8060348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth, J. P. , Woodruff, B. A. , Engle‐Stone, R. , Namaste, S. M. , Temple, V. J. , Petry, N. , Macdonald, B. , Suchdev, P. S. , Rohner, F. , & Aaron, G. J. (2017). Predictors of anemia in women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. The American Journal of Clinical Nutrition, 106(suppl_1), 416S–427S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2011). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Cold and hot‐spots of any, moderate and severe anemia
Table S1 Clusters of any anemia among women of reproductive age, 2011 and 2016
Table S2 Clusters of anemia among women of reproductive age by severity, 2016
Table S3 Multi‐level regression analyses identifying individual factors associated with anemia in high (> 20%) and low (< 20%) prevalence areas, 2016
Data Availability Statement
The data that support the findings of this study are available on request from the https://dhsprogram.com/data/.


